Highlights
-
•
T. ferdinandiana extracts impact in vitro cell viability less than ellagic acid.
-
•
T. ferdinandiana extracts impact intestinal cell viability less than HepG2 viability.
-
•
T. ferdinandiana seedcoat extracts impact cell viability less than fruit extracts.
Keywords: Cell viability, CyQUANT® NF Cell Proliferation Assay, Ellagic acid, HepG2, Kakadu plum, Terminalia ferdinandiana
Abstract
Terminalia ferdinandiana (Kakadu plum) is a native Australian fruit consumed by Indigenous Australians for centuries. Commercial interest in T. ferdinandiana has increased in recent years due to its high vitamin C content, however, food safety assessments are lacking. To explore the safety of extracts prepared from T. ferdinandiana using different solvents, in vitro cell viability of undifferentiated and differentiated Caco-2, HT29-MTX-E12, and HepG2 cells was measured using the CyQUANT® NF Cell Proliferation Assay. Changes to cell viability produced IC50 values between 3650 and 14400 µg/mL for all extracts and cell lines tested with HepG2 cells impacted the most by T. ferdinandiana extracts, followed by HT29-MTX-E12 cells, and undifferentiated and differentiated Caco-2 cells. Different solvents also produced extracts with variable effects on cell viability that were dependent on tissue source, however, extracts from seedcoats appeared to impact cell viability less than fruit extracts. The IC50 values for ellagic acid, an abundant phytochemical in T. ferdinandiana, varied from 1190 to 2390 µg/mL across different cells and were significantly lower than extract IC50 values. Findings from this study will help to inform future safety studies, select which solvents to use when preparing T. ferdinandiana extracts, and decide whether fruit flesh should be separated from seeds during extract preparation.
1. Introduction
The use of plants as whole foods and ingredients is increasing based on public perception that plants are mostly safe and without risk to human health. However, any new food or ingredient must be assessed for safety regardless of its origin (Berg, Coppens, & Rietjens, 2011). Terminalia ferdinandiana (Kakadu plum) is a native Australian fruit consumed by Indigenous Australians and used as a traditional medicine for treatment of skin infections, colds, and flus (Akter, Netzel, Fletcher, Tinggi, & Sultanbawa, 2018). Commercial T. ferdinandiana products are also available and composed of fruit pulp powders and mixtures of powdered fruits and seeds. Although T. ferdinandiana fruits and extracts are widely used by Indigenous Australians and included in modern Australian cuisine, scientific validation of safety and efficacy is lacking.
The demand for T. ferdinandiana fruit is steadily increasing due to new product lines emerging in mainstream supermarkets and the growing interest in Australian native food products in tourism and food service industries (Gorman, Wurm, Vemuri, Brady, & Sultanbawa, 2020). Previous studies conducted by our research group revealed that extracts from T. ferdinandiana are rich sources of polyphenols with significant antioxidant capacity and antimicrobial potential (Akter, et al., 2019). Other studies have highlighted the anti-inflammatory and anticancer potential of T. ferdinandiana in vitro (Tan et al., 2011, Tan et al., 2011) and revealed the presence of compounds, like ellagic acid, in extracts from T. ferdinandiana fruits that can elicit a diverse range of biological activities. However, despite these previous studies into the bioactivity of T. ferdinandiana extracts and constituents, research into the safety of T. ferdinandiana fruits and seedcoats is lacking. Furthermore, research studies have focussed on the various nutritional and health promoting effects of phytochemicals in fruits and have not yet investigated seeds, even though seeds and fruits are often processed together in some commercial T. ferdinandiana products.
Precautionary instructions prescribed by current T. ferdinandiana fact sheets restrict consumption of seeds by specifying ‘not to eat too many of the seeds as they contain some toxins’ (RIRDC & ANFIL, 2012). Concerns about the safety of T. ferdinandiana products have been raised by some consumers in response to these instructions. Therefore, a comprehensive risk assessment is needed to assess the safety of T. ferdinandiana products and ingredients. In the risk assessment of foods and food ingredients, potential toxic compounds must be identified along with the biological mechanisms that cause toxicity and concentration-dependent effects. Therefore, to determine potential health risks that could be associated with T. ferdinandiana consumption, the present study investigated the impact of methanolic, ethanolic, and water extracts prepared from T. ferdinandiana fruits and seedcoats on the viability of various human cell lines in vitro.
The in vitro assessment of cell viability in response to new compounds is important in biomedical research to identify potential toxicities (Ramirez, Antczak, & Djaballah, 2010). In vitro cell viability assays investigate the impact of test compounds on the viability of cells grown in culture, usually by determining the number of viable cells remaining after an incubation period. These assays are performed to predict in vivo cytotoxicity of a test compound by estimating the number of viable cells remaining compared to untreated controls (Ramirez, Antczak, & Djaballah, 2010). These observations are often compared to a reference control or commercial standard.
