Abstract

Quantitative characterization of the change in the cleat and pore structures and fractal dimensions in anthracite after electrochemical modification is crucial for better understanding of the modification effect. Thus, lump anthracite samples were electrochemically modified in our manufactured device with 0, 0.5, 1, and 2 V/cm potential gradients. The changes in heterogeneity and porosity after modification were tested and analyzed by mercury intrusion porosimetry (MIP) and fractal theory. The results indicated that the total volume of the pores increased after electrochemical treatment and continuously increased with increasing potential gradient during the treatment process. After modification, the number of pores or fractures with a pore size between 6 and 20 μm in coal after modification increases significantly. According to the intrusion pressure, three stages were defined as lower (PM < 0.1 MPa), intermediate (0.1 ≤ PM < 10 MPa), and higher regions (PM ≥ 10 MPa), which are characterized by fractal dimensions D1, D2, and compression stages, respectively. After modification, the fractal dimension D1 showed an increasing trend, while the fractal dimension D2 showed a decreasing trend, indicating that the fracture system became more complicated and that the pore system became more regular after electrochemical treatment. The evolution mechanism of heterogeneity and porosity and their fractal dimensions were explained by the dissolution of minerals, change in pH values, and dynamics of temperatures during the process of modification. The results obtained in this work are of important guiding significance for coalbed methane (CBM) extraction via in situ modification by electrochemical treatment.
1. Introduction
In recent years, unconventional resources such as coalbed methane (CBM), shale gas, and tight sandstone gas have attracted widespread attention, and the exploitation theory and technology are developing rapidly.1 However, commercial gas production has been hampered by the low permeability of coal seams in China.2,3 Thus, permeability-improving methods, including protective seam mining,4 hydraulic fracturing,5,6 hydraulic cutting,7,8 microwave modification,9 and blasting-induced fracturing,10,11 increase massive fractures in coal seams, which greatly reduce gas pressure in the early stage of extraction and improve the gas extraction rate. However, extraction is difficult in the later stage because of little variation in gas pressure and a lack of driving force,12 and the generated coal fines block fractures and boreholes and further cause permanent damage to permeability.13 In addition, the lump coals cut by cracks are still large, and some studies indicate that even an inch of lump coal requires months or years to release most of its gas.14,15 Eddy et al. reported that the residual gas content can be as high as 38%.16
To accelerate CBM extraction, Kang reported an exploratory study on intensifying methane desorption and seepage from coal modified by electrochemical treatment.17 Zhang et al. found that the amount of adsorbed CH4 on coal samples decreased after electrochemical modification due to the change in functional groups on the coal surface.18 Guo et al. found that the average desorption rate of methane from 130 to 140 mm lump coal after electrochemical treatment increased by 1.68 times, reaching desorption equilibration.19 The desorption ratio and diffusion coefficient of CH4 on Jincheng anthracite increased after electrochemical modification in ion electrolytes.20 In addition, Kang and Zhang et al. both found that the pores and fractures increased after electrochemical treatment of electrolytic dissolution of minerals and electrophoresis migration of charged particles such as coal powder and clay, which provides a gas migration pathway.21,22 Additionally, the adsorbed gas can be stripped from the coal surface and driven by electro-osmosis and heat, which provides an impetus for gas migration. Mercury intrusion porosimetry (MIP), low-pressure nitrogen gas adsorption (LP-N2GA), and CO2 adsorption have been used to characterize the macropores, mesopores, and micropores in the coal matrix, as well as the secondary porosity composed of cleats.22−25 The total porosity of coal varies with the coal rank26,27 and can be up to 20%, exceptionally as high as 50% due to the development of microfractures in high-rank coal.28,29 Li et al. observed the nanoscale pore structures of coals by combined atomic force microscope (AFM) and SEM technologies and reported that it is effective in revealing pore structures and mechanical properties.30 Li et al. reported that, for CO2 displacing CH4, more CO2 can be adsorbed on the nanoscale pore surface of coal, resulting in higher CH4 desorption.31 The pore structures change after electrochemical modification (mentioned above) discussed mainly the evolution of surface area, pore volume, and pore size, but the quantitative characterization of the change of pore fractal dimensions still needs better understanding.
The fractal dimension (D), a dimensionless index, is used to quantify the complexity of the pore/fracture distribution.32 Combining LP-N2GA test data and MIP test results, Steele and Angulo et al. reported the Frenkel–Halsey–Hill (FHH) model and fractal dimension of pore structures in coals.33,34 Sarkar and Chaudhuri proposed that the heterogeneity of pore distribution can also be obtained by digital images by scanning electron microscopy (SEM) and CT.35 The change in fractal characteristics of pores in coals after cyclical modification has been achieved by Su et al. with nuclear magnetic resonance techniques and LP-N2GA, and the results showed that the fractal dimensions of the adsorption pores decreased after modification.36
However, research on the effects of electrochemical modification on the pore structures and fractal dimensions of lump anthracite is limited. In this work, we electrochemically modified lump anthracite samples with 0, 0.5, 1, and 2 V/cm electric potential gradients. The pore structures and fractal dimensions of lump anthracite samples before and after modification were tested by MIP and SEM techniques. The pores were reclassified into four zones according to their degree of complexity, which was reasonable to characterize the pore structures of electrochemically modified lump anthracite. On the basis of fractal theory, the changes in heterogeneity and porosity were further analyzed.
