Abstract
Pioglitazone, an agonist at peroxisome proliferator-activated receptor gamma, is FDA-approved for the treatment of insulin resistance in type 2 diabetes. Numerous studies in male rodents suggest that pioglitazone inhibits inflammatory and neuropathic pain, but few included female subjects. To address this gap, we compared the effects of pioglitazone in both sexes in the intraplantar methylglyoxal model (MG) model of chemical pain and painful diabetic neuropathy (PDN), the plantar incision model (PIM) of postoperative pain, the spared nerve injury (SNI) model of traumatic nerve injury, and the ZDF rat and db/db mouse models of PDN. We administered pioglitazone by one-time intrathecal or intraperitoneal injection or by adding it to chow for 6 weeks, followed by measurement of hypersensitivity to non-noxious mechanical, noxious mechanical, heat, and/or cold stimuli. In all mouse models, injection of pioglitazone decreased pain-like behaviors with greater potency and/or efficacy in females as compared to males: heat and mechanical hypersensitivity in the MG model (0.1–10 mg/kg); mechanical hypersensitivity in the PIM model (10 μg); mechanical and cold hypersensitivity in the SNI model (100 mg/kg); and heat hypersensitivity in the db/db model (100 mg/kg). Furthermore, co-administration of low doses of morphine (1 mg/kg) and pioglitazone (10 mg/kg) decreased SNI-induced mechanical and cold hypersensitivity in female but not male mice. In the ZDF rat, pioglitazone (100 mg/kg) decreased heat and mechanical hypersensitivity with no sex difference. In the db/db model, pioglitazone had no effect when given into chow for 6 weeks at 0.3, 3 or 30 mg/kg doses. We conclude that females exhibit greater anti-hyperalgesic responses to pioglitazone in mouse models of chemical-induced nociception, postsurgical pain, neuropathic pain, and PDN. These findings set the stage for clinical trials to determine whether pioglitazone has analgesic properties across a broad spectrum of chronic pain conditions, particularly in women.
Keywords: Pioglitazone, Morphine, PPAR gamma, Methylglyoxal, sex-difference, postsurgical pain, neuropathic pain, painful diabetic neuropathy
Graphical Abstract
1. BACKGROUND
Pioglitazone is approved by the U.S. Food and Drug Administration for the treatment of insulin resistance in type 2 diabetes. Upon binding to peroxisome proliferator-activated receptors gamma (PPARγ), a member of the nuclear receptor superfamily, pioglitazone stimulates glucose uptake into muscle cells, decreases adipocyte hypertrophy, and sensitizes insulin receptors to enhance insulin signaling (Takada and Genda, 2019). Furthermore, a steadily growing body of preclinical evidence indicates that PPARγ agonists such as pioglitazone exert therapeutic actions in numerous neural disorders. For example, numerous studies indicate that pioglitazone decreases behavioral signs of hyperalgesia in animal models of inflammatory and peripheral neuropathic pain (de Guglielmo et al., 2014; Griggs et al., 2016; Griggs et al., 2015; Morgenweck et al., 2013; Okine et al., 2018; Zhong et al., 2019). For example, pioglitazone attenuates behavioral signs of neuropathic pain associated with peripheral nerve injury (Griggs et al., 2015) or chemotherapy (Khasabova et al., 2019). However, an overwhelming majority of these studies have been restricted to males.
Biological sex is an important risk factor for chronic pain. For example, the prevalence of chronic post-surgical pain (Pereira and Pogatzki-Zahn, 2015), neuropathic pain (Bouhassira et al., 2008), and painful diabetic neuropathy (Abraham et al., 2018; Raputova et al., 2017) is higher in women. Biological sex is also an important determinant of the analgesic efficacy of opioid analgesic drugs such as morphine (Abraham et al., 2018; Raputova et al., 2017). With regards to sex differences in the analgesic effects of pioglitazone, the available literature is limited, incomplete, and inconclusive. (Grace et al., 2021). Two studies in mouse models of peripheral nerve injury (Sorge et al., 2015) and spinal cord injury (Gensel et al., 2019) reported that pioglitazone reduced pain-like behavior in females but not males; however, these studies were limited to a single dose and a single route of administration. No studies have evaluated the interaction of sex and pioglitazone analgesia in models of acute chemical pain or inflammatory pain. To address these gaps, we administered pioglitazone to males and females in the plantar incision model (PIM) of postsurgical pain, the spared nerve injury (SNI) model of traumatic nerve injury, the db/db mouse and ZDF rat models of painful diabetic neuropathy (PDN), and the intraplantar methylglyoxal (MG) model of chemical pain and PDN. Our results indicate that pioglitazone exerts a greater anti-nociceptive effect in female rodents in multiple pre-clinical models of pain, promoting pioglitazone as a potential pharmacological strategy for a broad spectrum of pain conditions, particularly in women.
2. METHODS
2.1. Animals
All experiments were carried out in accordance with guidelines from the International Association for the Study of Pain (Zimmermann, 1983) and with the approval of the Institutional Animal Care and Use Committees of the University of Pittsburgh and the University of Kentucky. Rodents were housed in a temperature- and humidity-controlled room with food and water available ad libitum. Mice were on a 14-hour light–10-hour dark cycle with lights on from 6:00 AM to 8:00 PM, while rats were on a 12:12 cycle from 7:00 am to 7:00 pm. Mice and rats were housed at a maximum five and two per cage, respectively. We restricted behavioral studies to the hours of 8:00 am – 6:00 pm to avoid steep changes in physiological rhythms surrounding lights-on or lights-off transitions. We acknowledge that hormonal changes during the estrous cycle can influence pain in women. However, we used females without consideration of their stage in the estrous cycle, in accordance with the consensus report of the Sex, Gender and Pain Special Interest Group of the International Association for the Study of Pain, which states that “The value of testing female rodents at different stages of the estrous cycle is debatable”, in part because “an influence of estrous cycle stage is not necessarily indicated by larger observed variance” and because “measurement of estrous cycle would require daily handling, and the ensuing stress could affect nociception or sensitivity to drugs” (Greenspan et al., 2007).
C57Bl/6 mice.
Mice ordered from Charles Rivers Laboratories (Wilmington, MA) at 8–12 weeks were used to evaluate any effects of pioglitazone in the MG, PIM and SNI models.
ZDF Rats.
ZDF rats (Strain #370; Obese) are homozygous for the loss-of-function “fatty” mutation in the leptin receptor (fa/fa) that results in the development of type 2 diabetes, as indicated by hyperglycemia, hyperlipidemia, impaired glucose tolerance, and insulin insensitivity. ZL rats (Strain #371; Lean fa/+) are the heterozygous (fa/+) genetic controls for ZDF (Clark et al., 1983). ZDF and ZL rats (Charles Rivers Laboratories) arrived at seven weeks of age and housed as same-sex littermates. Full development of the diabetes phenotype requires specific diets for male and female ZDF and ZL: males received the Formulab 5008 diet (TestDiets; Purina Mills, Richmond, IN, USA) containing 23% protein, 6.5% fat, 58.5% carbohydrates, 4% fiber and 8% vitamins and minerals; females received the D12468 diet (Research Diets, Inc; New Brunswick, NJ, USA) containing 12% protein, 25.5% fat, 51% carbohydrates, 5.93% fiber and 5.57% vitamins and minerals.
Db/db mice.
Diabetic db/db (BKS.Cg-Dock7m+/+Leprdb/J) mice are homozygous for the diabetes spontaneous mutation (Leprdb) and manifest morbid obesity, chronic hyperglycemia, pancreatic beta cell atrophy, and hypoinsulinemia. BKS Dock7m +/+ Leprdb, also referred as C57BLKS/J (BKS), are heterozygous (db/+), do not develop a diabetic phenotype, and are genetic controls for db/db mice (Coleman, 1978). Diabetic db/db mice were purchased from Jackson Laboratories (Bar Harbor, ME) for the studies conducted at University of Kentucky. Mice for the experiments conducted at University of Pittsburgh were bred in-house. To this end, we interbred the heterozygous mice (db/+) with C57BL/6 background purchased from Jackson Laboratories (Bar Harbor, ME) to generate +/+, db/+ and db/db mice as genotyped as previously described (Peng et al., 2018). Mice were housed with a maximum of 4 same-sex littermates per cage. No specific diet is required for db/db mice to develop type 2 diabetes.
2.2. Glucose Testing
We lightly restrained the animals in a towel and wiped the tail clean with an alcohol wipe. Using a #11 scalpel blade, we made a small nick in the side of the tip of the tail. For the non-fasting glucose test, we placed a single drop of blood on a standard glucose monitor strip, which we then inserted into the monitor (Truetrack, Walgreens). For the hemoglobin A1c measurement, drops of blood were loaded into a HbA1c cartridge and analyzed using a DCA Vantage Analyzer (Siemens, Munich, Germany).
2.3. Surgery
Surgical models of postsurgical and neuropathic pain we conducted under isoflurane anesthesia (5% induction followed by 1.5%–2.0% maintenance using a nose cone). After suturing of the skin, triple antibiotic ointment (Neosporin, Johnson and Johnson) was applied to the surgical area.
2.3.1. Plantar Incision
Post-operative hyperalgesia was induced by longitudinal incision of the plantaris muscle as previously described (Jang et al., 2011; Pogatzki and Raja, 2003). Following antisepsis of the left hind paw with Chlorascrub® then alcohol, a #11 scalpel blade was used to make a 5mm incision through the skin and fascia, beginning 2mm from the proximal edge of the heel, and extending towards the digits. The underlying muscle was raised with a curved forceps, extended 4 mm and then incised longitudinally with a #11 scalpel blade, leaving the origin and insertion of the muscle intact. The overlying skin was closed with synthetic 5–0 sutures (PDS*II, Ethicon). Surgery was typically completed within 5–10min.
2.3.2. Spared Nerve Injury (SNI) surgery
As previously described (Decosterd and Woolf, 2000; Fu et al., 2020), an incision of the inguinal skin was followed by retraction of muscles to expose the common peroneal, tibial, and sural branches of the left sciatic nerve. With care taken not to disturb the sural nerve, the common peroneal and tibial nerves were ligated with a 6–0 silk suture (Ethicon, Somerville, NJ) and then transected 1mm proximal and 1mm distal to the ligation. The muscle and skin were closed with loosely tied 5–0 absorbable sutures (Ethicon, Somerville, NJ) and 9-mm stainless steel wound clips, respectively. Clips were removed on postsurgical day (POD) 13, and animals were tested on POD 14. All animals developed robust mechanical and cold hypersensitivity without adverse effects. Substantial doses of pioglitazone do not change mechanical thresholds or cold response duration in rats (Griggs et al., 2015) or mice (Maeda et al., 2008) with sham nerve injury surgery, and therefore we did not include sham groups here.
2.4. Drugs
Pioglitazone potassium salt (10028, Cayman Chemical, Ann Arbor, MI) was dissolved in either 0.9% saline for intraperitoneal administration, or in a mixture of 10% ETOH, 10% castor oil and 80% saline for intrathecal administration. Actos® tablets (25% pioglitazone hydrochloride; Pharmaceutical Takeda., Inc., Deerfield, IL, USA) were incorporated into mouse chow by TestDiets (Purina Mills, Richmond, IN, USA) and administered for 6 weeks at doses (0.3, 3 and 30 mg/kg/day) that we described previously (Griggs et al., 2016). Body weight and food consumption, measured by subtracting the amount of food left in the cage from that provided the previous day, were monitored weekly. The concentration of Actos® was calculated according to expected average daily body weight and food consumption over the course of the study. The control group received the same diet but without pioglitazone. “For single injection studies, pioglitazone or vehicle was administered in unanesthetized mice either by the intraperitoneal route (0.1–100 mg/kg in a volume of 10 ml/kg as we described (Griggs et al., 2015; Morgenweck et al., 2013), or by the intrathecal route using a 30-gauge needle at the maximum soluble dose (10 μg in 5μL of 10% DMSO, 10% castor oil, and 80% saline). To account for the rapid actions of pioglitazone (Griggs et al., 2015) and its 2.6 hour half-life (Maeshiba et al., 1997), we evaluated behavioral measures of mechanical and thermal sensitivity as early as 15 minutes and as late as 120 minutes. Morphine hydrochloride (Sigma, USA) was administered at a submaximal dose by the intraperitoneal route at 1 mg/kg as described by Zhao et al (Zhao et al. 2004).”
Methylglyoxal (MG; M0252, Sigma-Aldrich, St. Louis, MO) was diluted in 0.9% sterile saline. In unanesthetized mice using mild restraint, 30 μg MG was injected in a volume of 10μL under the skin of the plantar hindpaw using a 30-gauge needle as described previously (Griggs 2017).
2.5. Experiment Design and Timelines
The timelines of each experiment are illustrated with Panel A of each figure. Presurgical and/or pre-injection baselines are denoted as time =0. For the methyglyoxal studies, baseline mechanical and thermal baseline responses were immediately followed by an intraperitoneal injection of pioglitazone or vehicle. 30 min later, methylglyoxal was injected at t=0 followed by behavioral measurements at 15, 30, 45, 60 and 120 minutes in a repeated measures design. For the PIM and SNI studies, behavioral measurements at peak of hypersensitivity (Day 2 or Day 14 post-surgery, respectively), were following by drug injection and then repeated behavioral measurements. Other than the morphine plus pioglitazone study, we targeted N=6–10 a priori based on our previous publications using these pain models, behavioral assays, and experimental designs. The low variability observed in our SNI pioglitazone study justified the design of an experiment with smaller group sizes for the SNI morphine plus pioglitazone study. While keeping the experimenter blind to sex, pain condition and drug treatment, another experimenter randomly assigned animals to groups with the caveat that this was done in a balanced fashion to avoid significant differences in baseline thresholds. Gross obesity in db/db mice and ZDF rats prevented blinding by strain in the painful diabetic neuropathy studies.
2.6. Behavioral Testing
Before commencement of each von Frey and acetone testing session, we acclimated the animals within individual Plexiglas boxes placed on the top of a stainless-steel mesh platform for 45 min. Before commencement of the first hotplate or coldplate session, we acclimated the animals to the apparatus over 3 days. On the first day, they spent 5 minutes on the hotplate or coldplate in the OFF position. On the two consecutive days, we performed three trials with the devices turned to the ON position with an inter-trial interval of at least 10 min. Animals were removed immediately after a withdrawal response.
2.6.1. Mechanical sensitivity
Mouse:
Sensitivity to a non-noxious stimulus was tested with an incremental series of 8 von Frey monofilaments of logarithmic stiffness (Stoelting, Wood Dale, IL), ranging in gram force from 0.008g to 6g. The location of stimulation at the plantar hindpaw varied with the model: the central region in the MG model; lateral to the suture line in the plantar incision model; and at the sural receptive field (lateral) in the spared nerve injury model. Filaments were applied to the skin with slightly bending for a maximum of 5 seconds. A clear withdrawal of the paw away from the stimulus was recorded as a positive response. The 50% withdrawal threshold was determined using the up-down method of Dixon (1965), modified by Chaplan (Chaplan et al., 1994).
Rat:
Sensitivity to a noxious mechanical stimulus was tested in the ZDF model with an electronic force transducer (IITC Life Science Inc, Woodland Hills, CA) as previously described (Griggs et al., 2016). The force exerted on the surface of the hindpaw was gradually increased, and the gram force required to elicit a withdraw response was recorded. The force until paw withdraw (paw pressure threshold) was measured three times for each hindpaw and then averaged.
2.6.2. Cold sensitivity
2.6.2.1. Acetone test.
To test for cold sensitivity in the SNI model, a 3-mL syringe attached to an 8-cm length of PE-10 tubing flared to a diameter of 3.5 mm at the distal end was used to apply a drop of acetone (surface tension maintained the volume of the drop to 10–12 μL) to the sural receptive field (Griggs et al., 2015). The duration of time spent licking or flicking the ipsilateral hindpaw was recorded over a 1 min period. Three trials, with an inter-trial interval of at least 5 min, were averaged for each time point.
