Abstract
Gemifloxacin (known as SB-265805 or LB-20304) is a potent, novel fluoroquinolone compound with a broad spectrum of antibacterial activity. The pharmacokinetics and tolerability of oral gemifloxacin were characterized in healthy male volunteers after a single dose of 20, 40, 80, 160, 320, 600, or 800 mg. Multiple serum and urine samples were collected and analyzed for gemifloxacin using high-performance liquid chromatography with fluorescence detection. Safety assessments included vital signs, 12-lead electrocardiogram readings, hematology, clinical chemistry, urinalysis, and adverse-experience monitoring. Gemifloxacin was rapidly absorbed after all doses. Maximum concentrations of gemifloxacin in serum (Cmax) were achieved approximately 1 h after dosing, after which concentrations in serum declined in a biexponential manner. Values of Cmax and the area under the concentration-time curve in serum from 0 h to infinity (serum AUC0–∞) increased linearly with dose. Serum AUC0–∞ values (mean ± standard deviation) were 0.65 ± 0.01, 1.28 ± 0.22, 2.54 ± 0.31, 5.48 ± 1.24, 9.82 ± 2.70, 24.4 ± 7.1, and 31.4 ± 7.6 μg · h/ml following 20-, 40-, 80-, 160-, 320-, 600-, and 800-mg doses, respectively. The terminal phase elimination half-life was independent of dose, with an overall mean of 7.4 ± 2.0 h. The profiles indicated that the pharmacokinetic profile is suitable for a once-daily dosing regimen. Approximately 25 to 40% of the administered dose was excreted unchanged in the urine, and renal clearance (ca. 150 ml/min) was independent of dose. There were no significant changes in clinical chemistry, hematology, or urinalysis parameters, vital signs, or 12-lead electrocardiogram readings in subjects, irrespective of dose. The results of these studies support the further investigation of once-daily administration of gemifloxacin.
Gemifloxacin (R,S)-7-(3-aminomethyl-4-syn-methoxyimino-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8- naphthyridine-3-carboxylic acid methanesulfonate) (known as SB-265805 or LB-20304) is a new fluoronaphthyridone antibacterial agent with a broad spectrum of activity (1, 4, 6). It has shown potent activity against clinical isolates and reference strains in both in vitro studies and experimental infections in animals (2, 5; V. Berry, R. Page, J. Satterfield, C. Singley, R. Straub, and G. Woodnutt, Abstr. 21st Int. Congr. Chemother., abstr. 492, J. Antimicrob. Chemother. 44[Suppl.]:A148, 1999, and V. Berry, R. Page, J. Satterfield, R. Straub, and G. Woodnutt, Abstr. 21st Int. Congr. Chemother., abstr. 484, J. Antimicrob. Chemother. 44[Suppl.]:A146, 1999). Activity against gram-positive organisms is particularly enhanced, with gemifloxacin displaying levels of activity fourfold higher than that of moxifloxacin against Streptococcus pneumoniae in vitro (5). Gemifloxacin is also highly potent against penicillin-resistant strains of S. pneumoniae (D. Hardy, D. Amsterdam, L. Mandell, and C. Rotstein, Abstr. 21st Int. Congr. Chemother., abstr. 482, J. Antimicrob. Chemother. 44[Suppl.]:A146). In addition, gemifloxacin has high activity against the other major pathogens involved in respiratory tract infections, i.e., Haemophilus influenzae and Moraxella catarrhalis, and the atypical organisms, Legionella pneumophila, Chlamydia spp., and Mycoplasma spp. (A. D. Felmingham, M. Robbins, C. Dencer, H. Salman, I. Mathias, and G. Ridgeway, Abstr. 21st Int. Congr. Chemother., abstr. 408, J. Antimicrob. Chemother. 44[Suppl.]:A131, 1999, and P. Hannan and G. Woodnutt, 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 101, 1998). Gemifloxacin has also been shown to be highly active against many of the bacteria involved in sexually transmitted diseases, such as Neisseria gonorrhoeae, and against organisms isolated from urinary tract infections (1; K. G. Naber, K. Hollauer, D. Kirchbauer, and W. Witte, Abstr. 9th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. 222, Clin. Microbiol. Infect. 5[Suppl. 3]:144, 1999). These data indicate that clinical studies should be conducted to investigate the pharmacokinetics and tolerability of gemifloxacin in humans. This summary represents the initial investigations of gemifloxacin pharmacokinetics and tolerability in humans. Gemifloxacin was administered orally to healthy male volunteers as single doses of 20 to 800 mg in two studies. A preliminary account of the pharmacokinetics from these studies has been presented previously (A. Allen, E. Bygate, M. Teillol-Foo, S. Oliver, M. Johnson, and C. Ward, Abstr. 21st Int. Congr. Chemother., abstr. 440, J. Antimicrob. Chemother. 44[Suppl.]:A137, 1999).