2. Results and Discussion
2.1. Mercury Intrusion Curves
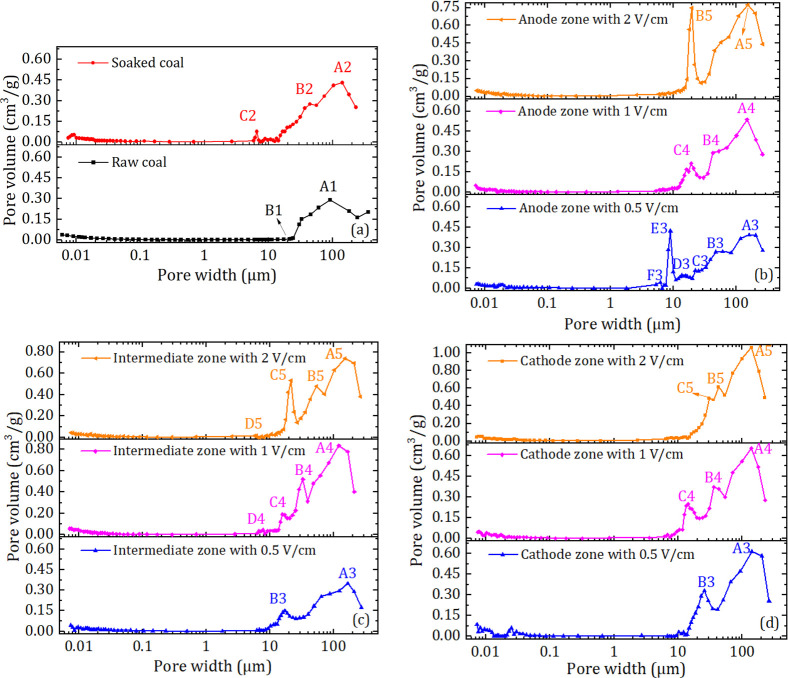
Figure 1 shows the mercury intrusion curves of lump anthracite samples before and after electrochemical treatment. The maximum mercury intrusion of raw coal samples was 0.28 cm3/g, increasing to 0.35 cm3/g after immersion in a Na2SO4 electrolyte for 120 h (Figure 1a), and further increasing to 0.39, 0.43, and 0.47 cm3/g after modification with 0.5, 1, and 2 V/cm electric potential gradients in the anode zone (Figure 1b), increasing by 39.3%, 53.6%, and 67.9%, respectively. Figure 1c and d also show that the maximum mercury intrusion increases after electrochemical modification and further increases with increasing electric potential gradients. In addition, the modification effect at the cathode zone is most obvious, followed by the intermediate zone and the anode zone. The MIP test results indicate that the total volume of the pores increased after electrochemical treatment and continuously increased with increasing potential gradient during the treatment process.
Figure 1.
Mercury intrusion curves of lump anthracite samples before and after electrochemical treatment: (a) raw and soaked sample, (b) modified samples at the anode zone, (c) modified samples at the intermediate zone, and (d) modified samples at the cathode zone.
2.2. Change of Pore Size Distribution
Figure 2 shows the pore size distributions of raw anthracite, soaked anthracite, and modified anthracite with different electric potential gradients in the anode zone, intermediate zone, and cathode zone. As seen from Figure 2a, with decreasing pore width, the pore volume of raw anthracite increases sharply and reaches a peak value at a pore size of approximately 91.2 μm (A1), while the pore volume decreases sharply and reaches a low value at a pore size of approximately 20 μm (B1), and the pore volume increases slightly when the pore size is greater than 0.1 μm. After soaking in Na2SO4 solution for 120 h and drying at 378–383 K, there are two new peak values at pore sizes of approximately 44.6 and 6.6 μm (C2 and B2, respectively), indicating that the number of pores and fractures in soaked coal increases. The same results were also found by Xia et al.,37 who observed new holes on the anthracite coal surface via SEM images. Figure 2b, c, and d show the pore size distributions of coal samples modified in the anodic, intermediate, and cathodic zones, respectively. After electrochemical modification, the number and value of pore volume peaks increased. The values of the A1 peak of raw anthracite coal increased from 0.289 cm3/g to 0.35–1.06 cm3/g, and the pore width increased from approximately 91.2 μm to 120–165 μm, which indicated that the number of fractures obviously increased.19 After modification, the new peaks at pore sizes ranging from 6 to 50 μm indicate that the number of pores or fractures with a pore size between 6 and 20 μm in coal after modification increases significantly.38
Figure 2.
Pore size distribution of coal samples modified with 0 V/cm (a) and 0.5, 1, and 2 V/cm at the anode zone (b), intermediate zone (c), and cathode zone (d).
