Graphical abstract
Keywords: Tocopherol, Grapefruit, Chilling injury, Fruit shading, Postharvest storage, Citrus
Highlights
-
•
Tocopherol content in the flavedo of grapefruit increase during fruit maturation.
-
•
TAT1 and genes of the tocopherol-core pathway are up-regulated during fruit maturation.
-
•
Light avoidance reduces γ-tocopherol and expression of GGDR and tocopherol-core pathway genes.
-
•
Cold up-regulated genes involved in precursors supply but repressed those of the core pathway.
-
•
Changes in tocopherols during storage appears to be cold-mediated and not related to CI tolerance.
Abstract
The aim of this study was to investigate the role of tocopherols in the susceptibility of Star Ruby grapefruit to postharvest chilling injury (CI). Fruit exposed to normal sunlight (NC, non-covered) and deprived of light (C, covered) in the last stages of development were used. Tocopherol contents increased in the flavedo of both NC and C fruit during development, concomitantly with the up-regulation of TAT1 and most genes of the tocopherol-core pathway. Fruit shading reduced total contents by repressing γ-tocopherol accumulation, associated to a down-regulation of GGDR and VTE1 and, to a lesser extent, of VTE2, VTE3a and VTE4. During cold storage, total and α-tocopherol contents increased in NC and C fruit, and no direct relationship between tocopherol accumulation and CI tolerance was found. Cold stress up-regulated most genes involved in the synthesis of tocopherol precursors and down-regulated those of the tocopherol-core pathway, but changes seemed to be cold-mediated and not related to CI development.
1. Introduction
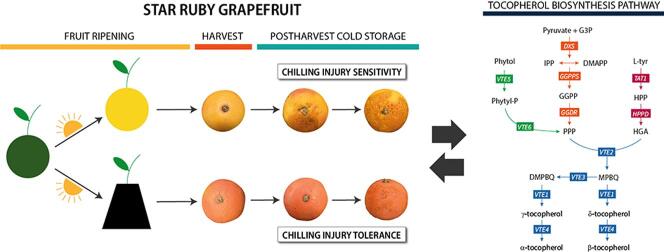
Chilling injury (CI) is an economically important postharvest disorder that reduces external fruit quality, marketability and consumer acceptance, culminating in important economic losses. Because of their subtropical origin, fruit of many species and cultivars of Citrus are prone to develop peel injuries during storage at low temperatures (Lado et al., 2019, Zacarias et al., 2020). Fruit of grapefruit cultivars are highly susceptible to CI when stored at temperatures below 10 °C (Lado et al., 2015, Schirra, 1993). CI in the peel of grapefruit is manifested as a series of symptoms, referred to as peel pitting, which initiates as small brown pit-like depressions that expand forming large necrotic and depressed areas after prolonged cold storage (Lado et al., 2019, Lado et al., 2015). Susceptibility to CI is influenced by many factors, including the Citrus genotype and environmental and agronomical conditions, such as the harvest season, maturity, growing conditions, rootstock, fruit position on the tree canopy and peel pigmentation, among others, that markedly define the initiation and development of CI during postharvest cold storage (Lado et al., 2019, Zacarias et al., 2020).
Oxidative stress is a primary mechanism in the response of Citrus fruit against stress caused by low temperature, and/or by the damage induced under these conditions (Toivonen, 2004). The capability of certain species and cultivars to counteract these processes appears to be essential to determine fruit tolerance or susceptibility to CI under cold stress conditions (Lado et al., 2019, Zacarias et al., 2020). Antioxidant defense mechanisms in plants include detoxifying enzymes and low molecular weight compounds such as ascorbic acid, glutathione, carotenoids and tocopherols (Decros et al., 2019, Toivonen, 2004). Enhanced activity of enzymes of the antioxidant system has been associated with the natural tolerance to CI of several mandarin cultivars (Sala, 1998) and also with the tolerance induced by heat-conditioning treatments (Lafuente, Establés-Ortíz, & González-Candelas, 2017). Among them, a protective role of catalase against chilling has been proposed, as higher catalase activity and increased catalase transcripts have been detected in the peel of tolerant fruit of mandarin and grapefruit (Lado et al., 2016, Maul et al., 2011, Sala, 1998). Ascorbic acid (vitamin C) and glutathione are potent water-soluble antioxidants (Decros et al., 2019) but their relation to CI in Citrus fruit is controversial, as contents in grapefruit and mandarin fruit did not support a direct relationship with CI tolerance (Lado et al., 2016, Rey et al., 2020). Carotenoids, on the other hand, are considered to be the first line of defense against singlet oxygen (Decros et al., 2019) and have been associated with higher tolerance to CI and other postharvest disorders (Cronje et al., 2011, Lado et al., 2015, Rey et al., 2020). In Star Ruby grapefruit, it has been shown that light avoidance at later stages of fruit development led to the accumulation of the red carotene lycopene, which resulted in fruit with a uniform red coloration of the peel and markedly induced fruit tolerance to CI after subsequent storage at low temperature (Lado et al., 2015, Lado et al., 2016). Interestingly, in the red peel of shaded grapefruit, the capacity to quench singlet oxygen was 2–3 times higher than in fruit exposed to light, which were susceptible to CI (Lado et al., 2016). Similarly, in fruit of mandarin cultivars with contrasting susceptibility to CI (Fortune, Nova and Nadorcott), a higher β-cryptoxanthin and violaxanthin content and capacity to quench singlet oxygen was detected in the CI-tolerant cultivars (Rey et al., 2020). Therefore, increased concentrations of carotenoids with enhanced antioxidant capacity may induce tolerance to CI in Citrus fruit. However, little is known about the role of tocopherols, which are potent lipid-soluble antioxidants, on the susceptibility of Citrus fruit to CI.
Tocopherols are plant-derived isoprenoids belonging to the chemical group of tocochromanols, which also includes tocotrienols, plastocromanol 8 and tocomonoenols. Their structure consists of a polar chromanol ring and a lipophilic polyprenyl side chain and, according to the position and degree of methylation of the chromanol ring, four isoforms exist: α-, β-, γ- and δ-tocopherols. Tocopherols are mainly produced in photosynthetic organisms and, together with tocotrienols, are the only organic molecules with vitamin E activity in animals (Mène-Saffrané, 2017, Muñoz and Munné-Bosch, 2019). These compounds have diverse physiological functions in plants, of which their role as antioxidants is among the most relevant, with the capability to scavenge free radicals and quench singlet oxygen, protecting cell membranes from lipid peroxidation and reducing oxidation of cellular components (Falk & Munné-Bosch, 2010). Additionally, the disposition and mobility of tocopherols within cell membranes helps to maintain stability and conformation of membrane’s structure (Boonnoy, Karttunen, & Wong-ekkabut, 2018). Since chilling in plants is associated with oxidative processes and the loss of cell membranes stability/fluidity (Lado et al., 2019, Zacarias et al., 2020), tocopherols could play a role in the protection of fruit against cold stress during postharvest storage by protecting cells against oxidative processes (Boonnoy et al., 2018, Falk and Munné-Bosch, 2010, Ma et al., 2020).
The synthesis of tocopherols in plants occurs mainly in plastids and can be divided into two stages. The first stage consists in the independent synthesis of the precursors homogentisate (HGA) and phytyl pyrophosphate (PPP), while the second stage involves the specific steps and modifications leading to tocopherol formation (Mène-Saffrané, 2017, Muñoz and Munné-Bosch, 2019). The chromanol ring of all tocochromanols derives from the precursor HGA, which results from the degradation of L-tyrosine formed in the Shikimate (SK) pathway (Riewe et al., 2012, Tsegaye et al., 2002). The precursor PPP is specific for tocopherol synthesis, and can be formed directly from GGPP (geranylgeranyl diphosphate) (Ruiz-Sola et al., 2016), produced in the methylerythritol 4-phosphate (MEP) pathway, or alternatively from the recycling of free phytol (Mène-Saffrané, 2017, Pellaud and Mène-Saffrané, 2017). The regulation of key enzymes involved in these steps define HGA and PPP availability and have a direct impact in the final amount of tocopherols accumulated (Pellaud & Mène-Saffrané, 2017). The specific tocopherol pathway, known as the tocopherol-core pathway, starts with the condensation of HGA and PPP, catalyzed by homogentisate phytyl transferase (HPT; VTE2), which forms 2-methyl-6-phytyl-1,4-benzoquinol (MPBQ). MPBQ can produce δ-tocopherol by the action of tocopherol cyclase (TC; VTE1) and later β-tocopherol by the tocopherol methyl transferase (γ-TMT; VTE4); or, it can be methylated into 2,3-dimethyl-6-phytyl-1,4-benzoquinol (DMPBQ) by a MPBQ methyltransferase (MPBQ-MT; VTE3). DMPBQ then produces γ-tocopherol and α-tocopherol by the same TC and γ-TMT. Tocopherol accumulation in vegetative tissues is limited by VTE2 (Collakova and DellaPenna, 2003, Maeda et al., 2006), and also highly influenced by the pathways supplying the precursors HGA and PPP controlling the influx into the tocopherol core-pathway (Muñoz and Munné-Bosch, 2019, Pellaud and Mène-Saffrané, 2017). Nonetheless, other studies have observed that the accumulation of tocopherols in fruit is not markedly influenced by the expression of the VTE2 gene, and that VTE3 seems to play a more pivotal role controlling tocopherol contents (Quadrana et al., 2013).
