Highlights
-
•
A coenzyme-A free multi-enzyme cascade was constructed in Escherichia coli.
-
•
4-Hydroxybenzoic acid was produced from biobased L-tyrosine.
-
•
150 mM of L-tyrosine afforded the synthesis of 128 ± 1 mM of 4-hydroxybenzoic acid (17.7 ± 0.1 g/L).
-
•
Benzoic acid was synthesized from L-phenylalanine via the same enzyme cascade with good productivity.
Keywords: Multi-enzyme cascade, Whole-cell biocatalyst, L-Tyrosine, 4-Hydroxybenzoic acid, Benzoic acid
Abstract
4-Hydroxybenzoic acid (4HBA) and its esterified forms can be used as preservatives in the pharmaceutical and food industries. Here, we reported the establishment of a coenzyme-A (CoA) free multi-enzyme cascade in Escherichia coli to utilize biobased L-tyrosine for efficient synthesis of 4HBA. The multi-enzyme cascade contains L-amino acid deaminase from Proteus mirabilis, hydroxymandelate synthase from Amycolatopsis orientalis, (S)-mandelate dehydrogenase and benzoylformate decarboxylase from Pseudomonas putida, and aldehyde dehydrogenase from Saccharomyces cerevisiae. The whole-cell biocatalysis afforded the synthesis of 128 ± 1 mM of 4HBA (17.7 ± 0.1 g/L) from 150 mM L-tyrosine with > 85% conversion within 96 h. In addition, the artificial enzymatic cascade also allowed the synthesis of benzoic acid from 100 mM L-phenylalanine with a conversion ∼ 90%. In summary, our research offers a sustainable alternative for synthesizing 4HBA and benzoic acid from renewable feedstocks.
1. Introduction
4-Hydroxybenzoic acid (4HBA) can be used for synthesis of parabens such as ethyl 4-hydroxybenzoate (INS NO. 214) and methyl 4-hydroxybenzoate (INS NO. 218), which serve as preservatives in cosmetics, pharmaceuticals and in food and beverages (Kromer et al., 2013, Sytar et al., 2012). 4HBA can also be used as a monomer for synthesis of liquid crystalline polymers. In addition, 4HBA also has various biological properties including hypoglycemic, anti-inflammatory, antiviral and antioxidative activities (Sytar et al., 2012). 4HBA is currently synthesized from petroleum-derived phenol using the Kolbe-Schmitt reaction under high temperature and pressure (Lindsey & Jeskey, 1957). Although chemical synthesis has the advantage of high yields, it often requires high temperatures (usually 140–165 °C), high-pressure conditions and toxic catalysts such as cobalt or manganese naphthenate (Maki & Takeda, 2000).
With the recent advancement of synthetic biology and metabolic engineering, there is a growing interest in utilizing microbial hosts for synthesis of fine chemicals via fermentation processes (J. Yuan, Chen, Mishra, & Ching, 2017; J. F. Yuan, Lukito, & Li, 2019). 4HBA is a natural compound in bacteria that serves a precursor for ubiquinone synthesis (Barker and Frost, 2001, Siebert et al., 1994), and it can also be synthesized in plants via the phenylpropanoids pathway (Wildermuth, 2006) (Fig. 1A). Previously, microbial synthesis of 4HBA has been demonstrated in metabolically designed microorganisms such as Pseudomonas putida S12 (Verhoef, Ruijssenaars, de Bont, & Wery, 2007), Corynebacterium glutamicum (Kitade, Hashimoto, Suda, Hiraga, & Inui, 2018) and Escherichia coli D2704 (Barker & Frost, 2001). For example, the maximum titer of 4HBA achieved in a genetically engineered E. coli was 12 g/L (Barker & Frost, 2001). In P. putida, a final titer of 1.8 g/L 4HBA was obtained under a glycerol-limited fed-batch fermentation (Verhoef, Ruijssenaars, de Bont, & Wery, 2007).
Fig. 1.