2.3. Change of Fractal Dimension
The Menger sponge model is suitable for calculating the fractal dimension D of pores based on mercury injection tests.39−41 According to the Washburn equation, the radius r of pores filled with mercury at pressure PM is expressed as42
| 1 |
where σ is the surface tension of mercury (0.48 N/m) and θ is the mercury–coal contact angle (130°). The relationship between the pore size distribution dVM/dr and the fractal dimension D is43
| 2 |
Combining eqs 1 and 2, we get:
| 3 |
That is
| 4 |
where VM is the volume of cumulative mercury injection, which is approximated by pore volume in cm3/g; PM is the pressure of mercury injection, MPa; and r is the radius of pores, μm. Figure 3 shows the fractal region curves of lump anthracite samples using the Menger sponge model. All of the curves were divided into three regions: lower (PM < 0.1 MPa) and intermediate (0.1 ≤ PM < 10 MPa), which are characterized by D1 and D2, respectively. Although the higher regions (PM ≥ 10 MPa) had strong piecewise fractal characteristics, the fractal dimensions of the pores underwent a drastic change to values higher than 3. A similar conclusion was also found by Toda and Toyoda, and Zwietering and Krevelen reported that the phenomenon can be attributed to the compressibility of the coal samples.44,45
Figure 3.
Relationship between log(dVM/dPM) and log(PM) based on anthracite sample MIP data after electrochemical modification with 0 V/cm (a) and 0.5, 1, and 2 V/cm at the anode zone (b), intermediate zone (c), and cathode zone (d).
The change in anthracite fractal dimensions (D1 and D2) after electrochemical modification with different electric potential gradients at the anode zone, intermediate zone, and cathode zone is shown in Figure 4. As shown in Figure 4, the fractal dimension D1 was comparatively low and showed an increasing trend after electrochemical modification, while the fractal dimension D2 showed a decreasing trend. The comparatively low fractal dimension of D1 indicates that the fractures in anthracite are developed in an orderly manner. Similar results were also illustrated in previous work that anthracite coal has a mutually orthogonal and fully developed structure of fractures, which was observed by optical microscopy.46 The fractal dimension D2 is 2.86, which is consistent with the calculation result of Zhao et al.47 that the range of fractal dimensions is 2.27–2.97, and the pore structure of tested anthracite coal is relatively complicated. After modification, the fractal dimensions D1 approximately showed an increasing trend with increasing electric potential gradients, while the fractal dimensions D2 showed a decreasing trend, which indicates that the fracture system became more complicated and the pore system became more regular after electrochemical treatment because the filled minerals in fractures are dissolved and macropores are formed.
Figure 4.
Change of fractal dimension after electrochemical modification at different anthracite zones: (a) anode zone, (b) intermediate zone, and (c) cathode zone.
In the higher-pressure regions (PM ≥ 10 MPa), there is a rectilinear relationship between high pressure and the volume of mercury intrusion. The intercept at the ordinate of the linear fitting results represents the pore volume that excludes compressibility, and the change in intercept after modification is shown in Figure 5. The volume of injected mercury at pressure 0.2 MPa is the cleat volume, and the pore volume of the coal matrix can be calculated by eq 15. The compressibility of the original and soaked samples was 1.342 × 10–4 and 1.414 × 10–4 MPa–1, respectively. After modification, the compressibility changes to (0.91–1.698) × 10–4 MPa–1. In general, the compressibility of the sample modified in the anodic zone decreases, while the compressibility of the sample modified in the intermediate and cathodic zones increases because the electrophoresis treatment is more obvious in the intermediate and cathodic zones, which migrates the coal particles and clay and makes the coal matrix disperse. Guo et al.46 also found a positive relationship between the porosity and coal matrix compressibility. Combined with the analysis in section 3 and the fact that the incremental instruction is very low when the pore size is larger than 6 μm, the increase in pore volume is due primarily to mercury intrusion into the cleats >25 μm wide, cleats ranging in width from 6 to 25 μm, and coal matrix pores ranging in aperture from 100 nm to 6 μm at lower, low, and intermediate pressures, respectively. Gamson et al.48 also found that the cleats in coal are commonly 5–20-μm-wide and infilled by minerals using SEM.
Figure 5.
Linear fitting results of intercept and slope at the anode zone (a), intermediate zone (b), and the cathode zone (c) and true density and compressibility (d).
2.4. Effect of Electrochemical Treatment on the Porosity of Anthracite Coal
Table 1 shows the pore structure parameters of the raw coal and modified coal samples. The cleat volume, average cleat width, and cleat porosity of the original sample are 0.129 mL/g, 27.51 μm, and 15.2%, respectively, similar to the research results of other scholars.27,28 In addition, the average diameter of pores ranging from 100 nm to 6 μm is 114 nm, and the coal matrix porosity is 0.12%, indicating that endogenous fractures (cleats) in anthracite occupy a considerable proportion of the total porosity. After soaking in Na2SO4 solution and drying, the average cleat width of coal increase to 38.73 μm, which increases the cleat porosity to 19.24%, and the average pore size of the coal matrix increases to 308 nm, which increases the matrix porosity to 0.42%. After modification, the average cleat diameter, cleat porosity, coal matrix porosity, and average pore size of the modified samples increase to 51.97–69.86 μm, 398–827 nm, 20.92–37.01%, and 0.56–1.2%, respectively, indicating that the cleat porosity and coal matrix porosity of the sample obviously increase by electrochemical modification and are positively related to the potential gradient.