2.6.2.2. Cold plate test.
Cold sensitivity was assessed by placing the animal on a cooled surface (4°C ± 0.5°C) within an acrylic enclosure (Coldplate; IITC Life Science Inc., Woodland Hills, CA) for five minutes. The combined number of nociceptive responses (e.g. jumping, licking, flicking) for both hindpaws during the 5 min period is reported, as previously described (Griggs et al., 2015)
2.6.3. Heat sensitivity
Heat hypersensitivity was tested on a heated surface (52.5 °C) within an acrylic enclosure (Hotplate; Columbus Instruments, Columbus, OH). The latency to hindpaw withdraw response (jumping, hindpaw licking, or hindpaw flinching) was recorded as previously described (Griggs et al., 2019). Three trials, with an inter-trial interval of at least 10 min, were averaged for each time point. The rodents were immediately removed after paw withdraw or a cutoff of 25 s to avoid tissue injury.
2.7. Statistical Analysis
Data were graphed and analyzed using Prism software (version 8.4.0, GraphPad, La Jolla, CA). We conducted experimental designs that balanced multiple factors: Sex, Strain, Drug, and Time as a repeated measure. To analyze these results, we conducted analysis of variance (ANOVA). For each study, we tested post-drug injection data for normal distribution using the Shapiro-Wilkes test and for homogeneity of variance using Levene’s test. In all cases, p>0.05, thus supporting the null hypotheses of normal distribution and equal variance across groups. To analyze studies involving Sex, Drug and/or Time, we used 2-way ANOVA with Time integrated across timepoints using the trapezoidal method for calculation of area under the curve (AUC). Time was also treated as a repeated measure and if a main effect was found, these were followed by Sidak multiple comparisons test at each timepoint. To analyze studies involving Strain, Sex, Drug and/or Time, we began with 3-way ANOVA to evaluate interactions between Time, Sex and Strain or Time, Strain, and Drug, with Time integrated across timepoints using AUC. If a main effect was found, these were followed by 2-way ANOVA. Due to the large number of possible values using the up-down method, von Frey data were analyzed with parametric statistics (Scheff et al., 2002). Statistical significance was set at p<0.05. Percentage of maximum possible effect (MPE) in the methylglyoxal study was calculated as: (post-drug threshold – post-vehicle threshold) / (baseline threshold – post-drug threshold) *100. All data are presented as mean ± SEM..
3. RESULTS
3.1. Pioglitazone inhibits chemical nociception with greater potency in female as compared to male mice
PDN is associated with increased levels of glucose-derived dicarbonyl metabolites such as MG (Becker et al., 2020; Bierhaus et al., 2012). In males, MG is both necessary and sufficient to the pain of PDN: methylglyoxal scavengers reduce behavioral signs of nociception in db/db mice, and either intraplantar or intrathecal administration of methylglyoxal induces nociception (Bierhaus et al., 2012; Griggs et al., 2019). Here we tested the hypothesis that pioglitazone would inhibit mechanical and heat hypersensitivity in the intraplantar methylglyoxal model of chemical pain. Figure 1 illustrates that MG induced a robust increase in both mechanical (Fig 1A–D) and heat (Fig 1E–H) sensitivity that lasted throughout the 120 min period of testing.
Figure 1. Pioglitazone inhibits chemical pain with greater potency in female as compared to male mice.
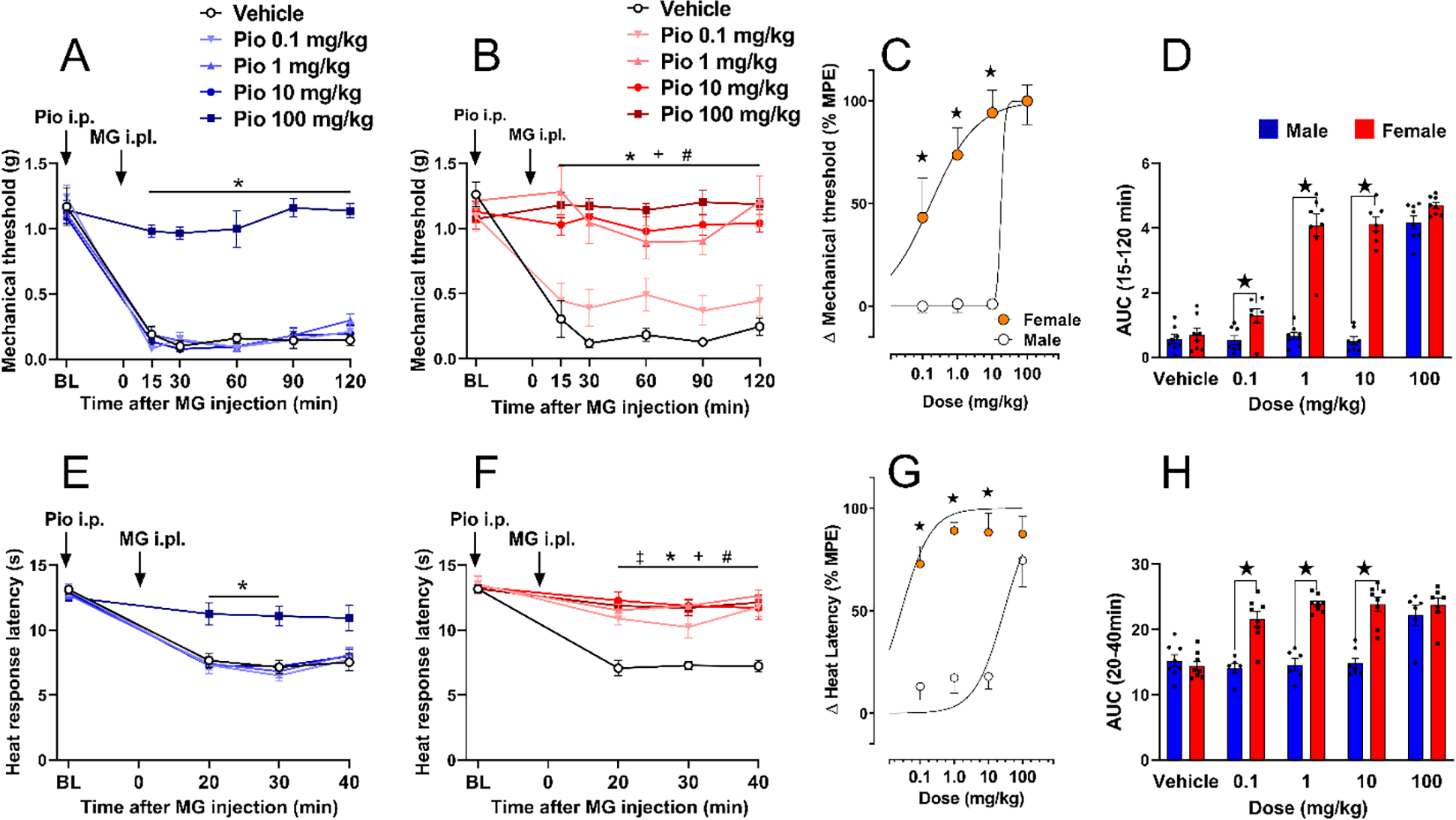
(A) Only the 100 mg/kg dose of pioglitazone prevented mechanical hypersensitivity in male mice. (B) 1–100 mg/kg pioglitazone decreased mechanical hypersensitivity in female mice. (C) Dose-response curve indicating that pioglitazone decreased MG-induced mechanical hyperalgesia in a dose-dependent manner with a greater effect in females (ED50: 0.17 mg/kg) as compared to males (ED50: 17.85 mg/kg). (D) Analysis of the data with Time collapsed across post-injection timepoints illustrates that pioglitazone exerted greater decreases in mechanical hypersensitivity in females as compared to males at 0.1, 1, and 10 mg/kg. (E) Only the 100 mg/kg dose of pioglitazone prevented heat hypersensitivity in male mice. (F) Pioglitazone doses of 1, 10, and 100 mg/kg decreased heat hypersensitivity in female mice. (G) Dose-response curve showing that pioglitazone decreased MG-induced heat hyperalgesia in a dose-dependent manner with a greater effect in females (ED50: 0.028 mg/kg) as compared to males (ED50: 28.8 mg/kg). (H) Analysis of the data with Time collapsed across post-injection timepoints illustrates that pioglitazone exerted greater decreases in mechanical hypersensitivity in females as compared to males at 0.1, 1, and 10 mg/kg. Two-way ANOVA was performed followed by Sidak’s multiple comparisons test for figures 1B–C, 1E–G and 1I. Non-linear regression analysis log(agonist) vs. normalized response was performed for figures 1D and 1H. %MPE = maximum possible effect. Symbol * P<0.05 Pio 100mg vs vehicleicle; + P<0.05 Pio 10mg vs vehicleicle; # P<0.05 Pio 1mg vs vehicleicle; ‡ P<0.05 Pio 0.1mg vs vehicleicle. ★ P<0.05 male vs female. Values represent mean ± SEM. N=6–8.
Mechanical hypersensitivity.
Pioglitazone reduced mechanical hypersensitivity following MG administration in both male and female mice, with a much stronger effect in females. In males, only the highest dose of pioglitazone (100 mg/kg, i.p.) prevented mechanical hypersensitivity from 15 to 120 minutes after intraplantar MG [Fig 1A; Dose × Time F (20, 175) = 8.0, P< 0.0001; Dose F (4, 35) = 107, P<0.0001, Table 1 lines #1–3]. In females, pioglitazone dose-dependently reduced mechanical hypersensitivity from 15 to 120 minutes after intraplantar MG [Fig 1B; Dose × Time F (20, 170) = 5.054 P< 0.0001; Dose F (4, 34) = 30.99 P<0.0001, Table 1 lines #4–6]. As shown in Figs 1D–E, pioglitazone was 100-fold more potent in reducing MG-induced mechanical hypersensitivity in females (ED50=0.17 mg/kg) than in males (ED50=17.85 mg/kg).
Table 1.
Two-Way ANOVA
| Two-way ANOVA | ||||||
|---|---|---|---|---|---|---|
| Figure | Sex | Assay | Line # | Factors | F | P value |
| 1 | Time × Dose | (20, 175) = 8.035 | <0.0001 | |||
| 1A | Male | vF | 2 | Time | (3, 111) = 153.0 | <0.0001 |
| 3 | Dose | (4, 35) = 107.4 | <0.0001 | |||
|
| ||||||
| 4 | Time × Dose | (20, 170) = 5.054 | <0.0001 | |||
| 1B | Female | vF | 5 | Time | (4, 137) = 14.56 | <0.0001 |
| 6 | Dose | (4, 34) = 30.99 | <0.0001 | |||
|
| ||||||
| Male vs Female | 7 | Dose × Sex | (4, 68) = 39.70 | <0.0001 | ||
| 1D | vF | 8 | Dose | (4, 68) = 129.6 | <0.0001 | |
| 9 | Sex | (1, 68) = 198.3 | <0.0001 | |||
|
| ||||||
| 10 | Time × Dose | (12, 87) = 4.785 | <0.0001 | |||
| 1E | Male | HP | 11 | Time | (2, 71) = 154.9 | <0.0001 |
| 12 | Dose | (4, 29) = 7.088 | 0.0004 | |||
|
| ||||||
| 13 | Time × Dose | (12, 102) = 4.579 | <0.0001 | |||
| 1F | Female | HP | 14 | Time | (2, 79) = 33.11 | <0.0001 |
| 15 | Dose | (4, 34) = 18.84 | <0.0001 | |||
|
| ||||||
| Male vs Female | 16 | Dose × Sex | (4, 62) = 11.76 | <0.0001 | ||
| 1H | HP | 17 | Dose | (4, 62) = 18.31 | <0.0001 | |
| 18 | Sex | (1, 62) = 74.83 | <0.0001 | |||
|
| ||||||
| 19 | Time × Drug | (5, 90) = 3.137 | P=0.0118 | |||
| 2A | Male | vF | 20 | Time | (3, 62) = 1.276 | 0.29 |
| 21 | Drug | (1, 18) = 8.300 | 0.0099 | |||
|
| ||||||
| 22 | Time × Drug | (5, 90) = 3.491 | 0.0063 | |||
| 2B | Female | vF | 23 | Time | (2, 44) = 4.177 | 0.0158 |
| 24 | Drug | (1, 18) = 35.83 | <0.0001 | |||
|
| ||||||
| Male vs Female | 25 | Drug × Sex | (1, 18) = 6.431 | 0.0207 | ||
| 2C | vF | 26 | Drug | (1, 18) = 53.06 | <0.0001 | |
| 27 | Sex | (1, 18) = 1.410 | 0.2505 | |||
|
| ||||||
| 28 | Time × Dose | (6, 78) = 1.584 | 0.163 | |||
| 3A | Male | vF | 29 | Time | (2, 38) = 2.513 | 0.073 |
| 30 | Dose | (1, 13) = 4.781 | 0.0477 | |||
|
| ||||||
| 31 | Time × Dose | (6, 102) = 7.490 | <0.0001 | |||
| 3B | Female | vF | 32 | Time | (2, 43) = 10.87 | <0.0001 |
| 33 | Dose | (1, 17) = 28.00 | <0.0001 | |||
|
| ||||||
| Male vs Female | 34 | Dose × Sex | (1, 30) = 7.854 | 0.0088 | ||
| 3C | vF | 35 | Dose | (1, 30) = 33.18 | <0.0001 | |
| 36 | Sex | (1, 30) = 5.184 | 0.0301 | |||
|
| ||||||
| 37 | Time × Dose | (6, 72) = 0.4550 | 0.8392 | |||
| 3D | Male | Act | 38 | Time | (3, 36) = 0.9631 | 0.4214 |
| 39 | Dose | (1, 12) = 2.480 | 0.1413 | |||
|
| ||||||
| 3E | Female | Act | 40 | Time × Dose | (6, 96) = 2.553 | 0.0245 |
| 41 | Time | (3, 54) = 4.441 | 0.0054 | |||
| 42 | Dose | (1, 16) = 47.70 | <0.0001 | |||
|
| ||||||
| Male vs Female | 43 | Dose × Sex | (1, 28) = 7.723 | 0.0096 | ||
| 3F | Act | 44 | Dose | (1, 28) = 35.91 | <0.0001 | |
| 45 | Sex | (1, 28) = 11.23 | 0.0023 | |||
|
| ||||||
| 46 | Time × Drug | (10, 70) = 0.4995 | 0.8847 | |||
| 4A | Male | vF | 47 | Time | (2, 31) = 1.181 | 0.3246 |
| 48 | Drug | (2, 14) = 1.331 | 0.2956 | |||
|
| ||||||
| 49 | Time × Drug | (10, 95) = 1.212 | 0.2936 | |||
| 4B | Female | vF | 50 | Time | (3, 63) = 4.286 | 0.0063 |
| 51 | Drug | (2, 19) = 4.712 | 0.0218 | |||
|
| ||||||
| Male vs Female | 52 | Sex × Drug | (2, 33) = 2.495 | 0.0979 | ||
| 4C | vF | 53 | Drug | (2, 33) = 1.286 | 0.2898 | |
| 54 | Sex | (1, 33) = 4.205 | 0.0483 | |||
|
| ||||||
| 55 | Time × Drug | (10, 60) = 0.6296 | 0.7827 | |||
| 4D | Male | Act | 56 | Time | (2, 31) = 2.162 | 0.1186 |
| 57 | Drug | (2, 12) = 1.014 | 0.3919 | |||
|
| ||||||
| 58 | Time × Drug | (10, 90) = 1.247 | 0.273 | |||
| 4E | Female | Act | 59 | Time | (3, 61) = 2.143 | 0.0966 |
| 60 | Drug | (2, 18) = 2.662 | 0.0971 | |||
|
| ||||||
| Male vs Female | 61 | Sex × Drug | (2, 30) = 2.418 | 0.1062 | ||
| 4F | Act | 62 | Drug | (2, 30) = 3.854 | 0.0324 | |
| 63 | Sex | (1, 30) = 3.383 | 0.0758 | |||
|
| ||||||
| 64 | Strain × Drug | (3, 28) = 3.545 | P=0.0271 | |||
| 6A | Male | HP | 65 | Drug | (3, 28) = 1.502 | P=0.2358 |
| 66 | Strain | (1, 28) = 38.03 | P<0.0001 | |||
|
| ||||||
| 67 | Strain × Drug | (3, 28) = 9.185 | P=0.0002 | |||
| 6B | Male | CP | 68 | Drug | (3, 28) = 13.28 | P<0.0001 |
| 69 | Strain | (1, 28) = 45.09 | P<0.0001 | |||
|
| ||||||
| 70 | Strain × Drug | (3, 32) = 0.5121 | P=0.6768 | |||
| 6C | Male | Glucose | 71 | Drug | (3, 32) = 1.046 | P=0.3856 |
| 72 | Strain | (1, 32) = 1.417 | P=0.2426 | |||
|
| ||||||
| Male vs Female | 73 | Sex × Drug | (1, 40) = 2.695 | 0.1085 | ||
| 6F | HP | 74 | Drug | (1, 40) = 59.53 | <0.0001 | |
| 75 | Sex | (1, 40) = 1.096 | 0.3015 | |||
|
| ||||||
| Male vs Female | 76 | Sex × Drug | (1, 40) = 2.946 | 0.0938 | ||
| 6I | PP | 77 | Drug | (1, 40) = 40.99 | <0.0001 | |
| 78 | Sex | (1, 40) = 0.3639 | 0.5497 | |||
|
| ||||||
| Male vs Female | 79 | Sex × Drug | (1, 36) = 4.876 | 0.0337 | ||
| 8C | HP | 80 | Drug | (1, 36) = 56.38 | <0.0001 | |
| 81 | Sex | (1, 36) = 1.812 | 0.1867 | |||
|
| ||||||
| 82 | Time × Dose | (15, 95) = 0.9696 | 0.4928 | |||
| 9A | Male | HP | 83 | Time | (3, 71) = 5.808 | 0.0006 |
| 84 | Dose | (3, 19) = 0.7648 | 0.5278 | |||
|
| ||||||
| 85 | Time × Dose | (15, 90) = 1.232 | 0.2633 | |||
| 9B | Female | HP | 86 | Time | (3, 68) = 5.721 | 0.0006 |
| 87 | Dose | (3, 18) = 1.805 | 0.1824 | |||
|
| ||||||
| 9C | Male vs Female | HP | 88 | Dose × Sex | (3, 38) = 1.329 | 0.2793 |
| 89 | Dose | (3, 38) = 0.5167 | 0.6733 | |||
| 90 | Sex | (1, 38) = 0.1684 | 0.6839 | |||
|
| ||||||
| Food intake | 91 | Time × Dose | (12, 32) = 3.299 | 0.0034 | ||
| 9D | Male | 92 | Time | (1, 12) = 2.826 | 0.1042 | |
| 93 | Dose | (3, 8) = 0.5317 | 0.6732 | |||
|
| ||||||
| Food intake | 94 | Time × Dose | (12, 40) = 7.158 | <0.0001 | ||
| 9E | Female | 95 | Time | (1, 12) = 5.565 | 0.0313 | |
| 96 | Dose | (3, 10) = 1.177 | 0.3667 | |||
|
| ||||||
| Male vs Female | Food intake | 97 | Dose × Sex | (3, 24) = 0.9784 | 0.4194 | |
| 9F | 98 | Dose | (3, 24) = 6.669 | 0.002 | ||
| 99 | Sex | (1, 24) = 13.65 | 0.0011 | |||
|
| ||||||
| 100 | Time × Dose | (18, 114) = 28.95 | <0.0001 | |||
| 9G | Male | Weight | 101 | Time | (1, 32) = 13.10 | 0.0001 |
| 102 | Dose | (3, 19) = 2.476 | 0.0926 | |||
|
| ||||||
| 103 | Time × Dose | (18, 108) = 2.660 | 0.001 | |||
| 9H | Female | Weight | 104 | Time | (1, 23) = 2.000 | 0.1685 |
| 105 | Dose | (3, 18) = 0.7926 | 0.5138 | |||
|
| ||||||
| Male vs Female | 106 | Dose × Sex | (3, 40) = 4.008 | 0.0138 | ||
| 9I | Weight | 107 | Dose | (3, 40) = 26.60 | <0.0001 | |
| 108 | Sex | (1, 40) = 2.142 | 0.1512 | |||
|
| ||||||
| 109 | Time × Dose | (18, 114) = 5.692 | <0.0001 | |||
| 9J | Male | Glucose | 110 | Time | (4, 80) = 13.36 | <0.0001 |
| 111 | Dose | (3, 19) = 6.770 | 0.0027 | |||
|
| ||||||
| 112 | Time × Dose | (18, 108) = 2.754 | 0.0006 | |||
| 9K | Female | Glucose | 113 | Time | (3, 68) = 4.003 | 0.0065 |
| 114 | Dose | (3, 18) = 1.110 | 0.3708 | |||
|
| ||||||
| Male vs Female | 115 | Dose × Sex | (3, 40) = 0.7509 | 0.5283 | ||
| 9L | Glucose | 116 | Dose | (3, 40) = 8.885 | 0.0001 | |
| 117 | Sex | (1, 40) = 0.6510 | 0.4245 | |||
vF: von Frey; HP: Hotplate; Act: Acetone testing; PP: Paw pressure
Heat hypersensitivity.
