Highlights
-
•
Peptide release methods influence its bioactivity by generating different sequences.
-
•
The absorption, toxicity and taste of peptides is influenced by the production method.
-
•
The most used methods are enzymatic hydrolysis and microbial fermentation.
-
•
The most used methods are biotechnological and differ in their process.
Keywords: Bioactive peptides, Biotechnology, Enzymatic hydrolysis, Microbial fermentation
Abstract
Bioactive peptides are biomolecules derived from proteins. They contain anywhere from 2 to 20 amino acids and have different bioactivities. For example, they have antihypertensive activity, antioxidant activity, antimicrobial activity, etc. However, bioactive peptides are encrypted and inactive in the parental protein, so it is necessary to release them to show their bioactivity. For this, there are different methods, where biotechnological methods are highly favorable, highlighting enzymatic hydrolysis and microbial fermentation.
The choice of the method to be used depends on different factors, which is why it is essential to know about the process, its principle, and its advantages and disadvantages. The process of peptide release is critical to generate various peptide sequences, which will produce different biological effects in the hydrolysate. This review focuses on providing extensive information on the enzymatic method and microbial fermentation to facilitate selecting the method that provides the most benefits.
1. Introduction
Bioactive peptides are molecules that show important beneficial effects on health because they have potential biological activities such as antimicrobial (Zanutto-Elgui et al., 2019), antihypertensive (Amorim et al., 2019), antioxidant (Tonolo et al., 2019), anticancer (Karami et al., 2019), immunomodulatory (Fernández-Tomé et al., 2019), anti-inflammatory (Hao et al., 2019), etc.
They are considered an alternative for preventing different metabolic diseases, as they have a broad spectrum of action, are less allergenic, and show high biospecificity activity and structural diversity (Agyei et al., 2016). In addition, they do not accumulate in the organism and are quickly degraded in the environment (Janser et al., 2015). They usually contain between 2 and 20 amino acid residues and are encoded in the primary structure of animal and plant proteins in an inactive form (Hayes & Mora, 2015).
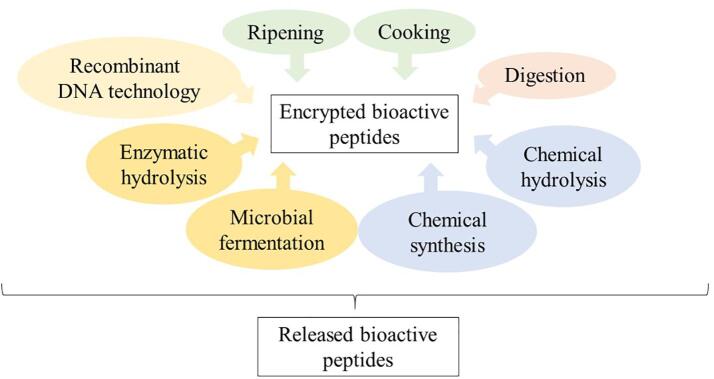
Bioactive peptides must be released from the parental protein for their activation. There are different methods, such as digestive enzymes, commercial proteolytic enzymes, chemical hydrolysis, and food processing through curing, fermentation, or maturation (Chauhan and Kanwar, 2020, Daliri et al., 2017). These methods involve the breakdown of the protein by enzymes, chemical reagents, and the action of temperature or time. On the other hand, through modern methods of cloning and gene expression in microorganisms, recombinant DNA technology has achieved the production of peptides.
The most used methods are enzymatic hydrolysis and microbial fermentation because they have more advantages than the other methods mentioned above. An example of one of these advantages is their GRAS nature (He et al., 2019). However, both have several differences in obtaining peptides, yield, bioactivities presented, the substrate used, process, cost, etc. Therefore, it is essential to know the most used methods since, in this way, the most appropriate one can be selected. Therefore, the objective of this work is to provide a wide range of information that will allow us to distinguish the method that provides the most significant benefits.
2. Bioactive peptides
Bioactive peptides are fragments of specific proteins, composed of 2 to 20 amino acid residues, have a molecular weight lower than 6000 Da, and stand out for promoting positive health effects in the consumer (Chalamaiah et al., 2019, He et al., 2019), which occur when the peptides are released as they are encrypted and inactive in the parental protein (Barberis et al., 2018). Furthermore, they are associated with important biological activities such as antihypertensive, antioxidant, anti-inflammatory, immunomodulatory, hypolipemic, antidiabetic, anticancer, antiadhesive, etc. (Xu et al., 2019). Therefore, they are considered therapeutic agents for treating and preventing certain diseases, with high specificity, a broad spectrum of action, low toxicity, high structural diversity, and small size (Mason, 2010). For all these reasons, bioactive peptides are ideal candidates to be applied as nutraceuticals or functional foods (Li-Chan, 2015).
However, to obtain this recognition, they must meet specific requirements: to maintain their bioactivity, be absorbed, have low or no toxicity, preserve an acceptable taste, and submit to the country’s regulations.
There are currently bioactive peptides on the market sold in food and drink, tablets, capsules, powders, and liquids (Chalamaiah et al., 2019). Table 1 shows some of the peptides commonly found in the market.
Table 1.
Bioactive peptides found in the market.
| Brand name | Source | Amino acid sequence | Beneficial effects | Product | Method of production | References |
|---|---|---|---|---|---|---|
| Calpis | Sour milk | VPP y IPP | Antihypertensive effect | Drink | Fermentation with L. helveticus CP790 and S. cerevisiae | (Siltari, Kivimäki, Ehlers, Korpela, & Vapaatalo, 2012) |
| Evolus® | Milk | VPP y IPP | Antihypertensive effect | Drink | Fermentation with L. helveticus LBK-16H | (Flynn, Korhonen, Marchelli, & Palou, 2008) |
| Lacprodan® Whey protein | Whey | N/A | Regulates blood sugar level | Powder product | Enzymatic hydrolysis with alcalse | (Chalamaiah et al., 2019) |
| Bonito peptide | Bonito fish | LKPNM | Helps regulate angiotensin converting enzyme | Capsules | Enzymatic hydrolysis with thermolysin | (Fujita & Yoshikawa, 1999) |
| Lacitum® | Milk | YLGYLEQLLR | Stress relief | Drink and capsules | Enzymatic hydrolysis with trypsin | (Nagai, Suzuki, & Nagashima, 2006) |
| Capolac® | Milk | CPP | Calcium absorption | Ingredient | Isolation process | (Chalamaiah et al., 2019) |
| Vasotensin® | Bonito fish | LKPNM | Antihypertensive effect | Tablet | Enzymatic hydrolysis with thermolysin and converted to “LKP” in the digestive system | (Fujita & Yoshikawa, 1999) |
| Cholesterol block | Soy | CSPHP | Cholesterol reduction | Drink | Enzymatic hydrolysis | (Chalamaiah et al., 2019) |
2.1. Signaling pathways and absorption
Bioactive peptides can exert their beneficial effects through 1) their uptake at the apical side of the polarized epithelial cell layer of the upper small intestine, or 2) by activation of different metabolic and sensory signaling pathways (Duca et al., 2021, Xu et al., 2019).
The first step is that the bioactive peptides consumed resist the digestion process and therefore must be resistant to gastrointestinal enzymes (Lu et al., 2021). Such as pepsin, and gastricin which are found in the stomach and are activated by hydrochloric acid, or to trypsin and chymotrypsin located in the small intestine and secreted in the duodenum (Sauer & Merchant, 2018).
When absorbed, they are subsequently transported through the epithelial cell monolayer into the blood vessels. For this, they use one or more of the following routes: carrier-mediated permeation, paracellular transport, transcytosis, and passive transcellular diffusion as summarized in Table 2 (Xu et al., 2019).
Table 2.
Transports used by bioactive peptides after absorption.
| Transport | Transport characteristics | Characteristics of the transported bioactive peptides | Examples of transported bioactive peptides | References |
|---|---|---|---|---|
| Paracellular transport | -Passive transport -Mediated by tight barrier |
-Hydrophilic -Neutral or negative charge -Oligopeptides |
VPP LSW GLLLPH and YFCLT |
(Ding, Wang, Zhang, Yu, & Liu, 2018, Satake et al., 2002, Lin et al., 2017) |
| Passive transcellular diffusion | -Passive transport -Passive uptake into cells -Basolateral secretion |
-Hydrophobic -Positive charge |
Asp-angiotensin I (nonapeptide) | (Chua, Jois, Sim, & Go, 2004) |
| PepT1 | -Active transport -H+/Na + exchanger |
-High hydrophobicity -Neutral charge -Short-chain |
IPP LKP IQW |
(Gleeson, Brayden, & Ryan, 2017, Xu et al., 2017) |
| Transcytosis | -Active transport -Diffusion through the apical and basolateral membranes -Transcytotic transport via internalized vesicles |
-High hydrophobicity -Long-chain |
AHLL VLPVPQK and YPFPG |
(Li et al., 2017, Vig et al., 2006) |
Paracellular transport is an energy-independent passive diffusion process (Matsui, 2018). It is mediated by tight junctions that are mainly composed of occludin, zonula occludens-1, and claudin proteins, which form a tight barrier with selective permeability (Marchiando et al., 2010). It transports mainly hydrophilic oligopeptides, with neutral or negative charge (Aluko, 2015).
Passive transcellular diffusion is the transport pathway involving passive uptake into cells, intracellular transport, and basolateral secretion (Pappenheimer & Michel, 2003). The transport of bioactive peptides depends on their size, charge, and hydrophobicity (Liu et al., 2009). Preferentially transports hydrophobic and positively charged bioactive peptides (Miguel et al., 2008). At present, the mediators involved in this pathway have not been identified for bioactive peptides, making their quantification difficult (Xu et al., 2019).
PepT1, an H + -dependent transporter, is present on the intestinal brush-border membrane (Lu et al., 2021). Through a proton gradient maintained by the H+/Na + exchanger, it can transport bioactive peptides from the gastrointestinal lumen into the intestinal epithelium (Xu et al., 2019). PepT1 binds mainly to short-chain bioactive peptides (dipeptides and tripeptides) of neutral charge and high hydrophobicity (Vig et al., 2006). Peptides transported by PepT1, are shown to have higher bioavailability compared to peptides transported by the paracellular pathway (Karaś, 2019).
Transcytosis is characterized as an energy-dependent transcellular transport pathway (Komin et al., 2017). It achieves the transport of bioactive peptides via diffusion through the apical and basolateral membranes. Highly hydrophobic and long-chain peptides have a higher affinity to this transport because the cell membrane is composed of a lipid bilayer and the absorption of lipophilic peptides is easier (Renukuntla et al., 2013).
As mentioned before, bioactive peptides can also exert their beneficial effect through the activation of different signaling pathways. For example, they can have anti-diabetic effects by binding to calcium-sensing receptors or G protein-coupled receptors (GPCRs), promoting gene transcription and subsequently the release of enteric peptide hormones such as glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptides 1 and 2 (GLP-1 and GLP-2), cholecystokinin (CCK), secretin, among others. These are responsible for regulating gastrointestinal motility, glucose and energy homeostasis, food intake and the postprandial increase of glucose, lipids, and amino acids (Bao and Wu, 2021, Duca et al., 2021).
Bioactive peptides have been shown to have antioxidant effects in intestinal cells via the Keap1-Nrf2 signaling pathway, which promotes the regulation of cellular antioxidant enzyme expression and the inhibition of pro-inflammatory cytokines. Bioactive peptides such as poly-L-lysine bind to calcium-sensing receptors and stimulate the interaction of β-arrestin2 with signaling proteins via the TRAF-NF-κB/MAPK pathways and thus produce anti-inflammatory effects (Bao & Wu, 2021).
Also, it is now known that tight junctions also serve as bidirectional signaling components and regulate different signaling pathways. Bioactive peptides interact with tight junctions and activate different signaling pathways to modulate gastrointestinal barrier functions (physical, chemical, biological and immunological), however the mechanism by which these molecules modulate signaling pathways is not yet understood (Zihni et al., 2016).
