Abstract
Candida tropicalis is less commonly isolated from clinical specimens than Candida albicans. Unlike C. albicans, which can be occasionally found as a commensal, C. tropicalis is almost always associated with the development of fungal infections. In addition, C. tropicalis has been reported to be resistant to fluconazole (FLC). To analyze the development of FLC resistance in C. tropicalis, an FLC-susceptible strain (ATCC 750) (MIC = 1.0 μg/ml) was cultured in liquid medium containing increasing FLC concentrations from 8.0 to 128 μg/ml. The strain developed variable degrees of FLC resistance which paralleled the concentrations of FLC used in the medium. The highest MICs of FLC were 16, 256, and 512 μg/ml for strains grown in medium with 8.0, 32, and 128 μg of FLC per ml, respectively. Development of resistance was rapid and could be observed already after a single subculture in azole-containing medium. The resistant strains were cross-resistant to itraconazole (MIC > 1.0 μg/ml) and terbinafine (MIC > 512 μg/ml) but not to amphotericin B. Isolates grown in FLC at concentrations of 8.0 and 32 μg/ml reverted to low MICs (1.0 μg/ml) after 12 and 11 passages in FLC-free medium, respectively. The MIC for one isolate grown in FLC (128 μg/ml) (128 R) reverted to 16 μg/ml but remained stable over 60 passages in FLC-free medium. Azole-resistant isolates revealed upregulation of two different multidrug efflux transporter genes: the major facilitators gene MDR1 and the ATP-binding cassette transporter CDR1. The development of FLC resistance in vitro correlated well with the results obtained in an experimental model of disseminated candidiasis. While FLC given at 10 mg/kg of body weight/day was effective in reducing the fungal burden of mice infected with the parent strain, the same dosing regimen was ineffective in mice infected with strain 128 R. Finally, the acquisition of in vitro FLC resistance in strain 128 R was related to a loss of virulence. The results of our study elucidate important characteristics and potential mechanisms of FLC resistance in C. tropicalis.
The risk of opportunistic infections is greatly increased in patients who are severely immunocompromised due to cancer chemotherapy, organ or bone marrow transplantation, and to human immunodeficiency virus infection (37–39). Although Candida albicans is the organism most often associated with serious fungal infections, other Candida species have emerged as clinically important pathogens associated with opportunistic infections (5, 37–39). This is due to several reasons. First, a major effort in yeast species identification has been made in diagnostic laboratories. Second, the introduction of particular surgical devices, prostheses, and indwelling intravenous catheters has increased the risk of yeast infections due to Candida species which originate mainly from the environment (37, 38). Third, the widespread use of new antifungal molecules, especially fluconazole (FLC), has selected Candida species that are intrinsically resistant to this triazole, such as Candida krusei, or whose resistance is easily inducible, such as Candida glabrata (2, 10, 23, 26, 28, 37–39). Another non-C. albicans species of considerable clinical importance is Candida tropicalis (39). This species of Candida is less commonly isolated from clinical specimens than C. albicans. Unlike C. albicans, which can be occasionally found as a commensal, C. tropicalis is almost always associated with the development of fungal infections (39). In addition, C. tropicalis has been reported to be often resistant to FLC (14, 21).
So far, three mechanisms of azole-resistance have been described in C. albicans and C. glabrata: failure to accumulate drug intracellularly, increased production of the azole target enzyme, a lanosterol 14-α-demethylase called Erg11, and point mutations in the ERG11 gene, the products of which have reduced affinity to azoles (1, 11, 12, 15–19, 29–36). The first mechanism of azole resistance may be caused by a lack of drug penetration due to change in membrane lipids or sterols or by active efflux of drugs resulting from upregulation of either CDR genes (encoding ABC transporters), effective against many azole drugs, or MDR (encoding major facilitators), specific for FLC (1, 11, 12, 15–19, 29–36). Few data are yet available on the mechanisms of azole resistance in C. tropicalis (9).
In this study, we developed an in vitro model to analyze the development of FLC resistance in C. tropicalis.
MATERIALS AND METHODS
Isolates.
C. tropicalis ATCC 750 was used throughout this study. C. krusei ATCC 6258 was used as control organism for experiments of antifungal susceptibility.
Drugs.
Standard antifungal powders of FLC, itraconazole (ITC), terbinafine (TRB), and amphotericin B (AMB) were obtained from their respective manufacturers. Stock solutions were prepared in water (FLC), polyethylene glycol (ITC and TRB), and dimethyl sulfoxide (AMB). Antifungal agents were diluted with RPMI 1640 medium containing 2% glucose (RPMI 1640-G; Sigma Chemical, Milano, Italy) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid buffer (MOPS; Sigma).
Development of FLC resistance. (i) Strategy for induction of FLC resistance.
A single C. tropicalis colony was used to inoculate 10 ml of RPMI 1640-G which was incubated overnight in a rotating drum at 35°C. An aliquot of this culture containing 106 cells was transferred to 10 ml of medium containing 8.0, 32, or 128 μg of FLC per ml, and the cells were incubated as described above. When the cultures reached a density of approximately 108 cells/ml, aliquots containing 106 cells were transferred into fresh medium containing the same respective FLC concentration and reincubated. At each passage, a 1-ml aliquot of the suspension was mixed with 0.5 ml of 50% glycerol, and the mixture was frozen at −70°C for antifungal susceptibility testing as described below.
(ii) Stability of FLC resistance in vitro.