Accumulation of tocopherols and transcriptional changes of their biosynthetic genes in response to different abiotic stresses have been widely studied in many plant species (Ma et al., 2020, Sadiq et al., 2019). A role of tocopherols in the tolerance of plant species to low temperatures has been suggested, as increases in tocopherol contents have been detected in vegetative tissues in response to low temperatures, and plants lacking tocopherols exhibit a hypersensitive phenotype under cold stress (Bergmüller et al., 2003, Maeda et al., 2006, Wang et al., 2017). The involvement of tocopherols in the response of fruit to postharvest cold storage has received little attention, and only few studies have addressed such relationship in cherry (Tijero, Teribia, Muñoz, & Munné-Bosch, 2016), avocado (Vincent, Mesa, & Munné-Bosch, 2020) and squash fruit (Rodov, Paris, Friedman, Mihiret, Vinokur, & Fennec, 2020). In Citrus fruit, we have recently observed that the natural tolerance of mandarin fruit to postharvest CI is associated with increased tocopherol contents (Rey, Rodrigo, & Zacarías, 2021), and that changes during storage appears to be independent of the development of CI. In order to gain further insights into the potential function of tocopherols in the response of other Citrus, fruit to postharvest cold temperatures, we took advantage of the chilling tolerance of Star Ruby grapefruit induced by fruit shading during the last stages of development (Lado et al., 2015, Lado et al., 2016). Using such system, the objective of the present work was to study the effects of shading and storage at 2 °C on tocopherol concentration, and on the expression of genes of the different tocopherol biosynthetic pathways in Star Ruby grapefruits.
2. Materials and methods
2.1. Plant material and storage conditions
Fruit of Star Ruby grapefruit (Citrus paradisi Macf.) were used throughout this study. Fruit of this cultivar are characterized by their red-colored flesh (due to lycopene accumulation), and they may also develop red coloration in areas of the peel. Accumulation of lycopene in the peel (external red coloration) is stimulated in this genotype by covering fruit and avoiding direct light exposure in the last stages of fruit development, which results in fruit tolerant to the development of CI, in contrast to CI-sensitive control fruit (Lado et al., 2015, Lado et al., 2016). Therefore, in this work we used non-covered (NC) and covered (C) fruit of Star Ruby to explore the involvement of tocopherols in the susceptibility/tolerance to CI. For this purpose, 100 fruits of Star Ruby grapefruit located outside of the tree canopy from four adult trees were covered with black polyethylene plastic bags 5 months before harvest at the immature-green stage (last week of July), leaving the bottom open to allow air circulation as described by Lado et al. (2015). Uncovered control fruit (NC) were located outside the canopy of the same trees, adjacent to C fruit, and exposed to direct sunlight. Orchards were located in the Citrus Germplasm Bank at the Instituto de Investigaciones Agrarias (IVIA, Moncada, Valencia, Spain) and grown under normal agro-ecological conditions. NC and C fruit were harvested at commercial maturity in the first week of January, being the average for maximum and minimum field temperature for the four weeks previous to harvest of 16.8 ± 0.4 °C and 4.3 ± 0.6 °C, respectively. Fruit was delivered to the lab, inspected for color uniformity and external damages, and fruit free of any defect or damage, of both NC and C grapefruits, were randomly separated into two lots and stored at 2 °C and 85% RH for up to 8 weeks. The first lot of fruit was used for collecting samples of flavedo tissue, while the second lot was divided in three replicates of ten fruit (for each NC or C condition) and used for the periodic evaluation of CI. Flavedo samples were collected from the equatorial section of 6–8 fruits at the covering time (CT), harvest (H) and periodically throughout storage (1, 3, 5, 8 weeks). Flavedo tissue was excised with a scalpel, frozen in liquid nitrogen and ground to a fine powder. Samples were stored at −80 °C until analysis. At each date, peel color was measured using a Minolta CR-330 colorimeter (Minolta, Osaka, Japan) on three locations around the equatorial plane of the fruit, using three replicates of 10 fruit for each condition. Color was expressed as the a/b Hunter ratio, which is negative for green fruit, around zero for yellow fruit at color break, and positive for orange and red colored fruit.
2.2. Evaluation of chilling injury
During cold storage, CI was evaluated by inspecting and scoring fruit on a scale according to the extension and severity of the peel damage: 0 (no CI), 1 (low CI, <25% peel surface), 2 (high CI, 25–50% peel surface) and 3 (severe CI > 50% peel surface). Results were expressed as CI index, which was calculated by adding the product of the number of fruit in each category multiplied by the score for each category and afterwards dividing it by the number of total fruits evaluated (Sala, 1998). CI was evaluated in 3 replicates of 10 fruit for each NC and C condition.
2.3. Tocopherol extraction and quantification
Tocopherols were extracted from the flavedo tissue following the protocol described in Fraser, Pinto, Holloway, and Bramley (2000). Briefly, 200 mg of flavedo tissue was extracted with methanol (MeOH, HPLC grade, Scharlau, Barcelona, Spain), Tris buffer plus NaCl (50 mM Tris-HCl pH 7.5/1 M NaCl) and dichloromethane (HPLC grade, Scharlau, Barcelona, Spain). Samples were grounded with a mortar and pestle with sea sand (PanReac AppliChem, Barcelona, Spain) as an abrasive, and the mixture was sonicated in a XUBA3 ultrasonic water bath (Grant Instruments, Cambridge, England) for 5 min. The homogenized sample was centrifuged for 10 min at 3000g and 4 °C for phase separation. The dichloromethane phase was recovered in a glass tube and the methanol phase and sediment were re-extracted with dichloromethane. The pooled dichloromethane extracts were dried under nitrogen gas and stored at −20 °C in a free O2 atmosphere until analysis. For tocopherol quantification, dry extracts were re-suspended in 500–700 µl of ethyl acetate (HPLC grade, Merck, Madrid, Spain) and an aliquot of 20 µl was injected directly in a HPLC system (Acquity® Arc™, Waters, Barcelona, Spain) coupled with a fluorescence detector (2475 FLR Detector, Waters, Barcelona, Spain), and a YMC C30 column (150 × 4.6 mm, 3 µm) (Teknokroma, Barcelona, Spain) at 25 °C. A ternary gradient elution at a flow rate of 1 ml/min was used for tocopherol separation. The initial solvent composition consisted of 90% of MeOH, 5% water and 5% methyl tert-butyl ether (MTBE, HPLC grade, Scharlau, Barcelona, Spain). After 12 min at these conditions, the solvent composition changed linearly to 95% MeOH and 5% MTBE, and after 2 min to 50% MeOH and 50% MTBE. These conditions were maintained for 5 min, after which the solvent composition was gradually re-established to initial conditions (min 20), and equilibrated for 10 min before the next injection. Compounds were detected by fluorescence with excitation at 296 nm and emission at 340 nm, and standards for δ-, γ- and α-tocopherol (SigmaAldrich, Barcelona, Spain) were used for the identification and quantification of tocopherols. All procedures were carried out on ice and under dim light to prevent degradation. Total tocopherol content was calculated as the sum of the tocopherol isoforms detected, and concentrations are expressed as µg/g of fresh weight. Samples were extracted twice and results are the mean of the replicates (mean ± standard error).
2.4. Total RNA extraction, cDNA synthesis and q-PCR conditions
Total RNA was extracted from flavedo tissue using the RNeasy Plant Mini Kit (Qiagen, Madrid, Spain), following manufacturer’s instructions. For cDNA synthesis, total RNA was treated with DNase I (DNA free, DNase treatment & removal, Ambion, Barcelona, Spain) and RNA concentration was measured by spectrophotometric analysis (Nanodrop, Thermo Fisher Scientific, Barcelona, Spain). The quality of the RNA was verified by agarose gel electrophoresis with GoodView™ Nucleic Acid Stain (SBS Genetech, Beijing, China). Five µg of total RNA were reverse-transcribed using the SuperScript III Reverse Transcriptase (Invitrogen, Barcelona, Spain) in a total volume of 20 µl, following the manufacturer’s procedure. First-strand cDNA samples were diluted 1:5 to a final concentration of approximately 100 ng/µl for each amplification reaction.
