Highlights
-
•
We investigated the effect of sodium butyrate on ER stress and inflammation in DIO mice model.
-
•
Sodium butyrate alters the microenvironment of adipose tissue, reducing ER stress and autophagy dysfunction.
-
•
Sodium butyrate modulates the polarization of macrophages in adipose tissue thereby increasing the anti-inflammatory M2 macrophage population.
-
•
Sodium Butyrate varies the immune cell population by increasing Tregs population.
Keywords: SCFA, ER stress, Adipose tissue, Immune cell population, Obesity
Abstract
Over the past decade, the gut microbiome has been linked to several diseases including gastrointestinal diseases, cancer, immune disorder and metabolic syndrome. Shifts in the gut bacterial population affect the overall metabolic health status leading towards obesity and Type II diabetes mellitus. Secondary metabolites secreted by the gut microbiome interact with various host-sensing signalling pathways and are responsible for functional modulation of immune resident cells in metabolic tissues (Blüher, 2019). Of these, short- chain fatty acids (SCFAs) i.e., acetate, propionate and butyrate have been significantly correlated with the disposition of diabetes and metabolic disorder. The altered gut microbial population depletes the intestinal barrier causing entry of LPS into circulation and towards metabolic tissues triggering pro-inflammatory responses. As butyrate has been known to maintain intestinal integrity, we aimed to assess the apparent effect of externally given sodium butyrate [NaB] on immuno-metabolic profiling of adipose tissue, and its association with metabolic and inflammatory status of adipose tissue. To assess this, we put groups of C57BL/6 mice i.e., Control fed with a regular chow diet and another group that was fed on a high fat diet (HFD, 60%) for 8 weeks. Following this, the HFD group were further subdivided into two groups one fed with HFD and the other with HFD + NaB (5%w/w) for another 8 weeks. Body composition, weight gain, body adiposity and biochemical parameters were assessed. NaB fed group showed an improved metabolic profile compared to HFD fed group. Administration of NaB also improved glucose tolerance capacity and insulin sensitivity as determined by IPGTT and ITT profiles. Earlier reports have shown gut leakage and increased LPS in circulation is the primary cause of setting up inflammation at the tissue level. Our studies exhibited that, NaB increased the expression of tight junction proteins of intestinal linings and thereby enhanced intestinal barrier integrity. The FITC dextran permeability assay further confirmed this enhanced intestinal barrier integrity. We assessed the quantitative and relative population of different types of resident immune cells from a stromal vascular fraction of adipose tissue. Flow cytometry studies revealed significantly increased M2 (CD206+ ) macrophages and Tregs (CD25+ ) relative to the M1 macrophage population and CD4+ T cells respectively in NaB treated mice, suggesting its potential role in alleviating the inflammatory profile. In a nutshell, taken together better glucose tolerance, better gut health, reduced inflammatory adipose tissue immune cells, suggest potential beneficial role of sodium butyrate in alleviating overall inflammation and metabolic dysfunction associated with obesity.
1. Introduction
The prevalence of obesity over the century has increased worldwide to a pandemic proportion. According to WHO, excessive fat accumulation leads to obesity which might impair health and is diagnosed at a BMI >25 kg/m2 (Bridgeman et al., 2020). Obesity leads to many metabolic complications such as Type II diabetes mellitus (T2DM), Non-Alcoholic Fatty Liver Disease (NAFLD) and cardiovascular diseases (CVD). An imbalance between the ratio of calories consumed and calories expended is the fundamental cause of obesity (Burhans, Hagman, Kuzma, Schmidt, & Kratz, 2018). The pathophysiology of metabolic syndrome comprises abdominal obesity, elevated fasting blood glucose, hypertriglyceridemia and insulin resistance which culminates into T2DM or CVD (Cani et al., 2009).
Evolutionarily, fat cells have energy storage mechanisms to mitigate the scarcity of food. In recent years researchers have demonstrated that fat cells are also major endocrine organs under their capability to secret more than 300 pro- and anti-inflammatory adipocytokines depending on tissue level microenvironment (Ellington, Berhow, & Singletary, 2006). In conditions where excess fat accumulation, adipocytes become hypertrophied, which further leads to the dysregulated secretion of various types of inflammatory adipokines such as TNFα (Tumor necrosis factor-alpha), leptin, visfatin, MCP-1 (macrophage chemoattractant protein), etc. This results in chronic low-grade inflammation parallel to obesity and associated metabolic disorders (Catrysse & van Loo, 2018). Tissue-resident macrophages act as surveillance to maintain the homeostatic condition. During obesity, these macrophages get recruited surrounding the hypertrophied adipocytes forming crown-like structures (CLNs). At this juncture, the macrophage population gets polarized towards the M1 macrophage population which in turn acts as a prominent source of pro-inflammatory cytokines that trigger the inflammatory gene expression in adipose tissue (Chelakkot, Ghim, & Ryu, 2018).
Dietary intake habits have been linked to overall gut health. Current nutritional practices differ from the primitive human generation and are tilted towards more processed, high sugar and high fat ready to eat a high calorie diet. These food habits are considered to be causing chronic low-grade inflammation due to the alteration in the population of specific microbial cells residing inside the gut. Changes in the microbial population lead to alteration in secondary metabolites and increased formation and release of lipopolysaccharides (LPS), which disrupt intestinal barrier integrity and form leaky gut (Chriett, Zerzaihi, Vidal, & Pirola, 2017). The microbiome forms various microbial metabolites such as short-chain fatty acids (SCFA). These metabolites act as a signalling molecule and have been extensively studied for their potential role on colonic health, glucose and lipid metabolism (den Besten et al., 2013). One such microbial metabolite i.e., butyrate has been known to maintain intestinal integrity and alleviate inflammation in high-fat diet-induced obesity (den Besten et al., 2013). Studies by Gao et al. gave mechanistic into how butyrate improved insulin sensitivity and diet induced obesity by promoting energy expenditure and activation of mitochondrial function (Gao et al., 2009). Although multiple benefits of sodium butyrate on overall metabolic health have been explained in detail, there is minimal information on adipose tissue level in terms of corrective pathophysiological alteration, resident immune cell population and its association with chronic inflammation. Considering the importance of adipose tissue as endocrine as well as fat turnover tissue, it warrants further studies concerning beneficial effects at this tissue level.
We hypothesize that sodium butyrate (NaB) administration can cause favorable immunometabolic alterations, leading to decreased inflammation and associated ER stress in HFD fed mice model of metabolic dyshomeostasis. Our data showed that NaB treatment increased the Tregs and M2 macrophage population in epididymal white adipose tissue (eWAT) as compared to HFD fed mice. Furthermore, NaB treated mice attenuated eWAT mass, adipose tissue hypertrophy, inflammation and also improvement in reducing ER stress and hepatic cholesterol metabolism by activating the reverse cholesterol transport mechanism.
