Abstract
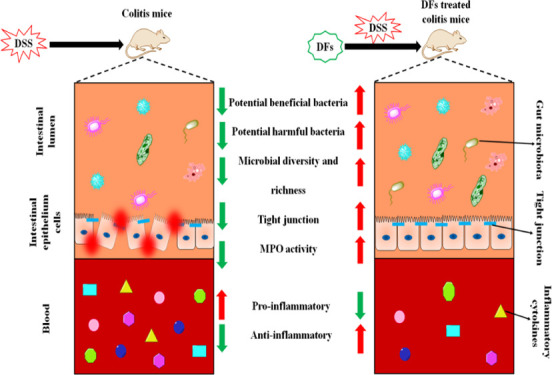
Several studies have reported that dietary fibers (DFs) from plants may exert beneficial effects on inflammatory bowel disease. In the present study, we investigated the structural differences of soluble DF (inulin) and insoluble DF (microcrystalline cellulose, MCC) and their effects on the intestinal barrier integrity, gut microbiota community, and inflammation response in mice with dextran sodium sulfate (DSS)-induced colitis. Mice were fed for 21 days with diets containing inulin or MCC (2.5 g/kg body weight), and colitis was induced by administration of DSS (4% w/v) in drinking water during the last 8 days of experimentation. The results showed that inulin and MCC differ in morphology and structure. MCC exhibited a smaller particle size, a larger specific surface area, and higher thermal stability than inulin. In addition, both inulin and MCC restored various physical signs (body weight, colon weight and length, disease activity index score, and infiltration of inflammatory cells), gut barrier function (as evidenced by the increased expression of claudin-3, claudin-7, ZO-2, occludin, JAM-2, and MUC-3 and the decreased activity of myeloperoxidase activity), downregulation of mRNA expression of proinflammatory cytokines (caspase-1, NLPR3, TLR4, TNF-α, and IL-1β), and modulation of colon microbiota community. Taken together, the present study demonstrates that DFs differ in morphology and structure and ameliorate DSS-induced colitis in mice by blocking proinflammatory cytokines, reinforcing gut barrier integrity, and modulating gut microbiota. Therefore, DFs, especially inulin, are promising dietary supplements to alleviate intestinal inflammation.
1. Introduction
Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), affects up to 500 per 10000 people in the Western world.1 In China, the morbidity of UC shows a year-by-year increasing trend.2 Classical symptoms of IBD include prolonged periods of abdominal pain, diarrhea, hematochezia, and other gastrointestinal symptoms, and these are usually difficult to cure due to recurrent attacks.3 Moreover, IBD not only affects the patients’ quality of life but also frequently progresses to colon cancer.4
Growing evidence indicates that gut bacteria play an important role in the pathogenesis of IBD and its further development into chronic disease.5 A decrease in Bacteroidetes and Firmicutes species together with an increase in virulent Escherichia coli (E. coli) species has been found in the intestinal flora of IBD patients.6 The interaction between enteric microorganisms and their metabolites and the mucosal immune system plays a pivotal part in gut health and in preventing the progression of IBD.6 The changes in mucosal flora, associated with damage to the intestinal mucosal barrier caused by toxins, initiate pathologic immune responses leading to acute and chronic colitis.5 Thus, the reduction of gut inflammation may be realized by ameliorating the integrity of the gut mucosal barrier. Due to the undesirable side effects of long-term use of traditional treatments of IBD including antibiotics, aminosalicylates, and glucocorticoids, improved therapeutic strategies for IBD are urgently needed.
Dietary intervention can regulate the diversity of the bacterial flora and the mucosal immune system to improve the intestinal health and overall health of the body and has shown great potential for the anesis or prevention of IBD.7 Dietary fiber (DF) consists of nondigestible edible carbohydrates and their analogues. While these substances cannot be digested or absorbed in the small intestine, they can be fermented in whole or in part by microorganisms in the hindgut.8 DFs have been reported to have numerous biological activities, including antioxidant,9 anti-inflammatory,10 and antitumor,11 and are commonly classified into insoluble (IDF) and soluble (SDF) dietary fiber according to their solubility in water.12 The SDFs include inulin, pectin, and guar gum, and part hemicellulose, while IDFs mainly include cellulose, hemicellulose, and lignin. Intake of DFs may have various physiological effects on human health based on different physiochemical properties.3 Inulin, a β-(2–1) linked fructan polymer, can be found in chicory roots, beet roots, leeks, and other natural sources.13 In addition, as a prebiotic additive in food, inulin has been found to effectively protect mice from developing colon cancer,14 directly modulate immune responses, and attenuate proinflammatory responses in dendritic cells.13 Microcrystalline cellulose (MCC, IDF), a novel food additive found in hardwood, softwood, cotton linter, and lignocellulosic materials, especially agricultural residues,15 can promote the growth of specific flora in the gut and regulate the host microbiota.16 Moreover, MCC has been shown to have positive effects on the gastrointestinal physiology and hypolipidemia, influencing the expression of enzymes involved in lipid metabolism.17,18 Inulin and MCC are both plant source fibers that are used as functional additives of yogurt, sausages, DF supplements during pregnancy, and so forth and play an important role in the food and health product industry.14 In animals, dietary inulin and MCC cannot be hydrolyzed by endogenous enzymes, but they are readily fermented by microbes in the colon and caecum and are beneficial to bifidobacterial growth in the gut.6 The combined soluble fibers, with high viscosity and fermentability, have been found to attenuate systemic inflammation, promote intestinal microbiota homeostasis, and improve the intestinal morphological integrity of mice suffering from obesity induced by a high-fat diet.19 Though some studies have found an anti-inflammatory action of DFs,20 it is not clear if DFs can promote anesis or prevention of gut injury.
The objective of the current study was to investigate whether inulin and MCC have an inhibitory effect on gut inflammation. To this end, diarrhea in BALB/c mice caused by the administration of dextran sodium sulfate (DSS) was used as a mouse model of IBD. The effects of inulin and MCC on gut barrier repair and their anti-inflammatory mechanism of action were assessed by qRT-PCR. Furthermore, the microbial community in the colons of mice was analyzed by 16S rRNA sequencing.
2. Results and Discussion
2.1. Particle Size Distribution of DFs
Particle size, including porosity and surface area, plays an important role in the physicochemical and functional properties of DF. By affecting fermentation and fecal bulking, it can promote the body’s absorption of glucose and cholesterol and intestinal health.21,22 The particle size distribution and specific surface area of different DFs are shown in Table 1. The results showed that the d (0.5) and d (0.9) of MCC (59.204 ± 3.840 and 128.284 ± 3.976 μm, respectively) were smaller than those of inulin (95.919 ± 3.215 and 428.326 ± 14.083 μm, respectively), which means inulin presents the larger average particle size. The particle size and specific surface area are usually negatively correlated,22 so MCC likely has a larger specific surface area than inulin.
Table 1. Mean Particle Diameter and Specific Surface Areas of DFsa.
| samples | d (0.1) (μm) | d (0.5) (μm) | d (0.9) (μm) | homogeneity | specific surface area (m2/g) |
|---|---|---|---|---|---|
| inulin | 21.473 ± 0.658a | 95.919 ± 3.215a | 428.326 ± 14.083a | 1.190 ± 0.556a | 0.404 ± 0.019b |
| MCC | 22.558 ± 0.596a | 59.204 ± 3.840b | 128.284 ± 3.976b | 0.561 ± 0.184b | 0.883 ± 0.014a |
MCC: microcrystalline cellulose. Different letters indicate significant differences (p < 0.05).
2.2. Monosaccharide Composition
The results (Table 2) of monosaccharide composition analysis showed that the main monosaccharide compositions of inulin were fructose, glucose, xylose, and galactosamine at a ratio of 81.6:5.9:4:3.6, which is similar to the previous report that fructose is the main composition in inulin.23 The monosaccharide compositions of MCC were glucose, arabinose, galacturonic acid, and mannose at a ratio of 87.3:6:4:0.6, which is similar to the sesame MCC that glucose makes up nearly 90% of total sugar.24 The monosaccharide composition and proportion between inulin and MCC are significantly different, which further leads to the differences in the structure and function of DFs.
Table 2. Monosaccharide Composition of DFs (%)a.
| items | inulin | MCC |
|---|---|---|
| mannose | 1.9 | 1.8 |
| ribose | ND | ND |
| rhamnose | 0.1 | ND |
| glucuronic acid | ND | 0.1 |
| galacturonic acid | 0.3 | 4.0 |
| glucosamine | 0.5 | 0.1 |
| glucose | 5.9 | 87.3 |
| galactosamine | 3.6 | ND |
| galactose | ND | 0.6 |
| xylose | 4.0 | ND |
| arabinose | 2.2 | 6.0 |
| fructose | 81.6 | ND |
MCC: microcrystalline cellulose. ND means not determined.
