Abstract
The main breakthrough in tumor immunotherapy was the discovery of immune checkpoint (IC) proteins, which act as a potent suppressor of the immune system by a myriad of mechanisms. After that, scientists focused on the immune checkpoint molecules mainly. Thereby, much effort was spent to progress novel strategies for suppressing these inhibitory axes, resulting in the evolution of immune checkpoint inhibitors (ICIs). Then, ICIs have become a promising approach and shaped a paradigm shift in tumor immunotherapies. CTLA-4 plays an influential role in attenuation of the induction of naïve and memory T cells by engagement with its responding ligands like B7-1 (CD80) and B7-2 (CD86). Besides, PD-1 is predominantly implicated in adjusting T cell function in peripheral tissues through its interaction with programmed death-ligand 1 (PD-L1) and PD-L2. Given their suppressive effects on anti-tumor immunity, it has firmly been documented that ICIs based therapies can be practical and rational therapeutic approaches to treat cancer patients. Nonetheless, tumor inherent or acquired resistance to ICI and some treatment-related toxicities restrict their application in the clinic. The current review will deliver a comprehensive overview of the ICI application to treat human tumors alone or in combination with other modalities to support more desired outcomes and lower toxicities in cancer patients.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-022-00854-y.
Keywords: Immune checkpoint inhibitors, CTLA-4, PD-1/PD-L1, Cancer, Immunotherapy
Background
The promising rise and achievement of cancer immunotherapy over the past decade has developed the clinical management of many malignancies that were beforehand endowed with poor prognosis [1, 2]. Immune-checkpoint inhibitors (ICIs) are the leading approaches in tumor immunotherapy. They have been considered in the treatment of tumors due to their comprehensive bioactivity in various histological tumors, the stability of their response, and therapies that are evident even in metastatic and chemotherapy-resistant malignancies [3]. Interaction between immune checkpoints and their ligands negatively modifies T cell function and responding pathways complicated in the physiological immune response against tumor-associated antigens (TAAs). The immune checkpoints and their responding ligands are commonly upregulated in the TME of many human malignancies, and they signify substantial barricades for initiation of effective anti-tumor immune reaction [4, 5].
Among the checkpoint-blocking approaches, the two most eminent are blocking cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4 or CD152) and targeting the interaction between programmed cell death 1 (PD-1 or CD279) and programmed cell death ligand 1 (PD-L1 or CD274 or B7 homolog 1) [6]. Co-stimulation of CD80/CD86 via CD28 provides essential stimulus signals that support T cell proliferation and effective differentiation throughout the induction phase of the immunological response [7]. Structurally, the CTLA-4 has substantial homology to the costimulatory molecule CD28. It can also bind B7 molecules on antigen-presenting cells (APCs) with much higher affinity and avidity than CD28 [8]. The CTLA-4 co-inhibitory receptor is found on lately induced T cells and interacts with the identical ligands as CD28 but with higher affinity [9, 10]. It has a unique YVKM motif at the cytoplasmic domain, which binds to the SHP-2 and elicits inhibitory signaling like PD-1 (Fig. 1) [11]. Conversely, Src homology two domain-containing protein tyrosine phosphatase 2 (SHP-2) inhibitors typically provoke anti-tumor immunity, such as enhancing T cell cytotoxic activities and immune-mediated tumor regression [12]. CTLA-4 on T cells throughout the induction phase of an anti-cancer immune reaction obstructs T cell activation by inhibiting the formation of interaction between CD80/CD86 and CD28 and conveying inhibitory signals, directly suppressing T cell activation [13, 14]. The information respecting the CTLA-4 activities has led to the theory that hindering its action could ease T cell responses to persist, which has implications for progressing an understanding of tumor immunology around that time [11]. Many preclinical proofs have sustained this theory, inspiring the manufacture of ipilimumab, a monoclonal antibody (mAb) against human CTLA-4, to use in the clinic [15]. CTLA-4 blocker therapy, despite documented activities in concomitant activation, can also deplete regulatory T cells (Treg) from the tumor microenvironment (TME) as a result of high CTLA-4 expression at the Treg level [16]. As loss of CTLA-4 may perturb immunosuppressive effects of Treg on tumor-infiltrating lymphocytes (TILs) [17], it has been robustly proved that CTLA-4 blockade can support a paradigm shift in tumor therapy. Besides, the activity of PD-1 as an immune checkpoint was recognized following the detection of one of its ligands, PD-L1 [18]. Like CTLA-4, the PD-1is expressed on activated T cells, and its functions have been found to abolish signaling mediated on antigen recognition by the T cell receptor [19]. PD-1 has two ligands, including PD-L1 and PD-L2. While PD-L2 is mainly expressed on APCs, PD-L1 can be found on various cell types, comprising tumor cells, immune cells, epithelial cells, and endothelial cells [20]. The cytoplasmic tail of PD-1 shall consist of two tyrosine-based structural motifs, an immunoreceptor tyrosine-based inhibitory motif (ITIM) (V/L/I/XpYXX/L/V) and an immunoreceptor tyrosine-based switch motif (ITSM) (TXpYXXV/I) (Fig. 2) [21]. There is clear evidence showing that PD-1 inhibitory function relies on the ITSM phosphotyrosine, which in turn recruits SHP2 and consequently suppresses downstream signaling axes [21, 22]. PD-L1 expression has been allied with exposure to interferon-γ (IFN-γ), for instance upon anti-tumor T helper type 1 (Th1) cell responses. It leads potently to tumor cells evasion from T cell immunosurveillance [23–25]. Indeed, the activities of PD-1 in immune cells comprise the stimulation of the maintenance of peripheral immune tolerance, defending tissue from immune attack, and diminishing infectious immunity and also tumor immunity [20]. Like anti-CTLA-4 antibody ipilimumab, PD-1/PD-L1 inhibitors nivolumab, pembrolizumab, cemiplimab, atezolizumab, avelumab, and durvalumab have received FDA approvals since 2011 [26]. Notwithstanding, tumor resistance to ICIs [27, 28] along with the ICIs mediated toxicities [29] hinder their clinical application. For instance, the response rate for melanoma patients treated with pembrolizumab (anti-PD-1) was only 33%, and also about 20–30% of patients with lung carcinoma mainly experienced desired outcomes upon ICIs blockade therapy [30].
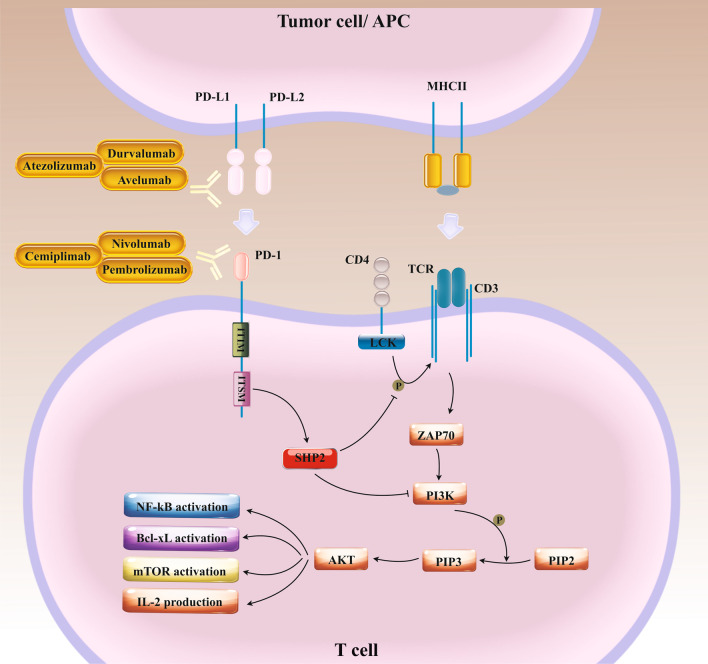
Fig. 1.
The inhibitory effects of the CTLA-4/B7 on T cell anti-tumor activities. CTLA-4 is expressed on activated T cells, is about 30% homologous with CD28 and binds to the same ligands as CD28, known as B7-1 and B7-2 expressed on APCs or tumor cells. This interaction results in activation of SHP2 and so down-regulation of PI3K/AKT axis. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), SH2 containing protein tyrosine phosphatase-2 (SHP2), Phosphoinositide 3-kinases (PI3Ks), Phosphatidylinositol-4,5-bisphosphate (PIP2), Phosphatidylinositol (3,4,5)-trisphosphate (PIP3), Lymphocyte-specific protein tyrosine kinase (LCK), T cell receptor (TCR), Nuclear factor-κB (NF-κB), Mammalian target of rapamycin (mTOR), B-cell lymphoma-extra large (Bcl-xL), Major histocompatibility complex class II (MHCII), Interleukin-2 (IL-2)
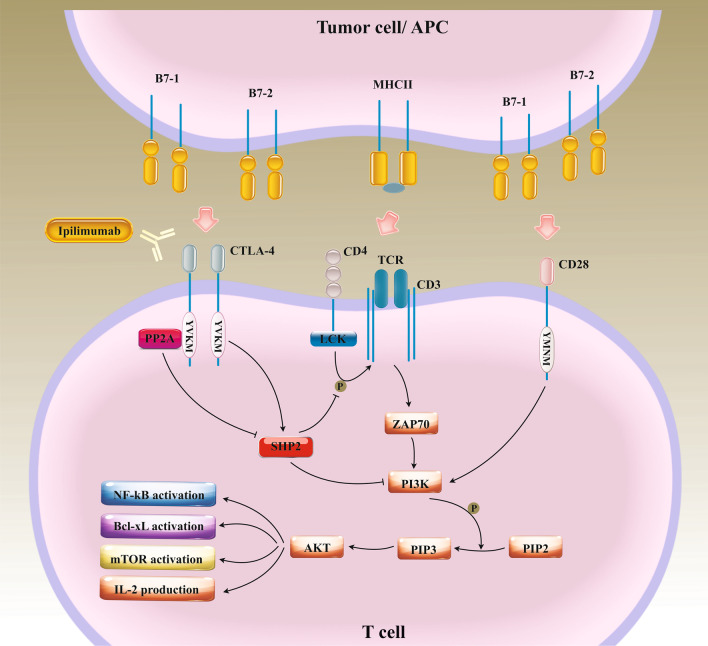
Fig. 2.
The inhibitory effects of the PD-1/PD-L interactions on T cell anti-tumor activities. PD-L1 expressed on APCs or tumor cells following interaction with PD-1 dysregulated on the surface of activated T cell limits self-reactive T cell proliferation and cytokine production as a result of activation of SHP2, which down-regulates PI3K/AKT axis. Programmed cell death protein 1(PD-1), Programmed death-ligand 1 and 2 (PD-L1, PD-L2), Antigen-presenting cells (APCs), SH2 containing protein tyrosine phosphatase-2 (SHP2), Phosphoinositide 3-kinases (PI3Ks), Phosphatidylinositol-4,5-bisphosphate (PIP2), Phosphatidylinositol (3,4,5)-trisphosphate (PIP3), Lymphocyte-specific protein tyrosine kinase (LCK), T cell receptor (TCR), Nuclear factor-κB (NF-κB), Mammalian target of rapamycin (mTOR), B-cell lymphoma-extra large (Bcl-xL), Major histocompatibility complex class II (MHCII), Interleukin-2 (IL-2)
Here, we've focused on the therapeutic application of ICIs as a pioneering approach in the field of tumor immunotherapy. Moreover, recent findings on combination therapy with ICIs to defeat tumor resistance against them will be stated.
Association between immune checkpoints (IC) proteins and tumor prognosis
To date, the predictive value of the ICs has been documented in a diversity of human malignancies. A study in 773 patients with stage I-III colorectal cancer (CRC) patients indicated that high tumoral immune checkpoints (lymphocyte-activation gene 3) LAG-3 and PD-1 were associated with poor survival. In contrast, patients with high T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), LAG-3, and PD-1 on immune cells within the stroma showed better survival [31]. Another investigation on 398 tumor tissues from stage I to IV gastric cancer patients revealed that higher tumor-infiltrating lymphocyte (TIL) frequency was associated with a lower risk of tumor development and showed survival advantages in gastric cancer patients [32]. Also, PD-L1 directly displayed a significant correlation with high TIL infiltration and tumor regression [32]. These findings signified that induction of immune checkpoint within gastric cancer patients could bring about a high immune infiltration frequency, directing patient selection for checkpoint blockade therapy [32]. The high densities of TILs, high ratios of PD-1+/CD8+ cells, and high levels of PD-L1 have been suggested that are negatively associated with melanoma brain metastases size, and high levels of PD-L1 may support a marked trend towards better survival [33]. However, there are some other inconsistent reports. For instance, studies in NSCLC patients treated with concurrent chemoradiotherapy (CRT) revealed that PD-L1 expression < 1% on tumor cells was related to improved overall survival (OS), and also patients with low CD8+ TIL density exhibited better OS [34]. Gennen et al. found that longest and shortest OS were attained in patients with type I (PD-L1neg/CD8low) and type IV (PD-L1pos/CD8low) tumors, respectively [34]. Similarly, evaluation of CD8+ T cell infiltration and expression of immune checkpoints, such as PD-1, PD-L1, PD-L2, and T cell immunoreceptor with Ig and ITIM domains (TIGIT) in 154 patients with primary esophageal squamous cells carcinoma (ESCCs) suggested that the number of PD-1+ TILs was positively correlated with CD8+ TILs [35]. The analysis demonstrated that enhanced quantities of PD-1+ and TIGIT+ TILs and PD-L1 and PD-L2 expression were related to a shorter OS [35]. Other studies in this regard also revealed that PD-1, PD-L1, TIM-3, LAG-3 immune checkpoints were positively associated with epithelial-mesenchymal transition (EMT) status in ESCC [36], and also tumor progress in breast cancer [37]. In metastatic breast tumors, PDL-L1high circulating tumor cells (CTC) were accompanied with the disease progression and shorter progression-free survival (PFS) [38]. PD-L1 has independent worse prognostic implications in metastatic breast cancer, indicating a possible role of innate and adaptive immune escape mechanisms in breast cancer metastatic potential [38]. Other studies in 135 primary clear cell renal cell carcinoma (ccRCC) tumors have also represented that high expression of ICs in the lack of fully functional mature dendritic cells (DCs) was in association with promoted risk of disease progression [39]. In contrast, low expression of ICs and also localization of mature DC in peritumoral immune aggregates might indicate desisted prognosis [39]. Indeed, expression of ICs expression and DC localization in the TME seems to hinder the clinical activities of CD8+ T cells in ccRCC [39].
These inconsistent reports have outlined the significance of patient selection based on tumor microenvironment characteristics for checkpoint blockade therapy.
The U.S. Food and Drug Administration (FDA)-approved ICIs
Tumor cells can trigger diverse ICs pathways to harbor immunosuppressive functions. Recent developments in our knowledge respecting T cell immunobiology have been chiefly involved in designing therapeutic strategies or molecules to circumvent tumor immune evasion mechanisms. Meanwhile, monoclonal antibodies (mAb) targeting ICs have delivered a massive breakthrough in tumor therapeutics [40–42]. Among the ICIs, PD-1/PD-L1 and CTLA-4 inhibitors have exhibited encouraging therapeutic outcomes, and some have been indicated for various tumor treatments since 2011 (Table 1), whereas others are under clinical trials [26]. In the context of cancer, where adverse T cell regulatory pathways are often overactive, immune checkpoint blockade has proven to be an effective strategy for enhancing the effector activity and clinical impact of anti-tumor T cells [26]. These ICIs have persuaded desired and durable responses in a significant proportion of cancer patients.
Table 1.
Current FDA-approved immune checkpoint inhibitors (ICIs)
| Agent | Target IC | Approved conditions |
|---|---|---|
| Ipilimumab | CTLA-4 | Melanoma, MSI-H/dMMR colorectal cancer (CRC), renal cell carcinoma (RCC) (in combination with nivolumab) |
| Nivolumab | PD-1 | MSI-H or dMMR colorectal cancer (CRC), head and neck squamous cell carcinomas (HNSCC), hepatocellular carcinoma (HCC), melanoma, Classic Hodgkin lymphoma (cHL), non-small-cell lung carcinoma (NSCLC), renal cell carcinoma (RCC), urothelial cancer, small-cell lung carcinoma (c-SCLC) |
| Pembrolizumab | PD-1 | Cervical cancer, gastric cancer, head and neck squamous cell carcinomas (HNSCC), hepatocellular carcinoma (HCC), Classic Hodgkin lymphoma (cHL), melanoma, Merkel cell carcinoma (MCC), MSI-H or dMMR colorectal cancer (CRC), non-small-cell lung carcinoma (NSCLC), diffuse large B-cell lymphoma (DLBCL), urothelial cancer |
| Cemiplimab | PD-1 | Cutaneous squamous cell carcinoma (cSCC) |
| Atezolizumab | PD-L1 | Non-small-cell lung carcinoma (NSCLC), urothelial cancer |
| Avelumab | PD-L1 | Merkel cell carcinoma (MCC), urothelial cancer |
| Durvalumab | PD-L1 | Non-small-cell lung carcinoma (NSCLC), urothelial cancer |
Programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4), microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR)
CTLA-4 inhibitors
Ipilimumab is a fully human IgG1 monoclonal antibody (mAb) targeting CTLA-4; it was the first FDA-approved ICI in 2011 for patients suffering from advanced melanoma [43]. Ipilimumab avoids T-cell suppression and stimulates the effector T cell’s activation and proliferation. This antibody, in combination with nivolumab as the PD-1 inhibitor, has been approved for the treatment of patients with metastatic colorectal cancer (CRC) with microsatellite instability-high (H-MSI) or mismatch repair (MMR) deficiencies [44]. Besides, its application along with nivolumab has been approved in patients with intermediate- or poor-risk renal cell carcinoma (RCC) regardless of PD-L1 status [45], as well as in patients with hepatocellular carcinoma (HCC) who have previously been treated with sorafenib [46]. In addition, ipilimumab plus nivolumab has also been indicated for the first-line treatment of patients with non-small-cell lung carcinoma (NSCLC) whose tumors express PD-L1 (≥ 1%) [47], and also for malignant pleural mesothelioma (MPM) [48].
PD-1 inhibitors
In addition to the nivolumab, which is a fully human IgG4 mAb, two other PD-1 inhibitors, pembrolizumab (IgG4 mAb) and cemiplimab (IgG4 mAb), have demonstrated promising outcomes in melanoma and NSCLC patients [49]. Interfaces between PD-1 and its ligands, B7-H1/PD-L1 and B7-DC/PD-L2, ultimately lead to T cells' inactivation to support immune homeostasis and prevent autoimmunity [49]. Apart from the combination therapy, monotherapy with nivolumab is also the first FDA-approved immunotherapy for gastric cancer's first-line treatment and is an effective treatment for NSCLC, classic Hodgkin's lymphoma (cHL), and melanoma [50]. Since 2019, pembrolizumab has been approved for the treatment of patients with metastatic melanoma, metastatic NSCLC in certain situations [51], as a first-line treatment for metastatic bladder cancer [52], as a second-line treatment for head and neck squamous cell carcinomas (HNSCC) [53], and also for refractory cHL [54], and metastatic ESCC [55]. Besides, cemiplimab was approved to treat basal cell carcinoma, cutaneous squamous cell carcinoma (CSCC), and non-small cell lung cancer (NSCLC).