2. Materials & methods
2.1. Animal study
Male C57BL/6J mice, aged between 6 and 8 weeks old were procured from the Laboratory Animal facility of CSIR-Central Drug Research Institute, Lucknow. Mice were kept at 25 ± 1 °C, 45–55% RH and a 12:12 light/dark cycle and acclimatized for 7 days. All the experiments were conducted according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEAs), India. They were approved by the Institutional Animal Ethics Committee (IAEC/2019/70). Mice were divided into 2 groups and were fed with a chow diet and a 60% High-Fat diet (HFD) (cat. no.: D12492, procured from Research Diets Inc., USA) for 8 weeks. Further, the HFD group was randomized after 8 weeks and divided into 2 groups as HFD and HFD + NaB (5%w/w) (Sigma Aldrich, cat no. 303410, India) for another 8 weeks. Body weight and food intake were measured weekly. Before sacrifice, mice were fasted and serum collection was done from the blood for lipid parameters.
2.2. Glucose tolerance test (GTT)
Briefly, mice were fasted for 8 h to carry out the GTT. After the fasting glucose measurement, glucose (2 g/kg) (Sigma Aldrich cat no. G8270, U.S.A) was administered intraperitoneally and blood glucose levels were measured at 15, 30, 60, 90 and 120 minutes (min) post-administration using a glucometer (Accu-Chek, Roche Diagnostic, Switzerland).
2.3. Insulin tolerance test (ITT)
To carry out ITT, mice fasted for 5 h and fasting insulin was measured at 0 min time point. Insulin (0.75 IU/kg) (Sigma, cat no. I5500, U.S.A) was injected and blood glucose was measured at 15, 30, 60, 90 and 120 min time intervals post insulin injection.
2.4. Body composition analysis
Echo Magnetic Resonance Imaging (echoMRI) was utilized to assess the effect of HFD and HFD + NaB on body composition. After the post treatment protocol, mice were placed into an echoMRI body analyzer (echoMRI 3- in-1, Echo Medical Systems LTD, Houston, TX). Total lean and fat mass were quantified.
2.5. Flourescein isothiocyanate (FITC) dextran permeability assay
FITC Dextran-4000 (Sigma Aldrich cat no. 46944, Sweden) solution was administered to mice at a 40 mg/100 g body weight concentration. Mice were kept on fasting and subsequently oral gavaged the solution. After completion of 4 h, blood was withdrawn and serum was collected. A standard curve was made using a serial dilution of FITC-Dextran solution in PBS and the concentration of FITC was measured in serum using a fluorescence spectrophotometer (excitation 490 nm; emission 530 nm; Infinite M200 PRO, Tecan, Crailsheim, Germany)
2.6. Protein estimation and immunoblotting
The sample was homogenized and lysed in cell lysis buffer (Mammalian cell lysis buffer, Gbiosciences cat no. 786-180, U.S.A) on ice and centrifuged at 12000 × g for 30 min at 4 °C. Protein estimation was carried out through the Bicinchoninic Acid method (BCA) (Gbiosciences cat no. 786846, U.S.A). An equal amount of protein samples were loaded on SDS-PAGE and transferred to the nitrocellulose membrane. 5% skim milk was used for blocking after which it was probed with primary antibodies. Subsequently, the membrane was washed and labeled with a horseradish peroxidase-conjugated secondary antibody. The image was captured using enhanced chemiluminescence western blotting substrate (Millipore cat no. WBKLS0100, U.S.A) and detected with ImageQuant LAS 4000 (GE Healthcare Life sciences). The list of antibodies is mentioned in Table S2.
2.7. Quantitative real-time PCR
Total cellular RNA has been isolated using TRIZOL reagent (Takara cat no. 9108, Japan) and first-strand cDNA synthesis was made through cDNA reverse transcription kit (Thermo Scientific, cat no. 00752219, Lithuania). The cDNA samples were then subjected to qPCR analysis by Light Cycler 480 (Roche Diagnostics, Switzerland) using SYBR Green master mix (Roche diagnostics, Switzerland). The list of primers is mentioned in Table S1.
2.8. Bone marrow-derived macrophages (BMDMs) isolation, culture and polarization
Bone marrow-derived monocytes were isolated from the femur of C57BL/6 mice. Mice were anesthesized and bone marrow was flushed from mouse femur and plated in RPMI (Roswell Park Memorial Institute, Sigma Aldrich cat no. R7388, U.S.A) supplemented with 10% heat-inactivated fetal bovine serum. A supernatant of L929 medium was added along with RPMI (1:4) to differentiate into macrophages. LPS (1 μg/ml) and IFN-γ (20 ng/ml) were given for 24 hrs to induce the polarization of macrophages. After this, NaB treatment was given for 24 hrs. BMDMs were tagged with antibodies CD11c, F4/80, and CD206 to conduct flow cytometry.
2.9. Flow cytometry
Stromal Vascular Fraction (SVF) was isolated for profiling of the immune cell population in eWAT (Kumar et al., 2019). Briefly, eWAT was minced in PBS/Penicillin:streptomycin solution (GIBCO cat no. 15140-122, U.S.A) after which it was digested with collagenase type-1 (1 mg/ml) with constant agitation at 200 rpm for 45 mins. The filtrate was passed through 70 μm (BD Falcon, U.S.A) and centrifuged at 400 rpm for 10 mins. The supernatant was discarded and the pellet was dissolved in 1% BSA (Sigma). Fluorescently labeled antibodies were incubated for 20 mins followed by washing with PBS. The list of antibodies is mentioned in Table 1.
Table 1.
List of flow cytometry antibodies.
| Cells | Markers | Manufacturer |
|---|---|---|
| M1 | CD11c, F4/80 | Anti-F4/80 (BD Horizon #565411) Anti-CD11c (BD Horizon #562454) |
| M2 | CD206, F4/80 | Anti-CD206 (BD Pharmingen #565250) |
| Helper T cells | CD4 | Anti-CD4 (BD Pharmingen #550954) |
| Cytotoxic T cells | CD8 | Anti-CD8a (BD Pharmingen #560182) |
| Regulatory T cells | CD25 | Anti-CD25 (BD Pharmingen #557192) |
2.10. Cell culture
3T3-L1 preadipocyte cell line were treated with 0.5 mM 3-isobutyl-1-methylxanthine (Sigma Aldrich cat no. I5879, U.S.A), 250 nM Dexamethasone (Sigma Aldrich cat no. D4902, U.S.A) and 5 µg/ml insulin (Sigma cat no. I6634) for 72 h. After which, the media was changed, and fresh medium and 5 µg/ml insulin were added for another 48 h. Finally, the media was replaced with DMEM + 10% Fetal bovine serum for 48 h to convert it into adipocytes. Palmitate treatment (600 µM) was given for 24 h to generate the ER stress model. NaB treatment (1 mM) was given for another 24hrs. Protein isolation was carried out after the treatment.