Schematic diagram of the natural and synthetic 4HBA biosynthetic routes. (A) Chorismate pyruvate-lyase (ubiC) mediated 4HBA in bacteria and the CoA-dependent 4HBA pathway in plants. E4P, D-erythrose-4-phosphate; PEP, phosphoenolpyruvate; DHAP, dihydroxyacetone phosphate; CHOR, chorismite; L-TYR, L-tyrosine; 4HBA, 4-hydroxybenzoic acid. (B) The synthetic CoA-independent 4HBA pathway. L-amino acid deaminase (LAAD) from P. mirabilis; HmaS, hydroxymandelate synthase (HmaS) from A. orientalis; (S)-mandelate dehydrogenase (SMDH) from P. putida; benzoylformate decarboxylase (BFD) from P. putida; aldehyde dehydrogenase (ALDH) from S. cerevisiae.
Besides glucose and glycerol as substrates, amino acids represent another class of relatively inexpensive feedstock that is readily available from protein waste hydrolysate (K. Y. Choi et al., 2014, Huo et al., 2011). At present, millions of tons of biological protein waste are produced every month from agriculture and food processing, such as meat waste in slaughterhouses and production residues in fermentation industry (I. S. Choi et al., 2015, Huo et al., 2011, Lafarga and Hayes, 2014). The upcycling of these biological wastes is a main trend of global sustainable development. Biocatalytic processes with natural microbial cells as biocatalysts have been developed for converting renewable feedstocks to target products under mild conditions without using toxic catalysts (Pontrelli, Chiu, Lan, Chen, Chang, & Liao, 2018; A. J. Straathof, 2014). For instance, a number of studies have demonstrated chemical productions from renewable feedstocks via recombinant E. coli based multi-enzyme cascades (Liu et al., 2020, Mao et al., 2020, Pontrelli et al., 2018; A. J. J. Straathof, 2014).
In this study, we sought to utilize biobased L-tyrosine as the substrate for 4HBA synthesis via the recombinant E. coli based whole-cell biotransformation. As depicted in Fig. 1B, a synthetic coenzyme-A (CoA) independent pathway was devised toward bioconverting L-tyrosine into 4HBA. The multi-enzyme cascade contains L-amino acid deaminase (LAAD) from Proteus mirabilis (Massad, Zhao, & Mobley, 1995), hydroxymandelate synthase (HmaS) from Amycolatopsis orientalis (Müller, van Assema, Gunsior, Orf, Kremer, Schipper, et al., 2006), (S)-mandelate dehydrogenase (SMDH) and benzoylformate decarboxylase (BFD) from P. putida (Iding, Dunnwald, Greiner, Liese, Muller, Siegert, et al., 2000), and aldehyde dehydrogenase (ALDH, encoded by HFD1 gene) from Saccharomyces cerevisiae (Payet, Leroux, Willison, Kihara, Pelosi, & Pierrel, 2016). To the best of our knowledge, this work represents a pioneering study to explore a biocatalytic approach for synthesis of the food preservative 4HBA from biobased L-tyrosine.
2. Materials and methods
2.1. Strains, chemicals and reagents
E. coli DH5α was used for general plasmid constructions, E. coli MG1655 RARE (MG1655 DE3 ΔendA ΔrecA derivative with ΔdkgB ΔyeaE Δ (yqhC-dkgA) ΔyahK and ΔyjgB), was a gift from Prof. Kristala Prather. E. coli strains were cultivated at 37 °C in Luria-Bertani media. All enzymes were purchased from New England Biolabs (Beverly, MA, USA). Gel extraction kit, PCR purification kit and plasmid purification kit were purchased from BioFlux (Shanghai, China).
2.2. Plasmid and strain construction
All the oligonucleotides in the present study are listed in Table S1. High-fidelity Phusion polymerase was used for standard PCR amplification of genes-of-interest. Both LAAD from P. mirabilis and HmaS from A. orientalis were synthesized by GenScript (Nanjing, China). Genomic DNA of P. putida KT2440 was used for PCR amplification of SMDH encoded by mdlB and BFD encoded by mdlC. Genomic DNA of S. cerevisiae BY4741 was used for PCR amplification of HFD1. PCR program was set as the following: 1 cycle of 98 °C for 30 s; amplification, 30 cycles of 98 °C for 15 s, 56 °C for 30 s and 72 °C for 90 s; 1 cycle of 72 °C for 3 min. All the plasmids were constructed in a similar way via restriction enzyme digestion, followed by T4-ligase ligation (Lessard, 2013). Standard heatshock approach or electroporation was used to transform plasmids into E. coli MG1655 RARE strain (Kunjapur, Tarasova, & Prather, 2014). All the relevant plasmids and strains used in the present study are listed in Table S2.
