Abstract

We report that the polymerization of cyclodextrin (CD) with epichlorohydrin (ECH) dramatically increases the binding constant of CD to vanillin, from 55 to 8.4 × 103 M–1, by approximately 100 times, as determined by diffusion ordered spectroscopy (DOSY)–1H NMR. The binding constant increased with an increase of the ECH content of the polymer, although ECH polymers without CDs showed no affinity at all, suggesting that the hydrophobicity of the ECH network outside of CDs helps to enhance the binding. This increased binding constant allows CD–ECH polymers to increase the drug loading ratio, which may be one of the most critical issues for drug delivery systems.
Introduction
Cyclodextrins (CDs) are cyclic oligosaccharides composed of α-1,4-linked glucoses that are produced in the enzymatic treatment of starch.1 CDs exist in nature, albeit in minute amounts, and are found in various fermented products, including beer and bread. Among them, three types of CDs are synthesized at a large scale and commercially available, which are six- (αCD), seven- (βCD), and eight-membered rings (γCD). Applications of CDs have been established in various fields including pharmacy, food, chromatography, catalysis, agriculture, cosmetics, medicine, and textiles.2−4 CDs are now attracting significant attention as key materials for environmental chemistry.5 They have a hydrophilic outer surface and a hydrophobic central cavity, providing the ability to form complexes with many hydrophobic compounds; as a result, these hydrophobic compounds can be dispersed in water as a complex with CDs, providing various physicochemical advantages, including stability and bioavailability. This is the main reason for the broad application of CDs.2 This remarkable encapsulation is one of the most well-known and -studied “host–guest”-type supramolecular interactions. In the pharmaceutical field, CDs have been applied as solubilizers for hydrophobic drugs to increase bioavailability and to control pharmacokinetics.4 CDs have become particularly indispensable in the formulation of steroids and prostanoid, which indeed has revolutionized anesthesia and obstetric medicine.6
Recently, numerous CD-containing polymers have been designed to control the particle size and the binding affinity of drugs.7−9 The polymerization of CDs is carried out via a direct reaction between their hydroxyl groups and a coupling agent to form water-soluble or -insoluble polymers. Among them, epichlorohydrin (ECH) is considered as the most commonly used cross-linking agent. Its widespread use is due to its high reactivity with CDs, simple synthesis reaction, and flexibility allowing various types of materials to be obtained, including hydrogels/gels, powders/particles, beads/resins, and nanoparticles/nanobeads.10 For example, water-insoluble cyclodextrin polymers have been developed as materials for adsorbing organic pollutants such as methylene blue, bisphenol A, and metal ions.11−17 In pharmaceutical applications, the water-soluble CDs polymers are of particular interest as a drug delivery system (DDS) given their ability to improve the solubility in aqueous solution as well as the bioavailability of some drugs beyond those with native CDs.18−20 For example, the aqueous solubility of glipizide was reported to increase in the presence of the βCD–ECH polymer and the dissolution rate of glipizide from the β-CD–ECH polymer complex was significantly greater than that of the drug itself.21 In general, a large binding constant (>104 M–1) between the DDS carrier and the drug is required in order to maintain a stable complex and improve bioavailability after intravenous administration. However, it is rare to form a CD complex with such a large binding constant. Recently, we synthesized various CD–ECH polymers (hereinafter referred to as CDNPs) with different CD contents and found that CDNPs dramatically increased the binding constants of α-mangostin (MGS) to nearly 100-fold compared to CDs themselves,22 where MGS is a xanthone derivative and well-known as an antioxidant and antigenotoxic agent. In these works, we measured the absolute weight-averaged molecular weight (Mw), hydrodynamic radius (Rh), and CD content and found how to control these physical characteristics by changing the reaction time, ECH/CD ratio, and pH of the polymerization solution. In some cases, the addition of amphiphilic molecules as a phase transfer catalyst is useful to control the properties.23 We also investigated the inclusion complex with MGS as a potential anticancer drug24 and an agent for removal of reactive oxygen species during ischemia-reperfusion (paper and patent in preparation), but the molecular mechanism of the inclusion of MGS by CDNPs still remains unclear. Although not for epichlorohydrin-linked CDs, Ma and Li found that the binding constant dramatically increased by about 5 orders of magnitude for diisocyanate-linked CDs by measuring UV absorbance.25 As far as we know, this is the first paper to report the dramatic enhancement of the binding due to the polymerization of CDs. Before the work of Ma and Li, there were several papers in which the water solubility of hydrophobic molecules was considerably improved by the polymerization of CDs. However, they did not observe a dramatic increase of the binding constant.26,27 Karoyo and Wilson claimed that the results of Ma and Li may be due to an artifact resulting from the presence of an additional binding site created at the outside of CD or the linker molecule.28 It appears that, within the field of cyclodextrin chemistry, no definitive conclusions can yet be drawn about the increased binding of CD polymers and their molecular mechanism.
Numerous analytical methods are available for the accurate determination of the binding constant of the inclusion complexes made from CDs and guests.29 The most commonly used and easiest approaches are the phase solubility and titration methods.30 In the solubility method, the solubility of the guest is measured after mixing CD and the guest molecule, and the binding constant is determined from the increment of the solubility from that of the solution saturated with the guest molecule alone. The phase solubility method is useful and convenient. However, the accuracy of the binding constant is strongly affected by the presence of small aggregates dispersed in water, which is sometimes difficult to distinguish from solubilized molecules. In fact, it is difficult to determine the saturated concentration of some organic compounds accurately. Generally, in the case of low intrinsic solubility, the error of the binding constant becomes large.30 In the titration method, one component (usually the guest) is gradually added to the solution containing the host while monitoring a physical property such as heat flow, specific chemical resonance (in NMR), absorption band (in UV), or fluorescence intensity that is sensitive to the interaction of interest.31 Recently, diffusion ordered NMR spectroscopy (DOSY–NMR) has been developed and provides a new additional NMR-based method and an alternative method to investigate the binding constant between CDs and guest molecules.32 An interesting feature of this technique is that the binding constant can be derived from the diffusion coefficient. In a fast exchange process between free and bound states of the component, its observed diffusion coefficient represents the population weight-average of the diffusion properties of these two states.32 Therefore, the fraction of each component can be evaluated from DOSY and thus the binding constant can be determined. Advances in NMR spectroscopy and computing have made high-resolution DOSY more readily available. High-resolution DOSY can distinguish small differences of about 1% in diffusion coefficient, and overlapping signals coming from two different objects that differ in diffusion coefficient by at least 30% can be distinguished.33 Furthermore, NMR does not require a chromophore for either the host or the guest and can provide molecular information on the binding model. Recently, DOSY has been applied to study the binding behavior of the inclusion complexes of CDs, but there has been no work to investigate CD-based polymers by use of DOSY.34,35
This study investigates the inclusion complexes of CDNPs with vanillin (4-hydroxy-3-methoxy benzaldehyde, see Figure 1A for its chemical structure) as a representative guest molecule, compared with CDs themselves. Vanillin was chosen instead of MGS because the solubility of vanillin in water is quite suitable for NMR experiments, whereas that of MGS is too small to allow accurate NMR experiments. Additionally, the interaction between vanillin and CDs has been studied by various groups and it is now well-established that (1) vanillin forms a stable 1:1 complex with βCD,36−38 (2) the binding constant between them in water at ambient temperate ranges from 10 to 2 × 102 M–1, depending on the method and reseachers,34,37,38 and (3) vanillin is oriented with the phenolic end nearer to the narrower end (or lower rim) and the aldehyde end near to the wider end (upper rim) of βCD.38 By use of such well-studied vanillin, we should be able to clarify the molecular mechanism of the CDNP–vanillin interaction by two-dimensional (2D) ROESY and NOESY and examine the binding constant for various types of CDNPs using DOSY.
