Abstract
Background:
Adrenal and sex hormone dysregulation have been independently associated with increased depression and anxiety. Cortisol can modify production of sex hormones and hormone-mood associations. This study evaluated associations and interplay of sex and adrenal hormones with depression and anxiety.
Methods:
We assessed 545 Ecuadorian adolescents (11–17y, 50.4% female, ESPINA) for depression and anxiety symptoms using standardized scales. Testosterone, cortisol, dehydroepiandrosterone (DHEA), and estradiol (boys only) were measured in saliva. We performed logistic regression modeling to calculate odds ratios (OR) of elevated depression or anxiety (scores ≥60) comparing participants with low (<10th percentile) and elevated hormones (≥90th percentile) to normal concentrations (10th-90th percentile). Effect modification by cortisol and testosterone was assessed. Models adjusted for demographic, anthropometric, and circadian measures.
Results:
In all participants, elevated testosterone (OR [95%CI:]=1.78 [0.98, 3.23]) and cortisol (OR=1.69 [0.95, 2.99]) were marginally associated with elevated anxiety scores. In boys, elevated estradiol was associated with elevated depression (OR=4.75 [1.95, 11.56]) and anxiety scores (OR=2.43 [1.01, 5.84]). In linear regression, estradiol was positively associated with depression (difference/10% hormone increase (β=0.45 [0.15, 0.75]) and anxiety scores (β=0.42 [0.13, 0.72]). Higher cortisol levels strengthened the depression association with estradiol in boys (β=0.54 [0.12, 0.96]), and with testosterone (β= −0.19 [−0.35, −0.03]) and DHEA (β= −0.12 [−0.22, −0.02]) in girls. Testosterone also modified associations.
Limitations:
This was a cross-sectional analysis.
Discussion:
Elevated testosterone, cortisol, and estradiol (≥90th percentile) were associated with altered mood. Cortisol and testosterone were considerable effect modifiers to the associations of most hormones with depression and anxiety.
Keywords: Depression, anxiety, adrenal hormones, gonadal hormones, adolescents, Ecuador
1. Introduction
Nearly 20% of children are affected with mental health disorders, in particular anxiety and depression, yet the underlying causes of these mood disorders remain generally unknown.1–3 Given childhood depression and anxiety incidence is correlated with both reoccurrence in adulthood and lifetime disease, it is a key stage that can be targeted to identify factors which lead to the development of these conditions.4,5 Hyperactivity of the hypothalamus-pituitary-adrenal (HPA) axis is one of the most consistent biological findings in major depression and anxiety, and is thought to be related to increased secretion of cortisol.6,7 However, the adrenal hormone dehydroepiandrosterone (DHEA) has not been studied thoroughly on its association with depression and anxiety and has yielded inconsistent associations: positive,8 null, 9 and negative.10 The hypothalamus-pituitary-gonadal (HPG) axis is also associated with internalizing behaviors, varying by gender and age.
For females, there is a negative association between serum 17-β estradiol (estradiol) concentrations and severity of depressive syndromes,11,12 while in adult men younger than 60 years old, higher estradiol level was associated with increased depressive symptomology, whereas in older men there were no differences.13 Testosterone has also been found to modulate depression and anxiety symptoms. In women, a parabolic (U-shaped) association between testosterone concentration and depression has been observed, as both low and high testosterone concentrations were previously associated with depression.14 Although there have been some conflicting findings, most studies identify that testosterone replacement therapy can significantly improve ratings of depression and anxiety in androgen-deficient men,15 and has a generally positive anti-depressant effect when compared to placebo in adult men and women. 16
The HPA and HPG endocrine axes function in tandem and in a bi-directional manner (activation of one axis can modulate the effect of the other) in order to ensure proper homeostasis, thus, it is important to take a dual systems approach to determine the potential role of effect modification.17–19 It is important to understand the role of HPA and HPG interplay on depression and anxiety in adolescence, as the transition from puberty to adolescence can be described as an organizational window, which can influence brain development and its subsequent response to hormone levels in adulthood. The purpose of this study was to evaluate the independent relationship between levels of testosterone, estradiol, DHEA, and cortisol on depression and anxiety symptoms in adolescents, and to assess possible interactions between these hormones in such associations.