2.3. SEM Analysis
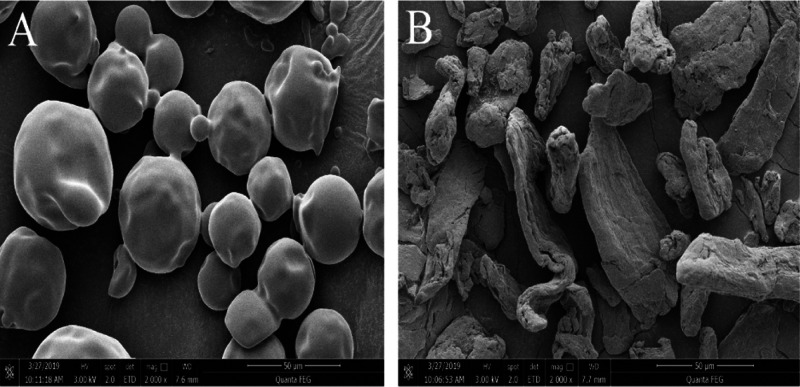
The morphology of DFs was observed by scanning electron microscopy (SEM). Clear differences in microstructures were observed between inulin and MCC at 2000-fold magnification. The image of inulin showed a spherical shape with a smooth surface (Figure 1A), while MCC displayed folds with a smaller particle size that afforded it a larger specific surface area (Figure 1B). Micropores increased the surface area of MCC, leading to capillary action, which was important for glucose absorption, water retention capacity, oil absorption capacity, and so forth.25
Figure 1.
SEM analysis of DFs at 2000× magnification. (A) Inulin. (B) MCC.
2.4. FT-IR Analysis
The chemical structures of the DFs were analyzed by Fourier transform infrared (FT-IR) spectroscopy (Figure 2). The absorption band in the range 3000–3600 cm–1 for inulin and MCC corresponds to hydroxyl groups (−OH) and the band around 2927 cm–1 corresponds to C–H stretching in cellulose, hemicellulose, and lignin.26,27 The carbonyl group band at 1647.21 cm–1 corresponds to the characteristic bond vibrations of the ester and carboxyl groups (−COOCH3 and −COOH) of ferulic and p-coumaric acids in hemicellulose.26 The band at 1028 cm–1 in DFs refers to C–O–C stretching of β-1,4-glycosidic bonds among d-glucose units in cellulose.28 In addition, the peak strength increased around 1130 and 3354 cm–1, indicating the presence of cellulose, which was more pronounced in the MCC.29
Figure 2.
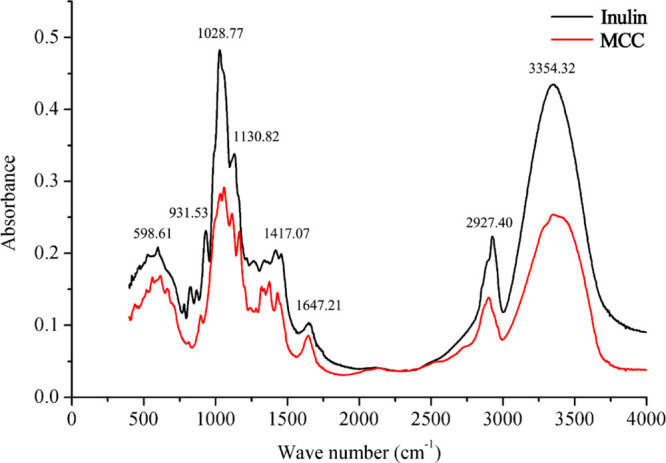
FT-IR spectra of DFs.
2.5. XRD Analysis
The X-ray diffraction (XRD) patterns of DFs are shown in Figure 3. There were no sharp peaks found in inulin around 20°, indicating its amorphous pattern.30 Such amorphization plays a critical role in the enhanced water solubility of inulin.31 In contrast, the XRD spectra of MCC displayed several sharp peaks at 2θ = 22.3 and 34.46°, indicating that MCC was semicrystalline.32 The crystalline structure of MCC will guarantee a natural role and proper stimulation of microorganisms in the colon.30
Figure 3.
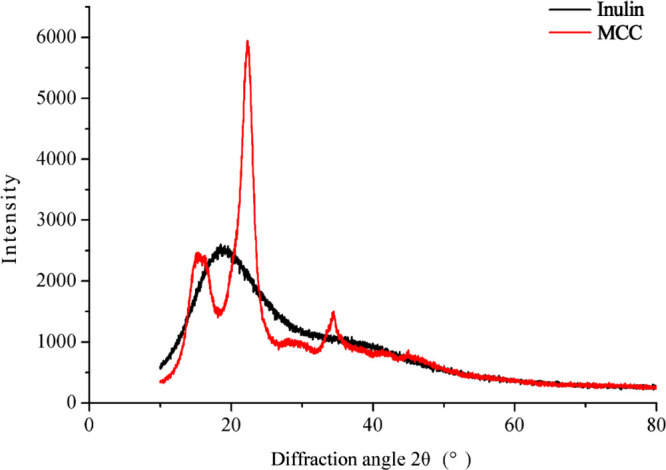
XRD patterns of DFs.
2.6. Thermal Analysis
The thermal stability of different DFs is shown in Figure 4. Thermogravimetry (TG) curves showed that thermal degradation of inulin and MCC was a multistage thermal degradation process. The first degradation peaks of inulin and MCC occurred at 55.30 and 97.91 °C and ended at 125.41 and 150 °C, respectively. The main cause of heat loss at this stage may be water loss in DFs.33 The second thermal degradation occurred in the range of 264.8 and 300 °C, possibly due to the breakdown of glycosidic bonds and the decomposition of carbohydrates.34 Compared to inulin, the degradation peak of the differential TG (DTG) curves of MCC exhibited polysaccharide characteristics and was exothermic. The fluctuation of the thermal degradation temperature may be caused by the different molecular properties and physical states of DFs.35 As shown in Figure 4, the thermal characteristics of DFs are relatively complex, and there were differences in the differential scanning calorimetry (DSC) spectra between different DFs. The degradation peak corresponding to water release appeared near 120 °C. Compared with inulin, MCC showed fewer endothermic peaks and exothermic peaks below 250 °C, indicating that MCC has better thermal stability than inulin.
Figure 4.
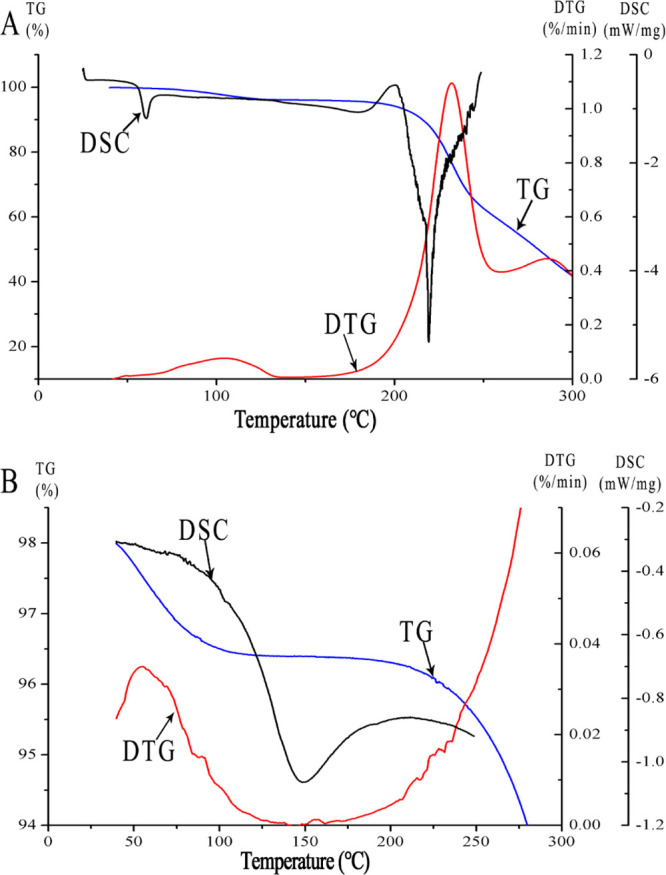
Thermal analysis of DFs. (A) Inulin. (B) MCC.