Table 1. Pore Structure Parameters of Coal Samples before and after Modification.
| coal samples | cleat volume (m/g) | average cleat width (μm) | cleat porosity (%) | pore volume (m/g) | average pore width (nm) | porosity (%) |
|---|---|---|---|---|---|---|
| raw coal | 0.1290 | 27.51 | 15.20 | 0.0012 | 114 | 0.12 |
| soaked coal | 0.1649 | 38.73 | 19.24 | 0.0043 | 308 | 0.42 |
| S3a | 0.1835 | 68.09 | 26.15 | 0.005 | 437 | 0.56 |
| S4a | 0.2035 | 61.79 | 29.87 | 0.0061 | 530 | 0.69 |
| S5a | 0.2234 | 61.21 | 30.57 | 0.0062 | 398 | 0.65 |
| S3i | 0.1396 | 51.97 | 20.92 | 0.0059 | 827 | 0.73 |
| S4i | 0.2867 | 60.35 | 33.24 | 0.0087 | 497 | 0.76 |
| S5i | 0.2901 | 58.51 | 36.92 | 0.0096 | 592 | 0.87 |
| S3c | 0.2332 | 60.51 | 28.22 | 0.0086 | 731 | 0.81 |
| S4c | 0.2447 | 63.18 | 34.02 | 0.0087 | 564 | 0.90 |
| S5c | 0.3217 | 69.86 | 37.01 | 0.0153 | 765 | 1.20 |
2.5. Modification Mechanism Discussion of Anthracite Pore Structure
Figure 6 shows the SEM images of the dissolution and disappearance of minerals filled in the fractures of coal in the anodic and immediate zones before and after electrochemical treatment and the appearance of fractures of coal in the cathodic zone. Figure 6a shows that two fractures with widths of 10–30 μm cross each other at an approximately 80° angle, and the cleat is filled with minerals, such as carbonate and sulfide. After treatment, the minerals disappear (shown in Figure 6b). The carbonate in the cleats is dissolved by hydrogen ions produced in the anodic zone during electrochemical processes because of the oxidation reaction.50 The reaction equation is as follows:
| 5 |
Figure 6c and d show SEM images of the disappearance of minerals filled in cleats and pores of coal in the immediate zone before and after treatment. Figure 6c shows that there is a mineral-filled cleat with a width of 1–20 μm, and some minerals are embedded in the coal matrix. Gamson et al.45 measured the microstructures in coals using SEM and observed that there are five types of microfractures recognized between the larger cleats: vertical cleats, horizontal cleats, blocky fractures, conchoidal fractures, and striae. These microfractures are often mineralized. After treatment, the microfractures and pores were unblocked (see Figure 6d) due to the electrolysis dissolution and electrophoresis migration of minerals.18 The emergence of these microfractures can build pathways for gas migration from pores to fractures. Figure 6e and f show SEM images of the appearance of fractures of coal in the cathodic zone. Figure 6e shows that the coal matrix surface is tight and flat. After treatment, mesh fractures with widths of 5–50 μm appeared on the coal surface (see Figure 6f), which may be due to the removal of coal or clay particles in the coal matrix in the action of electrophoresis or washing out by the electrolyte in the action of electroosmosis.
Figure 6.
SEM images of the dissolution and disappearance of minerals filled in cleats of coal in the anode zone (a and b) and intermediate zone (c and d) and the appearance of fractures of coal in the cathode zone (e and f).
3. Conclusions
To accelerate the CBM exploration process, the application of electrochemical modification makes the pore system of the lump anthracite more regular and the fracture system more complex, and the modification effect becomes more obvious with increasing potential gradient.
After modification, the porosity and the cleats with widths of 9 and 20 μm obviously increase due to the dissolution of carbonate in the cleats, and the coal/clay particles in the coal matrix in the cathodic zone are washed out by electrophoresis and electroosmosis.
The effect of modification with different potential gradients on Qinshui Basin anthracite coal is discussed above, which is not enough for the guiding of CBM extraction. More of the effect of electrochemical modification parameters like electrode materials and electrolyte types on coal pore and fracture structures and more test means for the characterization of pore and fracture structures like CO2/N2 adsorption and small X-ray scattering will be analyzed in future research.
4. Experimental Section
4.1. Coal Sample
The anthracite used was collected from the No. 15 coal seam in the southeastern Qinshui Basin from the Sihe Coal Mine (Shanxi Province, China). Table 2 and Table 3 summarize the sample details, including petrologic and chemical analyses. Sampling has to be based on vitrinite band. Some cubes with side lengths of approximately 1 cm were processed from the samples to document microstructures in at least three principal planes: the face cleat, the butt cleat, and the bedding. The sample cubes had a total mass of 2.5 kg and were divided randomly into five groups for electrochemical modification at different potential gradients.
Table 2. Coal Properties.
| analysis
wt % |
ultimate analysis % |
maceral composition % |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| coal sample | vitrinite reflectance % | moisture | ash yield | volatile matter | C | H | O | S | vitrinite | inertinite | liptinite |
| anthracite | 2.86 | 1.65 | 5.21 | 6.12 | 86.52 | 2.64 | 6.83 | 3.32 | 86.3 | 13.7 | 0.0 |
Table 3. Composition of the Ash Yield.
| ash composition % |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| coal sample | SiO2 | Al2O3 | CaO | Fe2O3 | SO3 | MgO | TiO2 | Na2O | P2O5 |
| anthracite | 24.83 | 22.14 | 11.87 | 26.27 | 10.81 | 0.9 | 0.09 | 0.19 | 0.13 |
4.2. Experimental Apparatus
The electrochemical modification equipment shown schematically in Figure 7 is composed of a direct current (DC) power source, current meter, wire, electrolyzer, electrolyte, electrodes, and coal samples. The output voltage of the DC power source is 0–250 V. The electrolyte is a solution of Na2SO4. The electrode is a square graphite sheet with a thickness of 5 mm and side lengths of 100 mm.