Quantitative real-time PCR was performed using a LightCycler 480 instrument (Roche, Madrid, Spain), using the LightCycler 480 SYBRGreen I Master kit (Roche, Madrid, Spain) and following the manufacturer’s instructions. The relative expression was measured for 14 genes related to: i) the SK pathway supplying the tocopherol precursor HGA (TAT1 and HPPD); ii) the MEP pathway supplying the precursor PPP (DXS1 and DXS2, GGPPS1 and GGPPS6 and GGDR); iii) the recycling of free phytol into PPP (VTE5 and VTE6); iv) and to the tocopherol-core pathway (VTE2, VTE3a and VTE3b, VTE1 and VTE4). These genes were selected on the basis of their putative function and the activity of the corresponding enzyme in the metabolic pathway of tocopherol biosynthesis in plants (Mène-Saffrané, 2017, Pellaud and Mène-Saffrané, 2017, Quadrana et al., 2013). Moreover, the selected genes were previously identified in the Citrus genome based on homology to Arabidopsis thaliana and Solanum lycopersicum protein sequences and the primers designed for each gene were successfully used for analysis of gene expression in the peel of mandarin fruits (Rey et al., 2021). Details of the genes and primers sequences used are listed in Table S1. The cycling protocol, for all genes analyzed, consisted of 10 min at 95 °C for pre-incubation, 40 cycles of 10 s at 95 °C for denaturation, 10 s at 59 °C for annealing and 10 s at 72 °C for extension. Specificity of the PCR reaction was assessed by the presence of a single peak in the dissociation curve performed after the amplification steps. For expression measurements, we used the LightCycler 480 Software release 1.5.0, version 1.5.0.39 (Roche, Madrid, Spain) and calculated expression levels relative to values of a reference sample using the Relative Expression Software Tool (Pfaffl, Horgan, & Dempfle, 2002). For all genes and all dates analyzed the reference sample used to calculate relative expression was the expression value for covered fruit of Star Ruby at harvest, which was set as 1. The housekeeping gene used for normalization was ACTIN (Alós, Rodrigo, & Zacarías, 2014). The reference sample used for all genes was the expression values for covered fruit of Star Ruby at harvest.
2.5. Statistical analyses
Unpaired Student’s t-test was used to determine statistically mean differences between NC and C fruit in each sample date (significance level at p ≤ 0.05). Additionally, a one-way ANOVA was carried out and Tukey’s test (significance level at p ≤ 0.05) was used for mean comparisons among dates for each fruit condition (NC or C fruit). Analyses were performed using SigmaPlot version 14.0 (Systat Software, USA).
3. Results and discussion
3.1. Fruit shading and cold storage influenced tocopherol accumulation in the flavedo of Star Ruby grapefruit
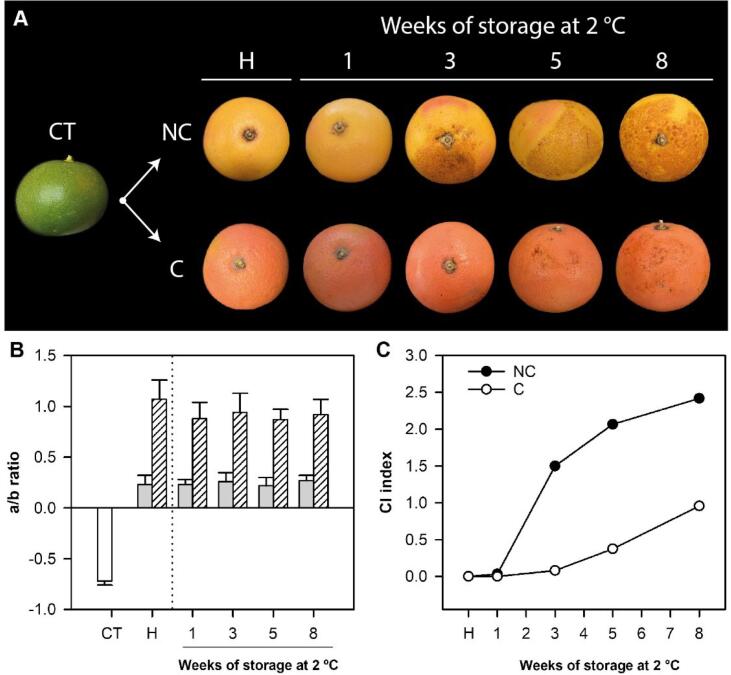
As a first step to understand the involvement of tocopherols, as potent lipid-soluble antioxidants, in the tolerance of Citrus fruit to CI, the concentration of tocopherols was determined in the flavedo of NC and C Star Ruby grapefruits, which displayed contrasting susceptibility to CI. For that purpose, immature fruit of Star Ruby grapefruit with an external green coloration were covered (C) in July with black bags to avoid light exposure during fruit ripening, whereas non-covered (NC) fruit were located outside of the fruit canopy and exposed to direct sunlight. Both fruit were harvested at the first week of January and were similar in size but with different internal maturity. Total soluble solids and acidity were higher in the juice of NC than in C fruit, resulting in a lower internal maturity index, 5.48 and 7.14, respectively (Table S2). These results may be indicative of an accelerated internal maturation in the pulp in covered conditions, similarly to the effect of shading on accelerating peel degreening, triggering the earlier degradation of chlorophylls and the accumulation of carotenoids (Lado, Cronje, et al., 2015). Moreover, at harvest, marked differences in peel coloration (Fig. 1A and B) between NC and C fruit were detected. C fruit developed a uniform red peel coloration, whereas peel color of NC fruit was pale-yellow with pale red shades in some areas of the fruit surface less exposed to sunlight (Fig. 1A). This effect was clearly reflected by the a/b Hunter ratio, which was around 1 for C fruit and 0.2, typical for yellow coloration, for NC fruit (Fig. 1B). During storage at 2 °C, peel coloration remained nearly constant in both NC and C fruit (Fig. 1B). NC fruit were highly sensitive to CI, with symptoms appearing after 1 week and increasing substantially during storage, reaching a CI index near 2.5 (severe damage) after 8 weeks at 2 °C. On the other hand, and as observed in previous studies (Lado et al., 2015, Lado et al., 2016), C fruit developed chilling symptoms at a much lower rate and, after 8 weeks, CI index was less than 1 (slight damage) (Fig. 1A and C).
Fig. 1.
External appearance (A), rind color expressed as a/b ratio (B) and development of CI expressed as CI index (C) of immature-green fruit at the covering time (CT), and of non-covered (NC) and covered (C) fruits of Star Ruby grapefruit at harvest (H) and during postharvest storage at 2 °C for up to 8 weeks. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
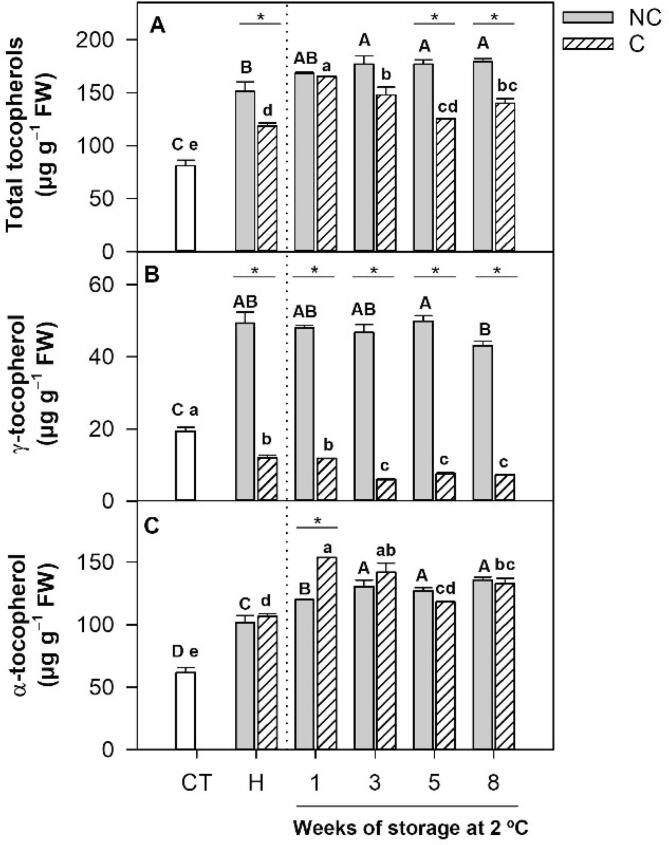
The HPLC-FLR analysis of tocopherols revealed that only the isoforms α- and γ-tocopherol were accumulated in the flavedo of NC and C Star Ruby grapefruit, and that α-tocopherol was the predominant form accumulated (Fig. 2). In other Citrus species, such as mandarin (Rey et al., 2021) and orange (Assefa, Saini, & Keum, 2017), α-tocopherol has been reported as the main tocopherol isoform, although the predominance of γ-tocopherol has also been described in the peel of fruit of other less common Citrus species (Assefa et al., 2017). Fruit shading significantly reduced total tocopherol content at harvest (Fig. 2A). In immature-green fruit, just before covering, total tocopherols were 81 µg/g, with α-tocopherol accounting for 76% and γ-tocopherol for 24% of total contents. During fruit development and maturation, α-tocopherol increased to a similar extent in NC and C (Fig. 2C), but fruit shading drastically impaired the increment of γ-tocopherol in flavedo of mature fruit, which was 4-times lower in C fruit (Fig. 2B). As a result of these changes, total tocopherols were lower in the flavedo of C than in NC fruit at harvest, and the proportion of α- and γ-tocopherol was substantially altered, with α-tocopherol accounting for 67–76% of the total in NC fruit in comparison to 89–95% in C fruit (Fig. 2). These results clearly indicate a differential effect of light on the accumulation of the different tocopherols isoforms in the peel of Star Ruby grapefruit. Previous experiments in Star Ruby revealed that shading altered the accumulation of carotenoids and vitamin C, and also influenced the expression of key genes of the biosynthetic pathways of these compounds (Lado et al., 2015, Lado et al., 2015, Lado et al., 2016). A similar effect of light on tocopherol synthesis has been recently reported in tomato fruit, in which the accumulation of tocopherols was reduced in fruit grown under darkness, associated with a down-regulation of several genes of their synthesis (Gramegna et al., 2019). Then, the concentration of tocopherols increased in the peel of grapefruit during the transition from fruit development to maturation, and it is likely that light avoidance affects metabolic steps regulating the accumulation of γ-tocopherol.
