Highlights
-
•
pH and temperature are the main variables affecting recovery and separation.
-
•
The selection of the adsorbent is critical for the recovery of less polar compounds.
-
•
Excellent separation of compounds in different fractions was achieved.
-
•
The use of a UV–Vis detector allowed monitoring the process in real-time.
-
•
The developed method provided higher recoveries than conventional methods.
Keywords: Pressurized-liquid extraction, Solid-phase extraction, On-line, UV detection, Phenolic acids, Caffeine, Flavonoids, Mate
Abstract
The in-line coupling of the pressurized liquid extraction with a solid-phase adsorbent and a UV–Vis detector for the simultaneous extraction and separation of bioactive compounds from yerba mate (PLE-SPE-UV) was carried out in two stages. In the first stage, water was used as a solvent, while in the second stage, ethanol was used. For the optimization of the method, different adsorbents (Sepra C18-E, Isolute C18-EC, and Strata-X C18), temperatures (40–80 °C), solvent flow-rate (1–3 mL/min), and pH (4.0 and 8.0) were evaluated. By using a UV–Vis detector on-line, it is possible to monitor the process in real-time. The developed method allowed obtaining similar or higher recoveries of all the compounds classes than other methods, such as ultrasound-assisted extraction, stirring, maceration, and pressurized liquid extraction alone, in addition to separating them into fractions. The developed method could be used as sample preparation for the analysis of different compounds classes from mate.
1. Introduction
Yerba mate (Ilex paraguariensis) is a plant widely consumed in several regions of South America through the tea made from the infusion of the dry leaves in hot water. In addition to cultural and sensory aspects, there is an increased interest in its consumption due to the potential health benefits associated with its chemical composition (Cardozo Junior and Morand, 2016, Gómez-Juaristi et al., 2018).
There is a wide variety of compounds present in the leaves of yerba mate, such as phenolic acids, alkaloids, and flavonoids (Kungel et al., 2018). Many studies suggest that some of the compounds present in yerba mate can affect lipid metabolism and oxidative stress, and be explored for the prevention and treatment of diseases, such as cardiovascular disease and cancer (Arçari et al., 2013, Bracesco et al., 2011, Cardozo Junior and Morand, 2016). Also, the antimicrobial action has been identified (Fernandes et al., 2017, Kungel et al., 2018), as well as their ability to prevent oxidative processes in meat (Jongberg, Racanicci, & Skibsted, 2019). On the other hand, caffeine is present in large quantities and is responsible for the stimulating effects derived from the consumption of the mate infusion. Caffeine is a potent stimulant of the central nervous system and has many applications in the pharmaceutical and food industry (Grosso, Godos, Galvano, & Giovannucci, 2017).
For the analysis of these compounds from mate leaves, an extraction step is required to remove them from the matrix of the raw material, which can be accomplished by several techniques and methods (Linares et al., 2010, Negrão Murakami et al., 2011, Riachi et al., 2018, Santos et al., 2020). Despite the availability of several techniques, some have stood out in recent decades as being able to extract these compounds more quickly and efficiently, as is the case with extraction with pressurized liquids (PLE). PLE combines high temperatures and high pressures without exceeding the critical points of a solvent, which remains in a liquid state. By employing high temperatures during the extraction process, it is possible to increase the mass transfer rate of the matrix compounds to the extraction solvent. By using high pressures, the system allows operating at temperatures above the boiling point of the solvents and avoids phase transition. Other significant advantages of PLE include the elimination of post-extraction steps, such as filtration and centrifugation, the high level of automation, and the possibility of coupling with different techniques, such as ultrasound (Rostagno et al., 2009, Rostagno et al., 2010, Santos et al., 2019, Sumere et al., 2018).
Despite the development of more efficient techniques, the process is not selective, and the extracts contain a complex mixture of compounds with different chemical characteristics. In the case of mate, the presence of caffeine in the extracts is particularly problematic due to its high concentration.
For the removal of unwanted compounds and purification of complex samples, other post-extraction techniques can be used. One of the most used techniques is solid-phase extraction (SPE). The separation is based on differential interaction of compounds with an adsorbent and different solvents in a process similar to liquid chromatography. In addition to being very efficient in the separation of phenolic compounds, SPE allows a high degree of automation and can be coupled with other sample preparation and analysis techniques (Płotka-Wasylka et al., 2016, Tian et al., 2010).
A recent trend consists of combining different extraction and purification techniques (Rostagno et al., 2010). Specifically, there are a few applications of PLE-SPE in-line for the simultaneous extraction and purification of compounds present in black tea and industrial residue from apple juice production, among others (da Silva et al., 2020, Souza et al., 2020). These studies provide evidence that the coupling of techniques is feasible and that it is possible to achieve high selectivity and separate several classes of compounds simultaneously without impairing the extraction yield, even when using green solvents. It is also essential to highlight the possibility of attaching an on-line detector to these techniques, which would allow monitoring the process in real-time and defining the fraction collection points.
Thus, the objective of this work was to evaluate the coupling of PLE and SPE for the simultaneous extraction and separation of the compounds present in yerba mate and to verify if it was possible to use an on-line UV detector to monitor the process.
2. Material and methods
2.1. Sample, chemicals, solvents, and adsorbents
The standards of the compounds analyzed by liquid chromatography (chlorogenic acid, caffeine, and rutin) were provided by Sigma Aldrich (St. Louis, MO, USA). HPLC-grade acetonitrile and methanol were supplied by the company Merck (São Paulo, Brazil). The ultra-pure water was provided by a Purelab Flex 3 purifying system (Elga Veolia, High Wycombe, United Kingdom). Ethanol (99.5%), phosphoric acid (85%), hydrochloric acid, and sodium hydroxide were purchased from Labsynth (São Paulo, Brazil). The adsorbents used in this study were: Sepra C18-E, (particle size: 50 μm, pore size: 85 Å, Phenomenex, Torrance, CA, USA); Isolute C18-EC, (particle size: 50 μm, poresize: 60 Å, Biotage, Sweden) and Strata X C18 (particle size: 33 μm, pore size: 85 Å, Phenomenex, Torrance, CA, USA).Yerba mate leaves were acquired in a local supermarket and stored at −20 °C until used as a sample. The particle size of the sample used for the experiments was between 1.41 and 2.00 mm.
2.2. Pressurized liquid extraction coupled on-line with solid-phase extraction and UV detection (PLE-SPE-UV)
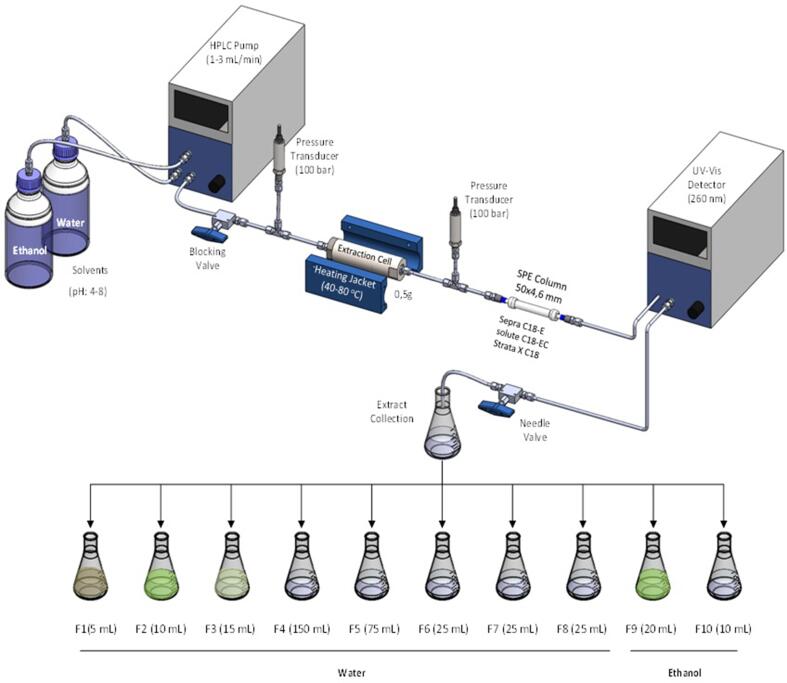
The experiments were carried out in the EXTRACT-US integrated extraction and analysis system (patent pending). The system consists of a liquid pump (PU-2080, Jasco, Tokyo, Japan), degasser, solvent mixer, UV detector (UV-2075, Jasco, Tokyo, Japan), five two-position and ten ports valves (Waters, Milford, MA, USA), an extraction cell (Sumere et al., 2018). The basic configuration of the system and conditions used are shown in Fig. 1.
