Abstract
The effects of prolonged itraconazole exposure on the susceptibility of Candida albicans isolates to itraconazole and fluconazole have not been well characterized. A recent placebo-controlled study of long-term itraconazole antifungal prophylaxis in persons with advanced human immunodeficiency virus infection afforded the opportunity to address this question. Mucosal Candida sp. isolates were obtained from subjects who developed oropharyngeal or esophageal candidiasis, and in vitro susceptibilities of the last isolate obtained at removal from the study as a prophylaxis failure were compared in itraconazole and placebo recipients. More subjects in the placebo group (74 of 146 [51%]) than in the itraconazole group (51 of 149 [34%]) developed mucosal candidiasis (P = 0.004). A total of 112 isolates were recovered from 56 of the 74 (76%) subjects with mucosal candidiasis assigned to the placebo group, compared to 97 isolates from 45 of the 51 (88%) subjects in the itraconazole group. C. albicans accounted for 98% of isolates in the placebo group and 89% of isolates in the itraconazole group. The itraconazole MIC at which 50% of the isolates tested were inhibited (MIC50) for last-episode isolates from the itraconazole group was 0.125 μg/ml compared to 0.015 μg/ml for the placebo group subjects, P = 0.0001. The MIC50 of fluconazole for the last isolates from the itraconazole group was 1.5 μg/ml compared to 0.5 μg/ml for the placebo subjects (P = 0.005). A lower proportion of isolates recovered from subjects on itraconazole therapy were classified as susceptible to itraconazole (63%) compared to isolates from the placebo group (96%) (P = 0.001). Similarly, a lower proportion of C. albicans isolates from subjects on itraconazole therapy were susceptible to fluconazole (78%) compared to isolates from the placebo group (96%) (P = 0.01). Also, the proportion of isolates that were not fully susceptible to itraconazole or fluconazole was greater in patients assigned to the itraconazole group than the placebo group (itraconazole susceptibility, 37 and 4%, respectively (P = 0.001); fluconazole susceptibility, 23 and 4%, respectively (P = 0.01). In conclusion, long-term itraconazole prophylaxis in patients with AIDS is associated with reduction in susceptibility to itraconazole and cross-resistance to fluconazole.
Fluconazole has been widely used in the treatment of mucosal candidiasis in patients with human immunodeficiency virus (HIV) infection. For HIV-infected persons with advanced immunosuppression, the risk of developing mucosal candidiasis with Candida albicans with reduced susceptibility to fluconazole has been associated with greater duration and cumulative prior exposure to fluconazole (1, 4, 7). Fluconazole-resistant isolates of C. albicans recovered from fluconazole-treated HIV-infected patients demonstrating reduced susceptibility to itraconazole have been described, implicating the development of cross-resistance to itraconazole in fluconazole-treated patients (3). As itraconazole has been used less often for the treatment and prevention of mucosal Candida sp. infections, the effects of prolonged itraconazole exposure on the susceptibility of C. albicans isolates to itraconazole and fluconazole have not been well characterized.
A recent study has afforded the opportunity to evaluate the effect of long-term itraconazole exposure on the susceptibility of Candida sp. strains obtained from patients with AIDS who participated in a trial of antifungal prophylaxis (2). This multicenter, randomized, double-blind study enrolled subjects living in areas of endemicity for histoplasmosis with CD4 cell counts of ≤150/mm3. Subjects were randomized to either a group receiving itraconazole capsules (200 mg daily) or a group receiving placebo, and the median duration of follow-up was 16 months. Itraconazole greatly reduced the incidence of histoplasmosis and cryptococcosis but did not improve patient survival. While fewer subjects assigned to itraconazole prophylaxis experienced mucosal candidiasis than placebo recipients, itraconazole prophylaxis did not reduce the incidence of recurrent or refractory mucosal Candida infection, another study end point. In order to investigate the possibility of resistance to itraconazole as a mechanism for a lack of efficacy in preventing recurrent oropharyngeal candidiasis and to examine the potential for development of cross-resistance to fluconazole in subjects treated with long-term itraconazole, we measured the in vitro susceptibilities to itraconazole and fluconazole of mucosal Candida sp. isolates from patients enrolled in this study.
MATERIALS AND METHODS
Description of patients and study design.
