Abstract
In the light of clean beauty and sustainability requirements emerging in the personal care market, the urgent need for the replacement of silicones in hair conditioners—with comparable performance and customer experience—has been highlighted in the industry. In this context, the goal of the present study was to investigate the physical effects of different silicone-free conditioner formulations on Mongolian hair after damage due to bleaching and compare the results to property changes induced by a classical silicone-containing formulation. To that end, the morphology, structure, and composition of strands and individual fibers of this hair type were characterized before and after bleaching by means of optical microscopy, atomic force microscopy (AFM), and X-ray photoelectron spectroscopy (XPS). It is shown that oxidative bleaching causes significant damage to the native hair surface, leading to local depletion or even large-area removal of the outer hydrophobic lipid layer. This results in enhanced wettability of the bleached hair by water (as confirmed by contact angle measurements) and is accompanied by an undesired loss of hair gloss and softness. Upon treatment with suitable cosmetic emulsions, the natural hydrophobicity of intact Mongolian hair can be partially or fully restored, with silicone-free formulations having effects similar to those of established silicone-containing products. The successive influence of bleaching and conditioning was further monitored using inverse gas chromatography (iGC), a technique that probes changes in surface energetics and polarity over an ensemble of an entire hair strand through interactions with specific molecules at the solid/gas interface. The resulting data mirror the macroscopic behavior of the bleached/conditioned hair and provide a quantitative scale for measuring damage and repair effects. Most notably, the effect of bleaching and subsequent conditioning on the haptic perception of hair strands could also be quantified with the aid of a biomimetic measurement system, which identifies increased friction (both tactile and sliding) as the major cause for the strawy feel of bleached hair and indicates successful relubrication after treatment with suitable conditioner formulations. Finally, the different physical properties determined for native, bleached, and reconditioned Mongolian hair are found to be reflected in application-oriented tests, namely in vitro measurements of wet and dry combing work. Overall, the data collected in this work shed novel light on the surface properties of Mongolian hair and highlight that effective hair conditioning after damage can be achieved without silicones in advanced cosmetic emulsions based on octyldodecyl myristate and glyceryl oleate.
Introduction
Human hair is a complex material with hierarchical structures based on fibrous keratin building blocks, i.e. α-helical protein filaments rich in cysteine units, which aggregate into larger textures and interconnect via disulfide bridges.1 The outer part of the hair, the so-called “cuticle”, consists of three sublayers (epi-, exo-, and endocuticle) and generally serves the purpose of protection against environmental stresses.2 The outermost epicuticular domain is additionally covered by a layer of 18-methyleicosanoic acid (18-MEA, the so-called “β-layer”), long-chain lipids that are bound to the protein network via thioester linkages with cysteine units. The β-layer imparts natural hydrophobicity to the hair surface and thus prevents ample uptake of water (swelling), which in turn facilitates the transition from wet to dry hair (by reducing capillary forces and favoring parallel alignment over entangling).3 It also accounts for other properties of virgin hair, such as sensory perception (softness),4 appearance (shine),5 and lubricity.6 In turn, when the β-layer is disintegrated or completely lost through natural stresses or cosmetic treatments (e.g., heat-induced damage during perming or oxidative degradation in the course of bleaching and dyeing), the physical properties of hair surfaces change substantially and undesired aesthetic effects such as hair tangling or optical dullness may occur.
Hair conditioners and cosmetic treatments are widely used to recover the different types of damage and restore the native properties as much as possible, providing the hair with pleasant features such as easy combing, smoothness, softness, and glossy appearance.7 Traditionally, silicone oils have been applied as active ingredients in hair care products due to their superior lubricating properties and the characteristic soft and smooth feel they impart upon treatment with corresponding formulations.8 With the recent advent of “clean beauty” trends and the increasing societal demand for sustainable solutions, there is a strong need in the personal care market to replace silicones in conditioner formulations while the product performance is maintained or even outperformed.
The effects of different types of damage and subsequent “repair” by conditioner formulations on the morphological, structural, and physicochemical properties of human hair have been studied extensively in the past, using a wide range of sophisticated characterization techniques such as optical microscopy, atomic force microscopy, X-ray photoelectron spectroscopy or wetting experiments.9−12 In the present work, we built upon this knowledge and investigated the overall effect of oxidative bleaching on hair surfaces and the (partial) recovery of native properties by treatment with cosmetic emulsions, highlighting three distinct aspects: first, all our studies were focused on Mongolian hair, a subclass of the Asian type, which has received much less attention in past research in comparison to the Caucasian type despite its high relevance for the global personal care market. Although human hair is commonly divided into three major categories (Asian, African, and Caucasian), such broad classifications fail to adequately describe the extreme biological diversity of hair types found in local ethnic groups of different or even mixed origin.13−15 Asian hair is generally known for its dark color, straight to wavy curvature, and large diameter, which together with compact cuticle structures render this type of hair mechanically very robust.16 The Mongolian subclass chosen in the present work is relatively soft, shiny, and fine, i.e., thinner and not as coarse as typical Asian hair.
Second, we employed a combination of methods to gain a complete picture of surface property changes, including the techniques mentioned above but also introducing novel approaches to hair characterization—most notably inverse gas chromatography (iGC)17−19 and haptic assessment based on a biomimetic measurement system.20,21 While iGC provides fundamental thermodynamic parameters (such as disperse surface energy) reflecting and quantifying physical consequences of hair treatment, sensory evaluation by an “artificial finger” delivers unbiased scientific data describing changes in haptic properties due to modulated surface friction and/or texture, which might have the potential to complement or even replace human panel tests for hair care products.
Third and most importantly, we used the different characterization techniques to study the effects of silicone-free conditioners based on octyldodecyl myristate, with and without glycerol oleate as an emulsifier, on bleached Mongolian hair in direct comparison to conventional formulations comprising silicone oil. The results obtained from the various analyses paint a consistent picture and suggest that silicone-free products can compete with silicone-containing systems when they are formulated properly—a notion that is sustained by in vitro combing tests performed on the same set of samples under application-near conditions.
Results and Discussion
Effects of Bleaching on the Physical Properties of Hair Surfaces
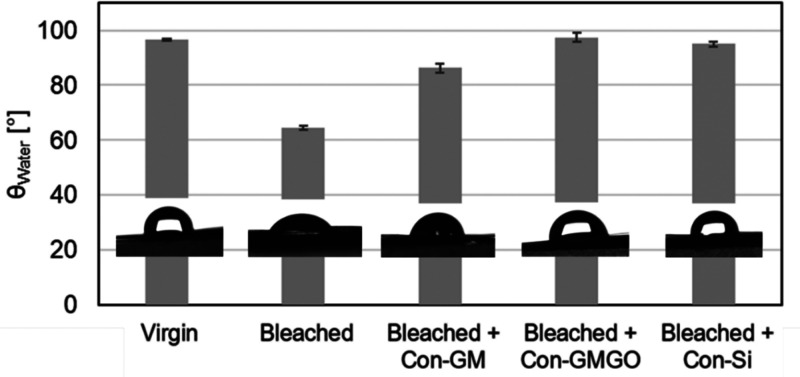
The hydrophobicity of virgin hair becomes immediately evident in the wetting behavior of water droplets on multiple hair fibers, as shown in Figure 1. The contact angle (θWater) determined for native Mongolian hair in five independent measurements is 97.0 ± 1.7° (Figure 1a). Upon oxidative bleaching, the natural hydrophobicity is partially lost, leading to better wetting with water (θWater = 62.5 ± 0.8°, Figure 1b) and rendering hair drying more difficult (as described below).
