Abstract
Purpose
Lunotriquetral (LT) instability is uncommon and few biomechanical analyses of the condition exist. For chronic LT instabilities, arthrodesis has long been the treatment of choice but has a high risk for nonunion. The aim of this study was to evaluate an alternative treatment option using a bone–ligament–bone graft in a cadaver model and compare it with a conventional arthrodesis.
Methods
We used 10 cadaveric forearms with different loading positions. We employed computed tomography scans to evaluate the LT joint. Scans were performed with the joint intact after we sectioned the dorsal LT ligament and the palmar LT ligament. The joints were then reconstructed using a bone–ligament–bone graft from the capitate–hamate joint as well as with a compression screw simulating arthrodesis. The joints were then rescanned and 3-dimensional analysis was performed using specialized 3-dimensional software.
Results
Sectioning the dorsal part of LT ligament had little effect on kinematics; however, additional division of the palmar LT ligament resulted in increased mobility. Restoration of physiological kinematics could be partially achieved after bone–ligament–bone reconstruction. Arthrodesis showed increased intercarpal motion in the adjacent scapholunate and lunocapitate joints compared with the bone–ligament–bone reconstruction.
Conclusions
The bone–ligament–bone reconstruction displayed physiologic carpal kinematics in the adjacent joints compared with arthrodesis. It provided enough stability but still some mobility in the LT joint to be able to use it as a treatment modality for chronic LT instability without the risk for nonunion. Decreased intercarpal motion was not statistically significant although there appeared to be a trend toward it.
Type of study/level of evidence
Therapeutic IV.
Key words: Biomechanical study, Bone–ligament–bone reconstruction, Lunotriquetral ligament, Wrist instability
Carpal injuries resulting in instability are a common cause of wrist pain. Although scapholunate injuries are more frequent and thus have received greater attention in the past, lunotriquetral (LT) injuries are not negligible. There is a wide spectrum of injury patterns, which range from stable partial tears to extensive dissociation.1 Anatomically, the proximal carpal row lacks direct tendon insertions and therefore acts as an intercalated segment, with stability depending mainly on the geometry of the articular surfaces and ligaments.2,3
The stabilizing ligaments of the wrist can be divided into extrinsic and intrinsic. The extrinsic ligaments connect the carpals to the radius, ulna, and midcarpus whereas the intrinsic ligaments interconnect 2 carpals. The most important intrinsic ligaments of the proximal carpal row are the scapholunate (SL) and LT ligaments. Like the SL ligament, the LT ligament is C-shaped. It is composed of 3 different regions including the dorsal and palmar true ligaments and a proximal membrane. Whereas the SL ligament is strongest dorsally, the LT ligament is the thickest and therefore strongest palmarly.4
In the uninjured wrist, the lunate is held in balance by a flexion moment provided by the scaphoid and an extension moment provided by the triquetrum, transmitted through the corresponding intrinsic as well as the extrinsic ligaments.5 With a loss of the integrity of the LT ligament, the triquetrum tends to extend, whereas the lunate, which is still connected to the scaphoid, attempts to flex. Complete rupture of an intrinsic ligament alone does not lead to malalignment in unloaded wrists, because the extrinsic ligaments keep the carpals in position.6 With additional load on the wrists, however, the motion is greater, forcing the carpals into malalignment as a result of the missing constraint of the intrinsic ligaments. This results in a dynamic instability, seen on dynamic stress views. For static instability, or instability seen on static radiographs, an additional tear or failure of the extrinsic ligaments is necessary.6
In the wrist, 2 major instability patterns exist, depending on which carpal ligament is torn. When the proximal and palmar components of the LT ligament fail, the lunate flexes, leading to volar intercalated segment instability.7 If instead the SL ligaments fail, the scaphoid tends to collapse into an abnormal flexed position, whereas the lunate and triquetrum extend, leading to dorsal intercalated segment instability.7
Diagnosis of a dynamic instability is difficult because symptoms such as ulnar-sided wrist pain and loss of grip strength are nonspecific with normal static radiographs.1 Depending on the chronicity, instability, and anatomy of the wrist, several therapeutic approaches are described in the literature. In acute cases, therapy options include immobilization, arthroscopically assisted stabilization, and direct open LT repair.8 In chronic cases, treatment options range from arthroscopic debridement9,10 to LT ligament repair,8,11 ligament augmentation using either part of the flexor carpi ulnaris (FCU)8 or extensor carpi ulnaris (ECU)11,12 or palmaris longus tendon13 to capsulodesis,14 arthrodesis,8,11 ulnar shortening osteotomy,15 and anterior pr posterior interosseous nerve neurectomy.16 However, there are no uniform therapy recommendations.
At our institution, we prefer a bone–ligament–bone reconstruction technique using a capitate–hamate bone–ligament–bone graft in patients with chronic LT instability, with promising early clinical results. This reconstruction technique is based on a single dorsal approach for harvesting the graft as well as reconstruction of the LT ligaments with only minimal change to the natural anatomy. Moreover, as observed for the bone–patellar tendon–bone graft for anterior cruciate ligament reconstruction, a bone–ligament–bone graft provides rigid fixation and quicker graft osteointegration than only tendon-in-bone healing using a tendon graft.17,18
van Kampen et al19 introduced a capitate–hamate bone–ligament–bone graft to reconstruct carpal ligament disruptions to treat SL interosseous ligament disruption. Compared with other reported transplants in the literature for SL ligament disruptions, the dorsal capitate–hamate graft was the strongest and therefore the best suited one.20
Based on clinical experience and the literature showing altered kinematics and increased strain in adjacent joints after arthrodesis in the ankle and spine,21,22 we expected our less rigid dorsal bone–ligament–bone reconstruction technique to restore the natural kinematics of the LT joint better and decrease strain on the adjacent joints, thus decreasing the risk for degenerative osteoarthritis compared with an arthrodesis.