Fig. 2.
Total tocopherol (A), γ-tocopherol (B) and α-tocopherol content (C) in the flavedo of non-covered (NC) and covered (C) fruits of Star Ruby grapefruit, at the covering time (CT), harvest (H) and during postharvest storage at 2 °C for up to 8 weeks. Letters indicate significant differences in tocopherol contents among dates for each fruit condition, capital letters for NC fruit and lowercase letters for C fruit (p ≤ 0.05, Tukey test). Asterisks indicate significant differences between NC and C fruit (p ≤ 0.05, t-test). Results are expressed as µg/g of fresh weight.
Exposure of Star Ruby grapefruits to cold storage provoked a moderate increase in total tocopherol content in both NC and C fruit (Fig. 2A), although the effects on α- and γ-tocopherol accumulation were different. The concentration of γ-tocopherol remained nearly constant in NC fruit while the low content in C fruit decreased during storage (50% after 3 weeks) (Fig. 2B). α-Tocopherol increased progressively during the whole storage period in both NC and C (30% after 3 weeks), but with minor differences between both conditions (Fig. 2C). Because of these changes, total tocopherol content increased during cold storage in both conditions, but were still higher in NC than in C fruit (Fig. 2A). Increases in tocopherol content in response to low temperatures have been observed in vegetative tissues (Bergmüller et al., 2003, Ma et al., 2020, Maeda et al., 2006), but information in fruit is scarce and variable. While cold storage increased α-tocopherol content in cherries (Tijero et al., 2016) it decreased them in fruit of different avocado cultivars (Vincent et al., 2020).
Our results in Star Ruby grapefruits indicate that: (i) fruit shading reduced total tocopherols in the peel of C fruit, which were more tolerant to CI than NC fruit; (ii) this effect was more notable in γ-tocopherol contents, which were 4- to 8-times lower in C fruit; and (iii) changes in α- and total tocopherol contents during storage were similar in both conditions. Together, it appears that the changes in tocopherols during cold storage are a response of the fruit to low temperature stress, and that the positive relation between shading and the tolerance to CI of Star Ruby grapefruit appears to be independent of tocopherol levels at harvest and during the storage period. In mandarin fruit, a direct association between tocopherol content at harvest and the tolerance to CI during cold storage has been observed (Rey et al., 2021). Similarly, in squash fruit stored at 4 °C, a higher resistance to CI was detected in genotypes accumulating higher tocopherol contents (Rodov et al., 2020). A possible explanation for the differences observed among Citrus species would take into account a differential function of tocopherols in the response of fruit to CI or, alternatively, that differences in tocopherol concentrations may be associated with the natural tolerance to CI (such as in mandarin varieties) but not with that induced by environmental stimuli, as light avoidance in Star Ruby grapefruits. It is important to remark that light deprivation in Star Ruby grapefruit triggers the accumulation of the carotene lycopene, which is detected at trace amounts in the peel of control fruit (Lado, Rodrigo et al., 2015). Lycopene shares similar antioxidant properties with tocopherols and both compounds are considered highly efficient singlet oxygen quenchers (Decros et al., 2019). Therefore, the tolerance of Star Ruby C fruit to CI is primarily related to the differential accumulation of this carotene (Lado et al., 2015, Lado et al., 2016) rather than to the tocopherol content.
3.2. Transcriptional regulation of tocopherol biosynthesis in the flavedo of Star Ruby grapefruit
In order to understand the effect of fruit shading during development and ripening and of cold storage on the regulation of tocopherol synthesis, and whether the alterations in gene expression are associated with the changes in tocopherol concentration, the expression of 14 genes involved in different steps of tocopherol synthesis were analyzed in the flavedo of NC and C grapefruits. Tocopherol biosynthesis requires the condensation of the precursors PPP and HGA, and the steps controlling their availability have been described to be as important as the specific steps of tocopherol synthesis in determining the final tocopherol content (Mène-Saffrané, 2017, Pellaud and Mène-Saffrané, 2017). Therefore, we analyzed the expression of genes regulating HGA supply through the SK pathway (TAT1 and HPPD), genes regulating PPP supply through the recycling of phytol (VTE5 and VTE6) and through the MEP pathway (DXS1, DXS2, GGPPS1, GGPPS6 and GGDR), and genes belonging to the tocopherol-core pathway (VTE2, VTE3a, VTE3b, VTE1 and VTE4) regulating the final concentration and composition of tocopherols.
3.2.1. Effect of fruit shading
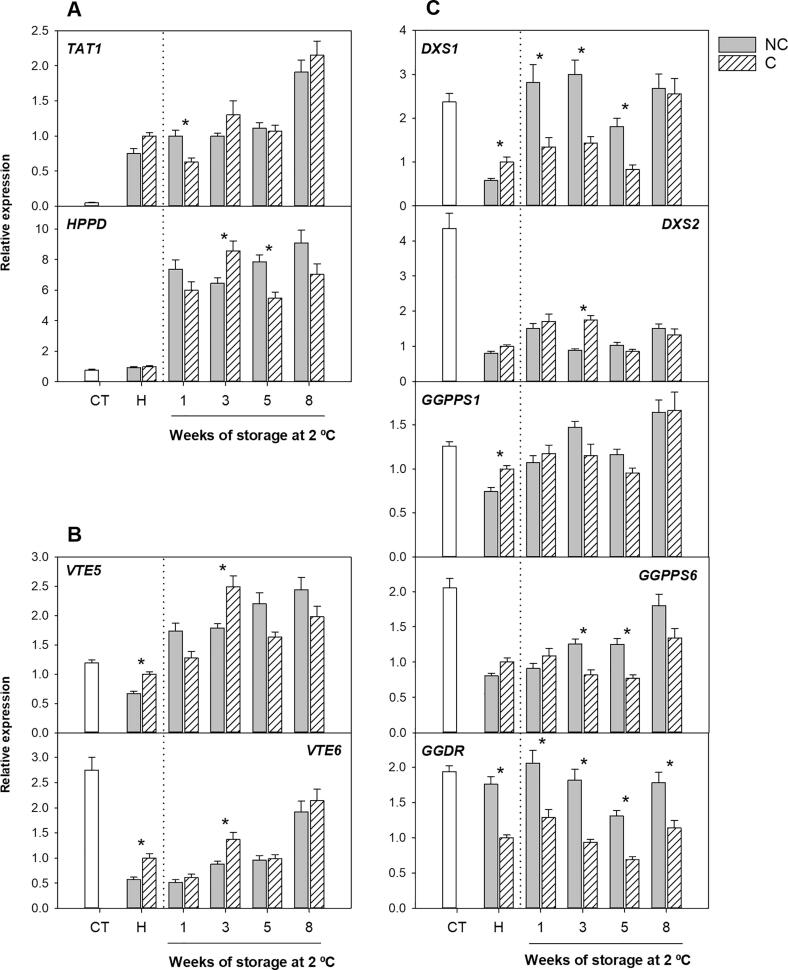
Fruit shading did not affect the expression of the two genes involved in the supply of HGA, but these genes were differentially expressed during fruit ripening (Fig. 3A). Transcripts of TAT1 experienced a 15-fold increase from immature (CT) to ripe fruit, whereas those of HPPD remained nearly constant. Interestingly, these changes were similar in both NC and C, indicating that the regulation of these genes was independent of light exposure. On the other hand, the expression of VTE5 and VTE6 genes, which are involved in the supply of PPP by the recycling of phytol, were slightly or markedly down-regulated during development and ripening, respectively, but transcript levels were higher in fruit kept in the darkness indicating that light may play a negative effect on the expression of these genes (Fig. 3B). It is interesting that, of these genes, only TAT1 was enhanced during fruit development and maturation with minor differences in NC and C fruit, suggesting that this step may contribute to the increase in total tocopherols observed at harvest in both conditions (Fig. 2A). Transcriptional levels and enzymatic activity of TAT have been related to tocopherol content in leaves of Arabidopsis (Riewe et al., 2012), and also appear to be involved in tocopherol accumulation in tomato fruit (Quadrana et al., 2013).