Cell viability assays usually include simple endpoint measurements based on DNA labelling, assessing metabolic function, or evaluating membrane integrity (Ramirez, Antczak, & Djaballah, 2010). For example, measuring cell viability through DNA involves estimating cellular DNA content via fluorescent dye binding after incubating cells with a test compound over a defined period. Cellular DNA content is highly regulated and closely proportional to cell number, therefore the impact of test compounds on cell viability is determined by comparing cellular DNA content in treated cells to untreated control cells (Akter, et al., 2019).
The small intestine and liver are two vital organs in the digestive system. During intestinal digestion and absorption, polyphenols directly interact with the epithelium of the small intestine (Yu & Huang, 2013) and may be transported to the liver after absorption. Furthermore, previous reports attribute ellagitannins (like punicalagin and castalagin) in T. oblongata (another native Australian species of Terminalia) to hepatotoxicity in cattle and sheep (FILIPPICH and CAO, 1993, Filippich et al., 1991). Therefore, potential cytotoxicity of polyphenols present in T. ferdinandiana extracts should be explored in the intestine and liver. Based on the reported abundance of ellagic acid in T. ferdinandiana fruits (Williams et al., 2016), and on the potential of this polyphenol as a promising health promoting phytochemical (Singh, Singh, Kaur, & Singh, 2018), ellagic acid should also be included in the assessment of T. ferdinandiana cytotoxicity.
Human cancer-derived cell lines are often used to investigate cytotoxicity in vitro. The human Caco-2 cell line has been widely used as a model of the intestinal epithelial barrier. The human HT29-MTX-E12 cell line secretes mucus and is also commonly used in human intestinal cell models. The human HepG2 cell line is derived from a hepatocellular carcinoma and widely used as an in vitro model of human liver epithelial cells. To model the small intestine in vitro and mimic exposure of the small intestine to polyphenols present in the T. ferdinandiana extracts, undifferentiated and differentiated Caco-2 cells and HT29-MTX-E12 cells were used in this study. To investigate the cytotoxicity of polyphenols present in T. ferdinandiana extracts in liver epithelial cells in vitro, HepG2 cells were treated with extracts before cell viability was measured.
Cell viability was measured via the CyQUANT® NF Cell Proliferation Assay in response to polyphenol-rich extracts prepared from T. ferdinandiana (using accelerated solvent extractions with methanol, ethanol, and water) in comparison to standard ellagic acid. The CyQUANT® NF Cell Proliferation Assay, an assay that measures cell viability through cellular DNA content, was selected for assessment of T. ferdinandiana extracts based on incompatibilities previously observed between T. ferdinandiana extracts and other cell proliferation assays (Akter, et al., 2019). These in vitro studies are the first attempts to explore possible cytotoxicity associated with polyphenol-rich extracts of T. ferdinandiana fruits and seedcoats.
2. Materials and methods
2.1. Materials
Ellagic acid, PEG 400, Nunc cell culture flasks, and 96 well plates were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia). Dulbecco’s modified eagle medium (DMEM), Dulbecco’s phosphate buffered saline without calcium and magnesium (PBS), Hank’s Balanced Salt Solution (HBSS), penicillin and streptomycin, fetal bovine serum (FBS), glutamax, trypsin-EDTA, non-essential amino acids (NEAA), and trypan blue exclusion dye were purchased from Invitrogen (Thermo Fisher Scientific Corporation, Waltham, MA, USA). CyQUANT® NF Cell Proliferation Assay reagent was purchased from Invitrogen (Molecular Probes™, Thermo Fisher Scientific Corporation, Waltham, MA, USA). The Caco-2 cell line was purchased from the American Type Culture Collection (Manassas, Virginia, USA). The HT29-MTX-E12 and HepG2 cell lines were purchased from Sigma-Aldrich.
2.2. Plant materials and preparation of T. Ferdinandiana extracts
The collection and preparation of the plant materials and the accelerated solvent extraction (ASE) method performed to prepare the extracts were described previously by Akter, Netzel, Tinggi, Osborne, Fletcher, and Sultanbawa (2019). Methanol, ethanol, and distilled water were used as solvents. The concentrated extracts were weighed and stored at −20 °C.
2.3. Extract information and in vitro treatment concentration ranges
The accelerated solvent extracts of T. ferdinandiana fruits prepared using methanol, ethanol and water are denoted as FM, FE, and FW respectively. Similarly, accelerated solvent extracts of T. ferdinandiana seedcoats prepared using methanol, ethanol, and water are denoted as SM, SE, and SW respectively. Treatments for in vitro assays were prepared in HBSS across a range of concentrations.