2.11. Statistical analysis
Data were presented as mean ± SEM and analyzed using Graphpad prism version 5.01. Comparisons were made by unpaired student t-test, One-way ANOVA followed by Bonferroni's correction as appropriate. *P < 0.05, **P < 0.01, * **P < 0.001 was considered statistically significant. For analysis of GTT and ITT, Two-way ANOVA followed by Bonferroni's correction was used.
3. Results
3.1. Sodium butyrate improved the serum lipid profile and reduced body obesity
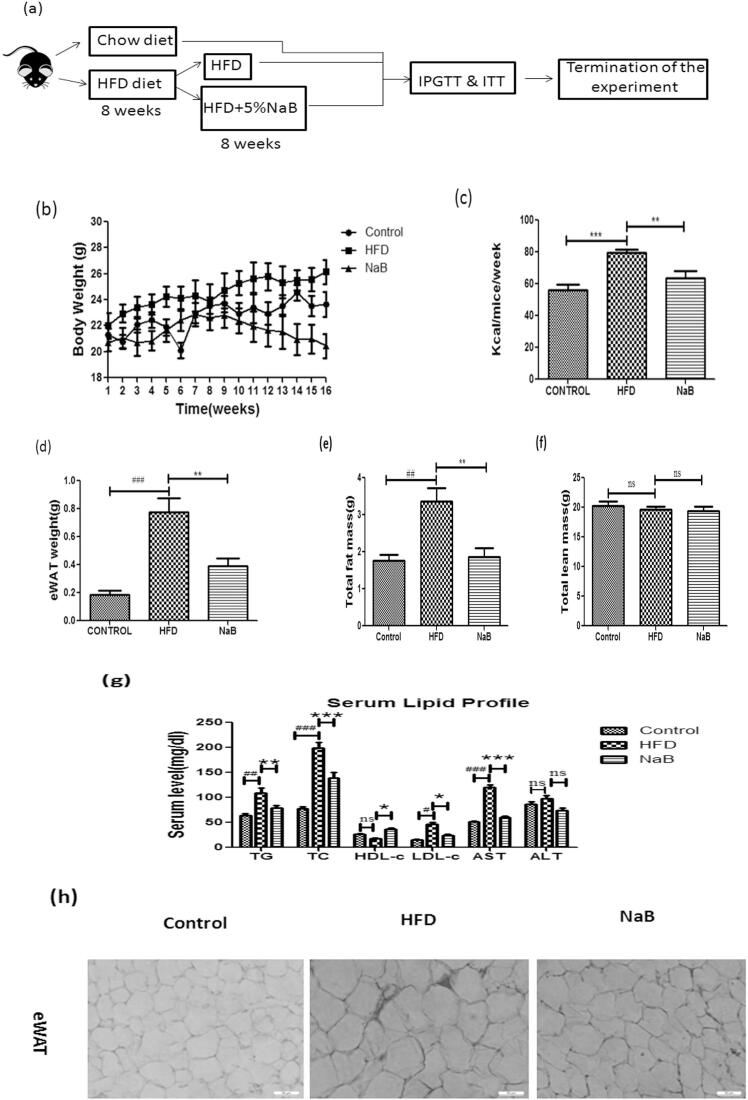
C57BL/6J mice were given an HFD for 8 weeks and then treated with sodium butyrate for another 8 weeks to produce the diet-induced obesity (DIO) model (Fig. 1a). Compared to the HFD group, the NaB treated group had lower body weight and food intake, and a substantial drop in eWAT weight (Fig. 1b–d). EchoMRI body composition study revealed a considerable reduction in fat content between the two groups, but lean mass remained unchanged (Fig. 1e & f). The HFD group had dyslipidemia, with significant increases in triglycerides (TG), total cholesterol (TC), and low density lipoprotein (LDL), whereas the NaB group had significant reductions in all of these lipid markers. The level of circulating aspartate aminotransferase (AST) was significantly reduced in sodium butyrate-treated mice, while the level of circulating serum alanine aminotransferase (ALT) showed a lowering tendency but was not significant (Fig. 1g). HFD mice showed hypertrophied adipose tissue as compared to NaB (Fig. 1h) treated mice which indicates that NaB restored the adipose tissue plasticity by improving the overall metabolic health. These data revealed that administration of NaB significantly rescued high fat diet feeding associated metabolic alterations.
Fig. 1.
Effect of Sodium butyrate administration on body composition and biochemical parameters in blood. (a) Schematic representation of the overall study. (b &c) Changes in weight gain and food intake comparison between Control, HFD and NaB groups. (d & e) Decrease in eWAT weight in NaB treated mice as compared to HFD group (f) Body composition analysis done by echoMRI exhibits a decrease in fat mass but no change in lean mass as compared to the HFD fed group. (g) Serum lipid profile showed a decrease in triglycerides, total cholesterol and LDL-c as compared to the HFD group. Data were presented as mean ± S.E.M. (N = 6). #P < 0.05, ##P < 0.01, ###P < 0.001 (comparison between control and HFD), *P < 0.05, **P < 0.01, * **P < 0.001 (comparison between HFD and NaB) indicated significance and ns indicated non-significant. Data were analysed by One-way ANOVA followed by Bonferroni's post hoc analysis.