In this technique, we decided to reconstruct only the dorsal ligament. Although the palmar ligament is known to be the strongest,4 we assumed that reconstruction of the dorsal ligament would suffice to restore adequate stability especially as a rotational constraint,4 because clinical cases at our institution already demonstrated this outcome. This allowed a single dorsal approach to harvest and implant the graft. Consequently, we expected lower morbidity compared with an additional palmar approach by leaving the palmar extrinsic ligaments intact; moreover, it would be easier to perform. Previous studies suggested that palmar extrinsic ligaments are required to maintain joint stability,23,24 and that scarring of the ligaments may contribute to loss of wrist extension, as reported in a few cases after volar plating of distal radius fractures.25 Furthermore, in our experience, combined dorsal and volar approaches lead to more stiffness, unless they are performed arthroscopically.
The aim of this study was to assess this reconstruction technique of LT repair biomechanically and compare it with arthrodesis. A secondary aim was to examine the LT ligament by sequential sectioning. According to the results of Ritt et al,7 only complete sectioning should show a significant change in carpal alignment. We hypothesized that the dorsal bone–ligament–bone reconstruction would restore the natural kinematics of the LT joint and decrease strain on the adjacent joints compared with an arthrodesis.
Materials and Methods
We used 10 Thiel-fixated upper-extremity cadavers provided by the Anatomic Institute of the University of Bern, Bern, Switzerland (7 females and 3 males, aged 78–93 years). We examined all specimens using an x-ray image intensifier to detect anatomic changes that would affect the results, including testing for existing dissociations. Tendons involved in wrist motion and fist closure were identified and dissected. These included the tendons of the extensor carpi radialis longus and brevis (ECRL/B), ECU, flexor carpi radialis (FCR), FCU, flexor digitorum profundus (FDP), and flexor pollicis longus (FPL). We armed the tendons securely with a 1-0 polyglactin 910 thread using the Krackow suture technique26 (Fig. 1A). The tendons of the ECRL and ECRB were attached together, as well as the tendons of the FDP and FPL. We then fixated the forearms in neutral rotation with 2 screws running through the proximal end of the radial and ulnar bone (Fig. 1B, C).
Figure 1.
Arming of the tendons and fixation in the test model. A Krackow suture technique. B, C Fixation in the test model.
We performed computed tomography in 5 different wrist positions including flexion, extension, fist closure, radial deviation, and ulnar deviation. For each position, we attached calibrated tension springs to the corresponding tendons with a predefined tension of 50 N; the tension was chosen based on the study of Pollock et al.27
For wrist flexion, the spring was attached to the tendons of the FCU and FCR; for extension, ECRL/B and ECU; for radial deviation, ECRL/B and FCR; and for ulnar deviation, FCU and ECU. To simulate fist closure, the FDP, FPL, and ECRL/B and ECU tendons were all attached and loaded.27
In total, the protocol was repeated 5 times (5 series, 0–4) for each wrist. The first series of computed tomography tests was conducted in the native state (0) before surgery.
For the second series (1), we approached the LT ligament through a longitudinal incision, partially dividing the extensor retinaculum distally between the fourth and fifth tendon compartments and performed an arthrotomy exposing the intact intercarpal ligaments. We then sectioned the dorsal and proximal part of the LT ligament with a scalpel under visual control, leaving the palmar portion of the ligament as well as the dorsal radiotriquetral ligament intact. Because of the limited number of cadaveric specimens, and so as not to open and manipulate the joint from the palmar side, we decided to perform only a sequential sectioning rather than divide the specimens into 2 groups and compare sectioning of the palmar part with sectioning of the dorsal part.
For the third series (2), we also sectioned the palmar portion, simulating total disruption of the LT ligament.
For the fourth series (3), we incised the extensor retinaculum over the fourth tendon compartment and performed an arthrotomy according to Berger et al,28 exposing the already cut LT ligament. The capitate–hamate joint and ligament were identified distally to the dorsal intercarpal ligament, and borders of the resection were marked, preserving at least 50% of the ligament to ensure normal kinematics in the capitate–hamate joint.
Through an ulnar incision, we inserted a cannulated compression screw (CCS) (APTUS 3.0, Medartis, Switzerland), stabilizing the LT joint under direct visual as well as fluoroscopy control to align the LT joint correctly.
After that procedure, we harvested the bone–ligament–bone graft of the capitate–hamate joint, similar to the technique described by Weiss.29 A corresponding bone window of approximately 4 × 4 × 4 mm was removed with a chisel from the dorsal ridge of both the lunate and triquetrum, and the bone–ligament–bone graft was fitted in. After insertion, the graft was fixed using 2 1.2-mm screws (APTUS 1.2 cortical screw) (Fig. 2A, B). To withstand biomechanical testing, we also attached the bone–ligament–bone graft to the lunate and triquetrum with a cerclage using 1-0 polyglactin 910 suture, simulating consolidation. This was performed without disrupting additional structures or strengthening the ligament. To leave the dorsal extrinsic ligaments intact, we did not perform a capsule repair.
Figure 3.
The x axis shows pronation-supination; the y axis, flexion-extension; and the z axis, ulnar-radial deviation. (From CASPA, Balgrist CARD AG.)
For the last series (4), we removed the CCS and tested the reconstruction on its own (Fig. 2C, D).
After testing was completed on all 10 specimens, the CT images were reconstructed into 3-dimensional models using OsiriX Lite software (OsiriX Foundation, Geneva, Switzerland). Using the software Meshmixer (Autodesk, Inc. San Rafael, CA), the 3-dimensional reconstructions of the examined hand could be further separated into single carpals. The specialized 3-dimensional software CASPA (Balgrist CARD AG, Zurich, Switzerland) then calculated relative movements of the carpals compared with the normal wrist after each step (Fig. 3). Intercarpal motion was calculated by subtracting differences in change among different carpals (SL, LT, and lunocapitate [LC]) using statistical software (IBM SPSS Statistics, IBM Corporation, Armonk, NY).