2.7. Effects of DFs on Body Weight and Intestinal Histology
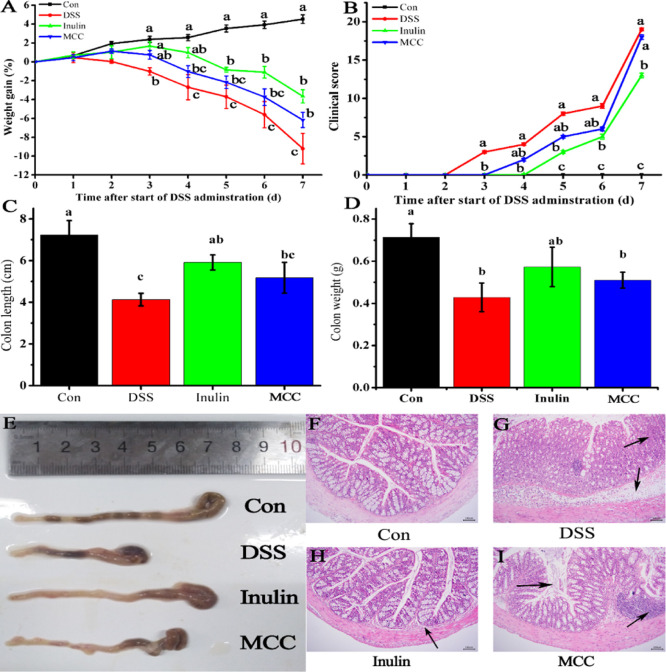
Inulin DF possesses high solubility and high fermentability, while MCC exhibits the opposite, that is, low solubility and low fermentability.3 DSS, a water-soluble polysaccharide sulfate with direct toxic effects on intestinal epithelial cells, can destroy the integrity of intestinal barrier, resulting in the onset of acute colitis.36 To directly assess the effect of solubility and fermentability of DFs on the amelioration of DSS-induced colitis in mice, we compared the body weight and intestinal histology of mice that were fed inulin or MCC in their diet. As shown in Figure 5A, mice in the inulin, MCC, and DSS groups suffered significant weight losses (p < 0.05) compared with the control group, and this trend continued until the end of the experiment. However, administration of inulin significantly reduced the weight loss of DSS-induced mice from day 3 to day 7 by 264.32, 136.11, 77.05, 80.32, and 60.25%, respectively. A similar trend was seen for the MCC group, although in this case, the difference with the DSS group was only significant at day 7, reaching 32.93%. Weight changes between the two DF groups were not significantly different.
Figure 5.
DFs ameliorate DSS-induced colitis in mice. (A) Bodyweight change, (B) clinical score, (C) colon length, (D) colon weight, (E) morphological characteristics of colons, (F) histological analysis of normal colonic mucosa, (G) histological analysis of colitis group, (H) histological analysis of 2.5 g/kg body weight inulin, and (I) histological analysis of 2.5 g/kg body weight MCC. Con, control mice fed regular diets; DSS, diet included 4% (w/v) DSS during the last 8 days of the experiment; inulin, diet included 2.5 g/kg body weight inulin and 4% (w/v) DSS; MCC, diet included 2.5 g/kg body weight MCC and 4% (w/v) DSS. Different letters above the graph points or columns in panels A–D indicate significant differences (p < 0.05).
As shown in Figure 5B, the disease activity index (DAI) clinical scores of the inulin group at day 3 and beyond were lower than those of the DSS group (p < 0.05) decreasing by 100, 100, 62.5, 44.44, and 31.58%. In addition, the clinical scores of the MCC groups at day 3 and beyond were lower than those of the DSS group with clinical scores decreasing by 100, 75, 50, 33.33, and 5.26%, respectively. Overall, inulin was better at preventing weight loss than MCC.
Colon length is generally regarded as a morphologic marker for inflammation degree, and the shortening of the colon in mice has been correlated with histologic changes.37 Oral administration of DSS decreased the length and weight of the colon in the mice of the DSS group, while feeding inulin partially prevented the decrease in the colon length (by 42.99%, p < 0.05) and the decrease in the colon weight (by 33.67%, p < 0.05) compared with the DSS group (Figure 5C–E). The reason may be that DSS induced goblet cell depletion in the distal side of the colon, resulting in the contraction of colon length and loss of colon weight.38 Furthermore, Figure 5E represents the morphological characteristics of colons. The colon length of DSS-treated mice was significantly shorter than that in mice in normal groups (p < 0.05). Meanwhile, feeding MCC also somewhat alleviated the decrease in colon length (by 25.27%) and colon weight (by 19.02%), although the difference was not statistically significant.
Histological comparison of colonic tissues from the control and DSS groups revealed multiple intestinal mucosal lesions in the latter accompanied by infiltration of inflammatory cells (Figure 5F–I). After 7 days of DSS administration, the mice in the DSS group exhibited a severe inflammatory response, while coadministration of inulin showed an inhibiting effect on DSS-induced inflammatory response which was greater than in the case of MCC coadministration. A similar result was reported by Ogata et al.39 who found that 5–10% psyllium fiber could prevent both colitis-induced shortening of colon length as well as the ulceration or erosion of intestinal mucosa. The colitis inflammation and histological damage scores were reduced by orally administered inulin, which is consistent with the result reported by Akram et al.40 Therefore, supplemental DFs, especially inulin, might have ameliorative potential in colitis,40,41 though the exact mechanisms are not clear.
2.8. Effect of DFs on Barrier Function
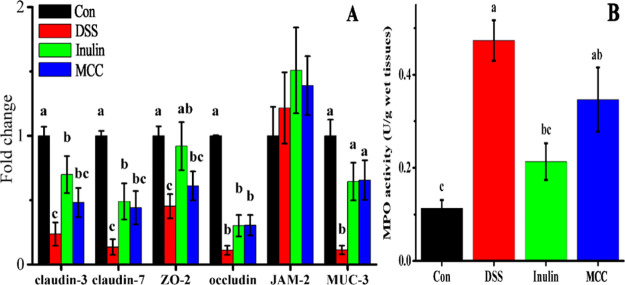
The internal environment of the body is isolated from the external environment, and the integrity of tissues is maintained by the intestinal barrier through the adhesion and intercellular junction connection system that is formed by epithelium cells.42 Intestinal permeability is regulated by epithelial adherens junctions (JAMs) and tight junctions (TJs, including ZOs, occludin, claudins, etc.), and these can be compromised by enteric dysbacteriosis or other stimuli.43 Increased intestinal permeability can aggravate certain diseases such as IBD and irritable bowel syndrome.44 Furthermore, MUCs, critical intestinal mucosal proteins, act as an important component of the mucus layer and provides an insoluble barrier to protect intestinal epithelium.45 MUC3 is expressed in goblet and columnar cells of the surface epithelium of the colon, is associated with the maturation of intestinal epithelial, and may be necessary to maintain normal epithelial cell function during inflammation.46 In this study, the relative expression of TJ and JAM genes, including claudin-3, claudin-7, ZO-2, occludin, JAM-2, and MUC-3, was found to be lower in colons of the DSS group than in the control group (p < 0.05) (Figure 6A), which means the intestinal barrier function was impaired while the intestinal permeability was increased. Feeding inulin restored the expressions of claudin-3, claudin-7, and ZO-2 mRNA, which were increased by 194.59, 258.72, and 102.86%, respectively, compared with the DSS group (p < 0.05), which means inulin can repair the intestinal barrier function and restore intestinal permeability.41 In addition, MUC-3 mRNA expression was higher in the inulin and MCC groups by 456.28 and 474.26%, respectively, than in the DSS group (p < 0.05). MUC3 is an important intestinal mucin that can bind many intestinal pathogens and viruses, preventing them from attaching to the surface of intestinal cells.47 The results showed that oral administration of inulin and MCC can increase the expression of MUC3, thus inhibiting enteropathogenic E. coli adherence.48 The overexpression of TJ genes and of the MUC3 protein has been reported to result in an amelioration of DSS-induced colitis,3,41,49 consistent with our results.
Figure 6.
DFs regulate the intestinal barrier integrity during DSS-induced colitis in mice. (A) Relative mRNA expressions of genes associated with intestinal barrier integrity (claudin-3, claudin-7, ZO-2, occludin, JAM-2, and MUC-3) in colon tissues. (B) MPO activities of colon tissues. Con, control mice fed regular diets; DSS, diet included 4% (w/v) DSS; inulin, diet included 2.5 g/kg body weight inulin and 4% (w/v) DSS; MCC, diet included 2.5 g/kg body weight MCC and 4% (w/v) DSS. Different letters above the columns in each panel indicate significant differences (p < 0.05).
Myeloperoxidase (MPO) is considered a potential marker of tissue inflammation, tissue damage, and neutrophil infiltration.50 DSS-induced colitis is characterized by diffuse inflammation and the accumulation of neutrophils in the colonic mucosa with high expression of MPO.3 The MPO activity in the colons of the DSS group had increased by 317.66 and 121.88% compared with the control group and the inulin group, respectively (p < 0.05) (Figure 6B), indicating that inulin intake reduced the infiltration of colonic mucosa by neutrophils. Another study similarly found that feeding 10% guar gum decreased the level of MPO activity in mice with DSS-induced colitis (p < 0.05) and increased the expression of TJ proteins.3 On the other hand, the MPO activity in the MCC group did not differ significantly from that in the inulin or the DSS groups. These results indicate that DFs, especially inulin, protected the mice from the DSS-induced increase in intestinal permeability.