Figure 1.
Wettability of hair fibers, as probed by depositing a water droplet on a fiber bundle of (a) virgin and (b) bleached Mongolian hair. Note that the inclination of the hair substrate was accounted for in the evaluation of contact angles by setting an appropriate baseline.
This indicates that the 18-MEA layer is removed (at least in part) from the hair surface during the bleaching process—a notion that is consistent with results reported in previous studies12 and XPS analyses performed in the present work (see Table S1 in the Supporting Information). Generally, the hair surfaces contain carbon, oxygen, nitrogen, and sulfur as the main elements expected on the basis of the composition discussed above, as well as smaller amounts of calcium (ubiquitous in living systems) and silicone (presumably from prior treatment of the hair samples with care products). Bleaching results in a decrease in the amount of carbon and concomitant increases in the detected concentrations of oxygen and nitrogen—which reflects the removal of long-chain lipids and the exposure of proteinaceous material deeper down in the cuticle. Detailed analyses of the carbon binding states confirm this conclusion, as the fractions of hydrocarbon-like species (C–C, C–H) and carboxylate functions decrease while those of amino acid related species (C–O, C–N) increase. However, the removal of the 18-MEA is not the only process occurring upon bleaching, as revealed by the distribution of sulfur binding states: after bleaching, the fraction of (di)sulfide groups has decreased while more sulf(on)ated functionalities are detected. Although this trend is in part due to the removal of the 18-MEA chains from their thioester anchor, it can also be traced back to the cleavage of disulfide bonds linking the protein fibers into cysteic acid species. The removal of the protective β-layer and the exposure of more polar entities explains the macroscopically observed increase in wettability by water (cf. Figure 1b).
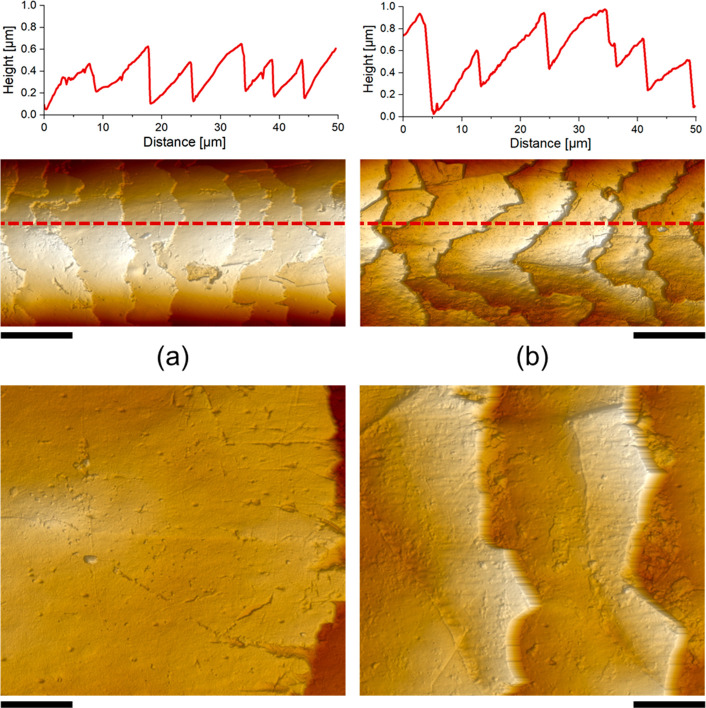
The effect of bleaching on the structure and morphology of hair surfaces can be clearly observed in AFM images, as shown in Figure 2. In comparison to virgin hair (Figure 2a), the bleached surface exhibits higher roughness on individual cuticular terraces and the edges of steps separating adjacent terraces appear sharper and somewhat corroded (Figure 2b). Both the step heights (ca. 300–600 nm; cf. height profiles in Figure 2) and morphologies also seem to be affected by the bleaching, although some of the observed features may also be due to natural variability between the fibers investigated before and after bleaching. In some cases, parts of the terraces have been lifted off the surface, leaving voids or smaller defects; however, removal of entire cuticles and excavation of exo- or endocuticular layers did not occur after the singular bleach applied here. A closer look at the terraces (bottom panels in Figure 2) reveals relatively smooth and homogeneous topographies on virgin hair, whereas after bleaching particulate deposits seem to be scattered across the surface. Most likely, these deposits are fragments of the original outer hydrophobic β-layer, remaining after its oxidative removal. All of these structural changes affect the macroscopic properties of the bleached hair fibers, which appear less shiny and feel strawier.
Figure 2.
AFM height images of (a) virgin and (b) bleached Mongolian hair at lower (50 μm × 25 μm, top, with the corresponding linear height profiles along the dotted red lines) and higher (10 μm × 10 μm, bottom) magnifications. Scale bars are 10 μm (top panels) and 2 μm (bottom panels).
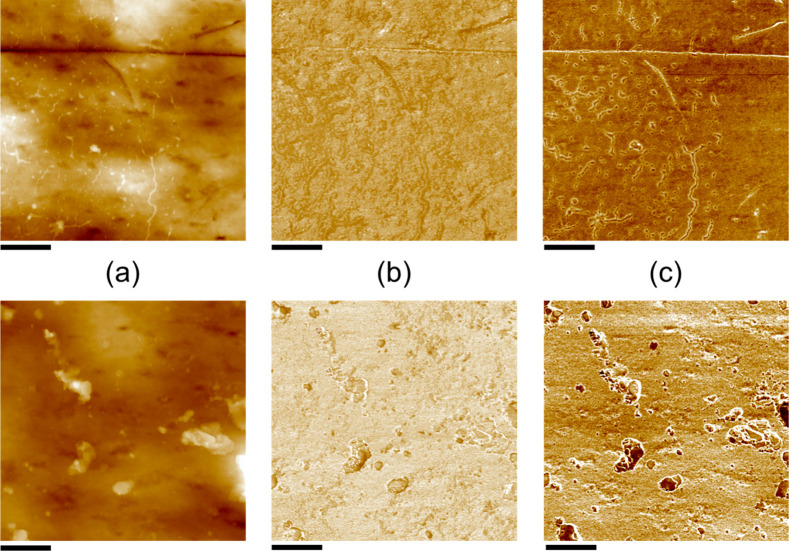
Further insights into the changes induced by bleaching can be gained by AFM-based nanomechanical analyses. Figure 3 shows height images of local areas on the surface of individual terraces, along with qualitative mappings of two different properties (adhesion and elastic modulus) across the same region. Again, numerous particulate species of different sizes and shapes are observed on the surfaces after bleaching (lower panels in Figure 3). In terms of adhesion, the base parts of the bleached cuticles exhibit increased stickiness in comparison to the virgin state (Figure 3c)—in line with the notion that rather weakly adhesive lipid layers are removed and more “sticky” proteinaceous material is exposed. Accordingly, the particles found on the bleached hair show significantly lower adhesion in comparison to their underlying parts, confirming them to be remainders of the original 18-MEA layer. Finally, a comparison of the elastic properties suggests that bleaching results in stiffer surfaces (i.e., higher E modulus; Figure 3b), presumably because the 18-MEA layer provides softness and flexibility in the native state. In many ways, the morphological and nanomechanical features observed before and after damage in Figures 2 and 3 are consistent with previous AFM-based studies on Caucasian human as well as animal hair,2,6,22,23 suggesting distinct similarities in surface properties and damage mechansism for different hair types.