Figure 7.
Diagram of electrochemical modification equipment.
4.3. Experimental Schemes
To investigate the change in the pore-fracture structure of the samples under different electrochemical treatments, 11 experimental schemes were designed, as shown in Table 4. Scheme S1 was used to investigate the pore-fracture structure of the original sample. Scheme S2 was used to investigate the pore-fracture structure with electrolyte soaking and power off, and schemes S3a–S5c were used to investigate the pore-fracture structure after modification under different electric gradients from 0.5 to 2 V/cm.
Table 4. Experimental Schemes.
| scheme | coal sample | potential gradient (V/cm) | time (h) | He denisity (g/cm3) |
|---|---|---|---|---|
| S1 | raw coal | 1.5501 | ||
| S2 | soaked coal | 0 | 120 | 1.5415 |
| S3a | modified coal at anode zone | 0.5 | 120 | 1.5269 |
| S4a | modified coal at anode zone | 1 | 120 | 1.5161 |
| S5a | modified coal at anode zone | 2 | 120 | 1.5283 |
| S3i | modified coal at intermediate zone | 0.5 | 120 | 1.4933 |
| S4i | modified coal at intermediate zone | 1 | 120 | 1.5344 |
| S5i | modified coal at intermediate zone | 2 | 120 | 1.5265 |
| S3c | modified coal at cathode zone | 0.5 | 120 | 1.5175 |
| S4c | modified coal at cathode zone | 1 | 120 | 1.5095 |
| S5c | modified coal at cathode zone | 2 | 120 | 1.5160 |
4.4. Experimental Process
The samples were modified with the experimental apparatus, according to the experimental schemes shown in Table 4. In the process of modification, the pH values and temperature of the electrolyte in the anodic, intermediate, and cathodic zones were measured using a pH meter and thermometer, respectively. After 120 h, the samples were labeled Sna, Sni, and Snc sequentially according to anodic, intermediate, and cathodic zones, where n is 3, 4, and 5. In addition, the original sample and modified sample were labeled S1 and S2, respectively. Then, the samples were cleaned with distilled water and dried in an oven at 378–383 K until a constant weight was achieved. True densities of the samples were measured by helium displacement using a Quantachrome UltraPyc 1000 helium pycnometer, and the helium density results are shown in Table 4.
4.5. Experimental Phenomenon
In the process of modification, an oxidizing reaction occurs at the anode where oxygen is formed, and a reduction reaction occurs at the cathode where hydrogen is formed. The reaction equations are as follows:
| 6 |
| 7 |
Because of the electrolytic reaction, the electrolyte pH in the anodic zone decreases while the pH in the cathodic zone increases. The H+ ions travel to the cathode, and the OH– ions travel to the anode under the action of an electric field. The electrolyte pH in the intermediate zone is between the pH in the anodic and cathodic zones. The electrolyte pH values in the three zones in schemes 3–11 after modification are shown in Figure 8 and were 2.1–3.2, 6–8.3, and 8.3–11.2.
Figure 8.
pH of three zones of electrolyte in different schemes after modification.
Figure 9 shows the brown precipitate produced in the process of modification of coal samples in the cathodic zone. The appearance of this precipitate is due primarily to Fe3+ generated by electrochemical dissolution of pyrite in anthracite in the anodic zone traveling to the cathode and generating Fe(OH)3 in combination with OH–. The reaction equations are as follows:
| 8 |
| 9 |
Figure 9.

Brown precipitate produced in the process of coal modification.
Figure 10 shows the temperature histogram of the electrolyte when the modification has just finished. The temperature increases from 13.4 °C to 17.3–35.1 °C and increases with increasing potential gradient.
Figure 10.

Temperature of electrolyte in different schemes after modification.
To gain insight into the change in pore structure of coal samples after modification, fractal analysis of the samples was performed. Pfeifer and Avnir51 stated that the injection curve of pore structure with a fractal characteristic obeys the following relationship:
| 10 |
where Vp is the cumulative injection volume at a given pore radius r and Ds is the surface fractal dimension. The relational equation will be obtained between the cumulative injection volume Vp derivative with respect to pressure p and the surface fractal dimension Ds.
| 11 |
Using this equation, the fractal dimension can be calculated.
5. Pore-Fracture Structure of Coal by MIP and SEM
5.1. MIP
MIP is based on the gradual injection of mercury into an evacuated pore system with external pressures. When the pressure is greater than 20 MPa, coal can be compressed, and compressibility affects the test results. Friesen and Mikula52 found that the porosity of coal particles can be corrected based on fractal theory. For the coal particles, the pressure regime is trisected by three different fractal dimensions. In order of increasing pressure, the injection volume corresponds to interparticle penetration, pore penetration, and compressibility.