2.9. Effect of DFs on Inflammatory Cytokine Expression in Colon
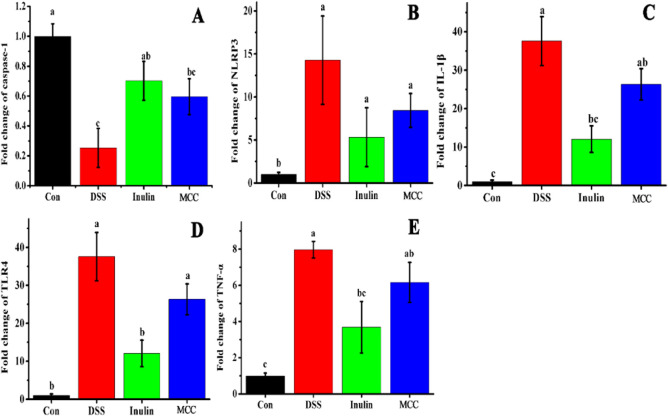
Upon the onset of enteritis, proinflammatory cytokines are critical biomarkers that are released in the gut lumen when immune cells are overactivated.51 Present evidence indicates that the inhibition of inflammatory cytokines can decrease neutrophil and macrophage migration, relieving the inflammation and progression of colitis.52 In order to study the mechanism of the anti-inflammatory effect of DFs in the gut, it was essential to determine how DFs regulate the expression of proinflammatory cytokines. The mRNA expression of the proinflammatory cytokines, NLRP3, IL-1β, TLR4, and TNF-α, had increased significantly in the colonic tissues of the DSS group compared with the control (p < 0.05) (Figure 7A–E). Moreover, DFs, especially inulin, effectively decreased the production of IL-1β, TLR4, and TNF-α in DSS-treated mice (p < 0.05), which were 47.45, 29.96, and 53.72% lower, respectively, than in the DSS group. Additionally, the addition of MCC also led to a downward trend in the expression of those inflammatory cytokines which failed to reach statistical significance. Depending on its gene activation level, caspase-1 can either cause programmed cell death or promote cell survival, and it can stimulate cell survival response and is beneficial for cell growth when expressed at low levels.53 Our results show that inulin and MCC increased the mRNA expression of caspase-1 by 177.13 and 134.97%, respectively, compared with the DSS group, which is attributed to the repairing of damaged intestinal mucosa. The inflammatory cytokines (IL-1β, TLR4, and TNF-α) involved in several cell death pathways, such as apoptosis and pyroptosis, could promote cell death and tissue damage.54,55 Furthermore, the epithelial TJ barrier could be destroyed by these proinflammatory cytokines in inflammatory immune cells through the reduction and redistribution of TJ proteins.3,56 This trend was decreased upon feeding inulin or MCC. These results indicated that mice treated with DSS showed more severe colitis that resulted in the activation of more proinflammatory factors for an inflammatory response.10 The trends of inflammatory cytokine expressions in the four groups were similar to the extent of immune cell infiltration of colon tissues. A possible reason might be that infiltrating immune cells are the major producers of a series of inflammatory cytokines.57
Figure 7.
Relative mRNA expression of proinflammatory cytokines in colon tissues. (A) Caspase-1, (B) NLRP3, (C) IL-1β, (D) TLR4, and (E) TNF-α. NLRP3, NOD-like receptor protein 3; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor α. Different letters above the columns in each panel indicate significant differences (p < 0.05).
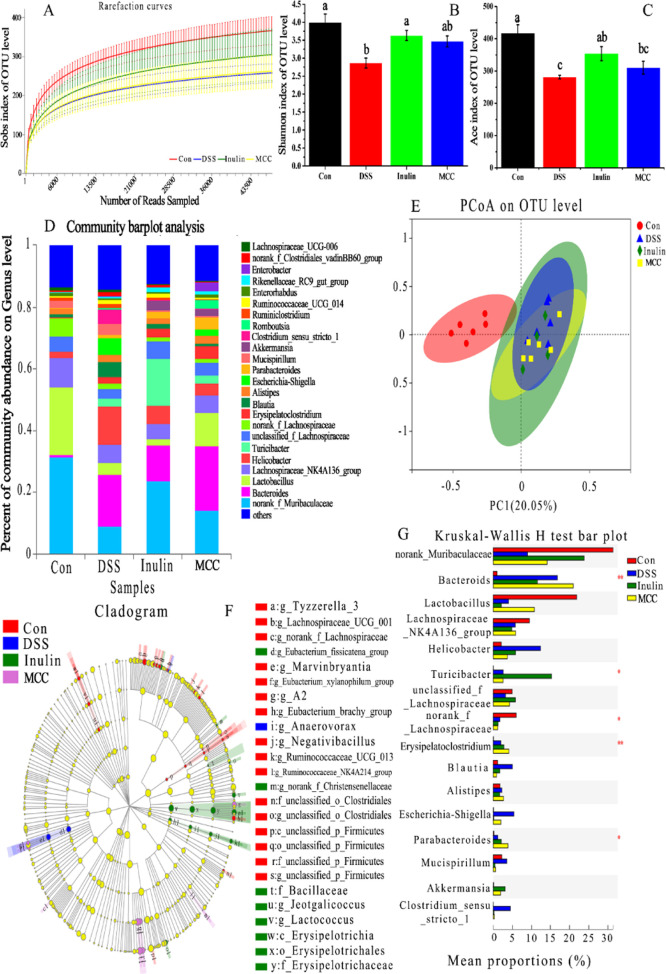
2.10. Effect of DFs on Colon Microbiota
The compositions of colon microbiota are diverse and complex and mainly include bacteria and eukaryotes. The colon microbiota are critical for human and animal health, including enhancement of the intestinal epithelial barrier, development of the mucosal immune system, and digestion of nutrients.58 The effect of adding different DFs in colon microbiota composition in colitic mice was evaluated using 16S rRNA sequencing. The rarefaction curves tended to reach a saturation plateau (Figure 8A), indicating that the sequencing coverage was sufficient for subsequent analysis. As seen in Figure 8B,C, mice in the DSS group displayed a significantly reduced microbial diversity (Shannon index) and microbial richness (ACE index) compared with the control mice. However, administration of inulin, but not MCC, resulted in a significantly greater microbial diversity and richness compared with mice treated with DSS only, which were increased by 26.74 and 15.54%, respectively. As shown in Figure 8D, DSS and DF treatments changed the composition of the colon microflora. The differences in colon microbiota composition between the four groups can also be intuitively confirmed by principal component analysis (PCoA) (Figure 8E). The results indicated that there was an obvious clustering relationship in the DSS group versus the control group with respect to the intestinal flora. The significant difference between the inulin group and the DSS group indicated that inulin had a regulatory effect on the microbial community composition of mice with DSS-induced colitis. Moreover, administration of MCC also resulted in a visible improvement of microbial community composition.
Figure 8.
DFs modulated the gut microbiota composition of mice with DSS-induced colitis. (A) Rarefaction curves of mice colon microbiota. (B) Shannon diversity index of bacterial communities in mice colon. (C) ACE diversity index of bacterial communities in mice colon. (D) Relative abundance of microbial genera among the four experimental groups. (E) PCoA of the gut microbiota communities based on OTU levels. (F) Differential abundance taxa of LEfSe cladogram analysis. (G) Relative abundance of the gut microbial community members. Different letters above the columns in panels B and C indicate significant differences (p < 0.05).