Figure 2.
A, B Reconstruction with CCS simulating arthrodesis. C, D Reconstruction after removal of CCS.
Statistical methods
Data are presented as means (SD). Differences between series were calculated using t test; P < .05 indicated statistical significance.
Results
Carpal motion relative to intact state
Calculation of the change in angle of individual carpals relative to the intact state resulted in a variety of results, depending on the position of the wrist (Table 1, Table 2, Table 3).
Table 1.
Mean Carpal Motion Relative to Intact State∗
| Series | x |
y |
z |
x |
y |
z |
|---|---|---|---|---|---|---|
| Wrist Extension | Wrist Flexion | |||||
| Lunate | ||||||
| Cut dorsal | Supination –0.3 (0.7) | Extension –2.4 (1.1) | Ulnar deviation 1.5 (1.7) | Supination –0.3 (1.4) | 0.0 (2.2) | Radial deviation –0.7 (1.3) |
| Cut palmar | Supination –1.3 (1.1) | Extension –2.2 (1.5) | Ulnar deviation 0.1 (1.6) | 0.0 (1.6) | Flexion 0.4 (1.7) | Radial deviation –0.2 (1.7) |
| CCS | Supination –1.7 (3.0) | Extension –2.1 (2.4) | Ulnar deviation 2.6 (2.0) | Supination –4.8 (2.7) | Flexion 8.7 (6.7) | Ulnar deviation 8.1 (4.4) |
| BLB graft | Supination –3.0 (1.9) | Extension –3 (3) | Ulnar deviation 1.1 (1.3) | Supination –5.3 (3.9) | Flexion 4.1 (5.2) | Ulnar deviation 2.3 (3.5) |
| Triquetrum | ||||||
| Cut dorsal | Supination –0.1 (1.0) | Extension –2.7 (1.5) | Ulnar deviation 1.6 (2.7) | Supination –0.6 (1.5) | 0.0 (2.7) | Ulnar deviation 0.1 (1.6) |
| Cut palmar | Pronation 1.3 (1.3) | Extension –4.2 (2.5) | Ulnar deviation 0.2 (2.5) | Supination –0.9 (1.4) | Flexion 1.8 (1.0) | Ulnar deviation 0.1 (0.7) |
| CCS | Supination –4.3 (2.8) | Extension –0.5 (7.6) | Ulnar deviation 1.5 (5.3) | Supination –4.1 (6.3) | Flexion 3.0 (4.9) | Ulnar deviation 4.9 (4.5) |
| BLB graft | Supination –1.5 (2.8) | Extension –4.6 (4.8) | Radial deviation –0.2 (4.0) | Supination –5.8 (3.2) | Flexion 4.9 (5.4) | Ulnar deviation 3.8 (5.6) |
| Ulnar Deviation | Radial Deviation | |||||
| Lunate | ||||||
| Cut dorsal | 0.0 (1.0) | Extension –0.9 (2.4) | Radial deviation –0.2 (2.0) | Supination –0.2 (2.0) | Flexion 0.5 (1.7) | Radial deviation –0.1 (0.6) |
| Cut palmar | Supination –0.8 (3.2) | Extension –3.1 (3.7) | Radial deviation –3.3 (2.7) | Supination –1.0 (1.7) | Flexion 0.4 (3.5) | Radial deviation –0.4 (2.7) |
| CCS | Supination –4.7 (3.5) | Extension –1.9 (5.2) | Ulnar deviation 2.3 (6.2) | Pronation 0.8 (2.4) | Extension –2.0 (4.7) | Radial deviation –2.0 (2.9) |
| BLB graft | Supination –3.0 (3.6) | Extension –4.1 (5.5) | Radial deviation –3.7 (4.6) | Supination –1.8 (2.3) | Extension –1.5 (4.3) | Radial deviation –0.1 (1.7) |
| Triquetrum | ||||||
| Cut dorsal | Supination –0.4 (1.9) | Extension –3.0 (5.1) | Radial deviation –0.7 (2.7) | Pronation 0.4 (1.0) | Flexion 0.1 (2.4) | Ulnar deviation 0.3 (1.4) |
| Cut palmar | Pronation 1.5 (1.4) | Extension –4.6 (4.6) | Radial deviation –2.5 (2.7) | Pronation 1.1 (1.5) | Extension –0.4 (2.9) | Radial deviation –0.8 (1.2) |
| CCS | Supination –3.0 (6.3) | Extension –5.2 (11.6) | Radial deviation –1.2 (6.2) | Supination –2.7 (3.0) | Extension –2.5 (4.3) | Radial deviation –0.6 (3.3) |
| BLB graft | Supination –0.9 (6.5) | Extension –5.8 (10.4) | Radial deviation –3.0 (5.2) | Supination –2.5 (2.6) | Extension –2.4 (3.9) | Radial deviation –2.3 (2.1) |
| Fist | ||||||
| Lunate | ||||||
| Cut dorsal | Pronation 0.7 (1.3) | Flexion 6.1 (8.4) | Radial deviation –1.3 (1.7) | |||
| Cut palmar | Supination –0.6 (1.4) | Extension –1.0 (7.9) | Radial deviation –1.0 (2.1) | |||
| CCS | Supination –0.6 (3.0) | Flexion 1.9 (7.1) | Ulnar deviation 1.5 (3.3) | |||
| BLB graft | Supination –2.1 (2.7) | Extension –0.4 (7.9) | Radial deviation –1.7 (1.5) | |||
| Triquetrum | ||||||
| Cut dorsal | Pronation 0.2 (1.4) | Flexion 4.6 (7.3) | Radial deviation –2.1 (2.5) | |||
| Cut palmar | Supination –0.1 (1.5) | Extension –2.0 (7.2) | Radial deviation –1.5 (3.1) | |||
| CCS | Supination –3.1 (4.2) | Extension –1.0 (7.5) | Radial deviation –2.2 (3.7) | |||
| BLB graft | Supination –1.7 (3.1) | Extension –3.1 (9.0) | Radial deviation –3.5 (3.3) | |||
Data are shown in degrees as mean (SD). Bold numbers indicate statistically significant differences compared with the intact state. Results are displayed in 3 planes (x axis, pronation-supination; y axis, flexion-extension; and z axis, ulnar-radial deviation).