Figure 1.
Chemical structures and proton numbers of vanillin and cyclodextrin (CDs) as well as the three-dimensional structure of CDs and 1H NMR spectra of vanillin, βCD, βCDNP2.7, and vanillin−βCD and vanillin−βCDNP2.7 mixtures. The intermediate δ regions of the spectra are enlarged in part E. A: The chemical structure of vanillin. B: The chemical structure and three-dimensional structure of CDs. C: 1H NMR spectra of vanillin, βCD, βCDNP2.7, vanillin−βCD, and vanillin−βCDNP2.7 mixtures. D: Synthesis scheme of cyclodextrin-based hyperbranched polymers (CDNPs) through polyaddition reactions with ECH. E: The intermediate δ regions from 6.85 to 9.75 ppm of the 1H NMR spectra of vanillin and vanillin−βCD and vanillin−βCDNP2.7 mixtures.
Results
CDNPs and Their Characteristics
Table 1 shows all samples used in this experiment; all of them were newly synthesized following the procedure in the previous works.39 We synthesized a dextran-based ECH hyperbranched polymer as a comparison material (denoted by DXNP). In the sample codes of CDNPs, the prefix and suffix indicate the type of CD and the ECH/CD weight ratio in the polymer, respectively. Typical examples of a gel permeation chromatography coupled with multi angle light scattering (GPC-MALS) chromatogram and the angular dependence of static light scattering to determine Mw are shown in the Supporting Information (Figure S1). The obtained values of the averaged molecular weight (Mw) are summarized in the third column. The angular dependence of the excess Rayleigh ratio was too small to obtain reliable values on the radius of gyration, so it is not shown. The fourth and fifth columns show the hydrodynamic radius (Rh) determined by dynamic light scattering and DOSY, respectively. As shown in the Supporting Information, the distribution of Rh was unimodal because we removed small particles by dialysis. The weight percent of CDs was determined by the phenol sulfuric acid method40 and converted into the number of CDs in one CDNP particle (NCD) using Mw. As we reported already, the particle sizes remained less than 10 nm. The weight ratios of ECD to CDs in CDNPs were calculated from the weight percent of CDs and Mw. We confirmed that the phenol sulfuric acid method could determine the weight percent of CDs within ±5% and that the ECH polymer without CD showed 0% of carbohydrates by use of this method.
Table 1. Sample Lists Used in This Work, Including the Molecular Characteristics Determined by GPC-MALS and the Phenol Sulfuric Acid Method.
| sample code | feeding weight ratioa | Mwb (g/mol) | Rhc (nm) | Rhd (nm) | NCDe | wt % CD (%) | ECH/CD in polymer (w/w) |
|---|---|---|---|---|---|---|---|
| βCDNP0.9 | 2.83 | 9.37 × 104 | 4.0 | 5.6 ± 0.1 | 43.8 | 53.0 | 0.9 |
| βCDNP2.0 | 4.25 | 2.74 × 105 | 7.1 | 8.8 ± 0.2 | 79.4 | 32.9 | 2.0 |
| βCDNP2.7 | 4.25 | 7.29 × 104 | 3.5 | 3.9 ± 0.2 | 17.1 | 26.7 | 2.7 |
| βCDNP3.0 | 5.66 | 1.55 × 105 | 5.6 | 8.0 ± 0.5 | 34.3 | 25.1 | 3.0 |
| αCDNP3.2 | 4.25 | 2.43 × 104 | 2.1 | 2.6 ± 0.2 | 5.9 | 23.8 | 3.2 |
| γCDNP3.3 | 4.25 | 1.23 × 105 | 4.0 | 6.6 ± 1.0 | 21.9 | 23.2 | 3.3 |
| DXNP | 2.83 | 1.98 × 105 | 3.8 | 38.5f | 0.89 |
The feeding weight ratio is the weight ratio between ECH and CD in the synthesis reaction. ECH/CD in the polymer is the weight ratio between ECH and CD in the polymer.
Determined by GPC coupled with SLS, the estimated error range is about ±3–5%.
Determined with DLS
Determined with DOSY.
From the phenol sulfuric acid method, the estimated error range is about ±5%.
Glucose weight percent instead of CD.
We obtained 1H NMR spectra of βCDNP0.9 in DMSO, and the results showed the disappearance of all −OH peaks, as shown in Figure S2. βCDNP0.9 had the lowest ECH ratio and even this sample showed no sugar hydroxyl protons, suggesting that most of the hydroxyl groups of CDs had reacted with ECH.