The cladogram, derived from the linear discriminant analysis effect size (LEfSe) analysis, showed a distinct gut microbiota composition for each of the four groups of mice (Figure 8F). The DSS treatment resulted in a significantly decreased relative abundance of norank_f_Muribaculaceae (Figure 8G), while inulin treatment resulted in the attenuation of this decrease with an abundance gain of 164.99% compared with the DSS group. Muribaculaceae, a novel name that includes members of the S24-7 family, contains a large number of multifunctional carbohydrate-active enzymes in the genome,59 which endow the host with the ability to digest DFs. The relative abundance of Muribaculaceae is negatively correlated with proinflammatory cytokines and positively correlated with the expression of TJ proteins and MUC3.36 Compared with the control group, the abundance of Lactobacillus in the DSS group had also decreased significantly (Figure 8G). However, compared with the DSS group, in the MCC-administered group, a significant attenuation of the DSS-induced decrease in Lactobacillus was observed, with the abundance of Lactobacillus increased by 167.96%. In a mouse model of colitis, Lactobacillus paracasei was found to reduce intestinal inflammation and expression of proinflammatory factors in the mucosa.60 Our finding that the relative abundance of Lactobacillus was decreased by DSS administration, which could, at least in part, be alleviated by MCC treatment is in agreement with Zhang et al.,61 who found that MCC had a tendency to increase the relative abundance of Lactobacillus in the colon of mice, which may be related to the fact that MCC can promote the defecation and bacterial community renewal of mice, reduce the adhesion of pathogenic bacteria, and indirectly increase the abundance of beneficial bacteria. The intestinal permeability and integrity of colitic mice can be broken by Bacteroides, while Helicobacter is thought to promote gastrointestinal disease.7,62 Here, we found that the relative abundance of Bacteroides and Helicobacter was the highest in the DSS group (Figure 8G). The administration of DFs (inulin and MCC) reduced the relative abundance of Helicobacter in colitic mice by 52.79 and 69.95%, respectively, compared with the DSS group, which might contribute to the reversal of the higher gut permeability and lower integrity of DSS-treated mice. A recent study found that bacteria from the relatively unknown Turicibacter genus settled in the rumen and feces of cattle.63 Furthermore, the density of Turicibacter was correlated with the level of glycylproline, which can reduce the permeability of cefadroxil, a bactericidal antibiotic.63,64 Therefore, we speculated that Turicibacter might play a role in preventing toxic chemicals such as DSS to penetrate the intestinal barrier and induce an inflammatory response. Oral administration of inulin significantly (by 483.49%, p < 0.05) increased the abundance of Turicibacter compared with the DSS groups, and this might be related to a special function of inulin in maintaining gut health (p < 0.05). Significant attenuations of DSS-induced increase in Escherichia-Shigella were observed in inulin and MCC groups (Figure 8G), which were decreased by 97.37 and 63.07%, respectively, compared with the DSS group. Elevated expression of Escherichia-Shigella has been identified in the feces of UC patients and rectal biopsies of CD patients and is regarded as the microorganism that reinforces intestinal inflammation of IBD.65 A tendency to first rise and then fall of Blautia, Alistipes, Mucispirillum, and Clostridium_sensu_stricto_1 was seen in the two DF groups, but there was no statistical difference between the DSS group with or without DFs (Figure 8G). In general, treatment with DFs can reverse, at least partially, the increase of colitis-associated microbiota in the colon of mice treated with DSS by decreasing the abundance of Bacteroides, Helicobacter, Blautia, Alistipes, Escherichia-Shigella, Mucispirillum, and Clostridium_sensu_stricto_1 while increasing the abundance of norank_f_Muribaculaceae, Lactobacillus, and unclassified_f_Lachnospiraeae.
These results indicated that DFs can exert an anti-inflammatory effect by reducing the invasion of immune cells and suppressing the expression of proinflammatory cytokines in the colon.57 A possible mechanism for the beneficial effects of DFs, particularly fermentable fibers, could be that they promote the growth of beneficial bacteria and are metabolized into substances that have a range of anti-inflammatory and other beneficial effects in the host.66 Although MCC has a smaller particle size, a larger specific surface area, and better thermal stability than inulin, in most cases, inulin showed better protection against DSS-induced colitis than MCC. A possible reason is that inulin, as a soluble fiber, is easily fermented by microorganisms, can stimulate the colon bacteria to produce short-chain fatty acids (butyric acid and propionic acid), promote the growth of Bifidobacterium and Lactobacillus, and offset the proinflammatory activity of other known symbiotic bacteria (Bacteroides or E. coli),67 and therefore, compared with MCC, inulin is better at easing colonic mucosa inflammation.
3. Conclusions
MCC exhibited a smaller particle size, a larger specific surface area, and higher thermal stability than inulin. It exhibited anti-inflammatory activities by preventing weight loss and reducing the DAI of DSS-treated mice. Additionally, DFs enhanced the gut barrier function by repairing colon mucosa and reducing the invasion of immune cells, increasing the expression of TJ proteins, and regulating the number of probiotics in the colon. Moreover, DFs downregulated the proinflammatory cytokines. Therefore, we propose that DFs are promising functional additives for relieving intestinal inflammation. However, the exact metabolites produced by inulin or MCC and how they impact the host remain to be determined, and further work should characterize the metabolome of the intestinal contents in mice fed with different DFs to investigate the potential relationship between metabolism of DFs and gut flora.
4. Materials and Methods
4.1. Chemicals
DSS (molecular weight 36000–50000) was purchased from MP Biomedicals (CA, USA). Inulin and MCC were obtained from Runyan Trading Market Co., Ltd. in Zhengzhou. Primers were designed according to the gene sequences in GenBank and synthesized by Shenggong Co. (Shanghai, China). Hematoxylin and eosin (H&E) staining solution was purchased from Sigma Co. (MO, USA). All chemicals were of analytical grade.
4.2. Particle Size Distribution
The particle size and particle size distribution were measured with a Mastersizer-2000 (Malvern Instruments Ltd., Malvern, UK) analyzer. Particle size distribution was obtained by measuring the particle scattering angular pattern intensity.22
4.3. Monosaccharide Composition
The monosaccharide composition was measured according to the method reported by Wang et al.68 with some modifications. Briefly, DFs (5 mg) were dissolved in trifluoroacetic acid (2 M) and hydrolyzed at 121 °C for 16 h. The residue was redissolved in deionized water and filtered through a 0.22 μm membrane, the supernatant of which was analyzed by high-performance anion-exchange chromatography on a CarboPac PA-20 anion-exchange column (3 × 150 mm, Dionex) using a pulsed amperometric detector (Dionex ICS 5000 system).
4.4. SEM
The surface morphology of the DFs was investigated using a JSM-6390/LV SEM (NTC, Japan) microscope using a gold layer coating for 50 s after being dried to a constant weight. Images at 2000 × magnifications were obtained at an accelerating voltage of 15 kV.21
4.5. FT-IR
A Nicolet 6700 spectrometer (Nicolet Instrument Corporation, USA) was used to record FT-IR spectra in the range of 400–4000 cm–1. Samples (2 mg) were dried and ground with KBr powder (spectroscopic grade) and then pressed into pellets (1 cm) for FT-IR analysis.28
4.6. XRD Analysis
XRD studies of DF samples were carried out using an X-ray diffractometer (Bruker AXS, Karlsruhe, Germany) with Cu Kα radiation (λ = 0.15418 nm) operating at a voltage of 40 kV and a current of 40 mA.30 The scanning speed was set at 1°/min, and the diffraction angle (2θ) ranged from 10 to 80°.
4.7. Thermal Analysis
DSC and TG were performed by a STA 449 C device (Netzsch, Selb, Germany) with a heating rate of 10 °C/min in a nitrogen environment.33 The DFs (about 15 mg) were weighed and heated from 20 to 300 °C. The nitrogen gas was injected into the device at the speed of 80 mL/min, and the data were recorded and analyzed using Netzsch software (Netzsch Inc., Selb, Germany).
4.8. Animals and Diets
All animal experiments were reviewed and approved by the Henan University of Technology IRB. A total of 32 female BALB/c mice (7 weeks old; weight 21 ± 0.5 g) were purchased from the laboratory animal center of Zhengzhou University. All experimental procedures were approved by the Animal Care and Use Committee of Henan University of Technology. The mice were housed under controlled temperature (22 °C), humidity (40–60%), and lighting (800–2000 l×) conditions. The mice were housed singly and acclimatized to the new environment while being fed an AIN-93 G diet and distilled water ad libitum for 1 week before formal experimentation began. The mice (n = 8) were randomly assigned to 4 groups: control, 4% (w/v) DSS (DSS group), 4% (w/v) DSS + 2.5 g/kg body weight inulin (inulin group), and 4% (w/v) DSS + 2.5 g/kg body weight MCC (MCC group). The dose selection of DFs was based on the World Health Organization’s recommended DF intake for normal adults.69 The control and DSS groups were fed the control diet, whereas the inulin and MCC groups were provided with diets containing inulin and MCC (both 2.5 g/kg body weight), respectively. Five days following the start of the experiment, the mice in the inulin and MCC groups were provided water containing 4% (w/v) DSS for 8 d, while the control groups were provided distilled water. Mice were assessed daily for the severity of colitis using a clinical score. At the end of the experiment, the mice were exsanguinated, and blood from the eyeballs and colon tissue was collected. The colons were dissected, and their weight and length were measured. Then, the colon tissues were quickly frozen in liquid nitrogen and used for qRT-PCR analysis. The MPO activity of colon tissues was analyzed using an MPO assay kit (JianCheng Bioengineering Institute, NanJing, China). Fresh colon contents were collected and were immediately stored at −80 °C to be later analyzed for microbiota using 16S rRNA gene sequencing.
4.9. H&E Staining
After the mice were sacrificed, colon tissues (8 μm) from the same area were harvested for H&E staining to observe the degree of intestinal injury and inflammatory cell infiltration.37 In brief, the samples were fixed in 10% neutral formalin solution for at least 24 h at room temperature following resection. The tissue sections were obtained by traditional techniques and were viewed with an Olympus CX41 microscope (Germany) at 200× magnification after staining with H&E.