Pioglitazone also reduced heat hypersensitivity following MG administration in both male and female mice, with a much stronger effect in females. In males, only the highest dose of 100 mg/kg prevented heat hypersensitivity from 20 to 40 minutes after intraplantar MG [Fig 1E; Dose × Time F (12, 87) = 4.785 P< 0.0001; Dose F (4, 29) = 7.088 P=0.0004, Table 1 lines #10–12]. In females, pioglitazone prevented heat hypersensitivity at all doses [Fig 1F; Dose × Time F (12, 102) = 4.579 P< 0.0001; Dose F (4, 34) = 18.84 P<0.0001. n=7–8, Table 1 lines #13–15]. As illustrated in Figs 1G–H, intraperitoneal administration of pioglitazone was 1000-fold more potent in reducing MG-induced mechanical hypersensitivity in females (ED50=0.028 mg/kg) than in males (ED50=28.8 mg/kg).
3.2. Pioglitazone decreases postsurgical hypersensitivity with greater efficacy in female mice
PPARγ agonists reduce hypersensitivity in male models of inflammatory or incision pain. In males, the PPARγ agonist 15d-PGJ2 decreased mechanical hypersensitivity in the rat intraplantar PGE2 model of inflammatory pain (Napimoga et al., 2008), and another PPARγ agonist, rosiglitazone, attenuated heat and mechanical hypersensitivity in the mouse plantar incision model of postsurgical pain (Hasegawa-Moriyama et al., 2012). To determine the sex dependence of PPARγ agonist analgesia in the PIM model, we administered pioglitazone in both male and female C57Bl/6 mice.
Figure 2 illustrates that PIM increased mechanical sensitivity at 2 days after incision, and intrathecal pioglitazone reduced this hypersensitivity. In males, intrathecal administration of pioglitazone (10 μg) slightly decreased mechanical hypersensitivity with a significant effect of Treatment × Time [Fig 2A; F (5, 90) = 3.137 P=0.0118] and Treatment [F (1, 18) = 8.300 P=0.0099, Table 1 lines #19–21]. Further analysis of Time collapsed across 15–120 min again suggest an overall effect of pioglitazone in males (Panel 2C), although this did not reach statistical significance at any individual timepoint. In females, pioglitazone (10 μg, i.t.) also decreased mechanical hypersensitivity [Fig 2B; Treatment × Time F (5, 90) = 3.491 P=0.0063, Treatment F (1, 18) = 35.83 P<0.0001, Table 1 lines #22–24]. With Time collapsed across the 15–120 measurements, Fig 2C reveals that pioglitazone reduced mechanical hypersensitivity to a greater extent in females as compared to males [Sex × Treatment F (1, 18) = 6.431 P=0.0207; Treatment F (1, 18) = 53.06 P<0.0001, Table 1 lines #25–27].
Figure 2. Pioglitazone inhibits postsurgical hypersensitivity with greater efficacy in female mice.
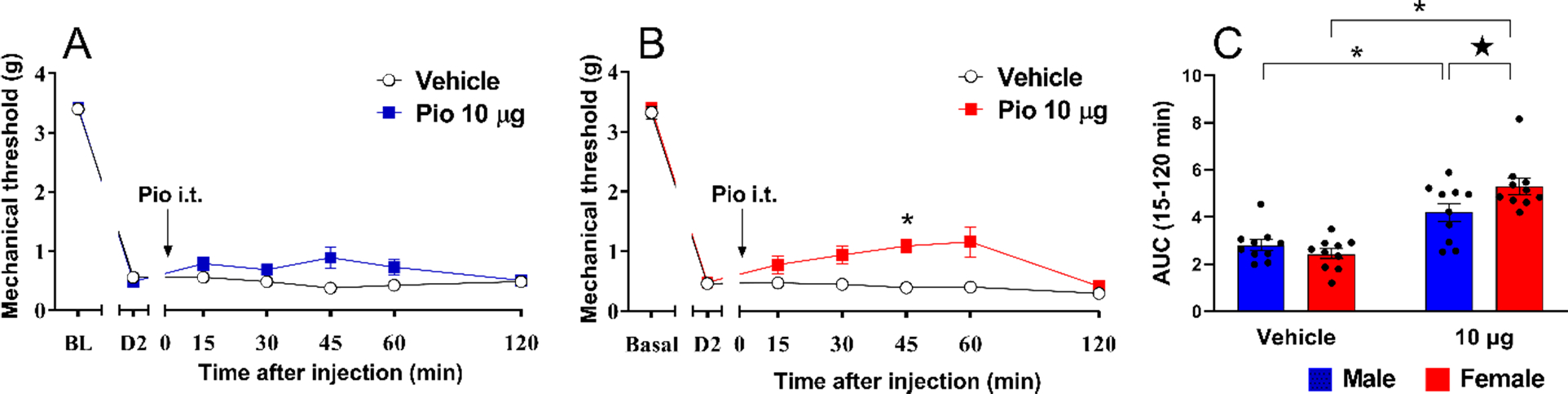
Pioglitazone decreased mechanical hypersensitivity in (A) male and (B) female mice. (C) Analysis of the data with Time collapsed across post-injection timepoints indicates greater efficacy in females at the 10 μg dose. Two-way ANOVA followed by Sidak’s multiple comparisons test). Symbol * P<0.05 Pio 10 μg vs vehicle; ★ P<0.05 male vs female. Values represent mean ± SEM. N=10.
3.3. Pioglitazone inhibits neuropathic pain with greater efficacy in female mice
Previous studies of the antihyperalgesic effects of pioglitazone after peripheral nerve injury were limited to either one sex or a single dose and yielded contradictory results (Sorge et al., 2015)(Gensel et al., 2019). To resolve this discrepancy, we administered multiple doses of pioglitazone to both male and female C57Bl/6 mice. Figure 3 illustrates that SNI induced a robust increase in both mechanical and cold sensitivity at 14 days after surgery. Pioglitazone reduced mechanical and cold hypersensitivity in both male and female mice following SNI, with a relatively stronger effect in female mice.
Figure 3. Pioglitazone inhibits neuropathic pain with greater efficacy in female mice.
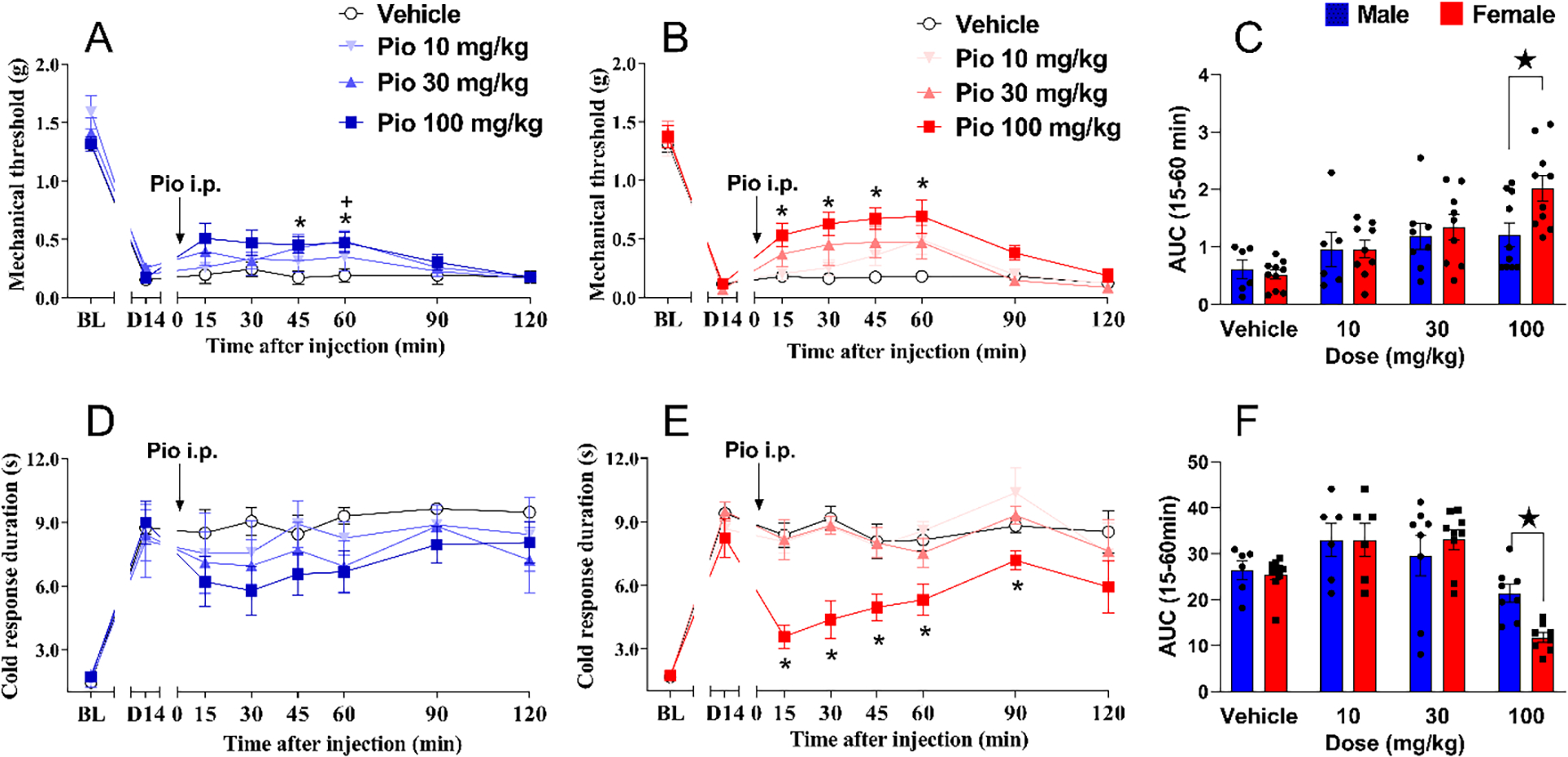
(A) 100 mg/kg pioglitazone decreased mechanical hypersensitivity in male mice. (B) 100 mg/kg pioglitazone decreased mechanical hypersensitivity in female mice. (C) Analysis of the data with Time collapsed across post-injection timepoints illustrates that 100 mg/kg pioglitazone exerted a greater decrease in mechanical hypersensitivity in females as compared to males. (D) Pioglitazone did not decrease cold hypersensitivity in male mice. (E) 100 mg/kg pioglitazone decreased cold hypersensitivity in female mice. (F) Analysis of the data with Time collapsed across post-injection timepoints illustrates that 100 mg/kg pioglitazone exerted a greater decrease in cold hypersensitivity in females as compared to males. Two-way ANOVA was performed followed by Sidak`s multiple comparisons test. Symbol * P<0.05 Pio 100mg/kg vs vehicle; ★ P<0.05 male vs female. Values represent mean ± SEM. N=6–10.
Mechanical sensitivity.
In males, pioglitazone decreased mechanical hypersensitivity [Fig 3A; Dose F (1, 13) = 4.781 P=0.0477, Table 1 line #30] (albeit our alternative analysis using AUC did not reveal a difference between vehicle and drug), and post-hoc analysis revealed a significant effect of 100 mg/kg, i.p. at the 45- and 60-minute timepoints.” In females, 100 mg/kg pioglitazone significantly decreased mechanical hypersensitivity from 15 to 60 minutes after the injection [Fig 3B; Dose × Time F (6, 102) = 7.490 P<0.0001; Dose F (1, 17) = 28.00 P<0.0001, Table 1 lines #31–33]. In Fig 3C, we compared dose by sex and found that pioglitazone 100 mg/kg was more potent in reducing SNI-induced mechanical hypersensitivity in females than in males [Dose F (1, 30) = 33.18 P<0.0001; Sex F (1, 30) = 5.184 P=0.0301, Table 1 lines #34–36].