Figure 3.
Nanomechanical characterization of virgin (top panels) and bleached (bottom panels) Mongolian hair yielding 2 μm × 2 μm mappings with (a) height, (b) DMT modulus, and (c) adhesion contrast. Note that the probed properties can be compared on a relative level (as the virgin and bleached samples were measured with the same cantilever) but cannot be quantified in absolute numbers, as the cantilever was not calibrated against known standards.
Taken together, our analyses of the structure, composition, and wettability of the Mongolian hair used in this work demonstrate that bleaching essentially causes significant damage, depletion, or even large-area removal of the lipid β-layer present on virgin hair. As a consequence, the natural hydrophobicity of the hair surface is partially lost, because the more hydrophilic material constituting the inner cellular structure becomes exposed. This not only should increase the propensity of the hair to take up water (or, vice versa, impede drying) and weaken the protective barrier against pollutants but will also result in substantial changes of other key properties. Effects explicitly observed here include reduced softness and lower lubricity (stronger adhesion), both of which should affect the haptic perception, as discussed in more detail below (note that the optical appearance of the hair also changes upon bleaching, although this effect will not be addressed further here).
Effects of Cosmetic Emulsions on the Properties of Bleached Hair Surfaces
In the light of the observations described above, it is evident that the task of a conditioner formulation should be to modify damaged hair surfaces in such a way that as many of the functions of the native 18-MEA layer are being restored. It will be shown in the following that the cosmetic emulsions given in Table 1 accomplish this task in various aspects and that the silicone-free formulations Con-GM and Con-GMGO are able to compete with or even outperform conventional silicone-containing systems such as Con-Si.
Table 1. Combinations of Emollients and W/O Emulsifier Used in Cosmetic Emulsions for Hair Treatment.
| code | emollient | W/O emulsifier |
|---|---|---|
| Con-Si | 2% dimethicone, 50 cst | |
| Con-GM | 2% Eutanol GM | |
| Con-GMGO | 2% Eutanol GM | 0.5% Monomuls 90-O18 |
With regard to wettability, treatment of the bleached Mongolian hair results in an increased water contact angle for all three formulations studied (Figure 4), with Con-Si and especially Con-GMGO recovering the value of the virgin hair and thus substantiating the claim of a “repair” function. This effect is ascribed to efficient depositioning and spreading of the hydrophobic active ingredients (i.e., silicone oil and octyldodecyl myristate, respectively) on the damaged hair surface.
Figure 4.
Comparison of average contact angles (with corresponding standard deviations resulting from at least five independent measurements) determined for water droplets on virgin and bleached hair as well as on bleached hair after treatment with different conditioner formulations as indicated. Inserted photographs show the typical shape of sessile water droplets on the different hair surfaces.
While contact angle experiments with water droplets provide the advantage of delivering a direct measure of the hair hydrophobicity, they are associated with drawbacks related to the fact that the hair surface is probed only locally and that variations in surface roughness may affect the results. Therefore, in an alternative approach, we used inverse gas chromatography (iGC) to probe changes in hair surface energetics (and polarity) caused by bleaching and subsequent conditioner treatment (Figure 5). In an iGC experiment, the entire surfaces of hair strands packed into a GC column are being sampled and, since the technique uses gases to probe the surfaces of interest, roughness effects such as those encountered with the wetting of liquids do not play a major role (except for size exclusion phenomena at the molecular scale).
Figure 5.
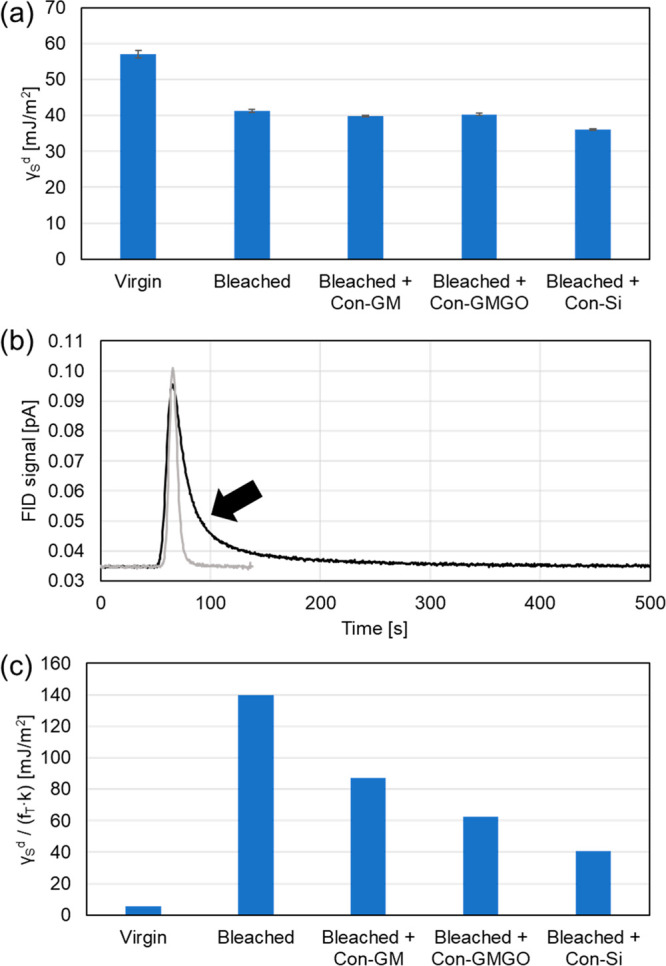
Results of iGC analyses on the surface energetics of hair in the virgin state, after bleaching, as well as after bleaching and subsequent treatment with different conditioner formulations. (a) Dispersive surface energy values obtained from a series of pulsed injections of n-alkanes, using the peak center of mass for evaluation according to the model of Dorris and Gray. (b) Chromatograms showing the elution of n-octane from columns filled with virgin (black) and bleached (gray) hair. The arrow highlights strong peak tailing in the case of the native hair. (c) Trends in dispersive surface energy after correction for peak tailing (fT) and different probe residence times (as given by the retention factor k).