Fig. 3.
Relative expression of the genes involved in the synthesis of HGA (homogentisate) through the SK pathway (A), and in the synthesis of PPP (phytyl pyrophosphate) through the recycling of free phytol (B) and MEP pathway (C) in the flavedo of non-covered (NC) and covered (C) fruits of Star Ruby grapefruit, at the covering time (CT), harvest (H) and during postharvest storage at 2 °C for up to 8 weeks. The genes analyzed were: TAT1 (tyrosine aminotransferase), HPPD (4-hydroxyphenylpyruvate dioxygenase), VTE5 (phytol kinase), VTE6 (phytyl-P kinase), DXS1 and DXS2 (1-deoxy-D-xylulose-5-phosphate synthase 1 and 2), GGPPS1 and GGPPS6 (geranylgeranyl pyrophosphate synthase 1 and 6) and GGDR (geranylgeranyl diphosphate reductase). An expression value of 1 was arbitrarily assigned to the values obtained in the flavedo of C fruit at harvest. The data are mean ± S.E of at least three replicates.
Most genes involved in the MEP pathway, the other source of PPP, were substantially down-regulated during fruit development, and GGDR was the only gene strongly repressed by light deprivation (Fig. 3C). From immature to ripe fruit, DXS1 and DXS2 experienced a dramatic reduction in their expression (2.5- and 5-times, respectively), and interestingly, transcripts accumulation of DXS1 and GGPPS1 were significantly higher in the flavedo of C than in NC fruit at harvest. The enzyme DXS limits the influx into the MEP pathway by determining the amount of IPP available (Estévez et al., 2001, Rodríguez-Concepción and Boronat, 2015), while GGPPS regulates the synthesis of GGPP necessary for PPP (Ruiz-Sola et al., 2016). Many GGPPS paralogues have been identified in Arabidopsis and tomato, but AtGGPPS11 (the orthologous of GGPPS6 in Citrus), and SlGGPPS1, 2 and 3 seem to be the main forms responsible for the synthesis of GGPP (Barja et al., 2021, Ruiz-Sola et al., 2016). Genes of MEP pathway are subject to multiple levels of regulation, including several environmental signals, and light has been demonstrated to be an essential promotor of their transcriptions (Rodríguez-Concepción & Boronat, 2015). Therefore, the higher expression of DXS1 and GGPPS1 in the flavedo of C fruit was an unexpected result. Previous experiments have shown an early repression of DXS and GGPPS genes in bagged fruit of grapefruit, although differences were not maintained in ripe fruit (Lado, Cronje, et al., 2015). Nonetheless, it is important to highlight that many intermediates of the MEP pathway are metabolic precursors for the synthesis of other plant isoprenoids (Rodríguez-Concepción & Boronat, 2015), and that many genes of this pathway are post-transcriptionally regulated (Hemmerlin, 2013). Thus, it is reasonable to assume that the down-regulation of genes of the MEP pathway during maturation does not necessarily implicate a reduction in tocopherol concentration, or that the higher expression of DXS1 and GGPPS1 in C fruit directly translates in higher contents.
The most remarkable effect of light deprivation during development and maturation on the genes of the MEP pathway was the reduction of GGDR (Fig. 3C). This gene controls the reduction of GGPP into PPP and thus, directly regulates the availability of PPP for tocopherol synthesis (Pellaud & Mène-Saffrané, 2017). A recent study in tomato fruit found a substantial down-regulation of this gene in plants grown under darkness (Gramegna et al., 2019). This effect was associated with the interaction of a phytochrome-interacting factor with the promoter region of GGDR in the absence of light, which negatively regulates GGDR expression in tomato fruit (Gramegna et al., 2019). Our results indicate that the expression of GGDR is also light-regulated in the peel of Star Ruby grapefruit and correlated with tocopherol accumulation, supporting the notion that the expression of GGDR gene may be a key mechanism regulating tocopherol contents in the peel of Citrus fruit (Rey et al., 2021).
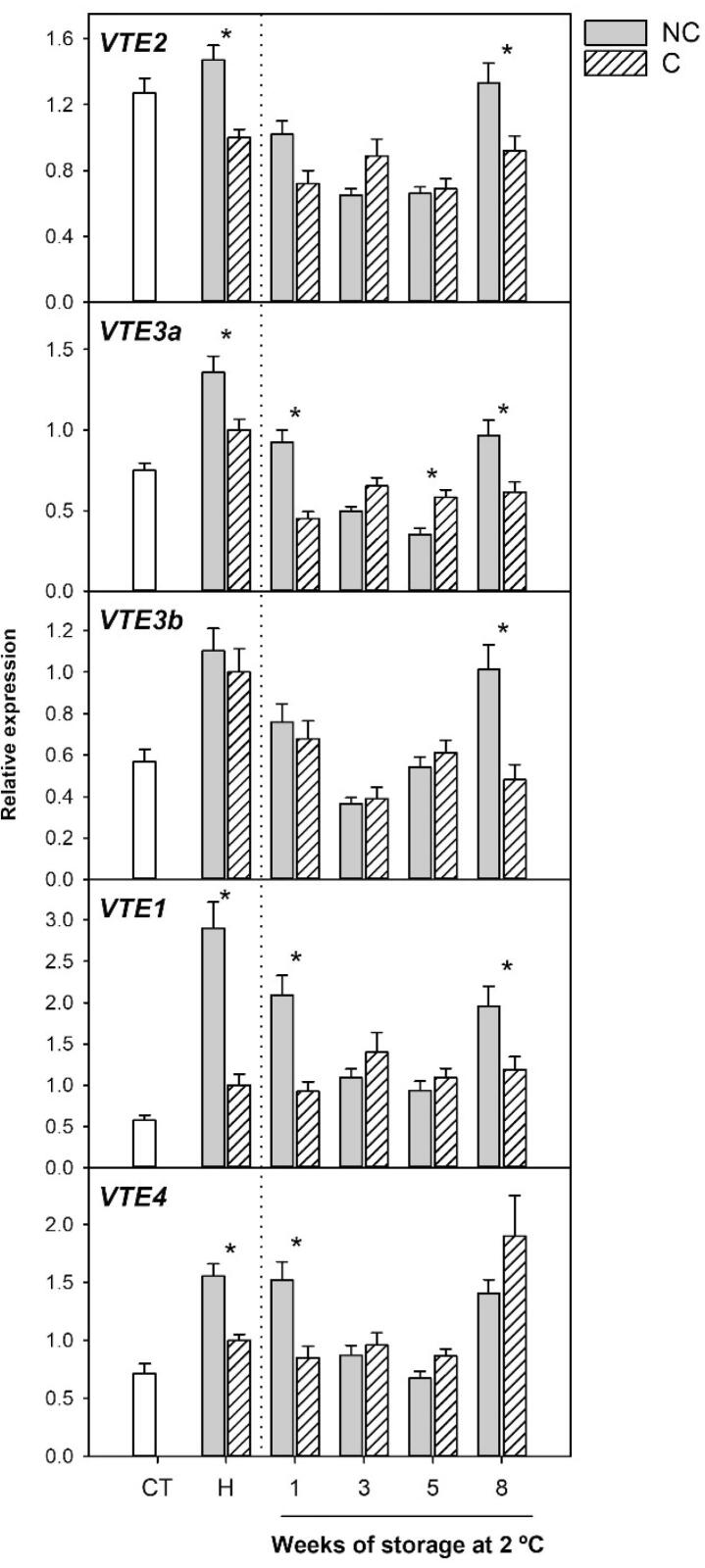
Regarding the genes of the tocopherol-core pathway, their expressions were in general up-regulated in the flavedo of NC grapefruit during maturation and, with the exception of VTE3b, accumulation of the transcripts was significantly lower in C fruit than in NC (Fig. 4). These results indicate that the genes of the tocopherol-core pathway are stimulated during development and ripening of grapefruit under normal environmental conditions, in accordance with the increase in tocopherol content (Fig. 2). Interestingly, the absence of light impaired the enhancement of VTE2, VTE3a, VTE1 and VTE4 genes in C fruit, as expression levels were similar to those before fruit bagging. Likewise, in tomato, a down-regulation of the genes VTE2, VTE1 and VTE4 has also been observed in dark-grown fruit (Gramegna et al., 2019).
Fig. 4.