Subjects were eligible for participation if they met the following criteria: CD4 lymphocyte count of <150/mm3 within 1 year prior to study entry and residence in a city with a high prevalence of histoplasmosis. Subjects were randomized in a 1:1 ratio to take either two 100-mg itraconazole capsules once daily or two identical placebo capsules. Clinical and laboratory assessments were undertaken at baseline, 6 weeks, and 3 months and then every 3 months for the remainder of the study. The study end points for efficacy included development of histoplasmosis, cryptococcosis, aspergillosis, or other proven or probable systemic fungal infection; oropharyngeal or vaginal candidiasis requiring more than 2 weeks of systemic antifungal treatment or such treatment on more than one occasion; or esophageal candidiasis requiring more than 3 weeks of systemic treatment.
Isolate processing and laboratory evaluation.
Cultures were obtained from patients who had clinical evidence of mucosal or systemic fungal infections and were processed at local hospital laboratories. Isolates were shipped to the Histoplasmosis Reference Laboratory, Indianapolis, Ind., for susceptibility testing. Isolates were subcultured once, and the freshly grown cultures were suspended in lactose glycerol freezing medium and stored frozen in liquid nitrogen. After completion of the clinical study, isolates were thawed and identification to the species level was performed for all Candida sp. isolates using CHROMagar (Hardy Diagnostics, Santa Maria, Calif.). For isolates which we were unable to identify to the species using CHROMagar, additional testing was performed with API 20 C AUX (bioMérieux, Marcy-l'Etoile, France) strips.
C. albicans isolates were evaluated in vitro for susceptibility to itraconazole and fluconazole using a broth microdilution method (9). The MIC was defined, as the concentration of drug required to inhibit 80% of the organism's growth as detailed in the NCCLS methodology. When the endpoint dilution causing 80% inhibition showed severe trailing (four or more wells), the MIC was defined as the drug concentration in the first well showing a prominent decrease in turbidity, according to the NCCLS procedure.
Statistical analysis.
Analysis of characteristics of the patient sources used in susceptibility testing for the two randomized regimens was conducted by utilizing chi-square tests for categorical measurements and the Kruskal-Wallis test for ordered measurements. The distribution of the susceptibility results, indicated by MICs of itraconazole and fluconazole, were compared for C. albicans isolates available from the last mucosal infection occurring in subjects assigned to the itraconazole prophylaxis group and isolates from last mucosal infection for subjects assigned to the placebo group, using the Kruskal-Wallis nonparametric test. A chi-square test (8) was applied in an analysis following the categorization of these isolates as susceptible, susceptible-dose dependent, and resistant to itraconazole and/or fluconazole (6), according to treatment group.
RESULTS
Description of patients.
Two hundred ninety-eight patients were enrolled, of whom three were ineligible and 295 were evaluable. Among evaluable cases, 146 were randomized to the placebo group and 149 were randomized to the itraconazole group. The baseline characteristics of the two treatment groups, described in detail elsewhere, were comparable (2). Of those whose isolates were analyzed for in vitro susceptibility to itraconazole and fluconazole, there was no difference with respect to CD4 cell counts or proportion of patients who had received fluconazole during the study period. Significantly more subjects in the placebo group (74 of 146 [51%]) had documented mucosal candidiasis compared to the itraconazole prophylaxis group (51 of 149 [34%]). A total of 112 isolates were recovered from 56 of the 74 (76%) subjects with mucosal candidiasis assigned to the placebo group, with 97 isolates recovered from 45 of the 51 (88%) subjects with mucosal candidiasis in the itraconazole prophylaxis group. The duration on study medication (14.35 months on itraconazole; 9.02 months on placebo [P = 0.10]) and duration of follow-up were longer for patients assigned to the itraconazole prophylaxis arm than those assigned to the placebo arm.
Identification of last mucosal isolate to the species level.
Identification to the species level of Candida isolates from the last mucosal infection revealed that the majority of recovered isolates were C. albicans. In the placebo group, C. albicans was identified in 98% of final infections while C. glabrata was found in 2% of final infections. C. albicans accounted for 89% of final isolates in the itraconazole prophylaxis group, with C. glabrata and C. tropicalis accounting for 9 and 2% of isolates respectively (P = 0.134).
Susceptibility to itraconazole.