PD-L1 inhibitors
Three anti-PD-L1 antibodies have been approved by the FDA: atezolizumab (IgG4 mAb), durvalumab (IgG1 mAb), and avelumab (IgG1 mAb) [56]. Atezolizumab, as the first FDA-approved PD-LI inhibitor, was approved in 2016 to treat patients with advanced or metastatic urothelial carcinoma [57]. This anti-body also has been approved for patients with metastatic NSCLC whose disorder developed throughout or upon platinum-containing chemotherapy [58]. Moreover, the FDA approved atezolizumab plus bevacizumab, an antiangiogenic drug, for people with unresectable or metastatic HCC [59]. Finally, atezolizumab, in combination with mitogen-activated extracellular kinase (MEK) inhibitor cobimetinib and B-Raf enzyme inhibitor called vemurafenib, has been approved for patients with BRAF V600 mutation-positive unresectable or metastatic melanoma [60]. In 2017, durvalumab was first approved for the treatment of locally advanced or metastatic urothelial carcinoma [61] and also for metastatic Merkel cell carcinoma (MCC), a rare and aggressive skin cancer [62]. Durvalumab, in combination with etoposide and carboplatin or cisplatin, has been approved as the first-line treatment for patients with advanced NSCLC [63]. In 2017, avelumab was approved for MCC [62] and metastatic urothelial carcinoma therapy [64]. Moreover, FDA has approved avelumab in combination with tyrosine kinase inhibitor axitinib for the first-line treatment of patients with advanced RCC in 2019 [65].
Monotherapy using immune checkpoint inhibitors in preclinical models
As described, six drugs targeting PD-1 or its ligand PD-L1 and one targeting CTLA-4 have been approved to treat diverse types of solid tumors and cHL. When used as monotherapy, the drugs mainly have a remarkable increase in objective response rate (ORR) and demonstrate a manageable safety profile. However, more than 50% of patients failed to respond to treatment. This section exclusively focuses on animal studies evaluating the therapeutic potential of ICIs therapy as monotherapy (Table 2).
Table 2.
Monotherapy using immune checkpoint inhibitors (ICIs) in preclinical models (animal study)
| Tumor | Target ICs | Main results | References |
|---|---|---|---|
| Glioma | CTLA-4 |
Induction of long-term survival in 80% of treated mice Reduction of CD4+ CD25+ Foxp3+ GITR+ Treg cell density |
[69] |
| Mesothelioma | CTLA-4 |
Inhibition of tumor development at the early stage of tumor development Improving frequency of CD4 and CD8 T cells infiltrating the tumor |
[73] |
| Hepatocellular carcinoma (HCC) | CTLA-4 |
Simulating longer survival in treated mice than control mice Amelioration of expression of CD4+ lymphocytes in residual tumors and IFN-γ generation |
[74] |
| NA | CTLA-4 | Inhibition of CD4+ CD25+ Treg function | [17] |
| Melanoma | CTLA-4 |
Augmentation of intratumoral T effector cell density in TME Reducing intratumoral Treg density in TME |
[258] |
| Colon adenocarcinoma | CTLA-4 |
Enhancement of intratumoral T effector cell density in TME Plummeting intratumoral Treg density in TME |
[70] |
| Colon adenocarcinoma | CTLA-4 | Inspiring anti-tumor response by immune cell | [259] |
| Prostate cancer | CTLA-4 | Modification of Treg activities is required for the anti-tumor impacts of the CTLA-4 blockade | [260] |
| Sarcomas | CTLA-4 | Anti-tumor immunotherapy by CTLA-4 blockade depends on the gut microbiota | [261] |
| Melanoma | CTLA-4 | Loss of IFN-γ axes in tumor cells is contributed to the cell resistance to anti-CTLA-4 therapy | [262] |
| Melanoma | CTLA-4 | Suppression of melanoma stem cells tumourigenesis | [72] |
| Melanoma | PD-1/PD-L1 | Tumors tempering the mitochondrial function in T cells show resistance to PD-1 blockade therapy | [263] |
| Oral squamous cell carcinoma (OSCC) | PD-1/PD-L1 |
Provoking the IFNγ, STAT1 activation and the making of the T-cell effector granzyme B in infiltrating cells Triggering apoptosis in the epithelial cells of the oral lesions |
[82] |
| Pancreatic ductal adenocarcinoma (PDA) | PD-1/PD-L1 | Mobilization of CD8+ T Cells by CXCR4 inhibition enables PD-1 checkpoint therapy | [86] |
| Myeloma | PD-1/PD-L1 | Inhibition of tumor cell growth transiently | [84] |
| Melanoma | PD-1/PD-L1 | Inhibition of tumor cell growth | [264] |
Programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4), interferon-gamma (IFNγ), signal transducer and activator of transcription (STAT1), Forkhead box P3 (Foxp3), glucocorticoid-induced tumor necrosis factor receptor (GITR), regulatory T cells (Tregs), C-X-C chemokine receptor type 4 (CXCR4)
CTLA-4 blockade
The constitutively expressed protein, CD28, arbitrates one of the best-recognized T cell costimulatory signals. CD28 binding to ligands B7-1 and B7-2 (CD80 and CD86) on APCs results eventually in T cell proliferation by stimulation of the production of interleukin-2 (IL-2) and anti-apoptotic factors [66]. CTLA-4 is expressed on activated T cells and has about 30% homologous with CD28 and also creates interaction with the ligands B7-1 and B7-2, thereby transmitting an inhibitory signal to T cells [67]. It has been found that monotherapy with antibodies to CTLA-4 can effectively instigate tumor regression of transplantable murine tumors. It seems that induction of effector T cells functions and suppressing Treg activities play central roles in this regard [17, 68].
Recently, studies in glioma xenograft models revealed that systemic administration of monoclonal antibody (9H10) to target CTLA-4 served prolonged survival in 80% of treated mice without exhibiting allergic encephalomyelitis [69]. Treatment caused diminished CD4+ CD25+ Foxp3+ GITR+ regulatory T cell fraction, supported improved CD4+ T-cell proliferative capacity, and provoked cervical lymph node antitumor response. These consequences indicated that CTLA-4 blockade could be rational modalities of restoring glioma-induced variations to the CD4 compartment and eliciting antitumor immunity [69]. Also, treatment with anti-CTLA-4 antibodies has shown promising anti-tumor effects in MC38 and CT26 tumor adenocarcinoma tumor models induced by selective attenuation of intra-tumor Treg with active T cell activation [70]. In addition, anti-CTLA-4 antibodies could ameliorate antitumor immunity through promoting melanoma-specific T-cell motility, according to Pentcheva et al. reports [71]. They suggested that CTLA-4 blockade could induce tumor immunity either by ameliorating effector T-cell activities or by depletion of Treg [71]. Importantly, treatment could give rise to improved T-cell motility, thereby supporting these T cells' enhanced frequencies in tumor-draining lymph nodes [71]. Remarkably, blocking CTLA‑4 in melanoma cells could also inhibit the particular competencies of melanoma stem‑like cells in vivo, comprising the capability for tumorigenesis [72]. CTLA-4 blocking antibodies also could hinder tumor growth at the early stage of murine mesothelioma [73]. Administration of anti-CTLA-4 antibody gave rise to an enhanced density of tumor-infiltrating CD4 and CD8 T cells. Also, it sustained the IL-2, IFN-γ, perforin, and granzyme B levels in TME in treated mice [73]. Moreover, anti-CTLA-4 therapy induced tumor regression and promoted survival after insufficient radiofrequency ablation (RFA) in the murine HCC model [74]. The analysis presented that expression of CD4+ T cells in residual tumors and IFN-γ generation in response to tumor cells were considerably higher in mice treated with anti-CTLA-4 than in the control group [74]. Other studies also have documented the importance of IFNγ in the CTLA-4 blockade-mediated anti-tumor response [75]. In a fibrosarcoma mice model, administration of anti-CTLA-4 anti-body caused raised levels of the IFN-inducible enzyme 2′,5′-oligoadenylate synthetase (OAS), a positive regulator of anti-tumor response, in draining lymph nodes concurrent with augmented levels of IFNγ in tumor lysates [75]. The prominence of IFNγ was confirmed through the aptitude of neutralizing antibodies to abolish the anti-tumor impacts of anti-CTLA-4 [75] wholly. In another study, Hanani et al. found that neutralizing IL-2 or blocking its receptor eliminated the antitumor effects and progression associated with the ratio of intra-tumor T effect versus Tregs, commonly induced by CTLA-4 blockade in melanoma mouse models [76]. In contrast, the administration of recombinant IL-2 led to the intensified therapeutic efficacy of CTLA-4 blockade [76]. The anti-CTLA-4 antibody also caused the abrogated Treg function and simultaneously improved IL-2-secreting effector T cell activities in vivo [76]. This study provides clear evidence illustrating the fundamental role of IL-2 and IL-2 receptors in the anti-tumor efficacy of CTLA-4 blockade [76]. Furthermore, Fransen et al. supposed that controlled local delivery of anti-CTLA-4 anti-body could trigger CD8+ T cell-dependent tumor elimination and reduced the risk of toxic side effects [77]. They found that lower dose and slow release of the anti-CTLA-4 anti-body gave rise to thousand-fold reduced levels of antibody in the serum, plummeting opposing events and the risk of autoimmunity in vivo [77]. Besides, they implied that CD4+ T cells do not play a noticeable role in the antibody-induced tumor regression [77].
PD-1/PD-L1 blockade
Preclinical and clinical evidence have delivered the rationale for PD-1/PD-L1blockade as a millstone in cancer immunotherapy, rendering that induction of the PD-1/PD-L1 axis is respected as an efficient tool for tumor escape host tumor antigen-specific T-cell immunity. Indeed, the binding of PD-L1 on the tumor cells with PD-1 on a T-cell obstructs T-cell proliferation as well as activation and consequently restrain immune cell-mediated antitumor function [78].
Clear proof indicates that PD-1/PD-L1 blockade could ease T-cell migration to tumors by inspiring IFN-γ inducible chemokines like CTLA-4 inhibitors [79]. Meanwhile, Peng et al. found that anti-PD-1 antibody could not affect the frequency of immunosuppressive cells, such as Treg and myeloid-derived suppressor cells (MDSCs), during tumor progression in tumor-bearing mice. At the same time, it could augment the expression of IFN-γ and C-X-C motif chemokine ligand 10 (CXCL10) at the tumor zone [80]. Blocking the PD-1 pathway could stimulate IFN-γ at the tumor tissue, thus enhancing chemokine-dependent infiltration of immune cells into malignant disease zone [80]. Moreover, studies in BRAFV600E mutation replace V600 valine-driven YUMM1.1 and YUMM2.1 melanomas, and the carcinogen-induced murine colon adenocarcinoma MC38 models clarified the critical competence of anti-PD-1 or anti-PD-L1 treatment to provoke potent antitumor effects versus tumor tissue in experimental models [81]. Anti-PD-1 therapy was also able to attenuate the number of oral lesions that developed in -nitroquinoline-1-oxide (4-NQO) mouse model of oral carcinogenesis and impede malignant progression in treated murine [82].
Meanwhile, low-grade dysplastic lesions reacted to anti-PD-1 therapy with a special promotion in the infiltration of CD8+ and CD4+ T cells and the accumulation of CTLA-4+ T cells in their TME [82]. In addition, PD-1 inhibition was associated with stimulation of IFNγ and signal transducer and activator of transcription 1 (STAT1) activation, production of granzyme B by infiltrating cells, and stimulation of apoptosis in the epithelial cells oral lesions [82]. These findings elucidated that T-cell activation arose from the anti-PD-1 therapy and suggested that CTLA-4 inhibitors may augment the preventive belongings of anti-PD-1 [82]. Monotherapy using anti-PD-1 or anti-PD-L1 antibody could also boost systemic T cell expansion, trigger objective responses, and convince the persistent neoantigen-specific T cell-mediated immunity in pancreatic ductal adenocarcinoma (PDA) murine model [83]. Furthermore, anti-PD-L1 administration suppressed myeloma cells' development in P815 tumor cell-bearing mice [84]. Also, transgenic expression of PD-L1 in P815 tumor cells maintained CTL-mediated tumor lysis in-vitro and increased tumorigenesis and invasiveness in-vivo [84].
Similarly, Zeng et al. disclosed that anti-PD-1 therapy-induced tumor regression inspired long-term survival of ovarian tumor-bearing mice [85]. They also found that AMD3100 could enhance these events, a particular C-X-C chemokine receptor type 4 (CXCR4), mainly by increasing the penetration and function of effective T cells, growing memory T cells in TME, and decreasing intratumoral Treg and MDSCs [85]. Thereby, it has been proven that PD-1 blocked therapy could be rational and practical candidates in ovarian cancer and could be clinically relevant to ovarian cancer patients [85]. Likewise, another report has shown that mobilization of CD8+ T cells through CXCR4 blockade supports anti-PD-1 therapy in PDA models in vivo [86].
Predictive biomarkers in ICIs therapy
Though ICIs therapy has provided robust anti-tumor effectiveness, some patients do not show desired response to this therapeutic intervention [87]. Thus, more consideration has been compensated for identifying and advancing predictive biomarkers for ICI's reaction. Currently, with the progress of high-throughput sequencing and microarray methods, a diversity of biomarker plans have been discovered and offered the process from the detection of a single marker to the advance of multifactorial synergistic predictive markers [88]. Now, PD-L1 expression, high tumor mutational burden (TMB), microsatellite instability (MSI), CD8 infiltration, and PD-L1 amplification are considered as primary predictive markers for ICIs response [89–91].
TMB is the total number of mutations found in the DNA of cancer cells and is recently being used as a type of biomarker [92]. High numbers of mutations seem to be more probable to respond to certain types of immunotherapy [92]. Dramatic association between high TMB and response to ICIs have been verified in various cancer types, such as urothelial carcinoma [93], NSCLC [94, 95], melanoma [96, 97], human papillomavirus (HPV)-negative HNSCC [98], biliary tract cancer [99], small-cell lung cancer (SCLC) [100] and CRC cancers [101, 102]. For example, studies on 22 patients with metastatic CRC treated with PD-1/L1 inhibitors verified the existence of a close association between TMB and objective response. Meanwhile, all 13 TMBhigh cases responded, while 6/9 TMBlow cases experienced progressive cancer [101]. On the other hand, any deregulation in mismatch repair (MMR) genes functions bring about high MSI (MSI-H), indicating a high amount of instability in the tumor. MSI-H tumors can mainly attract higher densities of TILs than low MSI (MSI-L), thereby showing a more favorable prognosis [103]. As cited, pembrolizumab has been approved in solid tumors with high MSI, based on a biomarker valuation of MSI status. High MSI correlates with the increased density of mutations in tumoral DNA, which is associated with more excellent rates of TMB and the enhanced presence of TILs and neoantigens [104]. Patients with MSI-H/deficient MMR tumors can usually benefit from ICIs therapy, and MSI can be applied as a genetic instability of a tumor detection index in various tumors, such as biliary tract cancer [99], NSCLC [90], gastroesophageal cancers [105, 106], breast cancer [104] and CRC [107]. Another study in 149 patients with endometrial cancer revealed that CD8+ T cells and PD-L1/PD-1 expression was considerably higher in the MSI group than in the microsatellite-stable group. Thereby, ICIs could be effective in endometrial cancers with MSI, and the existence of MSI may also be a biomarker for desired response to PD-1/PD-L1 immunotherapy [108]. Similarly, Xiao et al. suggested that MMR deficiency is accompanied by MSI phenotype, augmented TILs, and PD-L1 expression in immune cells in ovarian cancer [109]. Kumagai and coworkers have also supposed that PD-1 expression by CD8+ T cells and Treg cells negatively affect effector and immunosuppressive activities, respectively [110]. They pronounced that PD-1 blockade inspires both recovery of dysfunctional PD-1+ CD8+ T cells and improved PD-1+ Treg cell-elicited immunosuppression [110]. Given that a deep reactivation of effector PD-1+ CD8+ T cells rather than PD-1+ Treg cells by anti-PD-1 antibodies is required for tumor regression, they suggested that PD-1 expression could be used as a predictive biomarker for PD-1 blockade therapies [110]. Besides, the fact that ipilimumab plus nivolumab has been approved for the first-line treatment of patients with NSCLC whose tumors express PD-L1 (≥ 1%) [47], highlights the importance of the PD-L1 expression as a pivotal predictive biomarker in this regard. In addition, other studies have shown that PD-L1, LAG3, and indoleamine 2, 3-dioxygenase 1 (IDO1) expressions in TILs presented a more appropriate prognosis for patients with MSI-H colon cancer [111]. Also, MDM2/4 amplification (AMP) is correlated with hyperprogression during ICI therapy in various tumors types, in particular NSCLC, and predicts poor response to ICIs [112]. In sum, assessment of the combination of several parameters is of paramount importance for the successful prediction of the cancer patient’s responses to ICIs.
Clinical trials
Approved ICIs are the anti-PD1 antibodies, including nivolumab, cemiplimab, and pembrolizumab; anti-PD-L1 antibodies comprised of atezolizumab, avelumab, and durvalumab; and the anti-CTLA-4 antibody called ipilimumab. Many trials have been accomplished or are ongoing to evaluate the safety and efficacy of ICIs in human tumors, including melanoma, NSCLC, RCC, MCC, CRC, cHL, urothelial cancer, and various deficient MMR/MSI-H solid tumors, etc. (Fig. 3) (Tables 3, 4).
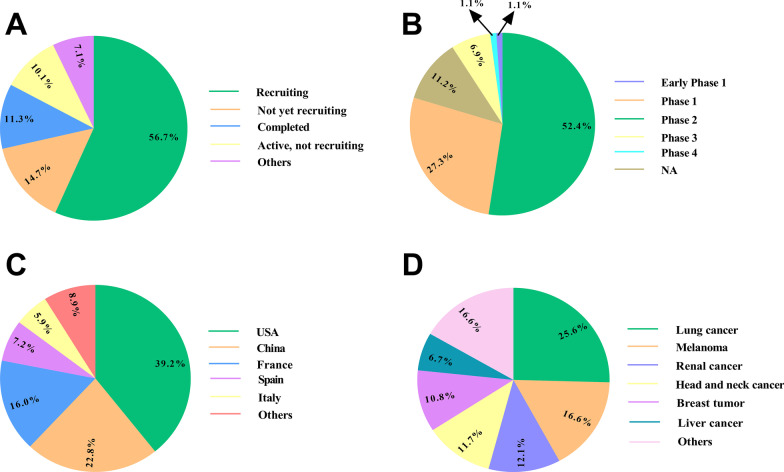
Fig. 3.
Clinical trials based on tumor immunotherapy using immune checkpoint inhibitors (ICIs) registered in ClinicalTrials.gov (November 2021). The schematic illustrates clinical trials using ICIs depending on the study phase (A), study status (B), conditions (C), and agents (D) in cancer patients
Table 3.