1D and 2D 1H NMR and the Stoichiometry of the Complex
The 1H NMR spectra of vanillin, βCD, βCDNP2.7, vanillin−βCD mixture, and vanillin−βCDNP2.7 (βCD/vanillin molar ratio of 1:1 for both samples) in D2O are shown in Figure 1. Ferrazza et al. showed that, when the concentration of vanillin was increased, a significant upfield shift was observed for the positions of all proton peaks owing to the water-dispersing aggregates of vanillin. We confirmed no such shift in vanillin. The peak assignments of free vanillin and βCD are shown in Figure 1C (the proton numberings are shown in Figure 1A and B). Referring to the free vanillin and previous work, we also assigned the vanillin peaks in βCD and βCDNP2.7. The magnified peaks in this region are shown in Figure 1E. It is clear that the complexation with βCD and βCDNP2.7 caused a downfield shift and no peak splitting was observed. In the mixtures, there are two types of vanillin, free and complexed states, and the absence of new splitting resonances indicates that the interconversion between the free and complexed states is faster than the NMR time scale. The downfield shift due to the complexation with CD has been observed by other groups and can be ascribed to less shielding as a result of the lower-electron-density environment in the complexed state than in the free state. This type of downfield shift is normally observed in CD inclusion complexes such as βCD–nicardipine hydrochloride, sodium picosulfate, and d(−)-chloramphenicol.41−43
Table 2 compares the chemical shifts (δVal) for four protons in vanillin among the three solutions: vanillin itself, vanillin in a mixture with βCD, and vanillin in a mixture with βCDNP2.7. The differences between the vanillin alone and the mixtures are shown in the fourth and sixth columns denoted by δVal/CD and δVal/CDNP, respectively. δVal/CD for H-5 and H-6 were 0.02–0.04 ppm, while those of H-1 and H-2 were lower. These features indicate that the H-5 and H-6 side of the aromatic ring digs more deeply into the CD cavity than the H-1 and H-2 side. The small change in H-1 suggests that the aldehyde group is most likely located at the upper rim or outside of the CD (as confirmed later by NOESY). This molecular arrangement is consistent with the previous work by Divakar.44 In βCDNP2.7–vanillin, H-5 showed a larger change in δVal than in βCD–vanillin. Even H-1 showed an appreciable change. These differences between βCD and βCDNP suggest that the binding mode is different between them. The larger δVal can be explained in two ways: the population of bound vanillin is increased, and/or a less shielded environment is created. Based only on 1D 1H NMR, we cannot draw conclusions about which is more likely. H-6 and H-5 are neighboring protons, and it seems difficult to understand why H-6 showed a small shift while H-1 and H-5 showed a clear increase.
Table 2. Chemical Shifts of Four Vanillin Protons before and after Mixing with βCD or βCDNP2.7.
| δVal | δVal mixed with βCD | ΔδVal/CD | δVal mixed with βCDNP2.7 | ΔδVal/CDNP | |
|---|---|---|---|---|---|
| H-5 | 6.907 | 6.949 | 0.042 | 7.013 | 0.106 |
| H-2 | 7.347 | 7.324 | –0.022 | 7.346 | –0.001 |
| H-6 | 7.388 | 7.411 | 0.023 | 7.416 | 0.028 |
| H-1 | 9.543 | 9.555 | 0.013 | 9.604 | 0.061 |
The stoichiometric ratio of the complexation of βCD and vanillin has been studied by many groups, and it is well established that they form a 1:1 complex.45 To obtain the stoichiometric ratio between βCD in βCDNP0.9 and vanillin, we constructed a Job plot from the H-5 proton chemical shift by changing the composition (Figure 2). Figure 2 plots [G]iΔδG against r, where [G]i and ΔδG are the total (free plus complexed) guest molecule concentration and the difference in chemical shifts of guest in the absence and presence of host for a given ratio r, and r is the composition ratio defined by r = [G]i/([G]i + [H]i), with [H]i being the total host site concentration, which is the βCD concentration of the solution. In this work, we regard the βCD in βCDNP as the host and [H] can be calculated from the polymer concentration and the number of CDs in polymers. The total concentration of [G]i + [H]i was kept constant at 4.9 mM. The [G]iΔδG value peaked at r = 0.5, indicating 1:1 binding. Therefore, the data points were fitted by the following equation for this binding mode:46
| 1 |
Here, ΔδHG = δHG – δG is the difference in chemical shifts of complex and guest in the absence of host. The data points were nicely fitted by the curve based on 1:1 binding, and the best fitting gave the value of K = 103 M–1. Compared with the fitted curve, which appears to show the maximum and minimum K values, the error range of this estimation is K = (0.8–1.25) × 103 M–1. The obtained value is consistent with that from DOSY. We constructed a Job plot for βCD and vanillin and compared it with that of βCDNP0.9 and vanillin, confirming that the K value is around 95 M–1 and there is 1:1 binding. To sum up the result of the Job plot, the main binding mode between βCDNP0.9 and vanillin is 1:1 complexation similarly to that of βCD and vanillin and the binding constant between βCDNP0.9 and vanillin appeared to be much larger than that of βCD and vanillin.
Figure 2.

Job plot for vanillin and βCDNP0.9 in D2O at 25 °C, compared with βCD. The total concentration [G]i + [H]i was fixed at 4.9 mM. The dotted and solid lines are calculated from eq 1 assuming K = 1.25 × 103, 1.0 × 103, 0.8 × 103, and 95 M–1 (from the top), respectively.
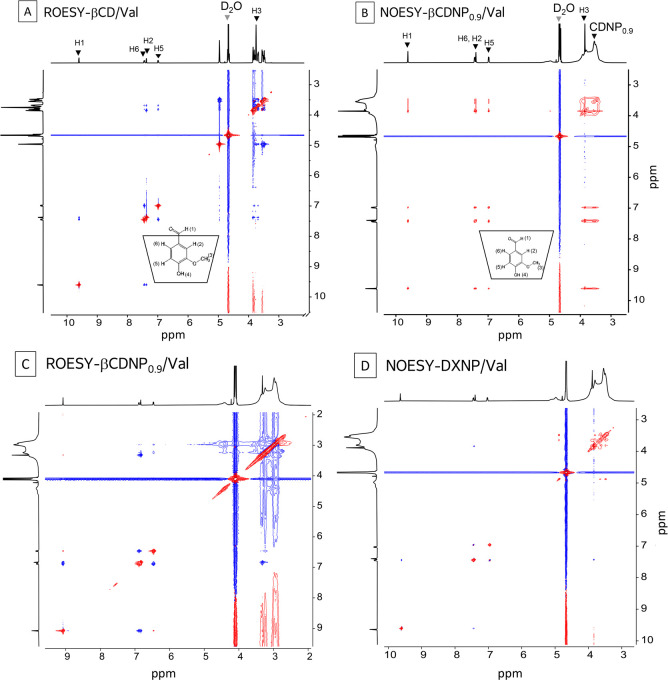
Figure 3 shows the 2D NMR spectra of βCD/Val (ROESY), βCDNP0.9/Val (NOESY and ROESY), and DXNP/Val (NOESY). βCD-Val showed clear intermolecular dipolar interactions between the phenyl ring protons and the βCD cavity protons, while the correlation between H-1 of vanillin and βCD was too moderate to be observed. This suggests that the vanillin molecule has the phenol side inside the cavity and the aldehyde part outside of it. This is consistent with the previous discussion as well as other studies.37,38 DXNP/Val showed no correlation between Val and DXNP at all; the off-diagonal signals are due to the Val–Val interactions. βCDNP0.9–Val shows the correlation between βCDNP and all phenyl ring protons including H-1, but it was difficult to distinguish the interaction between CD in CDNP and Val from that of Val and the polymerized ECH matrix. Therefore, there is a possibility that Val may be trapped by the polymerized ECH matrix instead of the host–guest interaction between CD in CDNP and Val. However, we can rule out this possibility based on the fact that there is no interaction between Val and DXNP. We can presume that all of the interactions observed between βCDNP0.9 and Val can be ascribed to the interactions between CD in CDNP and Val. This speculation is consistent with the result of the Job plot that the stoichiometry between CD and Val was maintained after the polymerization with ECH.