Table 2.
Carpal Motion Relative to Intact State in Wrist Extension and Flexion∗
| Series | Wrist Extension |
Wrist Flexion |
||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Rotation | x | y | z | Rotation | |
| Scaphoid | ||||||||
| Cut dorsal | −0.6 (0.9) | −2.4 (1.2) | 2.2 (1.9) | 3.7 (1.7) | −0.5 (1.0) | 0.5 (3.0) | −0.3 (1.1) | 2.4 (2.3) |
| Cut palmar | −0.3 (1.2) | −2.4 (2.6) | 1.1 (1.7) | 3.9 (1.4) | −0.4 (0.9) | 2.2 (1.4) | −0.1 (1.2) | 2.8 (1.1) |
| CCS | −1.3 (2.0) | −0.3 (4.1) | 0.6 (3.5) | 5.0 (2.8) | −3.6 (2.2) | 4.2 (2.6) | 1.8 (1.7) | 6.4 (2.4) |
| BLB graft | −2.5 (2.5) | −3.1 (2.5) | 0.1 (2.5) | 5.3 (2.3) | −2.9 (2.1) | 2.3 (4.8) | 0.9 (1.9) | 6.1 (2.2) |
| Lunate | ||||||||
| Cut dorsal | −0.3 (0.7) | −2.4 (1.1) | 1.5 (1.7) | 3.2 (1.6) | −0.3 (1.4) | 0.0 (2.2) | −0.7 (1.3) | 2.0 (2.1) |
| Cut palmar | −1.3 (1.1) | −2.2 (1.5) | 0.1 (1.6) | 3.0 (1.8) | 0.0 (1.6) | 0.4 (1.7) | −0.2 (1.7) | 2.3 (1.6) |
| CCS | −1.7 (3.0) | −2.1 (2.4) | 2.6 (2.0) | 5.3 (1.9) | −4.8 (2.7) | 8.7 (6.7) | 8.1 (4.4) | 13.2 (7.0) |
| BLB graft | −3.0 (1.9) | −3.0 (2.7) | 1.1 (1.3) | 5.1 (2.4) | −5.3 (3.9) | 4.1 (5.2) | 2.3 (3.5) | 8.6 (4.9) |
| Triquetrum | ||||||||
| Cut dorsal | −0.1 (1.0) | −2.7(1.5) | 1.6 (2.7) | 4.0 (1.9) | −0.6 (1.5) | 0.0 (2.7) | 0.1 (1.6) | 2.4 (2.4) |
| Cut palmar | 1.3 (1.3) | −4.2 (2.5) | 0.2 (2.5) | 5.3 (2.1) | −0.9 (1.4) | 1.8 (1.0) | 0.1 (0.7) | 2.5 (1.3) |
| CCS | −4.3 (2.8) | −0.5 (7.6) | 1.5 (5.3) | 9.6 (4.1) | −4.1 (6.3) | 3.0 (4.9) | 4.9 (4.5) | 9.6 (5.4) |
| BLB graft | −1.5 (2.8) | −4.6 (4.8) | −0.2 (4.0) | 7.6 (3.1) | −5.8 (3.2) | 4.9 (5.4) | 3.8 (5.6) | 10.1 (5.5) |
| Capitate | ||||||||
| Cut dorsal | −2.0 (2.1) | −1.4 (3.0) | 5.4 (5.5) | 7.9 (3.8) | −1.2 (2.5) | 0.4 (5.6) | −0.1 (1.8) | 4.1 (4.9) |
| Cut palmar | 0.2 (1.5) | −3.1 (2.7) | 1.8 (5.2) | 6.3 (2.6) | −0.4 (1.1) | 2.6 (2.2) | 0.4 (1.2) | 3.3 (1.8) |
| CCS | −2.7 (1.5) | 0.5 (4.0) | 1.6 (5.7) | 7.0 (2.8) | −6.1 (2.8) | 6.1 (3.8) | 2.4 (3.1) | 9.5 (4.2) |
| BLB graft | 0.0 (4.8) | −5.0 (1.5) | 1.9 (6.8) | 8.4 (4.6) | −3.7 (2.6) | 4.0 (6.8) | 2.4 (2.6) | 8.7 (3.6) |
| MC3 | ||||||||
| Cut dorsal | −2.1 (2.2) | −1.5 (3.3) | 6.3 (6.3) | 9.0 (4.1) | −1.7 (3.6) | 0.2 (6.8) | −0.3 (2.7) | 4.9 (6.3) |
| Cut palmar | −0.2 (1.6) | −4.0 (2.4) | 2.0 (6.1) | 7.2 (3.3) | −0.5 (1.5) | 2.8 (2.7) | 0.8 (1.3) | 3.7 (2.3) |
| CCS | −2.6 (1.7) | 0.5 (4.3) | 2.2 (6.6) | 7.8 (3.3) | −5.7 (2.7) | 4.4 (4.1) | 2.1 (2.4) | 8.2 (3.7) |
| BLB graft | −1.5 (1.8) | −4.8 (1.7) | 0.5 (6.7) | 7.9 (3.4) | −3.3 (2.6) | 1.1 (7.5) | 1.7 (2.1) | 7.9 (3.6) |
Data are shown in degrees as mean (SD). Bold numbers indicate statistically significant differences compared with the intact state. For the x axes, positive values indicate pronation and negative values, supination. For the y axes, positive values indicate flexion and negative values, extension. For the z axes, positive values indicate ulnar deviation and negative values, radial deviation.