MATERIALS AND METHODS
Subjects.
A total of 19 (13 in study 1; 6 in study 2) healthy adult male volunteers participated in two studies and completed at least one study session. Their ages ranged from 21 to 41 years (mean, 27.6 ± 5.8 years and 29.0 ± 7.6 years for study 1 and 2, respectively), and their average body weights were 71.4 ± 11.1 kg and 75.1 ± 10.4 kg for study 1 and 2, respectively. Before the study, all subjects provided a complete medical history and were subjected to physical examination and a 12-lead resting electrocardiogram (ECG). Blood samples were collected for clinical chemistry and hematology studies. Urine was collected for urinalysis and a drug screen. Subjects were excluded from the study if they had used any prescription drug within 14 days, any over-the-counter drug within 7 days, or any investigational drug within 4 months before participation in the study. Also excluded were subjects who smoked more than the equivalent of five cigarettes a day and subjects with a known drug or alcohol dependence or drug allergy. All subjects fully satisfied the inclusion and exclusion criteria. The study protocol and consent forms were reviewed and approved by the Besselaar Covance Independent Ethics Committee, Leeds, United Kingdom.
Study design.
Two double-blind, randomized, ascending, single-dose, placebo-controlled studies were conducted. In study 1, seven subjects in group 1 received at least one single oral dose of gemifloxacin (in capsule form) and a randomized placebo (i.e., two subjects received three doses and the placebo, three subjects received two doses and the placebo, one subject received one dose and the placebo, and one subject received one dose of gemifloxacin only) and four subjects in group 2 received 160 mg of gemifloxacin only. Treatment periods were separated by 7-day washouts. Subjects received single oral doses of 20 mg (n = 2), 40 mg (n = 4), 80 mg (n = 4), 160 mg (n = 4), or 320 mg (n = 4) of gemifloxacin or placebo (n = 8) in a fasting state. In study 2, six subjects received single oral doses of gemifloxacin at doses of 600 mg (n = 6; two of these subjects received a second dose of 600 mg on another occasion) and 800 mg (n = 4) and a placebo, administered in an escalating manner in the fasting state, with a washout period of at least 7 days. Gemifloxacin or placebo was administered with 240 ml of water after an overnight fast. Subjects were required to avoid lying down until 2 h after receiving the dose and eating or drinking beverages other than water until 4 h after dosing, after which a standard meal was served. Subjects abstained from smoking, alcohol, and caffeine-containing foods and beverages until 48 h after each dose and from sunbathing or using sun beds or sun lamps from 7 days prior to the last dose until after the final assessment (within 1 week of the last dose).
Safety.
All subjects were under close, continuous observation in the study unit throughout each 48-h investigation period. Any adverse events and remedial action were promptly recorded, together with the project physician's opinion of the event's relationship to drug administration. In addition to monitoring of adverse experience, safety assessments taken throughout the study day included vital signs, 12-lead ECG recordings, hematology, clinical chemistry, and urinalysis. In order to assess the potential for gemifloxacin to cause crystalluria in humans, urine samples were examined microscopically during the studies.
Sampling.
Blood samples (10 ml each) were taken by venipuncture of the antecubital veins (or via an indwelling catheter), before dosing and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 18, 24, 36, and 48 h after dosing. The blood samples were allowed to clot at room temperature and in the dark for at least 30 min prior to centrifugation for 15 min at 2,000 × g and at 4°C. Separated serum was frozen at approximately −20°C. The urine samples were collected 2 to 0 h prior to dosing and at 0 to 2, 2 to 4, 4 to 6, 6 to 8, 8 to 12, 12 to 24, 24 to 36, and 36 to 48 h after dosing for 20-, 80-, 160-, and 320-mg gemifloxacin doses (study 1). For study 2, urine samples were collected 2 to 0 h prior to dosing and at 0 to 6, 6 to 12, 12 to 24, and 24 to 48 h after dosing for 600- and 800-mg gemifloxacin doses. A 10-ml aliquot of each sample was transferred into a polystyrene tube and immediately frozen at approximately −20°C. Gemifloxacin has been shown to be stable for up to 6 months in serum and urine under these storage conditions (SmithKline Beecham, unpublished data).