One standard property measured by iGC in the so-called “infinite dilution” regime is the dispersive part of the surface energy of the studied material, γSd, which is commonly obtained from a series of pulsed injections of homologous n-alkanes. The results of such types of analyses are shown in Figure 5a. Intuitively, one would expect the (hydrophobic) native hair surface to exhibit low interfacial energies with air (or helium in the given experimental setup), whereas bleaching should lead to an increase in γSd as high-energy (polar) sites are being formed (cf. Table S1 in the Supporting Information). However, an opposite trend is observed for dispersive surface energies determined as described by eqs 2 and 3, while treatment with any of the cosmetic emulsions does not change the γSd value of the bleached hair noticeably. This unexpected behavior can be understood when considering that the native hair surface is not a “hard” two-dimensional interface but exhibits “soft” character (due to the 18-MEA layer) that allows gaseous probe molecules to undergo 3D sorption into the bulk volume. Clearly, such effects will lead to longer elution times and thus stronger apparent interactions, as desorption from the bulk volume should be significantly slower than that from sites at the outermost surface. After bleaching, the absence of the “soft” 18-MEA layer will result in less pronounced 3D sorption and therefore weaker apparent interactions. These circumstances become immediately evident in corresponding chromatograms (Figure 5b), where distinct tailing is observed for virgin hair while bleached hair gives a more Gaussian shape under the same conditions. Since γSd values are derived from the center of mass (COM) of the elution peaks, any asymmetry caused by 3D sorption will affect the result and lead to higher “apparent” dispersive surface energies (note that the same trend is observed for polar probe molecule—i.e. stronger interactions with the less polar native hair surface—for the same reason, data not shown). To account for these phenomena and extract a trend for the “true” energetics of the outer surface, we have determined the dimensionless tailing factors (fT) for the individual alkane peaks (fT = (a + b)/2a, where a and b are the widths of the peak to the left and right, respectively, of the peak maximum at a relative peak height of 5%) as a measure of peak tailing due to the ability of the material to allow 3D sorption of probe molecules. Another parameter to consider is the net retention time, or the (dimensionless) retention factor (k = tN/t0, where t0 is the dead time of the column), because longer dwelling of probe molecules at the surface will increase the probability for 3D sorption. Thus, we propose to correct the measured dispersive surface energy for these two effects and use the ratio γSd/(fTk) in order to separate 2D from 3D effects. The results of this semi-empirical approach to correction are shown in Figure 5c, where fT and k values of the n-octane peaks were employed exemplarily. Although the absolute magnitude of γSd/(fTk) per se has no physical meaning, the relative trends among the different studied hair samples appear reasonable: bleaching leads to a drastic increase in (corrected) surface energy, while treatment with conditioner formulations partially (though not entirely) restores the value of the virgin hair with an apparent “repair” efficiency of Con-Si > Con-GMGO > Con-GM—in good agreement with the data obtained by contact angle measurements with water (cf. Figure 4).
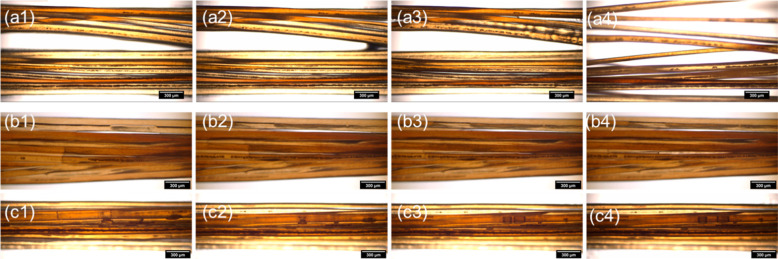
The impact of the physical effects described above on end-consumer-relevant hair properties was assessed in three ways. First, bundles of wet hair fibers were treated with pure octyldodecyl myristate or glyceryl oleate and the subsequent drying process was monitored by acquiring time-lapse optical micrographs, as shown in Figure 6. On bleached hair without any treatment (Figure 6a), water is spread as thin films over the relatively hydrophilic surfaces (cf. Figures 1b and 4) and closely connects individual fibers via capillary forces. With progressive drying, menisci are formed and the concurrent stresses cause fiber misalignment and increasing disorder. After treatment with the emollient octyldodecyl myristate (Figure 6b), the wet hair fibers remain closely aligned during drying and seem to be “glued” together by the hydrophobic oil. However, the time needed for complete drying under these conditions is significantly longer than that without prior treatment. Most probably, the oil is not able to displace the water films from the bleached hair surface and thus traps them along oil/water interfaces. In the presence of glyceryl oleate (the key active ingredient in Con-GMGO), the drying behavior of bleached hair is fundamentally different (Figure 6c): here, no extended water films are observed and, instead, the remaining aqueous islands shrink toward spherical droplets, which leads to significantly faster overall drying and avoids the adverse influence of capillary forces. Due to its amphiphilic character, glyceryl oleate likely adsorbs on the bleached hair via its hydrophilic head, displacing the water and rendering the surface hydrophobic via the exposed nonpolar tails. This highlights the importance of a suitable emulsifier in a conditioner formulation and shows that it not only contributes to formulation stability but also acts during application thanks to its surface activity (and chemical compatibility with the oil).
Figure 6.
Optical micrographs showing the progressive drying (from left to right) of bleached Mongolian hair fibers that were wetted with water after (a1–a4) no prior treatment, (b1–b4) prior treatment with octyldodecyl myristate, and (c1–c4) prior treatment with glyceryl oleate.
In a second set of experiments, the undesired “strawy” feel of bleached hair and possible “repair” effects of the different cosmetic emulsions were assessed. Instead of relying on subjective impressions, we used a biomimetic haptics measurement system (see Figure S3 in the Supporting Information) to quantify changes on a scientific level. On the basis of the sample configuration and the sliding motion profile of the artificial finger employed for this purpose, the following seven haptic properties could be derived (all scaled in relative responses from 0 to 100; see Table S2 in the Supporting Information for a more detailed description):20,21,24 macrotexture (mTX), macrotexture coarseness (mCO), macrotexture regularity (mRG), microtexture roughness (uRO), microtexture coarseness (uCO), tactile stiction (fST), and sliding resistance (fRS). Among these parameters, only those related to friction (i.e., fST and fRS) showed differentiation for the distinct types of hair investigated, whereas all responses belonging to the category of texture did not vary significantly between hair surfaces that were bleached and/or treated with cosmetic emulsions. Values obtained for fST and fRS are summarized in Figure 7. In terms of tactile stiction (or initial grip, Figure 7a), bleaching leads to an increase in the fST parameter, indicating a change from weaker resistance against the onset of a sliding movement (as observed e.g. for Teflon) toward a stronger initial grip (i.e., more rubber-like)—in line with the notion of the native 18-MEA layer providing softness and lubrication to the hair surface. Treatment with the cosmetic emulsions results in a decrease of fST back to or below the value of the virgin hair in all cases, suggesting a trend of “repair” efficiency according to Con-Si > Con-GMGO > Con-GM (when the measurement with a large error bar for Con-Si is ignored in Figure 7a). Essentially the same trends, with significantly lower standard deviations, are observed for the second friction-related parameter fRS, i.e. the sliding resistance (Figure 7b), which is conceptually similar to dynamic friction and indicates a change from a more slippery virgin hair surface to a more resistive (“sandpaper-like”) bleached surface. Again, this result of the damage due to the bleaching process is “repaired” after treatment with cosmetic emulsions, whereby Con-Si and Con-GMGO lend an even more slippery character in comparison to that observed for virgin hair. When the data are taken together, this quantitative haptic evaluation substantiates the claim that the conditioner formulations used—both silicone-containing and silicone-free—are able to restore or even surpass the lubricity characteristic of Mongolian hair in its native state.
Figure 7.