2. Methods
2.1. Participants
The study of Secondary Exposure to Pesticides among Children and Adolescents (ESPINA: Estudio de la Exposición Secundaria a Plaguicidas en Niños y Adolescentes) is a prospective cohort of children living in Pedro Moncayo County, Pichincha, Ecuador. ESPINA was established in 2008 to examine the associations of subclinical pesticide exposures on child development. In 2008, ESPINA included 313 children aged 4–9 years who were recruited using the 2004 Survey of Access and Demand of Health Services in Pedro Moncayo County (SADHS-PM), a representative survey of the region, or through community announcements. All participants were selected regardless of health status, but were primarily healthy volunteers.20 In July through October 2016, we carried out a follow-up examination of participants (n=238) and recruited new adolescent volunteers for a total of 535 participants of ages 11–17 years, using the System of Local and Community Information (SILC), a large geospatial database that contains information of the 2016 Pedro Moncayo County Community Survey (formerly the SADHS-PM). Details about both examinations have been published previously.20–22
The present analyses included 522 participants examined in 2016 who had all covariates of interest. These included age, z-score for body mass index (BMI)-for-age, parental education, household monthly income, hemoglobin concentration, awakening time, saliva collection time, and Tanner staging. One female participant reported being pregnant, and one female participant reported having leukemia. These participants were excluded because their endocrine levels were substantially different than the rest of the study population. Of this group, 512 completed the Multidimensional Anxiety Scale for Children 2nd Edition (MASC-2) (MHS Inc, North Tonawanda, NY) and 510 completed the Children’s Depression Inventory 2nd Edition (CDI-2) (MHS Inc, North Tonawanda, NY).
We acquired informed consent for participation from parents, parental permission for their children’s participation, and child assent from all adolescent participants. This study was approved by the institutional review boards at the University of California San Diego, Universidad San Francisco de Quito and the Ministry of Public Health of Ecuador.
2.2. Data collection
In 2016, children were examined in schools between July and October during the summer closure or during weekends. Height was measured to the nearest 1 mm following recommended procedures,23 and weight was measured using a digital scale (Tanita 0108MC; Corporation of America, Arlington Heights, IL, USA).23 Hemoglobin concentration was measured with the EQM Test-mate ChE Cholinesterase Test System 400 (EQM AChE Erythrocyte Cholinesterase Assay Kit 470; EQM research, Inc, Cincinnati, OH) from a finger-stick blood sample. Tanner staging measured sexual maturation based on self-reports of breast and pubic hair growth for girls, and pubic hair growth for boys using standard Tanner drawings. Information on income, education level, and other demographic information was reported by participants’ parents.
Anxiety and Depression Symptom Assessments.
Depression symptoms, were assessed using the CDI-2 short assessment, Spanish Version.24 The CDI-2 short has excellent psychometric properties and yields a score that is comparable to the score of the full-length version.24,25 Anxiety symptoms were assessed using the Spanish translated MASC-2 Child self-report. The MASC-2 indexes the range and severity of anxiety symptoms, has good psychometric properties and clinical utilities in identifying youth with anxiety disorders in Hispanic children.26,27 The MASC-2 English was translated into Spanish with input from community members and was approved by MHS Inc. Completed questionnaires were scored using the CDI-2 or MASC-2 Scoring Software (Multi-Health Systems [MHS] Inc). The scaled T-scores, standardized for age and gender for both the CDI-2 and MASC-2 were used in analyses.24
Sex and adrenal hormones.
Salivary concentrations of estradiol, testosterone, cortisol, and DHEA were measured using enzyme-linked immunosorbent assays (ELISA) (Salimetrics, Carlsbad, CA) at the University of California, San Diego Integrative Health and Mind-Body Biomarker Laboratory. Participants collected saliva samples through passive-drool upon awakening and the samples were stored at −80°C until assayed. Levels of cortisol, testosterone, and DHEA were measured in both girls and boys, while estradiol was only measured in boys, as the levels of estradiol in women vary according to the stage of the menstrual cycle.