High-performance liquid chromatography assay.
The concentrations of gemifloxacin in serum and urine were determined by a reverse-phase high-performance liquid chromatography method with fluorescence detection (SmithKline Beecham, unpublished data). Following liquid-liquid extraction into chloroform and back extraction into phosphate buffer, chromatographic separation was carried out using a PLRP-S column (Polymer Laboratories, Church Stretton, Shropshire, United Kingdom) and a trifluoroacetic acid-acetonitrile mobile phase. Gemifloxacin and the internal standard (a chemical analog of gemifloxacin) were detected by fluorescence with excitation and emission wavelengths of 337 and 460 nm, respectively. The calibration curve of the serum method was linear over a concentration range in serum of 0.01 to 5 μg/ml (correlation coefficient, 0.99085). Intra- and interassay coefficients of variation over a range of 0.02 to 5 μg/ml were less than 3 and 12%, respectively. At 0.01 μg/ml, the limit of quantitation, intra- and interassay coefficients of variation were less than 3 and 20%, respectively. The calibration curve of the urine method was linear over a concentration range in urine of 1 to 100 μg/ml (correlation coefficient, 0.98132), and the intra- and interassay coefficients of variation over this range were less than 11 and 14%, respectively. The lower limit of quantification for gemifloxacin was 0.01 μg/ml in serum and 1.0 μg/ml in urine with a 1-ml aliquot.
Pharmacokinetic analysis.
Concentration-time data of gemifloxacin in serum were analyzed by noncompartmental methods (3) using an in-house program written and validated for SAS software, version 6.12. The apparent terminal elimination rate constant (λz) was derived from the log linear disposition phase of the concentration-time curve using linear least-squares regression with visual inspection of the data to determine the appropriate number of terminal points needed to calculate λz. The corresponding terminal phase elimination half-life (t1/2) was calculated as ln(2)/λz. The area under the concentration-time curve in serum from time zero to the last quantifiable concentration (serum AUC0–t) was determined using the linear trapezoidal rule for each incremental trapezoid and the log trapezoidal rule for each trapezoid postmaximal concentration of the drug in serum (Cmax). The area under the concentration-time curve in serum extrapolated to infinity (serum AUC0–∞) was calculated as the sum of AUC0–t and C(t)/λz, where C(t) was the predicted concentration C from the log linear regression analysis at the last quantifiable time point and λz was the elimination rate constant. The first occurrence of Cmax and the time to Cmax were obtained directly from the serum concentration-time data. Apparent volume of distribution (V) was calculated as the ratio of the apparent clearance of gemifloxacin in serum (dose/AUC) to the elimination rate constant. Renal clearance was calculated as the ratio of the amount of gemifloxacin excreted in the urine to the serum AUC.
RESULTS
Pharmacokinetics of gemifloxacin.