Cold sensitivity.
Pioglitazone also reduced cold hypersensitivity following SNI in female mice but had no effect in males. In males, pioglitazone did not decrease cold hypersensitivity [Fig 3D; P>0.05, Table 1 lines #37–39]. By contrast, in females, 100 mg/kg pioglitazone decreased cold hypersensitivity from 15 to 60 minutes after injection [Fig 3E; Dose × Time F (6, 96) = 2.553 P=0.0245; Dose F (1, 16) = 47.70 P<0.0001, Table 1 lines #40–42]. Further Dose × Sex analysis in Fig 3F confirmed that pioglitazone 100 mg/kg was more potent in reducing SNI-induced cold hypersensitivity in females than in males [Dose F (1, 28) = 35.91 P<0.0001; Sex F (1, 28) = 11.23 P=0.0023, Table 1 lines #43–45].
3.4. Pioglitazone plus morphine inhibits neuropathic pain with greater efficacy in female mice
Previous studies indicated that the analgesic effects of morphine and the anti-hyperalgesic actions of pioglitazone are both sex-dependent, suggesting that their combined effect would also be sexually dimorphic. Morphine exerts more potent analgesic effects in women volunteers (Craft, 2003; Niesters et al., 2010; Sarton et al., 2000), while pioglitazone exerts greater anti-hyperalgesic effects in female mice with spinal cord injury (Gensel et al., 2019). Pioglitazone delays the behavioral tolerance that develops after repeated morphine administration through a PPARγ-dependent mechanism (de Guglielmo et al., 2014; Ghavimi et al., 2014), indicating that pioglitazone interacts with the morphine tolerance and withdrawal response in rodents. To test the hypothesis that pioglitazone and morphine interact to decrease mechanical and cold hypersensitivity in a sex-dependent manner, we administered them together to male and female mice in the SNI model. We combined a low dose of pioglitazone (10 mg/kg which is close to the therapeutic dose of 3 mg/kg used for clinical diabetes) with a low dose of morphine (1 mg/kg) to avoid adverse effects associated with high doses of pioglitazone (e.g. 500 mg/kg) such as ventricular hypertrophy and congestion of liver and kidneys (Chinnam et al., 2012), and adverse effects associated with high doses of morphine (10–50 mg/kg) such as sedation, inhibition of gastrointestinal transit, and respiratory suppression (Raehal et al., 2005).
Mechanical sensitivity.
Figure 4 again illustrates that SNI induced a robust increase in both mechanical and cold sensitivity at 14 days after surgery. In males, morphine (1 mg/kg) did not change mechanical hypersensitivity when given alone or in combination with 10 mg/kg pioglitazone [Fig 4A; P>0.05, Table 1 lines #46–48]. By contrast, in females, morphine (1 mg/kg) decreased mechanical hypersensitivity at 45 minutes after injection when given alone, and at 30 and 60 minutes after injection when given in combination with pioglitazone [Fig 4B; F (2, 19) = 4.712 P=0.0218, Table 1 lines #49–51]. Subsequent Treatment × Sex analysis in Fig 4C confirmed that morphine + pioglitazone more efficaciously reduced SNI-induced mechanical hypersensitivity in females than in males [Sex: F (1, 33) = 4.205 P=0.0483, Table 1 lines #52–54].
Figure 4. Pioglitazone / morphine inhibits neuropathic pain with greater efficacy in female mice.
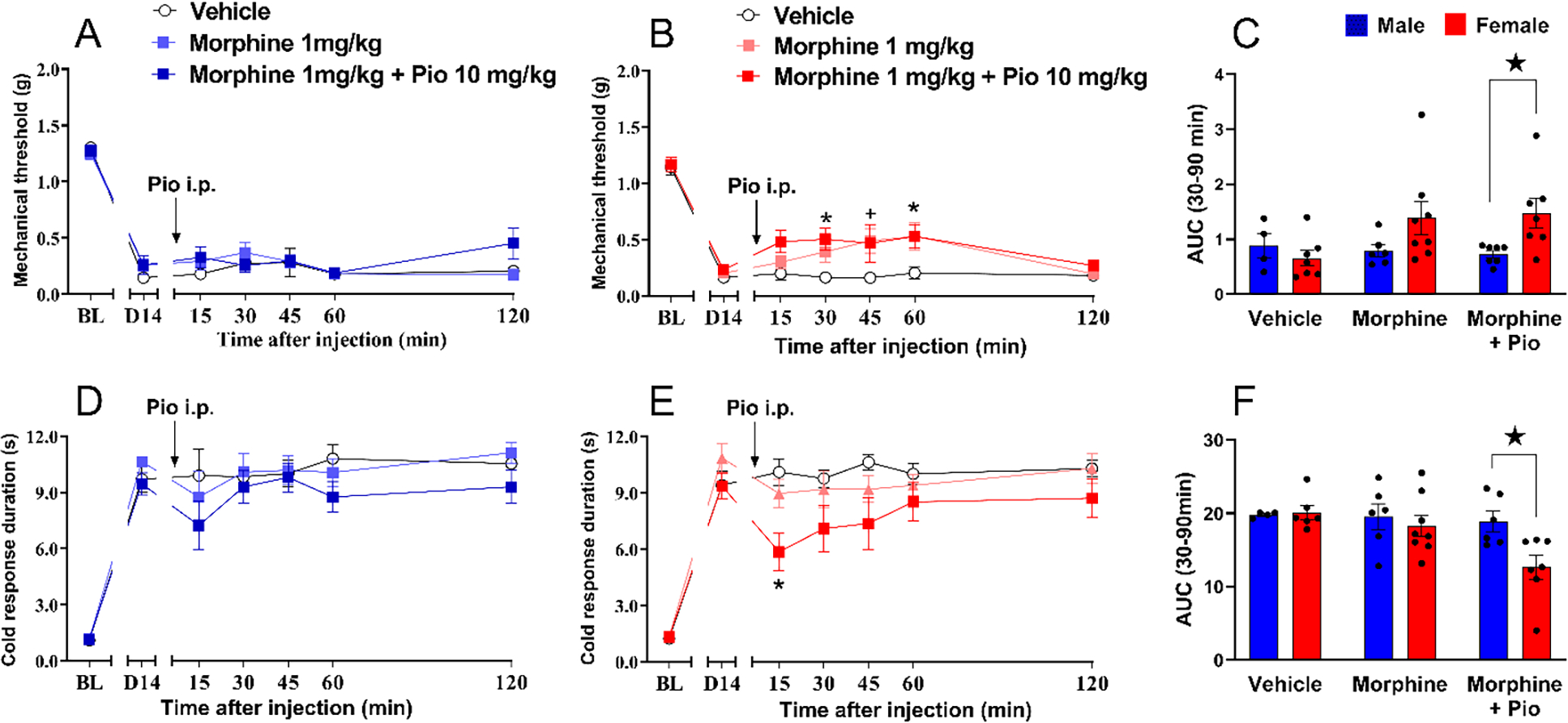
(A) Neither morphine 1 mg/kg alone nor morphine 1 mg/kg combined with pioglitazone 10 mg/kg decreased mechanical hypersensitivity in male mice. (B) Morphine alone or combined with pioglitazone decreased mechanical hypersensitivity in female mice. (C) Analysis of the data with Time collapsed across post-injection timepoints illustrates that the combination of morphine and pioglitazone exerted greater decrease in mechanical hypersensitivity in females as compared to males. (D) Neither morphine 1 mg/kg alone nor morphine 1 mg/kg combined with pioglitazone 10 mg/kg decreased cold hypersensitivity in male mice. (E) Morphine alone or combined with pioglitazone decreased cold hypersensitivity in female mice. (F) Analysis of the data with Time collapsed across post-injection timepoints illustrates that the combination of morphine and pioglitazone exerted greater decrease in cold hypersensitivity in females as compared to males. Two-way ANOVA was performed followed by Sidak’s multiple comparisons test for figures 4A–B and, Tukey`s multiple comparison test for figures 4D–E and Sidak’s multiple comparisons test for figure 3C and 3F. Symbol: + P<0.05 morphine 1 mg/kg alone vs vehicleicle; * P<0.05 morphine 1 mg/kg combined with pio 10mg/kg vs vehicle; ★ P<0.05 male vs female. Values represent mean ± SEM. N=4–8.
Cold sensitivity.
Combined morphine + pioglitazone treatment also decreased cold hypersensitivity following SNI in female mice, but not in male mice. In males, morphine (1 mg/kg) did not change mechanical hypersensitivity when given alone or in combination with 10 mg/kg pioglitazone [Fig 4D; P>0.05, Table 1 lines #55–57]. In females, the combination of morphine with pioglitazone tended to decrease cold hypersensitivity 15 minutes after intraperitoneal injection [Fig 4E; Table 1 lines #58–60]. Subsequent analysis of Treatment × Sex in Fig 4F confirmed that pioglitazone + morphine more efficaciously reduced cold hypersensitivity in females than in males [Treatment F (2, 30) = 3.854 P=0.0324, Table 1 lines #61–63].
3.5. Development of type 2 diabetes and cutaneous hypersensitivity in male and female ZDF rats
ZDF rats are homozygous for the fatty (fa/fa) leptin receptor mutation and develop a diabetic metabolic profile that includes an increase in obesity and hyperglycemia. We previously reported that male ZDF rats develop heat and mechanical hypersensitivity (Griggs et al., 2016). Here we determined whether hyperalgesia develops in a sex-dependent manner.
As illustrated in Figure 5, we first monitored the progression of type 2 diabetes in ZDF rats and their ZL controls. As expected, male and female ZDF rats showed time dependent increase at 7–16 weeks of age in body mass compared to male and female ZL rats, respectively [Fig 5A; Strain F (1, 168) = 480.0 P<0.0001, Table 2 lines #1–7]. Male and female ZDF rats showed time dependent increase at 7–16 weeks of age in blood glucose levels compared to male and female ZL rats, respectively [Fig 5B; Time × Strain F (5, 134) = 27.32 P<0.0001, Table 2 lines #8–14]. Male and female ZDF rats showed time dependent increases in heat hypersensitivity at 15–16 weeks of age compared to male and female ZL rats, respectively [Fig 5C; Time × Strain F (5, 168) = 4.309 P=0.0010, Table 2 lines #15–21]. Male and female ZDF rats also showed a time-dependent increase in mechanical hypersensitivity at 15–16 weeks of age compared to male and female ZL rats, respectively [Fig 5D; Time × Strain F (5, 168) = 4.309 P=0.0010, Table 2 lines #22–28]. Interestingly, male control ZL rats also showed increased in body weight compared to ZL female control rats [Sex × Strain F (1, 168) = 63.98 P<0.0001, Table 2 lines #1–7]. However, no sex difference was found in the development of blood glucose levels [P>0.05, Table 2 lines #8–14], heat hypersensitivity [P>0.05, Table 2 lines #15–21] or mechanical hypersensitivity [P>0.05, Table 2 lines #22–28].
Figure 5. Development of type 2 diabetes and cutaneous hypersensitivity in male and female ZDF rats.
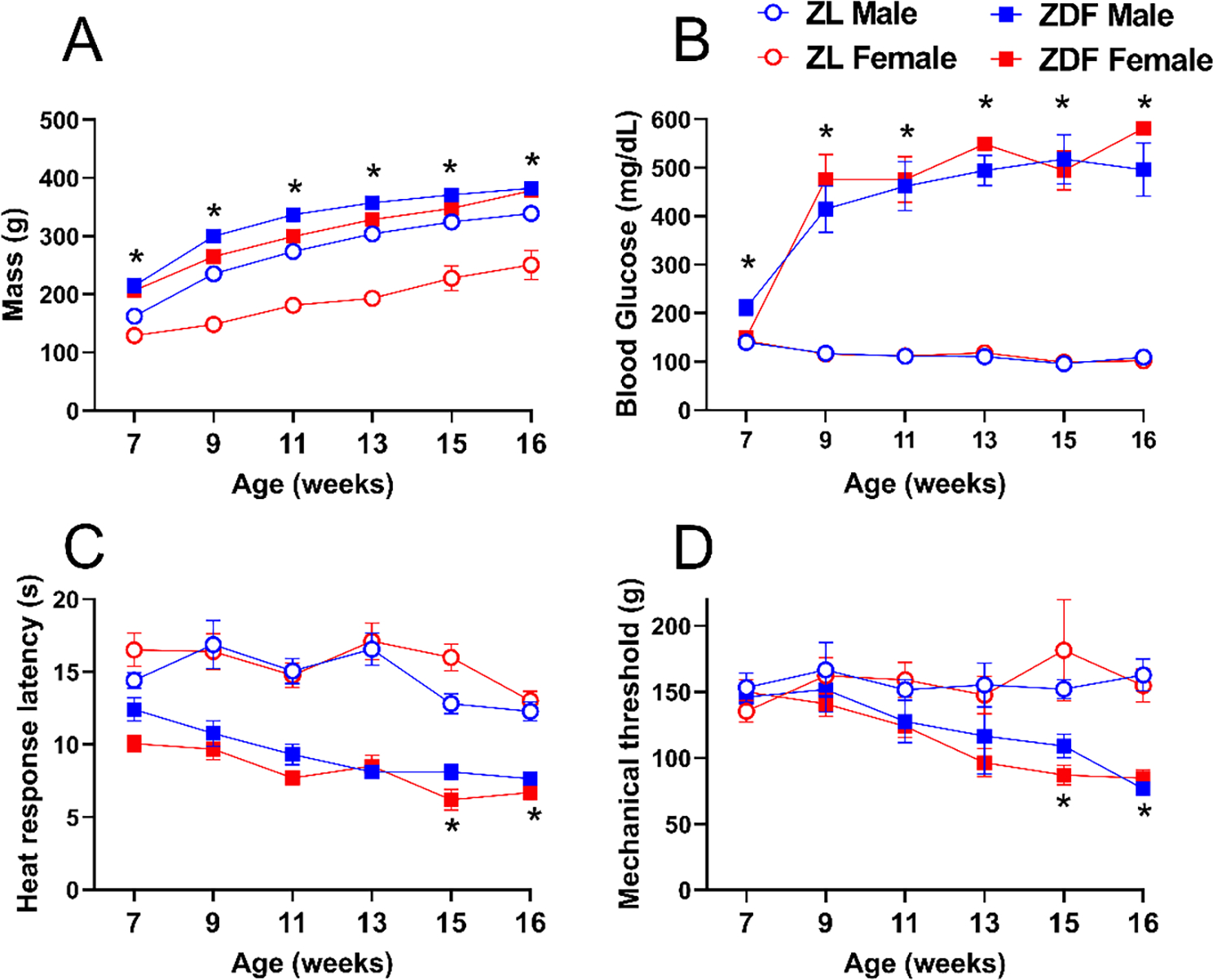
Compared to Zucker Lean (ZL) controls, male and female Zucker Diabetic Fatty (ZDF) rats exhibited a time-dependent increase in (A) body mass, (B) blood glucose, (C) heat hypersensitivity, and (D) mechanical hypersensitivity. Symbol * P<0.05 same-sex ZL vs ZDF, three-way ANOVA followed by Tukey`s multiple comparison test. Values represent mean ± SEM. N=8.
Table 2.