2.2. Taste
Bioactive peptides are characterized by a bitter taste, implying that their acceptability is low in the society that consumes them in therapeutic or functional products (Maehashi & Huang, 2009).
According to Acquah et al., (2018), the bitter taste is positively correlated with the general hydrophobicity of peptide molecules. Furthermore, it is also reported that the intensity of bitterness is related to molecular mass, as 4 kDa peptides were shown to have higher bitterness than those of 1 kDa (Görgüç et al., 2020).
Another factor that influences this flavor is the length of the peptide chain since it has been observed that the longer it is, the bitterness also increases (Fu et al., 2019). And the presence of amino acids with α-amino groups is also a determinant (Zhang et al., 2011).
Different solutions have been developed to reduce bitterness, such as the use of enzymes to reduce the bitter peptide content (Fu et al., 2011), the “removal” of these peptides using specific techniques (gel separation, alcohol extraction, silica gel chromatography, and isoelectric precipitation) individually or in combination (Saha & Hayashi, 2001), and the transformation, modulation, or masking of the flavor, through the use of flavor modifying agents such as sugars, salts, and nucleotides or through the fermentation of the product since during this process the flavor changes (Leksrisompong et al., 2012).
2.3. Toxicity
It is known that bioactive peptides are safe for the consumer, however, according to other research, several cases of toxic proteins/peptides can occur naturally (Leksrisompong et al., 2012, Saha and Hayashi, 2001).
For example, two peptides considered toxic are amatoxins and phallotoxins, which occur naturally in fungi and can cause hepatotoxicity by inhibiting RNA polymerase II, leading to impaired protein synthesis and cell necrosis, acute liver failure, and consequently death (Santi et al., 2012).
In recent years there has been an increase in silico methods to estimate toxicity, allergenicity, and bioactivity in peptides (Tu et al., 2018). In terms of toxicity, it has been identified that certain amino acids such as Cys, His, Asn, and Pro are usually part of the peptides considered toxic. The same applies to amino acid fragments such as Phe-Lys-Lys, Leu-Lys-Leu, Lys-Lys-Leu-Leu, Lys-Trp-Lys, and Cys-Tyr-Cys-Arg (Chaudhary et al., 2016).
Peptide toxicity can also be derived during food processing, pretreatment, or extraction, as undesirable derivatives such as lysinoalanine, D-amino acids, or biogenic amines can be formed that produce adverse health effects (Liu et al., 2020).
On commercial bioactive peptides, such as the antihypertensive peptides IPP and VPP (obtained by fermentation of milk with Lactobacillus helveticus LBK-16H), toxicological studies have been performed with hypertensive patients, who consumed liquid yogurts (150 g) with 0.79 mg IPP and 1.12 mg VPP for 10 weeks. No adverse effects such as dry cough, digestive tract symptoms, or abnormal changes were observed (Kotaro et al., 2005).
In the bioactive peptide product Lactermin®, a proprietary whey growth factor extract containing peptides, lactoferrin, lactoperoxidase, and proteins was subjected to in vitro genotoxicity tests, including Ames (test to identify gene mutation), mouse lymphoma, and micronucleus. 300–3000 mg/kg/day was administered for 90 days in mice, no genotoxicity or toxic effects of the product were found (Dyer et al., 2008).
However, more research is needed on the toxicity exerted by high doses of bioactive peptides and long-term studies in humans (Beltrán-Barrientos et al., 2017).
Before human consumption of bioactive peptides, in vitro and in vivo toxicity studies are recommended. Generally, in vitro cytotoxicity, hemolytic activity and genotoxicity tests have been performed for these molecules. As for in vivo tests, they have been tested in animals such as rats, rabbits, and dogs, performing acute oral toxicity tests, subacute oral toxicity tests, repeated dose toxicity tests, and chronic oral toxicity tests (Shivanna & Nataraj, 2020).
2.4. Regulation
In most countries, there are no specific regulations for bioactive peptides; only the regulations conferred for functional food or nutraceutical apply, except for Japan and the European Union (EU).
In Japan, a legal system includes health claims allowed on functional foods due to the creation of the Food for Specific Health Use (FOSHU) licensing system (Barberis et al., 2018). Bioactive peptides have been included as active food ingredients through this system, and therefore, Japan is recognized as the country with more commercialization and consumption of bioactive peptides.
In the EU, foods can have two types of claims: nutrition claims and health claims. Bioactive peptides are classified under health claims and can be divided into three types. i) General function claims, e.g., fish peptides support the normal function of the nervous system; ii) disease risk reduction claims, e.g., dairy peptides have shown to reduce hypertension; iii) claims relating to childreńs development or health, e.g., fish peptides help the cognitive development of children (Chalamaiah et al., 2019).
Bioactive peptides are regulated by the European Food Safety Authority (EFSA). An application must be submitted to this authority and then evaluated and approved by the EFSA expert group to support the commercialization of bioactive peptides on dietetic products, nutrition, and allergies based on different scientific evidence. It is a process of approximately 6 months, but the period can be longer depending on the opinions (Chalamaiah et al., 2019).
An example of bioactive peptides approved by EFSA is the dipeptide Val-Tyr. This peptide product, obtained from sardine muscle by hydrolysis with alkaline protease, has been shown to have angiotensin-converting enzyme inhibitory activity. EFSA approved it as a new functional food ingredient. Because in evaluating the scientific basis of the relationship between the peptides and the claimed effect it met the criteria of “strength”, “consistency”, “dose-response relationship”, “specificity”, and “biological plausibility of the association”, according to human (for EFSA these tests are essential), animal and in vitro studies (EFSA NDA Panel, 2016).
2.5. Sources of obtaining
Bioactive peptides can be obtained from both animal and plant proteins (Hernández-Almanza et al., 2017). Over the years animal proteins have been mostly investigated for their high protein content, high content of essential amino acids with the right balance, and high yield (Wen et al., 2019). Dairy products and milk have been identified as potential sources for bioactive peptides (Chauhan & Kanwar, 2020).
Also, several investigations report that they can be produced from whey, eggs, (Chalamaiah et al., 2019), meat, and various species of fish such as tuna, sardine, herring, and salmon (Kouhdasht & Moosavi-nasab, 2018).
Various by-products have also been used, such as blood (Hu et al., 2016), bones (Chiang et al., 2019), collagen (Ryder et al., 2016), liver (Verma et al., 2019), lungs (Lafarga & Hayes, 2017), placenta (Teng et al., 2011), and skin (Onuh et al., 2014).
Currently, there is a tendency to use edible insects because they are considered a good source of protein and have generated peptides with important biological activities (Jakubczyk et al., 2020).
Since plant sources have greater sustainability, low cost, and demonstrate an essential role in the diet of the current population, the search for obtaining peptides through these has increased (Rizzello et al., 2017). The same bioactivity has been observed in peptides from animal protein as in peptides obtained from plant protein. An example is VY multifunctional dipeptide released from a protein source such as brewed sake or milk (Jakubczyk et al., 2020, Rizzello et al., 2017).
Within the vegetable protein sources, favorable results have been generated when using rice, soy, peanuts, peas, corn, algae (Chalamaiah et al., 2019), pseudocereals, garlic, turmeric, spinach, and cocoa (García et al., 2013).
2.6. Methods of obtaining
To obtain bioactive peptides, these are required to be released from the parental protein in which they are encrypted and in an inactive form (Barberis et al., 2018). Different methods exist that are responsible for performing this action through specific mechanisms that influence the composition, sequence, and length of the amino acids that make up the peptide, causing the bioactivity to change according to the method used.
Bioactive peptides can be obtained from food processing through methods such as maturation and cooking.
The maturation method in foods such as meat involves enzymes such as endopeptidases and exopeptidases participating in the proteolysis process. It is known that endopeptidases such as calpains and cathepsins are responsible for hydrolyzing proteins, generating large fragments and oligopeptides. Then exopeptidases such as aminopeptidases and carboxypeptidases produce small peptides and free amino acids. During this process and by the effect of enzymes, the texture and taste of the food change (Mora et al., 2018). Recently the obtainment of a peptide with antioxidant activity in cured Spanish ham using this method has been reported (Gallego et al., 2018).
The method of cooking some type of food at high temperatures (60–80 °C) induces proteins to be more accessible and, in this way, the endogenous enzymes hydrolyze them more quickly and efficiently. However, suppose the temperature is higher than 100 °C. In that case, proteins are usually less accessible as they experience the phenomenon of denaturation where they can be irreversibly deployed, aggregated, fragmented or, chemically modified, resulting in bioactive peptides that cannot be obtained (Soladoye et al., 2015).
Another method that distinguishes itself by producing consumer-safe bioactive peptides due to its process is in vitro digestion. The bioactive peptides are released from the parent protein by sequential digestion by enzymatic proteolysis (Raju, 2019). It is performed using the food protein and simulating ingestion and gastrointestinal digestion with trypsin and/or chymotrypsin (or other gastrointestinal enzymes) developing the conditions of all the steps in real digestion (Mora et al., 2018).
Novel antihypertensive peptides (TAA, ATT, ITT, and TL) have been produced by in vitro digestion of cobia fish (Rachycentron canadum) skin. For this, the hydrolysate was incubated with potassium chloride buffer and 0.1 M hydrochloric acid (pH 2)/pepsin (1:2, w/w) at 37 °C for 4 h. This is because in real conditions the protein food is hydrolyzed by the action of pepsin, which is activated by hydrochloric acid. Subsequently, pancreatin was added at the same temperature and duration. Since in the intestinal compartment, peptidases such as pancreatin can hydrolyze the polypeptide generated by pepsin (Bechaux et al., 2019, Lin et al., 2019).
In vitro digestion of bioactive peptides involves the static model, where glass containers are used to mimic digestion. These models are preferred because they are practical, economical, and feasible. They have the disadvantage that they do not produce the mechanical forces and dynamic conditions that are generated in the digestive system when consuming a protein food. However, the objective is to generate bioactive peptides, so these disadvantages do not impair this process (Sensoy, 2021).
Because the dynamic system is more complex and expensive, it is not commonly used to produce bioactive peptides.
One of the major disadvantages of producing bioactive peptides through the in vitro digestion method is that, as with maturation and cooking, they generally fail to induce biological effects in the consumer (Bechaux et al., 2019, Gauthier et al., 2006).
However, in vitro digestion, simulates the gastrointestinal digestion of peptides obtained through this or other methods (such as enzymatic hydrolysis or microbial fermentation) to evaluate their absorption and bioavailability in food matrices or nutraceuticals. First, the bioactive peptides are subjected to static or dynamic model protocols (the choice of model depends on the research objectives, and the advantages and disadvantages of these models) to mimic normal human digestion (Udenigwe & Aluko, 2012).
In the static model it is advisable to use the protocol published by the INFOGEST multidisciplinary project, since it is considered a harmonized protocol and the results generated have been very similar to those identified in vivo (Brodkorb et al., 2019, Minekus et al., 2014). As for the dynamic model, several protocols can be used, since some focus only on simulating chemical conditions, mechanical conditions, or chemical, mechanical and dynamic conditions of the digestive system. For example, the TNO gastrointestinal model (TIM), the simulator of the human intestinal microbial ecosystem (SHIME), in vitro dynamic system (DIDGI), among others, have been used (Sensoy, 2021).
Following this, the peptides are purified, bioactivity tests are used to verify whether the physiological effects demonstrated prior to in vitro digestion are still maintained and finally these molecules are sequenced (Udenigwe & Aluko, 2012). For example, Baptista et al., (2020) evaluated the bioaccessibility and bioactivity of peptides obtained from Prato cheese through the addition of Lactobacillus helveticus LH-B02 after in vitro digestion with the static model and the dynamic model. In the static model the INFOGEST protocol was used and in the dynamic model the SHIME protocol was used. The peptides were extracted after ultrafiltration through mycorextraction with stop tips, evaluated whether the angiotensin inhibitory activity was still maintained, and finally bioactive peptides were identified using MALDI-TOF/TOF. In vitro digestion, in addition to releasing bioactive peptides from food proteins, also serves to evaluate in a more flexible, accurate and reproducible way the resistance of bioactive peptides to gastrointestinal digestion.