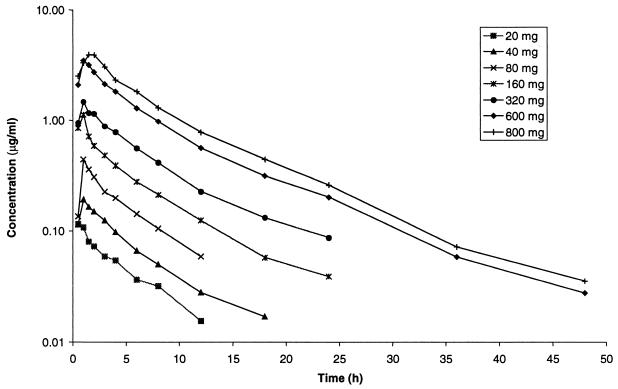
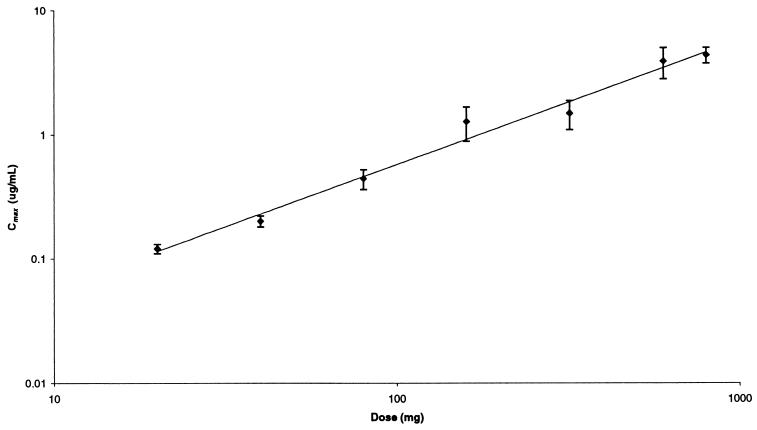
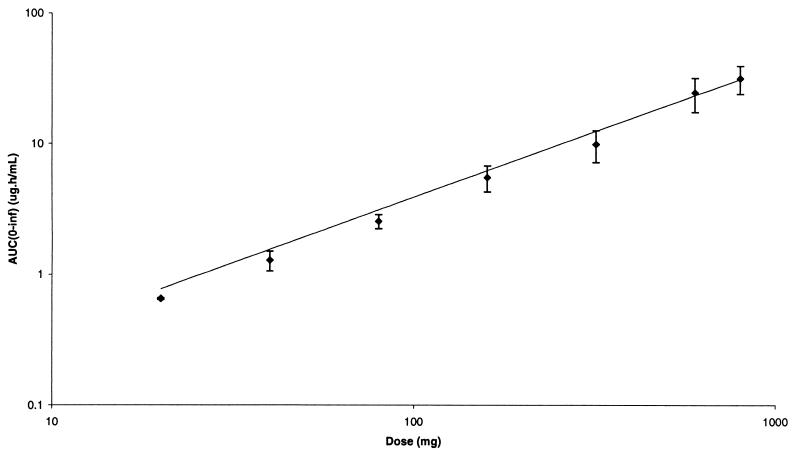
Serum gemifloxacin concentrations increased in a dose-dependent fashion following oral administration to healthy male volunteers in the dose range 20 to 800 mg. Gemifloxacin was rapidly absorbed after all doses, with Cmax achieved approximately 1 h after dosing, after which concentrations in serum declined in a biexponential manner (Fig. 1). The pharmacokinetic parameters for gemifloxacin in healthy male subjects following a single oral dose are given in Table 1. The mean Cmax values increased with increasing dose (Table 1; Fig. 2), and mean ± standard deviation (SD) values were 0.12 ± 0.01, 0.20 ± 0.02, 0.44 ± 0.08, 1.27 ± 0.39, 1.48 ± 0.39, 3.86 ± 1.09, and 4.33 ± 0.63 μg/ml following 20-, 40-, 80-, 160-, 320-, 600-, and 800-mg doses, respectively. The mean AUC0–∞ also increased with increasing dose (Fig. 3), and values (mean ± SD) were 0.65 ± 0.01, 1.28 ± 0.22, 2.54 ± 0.31, 5.48 ± 1.24, 9.82 ± 2.70, 24.4 ± 7.1, and 31.4 ± 7.6 μg · h/ml following 20-, 40-, 80-, 160-, 320-, 600-, and 800-mg doses, respectively. The t1/2 was independent of dose, with an overall mean of 7.4 ± 2.0 h. Volume of distribution values were independent of dose, with an overall mean of 4.2 ± 0.8 liters/kg, and exceeded total body water, indicating substantial distribution into tissues.
FIG. 1.
Mean concentrations of gemifloxacin in serum following single oral administration in healthy male volunteers.
TABLE 1.