2.3. Cultivating recombinant E. coli strain
E. coli strains harboring the corresponding plasmids were firstly inoculated in Luria-Bertani medium (10 g/L tryptone, 5 g/L yeast extract and 10 g/L sodium chloride) containing 50 μg/mL ampicillin, 25 μg/mL kanamycin, and 25 μg/mL streptomycin. The initial seeding cultures were cultivated on a rotary shaking incubator at 37 °C. Next day, the overnight cultures were subsequently inoculated into 250 mL baffled culture flasks containing 100 mL Terrific Broth (12 g/L tryptone, 24 g/L yeast extract, 0.4% glycerol, 12.54 g/L K2HPO4·3H2O, 2.31 g/L KH2PO4) with appropriate antibiotics to a final concentration of 1%. The E. coli strains were cultivated at 37 °C until the optical density at 600 nm (OD600) of the culture reached 0.6–0.8. 200 μL of cell broth was used to measure the OD600 by a microplate reader Synergy H1 (Biotek, Winooski, VT, USA) to determine the cell density. Then, isopropyl β-D-1-thiogalactopyranoside (IPTG, 1 mM) was added to induce the protein expression and the cell cultures were further incubated at 20 °C for 16–18 h. The cells were harvested by centrifugation at 4 °C (7000 rpm, 10 min) (Eppendorf 5810R, Hamburg, Germany).
2.4. Synthesis of 4HBA and benzoic acid
The harvested cells were washed with ddH2O for twice, and then resuspended in potassium phosphate (KP) buffer (200 mM, pH 8.0). For 4HBA production, 10 g cdw/L (cdw = cell dry weight) final density of cells, different concentrations of L-tyrosine (10, 50, 100 and 150 mM), 20 g/L glucose were combined together to give a 2 mL reaction mixture in KP buffer. For benzoic acid production, 10 g cdw/L (cdw = cell dry weight) final density of cells, 50 mM or 100 mM L-phenylalanine, 20 g/L glucose were combined together to give a 2 mL reaction mixture in KP buffer. All reactions were carried out at 30 °C and 250 rpm on a rotary shaking incubator. The samples (50 μL) were periodically collected, and diluted with ddH2O (950 μL). After centrifugation, the supernatant was analyzed by high-performance liquid chromatography (HPLC).
2.5. Analytical method
All samples were analyzed by Shimadzu Prominence LC-20A system (Shimadzu, Kyoto, Japan) equipped with a reversed phase column (Shimadzu Insert Sustain C18 column: 150 mm × 4.6 mm × 5 µm) and a photodiode array detector (DAD). Mobile phase for isocratic analysis: 70% water with 0.1% trifluoroacetate + 30% acetonitrile. Flow rate: 1 mL/min. Column temperature: 40 °C. Injection volume: 5 μL. Retention time: L-tyrosine 1.7 min; L-phenylalanine 2.1 min; 4HBA 2.9 min; benzoic acid 6.1 min. Authentic standards were used to plot the standard curves and the product concentrations were calculated accordingly.
3. Results and discussion
3.1. 4HBA synthesis via the CoA-Free multi-enzyme cascade
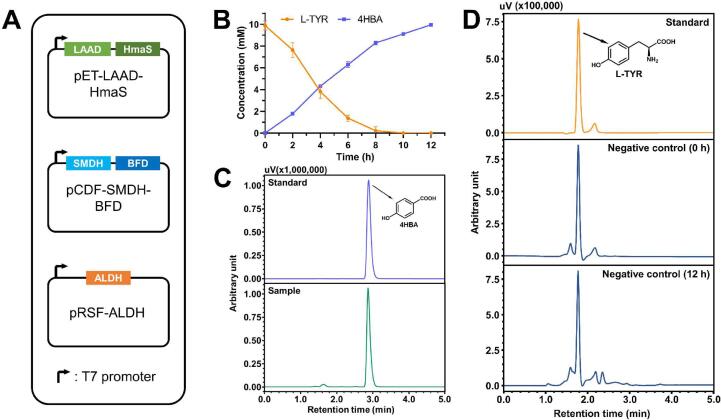
Recently, our group has demonstrated that LAAD from P. mirabilis and HmaS from A. orientalis coupled with SMDH/BFD from P. putida effectively synthesize 4-hydroxybenzyl alcohol and 4-hydroxybenzylamine from L-tyrosine (Liu et al., 2020, Zhu et al., 2020). Therefore, it is likely to produce 4HBA by simply introducing a functional ALDH to the previously established enzyme cascade. As shown in Fig. 2A, a three-plasmid system was used for expressing the CoA-free enzymatic cascade. As the reduction of 4-hydroxybenzaldehyde would divert the flux toward 4-hydroxybenzyl alcohol byproduct, E. coli MG1655 RARE strain with reduced aromatic aldehyde reduction (Kunjapur, Tarasova, & Prather, 2014) was chosen for expressing the above-mentioned enzymatic cascade.