The bioactivity of peptides after in vivo digestion has also been evaluated, as these methods provide more accurate results compared to in vitro methods (Yap & Gan, 2020). They are performed on animal or human models (Lucas-González et al., 2018). Peptides are extracted from plasma where they have been identified as being in the micromolar or nanomolar range, then purified, evaluated for bioactivity and finally identified (Xu et al., 2019). These methods are not commonly used because they are difficult, expensive, and limited by ethical issues, so in vitro digestion methods are preferred (Minekus et al., 2014). However, these methods must be done when bioactive peptides are intended to be commercialized, as it is a regulatory requirement.
Other methods use compounds or chemical techniques to obtain bioactive peptides, such as chemical hydrolysis. In this method, two types of hydrolysis are distinguished: acid hydrolysis, where HCl is commonly added. And alkaline hydrolysis, where NaOH is frequently used. Together with high temperatures, these reagents can produce chemical reactions in proteins that result in the cleavage of peptides. However, chemical hydrolysis is not a suitable method for obtaining bioactive peptides because it is non-specific, generates low yields, and causes denaturation of amino acids (Bechaux et al., 2019).
However, although there are various processes for obtaining bioactive peptides (Fig. 1), biotechnological methods such as microbial fermentation and enzymatic hydrolysis are the most used. They have more advantages and applications than the methods mentioned above. These will be described separately for your understanding.
Fig. 1.
Diagram of the different methods used for the release of encrypted bioactive peptides.
3. Enzymatic hydrolysis
3.1. Process for bioactive peptide production
This method involves incorporating commercial enzymes to obtain bioactive peptides since these are responsible for cleaving the peptide bonds established in the protein and thus releasing the encrypted peptide (Wouters et al., 2002).
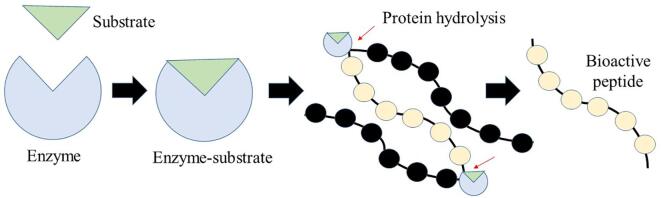
For the enzyme to carry out its activities, it is essential that it first binds to the substrate and then continue with enzymatic catalysis. For this, the enzyme has specific active sites containing residues that form temporary bonds with the substrate and residues that catalyze the reaction with the substrate. In this way, binding sites and catalytic sites are formed, respectively. The bonds forming the enzyme-substrate complex are usually hydrogen bonds, hydrophobic bonds, or Van der Waals interactions. Finally, when the enzyme-substrate complex is in a specific conformation, it can ensure protein hydrolysis (Fig. 2) (Arshad et al., 2019).
Fig. 2.
Graphic representation of the enzymatic hydrolysis process in the release of bioactive peptides.
Enzymatic hydrolysis can generally be carried out in three ways: i) under traditional batch conditions, ii) using immobilized enzymes, or iii) using ultrafiltration membranes. The least used, due to its disadvantages, is the one carried out under traditional batch conditions. Since the cost of enzymes is high, it has low yields and productivity, and undesirable secondary metabolites are obtained due to the enzymatic autolysis generated.
The proposed solution for this is to use the immobilized enzymes in a two-phase system, one phase where only the enzyme will be and the other where only the product will be (Rizzello et al., 2016). According to Michalak et al., (2017), enzyme immobilization has more significant advantages than the technique mentioned above because it is an economical process, it facilitates the separation of the enzyme and the product, so there is a low risk of product contamination and allows the enzyme to be reused, thus reducing costs.
Finally, the enzyme hydrolysis technique using ultrafiltration membranes has become the most efficient method for obtaining peptides, this consists of continuously pumping from a reaction vessel to a membrane filter, the enzyme-substrate mixture, where only small and hydrolyzed fractions pass, and large particles (polypeptides, non-hydrolyzed substrate, enzyme) are recycled back into the hydrolysis tank. What gives this technique more selectivity and speed (Rizzello et al., 2016).
At present, the hydrolysis process has been improved through pretreatment methods of the parental protein, thus produce higher hydrolysis rates to minimize the amount of enzyme used. Techniques such as microwave, ultrasound, high-voltage pulsed electric field, and hydrostatic high-pressure assisted enzymatic hydrolysis have been used (Mikhaylin et al., 2017, Rizzello et al., 2016).
3.2. Enzyme technology for bioactive peptide production
Proteolytic enzymes can be obtained from animal, plant, or microbial sources; however, the first two have certain limitations (Arshad et al., 2019).
For example, animal sacrifice should be considered when animal sources are used to extract such enzymes since proteases are commonly produced in tissues and organs. In vegetable sources, the proteases can be obtained from the shell or trunk, but they are influenced by viability, cultivation, climatic conditions, and long extraction processes. Therefore, it is preferred to obtain them from microbial sources because they have more advantages: the enzymes produced are more stable, faster production processes, simple nutritional requirements, and the microorganisms can be genetically and environmentally manipulated to generate the desired characteristics, increase their activity, as well as performance (Rani et al., 2012).
Numerous proteolytic enzymes are used to produce bioactive peptides, such as those listed in Table 3, but the most popular commercially are Alcalase ™, Protamex ™, and Flavourzyme ™. It is also common to use enzymes found in the human body with digestive activities such as pepsin, trypsin, and chymotrypsin (Boukil et al., 2018).
Table 3.
Commercial Enzymes.
| Enzyme | Source |
|---|---|
| Alcalase | Bacillus licheniformis |
| Biofeed pro | B. licheniformis |
| Durazym | Bacillus sp. |
| Esperase | B. lentus |
| Everlase | Bacillus sp |
| Flavorzyme | Aspergillus oryzae |
| Kannase | Bacillus sp. |
| Kojizyme | Not specified |
| Neutrase | B. subtilis |
| NovoBate WB | Bacillus sp. |
| Novozyme 243 | B. licheniformis |
| NUE | Bacillus sp. |
| Ovozyme | Bacillus sp. |
| Protamex | Bacillus protease Complex |
| Savinase | Bacillus sp. |
| Subtilisin A | Genetically modified Bacillus sp. |
| Fermgen | Bacillus sp. |
| Fungal protease | Aspergillus sp. |
| HT proteolytic | Bacillus sp. |
| Primatan | Bacterial source |
| Properase | Bacillus sp. |
| Purafect | B. lentus |
| Fromase | Rhizomucor miehei |
| Maxiren | Recombinant Kluyveromyces lactis |
| Suparen/Surecurd | Cryphonectriap arasítica |
| Collagenase | Clostridium sp. |
| Newlase F | Rhizopus niveus |
| Proleather | Bacillus sp. |
| Protease S | A. stearothermophilus |
| Protin | Bacillus sp. |
| Prozyme | A. mellius |
| Streptokinase/Streptodornase | Streptococcus sp. |
| Thermoase | Bacillus sp. |
| Enzeco neutral bacterial protease | B.subtilis |
| Chorolase PP | A. oryzae |
| Papain | Carica papaya |
| Pepsin | Porcine gastric mucosa |
| Pancreatin trypsin Novo | Porcine pancreatic glands |
| Trypsin | Bovine pancreas |
| Chimotrypsin A | Bovine pancreas |
| Seabzyme L 200 | Carica papaya |
| Bromelain | Pineapple stem |
| Collupulin | Carica papaya |
| Ficain | Figs latex |
3.3. Advantages of the use of the enzymatic hydrolysis for bioactive peptide production
This method is characterized by certain advantageous qualities in obtaining bioactive peptides that other techniques such as fermentation, chemical hydrolysis, or digestion do not offer. Among these is the improvement in reaction rate. When the enzyme is added to cleave the proteins, they also catalyze the reaction, and the speed is greater about 106 to 1012 times than when it is not catalyzed (Martínez-Medina et al., 2019).
Likewise, enzymatic hydrolysis is performed under mild temperature and pH conditions (Chew et al., 2019). Generally, the temperatures used are below 100 °C, and the pH is handled close to neutrality, which benefits in savings of cost and energy (Martínez-Medina et al., 2019).
Another advantage that distinguishes this method is the remarkable regioselectivity and good stereoselectivity (Chauhan & Kanwar, 2020). It is proposed that enzymatic hydrolysis using immobilized enzymes allows the extraction of peptide sequences already directed or desired due to the high specificity of the enzyme (Michalak et al., 2017).
It does not usually generate secondary products, has an environmentally friendly concept as it does not use synthetic chemicals, and is one of the preferred methods in the food industry (Carrasco-Castilla et al., 2012).
With enzymatic hydrolysis, in addition, higher peptide yields are obtained, and with better quality, enzymes are easy to inactivate, and in general, the process is simple (Martínez-Medina et al., 2019).
3.4. Disadvantages of the use of enzymatic hydrolysis for bioactive peptide production
Different factors such as the enzyme-substrate ratio, hydrolysis time, pH, and reaction temperature must be monitored and managed in optimal parameters since these can affect various properties in the peptides such as hydrolysate, length, molecular weight, and amino acid composition causing that its bioactivity to be affected (Li-Chan, 2015).
It is also essential to consider the specificity of the enzyme since, according to certain investigations, the bioactivity of the peptides obtained is influenced by the type of enzyme used. For example, in the study conducted by Ferri et al., (2017), when hydrolyzing rice proteins with bacillolysin, they showed better anti-inflammatory and anti-tyrosinase activity compared to subtilisin and papain, where the activity was lower.
When enzymatic hydrolysis is used to produce bioactive peptides, it is common to pre-treat the substrate to improve hydrolysis since the enzyme cleavage sites are exposed. However, sometimes thermal treatments are used, which denature the proteins and depending on the warming severity. As a result, some amino acids are often damaged, such as some nutritional amino acids (tryptophan, lysine, arginine, asparagine, cysteine, and methionine) and other amino acids (serine, threonine, aspartic acid, and glutamic acid) that can be broken down, dehydrated or cycled (Borad et al., 2017).
It is a method that has had problems at an industrial level because it can generate low yields and high costs. However, research is being carried out to combine this method with new processing technologies (high hydrostatic pressure, microwave processing, and pulsed electric field) to improve their performance and cost (Tadesse & Emire, 2020).
Finally, this method is expensive compared to other processes such as fermentation because the cost of enzymes is high and is required special to keep it active.
4. Microbial fermentation
4.1. Process for bioactive peptide production
Microbial fermentation is a biotechnological process through which bioactive peptides can be obtained. This process involves using microorganisms capable of producing proteolytic enzymes with the objective that these enzymes hydrolyze proteins into shorter peptides (Onuh et al., 2014). The microorganisms generally used are bacteria, fungi, or yeasts, which may be present in the substrate indigenously or added as a starter culture.
The microbial fermentation process can be divided into several systems. However, submerged fermentation and solid-state fermentation are the most widely used.
Submerged fermentation corresponds to a culture of microorganisms in a liquid medium, which contains nutrients. This system is suitable for microorganisms with high water level activities, such as bacteria, and offers the advantage that the generated bioactive peptides are easy to purify.
On the other hand, solid-state fermentation consists of microbial growth on nutrient-rich solid substrates. It has the advantage of releasing nutrients in a controlled way and is suitable for fungi and microorganisms with fewer moisture requirements (Subramaniyam & Vimala, 2012).
During the microbial fermentation process, it is essential to handle the appropriate substrate, suitable microorganism, and optimal environmental conditions, such as pH, temperature, and humidity, to generate peptides with better bioactivity (Melini et al., 2019).