Isolates found to exhibit FLC resistance were serially cultured in FLC-free medium. After each subculture, antifungal susceptibility testing was performed as described below. Passages were continued until the FLC MIC returned to the level of the parental strain.
Antifungal susceptibility testing. (i) Procedure.
Susceptibility testing was performed exactly as outlined by the NCCLS method (20). Briefly, testing was performed in RPMI 1640-G buffered to pH 7.0 with MOPS. Two sets of FLC microtiter plates were prepared: in the first set the drug was tested at concentrations ranging from 0.125 to 64 μg/ml; in the other set the drug was tested at concentrations ranging from 1.0 to 512 μg/ml. ITC, TRB, and AMB were tested at concentrations ranging from 0.007 to 4.0 μg/ml, from 0.5 to 256 μg/ml, and from 0.03 to 16 μg/ml, respectively. Before reading, microtiter plates were sealed and then agitated for 5 min on a microtiter plate shaker. Readings were performed spectrophotometrically with an automatic plate reader (model MR 700; Dynatech) set at 490 nm. For both triazoles and TRB the MICs were defined as the first concentration of drug at which turbidity in the well was ≤80% of that in the control well. For AMB the MIC was defined as the first concentration of drug at which turbidity in the well was ≤90% of that in the control well (20).
(ii) Definition.
According to the recent proposed breakpoints (20, 27), the isolates were defined as follows: FLC and ITC susceptible if the MICs were ≤8.0 μg/ml and ≤0.125 μg/ml, respectively; FLC and ITC susceptible-dose dependent if the MICs ranged from 16 to 32 μg/ml and from 0.25 to 0.5 μg/ml, respectively; or FLC and ITC resistant if the MICs were ≥64 and ≥1.0 μg/ml, respectively (20, 27).
Phenotypic analysis of isolates.
The parent strain (ATCC 750) and one drug-resistant and revertant isolate from each drug exposure group were analyzed phenotypically.
(i) Sugar assimilation.
The biochemical patterns of sugar assimilation were determined with the API 20C system (bioMérieux, Marcy l'Etoile, France) as specified by the manufacturer.
(ii) Enzymatic analysis.
Suspensions of cells grown for 48 h in RPMI 1640-G or the cell supernatants were tested for enzymatic activity with the API ZYM system (bioMérieux) as specified by the manufacturer.
(iii) Growth curves.
The growth rates were determined by incubating the isolates at 35°C in RPMI 1640-G with shaking. Absorbances were measured at 650 nm, and the cell doubling time was calculated for each isolate.
Northern blot analysis.
The parent strain and selected FLC-resistant and FLC-susceptible isolates from each drug exposure group were analyzed for overexpression of FLC resistance genes. Briefly, total RNA from different isolates grown to mid-logarithmic phase in YEPD medium (1% yeast extract, 2% glucose, 2% peptone) was obtained by using the RNAeasy mini kit (Qiagen Inc., Santa Clara, Calif.) following the manufacturer's instructions. RNA was separated by electrophoresis and subsequently transferred to Crenescrem Plus nylon membranes (Dupont NEN). CtMDR1 (GenBank accession no. AF194419) was isolated from genomic DNA by PCR amplification using primers designed from the C. albicans CaMDR1 gene. The primers used were BEN 36 (5′ ACCCCAWGCHACWGGATADT 3′) and BEN 56 (5′ TTATWYGTTMTTGGTTATGGTSTWGG 3′). Primer amplification was carried out with AmpliTaq (Perkin-Elmer) under the following conditions. A first cycle of denaturation for 4 min at 94°C which was followed by 30 cycles of annealing at 50°C for 2 min, elongation at 72°C for 2 min, and denaturation at 94°C for 30 s. A final elongation step at 72°C for 10 min completed the PCR. Hybridization with the C. albicans CDR1 gene was performed with a 32P-labeled probe corresponding to the entire CDR1 open reading frame. Low-stringency hybridization with the CDR1 labeled probe was performed at 42°C in order to detect the CDR1-like mRNA in C. tropicalis. The low-stringency buffer consisting of 20% formamide, 1% sodium dodecyl sulfate (SDS), 4× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 10% dextran sulfate, and salmon sperm DNA (100 μg/ml). Other hybridizations were carried out under high-stringency conditions with the same buffer but containing 50% formamide. The TEF3 gene from C. albicans was used as the RNA loading control as previously described (32). Probes were labeled by random priming (Amersham), and hybridizations were performed as described by Sanglard et al. (32). After hybridizations, blots were washed at 60°C as recommended by the manufacturer and subjected to autoradiography.
Animal studies.
The parent strain ATCC 750 and the isolate grown in FLC (128 μg/ml) were tested in a murine model of disseminated candidiasis. Inbred BALB/c female mice weighing 23 to 26 grams were used throughout the study. One day before infection the organisms were inoculated into brain heart infusion broth and were incubated for 24 h at 35°C on a gyratory shaker (200 rpm). Organisms were harvested by low-speed centrifugation (1,500 × g), washed three times in sterile 0.9% saline, counted with a hemacytometer, and suspended in sterile saline to the desired concentrations. All studies were performed by challenging the mice with an inoculum given in 0.2 ml of sterile saline administered in the lateral tail vein. Inoculum sizes were confirmed by quantitative cultures on Sabouraud dextrose agar (SDA) plates.
(i) Virulence.