Figure 3.
2D NMR spectra of βCD/Val, βCDNP0.9/Val, and DXNP/Val solutions at 25 °C: the molar ratio of vanillin:βCD was 1:1, and the concentrations of vanillin, βCD, and βCD in βCDNP0.9 were 10 mM. A: ROESY-βCD/Val (mixing time 300 ms). B: NOESY-βCDNP0.9/Val (mixing time 900 ms). C: ROESY-βCDNP0.9/Val (mixing time 300 ms). D: NOESY-DXNP/Val (mixing time 900 ms). The red is negative, and the blue is positive.
The correlation between Val and CDNP was observed in NOESY, and the signs of them were negative. We observed the corresponding correlations in ROESY, except for H-1. These results confirm that Val molecules interact with CDNP and are most likely ingested by the CD of the CDNP.
Determining the Binding Constant of CDNP–Vanillin with DOSY-NMR
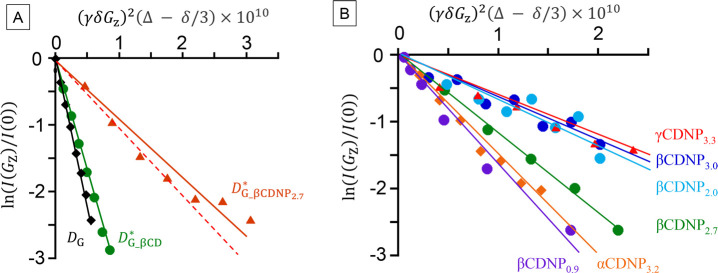
Figure 4 plots ln I(0)/I(Gz) against (γδGz)2 for the H-5 proton of vanillin of several samples. The diffusion coefficients (D) were determined from the slope, and the binding constant (K) was determined using eq 5. We carried out a similar fitting as for H-5 shown in the figure for the other protons, and the obtained values coincided within ±5%. In some cases, the data points had a relatively large error, as shown in Figure 4A. For such cases, the error range can be estimated as at most ±10%, which affected the final binding constant by ±21%. Table 3 summarizes all of the data including HVA and VMA: vanillin analogues are described later. The obtained K values for Val−βCDNP2.7 at different concentrations are presented in the Supporting Information (Figure S4).
Figure 4.
A: The signal intensity ratio ln I(0)/I(Gz) plotted against the exponential term in eq 3, where I(Gz) is the signal intensity at the end of the spin–echo after applying the gradient intensity of Gz and I(0) is the signal intensity at the end of the spin–echo in the absence of the gradient pulse. Here, the intensity of the H-5 proton of vanillin is plotted. The red dotted line fitted to βCDNP2.7 shows the upper limit of the estimated diffusion coefficient (D = 10–10 m2/s, K = 4.4 × 103 M–1), while the red solid line shows the result fitted by the least-squares method (D = 9 × 10–11 m2/s, K = 6.8 × 103 M–1). B: The diffusion profile of the H-5 proton of vanillin in the mixture with different CDNP samples.
Table 3. Obtained Values of the Diffusion Coefficient of DG* in eq 6 and Determined K Values for All Samples.
| guest | host | DG* (10–10 m2/s) | K (M–1) |
|---|---|---|---|
| Val | αCD | 4.01 ± 0.04 | 16 |
| βCD | 3.77 ± 0.44 | 55 | |
| γCD | 3.47 ± 0.25 | 97 | |
| βCDNP0.9 | 1.54 ± 0.33 | 1.07 × 103 | |
| βCDNP 2.0 | 0.78 ± 0.05 | 4.43 × 103 | |
| βCDNP2.7 | 0.90 ± 0.04 | 6.88 × 103 | |
| βCDNP3.0 | 0.68 ± 0.04 | 8.35 × 103 | |
| αCDNP3.2 | 1,75 ± 0.16 | 0.93 × 103 | |
| γCDNP3.3 | 0.67 ± 0.05 | 1.00 × 104 | |
| HVA | βCD | 3.65 ± 0.40 | 0.13 × 103 |
| βCDNP0.9 | 2.33 ± 0.07 | 0.48 × 103 | |
| βCDNP3.0 | 1.79 ± 0.08 | 1.20 × 103 | |
| VMA | βCD | 3.78 ± 0.13 | 0.12 × 103 |
| βCDNP0.9 | 2.13 ± 0.53 | 0.84 × 103 | |
| βCDNP3.0 | 1.63 ± 0.55 | 1.88 × 103 |
Based on the D values for each peak, we constructed a 2D DOSY plot in Figure 5 for βCD–vanillin and βCDNP2.7–vanillin with a peak width determined by the estimated error of the diffusion coefficient obtained from the fitting process. The vertical axis shows the chemical shift of 1H NMR of the mixtures, and the horizontal one is the obtained D value. The green and orange solid lines represent DG* ≡ DVal and DG* ≡ DCD or CDNP, and the green and orange dashed lines show DH and DG, respectively. The definitions of these symbols are shown in eq 6. From the dashed and solid orange lines of Figure 5, DH and DH* remain almost invariant to the binding upon consumption of the small molecular weight guest into the large molecular weight host. On the other hand, DG was dramatically decreased upon the binding. When the decrease in DG* is defined by ΔDG = DG – DG, Figure 5 shows ΔDG of βCDNP ≫ ΔDG of βCD.
Figure 5.
Examples of 2D DOSY comparing βCD/Val (A) and βCDNP2.7/Val (B) systems. The horizontal axis represents chemical shifts, whereas the vertical axis, diffusion coefficients; the dark spots are the resonances of the aqueous solution of the inclusion complex spread in the second dimension according to their measured diffusion coefficient.