4.2. Microbial technology for bioactive peptide production
To achieve the optimal production of peptides and high bioactivity of these, the type of microorganism used is crucial for microbial fermentation (Hayes & García-Vaquero, 2016). As already mentioned, the use of bacteria, fungi, and yeasts that have a high proteinase activity and a variety of peptidases is common (Gobbetti et al., 2007). However, microbial fermentation differs for each type of microorganism (bacteria, fungi, or yeasts) since the proteolytic systems with which they produce bioactive peptides are unique.
Among the bacteria, lactic acid bacteria (LAB) stand out, recognized among the most valuable microorganisms for obtaining bioactive peptides due to their high adaptability to different environments, as well as to animal and plant substrates; safety, since these bacteria are known as “friendly bacteria” because several strains are identified as “generally recognized as safe” (GRAS); and also, due to the efficient proteolytic system that characterizes them (Lafarga and Hayes, 2017, Melini et al., 2019).
The proteolytic system begins when the proteases associated with the cell envelope (CEP) break the proteins and transforms them into oligopeptides. In this first step, they become extracellular bioactive peptides considered protein residues, as the proteolytic system does not use them to assimilate nitrogen. Through active transporters, the oligopeptides are led to the cytoplasm. These transporters belong to 3 groups: the permeases that handle the proton motive force called PTR, the ABC transporters that obtain energy from the hydrolysis of ATP, and the antiports that use gradients of concentration to import a molecule against another exporter. Finally, intracellular endopeptidases generate free amino acids or low molecular weight peptides (Juillard et al., 2016). Some of the LABs mostly reported for their effective production of bioactive peptides are: Lactobacillus helveticus, Lactobacillus delbrueckii ssp. bulgaricus, Lactococcus lactis ssp. diacetylactis, Lactococcus lactis ssp. cremoris, and Streptococcus salivarius ssp. thermophylus (Hernández-Ledesma et al., 2011).
There are also fungi with important applications in the generation of bioactive peptides, such as Aspergillus egypticus and A. oryzae (Hernández-Ledesma et al., 2011, Juillard et al., 2016). These fungi do not have a proteolytic system as detailed as that of the LAB. Still, they contain various proteases that participate in the hydrolysis of proteins through the breakdown of peptide bonds, whereby means of limited proteolysis, it is common to obtain bioactive peptides because proteases act by breaking specific peptide bonds without reducing proteins in their constituent amino acids. However, the microorganism can use unlimited proteolysis to reduce the protein into amino acids, which can be used for its growth (Martínez-Medina et al., 2019).
Examples of yeast uses are Kluyveromyces marxianus and Saccharomyces cerevisiae (Li et al., 2015) which are considered GRAS. Yeasts, such as those mentioned above, possess a set of proteases and peptidases responsible for degrading proteins; they are mainly used in dairy products to break down milk proteins and obtain peptides and amino acids for growth (Melville et al., 2011). Some yeasts possess even better proteolytic activity than LAB. For example, according to Klein et al., (2002), it was found that yeasts were more efficient in degrading β-casein compared to L. helveticus.
Some of the microorganisms used to obtain bioactive peptides through microbial fermentation are listed in Table 4.
Table 4.
Microorganisms used in microbial fermentation.
| Microorganism | Peptide sequence | Bioactivity | References |
|---|---|---|---|
| Enterococcus faecalis | LVYPFPGPIPNSLPQNIPP, LHLPLP, LHLPLPL, VLGPVRGPFP, VRGPFPIIV | Antihypertensive | (Quirós et al., 2007) |
| Lactococcus lactis NRRL-B-50571 | DDQNPH, LDDDLTDDI, YPSYGL, HPHPHLSFMAIPP, YDTQAIVQ, DDDLTDDIMCV, YPSYG | ACE inhibitory | (Rodríguez-Figueroa et al., 2013) |
| Bifidobacterium bifidum MF 20/5 | LVYPFP | ACE inhibitory | (Gonzalez-Gonzalez, Gibson, & Jauregi, 2013) |
| Lactobacillus helveticus | VPP, IPP | Antihypertensive, antiinflammatory, antiadipogenic, antiatherosclerotic | (Fekete, Ian Givens, & Lovegrove, 2015) |
| Saccharomyces cerevisiae | VPP, IPP | Antihypertensive, antiinflammatory, antiadipogenic, antiatherosclerotic | (Chakrabarti & Wu, 2015) |
| Lacticaseibacillus casei shirota y Streptococcus thermophiles | YQEPVLGPVRGPFPIIV | ACE inhibitory, antithrombotic | (Rojas-Ronquillo et al., 2012) |
| Lactobacillis delbrueckii ssp. bulgaricus, Streptococcus thermophilus | VPYPQ, KAVPYPQ, KVLPVPE, GVRGPFPII, IPIQY, QQPVLGPVRGPFPIIV GVSKVKEAMAPKHKEMPFPKYPVEPFTESQ QEPVL, QEPV |
AntioxidantMucin stimulating, ACE inhibitory, opioidImmunomodulating | (Jiehui et al., 2014) |
| Bacillus licheniformis B1 | LE, EW, SP, VE, VL, VT, EF | Antidiabetic | (Yang et al., 2013) |
| Aspergillus aegypticus | Peptide containing Phe, Ile, and Gly in the ratio 1:2:5 | ACE inhibitory | (Zhang, Tatsumi, Ding, & Li, 2006) |
| Lacticaseibacillus casei spp. pseudoplantarum | LIVTQ, LIVT | ACE inhibitory | (Vallabha & Tiku, 2014) |
| Aspergillus sojae | AW, GW,AY, SY, GY, AF, VP, AI, VG | Antihypertensive | (Nakahara et al., 2011) |
| Lactobacillus sanfranciscensis and Candida humilis | VPFGVG | ACE inhibitory | (Nakamura, Yoshida, Komatsuzaki, Kawasumi, & Shima, 2007) |
| Lactiplantibacillus plantarum LB1; L. rossiae LB5 | VLHEPLF – YNNPIIYVTENGIAEGNNKSLPITEAL – ALKAAPSPA – AILIIVMLFGR – AAAAVFLSLLAVGHCAAADFNATDADADFAG NGVDFNSSDAAVYWGPWTKAR |
Antifungal | (Rizzello, Cassone, Coda, & Gobbetti, 2011) |
| Lactobacillus acidophilus ATCC 4356 | Unidentified | Inmunomodulatory | (Stuknyte, De Noni, Guglielmetti, Minuzzo, & Mora, 2011) |
| Pichia kudriavzevii, K. marxianus | Unidentified | ACE-I inhibition | (Chaves-López et al., 2014) |
| Aspergillus oryzae | VPP, IPP | Antihypertensive | (Inoue et al., 2009) |
4.3. Advantages of microbial fermentation for bioactive peptide production
Microbial fermentation provides certain benefits that are not present in other peptide delivery techniques. For example, they have a high diversity of microbial proteases, which generates peptides of different sizes and sequences, and because of this, greater biological activities.
Likewise, they have high levels of protease activity since the entire set of proteases characteristic of the microorganism act and not only one protease as in enzymatic hydrolysis, which leads to an adequate release of the peptide from the precursor protein. As a result, production cycles are shorter, depending on the microorganism and conditions used (LeBlanc et al., 2002).
The process is less costly than enzymatic hydrolysis since commercial enzymes are not used because their price is high, and the culture costs are also relatively low. In addition, when LAB is used, the proteases are expressed in the cell membrane, which results in simple purification processes for the bioactive peptides generated. (Agyei & Danquah, 2011).
The proteolytic activity exerted by LAB is highly variable from one strain to another. Due to factors such as differences in CEP gene expression, CEP gene mutations, differences in the optimal conditions for enzymatic activity, differences in the type of protein that is hydrolyzed, or differences in the growth phase in which the microorganism is found (proteolytic activity is maintained in exponential phase and in the initial period of stationary phase) (Ayyash et al., 2018). This characteristic of LAB offers the advantage to the fermentation process to produce different amino acid sequences and therefore peptides can have a high diversity of bioactivities.
So far, no reports have been found of genetic modifications specifically in the CEP gene of LAB to improve their enzymatic activity and produce peptides with higher biological effects. Only factors such as optimal growth parameters of LAB and consequently of enzymes have been improved; and the use of co-cultures has been studied, where the results indicate that it is a better way to produce peptides with higher biological activities (Ayala-Niño et al., 2019).
Some reports mention that it produces peptides with high levels of bioactivity, and this favors having better health-related benefits and better functional properties (Elfahri et al., 2016), such as the most studied VPP and IPP peptides that demonstrate high antihypertensive activity and are components of commercial dairy products, these were obtained by fermentation with Lactobacillus helveticus where they were released from β- and k-casein by the action of proteases and peptidases (Rodríguez-Figueroa et al., 2013).
The bioactive peptides released by microbial fermentation are considered safer and healthier, with no side effects because they are obtained from edible food proteins, and GRAS microorganisms are also used (Fan et al., 2019).
Microbial fermentation can give added value to the substrate used, contributing to microbial safety and improving organoleptic, technological, nutritional, and health properties (Leroy & De Vuyst, 2004). It is also an environmentally friendly process, as no toxic residues are produced.
4.4. Disadvantages of microbial fermentation for bioactive peptide production
Microbial fermentation can generate other compounds in addition to bioactive peptides such as the cells of living and dead bacteria, exopolysaccharides, and bacteriocins which also exert biological properties and therefore raise doubts as to whether the bioactivities showed are an effect of the peptides or these compounds (Martínez-Augustin et al., 2014).
Factors such as the type of sugar to be used, limited or unlimited availability of nutrients and oxygen, the presence of competitive microorganisms, and time affect negatively if they are not applied correctly. Therefore, it is recommended to investigate the appropriate parameters before carrying out microbial fermentation (Melini et al., 2019).
The microorganism's enzymes are essential for achieving the release of bioactive peptides. Still, these are sensitive to environmental conditions, and small changes in pH, temperature, and pressure during the process can cause undesirable results.
Finally, microbial fermentation, being a biotechnological process, is susceptible to problems of poor reproducibility between batches compared to enzymatic hydrolysis, which is better because it use commercial enzymes, but also has shortcomings due to variations in the activity of certain enzymes when they are not working under optimal conditions (Toldrá et al., 2017). In addition, microbial fermentation presents unexpected deviations in the process, and inconsistent quality of the final product, so it is necessary to adequately monitor the process when it is carried out industrially so that it complies with the standards quality (Rudnitskaya et al., 2016).
5. Purification and identification
After hydrolysis of the protein, through enzymatic hydrolysis or microbial fermentation, the bioactive peptides are released. However, efficient purification of this molecule is necessary to identify its structure (Sridhar et al., 2021). In addition, the purification process should be performed because it allows the separation of peptides with different functionalities from the desired bioactive peptide, as some of them can have antagonistic effects. By purifying the peptides, a higher concentration of these molecules can be generated, thus obtaining high-yield products that can be sold as functional food ingredients or nutraceuticals (Fernández et al., 2014).
The purification process is carried out through a series of steps based on the physical and chemical characteristics of the bioactive peptides, such as molecular size, charge, polarity, solubility, and specific covalent or non-covalent interactions (Arumugam et al., 2018).
Membrane systems such as microfiltration, ultrafiltration, nanofiltration, and reverse osmosis are used in purification processes (Suwal et al., 2015). For bioactive peptides, ultrafiltration is the technique commonly applied. This technique is based on the transmission and retention of peptides according to their hydrodynamic volume and the membrane pore diameters driven by a pressure gradient supplied by a pump (Bazinet & Firdaous, 2009). Allows the separation of peptides with specific molecular weight cut-off, in a fast, economical, and environmentally friendly way. It preserves the physiological properties of the peptide and is easy to scale up (Dullius et al., 2018, Sridhar et al., 2021).
Some of the disadvantages of this technique are poor reproducibility, peptides below the desired molecular weight can be removed, interactions may form between hydrophobic peptides and the membrane, leading to their absorption, membrane fouling and clogging, difficulty in obtaining pure peptides and a large sample volume is required (Acquah et al., 2019, Dlask and Václavíková, 2018). To solve these problems, it has been proposed to combine them with other techniques such as electrodialysis, multi-step recycle membrane reactor, and chromatographic techniques (Acquah et al., 2019, Cheung et al., 2015, Lafarga et al., 2020).