Table 3.
Carpal Motion Relative to Intact State in Ulnar and Radial Deviation∗
| Series | Ulnar Deviation |
Radial Deviation |
||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Rotation | x | y | z | Rotation | |
| Scaphoid | ||||||||
| Cut dorsal | 0.7 (1.1) | −1.6 (4.4) | 0.0 (1.8) | 4.2 (2.8) | 0.1 (1.4) | 0.2 (4.3) | 0.0 (1.3) | 3.7 (2.7) |
| Cut palmar | 1.6 (1.3) | −6.2 (4.7) | −1.3 (1.6) | 6.8 (4.8) | −0.3 (1.1) | −0.4 (4.4) | −0.2 (1.0) | 3.8 (2.4) |
| CCS | −0.1 (3.8) | −5.3 (11.3) | −1.1 (3.8) | 10.9 (7.5) | 0.5 (1.2) | −2.9 (3.7) | −0.9 (1.7) | 3.9 (3.5) |
| BLB graft | 1.5 (3.4) | −8.5 (12.4) | −1.1 (3.5) | 14.7 (4.7) | −1.3 (1.5) | −2.2 (4.9) | −1.2 (1.9) | 5.7 (1.6) |
| Lunate | ||||||||
| Cut dorsal | 0.0 (1.0) | −0.9 (2.4) | −0.2 (2.0) | 2.7 (2.0) | −0.2 (1.3) | 0.5 (1.7) | −0.1 (0.6) | 2.0 (1.0) |
| Cut palmar | −0.8 (3.2) | −3.1 (3.7) | −3.3 (2.7) | 5.2 (4.8) | −1.0 (1.7) | 0.4 (3.5) | −0.4 (2.7) | 3.8 (2.7) |
| CCS | −4.7 (3.5) | −1.9 (5.2) | 2.3 (6.2) | 8.7 (5.0) | 0.8 (2.4) | −2.0 (4.7) | −2.0 (2.9) | 5.4 (3.6) |
| BLB graft | −3.0 (3.6) | −4.1 (5.5) | −3.7 (4.6) | 8.9 (4.3) | −1.8 (2.3) | −1.5 (4.3) | −0.1 (1.7) | 5.1 (2.1) |
| Triquetrum | ||||||||
| Cut dorsal | −0.4 (1.9) | −3.0 (5.1) | −0.7 (2.7) | 5.2 (4.1) | 0.4 (1.0) | 0.1 (2.4) | 0.3 (1.4) | 2.6 (1.2) |
| Cut palmar | 1.5 (1.4) | −4.6 (4.6) | −2.5 (2.7) | 6.0 (4.9) | 1.1 (1.5) | −0.4 (2.9) | −0.8 (1.2) | 3.1 (1.9) |
| CCS | −3.0 (6.3) | −5.2 (11.6) | −1.2 (6.2) | 12.9 (7.9) | −2.7 (3.0) | −2.5 (4.3) | −0.6 (3.3) | 6.1 (3.7) |
| BLB graft | −0.9 (6.5) | −5.8 (10.4) | −3.0 (5.2) | 12.9 (5.8) | −2.5 (2.6) | −2.4 (3.9) | −2.3 (2.1) | 5.9 (2.5) |
| Capitate | ||||||||
| Cut dorsal | −0.1 (1.9) | −2.2 (4.8) | 0.4 (2.2) | 5.1 (2.8) | 0.1 (1.1) | 0.3 (4.0) | 0.6 (1.5) | 3.7 (2.2) |
| Cut palmar | 1.9 (1.7) | −7.1 (5.0) | −1.3 (2.1) | 7.8 (5.3) | 0.5 (1.2) | −0.5 (4.7) | −0.1 (1.2) | 4.3 (2.4) |
| CCS | −1.7 (4.6) | −3.4 (11.7) | 0.1 (4.3) | 11.6 (6.4) | −0.3 (1.1) | −2.9 (3.1) | 0.4 (2.2) | 4.0 (2.8) |
| BLB graft | 0.0 (4.6) | −6.9 (13.3) | −0.2 (4.1) | 14.5 (5.9) | −1.5 (1.5) | −1.9 (5.0) | −0.8 (2.3) | 5.6 (2.3) |
| MC3 | ||||||||
| Cut dorsal | 0.4 (1.6) | −1.8 (4.9) | 0.7 (2.3) | 5.0 (2.9) | −0.1 (1.3) | 0.7 (3.7) | 0.6 (1.3) | 3.6 (1.9) |
| Cut palmar | 2.0 (1.7) | −7.1 (5.0) | −1.0 (1.8) | 7.7 (5.2) | 0.3 (0.9) | −0.4 (4.7) | −0.1 (1.4) | 4.1 (2.6) |
| CCS | −1.1 (4.4) | −3.3 (11.5) | 0.4 (3.9) | 11.2 (6.3) | −0.3 (1.0) | −2.9 (2.6) | 0.0 (1.5) | 3.6 (2.1) |
| BLB graft | 0.6 (4.3) | −7.2 (13.5) | 0.2 (3.6) | 14.5 (6.0) | −1.3 (1.5) | −1.8 (5.0) | −0.5 (1.9) | 5.1 (2.7) |
Data are shown in degrees as mean (SD). Bold numbers indicate statistically significant differences compared with the intact state. For the x axes, positive values indicate pronation and negative values supination. For the y axes, positive values indicate flexion and negative values extension. For the z axes, positive values indicate ulnar deviation and negative values, radial deviation.