Discussion
Based on the results of the Job plot in Figure 2, it can be concluded that vanillin is taken up by βCDNP in the same mode (i.e., 1:1 binding) as βCD. DOSY indicated the large increase in K due to the polymerization. The same increase in K was confirmed by fitting the Job plot with eq 1. ROESY and NOESY in Figure 3 indicated that the vanillin molecule enters more deeply into the βCD cavity in βCDNP than in βCD, while its molecular orientation (the phenolic end nearer to the lower rim and the aldehyde end nearer to the upper rim of βCD) may be maintained in βCDNP. In this section, we discuss how the K value is affected by the nature of the host molecules: differences of CD ring and ECH/CD. Based on the obtained data, we speculate on the molecular origin of the increase in K. Incidentally, it is well-known that K can be expressed by the ratio of the association (ka) and dissociation (kd) rate constants in the 1:1 binding model:
| 2 |
Figure 6A compares the K values among three types of CDs and CDNPs. K of CD increased in the order of α < β < γ, namely, 16, 55, and 97 M–1, respectively. Several works have determined the K value between βCD and vanillin (hereinafter denoted as KβCD/Val). Ferrazza et al.34 obtained KβCD/Val = 142 ± 9 M–1 using DOSY in the solution similar to those in the present study. Two other groups carried out 1H NMR titration, similarly to the present Job plot, to obtain KβCD/Val = 74–172 M–1.38,45 Their values are at most 3 times larger than ours, but we do not know the reason for this discrepancy. On the other hand, Karathanos et al.37 obtained KβCD/Val = 5.3 M–1 using the solubility method, which is much smaller than our values. As shown in Figure 2, we obtained KβCD/Val = 95 M–1 using a Job plot of the NMR. We suppose that the absolute value of K may depend on the method and experimental conditions used. In particular, vanillin tends to aggregate even at a low concentration, which may affect the ability to determine K accurately. A notable feature in Figure 6A is the dramatic increase in K value due to the polymerization. The increment of K for βCDNP2.7 (denoted by KβCDNP2.7/Val) is approximately 125 times. αCDNP3.2 and γCDNP3.3 also showed large increases of around 60 and 100 times, respectively.
Figure 6.
Summary of DOSY experiments. A: The binding constants are plotted for the three CDs (green) and αCDNP3.2, βCDNP3.0, and γCDNP3.0 (blue). B: KβCDNP/Val values were divided by KβCD/Val and plotted against ECH weight percent in CDNPs; the inset shows the increase of KβCDNP/Val. C: The same plot as in part B was constructed for MGS-CDNPs using the published data.22
Figure 6B plots the ratio of KβCDNP/Val/KβCD/Val against ECH/CD. It is worth noting here that K increases almost exponentially with the increase in ECH/CD, consistent with our previous study on the MGS/βCDNP system, as shown in Figure 6C.22 This means that the ECH matrix in the particles significantly contributes to the increase in K. However, DXNP (dextran-based ECH polymer) has no ability to interact with vanillin (see Figure S5 in the Supporting Information) and βCDNP shows the same 1:1 binding mode with βCD. These two facts indicate that CD is essential in the interaction and the ECH matrix does not directly ingest the guest, but it helps increase the CD/guest interaction.
Figure 7 compares the K values of βCD, βCDNP0.9, and βCDNP2.7 for vanillin and its two analogues. Vanillin has an aldehyde group, while the others have a carboxyl group and VMA has an additional hydroxyl group. Therefore, the order of hydrophobicity would be as follows: vanillin > HVA > VMA. The most hydrophobic vanillin shows the largest increment of K due to the ECH polymerization, suggesting that the hydrophobic interaction may play an important role in the enhancement of K. The native CD has several hydroxyl groups on the upper rim, which may obstruct the hydrophobic guest from entering the inner cavity of CD. 1H NMR of βCDNP0.9 in DMSO showed that the polymerization converted all of the hydroxyl groups to esters. Therefore, there is nothing obstructing ingestion of the hydrophobic guests for CDNPs. This factor may increase ka in eq 2. This may be one reason for the enhancement of K. CD molecules are connected by the ECH network in CDNPs. Before the guest molecules are captured by CD, they may be located at the ECH network. The polymerized ECH is hydrophobic and thus may attractively interact with the guest molecules, which increases the local concentration of the guest molecules in the vicinity of the CDs in CDNPs. Furthermore, the hydrophobic interactions of the ECH network slow down the diffusion of vanillin and make the guest readily available for interaction with the CD cavity. These factors may decrease kd in eq 2 and thus increase K. We did not see any direct interaction between the ECH network and vanillin. However, the large ECH/CD polymers show deviation from the 1:1 binding model and further increase in K, as shown in the Supporting Information (Figure S3), which suggests that the ECH network can interact with vanillin.
Figure 7.

Increase of the binding constants for vanillin analogues.
Before concluding, we would like to present a good example of the enhanced binding of βCDNP. Alsbaiee et al.48 reported in Nature that a polymerized CD showed dramatically increased absorption of bisphenol A (BPA), comparable to that of active carbons. This finding made a profound impact on water-purification technology and green chemistry. They used 2,3,5,6-tetrafluoroterephthalonitrile as a linker of CDs, which is not environmentally friendly because it contains fluorine and is also expensive. We recently found that our βCDNP polymers (although we used water-insoluble βCDNP, which is synthesized by the cross-linking reaction between βCD and ECH in the presence of sodium dodecyl sulfate in this case, denoted as βCDNP–SDS49) can exhibit comparable performance to their polymers, as shown in Figure 8. Interestingly, βCDNP shows better adsorption behavior with granular activated charcoal; however, its surface area is much smaller than that of activated charcoal.
Figure 8.

Time-dependent adsorption of aqueous bisphenol A (0.1 mM) by granular activated charcoal (Cat. No. 01084-12, supplied by Kanto Chemical Co.) and βCDNP-SDS (1 mg/mL). The BPA encapsulation experiments were performed under the following conditions: βCDNP-SDS content (or granular activated charcoal) of 18 mg and temperature of 298 K. The Brunauer–Emmett–Teller (BET) surface areas of the granular activated charcoal and βCDNP-SDS are 1400 and 0.2 m2 g–1, respectively.
Conclusion
Several water-soluble CDNPs were produced by cross-linking reactions between CD molecules and different amounts of ECH in aqueous solutions. Detailed structural characterization and investigation of the binding constants of the CDNPs for vanillin and its analogues in aqueous solutions were performed. Our NMR findings clearly indicate that vanillin is more efficiently complexed with CDNPs than native CDs, with stronger binding affinity. This demonstrates that these water-soluble cyclodextrin polymers have great potential for various food, environmental, and pharmaceutical applications. In addition, DOSY NMR is useful as an alternative tool for the initial assessment of the enhanced binding constant of CD-based materials.
Experimental Section
Materials
Vanillin (catalog number 44020-30, purity >98%) was supplied by Kanto Chemical Co., Inc. (Tokyo, Japan) and used without further purification. α-, β-, and γ-cyclodextrins and ECH (purity >99%) were purchased from Tokyo Chemical Industry Co. (Tokyo, Japan) and used without further purification. Deuterium oxide (99.8%) was obtained from Fujifilm Wako Pure Chemical Co. (Osaka, Japan). We compared the inclusion phenomena between vanillin and its analogues: homovanillic acid (HVA) and vanillyl mandelic acid (VMA); both of these latter compounds were supplied by Tokyo Chemical Industry Co. (Tokyo, Japan; purity >98%) and used without further purification. All other chemicals and solvents were of commercial grade and used as received.