Three-Way ANOVA
| Figure | Sex | Assay | Line # | Factors | F | P value |
|---|---|---|---|---|---|---|
| 1 | Time | (5, 168) = 150.6 | <0.0001 | |||
| 2 | Sex | (1, 168) = 192.5 | <0.0001 | |||
| 5A | Male vs Female | Body mass | 3 4 |
Strain Time × Sex |
(1, 168) = 480.0 (5, 168) = 3.609 |
<0.0001 0.004 |
| 5 | Time × Strain | (5, 168) = 1.257 | 0.2848 | |||
| 6 | Sex × Strain | (1, 168) = 63.98 | <0.0001 | |||
| 7 | Time × Sex × Strain | (5, 168) = 1.413 | 0.2221 | |||
|
| ||||||
| 8 | Time | (5, 134) = 18.54 | <0.0001 | |||
| 9 | Sex | (1, 134) = 0.8059 | 0.371 | |||
| Male vs Female | Blood Glucose | 10 | Strain | (1, 134) = 692.4 | <0.0001 | |
| 5B | 11 | Time × Sex | (5, 134) = 0.8510 | 0.516 | ||
| 12 | Time × Strain | (5, 134) = 27.32 | <0.0001 | |||
| 13 | Sex × Strain | (1, 134) = 0.6879 | 0.4083 | |||
| 14 | Time × Sex × Strain | (5, 134) = 0.9537 | 0.4487 | |||
|
| ||||||
| 15 | Time | (5, 140) = 12.81 | <0.0001 | |||
| 16 | Sex | (1, 28) = 0.1101 | 0.7425 | |||
| 5C | Male vs Female | HP | 17 18 |
Strain Time × Sex |
(1, 28) = 214.0 (5, 140) = 0.6481 |
<0.0001 0.6634 |
| 19 | Time × Strain | (5, 140) = 3.431 | 0.0059 | |||
| 20 | Sex × Strain | (1, 28) = 6.489 | 0.0166 | |||
| 21 | Time × Sex × Strain | (5, 140) = 1.671 | 0.1454 | |||
|
| ||||||
| 22 | Time | (5, 168) = 2.939 | 0.0143 | |||
| 23 | Sex | (1, 168) = 0.3942 | 0.5309 | |||
| 5D | Male vs Female | PP | 24 25 |
Strain Time × Sex |
(1, 168) = 41.49 (5, 168) = 0.2074 |
<0.0001 0.959 |
| 26 | Time × Strain | (5, 168) = 4.309 | 0.001 | |||
| 27 | Sex × Strain | (1, 168) = 0.3558 | 0.5517 | |||
| 28 | Time × Sex × Strain | (5, 168) = 0.7538 | 0.5844 | |||
|
| ||||||
| 29 | Time | (2, 90) = 2.437 | 0.0932 | |||
| 30 | Strain | (1, 45) = 79.43 | <0.0001 | |||
| 31 | Drug | (1, 45) = 17.50 | 0.0001 | |||
| 6D | Male | HP | 32 | Time × Strain | (2, 90) = 0.8989 | 0.4106 |
| 33 | Time × Drug | (2, 90) = 6.504 | 0.0023 | |||
| 34 | Strain × Drug | (1, 45) = 1.735 | 0.1944 | |||
| 35 | Time × Strain × Drug | (2, 90) = 0.3000 | 0.7415 | |||
|
| ||||||
| 36 | Time | (2, 82) = 2.594 | 0.0809 | |||
| 37 | Strain | (1, 41) = 108.2 | <0.0001 | |||
| 38 | Drug | (1, 41) = 15.24 | 0.0003 | |||
| 6E | Female | HP | 39 | Time × Strain | (2, 82) = 3.787 | 0.0267 |
| 40 | Time × Drug | (2, 82) = 7.700 | 0.0009 | |||
| 41 | Strain × Drug | (1, 41) = 4.647 | 0.037 | |||
| 42 | Time × Strain × Drug | (2, 82) = 0.4271 | 0.6538 | |||
|
| ||||||
| 6G | Male | PP | 43 | Time | (2, 86) = 5.997 | 0.0036 |
| 44 | Strain | (1, 43) = 268.9 | <0.0001 | |||
| 45 | Drug | (1, 43) = 12.69 | 0.0009 | |||
| 46 | Time × Strain | (2, 86) = 2.513 | 0.087 | |||
| 47 | Time × Drug | (2, 86) = 2.950 | 0.0576 | |||
| 48 | Strain × Drug | (1, 43) = 2.641 | 0.1114 | |||
| 49 | Time × Strain × Drug | (2, 86) = 0.6298 | 0.5351 | |||
|
| ||||||
| 50 | Time | (2, 82) = 0.08181 | 0.9215 | |||
| 51 | Strain | (1, 41) = 82.62 | <0.0001 | |||
| 52 | Drug | (1, 41) = 8.638 | 0.0054 | |||
| 6H | Female | PP | 53 | Time × Strain | (2, 82) = 3.253 | 0.0437 |
| 54 | Time × Drug | (2, 82) = 1.064 | 0.3496 | |||
| 55 | Strain × Drug | (1, 41) = 2.831 | 0.1001 | |||
| 56 | Time × Strain × Drug | (2, 82) = 7.442 | 0.0011 | |||
|
| ||||||
| 57 | Time | (2, 52) = 24.79 | <0.0001 | |||
| 58 | Strain | (1, 26) = 206.3 | <0.0001 | |||
| 7A | Male vs Female | Weight | 59 60 |
Sex Time × Strain |
(1, 26) = 10.81 (2, 52) = 3.369 |
0.0029 0.0421 |
| 61 | Time × Sex | (2, 52) = 1.008 | 0.3719 | |||
| 62 | Strain × Sex | (1, 26) = 0.05295 | 0.8198 | |||
| 63 | Time × Strain × Sex | (2, 52) = 1.790 | 0.1771 | |||
|
| ||||||
| 64 | Time | (1, 50) = 233.8 | <0.0001 | |||
| 65 | Strain | (1, 27) = 393.6 | <0.0001 | |||
| 7B | Male vs Female | HbAlc | 66 67 |
Sex Time × Strain |
(1, 27) = 0.1071 (2, 54) = 168.3 |
0.746 <0.0001 |
| 68 | Time × Sex | (2, 54) = 1.468 | 0.2394 | |||
| 69 | Strain × Sex | (1, 27) = 0.02360 | 0.879 | |||
| 70 | Time × Strain × Sex | (2, 54) = 0.006281 | 0.9937 | |||
|
| ||||||
| 71 | Time | (1, 50) = 60.23 | <0.0001 | |||
| 72 | Strain | (1, 26) = 20.03 | 0.0001 | |||
| 7C | Male vs Female | HP | 73 74 |
Sex Time × Strain |
(1, 26) = 0.007916 (2, 52) = 5.446 |
0.9298 0.0071 |
| 75 | Time × Sex | (2, 52) = 0.2999 | 0.7422 | |||
| 76 | Strain × Sex | (1, 26) = 2.385 | 0.1346 | |||
| 77 | Time × Strain × Sex | (2, 52) = 0.08583 | 0.9179 | |||
|
| ||||||
| 78 | Time | (2, 82) = 1.276 | 0.2877 | |||
| 79 | Strain | (1, 32) = 565.8 | <0.0001 | |||
| 80 | Drug | (1, 32) = 17.42 | 0.0002 | |||
| 8A | Male | HP | 81 | Time × Strain | (3, 96) = 5.855 | 0.001 |
| 82 | Time × Drug | (3, 96) = 2.172 | 0.0963 | |||
| 83 | Strain × Drug | (1, 32) = 23.10 | <0.0001 | |||
| 84 | Time × Strain × Drug | (3, 96) = 0.1530 | 0.9275 | |||
|
| ||||||
| 85 | Time | (2, 77) = 16.24 | <0.0001 | |||
| 86 | Strain | (1, 32) = 174.4 | <0.0001 | |||
| 8B | Female | HP | 87 | Drug | (1, 32) = 8.973 | 0.0053 |
| 88 | Time × Strain | (3, 96) = 9.445 | <0.0001 | |||
| 89 | Time × Drug | (3, 96) = 7.931 | <0.0001 | |||
| 90 | Strain × Drug | (1, 32) = 9.866 | 0.0036 | |||
| 91 | Time × Strain × Dru | (3, 96) = 8.165 | <0.0001 | |||
HP: Hotplate; PP: Paw pressure
3.6. Pioglitazone reduces heat and mechanical hyperalgesia in both male and female ZDF rats
Figure 6 illustrates that pioglitazone 10, 30 and 100 mg/kg decreased heat hypersensitivity assessed in hotplate (Fig 6A; Interaction F (3, 28) = 3.545 P=0.0271) and Hargreaves tests (Supplemental Figure S1), as well as cold hypersensitivity in a coldplate test (Fig 6B; Interaction F (3, 28) = 9.185 P=0.0002).
Figure 6. Pioglitazone reduced heat and mechanical hyperalgesia in male and female ZDF rats.
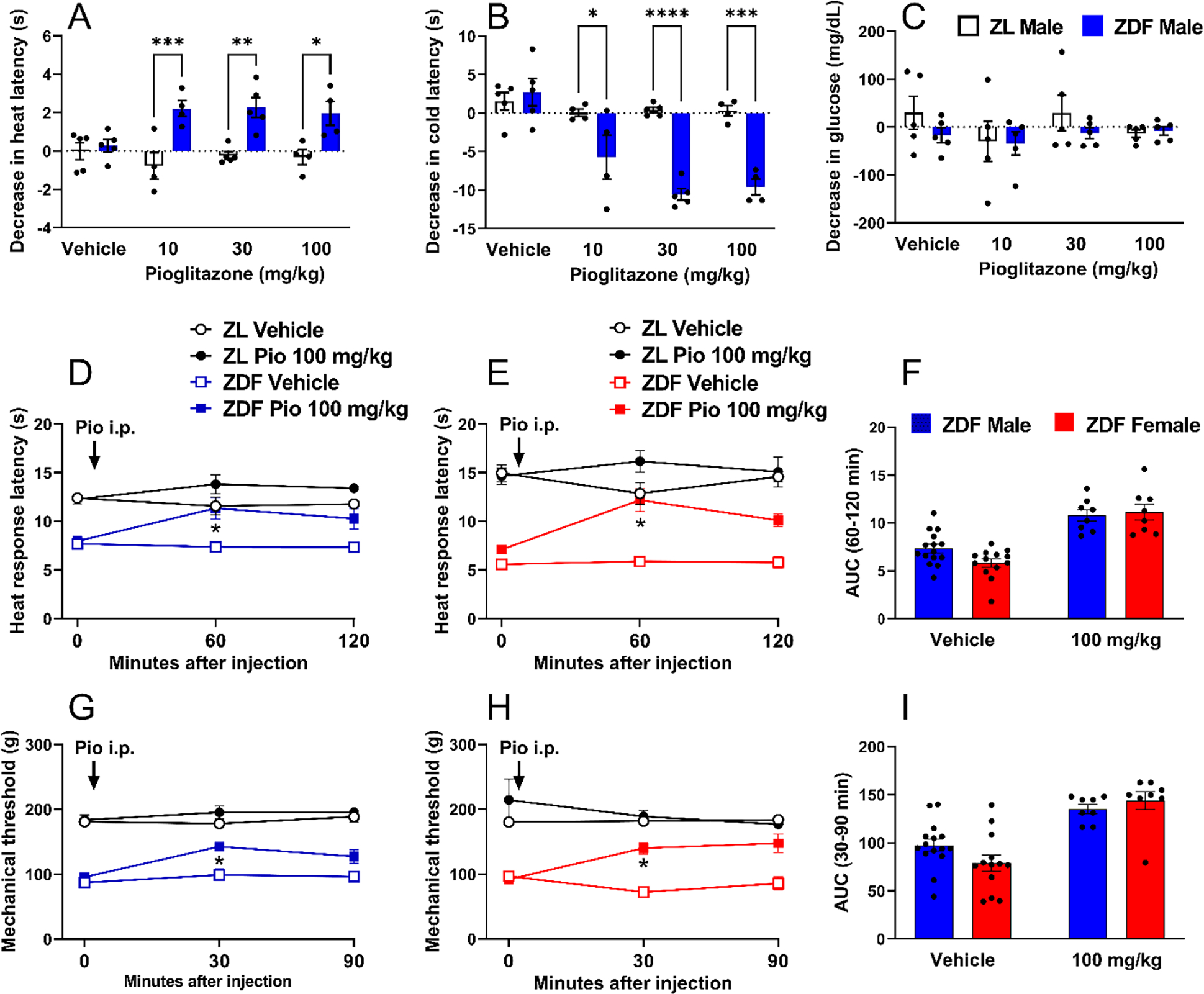
Values are plotted as the difference between pre-drug baseline values and 1–2 hour post-drug values collected in ZDF and ZL genetic controls after i.p. injection of pioglitazone at doses of 0, 100, 300 mg/kg, with respect to changes from baseline in (A) Hotplate latency, (B) cold plate responses and (C) glucose. Further studies comparing males and females illustrate that 100 mg/kg pioglitazone decreased heat hypersensitivity in (D) male and (E) female ZDF rats. (F) Analysis of the data with Time collapsed across post-injection timepoints illustrates that pioglitazone exerted a similar decrease in heat hypersensitivity in ZDF females and males at 100 mg/kg. 100 mg/kg pioglitazone decreased mechanical hypersensitivity in (G) male and female (H) ZDF rats. (I) Analysis of the data with Time collapsed across post-injection timepoints illustrates that pioglitazone exerted a similar decrease in mechanical hypersensitivity in males and females. Three-way ANOVA and Tukey’s multiple comparisons test for figures 6D–E and 6G–H. Two-way ANOVA and Sidak’s multiple comparisons test for figures 6A–C, 6F and 6I. Symbol: Panels A-C *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 pre- vs post-injection value; Panels D, E, G, H *p<0.05 ZDF vehicle vs ZDF Pio 100 mg/kg. Panels F and I * P<0.05 vehicle vs Pio 100 mg/kg. Values represent mean ± SEM. N=8–15.
In male ZDF rats, repeated administration of pioglitazone provides the combined benefit of reduced hyperglycemia, hyperalgesia, and central sensitization (Griggs et al., 2016). Single administration of pioglitazone also reduces behavioral signs of neuropathic pain after peripheral nerve injury (Griggs et al., 2015; Iwai et al., 2008; Morgenweck et al., 2013), but these studies did not test the null hypothesis antihyperalgesic effects were secondary to changes in glucose levels. Fig 6C demonstrates that pioglitazone 10, 30 and 100 mg/kg did not change non-fasting glucose levels when tested one hour after injection (Interaction; F (3, 32) = 0.5121 P=0.6768). These results indicate that pioglitazone decreases behavioral signs of PDN, without changes in glucose levels, and establish a dose to compare not only heat hypersensitivity but also mechanical hypersensitivity in both sexes of ZDF rats. Pioglitazone (100 mg/kg) decreased heat hypersensitivity 60 minutes after intraperitoneal injection in both males [Fig 6D Treatment × Time F (2, 90) = 6.504 P=0.0023; Treatment F (1, 45) = 17.50 P=0.0001, Table 2 lines #29–35] and females [Fig 6E Treatment × Time F (2, 82) = 7.700 P=0.0009; Treatment F (1, 41) = 15.24 P=0.0003, Table 2 lines #36–42]; Fig 6F did not reveal a sex difference (Sex × Treatment P>0.05, Table 1 lines #64–66). Similarly, pioglitazone decreased mechanical hypersensitivity in both males [Fig 6G Treatment F (1, 43) = 12.69 P=0.0009, Table 2 lines #43–49] and females [Fig 6H Treatment F (1, 41) = 8.638 P=0.0054, Table 2 lines #50–56]; Figure 6I did not reveal a sex difference (P>0.05, Table 1 lines #67–69). These results indicate that pioglitazone reduced heat and mechanical hypersensitivity in ZDF rats as compared to ZL rats, but with no difference between male and female cohorts.
3.7. Development of diabetes and cutaneous hypersensitivity in male and female db/db mice
As is true for ZDF rats, db/db mice are homozygous for a leptin receptor mutation (Leprdb) and develop obesity and hyperglycemia. In db/db mice, we previously reported that heat hypersensitivity typically develops within 8 weeks of age and lasts until at least 13 weeks of age, but these studies were restricted to male subjects (Griggs et al., 2019). Here we demonstrate in Figure 7 that not only male, but also female db/db mice exhibit a time-dependent increase across 5–11 weeks of age in body mass [Fig 7A Strain × Time F (2, 52) = 3.369 P=0.0421, Table 2 line #57–63], hemoglobin A1c levels [Fig 7B Strain × Time F (2, 54) = 168.3 P<0.0001, Table 2 lines #64–70], and heat hypersensitivity [Fig 7C Strain × Time F (2, 52) = 5.446 P=0.0071, Table 2 lines #71–77]. However, 3-way ANOVA did not reveal a sex difference in any of the outcome measures (P>0.05, Table 2 lines #57–77).