To assess the possible differences between isolates in levels of virulence, mice were infected with the same inoculum size of both the parent and the resistant strain. Mice were observed daily for 30 days, and survival was recorded. In other experiments, infected mice were sacrificed 5 and 30 days postinfection, both kidneys were excised by a sterile technique, weighed, and homogenized in 2.0 ml of sterile 0.9% saline. The homogenates were diluted by serial 10-fold dilution in sterile saline, and 0.1 ml of each dilution and the undiluted homogenate were cultured in triplicate on SDA. Culture plates were incubated for 48 h at 35°C, the numbers of CFU were then counted, and the number of CFU per gram of tissue was calculated.
(ii) Stability of FLC resistance.
Mice infected with the FLC-resistant strain were sacrificed at predetermined time intervals, and both kidneys and the spleen were excised, homogenized, and plated onto SDA. After 48 h of incubation at 35°C, multiple colonies were selected from each plate and tested against FLC as described above.
(iii) Azole treatment.
Mice infected with both the parent and the resistant strains were randomized into control and treatment groups, and FLC therapy was initiated at 24 h postchallenge. FLC was administered at 0.2 ml by intraperitoneal injection (25). Treatment was given once a day at 10 mg/kg of body weight and continued for 5 days. Control mice were given 0.2 ml of sterile saline. At 24 h after the administration of the last dose of FLC, the mice were sacrificed; both kidneys were excised, weighed, and homogenized; and the number of CFU per gram of tissue was calculated as described above.
(iv) Statistical analysis.
Data from survival and organ clearance studies were analyzed by the Mann-Whitney U test, and significance was defined as P < 0.05.
RESULTS
Table 1 shows FLC, ITC, TRB, and AMB MICs for C. tropicalis ATCC 750 and C. krusei ATCC 6258. Median FLC and ITC MICs for C. tropicalis ATCC 750 were 1.0 μg/ml and 0.125 μg/ml, respectively. According to NCCLS standards this isolate is susceptible to both triazoles (20, 27).
TABLE 1.
In vitro activities against C. tropicalis ATCC 750 and C. krusei ATCC 6258a
| Isolate | MICsb (μg/ml) of:
|
|||
|---|---|---|---|---|
| FLC | ITC | TRB | AMB | |
| C. tropicalis | 1.0 | 0.125 | 32 | 0.5 (0.25–0.5) |
| C. krusei | 32 (16–32) | 0.5 | 32 | 0.5 (0.5–1.0) |
Each antifungal agent was tested from 9 to 29 times against both isolates.
Values are medians (ranges shown in parentheses).
Development of FLC resistance.
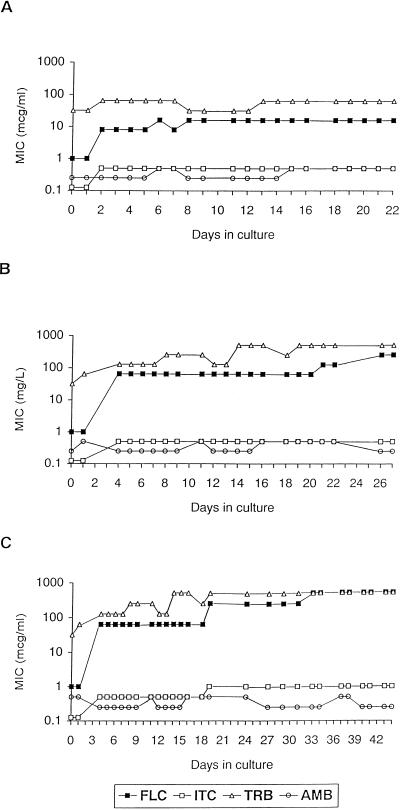
To analyze the development of FLC resistance, C. tropicalis ATCC 750 was cultured in medium containing FLC at concentrations of 8.0, 32, or 128 μg/ml. In general, isolates developed variable degrees of FLC resistance depending on the concentrations of FLC used in the medium (Fig. 1). The MICs for organisms grown in FLC at 8.0 μg/ml rose from 1.0 to 8.0 μg/ml after two passages, which was equivalent to 2 days of drug exposure. The highest MIC of FLC for this treatment group was 16 μg/ml, which was reached after 6 days of drug exposure. This value remained stable from day 8 to day 22 (Fig. 1A). Similarly, the MICs for organisms grown in FLC at 32 μg/ml rose rapidly, from 1.0 to 64 μg/ml after two passages, or 4 days of drug exposure. However, for this treatment group two further increases of FLC MIC were measured: from 64 to 128 μg/ml and from 128 to 256 μg/ml, after 21 and 26 days of drug exposure, respectively (Fig. 1B). The parent strain cultured directly in FLC at 128 μg/ml failed to reach an appropriate optical density, even after a prolonged incubation time. Therefore, the organisms grown in FLC at 32 μg/ml were passaged after 18 days of drug exposure (FLC MIC, 64 μg/ml) to FLC at 128 μg/ml (Fig. 1C). This strategy of induction of resistance resulted in an increase of FLC MIC from 64 to 256 μg/ml within 24 h. After 14 days of drug exposure at this concentration, the organism showed a further increase of FLC MIC (from 256 to 512 μg/ml) and was stable for up to 11 days in drug-containing medium (Fig. 1C).
FIG. 1.
Variations of FLC, ITC, TRB, and AMB MICs for isolates grown in medium containing 8.0 (A), 32 (B), and 128 (C) μg of FLC per ml. Each datum point represents one passage.
Susceptibilities to ITC, TRB, and AMB.