3.2. Sodium butyrate improved insulin sensitivity, glucose tolerance as well as maintained intestinal barrier integrity in the DIO model
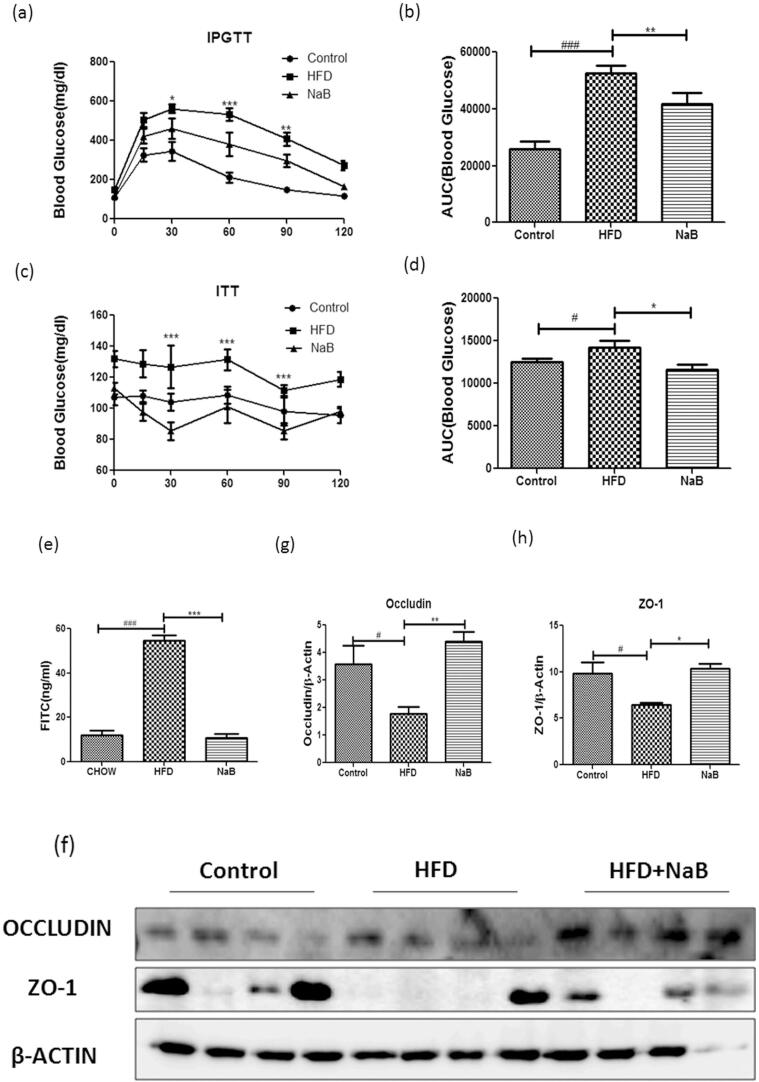
To investigate the potential role of NaB in the regulation of insulin sensitivity and glucose homeostatsis, we performed IPGTT and ITT. When compared to the HFD group, the glucose tolerance of the NaB group improved dramatically (Fig. 2a). Similarly, insulin tolerance test suggested an increase in insulin sensitivity (Fig. 2c). The AUCglucose and AUCinsulin curve showed a significant reduction in NaB treated group (Fig. 2b & d). Considering these benefits and the protective effect of NaB and its response towards insulin sensitivity, we further explored its potential effect on intestinal barrier integrity. The concentration of FITC dextran in the serum was considerably reduced in the NaB treated group compared to the HFD group in a FITC dextran permeability assay (Fig. 2e). We also looked at the effect of NaB on tight junction proteins, including occludin and ZO-1 (zona occludens) in the ileum, where the levels of both were considerably higher in the NaB group than in the HFD group. (Fig. 2f–h).
Fig. 2.
Sodium butyrate improved glucose homeostasis profile. (a) Blood glucose level during intraperitoneal glucose tolerance test (IPGTT) done by two way ANOVA along with Bonferroni post-hoc analysis (N = 6) (b) Area under the curve during ipgtt done by student’s t-test. (c) Blood insulin level during insulin tolerance test (N = 10) (d) Area under the curve during ITT. (e) FITC dextran permeability assay showed better intestinal integrity in NaB treated mice as compared to HFD mice. (f) Immuno-blots of epididymal WAT of HFD and NaB treated mice using antibodies specific for tight junction protein .i.e., occludin and ZO-1, β-Actin served as a loading control. (g & h) Quantification of protein level normalized by β-actin. #P < 0.05, ##P < 0.01, ###P < 0.001, *P < 0.05, **P < 0.01, * **P < 0.001 comparison between HFD and NaB) indicated significance and ns indicated non-significant.
3.3. Sodium butyrate ameliorated adipose tissue inflammation
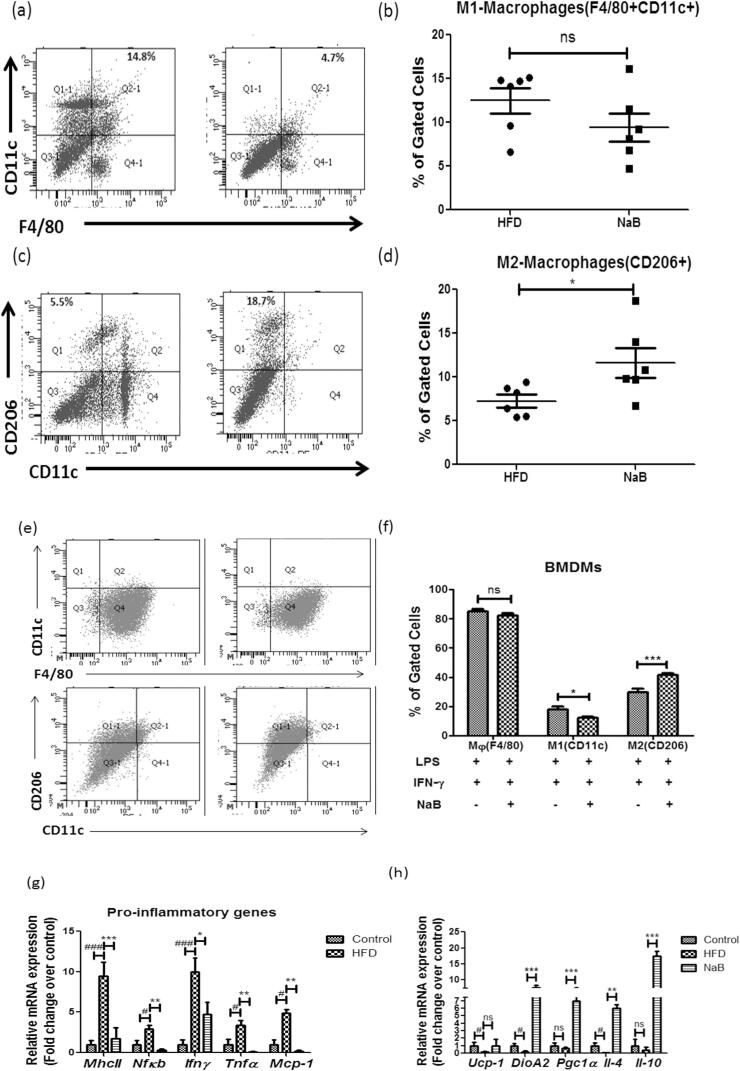
Macrophage infiltration is the major driver towards eWAT inflammation as CD11c+, pro-inflammatory Adipose Tissue Macrophages (ATMs) get accumulated surrounding the eWAT and causes activation of pro-inflammatory gene expression profile. We analyzed M1(CD11c+ ) and M2(CD206+ ) macrophages derived from stromal vasular fractions to further assess the influence of NaB on adipose tissue inflammation. When compared to the HFD alone fed group, the M1 macrophage population in the NaB treated group did not show a significant decrease (Fig. 3a & b), but the M2 macrophage population was found to be increased in NaB treated group (Fig. 3c & d). At tissue level profiling studies, the pro-inflammatory gene expression such as MhcII, Nfκb, Ifnγ, Tnfα, and Mcp-1 was significantly decreased compared to that of HFD alone fed group (Fig. 3g). In the NaB-treated group, M2 macrophage markers like IL-10, and anti-inflammatory genes including Ucp-1, DioA2, Pgc1, and Il-4, were elevated (Fig. 3h). To assess whether the supplementation of NaB plays a role in the polarization of macrophages, BMDMs were treated with LPS and IFN-γ which causes the conversion of macrophages towards the M1 profile. Flow cytometry data suggests that NaB supplemented group showed an increased population of M2 macrophages in comparison to M1 macrophages (Fig. 3e & f).