Because the test sample is a piece of coal and microfractures are developed, the injected mercury volume at low pressure can represent the fracture volume. Laubach et al.53 found that endogenous fractures (cleats) are developed in a more orderly manner than fractures in adjacent noncoal rocks and that the cumulative frequency (f) of cleats having apertures of e or larger follows a power law:
| 12 |
where b is a general measure of the cleat intensity and c is a constant (referred to as the fractal dimension). The formula indicates that the cleat system of coal has self-similarity. Therefore, the fractal dimension of cleats in coal can be calculated using MIP results. In combination with the research results of other scholars on the fractal dimension of coal particles, for the lump coal, at least three fractal dimensions such as D1 and D2 can be obtained. In order of increasing pressure, these fractal dimensions correspond, respectively, to cleat penetration, pore penetration, and coal matrix compressibility. If the values of D1 and D2 are distinctively different, the cleat and pore can be distinguished according to the intersection of their corresponding curves, and cleat structure parameters such as cleat volume, average cleat width, and cleat porosity can be further calculated. The cleat volume is the volume of injected mercury corresponding to the curve intersection. The average cleat width df can be calculated as follows:
| 13 |
where Vf is the cleat volume, mL/g, and Sf is the surface area of the cleat, m2·g–1. The cleat porosity is calculated as follows:
| 14 |
where Vm is the coal matrix skeleton volume, which can be calculated by coal mass and helium density,49 and Vmp is the coal matrix pore volume, which can be calculated as follows:
| 15 |
where Vt is the total injected mercury volume, mL, and Vco is the compressibility, which can be calculated by the helium density and slope of the linear relationship between high pressure and injected mercury volume. The coal matrix porosity can be calculated as follows:
| 16 |
5.2. The Equipment and Process of Testing
The pore-fracture structure of the original and modified samples was measured by mercury porosimetry. The experiments were performed using a PoreMaster 33G instrument (Quantachrome, US), which permits mercury filling at as low as 0.0056, up to 204 MPa. The dried sample is evacuated to 50 mmHg or below. The volume of mercury penetration into the lump coal is measured in real time with increasing pressure. To determine the pore size distribution, a surface tension of 485 dyn/cm with a contact angle of 130° was used in the Washburn equation, eq 17.
| 17 |
where P is the mercury pressure, MPa, and r is the pore diameter corresponding to pressure P, μm. All this work is in the absence of confining stress.
In addition, the surface structure of the polished cube samples in the anodic, intermediate, and cathodic zones both before and after treatment was measured by SEM analysis. The experiments for SEM analysis were performed using a TM-1000 Instrument (Hitachi, Japan).
Acknowledgments
This research was supported financially by the National Natural Science Foundation of China (Grant Nos. 51674173, U1810102, 42072203, 51974193), Basic Research Project of Shanxi Province (Grant No. 20210302123148), and Shanxi General Youth Fund Project (Grant No. 201901D211452).
The authors declare no competing financial interest.
References
- Li Y.; Yang J.; Pan Z.; Meng S.; Wang K.; Niu X. Unconventional natural gas accumulations in stacked deposits:a discussion of upper paleozoic coal-bearing strata in the east margin of the Ordos Basin, China. Acta Geologica Sinica 2019, 93 (1), 111–129. 10.1111/1755-6724.13767. [DOI] [Google Scholar]
- Lu Y.; Liu Y.; Li X.; Kang Y. A new method of drilling long boreholes in low permeability coal by improving its permeability. Int. J. Coal Geol. 2010, 84, 94–102. 10.1016/j.coal.2010.08.009. [DOI] [Google Scholar]
- Close J.; Law B. E.; Rice D.D.1 Natural fractures in coal. AAPG Stu. Geol. 1993, 38, 119–132. [Google Scholar]
- Yuan L. Theory of pressure-relieved gas extraction and technique system of integrated coal production and gas extraction. J. China Coal Soc. 2009, 34, 1–8. [Google Scholar]
- Zhang J. Numerical simulation of hydraulic fracturing coalbed methane reservoir. Fuel 2014, 136, 57–61. 10.1016/j.fuel.2014.07.013. [DOI] [Google Scholar]
- Wang T.; Zhou W.; Chen J.; Xiao X.; Li Y.; Zhao X. Simulation of hydraulic fracturing using particle flow method and application in a coal mine. Int. J. Coal Geol. 2014, 121, 1–13. 10.1016/j.coal.2013.10.012. [DOI] [Google Scholar]
- Zhao Y.; Yang D.; Hu Y.; Duan K.; Feng Z.; Zhao L. Study on the effective technology way for mining methane in low permeability coal seam. J. China Coal Soc. 2001, 26, 455–458. [Google Scholar]
- Yan F.; Lin B.; Zhu C.; Shen C.; Zou Q.; Guo C.; Liu T. A novel ECBM extraction technology based on the integration of hydraulic slotting and hydraulic fracturing. J. Natural Gas Sci. Eng. 2015, 22, 571–579. 10.1016/j.jngse.2015.01.008. [DOI] [Google Scholar]