Clinical trials based on immune checkpoint inhibitors (ICIs) therapy in human malignancies registered in ClinicalTrials.gov (November 2021)
| Condition | Drug | Phase | Participant number | Location | Status | NCT number |
|---|---|---|---|---|---|---|
| Hepatocellular carcinoma (HCC) |
Camrelizumab Apatinib |
2 | 40 | China | Recruiting | NCT04826406 |
| Oral squamous cell carcinoma (OSCC) | Avelumab | 2 | 240 | Italy | Recruiting | NCT04504552 |
|
Solid tumor Lymphoma |
Nivolumab Pembrolizumab Atezolizumab Durvalumab |
2 | 40 | USA | Recruiting | NCT03544723 |
| Brain cancer | Nivolumab | 2 | 180 | USA | Recruiting | NCT03173950 |
| Squamous cell carcinoma of head and neck (SCCHN) | Nivolumab | 2 | 24 | USA | Recruiting | NCT03878979 |
| Thymic carcinoma | KN046 | 2 | 29 | USA | Not yet recruiting | NCT04925947 |
| Gastric adenocarcinoma | Nivolumab | 2 | 124 | China | Recruiting | NCT04908566 |
| Cholangiocarcinoma |
Lenvatinib Sintilimab |
2 | 25 | China | Not yet recruiting | NCT05010681 |
|
Gastric cancer Liver cancer |
IMC-001 | 2 | 48 | S. Korea | Recruiting | NCT04196465 |
| Non-small-cell lung carcinoma (NSCLC) | Durvalumab | 2 | 55 | USA | Recruiting | NCT04062708 |
| Renal transitional cell carcinoma (TCC) | Pembrolizumab ramucirumab | 2 | 28 | USA | Recruiting | NCT04179110 |
| HCC |
Pembrolizumab Regorafenib |
2 | 119 | International | Recruiting | NCT04696055 |
| Renal cell carcinoma (RCC) |
Atezolizumab Cabozantinib |
3 | 500 | International | Recruiting | NCT04338269 |
| Solid tumors |
Nivolumab Pembrolizumab |
1/2 | 104 |
USA Canada |
Recruiting | NCT03311334 |
|
NSCLC Melanoma |
Infliximab Vedolizumab |
1/2 | 100 | USA | Recruiting | NCT04407247 |
| Pancreatic cancer | M7824 | 1/2 | 52 | USA | Recruiting | NCT04327986 |
| NSCLC | Pembrolizumab | 1/2 | 30 | China | Completed | NCT04670107 |
| NSCLC |
Atezolizumab Tocilizumab |
1/2 | 28 | USA | Not yet recruiting | NCT04691817 |
| Advanced cancers |
Nivolumab Ipilimumab Pembrolizumab |
1/2 | 104 | USA | Completed | NCT02467361 |
| Prostate cancer | Pembrolizumab | 2 | 100 | UK | Recruiting | NCT03506997 |
| NSCLC | Atezolizumab | 2 | 21 | USA | Active, not recruiting | NCT03689855 |
| Gastric carcinoma | Toripalimab | 2 | 70 | China | Not yet recruiting | NCT04891016 |
| RCC |
Tivozanib Nivolumab |
3 | 326 | USA | Active, not recruiting | NCT04987203 |
| NSCLC |
Ipilimumab Nivolumab |
3 | 1360 | France | Recruiting | NCT03469960 |
| Advanced cancers | INCB086550 | 2 | 150 |
Bulgaria Ukraine |
Not yet recruiting | NCT04629339 |
| Prostate cancer | Pembrolizumab | 2 | 33 | USA | Recruiting | NCT03406858 |
| HCC | Cabozantinib | 2 | 46 | Italy | Recruiting | NCT04435977 |
| Brain tumor | Pembrolizumab | 2 | 30 | USA | Recruiting | NCT04479241 |
| SCCHN |
Monalizumab Cetuximab |
3 | 600 | International | Recruiting | NCT04590963 |
| Cervical cancer | Durvalumab | 2 | 37 | S. Korea | Not yet recruiting | NCT04800978 |
| Melanoma |
Nivolumab Ipilimumab |
1/2 | 72 | Australia | Recruiting | NCT03161756 |
| Melanoma |
Indoximod Ipilimumab Nivolumab Pembrolizumab |
1/2 | 132 | USA | Completed | NCT02073123 |
| Solid tumor |
Biological Nivolumab |
1/2 | 102 | USA | Recruiting | NCT04317105 |
| Colorectal cancer | Atezolizumab | 2 | 52 | France | Recruiting | NCT04659382 |
| Oesophageal cancer |
Nivolumab Ipilimumab |
2 | 130 |
France Spain |
Recruiting | NCT03437200 |
| HCC | Durvalumab | 2 | 37 | Hong Kong | Recruiting | NCT04913480 |
| NSCLC | Camrelizumab | 2 | 62 | China | Recruiting | NCT04167774 |
| Advanced cancers | Toripalimab | 2 | 35 | China | Recruiting | NCT03810339 |
| Prostate cancer |
Ipilimumab Nivolumab |
2 | 75 | USA | Recruiting | NCT04717154 |
| NSCLC | Atezolizumab | 4 | 100 | S. Korea | Recruiting | NCT04059887 |
| Pancreatic cancer | Pembrolizumab | 2 | 16 | Denmark | Recruiting | NCT04835402 |
| Nasopharyngeal neoplasms | Camrelizumab | 3 | 442 | China | Recruiting | NCT03427827 |
|
Colorectal neoplasms Breast neoplasms |
Durvalumab | 2 | 384 | USA | Recruiting | NCT02484404 |
| Nasopharyngeal carcinoma | Toripalimab | 3 | 494 | China | Not yet recruiting | NCT04907370 |
| Mesothelioma | Durvalumab | 3 | 480 | International | Recruiting | NCT04334759 |
| Immune-mediated colitis | Tofacitinib | 2 | 10 | Canada | Not yet recruiting | NCT04768504 |
| Anal cancer | Durvalumab | 2 | 178 |
Germany Switzerland |
Recruiting | NCT04230759 |
| Metastatic solid tumor | Cemiplimab | 2 | 38 | Netherlands | Not yet recruiting | NCT04706715 |
|
Hepatocellular carcinoma Colorectal neoplasms Gastric cancer Lung cancer |
Nivolumab pembrolizumab | 2 | 80 | USA | Recruiting | NCT03259867 |
| Nasopharyngeal carcinoma | Durvalumab | 2 | 118 | Hong Kong | Recruiting | NCT04447612 |
| NSCLC | Camrelizumab | 2 | 40 | China | Recruiting | NCT04541251 |
|
Esophageal cancer Metastatic cancer Squamous cell carcinoma |
Cabozantinib atezolizumab | 2 | 37 | Taiwan | Recruiting | NCT05007613 |
| Cervical cancer | Atezolizumab | 2 | 189 | France | Recruiting | NCT03612791 |
| Pancreatic cancer | Pembrolizumab | 2 | 24 | USA | Completed | NCT03331562 |
| Nasopharyngeal carcinoma | Toripalimab | 2 | 126 | China | Recruiting | NCT04517214 |
| Breast cancer | Pembrolizumab | 2 | 46 | Germany | Recruiting | NCT03988036 |
| Breast cancer | Pembrolizumab | 2 | 15 | Israel | Recruiting | NCT03591276 |
| Solid tumors | Ipilimumab nivolumab pembrolizumab atezolizumab | 2 | 60 | USA | Recruiting | NCT03693014 |
| Gastrointestinal cancer | Atezolizumab | 2 | 175 | USA | Recruiting | NCT04214418 |
| Breast cancer | Nivolumab | 2 | 90 | S. Korea | Recruiting | NCT04061863 |
| Solid tumors |
Ipilimumab Nivolumab Pembrolizumab Atezolizumab Avelumab Durvalumab Cemiplimab |
2 | 126 | Netherlands | Recruiting | NCT04954599 |
| NSCLC | Pembrolizumab | 2 | 85 | USA | Recruiting | NCT03233724 |
Table 4.
The results of most important clinical trials based on immune checkpoint inhibitors (ICIs) therapy alone or in combination with other modalities in cancer patients
| Condition | Agents | Result | References |
|---|---|---|---|
| Untreated melanoma | Ipilimumab + nivolumab | Nivolumab alone or combined with ipilimumab caused significantly longer PFS than ipilimumab alone | [125] |
| Advanced melanoma | Nivolumab + ipilimumab | This combination had a controllable safety profile and provided clinical activity | [265] |
| Advanced UC | Nivolumab + ipilimumab | This combination provided an effective treatment strategy | [266] |
| NSCLC | Nivolumab + ipilimumab + chemotherapy | This combination provided a significantly longer OS against chemotherapy alone | [213] |
| Resectable NSCLC | Atezolizumab + carboplatin + nab-paclitaxel | This combination achieving a major pathological response, and controllable treatment-related toxic effects | [267] |
| Urothelial cancer | Pembrolizumab | Pembrolizumab has become a new treatment choice | [130] |
| Colorectal cancer | Nivolumab | Nivolumab provided strong responses | [124] |
| NSCLC, melanoma, renal-cell cancer | Nivolumab | Nivolumab is caused in objective responses | [123] |
| Recurrent glioblastoma | Pembrolizumab | Pembrolizumab enhances both the local and systemic antitumor immune response | [129] |
| Incurable human papillomavirus 16-related cancer | Nivolumab + ISA101 | This combination provided a clinical activity compared with nivolumab alone | [239] |
| Locally advanced and metastatic UC | Atezolizumab | Atezolizumab showed durable clinical activity and good tolerability | [268] |
| Unresectable hepatocellular carcinoma | Atezolizumab + bevacizumab | This combination made a longer PFS than with atezolizumab alone | [269] |
| NSCLC | Ipilimumab + radiation | This combination provided evidence that can be considered a treatment strategy | [270] |
| TNBC | Nivolumab + doxorubicin + cisplatin | They indicated that cisplatin and doxorubicin may increase the likelihood of response to nivolumab in TNBC | [271] |
| Extensive-stage small-cell lung cancer | Durvalumab + platinum-etoposide | This combination showed sustained OS improvement versus platinum-etoposide alone | [272] |
| NSCLC | Durvalumab + tremelimumab | This combination showed a controllable tolerability profile, with antitumor activity | [273] |
| Metastatic squamous cell carcinoma | Nivolumab | Nivolumab significantly improved OS | [119] |
| Resectable glioblastoma | Nivolumab | Nivolumab significantly improved OS | [120] |
| Advanced nonsquamous NSCLC | Nivolumab | Nivolumab significantly improved OS in patients that had progressed during or after chemotherapy | [121] |
| Advanced melanoma | Nivolumab and ipilimumab | Nivolumab plus ipilimumab or nivolumab alone significantly improved OS than ipilimumab alone | [274] |
| Recurrent squamous-cell carcinoma of the head and neck | Nivolumab | Nivolumab resulted in longer OS than treatment with standard, single-agent therapy | [122] |
| Advanced melanoma | Pembrolizumab against ipilimumab | The pembrolizumab prolonged PFS and OS and had less high-grade toxicity than did ipilimumab | [131] |
| Metastatic melanoma | Ipilimumab + glycoprotein 100 (Gp100) | This combination, as compared with gp100 alone, improved OS in patients | [275] |
| Squamous NSCLC | Pembrolizumab + chemotherapy | This combination resulted in significantly longer OS and PFS than chemotherapy alone | [276] |
| Metastatic NSCLC | Pembrolizumab + chemotherapy | This combination resulted in significantly longer OS and PFS than chemotherapy alone | [277] |
| Early TNBC | Pembrolizumab + chemotherapy | This combination resulted in a significantly higher pathological complete response than chemotherapy alone | [128] |
| Advanced UC | Pembrolizumab | This combination resulted in significantly longer OS than chemotherapy alone | [127] |
| Untreated metastatic nonsquamous NSCLC | Pembrolizumab + pemetrexed-platinum | This combination demonstrated substantially improved OS and PFS | [278] |
| MSI-H/dMMR noncolorectal cancer | Pembrolizumab | Pembrolizumab monotherapy demonstrated clinical benefits for the patients | [126] |
| Advanced CSCC | Cemiplimab | Cemiplimab induced a response in approximately half of the patients | [140] |
| Advanced CSCC | Cemiplimab | Cemiplimab showed antitumor activity and an acceptable safety profile | [139] |
| Metastatic CSCC | Cemiplimab | Cemiplimab produced substantial antitumor activity with a durable response and an acceptable safety profile | [138] |
| Advanced malignancies | Cemiplimab + radiotherapy and/or low-dose cyclophosphamide | Cemiplimab exhibited encouraging antitumor activity | [279] |
| Unresectable hepatocellular carcinoma | Atezolizumab + bevacizumab | Atezolizumab combined with bevacizumab resulted in better OS and PFS outcomes | [280] |
| NSCLC | Atezolizumab | Atezolizumab treatment resulted in significantly longer OS than platinum-based chemotherapy | [148] |
| NSCLC | Atezolizumab + bevacizumab + chemotherapy | This combination improved PFS and OS | [58] |
| Advanced TNBC | Atezolizumab + nab-paclitaxel | This combination prolonged PFS | [281] |
| Metastatic non-squamous NSCLC | Atezolizumab + carboplatin + nab-paclitaxel | This combination showed a significant and clinically meaningful improvement in OS and PFS | [282] |
| Early-stage TNBC | Atezolizumab + chemotherapy | This combination significantly resulted in pathological complete response rates with an acceptable safety profile | [283] |
| Metastatic urothelial cancer | Atezolizumab + chemotherapy | This combination prolonged PFS | [284] |
| Melanoma | Atezolizumab + vemurafenib, + cobimetinib | This combination significantly increased PFS and it was tolerable and safe | [285] |
| Advanced or metastatic UC | Avelumab | Avelumab with best supportive care significantly prolonged OS, as compared with best supportive care alone | [64] |
| Metastatic UC | Avelumab | Avelumab showed antitumor activity in the treatment of patients | [156] |
| Advanced or metastatic breast cancer | Avelumab | Avelumab exhibited a clinical activity and acceptable safety profile | [153] |
| Recurrent or refractory ovarian cancer | Avelumab | Avelumab demonstrated antitumor activity and acceptable safety | [155] |
| Relapsed or refractory extranodal NK/T-cell lymphoma | Avelumab | Avelumab showed single-agent activity | [154] |
| Advanced GC/GEJC | Avelumab + chemotherapy | Avelumab showed a more controllable safety profile than chemotherapy alone | [286] |
| NSCLC | Durvalumab | Durvalumab prolonged PFS than with placebo | [158] |
| NSCLC | Durvalumab | Durvalumab monotherapy caused significantly longer OS than placebo | [159] |
| NSCLC | Durvalumab | Durvalumab demonstrated durable PFS and sustained OS after chemoradiotherapy | [160] |
| Extensive-stage small-cell lung cancer (ES-SCLC) | Durvalumab + tremelimumab + platinum | Durvalumab plus platinum-etoposide demonstrated sustained OS improvement against platinum-etoposide alone | [287] |
| Recurrent or metastatic cervical cancer | Cemiplimab + radiation therapy | Cemiplimab demonstrated clinical activity | [288] |
| Advanced melanoma, NSCLC, bladder cancer | Nivolumab + NEO-PV-01 | This combination therapy was safe and feasible | [289] |
| Melanoma | Pembrolizumab + oncolytic virotherapy | The addition of oncolytic virotherapy might improve the value of pembrolizumab by changing the tumor microenvironment | [290] |
| Melanoma | Ipilimumab + talimogene laherparepvec | This combination was tolerated safely | [291] |
Non-small cell lung cancer (NSCLC), urothelial cancer (UC), progression-free survival (PFS), overall survival (OS), mismatch repair (MMR); high microsatellite instability (MSI-H), triple-negative breast cancer (TNBC), gastric cancer/gastro-oesophageal junction cancer (GC/GEJC), cutaneous squamous-cell carcinoma (CSCC)
CTLA-4 inhibitors
Ipilimumab is the most prominent member of the CTLA-4 inhibitors and also is defined as the first FDA-approved ICI. It has been approved for melanoma, MSI-H/dMMR CRC, intermediate or poor-risk RCC (in combination with nivolumab).
Based on trials recorded at ClinicalTrials.gov, 610 studies have been evaluating the antitumor effects of ipilimumab in participants suffering from melanoma, RCC, CRC, myeloma, NSCLC, glioblastoma, liver cancer, and prostate cancer. Of those, 62 ongoing studies are in phases 3 or 4. Among 610 studies, five trials (melanoma, lung cancer, kidney cancer, and RCC) are in phase 4. In addition, to strengthen the efficacy of ipilimumab in combination with nivolumab in NSCLC, this regimen could be an effective option for improving the median OS in patients with malignant pleural mesothelioma (MPM) [48]. Meanwhile, a phase 3 study (CheckMate 743) on 750 MPM patients reported the superiority of combination therapy with nivolumab (systemic 3 mg/kg) plus ipilimumab (1 mg/kg) on platinum plus pemetrexed chemotherapy in terms of improved median OS [48]. The median OS was 18.1 months in patients receiving combination treatment versus 14.1 months inpatient treated with a chemotherapy agent. Also, 2-year OS rates were 41% versus 27% in the nivolumab plus ipilimumab group and chemotherapy group, respectively. The results of this study supported the application of this regimen for previously untreated unresectable MPM from October 2020 [48].
Further, combination therapy with ipilimumab and nivolumab exhibited a more favored effect on PFS rate in patients with stage III or stage IV melanoma than monotherapy A 4 years follow-up showed a median PFS of 11.5 months in the nivolumab plus ipilimumab group, 6.9 months in the nivolumab group, and 2.9 months in ipilimumab group [113]. Further, the most common treatment-associated grade 3 side effects were diarrhea in the co-treated group (9%) and the nivolumab group (3%) and also colitis in the ipilimumab group (7%). Additionally, enhanced lipase (3–5%) was the most common grade 4 side effect in all three groups. The achieved outcomes demonstrated a durable and sustained survival benefit in patients with advanced melanoma who received ICIs alone or in combination [113].
Tremelimumab is another well-known CTLA-4 inhibitor. Unlike ipilimumab which is an IgG1 isotype, tremelimumab is an IgG2 isotype. According to trials registered in ClinicalTrials.gov, there exist 164 studies based on tremelimumab therapy in participants with various tumors. Meanwhile, 12 of them, including NSCLC, bladder cancer, head, and neck squamous cell carcinoma (HNSC), urothelial cancer, and HCC are in phase 3; however, there is no registered study in phase 4. In addition, a phase 2 study of tremelimumab (15 mg/kg intravenously) in advanced uveal melanoma patients who had not received prior immunotherapy demonstrated the safety and acceptable efficacy with survival benefits (median OS about 12.8 months) [114]. Also, administration of the tremelimumab (1 mg/kg) in combination with anti-PD-L1 monoclonal antibody durvalumab (20 mg/kg) elicited promising outcomes in mesothelioma patients [115]. The intervention caused improvement in median PFS (5.7 months) and median OS (16.6 months) irrespective of PD-L1 expression levels [115]. However, 18% of patients experienced high grade 3–4 treatment-associated adverse events. Thereby, it seems that comprehensive dose-escalation studies are required prior accomplishment of large scale phase 2 and 3 trials [115]. Furthermore, Pakkala et al. (2020) exhibited that combination therapy with durvalumab and tremelimumab could not be an effective therapeutic regimen for relapsed SCLC, highlighting the importance of the conduction of further studies to justify designing and conduction of phase 3 trials [116]. Besides, quavonlimab (MK-1308), a novel anti-CTLA-4 antibody, in conjunction with pembrolizumab, resulted in ORR about 40.0% in advanced NSCLC patients [117]. Importantly, PD-L1 expression and a total number of circulating CD4+ cells associated with ORR [117].