Fig. 1.
Schematics of the configuration of the Extract-US system, conditions used and fractions collected during the process.
The PLE-SPE extraction process was carried out as follows: The SPE column (50 mm × 4.6 mm) was filled with the selected adsorbent and connected to the system. The adsorbent was activated with 30 mL of methanol solvent and conditioned with 30 mL of water. The sample was weighed (0.5 g) and transferred to the extraction cell, which was then connected to the system. The system was pressurized (100 bar), and heating of the extraction cell was initiated. After reaching the experimental temperature, the pump was activated, and the extract was collected after passing through the detector. The process was divided into two stages. In the first stage of the extraction process, water was used as a solvent and eight fractions were collected: Fraction 1 (5 mL), Fraction 2 (10 mL), Fraction 3 (15 mL), Fraction 4 (150 mL), Fraction 5 (75 mL), Fraction 6 (25 mL), Fraction 7 (25 mL), Fraction 8 (25 mL). In the second stage, ethanol was used as a solvent to elute the compounds retained in the solid-phase column. Two fractions were collected in this stage: Fraction 9 (20 mL) and Fraction 10 (10 mL).
The optimization of the extraction/separation conditions was carried out using a step-by-step strategy. Initially, three different adsorbents (Sepra C18-E, Isolute C18-EC, and Strata X C18) were tested with the system operating at 40 °C and keeping the extraction and elution flow fixed at 2 mL/min. Afterward, different temperatures (40–80 °C) were tested with the selected adsorbent and keeping the extraction and elution flow set at 2 mL/min. With the chosen conditions, the pH of the aqueous phase was modified to improve the separation of compounds in the solid phase column. Finally, the use of different flow rates in the extraction and elution of the compounds (1–3 mL/ min) was tested to optimize the processing time, in addition to improving separation. The process was monitored by a UV-2075 Plus detector (Jasco, Tokyo, Japan) connected in-line to the SPE column output, registering the signal at 260 nm. All fractions were filtered through a syringe filter (nylon, 25 mm, 0.22 µm, Analitica, São Paulo, Brazil) for further chromatographic analysis. All extractions were done in duplicate.
2.3. Other extraction techniques
The ultrasound-assisted extraction (UAE) was performed in an ultrasound bath (P60H, Elmasonic, Singen, Germany), maintained at 40 °C and operating at 37 kHz and 100% power (150 W). The sample (0.5 g) was extracted in three sequential stages of 30 min, using water, 50% ethanol, and 100% ethanol, respectively (Supplementary Fig. 1). In the first stage, 25 mL of water was used as a solvent. After the process was completed, the sample was centrifuged at 10,000 rpm g 10 min, and the supernatant collected. The solid residue was extracted again with 50% ethanol, and after centrifuging, the residue was extracted once more using 100% ethanol. After the extractions, all extracts were combined, and the volume was brought up to 100 mL. The sample was filtered through a syringe filter (nylon, 25 mm, 0.22 µm, Analitica, São Paulo, Brazil) before analysis. The same process and conditions were used for the stirring, which was carried out on an IKA C-MAG HS 7 magnetic stirring (Staufen im Breisgau, Baden-Wurttemberg, Germany). The extraction using pressurized liquids (PLE) was also used sequential extractions in three 30-minute cycles, using water, 50% ethanol, and 100% ethanol, respectively. The pressure was maintained at 100 bar during the process. In addition to these techniques, extraction by maceration was also carried out, which consisted of leaving the same amount of sample in contact with 100 mL of 50% ethanol for three hours. The extractions were performed in duplicate.
2.4. Ultra-high performance liquid chromatography (UHPLC)
The UHPLC analyzes were performed in an Acquity UPLC H-Class system (Waters, Milford, MA, USA) using a method previously developed with some adaptations (Rostagno et al., 2011). The mobile phase was composed of water (solvent A) and acetonitrile (solvent B), both containing 0.1% acetic acid (v / v). The gradient used was as follows: 1 min (10% B), 2 min (20% B), 4 min (30% B), 5 min (90% B), 8 min (10% B). The separation was performed on a Kinetex C18 column (150 × 4.6 mm, 2.6 µm, Phenomenex Torrance, CA, USA) maintained at 55 °C with a mobile phase flow of 1.0 mL/min. The absorbance was monitored between 210 and 400 nm, and the peaks were integrated at 260 nm. The injection volume was 3 μL, and the conditioning time was 4 min. The identification of the compounds was carried out by comparing the retention times and UV spectra of the separated compound, as well as by co-elution with authentic standards. The acids were quantified as equivalents of chlorogenic acid (R2 = 0.999), in a concentration range of 0.37–190 mg/L, the alkaloid was quantified as caffeine equivalent (R2 = 0.999), in a concentration range of 0.48–250 mg/L and flavonoids were quantified as rutin equivalents (R2 = 0.999), in a concentration range of 0.35 – 90 mg/L. Peak identification was confirmed by the UV–Vis spectrum and by co-elution with standards. All other compounds were tentatively identified based on their UV–Vis spectra and relative retention times to known compounds. All analyzes were performed in duplicate.
2.5. Statistical analysis
First, all variables were analyzed to verify adherence to normal distribution and homoscedasticity. The Shapiro-Wilk and Levene tests were used, respectively. To compare the recovery among different adsorbents, temperatures, and flow ratio, the one-way analysis of variance with Tukey’s posthoc test was used. It was used Student’s t-test to compare the recovery amount between two pH and flow-rate.All tests were two-sided, and p < 0.05 was considered statistically significant. The analyses were processed in the Statistical Package for the Social Sciences – SPSS Statistics, version 2013.22.0.
3. Results and discussion
3.1. Identification of compounds in the sample
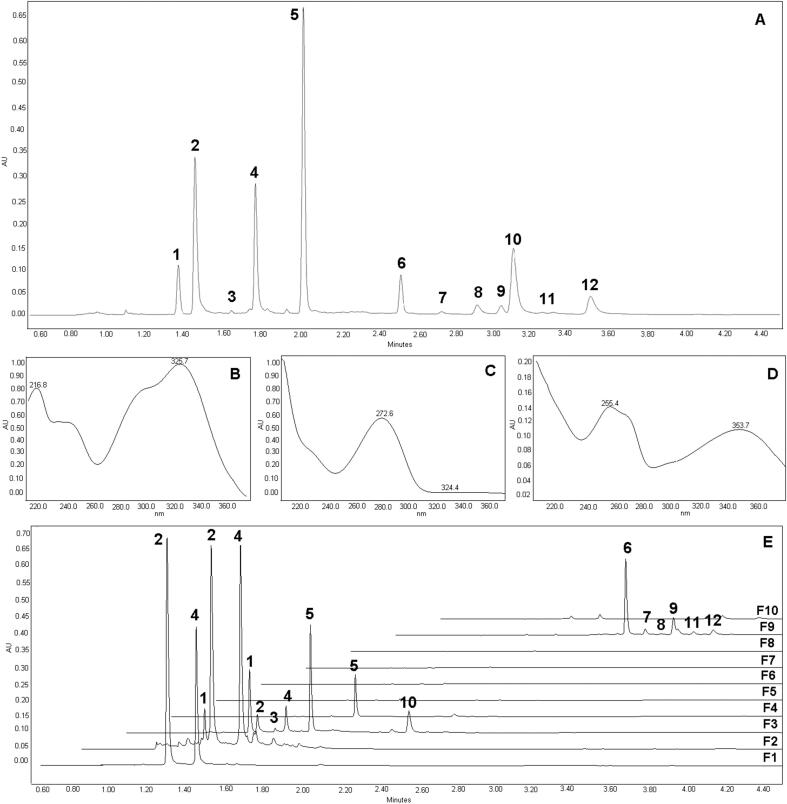
A representative chromatogram of the mate sample is shown in Fig. 2A. Twelve main compounds were detected and classified according to their UV absorption spectrum into three groups: acids (Fig. 2B), alkaloids (Fig. 2C) and flavonoids (Fig. 2D), represented by chlorogenic acid (peak # 4), caffeine (peak # 5) and rutin (peak # 6), respectively. Compound #1, was tentatively identified as theobromine, presenting a UV–Vis spectrum with a single peak and ʎmax at 272 nm. Compounds #2, #3, #8, #10, and #12 were tentatively identified as 5-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid, respectively. These compounds revealed a similar spectrum to chlorogenic acid, with a characteristic ʎmax at 325 nm and shoulders at 250 and 290 nm. Finally, compounds #7, #9, and #11 were tentatively identified as quercetin-3-O-glycoside, kaempferol-3-O-rhamnoglucoside, and kaempferol-3-O-rutinoside, respectively. These compounds presented an absorption spectrum with two peaks, one at 255 nm and another at 350 nm, which is characteristic of flavonoids compounds. The identification of the compounds present is in accordance with the profile reported in the literature for mate samples and with the reference method used (Bravo et al., 2007, da Silveira et al., 2016, Gonçalves et al., 2015, Lima et al., 2016; Rostagno et al., 2011).
Fig. 2.
(A) Representative chromatogram of the sample (270 nm). Peaks: #1: theobromine; #2: 5-O-Caffeoylquinic acid; #3: caffeoylquinic acid; #4: chlorogenic acid; #5: caffeine; #6: rutin; #7: quercetin-3-O-glycoside; #8: 3,4-Dicaffeoylquinic acid; #9: kaempferol-3-O-rhamnoglucoside; #10: 3,5-Dicaffeoylquinic acid; #11: kaempferol-3-O-rutinoside; #12: 4,5-Dicaffeoylquinic acid. (B) UV–Vis spectrum of chlorogenic acid; (C) UV–Vis spectrum of caffeine, (D) UV–Vis spectrum of rutin, (E) Staked chromatograms of the collected fractions. F1- fraction 1, F2- fraction 2, F3- fraction 3, F4- fraction 4, F5- fraction 5, F6- fraction 6, F7- fraction 7, F8- fraction 8, F9- fraction 9, F10- fraction 10.
3.2. Comparison of SPE adsorbents
The PLE-SPE-UV extraction and purification process was initially carried out using fixed operating conditions to compare the efficiency of different adsorbents (Sepra C18-E, Isolute C18-EC, and Strata X C18) for the separation and recovery of compounds present in the mate sample. The temperature was constant (40 °C), and the extraction and elution flow rate fixed at 2 mL/min.
As can be seen in Fig. 2E, it was possible to obtain a good separation of the compounds in different fractions. In the first fractions (F1 and F2), 5-O-caffeoylquinic acid and chlorogenic acid were the only compounds present while the other compounds were retained in the adsorbent or were not extracted from the sample matrix. As more solvent passed through the extraction and the SPE columns, alkaloids (theobromine and caffeine) were also detected in the extracts. It is also observed that 3,5-dicaffeoylquinic acid is not well retained, and it is detected in the F3 fraction. Afterward, F3, caffeine is the only compound recovered in the extracts, whose concentration gradually decreases. Finally, by changing the extraction solvent from water to ethanol, the compounds that were still present in the sample were extracted, and the compounds retained in by the adsorbent were simultaneously eluted. All flavonoids (rutin, quercetin-3-O-glycoside, kaempferol-3-O-rhamnoglucoside, and kaempferol-3-O-rutinoside) were recovered in F9, in addition to small amounts of dicaffeoylquinic acids (3,4-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid). In the F10 fraction, only trace peaks were detected in the chromatogram suggesting the complete removal of all phenolic acids, alkaloids, and flavonoids from the sample and adsorbent.
Therefore, the separation of the compounds was controlled by polarity following a conventional reverse phase separation. It is essential to highlight that the acid character of phenolic acids is also influencing the interaction of adsorbents with these compounds. It can be seen that the highly polar theobromine is only detected in higher amounts in the F3 fraction, while chlorogenic acid is detected in the first fraction (F1). Another factor that should not be overlooked is the amount of the compound present in the sample. As the amount of a compound in the sample increases, it is expected that it will appear in more fractions throughout the extraction process since the kinetic curve of the extraction is extended (Rostagno, Prado, & Kraus, 2013). This aspect is evident in the case of caffeine, and its recovery is controlled by the two mechanisms (kinetic curve of extraction and retention by the adsorbent as a function of polarity). High concentration compounds can also cause adsorbent saturation and leading to the detection in the fractions along with more polar compounds.
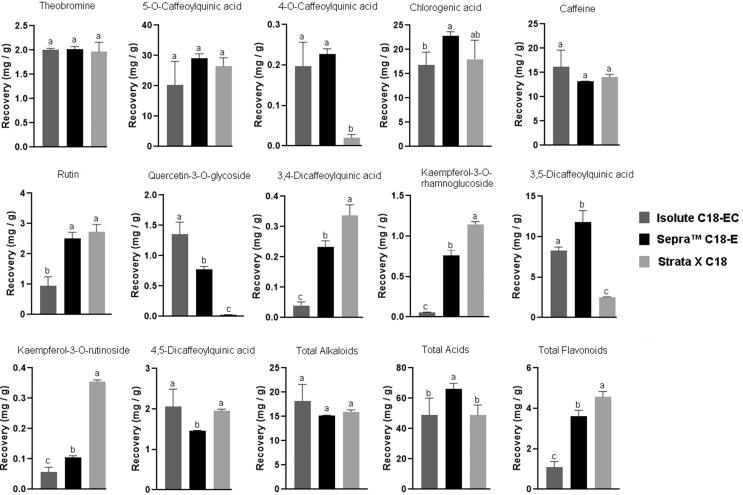
However, some significant differences were observed in the retention capacity and separation of the compounds when the adsorbents were compared using the same extraction conditions. The Sepra C18-E adsorbent was the adsorbent that provided the best results in terms of recovery and separation of compounds (Fig. 2E). With this adsorbent, it was possible to achieve a higher recoveryof most compounds (Fig. 3). The highest total recovery of phenolic acids was obtained with this adsorbent, while the Strata X C18 adsorbent allowed the highest recovery of flavonoids. There was no significant difference in the recovery of alkaloids between the three adsorbents tested.
Fig. 3.
Recovery (mg / g) of individual and total alkaloids, phenolic acids and flavonoids with different adsorbents (Isolute C18-EC, Sepra™ C18-E e Strata X C18).Different lower case letters indicate significant difference (p < 0.05 - Tukey HSD test).
When the individual behavior of the compounds is analyzed (Fig. 3), it is possible to identify significant differences in the recovery depending on the compound and the adsorbent. Although the Strata X C18 adsorbent allowed recovering a higher total amount of flavonoids than the other adsorbents, it did not allow the recovery of quercetin-3-O-glycoside. Similar behavior was observed for some phenolic acids, which affected the total recovery of these compounds. The other adsorbents also have deficiencies in the recovery of some compounds (i.e., rutin by the adsorbent Isolute and kaempferol-3-O-rutinoside by the adsorbent Sepra C18-E).
These differences are basically due to the different interactions of the compounds extracted with the adsorbent since the same extraction conditions were used. The recovery is mainly affected by the intensity of the retention of the compounds by the adsorbent. These results indicate that the selection of the adsorbent is critical in the recovery of compounds, especially less polar ones, such as flavonoids and dicaffeoylquinic acids. When these compounds are targeted, longer SPE columns could be used to improve retention and minimize breakthrough leading to lower recovery. Compounds that are not fully retained by the adsorbent and are gradually collected throughout the process may be in low concentration. If they are below the detection limit of the compounds, they will not be quantified, resulting in less total recovery and despite the same extraction conditions having been used. Although the polymeric adsorbent Strata X C18 has shown excellent results, the conventional adsorbent Sepra C18-E has a much lower cost. Itis also capable of achieving an excellent separation between the compounds despite the lower recovery of flavonoids.
3.3. Extraction temperature
Temperature is an essential factor in the extraction process because it influences the solvent, the sample, and the phenomena involved in removing the compounds present in a complex matrix. In this context, to reduce the processing time, increase the recovery, and improve the separation of the compounds between the fractions, different extraction temperatures (40–80 °C) were evaluated (Table 1) using the adsorbent Sepra C18-E.
Table 1.
Recovery of analyzed compounds using different extraction temperatures.
| Peak | Compound | Recovery (mg/g) |
||||
|---|---|---|---|---|---|---|
| 40 °C | 50 °C | 60 °C | 70 °C | 80 °C | ||
| 1 | Theobromine | 2.02 ± 0.06 a | 1.96 ± 0.08 a | 1.91 ± 0.06b | 2.12 ± 0.11 a | 1.93 ± 0.08b |
| 2 | 5-O-Caffeoylquinic acid | 29.10 ± 1.44 a | 27.20 ± 2.27 a | 25.83 ± 2.24a,b | 22.44 ± 1.75b | 25.06 ± 1.50a,b |
| 3 | 4-O-Caffeoylquinic acid | 0.23 ± 0.01c | 0.82 ± 0.10 a | 0.66 ± 0.04b | 0.72 ± 0.02a,b | 0.69 ± 0.03b |
| 4 | Chrologenicacid | 22.80 ± 0.87a,b | 23.77 ± 0.46a,b | 21.66 ± 0.23b | 23.61 ± 2.14a,b | 24.97 ± 0.21 a |
| 5 | Caffeine | 13.18 ± 0.04b | 13.89 ± 0.52a,b | 13.31 ± 0.08ab | 14.53 ± 0.88 a | 14.33 ± 0.83a,b |
| 6 | Rutin | 2.51 ± 0.20 a | 1.09 ± 0.03c | 1.07 ± 0.06c | 1.65 ± 0.18b | 0.61 ± 0.07 d |
| 7 | Quercetin-3-O-glycoside | 0.23 ± 0.02 a | 0.20 ± 0.01b | 0.11 ± 0.01c | 0.07 ± 0.01 d | 0.04 ± 0.00 d |
| 8 | 3,4-Dicaffeoylquinic acid | 0.77 ± 0.05 d | 1.74 ± 0.07c | 0.36 ± 0.03 e | 2.36 ± 0.14 a | 2.28 ± 0.04b |
| 9 | Kaempferol-3-O-rhamnoglucoside | 0.76 ± 0.07b | 0.86 ± 0.01 a | 0.34 ± 0.01c | 0.76 ± 0.03b | 0.31 ± 0.05c |
| 10 | 3,5-Dicaffeoylquinic acid | 11.80 ± 1.42 a | 14.99 ± 1.71 a | 11.12 ± 2.74 a | 12.06 ± 0.59 a | 13.47 ± 1.90 a |
| 11 | Kaempferol-3-O-rutinoside | 0.11 ± 0.01b | 0.04 ± 0.01 d | 0.05 ± 0.01c | 0.12 ± 0.01 a | 0.04 ± 0.01 d |
| 12 | 4,5-Dicaffeoylquinic acid. | 1.46 ± 0.01 d | 2.51 ± 0.16b,c | 2.70 ± 0.15a,b | 2.40 ± 0.10c | 2.78 ± 0.09 a |
| – | Total alkaloids | 15.19 ± 0.08b | 15.84 ± 0.59 a, b | 15.22 ± 0.13 a, b | 16.65 ± 0.99 a | 16.26 ± 0.91 a, b |
| – | Total acids | 66.16 ± 3.79a,b | 71.03 ± 4.78 a | 62.34 ± 5.42b | 63.58 ± 4.75a,b | 69.25 ± 3.76a,b |
| – | Total flavonoids | 3.61 ± 0.30 a | 2.20 ± 0.06c | 1.57 ± 0.08 d | 2.61 ± 0.22b | 1.00 ± 0.13 e |
Different lower case letters between columns indicate significant difference (p < 0.05 - Tukey HSD test)
The results indicate that compounds present in the sample are affected differently by the increase in temperature. While the recovery of some compounds is not affected, others show significant differences. For the recovery of caffeine, the best result was observed at 70 °C (14.53 ± 0.88 mg/g), indicating that higher temperatures are required for the extraction of this compound, which has been reported in other studies (Sökmen et al., 2018, Xu et al., 2019). Quantitative extractions may have already been reached at 70 °C, which would explain the lack of increase in recovery with an increase in temperature to 80 °C. Acids, in turn, are better recovered at relatively low temperatures (40–50 °C). Chlorogenic acid had the highest average at a temperature of 80 °C (24.97 ± 0.21 mg/g), but it was not significant when compared to recovery at 50 °C (23.77 ± 0.46 mg/g).The flavonoid group also shows significantlybetter results with milder temperatures. The recovery of rutin was higher at 40 °C (2.51 ± 0.20 mg/g), while the temperature increase negatively affected the recovery. At 80 °C, the recovery was 4.12 times lower than at 40 °C. The results indicate that better results are obtained with lower temperaturesfor the other flavonoids present in lower concentrations.
The results shown in Table 1 reflect an overlap of the effects of temperature on the extraction process and on the capacity of the adsorbent in retaining extracted compounds. On the one hand, the temperature favors the mass transfer of the sample matrix compound to the solvent, decreases the viscosity of the solvent, and increases the penetration power. The increase in temperature also increases the solubility of the compounds in the solvents, favoring diffusion. Thus, increasing the temperature can increase the extraction yield of compounds. On the other hand, temperature also affects the process of separating mixtures in an adsorbent precisely by increasing the mass transfer rate, which causes a reduction in the adsorbent's retention capacity (Rostagno et al., 2013, Souza et al., 2020). By not properly retaining the compounds, they are gradually released throughout the process, being present in low concentration in various fractions. These compounds end up not being quantified because they are not detected and reduce the total recovery.
This overlapping effect occurs because we are carrying out a coupled process, where the temperature can simultaneously accelerate the extraction of the compounds. Still, it can also reduce the separation capacity of the adsorbent, reducing the separation of the compounds between collected fractions. These effects may be caused by the higher temperature of the solvent when it leaves the extraction cell, there by reaching the SPE column hotter, affecting the adsorbent's ability to retain the extracted compounds. An alternative to mitigate this effect is to work with the temperature control of the extraction cell and the retention column of the compounds independently, thus being able to employ higher temperatures for the extraction of the compounds and employ lower temperatures for the separation of the compounds in the solid phase column.
The data also suggests the role of the adsorbent's retention capacity in the recovery since the most affected compounds are precisely the flavonoids. The flavonoids are recovered mainly in the last fraction. They are retained in the adsorbent as they are extracted along the process and pass through the SPE column. Alkaloids and acids were less affected (there is a mixture of acids with different polarities), and their recovery does not entirely depend on retention since most compounds are recovered in the first fractions and interact little with the adsorbent.
Another factor that may be influencing the results and that cannot be overlooked is the stability of the compounds present, since the use of high temperatures can also lead to the degradation of some thermolabile phenolic compounds (Alvarez-Rivera et al., 2019, Pereira et al., 2019).
In general, more than 50% of the compounds present in the mate showed the highest recoveries at 40 °C, and the use of higher temperatures drastically affected the recovery of flavonoids. The low temperature also reduces costs, heating time, and minimizes the risk of degradation of the compounds present.
3.4. pH and flow-rate
Modification of the pH can be an interesting strategy to improve the separation of ionizable compounds since it can affect the retention and elution properties of an adsorbent. The retention of a compound is stronger in its neutral form as it becomes more hydrophobic. If pH is adjusted to values above or below pKa of a compound, there may be differences in the adsorbent ability to retain it. The pH can also affect the interaction between the functional groups of the adsorbent and the compound, facilitating its elution (Berrueta, Gallo, & Vicente, 1995). There is also evidence that pH may improve the extraction of some phenolic compounds, such as anthocyanins (Garcia-Mendoza et al., 2017), catechin, and dihydromyricetin (Mai et al., 2020), among other flavonoids (Motikar et al., 2020, Soquetta et al., 2019). Thus, the pH of the extraction solvent was adjusted to 4.0 and 8.0 to assess the effect on the process (Table 2).
Table 2.