The range of treatment concentrations selected for each extract were irrespective of biological relevance and based on preliminary experiments to determine concentration ranges that might produce 0–100% cell viability and enable accurate calculation of half maximal inhibitory concentrations. The half-maximal inhibitory concentration, or IC50, is a commonly used measure defined as a treatment concentration that inhibits 50% of a specific biological or biochemical function. In this study, IC50 refers to the treatment concentration that decreased cell viability by 50%. Lower IC50 values indicate that less compound is needed to produce cytotoxicity. Conversely, higher IC50 values indicate that more of the compound can be added to the cells without impacting cell viability. Preliminary experiments trialling broad treatment concentration ranges were performed initially to narrow the treatment range and allow accurate calculations of IC50 values. In the CyQUANT® NF Cell Proliferation Assay, the treatment weight range of solubilised dried T. ferdinandiana material for FM was 66–50000 µg/mL, SM was 66–38340 µg/mL, FW was 66–135900 µg/mL, SW was 66–113200 µg/mL, FE was 66–200000 µg/mL, and SE was 33–200000 µg/mL.
2.4. Cell culture
DMEM (supplemented with 10% FBS (v/v)), 1X NEAA, penicillin 100 U/mL, streptomycin 100 μg/mL, and Glutamax 2 mM was used to culture Caco-2 and HT29-MTX-E12 cells. HepG2 cells were maintained in DMEM (supplemented with 10% FBS (v/v)), penicillin 100 U/mL, streptomycin 100 μg/mL, and Glutamax 2 mM. Cells were grown in vented culture flasks at 37 °C and 5% CO2. Cells were passaged at 90% confluency and every 2–3 days. Sub-culturing of cells was performed by washing cells with PBS followed by incubation with 0.25% (v/v) trypsin-EDTA for 2–4 min to detach the cells from the flask. Growth media was then added to neutralize trypsin before centrifugation at 1500 × g for 5 min. Centrifugation was used to pellet the cells and enable the supernatant to be replaced with fresh growth media for cell suspension. Cells were counted using trypan blue exclusion staining with the cell suspension appropriately diluted for cell passage. For the in vitro assays, cell plating, and media and buffer changes, were performed by an epMotion® 5075 t liquid handling system (Eppendorf, Hamburg, Germany).
2.5. Cell line information and cell numbers for in vitro assays
All cell numbers and growth conditions were adapted from Sultan et al. (Sultan, Osborne, Addepalli, Netzel, Netzel, & Fletcher, 2017). For the undifferentiated Caco-2 cell model, 4 × 104 cells/well were grown in 96 well plates for 24 h prior to treatment producing 90% cell confluency. For the differentiated Caco-2 cell model, 1 × 104 cells/well were grown in 96 well plates for 7 days prior to cell treatment and viability measurements. For the HT29-MTX-E12 cell model, 4 × 104 cells/well were grown in 96 well plates for 24 h prior to cell treatment and viability measurements. For the HepG2 cell model, 5 × 104 cells/well were grown in 96 well plates for 24 h prior to cell treatment and viability measurements.
2.6. Preparation of ellagic acid standard
Ellagic acid (30 mg) was used as the positive control and dissolved in 4 mL PEG 400 to prepare a stock solution of 7.5 mg/mL. The stock solution was diluted in HBSS to achieve the following treatment concentrations: 100 µg/mL, 500 µg/mL, 1500 µg/mL, 3000 µg/mL, 6000 µg/mL, 9000 µg/mL, 12000 µg/mL, 20000 µg/mL, and 24000 µg/mL. These nine treatment concentrations were applied to all cell assays to compare to cell viability in response to the T. ferdinandiana extracts.
2.7. Cytotoxicity assay protocol
CyQUANT® NF Cell Proliferation Assay
Assay protocol
Nunc™ F96 MicroWell™ Black polystyrene plates were purchased from Thermo Scientific™ (Waltham, MA, USA). Plates were prepared with 100 µL mammalian cell suspensions per well. Control wells were prepared by adding 100 µL medium without cells to obtain a value for background fluorescence. The plates were incubated for 24 h at 37 °C and 95% CO2. On the day of experiment, culture media was removed and replaced with 100 µL HBSS and incubated for 2 h. After 2 h, HBSS was removed and replaced with 50 µL test compounds or HBSS for the control wells. The plates were incubated for 2 h at 37 °C and 95% CO2. After 2 h, the test compounds were removed, wells were washed with 100 µL HBSS. The HBSS was removed before 74 μL 1 X dye binding solution was added to each well using a manual multichannel pipette. The plate was covered and incubated at 37 °C for 60 min. Fluorescence was measured with excitation at 485 nm and emission at 530 nm using a Spectramax M3 multi-mode microplate reader (Molecular Devices, San Jose, CA, USA). Cell-free experiments were also performed to detect possible interactions between the extracts and assay reagents.