PD-1 inhibitors
As a human IgG4 monoclonal antibody, Nivolumab is the second FDA-approved systemic treatment for mesothelioma and the first-line FDA-approved immunotherapy for gastric cancer [118]. Regarding trials registered in ClinicalTrials.gov, there are 1152 documented studies conducted or ongoing to address nivolumab's safety and efficacy in many tumors, 115 of which (melanoma, NSCLC, mesothelioma, breast cancer, RCC, lymphoma, HCC, urothelial cancer, etc.) are in phases 3 or 4 while 6 of which (NSCLC, kidney cancer, and RCC) are in phase 4. There have been several studies in the literature reporting that the nivolumab monotherapy has beneficial effects in the treatment of patients with metastatic squamous cell carcinoma, glioblastoma, NSCLC, melanoma, colorectal cancer, renal cell cancer, and recurrent squamous-cell carcinoma of the head and neck [119–124] (NCT02105636) (NCT02550249) (NCT01673867) (NCT02105636) (NCT00730639) (NCT02060188). The findings of these studies confirm the association between PD-L1 gene expression in tumor cells and the objective responses. Larkin et al. showed that using nivolumab alone or combined with the ipilimumab resulted in a significantly longer PFS than ipilimumab alone in patients with unresectable stage III or IV melanoma [125] (NCT01844505). The results of this study showed that the combination of nivolumab and ipilimumab had more beneficial effects in patients with PD-L1 negative tumors than in patients treated with either of these drugs alone [125].
Pembrolizumab, another PD-1 inhibitor, has been approved for cervical cancer, gastric cancer, HNSCC, HCC, cHL, melanoma, MCC, NSCLC, diffuse large B-cell lymphoma (DLBCL), and urothelial cancer. Regarding trials registered in ClinicalTrials.gov, 1355 registered trials assess the safety and efficacy of pembrolizumab in human cancers. The 140 (NSCLC, RCC, lymphoma, HCC, endometrial cancers, melanoma, biliary tract carcinoma, urothelial cancer, etc.) are in phases 3 or 4. 4 trials (NSCLC, melanoma, thymoma and thymic carcinoma, and SCC) are in phase 4. Pembrolizumab monotherapy has shown a survival benefit for several cancers, including Recurrent glioblastoma, early TNBC, advanced urothelial cancer (UC), and MSI-H/dMMR solid tumors (NCT02628067) (NCT03036488) (NCT02256436) [126–129]. Balar et al. reported that pembrolizumab had become a new treatment strategy for UC patients [130] (NCT02335424). The other study done by Robert et al. demonstrated that the pembrolizumab prolonged PFS and OS and had less toxicity than ipilimumab in patients with melanoma [131] (NCT01866319). During Phase 2, 259 patients with advanced gastrointestinal or esophageal cancer received 200 mg of pembrolizumab intravenously. Pembrolizumab monotherapy exhibited auspicious activity (durable responses) and a manageable safety profile in these patients [132]. Pembrolizumab also was well tolerated and elicited significant antitumor effects in patients with BCG-unresponsive non-muscle-invasive bladder cancer [133]. The standard grade 3 or 4 treatment-related adverse events were arthralgia (2%) and hyponatremia (3%) [133]. Thereby, pembrolizumab monotherapy can be suggested as a potential non-surgical therapeutic approach in difficult-to-treat bladder cancer patients. In addition, pembrolizumab monotherapy was associated with durable antitumor effects in metastatic triple-negative breast cancer (mTNBC) patients [134]. The ORR and disease control rate (DCR) was significantly but not strongly higher in the PD-L1-positive populations than the total population [134]. These results heightened the importance of defining and assessing predictive biomarkers before conducting ICIs therapies to distinguish between responder and non-responder patients [134]. Significantly, systemic administration of pembrolizumab 200 mg every three weeks improved PFS in HL patients who have relapsed post-autologous HSCT [135]. In high-risk stage III melanoma, pembrolizumab promoted 3.5-year distant metastasis-free survival and recurrence-free survival more efficiently in PD-L1-positive tumors [136]. Hence, the outcome of this phase 3 trial supported the indication to exploit adjuvant pembrolizumab therapy in advanced cutaneous melanoma patients. Similar results were obtained in patients with kidney cancer at high risk for recurrence following pembrolizumab monotherapy [137].
Another PD-1 inhibitor, cemiplimab, has been approved for cutaneous SCC. 1355 registered trials assess the safety and efficacy of cemiplimab in various human tumors. There are 55 documented studies based on cemiplimab therapy for human tumors, of which 3 of them (cutaneous SCC and NSCLC) are in phase 3, while there is no registered trial in phase 4. The cemiplimab monotherapy also provided a survival benefit and acceptable safety in patients with advanced and metastatic cutaneous squamous cell carcinoma (CSCC) [138–140] (NCT02383212 and NCT02760498) (NCT02760498) (NCT02760498). Among patients with advanced CSCC, cemiplimab stimulated a response in approximately half the patients and was associated with severe events that usually occur ICIs [138–140]. Cemiplimab monotherapy (3 mg/kg intravenously) encouraged antitumor effects due to its 44% objective response (34 out of 78 patients) and an acceptable safety profile in patients with CSCC [139]. Indeed, 10 patients exhibited complete response, and 24 participants displayed partial response, suggesting cemiplimab as a potential treatment option for CSCC therapy [139]. The promising results of this study and similar studies led to the approval of this cemiplimab for CSCC therapy in September 2018. Another phase 3 trial also signified the more prominent anti-tumor effects of cemiplimab monotherapy versus chemotherapy in NSCLC patients with PD-L1 of at least 50% [141]. Accordingly, the median PFS was 8.2 months versus 5.7 months in the cemiplimab group versus with chemotherapy group [141]. Further, serious adverse events happened in 28% of patients who received cemiplimab ad 39% of patients in the chemotherapy group. Thereby, in terms of safety and efficacy, it appears that cemiplimab is a more favored treatment than conventional chemotherapies. Thus, it seems that cemiplimab can receive approval from FDA for NSCLC with PD-L1 of at least 50% [141].
Sintilimab, a humanized IgG4 anti-PD-1 monoclonal antibody, is one of the most important PD-1 inhibitor that has not yet been approved by FDA despite promising clinical results. Sintilimab administration (200 mg/patient intravenously) has exhibited major pathologic response (MPR) (40.0%) and also objective response (20.0%) in NSCLC patients [142]. Also, in another phase 1b trial (NCT03628521) on 22 NSCLC patients, it improved median PFS rate to 15 months with no grade 4 treatment-related adverse events [143]. In addition, tislelizumab (BGB-A317), a humanized IgG4 anti-PD-1 monoclonal antibody, has shown anti-tumor potential in advanced solid tumors more evidently at the 5 mg/kg dose [144]. With respect to the observed results, various studies are underway to prove the safety and efficacy of tislelizumab in patients suffering from ESCC, gastric cancer, HCC, lung cancer and UC [144].
Recently, MDX-1106, a humanized IgG4 anti-PD-1 monoclonal antibody, as another type of the PD-1 inhibitors exhibited antitumor activity in metastatic melanoma, CRC, castrate-resistant prostate cancer (CRPC), NSCLC and RCC patients [145]. As well, there is clear evidence indicating the beneficial effects of administration of prolgolimab [146] and toripalimab [147], two other types of the anti-PD-1 monoclonal antibody, in melanoma patients [146] and also in patients with chemorefractory metastatic nasopharyngeal carcinoma (NPC) [147], respectively. Nonetheless, the preliminary results have to be validated in the large scale phase 2 and 3 trials.
PD-L1 inhibitors
Atezolizumab is a human IgG1 approved for NSCLC and urothelial cancer. There are 488 trials based on atezolizumab therapy in the context of tumor therapy. 84 trials are in phases 3 or 4, and only 4 (NSCLC) are in phase 4. In a study conducted by Herbst et al., the efficacy and safety of the atezolizumab were investigated compared with the use of platinum-based chemotherapy [148]. Examination of the safety of atezolizumab in 615 patients with advanced NSCLC verified the benefit-risk profile of atezolizumab monotherapy in these patients [149]. Atezolizumab monotherapy also was well-tolerated in UC patients and resulted in an objective response of about 40% of patients with PD-L1 expression of at least 5% tumor-infiltrating immune cells [150]. Thereby, atezolizumab may offer durable anti-tumor activity in UC patients; however, further studies are warranted. Likewise, systemic administration of atezolizumab 1–20 mg/kg or 1200 mg led to objective response up to 50% of NSCLC patients with acceptable safety profile [151]. Notably, the ameliorated responses and survival rates were realized with augmenting baseline PD-L1 expression as expected [151].
Another PD-L1 inhibitor, avelumab, has been approved for MCC and UC. In 2019, the FDA approved avelumab combined with axitinib for the first-line treatment of people with advanced RCC [152]. Among 184 registered trials, 17 studies are in phase 3 (NSCLC, MCC, ovarian cancer, SCC, DLBCL, and RCC), while no study is in phase 4. Powles et al. used the avelumab in patients with UC and showed that the avelumab had a tolerable safety profile and clinical activity among these patients [64] (NCT02603432). Also, avelumab significantly improved the OS rate among patients with advanced or metastatic breast cancer [153] (NCT01772004). Moreover, Kim et al. determined the effect of avelumab in patients with relapsed or refractory extranodal natural killer/T-cell lymphoma [154] (NCT03439501). They found that the response to the avelumab was affected by the expression of PD-L1 in tumor tissues. They emphasized that assessing PD-L1 expression in tumor cells can help identify the responders to the PD-L1 antibody [154]. Also, this antibody has shown a significant antitumor effect and acceptable safety in patients with recurrent or refractory ovarian cancer [155, 156].
Durvalumab is another FDA-approved PD-L1 inhibitor, a monoclonal antibody of isotype IgG1, verified for NSCLC and urothelial carcinoma [61, 157]. Among 508 registered trials for this ICI, 47 studies are in phases 3 or 4, and 5 studies (NSCLC) are in phase 4. Antonia et al. assessed the durvalumab as consolidation therapy after the chemoradiotherapy in stage III NSCLS chemoradiotherapy (NCT02125461) (NCT02125461). This antibody made a significantly longer PFS in patients receiving the durvalumab than in patients receiving placebo [158, 159]. Also, durvalumab as consolidation therapy after the chemoradiotherapy has supported promising outcomes in patients with stage III NSCLC [160] (NCT02125461). Meanwhile, durvalumab monotherapy (10–20 mg/kg) was well-tolerated in Japanese patients with advanced solid tumors [161]. However, a significant anti-tumor effect was not observed [161]. Nonetheless, its administration into 70 patients with endometrial cancer provided an objective response in 47% of treated patients [162]. Also, the regimen led to improvement in OS rate12-month approximately 71% in endometrial cancer patients with dMMR with some managable grades 1–2 adverse events [162]. These findings delivered proof of the concepts that targeting PD-1/PDL-1 interaction can be a rational and effective therapeutic strategy for endometrial cancer. Notably, the higher antitumor efficacy of durvalumab administration in cancer with dMMR compared with cancers with MMR-proficient (MMRp) elucidates the robust rationale behind the application of immunotherapy in these subgroups of patients [162]. PD-L1 expression levels also impact therapeutic outcomes following durvalumab therapy, apart from MMR status. In this light, a recent phase 2 trial (NCT02207530) in recurrent/metastatic (R/M) HNSCC patients indicated that patients with ≥ 25% of tumor cells expressing PD-L1 exhibited favored responses to durvalumab therapy (10 mg/kg intravenously) [163]. Significant antitumour activity (e.g., ORR about 16.2%) along with acceptable safety profile in PD-L1-high patients with R/M HNSCC justified durvalumab ongoing evaluation in phase III trials [163]. Because the HPV-positive patients displayed better response and survival to durvalumab therapy than HPV-negative patients, it is highly recommended that the types of cancer (HPV-related HNSCCs and HPV-unrelated HNSCCs) be assessed before selecting patients for treatment irrespective of the PD-L1 expression patterns and MMR status [163].
Other PD-L1 inhibitors like envafolimab are in phase 1 and 2 clinical trials. Envafolimab is a first-in-class nanobody which its efficacy and also safety has recently been evidenced following subcutaneous injection in previously treated advanced dMMR/MSI-H solid tumors [164]. It was suggested that envafolimab could be an alternative to systemic administration of PD-1/PD-L1 inhibitors for advanced, refractory solid tumors therapy [165]. BMS-936559 (NCT01455103) and SHR-1316 (HTI-1088) (e.g., NCT04647357, NCT03474289, NCT05082545) (two fully humanized IgG4 monoclonal antibodies), and also CK-301 (NCT03212404 and NCT04786964), BGB-A333 (NCT03379259), CBT-502 (TQB-2450) (e.g., NCT05111366, NCT05013697, NCT04665609) and CS-1001 (e.g., NCT03744403, NCT04472858, NCT03789604), which are fully human monoclonal antibody of IgG1, are other PD-L1 inhibitors, which evaluation of their safety and efficacy is being studied. Meanwhile, SHR-1316 administration demonstrated promising outcomes in esophageal squamous cell carcinoma (ESCC) [166]. Of course, most ongoing studies are related to CBT-502, while the results are not yet available.
Current challenges
Although ICIs are at the forefront of immunotherapy for various cancers, they fail to modify tumor progress in a significant proportion of patients or arouse several serious adverse events.
Toxicities of immune checkpoint inhibitors
Unfortunately, ICIs therapy has also been associated with the occurrence of some immune-related untoward events, which diverge among patients based on the agent, malignancy, and individual susceptibilities [167]. Skin and colon are the most mutual organs, while the liver, lungs, kidneys, and heart are negatively affected by ICIs. Invariably, such toxicities are detected by excluding other secondary infectious or inflammatory underlies [168]. Corticosteroids are generally utilized to alleviate moderate and severe immune-related unwanted events, whereas additional immunosuppressive modalities may sometimes be required [169, 170]. The incidence of such toxicities may necessitate cessation of immunotherapy regarding the specific toxicity and its severity.
CTLA-4 adjusts the competence of immunologic response at early stages of T-cell induction, while PD-1 and PD-L1 pathways perform at later stages, preventive T-cell function in the peripheral tissues [171, 172]. Such differences partially explain anti-CTLA-4, anti-PD-1, and anti-PD-L1 [172, 173]. Overall, common ICIs related toxicities includes systemic toxicities (fatigue, fever, chills, and infusion reactions), dermatological toxicities (rash or pruritus), gastrointestinal (GI) toxicities (diarrhea, hepatitis and colitis), endocrine toxicities (thyroid dysfunction, hypophysitis, adrenal insufficiency and type 1 diabetes mellitus), pulmonary toxicities (dyspnea, cough, wheezing, and increased supplemental oxygen requirement), rheumatologic toxicities (inflammatory arthritis, inflammatory myositis, rhabdomyolysis, giant cell arteritis, and polymyalgia-like syndrome), neurologic toxicities (motor or sensory peripheral neuropathies, myasthenia gravis-like syndrome, aseptic meningitis, autoimmune encephalitis, posterior reversible encephalopathy syndrome, and transverse myelitis), ocular toxicities (conjunctivitis, episcleritis, keratitis, blepharitis, and uveitis), renal toxicities (acute interstitial nephritis, lupus-like nephritis, granulomatous nephritis, diffuse interstitial nephritis), cardiac toxicities (myocarditis, pericarditis, arrhythmias and heart block) and also hematologic toxicities (anemia) [29, 173].
Based on recent reports, the occurrence of extreme immune-related adverse events (irAEs) has been observed to be as high as 27% with the use of CTLA-4 blockade in comparison to 16% with PD-1 blockade. It may improve to 55% once both therapies are applied concomitantly [125]. The incidence of specific toxicities diverges according to the types of malignancy or used ICI. Meanwhile, patients with melanoma seem to demonstrate higher degrees of rash and colitis and lower pneumonitis than RCC and NSCLC [174]. Moreover, CTLA-4 blockade may result in higher degrees of colitis, hypophysitis, and inflammation, while pneumonitis, thyroiditis/hypothyroidism, arthralgias, and vitiligo are associated with PD-1 blockade [174, 175]. Notably, irAEs are dose associated with anti-CTLA-4 antibodies but not anti-PD-1 antibodies [176].
Tumor resistance to ICIs
Nowadays, it is universally accepted that transformed cells shape tight interactions with the extracellular matrix (ECM), stromal cells, and immune cells composing TME. These components of the TME organization facilitate a chronic inflammatory, immunosuppressive, and pro-angiogenic intratumoral milieu, which ultimately supports cancer cell escape and eradication by the host immune system [177, 178]. DCs must properly activate T cells in the peripheral lymph nodes to eliminate cancer cells in the tumor site and penetrate barriers (such as stromal tissue) [179, 180]. Emerging cancer frequently avert such requirements for T cell immunosurveillance to deter immune-mediated tumor regression. Because ICIs therapy's efficacy is primarily motivated by T cells, this efficient immune escape can finally underlie failures in ICIs treatment. A spectrum of studies has implied that upregulation of PD-L1 in the TME by cancer cells and APCs is one the most shared strategy by which cancers circumvents immune surveillance [181, 182]. Besides, the catabolism of tryptophan inside the TME plays an influential role in suppressing anti-tumor immune reactions. The IDO often catabolizes tryptophan in myeloid cells and tumor cells to produce immunosuppressive metabolites such as kynurenine [183]. The kynurenine activities associated with the exhaustion of the crucial amino acid tryptophan are broadly complicated in obstruction of clonal expansion of T cells. Also, they can provoke T cell anergy and apoptosis [183]. Therefore, a combination of IDO inhibitors and treatment of ICIs has been proposed to enforce TILs and their functional capabilities in TME and thus eradicate either IDO-expressing or nonexpressing poorly immunogenic cancer cells [184]. Some clinical trials are being conducted to address the safety and efficacy of IDO blockade plus ICIs (NCT02073123, NCT01604889, and NCT02327078). Besides, the presence of Treg cells, Th2 cells, and MDSCs is another pivotal obstacle to ICIs therapies by inhibition of ICI-mediated anti-tumor CTL and Th1 cell responses [185, 186]. Exhaustion of such cell types, as might be plausible for Treg cells [187], can be employed together ICIs therapies to offer more appropriate outcomes. In addition, activation of some oncogenic factors, such as the WNT-β-catenin signaling pathway, severely inhibits the infiltration of TILs and CD103+ DC into TME by suppressing β-catenin-associated CCL4 chemokine in melanoma, thereby facilitating tumor escape [188, 189]. These reports have outlined the importance of developing an innovative plan for combination therapy with ICIs and other therapeutic modalities.
Expression of cyclooxygenase (COX) at high levels is another mechanism contributed to immune suppression in TME by the production of prostaglandin E2 (PGE2), which offers an inflammatory environment favoring tumor proliferation [190, 191]. Assessment of tumor and immune cell glucose and glutamine metabolism has led to the raising evidence signifying that the metabolic interaction between the tumor cells and immune cells can sustain the poor response to ICIs [192]. Indeed, such metabolism promotes the expression of PD-L1 in tumor cells by the epidermal growth factor receptor (EGFR)/extracellular signal-regulated kinase (ERK)/C-Jun pathway [192]. Thereby, the targeting of tumor glucose or glutamine metabolism in combination with PD-1/PD-L1 targeting looks to serve new therapeutic chances for patients with tumors.