Recovery of compounds from the mate sample using different solvent pH and flow-rate. Flow (X:Y:Z); X = Flow-rate (ml/min) during the collection of fractions F1-F3; Y = Flow-rate (ml/min) during the collection of fractions F4-F8; Z = Flow-rate (ml/min) during the collection of fractions F9-F10.
| Peak | Compound | Recovery (mg/g) |
||||
|---|---|---|---|---|---|---|
| A |
B |
|||||
| pH 4 (Flow 2.2.2) | pH 8 (Flow 2.2.2) | pH 4 (Flow 1.3.3) | pH 4 (Flow 3.3.2) | pH 4 (Flow 3.3.3) | ||
| 1 | Theobromine | 2.03 ± 0.14 a | 2.12 ± 0.04 a | 1.79 ± 0.03b | 1.71 ± 0.07b | 1.67 ± 0.07b |
| 2 | 5-O-Caffeoylquinic acid | 24.22 ± 1.76 a | 23.83 ± 0.38 a, b | 22.49 ± 0.24 a, b | 20.22 ± 0.25c | 22.21 ± 0.82b |
| 3 | 4-O-Caffeoylquinic acid | 0.65 ± 0.04 a | 0.57 ± 0.13 a | 0.09 ± 0.01c | 0.22 ± 0.01b | 0.00 ± 0.00c |
| 4 | Chrologenic acid | 19.81 ± 1.30 a, b | 22.11 ± 1.64 a | 18.78 ± 0.20b | 18.18 ± 1.10b | 19.21 ± 2.01 a, b |
| 5 | Caffeine | 14.13 ± 0.54 a | 14.03 ± 0.02 a | 13.10 ± 0.45a,b | 11.23 ± 1.50c | 12.34 ± 0.27b, c |
| 6 | Rutin | 3.71 ± 0.02 a | 3.04 ± 0.07b | 1.69 ± 0.08c | 1.81 ± 0.11c | 3.80 ± 0.06 a, b |
| 7 | Quercetin-3-O-glycoside | 0.55 ± 0.09c | 0.25 ± 0.03b | 0.15 ± 0.01 d | 1.21 ± 0.03 a | 0.00 ± 0.00 e |
| 8 | 3,4-Dicaffeoylquinic acid | 0.41 ± 0.02 a | 0.72 ± 0.01c | 0.18 ± 0.02c | 0.16 ± 0.02c | 0.39 ± 0.01b |
| 9 | Kaempferol-3-O-rhamnoglucoside | 1.94 ± 0.04 a | 1.14 ± 0.03b | 0.94 ± 0.10c | 0.97 ± 0.12c | 1.01 ± 0.07b, c |
| 10 | 3,5-Dicaffeoylquinic acid | 5.87 ± 0.90b | 7.10 ± 0.50 a | 6.66 ± 0.40 a, b | 5.59 ± 0.26b | 5.75 ± 0.01b |
| 11 | Kaempferol-3-O-rutinoside | 0.07 ± 0.01c | 0.18 ± 0.00 a | 0.05 ± 0.00c | 0.15 ± 0.01 a, b | 0.15 ± 0.02b |
| 12 | 4,5-Dicaffeoylquinic acid. | 5.52 ± 0.36 a | 1.20 ± 0.08c | 0.76 ± 0.01 d | 1.81 ± 0.03b | 1.64 ± 0.04b |
| – | Total alkaloids | 16.16 ± 0.68 a | 16.15 ± 0.05 a | 14.89 ± 0.42 a, b | 12.94 ± 1.42c | 14.01 ± 0.28b, c |
| – | Total acids | 56.48 ± 4.37 a | 55.52 ± 2.72 a | 48.92 ± 0.88b | 47.23 ± 1.68b | 48.80 ± 2.88b |
| – | Total flavonoids | 6.27 ± 0.17 a | 4.61 ± 0.14c | 2.86 ± 0.19 d | 3.09 ± 0.27 d | 5.34 ± 0.17b |
Different lower case letters between columns indicate significant difference (p < 0.05)
The data obtained revealed interesting information that reinforces the evidence gathered by comparing the adsorbents. As can be seen, the class of compounds whose recovery was most affected by pH manipulation was that of the flavonoids. What draws the most attention is that with the reduction of the pH of the solvent to 4.0, the total recovery of flavonoids increased 43% relative to the results obtained without acidification. The use of acidified solvents improved individual recovery of flavonoids, except for kaempferol-3-O-rutinoside.
On the other hand, for most phenolic acids and all alkaloids, there was no significant difference in their recovery when the extraction was carried out using water with pH 4.0 or pH 8.0 as a solvent. The recovery of the major acid present, chlorogenic acid, was not affected by the change in pH. However, for some acids, the recovery was higher using the acidified pH solvent (e.g.,4,5-dicaffeoylquinic acid), while for others, the recovery was higher with the basic solvent (3,4-dicaffeoylquinic acid and 3,5-dicaffeoylquinic acid). Although there are individual differences, there is a trade-off between increases and decreases in recovery, which does not change the average total recovery of acids obtained at different pH values.
In the case of acids, it can also be observed that the most affected compounds were the less polar compounds and that they were more retained by the adsorbent, such as 4,5-dicaffeoylquinic acid. This behavior is similar to that of flavonoids. It also suggeststhe influence of pH on the adsorbent's retention capacity and the critical role of this characteristic in the recovery of compounds in a coupled extraction system. It is also possible that the change in pH affected the extraction process by disrupting the interactions between the compounds and the sample matrix and changing the hydrophobicity of the compounds. Possibly the data reflect an overlap of effects on the two processes, extraction and separation, and highlight the complexity of coupled systems and the difficulty in developing methods.
Another critical factor is the extraction time, which is defined according to the flow and volume used in the collection of fractions. Up to this point, all extractions have been carried out with a flow-rate of 2 mL/min. The solvent flow was reduced to 1 mL/min during the fractions collection (F1-F4) to improve the separation of compounds. On the other hand, to reduce the processing time, the solvent flow was also increased to 3 mL/min during the collection of fractions F5-F8 since only caffeine was present in low concentrations, as well as the flow of fractions F9-F10 (Table 2).
In general, the increased flow reduced the recovery of the compounds but did not affect their distribution between the fractions. Apparently, by increasing the flow while keeping the total volume constant, there is a reduction in the mass transfer efficiency in the extraction step. However, it is also possible that the higher flow may have negatively affected the retention of the more hydrophobic compounds that were recovered in the last fractions. The lower retention can cause some compounds to be gradually eluted from the SPE column in low concentrations. Cumulatively these small amounts translate into a reduction in the total recovery of the compounds. This effect is even more pronounced for flavonoid compounds, suggesting that flow-rate also affects the retention capacity of the adsorbent. Another explanation for the effect of the flow is the temperature of the solvent reaching the SPE column. The higher flow-rate can lead to higher solvent temperature since it will reach the SPE column faster.
Unfortunately, when reducing the flow for the collection of the first fractions, no differences were observed in the separation of compounds between fractions. For better separation of these more polar compounds, a larger SPE column (i.e., amount of adsorbent) may be necessary. Thus, the use of a higher or lower flow rate reduced the recovery, indicating that an intermediate flow-rate of 2 mL/min is the most adequate to provide a balance between time and recovery.
3.5. Characteristics of the method
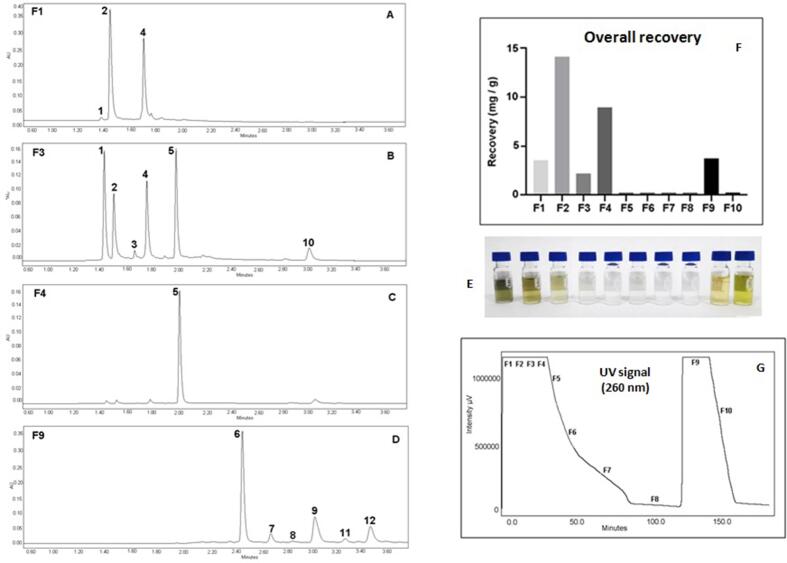
The developed PLE-SPE method allowed us to extract and simultaneously separate the compounds present in the mate sample and collect fractions with a different chemical composition (Fig. 4A - D). In the fraction F1, it was obtaineda dark green extract with a high concentration of acids5-O-Caffeoylquinic acid and Chrologenic acid– 1250.60 mg/L) (Fig. 4E). In F2, the level of these acids remained high (1368.28 mg/L), and the color of the extract was green/brown. Still, it was also possible to detect theobromine with a concentration of 58.75 mg/L. In F3 the color of the extract faded to light brown and a lower level of acids was observed (356.16 mg/L) while the concentration of caffeine and theobromine increased (131.17 mg/L); in F4 there was a lower acid concentration (9.21 mg / L) and the caffeine concentration reduced to 26.87 mg / L. In the fractions 5, 6 and 7 (F5, F6, F7) there was a gradual decrease in the concentration of caffeine in the sample (2.10, 1.48, 0.07 mg/L respectively), and no other compounds were detected. In F8, no compoundswere detected, indicating the depletion of compounds that can be extracted from the sample and removed from the adsorbent by the extraction solvent (water). Extracts collected in fractions 4–8 did not present any color, reflecting the low concentration of caffeine.
Fig. 4.
Chromatograms of the main fractions collected, UV–Vis detector signal and overall recovery of compounds during the extraction process: A: Chromatogram of Fraction 1 (F1); B: Chromatogram of Fraction 3 (F3); C: Chromatogram of Fraction 4 (F4); D: Chromatogram of Fraction 9 (F9); E: Recorded UV–Vis detector signal; F: total ∑ of compounds collected in each fraction. F1: ∑ of amount of compounds #2+#4; F2: ∑ of amount of compounds #1+#2+#4; F3: ∑ of amount of compounds #1+#2+#3+#4+#5+#10; F4-F8: amount of compound #4; F9: ∑ of amount of compounds #6+#7+#8+#9+#12+#12. compounds: #1: theobromine; #2: 5-O-Caffeoylquinic acid; #3: caffeoylquinic acid; #4: chlorogenic acid; #5: caffeine; #6: rutin; #7: quercetin-3-O-glycoside; #8: 3,4-Dicaffeoylquinic acid; #9: kaempferol-3-O-rhamnoglucoside; #10: 3,5-Dicaffeoylquinic acid; #11: kaempferol-3-O-rutinoside; #12: 4,5-Dicaffeoylquinic acid.
Afterward, the solvent composition is changed to ethanol, and fractions F9 and F10 are collected. Ethanol acts both as an extraction solvent and as an elution solvent for the compounds retained in the adsorbent. In F9, the main compounds obtained were flavonoids (76.41 mg/L) followed by acids (26.54 mg/L). The extract had a light brown/yellowish color. The flavonoids were collected only in F9, and the acids accumulated were different from those present in the F1-F3 fractions. The data confirm the role of the gradual elution of compounds throughout the process in low concentrations, affecting the total recovery due to an accumulative factor, which explains many of the differences observed with the tested conditions. Finally, in the F10, no compounds were detected, indicating a total depletion of compounds in the matrix of the mate sample and the compounds retained in the adsorbent. However, it is essential to notice theintense green-yellowish color of the F10 fraction (Fig. 4E), indicating the presence of other compounds that did not absorb light at 260 nm. Although the presence of these compounds would not interfere in the detection of phenolic acids, alkaloids, and flavonoids, they could damage the analytical column due to their high hydrophobicity, leading to shorter column life.
The ability to separate compounds from a complex sample in different fractions provides flexibility, and the developed method can be explored for the analysis of specific compounds classes with several advantages. The method can be used for the analysis of all compounds classes by collecting a single extract by combining fractions F1-F9. Post-extraction steps are not necessary, and a cleaner sample is obtained due to the retention of hydrophobic compounds. The method can also be used for the analysis of caffeine individually by combining fractions F3-F8. In these fractions, caffeine is the only primary compound, and only small contamination of phenolic acids was detected, while flavonoids and dicaffeoyl acids are still retained in the adsorbent. Therefore, the extract may allow the determination of caffeine by a more straightforward instrument in this sample, such as a UV spectrometer instead of UHPLC, since no separation is required to provide individual concentration (Navarra et al., 2017). A clean sample also implies lower requirements of the LC method to accurately quantify caffeine in less time, allowing fast isocratic analysis.
Another application of the method is the analysis of all phenolic acids through the combination of fractions F1, F2, F3, and F9. Caffeine is present in low concentration in these extracts, thus preventing the overload of the column with a concentrated concentration sample, or the need to dilute the sample before analysis. For a more focused analysis of caffeoyl acids, fractions F1, F2, and F3 could be combined, thereby eliminating caffeine, dicaffeoyl acids, and flavonoids from the sample, among other compounds. If the targets of the analysis are dicaffeoyl acids or flavonoids, only F9 needs to be analyzed.
In addition to excellent results in terms of recovery and separation, using a high-pressure technique and a dynamic process allowed the use of an in-line detector. It enabled to monitor in real-time the concentration of the compounds in the collected extracts. Most extraction and separation processes do not use real-time monitoring, and the process is based on the extraction time or the volume collected, which must be optimized for each different sample.
The overall recovery of compounds in the different fractions collected is shown in Fig. 4F. As can be seen in Fig. 4F, there is an evident correlation between the UV detector signal and the concentration of compounds present in the extracts that were collected. The detector’s signal was saturated in the first fractions, even though the minimum sensitivity of the detector was used to minimize this problem. The signal saturation is due to the high concentration of compounds that absorb at the selected wavelength (260 nm). From the moment that the concentration of the compounds present begins to decrease in the F4 fraction, the detector signal also begins to fall until it reaches a minimum in the F8 fraction, where compounds are no longer detected in the extracts. By changing the solvent to ethanol, the compounds that were retained are eluted with high concentration, causing the saturation of the detector signal once more. In the last step of the process, although there are no more compounds in the extracts, the detector signal takes a long time to return to minimum values, but they indicate that the elution process has ended.
As ethanol has a UV cutoff at 210 nm, the composition of the extraction solvent does not affect the UV signal since the detector is operating at 260 nm. This wavelength was selected since it allows the detection of all compounds in the sample. As one of the critical points of the process is the depletion of the compounds extracted/eluted with water, it is necessary to be able to detect the smallest possible amounts of the compounds present. In this case, only caffeine is detected after the F4 fraction. The concentration also identifies different stages of the caffeine extraction kinetic curve in the detector signal. In the graph of total recovery of the compounds, it is possible to see a drop in the rate of caffeine extraction from the F4 fraction, which indicates the end of the first stage of the curve, where there is a constant rate of extraction (CER- Constant extraction rate) (Rostagno et al., 2013). From this point on, the falling extraction rate phase (FER) begins between fractions F5 and F6. It is also possible to observe an inflection in the curve from the F7, suggesting the beginning of the phase controlled by the diffusion (DC), which is the longest stage of an extraction process.
As caffeine is the compound in greater quantity and is the only one detected in the last fraction, and therefore, its monitoring can be used to control the first stage of the process (aqueous extraction) and enable the application of the method developed for different samples. In this case, the process can be extended if a high signal is still detected in the last fraction in the aqueous extraction phase (F8) or the ethanol extraction phase (F10). Different wavelengths can also be used focusing on specific compounds, such as 325 nm for phenolic acids and 350 nm for flavonoids.
Due to the simplicity of implementing this strategy by connecting the detector to the outlet of the system, it can be easily explored more widely in research laboratories dealing with different samples with varying concentrations of compounds. Additionally, this type of strategy can be used for other types of samples and compounds, such as anthocyanins in fruits at 525 nm. The ideal in this type of application would be to use a DAD detector where it would be possible to monitor several wavelengths simultaneously. There is also the possibility of using other detectors in line, such as a fluorescence detector, exploring the characteristics of some compoundsto control the processselectively (Rostagno et al., 2011).
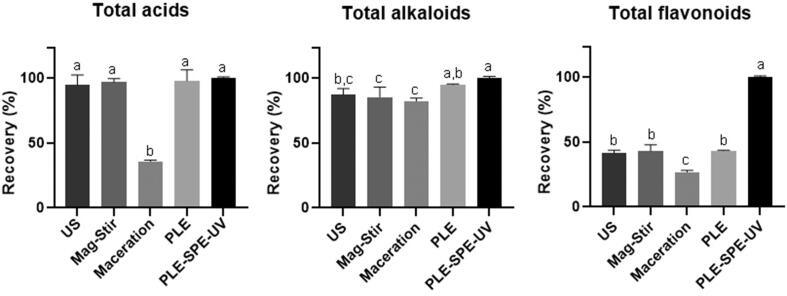
3.6. Comparison with other extraction techniques
The results obtained with the developed method (PLE-SPE-UV) were compared with other techniques: ultrasound (US), magnetic stirring (Mag-Stir), maceration, and pressurized liquids (PLE) (Fig. 5). In terms of total acids, maceration was the only technique returning a low recovery (30% of the highest recovery). At the same time, no significant difference was observed between the US, Mag-Stir, and PLE-SPE-UV methods. It is also observed that magnetic stirring and maceration provided the lowest recoveries of total alkaloids (13.85 ± 1.29 and 13.32 ± 0.34 mg/g, respectively). In contrast, PLE and PLE-SPE-UV showed the highest recovery (15.38 ± 0.05 and 16.16 ± 0.08 mg/g, respectively). The recovery obtained with US (14.14 ± 0.67 mg/g) was not statistically different from those produced with other techniques, except for PLE-SPE-UV.
Fig. 5.
Recovery (mg / g) of total alkaloids, phenolic acids and flavonoids using different extraction techniques. Ultrasound (US), Magnetic Stirring (Mag-Stir), Maceration, Pressurized Liquids Extraction (PLE) and Pressurized Liquids Extraction coupled on-line with solid phase extraction and UV–Vis detection (PLE-SPE-UV). Different lower case letters indicate significant difference (p < 0.05 - Tukey HSD test).
However, the most significant differences were observed in the recovery of flavonoids. It is noteworthy that the recovery of flavonoids with PLE-SPE-UV was more than double that of the other techniques. While US, Mag-Stir, maceration, and PLE produced recoveries between 1.79 and 2.87 mg/g of flavonoids, with PLE-SPE-UV, it was possible to recover 6.27 ± 0.17 mg/g. It is interesting to highlight that these methods use a sequential process with different solvents (first water, then 50% ethanol, and lastly, 100% ethanol), and therefore, they should provide quantitative recoveries. The higher recovery of flavonoids by PLE-SPE-UV may be related to the higher volume of solvent used (330 mL of water and 30 mL of ethanol, compared to 37.5 mL of water and 37.5 mL of ethanol) and the longer extraction time. The higher recovery may also be associated with the collection of flavonoids in a concentrated fraction, facilitating their detection and quantification. Modification of pH is likely to be the main factor behind the increased recovery of flavonoids, either by improving their retention capacity of the adsorbent or by improving their extraction from the sample matrix.
It is also important to note that the extraction techniques used to produce extracts with a complex mixture of compounds. On the other hand, the PLE-SPE-UV allows separating them into different fractions. Furthermore, the concentration of compounds in the fractions is higher than those found in the extracts obtained with the other tested methods. In this context, the proposed method can be used not only as an analytical tool but also insemi-preparative applications since it provides high recoveries and excellent separation of compounds.
4. Conclusions
The coupling of PLE with SPE and a UV detector was successful for the simultaneous extraction and separation of compounds from mate leaves. Using a two-stage extraction/separation process, allowed to separate phenolic acids, caffeine, and flavonoids. Although it was possible to separate caffeoyl from dicafeoyl acids, it was not possible to separate the later from the flavonoids. Caffeine can be isolated from other sample components, allowing to use a more straightforward analysis technique, such as spectrometry, or shorter/faster LC method. The coupling of a UV–Vis to the system proved to be simple and a powerfultool to monitor the process in real-time, thus allowing the application of the developed method to other samples with different concentrations of caffeine. Flavonoids and dicaffeoyl acids were the most affected compounds by modifying the main variables. Among the most significant variables in the process, pH and temperature seem to play significant roles in the process by affecting the extraction of compounds from the sample or the retention by the adsorbent. Finally, the developed method provided a similar or higher recovery of phenolic acids and alkaloids to those obtained with other techniques and methods. However, much higher recovery of flavonoids was obtained using the developed method, suggesting that conventional methods were subestimating the overall amount of these compounds in the sample.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants 2013/04304-4 and 2018/14582-5 from the São Paulo Research Foundation (FAPESP). MCS and LCS are grateful to FAPESP (2016/19930-6, 2018/17089-8, 2019/24537-0). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Process 88887.310558/2018-00 and Finance Code 001.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2020.100008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alvarez-Rivera G., Bueno M., Ballesteros-Vivas D., Mendiola J.A., Ibañez E. Liquid-Phase Extraction. 2020. Pressurized liquid extraction; pp. 375–398. [DOI] [Google Scholar]
- Arçari D.P., Santos J.C., Gambero A., Ribeiro M.L. The in vitro and in vivo effects of yerba mate (Ilex paraguariensis) extract on adipogenesis. Food Chemistry. 2013;141(2):809–815. doi: 10.1016/j.foodchem.2013.04.062. [DOI] [PubMed] [Google Scholar]
- Berrueta L.A., Gallo B., Vicente F. A review of solid phase extraction: Basic principles and new developments. Chromatographia. 1995;40(7-8):474–483. doi: 10.1007/BF02269916. [DOI] [Google Scholar]
- Bracesco N., Sanchez A.G., Contreras V., Menini T., Gugliucci A. Recent advances on Ilex paraguariensis research: Minireview. Journal of Ethnopharmacology. 2011;136(3):378–384. doi: 10.1016/j.jep.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Bravo L., Goya L., Lecumberri E. LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Research International. 2007;40(3):393–405. doi: 10.1016/j.foodres.2006.10.016. [DOI] [Google Scholar]
- Cardozo Junior E.L., Morand C. Interest of mate (Ilex paraguariensis A. St.-Hil.) as a new natural functional food to preserve human cardiovascular health – A review. Journal of Functional Foods. 2016;21:440–454. doi: 10.1016/j.jff.2015.12.010. [DOI] [Google Scholar]
- da Silva L.C., Souza M.C., Sumere B.R., Silva L.G.S., da Cunha D.T., Barbero G.F.…Rostagno M.A. Simultaneous extraction and separation of bioactive compounds from apple pomace using pressurized liquids coupled on-line with solid-phase extraction. Food Chemistry. 2020;318:126450. doi: 10.1016/j.foodchem.2020.126450. [DOI] [PubMed] [Google Scholar]
- da Silveira T.F.F., Meinhart A.D., de Souza T.C.L., Teixeira Filho J., Godoy H.T. Phenolic compounds from yerba mate based beverages – A multivariate optimisation. Food Chemistry. 2016;190:1159–1167. doi: 10.1016/j.foodchem.2015.06.031. [DOI] [PubMed] [Google Scholar]
- Fernandes C.E.F., Scapinello J., Bohn A., Boligon A.A., Athayde M.L., Magro J.D.…Tres M.V. Phytochemical profile, antioxidant and antimicrobial activity of extracts obtained from erva-mate (Ilex paraguariensis) fruit using compressed propane and supercritical CO2. J Food Sci Technol. 2017;54(1):98–104. doi: 10.1007/s13197-016-2440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mendoza M.D.P., Espinosa-Pardo F.A., Baseggio A.M., Barbero G.F., Maróstica Junior M.R., Rostagno M.A., Martínez J. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. The Journal of Supercritical Fluids. 2017;119:9–16. doi: 10.1016/j.supflu.2016.08.014. [DOI] [Google Scholar]