2.8. Statistical analysis
Cytotoxicity is expressed as the percentage of viable cells remaining after extract treatment compared to the HBSS control. Data is presented as the mean percentage of six replicates ± standard error of the mean (SEM) for each treatment. The half maximal inhibitory concentration (IC50) was calculated from the percentage of viable cells remaining after each extract treatment and in each cell line using four parameter, variable response curves fitted by GraphPad Prism version 8 (San Diego, CA, USA). Statistical comparisons were comprised of one-way ANOVAs with Dunnett’s multiple comparison test to compare with standards and Tukey’s multiple comparison tests to compare groups. P values ≤ 0.05 were considered significant. Unpaired t tests were also performed to determine the differences between two groups and P values ≤ 0.05 were considered significant.
3. Results and discussion
The CyQUANT® NF Cell Proliferation Assay measures cellular DNA content via fluorescent dye binding. Cellular DNA content is highly regulated and closely proportional to cell number, hence, the CyQUANT® NF Cell Proliferation Assay kit can be used to measure cell proliferation rate over a defined time period, or cell viability as a proportion of live cells within a population at a given time. In this study, the CyQUANT® NF Cell Proliferation Assay was used to determine cell viability following treatment with polyphenol-rich extracts prepared from T. ferdinandiana fruits and seedcoats.
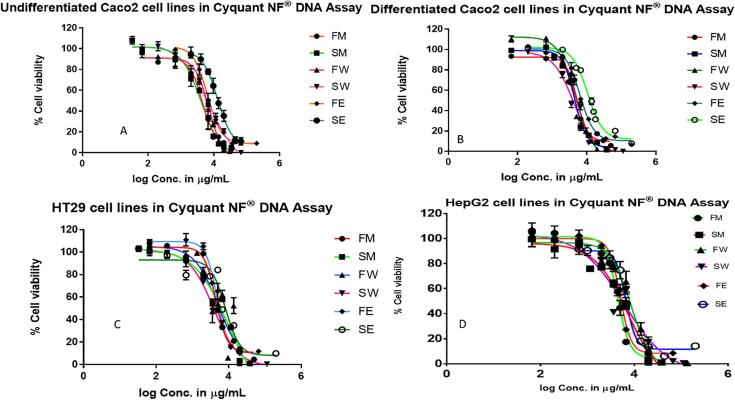
Treatment concentration–response curves are presented in Fig. 1. Each of the polyphenol-rich ASE extracts reduced cell viability of the tested cell lines in a treatment-dependent manner. The IC50 values calculated from these curves are presented in Table 1 and range from 3650 to 14400 µg/mL for all extracts. These values indicate a broad response in cell viability to treatment with the different extracts. Significant differences (p ≤ 0.05) were observed with the same extract across different cell types, and among different extraction methods when fruits and seedcoats were compared separately within the same cell type. Cell-free experiments were also performed to observe any interactions between T. ferdinandiana extracts and assay reagents. No interactions were detected between the extracts and assay reagents (data not shown).
Fig. 1.
Treatment concentration–response curve for fruit methanol (FM), seedcoat methanol (SM), fruit water (FW), seedcoat water (SW), fruit ethanol (FE) and seedcoat ethanol (SE) extract expressed as percentage viable intestinal and liver cells remaining after 2 h treatment compared to the HBSS control in (A) Undifferentiated Caco-2; (B) Differentiated Caco-2; (C) HT29-MTX-E12; and (D) HepG2 in CyQUANT® NF Cell Proliferation Assay. The data is presented as the mean percentage (±SEM) of six replicates for each treatment.
Table 1.
IC 50 values obtained using the CyQUANT® NF Cell Proliferation Assay.
| FM (µg/mL) | SM (µg/mL) | FW (µg/mL) | SW (µg/mL) | FE (µg/mL) | SE (µg/mL) | Standard ellagic Acid (µg/mL) | |
|---|---|---|---|---|---|---|---|
| Undifferentiated Caco-2 | 4550 ± 600 I, a | 11700 ± 360 I, x | 7350 ± 320 I, b, | 7040 ± 750 I, y | 6220 ± 330 I, c | 14400 ± 460 I, z | 1190 ± 180 |
| Differentiated Caco-2 | 5540 ± 470 II, a | 4120 ± 530 II, x | 5390 ± 600 II, IV, a | 3740 ± 400 II, V, x | 6420 ± 900 I, a | 10900 ± 100 II, y | 1210 ± 140 |
| HT29-MTX-E12 | 7170 ± 490 III, a | 8290 ± 240 III, x | 7110 ± 270 I, a | 3650 ± 280 III, V, y | 4820 ± 74 II, V, b | 7360 ± 600 III, V, z | 2390 ± 480 |
| HepG2 | 4300 ± 480 I, a | 7740 ± 190 III, x | 6000 ± 900 III, IV, b, c | 4700 ± 310 IV, y | 5000 ± 280 III, IV, a, c | 6700 ± 860 IV, V, z | 2000 ± 630 |
Results are expressed as mean ± SD; (n = 6); mean values of each column with different numerals and letters are significantly different (P ≤ 0.05); a, b, c, denotes significant differences of extraction solvents within fruits and ×, y, z denotes significant differences of extraction solvents in seedcoats within the same cell type; I – V denotes significant differences of the same extract across different cell types.