Combination therapy with ICIs
The success of ICIs in patients with a diversity of human malignancies has evolved tumor immune therapies. At the same time, combination therapies with ICIs are always required to circumvent resistance and expand the clinical application of immunotherapy. Making crucial progress in combination therapy with ICI is of paramount importance given that only a small proportion of patients respond to ICI therapy, and many will relapse [193, 194]. Numerous clinical trials have been conducted to address the safety and efficacy of ICI in combination with conventional cancer therapies, targeted molecular compounds, and new immunomodulatory therapies [195]. In addition to the combination therapy with other therapeutic modalities, the combination of CTLA-4 and PD-1 blockers has been suggested to have a synergistic impact on eliciting the anti-tumor activities and consequently lessening the response rates in cancer patients [196, 197]. The therapeutic efficacy of combinational schemes has been determined by recent FDA approval of nivolumab plus ipilimumab for patients with advanced melanoma [198]. In this regard, the nominated combination therapy regimens include nivolumab plus ipilimumab (e.g., NCT02905266, NCT02998528, NCT02872116, and NCT02477826), pembrolizumab plus ipilimumab (e.g., NCT03302234, NCT04571632, and NCT04571632), atezolizumab plus ipilimumab (NCT02174172 and NCT04084951), and tremelimumab plus durvalumab (NCT02701400 and NCT02516241). Such trials mainly have concentrated on survival and other treatment response indices. At the same time, a restricted number of them explore the safety profile and maximum tolerable dose (MTD) of combination therapy protocols [198, 199]. Also, various studies have inspected combination ICI with traditional treatment means and plans such as chemotherapy, radian therapy, angiogenesis-inhibitors, and cancer vaccines (e.g., oncolytic viruses).
Chemotherapy
Several investigations have been conducted or executed to evaluate the therapeutic potential of combination therapy with chemotherapy and ICIs. Also, a large number of studies have evidenced the therapeutic capacity of ICI plus chemotherapeutic agents, including cyclophosphamide [200, 201], fluorouracil [200], gemcitabine [202, 203], doxorubicin [204], oxaliplatin [205, 206], cis-platin [204, 207], paclitaxel [208], methotrexate [204] and vinblastine [204] in preclinical models (Table 5). It seems that chemotherapy with ICI may result in improved activation of APC, which elicits T-cell-related antitumor effect leading to the suppressed metastatic tumor growth, reducing the immunosuppressive M2 macrophage, Tregs and MDSCs population, and finally improving the expression of the IFNβ, and CCL5 and CXCL10 [204, 207, 209, 210]. Such events promote the OS rate in treated animals by enhancing the host immune system's recognition and eradication of tumor cells and concurrently condenses the immunosuppressive TME. Based on the result achieved from clinical trials, combination therapy is also mainly well-tolerated, and several trials have attained durable responses [211]. Though chemotherapy and ICB are mainly used concomitantly and at full doses, some trials have assessed the optimal dose or sequence of administration [211]. In 2018, the FDA approved atezolizumab combined with bevacizumab, paclitaxel, and carboplatin for the first-line treatment of patients with NSCLC [212]. Approval was concerning the open-label phase 3 study (NCT02366143) on 1202 patients with NSCLC who had not previously received chemotherapy [58]. As cited in previous sections, Socinski et al. showed that atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin considerably promoted PFS and OS among patients with metastatic NSCLC [58]. Another randomized, open-label, phase 3 trial (NCT03215706) has also shown that nivolumab plus ipilimumab with two cycles of chemotherapy may offer an acceptable improvement in OS compared with chemotherapy alone and also has a suitable risk–benefit profile in NSCLC patients [213]. As well, some other trials based on ICI plus chemotherapeutic agents, such as cisplatin and pemetrexed or docetaxel, are being carried out on patients with NSCLC, gastric carcinoma, head and neck cancer, and also human papillomavirus-associated oropharyngeal squamous cell carcinoma (HPV(+)OPSCC) (e.g., NCT04945200, NCT04997382, NCT04062708, NCT04867330, NCT03532737, NCT04908566, and NCT04891016).
Table 5.
Immune checkpoint inhibitors (ICIs) combination therapy with chemotherapy (animal study)
| Tumor | ICI type | Chemotherapeutic agent | Main result | References |
|---|---|---|---|---|
|
Breast tumor Ovarian tumor |
PD-L1 | Cyclophosphamide | Selective depletion of Treg in the tumor tissue in vivo | [201] |
|
Breast tumor Lymphoma |
PD-L1 |
Cyclophosphamide Fluorouracil Vinorelbine |
Activation of circulating and tumor-infiltrating immune cells in vivo | [200] |
| Breast cancer | PD-1 |
Cyclophosphamide Vinorelbine |
Activation of APC, and eliciting T-cell-related effect leading to the suppressed metastatic tumor growth in vivo | [209] |
| Pancreatic ductal adenocarcinoma (PDA) | PD-1 | Gemcitabine | Restoring the tumor cell sensitivity to ICI in vivo | [202] |
| Mesothelioma | PD-1 | Gemcitabine |
Hindrance of tumor development in vivo Improving the overall survival of treated models in vivo |
[203] |
| Lewis lung carcinoma (LLC) | PD-1 | Gemcitabine | Arousing strong anti-tumor effect in vivo | [292] |
|
Colon cancer Bladder cancer |
PD-1 PD-L1 |
Methotrexate Vinblastine Doxorubicin Cis-platin Cyclophosphamide |
Convincing robust anti-tumor response in vivo | [204] |
|
Colon cancer Renal carcinoma |
CTLA-4 | Cyclophosphamide | Augmentation of the antitumor effect of anti-CTLA-4 therapy in vivo | [293] |
| Pancreatic cancer | PD-1 | Gemcitabine | Enhancing the anticancer effect of M1 macrophages and the Th1 response in vivo | [294] |
| Small cell lung cancer (SCLC) | PD-L1 | Gemcitabine |
Restoring the antitumorigenic CD8+ cytotoxic T cells, dendritic cells, and M1 macrophage populations in vivo Reducing the immunosuppressive M2 macrophage and MDSCs population in vivo Increasing the expression of the IFNβ, and CCL5 and CXCL10 in vivo |
[210] |
| Lung cancer | PD-L1 | Oxaliplatin | Activation of dendritic cells (DCs CD80+ CD86+) and CD8+ T cells resulted in tumor regression in vivo | [206] |
| Colon cancer | PD-1 |
Cis-platin Oxaliplatin |
Triggering T cell activation and recruitment into tumors in vivo | [205] |
| Triple-negative breast cancer (TNBC) | PD-L1 | Paclitaxel | Provoking the tumor regression, and inhibition of tumor metastasis in vivo | [208] |
| TNBC | PD-L1 | Paclitaxel | Inducing the TILs infiltration into TME in vivo | [295] |
| Fibrosarcoma | PD-1 | Methotrexate | Robust therapeutic effect in vivo | [296] |
Programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4), interferon-beta (IFNβ), regulatory T cells (Tregs), C-X-C chemokine receptor type 10 (CXCR10), tumor microenvironment (TME), tumor-infiltrating lymphocytes (TILs), C–C chemokine receptor type 5 (CCR5), antigen-presenting cell (APC)
Radiotherapy
Previously, the aptitude of ionizing radiation underlies cell death, and the inflammatory response has been indicated [214]. However, such attributes of radiation have enticed the evolving attention to arouse or improve antitumor immunity [214]. Researchers have observed unexpected out-of-the-field (abscopal) responses in patients receiving radiation therapy throughout immunotherapy [215]. Increasing evidence has delivered the proof of the theory that ICIs combination therapy with radiotherapy can convince more preferred anti-tumor response versus tumor cell primarily by triggering immunogenic cell death and resultant infiltration and activities of T cells within the TME [216, 217]. Radiotherapy may restore the antitumor influence of immune ICIs by eliciting endogenous danger signals and cytokines, augmenting the presentation of tumor-associated antigens (TAA) on APC along with inspiring T cell anti-tumor immunity [218, 219]. Correspondingly, in the HCC mice model, radiotherapy plus anti-PD-L1 promoted CD8+ T cell infiltration and activation, supported enhanced interferon IFN-γ production potential of TILs, and enticed a lessening trend in Tregs and exhausted T cells [220]. Another report exemplified that PD-L1 and IDO were stimulated on tumor epithelia of pancreatic ductal adenocarcinoma (PDAC) cells following radiotherapy, suggesting that radiation therapy may prime PDAC for PD-1 blockade therapy or IDO inhibitor treatments. Moreover, such combination treatment led to the more excellent systemic IFN-γ response and a local expression of immune-activation genes, including CD28 and ICOS, than monotherapy [221]. Furthermore, evaluation of the safety and efficacy of the radiotherapy plus ICI in 59 patients, mainly with RCC or melanoma treated with radiotherapy during or within eight weeks of ICI administration, signified this therapeutic modality's safety and modest efficacy [222]. Similarly, another study on 133 cancer patients revealed that a combination of focal palliative radiation and CTLA-4 and/or PD-1 inhibitors were well tolerated, with manageable irAEs [223]. In contrast, other reports indicating that radiotherapy plus ICI may result in reduced median OS, median PFS, and median time‑to‑treatment failure compared with monotherapy with ICIs have described the importance of the execution of further studies [224]. Now, several studies based on ICIs plus radiotherapy are being carried out on patients with cHL, HNSCC, NSCLC, SCC, RCC, melanoma, pancreatic cancer, breast cancer, and also bladder cancer (e.g., NCT0441944, NCT04892849, NCT04454489, NCT04793737, NCT03275597, NCT04454528, NCT02311361, and NCT03693014).
Cancer vaccines
Therapeutic cancer vaccines facilitate tumor regression, elimination of minimal residual disease (MRD), and also establishing the long-term antitumor memory and avoiding non-specific or adverse reactions [225, 226]. To date, FDA has approved three vaccines, including Bacillus Calmette-Guérin (BCG) live, sipuleucel-T, and also talimogene laherparepvec (TVEC) for patients with early-stage bladder cancer, prostate cancer, and melanoma, respectively [227]. The FDA has approved the BCG Live (Intravesical) from 1990 in primary or recurrent UC upon transurethral resection [228]. Further, Sipuleucel-T gained approval from the FDA in April 2010 as autologous cellular immunotherapy used to treat metastatic castration-resistant prostate cancer (mCRPC) [229]. The FDA approved TVEC, a genetically modified oncolytic viral therapy in 2015 for advanced melanoma therapy [230]. Recently, shreds of preclinical evidence suggest that a combination of vaccines and ICIs may enhance immunogenicity and inhibit the immunosuppressive TME [231]. Cancer vaccines can bring about tumor-specific T cells in the periphery or situ tumors and ease infiltration of activated peripheral T cells into the TME [231]. Besides, vaccine-induced tumor cell elimination provokes the secretion of more cascade antigens and convinces more robust immune reaction specific to antigens not included within the vaccine, a phenomenon known as antigen cascade or epitope spreading [232]. So, the theory suggested that better ICI treatment efficacy may be attained by optimizing tumor immunogenicity or host immune reaction with vaccines [231].
In vivo, GM-CSF cell-based vaccines (GVAX) plus anti-CTLA-4 antibody has shown synergistic impacts in attenuating tumor size and restoring the antitumor immune reactions in melanoma [233] and also prostate [234] mice model. The DC tumor lysate-based vaccine plus PD-1 blockade also caused improved survival in glioma cell-bearing mice [235]. Besides, PD-1 blockade plus GVAX vaccine intensified survival and improved T-cell activity in mice with PDA [236]. On the other hand, a recent clinical trial (NCT01302496) showed that DC-based mRNA vaccination plus ipilimumab could lead to intense CD8+ T-cell responses in stage III or IV melanoma patients [237]. In addition, a study of the safety and efficacy of ipilimumab plus GVAX in 30 patients with advanced PDA previously treated showed that the intervention could, in some cases, improve the median OS compared to ipilimumab monotherapy [238]. Moreover, the efficacy of nivolumab was augmented by treatment with ISA 101, a synthetic long-peptide HPV-16 vaccine containing HPV-specific T cells, in patients with incurable HPV-16-positive tumors [239]. Based on the analysis, the overall response rate (ORR) of 33% and median OS of 17.5 months was more encouraging than monotherapy with nivolumab in similar patients [239].
Oncolytic viruses (OVs), as well-known types of cancer vaccines, are tumor-selective, multi-mechanistic antitumors [240, 241]. They lyse directly infected cancerous and endothelial cells while non-infected cells are destroyed by targeting cancer vessels and the bystander effect. Multimodal immunogenic cell death (ICD) accompanied with autophagy, which often is prompted by OVs, provides dominant danger signals to DCs and also supports cross-present tumor-associated antigens (TAAs) from tumor cells to DCs to T cells to trigger adaptive antitumor immunity [242]. Respecting the promising immune background, genetic engineering of OVs, and rational combinations efficiently can potentiate OVs as cancer vaccines [243, 244]. Notably, ICIs combination therapy with OVs has persuaded promising results in preclinical models primarily by stimulation of macrophage influx and M1-like polarization and stimulating recruitment and activities of T effector cells, improving IFN-γ levels in TME, and finally down-regulation of Treg and MDSCs density and activity [245, 246] (Table 6). Also, multiple ongoing clinical trials are currently combining OVs with ICIs (NCT02798406 and NCT03206073).
Table 6.
Immune checkpoint inhibitors (ICIs) combination therapy with oncolytic viruses (OVs) (animal study)
| Tumor | Target ICs | OVs type | Main result | References |
|---|---|---|---|---|
| Glioma |
CTLA-4 PD-1 |
IL-12-expressing oHSV | Induction of macrophage influx and M1-like polarization and improving T effector (CD4+ and CD8+ T cells) to T regulatory cell ratio | [297, 298] |
| Rectal cancer | PD-1 | hTERT-expressing oAd | Tumor regression by recruitment of CTLs | [299] |
| Osteosarcoma | PD-1 | hTERT-expressing oAd | Tumor regression by recruitment of CTLs | [299] |
| Breast cancer |
PD-1 CTLA-4 |
Soluble TGFβRIIFc-expressing oAd | Inhibition of tumor growth and lung and liver metastases | [300] |
|
Lung cancer Breast cancer Melanoma Lymphoma |
PD-1 PD-L1 CTLA-4 |
GM-CSF-expressing oHSV-1 | Tumor regression and also induction of immunological memory | [300] |
| Glioblastoma multiforme (GBM) | PD-1 | ZIKV | Improved survival of treated animals | [301] |
| Rhabdomyosarcoma | PD-1 | oHSV | Amelioration of incidence of CD4+ and CD8+ T cells but not Treg populations in the tumor | [302] |
| Melanoma | PD-L1 | oHSV |
Enhancing IFNγ-producing CD8+ TILs Improved survival of treated animals |
[303] |
| Lung adenocarcinoma | PD-1 | oAd | Inhibition of tumor cell dissemination in a CD8 T-cell-dependent manner | [304] |
| Melanoma |
PD-1 PD-L1 CTLA-4 |
CD40L-expressing oAd | Increasing the systemic level of tumor-specific CD8+ T cells, and also promoting the ratio of intratumoral CD8+ T cells to Treg | [245] |
| GBM | PD-L1 | CD40L-expressing oAd | Inhibition of tumor development associated with increased survival | [305] |
| Prostate cancer | PD-1 | oAd | Induction of antigen-specific CD8+ T-cell responses in mice | [306] |
| Melanoma | PD-1 | oAd | Delayed tumor growth leading to the boosted survival of treated animal | [307] |
| Melanoma | PD-1 | Reovirus | Enhanced capacity of NK cells to eliminate reovirus-infected tumor cells, abridged Treg activity and augmented the CD8+ T-cell-mediated antitumor response | [308] |
| GBM | PD-1 | Reovirus | Improving the expression of IFN-regulated gene expression, as well as the PD-1/PD-L1 axis in tumors | [309] |
| GBM |
PD-1 CTLA-4 |
HIF-2α, Sox-10, c-Myc, and TRP1-expressing VSV | Restoring the antitumor Th1 interferon-γ and Th17 T cell responses | [310] |
| Melanoma | PD-L1 | MV | Inducing tumor remission | [311] |
| GBM | PD-1 | EGFR-expressing MV |
Improved inflammatory cell influx into the brains of treated mice Enhanced overall survival in treated animal |
[312] |
Programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4), interferon-gamma (IFNγ), regulatory T cells (Tregs), tumor-infiltrating lymphocytes (TILs), natural killer (NK) cell, cytotoxic T lymphocytes (CTLs), oncolytic herpes simplex virus (oHSV), oncolytic adenovirus (oAd), measles virus (MV), vesicular stomatitis virus (VSV), zika virus (ZIKV), human telomerase reverse transcriptase (hTERT), transforming growth factor-beta receptor 2 fused with Fc protein (TGFβRIIFc), granulocyte–macrophage colony-stimulating factor (GM-CSF), Hypoxia-inducible factor-2α (HIF-2α), SRY-related HMG-box 10 (SOX10), epidermal growth factor receptor (EGFR)
Anti-angiogenic agents
Abnormal vasculature is one of the most renowned properties of solid tumors and contributes to tumor immune evasion [247]. This aberration arises from the enhancement in the expression of pro-angiogenic factors, chiefly contributed to the regulation of the function and migration of immune cells [247]. Anti-angiogenic compounds thereby can normalize blood vessels and transform the TME from immunosuppressive to immune-supportive by heightening the infiltration and stimulation of immune cell activities. Axitinib, bevacizumab, cabozantinib, everolimus, lenalidomide, lenvatinib mesylate, pazopanib, ramucirumab, regorafenib, sorafenib, sunitinib, thalidomide, vandetanib and Ziv-aflibercept are FDA-approved angiogenesis inhibitors [248]. Recently, Wang et al. supposed that anti-angiogenesis therapy may defeat the innate resistance to PD-1/PD-L1 blockade in vascular endothelial growth factor A (VEGF-A)-overexpressed mice tumor models [249]. The safety and efficacy of combination immunotherapies with ICIs and bevacizumab, a humanized monoclonal antibody binding to VEGF-A, has been evaluated in clinical trials to treat patients with NSCLC nasopharyngeal carcinoma and also metastatic CRC (NCT04997382, NCT04872582, and NCT04659382). As described, atezolizumab, in combination with bevacizumab, paclitaxel, and carboplatin, is the first-line FDA-approved treatment for patients with NSCLC [250]. Moreover, bevacizumab has exposed a synergistic effect with atezolizumab on the median OS of patients with RCC [251]. Also, evaluation of combined nivolumab and bevacizumab activity in 38 women with relapsed ovarian cancer during a phase 2 study indicated that 11 patients showed an objective response (OR) to nivolumab plus bevacizumab. In contrast, nine patients exhibited a grade 3 or higher treatment-associated unwanted event [252]. The efficacy of the intervention was more eminent in the platinum-sensitive setting, and thereby an alternative combinational strategy seems to be required in the platinum-resistant setting [252].
In addition to the chemotherapeutic agent, radiotherapy, OVs, other cancer vaccines, and anti-angiogenic agents, other therapies such as CXCR4 blockade [253, 254] have demonstrated great application capacity to combination with ICI, circumventing tumor resistance to ICIs.
Conclusion and prospect
As described, ICI has been pronounced as a game changeling toll to treat even metastatic or chemoresistant malignancies with unfavorable prognoses. New inhibitory checkpoints and their target molecules are being studied to evolve the application and efficacy of current immune checkpoint inhibition therapy. Notwithstanding, such novel treatments are not effective sufficient to be utilized alone but can improve the activity of existing therapy. Albeit, this synergism may cause a boosted occurrence and severity of irAEs. The irAEs associated with ICI therapies underlies significant morbidity for patients and the considerable cost to the healthcare system, restricting their utility in the clinic.