Pharmacokinetic parameters for gemifloxacin following administration of single oral doses to healthy male volunteersa
| Dose (mg)b | Cmax (μg/ml) | Tmaxc (h) | AUC0–∞ (μg · h/ml) | t1/2 (h) | V (liters/kg) | Aed (%) | Renal clearance (ml/min) |
|---|---|---|---|---|---|---|---|
| 20 (2) | 0.12 (0.01) | 0.5 (0.5–0.5) | 0.65 (0.01) | 4.75 (0.07) | 3.24 (0.26) | 31.7 (0.85) | 180 (3.1) |
| 40 (4) | 0.20 (0.02) | 1.0 (1.0–1.5) | 1.28 (0.22) | 7.58 (2.99) | 4.14 (0.64) | 25.9 (6.5) | 143 (28.2) |
| 80 (4) | 0.44 (0.08) | 1.0 (1.0–1.0) | 2.54e (0.31) | 5.88e (0.16) | 3.39 (0.40) | 25.9 (3.2) | 141 (7.1) |
| 160 (4) | 1.27 (0.39) | 1.0 (0.5–1.0) | 5.48 (1.24) | 8.77 (3.20) | 5.49 (1.81) | 28.8 (3.9) | 147 (19.5) |
| 320 (4) | 1.48 (0.39) | 1.0 (1.0–1.0) | 9.82 (2.70) | 6.65 (1.25) | 4.97 (0.96) | 27.5 (6.4) | 151 (8.2) |
| 600 (8) | 3.86 (1.09) | 1.0 (1.0–2.0) | 24.4 (7.1) | 8.33 (0.84) | 4.09 (0.87) | 32.0 (7.2) | 141 (43.1) |
| 800 (4) | 4.33 (0.63) | 1.8 (1.0–2.0) | 31.4 (7.6) | 8.02 (0.73) | 4.23 (0.76) | 39.4 (7.9) | 175 (39.1) |
Values are means (standard deviations in parentheses), except as noted.
Values in parentheses are the number of subjects receiving that dose.
Median and range of the time, T, to Cmax.
Amount excreted unchanged in urine.
n = 3.
FIG. 2.
Mean ± SD Cmax versus dose of gemifloxacin.
FIG. 3.
Mean ± SD serum AUC0–∞ versus dose of gemifloxacin.
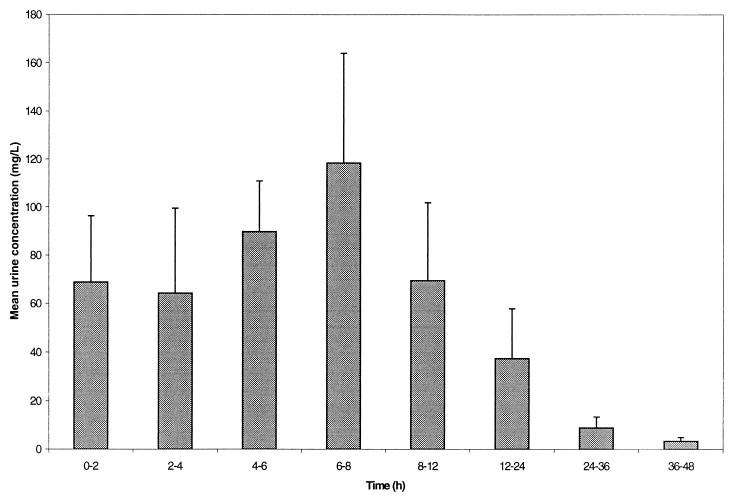
On average, approximately 25 to 40% of the administered gemifloxacin doses were excreted unchanged in the urine over 48 h, the vast majority after the first 12 h (Fig. 4). Renal clearance was independent of dose (ca. 150 ml/min) and was somewhat in excess of the glomerular filtration rate (120 ml/min), suggestive of active renal secretion.
FIG. 4.
Mean ± SD concentrations of gemifloxacin in urine in healthy male volunteers following single oral administration of 320-mg dose.
Tolerability.
All doses of gemifloxacin were very well tolerated over the single oral dose range of 20 to 800 mg. No deaths, serious adverse events, or withdrawals due to adverse events were reported. There was a low incidence of adverse events in the placebo- and gemifloxacin-treatment groups. There was no evidence of a drug- or dose-related increase in the incidence or severity of adverse events during the study. The most frequently reported adverse experiences were headache and rhinitis. The majority of adverse events were mild in severity and resolved without treatment. Coughing, influenza-like symptoms, dry mouth, myalgia, abrasions, anorexia, and maculopapular rash were each reported only once during the studies and were all considered unrelated or unlikely to be related to study medication. There were no clinically relevant changes in vital signs or results of laboratory investigations at the doses investigated. Drug-related crystals were observed in the urine of three volunteers receiving a 600-mg dose and of three volunteers receiving a 800-mg dose. Following refinement of the microscopic examination technique, an attempt was made to determine whether crystal formation was occurring in vivo or as the urine samples cooled ex vivo. The crystals seen in both studies were considered to be the result of ex vivo crystal formation as the samples cooled. There were no clinical signs or symptoms of renal damage in any of the subjects.