Arthrodesis compared with reconstruction
Compared with arthrodesis, the bone–ligament–bone reconstruction showed statistically significant changes: the triquetrum showed a significant change only in the extended position, with less supination and more extension. The lunate showed significant changes in flexion, extension, radial deviation, and ulnar deviation. However, the direction of movement differed depending on the position of the wrist (Table 4).
Table 4.
Mean Difference From Arthrodesis to Bone–Ligament–Bone Reconstruction∗
| Wrist Position | From Arthrodesis to Reconstruction | Mean Difference | P Value |
|---|---|---|---|
| Extension | |||
| Lunate | No statistically significant difference | ||
| Triquetrum | Less supination More extension |
x: +2.77° y: –4.17° |
.04 .02 |
| Flexion | |||
| Lunate | Less flexion Less ulnar deviation |
y: –4.61° z: –5.8° |
.006 .00002 |
| Triquetrum | No statistically significant difference | ||
| Fist | |||
| Lunate | More supination More radial deviation |
x: –1.47° z: –3.16° |
.03 .01 |
| Triquetrum | No statistically significant difference | ||
| Radial deviation | |||
| Lunate | Supination Less radial deviation |
x: –2.55° z: +1.89° |
.005 .011 |
| Triquetrum | No statistically significant difference | ||
| Ulnar deviation | |||
| Lunate | Less supination Radial deviation |
x: +1.7° z: –5.99° |
.002 .009 |
| Triquetrum | No statistically significant difference | ||
X axis, pronation-supination; y axis, flexion-extension; z axis, ulnar-radial deviation.
Intercarpal motion of SL, LC, and LT joints
After arthrodesis, intercarpal motion in the SL and LC joints was significantly increased in the extended and flexed position of the wrist compared with the bone–ligament–bone reconstruction. Removing the CCS, and thus testing the bone–ligament–bone reconstruction alone, partially restored natural kinematics in the adjacent joints; intercarpal motion approximated the value 0, corresponding to intercarpal motion in the native state (Table 5, Fig. 4).
Table 5.
Intercarpal Motion: Comparison of Arthrodesis Simulation Versus Bone–Ligament–Bone Reconstruction in SL and LC Joints and Fully Sectioned LT Ligament Versus Bone–Ligament–Bone Reconstruction in LT Joint
| Wrist Position | Arthrodesis With CCS | BLB Reconstruction | Difference | P Value |
|---|---|---|---|---|
| SL | ||||
| Flexion | –4.5° | –1.8° | 2.7° | .015 |
| Extension | 1.8° | –0.1° | –1.8° | .048 |
| LC | ||||
| Flexion | 2.6° | 0.1° | –2.5° | .066 |
| Extension | –2.5° | 2.0° | 4.5° | .002 |
| Sectioning LT ligament | ||||
| LT | ||||
| Flexion | –1.7° | –.08° | –0.9° | .464 |
| Extension | 2.0° | 1.6° | 0.4° | .771 |
Figure 4.
Differences in carpal motion changes relative to the intact state (degrees). BLB, bone–ligament–bone.
For the LT joint, sectioning of the entire LT ligament led to increased intercarpal motion in flexion and extension. In the extended wrist position, the triquetrum had a greater change in extension than did the lunate. In the flexed wrist position, the triquetrum flexed more than did the lunate. After bone–ligament–bone reconstruction, intercarpal motion in the LT joint decreased, but not statistically significantly (Table 5, Fig. 4).
Discussion
For LT instability, there is no general agreement regarding a treatment technique. Treatment options range from conservative therapy in more acute cases to surgical therapy including arthroscopic debridement, LT ligament repair, ligament augmentation, arthrodesis, ulnar shortening osteotomy, and anteroposterior interosseous neurectomy.8, 9, 10, 11, 12, 13, 14, 15, 16
For SL ligament reconstruction Nakamura et al30 report excellent clinical and radiographic results using a capitohamate bone–ligament–bone graft for SL instability and static SL dissociation.30 Long-term results of van Kampen et al19 using a capitohamate bone–ligament–bone autograft for SL reconstruction showed results comparable to other SL reconstructive options with no superiority to other techniques. At our institution, the technique of van Kampen et al was transferred to stabilize chronic unstable LT joints, although we recognized that the most stable part of the LT ligament is the palmar part with promising results, indicating possible advantages compared with an arthrodesis or other options. However, because the reconstruction was nonanatomic, we conducted the study for further evaluation.
To our knowledge, to date, there has been no biomechanical assessment of a bone–ligament–bone reconstruction technique for LT repair. The use of a bone–ligament–bone graft to restore carpal stability offers a simple option for treating LT instability with a single dorsal approach. Compared with an arthrodesis, the graft showed significantly less intercarpal motion in the adjacent SL and LC joints, thus partially restoring natural intercarpal motion. With regard to the LT joint, we found that intercarpal motion tended to be reduced after bone–ligament–bone reconstruction compared with a totally dissected LT ligament.