2.3. Statistical analysis
We calculated the means and standard deviation (SD) of participant characteristics or the proportions of participants with a certain characteristic (as appropriate) for all participants and stratified by gender (Table 1). Differences in baseline characteristics by gender was determined by t-tests or Mann-Whitney U tests for normal or skewed variables, respectively. Pearson’s correlation coefficient matrix was used to statistically evaluate the relationship between all hormones. We used multiple linear regression to estimate linear associations of estradiol, testosterone, DHEA and cortisol concentrations with depression and anxiety scores (Tables 2, 3). Statistical significance was defined using an alpha of 0.05.
Table 1.
Participant characteristics.
| All | Female | Male | P-diff | |
|---|---|---|---|---|
| N | 522 | 262 (50.1%) | 260 (49.8%) | |
|
| ||||
| Age in summer 2016 | 14.5 (1.76) | 14.5 (1.8) | 14.4 (1.73) | 0.39 |
| Household income, USD | 581 (400400) | 597 (450) | 565 (342) | 0.35 |
| Parental education, Years | 8.1 (3.5) | 7.9 (3.4) | 8.3 (3.5) | 0.23 |
| Tanner score | 2.89 (0.95) | 2.85 (0.75) | 2.93 (1.12) | 0.34 |
| BMI-for-age z-score, SD | 0.39 (0.85) | 0.54 (0.77) | 0.23 (0.91) | <0.001 |
| Saliva collection time, hh:mm | 07:11 (1:12) | 07:08 (1:13) | 07:14 (1:11) | 0.31 |
| Awakening time, hh:mm | 06:44 (0:55) | 06:42 (0:52) | 06:47 (0:58) | 0.32 |
| Hemoglobin, mg/dL | 13.0 (1.2) | 12.6 (0.9) | 13.4 (1.3) | <0.001 |
| Cortisol, μg/dL a | 0.21 (0.14 – 0.30) | 0.20 (0.14 – 0.30) | 0.21 (0.13 – 0.30) | 0.75 |
| 17β-estradiol, pg/mL b | 0.43 (0.31 – 0.59) | - | 0.43 (0.30 – 0.59) | - |
| Testosterone, pg/mL c | 39.9 (26.1 – 67.3) | 32.1 (23.2 – 42.9) | 58.5 (32.5 – 99.3) | <0.001 |
| DHEA, pg/mL d | 61.9 (30.2 – 105.9) | 81.5 (45.9 – 133.1) | 42.5 (21.7 – 78.5) | <0.001 |
| Depression score e | 52.9 (9.3232) | 53.8 (8.95) | 52.1 (9.60) | 0.04 |
| Anxiety score f | 57.7 (9.6363) | 58.5 (9.41) | 56.8 (9.80) | 0.05 |
Values are percent, mean (SD) or median (25th - 75th percentile). USD = United States Dollar, BMI= Body Mass Index, hh:mm= hours and minutes, DHEA = dehydroepiandrosterone
n=520
n=253
n=517
n=499
n=510
n=512
Table 2.
Adjusted associations of sex and adrenal hormones with depression and anxiety scores for boys, overall and then stratified by medians of cortisol and testosterone. Generalized linear regression was conducted to identify difference in assessment score per 10% change in hormone level (95% CI).