Meanwhile, overactivation of the immune system may result in autoimmune-like side effects, thus finally perturbing organ functions and necessitating discontinuation of therapy, hospital admission, or management with systemic immunosuppressive compounds [29, 172]. Understanding the corresponding mechanism of these untoward events and how they can be distinguished from the antitumor impacts of ICI and recognising additional biomarkers that predict the incidence of irAEs will enable further trials to hinder their onset facilitate patient outcomes. Also, ‘on-target off-tumor’ impacts have recently been pronounced, thereby the possible influences of ICIs on healthy tissue remain disquiet. Concerning the mechanism of action, earlier clinical trials usually excluded patients with an underlying autoimmune disorder such as inflammatory bowel diseases (IBD) and arthritis. It has not yet been proven that the existence of these diseases can cause complete contraindication. Whether patients with chronic immunosuppression will respond to treatment has not yet been answered. In addition, various types of ICIs did not exhibit robust activity in ‘cold’ TMEs. Approaches to stimulate a shift to ‘hot’ TMEs may augment the efficacy and grow the use of these therapies. Though numerous clinical trials evidenced that elevating the dose has no statistically substantial impact on tumor response, more investigation into optimal ICI dosing approaches and progress of dose escalation plans may be justified. Comprehensive studies are urgently required to identify biomarkers that could aid select patients who may advantage the most while also ducking robust toxicities. Respecting current evidence, tumors exhibiting a high PDL-1 expression level and TMI, MSI, or dMMR have higher response rates to PD-1 blockade [255].
Also, the frequency of tumor-infiltrating lymphocytes (TILs) is suggested as a potent indicator of response to ICIs. In this context, PD-L1 protein expression on tumor or immune cells arose as the chief potential prognostic biomarker for sensitivity to ICIs. A growing body of evidence infers that higher baseline PD-L1 and/or PD-1 on peripheral blood T cells is a sign that increases the probability of successful treatment with PD-1 inhibitors. Patients with a higher quantity of PD-L1+ T cells typically show a more favored objective response to PD-1 inhibitor therapy. In comparison, patients with a lower portion of regulatory T cells at baseline mainly demonstrate more irAEs [256]. 9 FDA drug approvals related to a specific PD-L1 threshold and companion diagnostic in various cancers, such as bladder cancer, NSCLC, TNBC, cervical cancer, and gastric/gastroesophageal junction cancer [257]. Of course, combination therapies using ICIs and chemotherapy or other therapeutic modalities may further constrain the predictive value of PD-L1 expression. Hence, additional studies are needed to institute a consistent and dynamic predictive biomarkers scheme that may vary across tumor types and indications. The scientist must be cautious about applying the ICIs linked to PD/PD-L1 status in the suitable, FDA-approved setting.
Acknowledgements
Not applicable.
Abbreviations
- ICIs
Immune checkpoint inhibitors
- CTLA-4
Cytotoxic T-lymphocyte antigen-4
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-ligand 1
- TME
Tumor microenvironment
- Tregs
Regulatory T cells
- MDSCs
Myeloid-derived suppressor cells
- TILs
Tumor-infiltrating lymphocytes
- CTLs
Cytotoxic T lymphocytes
- APCs
Antigen-presenting cells
- DCs
Dendritic cells
- IFNγ
Interferon-gamma
- OS
Overall survival
- irAEs
Immune-related adverse event
- PFS
Progression-free survival
Author contributions
All authors contributed to the conception and the main idea of the work. AN, AR, AVY, SC, WS, SK, NS and MNS drafted the main text, figures, and tables. ER supervised the work and provided the comments and additional scientific information. AN and ER also reviewed and revised the text. All authors read and approved the final version of the work to be published. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors certify that no competing interests exists in this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adel Naimi, Email: naeimia@medsab.ac.ir.
Rebar N. Mohammed, Email: rebar.mohammed@sulicihan.edu.krd
Ahmed Raji, Email: dr.ahmedraji@uobabylon.edu.iq.
Supat Chupradit, Email: supat.c@cmu.ac.th.
Alexei Valerievich Yumashev, Email: sfmsmu@mail.ru.
Wanich Suksatan, Email: wanich.suk@pccms.ac.th.
Mohammed Nader Shalaby, Email: dr.m.nader@suez.edu.eg.
Lakshmi Thangavelu, Email: Lakshmi@saveetha.com.
Siavash Kamrava, Email: kamrava.siavash@yahoo.com.
Navid Shomali, Email: shomali.navid@yahoo.com.
Armin D. Sohrabi, Email: arminsohrabi20@gmail.com
Ali Adili, Email: dradili@hotmail.com.
Ali Noroozi-Aghideh, Email: ali.agideh@gmail.com.
Ehsan Razeghian, Email: ehsan.razeghi@gmail.com.
References
- 1.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 3.Naidoo J, Page DB, Wolchok JD. Immune checkpoint blockade. Hematol Oncol Clin. 2014;28(3):585–600. doi: 10.1016/j.hoc.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Toor SM, Nair VS, Decock J, Elkord E, editors. Immune checkpoints in the tumor microenvironment. Seminars in cancer biology. Amsterdam: Elsevier; 2020. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen JF, Van Hecke W, Adriaansen EJ, Jansen MK, Bouma RG, Villacorta Hidalgo J, et al. Prognostic relevance of tumor-infiltrating lymphocytes and immune checkpoints in pediatric medulloblastoma. Oncoimmunology. 2018;7(3):e1398877. doi: 10.1080/2162402X.2017.1398877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195–207. doi: 10.1038/nrendo.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyersdorf N, Kerkau T, Hünig T. CD28 co-stimulation in T-cell homeostasis: a recent perspective. ImmunoTargets and therapy. 2015;4:111. doi: 10.2147/ITT.S61647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183(6):2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9(3):396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 10.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3(12):939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 11.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14(2):145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228(1):342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 14.Lee K-M, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282(5397):2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 15.Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nat Rev Drug Discov. 2011;10(6):411–413. doi: 10.1038/nrd3463. [DOI] [PubMed] [Google Scholar]
- 16.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, et al. Blockade of CTLA-4 on CD4+ CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177(7):4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Investig. 2015;125(9):3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okazaki T, Honjo T. The PD-1–PD-L pathway in immunological tolerance. Trends Immunol. 2006;27(4):195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Patsoukis N, Duke-Cohan JS, Chaudhri A, Aksoylar H-I, Wang Q, Council A, et al. Interaction of SHP-2 SH2 domains with PD-1 ITSM induces PD-1 dimerization and SHP-2 activation. Commun Biol. 2020;3(1):1–13. doi: 10.1038/s42003-020-0845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veluswamy P, Wacker M, Scherner M, Wippermann J. Delicate role of PD-L1/PD-1 axis in blood vessel inflammatory diseases: current insight and future significance. Int J Mol Sci. 2020;21(21):8159. doi: 10.3390/ijms21218159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Zeng Y, Qu Q, Zhu J, Liu Z, Ning W, et al. PD-L1 induced by IFN-γ from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int J Clin Oncol. 2017;22(6):1026–1033. doi: 10.1007/s10147-017-1161-7. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y, et al. Interferon-γ-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. 2012;217(4):385–393. doi: 10.1016/j.imbio.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Imai Y, Chiba T, Kondo T, Kanzaki H, Kanayama K, Ao J, et al. Interferon-γ induced PD-L1 expression and soluble PD-L1 production in gastric cancer. Oncol Lett. 2020;20(3):2161–2168. doi: 10.3892/ol.2020.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book. 2019;39:147–164. doi: 10.1200/EDBK_240837. [DOI] [PubMed] [Google Scholar]
- 28.Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 2020;37(4):443–455. doi: 10.1016/j.ccell.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marin-Acevedo JA, Chirila RM, Dronca RS, editors. Immune checkpoint inhibitor toxicities. Mayo Clinic proceedings. Amsterdam: Elsevier; 2019. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118(1):9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Badran SS, Grant L, Campo MV, Inthagard J, Pennel K, Quinn J, et al. Relationship between immune checkpoint proteins, tumour microenvironment characteristics, and prognosis in primary operable colorectal cancer. J Pathol Clin Res. 2021;7(2):121–134. doi: 10.1002/cjp2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai C, Geng R, Wang C, Wong A, Qing M, Hu J, et al. Concordance of immune checkpoints within tumor immune contexture and their prognostic significance in gastric cancer. Mol Oncol. 2016;10(10):1551–1558. doi: 10.1016/j.molonc.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harter PN, Bernatz S, Scholz A, Zeiner PS, Zinke J, Kiyose M, et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget. 2015;6(38):40836. doi: 10.18632/oncotarget.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gennen K, Käsmann L, Taugner J, Eze C, Karin M, Roengvoraphoj O, et al. Prognostic value of PD-L1 expression on tumor cells combined with CD8+ TIL density in patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. Radiat Oncol. 2020;15(1):1–12. doi: 10.1186/s13014-019-1453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J-J, Zhou Z-Q, Wang P, Chen C-L, Liu Y, Pan Q-Z, et al. Orchestration of immune checkpoints in tumor immune contexture and their prognostic significance in esophageal squamous cell carcinoma. Cancer Manag Res. 2018;10:6457. doi: 10.2147/CMAR.S181949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmoudian RA, Mozhgani S, Abbaszadegan MR, Mokhlessi L, Montazer M, Gholamin M. Correlation between the immune checkpoints and EMT genes proposes potential prognostic and therapeutic targets in ESCC. J Mol Histol. 2021;52(3):597–609. doi: 10.1007/s10735-021-09971-3. [DOI] [PubMed] [Google Scholar]
- 37.Barriga V, Kuol N, Nurgali K, Apostolopoulos V. The complex interaction between the tumor micro-environment and immune checkpoints in breast cancer. Cancers. 2019;11(8):1205. doi: 10.3390/cancers11081205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papadaki MA, Koutsopoulos AV, Tsoulfas PG, Lagoudaki E, Aggouraki D, Monastirioti A, et al. Clinical relevance of immune checkpoints on circulating tumor cells in breast cancer. Cancers. 2020;12(2):376. doi: 10.3390/cancers12020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giraldo NA, Becht E, Pages F, Skliris G, Verkarre V, Vano Y, et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res. 2015;21(13):3031–3040. doi: 10.1158/1078-0432.CCR-14-2926. [DOI] [PubMed] [Google Scholar]
- 40.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 41.Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018;154(6):1416–1423. doi: 10.1016/j.chest.2018.08.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HW, Cho KJ, Park JY. Current status and future direction of immunotherapy in hepatocellular carcinoma: what do the data suggest? Immune Netw. 2020;20(1):e11. doi: 10.4110/in.2020.20.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michel L, Rassaf T, Totzeck M. Cardiotoxicity from immune checkpoint inhibitors. Int J Cardiol Heart Vasculature. 2019;25:100420. doi: 10.1016/j.ijcha.2019.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sur D, Havasi A, Cainap C, Samasca G, Burz C, Balacescu O, et al. Chimeric antigen receptor T-cell therapy for colorectal cancer. J Clin Med. 2020;9(1):182. doi: 10.3390/jcm9010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheng IY, Ornstein MC. Ipilimumab and nivolumab as first-line treatment of patients with renal cell carcinoma: the evidence to date. Cancer Manag Res. 2020;12:4871–4881. doi: 10.2147/CMAR.S202017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsang J, Wong JSL, Kwok GGW, Li BCW, Leung R, Chiu J, et al. Nivolumab + ipilimumab for patients with hepatocellular carcinoma previously treated with sorafenib. Expert Rev Gastroenterol Hepatol. 2021;15(6):589–598. doi: 10.1080/17474124.2021.1899808. [DOI] [PubMed] [Google Scholar]
- 47.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 49.Twomey JD, Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021;23(2):39. doi: 10.1208/s12248-021-00574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pai-Scherf L, Blumenthal GM, Li H, Subramaniam S, Mishra-Kalyani PS, He K, et al. FDA approval summary: pembrolizumab for treatment of metastatic non-small cell lung cancer: first-line therapy and beyond. Oncologist. 2017;22(11):1392–1399. doi: 10.1634/theoncologist.2017-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aragon-Ching JB. Pembrolizumab use in bladder cancer: a tale of two trials. Nat Rev Urol. 2021;18:577–578. doi: 10.1038/s41585-021-00499-5. [DOI] [PubMed] [Google Scholar]
- 53.US F. FDA approves pembrolizumab for first-line treatment of head and neck squamous cell carcinoma. Food and Drug Administration, Silver Spring. 2019.
- 54.Maly J, Alinari L. Pembrolizumab in classical Hodgkin's lymphoma. Eur J Haematol. 2016;97(3):219–227. doi: 10.1111/ejh.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto S, Kato K. Pembrolizumab for the treatment of esophageal cancer. Expert Opin Biol Ther. 2020;20(10):1143–1150. doi: 10.1080/14712598.2020.1792881. [DOI] [PubMed] [Google Scholar]
- 56.Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27(1):39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 57.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 59.Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, Shen Y-L, et al. FDA approval summary: atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin Cancer Res. 2021;27(7):1836–1841. doi: 10.1158/1078-0432.CCR-20-3407. [DOI] [PubMed] [Google Scholar]
- 60.Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395(10240):1835–1844. doi: 10.1016/S0140-6736(20)30934-X. [DOI] [PubMed] [Google Scholar]
- 61.Syed YY. Durvalumab: first global approval. Drugs. 2017;77(12):1369–1376. doi: 10.1007/s40265-017-0782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker M, Cordes L, Brownell I. Avelumab: a new standard for treating metastatic Merkel cell carcinoma. Expert Rev Anticancer Ther. 2018;18(4):319–326. doi: 10.1080/14737140.2018.1445528. [DOI] [PubMed] [Google Scholar]
- 63.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 64.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 65.Choueiri TK, Larkin J, Oya M, Thistlethwaite F, Martignoni M, Nathan P, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018;19(4):451–460. doi: 10.1016/S1470-2045(18)30107-4. [DOI] [PubMed] [Google Scholar]
- 66.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14(1):233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 67.Brunner-Weinzierl MC, Hoff H, Burmester G-R. Multiple functions for CD28 and cytotoxic T lymphocyte antigen-4 during different phases of T cell responses: implications for arthritis and autoimmune diseases. Arthritis Res Ther. 2004;6(2):45. doi: 10.1186/ar1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–2167. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 70.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1(1):32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 71.Pentcheva-Hoang T, Simpson TR, Montalvo-Ortiz W, Allison JP. Cytotoxic T lymphocyte antigen-4 blockade enhances antitumor immunity by stimulating melanoma-specific T-cell motility. Cancer Immunol Res. 2014;2(10):970–980. doi: 10.1158/2326-6066.CIR-14-0104. [DOI] [PubMed] [Google Scholar]
- 72.Zhang B, Dang J, Ba D, Wang C, Han J, Zheng F. Potential function of CTLA-4 in the tumourigenic capacity of melanoma stem cells. Oncol Lett. 2018;16(5):6163–6170. doi: 10.3892/ol.2018.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu L, Yun Z, Tagawa T, Rey-McIntyre K, De Perrot M. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther. 2012;11(8):1809–1819. doi: 10.1158/1535-7163.MCT-11-1014. [DOI] [PubMed] [Google Scholar]
- 74.Zhang L, Wang J, Jiang J, Zhang M, Shen J. CTLA-4 blockade suppresses progression of residual tumors and improves survival after insufficient radiofrequency ablation in a subcutaneous murine hepatoma model. Cardiovasc Intervent Radiol. 2020;43(9):1353–1361. doi: 10.1007/s00270-020-02505-6. [DOI] [PubMed] [Google Scholar]
- 75.Paradis TJ, Floyd E, Burkwit J, Cole SH, Brunson B, Elliott E, et al. The anti-tumor activity of anti-CTLA-4 is mediated through its induction of IFNγ. Cancer Immunol Immunother. 2001;50(3):125–133. doi: 10.1007/s002620100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hannani D, Vetizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015;25(2):208–224. doi: 10.1038/cr.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fransen MF, Van Der Sluis TC, Ossendorp F, Arens R, Melief CJ. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin Cancer Res. 2013;19(19):5381–5389. doi: 10.1158/1078-0432.CCR-12-0781. [DOI] [PubMed] [Google Scholar]
- 78.Chen Y, Pei Y, Luo J, Huang Z, Yu J, Meng X. Looking for the optimal PD-1/PD-L1 inhibitor in cancer treatment: a comparison in basic structure, function, and clinical practice. Front Immunol. 2020;11:1088. doi: 10.3389/fimmu.2020.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Investig. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72(20):5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Homet Moreno B, Zaretsky JM, Garcia-Diaz A, Tsoi J, Parisi G, Robert L, et al. Response to programmed cell death-1 blockade in a murine melanoma syngeneic model requires costimulation, CD4, and CD8 T cells. Cancer Immunol Res. 2016;4(10):845–857. doi: 10.1158/2326-6066.CIR-16-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Xie T, Wang B, William WN, Jr, Heymach JV, El-Naggar AK, et al. PD-1 blockade prevents the development and progression of carcinogen-induced oral premalignant lesions. Cancer Prev Res (Phila) 2017;10(12):684–693. doi: 10.1158/1940-6207.CAPR-17-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burrack AL, Spartz EJ, Raynor JF, Wang I, Olson M, Stromnes IM. Combination PD-1 and PD-L1 blockade promotes durable neoantigen-specific T cell-mediated immunity in pancreatic ductal adenocarcinoma. Cell Rep. 2019;28(8):2140–55.e6. doi: 10.1016/j.celrep.2019.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng Y, Li B, Liang Y, Reeves PM, Qu X, Ran C, et al. Dual blockade of CXCL12–CXCR4 and PD-1–PD-L1 pathways prolongs survival of ovarian tumor-bearing mice by prevention of immunosuppression in the tumor microenvironment. FASEB J. 2019;33(5):6596–6608. doi: 10.1096/fj.201802067RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seo YD, Jiang X, Sullivan KM, Jalikis FG, Smythe KS, Abbasi A, et al. Mobilization of CD8(+) T cells via CXCR4 blockade facilitates PD-1 checkpoint therapy in human pancreatic cancer. Clin Cancer Res. 2019;25(13):3934–3945. doi: 10.1158/1078-0432.CCR-19-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020;8(1):1–17. doi: 10.1186/s40364-020-00209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang BW, Chau I. Current status and future potential of predictive biomarkers for immune checkpoint inhibitors in gastric cancer. ESMO Open. 2020;5(4):e000791. doi: 10.1136/esmoopen-2020-000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raimondi A, Sepe P, Zattarin E, Mennitto A, Stellato M, Claps M, et al. Predictive biomarkers of response to immunotherapy in metastatic renal cell cancer. Front Oncol. 2020;10:1644. doi: 10.3389/fonc.2020.01644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giustini N, Bazhenova L. Recognizing prognostic and predictive biomarkers in the treatment of non-small cell lung cancer (NSCLC) with immune checkpoint inhibitors (ICIs) Lung Cancer Targets Ther. 2021;12:21. doi: 10.2147/LCTT.S235102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyake M, Hori S, Owari T, Oda Y, Tatsumi Y, Nakai Y, et al. Clinical impact of tumor-infiltrating lymphocytes and PD-L1-positive cells as prognostic and predictive biomarkers in urological malignancies and retroperitoneal sarcoma. Cancers. 2020;12(11):3153. doi: 10.3390/cancers12113153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allgäuer M, Budczies J, Christopoulos P, Endris V, Lier A, Rempel E, et al. Implementing tumor mutational burden (TMB) analysis in routine diagnostics—a primer for molecular pathologists and clinicians. Transl Lung Cancer Res. 2018;7(6):703. doi: 10.21037/tlcr.2018.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bilgin B, Sendur MA, Hizal M, Yalçın B. An update on immunotherapy options for urothelial cancer. Expert Opin Biol Ther. 2019;19(12):1265–1274. doi: 10.1080/14712598.2019.1667975. [DOI] [PubMed] [Google Scholar]