Fig. 3.
Anti-inflammatory gene expression profile triggered by Sodium Butyrate. (a & b) Immune cells of eWAT were profiled using flow cytometry for pro-inflammatory M1(CD11c + ) macrophages. (c & d) Anti-inflammatory M2(CD206 + ) macrophages. (e & f) Polarization of macrophages (g) Decreased mRNA expression of pro-inflammatory gene expression profile after the treatment of NaB. (h) Anti-inflammatory gene expression profile showing increase in the mRNA expression of IL-4 and IL-10 as compared to HFD. Student’s t-test used to analyze the data. #P < 0.05, ##P < 0.01, ###P < 0.001 (comparison between control and HFD) *P < 0.05, **P < 0.01, * **P < 0.001 (comparison between HFD and NaB) indicated significance and ns indicated non-significant.
3.4. Sodium butyrate suppressed CD4 + T cells and induced Tregs population
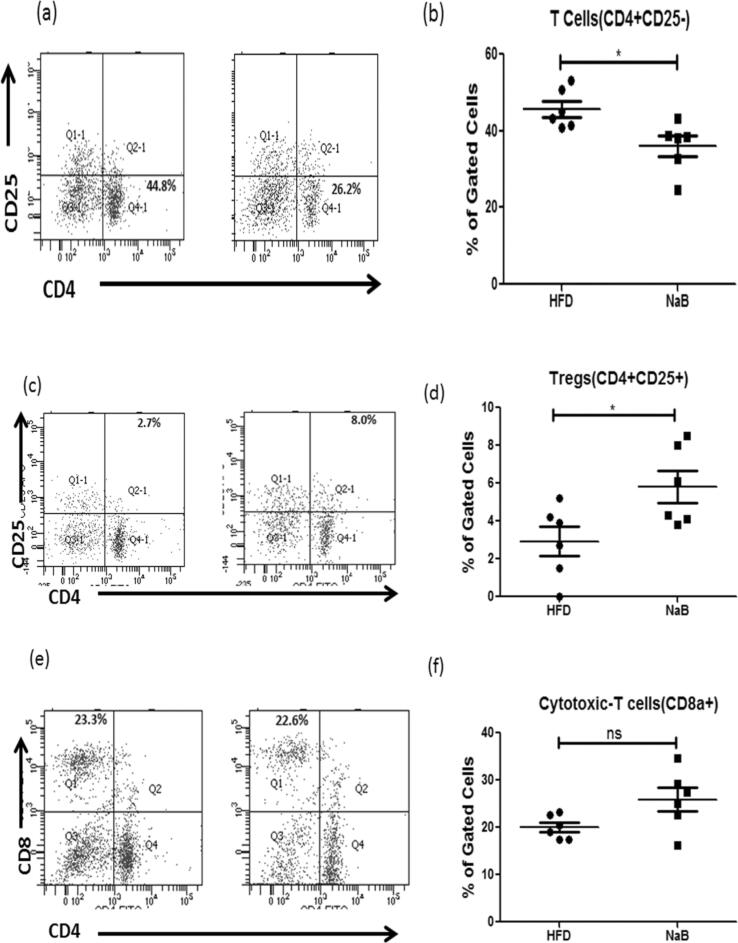
Given the considerable influence of NaB on macrophage infiltration as well as anti-inflammatory profile, we examined whether NaB regulates other immune cell populations such as CD4+ T cells and Tregs isolated from SVF of eWAT and assessed using flow cytometry. As shown in Fig. 4a–d, the number of Tregs in the NaB-treated group grew dramatically whereas the number of CD4+ T cells decreased. We have also profiled the CD8+ T cells but no significant change was observed in both the groups (Fig. 4e & f). Further, we evaluated the effect of NaB on reverse cholesterol transport machinery in the liver as obesity leads to the impairment of this mechanism. ABCA1 in adipose tissue was increased which mediates the secretion of cellular free cholesterol to the liver (Fig. S1). CETP gene expression was reduced in the liver, whereas LCAT gene expression was enhanced after NaB treatment (Fig. S1).
Fig. 4.
Sodium butyrate attenuated pro-inflammatory T cell population (a & b) Isolation of stromal vascular fraction (SVF) from eWAT of HFD and NaB showed increase in the immune cell population of Tregs (CD25 + ) in treated mice. (c & d) Pro-inflammatory CD4 + T cells population of NaB mice reduced as compared to HFD mice. (e & f) No significant change was observed in CD8 + T cells population. *P < 0.05, **P < 0.01, * **P < 0.001 indicated significance and ns indicated non-significant.
3.5. Sodium butyrate attentuated ER stress and defective autophagy
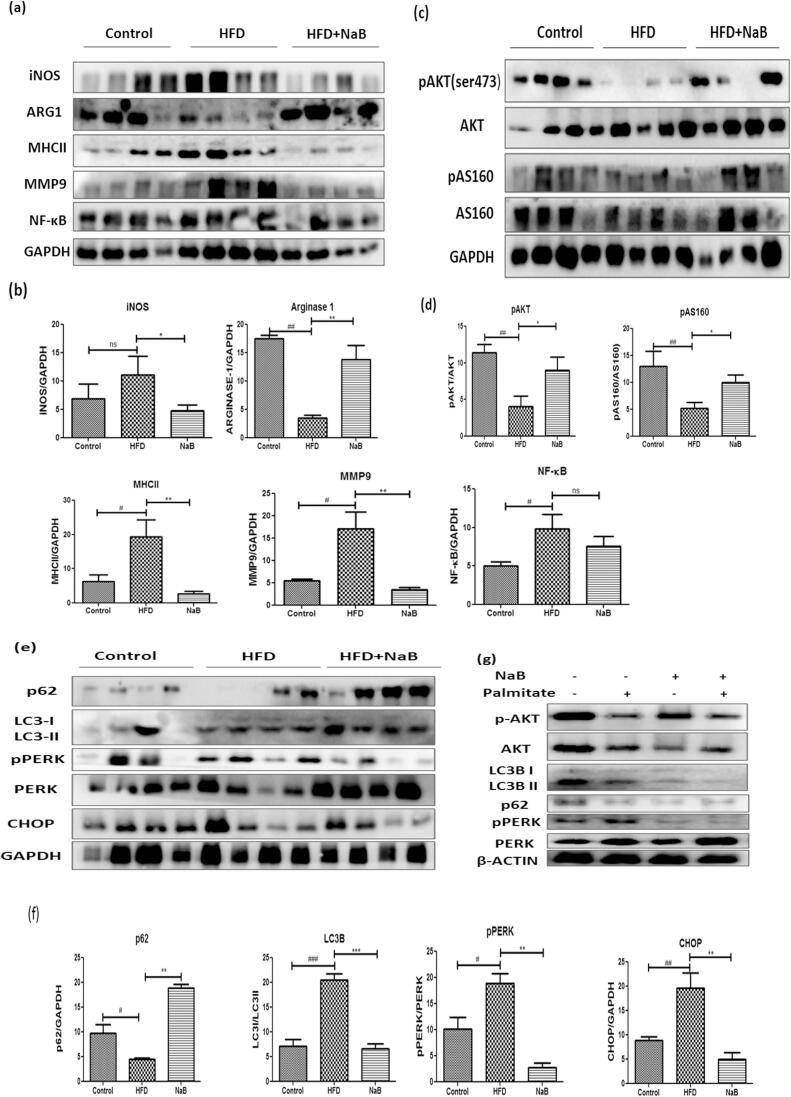
Nutrient overload will lead towards ER stress that converges with metabolic inflammation during obesity. To examine the effect of NaB on inflammation, we performed comparative profiling in HFD fed and NaB treated HFD fed group expression of pro-inflammatory markers such as iNOS, MHCII, NFκB in epididymal white adipose tissue (Fig. 5a & b). ER stress and inflammation are correlated, so we have also assessed the markers of ER stress and autophagy such as PERK, p-PERK, CHOP, LC3bII, p62 (Fig. 5e & f). As anticipated nutrient overload with HFD feeding lead to ER stress induced defective autophagy. Protein misfolding leads to the accumulation of p62/SQSTM1 as reported in earlier studies (Rocha, Apostolova, Diaz-Rua, Muntane, & Victor, 2020). Treatment with NaB reduced the expression of p62, as well as the ER stress markers pPERK and CHOP, and increased insulin sensitivity markers (Fig. 5c & d). The palmitate induced ER stress model was used to analyse NaB induced insulin sensitivity, as shown in Fig. 5g, to further determine the effect of NaB on ER stress.