Chromatographic techniques are able to separate and purify peptides with higher resolution, generating high quality (very pure) products (Dullius et al., 2018). They are based on the interaction of peptides with the stationary and mobile phases (Jahandideh, 2017). Techniques based on ion exchange, liquid–solid adsorption, liquid–liquid partition, and size exclusion are commonly used (Coskun, 2016).
Size-exclusion chromatography allows the separation of peptides based on their molecular weight. Ion-exchange chromatography allows the separation of peptides based on their charge. Reversed-phase high-performance liquid chromatography (RP-HPLC) allows the separation of peptides based on their hydrophobicity. Therefore, using these chromatographic techniques it is also possible to concentrate bioactive peptides of the desired molecular weight, charge and hydrophobicity (Issaq et al., 2002). Combining chromatographic techniques with UV, diode array, fluorescence, and mass detectors allows the identification of bioactive peptides (Sridhar et al., 2021).
The disadvantages of these techniques are that they are time-consuming and expensive, raising the price of commercializing bioactive peptides (Dullius et al., 2018).
Capillary electrophoresis is another technique for the identification and separation of bioactive peptides (Kartsova et al., 2021). It is based on m/z values and uses a fused silica capillary tube (Sridhar et al., 2021). It is characterized by high efficiency, low sample volume, simple sample preparation, high resolution and selectivity (Kartsova et al., 2021, Sridhar et al., 2021). It is sometimes complementary to chromatographic techniques; the problems of this technique are its low repeatability, low sensitivity and low detection limits (Polikarpova et al., 2020).
After the purification of the bioactive peptide, it is important to identify its sequence. There are several techniques that can be performed, for example, one of the most popular is liquid chromatography-tandem mass spectrometry (LC-MS/MS), which is characterized by its accuracy and sensitivity to detect bioactive peptides (Chen et al., 2019, Lafarga et al., 2020). You can analyze completely unknown peptides based on tandem mass spectrometry (Elam et al., 2021).
Another option is matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF-MS/MS) or surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) (Zhou et al., 2020). These are fast, efficient and highly sensitive techniques (Elam et al., 2021). MALDI-TOF-MS/MS is based on the analysis of ions, generated with UV laser pulses, as a function of their mass-to-charge ratio (Lu et al., 2015).
6. Future trends
The release of the peptides from the parent proteins is an essential step for these molecules to demonstrate their bioactive profile. For this, several methods can be used. However, it is important to choose the method where the amino acid sequences that show high bioactivity are generated. Two methods stand out: enzymatic hydrolysis and microbial fermentation. These must be understood before being used in the protein source, as there are many benefits, but they can also produce adverse effects if not used correctly. In recent years we have sought to improve these methods, for example, through in silico methods. But there are still areas of improvement, as in the use of bioreactors, since its scale-up to an industrial level is a problem that must be solved for the generation of marketable bioactive peptides.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors thank the National Council of Science and Technology (CONACYT) of Mexico and the Department of Food Research of the Universidad Autónoma de Coahuila.
Funding
This work was supported by the State Council for Science and Technology of Coahuila (COECYT) through the FONCYT project COAH-2020-C14-C078.
DECC thanks the National Council of Science and Technology (CONACYT) of Mexico for its postgraduate scholarship grant [grant number: 9994499].
Contributor Information
Dora Elisa Cruz-Casas, Email: elisacruz@uadec.edu.mx.
Cristóbal N. Aguilar, Email: cristobal.aguilar@uadec.edu.mx.
Juan A. Ascacio-Valdés, Email: alberto_ascaciovaldes@uadec.edu.mx.
Raúl Rodríguez-Herrera, Email: raul.rodriguez@uadec.edu.mx.
Adriana C. Flores-Gallegos, Email: carolinaflores@uadec.edu.mx.
References
- Acquah C., Chan Y.W., Pan S., Agyei D., Udenigwe C.C. Structure-informed separation of bioactive peptides. Journal of Food Biochemistry. 2019;43(1):1–10. doi: 10.1111/jfbc.12765. [DOI] [PubMed] [Google Scholar]
- Acquah, C., Stefano, E. Di, & Udenigwe, C. C. (2018). Role of hydrophobicity in food peptide functionality and bioactivity. Journal of Food Bioactives, 4, 88–98. 10.31665/jfb.2018.4164.
- Agyei D., Danquah M.K. Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnology Advances. 2011;29(3):272–277. doi: 10.1016/j.biotechadv.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Agyei, D., Ongkudon, C. M., Yi, C., Chan, A. S., & Danquah, M. K. (2016). Food and Bioproducts Processing Bioprocess challenges to the isolation and purification of bioactive peptides. 8, 244–246.
- Aluko R.E. Antihypertensive peptides from food proteins. Annual Review of Food Science and Technology. 2015;6(1):235–262. doi: 10.1146/food.2015.6.issue-110.1146/annurev-food-022814-015520. [DOI] [PubMed] [Google Scholar]
- Amorim, F. G., Coitinho, L. B., Dias, A. T., Friques, A. G. F., Monteiro, B. L., Rezende, L. C. D. de, Pereira, T. de M. C., Campagnaro, B. P., De Pauw, E., Vasquez, E. C., & Quinton, L. (2019). Identification of new bioactive peptides from Kefir milk through proteopeptidomics: Bioprospection of antihypertensive molecules. Food Chemistry, 282(December 2018), 109–119. 10.1016/j.foodchem.2019.01.010. [DOI] [PubMed]
- Arshad, N., Siow, H.-L., Ngoh, Y.-Y., Sofian, N. A. H. S., & Gan, C.-Y. (2019). Enzyme and Bioactive Peptides—A Strategy for Discovery and Identification of Antihypertensive Peptides. In Enzymes in Food Biotechnology. Elsevier Inc. 10.1016/b978-0-12-813280-7.00020-7.
- Arumugam V., Venkatesan M., Ramachandran S., Sundaresan U. Bioactive peptides from marine ascidians and future drug development–A review. International Journal of Peptide Research and Therapeutics. 2018;24(1):13–18. doi: 10.1007/s10989-017-9662-9. [DOI] [Google Scholar]
- Ayala-Niño, A., Rodríguez-Serrano, G. M., Jiménez-Alvarado, R., Bautista-Avila, M., Sánchez-Franco, J. A., González-Olivares, L. G., & Cepeda-Saez, A. (2019). Bioactivity of Peptides Released During Lactic Fermentation of Amaranth Proteins with Potential Cardiovascular Protective Effect: An In Vitro Study. 00(0), 1–6. 10.1089/jmf.2019.0039. [DOI] [PubMed]
- Ayyash M., Al-Nuaimi A.K., Al-Mahadin S., Liu S.Q. In vitro investigation of anticancer and ACE-inhibiting activity, α-amylase and α-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. Food Chemistry. 2018;239:588–597. doi: 10.1016/j.foodchem.2017.06.149. [DOI] [PubMed] [Google Scholar]
- Bao X., Wu J. Impact of food-derived bioactive peptides on gut function and health. Food Research International. 2021;147(May) doi: 10.1016/j.foodres.2021.110485. [DOI] [PubMed] [Google Scholar]
- Baptista D.P., Salgaço M.K., Sivieri K., Gigante M.L. Use of static and dynamic in vitro models to simulate Prato cheese gastrointestinal digestion: Effect of Lactobacillus helveticus LH-B02 addition on peptides bioaccessibility. LWT - Food Science and Technology. 2020;134:110229. doi: 10.1016/j.lwt.2020.110229. [DOI] [Google Scholar]
- Barberis, S. E., Origone, A. L., Adaro, M. O., & Bersi, G. (2018). Bioactive Peptides as Functional Food Ingredients. In Microbial Biotechnology: Principles and Applications, Third Edition. Elsevier Inc. 10.1142/9789814366830_0012.
- Bazinet L., Firdaous L. Membrane Processes and Devices for Separation of Bioactive Peptides. Recent Patents on Biotechnology. 2009;3(1):61–72. doi: 10.2174/187220809787172623. [DOI] [PubMed] [Google Scholar]
- Bechaux J., Gatellier P., Le Page J.F., Drillet Y., Sante-Lhoutellier V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food and Function. 2019;10(10):6244–6266. doi: 10.1039/c9fo01546a. [DOI] [PubMed] [Google Scholar]
- Beltrán-Barrientos L.M., García H.S., Torres-Llanez M.J., González-Córdova A.F., Hernández-Mendoza A., Vallejo-Cordoba B. Safety of milk-derived bioactive peptides. International Journal of Dairy Technology. 2017;70(1):16–22. doi: 10.1111/idt.2017.70.issue-110.1111/1471-0307.12338. [DOI] [Google Scholar]
- Borad S.G., Kumar A., Singh A.K. Effect of processing on nutritive values of milk protein. Critical Reviews in Food Science and Nutrition. 2017;57(17):3690–3702. doi: 10.1080/10408398.2016.1160361. [DOI] [PubMed] [Google Scholar]
- Boukil, A., Suwal, S., Chamberland, J., Pouliot, Y., & Doyen, A. (2018). Ultrafiltration performance and recovery of bioactive peptides after fractionation of tryptic hydrolysate generated from pressure-treated Β-lactoglobulin. Journal of Membrane Science, 556(November 2017), 42–53. 10.1016/j.memsci.2018.03.079.
- Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S., et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nature Protocols. 2019;14(4):991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- Carrasco-Castilla J., Hernández-Álvarez A.J., Jiménez-Martínez C., Gutiérrez-López G.F., Dávila-Ortiz G. Use of proteomics and peptidomics methods in food bioactive peptide science and engineering. Food Engineering Reviews. 2012;4(4):224–243. doi: 10.1007/s12393-012-9058-8. [DOI] [Google Scholar]
- Chakrabarti S., Wu J. Milk-derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro- Pro) promote adipocyte differentiation and inhibit inflammation in 3T3-F442A cells. PLoS ONE. 2015;10(2):1–15. doi: 10.1371/journal.pone.0117492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalamaiah M., Keskin S., Hong H., Wu J. Regulatory requirements of bioactive peptides (protein hydrolysates) from food proteins. Journal of Functional Foods. 2019;58(January):123–129. doi: 10.1016/j.jff.2019.04.050. [DOI] [Google Scholar]
- Chaudhary K., Kumar R., Singh S., Tuknait A., Gautam A., Mathur D., et al. A web server and mobile app for computing hemolytic potency of peptides. Scientific Reports. 2016;6(1) doi: 10.1038/srep22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan, V., & Kanwar, S. S. (2020). Bioactive peptides: synthesis, functions and biotechnological applications. In Biotechnological Production of Bioactive Compounds. Elsevier B.V. 10.1016/B978-0-444-64323-0.00004-7.
- Chaves-López C., Serio A., Paparella A., Martuscelli M., Corsetti A., Tofalo R., Suzzi G. Impact of microbial cultures on proteolysis and release of bioactive peptides in fermented milk. Food Microbiology. 2014;42:117–121. doi: 10.1016/j.fm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Chen M.-F., Zhang Y.Y., Di He M., Li C.Y., Zhou C.X., Hong P.Z., et al. Antioxidant peptide purified from enzymatic hydrolysates of isochrysis zhanjiangensis and its protective effect against ethanol induced oxidative stress of HepG2 cells. Biotechnology and Bioprocess Engineering. 2019;24(2):308–317. doi: 10.1007/s12257-018-0391-5. [DOI] [Google Scholar]
- Cheung R., Ng T., Wong J. Marine peptides: Bioactivities and applications. Marine Drugs. 2015;13(7):4006–4043. doi: 10.3390/md13074006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, L. Y., Toh, G. T., & Ismail, A. (2019). Application of Proteases for the Production of Bioactive Peptides. In Enzymes in Food Biotechnology. Elsevier Inc. 10.1016/b978-0-12-813280-7.00015-3.