In order to measure the development of cross-resistance to other antifungal agents, we performed MIC assays with three antifungal agents with the organisms grown in separate FLC concentrations. The MICs of ITC for organisms cultured in FLC at both 8.0 and 32 μg/ml rose from 0.125 to 0.5 μg/ml after 2 and 4 days of FLC exposure, respectively. The increase of ITC MICs paralleled exactly the increase of FLC MIC (Fig. 1A and 1B). The MICs of ITC for organisms grown in FLC at 128 μg/ml increased rapidly to 1.0 μg/ml. However, even for this FLC treatment group, the ITC MIC did not increase further and remained stable for up to 44 days (Fig. 1C). The organisms grown in FLC at 8.0 μg/ml showed stable TRB MICs, ranging from 32 to 64 μg/ml (Fig. 1A). The MICs of TRB for the organisms grown in FLC at 32 μg/ml increased from 32 to 128 μg/ml and paralleled the increases of FLC and ITC MICs. The highest TRB MIC (>256 μg/ml) was reached after 14 days of FLC exposure and remained stable up to 31 days (Fig. 1B and 1C). AMB MICs for all organisms did not vary significantly and ranged from 0.25 to 0.5 μg/ml.
Stability of azole resistance in vitro.
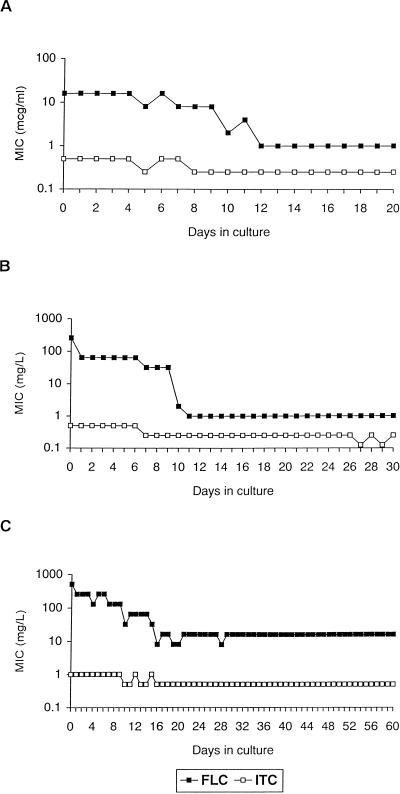
To assess the stability of FLC resistance, organisms grown in separate FLC concentrations were serially cultured in drug-free medium, and both FLC and ITC MICs were assayed after subcultures (Fig. 2). The isolates grown in FLC concentrations of 8.0 and 32 μg/ml reverted to the low MIC (1.0 μg/ml) after 12 and 11 days of growth in FLC-free medium, respectively (Fig. 2A and 2B). Although the MIC of FLC for the isolate grown in FLC at 128 μg/ml was significantly reduced (greater-than-fourfold dilutions) after 15 days of growth in FLC-free medium, the MIC of FLC for this isolate remained higher than that for the initial FLC-susceptible isolate up to 60 days (FLC MICs ranged from 8.0 to 16 μg/ml) (Fig. 2C). In general, ITC MICs decreased as FLU MICs decreased, and eventually all organisms reverted to the ITC-susceptible (ITC MIC, 0.125 μg/ml) or ITC-susceptible–dose-dependent (ITC MIC, 0.25 to 0.5 μg/ml) phenotypes. TRB MICs were determined for one organism in each FLC treatment group, and all reverted to the MIC observed for the parent strain (data not shown).
FIG. 2.
Variations of FLC and ITC MICs for isolates previously grown in medium containing 8.0 (A), 32 (B), and 128 (C) μg of FLC per ml. Each datum point represents one passage.
Phenotypic analysis.
The patterns of sugar assimilation for the parent strain and both resistant and revertant isolates from each FLC treatment group were similar, and the same code (2556175) was obtained for all organisms tested. Although the enzymatic patterns of whole cells were quite stable among the strains, the resistant phenotypes revealed lower activities than those observed in the susceptible phenotypes for the following enzymes: alkaline phosphatase, caprylate esterase lipase, myristate lipase, two arylamidases (leucine and cystine), naphthol-AS-BI-phosphohydrolase, and α-glucosidase. Furthermore, no cystine-arylamidase activity was detected for the organism grown in FLC at 128 μg/ml, even upon recovering it from the kidneys of infected mice. Acid phosphatase was the only enzyme detected in the filtrates of culture supernatants of all strains, with the exception of the filtrates obtained from the organisms grown in FLC at 128 μg/ml (data not shown). Growth curves revealed that doubling times for FLC-resistant phenotypes were significantly longer than those for FLC-susceptible counterparts (Table 2).
TABLE 2.
Growth rates of FLC-susceptible and -resistant phenotypes of C. tropicalis ATCC 750
| Straina | Doubling time (h) |
|---|---|
| Parent | 2.2 |
| 8-R | 3.7 |
| 8-S | 2.2 |
| 32-R | 3.8 |
| 32-S | 2.3 |
| 128-R | 4.5 |
| 128-S | 2.5 |
| 128-mouseb | 5.0 |
The numbers indicate FLC concentrations (micrograms per milliliter) in medium; S, susceptible phenotype; R, resistant phenotype.
This isolate, previously grown in FLC at 128 μg/ml, was recovered from the kidney of a mouse sacrificed 30 days postinfection (FLC MIC, 256 μg/ml).
Expression of CtMDR1 and CDR1.