DISCUSSION
Gemifloxacin was rapidly absorbed, and Cmax increased linearly with dose. Mean AUC0–∞ values did not deviate notably from linearity over the range of doses studied (20 to 800 mg). However, it should be noted that this overview summarizes data from two studies, neither of which had a crossover design, and thus pharmacokinetic parameters were not strictly comparable between the different dosing groups. Following once-daily dosing at doses in the range studied, accumulation would be predicted to be minimal, while trough concentrations in serum would still be at measurable levels. The t1/2 of, on average, 7 h, the relatively high concentrations in serum observed over 24 h compared with MIC values for the relevant pathogens (AUC:MIC ratio) and the good activity against gram-positive bacteria suggests that gemifloxacin administered once daily is likely to be useful for the treatment of respiratory tract infections. At the lowest dose studied, 20 mg, the t1/2 estimate was shorter than for the other doses, but this was probably due to the assay not being sufficiently sensitive to follow the serum profile for long enough at this dose. Concentrations in urine (mean range, 30 to 120 μg/ml) of gemifloxacin over 0 to 24 h (Fig. 4) for the 320-mg dose are sufficient to kill most gram-negative bacteria responsible for urinary tract infections (Naber et al., 9th Eur. Congr.). This observation suggests that gemifloxacin administered once daily is also likely to be useful in the treatment of a wide range of urinary tract infections.
Whilst most fluoroquinolones at a dose of 400 mg produce an AUC of 30 to 40 μg · h/ml, it should be noted that the serum gemifloxacin AUC for a 400-mg dose would be approximately 15 μg · h/ml. Since bioavailability of gemifloxacin is approximately 70% (SmithKline Beecham, unpublished data), this lower AUC of gemifloxacin in serum is likely due to the high volume of distribution, suggestive of extensive distribution into tissues.
Following oral administration of 20- to 800-mg single doses, there were no clinically significant changes in clinical chemistry (including liver function tests), hematology or urinalysis parameters, vital signs, or 12-lead ECG readings in subjects, irrespective of dose. Elevations of liver function test parameters are reported with many fluoroquinolones; however, there were no clinically significant changes in liver function test results during either single-dose study. Similarly, older quinolones have been associated with dermatological reactions, such as skin rashes; however, during both studies there was no occurrence of an erythematous or macropapular rash of likely, suspected, or probable relationship to the study medication. In these limited numbers of healthy male subjects, all doses of gemifloxacin were very well tolerated.
In conclusion, the results of these studies, combined with the antibacterial spectrum and potency of gemifloxacin (1, 2, 5, 6; Felmingham et al., 21st Int. Congr. Chemother.), support the further investigation of once-daily administration of this antimicrobial for indications such as respiratory tract and urinary tract infections.
REFERENCES
- 1.Cormican M G, Jones R N. Antimicrobial activity and spectrum of LB20304, a novel fluoronaphthyridone. Antimicrob Agents Chemother. 1997;41:204–211. doi: 10.1128/aac.41.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erwin M E, Jones R N. Studies to establish quality control ranges for SB-265805 (LB20304) when using National Committee for Laboratory Standards antimicrobial susceptibility test methods. Quality Control Study Group. J Clin Microbiol. 1999;37:279–280. doi: 10.1128/jcm.37.1.279-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. pp. 45–111. [Google Scholar]
- 4.Hohl A F, Frei R, Pünter V, von Graevenitz A, Knapp C, Washington J, Johnson D, Jones R N. International multicenter investigation of LB20304, a new fluoronaphthyridone. Clin Microbiol Infect. 1998;4:280–284. doi: 10.1111/j.1469-0691.1998.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson D M, Jones R N, Erwin M E. Anti-streptococcal activity of SB-265805 (LB20304) a novel fluoronaphthyridone, compared with five other compounds, including quality control guidelines. Diagn Microbiol Infect Dis. 1999;33:87–91. doi: 10.1016/s0732-8893(98)00104-7. [DOI] [PubMed] [Google Scholar]
- 6.Oh J-I, Paek K-S, Ahn M-J, Kim M-Y, Hong C Y, Kim I-C, Kwak J-H. In vitro and in vivo evaluations of LB20304, a new fluoronaphthyridone. Antimicrob Agents Chemother. 1996;40:1564–1568. doi: 10.1128/aac.40.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]