4.10. DAI Assessment
The clinical scoring to assess the severity of colitis in mice is based on a standard scoring system according to Miles et al.66 In brief, the DAI score was recorded daily for all mice by assessing stool consistency, blood stool, and weight loss. Each score was determined as shown in Table 3. Bodyweight loss was calculated as the percentage of the difference between the initial body weight (day 0) and the weight on any given day.
Table 3. DAI Assessmenta.
| score | weight loss (%) | stool consistency | stool blood |
|---|---|---|---|
| 0 | none | normal | normal |
| 1 | 1–5 | ||
| 2 | 5–10 | loose stools | visible blood |
| 3 | 10–20 | ||
| 4 | >20 | diarrhea | rectal bleeding |
DAI, disease activity index.
4.11. qRT-PCR Analysis
The RNA was isolated from the colon tissue using the MiniBEST Universal RNA Extraction kit (Takara, Japan) following the manufacturer’s instructions. First strand cDNA was synthesized by using PrimeScript Reverse Transcriptase (Takara, Japan) during incubation at 42 °C for 15 min followed by deactivation at 85 °C for 5 s.
The one-step RT-PCR Kit (Toyobo, Japan) was used to perform qRT-PCR on a 7500 real-time PCR system (Applied Biosystems) under the following conditions: 94 °C for 30 s and then 40 cycles of 94 °C for 30 s followed by 60 °C for 30 s. The PCR primer sets for gene identification are listed in Table 4. The relative mRNA expression levels of genes were normalized to β-actin in each sample using the 2–ΔΔCT method with three replicates. Genes associated with intestinal barrier integrity (claudin-3, claudin-7, ZO-2, occludin, JAM-2, and MUC-3) and proinflammatory cytokines (caspase-1, NLPR3, TLR4, TNF-α, IL-1β) were detected in the colon tissue.
Table 4. Sequences of Primers Used for qRT-PCRa.
| target gene | forward (5′–3′) | reverse (5′–3′) |
|---|---|---|
| claudin-3 | CCGCTCGAGGCATCTTTTGGGTACCTTTGCG | GAAGATCTCCAGATGTTCTGCGACGTGATG |
| claudin-7 | GGATTGGTCATCAGATTGTCACA′ | TGGCAGGTCCAAACTCGTACT |
| ZO-2 | CTGGTGGCAATGATGTCG | CCCGCACTAATCCTCTGAAA |
| occludin | TGGCTATGGAGGCGGCTATGG | AAGGAAGCGATGAAGCAGAAGGC |
| JAM-2 | ATGCTGCTGCTGCTACACTACTT | TGACTTCTTGACGGTGGTCTTTT |
| MUC-3 | CGTGGTCAACTGCGAGAATGG | CCTCTCGCATCTCTATCTCGGC |
| caspase-1 | CTTGGAGACATCCTGTCAGGG | AGTCACAAGACCAGGCATATTCT |
| NLRP3 | CCATCAATGCTGCTTCGACA | GAGCTGCCTAAGGGAGGTAG |
| IL-1β | CAACCAACAAGTGATATTCTCCATG | GATCCACACTCTCCAGCTGCA |
| TLR4 | GCTCTCAGCCATCCACAAAG | GAGTCGGGAAGAGGAAGAGG |
| TNF-α | CTCATGCACCACCATCAAGG | ACCTGACCACTCTCCCTTTG |
| β-actin | GTGAAGGTGACAGCAGTCGGTT | GAGAAGTGGGGTGGCTTTTAGGA |
ZO-2, tight junction protein 2; JAM-2, junctional adhesion molecule 2; MUC-3, mucins 3; NLRP3, NOD-like receptor protein 3; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor α.
4.12. DNA Extraction and Analysis of Microbiome
Proximal colonic contents were collected into a sterile centrifuge tube immediately after the mice were sacrificed for further 16S rDNA analysis according to Liu et al.7 In brief, the samples were stored at −80 °C until needed for DNA extraction. Aliquots (10 ng) of extracted DNA were used for amplifying and sequencing the V3–V4 hypervariable regions of the 16S rDNA. The operational taxonomic units (OTUs), ribosomal database project, fifth level of taxa assignment (genus), PCoA, and the distance matrix were analyzed on the online platform of Majorbio Cloud Platform (Majorbio Biopharm Technology Co., Ltd., Shanghai, China).
4.13. Statistical Analysis
All data are presented as mean ± SEM. Data of samples were compared using ANOVA with Duncan’s test (SAS version 9.2, SAS Inc., Cary, NC, USA). p < 0.05 indicated statistical significance.
Acknowledgments
This work is supported by the National Key Research and Development Program of China (2021YFD1300300), the Natural Science Foundation of Henan Province (202300410104), the Innovation Fund of Henan University of Technology (2020ZKCJ25), and the High Level Research Fund for Qualified People of Henan University of Technology (2018QNJH16, 31401132).
Glossary
Abbreviations
- DFs
dietary fibers
- IBD
inflammatory bowel disease
- DSS
dextran sodium sulfate
- MCC
microcrystalline cellulose
- CD
Crohn’s disease
- UC
ulcerative colitis
- SDF
soluble dietary fiber
- IDF
insoluble dietary fiber
- TJs
tight junctions
- LEfSe
linear discriminant analysis effect size
- SEM
scanning electron microscopy
- FT-IR
Fourier-transform infrared spectroscopy
- XRD
X-ray diffraction
- TA
thermal analysis
- DSC
differential scanning calorimetry
- TG
thermo-gravimetry
- MPO
myeloperoxidase
- H&E
hematoxylin and eosin
- DAI
disease activity index
- E. coli
Escherichia coli
- OTU
operational taxonomic units
- RDP
ribosomal database project
- PCoA
principal component analysis
- NLRP3
NOD-like receptor protein 3
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor α
Author Contributions
H.Q., T.Z., Y.J., and Y.C.: writing-original draft, data curation, investigation, and resources. H.R., S.C., and Z.W.: conceptualization, methodology, and formal analysis. R.Z. and X.W.: funding acquisition and supervision. J.W. and L.G.: writing-review and editing and project administration. All authors adhered to the Committee on Publication Ethics guideline.
The authors declare no competing financial interest.