Figure 7. Development of diabetes and cutaneous hypersensitivity in male and female db/db mice.
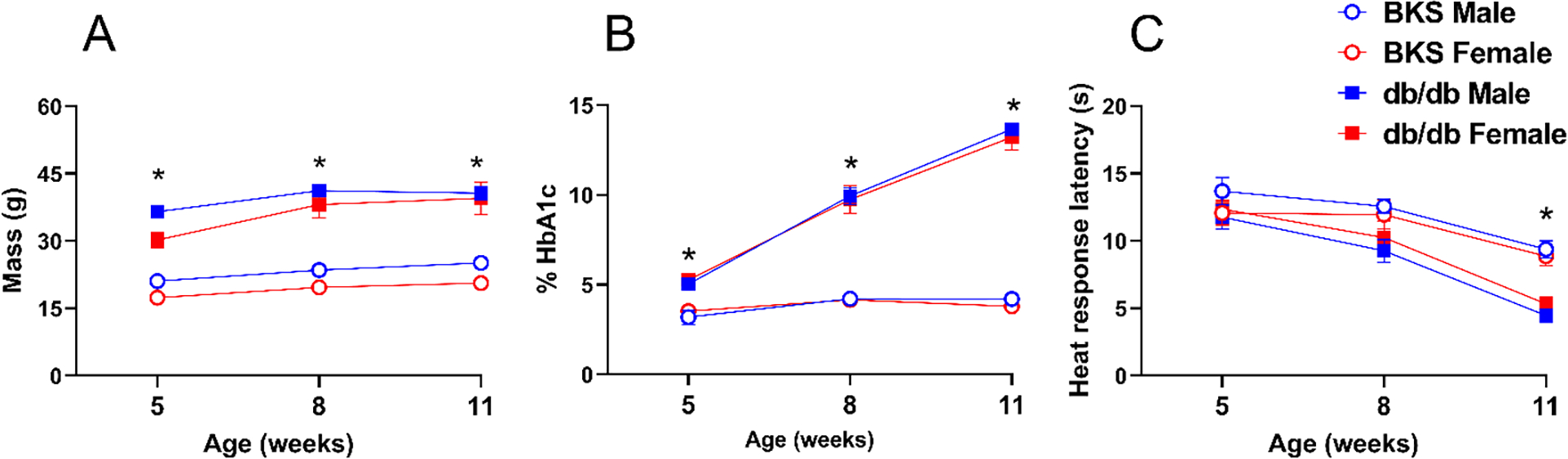
Male and female db/db exhibited a time-dependent increase in (A) body mass, (B) blood glucose, and (C) heat hypersensitivity. Symbol * P<0.05 same-sex C57BLKS/J (BKS) vs db/db, three-way ANOVA followed by Tukey`s multiple comparison test. Values represent mean ± SEM. N=7–9.
3.8. Pioglitazone decreased heat hypersensitivity with greater efficacy in females in the db/db model of PDN
Pioglitazone reduced heat hypersensitivity in both male and female db/db mice, with a stronger effect in females. Figure 8 illustrates that pioglitazone (100 mg/kg) decreased heat hypersensitivity 60 minutes after intraperitoneal injection in 12-week-old males [Fig 8A; Strain × Treatment F (1, 32) = 23.10 P<0.0001; Treatment F (1, 32) = 17.42 0.0002 P=0.0002, Table 2 lines #78–84] and from 60 to 90 minutes in females, [Fig 8B; Strain × Treatment F (1, 32) = 9.866 P=0.0036; Treatment F (1, 32) = 17.42 P=0.0002, Table 2 lines #85–91]. Subsequent analysis in Fig 8C indicates that pioglitazone more efficaciously reduced heat hypersensitivity in females than in males [Treatment F (1, 32) = 8.973 P=0.0053, Table 1 Lines #79–81].
Figure 8. Pioglitazone decreased heat hypersensitivity in db/db mice with greater efficacy in female mice.
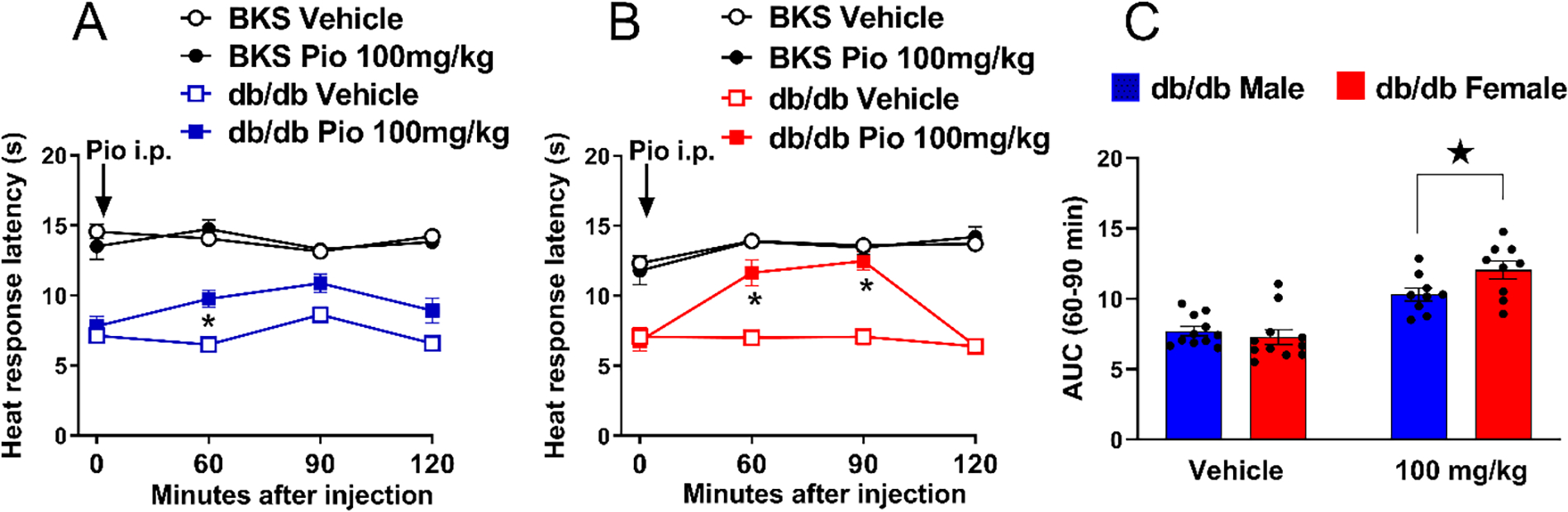
100 mg/kg pioglitazone decreased heat hypersensitivity in male (A) and female (B) db/db mice. Three-way ANOVA was performed followed by Tukey’s multiple comparisons test. (C) pioglitazone exerted a greater decrease in heat hypersensitivity in db/db females as compared to males. Sidak’s multiple comparisons test. Symbol * P<0.05 ZDF Pio 100 mg/kg vs vehicle; ★ P<0.05 ZDF male vs ZDF female. Values = mean ± SEM. N=8–15.
3.9. Six weeks administration of pioglitazone does not change heat hypersensitivity in db/db mice
Griggs et al., 2016 reported that repeated administration of pioglitazone decreased the development of heat hypersensitivity in male ZDF rats (Griggs et al., 2016). Here we asked whether chronic administration of pioglitazone could exert the same effect in male and/or female db/db mice. To test this hypothesis, we provided pioglitazone in the food and evaluated heat response thresholds. Figure 9 illustrates that the 30 mg/kg/d dose of pioglitazone was bioactive as it decreased food intake in both males [Fig 9D; Time × Dose F (12, 32) = 3.299 P=0.0034, Table 1 Lines #91–93] and females [Fig 9E; Time × Dose F (12, 40) = 7.158 P<0.0001, Table 1 Lines #94–96], with greater effect in females [Fig 9F; Sex F (1, 16) = 5.945 P=0.0268, Table 1 Lines #97–99]. Pioglitazone 30 mg/kg/d also increased body weight in males Fig 9G; [Time × Dose F (18, 114) = 28.95 P<0.0001, Table 1 Lines #100–102] and females [Fig 9H; Time × Dose F (18, 108) = 2.660 P=0.001, Table 1 Lines #103–105] with greater effect in males [Fig 9I; Dose F (3, 40) = 26.60 P<0.0001, Table 1 Lines #106–108]. Pioglitazone 30 mg/kg/d also decreased blood glucose levels in in males [Fig 9J; Time × Dose F (18, 114) = 5.692 P<0.0001, Table 1 Lines #109–111] and females [Fig 9L; Time × Dose (18, 108) = 2.754 P=0.0006, Table 1 Lines #112–114] with greater effect in males [Fig 9M; Dose F (3, 40) = 8.885 P=0.0001]. However, neither 0.3, 3.0 nor 30 mg/kg/day pioglitazone decreased heat hypersensitivity in males [Fig 9A P>0.05, Table 1 lines #82–84] or females [Fig 9B Table 1 lines #85–87]. This contrasts with our previous study in ZDF rats that reported that oral pioglitazone (10 mg/kg/day for six weeks) reduced hypersensitivity and spinal pERK expression in male ZDF rats (Griggs et al., 2016); perhaps species differences in analgesic or pharmacokinetic profile might explain this discrepancy (Matheson and Le Foll, 2020; O’Brien et al., 2014). Thus, it remains possible that higher doses of pioglitazone will exert anti-hyperalgesic effects. Future studies are needed to determine the pharmacotherapeutic effectiveness of pioglitazone for the treatment of hyperalgesia and ongoing pain in PDN.
Figure 9. Six weeks of pioglitazone does not change heat hypersensitivity in db/db mice.
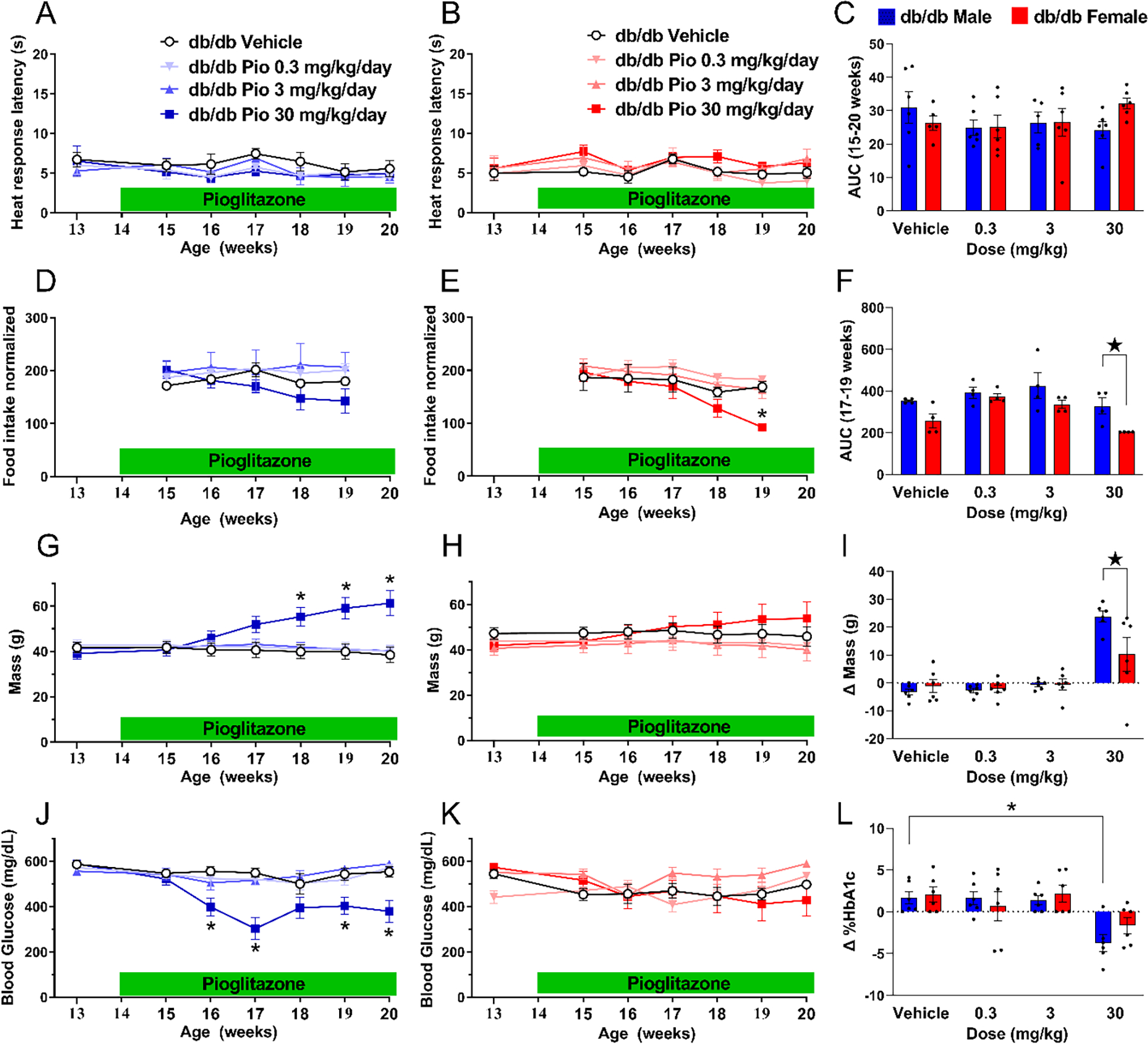
Pioglitazone did not change heat hypersensitivity in (A) male or (B) female db/db mice. (C) Analysis of the data with Time collapsed across post-injection timepoints illustrates that pioglitazone did not change heat hypersensitivity. Pioglitazone decreased food consumption in (D) males and (E) female mice. (F) Analysis of the data with Time collapsed across post-injection timepoints illustrates that the 30 mg/kg/day dose of pioglitazone decreased food consumption in females. Pioglitazone significantly increased body weight in (G) males but not (H) females. (I) Pioglitazone 30 mg/kg/d increased body weight (change from 13 to 20 weeks of age) to a greater extent in males as compared to females. Pioglitazone decreased non-fasting glucose levels in (J) males but not females (K). (L) Pioglitazone 30 mg/kg/d increased HbA1c (change from 13 to 20 weeks of age) in males but not females. 30 mg/kg/d produced greater increases in males. Two-way ANOVA was performed followed by Sidak’s multiple comparisons test for figures 9A–B, 9D–E, G–H and J–k. Tukey’s multiple comparisons test was performed for figure 9C, F, I and L. *p<0.05, **p<0.01. Values = mean ± SEM. N=5–6.
4. DISCUSSION
4.1. Pioglitazone inhibits acute chemical pain with greater potency in females
The current results indicate that systemic administration of pioglitazone inhibits MG-evoked mechanical and heat hypersensitivity. Remarkably, pioglitazone was 100-fold more potent in females than in males in reducing MG-induced mechanical hypersensitivity, and 1000-fold more potent in reducing MG-induced heat hypersensitivity. Methylglyoxal is emerging as an important mediator of pain (Feldman et al., 2019). High serum levels of MG correlate with PDN in humans compared to diabetic patients without pain, as well as in multiple animal models of diabetes-associated cutaneous hypersensitivity including the db/db mouse (Bierhaus et al., 2012; Griggs et al., 2016; Griggs et al., 2019). Conversely, downregulation of spinal MG with intrathecal administration of an MG scavenger reduced heat hypersensitivity in db/db mice (Griggs et al., 2019). Intravenous, intrathecal, and intraplantar administration of MG evokes mechanical and thermal hypersensitivity as well as behavioral signs of the affective component of pain (Bierhaus et al., 2012; Griggs et al., 2019).
We previously demonstrated that pharmacological inhibitors of TRPA1, adenylyl cyclase type 1 (AC1), and Exchange protein directly activated by cAMP (Epac) prevent MG-induced hypersensitivity, indicating that MG triggers a TRPA1➔AC1➔Epac signaling pathway to produce nociception (Griggs et al., 2019). An important question raised by the current results is whether pioglitazone sex-dependently inhibits one or more of the TRPA1, AC1, or Epac signaling elements to inhibit MG-evoked pain.