- Chiang J.H., Loveday S.M., Hardacre A.K., Parker M.E. Effects of enzymatic hydrolysis treatments on the physicochemical properties of beef bone extract using endo- and exoproteases. International Journal of Food Science and Technology. 2019;54(1):111–120. doi: 10.1111/ijfs.2019.54.issue-110.1111/ijfs.13911. [DOI] [Google Scholar]
- Chua H.L., Jois S., Sim M.K., Go M.L. Transport of angiotensin peptides across the Caco-2 monolayer. Peptides. 2004;25(8):1327–1338. doi: 10.1016/j.peptides.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Coskun, O. (2016). Separation Tecniques: Chromatography. Northern Clinics of Istanbul, 3(2), 156–160. 10.14744/nci.2016.32757. [DOI] [PMC free article] [PubMed]
- Daliri E., Oh D., Lee B. Peptides. 2017;6(5):32. doi: 10.3390/foods6050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Wang L., Zhang T., Yu Z., Liu J. Hydrolysis and transepithelial transport of two corn gluten derived bioactive peptides in human Caco-2 cell monolayers. Food Research International. 2018;106:475–480. doi: 10.1016/j.foodres.2017.12.080. [DOI] [PubMed] [Google Scholar]
- Dlask O., Václavíková N. Electrodialysis with ultrafiltration membranes for peptide separation. Chemical Papers. 2018;72(2):261–271. doi: 10.1007/s11696-017-0293-6. [DOI] [Google Scholar]
- Duca F.A., Waise T.M.Z., Peppler W.T., Lam T.K.T. The metabolic impact of small intestinal nutrient sensing. Nature Communications. 2021;12(1):1–12. doi: 10.1038/s41467-021-21235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dullius, A., Goettert, M. I., & de Souza, C. F. V. (2018). Whey protein hydrolysates as a source of bioactive peptides for functional foods – Biotechnological facilitation of industrial scale-up. Journal of Functional Foods, 42(December 2017), 58–74. 10.1016/j.jff.2017.12.063.
- Dyer A.R., Burdock G.A., Carabin I.G., Haas M.C., Boyce J., Alsaker R., et al. In vitro and in vivo safety studies of a proprietary whey extract. Food and Chemical. Toxicology. 2008;46(5):1659–1665. doi: 10.1016/j.fct.2007.12.029. [DOI] [PubMed] [Google Scholar]
- EFSA, Nda Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) General scientific guidance for stakeholders on health claim applications. EFSA Journal. 2016;14(1):4367. doi: 10.2903/j.efsa.2021.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam E., Feng J., Lv Y.-M., Ni Z.-J., Sun P., Thakur K., et al. Recent advances on bioactive food derived anti-diabetic hydrolysates and peptides from natural resources. Journal of Functional Foods. 2021;86:104674. doi: 10.1016/j.jff.2021.104674. [DOI] [Google Scholar]
- Elfahri K.R., Vasiljevic T., Yeager T., Donkor O.N. Anti-colon cancer and antioxidant activities of bovine skim milk fermented by selected Lactobacillus helveticus strains. Journal of Dairy Science. 2016;99(1):31–40. doi: 10.3168/jds.2015-10160. [DOI] [PubMed] [Google Scholar]
- Fan M., Guo T., Li W., Chen J., Li F., Wang C., et al. Isolation and identification of novel casein-derived bioactive peptides and potential functions in fermented casein with Lactobacillus helveticus. Food Science and Human Wellness. 2019;8(2):156–176. doi: 10.1016/j.fshw.2019.03.010. [DOI] [Google Scholar]
- Fekete Á.A., Ian Givens D., Lovegrove J.A. Casein-derived lactotripeptides reduce systolic and diastolic blood pressure in a meta-analysis of randomised clinical trials. Nutrients. 2015;7(1):659–681. doi: 10.3390/nu7010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández A., Zhu Y., FitzGerald R.J., Riera F.A. Membrane fractionation of a β-lactoglobulin tryptic digest: Effect of the membrane characteristics. Journal of Chemical Technology and Biotechnology. 2014;89(4):508–515. [Google Scholar]
- Fernández-Tomé, S., Montalban-Arques, A., Díaz-Guerra, A., Galvan-Roman, J. M., Marin, A. C., Mora-Gutiérrez, I., Ortega Moreno, L., Santander, C., Sánchez, B., Chaparro, M., Gisbert, J. P., & Bernardo, D. (2019). Peptides encrypted in the human intestinal microbial-exoproteome as novel biomarkers and immunomodulatory compounds in the gastrointestinal tract. Journal of Functional Foods, 52(November 2018), 459–468. 10.1016/j.jff.2018.11.036.
- Ferri M., Graen-Heedfeld J., Bretz K., Guillon F., Michelini E., Calabretta M.M., et al. Peptide fractions obtained from rice by-products by means of an environment-friendly process show in vitro health-related bioactivities. PLoS ONE. 2017;12(1):e0170954. doi: 10.1371/journal.pone.0170954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn A., Korhonen H., Marchelli R., Palou A. Evolus® and reduce arterial stiffness - Scientific substantiation of a health claim related to Lactobacillus helveticus fermented Evolus® low-fat milk products and reduction of arterial stiffness pursuant to Article 14 of the Regulation (EC) No 1924/2006. EFSA Journal. 2008;6(10) doi: 10.2903/j.efsa.2008.824. [DOI] [Google Scholar]
- Fu J., Li L., Yang X.Q. Specificity of carboxypeptidases from actinomucor elegans and their debittering effect on soybean protein hydrolysates. Applied Biochemistry and Biotechnology. 2011;165(5–6):1201–1210. doi: 10.1007/s12010-011-9338-4. [DOI] [PubMed] [Google Scholar]
- Fu Y.u., Chen J., Bak K.H., Lametsch R. Valorisation of protein hydrolysates from animal by-products: Perspectives on bitter taste and debittering methods: A review. International Journal of Food Science and Technology. 2019;54(4):978–986. doi: 10.1111/ijfs.2019.54.issue-410.1111/ijfs.14037. [DOI] [Google Scholar]
- Fujita H., Yoshikawa M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology. 1999;44(1–2):123–127. doi: 10.1016/S0162-3109(99)00118-6. [DOI] [PubMed] [Google Scholar]
- Gallego M., Mora L., Toldrá F. Characterisation of the antioxidant peptide AEEEYPDL and its quantification in Spanish dry-cured ham. Food Chemistry. 2018;258:8–15. doi: 10.1016/j.foodchem.2018.03.035. [DOI] [PubMed] [Google Scholar]
- García M.C., Puchalska P., Esteve C., Marina M.L. Vegetable foods : A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta. 2013;106:328–349. doi: 10.1016/j.talanta.2012.12.041. [DOI] [PubMed] [Google Scholar]
- Gauthier, S., Pouliot, Y., & Saint-Sauveur, D. (2006). Immunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. 16, 1315–1323. 10.1016/j.idairyj.2006.06.014.
- Gleeson J.P., Brayden D.J., Ryan S.M. Evaluation of PepT1 transport of food-derived antihypertensive peptides, Ile-Pro-Pro and Leu-Lys-Pro using in vitro, ex vivo and in vivo transport models. European Journal of Pharmaceutics and Biopharmaceutics. 2017;115:276–284. doi: 10.1016/j.ejpb.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Gobbetti M., Minervini F., Rizzello C. Bioactive peptides in dairy products. International Journal of Dairy Technology. 2007;65(1):1–12. doi: 10.1111/j.1471-0307.2011.00725.x. [DOI] [Google Scholar]
- Gonzalez-Gonzalez C., Gibson T., Jauregi P. Novel probiotic-fermented milk with angiotensin I-converting enzyme inhibitory peptides produced by Bifidobacterium bifidum MF 20/5. International Journal of Food Microbiology. 2013;167(2):131–137. doi: 10.1016/j.ijfoodmicro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Görgüç A., Gençdağ E., Yılmaz F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments – A review. Food Research International. 2020;136:109504. doi: 10.1016/j.foodres.2020.109504. [DOI] [PubMed] [Google Scholar]
- Hao Y., Fan X., Guo H., Yao Y., Ren G., Lv X., et al. Overexpression of the bioactive lunasin peptide in soybean and evaluation of its anti-inflammatory and anti-cancer activities in vitro. Journal of Bioscience and Bioengineering. 2019;xxx(xxx):1–10. doi: 10.1016/j.jbiosc.2019.11.001. [DOI] [PubMed] [Google Scholar]
- Hayes M., García-Vaquero M. Bioactive compounds from fermented food products. Food Engineering Series. 2016;293–310 doi: 10.1007/978-3-319-42457-6_14. [DOI] [Google Scholar]
- Hayes, M., & Mora, L. (2015). Cardioprotective cryptides derived from fish and other food sources : Generation, application and future markets. 10.1021/jf505019z. [DOI] [PubMed]
- He, Y., Pan, X., Chi, C. F., Sun, K. L., & Wang, B. (2019). Ten new pentapeptides from protein hydrolysate of miiuy croaker (Miichthys miiuy) muscle: Preparation, identification, and antioxidant activity evaluation. LWT - Food Science and Technology, 105(November 2018), 1–8. 10.1016/j.lwt.2019.01.054.
- Hernández-Almanza, A., Muñiz-Márquez, D. B., De la Rosa, O., Navarro, V., Martínez-Medina, G., Rodríguez-Herrera, R., & Aguilar, C. N. (2017). Microbial Production of Bioactive Pigments, Oligosaccharides and Peptides. In Food Biosynthesis. Elsevier Inc. 10.1016/B978-0-12-811372-1/00004-X.
- Hernández-Ledesma B., Del Mar Contreras M., Recio I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Advances in Colloid and Interface Science. 2011;165(1):23–35. doi: 10.1016/j.cis.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Hu F., Wu Q., Song S., She R., Zhao Y., Yang Y., et al. Antimicrobial activity and safety evaluation of peptides isolated from the hemoglobin of chickens. BMC Microbiology. 2016;16(1) doi: 10.1186/s12866-016-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Gotou T., Kitajima H., Mizuno S., Nakazawa T., Yamamoto N. Release of antihypertensive peptides in miso paste during its fermentation, by the addition of casein. Journal of Bioscience and Bioengineering. 2009;108(2):111–115. doi: 10.1016/j.jbiosc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Issaq H.J., Conrads T.P., Janini G.M., Veenstra T.D. Methods for fractionation, separation and profiling of proteins and peptides. Electrophoresis. 2002;23(17):3048–3061. doi: 10.1002/1522-2683(200209)23:17<3048::AID-ELPS3048>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Jahandideh F. University of Alberta; Edmonton: 2017. Effects of egg white derived peptides on metabolic syndrome complications: Hypertension, inflammation, and insulin resistance. [Google Scholar]
- Jakubczyk A., Karas M., Rybczynska-Tkaczyk K., Zielinska E., Zielinski D. Current trends of bioactive peptides – New sources and therapeutic effect. Foods. 2020;9(7):1–28. doi: 10.3390/foods9070846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janser R., Castro S.D., Sato H.H. Biologically active peptides: Processes for their generation, puri fi cation and identi fi cation and applications as natural additives in the food and pharmaceutical industries. FRIN. 2015;74:185–198. doi: 10.1016/j.foodres.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Jiehui Z., Liuliu M., Yang G., Yingkai J., Lun Z., Li D.X.A.…Shaohui Z. Immunomodulating effects of casein-derived peptide QEPVL and QEPV on lymphocytes in vitro and in vivo. Food and Function. 2014 doi: 10.1039/c3fo60657k. [DOI] [PubMed] [Google Scholar]
- Juillard, V., National, I., Recherche, D., Inra, A., & Cedex, J. (2016). Proteolytic Systems of Lactic Acid Bacteria (LAB). 1–7. 10.1016/B978-0-08-100596-5.00862-3.