Preparation and Characterization of CDNPs
Four types of βCDNP with different ECH/CD weight ratios were prepared by changing the ECH feeding amount, as shown in Table 1. This polymerization reaction is a nucleophilic addition reaction of hydroxyl groups under basic conditions, the details of which are described elsewhere.39 The obtained solution was purified by dialysis for 2 days using a membrane (molecular weight cutoff, 3500). During the dialysis, water-undissolved components were precipitated, and we used the supernatant that contains CDNP. Occasionally, we filtered the solution to remove large particles. The weight-averaged molecular weight (Mw) was determined by static light scattering coupled with gel chromatography. The hydrodynamic radius (Rh) was determined by dynamic light scattering. The detailed procedures of these scattering experiments are described in our previous paper39 as well as in the Supporting Information. The CD content in CDNPs was determined by the phenol sulfuric acid method.40 As a negative control of CDNPs, we prepared an ECH polymer from dextran (Mw = 40 kDa; denoted DXNP), whose molecular characteristics are also listed in Table 1. The inclusion phenomena of βCDNPs and HVA or VMA were also studied in a similar manner as for vanillin.
NMR Measurements
All NMR experiments were performed on a JEOL JNM-ECZ500R spectrometer operating at 500 MHz with a stationary magnetic field of strength 11.74 T at the analytical center of the University of Kitakyushu. All tested solutions were prepared 1 day before the measurements to ensure complete solubilization. In the 1H NMR experiment, the concentrations of vanillin and CDs were all set to 10 mM. For the case of CDNP, the concentration of CDNPs was set so that the CD concentration was 10 mM. Two-dimensional nuclear Overhauser effect spectroscopy (NOESY) and rotating frame Overhauser effect spectroscopy (ROESY) experiments were performed to characterize the structure of βCD and βCDNP0.9 complexes as well as DXNP formed with vanillin. ROESY and NOESY NMR spectra were acquired using a standard pulse sequence from the JEOL library at 25 °C. The relaxation delay between successive pulse cycles was 3.9 s for βCD–Val ROESY and 2.5 s for βCDNP0.9–Val NOESY. Mixing times of 0.3 and 0.9 s were used for ROESY and NOESY, respectively.
Diffusion coefficients were obtained through DOSY experiments using a BPP longitudinal eddy current delay (BPP-LED) sequence at 20 ± 0.5 °C in 5 mm tubes. The duration of the magnetic field pulse gradients δ was 3–4 ms, depending on the sample. The pulse gradient strength was increased from 30 to 300 T/m. The diffusion times were optimized between 0.1 s for vanillin and 0.15–0.4 s for each host sample to completely decrease the signals by a factor of 10–20 and better analyze the exponential signal decay. After measurement, the DOSY experimental data were processed using the software Delta 5.3 (JEOL Ltd., Tokyo Japan). After baseline correction, phase correction, and Fourier transformation, the diffusion coefficients were obtained from the peak intensity I(Gz) and exponential fitting to satisfy the Stejskal–Tanner equation50
| 3 |
where I(Gz) is the NMR spectral intensity at a gradient with strength Gz, I(0) is the spectral intensity at zero gradient in Gauss/cm units, γ is the gyromagnetic ratio, Δ is the time interval between the first and second gradient pulses, and D is the translational diffusion coefficient. The D value for the given solution was obtained by taking the average of the diffusion coefficients of several different protons. To display the data two-dimensionally, the inverse Laplace transformation of the Y-axis was performed on every point of data that was Fourier-transformed along the X-axis. The translational diffusion coefficient is related to the hydrodynamic radius (Rh) by the following Stokes–Einstein equation
| 4 |
where kB is the Boltzmann constant, T is the absolute temperature, and η is the viscosity of the medium (in the present experiment, we used η = 1.2467 mPa·s). Dynamic light scattering also provides D, although its accuracy is not as good as DOSY. We compared the Rh values obtained by these two methods.
Job Plot to Determine the Stoichiometry
We determined the stoichiometry of the complexation between βCDNP and vanillin by the continuous variation method (Job plot) with NMR (for details, see the Supporting Information). We chose the H-5 proton of vanillin because it was most affected by the complex formation. A series of vanillin/βCDNP solutions were prepared by varying the molar fraction of the vanillin within the range of 0–1. The stock concentrations of both vanillin and βCDNP were 4.9 mM. Here, the concentration of βCDNP is calculated based on the βCD units in βCDNP. All of the 1H NMR measurements were performed at 25 °C.
Estimating the Binding Constant (K) from DOSY Experiment
The 1:1 host–guest complexation, H + G ⇄ HG, determines the binding constant (K):
| 5 |
Here, [H], [G], and [HG] are the equilibrium concentrations of the host, guest, and complex, respectively. In the vanillin and CD system, vanillin is the guest and CDs or CDNPs are the host. When we denote the initial concentrations of the host and guest as [H]i and [G]i, the relations of [H]i = [H] + [HG] and [G]i = [G] + [HG] hold. When the molecular exchange between complexed and uncomplexed states occurs much faster than the NMR time scale, the observed diffusion coefficient represents the molar fraction weighted average of the diffusion properties of these two states:32
| 6 |
Here, DH* and DG are the observed diffusion coefficients of host and guest in their mixtures and fH and fG are the molar fractions of free guest and host in the mixture, which are related to the initial and equilibrium concentrations such as fH = ([H]i – [HG])/[H]i. DH, DG, and DHG are the diffusion coefficients of the host-alone state, guest-alone state, and inclusion complex, respectively. DH and DG are determined by measuring individual solutions. DHG and [HG] can be obtained by solving the simultaneous equation of eq 6. Consequently, the value of K can be determined using eq 5.
Acknowledgments
This work was financially supported by JST CREST and Grant-in-Aid for Scientific Research A (20H00668) and Exploratory Research (19H05531) programs.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06194.