To determine whether viability of a specific cell type decreased more in response to treatment with the different extracts, the impact of each extract on the viability of the different cell types was compared. Statistical comparisons showed that viability of HepG2 and undifferentiated Caco-2 cells was more affected by FM compared to the other cell types, whereas SM impacted differentiated Caco-2 cells the most. FW impacted viability of differentiated Caco-2 and HepG2 cells more than other cell types, whilst SW impacted differentiated Caco-2 and HT29-MTX-E12 cells the most. Finally, FE impacted HT29-MTX-E12 cell viability the most whilst SE decreased cell viability similarly for both HepG2 and HT29-MTX-E12 cells. Overall, viability of HepG2 cells appeared to be more affected than other cell types by treatment with the different extracts followed by differentiated Caco-2 cells, HT29-MTX-E12 cells, and undifferentiated Caco-2 cells.
To determine if extraction with a specific solvent decreased cell viability more than other solvents, the impact of methanol, water, and ethanol extraction was compared within each cell line, and within fruit and seeds separately. From the fruit, methanol extraction decreased cell viability the most in undifferentiated Caco-2 cells, whilst ethanol extraction impacted HT29-MTX-E12 cell viability more than the other solvents. In HepG2 cells ethanol and methanol extraction decreased cell viability more whilst differentiated Caco-2 cells responded similarly to all extracts. From the seedcoats, extracts prepared in water decreased cell viability the most in all cell lines, except for differentiated Caco-2 cells where extraction with methanol and water impacted cell viability in a similar way.
To determine if extracts from fruit had impacted cell viability more or less than extracts from seedcoats, comparisons were made between each extract within each cell line. Overall, extracts from seedcoats appeared to impact cell viability less with IC50 values significantly higher than those in response to fruit extracts in 7 out of 12 comparisons (Table 2). Furthermore, methanol and ethanol extraction appeared to decrease the impact of seedcoat extracts on cell viability, with water extraction significantly decreasing IC50 values compared to fruit extracts in all cell types, except for undifferentiated Caco-2 cells that were comparable.
Table 2.
Unpaired t test values for the difference of means between the IC50 of fruit extracts and seedcoat extracts of T. ferdinandiana in various cell lines using CyQUANT® NF Cell Proliferation Assay.
| Fruits (µg/mL) (Mean ± SD) |
Seedcoats (µg/mL) (Mean ± SD) |
Mean Difference Fruits/Seedcoats | 95% CI | p value | R squared | |
|---|---|---|---|---|---|---|
| Undifferentiated Caco-2 | ||||||
| Methanol | 4550 ± 600 | 11700 ± 360 | 7150 | 6430 to 7880 | <0.0001 | 0.99 |
| Water | 7350 ± 320 | 7040 ± 750 | −300 | −1150 to 540 | 0.44 | 0.08 |
| Ethanol | 6220 ± 330 | 14400 ± 460 | 8220 | 7640 to 8800 | <0.0001 | 0.99 |
| Differentiated Caco-2 | ||||||
| Methanol | 5540 ± 470 | 4120 ± 530 | −1420 | −2150 to −680 | 0.002 | 0.71 |
| Water | 5390 ± 600 | 3740 ± 400 | −1650 | −2400 to −890 | 0.001 | 0.76 |
| Ethanol | 6420 ± 900 | 10900 ± 100 | 4500 | 3550 to 5450 | <0.0001 | 0.94 |
| HT29-MTX-E12 | ||||||
| Methanol | 7170 ± 490 | 8290 ± 240 | 1120 | 550 to 1700 | 0.0018 | 0.72 |
| Water | 7110 ± 270 | 3650 ± 280 | −3450 | −3860 to −3050 | <0.0001 | 0.98 |
| Ethanol | 4820 ± 74 | 7360 ± 600 | 4500 | 1900 to 3170 | <0.0001 | 0.92 |
| HepG2 | ||||||
| Methanol | 4300 ± 480 | 7740 ± 190 | 3440 | 2900 to 3970 | <0.0001 | 0.97 |
| Water | 6000 ± 900 | 4700 ± 310 | −1300 | −2290 to −310 | 0.016 | 0.54 |
| Ethanol | 5000 ± 280 | 6700 ± 860 | 1700 | 760 to 2630 | 0.0031 | 0.69 |
A detailed characterisation of phenolic compounds in extracts prepared from T. ferdinandiana fruits and seedcoats has been previously reported (Akter, Netzel, Tinggi, Osborne, Fletcher, & Sultanbawa, 2019) revealing that total phenolic content (TPC) in T. ferdinandiana fruits and seedcoats extracts can range from 0.2 to 12 g gallic acid equivalent (GAE)/100 g dry weight (DW). Among the extracts tested, methanol extracts of fruits exhibited the highest TPC (12.2 g GAE/100 g DW), whereas methanol extracts prepared from seedcoats contained only 0.3 g GAE/100 g DW. Condensed tannin contents of fruits and seedcoats were reported as 0.8 and 0.1 g catechin equivalent (CaE)/100 g DW respectively, 0.4 g quillaja saponins equivalent (QSE)/100 g DW fruit, and hydrolysable tannin content, in methanol extracts of fruits and seedcoats, as 55.3 and 0.9 g tannic acid equivalent (TAE)/100 g DW respectively. The differences in total phenolic and tannin contents in different polyphenol-rich extracts prepared from T. ferdinandiana fruits and seedcoats may help to explain the differences in cell viability observed in response to different extracts.