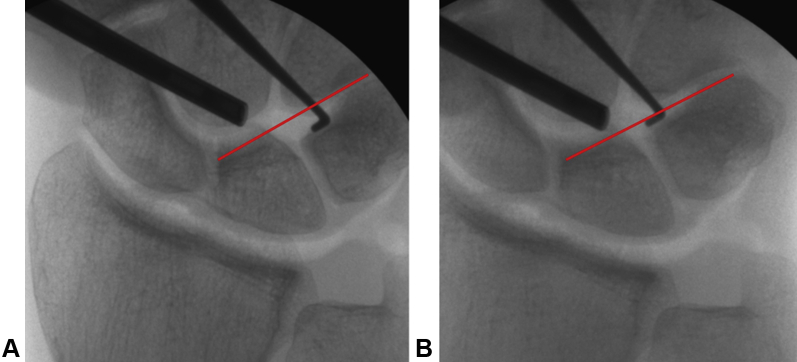
One example of a clinical case is a 33-year-old man who sustained a bicycle accident 1 year before surgery. A stressed x-ray showed malrotation of the LT joint as well as a lesion of the palmar part of the LT ligament. He had pain with rotation and weight-bearing. The diagnosis was confirmed arthroscopically and under an image intensifier (Fig. 5). A reconstruction was performed; 3 months later, the compression screw was removed after healing of the graft was confirmed (as is typically done). So as not to manipulate the joint further, the cortical screws were left in situ. The patient returned to work without pain when rotation the arm and bearing weight upon it. After 6 months, at the final follow-up, the Disabilities of the Arm, Shoulder, and Hand questionnaire score improved from 69.8 to 36.2 and the final Michigan Hand Outcomes Questionnaire score was 77.6. The final grip strength improved to 40.5 from 30 kg before surgery.
Figure 5.
Clinical case: A 33-year-old man with an LT injury and instability on arthroscopic and radiologic examination. A Pressure application leading to widening of the LT joint. B Without pressure, return to normal alignment.
We acknowledge several limitations to this study. With 10 cadaveric forearms, there was only a small sample size to test different treatment options. To avoid opening and manipulating the LT joint from palmar, we decided to perform only sequential sectioning rather than dividing the specimens into 2 groups and comparing sectioning of the palmar part with sectioning of the dorsal part. Complete dissection of the LT ligament showed only a small amount of change in position; hence, the comparison of the reconstruction and arthrodesis with the dissected state is less significant. Additional cycles of motion might have increased the change after LT sectioning.7 Also, some of the statistically significant differences were relatively small (1° to 4°); therefore, their clinical relevance is unclear.
With computed tomography imaging and 3-dimensional software calculating exact position changes in an entire body compared with only a few points, we used a clinically relevant method to evaluate carpal kinematics. However, owing to this new method, it is difficult to compare with those of previous studies. All results are given as relative data compared with the native state of each position, rather than demonstrating absolute data or compared with the neutral position. This may compensate for small irregularities in fixation in the jig, but it makes comparisons with other studies more difficult. Testing was done with cadaver forearms; therefore, we cannot evaluate the outcome of the reconstruction after tissue healing. However, to simulate consolidation, we also attached the bone–ligament–bone graft to the lunate and triquetrum with a cerclage. We did not perform a suture of the distally incised retinaculum because of a lack of literature on providing stability to the proximal carpal row and because a suture is not as stable as a healed retinaculum. Whether the distally incised retinaculum has an influence on the stability is unknown.
This study did not confirm biomechanical restoration of LT stability after reconstruction with a bone–ligament–bone graft. However, compared with arthrodesis, the bone–ligament–bone reconstruction displayed more physiologic carpal kinematics in the adjacent joints with enough stability but some mobility in the LT joint to treat chronic LT instability without the risk for nonunion. Indeed, compared with arthrodesis, less intercarpal motion could be observed in the adjacent joints. Therefore, in addition to promising clinical short-term results, we expect less strain on the adjacent joints with the bone–ligament–bone graft in the long term as a result of more physiological motion. Further clinical data with long-term follow-up are necessary to evaluate the advantage and validity of this treatment option in chronic LT instability.
Acknowledgments
We thank Medartis for kindly supplying the screws, and the Computer Assisted Research & Development team of Balgrist University Hospital for providing the CASPA software.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received by the authors related directly or indirectly to the subject of this article.
References
- 1.Shin A.Y., Battaglia M.J., Bishop A.T. Lunotriquetral instability: diagnosis and treatment. J Am Acad Orthop Surg. 2000;8(3):170–179. doi: 10.5435/00124635-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kauer J.M., de Lange A. The carpal joint: anatomy and function. Hand Clin. 1987;3(1):23–29. [PubMed] [Google Scholar]
- 3.Jacob H.A., Kunz C., Sennwald G. Biomechanics of the carpus--functional anatomy and movement analysis of the carpal bones [in German] Orthopade. 1992;21(1):81–87. [PubMed] [Google Scholar]
- 4.Ritt M.J., Bishop A.T., Berger R.A., Linscheid R.L., Berglund L.J., An K.N. Lunotriquetral ligament properties: a comparison of three anatomic subregions. J Hand Surg Am. 1998;23(3):425–431. doi: 10.1016/S0363-5023(05)80460-5. [DOI] [PubMed] [Google Scholar]
- 5.Berger R.A. The anatomy of the ligaments of the wrist and distal radioulnar joints. Clin Orthop Relat Res. 2001;383:32–40. doi: 10.1097/00003086-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Horii E., Garcia-Elias M., An K.N., et al. A kinematic study of luno-triquetral dissociations. J Hand Surg Am. 1991;16(2):355–362. doi: 10.1016/s0363-5023(10)80126-1. [DOI] [PubMed] [Google Scholar]