Fig. 2.
Synthesis of 4HBA via the CoA independent enzymatic route. (A) Schematic diagram of plasmids used for CoA-free synthesis of 4HBA. (B) Time course of whole-cell biotransformation of L-tyrosine (10 mM) to 4HBA. The reactions were conducted in KP buffer (200 mM, pH 8.0) at 30 °C with 10 g cdw/L resting cells of E. coli MG1655 RARE cells. The data are the mean values with standard deviations from triplicate experiments. (C) Representative HPLC result showing the 4HBA production. The retention time for 4HBA is 2.9 min. (D) HPLC result of the negative control. E. coli carrying empty plasmids under the same reaction condition was used as the negative control. The retention time for L-tyrosine is 1.7 min.
We first examined the endogenous ALDH encoded by feaB gene from E. coli. Only a negligible amount of 4HBA was obtained after 12 h of reaction (data not shown), suggesting this ALDH had a poor catalytic activity toward 4-hydroxybenzaldehyde. Next, ALDH encoded by HFD1 gene from S. cerevisiae (Payet, Leroux, Willison, Kihara, Pelosi, & Pierrel, 2016) was selected for the 4HBA synthesis from 4-hydroxybenzaldehyde. After supplementing the recombinant E. coli resting cells with 10 mM L-tyrosine, 4HBA reached a concentration of 1.37 ± 0.02 g/L after 12 h, with a conversion > 98% (Fig. 2B). In comparison, the negative control without expressing the enzyme cascade gave no appreciable amount of 4HBA and the L-tyrosine substrate remained unconsumed (Fig. 2D).
3.2. High-yielding 4HBA production from increased L-tyrosine concentrations
Next, we sought to investigate the dose effect of L-tyrosine on 4HBA productions. As shown in Fig. 3A, 50 mM L-tyrosine still afforded an efficient synthesis of 49 ± 2 mM (6.9 ± 0.3 g/L) 4HBA after 48 h with a conversion > 98% (Fig. 3A). However, when 100 mM L-tyrosine was used, only 59 ± 4 mM 4HBA was produced after 72 h, which corresponds to ∼ 59% conversion (Fig. 3A). As 4HBA is an organic acid, the accumulation of organic acid could lead to a pH decrease of the reaction system, resulting in an unfavorable environment to the enzyme activities (Chen, Fu, Xiao, Ko, Kao, Fan, et al., 2021). Next, we used the pulse-feeding of L-tyrosine to mitigate the accumulation of intermediates, and the biocatalytic system at pH 8.0 was periodically adjusted by NaOH (2.5 M). However, both strategies did not lead to a substantial improvement of the 4HBA productivity (Table 1).
Fig. 3.
Optimizing the buffer pH conditions for enhanced production of 4HBA. (A) Dose effect of different L-tyrosine concentrations on 4HBA production at pH 8.0. (B) The effect of pH conditions on 4HBA productions. All the reactions were conducted at 30 °C with 10 g cdw/L resting cells of E. coli MG1655 RARE cells. The data are the mean values with standard deviations from triplicate experiments.
Table 1.