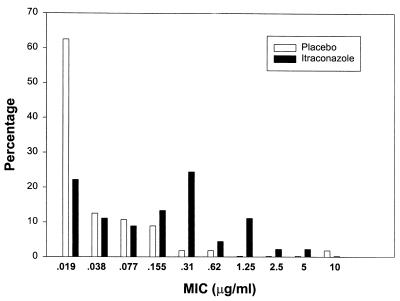
C. albicans isolates obtained from the last mucosal infection occurring while subjects were participating in this study were tested for susceptibility to itraconazole. A graphic representation of the itraconazole MIC distribution is shown in Fig. 1. The MICs for the last mucosal isolate were higher for patients randomized to the itraconazole group than for those randomized to the placebo group. The itraconazole MIC at which 50% of the isolates tested were inhibited (MIC50) was 0.015 μg/ml in the placebo group, compared to 0.125 μg/ml in the itraconazole group (P = 0.001). The MIC90 of itraconazole in the placebo group was 0.125 μg/ml, compared to 0.5 μg/ml in the itraconazole group. Thus, itraconazole prophylaxis was associated with a reduction in the susceptibility of candida isolates to itraconazole.
FIG. 1.
Distribution of itraconazole MICs for isolates from the last episode of mucosal candidiasis in itraconazole and placebo recipients.
Using the antifungal breakpoint categories for itraconazole of susceptible (MIC, ≤0.125 μg/ml), susceptible-dose dependent (MIC, 0.25 to 0.5 μg/ml), and resistant (MIC, ≥1 μg/ml) (5), a lower proportion of isolates recovered from subjects on itraconazole prophylaxis were classified as susceptible (25 of 40 [63%]) than those from the placebo group (53 of 55 [96%]) (P = 0.001).
Susceptibility to fluconazole.
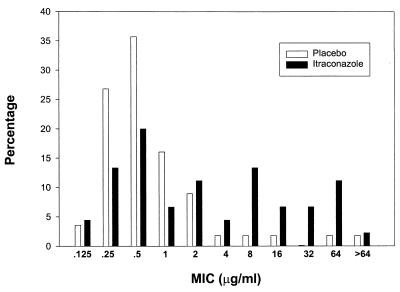
The fluconazole MIC data for the last isolates are shown in Fig. 2. The MICs for the last mucosal isolate were higher in patients randomized to the fluconazole group than in those randomized to the placebo group. The MIC50 of fluconazole was 0.5 μg/ml in the placebo group, compared to 1.5 μg/ml in the fluconazole group (P = 0.001). The MIC90 of fluconazole in the placebo group was 2.0 μg/ml, compared to 64 μg/ml in the fluconazole group. Using the antifungal breakpoint categories for fluconazole of susceptible (MIC, ≤8 μg/ml), susceptible-dose dependent (MIC, 16 to 32 μg/ml), and resistant (MIC, ≥64 μg/ml) (5), a significantly lower proportion of C. albicans isolates recovered from subjects on itraconazole prophylaxis (31 of 40 [78%]) were susceptible to fluconazole compared to isolates from the placebo group (53 of 55 [96%]) (P = 0.01). Thus, itraconazole prophylaxis was associated with a reduction in susceptibility to fluconazole.
FIG. 2.
Distribution of fluconazole MICs for isolates from the last episode of mucosal candidiasis in itraconazole and placebo recipients.
DISCUSSION
Itraconazole (200 mg daily) used as prophylaxis for systemic fungal infection in persons with AIDS who had CD4 counts below 150/mm3 (2) was associated with a reduction in the number of subjects who experienced mucosal candidiasis. Approximately one-third of subjects who received itraconazole, however, developed at least one episode of mucosal candidiasis. Prophylaxis did not reduce the incidence of recurrent candidiasis requiring treatment with systemically absorbed antifungal agents (2).
In this report, we assessed the effect of long-term antifungal treatment on the susceptibility of oral C. albicans isolates to both itraconazole and fluconazole in patients randomized to either an itraconazole or a placebo group for the prevention of systemic mycoses (2). This study is unique in that it examined the effect of prophylaxis on susceptibility of C. albicans isolates in the largest cohort of HIV-infected subjects treated with long-term itraconazole and allowed for a comparison to a placebo group. Reduced susceptibility to itraconazole was observed in isolates from the itraconazole group compared to the placebo group. Also, the proportion of isolates from subjects on itraconazole prophylaxis that were fully susceptible to itraconazole was reduced.