- 94.Ramalingam S, Hellmann M, Awad M, Borghaei H, Gainor J, Brahmer J, et al. Tumor mutational burden (TMB) as a biomarker for clinical benefit from dual immune checkpoint blockade with nivolumab (nivo)+ ipilimumab (ipi) in first-line (1L) non-small cell lung cancer (NSCLC): identification of TMB cutoff from CheckMate 568. Cancer Res. 2018;78(13S):CT078-CT. [Google Scholar]
- 95.Galvano A, Gristina V, Malapelle U, Pisapia P, Pepe F, Barraco N, et al. The prognostic impact of tumor mutational burden (TMB) in the first-line management of advanced non-oncogene addicted non-small-cell lung cancer (NSCLC): a systematic review and meta-analysis of randomized controlled trials. ESMO Open. 2021;6(3):100124. doi: 10.1016/j.esmoop.2021.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang J, Ding Y, Wu M, Chen Y, Lyu X, Lu J, et al. Integrated genomic analysis identifies a genetic mutation model predicting response to immune checkpoint inhibitors in melanoma. Cancer Med. 2020;9(22):8498–8518. doi: 10.1002/cam4.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robert C, Lanoy E, Besse B. One or two immune checkpoint inhibitors? Cancer Cell. 2019;36(6):579–581. doi: 10.1016/j.ccell.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 98.Oliva M, Spreafico A, Taberna M, Alemany L, Coburn B, Mesia R, et al. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann Oncol. 2019;30(1):57–67. doi: 10.1093/annonc/mdy507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rizzo A, Ricci AD, Brandi G. PD-L1, TMB, MSI, and other predictors of response to immune checkpoint inhibitors in biliary tract cancer. Cancers. 2021;13(3):558. doi: 10.3390/cancers13030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Armstrong SA, Liu SV. Immune checkpoint inhibitors in small cell lung cancer: a partially realized potential. Adv Ther. 2019;36(8):1826–1832. doi: 10.1007/s12325-019-01008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schrock A, Ouyang C, Sandhu J, Sokol E, Jin D, Ross J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30(7):1096–1103. doi: 10.1093/annonc/mdz134. [DOI] [PubMed] [Google Scholar]
- 102.Song Y, Huang J, Liang D, Hu Y, Mao B, Li Q, et al. DNA damage repair gene mutations are indicative of a favorable prognosis in colorectal cancer treated with immune checkpoint inhibitors. Front Oncol. 2020;10:3349. doi: 10.3389/fonc.2020.549777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diaz LA, Marabelle A, Delord J-P, Shapira-Frommer R, Geva R, Peled N, et al. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. Am Soc Clin Oncol. 2017;35:3071. [Google Scholar]
- 104.Sivapiragasam A, Ashok Kumar P, Sokol ES, Albacker LA, Killian JK, Ramkissoon SH, et al. Predictive biomarkers for immune checkpoint inhibitors in metastatic breast cancer. Cancer Med. 2021;10(1):53–61. doi: 10.1002/cam4.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiao R, Luo H, Xu W, Ge H. Immune checkpoint inhibitors in esophageal squamous cell carcinoma: progress and opportunities. Onco Targets Ther. 2019;12:6023. doi: 10.2147/OTT.S214579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen C, Zhang F, Zhou N, Gu Y-M, Zhang Y-T, He Y-D, et al. Efficacy and safety of immune checkpoint inhibitors in advanced gastric or gastroesophageal junction cancer: a systematic review and meta-analysis. Oncoimmunology. 2019;8(5):e1581547. doi: 10.1080/2162402X.2019.1581547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li K, Luo H, Huang L, Luo H, Zhu X. Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int. 2020;20(1):1–13. doi: 10.1186/s12935-019-1091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamashita H, Nakayama K, Ishikawa M, Nakamura K, Ishibashi T, Sanuki K, et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget. 2018;9(5):5652. doi: 10.18632/oncotarget.23790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiao X, Dong D, He W, Song L, Wang Q, Yue J, et al. Mismatch repair deficiency is associated with MSI phenotype, increased tumor-infiltrating lymphocytes and PD-L1 expression in immune cells in ovarian cancer. Gynecol Oncol. 2018;149(1):146–154. doi: 10.1016/j.ygyno.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 110.Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. 2020;21(11):1346–1358. doi: 10.1038/s41590-020-0769-3. [DOI] [PubMed] [Google Scholar]
- 111.Lee SJ, Jun S-Y, Lee IH, Kang BW, Park SY, Kim HJ, et al. CD274, LAG3, and IDO1 expressions in tumor-infiltrating immune cells as prognostic biomarker for patients with MSI-high colon cancer. J Cancer Res Clin Oncol. 2018;144(6):1005–1014. doi: 10.1007/s00432-018-2620-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 114.Joshua AM, Monzon JG, Mihalcioiu C, Hogg D, Smylie M, Cheng T. A phase 2 study of tremelimumab in patients with advanced uveal melanoma. Melanoma Res. 2015;25(4):342–347. doi: 10.1097/CMR.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 115.Calabrò L, Morra A, Giannarelli D, Amato G, D'Incecco A, Covre A, et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study. Lancet Respir Med. 2018;6(6):451–460. doi: 10.1016/S2213-2600(18)30151-6. [DOI] [PubMed] [Google Scholar]
- 116.Pakkala S, Higgins K, Chen Z, Sica G, Steuer C, Zhang C, et al. Durvalumab and tremelimumab with or without stereotactic body radiation therapy in relapsed small cell lung cancer: a randomized phase II study. J Immunother Cancer. 2020;8(2):e001302. doi: 10.1136/jitc-2020-001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perets R, Bar J, Rasco DW, Ahn MJ, Yoh K, Kim DW, et al. Safety and efficacy of quavonlimab, a novel anti-CTLA-4 antibody (MK-1308), in combination with pembrolizumab in first-line advanced non-small-cell lung cancer. Ann Oncol. 2021;32(3):395–403. doi: 10.1016/j.annonc.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 118.Gunturi A, McDermott DF. Nivolumab for the treatment of cancer. Expert Opin Investig Drugs. 2015;24(2):253–260. doi: 10.1517/13543784.2015.991819. [DOI] [PubMed] [Google Scholar]
- 119.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, López-Janeiro A, Porciuncula A, Idoate MA, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–476. doi: 10.1038/s41591-018-0339-5. [DOI] [PubMed] [Google Scholar]
- 121.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 129.Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 131.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 132.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguié M, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22(7):919–930. doi: 10.1016/S1470-2045(21)00147-9. [DOI] [PubMed] [Google Scholar]
- 134.Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397–404. doi: 10.1093/annonc/mdy517. [DOI] [PubMed] [Google Scholar]
- 135.Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021;22(4):512–524. doi: 10.1016/S1470-2045(21)00005-X. [DOI] [PubMed] [Google Scholar]
- 136.Eggermont AMM, Blank CU, Mandalà M, Long GV, Atkinson VG, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(5):643–654. doi: 10.1016/S1470-2045(21)00065-6. [DOI] [PubMed] [Google Scholar]
- 137.Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683–694. doi: 10.1056/NEJMoa2106391. [DOI] [PubMed] [Google Scholar]
- 138.Rischin D, Migden MR, Lim AM, Schmults CD, Khushalani NI, Hughes BGM, et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer. 2020;8(1):e000775. doi: 10.1136/jitc-2020-000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21(2):294–305. doi: 10.1016/S1470-2045(19)30728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 141.Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 142.Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, et al. Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC. J Thorac Oncol. 2020;15(5):816–826. doi: 10.1016/j.jtho.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 143.Chu T, Zhong R, Zhong H, Zhang B, Zhang W, Shi C, et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol. 2021;16(4):643–652. doi: 10.1016/j.jtho.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 144.Desai J, Deva S, Lee JS, Lin CC, Yen CJ, Chao Y, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer. 2020;8(1):e000453. doi: 10.1136/jitc-2019-000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tjulandin S, Demidov L, Moiseyenko V, Protsenko S, Semiglazova T, Odintsova S, et al. Novel PD-1 inhibitor prolgolimab: expanding non-resectable/metastatic melanoma therapy choice. Eur J Cancer. 2021;149:222–232. doi: 10.1016/j.ejca.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 147.Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02) J Clin Oncol. 2021;39(7):704–712. doi: 10.1200/JCO.20.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 149.Ardizzoni A, Azevedo S, Rubio-Viqueira B, Rodríguez-Abreu D, Alatorre-Alexander J, Smit HJM, et al. Primary results from TAIL: a global single-arm safety study of atezolizumab monotherapy in a diverse population of patients with previously treated advanced non-small cell lung cancer. J Immunother Cancer. 2021;9(3):e001865. doi: 10.1136/jitc-2020-001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Petrylak DP, Powles T, Bellmunt J, Braiteh F, Loriot Y, Morales-Barrera R, et al. Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer: long-term outcomes from a phase 1 study. JAMA Oncol. 2018;4(4):537–544. doi: 10.1001/jamaoncol.2017.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Horn L, Gettinger SN, Gordon MS, Herbst RS, Gandhi L, Felip E, et al. Safety and clinical activity of atezolizumab monotherapy in metastatic non-small-cell lung cancer: final results from a phase I study. Eur J Cancer. 2018;101:201–209. doi: 10.1016/j.ejca.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 152.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim SJ, Lim JQ, Laurensia Y, Cho J, Yoon SE, Lee JY, et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood. 2020;136(24):2754–2763. doi: 10.1182/blood.2020007247. [DOI] [PubMed] [Google Scholar]
- 155.Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN Solid Tumor trial. JAMA Oncol. 2019;5(3):393–401. doi: 10.1001/jamaoncol.2018.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51–64. doi: 10.1016/S1470-2045(17)30900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang H, Shen K, Zhu C, Li Q, Zhao Y, Ma X. Safety and efficacy of durvalumab (MEDI4736) in various solid tumors. Drug Des Dev Ther. 2018;12:2085. doi: 10.2147/DDDT.S162214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 159.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 160.Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC—an update From the PACIFIC trial. J Thorac Oncol. 2021;16(5):860–867. doi: 10.1016/j.jtho.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 161.Fujiwara Y, Iguchi H, Yamamoto N, Hayama M, Nii M, Ueda S, et al. Tolerability and efficacy of durvalumab in Japanese patients with advanced solid tumors. Cancer Sci. 2019;110(5):1715–1723. doi: 10.1111/cas.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Antill Y, Kok PS, Robledo K, Yip S, Cummins M, Smith D, et al. Clinical activity of durvalumab for patients with advanced mismatch repair-deficient and repair-proficient endometrial cancer. A nonrandomized phase 2 clinical trial. J Immunother Cancer. 2021;9(6):e002255. doi: 10.1136/jitc-2020-002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with ≥ 25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. 2019;107:142–152. doi: 10.1016/j.ejca.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 164.Li J, Deng Y, Zhang W, Zhou AP, Guo W, Yang J, et al. Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors. J Hematol Oncol. 2021;14(1):95. doi: 10.1186/s13045-021-01095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Papadopoulos KP, Harb W, Peer CJ, Hua Q, Xu S, Lu H, et al. First-in-human phase I study of envafolimab, a novel subcutaneous single-domain anti-PD-L1 antibody, in patients with advanced solid tumors. Oncologist. 2021;26(9):e1514–e1525. doi: 10.1002/onco.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mu L, Song Y, Zhao K, Liu Y, Fan Q, Wang X, et al. SHR-1316, an anti-PD-L1 antibody, plus chemotherapy as the first-line treatment for advanced esophageal squamous cell carcinoma: a multicentre, phase 2 study. Thorac Cancer. 2021;12(9):1373–1381. doi: 10.1111/1759-7714.13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Johnson DB, Chandra S, Sosman JA. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320(16):1702–1703. doi: 10.1001/jama.2018.13995. [DOI] [PubMed] [Google Scholar]
- 168.Johnson DB, Reynolds KL, Sullivan RJ, Balko JM, Patrinely JR, Cappelli LC, et al. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol. 2020;21(8):e398–e404. doi: 10.1016/S1470-2045(20)30107-8. [DOI] [PubMed] [Google Scholar]
- 169.Agarwal K, Yousaf N, Morganstein D. Glucocorticoid use and complications following immune checkpoint inhibitor use in melanoma. Clin Med. 2020;20(2):163. doi: 10.7861/clinmed.2018-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZ, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141(24):2031–2034. doi: 10.1161/CIRCULATIONAHA.119.044703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 173.Naidoo J, Page D, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Khoja L, Day D, Chen TW-W, Siu L, Hansen AR. Tumour-and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–2385. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 175.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 176.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33(18):2092. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Laplane L, Duluc D, Larmonier N, Pradeu T, Bikfalvi A. The multiple layers of the tumor environment. Trends Cancer. 2018;4(12):802–809. doi: 10.1016/j.trecan.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 178.Tang H, Qiao J, Fu Y-X. Immunotherapy and tumor microenvironment. Cancer Lett. 2016;370(1):85–90. doi: 10.1016/j.canlet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yoo SY, Badrinath N, Jeong S-N, Woo HY, Heo J. Overcoming tumor resistance to oncolyticvaccinia virus with anti-PD-1-based combination therapy by inducing antitumor immunity in the tumor microenvironment. Vaccines. 2020;8(2):321. doi: 10.3390/vaccines8020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang Y, Ertl HC. Starved and asphyxiated: how can CD8+ T cells within a tumor microenvironment prevent tumor progression. Front Immunol. 2016;7:32. doi: 10.3389/fimmu.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jiang Y, Zhan H. Communication between EMT and PD-L1 signaling: new insights into tumor immune evasion. Cancer Lett. 2020;468:72–81. doi: 10.1016/j.canlet.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 182.Lucibello G, Mograbi B, Milano G, Hofman P, Brest P. PD-L1 regulation revisited: impact on immunotherapeutic strategies. Trends Mol Med. 2021;27:868–881. doi: 10.1016/j.molmed.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 183.Jiang Z, Hsu JL, Li Y, Hortobagyi GN, Hung M-C. Cancer cell metabolism bolsters immunotherapy resistance by promoting an immunosuppressive tumor microenvironment. Front Oncol. 2020;10:1197. doi: 10.3389/fonc.2020.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim C, Kim JH, Kim JS, Chon HJ, Kim J-H. A novel dual inhibitor of IDO and TDO, CMG017, potently suppresses the kynurenine pathway and overcomes resistance to immune checkpoint inhibitors. Am Soc Clin Oncol. 2019;37:e14228. [Google Scholar]
- 185.Hou A, Hou K, Huang Q, Lei Y, Chen W. Targeting myeloid-derived suppressor cell, a promising strategy to overcome resistance to immune checkpoint inhibitors. Front Immunol. 2020;11:783. doi: 10.3389/fimmu.2020.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.O’Donnell JS, Teng MW, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16(3):151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 187.Menetrier-Caux C, Faget J, Biota C, Gobert M, Blay J-Y, Caux C. Innate immune recognition of breast tumor cells mediates CCL22 secretion favoring Treg recruitment within tumor environment. Oncoimmunology. 2012;1(5):759–761. doi: 10.4161/onci.19680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li X, Xiang Y, Li F, Yin C, Li B, Ke X. WNT/β-catenin signaling pathway regulating T cell-inflammation in the tumor microenvironment. Front Immunol. 2019;10:2293. doi: 10.3389/fimmu.2019.02293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 190.Hutchinson L. Evading immune escape: synergy of COX and immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2015;12(11):622. doi: 10.1038/nrclinonc.2015.167. [DOI] [PubMed] [Google Scholar]
- 191.Pu D, Yin L, Huang L, Qin C, Zhou Y, Wu Q, et al. Cyclooxygenase-2 inhibitor: a potential combination strategy with immunotherapy in cancer. Front Oncol. 2021;11:248. doi: 10.3389/fonc.2021.637504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ma G, Li C, Zhang Z, Liang Y, Liang Z, Chen Y, et al. Targeted glucose or glutamine metabolic therapy combined with PD-1/PD-L1 checkpoint blockade immunotherapy for the treatment of tumors—mechanisms and strategies. Front Oncol. 2021;11:697894. doi: 10.3389/fonc.2021.697894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lai X, Stiff A, Duggan M, Wesolowski R, Carson WE, Friedman A. Modeling combination therapy for breast cancer with BET and immune checkpoint inhibitors. Proc Natl Acad Sci. 2018;115(21):5534–5539. doi: 10.1073/pnas.1721559115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hanfei G, Rilan B, Jiuwei C. Advances in combination therapy of immune checkpoint inhibitors for lung cancer. Chin J Lung Cancer. 2020;23(2):101–110. doi: 10.3779/j.issn.1009-3419.2020.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Patel SA, Minn AJ. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity. 2018;48(3):417–433. doi: 10.1016/j.immuni.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Puri S, Shafique M. Combination checkpoint inhibitors for treatment of non-small-cell lung cancer: an update on dual anti-CTLA-4 and anti-PD-1/PD-L1 therapies. Drugs Context. 2020;9:1–11. doi: 10.7573/dic.2019-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC) J Immunother Cancer. 2018;6(1):1–27. doi: 10.1186/s40425-018-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tsai KK, Daud AI. Nivolumab plus ipilimumab in the treatment of advanced melanoma. J Hematol Oncol. 2015;8(1):1–4. doi: 10.1186/s13045-015-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wan M, Ming M. Nivolumab versus ipilimumab in the treatment of advanced melanoma: a critical appraisal: ORIGINAL ARTICLE: Wolchok JD, Chiarion‐Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377:1345–56. Br J Dermatol. 2018;179(2):296–300. doi: 10.1111/bjd.16785. [DOI] [PubMed] [Google Scholar]
- 200.Orecchioni S, Talarico G, Labanca V, Calleri A, Mancuso P, Bertolini F. Vinorelbine, cyclophosphamide and 5-FU effects on the circulating and intratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br J Cancer. 2018;118(10):1329–1336. doi: 10.1038/s41416-018-0076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang L, Zhou J, Hu L, Han X, Zou X, Chen Q, et al. In situ formed fibrin scaffold with cyclophosphamide to synergize with immune checkpoint blockade for inhibition of cancer recurrence after surgery. Adv Funct Mater. 2020;30(7):1906922. [Google Scholar]
- 202.Principe DR, Narbutis M, Kumar S, Park A, Viswakarma N, Dorman MJ, et al. Long-term gemcitabine treatment reshapes the pancreatic tumor microenvironment and sensitizes murine carcinoma to combination immunotherapy. Cancer Res. 2020;80(15):3101–3115. doi: 10.1158/0008-5472.CAN-19-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.de Lara PT, Cecconi V, Hiltbrunner S, Yagita H, Friess M, Bode B, et al. Gemcitabine synergizes with immune checkpoint inhibitors and overcomes resistance in a preclinical model and mesothelioma patients. Clin Cancer Res. 2018;24(24):6345–6354. doi: 10.1158/1078-0432.CCR-18-1231. [DOI] [PubMed] [Google Scholar]
- 204.Grasselly C, Denis M, Bourguignon A, Talhi N, Mathe D, Tourette A, et al. The antitumor activity of combinations of cytotoxic chemotherapy and immune checkpoint inhibitors is model-dependent. Front Immunol. 2018;9:2100. doi: 10.3389/fimmu.2018.02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fu D, Wu J, Lai J, Liu Y, Zhou L, Chen L, et al. T cell recruitment triggered by optimal dose platinum compounds contributes to the therapeutic efficacy of sequential PD-1 blockade in a mouse model of colon cancer. Am J Cancer Res. 2020;10(2):473–490. [PMC free article] [PubMed] [Google Scholar]