- Karami, Z., Peighambardoust, S. H., Hesari, J., Akbari-Adergani, B., & Andreu, D. (2019). Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates. Food Bioscience, 32(April 2018), 100450. 10.1016/j.fbio.2019.100450.
- Karaś M. Influence of physiological and chemical factors on the absorption of bioactive peptides. International Journal of Food Science and Technology. 2019;54(5):1486–1496. doi: 10.1111/ijfs.14054. [DOI] [Google Scholar]
- Kartsova L.A., Makeeva D.V., Kravchenko A.V., Moskvichev D.O., Polikarpova D.A. Capillary electrophoresis as a powerful tool for the analyses of bacterial samples. TrAC – Trends in Analytical Chemistry. 2021;134:116110. doi: 10.1016/j.trac.2020.116110. [DOI] [Google Scholar]
- Klein N., Zourari A., Lortal S. Peptidase activity of four yeast species frequently encountered in dairy products – Comparison with several dairy bacteria. International Dairy Journal. 2002;12(10):853–861. doi: 10.1016/S0958-6946(02)00081-X. [DOI] [Google Scholar]
- Komin A., Russell L.M., Hristova K.A., Searson P.C. Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: Mechanisms and challenges. Advanced Drug Delivery Reviews. 2017;110-111:52–64. doi: 10.1016/j.addr.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Kotaro A., Osami K., Hiroshi H., Rei T., Yasunori N. Effect of powdered fermented milk with lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. Journal of the American College of Nutrition. 2005;24(4):257–265. doi: 10.1080/07315724.2005.10719473. [DOI] [PubMed] [Google Scholar]
- Kouhdasht, A. M., & Moosavi-nasab, M. (2018). Bioactive Peptides Derived from Fish By-Product Collagen. 13(2), 9–12. 10.19080/IJESNR.2018.13.55585.
- Lafarga T., Hayes M. Effect of pre-treatment on the generation of dipeptidyl peptidase-IV-and prolyl endopeptidase-inhibitory hydrolysates from bovine lung. Irish Journal of Agricultural and Food Research. 2017;56(1):12–24. doi: 10.1515/ijafr-2017-0002. [DOI] [Google Scholar]
- Lafarga T., Acién-Fernández F.G., Garcia-Vaquero M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Research. 2020;48:101909. doi: 10.1016/j.algal.2020.101909. [DOI] [Google Scholar]
- LeBlanc J.G., Matar C., Valdéz J.C., LeBlanc J., Perdigon G. Immunomodulating Effects of Peptidic Fractions Issued from Milk Fermented with Lactobacillus helveticus. Journal of Dairy Science. 2002;85(11):2733–2742. doi: 10.3168/jds.S0022-0302(02)74360-9. [DOI] [PubMed] [Google Scholar]
- Leksrisompong, P., Gerard, P., Lopetcharat, K., & Drake, M. (2012). Bitter taste inhibiting agents for whey protein hydrolysate and whey protein hydrolysate beverages. Journal of Food Science, 77(8). 10.1111/j.1750-3841.2012.02800.x. [DOI] [PubMed]
- Leroy F., De Vuyst L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends in Food Science and Technology. 2004;15(2):67–78. doi: 10.1016/j.tifs.2003.09.004. [DOI] [Google Scholar]
- Li Y., Zhao J., Liu X., Xia X., Wang Y., Zhou J. Transport of a Novel Angiotensin-I-Converting Enzyme Inhibitory Peptide Ala-His-Leu-Leu Across Human Intestinal Epithelial Caco-2 Cells. Journal of Medicinal Food. 2017;20(3):243–250. doi: 10.1089/jmf.2016.3842. [DOI] [PubMed] [Google Scholar]
- Lin, Q., Xu, Q., Bai, J., Wu, W., Hong, H., & Wu, J. (2017). Transport of soybean protein-derived antihypertensive peptide LSW across Caco-2 monolayers. Journal of Functional Foods, 39, 96–102. 10.1016/j.jff.2017.10.011. [DOI]
- Li-Chan E.C.Y. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Current Opinion in Food Science. 2015;1(1):28–37. doi: 10.1016/j.cofs.2014.09.005. [DOI] [Google Scholar]
- Li Y., Sadiq F.A., Liu T.J., Chen J.C., He G.Q. Purification and identification of novel peptides with inhibitory effect against angiotensin I-converting enzyme and optimization of process conditions in milk fermented with the yeast Kluyveromyces marxianus. Journal of Functional Foods. 2015;16:278–288. doi: 10.1016/j.jff.2015.04.043. [DOI] [Google Scholar]
- Lin Y.-H., Chen C.-A., Tsai J.-S., Chen G.-W. Preparation and identification of novel antihypertensive peptides from the in vitro gastrointestinal digestion of marine cobia skin hydrolysates. Nutrients. 2019;11(6):1351. doi: 10.3390/nu11061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L., Li, S., Zheng, J., Bu, T., He, G., & Wu, J. (2020). Safety considerations on food protein-derived bioactive peptides. Trends in Food Science and Technology, 96(February 2019), 199–207. 10.1016/j.tifs.2019.12.022.
- Liu Z., Wang S., Hu M. Developing solid oral dosage forms. Elsevier; 2009. Oral absorption basics; pp. 263–288. [DOI] [Google Scholar]
- Lu I.C., Lee C., Lee Y.T., Ni C.K. Ionization mechanism of matrix-assisted laser desorptionionization. Annual Review of Analytical Chemistry. 2015;8(May):21–39. doi: 10.1146/annurev-anchem-071114-040315. [DOI] [PubMed] [Google Scholar]
- Lu Y., Wang J., Soladoye O.P., Aluko R.E., Fu Y., Zhang Y. Preparation, receptors, bioactivity and bioavailability of γ-glutamyl peptides: A comprehensive review. Trends in Food Science and Technology. 2021;113(May):301–314. doi: 10.1016/j.tifs.2021.04.051. [DOI] [Google Scholar]
- Lucas-González R., Viuda-Martos M., Pérez-Alvarez J.A., Fernández-López J. In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges. Food Research International. 2018;107(2017):423–436. doi: 10.1016/j.foodres.2018.02.055. [DOI] [PubMed] [Google Scholar]
- Maehashi K., Huang L. Bitter peptides and bitter taste receptors. Cellular and Molecular Life Sciences. 2009;66(10):1661–1671. doi: 10.1007/s00018-009-8755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando A.M., Graham W.V., Turner J.R. Epithelial barriers in homeostasis and disease. Annual Review of Pathology: Mechanisms of Disease. 2010;5(1):119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- Martínez-Augustin O., Rivero-Gutiérrez B., Mascaraque C., de Medina F.S. Food derived bioactive peptides and intestinal barrier function. International Journal of Molecular Sciences. 2014;15(12):22857–22873. doi: 10.3390/ijms151222857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Medina, G. A., Barragán, A. P., Ruiz, H. A., Ilyina, A., Martínez Hernández, J. L., Rodríguez-Jasso, R. M., Hoyos-Concha, J. L., & Aguilar-González, C. N. (2019). Fungal Proteases and Production of Bioactive Peptides for the Food Industry. In Enzymes in Food Biotechnology. Elsevier Inc. 10.1016/b978-0-12-813280-7.00014-1.
- Mason J.M. Design and development of peptides and peptide mimetics as antagonists for therapeutic intervention. Future Science. 2010;2(12):1813–1822. doi: 10.4155/fmc.10.259. [DOI] [PubMed] [Google Scholar]
- Matsui T. Are peptides absorbable compounds? Journal of Agricultural and Food Chemistry. 2018;66(2):393–394. doi: 10.1021/acs.jafc.7b05589. [DOI] [PubMed] [Google Scholar]
- Melini F., Melini V., Luziatelli F., Ficca A.G., Ruzzi M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients. 2019;11(1189):1–24. doi: 10.3390/nu11051189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville P.A., Benites N.R., Ruz-Peres M., Yokoya E. Proteinase and phospholipase activities and development at different temperatures of yeasts isolated from bovine milk. Journal of Dairy Research. 2011;78(4):385–390. doi: 10.1017/S0022029911000434. [DOI] [PubMed] [Google Scholar]
- Michalak I., Dmytryk A., Śmieszek A., Marycz K. Chemical characterization of Enteromorpha prolifera extract obtained by enzyme-assisted extraction and its influence on the metabolic activity of Caco-2. International Journal of Molecular Sciences. 2017;18(3):1–20. doi: 10.3390/ijms18030479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel M., Dávalos A., Manso M.A., de la Peña G., Lasunción M.A., López-Fandiño R. Transepithelial transport across Caco-2 cell monolayers of antihypertensive egg-derived peptides. PepT1-mediated flux of Tyr-Pro-Ile. Molecular Nutrition and Food Research. 2008;52(12):1507–1513. doi: 10.1002/mnfr.v52:1210.1002/mnfr.200700503. [DOI] [PubMed] [Google Scholar]
- Mikhaylin S., Boussetta N., Vorobiev E., Bazinet L. High voltage electrical treatments to improve the protein susceptibility to enzymatic hydrolysis. ACS Sustainable Chemistry and Engineering. 2017;5(12):11706–11714. doi: 10.1021/acssuschemeng.7b03192. [DOI] [Google Scholar]
- Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food and Function. 2014;5(6):1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- Mora L., Aristoy M.C., Toldrá F. Bioactive peptides. Encyclopedia of Food Chemistry. 2018;381–389 doi: 10.1016/B978-0-08-100596-5.22397-4. [DOI] [PubMed] [Google Scholar]
- Mora L., Gallego M., Toldrá F. ACEI-inhibitory peptides naturally generated in meat and meat products and their health relevance. Nutrients. 2018;10(9):1–12. doi: 10.3390/nu10091259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T., Suzuki N., Nagashima T. Antioxidative activities and angiotensin I-converting enzyme inhibitory activities of enzymatic hydrolysates from commercial kamaboko type samples. Food Science and Technology International. 2006;12(4):335–346. doi: 10.1177/1082013206067933. [DOI] [Google Scholar]
- Nakahara T., Sugimoto K., Sano A., Yamaguchi H., Katayama H., Uchida R. Antihypertensive Mechanism of a Peptide-Enriched Soy Sauce-Like Seasoning: The Active Constituents and Its Suppressive Effect on Renin-Angiotensin-Aldosterone System. Journal of Food Science. 2011;76(8) doi: 10.1111/j.1750-3841.2011.02362.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Yoshida A., Komatsuzaki N., Kawasumi T., Shima J. Isolation and characterization of a low molecular weight peptide contained in sourdough. Journal of Agricultural and Food Chemistry. 2007;55(12):4871–4876. doi: 10.1021/jf0700. [DOI] [PubMed] [Google Scholar]
- Onuh J.O., Girgih A.T., Aluko R.E., Aliani M. In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chemistry. 2014;150:366–373. doi: 10.1016/j.foodchem.2013.10.107. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J.R., Michel C.C. Role of villus microcirculation in intestinal absorption of glucose: Coupling of epithelial with endothelial transport. Journal of Physiology. 2003;553(2):561–574. doi: 10.1113/jphysiol.2003.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikarpova D., Makeeva D., Kolotilina N., Dolgonosov A., Peshkova M., Kartsova L. Nanosized cation exchanger for the electrophoretic separation and preconcentration of catecholamines and amino acids. Electrophoresis. 2020;41(12):1031–1038. doi: 10.1002/elps.v41.1210.1002/elps.201900416. [DOI] [PubMed] [Google Scholar]
- Quirós A., Ramos M., Muguerza B., Delgado M.A., Miguel M., Aleixandre A., Recio I. Identification of novel antihypertensive peptides in milk fermented with Enterococcus faecalis. International Dairy Journal. 2007;17(1):33–41. doi: 10.1016/j.idairyj.2005.12.011. [DOI] [Google Scholar]
- Raju, T. S. (2019). Proteolysis of Proteins. Co and Post-Translational Modifications of Therapeutic Antibodies and Proteins, 183–202. 10.1002/9781119053354.ch15.