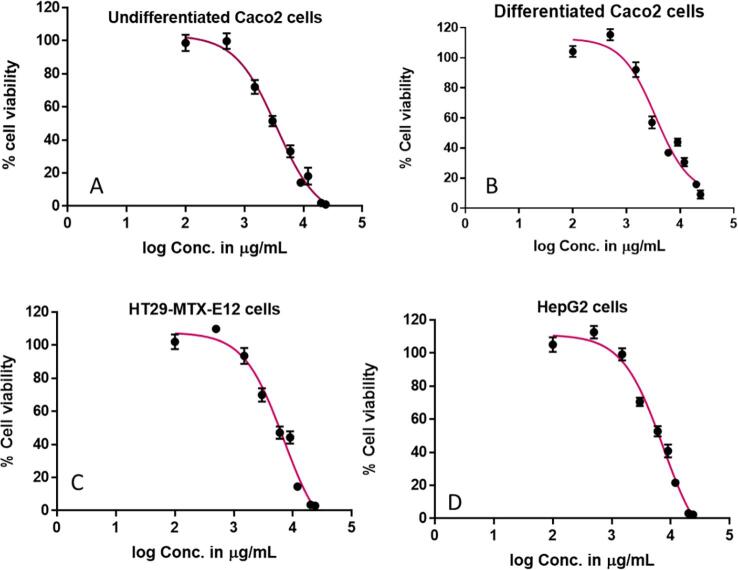
Ellagic acid is known to produce anti-proliferative effects in various cancer cell lines (Seeram et al., 2005). In this study, ellagic acid showed a treatment-dependent decrease in cell viability when cells were treated with a concentration range of 100–24000 µg/mL. The treatment concentration–response curves of standard ellagic acid produced by the CyQUANT® NF Cell Proliferation Assay are presented in Fig. 2. Cell viability results obtained using the CyQUANT® NF Cell Proliferation Assay produced an IC50 value of 1190 µg/mL for ellagic acid in undifferentiated Caco-2 cells followed by 1210, 2000, and 2390 µg/mL for differentiated Caco-2, HepG2, and HT29-MTX-E12 cells respectively (Table 1). Statistical comparisons within each cell type revealed IC50 values of ellagic acid were significantly lower than all other extracts indicating that standard ellagic acid impacts cell viability more than whole extracts containing ellagic acid.
Fig. 2.
Treatment concentration–response curves for ellagic acid using CyQUANT® NF Cell Proliferation Assay expressed as percentage viable intestinal and liver cells remaining after 2 h treatment compared to the HBSS control in (A) Undifferentiated Caco-2, (B) Differentiated Caco-2, (C) HT29-MTX-E12 and (D) HepG2. The data is presented as the mean percentage (±SEM) of six replicates for each treatment.
In previous studies, cell viability was measured in response to purified polyphenolic extracts from T. ferdinandiana fruits in AGS, HT29, CCD-18Co, and Hs 738.St/Int cells using MTT assays and in HL-60 and PBMC cultures using the Luminescent CellTiter-Glo ATP assay (Tan, Konczak, Ramzan, & Sze, 2011). The study reported different IC50 (µg/mL) values for AGS (872), HT29 (385), HL-60 (257), PBMC (239), CCD-18Co and Hs 738.St/Int (>2000) (Tan, Konczak, Ramzan, & Sze, 2011). The differences between IC50 values observed in previous research and in the current study might be due to the different cell types, experimental conditions, and the cell viability methods applied.
Based on the observations from the CyQUANT® NF Cell Proliferation Assay, the variable response by cells to different extracts may be due to several factors. For example, cell viability may be affected by chemical constituents of the extracts inducing pathological lesions in the cells resulting in cell necrosis (Alimba, Gandhi, Sivanesan, Bhanarkar, Naoghare, Bakare, et al., 2016). Viability of the HepG2 cells appeared to be impacted more than the other types and could be attributed to greater sensitivity of HepG2 cells to apoptotic processes that are affected by extract composition. Findings from this study suggest that HT29-MTX-E12 cells were the second most sensitive cell type investigated followed by undifferentiated and differentiated Caco-2 cells that responded similarly to the extracts.