References
- Shohdy K. S.; Rashad W.; Elmeligui A. Alopecia universalis associated with ulcerative colitis and the role of azathioprine. Middle East J. Digest. Dis. 2018, 10, 50–54. 10.15171/mejdd.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples J.; Evans K.; Chaumont N.; Strassle P.; Sadiq T.; Koruda M. Variant two-stage ileal pouch-anal anastomosis: An innovative and effective alternative to standard resection in ulcerative colitis. J. Am. Coll. Surg. 2017, 224, 557–563. 10.1016/j.jamcollsurg.2016.12.049. [DOI] [PubMed] [Google Scholar]
- Hung T. V.; Suzuki T. Dietary fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. J. Nutr. 2016, 146, 1970–1979. 10.3945/jn.116.232538. [DOI] [PubMed] [Google Scholar]
- Zheng K.; Shen H.; Jia J.; Lu Y.; Zhu L.; Zhang L.; Shen Z. Traditional Chinese medicine combination therapy for patients with steroid-dependent ulcerative colitis: Study protocol for a randomized controlled trial. Trials 2017, 18, 8–6. 10.1186/s13063-016-1763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praengam K.; Sahasakul Y.; Kupradinun P.; Sakarin S.; Sanitchua W.; Rungsipipat A.; Rattanapinyopituk K.; Angkasekwinai P.; Changsri K.; Mhuantong W.; et al. Brown rice and retrograded brown rice alleviate inflammatory response in dextran sulfate sodium (DSS)-induced colitis mice. Food Funct. 2017, 8, 4630–4643. 10.1039/c7fo00305f. [DOI] [PubMed] [Google Scholar]
- Koleva P. T.; Valcheva R. S.; Sun X.; Gänzle M. G.; Dieleman L. A. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br. J. Nutr. 2012, 108, 1633–1643. 10.1017/s0007114511007203. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Wang X.; Chen Q.; Luo L.; Ma M.; Xiao B.; Zeng L. Camellia sinensis and litsea coreana ameliorate intestinal inflammation and modulate gut microbiota in dextran sulfate sodium-induced colitis mice. Mol. Nutr. Food Res. 2020, 64, 1900943. 10.1002/mnfr.201900943. [DOI] [PubMed] [Google Scholar]
- Chen H.; Zhao C.; Li J.; Hussain S.; Yan S.; Wang Q. Effects of extrusion on structural and physicochemical properties of soluble dietary fiber from nodes of lotus root. LWT - Food Sci. Technol. 2018, 93, 204–211. 10.1016/j.lwt.2018.03.004. [DOI] [Google Scholar]
- Huang L.; Zhang W.; Cheng J.; Lu Z. Antioxidant and physicochemical properties of soluble dietary fiber from garlic straw as treated by energy-gathered ultrasound. Int. J. Food Prop. 2019, 22, 678–688. 10.1080/10942912.2019.1600544. [DOI] [Google Scholar]
- Zou J.; Chassaing B.; Singh V.; Pellizzon M.; Ricci M.; Fythe M. D.; Kumar M. V.; Gewirtz A. T. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 2018, 23, 41–53. 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.; Zhao Q.; Li X.; Zhao J.; Li P.; Lin S.; Wang H.; Zang J.; Xiao Y.; Xu W.; et al. Dietary fiber intake and endometrial cancer risk: A systematic review and meta-analysis. Nutrients 2018, 10, 945. 10.3390/nu10070945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y.; Rasmussen H.; Kamil A.; Du P.; Blumberg J. Orange pomace improves postprandial glycemic responses: An acute, randomized, placebo-controlled, double-blind, crossover trial in overweight men. Nutrients 2017, 9, 130. 10.3390/nu9020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman R.; Logtenberg M. J.; Beukema M.; de Haan B. J.; Faas M. M.; Zoetendal E. G.; Schols H. A.; de Vos P. Chicory inulin enhances fermentation of 2′-fucosyllactose by infant fecal microbiota and differentially influences immature dendritic cell and T-cell cytokine responses under normal and Th2-polarizing conditions. Food Funct. 2021, 10, 9018. 10.1039/d1fo00893e. [DOI] [PubMed] [Google Scholar]
- Ali M. S.; Hussein A. R. M.; Gaber Y.; Hammamd O. A.; Kandeile M. A. Modulation of JNK-1/β-catenin signaling by Lactobacillus casei, inulin and their combination in 1,2-dimethylhydrazine-induced colon cancer in mice. RSC Adv. 2019, 9, 29368. 10.1039/c9ra04388h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada A. M.; EI-Kady M. Y.; EI-Sayed E. S.; Amine F. M. Preparation and characterization of microcrystalline cellulose. BioResources 2009, 4, 1359–1371. [Google Scholar]
- Campbell J. M.; Fahey G. C.; Wolf B. W. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J. Nutr. 1997, 127, 130–136. 10.1093/jn/127.1.130. [DOI] [PubMed] [Google Scholar]
- Bartley G. E.; Yokoyama W.; Young S. A.; Anderson W. H. K.; Hung S.-C.; Albers D. R.; Langhorst M. L.; Kim H. Hypocholesterolemic effects of hydroxypropyl methylcellulose are mediated by altered gene expression in hepatic bile and cholesterol pathways of male hamsters. J. Nutr. 2010, 140, 1255–1260. 10.3945/jn.109.118349. [DOI] [PubMed] [Google Scholar]
- Takahashi T.; Karita S.; Ogawa N.; Goto M. Crystalline cellulose reduces plasma glucose concentrations and stimulates water absorption by increasing the digesta viscosity in rats. J. Nutr. 2005, 135, 2405–2410. 10.1093/jn/135.10.2405. [DOI] [PubMed] [Google Scholar]
- Xu C.; Liu J.; Gao J.; Wu X.; Cui C.; Wei H.; Zheng R.; Peng J. Combined soluble fiber-mediated intestinal microbiota improve insulin sensitivity of obese mice. Nutrients 2020, 12, 351. 10.3390/nu12020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishisono K.; Mano T.; Yabe T.; Kitaguchi K. Dietary fiber pectin ameliorates experimental colitis in a neutral sugar side chain-dependent manner. Front. Immunol. 2019, 10, 1–14. 10.3389/fimmu.2019.02979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M.-m.; Mu T.-h. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016, 194, 237–246. 10.1016/j.foodchem.2015.07.095. [DOI] [PubMed] [Google Scholar]
- Qiao H.; Shao H.; Zheng X.; Liu J.; Liu J.; Huang J.; Zhang C.; Liu Z.; Wang J.; Guan W. Modification of sweet potato (Ipomoea batatas Lam.) residues soluble dietary fiber following twin-screw extrusion. Food Chem. 2021, 335, 127522. 10.1016/j.foodchem.2020.127522. [DOI] [PubMed] [Google Scholar]
- Yang Z.Study on Eextraction, Refining and Oxidation Resistant Effects of Inulin from Jerusalem Artichoke; Gansu Agricultural University: Lanzhou, Gansu, China, 2009. [Google Scholar]
- Zhang R.-Y.; Liu H.; Hou J.; Yao Y.; Ma Y.; Wang X. Cellulose fibers extracted from sesame hull using subcritical water as a pretreatment. Arab. J. Chem. 2021, 14, 103178. 10.1016/j.arabjc.2021.103178. [DOI] [Google Scholar]
- Yang L.; Lin Q.; Han L.; Wang Z.; Luo M.; Kang W.; Liu J.; Wang J.; Ma T.; Liu H. Soy hull dietary fiber alleviates inflammation in BALB/C mice by modulating the gut microbiota and suppressing the TLR-4/NF-κB signaling pathway. Food Funct. 2020, 11, 5965–5975. 10.1039/d0fo01102a. [DOI] [PubMed] [Google Scholar]
- Zhang P. P.; Tong D. S.; Lin C. X.; Yang H. M.; Zhong Z. K.; Yu W. H.; Wang H.; Zhou C. H. Effects of acid treatments on bamboo cellulose nanocrystals. Asia-Pac. J. Chem. Eng. 2014, 9, 686–695. 10.1002/apj.1812. [DOI] [Google Scholar]
- Das K.; Ray D.; Bandyopadhyay N. R.; Sengupta S. Study of the properties of microcrystalline cellulose particles from different renewable resources by XRD, FTIR, nanoindentation, TGA and SEM. J. Polym. Environ. 2010, 18, 355–363. 10.1007/s10924-010-0167-2. [DOI] [Google Scholar]
- Julie C. C. S.; George N.; Narayanankutty S. K. Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydr. Polym. 2016, 142, 158–166. 10.1016/j.carbpol.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Guancha-Chalapud M. A.; Gálvez J.; Serna-Cock L.; Aguilar C. N. Valorization of colombian fique (Furcraea bedinghausii) for production of cellulose nanofibers and its application in hydrogels. Sci. Rep. 2020, 10, 11637. 10.1038/s41598-020-68368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolinário A. C.; de Carvalho E. M.; de Lima Damasceno B. P. G.; Da Silva P. C. D.; Converti A.; Pessoa A.; Da Silva J. A. Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind. Crop. Prod. 2017, 108, 355–362. 10.1016/j.indcrop.2017.06.045. [DOI] [Google Scholar]
- Fares M. M.; Salem M. t. S. Dissolution enhancement of curcuminviacurcumin-prebiotic inulin nanoparticles. Drug Dev. Ind. Pharm. 2015, 41, 1785–1792. 10.3109/03639045.2015.1004184. [DOI] [PubMed] [Google Scholar]
- Hu X.; Wang K.; Yu M.; He P.; Qiao H.; Zhang H.; Wang Z. Characterization and antioxidant activity of a low-molecular-weight xanthan gum. Biomolecules 2019, 9, 1–12. 10.3390/biom9110730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Wang Z.-W. Thermal analysis and physiological behavior of cellulose/pectin complex from Canna edulis Ker by-product. Carbohydr. Polym. 2012, 87, 1153–1158. 10.1016/j.carbpol.2011.08.089. [DOI] [Google Scholar]
- Zhang J.; Wang Z.-W. Soluble dietary fiber from Canna edulis Ker by-product and its physicochemical properties. Carbohydr. Polym. 2013, 92, 289–296. 10.1016/j.carbpol.2012.09.067. [DOI] [PubMed] [Google Scholar]