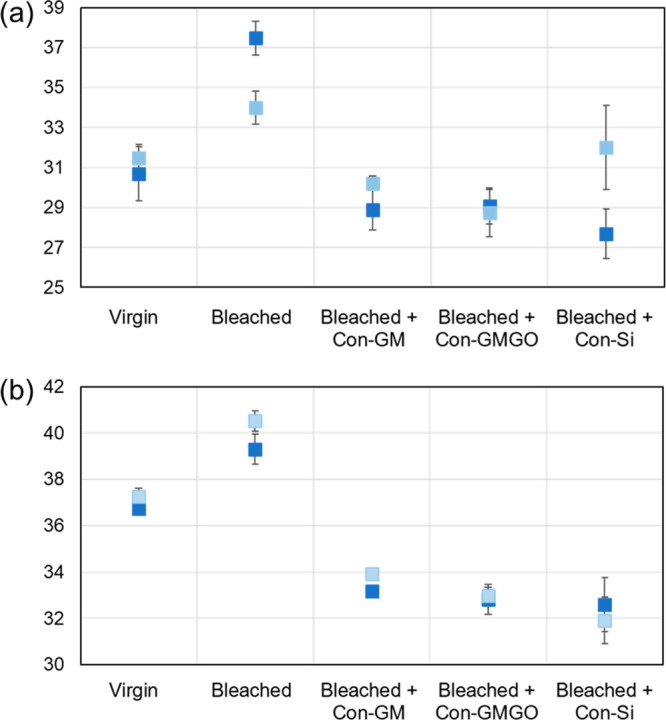
Haptic characterization of virgin hair, bleached hair, and bleached hair after treatment with different conditioner formulations: (a) tactile stiction (fST) and (b) sliding resistance (fRS) as major differentiating responses of the Toccare Haptics Measurement System in the case of human hair. Values on the y-axes provide relative scales for the degree of a given property, ranging from 0 to 100 and corresponding to weak-gripped and slippery-resistive for fST and fRS, respectively. For each sample, average responses (with their standard deviations) are given for two independent measurements (light and dark blue symbols).
Finally, we have investigated the effect of conditioner treatment on the properties of bleached Mongolian hair under “application” conditions using in vitro hair combing tests. Results for the residual combing work determined in such tests are summarized in Figure 8.
Figure 8.
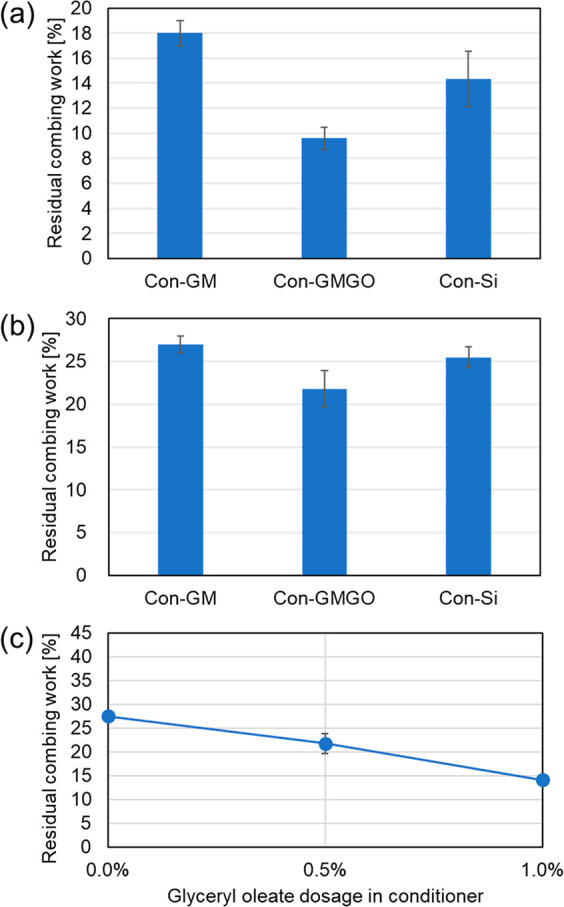
Results of in vitro hair combing tests, given as the residual combing work determined (a) in the wet state after treatment with different conditioner formulations, (b) in the dry state after treatment with different conditioner formulations, and (c) in the dry state after treatment with GM formulations containing different amounts of the emulsifier glyceryl oleate.
In both the wet (Figure 8a) and dry (Figure 8b) states, treatment with all three cosmetic emulsions leads to a substantial reduction in combing work (by respectively 80–90% and 70–80%, respectively, relative to untreated bleached hair). Interestingly, the silicone-free formulation Con-GMGO can compete with, or even outperform, the silicone-containing system Con-Si (especially in the wet combing test), while the reduction in combing work is somewhat less pronounced in the case of Con-GM, i.e., the silicone-free formulation lacking the emulsifier glyceryl oleate. The key role of this component in enhancing conditioning performance is further illustrated by dry combing tests with Con-GM formulations to which increasing amounts of glycerol oleate had been added (Figure 8c). Clearly, the combing effort decreases as more emulsifier is present—most likely because glyceryl oleate enables a more homogeneous distribution of octyldodecyl myristate across the damaged hair surface and/or the ingredient provides hair lubrication by itself after adsorption and hydrophobization of the bleached hair (as shown by the experiments depicted in Figure 6). Thus, the role of the emulsifier appears to be dual: fine(r) dispersion of emollient droplets in the formulation during application and hair surface modification to increase compatibility with the hydrophobic emollient oil.
The data presented above strongly suggest that cosmetic emulsions based on octyldodecyl myristate can modify damaged hair surfaces to an extent that is comparable to the effects of systems formulated using a classical silicone oil such as dimethicone. To shed further light on the reasons for the good performance of the silicone-free formulations, we have studied the wetting behavior of both oils and, for comparison, water on bleached Mongolian hair by means of the Washburn technique (as illustrated by Figure S2 in the Supporting Information). Time-dependent sorption profiles (see Figure S4 in the Supporting Information) indicate that dimethicone most readily wets the damaged hair surface, whereas less pronounced and rather poor wetting is observed for octyldodecyl myristate and water, respectively. This trend is commensurate with the surface tensions of the three liquids (γDimethicone/Air = 19.4 mN/m, γOctyldodecyl myristate/Air = 29.1 mN/m, γWater/Air = 72.8 mN/m) and suggests that the low interfacial energy between the silicone oil and air drives wetting and an even distribution of the oil over the hair surfaces. However, during application of a conditioner formulation, good spreading of an oil requires the interfacial energies between (i) oil and the hair surface and (ii) oil and the surrounding aqueous environment to be as low as possible. Therefore, we have measured the interfacial tensions of dimethicone and octyldodecyl myristate with water as a proxy for the second contribution. Indeed, the results confirm that the energetic penalty related to the interface between water and the silicone-free emollient is less severe (γOctyldodecyl myristate/Water = 28.9 mN/m) than in the case of the silicone oil (γDimethicone/Water = 43.7 mN/m). Although the interfacial energy between the two oils and the bleached hair surface is per se unknown and not straightforward to determine experimentally, it can be assumed that dimethicone will not show a better compatibility with the largely polar surface in comparison to octyldodecyl myristate, especially when the latter is combined with the interfacial “compatibilizer” glycerol oleate. These considerations are in line with the experimental results discussed above and suggest that different interfacial scenarios need to be taken into account when the conditioning effects of both silicone-containing and silicone-free formulations are interpreted.
Conclusion
In this work, the effects of common oxidative bleaching on the properties of Mongolian hair were investigated with respect to morphology, structure, composition, and wettability. In addition to well-established characterization techniques such as atomic force microscopy and X-ray photoelectron spectroscopy, we also employed novel and powerful methods to trace changes in terms of surface energies (using inverse gas chromatography) and haptic perception (using a biomimetic measurement system). The different analyses paint a consistent picture and identify the depletion or even large-area removal of the lipid β-layer present on the native hair surface as the major cause of damage and related changes in properties. This results in increased roughness and the exposure of more polar entities at the hair surface, which increases the wettability by water as well as the apparent surface energy. The damage also leads to a significant loss of hair shininess and softness, causing a “strawy” feeling, as reflected in the measured friction-related haptic parameters.