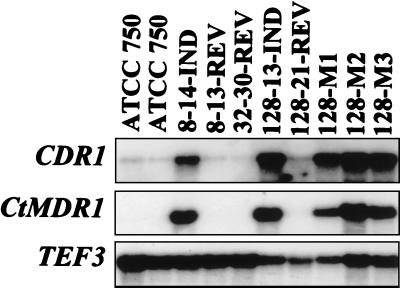
Since multidrug efflux transporters are known to be involved in azole resistance in C. albicans, this possibility was explored with the strain used in the present study. Multidrug efflux transporters from two different families (ATP-binding cassette [ABC] transporters and major facilitators) were first cloned by PCR with primers matching the consensus sequences of both families. A CDR-like fragment and a CaMDR1-like fragment were recovered from C. tropicalis. From Northern analysis with RNA extracted from the strain used in the present study, only the CaMDR1-like gene (CtMDR1) showed expression patterns consistent with an involvement in FLC resistance. Representative results are reported in Fig. 3. The parent strain did not show any constitutive level of expression of CtMDR1. The FLC-resistant isolates grown in FLC at 8.0, 32, and 128 μg/ml all revealed overexpression of CtMDR1, while in their respective revertant isolates gene expression was reduced to background levels. CtMDR1 was also upregulated in the strain grown in FLC at 128 μg/ml which was recovered from the kidneys of untreated mice 30 days postinfection (Fig. 3). However, upregulation of MDR1 genes from Candida species only confers resistance to FLC (30, 32). Since cross-resistance to other azole derivatives was observed in the FLC-resistant isolates of the present study, the single overexpression of CtMDR1 could not account for this feature. It was still possible that multidrug transporters of the ABC superfamily, which take as substrates different azole derivatives, could still be upregulated in the C. tropicalis azole-resistant isolates. Therefore, in order to evaluate this hypothesis, we performed low-stringency hybridizations with the entire open reading frame of the ABC transporter gene CDR1 from C. albicans. As shown in Fig. 3, the signal corresponding to the mRNA matching CDR1 increased in parallel to those of CtMDR1. These results strongly suggest that a C. tropicalis CDR1 gene homologous to CDR1 is upregulated in isolates with increasing FLC MICs. Thus, azole cross-resistance observed in the C. tropicalis isolates of the present study could be explained by the simultaneous upregulation of both multidrug transporters of two different families.
FIG. 3.
Variation of CtMDR1 and CDR1 expression in FLC-susceptible and -resistant phenotypes of C. tropicalis ATCC 750. Lanes: ATCC 750, parent strain; 8-14-IND, isolate cultured in FLC at 8.0 μg/ml (14th passage); 8-13-REV, isolate previously cultured in FLC at 8.0 μg/ml and then passaged in FLC-free medium (13th passage); 32-30-REV, isolate previously cultured in FLC at 32 μg/ml and then passaged in FLC-free medium (30th passage); 128-13-IND, isolate cultured in FLC at 128 μg/ml (13th passage); 128-21-REV, isolate previously cultured in FLC at 128 μg/ml and then passaged in FLC-free medium (21st passage); 128-M1, 128-M2, and 128-M3, isolates cultured in FLC at 128 μg/ml and recovered from the kidneys of three mice 30 days postinfection.
Animal experiments.
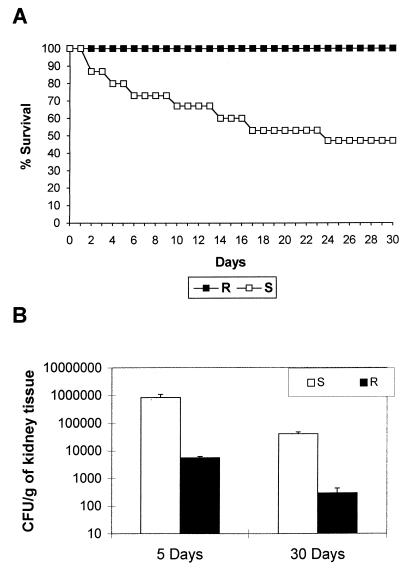
To assess the virulence of C. tropicalis ATCC 750, we performed initial experiments by challenging the mice (10 mice per group) with a broad range of inoculum sizes, i.e., 5 × 104, 5 × 105, and 5 × 106 CFU per mouse. Only in the third group we observed a mortality rate >50% within 30 days. In addition, while the first two groups of animals cleared the infection within few days, mice infected with the larger inoculum showed a fungal density of log 3 CFU/g of kidneys on day 30 after the challenge (data not shown). Therefore, further experiments were performed with an inoculum of 107 CFU per mouse. Experiments were performed with the parent strain and the strain grown in FLC at 128 μg/ml. There was a dramatic difference in the survival rate between animals injected with the parent strain (60% mortality) and those challenged with the resistant phenotype (0% mortality; P = 0.0001) (Fig. 4A). Mouse survival was mirrored in the kidney fungal burdens of infected mice (Fig. 4B). The fungal burdens in animals infected with the parent strain resulted in significantly higher counts than those observed in animals infected with the resistant phenotype either 5 (P = 0.019) or 30 days (P = 0.0001) postinfection.
FIG. 4.
Virulence of FLC-susceptible (S [parent strain]; FLC MIC, 1.0 μg/ml) and FLC-resistant (R; organism cultured in FLC at 128 μg/ml; FLC MIC, 256 μg/ml) phenotypes of C. tropicalis ATCC 750 in an immunocompetent mouse model. Mice were inoculated with 107 CFU via the lateral tail vein. (A) Survival curves. There were 15 mice per group (B) CFU of strain ATCC 750-infected kidneys recovered from animals sacrificed on days 5 and 30 postinfection. There were four to seven mice per time interval or group. Error bars show standard deviations from the mean.
Further experiments were carried out to address the stability of FLC resistance in vivo with the strain grown in FLC at 128 μg/ml. Groups of two to three mice each were sacrificed on days 5, 10, and 30 postinfection, and two to five random isolates per mouse recovered from both kidneys and spleens were tested for FLC susceptibility. All organisms retained the FLC-resistant phenotype over the test period (data not shown).
Table 3 shows fungal burdens of FLC treated mice. While FLC at 10 mg/kg of body weight was effective in reducing the number of CFU per gram of kidneys in mice infected with the parent strain (P < 0.05), the same dosing regimen was ineffective in mice infected with the resistant phenotype.
TABLE 3.
Fungal burden in kidney tissues of mice infected with C. tropicalis ATCC 750 and treated with FLCa
| Isolateb | FLC dosage (mg/kg) | Median counts ± SE (CFU/g [104]) |
|---|---|---|
| S | 0 | 211 ± 98.2 |
| 10 | 31c ± 3.1 | |
| R | 0 | 12 ± 5.1 |
| 10 | 6.5d ± 1.9 |
There were seven mice in each treatment group. The mice were randomized into control and treatment groups, and FLC therapy was initiated at 24 h postchallenge. FLC was administered at 0.2 ml by intraperitoneal injection. Treatment was given once a day at 10 mg/kg of body weight and continued for 5 days. At 24 h after the administration of the last dose of FLC, the mice were sacrificed.
S, C. tropicalis ATCC 750 FLC susceptible (FLC MIC, 1.0 μg/ml) (parent strain); R, C. tropicalis ATCC 750 FLC resistant (cultured in FLC at 128 μg/ml; FLC MIC, 256 μg/ml).
Treatment reduced the count significantly below that in the tissues of controls (P < 0.05).
Treatment did not reduce the count significantly below that in the tissues of controls (P = 0.323).
DISCUSSION
Mechanisms of azole resistance have been extensively investigated in strains of C. albicans isolated from patients with AIDS and oropharyngeal candidiasis (11, 16, 17, 29, 32, 34, 35). Recently, several studies elucidated the mechanisms of azole resistance in C. glabrata and C. krusei (12, 18, 22, 23). Little is known about azole resistance in C. tropicalis (9). This species has been reported as the second-most-frequent cause of candidemia in both neutropenic and nonneutropenic patients. Additionally, in contrast to C. albicans, which can be occasionally found as a commensal, C. tropicalis, when encountered, is almost always associated with diseases (14, 39). It has been reported that C. tropicalis azole susceptibility can be reduced by drug exposure in vitro or in vivo (2, 14, 39). The dynamics of development of FLC resistance in this Candida species is largely unknown.
The results of our study underline several characteristics of the in vitro acquisition of FLC resistance in C. tropicalis. We showed here that the development of FLC resistance occurs very rapidly and that the degree of resistance is strongly related to the drug concentration utilized in the growth medium. In particular, the MIC of FLC for the strain grown in FLC at 32 μg/ml increased from 1.0 to 64 μg/ml after 4 days of drug exposure. Similarly, the strain previously grown in FLC at 32 μg/ml and then passaged in FLC at 128 μg/ml developed a very high degree of resistance within 24 h: from 64 to 256 μg/ml. In addition, when the resistant strain grown in FLC at 32 μg/ml was passaged in FLC-free medium, the MICs for this strain reverted to low values comparable to the one measured in the initial strain (12 passages in FLC-free medium). On the other hand, the strain grown in FLC at 128 μg/ml retained a level of resistance (MIC, 16 μg/ml) that was higher than that measured in the parent strain up to 60 days of passages in FLC-free medium. This finding correlated well with the animal experiment results. Following challenge with the strain grown in FLC at 128 μg/ml, the yeasts recovered 30 days postinfection from the spleen or kidneys of untreated mice retained a high degree of FLC resistance.
All together these data indicate that the dynamics of development of FLC-resistance in this Candida sp. is quite different from that experimentally observed in C. albicans (8). For the latter species, the time required to develop FLC resistance as well as the time required to revert to baseline is longer. Calvet et al. (8) showed that even when the strain used in their study was originally passaged in FLC at 128 μg/ml it acquired a very high degree of FLC resistance. Contrarily to these authors, we failed to obtain an appropriate optical density for cells grown in FLC at 128 μg/ml, thus indicating that this drug concentration is very toxic to susceptible strains of C. tropicalis. In order to see whether the development of FLC resistance in C. tropicalis was characterized by a cross-resistance to other antifungals, we determined ITC, TRB, and AMB MICs for all the organisms grown in each FLC concentration. As repeatedly observed in C. albicans, ITC and TRB showed a progressive increase in their respective MICs which paralleled exactly the increase of FLC MICs (3, 17, 24, 29, 31, 34). As expected, AMB MICs remained stable over time.
The increase in FLC and ITC MICs was correlated with increases of both CtMDR1 and CDR1 expression. Because we did not clone a CDR1-like homologue from C. tropicalis, we assumed in this study that the C. albicans CDR1 probe would reveal the C. tropicalis homologous CDR1 gene in low-stringency hybridizations. As shown in Fig. 3, this was effectively the case. Strains for which the MICs of FLC reverted to low values did not express CtMDR1 at detectable levels. However, in these strains, basal CDR1 expression was still detected, as reported in most azole-susceptible C. albicans isolates (1, 11, 16, 17, 19). Interestingly, the resistant phenotype recovered 30 days postinfection from the kidneys of untreated mice showed the maintenance of the upregulation of both genes. Without excluding the possibility that other azole resistance mechanisms could operate in azole-resistant isolates of this study, these data show that, as seen in C. albicans, one of the possible mechanisms involved in FLC resistance is the active efflux of drug due to an upregulation of multidrug efflux transporter genes (1, 11, 16, 17, 31, 32, 34). Simultaneous upregulation of both types of transporters by in vitro exposure to FLC has not been yet described in other yeast species. Albertson et al. (1) obtained a FLC-resistant strain in C. albicans overexpressing CaMDR1; Marr et al. (19) were also able to transiently downregulate CDR1 following in vitro passages of azole-resistant C. albicans isolates in drug-free medium.
Although the resistant phenotype did not respond to 10 mg of FLC/kg/day in a mouse model of systemic candidiasis, this strain was dramatically less virulent than the parent strain as shown by the lack of mortality and the lower number of CFU/gram of infected tissues. The relationship between virulence and antifungal resistance is controversial, and the limited data available concern mainly C. albicans strains (4, 6, 8, 13). In an earlier study of two pairs of serial isolates of C. albicans with identical DNA patterns obtained from two patients with first responsive and then refractory thrush, we found that pre- and posttreatment isolates from both patients were equally virulent in an animal model of murine candidemia (4). Recently, Graybill et al. (13) showed that decreased virulence of serial C. albicans isolates is associated with increasing FLC MICs in some but not all cases. Interestingly, these authors found that the isolates for which the MICs of FLC were high which had low virulence were associated with FLC failure when they were tested in mice with candidal infections. In contrast, the in vitro resistant isolates which had high virulence, responded to FLC therapy with standard doses in the same animal model (13). The reduced virulence of the C. tropicalis resistant strain compared with that of the parent strain found in this study could be due to the experimental acquisition of FLC resistance. The phenotypic characteristics of the resistant strains were profoundly altered compared with those of the parent and the revertant strains. These data would indicate a reduced fitness of the resistant phenotype and would explain its reduced capability to establish an infection. Our data agree with those recently observed by Buckner et al. (7) in Trypanosoma cruzi. They found that the azole-resistant mutant was less virulent than the parent strain.
In summary, we evaluated an in vitro model to analyze the development of FLC resistance in C. tropicalis. In this model the acquisition of azole resistance occurs very rapidly. Multidrug transporter genes of two different families, the ABC transporters and the major facilitators, are upregulated in the resistant phenotypes, indicating that one of the mechanisms of resistance in this Candida sp. may be drug efflux from the cell. Similar to observations by others in several eukaryotes, the resistant phenotype appears less virulent than the parent strain.
ACKNOWLEDGMENTS
This work was in part supported by a grant from the Istituto Superiore di Sanità, Rome, Italy (II AIDS project [grant 50B.36]), and by a grant from M.U.R.S.T., 1998–1999. D.S. is supported by a grant (3100-055901) from the Swiss Research National Foundation.
REFERENCES
- 1.Albertson G D, Niimi M, Cannon R D, Jenkinson H F. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother. 1996;40:2835–2841. doi: 10.1128/aac.40.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barchiesi F, Arzeni D, Del Prete M S, Sinicco A, Falconi Di Francesco L, Pasticci M B, Lamura L, Nuzzo M M, Burzacchini F, Coppola S, Chiodo F, Scalise G. Fluconazole susceptibility and strain variation of Candida albicans isolates from HIV-infected patients with oropharyngeal candidosis. J Antimicrob Chemother. 1998;41:541–548. doi: 10.1093/jac/41.5.541. [DOI] [PubMed] [Google Scholar]
- 3.Barchiesi F, Colombo A L, McGough D A, Fothergill A W, Rinaldi M G. In vitro activity of itraconazole against fluconazole-susceptible and -resistant isolates of Candida albicans isolates from oral cavities of patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1994;38:1530–1533. doi: 10.1128/aac.38.7.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barchiesi F, Najvar L K, Luther M F, Scalise G, Rinaldi M G, Graybill J R. Variation in fluconazole efficacy for Candida albicans strains sequentially isolated from oral cavities of patients with AIDS in an experimental murine candidiasis model. Antimicrob Agents Chemother. 1996;40:1317–1320. doi: 10.1128/aac.40.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barchiesi F, Tortorano A M, Falconi Di Francesco L, Cogliati M, Scalise G, Viviani M A. In-vitro activity of five antifungal agents against uncommon clinical isolates of Candida spp. J Antimicrob Chemother. 1999;43:295–299. doi: 10.1093/jac/43.2.295. [DOI] [PubMed] [Google Scholar]
- 6.Becker J M, Henry L K, Jiang W, Koltin Y. Reduced virulence of Candida albicans mutants affected in multidrug resistance. Infect Immun. 1995;63:4515–4518. doi: 10.1128/iai.63.11.4515-4518.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckner F S, Wilson A J, White T C, Van Voorhis W C. Induction of resistance to azole drugs in Trypanosoma cruzi. Antimicrob Agents Chemother. 1998;42:3245–3250. doi: 10.1128/aac.42.12.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvet H M, Yeaman M R, Filler S G. Reversible fluconazole resistance in Candida albicans: a potential in vitro model. Antimicrob Agents Chemother. 1997;41:535–539. doi: 10.1128/aac.41.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Turi T G, Sanglard D, Loper D J. Isolation of the Candida tropicalis gene for P450 lanosterol demethylase and its expression in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1987;146:1311–1317. doi: 10.1016/0006-291x(87)90792-3. [DOI] [PubMed] [Google Scholar]
- 10.Como J A, Dismukes W E. Oral azole drugs as system antifungal therapy. N Engl J Med. 1994;330:263–272. doi: 10.1056/NEJM199401273300407. [DOI] [PubMed] [Google Scholar]
- 11.Franz R, Kelly S L, Lamb D C, Kelly D E, Ruhnke M, Morschhäuser J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother. 1998;42:3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geber A, Hitchocock C A, Swartz J E, Pullen F S, Marsden K E, Kwon-Chung K J, Bennett J E. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob Agents Chemother. 1995;39:2708–2717. doi: 10.1128/aac.39.12.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graybill J H, Montalbo E, Kirkpatrick W R, Luther M F, Revankar S G, Patterson T F. Fluconazole versus Candida albicans: a complex relationship. Antimicrob Agents Chemother. 1998;42:2938–2942. doi: 10.1128/aac.42.11.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graybill J H, Najvar L K, Holmberg J D, Luther M F. Fluconazole, D0870, and flucytosine treatment of disseminated Candida tropicalis infections in mice. Antimicrob Agents Chemother. 1995;39:924–929. doi: 10.1128/aac.39.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hitchcock C A, Barett-Bee K, Russel N J. The lipid composition of azole-sensitive and azole-resistant strains of Candida albicans. J Gen Microbiol. 1986;132:2421–2431. doi: 10.1099/00221287-132-9-2421. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Ribot J L, McAtee R K, Lee L N, Kirkpatrick W R, White T C, Sanglard D, Patterson T F. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother. 1998;42:2932–2937. doi: 10.1128/aac.42.11.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Ribot J L, McAtee R K, Perea S, Kirkpatrick W R, Rinaldi M G, Patterson T F. Multiple resistant phenotypes of Candida albicans coexist during episodes of oropharyngeal candidiasis in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1999;43:1621–1630. doi: 10.1128/aac.43.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marichal P, Vanden Bossche H, Odds F C, Nobels G, Warnock D W, Timmerman V, Van Broeckhoven C, Fay S, Mose-Larsen P. Molecular biological characterization of an azole-resistant Candida glabrata isolate. Antimicrob Agents Chemother. 1997;41:2229–2237. doi: 10.1128/aac.41.10.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marr K A, Lyons C N, Rustad T, Bowden R A, White T C. Rapid, transient, fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob Agents Chemother. 1998;42:2584–2589. doi: 10.1128/aac.42.10.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Reference method for broth dilution susceptibility testing of yeasts. Approved Standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 21.Odds F C. Resistance of yeasts to azole-derivative antifungals. J Antimicrob Chemother. 1993;31:463–471. doi: 10.1093/jac/31.4.463. [DOI] [PubMed] [Google Scholar]
- 22.Orozco A S, Higginbotham L M, Hitchcock C A, Parkinson T, Falconer D, Ibrahim A S, Ghannoum M A, Filler S G. Mechanism of fluconazole resistance in Candida krusei. Antimicrob Agents Chemother. 1998;42:2645–2649. doi: 10.1128/aac.42.10.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller M A, Rhine-Chalberg J, Redding S W, Smith J, Farinacci G, Fothergill A W, Rinaldi M G. Variations in fluconazole susceptibility and electrophoretic karyotype among oral isolates of Candida albicans from patients with AIDS and oral candidiasis. J Clin Microbiol. 1994;32:59–64. doi: 10.1128/jcm.32.1.59-64.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revankar S G, Kirkpatrick W R, McAtee R K, Dib O P, Fothergill A W, Redding S W, Rinaldi M G, Patterson T F. Detection and significance of fluconazole resistance in oropharyngeal candidiasis in human immunodeficiency virus-infected patients. J Infect Dis. 1996;174:821–827. doi: 10.1093/infdis/174.4.821. [DOI] [PubMed] [Google Scholar]
- 25.Rex J H, Nelson P W, Paetznick V L, Lozano-Chiu M, Espinel-Ingroff A, Anaissie E J. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42:129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rex J H, Pfaller M A, Barry A L, Nelson P W, Webb C D. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob Agents Chemother. 1995;39:40–44. doi: 10.1128/aac.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro/in vivo correlation data for fluconazole, itraconazole, and Candida infection. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 28.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14-α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanglard D, Isher F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 31.Sanglard D, Isher F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–2305. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanglard D, Kuchler K, Isher F, Pagani J L, Monod M, Bille J. Mechanisms of resistance to azole antifungal in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanden Bossche H, Marichal P, Odds F C, Le Jeune L, Coene M-C. Characterization of an azole-resistant Candida glabrata isolate. Antimicrob Agents Chemother. 1992;36:2602–2610. doi: 10.1128/aac.36.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with the increases in azole resistance in Candida albicans isolates from a human immunodeficiency virus-infected patient. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White T C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14-α-demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wingard J R, Merz W G, Rinaldi M G, Johnson T R, Karp J E, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;325:1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]
- 38.Wingard J R, Merz W G, Rinaldi M G, Miller C B, Karp J E, Saral R. Association of Torulopsis glabrata infections with fluconazole prophylaxis in neutropenic bone marrow transplant patients. Antimicrob Agents Chemother. 1993;37:1847–1849. doi: 10.1128/aac.37.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wingard J R, Merz W G, Saral R. Candida tropicalis: a major pathogen in immunocompromised patients. Ann Intern Med. 1979;91:539–543. doi: 10.7326/0003-4819-91-4-539. [DOI] [PubMed] [Google Scholar]