Relative expression of the genes of the tocopherol-core pathway in the flavedo of non-covered (NC) and covered (C) fruits of Star Ruby grapefruit, at the covering time (CT), harvest (H) and during postharvest storage at 2 °C for up to 8 weeks. The genes analyzed were VTE2 (homogentisate phytyl transferase), VTE3a and VTE3b (2-methyl-6-phytyl-1,4-benzoquinol methyltransferase a and b), VTE1 (tocopherol cyclase) and VTE4 (γ-TMT, γ-tocopherol methyltransferase. An expression value of 1 was arbitrarily assigned to the values obtained in the flavedo of C fruit at harvest. The data are mean ± S.E of at least three replicates.
Tocopherol accumulation is highly dependent on the availability of the precursors PPP and HGA, and the up-regulation of the genes involved in their biosynthetic pathways usually translates in higher availability of precursors and hence higher tocopherol contents (Pellaud & Mène-Saffrané, 2017). In vegetative tissues, the tocopherol-core genes (with the exception of VTE2 and VTE1) are thought to play a role in shaping tocopherol composition rather than defining the contents, but transcriptional studies in tomato fruit have revealed the importance of VTE3 in regulating tocopherol accumulation in fruit tissues (Quadrana et al., 2013). Our results in grapefruits suggest that the upsurge in total tocopherols in both NC and C fruit during maturation could be due to the combined up-regulation of TAT1 (Fig. 3A), involved in HGA synthesis, and most of the genes of the tocopherol-core pathway (Fig. 4).
One of the most remarkable effects of light avoidance in Star Ruby grapefruits was the reduction of γ-tocopherol during development and maturation, as the content in C fruit was 4-times lower than in NC fruit (Fig. 2B). Consequently, a moderated reduction in total tocopherols was also detected (Fig. 2A). These reductions in contents under dark conditions could be related to the reduced expression of GGDR, VTE1, VTE4, VTE3a and VTE2 in C fruit (Fig. 3C and 4). Similarly, Gramegna et al. (2019) concluded that the down-regulation of the upstream gene GGDR and downstream genes VTE2, VTE1 and VTE4 explained the low levels of tocopherols accumulating in dark-grown tomato fruit. Although the expression of many genes (DXS1, GGPPS1, VTE5 and VTE6) involved in the formation of PPP were significantly higher in C fruit (Fig. 3B and C), this was not translated into a higher tocopherol content. This suggests that the reduction in the levels of GGDR transcripts by light avoidance play a pivotal role controlling the supply of the precursor PPP for condensation with HGA. Therefore, the enzyme GGDR is revealed as a limiting step in tocopherol accumulation in grapefruit peel, reinforcing its role in tocopherol accumulation in fruit tissues of different species (Georgiadou et al., 2019, Gramegna et al., 2019, Quadrana et al., 2013, Rey et al., 2021). It is noteworthy that, besides the shade-induced repression of GGDR, four of the five genes of the tocopherol-core pathway analyzed were also affected by light avoidance. This effect was more prominent for VTE1 (transcript accumulation was 3-times lower in C than in NC fruit) than in any other gene of this pathway (Fig. 4). The gene VTE1 encodes for the enzyme tocopherol cyclase, which catalyzes the conversion of DMPBQ into γ-tocopherol, and vte1 mutant plants lack all tocopherol isoforms and accumulate the intermediate DMPBQ (Porfirova, Bergmuller, Tropf, Lemke, & Dormann, 2002). A reasonable hypothesis to explain the reduction of γ-tocopherol provoked by fruit shading would take into account the down-regulation of GGDR, and to a lesser extent of VTE2 and VTE3a, and the marked impairment of VTE1 induction during ripening (Fig. 2, Fig. 3, Fig. 4). Then, a reduction in the precursor supply and in the conversion of DMPBQ to γ-tocopherol, and a non-limiting rate of conversion into α-tocopherol by VTE4, may lead to a lower γ-tocopherol pool in C grapefruits.
3.2.2. Effect of cold storage
The expression profiling of the genes involved in the different pathways of tocopherol biosynthesis were analyzed in the peel of NC and C fruit stored at 2 °C for up to 8 weeks. In general, cold temperatures induced an up-regulation of the genes involved in the synthesis of tocopherol precursors in the flavedo of both NC and C fruit of SR grapefruit (Fig. 3).
The most marked effect of cold storage was in the expression of the HPPD gene, with a near 7-fold increment only 1 week after storage at 2 °C in both NC and C fruit. Levels of HPPD transcripts remained with minor variations after subsequent storage. The other gene of this pathway, TAT1, also increased but only at the end of the storage (Fig. 3A). Similarly, an induction of HPPD by cold storage has also been observed in other species, like alfalfa and lettuce, in response to other abiotic stresses, suggesting the involvement of HPPD in plant response to different stresses (Ma et al., 2020). Overexpression of this gene has increased tocopherol content in seeds and leaves of Arabidopsis and oilseed crops (Tsegaye et al., 2002), and thus could be contributing to the increased tocopherol contents in both NC and C fruit. However, it is important to indicate that the induction of HPPD under cold temperatures does not necessarily indicate a higher HGA availability for tocopherol production, since HGA is a common precursor for all tocochromanols. In relation to this, plastochromanol-8 and its precursor plastoquinone-9 are potent singlet oxygen quenchers and have also been induced in response to abiotic stresses (Liu & Lu, 2016), but their potential role under cold stress is still unclear.
The genes VTE5 and VTE6, involved in the supply of PPP through the recycling of phytol formed during chlorophyll degradation, were also cold-responsive. Both genes were stimulated by cold, at early stages of the storage period for VTE5 and at the end for VTE6 (Fig. 3B). In general, the effect of cold was similar in NC and C fruit, indicating that fruit shading does not affect PPP supply through this pathway during cold storage.
Genes of the MEP pathway were in general stimulated by postharvest cold storage, although each gene was induced at a different stage of storage. DXS1 was the only gene of this pathway that was more stimulated by cold in NC than in C fruit. Other genes, like GGPPS6 showed differences between both conditions after 3 weeks of storage. Interestingly, the differences in transcript levels of GGDR induced by shading were maintained during cold storage, and transcript accumulation was always higher in NC than in C fruit (Fig. 3C), similarly to the effect of cold stress on these genes in other species (Xu et al., 2019). Since this metabolic pathway, which provides the precursor PPP in combination with the supply through the recycling of phytol, is enhanced during storage it indicates that the metabolic precursor for tocopherol synthesis appears to be ensured during prolonged cold storage. These results reinforce the notion that in Citrus fruit the transcriptional changes in the genes of this pathway appear to be cold-responses rather than being associated with the susceptibility to CI (Rey et al., 2021).
Cold storage, in general, produced a similar effect in most genes of the tocopherol-core pathway in NC fruit: a decline at the middle of the storage period and a later increase by week 8 (Fig. 4). In C fruit, in which transcripts levels were lower than in NC before storage, low temperature reduced (VTE3a, VTE3b) or maintained (VTE2, VTE1 and VTE4) accumulation of the corresponding mRNAs, but differences between both conditions were in general maintained. In green tissues under high-light stress, drought or nutrient deficiency, changes in VTE2, VTE1 and VTE4 transcripts have been detected, supporting their role in the response of plants to stress conditions (Collakova and DellaPenna, 2003, Ma et al., 2020).
Collectively, the changes in gene expression during storage of NC and C grapefruits at low temperature indicated that the up-regulation of most genes involved in the synthesis of tocopherol precursor’s (Fig. 3) may explain the slight increase in total and α-tocopherol contents during cold storage (Fig. 2A and C). Moreover, the down regulation or maintenance of the tocopherol-core genes was not limiting for tocopherol accumulation. Finally, most of the changes in gene expression during cold storage appeared to be cold-mediated responses not related to the tolerance of Star Ruby grapefruits to CI.