4.2. Pioglitazone decreases behavioral signs of postsurgical pain in female mice
Antihyperalgesic actions of PPARγ agonists have been reported in multiple rodent models of inflammatory pain, and the present results indicate for the first time that intrathecal administration of pioglitazone decreased mechanical hypersensitivity in an incision model of postsurgical pain, with greater efficacy in female mice as compared to male mice. This is analogous to humans studies that reported that opioid analgesics such as nalbuphine (Gear et al., 2003), butorphanol (Gear et al., 1996b), pentazocine alone (Gear et al., 1996a) or pentazocine combined with naloxone (Ryan et al., 2008) exert greater analgesia in women. Moreover, lower doses of nalbuphine are more effective in women than in men (Gear et al., 1999) and interestingly, in a Chinese population, women used significantly less morphine than men to decrease postsurgical pain (Chia et al., 2002). However, the sexual dimorphism of analgesic drugs for postsurgical pain remains controversial. For instance, women requested greater doses of morphine than men for postsurgical pain relief (Aubrun et al., 2005), while another study using Sprague Dawley rats that underwent to a model of postsurgical pain showed no sex differences to the analgesics used, including systemic or intrathecal administration of Morphine, gabapentin, clonidine or neostigmine (Kroin et al., 2003). The results are similarly unclear for the non-steroidal anti-inflammatory drugs (NSAIDs): while indomethacin was used more frequently in women to decrease postsurgical pain (Uchiyama et al., 2006), ibuprofen was not (Averbuch and Katzper, 2000). These discrepancies might be due to differences in experimental protocol, which includes diverse types of surgery, area of incision, dose of analgesic, use or not of patient-controlled analgesia, or side effect profile.
4.3. Pioglitazone inhibits chronic neuropathic pain with greater efficacy in female mice
We report that systemic administration of pioglitazone decreased SNI-induced mechanical and cold hypersensitivity in mice. These results are consistent with previous studies describing the anti-allodynic actions of PPARγ agonists in traumatic nerve injury models of neuropathic pain in mice (Iwai et al., 2008; Khasabova et al., 2019; Maeda et al., 2008; Takahashi et al., 2011) and rats (Churi et al., 2008; Griggs et al., 2015; Zhong et al., 2019). For example, we reported that a single administration of systemic pioglitazone rapidly and dose-dependently reduced mechanical and cold hypersensitivity in the rat SNI model of neuropathic pain (Griggs et al., 2015). Although PPARγ is a transcription factor, the fast onset of action observed in our studies indicated that pioglitazone decreases hypersensitivity through a non-genomic mechanism of action (Griggs et al., 2015). We propose the following non-genomic mechanisms of action of the neuropathic pain-relieving actions of PPARγ agonists: 1) inhibition of neuronal sensitization, consistent with prevention of p-p38 and touch stimulus-evoked pERK expression in dorsal horn (Griggs et al., 2015; Morgenweck et al., 2013); 2) inhibition of astrocyte activation, consistent with inhibition of injury-induced GFAP expression in dorsal horn (Griggs et al., 2015; Zhong et al., 2019); 3) inhibition of microglial activation, consistent with inhibition of injury-induced Iba1 expression in dorsal horn (Iwai et al., 2008; Park et al., 2007), though it must be kept in mind that spinal microglia may not be required for neuropathic mechanical hypersensitivity in females (Sorge et al., 2015); 4) inhibition of neuroinflammatory cytokines in the injured nerve, consistent with normalization of TNFα and IL6 levels (Jia et al., 2013; Murad and Ayuob, 2015; Zhong et al., 2019). Support for these mechanisms comes from studies conducted in male rodents, and so future studies are necessary to determine whether each of these mechanisms also operate in females.
An emerging body of literature is revealing sex differences in the mechanisms by which neuropathic pain develops in male and female mice (Sorge et al., 2015) and rats (Mapplebeck et al., 2018). Here we included both male and female subjects and demonstrate that pioglitazone more efficaciously reduced SNI-induced mechanical and cold hypersensitivity in female mice. Our findings are consistent with a previous report indicating that pioglitazone (albeit at a very high dose of 300 μg, i.t.) reduced SNI-induced mechanical hypersensitivity in a PPARγ-dependent manner by less than 20% in males but by more than 60% in females (Sorge et al., 2015). These findings are reminiscent of a recent study in a spinal cord injury model of central neuropathic pain by Gensel et al (Gensel et al., 2019), who found that systemic administration of pioglitazone (10 mg/kg) decreased heat hypersensitivity in female but not male mice. Sorge et al posited that T cells or microglia are required for mechanical hypersensitivity in female or male mice, respectively (Sorge et al., 2015). In support of this, the endogenous levels of sex hormones in females up-regulate the expression of PPARγ in T cells to a greater extent in females as compared to males (Park and Choi, 2017; Park et al., 2016). However, the hypothesis that pioglitazone decreases neuropathic pain with greater efficacy in females due to the greater expression and activation of T cells requires definitive evidence of a causal link. This experimental direction has the potential to further our understanding of whether sex-specific differences in immune function contribute to the anti-allodynic mechanisms of pioglitazone in the setting of peripheral nerve injury.
4.4. Pioglitazone plus morphine inhibits neuropathic pain with greater efficacy in female mice
Pioglitazone may reduce dosage requirements of drugs that are commonly used for the treatment of pain. For example, the combination of pioglitazone with fluoxetine, a selective serotonin reuptake inhibitor, decreased behavioral and molecular signs of neuropathic pain after peripheral nerve injury (reversing heat hypersensitivity, spinal cord GFAP expression, serum TNF-α, IL-6, and MCP-1) to a greater degree as compared to either fluoxetine or pioglitazone alone (Murad and Ayuob, 2015). The current results indicate that pioglitazone might lower the dosage requirement for opioid analgesics as well. We found that the combination of pioglitazone with morphine decreased mechanical and cold hypersensitivity at doses that by themselves had no efficacy. This effect was restricted to females, consistent with sex differences in the analgesic actions of pioglitazone (Sorge et al., 2015) and opioids (Craft, 2003).
4.5. Pioglitazone decreased behavioral signs of PDN with greater efficacy in female db/db
Duloxetine and pregabalin are the only FDA-approved drugs available for the treatment of pain in PDN (Otto et al., 2011; Vincent et al., 2011), despite weak efficacy in just a small subset of patients, a new pharmacotherapeutic approach is needed. The current study indicates that a single injection of pioglitazone rapidly (within 30–60 minutes) decreased heat hypersensitivity in the ZDF rat and db/db mouse models of PDN in a sex-dependent manner, with greater efficacy in female db/db mice. Interestingly, pioglitazone accumulates in adipose tissue to a great extent in female rats compared to male rats after a single oral dose (10 mg/kg) or repeated oral administration (10 mg/kg for 21 consecutive days) (Fujita et al., 2003), perhaps leading to greater reductions in glycated hemoglobin (HbA1c) (Tajiri et al., 2007), and efficacy of pioglitazone in female as compared to male patients with nonalcoholic fatty liver disease or abnormal glucose metabolism in type 2 diabetes (Yan et al., 2021). Whether adipose tissue could be a target for the sex-dependent antihyperalgesic action of pioglitazone is an interesting question for future studies to address.
4.6. Neural sites of analgesic action of systemic pioglitazone
Pioglitazone has well-described binding affinity and efficacy at PPARγ. Thus it is not surprising that PPARg-selective receptor antagonists such as GW9662 prevent the antihyperalgesic actions of pioglitazone in experimental models of neuropathic pain (Griggs et al., 2015), bone cancer pain (Gu et al., 2020), chemical-induced pain (Mansouri et al., 2017), inflammatory joint pain (Ruiz-Miyazawa et al., 2018), and inflammatory muscle pain (Santos et al., 2021). PPARγ is expressed in numerous pain processing regions of the central nervous system that include peripheral nerves (Zhou et al., 2019 https://pubmed.ncbi.nlm.nih.gov/31360147/), spinal cord (Elkholy et al., 2020; Jiang et al., 2021) and supraspinal sites (Okine et al., 2018). Each of these sites could be a target of systemic pioglitazone. Pioglitazone is permeant through the blood-brain barrier, and therefore systemic administration can lead to significant concentrations not only in the periphery but also the spinal cord and brain.
Peripheral nerves.
PPARγ agonists inhibit behavioral signs of persistent pain when delivered to the plantar site of inflammation. For example, in the plantar formalin test, systemic administration of pioglitazone reduced the persistent phase of nociresponsive behaviors in mice, and both acute and persistent phase in rats (Oliveira et al., 2007). Likewise, local administration of rosiglitazone reduced mechanical and heat hypersensitivity in the plantar incision model in mice (Hasegawa-Moriyama et al., 2012), and mechanical hypersensitivity after intraplantar injection of CFA in rats (Hasegawa-Moriyama et al., 2013). Finally, local administration of the endogenous PPARγ agonist 15d-PGJ2 reduced: 1) mechanical hyperalgesia following the injection of carrageenan under that plantar skin (Napimoga et al., 2008) or prior to the injection of carrageenan into the gastrocnemius muscle of rats (Santos et al., 2021); 2) mechanical hyperalgesia induced by intra-articular collagen in rat (Carregaro et al., 2016); and 3) the persistent phase of ongoing nociresponsive behaviors after dilute formalin injection into the temporomandibular joint (but not into the paw) in rats (Napimoga et al., 2008; Pena-Dos-Santos et al., 2009).
In the setting of inflammation, immune cells can increase mechanical sensitivity at peripheral nerve terminals in the spinal cord through the release of pronociceptive neuropeptides and neurotransmitters (Pinho-Ribeiro et al., 2017). An alternative peripheral mechanism of analgesic action of PPARγ agonists includes the downregulation of pro-inflammatory signaling in local macrophages, as observed in the intraplantar carrageenan model (Napimoga et al., 2008). Furthermore, rosiglitazone downregulated NF-κB phosphorylation and decreased the M1-macrophage-associated marker IL-1β, while increasing the M2-macrophage-associated marker interleukin IL-10 in the plantar incision model in male mice (Hasegawa-Moriyama et al., 2012). These results suggest that PPARγ agonists may facilitate the polarization of macrophages from the M1 to the M2 phenotype, leading to inhibition of inflammation and pain. In summary, the antihyperalgesic actions of systemic pioglitazone, as we report here in the methylglyoxal and neuropathic pain models, might results from peripheral actions at the site of injury.
Dorsal horn.
Intrathecal administration of PPARg agonists decreases behavioral signs of persistent pain not only in the plantar incision model as described here, but also after nerve injury. For example, intrathecal pioglitazone decreased not only allodynia but also a natural stimulus-evoked activation of presumably nociresponsive dorsal horn neurons (Griggs et al., 2015). After nerve injury, the development of allodynia is correlated with the downregulation of PPARγ expression in the spinal cord dorsal horn (Jiang et al., 2021) in a time-dependent manner (Zhong et al., 2019). Further support for a spinal site of actions comes from reports that intrathecal administration of the PPARγ antagonist BADGE (Fehrenbacher et al., 2009) and GW9662 inhibited the anti-hyperalgesic effect of rosiglitazone or 15d-PGJ2 (Churi et al., 2008). In summary, the antihyperalgesic actions of intrathecal or systemic pioglitazone as described here in the methylglyoxal, postsurgical and neuropathic pain models might result from actions at peripheral nerve.
Brain.
We previously reported that intracerebroventricular administration of the PPARg agonists rosiglitazone or 15d-PGJ2 reduced plantar carrageenan-induced paw edema, hypersensitivity and dorsal horn expression of the immediate-early gene c-fos (Morgenweck et al., 2010). Since then, an emerging body of literature is beginning to identify specific brain regions (Okine et al., 2018). For example, microinjection of the PPARg inhibitor GW9662 in the anterior cingulate cortex reduced formalin-evoked nociceptive response, suggesting the endogenous PPARg agonists facilitate chemical pain (Okine et al., 2017).
4.7. Clinical Significance
More research is needed to identify peripheral, spinal and supraspinal mechanisms and sites of action of PPARg ligands in the setting of surgical incision, peripheral nerve injury, and painful diabetic neuropathy. Since pioglitazone is FDA approved and readily available, the current results promote its repurposing for chronic pain, particularly in women. Pioglitazone provides clinical benefit for patients with insulin resistance, and so repurposing it for patients who have PDN as well seems a reasonable goal.
Supplementary Material
Supplementary Figure 1. Pioglitazone reduced hat hyperalgesia in male ZDF rats. Decrease in heat latency in ZDF (open bar) and ZL rats (filled bar) after vehicle or pioglitazone (10–100 mg/kg, i.p). Values are plotted as the difference between values collected before injection and those collected 1–2 hour after injection. Two-way ANOVA followed by Sidak’s multiple comparisons test. Symbol *p<0.05. Values represent mean ± SEM. N=5.
Highlights.
Pioglitazone inhibits chemical nociception with greater potency in female mice
Pioglitazone inhibits post-surgical pain with greater potency in female mice
Pioglitazone inhibits neuropathic pain with greater efficacy in female mice
Pioglitazone inhibits PDN with greater efficacy in female db/db mice
These results promote the repurposing of pioglitazone for chronic pain in women
5. Acknowledgments
This study was supported by NIH grants R01NS62306, R01NS45954, and R01DA37621 to BKT, and R01NS107398 to Keichiro Susuki and BKT. The Graphical Abstract was created with BioRender.com. We thank Charles River Laboratories for providing the rodent images used in the Graphical Abstract.