Summary of 4HBA and benzoic acid levels under different conditions.[a]
| Product | Substrate conc. (mM) | Product conc. (mM) | Yield (g/L) | Conv. |
|---|---|---|---|---|
| 4HBA | 10 50 100 100[b] 100[c] 100[d] 150[d] |
9.9 ± 0.1 49 ± 2 59 ± 4 36 ± 1 40 ± 1 98 ± 3 128 ± 1 |
1.37 ± 0.02 6.9 ± 0.3 8.1 ± 0.5 5.0 ± 0.1 5.5 ± 0.1 13.6 ± 0.4 17.7 ± 0.1 |
>98% >98% 59.0% 36.0% 40.0% >98% 85.3% |
| Benzoic acid | 50[d] 100[d] |
41 ± 2 90 ± 3 |
5.0 ± 0.2 11.0 ± 0.4 |
82.0% 90.0% |
[a] MG1655 (DE3) ΔendA ΔrecA with ΔdkgB ΔyeaE Δ (yqhC-dkgA) ΔyahK and ΔyjgB was used.
[b] Experiments were carried out in a fed-batch mode.
[c] Experiments were carried out in a fed-batch mode with pH maintained at 8.0.
[d] Experiments were carried out in the phosphate buffer of pH 9.0.
As shown in Fig. 3B, we found that increasing the pH of phosphate buffer to 9.0 could effectively improve the 4HBA production. 100 mM L-tyrosine afforded the production of 98 ± 3 mM 4HBA (13.6 ± 0.4 g/L), with a conversion of > 98% (Fig. 3B). Notably, we noticed that there was an abrupt increase of 4HBA synthesis after 36 h, indicating that the accumulation of 4HBA might lead to a pH environment favourable to the enzyme activities. Further increasing the L-tyrosine concentration to 150 mM still afforded the synthesis of 128 ± 1 mM 4HBA (17.7 ± 0.1 g/L), with a conversion > 85% (Table 1). In addition, we also examined the phosphate buffer at pH 9.5. However, pH 9.5 did not improve the 4HBA production, and the rate of 4HBA formation was also slowed (Fig. 3B). Taken together, our work represented one of the pioneering studies to demonstrate whole-cell biocatalysis as an effective approach in achieving high-yielding production of 4HBA from L-tyrosine.
3.3. Synthesis of benzoic acid via the CoA-free multi-enzyme cascade
Benzoic acid is an important chemical that is often used as food preservative owing to its antioxidant and antimicrobial activities (Hazan et al., 2004, Wei et al., 2018). Natural benzoic acid synthesis has been discovered in plants through two major routes: β-oxidation-type pathway and nonoxidative pathway (Hertweck et al., 2001, Qualley et al., 2012). Currently, benzoic acid is commercially manufactured from petroleum-derived toluene through chemical methods such as toluene liquid phase oxidation, chlorination and hydrolysis of toluene (Maki & Takeda, 2000), which is not an environment-friendly method. The first example of microbial synthesis of benzoic acid was reported in a wild type strain of Streptomyces maritimus and a maximal level of 460 mg/L was achieved (Noda, Kitazono, Tanaka, Ogino, & Kondo, 2012). Recently, heterologous microbial cell factories such as E. coli and Pseudomonas taiwanensis have been used for manufacturing benzoic acid (Luo and Lee, 2020, Otto et al., 2020). For instance, fed-batch fermentation of the engineered E. coli strain harboring the β-oxidation pathway resulted in 2.37 ± 0.02 g/L benzoic acid from glucose, while the strain harboring a synthetic CoA-independent benzoic acid pathway produced 181.0 ± 5.8 mg/L benzoic acid from glucose (Luo & Lee, 2020). However, E. coli could only tolerate ∼ 2.0 g/L benzoic acid (Luo & Lee, 2020), which might limit further improvement of benzoic acid titer from glucose for industrial applications.
To further extend the synthetic utility of the CoA-independent multi-enzyme cascade, the recombinant E. coli was applied for the synthesis of benzoic acid from L-phenylalanine. As shown from Fig. 4, 41 ± 2 mM of benzoic acid was obtained from 50 mM L-phenylalanine after 72 h, which corresponds to 82% conversion. Further HPLC analysis revealed that mandelate (a peak at 3.0 min) was accumulated (data not shown), indicating that the intermediates were not fully converted to the end product. We also investigated the dose effect of L-phenylalanine on benzoic acid productions. We found that 100 mM L-phenylalanine could still afford 90 ± 3 mM of benzoic acid (11.0 ± 0.4 g/L, Fig. 4), with a conversion efficiency of 90%. Similar to the 4HBA production, we also observed a sharp increase in the benzoic acid titer between 36 and 48 h (Fig. 4), indicating that the change of pH condition during the biocatalytic process would also favor the benzoic acid production at a later stage.