Fig. 5.
Sodium butyrate ameliorated ER stress and defective autophagy. (a) Immuno-blots of epididymal WAT of HFD and NaB treated mice using antibodies specific for inflammation. i.e., iNos, Arg-1, MHCII, MMP9, and NF-κB, GAPDH as a loading control. (b) Quantification of protein level normalized by GAPDH. (c) Immunoblots of epididymal WAT of HFD and NaB treated mice using antibodies specific for insulin signaling. i.e., pAKT and pAS160. (d) Quantification of protein level normalized by GAPDH. (e) Immunoblots of epididymal WAT of HFD and NaB treated mice using antibodies specific for ER stress and autophagy .i.e., PERK, CHOP, Lc3b and p62. (f) Quantification of protein level normalized by GAPDH. The student’s t-test was used to analyze the data. #P < 0.05, ##P < 0.01, ###P < 0.001 (comparison between control and HFD)*P < 0.05, **P < 0.01, * **P < 0.001 (comparison between HFD and NaB) indicated significance and ns indicated non-significant.
4. Discussion
Short-chain fatty acids ameliorated the effects caused due to metabolic imbalance during the obesity condition. Butyrate is known for its overall beneficial metabolic health status. However, its role in modulating the adipose tissue micro-environment, ER stress and resident immune cell population has not yet been reported. In this study, we observed following oral sodium butyrate administration, a tissue resident immune cell population which belongs to inflammatory profile such as M1 and CD8 + cells decreased and anti- inflammatory population i.e., M2 and regulatory T cells (Tregs) increased. It was also confirmed that NaB improved overall metabolic health by lowering hyperglycemia, hyperinsulinemia, and hyperlipidemia. Our findings suggest that NaB may play a role in reducing ER stress by lowering the expression of ER stress indicators such as pPERK and CHOP and boosting insulin sensitivity as a result. Overall, we provide sufficient evidence that NaB decreases adipose ER stress and improves local anti-inflammatory immune cell regulation for the first time, resulting in improved glucose homeostasis.
In the DIO mice model, NaB supplementation reduces body weight gain and food intake over 8 weeks. It was postulated to explain the lean phenotype after HFD feeding by activating FFARs (Free Fatty Acid Receptors) on intestinal cells, linked to enhanced GLP-1 and PYY production after butyrate administration (Li et al., 2018). In these studies too, we observed that NaB prevented the fat mass gain without affecting the lean mass as reported in previous studies. In addition, NaB increases the expression of Ucp1, which promotes heat generation suggesting that it may increase thermogenesis and energy expenditure. Butyrate has been ascribed to improve the insulin sensitivity in skeletal muscle by increasing histone acetylation of IRS-1 promoter, suggesting a direct mechanism through HDAC inhibition (Chriett et al., 2017). In our studies, butyrate increased the phosphorylation of Akt marker of improved insulin signaling in white adipose tissue (WAT) which can further be corroborated with outcome insulin tolerance test in mice.
In this study, we demonstrated that NaB supplementation causes a reduction in circulating serum triglycerides, serum cholesterol, and LDL, which were consistent with earlier reports by Hong et al. (Du et al., 2020). Chronic high-fat diet feeding changes gut microbiota, causes leaky gut, and raises circulating LPS levels, which activate TLR4 signalling in numerous organs, including adipose tissue. With the growth of obesity, the extracellular matrix around adipocytes becomes denser, resulting in adipose fibrosis, which promotes diminished plasticity and adipose tissue malfunction. These processes, when combined, reduce local and systemic insulin sensitivity (Kumari, Heeren, & Scheja, 2018). A high-fat diet leads to disruption in intestinal permeability as it causes depletion of those gut bacteria which are involved in the production of butyrate as a secondary metabolite, as reported by Daniela et al. (Parada Venegas et al., 2019). Butyrate has been known to maintain this intestinal integrity. As a result, we observed the effect of NaB on total gut permeability. Using FITC-based gut permeability assays, we found that DIO associated intestinal permeability abnormalities were reduced in NaB-treated animals. To further confirm it, we performed protein expression alterations, if any, in tight junctions proteins such as occludin and zona occludens-1 (Cani et al., 2009, Guo et al., 2013). The NaB treated groups exhibited increased expression relative to that of the HFD group. Earlier reports suggest crosstalk between gut microbial metabolites and host immune response. These metabolites act as a signaling molecule, and their receptors are present in metabolic tissues (Samuel et al., 2008, Lu et al., 2019). Chronic macrophage infiltration in adipose tissue drives local and systemic inflammation in obesity and is a significant contributor to insulin resistance. The flexibility and remodelling potential of adipose tissue has been widely investigated in recent years in order to discover therapeutic targets to combat obesity and accompanying metabolic disorders. The formation of heat-producing beige adipocytes in white adipose tissue and the polarization of macrophages from an inflammatory to an anti-inflammatory phenotype have been identified as new potential intervention targets to investigate as we learn more about the potential of adipocytes and the contribution of macrophages and other immune cells to control immune-metabolism in disease states (Kumari et al., 2018). The relative population of tissue-resident inflammatory and anti-inflammatory cells aid in generating conditional micro-milieu in that tissue; therefore, we assessed stromal vascular fraction of adipose tissue using flow cytometry. Adipose tissue-resident macrophages present in lean phenotype are distributed between adipocytes and along with the vascular structure in adipose tissue. They play a crucial role in regulating adipocyte lipid metabolism. In lean phenotype, they express anti-inflammatory molecules such as IL-10, which is a marker of M2 phenotype (Lumeng, DelProposto, Westcott, & Saltiel, 2008). During obesity, phenotypic switch occurs between M2 to M1, a pro-inflammatory macrophage, and gets recruited to clusters surrounding the necrotic adipocytes. High fat diet-induced mice showed an increase in macrophage either by immune infiltration or by phenotypic polarization of macrophages (Catrysse & van Loo, 2018). In obese phenotype, tissue resident-macrophages increase in M1 phenotype, and it triggers the inflammatory gene expression profile. TNFα, Mcp1, iNOS are characteristic of theM1 phenotype, whereas IL-10, IL-4, and Arg1 are of the M2 phenotype (Lumeng, Bodzin, & Saltiel, 2007). In our study, we observed decrease in M1 specific cytokine profile and an increase in M2 specific cytokine profile in NaB treated mice. Lumeng et al have shown that lean mice contained less proportion of CD11c + cells as compared to obese mice, with a significant increase in the percentage of CD11c + cells (Lumeng et al., 2007, Weisberg et al., 2003). As lean mice have very less epidydimal adipose tissue, doing tissue resident immuno-metabolic cell proliferation in chow fed diet becomes major bottleneck. Our study revealed relatively the similar proportion of CD11c + cells in obese mice as shown by the earlier reports. For classical activation of M1 macrophages, LPS and IFN-γ were given to BMDMs derived macrophages (Orecchioni, Ghosheh, Pramod, & Ley, 2019). In-vitro polarization of BMDM revealed that NaB treated cells showed polarization of macrophages towards the M2 population.