4. Conclusions
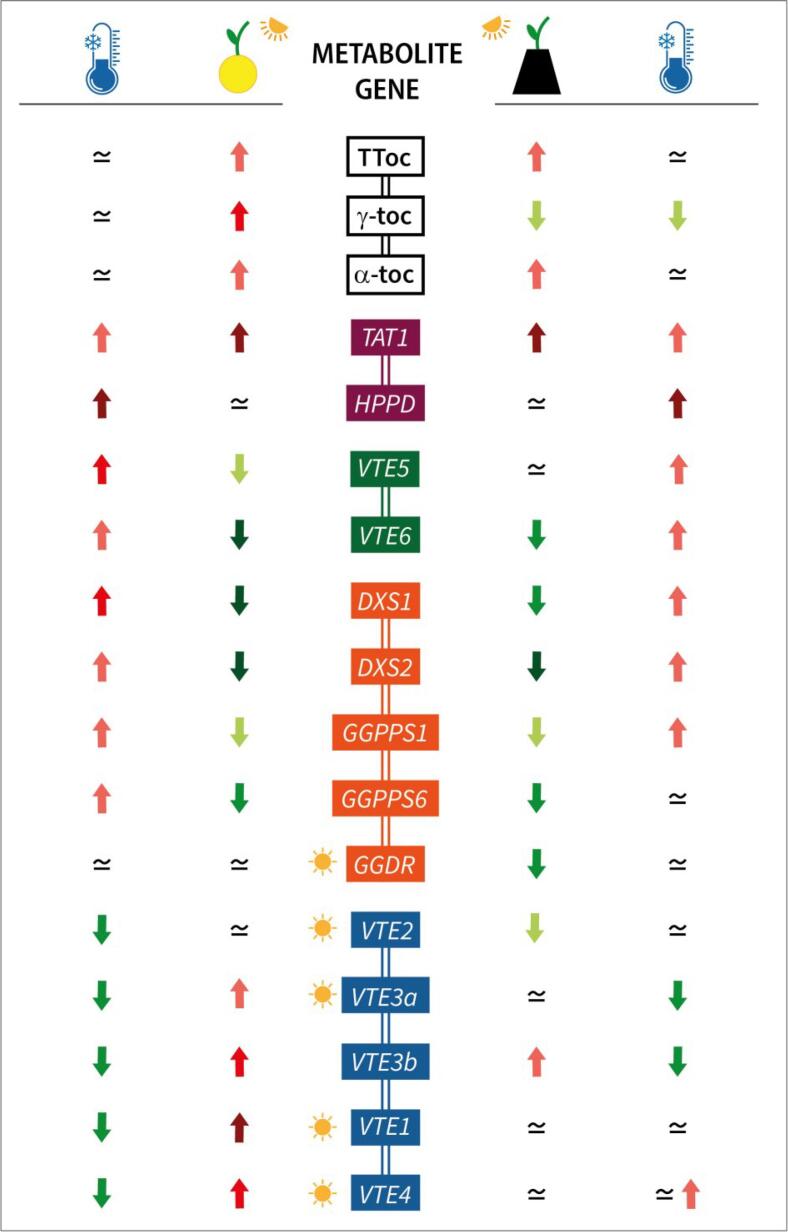
Fruit shading of immature green Star Ruby grapefruit was effective in conferring tolerance to CI during cold storage. Compared to immature grapefruit, total, α- and γ-tocopherol increased in mature Star Ruby fruit, but light avoidance during ripening repressed the accumulation of γ-tocopherol and reduced total content. The effect of light deprivation during maturation and cold storage on tocopherol contents and the transcriptional profiling of tocopherol biosynthetic genes are summarized in Fig. 5. During development and maturation, expression of TAT1 increased, while the expression of genes involved in PPP supply, either through the recycling of phytol or the MEP pathway, declined. On the other hand, genes of the tocopherol-core pathway were stimulated, and this was more significantly in light-exposed fruit. Genes specifically repressed in darkness (or light-stimulated) were GGDR, VTE1, VTE4, VTE3a, and VTE2, which appear to be key for tocopherol synthesis and accumulation. Interestingly, in both NC and C fruit cold enhanced the expression of genes involved in the synthesis of precursors, particularly HPPD, which were in general repressed during maturation (except for TAT1 and HPPD). By contrast, genes of the core-pathway were down-regulated by cold when they had been stimulated during development. To our knowledge, this is the first report describing the synthesis and accumulation of tocopherol in grapefruits during fruit ripening and during cold storage, and exemplified the complexity of the regulatory network and signals modulating tocopherol biosynthesis in the peel of grapefruits, with specific and common responses to fruit shading and cold stress.
Fig. 5.
Representation summarizing the changes in tocopherol contents (TToc, total tocopherols; α-toc, α-tocopherol; γ-toc, γ-tocopherol) and gene expression during fruit ripening (covering time vs. harvest) and cold storage (harvest vs. storage), in non-covered (left) and covered fruits (right) of Star Ruby grapefruits. Red arrows indicate up-regulation, while green arrows indicate down-regulation, and the color intensity of the arrows represents the magnitude of the change. The symbol ≃ indicates similar expression values. Genes particularly affected by light deprivation are highlighted with a sun symbol. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
CRediT authorship contribution statement
Conceptualization, G.D, M.J.R. and L.Z.; Methodology, F.R, M.J.R. and L.Z.; Experimental work, Formal analysis and Data curation, F.R; Writing – original draft preparation, F.R; Writing – review, F.R., G.D., M.J.R. and L.Z.; Supervision and Funding acquisition, M.J.R. and L.Z. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by research grants RTI2018-095131-B-I00 of the Ministry of Science and Innovation (Spanish Government). F. Rey is the recipient of a PhD scholarship (POS_EXT_2016_1_133720) from ANII (Uruguay). The authors acknowledge the Citrus Germplasm Bank of Instituto Valenciano de Investigaciones Agrarias (IVIA, Generalitat Valenciana) for the provision of fruits. The excellent technical assistance of Mª C. Gurrea and I. Carbonell is gratefully acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2021.100037.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alós E., Rodrigo M.J., Zacarías L. Differential transcriptional regulation of l-ascorbic acid content in peel and pulp of citrus fruits during development and maturation. Planta. 2014;239(5):1113–1128. doi: 10.1007/s00425-014-2044-z. [DOI] [PubMed] [Google Scholar]
- Assefa A.D., Saini R.K., Keum Y.-S. Fatty acids, tocopherols, phenolic and antioxidant properties of six citrus fruit species: A comparative study. Journal of Food Measurement and Characterization. 2017;11(4):1665–1675. doi: 10.1007/s11694-017-9546-x. [DOI] [Google Scholar]
- Barja M.V., Ezquerro M., Beretta S., Diretto G., Florez‐Sarasa I., Feixes E.…Rodríguez‐Concepción M. Several geranylgeranyl diphosphate synthase isoforms supply metabolic substrates for carotenoid biosynthesis in tomato. New Phytologist. 2021;231(1):255–272. doi: 10.1111/nph.v231.110.1111/nph.17283. [DOI] [PubMed] [Google Scholar]
- Bergmüller E., Porfirova S., Dörmann P. Characterization of an Arabidopsis mutant deficient in γ-tocopherol methyltransferase. Plant Molecular Biology. 2003;52(6):1181–1190. doi: 10.1023/B:PLAN.0000004307.62398.91. [DOI] [PubMed] [Google Scholar]
- Boonnoy P., Karttunen M., Wong-ekkabut J. Does α-tocopherol flip-flop help to protect membranes against oxidation? Journal of Physical Chemistry B. 2018;122(45):10362–10370. doi: 10.1021/acs.jpcb.8b09064. [DOI] [PubMed] [Google Scholar]
- Collakova E., DellaPenna D. The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regulation of tocopherol synthesis during abiotic stress. Plant Physiology. 2003;133(2):930–940. doi: 10.1104/pp.103.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronje P.J.R., Barry G.H., Huysamer M. Postharvest rind breakdown of ‘Nules Clementine’ mandarin is influenced by ethylene application, storage temperature and storage duration. Postharvest Biology and Technology. 2011;60(3):192–201. doi: 10.1016/j.postharvbio.2011.01.009. [DOI] [Google Scholar]
- Decros, G., Baldet, P., Beauvoit, B., Stevens, R., Flandin, A., Colombié, S., … Pétriacq, P. (2019). Get the Balance Right: ROS Homeostasis and Redox Signalling in Fruit. Frontiers in Plant Science, 10(September). DOI:10.3389/fpls.2019.01091. [DOI] [PMC free article] [PubMed]
- Estévez J.M., Cantero A., Reindl A., Reichler S., León P. 1-Deoxy-d-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. Journal of Biological Chemistry. 2001;276(25):22901–22909. doi: 10.1074/jbc.M100854200. [DOI] [PubMed] [Google Scholar]
- Falk J., Munné-Bosch S. Tocochromanol functions in plants: Antioxidation and beyond. Journal of Experimental Botany. 2010;61(6):1549–1566. doi: 10.1093/jxb/erq030. [DOI] [PubMed] [Google Scholar]
- Fraser P.D., Pinto M.E.S., Holloway D.E., Bramley P.M. Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. The Plant Journal. 2000;24(4):551–558. doi: 10.1046/j.1365-313x.2000.00896.x. [DOI] [PubMed] [Google Scholar]
- Georgiadou E.C., Koubouris G., Goulas V., Sergentani C., Nikoloudakis N., Manganaris G.A.…Wittstock U. Genotype-dependent regulation of vitamin E biosynthesis in olive fruits as revealed through metabolic and transcriptional profiles. Plant Biology. 2019;21(4):604–614. doi: 10.1111/plb.12950. [DOI] [PubMed] [Google Scholar]
- Gramegna G., Rosado D., Sánchez Carranza A.P., Cruz A.B., Simon‐Moya M., Llorente B.…Rossi M. PHYTOCHROME-INTERACTING FACTOR 3 mediates light-dependent induction of tocopherol biosynthesis during tomato fruit ripening. Plant Cell and Environment. 2019;42(4):1328–1339. doi: 10.1111/pce:13467. [DOI] [PubMed] [Google Scholar]
- Hemmerlin A. Post-translational events and modifications regulating plant enzymes involved in isoprenoid precursor biosynthesis. Plant Science. 2013;203–204:41–54. doi: 10.1016/j.plantsci.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Lado J., Cronje P., Alquézar B., Page A., Manzi M., Gómez-Cadenas A.…Rodrigo M.J. Fruit shading enhances peel color, carotenes accumulation and chromoplast differentiation in red grapefruit. Physiologia Plantarum. 2015;154(4):469–484. doi: 10.1111/ppl.12332. [DOI] [PubMed] [Google Scholar]
- Lado, J., Cronje, P. J., Rodrigo, M. J., & Zacarías, L. (2019). Citrus. In T. de F. Sergio & P. Sunil (Eds.), Postharvest Physiological Disorders in Fruits and Vegetables (pp. 377–398). DOI:10.1201/b22001-17.