Fig. 4.
Synthesis of benzoic acid via the CoA-independent enzymatic route. Dose effect of different L-phenylalanine concentrations on benzoic acid productions. Time course of benzoic acid synthesis from 50 mM or 100 mM L-phenylalanine with 10 g cdw/L resting cells of E. coli MG1655 RARE cells. All the reactions were conducted in KP buffer (pH 9.0) at 30 °C. The data are the mean values with standard deviations from triplicate experiments.
4. Conclusion
Taken together, our work represented one of the pioneering studies to demonstrate the whole-cell biocatalysis as an effective approach in achieving high-yielding production of 4HBA and benzoic acid from renewable feedstocks. The CoA-free multi-enzyme cascade allowed the synthesis of 4HBA and benzoic acid with good yields (85%∼99%, Table 1). The whole-cell biotransformation was performed under mild conditions without the use of toxic catalysts, which is a more environment-friendly approach than conventional chemical method. Unlike the fermentation approach that requires sophisticated skills to separate the product from the complex culture medium, our biocatalytic process might greatly simplify the procedure for product purification as only negligible amounts of intermediates were accumulated. Considering E. coli strain harboring a nine-step biocatalytic pathway that utilizes styrene as intermediate enabled 17.8 g/L benzoic acid from L-phenylalanine (Zhou, Sekar, Wu, & Li, 2020), fine-tune the individual enzyme components in our system to achieve a balanced flux might be required for the optimal catalytic efficiency of benzoic acid production. In summary, our work represents a showcase of biocatalysis as an alternative green and sustainable approach for synthesis of the food preservative of both 4HBA and benzoic acid.
Author contributions
J.Y. conceived and designed the project. YY.C., YF.C. and L.L. performed the experiments. J.Y. and Y.Z. interpreted the data and wrote the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Xiamen University under grant no. 0660-X2123310, XMU Training Program of Innovation and Entrepreneurship for Undergraduates no. 2020X902 and ZhenSheng Biotech, China.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2021.100059.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Barker J.L., Frost J.W. Microbial synthesis of p-hydroxybenzoic acid from glucose. Biotechnology and Bioengineering. 2001;76(4):376–390. doi: 10.1002/bit.10160. [DOI] [PubMed] [Google Scholar]
- Chen Y., Fu B., Xiao G., Ko L.-Y., Kao T.-Y., Fan C., Yuan J. Bioconversion of lignin-derived feedstocks to muconic acid by whole-cell biocatalysis. ACS Food Science & Technology. 2021;1(3):382–387. doi: 10.1021/acsfoodscitech.1c0002310.1021/acsfoodscitech.1c00023.s001. [DOI] [Google Scholar]
- Choi I.S., Cho E.J., Moon J.H., Bae H.J. Onion skin waste as a valorization resource for the by-products quercetin and biosugar. Food Chemistry. 2015;188:537–542. doi: 10.1016/j.foodchem.2015.05.028. [DOI] [PubMed] [Google Scholar]
- Choi K.Y., Wernick D.G., Tat C.A., Liao J.C. Consolidated conversion of protein waste into biofuels and ammonia using Bacillus subtilis. Metabolic Engineering. 2014;23:53–61. doi: 10.1016/j.ymben.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Hazan R., Levine A., Abeliovich H. Benzoic acid, a weak organic acid food preservative, exerts specific effects on intracellular membrane trafficking pathways in Saccharomyces cerevisiae. Applied and Environmental Microbiology. 2004;70(8):4449–4457. doi: 10.1128/AEM.70.8.4449-4457.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertweck C., Jarvis A.P., Xiang L., Moore B.S., Oldham N.J. A mechanism of benzoic acid biosynthesis in plants and bacteria that mirrors fatty acid beta-oxidation. Chembiochem. 2001;2(10):784–786. doi: 10.1002/1439-7633(20011001)2:10<784::Aid-cbic784>3.0.Co;2-k. [DOI] [PubMed] [Google Scholar]