- 7.Ritt M.J., Linscheid R.L., Cooney W.P., Berger R.A., An K.N. The lunotriquetral joint: kinematic effects of sequential ligament sectioning, ligament repair, and arthrodesis. J Hand Surg Am. 1998;23(3):432–445. doi: 10.1016/S0363-5023(05)80461-7. [DOI] [PubMed] [Google Scholar]
- 8.Reagan D.S., Linscheid R.L., Dobyns J.H. Lunotriquetral sprains. J Hand Surg Am. 1984;9(4):502–514. doi: 10.1016/s0363-5023(84)80101-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.I.L., Nha K.W., Lee G.Y., Kim B.H., Kim J.W., Park J.W. Long-term outcomes of arthroscopic debridement and thermal shrinkage for isolated partial intercarpal ligament tears. Orthopedics. 2012;35(8):e1204–e1209. doi: 10.3928/01477447-20120725-20. [DOI] [PubMed] [Google Scholar]
- 10.Weiss A.P., Sachar K., Glowacki K.A. Arthroscopic debridement alone for intercarpal ligament tears. J Hand Surg Am. 1997;22(2):344–349. doi: 10.1016/S0363-5023(97)80176-1. [DOI] [PubMed] [Google Scholar]
- 11.Shin A.Y., Weinstein L.P., Berger R.A., Bishop A.T. Treatment of isolated injuries of the lunotriquetral ligament. J Bone Joint Surg Br. 2001;83(7):1023–1028. doi: 10.1302/0301-620x.83b7.11413. [DOI] [PubMed] [Google Scholar]
- 12.Shahane S.A., Trail I.A., Takwale V.J., Stilwell J.H., Stanley J.K. Tenodesis of the extensor carpi ulnaris for chronic, post-traumatic lunotriquetral instability. J Bone Joint Surg Br. 2005;87(11):1512–1515. doi: 10.1302/0301-620X.87B11.16361. [DOI] [PubMed] [Google Scholar]
- 13.Harper C.M., Iorio M.L. Lunotriquetral ligament reconstruction utilizing a palmaris longus autograft. J Hand Surg Asian Pac Vol. 2017;22(4):544–547. doi: 10.1142/S0218810417710010. [DOI] [PubMed] [Google Scholar]
- 14.Omokawa S., Fujitani R., Inada Y. Dorsal radiocarpal ligament capsulodesis for chronic dynamic lunotriquetral instability. J Hand Surg Am. 2009;34(2):237–243. doi: 10.1016/j.jhsa.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Mirza A., Mirza J.B., Shin A.Y., Lorenzana D.J., Lee B.K., Izzo B. Isolated lunotriquetral ligament tears treated with ulnar shortening osteotomy. J Hand Surg Am. 2013;38(8):1492–1497. doi: 10.1016/j.jhsa.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Hofmeister E.P., Moran S.L., Shin A.Y. Anterior and posterior interosseous neurectomy for the treatment of chronic dynamic instability of the wrist. Hand (N Y) 2006;1(2):63–70. doi: 10.1007/s11552-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stecker S., Parker R.D. Graft selection in knee cruciate ligament surgery: autograft, allograft, and synthetic. Oper Tech Orthop. 1999;9(4):248–255. [Google Scholar]
- 18.Thaunat M., Fayard J.M., Sonnery-Cottet B. Hamstring tendons or bone-patellar tendon-bone graft for anterior cruciate ligament reconstruction? Orthop Traumatol Surg Res. 2019;105(suppl 1):S89–S94. doi: 10.1016/j.otsr.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 19.van Kampen R.J., Bayne C.O., Moran S.L., Berger R.A. Outcomes of capitohamate bone-ligament-bone grafts for scapholunate injury. J Wrist Surg. 2015;4(4):230–238. doi: 10.1055/s-0035-1556866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gay A.M., Thoreson A., Berger R.A. Biomechanical comparison of the hand-based transplant used in bone-tissue-bone scapho-lunate ligament reconstruction. Chir Main. 2014;33(1):23–28. doi: 10.1016/j.main.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Beyaert C., Sirveaux F., Paysant J., Molé J., André J.M. The effect of tibio-talar arthrodesis on foot kinematics and ground reaction force progression during walking. Gait Posture. 2004;20(1):84–91. doi: 10.1016/j.gaitpost.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Song J., Zhang Y.-X., Song K.-H., Wang H.-L., Zou F., Jiang J.-Y. Risk factors of adjacent segment disease after anterior cervical arthrodesis: a retrospective study of sagittal measurement of thoracic inlet parameters. World Neurosurg. 2018;114:e1094–e1100. doi: 10.1016/j.wneu.2018.03.152. [DOI] [PubMed] [Google Scholar]
- 23.Kamal R.N., Bariteau J.T., Beutel B.G., DaSilva M.F. Arthroscopic reduction and percutaneous pinning of a radiocarpal dislocation: a case report. J Bone Joint Surg Am. 2011;93(15):e84. doi: 10.2106/JBJS.J.01306. [DOI] [PubMed] [Google Scholar]
- 24.Ilyas A.M., Mudgal C.S. Radiocarpal fracture-dislocations. J Am Acad Orthop Surg. 2008;16(11):647–655. doi: 10.5435/00124635-200811000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kamal R.N., Ruch D.S. Volar capsular release after distal radius fractures. J Hand Surg Am. 2017;42(12):1034.e1–1034.e6. doi: 10.1016/j.jhsa.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Krackow K.A., Thomas S.C., Jones L.C. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909–917. doi: 10.3928/0147-7447-19880601-11. [DOI] [PubMed] [Google Scholar]
- 27.Pollock P.J., Sieg R.N., Baechler M.F., Scher D., Zimmerman N.B., Dubin N.H. Radiographic evaluation of the modified Brunelli technique versus the Blatt capsulodesis for scapholunate dissociation in a cadaver model. J Hand Surg Am. 2010;35(10):1589–1598. doi: 10.1016/j.jhsa.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Berger R.A., Bishop A.T., Bettinger P.C. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54–59. doi: 10.1097/00000637-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Weiss A.P. Scapholunate ligament reconstruction using a bone-retinaculum-bone autograft. J Hand Surg Am. 1998;23(2):205–215. doi: 10.1016/S0363-5023(98)80115-9. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T., Abe K., Iwamoto T., Ochi K., Sato K. Reconstruction of the scapholunate ligament using capitohamate bone-ligament-bone. J Wrist Surg. 2015;4(4):264–268. doi: 10.1055/s-0035-1566268. [DOI] [PMC free article] [PubMed] [Google Scholar]