Additional details of the experiment, 1H NMR of βCDNP0.9 in DMSO-d6, concentration dependence of D and K, complex formation ability of CDNP and DXNP, NOESY spectra of βCDNP0.9 in DMSO-d6, and 1H NMR spectra of the βCDNP0.9/βCD mixture at 10 mM in D2O (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Biwer A.; Antranikian G.; Heinzle E. Enzymatic production of cyclodextrins. Appl. Microbiol. Biotechnol. 2002, 59, 609–17. 10.1007/s00253-002-1057-x. [DOI] [PubMed] [Google Scholar]
- Crini G.; Fourmentin S.; Fenyvesi É.; Torri G.; Fourmentin M.; Morin-Crini N.. Fundamentals and Applications of Cyclodextrins. In Cyclodextrin Fundamentals, Reactivity and Analysis; Fourmentin S., Crini G., Lichtfouse E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp 1–55. [Google Scholar]
- Gonzalez Pereira A.; Carpena M.; García Oliveira P.; Mejuto J. C.; Prieto M. A.; Simal Gandara J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host-Guest Complexes. Int. J. Mol. Sci. 2021, 22 (3), 1339. 10.3390/ijms22031339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftsson T.; Duchêne D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329 (1), 1–11. 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- Köse K.; Tüysüz M.; Aksüt D.; Uzun L. Modification of cyclodextrin and use in environmental applications. Environ. Sci. Pollut. Res. 2022, 29, 182–209. 10.1007/s11356-021-15005-y. [DOI] [PubMed] [Google Scholar]
- Fenyvesi É.; Puskás I.; Szente L.. Cyclodextrin-Steroid Interactions and Applications to Pharmaceuticals, Food, Biotechnology and Environment. In Cyclodextrin Applications in Medicine, Food, Environment and Liquid Crystals; Fourmentin S., Crini G., Lichtfouse E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp 19–57. [Google Scholar]
- Yao X.; Huang P.; Nie Z. Cyclodextrin-based polymer materials: From controlled synthesis to applications. Prog. Polym. Sci. 2019, 93, 1–35. 10.1016/j.progpolymsci.2019.03.004. [DOI] [Google Scholar]
- Liu Y.; Lin T.; Cheng C.; Wang Q.; Lin S.; Liu C.; Han X. Research Progress on Synthesis and Application of Cyclodextrin Polymers. Molecules 2021, 26 (4), 1090. 10.3390/molecules26041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean M.; García-Zubiri I. X.; Isasi J. R. History of cyclodextrin-based polymers in food and pharmacy: a review. Environ. Chem. Lett. 2021, 19 (4), 3465–3476. 10.1007/s10311-021-01244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Crini N.; Winterton P.; Fourmentin S.; Wilson L. D.; Fenyvesi E.; Crini G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. 10.1016/j.progpolymsci.2017.07.004. [DOI] [Google Scholar]
- Li X.; Zhou M.; Jia J.; Ma J.; Jia Q. Design of a hyper-crosslinked β-cyclodextrin porous polymer for highly efficient removal toward bisphenol a from water. Sep. Purif. Technol. 2018, 195, 130–137. 10.1016/j.seppur.2017.12.007. [DOI] [Google Scholar]
- Wang J.; Cheng G.; Lu J.; Chen H.; Zhou Y. PDA-cross-linked beta-cyclodextrin: a novel adsorbent for the removal of BPA and cationic dyes. Water Sci. Technol. 2020, 81 (11), 2337–2350. 10.2166/wst.2020.286. [DOI] [PubMed] [Google Scholar]
- Wang J.-w.; Lan D.; Yong-Qiang L.; Li R.-f.; Yang X.-t.; Lan G.-h.; Qiu H.-y.; Xu B. Adsorption properties of β-cyclodextrin modified hydrogel for methylene blue. Carbohydr. Res. 2021, 501, 108276. 10.1016/j.carres.2021.108276. [DOI] [PubMed] [Google Scholar]
- Kono H.; Nakamura T.; Hashimoto H.; Shimizu Y. Characterization, molecular dynamics, and encapsulation ability of β-cyclodextrin polymers crosslinked by polyethylene glycol. Carbohydr. Polym. 2015, 128, 11–23. 10.1016/j.carbpol.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Zhao D.; Zhao L.; Zhu C.-S.; Huang W.-Q.; Hu J.-L. Water-insoluble β-cyclodextrin polymer crosslinked by citric acid: synthesis and adsorption properties toward phenol and methylene blue. J. Inclusion Phenom. Macrocyclic Chem. 2009, 63 (3), 195–201. 10.1007/s10847-008-9507-4. [DOI] [Google Scholar]
- Sikder M. T.; Islam M. S.; Kikuchi T.; Suzuki J.; Saito T.; Kurasaki M. Removal of Copper Ions From Water Using Epichlorohydrin Cross-Linked β-Cyclodextrin Polymer: Characterization, Isotherms and Kinetics. Water Environ. Res. 2014, 86 (4), 296–304. 10.2175/106143013X13807328848054. [DOI] [PubMed] [Google Scholar]
- Kitaoka M.; Hayashi K. Adsorption of Bisphenol A by Cross-Linked β-Cyclodextrin Polymer. J. Inclusion Phenom. Macrocyclic Chem. 2002, 44 (1), 429–431. 10.1023/A:1023024004103. [DOI] [Google Scholar]
- Poornima K.; Deveswaran R.; Bharath S.; Basavaraj B.; Madhavan V. Synthesis and evaluation of β-cyclodextrin-epichlorohydrin inclusion complex as a pharmaceutical excipient. J. Fundam. Appl. Sci. 2015, 7 (2), 203–221. 10.4314/jfas.v7i2.5. [DOI] [Google Scholar]
- Gidwani B.; Vyas A. Synthesis, characterization and application of epichlorohydrin-β-cyclodextrin polymer. Colloids Surf., B 2014, 114, 130–7. 10.1016/j.colsurfb.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Szeman J.; Ueda H.; Szejtli J.; Fenyvesi E. V. A.; Machida Y.; Nagai T. Complexation of Several Drugs with Water-Soluble Cyclodextrin Polymer. Chem. Pharm. Bull. 1987, 35 (1), 282–288. 10.1248/cpb.35.282. [DOI] [PubMed] [Google Scholar]
- Nie S.; Zhang S.; Pan W.; Liu Y. In vitro and in vivo studies on the complexes of glipizide with water-soluble β-cyclodextrin-epichlorohydrin polymers. Drug Dev. Ind. Pharm. 2011, 37, 606–12. 10.3109/03639045.2010.533277. [DOI] [PubMed] [Google Scholar]
- Van D. T. H.; Anh D. T. N.; Fujii S.; Sakurai K. Enhanced binding constant of cyclodextrin to alpha-mangostin in hyperbranched polymers. Chem. Lett. 2020, 49 (10), 1144–1146. 10.1246/cl.200210. [DOI] [Google Scholar]
- Jabbari A.; Sadeghian H.. Chapter 12 - Amphiphilic Cyclodextrins, Synthesis, Utilities and Application of Molecular Modeling in Their Design. In Recent Advances in Novel Drug Carrier Systems; Sezer A. D., Ed.; IntechOpen: London, 2012; pp 331–354. [Google Scholar]