The second focus of our study was to evaluate the ability of different cosmetic emulsions to restore the native properties by treatment after oxidative bleaching. Both classical silicone-containing formulations and alternative silicone-free recipes based on octyldodecyl myristate as an emollient were found to be able to modify the damaged hair surface in a way that the native hydrophobicity and low apparent surface energy were (partially) recovered, indicating a desired “repair” effect. These changes in physical properties become directly manifested in key performance criteria such as reduced tactile stiction and sliding resistance, as well as lower combing work in both the dry and wet states. The conditioning performance of the silicone-free formulation could be further enhanced by introducing glycerol oleate as an emulsifier, which proved to modify the hair surface by adsorption, rendering it highly hydrophobic and more compatible for lubrication with an oil such as octyldodecyl myristate. These findings highlight that silicones can successfully be replaced in personal care products by more sustainable solutions if the new ingredients are carefully selected and properly formulated. Last but not least, a comprehensive physical characterization as achieved with the different techniques applied in this work has proven to provide valuable guidance in the design of advanced hair care formulations.
Materials and Methods
Preparation of Cosmetic Emulsions
Cosmetic emulsions containing different types of emollients were prepared as follows: 1.87% behentrimonium chloride, 4% cetearyl alcohol, and 2% emollient and optionally 0.5% W/O emulsifier, were mixed and heated at 80–85 °C until melting. The homogeneous molten mixture was then emulsified with water to give the relevant hair conditioning emulsions. The pH of these formulations was adjusted with lactic acid solution to 4.0–4.5. Preservation was ensured by addition of 1,3-dimethylol-5,5-dimethylhydantoin (DMDM-Hydantoin) and iodopropynyl butylcarbamate. Table 1 describes the three different formulations studied in this work, while the chemical structures of the silicone-free emollient used (Eutanol GM, octyldodecyl myristate, supplied by BASF) and emulsifier (Monomuls 90-O18, glyceryl oleate, supplied by BASF) are shown in Figure S1 in the Supporting Information. Note that both Eutanol GM and Monomuls 90-O18 are qualified products for safe use on skin and hair in personal care formulations. Dimethicone was supplied by DowDuPont Inc.
Treatment of Human Hair with Cosmetic Emulsions
Virgin Mongolian hair strands (15 cm in length, 2 g in weight), collected from Chinese people of the Han ethnicity, were purchased from International Hair Importer Products Inc. (New York, USA). Bleached Mongolian hair strands were prepared by immersing the virgin hair twice in 17% hydrogen peroxide solution for 30 min each. Both virgin and bleached hair strands were brushed with 0.25 g of the cosmetic emulsions given in Table 1, subsequently rinsed under tap water for 1 min, and then dried in air.
Contact Angle Measurements
Wetting experiments were carried out by using a JC2000D1 contact angle meter in a sessile drop configuration. Several fibers of the hair strands (with or without prior conditioner treatment) were aligned on a glass slide and fixed horizontally by adhesive tape. Subsequently, a droplet of water was placed on the substrates through the dosing device, while the entire process of water deposition and spreading on the hair surface was monitored by means of a high-speed camera (Daheng Image DH-HV1303UM). From images taken at intervals of 0.05 s, the contact angles of the water droplets were determined at initial contact using drop shape analysis routines offered by the instrument software.
Interfacial Tension Measurements
The surface tension of the emollients was determined by the pendant drop technique, where free-hanging droplets of ca. 5 μL volume were formed in air at the outlet of a vertical needle and, after equilibration, analyzed with respect to their shape at 23 °C using a Krüss DSA100 tensiometer. The same setup was employed to measure oil/water interfacial tensions, with the sole difference that a free-hanging water droplet was not generated in air but rather a surrounding reservoir of the oil of interest. With the known density of the liquid phase(s), the static surface tension and oil/water interfacial tension could be calculated from the determined drop contour profile.
Washburn Sorption Experiments
In an alternative approach, the wetting properties of bleached Mongolian hair was studied using the Washburn technique,25,26 for which a defined amount of intact hair fibers was filled into glass columns in a reproducible way, as shown by Figure S2 in the Supporting Information. The column was then suspended vertically on the holder of a force tensiometer (Krüss K100). Subsequently, a reservoir filled with test liquid (water, dimethicone, or octyldodecyl myristate) placed underneath the suspended column was moved upward until contact between the liquid and the bottom outlet of the column was established. Due to wetting and sorption of the hair fibers, liquid ascends into the column and increases the weight, which was monitored over time by the tensiometer. The change in mass (m) per time (t) is linked to the contact angle (θ) of the liquid on the hair surfaces via the equation
| 1 |
where γ is the surface tension of the test liquid, ρ is density and η is viscosity. C represents the so-called capillary constant, which depends on the packing of the column and is per se unknown. Here we assume that the C value did not vary significantly among independent experiments with the same type of bleached hair and different test liquids. Hence, the product of m2/t (measured) and η/(γ·ρ2) (known for each liquid) serves as a measure for the contact angle (more specifically the product C·cos θ), with higher values indicating lower contact angles and therefore better wetting.
Inverse Gas Chromatography (iGC)
Inverse gas chromatography is a powerful technique to characterize the physicochemical properties of solid surfaces in contact with a gas phase (e.g., air).17−19 In particular, the method can deliver various relevant parameters such as surface energy, polarity, acid/base balance, roughness, adsorption, and/or solubility of interacting molecules, as well as specific surface areas for any such interacting molecules. Here, we used this method to determine the dispersive part of the surface energy of human hair as well as the effects of bleaching and conditioner treatment on this parameter. To this end, iGC experiments on virgin, bleached, and conditioner-treated hair strands were performed on a conventional gas chromatograph from PerkinElmer (Model Clarus 580) equipped with split/splitless (SSL) injectors, flame ionization (FI) detectors, and a PAL RSI system for automated injections. The GC instrument was controlled with proprietary software (Nucleus 2.2.3) developed specifically for iGC studies by Adscientis SARL (Wittelsheim, France). Measurements were performed under conditions of infinite dilution (ID), where very low amounts of probe molecules are injected to ensure that only individual probe/surface interactions occur and probe/probe interactions are avoided.17 Data were analyzed and processed using the SolID 3.2.2 software package provided by Adscientis SARL. For measurement, the hair strands were filled into standard GC columns made from stainless steel (inner diameter 4 mm, length 50 cm), using standardized column-packing procedures. The masses of hair per column used (m) varied from 4.0 to 4.6 g, a range that was previously optimized with respect to the interactivity of the samples. Prior to iGC analyses, the packed columns were mounted into the GC instrument and preconditioned with pure carrier gas (helium from Linde, 6.0 quality) at a flow rate of F = 10 mL/min and a temperature of 35 °C for 12 h, in order to remove any volatile contaminants present at the surfaces of interest. Measurements in the ID regime were performed at an analysis temperature of 30 °C and a corrected carrier gas flow rate of Dc = jF = 7 mL/min (where j is the James–Martin pressure correction factor). Probe molecules were injected in small concentrations as pulses at an injector temperature of 194 °C, together with small amounts of methane to determine the dead volume of the packed column. The interaction of the chosen probe molecules with the hair surfaces in the column was monitored in the form of a chromatogram by the FI detector (run at 200 °C) positioned at the outlet of the column. Retention times (tR) were determined from the peak center of mass, corrected for the dead volume (giving normalized retention time tN) and used to calculate the net retention volume (VN = DctN), which is related to the free energy of adsorption (ΔGa) of the probe molecules via27
| 2 |
where R is the universal gas constant, T the temperature, S the specific surface area of the material in the column, π0 the spreading pressure of the adsorbate, and p0 its saturation pressure in the gas phase. The probe molecules chosen in the present work comprised a homologous series of n-alkanes from heptane to decane. According to the model of Dorris and Gray,28 the change in ΔGa per CH2 unit, i.e. ΔGa(CH2), scales linearly with the first term on the right side of eq 2 and can be used to derive the dispersive part of the surface energy of the solid surface (γSd) according to
| 3 |
where γ(CH2) is the—purely dispersive—surface energy of a CH2 unit (assumed as the value of polyethylene), a(CH2) the adsorption cross-section of a methylene group (ca. 6 Å2), and NA Avogadro’s constant.17 By this approach, γSd values were determined for virgin, bleached, and conditioner-treated hair surfaces. In all cases, plots of −RT ln VN as a function of alkane carbon number gave linear trends with high correlation coefficients (R2 > 0.9999), from the slope of which ΔGa(CH2) and γSd were obtained in a robust way.