Different extraction methods also influenced cell viability with methanol and ethanol producing extracts that decreased cell viability more than water extracts. This may be due to different chemical profiles achieved by different solvent extractions. Changes to cell viability observed in response to the different T. ferdinandiana extracts and chemical profiles, may be associated with DNA damage caused by DNA single and double strands breaks, DNA adducts formations, DNA-DNA and DNA-protein cross-links, alkali-labile sites, or incomplete excision repair (Alimba, et al., 2016). DNA damage may be due to individual and/or interactions between polyphenols present in the extracts.
The present study suggests that polyphenolic compounds present in the fruit and seedcoat extracts, and possible interactions between these compounds, might also influence the efficacy of ellagic acid in reducing cell viability. Ingestion of ellagic acid within different food matrices, or in its native form as in fruits and seedcoats of T. ferdinandiana, may influence bioavailability, metabolism, and therapeutic efficacy of ellagic acid differently from consumption of ellagic acid alone.
This study presents the first in vitro assessment of cytotoxicity in response to a variety of extracts from T. ferdinandiana fruits and seedcoats. Undifferentiated and differentiated Caco-2 and HT29-MTX-E12 cells were used to provide in vitro models of intestinal enterocytes and goblet cells that would be in direct contact with ingested food ingredients in the intestinal lumen. HepG2 cells were used to provide an in vitro liver cell model to observe potential hepatotoxicity. However, like all in vitro models, these cell models have limitations and are at best an indicator of biological effects in vivo.
Intestinal cell assays have become one of the most common in vitro techniques to mimic intestinal absorption and provide a means to investigate the biological activity of new foods and ingredients. However, cell-based assays cannot replace in vivo studies as these models are not reflective of all human cells, tissues, and complexity of biological functions and processes. For example, the high concentration of polyphenols used in cell culture do not accurately reflect the actual dietary levels nor do these cell models fully mimic bioaccessibility, bioavailability, metabolism, and elimination of polyphenolic compounds (Wisman, Perkins, Jeffers, & Hagerman, 2008). Moreover, possible interactions between ingested polyphenols and endogenous molecules (carbohydrates, proteins, enzymes) may influence the bioactivity of polyphenols in the body that cannot be evaluated using in vitro cell culture techniques (Wisman, Perkins, Jeffers, & Hagerman, 2008).
4. Conclusion
This is the first study to assess the impact of polyphenols (such as ellagic acid) present in T. ferdinandiana fruits and seedcoats on the viability of intestinal and hepatic cells in vitro. Amongst all the cell lines investigated in this study, HepG2 cells were the most sensitive cell type with the greatest impact on cell viability observed in response to T. ferdinandiana fruit extracts. This study also showed that standard ellagic acid decreased cell viability more than any of the T. ferdinandiana extracts, suggesting that these extracts contain a diverse range of polyphenolic and non-polyphenolic components that may promote cell growth and function, or inhibit the efficacy of ellagic acid as an anti-proliferative compound.
Beyond assessing cytotoxicity, understanding the ‘mode of action’ of polyphenols in vitro and in vivo is the next crucial step in elucidating the potential roles of these compounds in preventing diseases, such as cardiovascular diseases and cancer. The possible interactions between ellagic acid, nutrients, and other plant matrix components, during ingestion, digestion and absorption in the gastrointestinal tract, as well as the mechanisms affecting polyphenol circulation (blood), distribution (tissue), and metabolism (liver, colon), warrant further investigation to gain a better understanding of the safety and health promoting potential of T. ferdinandiana.
To date, T. ferdinandiana seedcoats have been believed to be unsafe and removed as a waste product. However, this in vitro study has shown that T. ferdinandiana seedcoats do not pose any additional impact on cell viability compared to T. ferdinandiana fruits. This information provides important evidence for the food industry in assessing the safety of T. ferdinandiana food products where fruits and seeds are processed together.
Author contribution
SA, MN, MF, YS and SO conceived and designed the study. SA performed the experiments, analysed data and wrote the manuscript. RA helped with the experiments and in analysing data. MN, MF, YS and SO critically revised and edited the manuscript. All authors approved the final manuscript.
Funding
This project was funded by AgriFutures Australia Grant 201430161, and by the CSIRO Active Integrated Matter Future Science Platform. Saleha Akter’s PhD was supported by an Australian Government Research Training Program Scholarship and The University of Queensland.
Conflicts of interest
The authors declare that there is no competing financial, professional or personal interests that might have influenced the performance or presentation of the work described in this manuscript.