Declarations of interest:
This study was supported by R01NS62306, R01NS45954, R01DA37621 to BKT and R01NS107398 to K Susuki and BKT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6 REFERENCES
- Abraham A, Barnett C, Katzberg HD, Lovblom LE, Perkins BA, Bril V, 2018. Sex differences in neuropathic pain intensity in diabetes. J Neurol Sci 388, 103–106. [DOI] [PubMed] [Google Scholar]
- Aubrun F, Salvi N, Coriat P, Riou B, 2005. Sex- and Age-related Differences in Morphine Requirements for Postoperative Pain Relief. Anesthesiology 103, 156–160. [DOI] [PubMed] [Google Scholar]
- Averbuch M, Katzper M, 2000. A Search for Sex Differences in Response to Analgesia. Archives of Internal Medicine 160, 3424–3428. [DOI] [PubMed] [Google Scholar]
- Becker AK, Auditore A, Pischetsrieder M, Messlinger K, Fleming T, Reeh PW, Sauer SK, 2020. Reactive dicarbonyl compounds cause Calcitonin Gene-Related Peptide release and synergize with inflammatory conditions in mouse skin and peritoneum. J Biol Chem 295, 6330–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnolzer M, Lasitschka F, Neuhuber WL, Kichko TI, Konrade I, Elvert R, Mier W, Pirags V, Lukic IK, Morcos M, Dehmer T, Rabbani N, Thornalley PJ, Edelstein D, Nau C, Forbes J, Humpert PM, Schwaninger M, Ziegler D, Stern DM, Cooper ME, Haberkorn U, Brownlee M, Reeh PW, Nawroth PP, 2012. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med 18, 926–933. [DOI] [PubMed] [Google Scholar]
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C, 2008. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136, 380–387. [DOI] [PubMed] [Google Scholar]
- Carregaro V, Napimoga MH, Peres RS, Benevides L, Sacramento LA, Pinto LG, Grespan R, Cunha TM, da Silva JS, Cunha FQ, 2016. Therapeutic Treatment of Arthritic Mice with 15-Deoxy Delta(12,14)-Prostaglandin J2 (15d-PGJ2) Ameliorates Disease through the Suppression of Th17 Cells and the Induction of CD4(+)CD25(−)FOXP3(+) Cells. Mediators Inflamm 2016, 9626427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, 1994. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53, 55–63. [DOI] [PubMed] [Google Scholar]
- Chia Y-Y, Chow L-H, Hung C-C, Liu K, Ger L-P, Wang P-N, 2002. Gender and pain upon movement are associated with the requirements for postoperative patient-controllediv analgesia: a prospective survey of 2,298 Chinese patients. Canadian Journal of Anesthesia 49, 249. [DOI] [PubMed] [Google Scholar]
- Chinnam P, Mohsin M, Shafee LM, 2012. Evaluation of acute toxicity of pioglitazone in mice. Toxicol Int 19, 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churi SB, Abdel-Aleem OS, Tumber KK, Scuderi-Porter H, Taylor BK, 2008. Intrathecal rosiglitazone acts at peroxisome proliferator-activated receptor-gamma to rapidly inhibit neuropathic pain in rats. J Pain 9, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JB, Palmer CJ, Shaw WN, 1983. The diabetic Zucker fatty rat. Proc Soc Exp Biol Med 173, 68–75. [DOI] [PubMed] [Google Scholar]
- Coleman DL, 1978. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14, 141–148. [DOI] [PubMed] [Google Scholar]
- Craft RM, 2003. Sex Differences in Opioid Analgesia: “From Mouse to Man”. The Clinical Journal of Pain 19, 175–186. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Scuppa G, Stopponi S, Demopulos G, Gaitanaris G, Ciccocioppo R, 2014. Analgesic tolerance to morphine is regulated by PPARgamma. Br J Pharmacol 171, 5407–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ, 2000. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, 1965. The Up-and-Down Method for Small Samples. Journal of the American Statistical Association 60, 967–978. [Google Scholar]
- Elkholy SE, Elaidy SM, El-Sherbeeny NA, Toraih EA, El-Gawly HW, 2020. Neuroprotective effects of ranolazine versus pioglitazone in experimental diabetic neuropathy: Targeting Nav1.7 channels and PPAR-γ. Life Sci 250, 117557. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Loverme J, Clarke W, Hargreaves KM, Piomelli D, Taylor BK, 2009. Rapid pain modulation with nuclear receptor ligands. Brain Res Rev 60, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V, 2019. Diabetic neuropathy. Nat Rev Dis Primers 5, 41. [DOI] [PubMed] [Google Scholar]
- Fu W, Wessel CR, Taylor BK, 2020. Neuropeptide Y tonically inhibits an NMDAR➔AC1➔TRPA1/TRPV1 mechanism of the affective dimension of chronic neuropathic pain. Neuropeptides 80, 102024–102024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Yamada Y, Kusama M, Yamauchi T, Kamon J, Kadowaki T, Iga T, 2003. Sex differences in the pharmacokinetics of pioglitazone in rats. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 136, 85–94. [DOI] [PubMed] [Google Scholar]
- Gear RW, Gordon NC, Heller PH, Paul S, Miaskowski C, Levine JD, 1996a. Gender difference in analgesic response to the kappa-opioid pentazocine. Neuroscience Letters 205, 207–209. [DOI] [PubMed] [Google Scholar]
- Gear RW, Gordon NC, Miaskowski C, Paul SM, Heller PH, Levine JD, 2003. Sexual dimorphism in very low dose nalbuphine postoperative analgesia. Neuroscience Letters 339, 1–4. [DOI] [PubMed] [Google Scholar]
- Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD, 1996b. Kappa–opioids produce significantly greater analgesia in women than in men. Nature Medicine 2, 1248–1250. [DOI] [PubMed] [Google Scholar]
- Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD, 1999. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain 83. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Donahue RR, Bailey WM, Taylor BK, 2019. Sexual Dimorphism of Pain Control: Analgesic Effects of Pioglitazone and Azithromycin in Chronic Spinal Cord Injury. Journal of neurotrauma 36, 2372–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavimi H, Hassanzadeh K, Maleki-Dizaji N, Azarfardian A, Ghasami S, Zolali E, Charkhpour M, 2014. Pioglitazone prevents morphine antinociception tolerance and withdrawal symptoms in rats. Naunyn Schmiedebergs Arch Pharmacol 387, 811–821. [DOI] [PubMed] [Google Scholar]
- Grace PM, Tawfik VL, Svensson CI, Burton MD, Loggia ML, Hutchinson MR, 2021. The Neuroimmunology of Chronic Pain: From Rodents to Humans. J Neurosci 41, 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ, Consensus Working Group of the Sex, G., Pain, S. I. G. o. t. I., 2007. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132 Suppl 1, S26–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs RB, Donahue RR, Adkins BG, Anderson KL, Thibault O, Taylor BK, 2016. Pioglitazone Inhibits the Development of Hyperalgesia and Sensitization of Spinal Nociresponsive Neurons in Type 2 Diabetes. J Pain 17, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs RB, Donahue RR, Morgenweck J, Grace PM, Sutton A, Watkins LR, Taylor BK, 2015. Pioglitazone rapidly reduces neuropathic pain through astrocyte and nongenomic PPARgamma mechanisms. Pain 156, 469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs RB, Santos DF, Laird DE, Doolen S, Donahue RR, Wessel CR, Fu W, Sinha GP, Wang P, Zhou J, Brings S, Fleming T, Nawroth PP, Susuki K, Taylor BK, 2019. Methylglyoxal and a spinal TRPA1-AC1-Epac cascade facilitate pain in the db/db mouse model of type 2 diabetes. Neurobiol Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Sun Y, Gu W, Huang Y, Bo J, Zhou L, Ma Z, Gu X, Zhang W, 2020. The analgesic effects of pioglitazone in the bone cancer pain rats via regulating the PPARγ/PTEN/mTOR signaling pathway in the spinal dorsal horn. Biomed Pharmacother 131, 110692. [DOI] [PubMed] [Google Scholar]
- Hasegawa-Moriyama M, Kurimoto T, Nakama M, Godai K, Kojima M, Kuwaki T, Kanmura Y, 2013. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuates inflammatory pain through the induction of heme oxygenase-1 in macrophages. Pain 154, 1402–1412. [DOI] [PubMed] [Google Scholar]
- Hasegawa-Moriyama M, Ohnou T, Godai K, Kurimoto T, Nakama M, Kanmura Y, 2012. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuates postincisional pain by regulating macrophage polarization. Biochemical and biophysical research communications 426, 76–82. [DOI] [PubMed] [Google Scholar]
- Iwai S, Maeda T, Kiguchi N, Kobayashi Y, Fukazawa Y, Ozaki M, Kishioka S, 2008. Pioglitazone attenuates tactile allodynia and microglial activation in mice with peripheral nerve injury. Drug Discov Ther. 2, 353–356. [PubMed] [Google Scholar]
- Jang JH, Liang D, Kido K, Sun Y, Clark DJ, Brennan TJ, 2011. Increased local concentration of complement C5a contributes to incisional pain in mice. J Neuroinflammation 8, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HB, Wang XM, Qiu LL, Liu XY, Shen JC, Ji Q, Yang JJ, 2013. Spinal neuroimmune activation inhibited by repeated administration of pioglitazone in rats after L5 spinal nerve transection. Neurosci Lett 543, 130–135. [DOI] [PubMed] [Google Scholar]
- Jiang P, Jiang Q, Yan Y, Hou Z, Luo D, 2021. Propofol ameliorates neuropathic pain and neuroinflammation through PPAR γ up-regulation to block Wnt/β-catenin pathway. Neurol Res 43, 71–77. [DOI] [PubMed] [Google Scholar]
- Khasabova IA, Khasabov SG, Olson JK, Uhelski ML, Kim AH, Albino-Ramírez AM, Wagner CL, Seybold VS, Simone DA, 2019. Pioglitazone, a PPARγ agonist, reduces cisplatin-evoked neuropathic pain by protecting against oxidative stress. Pain 160, 688–701. [DOI] [PubMed] [Google Scholar]
- Kroin JS, Buvanendran A, Nagalla SKS, Tuman KJ, 2003. Postoperative pain and analgesic responses are similar in male and female Sprague-Dawley rats. Canadian Journal of Anesthesia 50, 904–908. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Kobayashi Y, Ozaki M, Kishioka S, 2008. Pioglitazone attenuates tactile allodynia and thermal hyperalgesia in mice subjected to peripheral nerve injury. J Pharmacol Sci 108, 341–347. [DOI] [PubMed] [Google Scholar]
- Maeshiba Y, Kiyota Y, Yamashita K, Yoshimura Y, Motohashi M, Tanayama S, 1997. Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittelforschung 47, 29–35. [PubMed] [Google Scholar]
- Mansouri MT, Naghizadeh B, Ghorbanzadeh B, Rajabi H, Pashmforoush M, 2017. Pharmacological evidence for systemic and peripheral antinociceptive activities of pioglitazone in the rat formalin test: Role of PPARγ and nitric oxide. European Journal of Pharmacology 805, 84–92. [DOI] [PubMed] [Google Scholar]
- Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, Assi S, Mogil JS, Trang T, Salter MW, 2018. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain 159, 1752–1763. [DOI] [PubMed] [Google Scholar]
- Matheson J, Le Foll B, 2020. Therapeutic Potential of Peroxisome Proliferator-Activated Receptor (PPAR) Agonists in Substance Use Disorders: A Synthesis of Preclinical and Human Evidence. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenweck J, Griggs RB, Donahue RR, Zadina JE, Taylor BK, 2013. PPARgamma activation blocks development and reduces established neuropathic pain in rats. Neuropharmacology 70, 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad H, Ayuob N, 2015. Co-Administration of Pioglitazone Improves Fluoxetine’s Antinociceptive, Neuroprotective, and Antidepressant Effects in Chronic Constriction Injury in Rats. Pain Physician 18, 609–620. [PubMed] [Google Scholar]
- Napimoga MH, Souza GR, Cunha TM, Ferrari LF, Clemente-Napimoga JT, Parada CA, Verri WA Jr., Cunha FQ, Ferreira SH, 2008. 15d-prostaglandin J2 inhibits inflammatory hypernociception: involvement of peripheral opioid receptor. J Pharmacol Exp Ther 324, 313–321. [DOI] [PubMed] [Google Scholar]
- Niesters M, Dahan A, Kest B, Zacny J, Stijnen T, Aarts L, Sarton E, 2010. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain 151, 61–68. [DOI] [PubMed] [Google Scholar]
- O’Brien PD, Sakowski SA, Feldman EL, 2014. Mouse models of diabetic neuropathy. ILAR journal 54, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okine BN, Gaspar JC, Finn DP, 2018. PPARs and pain. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okine BN, Gaspar JC, Madasu MK, Olango WM, Harhen B, Roche M, Finn DP, 2017. Characterisation of peroxisome proliferator-activated receptor signalling in the midbrain periaqueductal grey of rats genetically prone to heightened stress, negative affect and hyperalgesia. Brain Res 1657, 185–192. [DOI] [PubMed] [Google Scholar]
- Oliveira ACP, Bertollo CM, Rocha LTS, Nascimento EB, Costa KA, Coelho MM, 2007. Antinociceptive and antiedematogenic activities of fenofibrate, an agonist of PPAR alpha, and pioglitazone, an agonist of PPAR gamma. European Journal of Pharmacology 561, 194–201. [DOI] [PubMed] [Google Scholar]
- Otto KJ, Wyse BD, Cabot PJ, Smith MT, 2011. Longitudinal study of painful diabetic neuropathy in the Zucker diabetic fatty rat model of type 2 diabetes: impaired basal G-protein activity appears to underpin marked morphine hyposensitivity at 6 months. Pain Med 12, 437–450. [DOI] [PubMed] [Google Scholar]
- Park HJ, Choi JM, 2017. Sex-specific regulation of immune responses by PPARs. Exp Mol Med 49, e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Park HS, Lee JU, Bothwell AL, Choi JM, 2016. Gender-specific differences in PPARgamma regulation of follicular helper T cell responses with estrogen. Sci Rep 6, 28495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R, 2007. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther 320, 1002–1012. [DOI] [PubMed] [Google Scholar]
- Pena-Dos-Santos DR, Severino FP, Pereira SAL, Rodrigues DBR, Cunha FQ, Vieira SM, Napimoga MH, Clemente-Napimoga JT, 2009. Activation of peripheral [kappa]/[delta] opioid receptors mediates 15-deoxy-[Delta]12,14-prostaglandin J2 induced-antinociception in rat temporomandibular joint. Neuroscience 163, 1211–1219. [DOI] [PubMed] [Google Scholar]
- Peng BY, Wang Q, Luo YH, He JF, Tan T, Zhu H, 2018. A novel and quick PCR-based method to genotype mice with a leptin receptor mutation (db/db mice). Acta Pharmacol Sin 39, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MP, Pogatzki-Zahn E, 2015. Gender aspects in postoperative pain. Curr Opin Anaesthesiol 28, 546–558. [DOI] [PubMed] [Google Scholar]
- Pinho-Ribeiro FA, Verri WA Jr., Chiu IM, 2017. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends in immunology 38, 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogatzki EM, Raja SN, 2003. A mouse model of incisional pain. Anesthesiology 99, 1023–1027. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM, 2005. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314, 1195–1201. [DOI] [PubMed] [Google Scholar]
- Raputova J, Srotova I, Vlckova E, Sommer C, Üçeyler N, Birklein F, Rittner HL, Rebhorn C, Adamova B, Kovalova I, Kralickova Nekvapilova E, Forer L, Belobradkova J, Olsovsky J, Weber P, Dusek L, Jarkovsky J, Bednarik J, 2017. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. Pain 158, 2340–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Miyazawa KW, Staurengo-Ferrari L, Pinho-Ribeiro FA, Fattori V, Zaninelli TH, Badaro-Garcia S, Borghi SM, Andrade KC, Clemente-Napimoga JT, Alves-Filho JC, Cunha TM, Fraceto LF, Cunha FQ, Napimoga MH, Casagrande R, Verri WA, 2018. 15d-PGJ2-loaded nanocapsules ameliorate experimental gout arthritis by reducing pain and inflammation in a PPARgamma-sensitive manner in mice. Scientific Reports 8, 13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JL, Jureidini B, Hodges JS, Baisden M, Swift JQ, Bowles WR, 2008. Gender Differences in Analgesia for Endodontic Pain. Journal of Endodontics 34, 552–556. [DOI] [PubMed] [Google Scholar]
- Santos DFS, Melo-Aquino B, Jorge CO, Clemente-Napimoga JT, Taylor BK, Oliveira-Fusaro MCG, 2021. Prostaglandin 15d-PGJ2 targets PPARγ and opioid receptors to prevent muscle hyperalgesia in rats. Neuroreport 32, 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L, Dahan A, 2000. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology 93, 1245–1254; discussion 1246A. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Saucier DA, Cain ME, 2002. A statistical method for analyzing rating scale data: the BBB locomotor score. Journal of neurotrauma 19, 1251–1260. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS, 2015. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 18, 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri Y, Takei R, Mimura K, Umeda F, 2007. Indicators for the efficacy of pioglitazone before and during treatment in Japanese patients with type 2 diabetes. Diabetes Technol Ther 9, 429–437. [DOI] [PubMed] [Google Scholar]
- Takada N, Genda K, 2019. Pioglitazone (Actos, Glustin). In: Nagaoka S, (Ed), Drug Discovery in Japan: Investigating the Sources of Innovation. Springer; Singapore, Singapore, pp. 169–181. [Google Scholar]
- Takahashi Y, Hasegawa-Moriyama M, Sakurai T, Inada E, 2011. The macrophage-mediated effects of the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuate tactile allodynia in the early phase of neuropathic pain development. Anesth Analg 113, 398–404. [DOI] [PubMed] [Google Scholar]
- Uchiyama K, Kawai M, Tani M, Ueno M, Hama T, Yamaue H, 2006. Gender differences in postoperative pain after laparoscopic cholecystectomy. Surgical Endoscopy And Other Interventional Techniques 20, 448–451. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Callaghan BC, Smith AL, Feldman EL, 2011. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nature reviews. Neurology 7, 573–583. [DOI] [PubMed] [Google Scholar]
- Yan H, Wu W, Chang X, Xia M, Ma S, Wang L, Gao J, 2021. Gender differences in the efficacy of pioglitazone treatment in nonalcoholic fatty liver disease patients with abnormal glucose metabolism. Biology of Sex Differences 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Chen J, Chen J, Chen Y, Li L, Xie Y, 2019. Crosstalk between Cdk5/p35 and ERK1/2 signalling mediates spinal astrocyte activity via the PPARgamma pathway in a rat model of chronic constriction injury. J Neurochem 151, 166–184. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, 1983. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16, 109–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Pioglitazone reduced hat hyperalgesia in male ZDF rats. Decrease in heat latency in ZDF (open bar) and ZL rats (filled bar) after vehicle or pioglitazone (10–100 mg/kg, i.p). Values are plotted as the difference between values collected before injection and those collected 1–2 hour after injection. Two-way ANOVA followed by Sidak’s multiple comparisons test. Symbol *p<0.05. Values represent mean ± SEM. N=5.