Along with macrophages, T cells are also critical for the maintenance of immune homeostasis. T cell infiltration in obese adipose tissue has been linked to the regulation of macrophage quantity and polarization state, which helps to manage adipose tissue inflammation. CD4 + T cells, CD8 + T cells, and Foxp3 + regulatory T cells are three different types of T cells that play different roles in the fat pads of obese people. Obesity increases CD8 + T cells (also known as cytotoxic T cells), which boost the recruitment and activation of adipose macrophages. It's worth noting that changes in CD8 + T cells occur before changes in adipose tissue macrophages during obesity, implying that CD8 + T cells are involved in the onset of inflammation (Nishimura et al., 2009). Tregs are present in abundance in the lean phenotype as compared to the obese phenotype. Increasing or decreasing the WAT Tregs number improves or worsens the inflammation (Feuerer et al., 2009). Tregs increase the expression of IL-10 cytokine, which is known for anti-inflammatory profile and causes activation of M2 proliferation. IL-10 is known to phosphorylate Akt, thereby increasing insulin-stimulated glucose uptake. We observed that NaB treated mice showed an increase in anti-inflammatory gene expression i.e., IL-10 and IL-4, and a decrease in pro-inflammatory cytokines. It also exhibited an increase in M2 phenotype in the macrophage population and thereby increased expression of IL-10, which in turn reduced inflammation to maintain insulin sensitivity (Orliaguet, Dalmas, Drareni, Venteclef, & Alzaid, 2020). In our study, we observed an increased Tregs immune cell population in adipose tissue of NaB treated mice. Phenotypic switching between the inflammatory and anti-inflammatory macrophages has been linked with ER stress (Huang, Xing, & Liu, 2019). Nutrient overload leads to activation of IRE1α that represses the M2-macrophage population and enhances the M1-macrophage population, thus causing M1-M2 imbalance leading to exacerbation of insulin resistance (Shan et al., 2017). ER activates the canonical UPR (unfolded protein response) pathways in order to remove the misfolded protein with the help of autophagy machinery to maintain homeostasis (Gregor & Hotamisligil, 2007). During obesity, insulin signaling has been dampened due to nutrient overload, which in turn leads to defective autophagy as it is regulated by the integrated actions of insulin and mTOR (Ellington et al., 2006, Yang et al., 2010). Our study revealed that treatment of NaB (i) increases the M2 cell population (ii) decreases ER stress as indicated by the protein expression of ER stress markers such as PERK and CHOP (iii) increases insulin sensitivity. Pro-inflammatory markers such as iNOS, NF-κB, and MHCII were found to be decreased in the NaB treated group compared to the HFD fed mice, indicating that NaB treatment protects against DIO-induced inflammation. The ER's protective mechanism is known to control autophagy. ER stress is known to be caused by pro-inflammatory cytokines like TNFα, which leads to the activation of the autophagy mechanism via the unfolded protein response. This helps to clear out misfolded proteins, which reduces inflammation (Huang et al., 2019). Our studies exhibit NaB alleviates ER stress via suppressing the faulty autophagy mechanisms.
With reference to liver, we have also studied the reverse cholesterol transport machinery as the concentration of HDL-c significantly increased in NaB treated mice. Reduced serum cholesterol could be due to alterations in cholesterol biosynthesis, cholesterol import/export from cells, or dietary cholesterol uptake. Cholesterol biosynthesis was reduced as HMGCoA, a rate-limiting enzyme, was decreased in the NaB treated group. Conflicting evidence reported regarding cholesterol transport by butyrate as Kaptein et al. showed increased export of cholesterol ester in HepG2 cells. In contrast, Marcil et al. showed reduced cholesterol export in CaCO2 cell line (Kaptein et al., 1991, Marcil et al., 2003). Cholesterol transport requires lipoprotein particles as it is insoluble in an aqueous medium. ABCA1 was found to be increased in our study, suggesting transport of cholesterol ester from adipose tissue. LCAT, which promotes the transfer of cholesterol from cell membrane to HDL was found to be increased thus indicating that butyrate might contribute to reverse cholesterol transport machinery activation. Although multiple reports suggest butyrate's beneficial and protective role of butyrate, the physiological concentration measured in the circulation was significantly less. Sellin et al., reported that butyrate is readily metabolized and cleared up from the circulation (Sellin, 1999). The colonocytes and liver metabolize the majority of it. However, studies showing uptake of butyrate in particular tissues can vary (Kim et al., 2013). As a result, although systemic availability is low chronic supplementation of butyrate could exert beneficial effects on WAT. In summary, our data suggest that supplementation of NaB may alter the micro-milieu of adipose tissue by generating a favorable immuno-metabolic profile, reducing inflammation, thereby alleviating ER stress and correcting the defective autophagy. Our study revealed that NaB treated mice showed increased expression of ABCA1 and ABCA8 in adipose tissue as well as LCAT1 in the liver, thus exhibiting that NaB contributes to activation of reverse cholesterol transport machinery. However, our present studies are limited in addressing the mechanistic insights on how butyrate may cause epigenetic modulations or other signaling pathways that impact the gut-liver-adipose axis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We are grateful for the excellent instrumental and technical support from Achchhe Lal Vishwakarma institutional SAIF division.