Of note, the study was not designed or powered to address this question of whether prophylaxis leads to the development of resistance. Specifically, baseline cultures at the time of enrollment before initiation of itraconazole or placebo were not obtained. Rather, the first culture for each subject was performed at the initial episode of thrush that occurred during the study. Also, systemic or topical antifungal treatment, in addition to the study treatment, administered before and during the study, may have affected susceptibility. These caveats must be recognized in interpretation of the findings of the study.
Cross-resistance to fluconazole also was observed in individuals with prolonged itraconazole exposure. The MIC distribution of isolates of C. albicans recovered from infected mucosal sites from subjects assigned to itraconazole prophylaxis demonstrated significantly reduced susceptibility to fluconazole compared to isolates from subjects receiving placebo. Furthermore, significantly fewer isolates from subjects on the itraconazole prophylaxis arm were fully susceptible to fluconazole. Similar nonsignificant trends were observed for an increase in the MIC of fluconazole in the paired-isolate analysis.
In conclusion, we have shown a trend towards reduction in susceptibility to itraconazole and occurrence of cross-resistance to fluconazole in patients with AIDS receiving long-term itraconazole prophylaxis. As both triazoles suppress fungal multiplication by inhibition of C-14 α-demethylase, the occurrence of cross-resistance is not surprising. This potential for induction of resistance among C. albicans strains and the occurrence of recurrent and refractory mucosal candidiasis among prophylaxis recipients must be recognized in establishment of strategies to prevent systemic mycoses in patients with AIDS through long-term antifungal therapy.
ACKNOWLEDGMENTS
We are grateful to Richard Hafner, National Institute of Allergy and Infectious Disease, Division of AIDS, Rockville, Md., for collaboration in the development of the study and advice on preparation of the manuscript.
The Mycoses Study Group was funded in part by NIAID contract NO1-AI65296.
REFERENCES
- 1.Maenza J R, Keruly J C, Moore R D, Chaisson R E, Merz W G, Gallant J E. Risk factors for fluconazole-resistant candidiasis in human immunodeficiency virus-infected patients. J Infect Dis. 1996;173:219–225. doi: 10.1093/infdis/173.1.219. [DOI] [PubMed] [Google Scholar]
- 2.McKinsey D S, Wheat L J, Cloud G A, Pierce M, Black J R, Bamberger D M, Goldman M, Thomas C J, Gutsch H M, et al. Itraconazole prophylaxis for fungal infections in patients with advanced human immunodeficiency virus infection: randomized, placebo-controlled, double-blind study. Clin Infect Dis. 1999;28:1049–1056. doi: 10.1086/514744. [DOI] [PubMed] [Google Scholar]
- 3.Phillips P, Zemcov J, Mahmood W, Montaner J S G, Craib K, Clarke A M. Itraconazole cyclodextrin solution for fluconazole-refractory oropharyngeal candidiasis in AIDS: correlation of clinical response with in vitro susceptibility. AIDS. 1996;10:1369–1376. doi: 10.1097/00002030-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Revankar S G, Kirkpatrick W R, McAtee R K, Fothergill A W, Redding S W, Rinaldi M G, Hilsenbeck S G, Patterson T F. A randomized trial of continuous or intermittent therapy with fluconazole for oropharyngeal candidiasis in HIV-infected patients: clinical outcomes and development of fluconazole resistance. Am J Med. 1998;105:7–11. doi: 10.1016/s0002-9343(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 5.Rex J H, Pfaller M A, Barry A L, Nelson P W, Webb C D. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob Agents Chemother. 1995;39:40–44. doi: 10.1128/aac.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 7.Sangeorzan J A, Bradley S F, He X, Zarins L T, Ridenour G L, Tiballi R N, Kauffman C A. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994;97:339–346. doi: 10.1016/0002-9343(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 8.Snedecor G W, Cochran W G. Statistical methods. Ames: Iowa State University Press; 1980. [Google Scholar]
- 9.Waitz J A, Bartlett M S, Ghannoum M A, Espinel-Ingroff A, Lancaster M V, Odds F C, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J, Galgiani J N. Reference method of broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. pp. 1–29. [Google Scholar]