- Lado J., Rodrigo M.J., Cronje P., Zacarías L. Involvement of lycopene in the induction of tolerance to chilling injury in grapefruit. Postharvest Biology and Technology. 2015;100:176–186. doi: 10.1016/j.postharvbio.2014.10.002. [DOI] [Google Scholar]
- Lado J., Rodrigo M.J., López-Climent M., Gómez-Cadenas A., Zacarías L. Implication of the antioxidant system in chilling injury tolerance in the red peel of grapefruit. Postharvest Biology and Technology. 2016;111:214–223. doi: 10.1016/j.postharvbio.2015.09.013. [DOI] [Google Scholar]
- Lafuente M.T., Establés-Ortíz B., González-Candelas L. Insights into the molecular events that regulate heat-induced chilling tolerance in citrus fruits. Frontiers Plant Science. 2017;8(June) doi: 10.3389/fpls.2017.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Lu S. Plastoquinone and ubiquinone in plants: Biosynthesis, physiological function and metabolic engineering. Frontiers in Plant Science. 2016;7(DECEMBER2016):1–18. doi: 10.3389/fpls.2016.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Qiu D., Pang Y., Gao H., Wang X., Qin Y. Diverse roles of tocopherols in response to abiotic and biotic stresses and strategies for genetic biofortification in plants. Molecular Breeding. 2020;40(2) doi: 10.1007/s11032-019-1097-x. [DOI] [Google Scholar]
- Maeda H., Song W., Sage T.L., DellaPenna D. Tocopherols play a crucial role in low-temperature adaptation and phloem loading in arabidopsis. The Plant Cell. 2006;18(10):2710–2732. doi: 10.1105/tpc.105.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul P., McCollum G., Guy C.L., Porat R. Temperature conditioning alters transcript abundance of genes related to chilling stress in “Marsh” grapefruit flavedo. Postharvest Biology and Technology. 2011;60(3):177–185. doi: 10.1016/j.postharvbio.2010.06.007. [DOI] [Google Scholar]
- Mène-Saffrané L. Vitamin E biosynthesis and its regulation in plants. Antioxidants. 2017;7(1):2. doi: 10.3390/antiox7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz P., Munné-Bosch S. Vitamin E in plants: Biosynthesis, transport, and function. Trends in Plant Science. 2019;24(11):1040–1051. doi: 10.1016/j.tplants.2019.08.006. [DOI] [PubMed] [Google Scholar]
- Pellaud S., Mène-Saffrané L. Metabolic origins and transport of vitamin E biosynthetic precursors. Frontiers in Plant Science. 2017;8(November):1–8. doi: 10.3389/fpls.2017.01959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Research. 2002;30(9) doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porfirova S., Bergmuller E., Tropf S., Lemke R., Dormann P. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proceedings of the National Academy of Sciences. 2002;99(19):12495–12500. doi: 10.1073/pnas.182330899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrana L., Almeida J., Otaiza S.N., Duffy T., Corrêa da Silva J.V., de Godoy F.…Rossi M. Transcriptional regulation of tocopherol biosynthesis in tomato. Plant Molecular Biology. 2013;81(3):309–325. doi: 10.1007/s11103-012-0001-4. [DOI] [PubMed] [Google Scholar]
- Rey F., Rodrigo M.J., Zacarías L. Accumulation of tocopherols and transcriptional regulation of their biosynthesis during cold storage of mandarin fruit. Postharvest Biology and Technology. 2021;180(May) doi: 10.1016/j.postharvbio.2021.111594. [DOI] [Google Scholar]
- Rey F., Zacarías L., Rodrigo M.J. Carotenoids, vitamin C, and antioxidant capacity in the peel of mandarin fruit in relation to the susceptibility to chilling injury during postharvest cold storage. Antioxidants. 2020;9(12):1296. doi: 10.3390/antiox9121296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riewe, D., Koohi, M., Lisec, J., Pfeiffer, M., Lippmann, R., Schmeichel, J., … Altmann, T. (2012). A tyrosine aminotransferase involved in tocopherol synthesis in Arabidopsis. Plant Journal, 71(5), 850–859. DOI:10.1111/j.1365-313X.2012.05035.x. [DOI] [PubMed]
- Rodov, V., Paris, H. S., Friedman, H., Mihiret, M., Vinokur, Y., & Fennec, A. (2020). Chilling sensitivity of four near-isogenic fruit-color genotypes of summer squash (Cucurbita pepo, Cucurbitaceae) and its association with tocopherol content. Postharvest Biology and Technology, 168(November 2019), 111279. DOI:10.1016/j.postharvbio.2020.111279.
- Rodríguez-Concepción M., Boronat A. Breaking new ground in the regulation of the early steps of plant isoprenoid biosynthesis. Current Opinion in Plant Biology. 2015;25:17–22. doi: 10.1016/j.pbi.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Ruiz-Sola M.Á., Coman D., Beck G., Barja M.V., Colinas M., Graf A.…Vranová E. Arabidopsis GERANYLGERANYL DIPHOSPHATE SYNTHASE 11 is a hub isozyme required for the production of most photosynthesis-related isoprenoids. New Phytologist. 2016;209(1):252–264. doi: 10.1111/nph.13580. [DOI] [PubMed] [Google Scholar]
- Sadiq M., Akram N.A., Ashraf M., Al-Qurainy F., Ahmad P. Alpha-tocopherol-induced regulation of growth and metabolism in plants under non-stress and stress conditions. Journal of Plant Growth Regulation. 2019;38(4):1325–1340. doi: 10.1007/s00344-019-09936-7. [DOI] [Google Scholar]
- Sala, J. M. (1998). Involvement of oxidative stress in chilling injury in cold-storedmandarin fruits. Postharvest Biology and Technology, 13(3), 255–261. DOI:10.1016/S0925-5214(98)00011-8.
- Schirra M. Behaviour of “Star Ruby” grapefruits under chilling and non-chilling storage temperature. Postharvest Biology and Technology. 1993;2(4):315–327. doi: 10.1016/0925-5214(93)90036-3. [DOI] [Google Scholar]
- Tijero V., Teribia N., Muñoz P., Munné-Bosch S. Implication of abscisic acid on ripening and quality in sweet cherries: Differential effects during pre- and post-harvest. Frontiers in Plant Science. 2016;7(May):1–15. doi: 10.3389/fpls.2016.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivonen, P. M. A. (2004). Postharvest Storage Procedures and Oxidative Stress. Horticulture Research, 39(5), 938–942. DOI:10.13140.
- Tsegaye Y., Shintani D.K., DellaPenna D. Overexpression of the enzyme p-hydroxyphenolpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiology and Biochemistry. 2002;40(11):913–920. doi: 10.1016/S0981-9428(02)01461-4. [DOI] [Google Scholar]
- Vincent C., Mesa T., Munné-Bosch S. Identification of a New Variety of Avocados (Persea americana Mill. CV. Bacon) with High Vitamin E and Impact of Cold Storage on Tocochromanols Composition. Antioxidants. 2020;9(5):403. doi: 10.3390/antiox9050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.i., Wang Y., Long W., Niu M., Zhao Z., Teng X.…Wan J. SGD1, a key enzyme in tocopherol biosynthesis, is essential for plant development and cold tolerance in rice. Plant Science. 2017;260:90–100. doi: 10.1016/j.plantsci.2017.04.008. [DOI] [PubMed] [Google Scholar]
- Xu C., Wei H., Movahedi A., Sun W., Ma X., Li D.…Zhuge Q. Evaluation, characterization, expression profiling, and functional analysis of DXS and DXR genes of Populus trichocarpa. Plant Physiology and Biochemistry. 2019;142:94–105. doi: 10.1016/j.plaphy.2019.05.034. [DOI] [PubMed] [Google Scholar]
- Zacarias, L., Cronje, P. J. R., & Palou, L. (2020). Postharvest technology of citrus fruits. In M. Talon, M. Caruso, & F. G. Gmitter (Eds.), The Genus Citrus (pp. 421–446). DOI:10.1016/B978-0-12-812163-4.00021-8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.