- Huo Y.X., Cho K.M., Rivera J.G.L., Monte E., Shen C.R., Yan Y.J., Liao J.C. Conversion of proteins into biofuels by engineering nitrogen flux. Nature Biotechnology. 2011;29(4):346–U160. doi: 10.1038/nbt.1789. [DOI] [PubMed] [Google Scholar]
- Iding H., Dunnwald T., Greiner L., Liese A., Muller M., Siegert P.…Pohl M. Benzoylformate decarboxylase from Pseudomonas putida as stable catalyst for the synthesis of chiral 2-hydroxy ketones. Chemistry. 2000;6(8):1483–1495. doi: 10.1002/(sici)1521-3765(20000417)6:8<1483::aid-chem1483>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Kitade Y., Hashimoto R., Suda M., Hiraga K., Inui M., Kivisaar M. Production of 4-Hydroxybenzoic Acid by an Aerobic Growth-Arrested Bioprocess Using Metabolically Engineered Corynebacterium glutamicum. Applied and Environmental Microbiology. 2018;84(6) doi: 10.1128/AEM.02587-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer J.O., Nunez-Bernal D., Averesch N.J.H., Hampe J., Varela J., Varela C. Production of aromatics in Saccharomyces cerevisiae-A feasibility study. Journal of Biotechnology. 2013;163(2):184–193. doi: 10.1016/j.jbiotec.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Kunjapur A.M., Tarasova Y., Prather K.L. Synthesis and accumulation of aromatic aldehydes in an engineered strain of Escherichia coli. Journal of the American Chemical Society. 2014;136(33):11644–11654. doi: 10.1021/ja506664a. [DOI] [PubMed] [Google Scholar]
- Lafarga T., Hayes M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Science. 2014;98(2):227–239. doi: 10.1016/j.meatsci.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Lessard J.C. Molecular cloning. Methods in Enzymology. 2013;529:85–98. doi: 10.1016/b978-0-12-418687-3.00007-0. [DOI] [PubMed] [Google Scholar]
- Lindsey A.S., Jeskey H. The Kolbe-Schmitt Reaction. Chemical Reviews. 1957;57(4):583–620. doi: 10.1021/cr50016a001. [DOI] [Google Scholar]
- Liu L., Zhu Y., Chen Y., Chen H., Fan C., Mo Q., Yuan J. One-Pot Cascade Biotransformation for Efficient Synthesis of Benzyl Alcohol and Its Analogs. Chemistry – An AsianJournal. 2020;15(7):1018–1021. doi: 10.1002/asia.201901680. [DOI] [PubMed] [Google Scholar]
- Luo Z.W., Lee S.Y. Metabolic engineering of Escherichia coli for the production of benzoic acid from glucose. Metabolic Engineering. 2020;62:298–311. doi: 10.1016/j.ymben.2020.10.002. [DOI] [PubMed] [Google Scholar]
- Maki T., Takeda K. Benzoic Acid and Derivatives. In Ullmann's Encyclopedia of Industrial. Chemistry. 2000 doi: 10.1002/14356007.a03_555. [DOI] [Google Scholar]
- Mao Z., Liu L., Zhang Y., Yuan J. Efficient Synthesis of Phenylacetate and 2-Phenylethanol by Modular Cascade Biocatalysis. Chembiochem. 2020;21(18):2676–2679. doi: 10.1002/cbic.v21.1810.1002/cbic.202000182. [DOI] [PubMed] [Google Scholar]
- Massad G., Zhao H., Mobley H.L. Proteus mirabilis amino acid deaminase: Cloning, nucleotide sequence, and characterization of aad. Journal of Bacteriology. 1995;177(20):5878–5883. doi: 10.1128/jb.177.20.5878-5883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U., van Assema F., Gunsior M., Orf S., Kremer S., Schipper D.…Wubbolts M. Metabolic engineering of the E. coli L-phenylalanine pathway for the production of D-phenylglycine (D-Phg) Metabolic Engineering. 2006;8(3):196–208. doi: 10.1016/j.ymben.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Noda S., Kitazono E., Tanaka T., Ogino C., Kondo A. Benzoic acid fermentation from starch and cellulose via a plant-like β-oxidation pathway in Streptomyces maritimus. Microbial Cell Factories. 2012;11:49. doi: 10.1186/1475-2859-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M., Wynands B., Marienhagen J., Blank L.M., Wierckx N. Benzoate Synthesis from Glucose or Glycerol Using Engineered Pseudomonas taiwanensis. Biotechnology Journal. 2020;15(11):2000211. doi: 10.1002/biot.v15.1110.1002/biot.202000211. [DOI] [PubMed] [Google Scholar]