Optical Microscopy
Optical microscopy was used to study the behavior of native, bleached, and conditioner-treated hair strands during drying in transmission mode. The microscope used was a Zeiss Axio Imager 2 equipped with an EC Epiplan-Neofluar 5× objective (NA = 0.13). Images were captured by an attached Axiocam 506 CMOS camera. To observe the drying process, a small bundle of wet hair sample was fixed onto a glass slide and monitored continuously at 50% relative humidity (RH) and 20 °C until the hair fibers completely dried. Images were taken every 6.5 s. Polarized light was used to generate images with optimum contrast between the dry hair fibers and residues on the hair surfaces.
X-ray Photoelectron Spectroscopy (XPS)
The surface chemistry of human hair before and after bleaching was studied by means of X-ray photoelectron spectroscopy. The technique can detect all elements except hydrogen and helium, probes the surface of the sample to a depth of 5–7 nm, and has detection limits ranging from 0.1 to 0.5 atom-% depending on the element of interest. The XPS analyses were carried out with a Phi Versa Probe 5000 spectrometer using monochromatic Al Kα radiation (25 W). The instrument work function was calibrated to give a binding energy of 84.00 eV for the Au 4f7/2 line of metallic gold, while the spectrometer dispersion was adjusted to give a binding energy of 932.62 eV for the Cu 2p3/2 line of metallic copper. The built-in Phi charge neutralizer system was used on all specimens. To minimize the effects of differential charging, all samples were mounted insulated against ground. Survey scan analyses were carried out with an analysis spot size of 200 μm2, a pass energy of 117 eV, and an energy step size of 0.5 eV. High-resolution analyses were performed on the same analysis area with a pass energy of 23.5 eV and an energy step size of 0.1 eV. All acquired spectra were charge-corrected to the main line of the carbon 1s spectrum, set to 284.8 eV as a typical value for the binding energy corresponding to hydrocarbons. The detailed spectra were analyzed using CasaXPS software (version 2.3.22PR1.0) with a Shirley background correction for all regions. Relative sensitivity factors as provided by the instrument manufacturer were used for quantification.
Atomic Force Microscopy (AFM)
The topography and (nano)mechanical properties of virgin and bleached Mongolian hair surfaces were investigated using AFM in tapping and peak-force quantitative nanomechanics (QNM) modes, respectively. Measurements were performed under ambient conditions on a Bruker Dimension ICON instrument, equipped with Bruker NanoScope 9.3 measurement and Bruker NanoScope Analysis 1.8 analysis software. The hair surfaces were probed using Bruker RTESPA-150 cantilevers with a resonance frequency of 150 kHz and a spring constant of 6 N/m, at a peak force amplitude of 100 nm. From the acquired data, two-dimensional maps were generated to visualize changes in height across different areas (50 μm × 25 μm scanned at a rate of 0.05 Hz, 10 μm × 10 μm at 0.2 Hz, and 2 μm × 2 μm at 0.5 Hz) as well as relative differences in local adhesion and reduced elastic modulus (2 μm × 2 μm at 0.5 Hz), which was derived using the Derjaguin–Müller–Toporov (DMT) model. To be able to detect changes in the measured nanomechanical properties caused by bleaching, the native and damaged hair samples were investigated using the same cantilever. This approach permits a straightforward comparison of the observed effects on a relative level but does not give quantitative absolute values, for which a more elaborate calibration procedure would have to be applied. Detailed quantitative insights into nanomechanical properties of various types of (treated) hair surfaces can be found elsewhere.22,23
Haptic Characterization
The haptic properties of hair strands before and after bleaching, as well as after treatment with different cosmetic emulsions, were evaluated using a Toccare Haptics Measurement System from Syntouch Inc. (Montrose, USA). The device comprises a biomimetic finger that explores the surface of a material according to predefined, “human-like” motions and monitors the response of the material through a variety of embedded sensors.20 Using an algorithm, these multiple responses are translated into up to 15 quantitative “dimensions of touch”, meant to represent human perception of feel. For the case of hair strands, a special setup and modified motion profile had to be developed. The adapted setup consists of a 3D-printed channel, along which the hair strands were aligned and stretched in a way that the entire surface in the channel was covered by hair. At both ends, the strands were fixed with clamps and soft pads, as shown by Figure S3 in the Supporting Information. The biomimetic finger was programmed to perform a unidirectional stroking/sliding movement along the cuticles on the hair surfaces toward the tip (i.e., along the growth direction, indicated by the arrow in Figure S3) to avoid mechanical damage to the hair. This movement was performed three times in succession over a length of 40 mm at two different points, in order to check the reproducibility on previously undistorted areas of the sample. A force of 1.5 N was applied to the hair at a constant sliding speed of 3 cm/s. Prior to each measurement, the sensor was cleaned with ethanol to remove residual contaminants. Sensor calibration was automatically checked before each measurement and, if necessary, initiated again. The subsequent measurement took approximately 5 min. It is important to note that comparable results can only be obtained if identical motion profiles are used, since changes in location, force, speed, or other parameters will affect the outcome of the haptic measurement.
In Vitro Hair Combing Tests
Residual combing work was measured using a Zwick/Roell Z2.5 material testing machine (Zwick GmbH, Ulm, Germany). The combing work was determined before and after treatment of bleached hair with different conditioners in both the wet and dry states. For the wet combing test, 1 g of hair strands was treated with 0.125 g of the respective conditioner formulation, while 0.25 g of the formulation was applied to 2 g of hair strands for the dry combing test. The residual combing work was calculated as the ratio of measured combing work after conditioner treatment and the measured work before treatment (for both the dry and wet states).