- Gómez-Juaristi M., Martínez-López S., Sarria B., Bravo L., Mateos R. Absorption and metabolism of yerba mate phenolic compounds in humans. Food Chemistry. 2018;240:1028–1038. doi: 10.1016/j.foodchem.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Gonçalves I.L., Dartora N., Piovezan Borges A.C., Picolo A.P., Dallago R.M., Mera de Souza L., Valduga A.T. Accelerated maturation of processed yerba-mate under the controlled conditions of temperature and humidity. Nutrition & Food Science. 2015;45(4):564–573. doi: 10.1108/NFS-12-2014-0105. [DOI] [Google Scholar]
- Grosso G., Godos J., Galvano F., Giovannucci E.L. Coffee, caffeine, and health outcomes: An umbrella review. Annual Review of Nutrition. 2017;37(1):131–156. doi: 10.1146/annurev-nutr-071816-064941. [DOI] [PubMed] [Google Scholar]
- Jongberg S., Racanicci A.M.C., Skibsted L.H. Mate extract is superior to green tea extract in the protection against chicken meat protein thiol oxidation. Food Chemistry. 2019;300:125134. doi: 10.1016/j.foodchem.2019.125134. [DOI] [PubMed] [Google Scholar]
- Kungel P.T.A.N., Correa V.G., Corrêa R.C.G., Peralta R.A., Soković M., Calhelha R.C.…Peralta R.M. Antioxidant and antimicrobial activities of a purified polysaccharide from yerba mate (Ilex paraguariensis) International Journal of Biological Macromolecules. 2018;114:1161–1167. doi: 10.1016/j.ijbiomac.2018.04.020. [DOI] [PubMed] [Google Scholar]
- Lima J.d.P., Farah A., King B., de Paulis T., Martin P.R. Distribution of major chlorogenic acids and related compounds in Brazilian Green and Toasted Ilex paraguariensis (Maté) Leaves. Journal of Agricultural and Food Chemistry. 2016;64(11):2361–2370. doi: 10.1021/acs.jafc.6b00276. [DOI] [PubMed] [Google Scholar]
- Linares A.R., Hase S.L., Vergara M.L., Resnik S.L. Modeling yerba mate aqueous extraction kinetics: Influence of temperature. Journal of Food Engineering. 2010;97(4):471–477. doi: 10.1016/j.jfoodeng.2009.11.003. [DOI] [Google Scholar]
- Mai X., Liu Y., Tang X., Wang L., Lin Y., Zeng H., Luo L., Fan H., Li P. Sequential extraction and enrichment of flavonoids from Euonymus alatus by ultrasonic-assisted polyethylene glycol-based extraction coupled to temperature-induced cloud point extraction. Ultrasonics Sonochemistry. 2020;66:105073. doi: 10.1016/j.ultsonch.2020.105073. [DOI] [PubMed] [Google Scholar]
- Motikar P.D., More P.R., Arya S.S. A novel, green environment-friendly cloud point extraction of polyphenols from pomegranate peels: A comparative assessment with ultrasound and microwave-assisted extraction. Separation Science and Technology (Philadelphia) 2020;1–12 doi: 10.1080/01496395.2020.1746969. [DOI] [Google Scholar]
- Navarra G., Moschetti M., Guarrasi V., Mangione M.R., Militello V., Leone M. Simultaneous determination of caffeine and chlorogenic acids in green coffee by UV/Vis spectroscopy. Journal of Chemistry. 2017;2017:1–8. doi: 10.1155/2017/6435086. [DOI] [Google Scholar]
- Negrão Murakami A.N., de Mello Castanho Amboni R.D., Prudêncio E.S., Amante E.R., de Moraes Zanotta L., Maraschin M., Cunha Petrus J.C., Teófilo R.F. Concentration of phenolic compounds in aqueous mate (Ilex paraguariensis A. St. Hil) extract through nanofiltration. LWT - Food Science and Technology. 2011;44(10):2211–2216. doi: 10.1016/j.lwt.2011.06.002. [DOI] [Google Scholar]
- Pereira D.T.V., Tarone A.G., Cazarin C.B.B., Barbero G.F., Martínez J. Pressurized liquid extraction of bioactive compounds from grape marc. Journal of Food Engineering. 2019;240:105–113. doi: 10.1016/j.jfoodeng.2018.07.019. [DOI] [Google Scholar]
- Płotka-Wasylka J., Szczepańska N., de la Guardia M., Namieśnik J. Modern trends in solid phase extraction: New sorbent media. TrAC Trends in Analytical Chemistry. 2016;77:23–43. doi: 10.1016/j.trac.2015.10.010. [DOI] [Google Scholar]
- Riachi L.G., Simas D.L.R., Coelho G.C., Marcellini P.S., Ribeiro da Silva A.J., Bastos de Maria C.A. Effect of light intensity and processing conditions on bioactive compounds in maté extracted from yerba mate (Ilex paraguariensis A. St.-Hil.) Food Chemistry. 2018;266:317–322. doi: 10.1016/j.foodchem.2018.06.028. [DOI] [PubMed] [Google Scholar]
- Rostagno M.A., D’Arrigo M., Martínez J.A., Martínez J.A. Combinatory and hyphenated sample preparation for the determination of bioactive compounds in foods. TrAC Trends in Analytical Chemistry. 2010;29:553–561. doi: 10.1016/j.trac.2010.02.015. [DOI] [Google Scholar]
- Rostagno, M. A., Prado, J. M., & Kraus, G. A. (2013). Natural Product Extraction: Principles and Applications. 58-86.
- Rostagno M.A., Manchón N., D’Arrigo M., Guillamón E., Villares A., García-Lafuente A.…Martínez J.A. Fast and simultaneous determination of phenolic compounds and caffeine in teas, mate, instant coffee, soft drink and energetic drink by high-performance liquid chromatography using a fused-core column. Analytica Chimica Acta. 2011;685(2):204–211. doi: 10.1016/j.aca.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Rostagno M.A., Villares A., Guillamón E., García-Lafuente A., Martínez J.A. Sample preparation for the analysis of isoflavones from soybeans and soy foods. Journal of Chromatography A. 2009;1216(1):2–29. doi: 10.1016/j.chroma.2008.11.035. [DOI] [PubMed] [Google Scholar]
- Santos M.P., Souza M.C., Sumere B.R., da Silva L.C., Cunha D.T., Bezerra R.M.N., Rostagno M.A. Extraction of bioactive compounds from pomegranate peel (Punica granatum L.) with pressurized liquids assisted by ultrasound combined with an expansion gas. Ultrasonics Sonochemistry. 2019;54:11–17. doi: 10.1016/j.ultsonch.2019.02.021. [DOI] [PubMed] [Google Scholar]
- Santos L.F.D., Vargas B.K., Bertol C.D., Biduski B., Bertolin T.E., Santos L.R.D., Brião V.B. Clarification and concentration of yerba mate extract by membrane technology to increase shelf life. Food and Bioproducts Processing. 2020;122:22–30. doi: 10.1016/j.fbp.2020.04.002. [DOI] [Google Scholar]
- Sökmen M., Demir E., Alomar S.Y. Optimization of sequential supercritical fluid extraction (SFE) of caffeine and catechins from green tea. The Journal of Supercritical Fluids. 2018;133:171–176. doi: 10.1016/j.supflu.2017.09.027. [DOI] [Google Scholar]
- Soquetta M.B., Tonato D., Quadros M.M., Boeira C.P., Cichoski A.J., Marsillac Terra L., Kuhn R.C. Ultrasound extraction of bioactive compounds from Citrus reticulata peel using electrolyzed water. J Food Process Preserv. 2019;43(12) doi: 10.1111/jfpp.14236. [DOI] [Google Scholar]
- Souza M.C., Santos M.P., Sumere B.R., Silva L.C., Cunha D.T., Martínez J.…Rostagno M.A. Isolation of gallic acid, caffeine and flavonols from black tea by on-line coupling of pressurized liquid extraction with an adsorbent for the production of functional bakery products. LWT. 2020;117:108661. doi: 10.1016/j.lwt.2019.108661. [DOI] [Google Scholar]
- Sumere B.R., de Souza M.C., dos Santos M.P., Bezerra R.M.N., da Cunha D.T., Martinez J., Rostagno M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.) Ultrasonics Sonochemistry. 2018;48:151–162. doi: 10.1016/j.ultsonch.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Tian M., Yan H., Row K.H. Solid-phase extraction of caffeine and theophylline from green tea by a new ionic liquid-modified functional polymer sorbent. Analytical Letters. 2010;43(1):110–118. doi: 10.1080/00032710903276554. [DOI] [Google Scholar]
- Xu J.L., Kim T.J., Kim J.-K., Choi Y. Simultaneous roasting and extraction of green coffee beans by pressurized liquid extraction. Food Chemistry. 2019;281:261–268. doi: 10.1016/j.foodchem.2018.12.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.