- Rani K., Rana R., Datt S. Review on latest overview of proteases. International Journal of Current Life Sciences. 2012;2(1):12–18. [Google Scholar]
- Renukuntla J., Vadlapudi A.D., Patel A., Boddu S.H.S., Mitra A.K. Approaches for enhancing oral bioavailability of peptides and proteins. International Journal of Pharmaceutics. 2013;447(1–2):75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzello C., Lorusso A., Russo V., Pinto D., Marzani B., Gobbetti M. Improving the antioxidant properties of quinoa flour through fermentation with selected autochthonous lactic acid bacteria. International Journal of Food Microbiology. 2017;241:252–261. doi: 10.1016/j.ijfoodmicro.2016.10.035. [DOI] [PubMed] [Google Scholar]
- Rizzello C., Tagliazucchi D., Babini E., Rutella G., Taneyo D., Gianotti A. Bioactive peptides from vegetable food matrices: Research trends and novel biotechnologies for synthesis and recovery. Journal of Functional Foods. 2016;27:549–569. doi: 10.1016/j.jff.2016.09.023. [DOI] [Google Scholar]
- Rizzello C.G., Cassone A., Coda R., Gobbetti M. Antifungal activity of sourdough fermented wheat germ used as an ingredient for bread making. Food Chemistry. 2011;127(3):952–959. doi: 10.1016/j.foodchem.2011.01.063. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Figueroa J.C., González-Córdova A.F., Astiazaran-García H., Vallejo-Cordoba B. Hypotensive and heart rate-lowering effects in rats receiving milk fermented by specific Lactococcus lactis strains. British Journal of Nutrition. 2013;109(5):827–833. doi: 10.1017/S0007114512002115. [DOI] [PubMed] [Google Scholar]
- Rojas-Ronquillo R., Cruz-Guerrero A., Flores-Nájera A., Rodríguez-Serrano G., Gómez-Ruiz L., Reyes-Grajeda J.P.…García-Garibay M. Antithrombotic and angiotensin-converting enzyme inhibitory properties of peptides released from bovine casein by Lactobacillus casei Shirota. International Dairy Journal. 2012;26(2):147–154. doi: 10.1016/j.idairyj.2012.05.002. [DOI] [Google Scholar]
- Rudnitskaya, A., Kirsanov, D., & Legin, A. (2016). Monitoring of Fermentation and Biotechnological Processes. In Electronic Noses and Tongues in Food Science (Issue Ccd). Elsevier Inc. 10.1016/B978-0-12-800243-8.00022-6.
- Ryder K., Bekhit A.E.D., McConnell M., Carne A. Towards generation of bioactive peptides from meat industry waste proteins: Generation of peptides using commercial microbial proteases. Food Chemistry. 2016;208:42–50. doi: 10.1016/j.foodchem.2016.03.121. [DOI] [PubMed] [Google Scholar]
- Saha B.C., Hayashi K. Debittering of protein hydrolyzates. Biotechnology Advances. 2001;19(5):355–370. doi: 10.1016/S0734-9750(01)00070-2. [DOI] [PubMed] [Google Scholar]
- Santi L., Maggioli C., Mastroroberto M., Tufoni M., Napoli L., Caraceni P. Acute liver failure caused by amanita phalloides poisoning. International Journal of Hepatology. 2012;2012:1–6. doi: 10.1155/2012/487480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Enjoh M., Nakamura Y., Takano T., Kawamura Y., Arai S., Shimizu M. Transepithelial transport of the bioactive tripeptide, Val-Pro-Pro, in human intestinal Caco-2 cell monolayers. Bioscience, Biotechnology and Biochemistry. 2002;66(2):378–384. doi: 10.1271/bbb.66.378. [DOI] [PubMed] [Google Scholar]
- Sauer, J. M., & Merchant, H. A. (2018). Physiology of the Gastrointestinal System. In Comprehensive Toxicology: Third Edition (Third Edit, Vols. 3–15, Issue July 2017). Elsevier. 10.1016/B978-0-12-801238-3.99195-5.
- Sensoy I. A review on the food digestion in the digestive tract and the used in vitro models. Current Research in Food Science. 2021;4(February):308–319. doi: 10.1016/j.crfs.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna S.K., Nataraj B.H. Revisiting therapeutic and toxicological fingerprints of milk-derived bioactive peptides: An overview. Food Bioscience. 2020;38:100771. doi: 10.1016/j.fbio.2020.100771. [DOI] [Google Scholar]
- Siltari A., Kivimäki A.S., Ehlers P.I., Korpela R., Vapaatalo H. Effects of milk casein derived tripeptides on endothelial enzymes in vitro; A study with synthetic tripeptides. Arzneimittel-Forschung/Drug Research. 2012;62(10):477–481. doi: 10.1055/s-0032-1321846. [DOI] [PubMed] [Google Scholar]
- Soladoye O.P., Juárez M.L., Aalhus J.L., Shand P., Estévez M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Comprehensive Reviews in Food Science and Food Safety. 2015;14(2):106–122. doi: 10.1111/1541-4337.12127. [DOI] [PubMed] [Google Scholar]
- Sridhar K., Inbaraj B.S., Chen B.H. Recent developments on production, purification and biological activity of marine peptides. Food Research International. 2021;147(June) doi: 10.1016/j.foodres.2021.110468. [DOI] [PubMed] [Google Scholar]
- Stuknyte M., De Noni I., Guglielmetti S., Minuzzo M., Mora D. Potential immunomodulatory activity of bovine casein hydrolysates produced after digestion with proteinases of lactic acid bacteria. International Dairy Journal. 2011;21(10):763–769. doi: 10.1016/j.idairyj.2011.04.011. [DOI] [Google Scholar]
- Subramaniyam R., Vimala R. Solid state and submerged fermentation for the production of bioactive substances: A Comparative Study. International Journal of Science and Nature. 2012;3(3):480–486. [Google Scholar]
- Suwal S., Doyen A., Bazinet L. Characterization of protein, peptide and amino acid fouling on ion-exchange and filtration membranes: Review of current and recently developed methods. Journal of Membrane Science. 2015;496:267–283. doi: 10.1016/j.memsci.2015.08.056. [DOI] [Google Scholar]
- Tadesse S.A., Emire S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon. 2020;6(8):e04765. doi: 10.1016/j.heliyon.2020.e04765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng D., Fang Y., Song X., Gao Y. Optimization of enzymatic hydrolysis parameters for antioxidant capacity of peptide from goat placenta. Food and Bioproducts Processing. 2011;89(3):202–208. doi: 10.1016/j.fbp.2010.05.001. [DOI] [Google Scholar]
- Toldrá F., Reig M., Aristoy M.C., Mora L. Generation of bioactive peptides during food processing. Food Chemistry. 2017;267(June):395–404. doi: 10.1016/j.foodchem.2017.06.119. [DOI] [PubMed] [Google Scholar]
- Tonolo, F., Folda, A., Cesaro, L., Scalcon, V., Marin, O., Ferro, S., Bindoli, A., & Pia, M. (2019). Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. Journal of Functional Foods, March, 103696. 10.1016/j.jff.2019.103696.
- Tu, M., Liu, H., Zhang, R., Chen, H., Fan, F., Shi, P., Xu, X., Lu, W., & Du, M. (2018). Bioactive hydrolysates from casein: generation, identification, and in silico toxicity and allergenicity prediction of peptides. In Journal of the Science of Food and Agriculture (Vol. 98, Issue 9). 10.1002/jsfa.8854. [DOI] [PubMed]
- Udenigwe C.C., Aluko R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. Food Science. 2012;71 doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- Vallabha V., Tiku P.K. Antihypertensive peptides derived from soy protein by fermentation. International Journal of Peptide Research and Therapeutics. 2014;20(2):161–168. doi: 10.1007/s10989-013-9377-5. [DOI] [Google Scholar]
- Verma A.K., Chatli M.K., Kumar P., Mehta N. Antioxidant and antimicrobial activity of porcine liver hydrolysate in meat emulsion and their influence on physico-chemical and color deterioration during refrigeration storage. Journal of Food Science. 2019;84(7):1844–1853. doi: 10.1111/jfds.2019.84.issue-710.1111/1750-3841.14683. [DOI] [PubMed] [Google Scholar]
- Vig B.S., Stouch T.R., Timoszyk J.K., Quan Y., Wall D.A., Smith R.L., et al. Human PEPT1 pharmacophore distinguishes between dipeptide transport and binding. Journal of Medicinal Chemistry. 2006;49(12):3636–3644. doi: 10.1021/jm0511029. [DOI] [PubMed] [Google Scholar]
- Ward O.P., Rao M.B., Kulkarni A. Proteases, Production. Encyclopedia of Microbiology. 2009:495–511. doi: 10.1016/B978-012373944-5.00172-3. [DOI] [Google Scholar]
- Wen, C., Zhang, J., Yao, H., Zhou, J., Duan, Y., Zhang, H., & Ma, H. (2019). Advances in renewable plant-derived protein source: The structure, physicochemical properties affected by ultrasonication. Ultrasonics Sonochemistry, 53(August 2018), 83–98. 10.1016/j.ultsonch.2018.12.036. [DOI] [PubMed]
- Wouters J.T.M., Ayad E.H.E., Hugenholtz J., Smit G. Microbes from raw milk for fermented dairy products. International Dairy Journal. 2002;12(2–3):91–109. doi: 10.1016/S0958-6946(01)00151-0. [DOI] [Google Scholar]
- Xu F., Wang L., Ju X., Zhang J., Yin S., Shi J.…Yuan Q. Transepithelial Transport of YWDHNNPQIR and Its Metabolic Fate with Cytoprotection against Oxidative Stress in Human Intestinal Caco-2 Cells. Journal of Agricultural and Food Chemistry. 2017;65(10):2056–2065. doi: 10.1021/acs.jafc.6b04731. [DOI] [PubMed] [Google Scholar]
- Xu Q., Hong H., Wu J., Yan X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends in Food Science and Technology. 2019;86(February):399–411. doi: 10.1016/j.tifs.2019.02.050. [DOI] [Google Scholar]
- Yang H.J., Kwon D.Y., Moon N.R., Kim M.J., Kang H.J., Jung D.Y., Park S. Soybean fermentation with Bacillus licheniformis increases insulin sensitizing and insulinotropic activity. Food and Function. 2013;4(11):1675–1684. doi: 10.1039/c3fo60198f. [DOI] [PubMed] [Google Scholar]
- Yap P.G., Gan C.Y. In vivo challenges of anti-diabetic peptide therapeutics: Gastrointestinal stability, toxicity and allergenicity. Trends in Food Science and Technology. 2020;105(July):161–175. doi: 10.1016/j.tifs.2020.09.005. [DOI] [Google Scholar]
- Zanutto-Elgui M.R., Vieira J.C.S., Prado D.Z.d., Buzalaf M.A.R., Padilha P.d.M., Elgui de Oliveira D., et al. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases. Food Chemistry. 2019;278:823–831. doi: 10.1016/j.foodchem.2018.11.119. [DOI] [PubMed] [Google Scholar]
- Zhang J.H., Tatsumi E., Ding C.H., Li L.Te. Angiotensin I-converting enzyme inhibitory peptides in douchi, a Chinese traditional fermented soybean product. Food Chemistry. 2006;98(3):551–557. doi: 10.1016/j.foodchem.2005.06.024. [DOI] [Google Scholar]
- Zhang Y., Lu H., Lin Y., Cheng J. Water-soluble polypeptides with elongated, charged side chains adopt ultrastable helical conformations. Macromolecules. 2011;44(17):6641–6644. doi: 10.1021/ma201678r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Chen M., Wu S., Liao X., Wang J., Wu Q., et al. A review on mushroom-derived bioactive peptides: Preparation and biological activities. Food Research International. 2020;134:109230. doi: 10.1016/j.foodres.2020.109230. [DOI] [PubMed] [Google Scholar]
- Zihni C., Mills C., Matter K., Balda M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nature Reviews Molecular Cell Biology. 2016;17(9):564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]