- Zhang L.; Kang T.; Kang J.; Zhang X.; Zhang B.; Chai Z.; Zhang R.; Wang Y.; Kang G.; Zhao G. Effect of Cyclical Microwave Modification on the Apparent Permeability of Anthracite: A Case Study of Methane Extraction in Sihe Mine, China. ACS omega 2021, 6, 15001–15011. 10.1021/acsomega.1c01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H.; Lester E.; Kingman S.; Bourne R.; Avila C.; Jones A.; Robinson J.; Halleck P.; Mathews J. Inducing fractures and increasing cleat apertures in a bituminous coal under isotropic stress via application of microwave energy. Int. J. Coal Geol. 2011, 88, 75–82. 10.1016/j.coal.2011.07.007. [DOI] [Google Scholar]
- Guo D.; Pei H.; Song J.; Qin F.; Liu X. Study on splitting mechanism of coal bed deep hole cumulative blasting to improve permeability. J. China Coal Soc. 2008, 33, 1381–1385. [Google Scholar]
- Li Z.; Wang D.; Song D. Influences of temperature on dynamic diffusion coefficient of CH4 into coal particles by new diffusion model. J. China Coal Soc. 2015, 40, 1055–1064. [Google Scholar]
- Zou Y.; Zhang S.; Zhang J. Damage mechanism of coal powder on fracture conductivity. J. China Coal Soc. 2012, 37, 1890–1894. [Google Scholar]
- Bodden W. R.; Ehrlich R. Permeability of coal and characteristics of desorption tests: implications for coalbed methane production. Int. J. Coal Geol. 1998, 35, 333–347. 10.1016/S0166-5162(97)00039-6. [DOI] [Google Scholar]
- Masszi D. Cavity stress-relief method for recovering methane from coal seams. Rocky Mt. Assoc. Geologists 1991, 149–154. [Google Scholar]
- Eddy G. E.; Rightmire C. T.; Bryer C. W.. Relationship between methane content of coal rank and depth: theoretical vs. observed. Unconventional Gas Recovery Symposium; SPE: Pittsburgh, PA, 1982.
- Kang T.A Method of Enhancing Gas Desorption and Seepage from Coal Seam by Electrochemical Method. CN102296982-A [P]. China, 2013; ZL201110130935.8. [Google Scholar]
- Zhang X.; Kang T.; Hou M.; Kang J.; Guo J.; Li L.; Zhang R.; Hu Y. Experimental research on the effects of electrode materials on methane adsorption and desorption in anthracite modified by electrochemical treatment. Chin. J. Geophys-CH. 2020, 63, 2466–2477. [Google Scholar]
- Guo J.; Kang T.; Kang J.; Chai Z.; Zhao G. Accelerating methane desorption in lump anthracite modified by electrochemical treatment. Int. J. Coal Geol. 2014, 131, 392–399. 10.1016/j.coal.2014.06.011. [DOI] [Google Scholar]
- Zhang X.; Zhang R.; Kang T.; Hu Y. The adsorption and desorption behavior of CH4 on Jincheng anthracite modified in Fe3+ and Cu2+ ion electrolytes. Energy Fuels 2020, 34, 1251–1258. 10.1021/acs.energyfuels.9b02622. [DOI] [Google Scholar]
- Kang J. Ph.D. Thesis, Taiyuan University of Technology, 2018. [Google Scholar]
- Li Y.; Lu G.; Rudolph V. Compressibility and fractal dimension of fine coal particles in relation to pore structure characterisation using mercury porosimetry. Part. Part Syst. Char. 1999, 16, 25–31. . [DOI] [Google Scholar]
- Spitzer Z. Mercury porosimetry and its application to the analysis of coal pore structure. Powder Technol. 1981, 29, 177–186. 10.1016/0032-5910(81)85015-2. [DOI] [Google Scholar]
- Walker P. L. Jr; Verma S. K.; Rivera-Utrilla J.; Davis A. Densities, porosities and surface areas of coal macerals as measured by their interaction with gases, vapours and liquids. Fuel 1988, 67, 1615–1623. 10.1016/0016-2361(88)90204-9. [DOI] [Google Scholar]
- Yao Y.; Liu D.; Tang D.; Tang S.; Huang W.; Liu Z.; Che Y. Fractal characterization of seepage-pores of coals from China: An investigation on permeability of coals. Comput. Geo Sci. 2009, 35, 1159–1166. 10.1016/j.cageo.2008.09.005. [DOI] [Google Scholar]
- Gan H.; Nandi P.; Walker P. L. Nature of the porosity in American coals. Fuel 1972, 51, 272–277. 10.1016/0016-2361(72)90003-8. [DOI] [Google Scholar]
- Rodrigues C. F.; Lemos de Sousa M. J. The measurement of coal porosity with different gases. Int. J. Coal Geol. 2002, 48, 245–251. 10.1016/S0166-5162(01)00061-1. [DOI] [Google Scholar]
- Spitzer Z. Mercury porosimetry and its application to the analysis of coal pore structure. Powder Technol. 1981, 29, 177–186. 10.1016/0032-5910(81)85015-2. [DOI] [Google Scholar]
- Xie K.Coal Structure and Its Reactivity; Science Press: Beijing, 2002. [Google Scholar]
- Li Y.; Yang J.; Pan Z.; Tong W. Nanoscale pore structure and mechanical property analysis of coal: An insight combining AFM and SEM images. Fuel 2020, 260, 116352. 10.1016/j.fuel.2019.116352. [DOI] [Google Scholar]
- Li Y.; Wang Y.; Wang J.; Pan Z. Variation in permeability during CO2-CH4 displacement in coal seams: Part 1 - Experimental insights. Fuel 2020, 263, 116666. 10.1016/j.fuel.2019.116666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbrot B. B.The Fractal Geometry of Nature; Freeman: San Francisco, 1982. [Google Scholar]
- Steele W. A.The Interaction of Gases with Solid Surface; Pergamon: Oxford, 1974. [Google Scholar]
- Angulo R. F.; Alvarado V.; Gonzalez H.. Fractal dimensions from mercury intrusion capillary tests. Latin America Petroleum Engineering Conference; SPE, 1982.