References
- Akter S., Addepalli R., Netzel M.E., Tinggi U., Fletcher M.T., Sultanbawa Y., Osborne S.A. Antioxidant-rich extracts of Terminalia ferdinandiana interfere with estimation of cell viability. Antioxidants. 2019;8(6):191. doi: 10.3390/antiox8060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter S., Netzel M.E., Fletcher M.T., Tinggi U., Sultanbawa Y. Chemical and nutritional composition of Terminalia ferdinandiana (kakadu plum) kernels: A novel nutrition source. Foods. 2018;7(4):60. doi: 10.3390/foods7040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter S., Netzel M.E., Tinggi U., Osborne S.A., Fletcher M.T., Sultanbawa Y. Antioxidant rich extracts of Terminalia ferdinandiana inhibit the growth of foodborne bacteria. Foods. 2019;8(8):281. doi: 10.3390/foods8080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimba C.G., Gandhi D., Sivanesan S., Bhanarkar M.D., Naoghare P.K., Bakare A.A., Krishnamurthi K. Chemical characterization of simulated landfill soil leachates from Nigeria and India and their cytotoxicity and DNA damage inductions on three human cell lines. Chemosphere. 2016;164:469–479. doi: 10.1016/j.chemosphere.2016.08.093. [DOI] [PubMed] [Google Scholar]
- Berg S., Serra-Majem L., Coppens P., Rietjens I.M.C.M. Safety assessment of plant food supplements (PFS) Food and Function. 2011;2(12):760. doi: 10.1039/c1fo10067j. [DOI] [PubMed] [Google Scholar]
- FILIPPICH L.J., CAO G.R. Experimental acute yellow-wood (Terminalia oblongata) intoxication in sheep. Australian Veterinary Journal. 1993;70(6):214–218. doi: 10.1111/j.1751-0813.1993.tb03307.x. [DOI] [PubMed] [Google Scholar]
- Filippich L.J., Zhu J., Oelrichs P., Alsalami M.T., Doig A.J., Cao G.R., English P.B. Hepatotoxic and nephrotoxic principles in Terminalia oblongata. Research in Veterinary Science. 1991;50(2):170–177. doi: 10.1016/0034-5288(91)90101-s. [DOI] [PubMed] [Google Scholar]
- Gorman J.T., Wurm P.A.S., Vemuri S., Brady C., Sultanbawa Y. Kakadu Plum (Terminalia ferdinandiana) as a sustainable Indigenous agribusiness. Economic Botany. 2020;74(1):74–91. [Google Scholar]
- Ramirez C.N., Antczak C., Djaballah H. Cell viability assessment: Toward content-rich platforms. Expert opinion on drug discovery. 2010;5(3):223–233. doi: 10.1517/17460441003596685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIRDC, & ANFIL. (2012). Kakadu plum specification sheet. In). Australia: Rural Industries Research & Development Corporation.
- SEERAM N., ADAMS L., HENNING S., NIU Y., ZHANG Y., NAIR M., HEBER D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. The Journal of Nutritional Biochemistry. 2005;16(6):360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Singh B., Singh J.P., Kaur A., Singh N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chemistry. 2018;261:75–86. doi: 10.1016/j.foodchem.2018.04.039. [DOI] [PubMed] [Google Scholar]
- Sultan S., Osborne S.A., Addepalli R., Netzel G., Netzel M.E., Fletcher M.T. Indospicine cytotoxicity and transport in human cell lines. Food Chemistry. 2017 doi: 10.1016/j.foodchem.2017.08.029. [DOI] [PubMed] [Google Scholar]
- Tan A.C., Hou D.-X., Konczak I., Tanigawa S., Ramzan I., Sze D.-Y. Native Australian fruit polyphenols inhibit COX-2 and iNOS expression in LPS-activated murine macrophages. Food Research International. 2011;44(7):2362–2367. [Google Scholar]
- Tan A., Konczak I., Ramzan I., Sze D. Native Australian fruit polyphenols inhibit cell viability and induce apoptosis in human cancer cell lines. Nutrition and Cancer. 2011;63(3):444–455. doi: 10.1080/01635581.2011.535953. [DOI] [PubMed] [Google Scholar]
- Williams D.J., Edwards D., Pun S., Chaliha M., Burren B., Tinggi U., Sultanbawa Y. Organic acids in Kakadu plum (Terminalia ferdinandiana): The good (ellagic), the bad (oxalic) and the uncertain (ascorbic) Food Research International. 2016;89:237–244. doi: 10.1016/j.foodres.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Wisman K.N., Perkins A.A., Jeffers M.D., Hagerman A.E. Accurate assessment of the bioactivities of redox-active polyphenolics in cell culture. Journal of Agricultural and Food Chemistry. 2008;56(17):7831–7837. doi: 10.1021/jf8011954. [DOI] [PubMed] [Google Scholar]
- Yu H., Huang Q. Investigation of the cytotoxicity of food-grade nanoemulsions in Caco-2 cell monolayers and HepG2 cells. Food Chemistry. 2013;141(1):29–33. doi: 10.1016/j.foodchem.2013.03.009. [DOI] [PubMed] [Google Scholar]