Acknowledgments
The authors thank Prof. Xiaoyong Wang (East China University of Science and Technology) for support with contact angle measurements, Elisabeth Wagner (BASF SE) for performing Washburn sorption experiments, Werner Wacker (BASF SE) for interfacial tension measurements, as well as Pia Mühlbeier and Arkadius Boron (BASF) for XPS studies. We are further grateful to Dr. Sabine Hirth and Dr. Roelf-Peter Baumann for valuable discussions on XPS and haptic analyses, respectively. The work performed in this study was funded by BASF SE and BASF Advanced Chemicals Co., Ltd.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06526.
Additional tables and figures as described in the text (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Robbins C.Chemical and Physical Behavior of Human Hair; Springer: 1994. [Google Scholar]
- Smith J. R.; Swift J. A. Lamellar subcomponents of the cuticular cell membrane complex of mammalian keratin fibres show friction and hardness contrast by AFM. J. Microsc. 2002, 206, 182–193. 10.1046/j.1365-2818.2002.01028.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga S.; Tanamachi H.; Ishikawa K. Degradation of hair surface: Importance of 18-MEA and Epicuticle. Cosmetics 2019, 6, 31–48. 10.3390/cosmetics6020031. [DOI] [Google Scholar]
- Tanamachi H. Temperature as a moisture cue in haptics on hair. Int. J. Cosmet. Sci. 2011, 33, 25–36. 10.1111/j.1468-2494.2010.00586.x. [DOI] [PubMed] [Google Scholar]
- Tanamachi H.; Tokunaga S.; Tanji N.; Oguri M.; Inoue S. 18-MEA and hair appearance. J. Cosmet. Sci. 2010, 61, 147–160. [PubMed] [Google Scholar]
- Breakspear S.; Smith J. R.; Luengo G. Effect of the covalently linked fatty acid 18-MEA on the nanotribology of hair’s outermost surface. J. Struct. Biol. 2005, 149, 235–242. 10.1016/j.jsb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Guryanov I.; Naumenko E.; Fakhrullin R. Hair surface engineering: combining nanoarchitectonics with hair topical and beauty formulations. Appl. Surf. Sci. Adv. 2022, 7, 100188. 10.1016/j.apsadv.2021.100188. [DOI] [Google Scholar]
- Disapio A.; Fridd P. Silicones: use of substantive properties on skin and hair. Int. J. Cosm. Sci. 1988, 10, 75–89. 10.1111/j.1467-2494.1988.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Man Q.; Zhang L.; Cho Y. Efficient hair damage detection using SEM images based on convolutional neural network. Appl. Sci. 2021, 11, 7333–7335. 10.3390/app11167333. [DOI] [Google Scholar]
- Chen N.; Bushan B. Morphological, nanomechanical and cellular structural characterization of human hair and conditioner distribution using torsional resonance mode with an atomic force microscope. J. Microsc. 2005, 220, 96–112. 10.1111/j.1365-2818.2005.01517.x. [DOI] [PubMed] [Google Scholar]
- Lodge R. A.; Bushan B. Wetting properties of human hair by means of dynamic contact angle measurement. J. Appl. Polym. Sci. 2006, 102, 5255–5265. 10.1002/app.24774. [DOI] [Google Scholar]
- Okamoto M.; Ishikawa K.; Tanji N.; Aoyagi S. Investigation of the damage of on the outermost hair surface using ToF-SIMS and XPS. Surf. Interface Anal. 2012, 44, 736–739. 10.1002/sia.3878. [DOI] [Google Scholar]
- Robbins C. R.Chemical and Physical Behavior of Human Hair; Springer: 2012. [Google Scholar]
- Loussouarn G.; Lozano I.; Panhard S.; Collaudin C.; El Rawadi C.; Genain G. Diversity in human hair growth, diameter, colour and shape. An in vivo study on young adults from 24 ethnic groups observed in the five continents. Eur. J. Dermatol. 2016, 26, 144–154. 10.1684/ejd.2015.2726. [DOI] [PubMed] [Google Scholar]
- De La Mettrie R.; Saint-Leger D.; Loussouarn G.; Garcel A. L.; Porter C.; Langaney A. Shape variability and classification of human hair: a worldwide approach. Hum. Biol. 2007, 79, 265–281. 10.1353/hub.2007.0045. [DOI] [PubMed] [Google Scholar]
- Leerunyakul K.; Suchonwanit P. Asian hair: a review of structures, properties, and distinctive disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 309–318. 10.2147/CCID.S247390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendle E.; Papirer E.. Surface properties characterization by inverse gas chromatography (iGC) applications, in Powders and Fibers: Interfacial Science and Applications; CRC Press: 2006; pp 47–122. [Google Scholar]
- Mohammadi-Jam S.; Waters K. E. Inverse gas chromatography applications: A review. Adv. Colloid Interface Sci. 2014, 212, 21–44. 10.1016/j.cis.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Gholami F.; Tomas M.; Gholami Z.; Mirzaei S.; Vakili M. Surface characterization of carbonaceous materials using inverse gas chromatography: A review. Electrochem. 2020, 1, 367–387. 10.3390/electrochem1040024. [DOI] [Google Scholar]
- Wettels N.; Santos V. J.; Johansson R. S.; Loeb G. E. Biomimetic tactile sensor array. Adv. Robotics 2008, 22, 829–849. 10.1163/156855308X314533. [DOI] [Google Scholar]
- Tang W.; Zhang S. G.; Zhang J. K.; Chen S.; Zhu H.; Ge S. R. Ageing effects on the diameter, nanomechanical properties and tactile perception of human hair. Int. J. Cosm. Sci. 2016, 38, 155–163. 10.1111/ics.12269. [DOI] [PubMed] [Google Scholar]
- Cavallaro G.; Milioto S.; Konnova S.; Fakhrullina G.; Akhatova F.; Lazzara G.; Fakhrullin R.; Lvov Y. Halloysite/keratin nanocomposite for human hair photoprotection coating. ACS Appl. Mater. Interfaces 2020, 12, 24348–24362. 10.1021/acsami.0c05252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman N.; Scott F. H.; Lvov Y.; Stavitskaya A.; Akhatova F.; Konnova S.; Fakhrullina G.; Fakhrullin R. Clay nanotube immobilization on animal hair for sustained anti-lice protection. Pharmaceuticals 2021, 13, 1477. 10.3390/pharmaceutics13091477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski S.; Matsumoto J.; Gallagher D.; McConaughy S.; Fishel J.; Botticelli P. Application of a novel biomimetic tactile evaluation system to quantify/qualify desired product feel. J. Am. Acad. Dermatology 2018, 79, AB45. 10.1016/j.jaad.2018.05.221. [DOI] [Google Scholar]
- Washburn E. W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. 10.1103/PhysRev.17.273. [DOI] [Google Scholar]
- Alghunaim A.; Kirdponpattara S.; Newby B.-m. Z. Techniques for determining contact angle and wettability of powders. Powder Technol. 2016, 287, 201–215. 10.1016/j.powtec.2015.10.002. [DOI] [Google Scholar]
- Conder J. R.; Young C. L.. Physico-Chemical Measurement by Gas Chromatography; Wiley: 1979. [Google Scholar]
- Dorris G. M.; Gray D. G. Adsorption of n-alkanes at zero surface coverage on cellulose paper and wood fibers. J. Colloid Interface Sci. 1980, 77, 353–362. 10.1016/0021-9797(80)90304-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.