- Doan V. T. H.; Takano S.; Doan N. A. T.; Nguyen P. T. M.; Nguyen V. A. T.; Pham H. T. T.; Nakazawa K.; Fujii S.; Sakurai K. Anticancer efficacy of cyclodextrin-based hyperbranched polymer nanoparticles containing alpha-mangostin. Polym. J. 2021, 53 (3), 481–492. 10.1038/s41428-020-00441-3. [DOI] [Google Scholar]
- Ma M.; Li D. New Organic Nanoporous Polymers and Their Inclusion Complexes. Chem. Mater. 1999, 11 (4), 872–874. 10.1021/cm981090y. [DOI] [Google Scholar]
- Fenyvesi É. Cyclodextrin polymers in the pharmaceutical industry. J. Inclusion Phenom. 6 1988, 6 (5), 537–545. 10.1007/BF00660751. [DOI] [Google Scholar]
- Szeman J.; Fenyvesi E.; Szejtli J.; Ueda H.; Machida Y.; Nagai T. Water soluble cyclodextrin polymers: Their interaction with drugs. J. Inclusion Phenom. 5 1987, 5 (4), 427–431. 10.1007/BF00664098. [DOI] [Google Scholar]
- Karoyo A. H.; Wilson L. D. Nano-Sized Cyclodextrin-Based Molecularly Imprinted Polymer Adsorbents for Perfluorinated Compounds—A Mini-Review. Nanomaterials 2015, 5 (2), 981–1003. 10.3390/nano5020981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle E. M. M. Cyclodextrins and their uses: a review. Process Biochem. 2004, 39 (9), 1033–1046. 10.1016/S0032-9592(03)00258-9. [DOI] [Google Scholar]
- Loftsson T.; Hreinsdóttir D.; Másson M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302 (1–2), 18–28. 10.1016/j.ijpharm.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Hirose K. A Practical Guide for the Determination of Binding Constants. J. Inclusion Phenom. Macrocyclic Chem. 2001, 39 (3), 193–209. 10.1023/A:1011117412693. [DOI] [Google Scholar]
- Fielding L. Determination of Association Constants (Ka) from Solution NMR Data. Tetrahedron 2000, 56 (34), 6151–6170. 10.1016/S0040-4020(00)00492-0. [DOI] [Google Scholar]
- Colbourne A. A.; Meier S.; Morris G. A.; Nilsson M. Unmixing the NMR spectra of similar species – vive la différence. Chem. Commun. 2013, 49 (89), 10510–10512. 10.1039/c3cc46228e. [DOI] [PubMed] [Google Scholar]
- Ferrazza R.; Rossi B.; Guella G. DOSY-NMR and Raman Investigations on the Self-Aggregation and Cyclodextrin Complexation of Vanillin. J. Phys. Chem. B 2014, 118 (25), 7147–7155. 10.1021/jp504406j. [DOI] [PubMed] [Google Scholar]
- Simova S.; Berger S. Diffusion Measurements vs. Chemical Shift Titration for Determination of Association Constants on the Example of Camphor–Cyclodextrin Complexes. J. Inclusion Phenom. Macrocyclic Chem. 2005, 53 (3), 163–170. 10.1007/s10847-005-2631-5. [DOI] [Google Scholar]
- Meryem G.; Rabah K.; Fatiha M.; Leila N.; Aziz B. A.; Imane D.; Rachid M. Computational investigation of vanillin/β-cyclodextrin inclusion complex: Electronic and intermolecular analysis. J. Mol. Liq. 2021, 321, 114839. 10.1016/j.molliq.2020.114839. [DOI] [Google Scholar]
- Karathanos V. T.; Mourtzinos I.; Yannakopoulou K.; Andrikopoulos N. K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101 (2), 652–658. 10.1016/j.foodchem.2006.01.053. [DOI] [Google Scholar]
- Korytkowska-Wałach A.; Dubrawska B.; Śmiga-Matuszowicz M.; Bieg T. Spectroscopic study on the inclusion complexes of β-cyclodextrin with selected metabolites of catecholamines. J. Mol. Struct. 2017, 1127, 532–538. 10.1016/j.molstruc.2016.08.010. [DOI] [Google Scholar]
- Doan V. T. H.; Lee J. H.; Takahashi R.; Nguyen P. T. M.; Nguyen V. A. T.; Pham H. T. T.; Fujii S.; Sakurai K. Cyclodextrin-based nanoparticles encapsulating α-mangostin and their drug release behavior: potential carriers of α-mangostin for cancer therapy. Polym. J. 2020, 52 (4), 457–466. 10.1038/s41428-019-0296-y. [DOI] [Google Scholar]
- Albalasmeh A. A.; Berhe A. A.; Ghezzehei T. A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97 (2), 253–261. 10.1016/j.carbpol.2013.04.072. [DOI] [PubMed] [Google Scholar]
- Fernandes C.; Carvalho R.; Costa S. P. D.; Veiga F. Multimodal molecular encapsulation of nicardipine hydrochloride by β-cyclodextrin, hydroxypropy β-cyclodextrin and triacetyl β-cyclodextrin in solution. Structural studies by 1H NMR and ROESY experiments. Eur. J. Pharm. Sci. 2003, 18, 285–296. 10.1016/S0928-0987(03)00025-3. [DOI] [PubMed] [Google Scholar]
- Maheshwari A.; Sharma M.; Sharma D. Complexation of sodium picosulphate with beta cyclodextrin: NMR spectroscopic study in solution. J. Inclusion Phenom. Macrocyclic Chem. 2013, 77, 337. 10.1007/s10847-012-0251-4. [DOI] [Google Scholar]
- Mashhood Ali S.; Asmat F.; Maheshwari A. NMR spectroscopy of inclusion complex of D-(−)-chloramphenicol with beta-cyclodextrin in aqueous solution. Farmaco 2004, 59 (10), 835–8. 10.1016/j.farmac.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Divakar S. Structure of a. beta.-cyclodextrin-vanillin inclusion complex. J. Agric. Food Chem. 1990, 38 (4), 940–944. 10.1021/jf00094a005. [DOI] [Google Scholar]
- Pîrnau A.; Bogdan M.; Floare C. G. NMR spectroscopic characterization of β-cyclodextrin inclusion complex with vanillin. J. Phys.: Conf. Ser. 2009, 182, 012013. 10.1088/1742-6596/182/1/012013. [DOI] [Google Scholar]
- Thordarson P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40 (3), 1305–1323. 10.1039/C0CS00062K. [DOI] [PubMed] [Google Scholar]
- Alsbaiee A.; Smith B. J.; Xiao L.; Ling Y.; Helbling D. E.; Dichtel W. R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529 (7585), 190–194. 10.1038/nature16185. [DOI] [PubMed] [Google Scholar]
- Sakurai K.; Fujii S.; Doan V. T. H.; Nguyen P. T. M.; Nguyen V. A. T.; Pham H. T. T.. Particles, Method for Producing Particles, Drug, Method for Producing Drug, and Anti-cancer Agent. J190398T00, 2019. [Google Scholar]
- Claridge T. D. W.Chapter 10 - Diffusion NMR Spectroscopy. High-Resolution NMR Techniques in Organic Chemistry, 3rd ed.; Elsevier: Boston, MA, 2016; pp 381–419. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.