- 206.Sun F, Cui L, Li T, Chen S, Song J, Li D. Oxaliplatin induces immunogenic cells death and enhances therapeutic efficacy of checkpoint inhibitor in a model of murine lung carcinoma. J Recept Signal Transduction. 2019;39(3):208–214. doi: 10.1080/10799893.2019.1655050. [DOI] [PubMed] [Google Scholar]
- 207.Zuo S, Sun L, Wang Y, Chen B, Wang J, Ge X, et al. Establishment of a novel mesenchymal stem cell-based regimen for chronic myeloid leukemia differentiation therapy. Cell Death Dis. 2021;12(2):1–15. doi: 10.1038/s41419-021-03499-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Feng B, Niu Z, Hou B, Zhou L, Li Y, Yu H. Enhancing triple negative breast cancer immunotherapy by ICG-templated self-assembly of paclitaxel nanoparticles. Adv Funct Mater. 2020;30(6):1906605. [Google Scholar]
- 209.Falvo P, Orecchioni S, Hillje R, Raveane A, Mancuso P, Camisaschi C, et al. Cyclophosphamide and vinorelbine activate stem-like CD8+ T cells and improve anti-PD-1 efficacy in triple-negative breast cancer. Can Res. 2021;81(3):685–697. doi: 10.1158/0008-5472.CAN-20-1818. [DOI] [PubMed] [Google Scholar]
- 210.Sen T, Della Corte CM, Milutinovic S, Cardnell RJ, Diao L, Ramkumar K, et al. Combination treatment of the oral CHK1 inhibitor, SRA737, and low-dose gemcitabine enhances the effect of programmed death ligand 1 blockade by modulating the immune microenvironment in SCLC. J Thorac Oncol. 2019;14(12):2152–2163. doi: 10.1016/j.jtho.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Leonetti A, Wever B, Mazzaschi G, Assaraf YG, Rolfo C, Quaini F, et al. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist Update. 2019;46:100644. doi: 10.1016/j.drup.2019.100644. [DOI] [PubMed] [Google Scholar]
- 212.Dhillon S, Syed YY. Atezolizumab first-line combination therapy: a review in metastatic nonsquamous NSCLC. Target Oncol. 2019;14(6):759–768. doi: 10.1007/s11523-019-00686-w. [DOI] [PubMed] [Google Scholar]
- 213.Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 214.Ngiow SF, McArthur GA, Smyth MJ. Radiotherapy complements immune checkpoint blockade. Cancer Cell. 2015;27(4):437–438. doi: 10.1016/j.ccell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 215.Thangamathesvaran L, Shah R, Verma R, Mahmoud O. Immune checkpoint inhibitors and radiotherapy—concept and review of current literature. Ann Transl Med. 2018;6(8):155. doi: 10.21037/atm.2018.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lee YH, Tai D, Yip C, Choo SP, Chew V. Combinational immunotherapy for hepatocellular carcinoma: radiotherapy, immune checkpoint blockade and beyond. Front Immunol. 2020;11:2577. doi: 10.3389/fimmu.2020.568759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Eckert F, Zwirner K, Boeke S, Thorwarth D, Zips D, Huber SM. Rationale for combining radiotherapy and immune checkpoint inhibition for patients with hypoxic tumors. Front Immunol. 2019;10:407. doi: 10.3389/fimmu.2019.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vanpouille-Box C, Formenti SC, Demaria S. Toward precision radiotherapy for use with immune checkpoint blockers. Clin Cancer Res. 2018;24(2):259–265. doi: 10.1158/1078-0432.CCR-16-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sindoni A, Minutoli F, Ascenti G, Pergolizzi S. Combination of immune checkpoint inhibitors and radiotherapy: review of the literature. Crit Rev Oncol Hematol. 2017;113:63–70. doi: 10.1016/j.critrevonc.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 220.Sheng H, Huang Y, Xiao Y, Zhu Z, Shen M, Zhou P, et al. ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J Immunother Cancer. 2020;8(1):e000340. doi: 10.1136/jitc-2019-000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fujiwara K, Saung MT, Jing H, Herbst B, Zarecki M, Muth S, et al. Interrogating the immune-modulating roles of radiation therapy for a rational combination with immune-checkpoint inhibitors in treating pancreatic cancer. J Immunother Cancer. 2020;8(2):e000351. doi: 10.1136/jitc-2019-000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mohamad O, Diaz de Leon A, Schroeder S, Leiker A, Christie A, Zhang-Velten E, et al. Safety and efficacy of concurrent immune checkpoint inhibitors and hypofractionated body radiotherapy. Oncoimmunology. 2018;7(7):e1440168. doi: 10.1080/2162402X.2018.1440168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bang A, Wilhite TJ, Pike LR, Cagney DN, Aizer AA, Taylor A, et al. Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat Oncol Biol Phys. 2017;98(2):344–351. doi: 10.1016/j.ijrobp.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 224.Bersanelli M, Lattanzi E, D'Abbiero N, Buti S, Leonetti A, Canè MG, et al. Palliative radiotherapy in advanced cancer patients treated with immune-checkpoint inhibitors: the PRACTICE study. Biomed Rep. 2020;12(2):59–67. doi: 10.3892/br.2019.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pardoll DM. Cancer vaccines. Nat Med. 1998;4(5):525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 226.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3(8):630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 227.Gatti-Mays ME, Redman JM, Collins JM, Bilusic M. Cancer vaccines: enhanced immunogenic modulation through therapeutic combinations. Hum Vaccines Immunother. 2017;13(11):2561–2574. doi: 10.1080/21645515.2017.1364322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kresowik TP, Griffith TS. Bacillus Calmette—Guerin immunotherapy for urothelial carcinoma of the bladder. In: Fialho AM, Chakrabarty AM, editors. Emerging cancer therapy: microbial approaches and biotechnological tools. New York: Wiley; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 230.Khaddour K, Dowling J, Huang J, Council M, Chen D, Cornelius L, et al. Successful administration of sequential TVEC and pembrolizumab followed by temozolomide in immunotherapy refractory intracranial metastatic melanoma with acquired B2M mutation. Oncotarget. 2020;11(52):4836. doi: 10.18632/oncotarget.27848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhao J, Chen Y, Ding Z-Y, Liu J-Y. Safety and efficacy of therapeutic cancer vaccines alone or in combination with immune checkpoint inhibitors in cancer treatment. Front Pharmacol. 2019;10:1184. doi: 10.3389/fphar.2019.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gregor PD, Wolchok JD, Ferrone CR, Buchinshky H, Guevara-Patiño JA, Perales M-A, et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22(13–14):1700–1708. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 233.Van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Can Res. 2000;60(9):2444–2448. [PubMed] [Google Scholar]
- 235.Antonios JP, Soto H, Everson RG, Orpilla J, Moughon D, Shin N, et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight. 2016;1(10):e87059. doi: 10.1172/jci.insight.87059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T cell infiltration into pancreatic tumors. J Immunother (Hagerstown, Md: 1997) 2015;38(1):1. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.De Keersmaecker B, Claerhout S, Carrasco J, Bar I, Corthals J, Wilgenhof S, et al. TriMix and tumor antigen mRNA electroporated dendritic cell vaccination plus ipilimumab: link between T-cell activation and clinical responses in advanced melanoma. J Immunother Cancer. 2020;8(1):e000329. doi: 10.1136/jitc-2019-000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36(7):382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67–73. doi: 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gujar S, Pol JG, Kroemer G. Heating it up: oncolytic viruses make tumors ‘hot’and suitable for checkpoint blockade immunotherapies. London: Taylor & Francis; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rivadeneira DB, DePeaux K, Wang Y, Kulkarni A, Tabib T, Menk AV, et al. Oncolytic viruses engineered to enforce leptin expression reprogram tumor-infiltrating T cell metabolism and promote tumor clearance. Immunity. 2019;51(3):548–60.e4. doi: 10.1016/j.immuni.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12(1):1–16. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chaurasiya S, Chen NG, Fong Y. Oncolytic viruses and immunity. Curr Opin Immunol. 2018;51:83–90. doi: 10.1016/j.coi.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Scott EM, Duffy MR, Freedman JD, Fisher KD, Seymour LW. Solid tumor immunotherapy with T cell engager-armed oncolytic viruses. Macromol Biosci. 2018;18(1):1700187. doi: 10.1002/mabi.201700187. [DOI] [PubMed] [Google Scholar]
- 245.Singh M, Vianden C, Cantwell MJ, Dai Z, Xiao Z, Sharma M, et al. Intratumoral CD40 activation and checkpoint blockade induces T cell-mediated eradication of melanoma in the brain. Nat Commun. 2017;8(1):1–10. doi: 10.1038/s41467-017-01572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sivanandam V, LaRocca CJ, Chen NG, Fong Y, Warner SG. Oncolytic viruses and immune checkpoint inhibition: the best of both worlds. Mol Ther Oncolyt. 2019;13:93–106. doi: 10.1016/j.omto.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jubb AM, Oates AJ, Holden S, Koeppen H. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006;6(8):626–635. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 248.Al-Abd AM, Alamoudi AJ, Abdel-Naim AB, Neamatallah TA, Ashour OM. Anti-angiogenic agents for the treatment of solid tumors: potential pathways, therapy and current strategies—a review. J Adv Res. 2017;8(6):591–605. doi: 10.1016/j.jare.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang Q, Gao J, Di W, Wu X. Anti-angiogenesis therapy overcomes the innate resistance to PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models. Cancer Immunol Immunother. 2020;69(9):1781–1799. doi: 10.1007/s00262-020-02576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Reck M, Shankar G, Lee A, Coleman S, McCleland M, Papadimitrakopoulou VA, et al. Atezolizumab in combination with bevacizumab, paclitaxel and carboplatin for the first-line treatment of patients with metastatic non-squamous non-small cell lung cancer, including patients with EGFR mutations. Expert Rev Respir Med. 2020;14(2):125–136. doi: 10.1080/17476348.2020.1701439. [DOI] [PubMed] [Google Scholar]
- 251.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Liu JF, Herold C, Gray KP, Penson RT, Horowitz N, Konstantinopoulos PA, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(12):1731–1738. doi: 10.1001/jamaoncol.2019.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Starbuck K, McGray R, Masoumi-Moghaddam S, Francois A, Odunsi K, Zsiros E. CXCR4 in combination with immune check point inhibition in ovarian cancer mouse model demonstrates potential for novel therapeutic strategies. In: AACR; 2017.
- 254.Starbuck K, McGray R, Masoumi-Moghaddam S, Francois A, Odunsi K, Zsiros E. Novel oral small molecule CXCR4 inhibitor improves activity of immune checkpoint blockade in ovarian cancer mouse model. In: AACR; 2017.
- 255.Makuku R, Khalili N, Razi S, Keshavarz-Fathi M, Rezaei N. Current and future perspectives of PD-1/PDL-1 blockade in cancer immunotherapy. J Immunol Res. 2021;2021:6661406. doi: 10.1155/2021/6661406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Dart SJ, Cook AM, Millward MJ, McDonnell AM, Chin WL, Hakeem MU, et al. Changes in expression of PD-L1 on peripheral T cells in patients with melanoma and lung cancer treated with PD-1 inhibitors. Sci Rep. 2021;11(1):15312. doi: 10.1038/s41598-021-93479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hannani D, Vétizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015;25(2):208–224. doi: 10.1038/cr.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Son CH, Bae JH, Shin DY, Lee HR, Choi YJ, Jo WS, et al. CTLA-4 blockade enhances antitumor immunity of intratumoral injection of immature dendritic cells into irradiated tumor in a mouse colon cancer model. J Immunother. 2014;37(1):1–7. doi: 10.1097/CJI.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 260.Klyushnenkova EN, Riabov VB, Kouiavskaia DV, Wietsma A, Zhan M, Alexander RB. Breaking immune tolerance by targeting CD25+ regulatory T cells is essential for the anti-tumor effect of the CTLA-4 blockade in an HLA-DR transgenic mouse model of prostate cancer. Prostate. 2014;74(14):1423–1432. doi: 10.1002/pros.22858. [DOI] [PubMed] [Google Scholar]
- 261.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell. 2016;167(2):397–404.e9. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kumar A, Chamoto K, Chowdhury PS, Honjo T. Tumors attenuating the mitochondrial activity in T cells escape from PD-1 blockade therapy. Elife. 2020;9:e52330. doi: 10.7554/eLife.52330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162(6):1242–1256. doi: 10.1016/j.cell.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020;26(12):1839–1844. doi: 10.1038/s41591-020-1085-z. [DOI] [PubMed] [Google Scholar]
- 267.Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):786–795. doi: 10.1016/S1470-2045(20)30140-6. [DOI] [PubMed] [Google Scholar]
- 268.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–820. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 270.Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 272.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 273.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 275.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 277.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 278.Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 279.Papadopoulos KP, Johnson ML, Lockhart AC, Moore K, Falchook GS, Formenti SC, et al. First-in-human study of cemiplimab alone or in combination with radiotherapy and/or low-dose cyclophosphamide in patients with advanced malignancies. Clin Cancer Res. 2020;26(5):1025–1033. doi: 10.1158/1078-0432.CCR-19-2609. [DOI] [PubMed] [Google Scholar]
- 280.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 281.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 282.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 283.Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 284.Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 285.Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395(10240):1835–1844. doi: 10.1016/S0140-6736(20)30934-X. [DOI] [PubMed] [Google Scholar]
- 286.Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–2060. doi: 10.1093/annonc/mdy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 288.Rischin D, Gil-Martin M, González-Martin A, Braña I, Hou JY, Cho D, et al. PD-1 blockade in recurrent or metastatic cervical cancer: Data from cemiplimab phase I expansion cohorts and characterization of PD-L1 expression in cervical cancer. Gynecol Oncol. 2020;159(2):322–328. doi: 10.1016/j.ygyno.2020.08.026. [DOI] [PubMed] [Google Scholar]
- 289.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 290.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–19.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Puzanov I, Milhem MM, Minor D, Hamid O, Li A, Chen L, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB–IV melanoma. J Clin Oncol. 2016;34(22):2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Du B, Wen X, Wang Y, Lin M, Lai J. Gemcitabine and checkpoint blockade exhibit synergistic anti-tumor effects in a model of murine lung carcinoma. Int Immunopharmacol. 2020;86:106694. doi: 10.1016/j.intimp.2020.106694. [DOI] [PubMed] [Google Scholar]
- 293.Iida Y, Harashima N, Motoshima T, Komohara Y, Eto M, Harada M. Contrasting effects of cyclophosphamide on anti-CTL-associated protein 4 blockade therapy in two mouse tumor models. Cancer Sci. 2017;108(10):1974–1984. doi: 10.1111/cas.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ho TTB, Nasti A, Seki A, Komura T, Inui H, Kozaka T, et al. Combination of gemcitabine and anti-PD-1 antibody enhances the anticancer effect of M1 macrophages and the Th1 response in a murine model of pancreatic cancer liver metastasis. J Immunother Cancer. 2020;8(2):e001367. doi: 10.1136/jitc-2020-001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chen Q, Xia R, Zheng W, Zhang L, Li P, Sun X, et al. Metronomic paclitaxel improves the efficacy of PD-1 monoclonal antibodies in breast cancer by transforming the tumor immune microenvironment. Am J Transl Res. 2020;12(2):519–530. [PMC free article] [PubMed] [Google Scholar]
- 296.Lévesque S, Le Naour J, Pietrocola F, Paillet J, Kremer M, Castoldi F, et al. A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice. Oncoimmunology. 2019;8(11):e1657375. doi: 10.1080/2162402X.2019.1657375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32(2):253–67.e5. doi: 10.1016/j.ccell.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Saha D, Martuza RL, Rabkin SD. Oncolytic herpes simplex virus immunovirotherapy in combination with immune checkpoint blockade to treat glioblastoma. Immunotherapy. 2018;10(9):779–786. doi: 10.2217/imt-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kanaya N, Kuroda S, Kakiuchi Y, Kumon K, Tsumura T, Hashimoto M, et al. Immune modulation by telomerase-specific oncolytic adenovirus synergistically enhances antitumor efficacy with anti-PD1 antibody. Mol Ther. 2020;28(3):794–804. doi: 10.1016/j.ymthe.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yang Y, Xu W, Peng D, Wang H, Zhang X, Wang H, et al. An oncolytic adenovirus targeting transforming growth factor β inhibits protumorigenic signals and produces immune activation: a novel approach to enhance anti-PD-1 and anti-CTLA-4 therapy. Hum Gene Ther. 2019;30(9):1117–1132. doi: 10.1089/hum.2019.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Nair S, Mazzoccoli L, Jash A, Govero J, Bais SS, Hu T, et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. JCI Insight. 2021;6(1):e144619. doi: 10.1172/jci.insight.144619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Chen CY, Wang PY, Hutzen B, Sprague L, Swain HM, Love JK, et al. Cooperation of oncolytic herpes virotherapy and PD-1 blockade in murine rhabdomyosarcoma models. Sci Rep. 2017;7(1):2396. doi: 10.1038/s41598-017-02503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Du W, Seah I, Bougazzoul O, Choi G, Meeth K, Bosenberg MW, et al. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc Natl Acad Sci. 2017;114(30):E6157–E6165. doi: 10.1073/pnas.1700363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Woller N, Gürlevik E, Fleischmann-Mundt B, Schumacher A, Knocke S, Kloos AM, et al. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol Ther. 2015;23(10):1630–1640. doi: 10.1038/mt.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Jiang H, Rivera-Molina Y, Gomez-Manzano C, Clise-Dwyer K, Bover L, Vence LM, et al. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Can Res. 2017;77(14):3894–3907. doi: 10.1158/0008-5472.CAN-17-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Cappuccini F, Stribbling S, Pollock E, Hill AV, Redchenko I. Immunogenicity and efficacy of the novel cancer vaccine based on simian adenovirus and MVA vectors alone and in combination with PD-1 mAb in a mouse model of prostate cancer. Cancer Immunol Immunother. 2016;65(6):701–713. doi: 10.1007/s00262-016-1831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cappuccini F, Pollock E, Stribbling S, Hill AV, Redchenko I. 5T4 oncofoetal glycoprotein: an old target for a novel prostate cancer immunotherapy. Oncotarget. 2017;8(29):47474. doi: 10.18632/oncotarget.17666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rajani K, Parrish C, Kottke T, Thompson J, Zaidi S, Ilett L, et al. Combination therapy with reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responses. Mol Ther. 2016;24(1):166–174. doi: 10.1038/mt.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Samson A, Scott KJ, Taggart D, West EJ, Wilson E, Nuovo GJ, et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci Transl Med. 2018;10(422):eaam7577. doi: 10.1126/scitranslmed.aam7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Cockle JV, Rajani K, Zaidi S, Kottke T, Thompson J, Diaz RM, et al. Combination viroimmunotherapy with checkpoint inhibition to treat glioma, based on location-specific tumor profiling. Neuro Oncol. 2015;18(4):518–527. doi: 10.1093/neuonc/nov173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Engeland CE, Grossardt C, Veinalde R, Bossow S, Lutz D, Kaufmann JK, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther. 2014;22(11):1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Hardcastle J, Mills L, Malo CS, Jin F, Kurokawa C, Geekiyanage H, et al. Immunovirotherapy with measles virus strains in combination with anti-PD-1 antibody blockade enhances antitumor activity in glioblastoma treatment. Neuro Oncol. 2017;19(4):493–502. doi: 10.1093/neuonc/now179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.