- Einhorn-Stoll U.; Kunzek H.; Dongowski G. Thermal analysis of chemically and mechanically modified pectins. Food Hydrocolloids 2007, 21, 1101–1112. 10.1016/j.foodhyd.2006.08.004. [DOI] [Google Scholar]
- Yan S.; Yang B.; Zhao J.; Zhao J.; Stanton C.; Ross R. P.; Zhang H.; Chen W. A ropy exopolysaccharide producing strainBifidobacterium longumsubsp.longumYS108R alleviates DSS-induced colitis by maintenance of the mucosal barrier and gut microbiota modulation. Food Funct. 2019, 10, 1595–1608. 10.1039/c9fo00014c. [DOI] [PubMed] [Google Scholar]
- Kim M.; Chung K.; Hwang S.; Yoon Y. S.; Jang Y. P.; Lee J. K.; Lee K. Protective effect of Cicer arietinum L. (Chickpea) ethanol extract in the dextran sulfate sodium-induced mouse model of ulcerative colitis. Nutrients 2020, 12, 456. 10.3390/nu12020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S.; Ohara S.; Kubota T.; Saigenji K.; Hotta K. Compensatory response of colon tissue to dextran sulfate sodium-induced colitis. J. Gastroenterol. 1999, 34, 207–214. 10.1007/s005350050245. [DOI] [PubMed] [Google Scholar]
- Ogata M.; Ogita T.; Tari H.; Arakawa T.; Suzuki T. Supplemental psyllium fibre regulates the intestinal barrier and inflammation in normal and colitic mice. Br. J. Nutr. 2017, 118, 661–672. 10.1017/s0007114517002586. [DOI] [PubMed] [Google Scholar]
- Akram W.; Garud N.; Joshi R. Role of inulin as prebiotics on inflammatory bowel disease. Drug Discoveries Ther. 2019, 13, 1–8. 10.5582/ddt.2019.01000. [DOI] [PubMed] [Google Scholar]
- Leenen C. H. M.; Dieleman L. A. Inulin and oligofructose in chronic inflammatory bowel disease. J. Nutr. 2007, 137, 2572–2575. 10.1093/jn/137.11.2572s. [DOI] [PubMed] [Google Scholar]
- Choi W.; Yeruva S.; Turner J. R. Contributions of intestinal epithelial barriers to health and disease. Exp. Cell Res. 2017, 358, 71–77. 10.1016/j.yexcr.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm A. T.; Pedicord V. A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. 10.1111/imm.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechuga S.; Ivanov A. I. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1183–1194. 10.1016/j.bbamcr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani S.; Watanabe T.; Nadatani Y.; Sugimura N.; Shimada S.; Takeda S.; Otani K.; Hosomi S.; Nagami Y.; Tanaka F.; et al. NLRP3 inflammasome has a protective effect against oxazolone-induced colitis: A possible role in ulcerative colitis. Sci. Rep. 2016, 6, 39075–14. 10.1038/srep39075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A.; Babyatsky M. W.; Ogata S.; Chen A.; Itzkowitz S. H. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J. Histochem. Cytochem. 1996, 44, 1161–1166. 10.1177/44.10.8813081. [DOI] [PubMed] [Google Scholar]
- Larson M. A.; Wei S. H.; Weber A.; Mack D. R.; McDonald T. L. Human serum amyloid A3 peptide enhances intestinal MUC3 expression and inhibits EPEC adherence. Biochem. Biophys. Res. Commun. 2002, 300, 531–540. 10.1016/s0006-291x(02)02901-7. [DOI] [PubMed] [Google Scholar]
- Mack D. R.; Michail S.; Wei S.; McDougall L.; Hollingsworth M. A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 1999, 276, G941. 10.1152/ajpgi.1999.276.4.g941. [DOI] [PubMed] [Google Scholar]
- Engler D. B.; Leonardi I.; Hartung M. L.; Kyburz A.; Spath S.; Becher B.; Rogler G.; Müller A. Helicobacter pylori-specific protection against inflammatory bowel disease requires the NLRP3 inflammasome and IL-18. Inflamm. Bowel Dis. 2015, 21, 854–861. 10.1097/mib.0000000000000318. [DOI] [PubMed] [Google Scholar]
- Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Dong N.; Li X.; Xue C.; Wang C.; Xu X.; Bi C.; Shan A.; Li D. Astragalus polysaccharides attenuated inflammation and balanced the gut microflora in mice challenged with Salmonella typhimurium. Int. Immunopharmacol. 2019, 74, 105681–11. 10.1016/j.intimp.2019.105681. [DOI] [PubMed] [Google Scholar]
- Cui L.; Wang W.; Luo Y.; Ning Q.; Xia Z.; Chen J.; Feng L.; Wang H.; Song J.; Tan X.; et al. Polysaccharide from Scutellaria baicalensis Georgi ameliorates colitis via suppressing NF-κB signaling and NLRP3 inflammasome activation. Int. J. Biol. Macromol. 2019, 132, 393–405. 10.1016/j.ijbiomac.2019.03.230. [DOI] [PubMed] [Google Scholar]
- Wu H. C.; Chen Y. J.; Xu Y. C.; Shen P. P. The role of caspase-1 played in the process of inflammation and programmed cell death. Chin. J. Cell Biol. 2011, 2, 182–189. [Google Scholar]
- Green D. R. The coming decade of cell death research: Five riddles. Cell 2019, 177, 1094–1107. 10.1016/j.cell.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q.; Li L.; Sun Y.; Yang H.; Ye Z.; Zhao J. Effects of the TLR4/Myd88/NF-κB Signaling Pathway on NLRP3 Inflammasome in Coronary Microembolization-Induced Myocardial Injury. Cell. Physiol. Biochem. 2018, 47, 1497–1508. 10.1159/000490866. [DOI] [PubMed] [Google Scholar]
- Han J.; Wang X.; Tang S.; Lu C.; Wan H.; Zhou J.; Li Y.; Ming T.; Wang Z. J.; Su X. Protective effects of tuna meat oligopeptides (TMOP) supplementation on hyperuricemia and associated renal inflammation mediated by gut microbiota. FASEB J. 2020, 34, 5061. 10.1096/fj.201902597rr. [DOI] [PubMed] [Google Scholar]
- Han Y.; Song M.; Gu M.; Ren D.; Zhu X.; Cao X.; Li F.; Wang W.; Cai X.; Yuan B.; et al. Dietary intake of whole strawberry inhibited colonic inflammation in dextran-sulfate-sodium-treated mice via restoring immune homeostasis and alleviating gut microbiota dysbiosis. J. Agric. Food Chem. 2019, 67, 9168–9177. 10.1021/acs.jafc.8b05581. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Huang Y.; Wang Y.; Wang P.; Wang F. Severe burn injury alters intestinal microbiota composition and impairs intestinal barrier in mice. Burns Trauma 2019, 7, 20–14. 10.1186/s41038-019-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagkouvardos I.; Lesker T. R.; Hitch T. C. A.; Gálvez E. J. C.; Smit N.; Neuhaus K.; Wang J.; Baines J. F.; Abt B.; Stecher B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28–15. 10.1186/s40168-019-0637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad M. A. K.; Sarker M.; Li T.; Yin J. Probiotic species in the modulation of gut microbiota: An overview. BioMed Res. Int. 2018, 2018, 1–8. 10.1155/2018/9478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Chen D. W.; Yu B.; He J.; Yu J.; Luo J. Q.; Mao X. B.; Huang Z. Q.; Zheng P.; Luo Y. H. Two dietary fibers influence the bacterial community in the colon of Balb/c mice. Microbiol. China 2018, 45, 395–404. [Google Scholar]
- Seregin S. S.; Golovchenko N.; Schaf B.; Chen J.; Pudlo N. A.; Mitchell J.; Baxter N. T.; Zhao L.; Schloss P. D.; Martens E. C.; et al. NLRP6 Protects Il10 −/– Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep. 2017, 19, 733–745. 10.1016/j.celrep.2017.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S.; Tian P.; Luo Y.; Tian J.; Hua C.; Geng Y.; Cong R.; Ni Y.; Zhao R. Microbiome-metabolome responses to a high-grain diet associated with the hind-gut health of goats. Front. Microbiol. 2017, 8, 1764. 10.3389/fmicb.2017.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada M. M.; Smith D. E. Relevance of PepT1 in the intestinal permeability and oral absorption of cefadroxil. Pharm. Res. 2013, 30, 1017–1025. 10.1007/s11095-012-0937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Wang W.; Zhou R.; Ng S. C.; Li J.; Huang M.; Zhou F.; Wang X.; Shen B.; Kamm M.; et al. Characteristics of fecal and mucosa-associated microbiota in Chinese patients with inflammatory bowel disease. Medicine 2014, 93, e51 10.1097/md.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J. P.; Zou J.; Kumar M.-V.; Pellizzon M.; Ulman E.; Ricci M.; Gewirtz A. T.; Chassaing B. Supplementation of low- and high-fat diets with fermentable fiber exacerbates severity of DSS-induced acute colitis. Inflamm. Bowel Dis. 2017, 23, 1133–1143. 10.1097/mib.0000000000001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson P.; Medina D. A.; Ortúzar V.; Gotteland M.; Garrido D. Anti-inflammatory effect of microbial consortia during the utilization of dietary polysaccharides. Food Res. Int. 2018, 109, 14–23. 10.1016/j.foodres.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Wang L.; Zhang B.; Xiao J.; Huang Q.; Li C.; Fu X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. 10.1016/j.foodchem.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Gao M.; Li H.; Deng Z.; Zhang B.; Fan Y. Effects of soluble dietary fiber from sweet potato dregs on the structures of intestinal flora in mice. Food Biosci. 2021, 40, 100880. 10.1016/j.fbio.2021.100880. [DOI] [Google Scholar]