- Payet L.A., Leroux M., Willison J.C., Kihara A., Pelosi L., Pierrel F. Mechanistic Details of Early Steps in Coenzyme Q Biosynthesis Pathway inYeast. Cell Chemical Biology. 2016;23(10):1241–1250. doi: 10.1016/j.chembiol.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Pontrelli S., Chiu T.Y., Lan E.I., Chen F.Y., Chang P., Liao J.C. Escherichia coli as a host for metabolic engineering. Metabolic Engineering. 2018;50:16–46. doi: 10.1016/j.ymben.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Qualley A.V., Widhalm J.R., Adebesin F., Kish C.M., Dudareva N. Completion of the core beta-oxidative pathway of benzoic acid biosynthesis in plants. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):16383–16388. doi: 10.1073/pnas.1211001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert M., Severin K., Heide L. Formation of 4-hydroxybenzoate in Escherichia coli: Characterization of the ubiC gene and its encoded enzyme chorismate pyruvate-lyase. Microbiology (Reading, England) 1994;140(Pt 4):897–904. doi: 10.1099/00221287-140-4-897. [DOI] [PubMed] [Google Scholar]
- Straathof A.J.J. Transformation of Biomass into Commodity Chemicals Using Enzymes or Cells. Chemical Reviews. 2014;114(3):1871–1908. doi: 10.1021/cr400309c. [DOI] [PubMed] [Google Scholar]
- Sytar O., Brestic M., Rai M., Shao H. Plant phenolic compounds for food, pharmaceutical and cosmetics production. Journal of Medicinal Plant Research. 2012;6:2526–2539. doi: 10.5897/JMPR11.1695. [DOI] [Google Scholar]
- Verhoef S., Ruijssenaars H.J., de Bont J.A., Wery J. Bioproduction of p-hydroxybenzoate from renewable feedstock by solvent-tolerant Pseudomonas putida S12. Journal of Biotechnology. 2007;132(1):49–56. doi: 10.1016/j.jbiotec.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Wei Q., Wang X., Cheng J.H., Zeng G., Sun D.W. Synthesis and antimicrobial activities of novel sorbic and benzoic acid amide derivatives. Food Chemistry. 2018;268:220–232. doi: 10.1016/j.foodchem.2018.06.071. [DOI] [PubMed] [Google Scholar]
- Wildermuth M.C. Variations on a theme: Synthesis and modification of plant benzoic acids. Current Opinion in Plant Biology. 2006;9(3):288–296. doi: 10.1016/j.pbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Yuan J., Chen X., Mishra P., Ching C.B. Metabolically engineered Saccharomyces cerevisiae for enhanced isoamyl alcohol production. Applied Microbiology and Biotechnology. 2017;101(1):465–474. doi: 10.1007/s00253-016-7970-1. [DOI] [PubMed] [Google Scholar]
- Yuan J., Lukito B.R., Li Z. De Novo Biosynthesis of (S)- and (R)-Phenylethanediol in Yeast via Artificial Enzyme Cascades. ACS Synthetic Biology. 2019;8(8):1801–1808. doi: 10.1021/acssynbio.9b0012310.1021/acssynbio.9b00123.s001. [DOI] [PubMed] [Google Scholar]
- Zhou Y.i., Sekar B.S., Wu S., Li Z. Benzoic acid production via cascade biotransformation and coupled fermentation-biotransformation. Biotechnology and Bioengineering. 2020;117(8):2340–2350. doi: 10.1002/bit.v117.810.1002/bit.27366. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Yang T., Chen Y., Fan C., Yuan J. One-Pot Synthesis of Aromatic Amines from Renewable Feedstocks via Whole-Cell Biocatalysis. ChemistrySelect. 2020;5(45):14292–14295. doi: 10.1002/slct.202003807. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.