Funding
This research work is supported by CSIR-CDRI project. KV, NK and DK are supported by SRF-CSIR.SG is supported by SRF-DBT. SV is supported by SRF-ICMR. PR is supported by SRF-UGC. This manuscript bears CSIR-CDRI communication number-10357.
Author’s contribution
VK designed and performed in vivo experiments and wrote the manuscript. PR and SV performed in vivo experiments and histology. SG and NK performed the in vitro experiments. DK gave input in analysing flow cytometry data. ANG conceptualized the idea and gave critical input in manuscript writing and experimental design. All the authors participated in drafting and reviewing the manuscript.
Ethics approval
Animal experiments were conducted according to the guidelines of the Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEAs), India and were approved by the Institutional Animal Ethics Committee (IAEC/2019/70).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
This study did not generate/analyze datasets.
Code availability
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2022.100079.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Blüher M. Obesity: Global epidemiology and pathogenesis. Nature Reviews Endrocrinology. 2019;15(5):288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- Bridgeman S.C., Northrop W., Melton P.E., Ellison G.C., Newsholme P., Mamotte C.D.S. Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacological Research. 2020;160(105174):27. doi: 10.1016/j.phrs.2020.105174. [DOI] [PubMed] [Google Scholar]
- Burhans M.S., Hagman D.K., Kuzma J.N., Schmidt K.A., Kratz M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Comprehensive Physiology. 2018;9(1):1–58. doi: 10.1002/cphy.c170040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P.D., Possemiers S., Van de Wiele T., Guiot Y., Everard A., Rottier O.…Delzenne N.M. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrysse L., van Loo G. Adipose tissue macrophages and their polarization in health and obesity. Cellular Immunology. 2018;330:114–119. doi: 10.1016/j.cellimm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Chelakkot C., Ghim J., Ryu S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Experimental & Molecular Medicine. 2018;50(8):1–9. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chriett S., Zerzaihi O., Vidal H., Pirola L. The histone deacetylase inhibitor sodium butyrate improves insulin signalling in palmitate-induced insulin resistance in L6 rat muscle cells through epigenetically-mediated up-regulation of Irs1. Molecular and Cellular Endocrinology. 2017;439:224–232. doi: 10.1016/j.mce.2016.09.006. [DOI] [PubMed] [Google Scholar]
- den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Li X., Su C., Xi M., Zhang X., Jiang Z.…Hong B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. British Journal of Pharmacology. 2020;177(8):1754–1772. doi: 10.1111/bph.14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington A.A., Berhow M.A., Singletary K.W. Inhibition of Akt signaling and enhanced ERK1/2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis. 2006;27(2):298–306. doi: 10.1093/carcin/bgi214. [DOI] [PubMed] [Google Scholar]
- Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A.…Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature Medicine. 2009;15(8):930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M.…Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M.F., Hotamisligil G.S. Thematic review series: Adipocyte Biology. Adipocyte stress: The endoplasmic reticulum and metabolic disease. Journal of Lipid Research. 2007;48(9):1905–1914. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- Guo S., Al-Sadi R., Said H.M., Ma T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. American Journal of Pathology. 2013;182(2):375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Xing Y., Liu Y. Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease. Journal of Biological Chemistry. 2019;294(49):18726–18741. doi: 10.1074/jbc.REV119.007036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptein A., Roodenburg L., Princen H.M. Butyrate stimulates the secretion of apolipoprotein (apo) A-I and apo B100 by the human hepatoma cell line Hep G2. Induction of apo A-I mRNA with no change of apo B100 mRNA. The Biochemical Journal. 1991;278(Pt 2):557–564. doi: 10.1042/bj2780557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Hooker J.M., Otto N., Win K., Muench L., Shea C.…Fowler J.S. Whole-body pharmacokinetics of HDAC inhibitor drugs, butyric acid, valproic acid and 4-phenylbutyric acid measured with carbon-11 labeled analogs by PET. Nuclear Medicine and Biology. 2013;40(7):912–918. doi: 10.1016/j.nucmedbio.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Pandya S.K., Varshney S., Shankar K., Rajan S., Srivastava A.…Gaikwad A.N. Temporal immmunometabolic profiling of adipose tissue in HFD-induced obesity: Manifestations of mast cells in fibrosis and senescence. International Journal of Obesity. 2019;43(6):1281–1294. doi: 10.1038/s41366-018-0228-5. [DOI] [PubMed] [Google Scholar]
- Kumari M., Heeren J., Scheja L. Regulation of immunometabolism in adipose tissue. Seminars in Immunopathology. 2018;40(2):189–202. doi: 10.1007/s00281-017-0668-3. [DOI] [PubMed] [Google Scholar]
- Li Z., Yi C.X., Katiraei S., Kooijman S., Zhou E., Chung C.K.…Wang Y. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67(7):1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- Lu J., Zhao J., Meng H., Zhang X. Adipose Tissue-Resident Immune Cells in Obesity and Type 2 Diabetes. Frontiers in Immunology. 2019;10(1173) doi: 10.3389/fimmu.2019.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng C.N., DelProposto J.B., Westcott D.J., Saltiel A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcil V., Delvin E., Garofalo C., Levy E. Butyrate impairs lipid transport by inhibiting microsomal triglyceride transfer protein in Caco-2 cells. Journal of Nutrition. 2003;133(7):2180–2183. doi: 10.1093/jn/133.7.2180. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M.…Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature Medicine. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Orecchioni M., Ghosheh Y., Pramod A.B., Ley K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. [Comparative Study] Frontiers in Immunology. 2019;10(1084) doi: 10.3389/fimmu.2020.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orliaguet L., Dalmas E., Drareni K., Venteclef N., Alzaid F. Mechanisms of Macrophage Polarization in Insulin Signaling and Sensitivity. Frontiers in Endocrinology. 2020;11(62) doi: 10.3389/fendo.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G.…Hermoso M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Frontiers in Immunology. 2019;10(277) doi: 10.3389/fimmu.2019.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M., Apostolova N., Diaz-Rua R., Muntane J., Victor V.M. Mitochondria and T2D: Role of Autophagy, ER Stress, and Inflammasome. Trends in Endocrinology and Metabolism. 2020;31(10):725–741. doi: 10.1016/j.tem.2020.03.004. [DOI] [PubMed] [Google Scholar]
- Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K.…Gordon J.I. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(43):16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin J.H. SCFAs: The Enigma of Weak Electrolyte Transport in the Colon. News in Physiological Sciences. 1999;14:58–64. doi: 10.1152/physiologyonline.1999.14.2.58. [DOI] [PubMed] [Google Scholar]
- Shan B., Wang X., Wu Y., Xu C., Xia Z., Dai J.…Liu Y. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nature Immunology. 2017;18(5):519–529. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Li P., Fu S., Calay E.S., Hotamisligil G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metabolism. 2010;11(6):467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets.