Abstract
Purpose
Given the state of the opioid crisis and national pressure to minimize prescriptions, pain management after common hand procedures can pose a challenge for patients and providers. Despite the volume of recent literature on prescribing protocols and over-the-counter (OTC) medications, patient satisfaction has not been adequately assessed. The purposes of this study were (1) to investigate patient satisfaction with pain management using an opioid prescribing protocol after common hand procedures, and (2) to evaluate medication use in the postoperative period using this opioid prescribing protocol.
Methods
A prospective survey was administered to 100 consecutive patients undergoing common soft tissue hand procedures at a Level I academic institution over a 5-month period. The medical record was reviewed for demographics and the number or dosage of opioid pills prescribed. The survey was conducted at 2 time points within 2 weeks after the procedure and assessed the number of opioid pills taken, use of OTC medications, visual analog scale (VAS) pain score, and satisfaction with pain management and surgery.
Results
Mean number of opioid pills consumed at 2 weeks after the procedure was 1.5; 19 patients consumed all of their prescribed opioid pills. Acetaminophen was the most commonly used OTC medication and 84 patients reported using OTC medication in the postoperative period. The average VAS score at the end of the study period was 1.7. Nearly all patients were satisfied with the pain management and surgery; no patients received a second opioid prescription.
Conclusions
We found that patients consumed far fewer opioid pills than were prescribed to them. We also found that patients who took more opioid pills had higher VAS pain scores, with lower satisfaction in both categories. The cohort demonstrated effective control of pain with high satisfaction, indicating that an opioid protocol is a successful and patient-accepted tool for managing postoperative pain.
Type of study/level of evidence
Therapeutic IV.
Key words: Hand surgery, Opioids, Oxycodone, Pain management, Pain protocol
The current opioid crisis has led to shifts in postsurgical pain management across orthopedic specialties, with the goal of decreasing the quantity of opioids available for overuse, misuse, and diversion. Several studies have collectively reported that over 50% of opioid analgesics prescribed after common hand procedures go unconsumed1,2; one study noted that patients were given 5 times the appropriate amount of opioids after carpal tunnel release (CTR) surgery.1,2 Consequences of excessive prescribing have been cited as a driving force behind the opioid epidemic.3,4 Balancing concerns about overprescribing of opioids with the need to achieve adequate postoperative analgesia can be challenging for both the patient and the provider.
Variable prescribing protocols have emerged among providers3 to attempt to provide solutions to postoperative pain management. Implementing preoperative opioid counseling or educational sheets for patients has been effective in reducing the number of pills prescribed.4, 5, 6, 7 Opioid prescriptions, along with over-the-counter (OTC) analgesics, remain the standard of care for postoperative pain management in hand surgery.4,8, 9, 10 Recent evidence suggests that opioids result in worse pain control and overall poor satisfaction compared with OTC medications.10, 11, 12 For this reason, current studies have advocated for opioid-free protocols be implemented after surgery.8, 9, 10 In addition, the success of nonsteroidal anti-inflammatory drugs in managing postoperative pain has been demonstrated for CTR and distal radius fracture fixation.9,12
Despite data on the success of decreasing opioid use with postoperative opioid protocols, few data exist on patient satisfaction with these interventions. With the increase in on-line availability and use of physician rankings,13 efforts to increase patient satisfaction have become more notable and physicians may be concerned about the effect of pain management protocols on patients’ satisfaction. This concern may contribute to an overprescription of opioids.7,8,11,14
We sought to expand on current research by prospectively investigating patient satisfaction as well as medication use in the postoperative period using an opioid protocol for patients undergoing a variety of common soft tissue hand procedures. We hypothesized that patients would be satisfied with the pain management and care.
Materials and Methods
An institutional review board–approved survey was prospectively administered to 100 consecutive patients who underwent a predetermined selection of soft tissue hand procedures performed by a single orthopedic hand surgeon at a Level I academic institution over 5 months from September 2018 to January 2019. The selection of included procedures was chosen from previous work completed at our institution, which divided the most common upper-limb procedures into 5 tiers based on a consensus perception of how painful those procedures are and how many pills of 5-mg oxycodone would be prescribed after the procedures (Fig. 1).3 For this study, only patients having procedures in the Tier 1 class were evaluated, as those were the patients for whom the lowest level of postoperative opioid prescription was indicated.3 These tier 1 procedures included carpal tunnel release, trigger finger release, de Quervain release, and ganglion or mucous cyst excision. According to protocol, patients were each given a postoperative prescription for 5 tablets of oxycodone, or 7.5 mg morphine equivalents (MME), to be taken as needed for pain not controlled with OTC medication, which is our divisional counseling for opioid practice. Patients also received instructions for postoperative care (OTC medication use, ice packs, and elevation of the operative limb), as well as proper opioid pill disposal after recovery. Exclusion criteria included age less than 18 years, history of substance abuse, current prescription for opioids within 30 days of surgery, non–tier 1 procedures, procedures in which the index surgery during the study period was a revision surgery, and bilateral procedures.
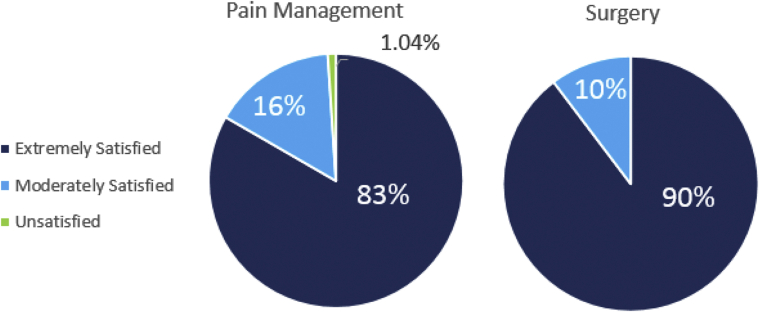
Figure 1.
Patient satisfaction with surgery and pain management 2 to 4 days after the procedure.
We evaluated the medical record for age, sex, type of procedure, and number and dosage of oxycodone pills specified on the immediate postoperative prescription, as well as any additional pain prescriptions. The survey was conducted by an independent provider not involved in the surgery or postoperative oxycodone prescription. The survey was administered at 2 postoperative time frames: 2 to 4 days (via a phone call) and 10 to 14 days (in person at the first postoperative visit), and assessed the number of prescribed oxycodone pills taken by having patients confirm the number of pills remaining from the prescription, the use and type of OTC pain medications if taken, visual analog scale (VAS) pain score, satisfaction with pain management, and overall satisfaction with the procedure. Satisfaction was classified as extremely satisfied, moderately satisfied, mildly satisfied, or unsatisfied. Descriptive statistics were used for analysis.
Continuous data are shown as mean (SD), and categorical data as counts (%). Descriptive comparisons in patient satisfaction are made between pain management and surgery. Next, we analyzed the number of opioids at 2 days with VAS pain score and patient satisfaction at both 2 to 4 days and 10 to 14 days. For this, we created 3 groups: 0, 1–3, and 4–5 pills taken; these values are also reported as MME. The current sample size has 80% power to detect a mean VAS distribution across 3 groups of 1.0, 2.0, and 2.5 with an SD of 2. We used Kruskal-Wallis test to test differences in pain scores owing to the non-normal distribution of the VAS pain score, and chi-square tests for patient satisfaction on the Likert scale.
Results
We enrolled 100 consecutive eligible patients who underwent tier 1 procedures. Four were receiving chronic baseline opioid pain medication at the time of surgery and were excluded. After exclusions, average patient age was 64 years (range, 27–88 years), with 56 female patients (58%) and 40 male patients (42%). Table 1 lists the procedure type breakdown.
Table 1.
Number and Type of Procedures Performed
| Procedure Type | n (%) |
|---|---|
| Carpal tunnel release | 41 (43%) |
| Trigger finger release | 26 (27%) |
| de Quervain release | 5 (5%) |
| Ganglion cyst excision | 4 (4%) |
| Mucous cyst excision | 11 (11%) |
| Combined procedure | 9 (9%) |
At 2 to 4 days after the procedure, the average number of oxycodone pills taken was one (7.5 MME; 1.83 SD); 13 patients reported taking 5 pills (37.5 MME) and 58 reported taking none. Seventy-five patients (78%) reported taking other OTC medications. The remaining 21 patients reported not taking any OTC pain medication (Table 2). Average VAS score was 2.3 (2.3 SD), and 19 patients reported a score greater than 5. Interestingly, of those 19 patients, only 5 had taken all of their prescribed oxycodone pills. Most patients reported that they were extremely satisfied with the pain management (83%) and surgery (90%) (Fig. 2). One patient reported dissatisfaction with the pain management; this patient reported having taken all 5 prescribed oxycodone pills, as well as acetaminophen and ibuprofen.
Table 2.
Pill Consumption, VAS Score, and OTC Medication Use at Study Time Points
| Variable | 2–4 d | 10–14 d |
|---|---|---|
| Average number of oxycodone pills consumed | 1; 7.5 MME (1.83 SD) | 1.5; 11.75 MME (2.02 SD) |
| Average VAS pain score | 2.3 (2.3 SD) | 1.7 (2.2 SD) |
| Number (%) of patients using | 75 | 84 |
| Acetaminophen | 34 (45) | 30 (36) |
| Ibuprofen | 26 (35) | 22 (26) |
| Acetaminophen and ibuprofen | 11 (15) | 20 (24) |
| Other OTC medication (naproxen, aspirin, etc) | 4 (5) | 12 (14) |
| No OTC medication | 21 | 12 |
| Number (%) of patients who consumed oxycodone pills | 41 | 44 |
| 5 pills (37.5 MME) | 12 (31) | 18 (42) |
| 4 pills (30 MME) | 6 (14) | 5 (11) |
| 3 pills (22.5 MME) | 2 (5) | 6 (13) |
| 2 pills (15 MME) | 10 (24) | 7 (16) |
| 1 pill (7.5 MME) | 11 (26) | 8 (18) |
| 0 pills | 55 | 52 |
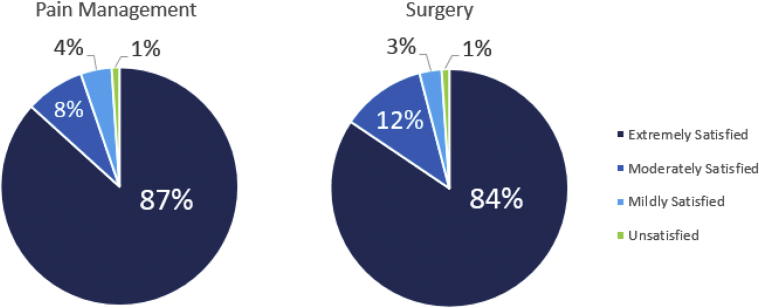
Figure 2.
Patient satisfaction with surgery and pain management 10 to 14 days after the procedure.
At 10 to 14 days after the procedure, the average number of oxycodone pills taken was 1.5 (11.25 MME; 2.02 SD); 6 additional patients reported taking all 5 pills (37.5 MME) and 53 continued to report taking none. Eighty-four patients (87%) reported taking other OTC medications. The remaining 12 patients reported not taking any OTC pain medication (Table 2). Average VAS score was 1.7 (2.2 SD), and 15 patients reported a score greater than 5. The vast majority of patients reported that they were extremely satisfied with the pain management (87%) as well as the surgery (84%) (Fig. 2). One patient reported dissatisfaction with the pain management and another reported dissatisfaction with the surgery.
On average, patients took an additional half of an oxycodone pill (3.75 MME) between the first and second postoperative surveys. Ate 2 to 4 days after the procedure, 88 patients (92%) did not take any additional oxycodone pills. Three patients requested but were declined an additional script for more oxycodone pills after surgery; of those 3, only 1 reported dissatisfaction with the pain management. No patients received a second prescription for oxycodone from the surgical provider or within the electronically available medical record.
When patients were grouped based on the number of oxycodone pills taken, the group that took no oxycodone pills had a statistically significant lower average VAS score at both time points compared with the group that took 4 to 5 pills (P < .05 and P < .05, respectively) (Table 3). Patient satisfaction with pain management was also significantly higher in the group that took no oxycodone than in the group that took 4 to 5 pills (P < .05 and P < .05, respectively). At the first postoperative visit at 10 to 14 days, patient satisfaction with the surgery was significantly associated with the number of oxycodone pills taken; of patients who took no oxycodone pills, 93% were extremely satisfied compared with 68% of patients who took 4 to 5 pills) (P < .05) (Table 3).
Table 3.
Statistical Analysis of Opioid Pills per MME Consumed, VAS Score, and Patient Satisfaction∗
| Variable | Number of Opioid Pills Taken |
P Value | ||
|---|---|---|---|---|
| 0 (n = 58) | 1–3 (7.5–22.5 MME) (n = 23) | 4–5 (30–37.5 MME) (n = 19) | ||
| 2–4 d | ||||
| VAS pain score (median [interquartile range]) | 0.8 (0.0–3.0) | 2.5 (1.0–4.0) | 3.5 (2.0–5.5) | < .001 K |
| Satisfaction with surgery, n (%) | < .001 | |||
| Unsatisfied | 55 (95) | 0 | 1 (5) | |
| Moderately satisfied | 3 (5) | 3 (13) | 9 (47) | |
| Extremely satisfied | 0 | 20 (87) | 9 (47) | |
| Satisfaction with pain management, n (%) | .197 | |||
| Moderately satisfied | 4 (7) | 2 (9) | 4 (21) | |
| Extremely satisfied | 54 (93) | 21 (91) | 15 (79) | |
| 10–14 d | ||||
| VAS pain score (median [interquartile range]) | 0.0 (0.0–2.0) | 1.0 (0.0–3.0) | 2.0 (1.0–5.0) | .003 K |
| Satisfaction with pain management, n (%) | < .001 | |||
| Unsatisfied | 0 | 0 | 1 (5) | |
| Mildly satisfied | 2 (3) | 1 (4) | 1 (5) | |
| Moderately satisfied | 1 (2) | 1 (4) | 6 (32) | |
| Extremely satisfied | 55 (95) | 21 (91) | 11 (58) | |
| Satisfaction with surgery, n (%) | .028 | |||
| Unsatisfied | 0 | 0 | 1 (5) | |
| Mildly satisfied | 2 (3) | 1 (4) | 0 | |
| Moderately satisfied | 2 (3) | 4 (17) | 5 (26) | |
| Extremely satisfied | 54 (93) | 18 (78) | 13 (68) | |
K, Kruskal-Wallis test.
Continuous variables were compared using one-way analysis of variance and categorical variables were compared using chi-square test except as noted.
Discussion
The current climate of opioid misuse, abuse, and diversion requires a multifaceted approach to postoperative care that manages both physician and patient goals and expectations. Early research on the use of opioids in hand surgery cited excessively high quantities of leftover or used pills, even after CTR.2,11,12 Several studies were conducted to assess opioid consumption for tier 1 hand procedures.2,4, 5, 6,9, 10, 11,15, 16, 17 Despite the opioid crisis, satisfaction remains a competing priority, and at times, it may be at odds with new prescribing protocols.18 Our findings suggest that high levels of pain control and satisfaction can be achieved by adhering to a postoperative pain protocol.
Past literature on opioid consumption after common hand procedures found that most pills are unused by 2 weeks after the procedure; 2 of those studies found that patients who underwent CTR had only taken one-fifth of pills that had been prescribed.2,4, 5, 6,9, 10, 11, 12 Recent studies demonstrated similar findings: Peters et al15 reported that during the first postoperative week, over half of patients who underwent carpal tunnel surgery took an average of 2 of 40 pills that were prescribed,. Our study found this to be similar, with slightly over half of patients using none of their prescribed opioids (55%) and most using less than 2 (71%). Waljee et al19 demonstrated that a prescribing protocol as short as 5 days can be a risk factor for prolonged opioid use in both nonopioid and opioid-naive patients. In our study, 92% of patients took no additional oxycodone pills after the first postoperative survey at 2 to 4 days after surgery. Similarly, in 2016, a study on common hand procedures both soft tissue and trauma-related reported that 13% of opioid-naive patients continued to fill their prescriptions past 3 months after the procedure.20
In response to these findings, many hand surgeons have created guidelines for prescribing and preoperative counseling. Several studies conducted recently showed that implementing a prescribing protocol markedly decreased opioid use after common hand surgery.4,6,10,16,17,21 Ilyas et al4 conducted a randomized controlled trial on opioids versus nonsteroidal anti-inflammatory drugs for pain management after CTR. High satisfaction was achieved regardless of pill type, which indicates that pain relief can be achieved without opioids after surgery.4 Weinheimer et al9 conducted a similar study on patients undergoing common soft tissue procedures. They found that ibuprofen and acetaminophen were also safe and effective in managing postoperative pain. Similarly, using preoperative educational materials, Dwyer et al10 demonstrated that patients undergoing CTR achieved high satisfaction with low consumption of opioids. Schommer et al16 also found that using a prescribing protocol after common hand surgery significantly reduced the number of pills consumed while maintaining high satisfaction.
We found that on average, patients initially consumed one pill, which is considerably less than what had been prescribed. The average number of pills taken by 14 days was 1.5 (11.25 MME), and 53% took no opioids at all. This is lower but consistent with prior studies of soft tissue procedures, which cited opioid consumption as high as 4.3 pills and as low as 3.2,4,5,9 We found that patients were highly satisfied with the pain management and surgical outcome, similar to the prior publications by Weinheimer et al9 and Ilyas et al.4
Interestingly, higher postoperative opioid consumption was associated with higher VAS scores and lower satisfaction with pain management at both time points, and lower satisfaction with surgery at the first postoperative visit. Some patients may experience more pain after surgery and therefore take more opioid pills, but this additional opioid use does not lead to improvement in VAS scores or improved satisfaction with pain management. These data may prove useful for preoperative counseling of patients on appropriate expectations for postoperative pain management.
This study had several limitations. First, it was based on a single surgeon at a single institution. Second, this was a small, prospective cohort and therefore may not represent the general population. Third, because the cohort was composed of 5 different procedures, the data may not reflect each individual procedure. Fourth, results may be affected by patient misreporting pain, pill consumption, and/or satisfaction, although by contacting them directly at 2 time points and having them check pill bottles, we aimed to minimize this. Fifth, further prescriptions could be confirmed only within the electronic medical record for our health system, and it is possible that patients went outside the system for further opioid management and failed to report this. Sixth, our study assessed satisfaction and opioid consumption only with this particular postoperative pain protocol, and thus we cannot comment on how patient satisfaction would have been affected by a different protocol. Seventh, categories within the satisfaction survey were limited and therefore may have affected patient responses. Finally, the study was not a randomized controlled trial and higher tiered procedures were not assessed.
There is a recognized need to minimize opioid prescribing, especially in the postoperative period, to decrease the risks of misuse, diversion, and dependency. Health care providers are assessing various methods to determine optimal management. Postoperative pain protocols have been shown to be successful in limiting the number of pills prescribed. Despite concerns about balancing patient satisfaction with adequate patient postoperative analgesia, this study demonstrates that a postoperative prescribing protocol can achieve both, with low opioid use and high patient satisfaction for soft tissue procedures of the hand.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received by the authors related directly or indirectly to the subject of this article.
References
- 1.Labrum J.T.I.V., Ilyas A.M. Perioperative pain control in upper extremity surgery: prescribing patterns, recent developments, and opioid-sparing treatment strategies. Hand (N Y) 2019;14(4):439–444. doi: 10.1177/1558944718787262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman T., Kim N., Maltenfort M., Ilyas A.M. Prospective evaluation of opioid consumption following carpal tunnel release surgery. Hand (N Y) 2017;12(1):39–42. doi: 10.1177/1558944716646765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earp B.E., Silver J.A., Mora A.N., Blazar P.E. Implementing a postoperative opioid-prescribing protocol significantly reduces the total morphine milligram equivalents prescribed. J Bone Joint Surg Am. 2018;100(19):1698–1703. doi: 10.2106/JBJS.17.01307. [DOI] [PubMed] [Google Scholar]
- 4.Ilyas A.M., Miller A.J., Graham J.G., Matzon J.L. Pain management after carpal tunnel release surgery: a prospective randomized double-blinded trial comparing acetaminophen, ibuprofen and oxycodone. J Hand Surg Am. 2018;43(10):913–919. doi: 10.1016/j.jhsa.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Alter T.H., Ilyas A.M. A Prospective randomized study analyzing preoperative opioid counseling in pain management after carpal tunnel release surgery. J Hand Surg Am. 2017;42(10):810–815. doi: 10.1016/j.jhsa.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Stepan J.G., London D.A., Osei D.A., Boyer M.I., Dardas A.Z., Calfee R.P. Perioperative celecoxib and postoperative opioid use in hand surgery: a prospective cohort study. J Hand Surg Am. 2018;43(4):346–353. doi: 10.1016/j.jhsa.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanek J.J., Renslow M.A., Kalliainen L.K. The effect of an educational program on opioid prescription patterns in hand surgery: a quality improvement program. J Hand Surg Am. 2016;40(2):341–346. doi: 10.1016/j.jhsa.2014.10.054. [DOI] [PubMed] [Google Scholar]
- 8.Gauger E.M., Gauger E.J., Desai M.J., Lee D.H. Opioid use after upper extremity surgery. J Hand Surg Am. 2018;43(5):470–479. doi: 10.1016/j.jhsa.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Weinheimer K., Michelotti B., Silver J., Taylor K., Payatakes A. A prospective, randomized, double-blinded controlled trial comparing ibuprofen and acetaminophen versus hydrocodone and acetaminophen for soft tissue hand procedures. J Hand Surg Am. 2019;44(5):387–393. doi: 10.1016/j.jhsa.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer C.L., Soong M., Hunter A., Dashe J., Tolo E., Kasparyan N.G. Prospective evaluation of an opioid reduction protocol in hand surgery. J Hand Surg Am. 2018;43(6):516–522. doi: 10.1016/j.jhsa.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Kim N., Matzon J.L., Abboudi J., et al. A prospective evaluation of opioid utilization after upper-extremity surgical procedures: identifying consumption patterns and determining prescribing guidelines. J Bone Joint Surg Am. 2016;98(20):e89. doi: 10.2106/JBJS.15.00614. [DOI] [PubMed] [Google Scholar]
- 12.Rodgers J., Cunningham K., Fitzgerald K., Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645–650. doi: 10.1016/j.jhsa.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Ramkumar P.N., Navarro S.M., Chughtai M., La T., Jr., Fisch E., Mont M.A. The patient experience: an analysis of orthopedic surgeon quality on physician-rating sites. J Arthroplasty. 2017;32(9):2905–2910. doi: 10.1016/j.arth.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 14.Kelley B.P., Shauver M.J., Chung K.C. Management of acute postoperative pain in hand surgery: a systematic review. J Hand Surg Am. 2015;40(8):1610–1619.e1. doi: 10.1016/j.jhsa.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Peters B., Izadpanah A., Islur A. Analgesic consumption following outpatient carpal tunnel release. J Hand Surg Am. 2018;43(2):189e1–189e5. doi: 10.1016/j.jhsa.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Schommer J, Allen S, Scholz N, Reams M, Bohn D. Evaluation of quality improvement methods for altering opioid prescribing behavior in hand surgery [e-pub ahead of print February 24, 2020]. J Bone Joint Surg Am. https://doi.org/10.2106/JBJS.19.01052. [DOI] [PubMed]
- 17.Sraj S. Narcotic-free, over-the-counter pain management after wide-awake hand surgery. J Am Acad Orthop Surg Glob Res Rev. 2019 Nov;3(11) doi: 10.5435/JAAOSGlobal-D-19-00137. e19.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ketonis C., Kim N., Liss F., et al. Wide awake trigger finger release surgery: prospective comparison of lidocaine, Marcaine, and Exparel. Hand (N Y) 2016;11(2):177–183. doi: 10.1177/1558944715627618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waljee J.F., Zhong L., Hou H., Sears E., Brummett C., Chung K.C. The use of opioid analgesics following common upper extremity surgical procedures: a national, population-based study. Plast Reconstr Surg. 2016;137(2):355e–364e. doi: 10.1097/01.prs.0000475788.52446.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson S.P., Chung K.C., Zhong L., et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947–957.e3. doi: 10.1016/j.jhsa.2016.07.113. [DOI] [PubMed] [Google Scholar]
- 21.Harrison R.K., DiMeo T., Klinefelter R.D., Ruff M.E., Awan H.M. Multi-modal pain control in ambulatory hand surgery. Am J Orthop (Belle Mead NJ) 2018;47(6):1–10. doi: 10.12788/ajo.2018.0042. [DOI] [PubMed] [Google Scholar]