- Sarkar N.; Chaudhuri B. B. An efficient differential box-counting approach to compute fractal dimension of image. IEEE. T. Syst. Man CY-S. 1994, 24, 115–120. 10.1109/21.259692. [DOI] [Google Scholar]
- Su E.; Liang Y.; Zou Q. Structures and fractal characteristics of pores in long-flame coal after cyclical supercritical CO2 treatment. Fuel 2021, 286, 119305. 10.1016/j.fuel.2020.119305. [DOI] [Google Scholar]
- Xia W.; Yang J. Changes in surface properties of anthracite coal before and after inside/outside weathering processes. Appl. Surf. Sci. 2014, 313, 320–324. 10.1016/j.apsusc.2014.05.209. [DOI] [Google Scholar]
- Fu X.; Qin Y.; Xue X. Research on fractals of pore and fracture structure of coal reservoirs. J. China U. Min. Technol. 2001, 20, 225–228. [Google Scholar]
- Liu C.; Sang S.; Zhang K.; Song F.; Wang H.; Fan X. Effects of temperature and pressure on pore morphology of different rank coals: Implications for CO2 geological storage. J. CO2 Util. 2019, 34, 343–352. 10.1016/j.jcou.2019.07.025. [DOI] [Google Scholar]
- Li Z.; Lin B.; Hao Z.; Gao Y.; Liu F. Fractal characteristics of porosity for porous media in coal mass. J. Min. Saf. Eng. 2013, 30, 437–442. [Google Scholar]
- Pfeifer P.; Wu Y. J.; Cole M. W.; Krim J. Multilayer adsorption on a fractally rough surface. Phys. Rev. Lett. 1989, 62, 1997–2000. 10.1103/PhysRevLett.62.1997. [DOI] [PubMed] [Google Scholar]
- Washburn E. W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. 10.1103/PhysRev.17.273. [DOI] [Google Scholar]
- Avinr D.; Farin D.; Pfeifer P. A discussion of some aspects of surface fractality and of its determination. New J. Chem. 1992, 16, 439–449. [Google Scholar]
- Toda Y.; Toyoda S. Application of mercury porosimetry to coal. Fuel 1972, 51, 199–201. 10.1016/0016-2361(72)90080-4. [DOI] [Google Scholar]
- Zwietering P.; Krevelen D. W. Chemical structure and properties of coal IV-Pore structure. Fuel 1954, 33, 331–333. [Google Scholar]
- Guo J.; Kang T.; Kang J.; Zhao G.; Huang Z. Effect of the lump size on methane desorption from anthracite. J. Natural Gas Sci. Eng. 2014, 20, 337–346. 10.1016/j.jngse.2014.07.019. [DOI] [Google Scholar]
- Zhao A.; Liao Y.; Tang X. Quantitative analysis of pore structure by fractal analysis. J. China Coal Soc. 1998, 23, 439–442. [Google Scholar]
- Gamson P. D.; Beamish B. B.; Johnson D. Coal microstructure and micropermeability and their effects on natural gas recovery. Fuel 1993, 72, 87–99. 10.1016/0016-2361(93)90381-B. [DOI] [Google Scholar]
- Guo X.; Yao Y.; Liu D. Characteristics of coal matrix compressibility: an investigation by mercury intrusion porosimetry. Energy Fuel 2014, 28, 3673–3678. 10.1021/ef5004123. [DOI] [Google Scholar]
- Steel K. M.; Besida J.; O'Donnell T. A.; Wood D. G. Production of ultra clean coal Part I—Dissolution behavior of mineral matter in black coal toward hydrochloric and hydrofluoric acids. Fuel Process. Technol. 2001, 70, 171–192. 10.1016/S0378-3820(01)00171-0. [DOI] [Google Scholar]
- Pfeifer P.; Avnir D. Chemistry in non integer dimensions between 2 and 3, I: fractal theory of heterogenous surface. J. Chem. Phys. 1983, 79, 3558–3565. 10.1063/1.446210. [DOI] [Google Scholar]
- Laubach S. E.; Marrett R. A.; Olson J. E.; Scott A. R. Characteristics and origins of coal cleat: A review. Int. J. Coal Geol. 1998, 35, 175–207. 10.1016/S0166-5162(97)00012-8. [DOI] [Google Scholar]
- Friesen W. I.; Mikula R. J. Fractal dimensions of coal particles. J. Colloid Interface Sci. 1987, 120, 263–271. 10.1016/0021-9797(87)90348-1. [DOI] [Google Scholar]