| Difference in assessment score (β) per 10% increase in hormone level (95% CI), overall and stratified by cortisol medians (range) |
|||||
|---|---|---|---|---|---|
| Boys | |||||
| All |
Cortisol |
Testosterone |
|||
| Below Median (0.002 – 0.19 μg/dL) |
Above Median (0.20 – 1.17 μg/dL) |
Below Median (1.28 – 4.08 pg/mL) |
Above Median (4.09 – 6.45 pg/mL) |
||
| Depression (n=253) | |||||
| Testosterone | −0.01 (−0.10, 0.07) | −0.01 (−0.13, 0.11) | 0.01 (−0.12, 0.14) | - | - |
| DHEA | 0.02 (−0.04, 0.07) | 0.003 (−0.08, 0.09) | 0.06 (−0.02, 0.15) | −0.01 (−0.10, 0.08) | 0.04 (−0.04, 0.13) |
| Estradiol | 0.45 (0.15, 0.75) a | 0.28 (−0.16, 0.72) | 0.54 (0.12, 0.96) a | 0.13 (−0.33, 0.59) | 0.80 (0.39, 1.22) a |
| Cortisol | 0.15 (−0.31, 0.61) | - | - | −0.05 (−0.76, 0.75) | 0.45 (−0.19, 1.08) |
| Anxiety (n=254) | |||||
| Testosterone | 0.04 (−0.05, 0.12) | 0.11 (−0.00001, 0.22) a | −0.01 (−0.15, 0.13) | - | - |
| DHEA | −0.03 (−0.09, 0.03) | −0.01 (−0.08, 0.07) | −0.03 (−0.12, 0.06) | −0.12 (−0.21, −0.03) a | 0.001 (−0.07, 0.10) |
| Estradiol | 0.42 (0.13, 0.72) a | 0.31 (−0.09, 0.71) | 0.37 (−0.08, 0.82) | 0.44 (0.00, 0.87) a | 0.44 (0.00, 0.89) a |
| Cortisol | 0.23 (−0.23, 0.69) | - | - | −0.49 (−1.27, 0.29) | 0.59 (−0.06, 1.23) |
DHEA = dehydroepiandrosterone
p<0.05
Table 3.
Adjusted associations of sex and adrenal hormones with depression and anxiety scores for girls, overall and stratified by medians of cortisol and testosterone.
| Score difference (β) per 10% increase in hormone level (95% CI) |
|||||
|---|---|---|---|---|---|
| Girls | |||||
| All |
Cortisol |
Testosterone |
|||
| Below Median (0.002 – 0.19 μg/dL) |
Above Median (0.20 – 1.17 μg/dL) |
Below Median (2.01 – 3.49 μg/mL) |
Above Median (3.50 – 4.96 pg/mL) |
||
| Depression (n=259) | |||||
| Testosterone | −0.06 (−0.16, 0.03) | 0.04 (−0.10, 0.17) | −0.19 (−0.35, −0.03) a | - | - |
| DHEA | −0.02 (−0.98, 0.04) | 0.05 (−0.04, 0.134) | −0.12 (−0.22, −0.02) a | −0.03 (−0.14, 0.08) | 0.02 (−0.09, 0.12) |
| Cortisol | 0.25 (−0.15, 0.65) | - | - | 0.94 (0.22, 1.66) a | 0.04 (−0.50, 0.59) |
| Anxiety (n=260) | |||||
| Testosterone | 0.03 (−0.05, 0.12) | 0.02 (−0.14, 0.17) | −0.06 (−0.22, 0.10) | - | - |
| DHEA | −0.03 (−0.09, 0.02) | −0.03 (−0.12, 0.07) | 0.06 (−0.04, 0.16) | 0.04 (−0.08, 0.15) | −0.02 (−0.12, 0.08) |
| Cortisol | 0.01 (−0.42, 0.43) | - | - | 0.50 (−0.30, 1.32) | −0.26 (−0.79, 0.27) |
Adjusted for hemoglobin, saliva time, awakening time, age, BMI-for-age z-score, Tanner score, parental education, and household monthly income.
DHEA = dehydroepiandrosterone
p<0.05
We adjusted models for potential confounders defined a-priori including age, z-score for BMI-for-age (based on World Health Organization standards), parental education, household monthly income, hemoglobin concentration, and Tanner staging. Awakening time, saliva collection time were also included in the model to account for circadian effects. 28–30 BMI-for-age was included as a marker of chronic and subacute nutritional status which has been found to be associated with mental health status in adolescents.28 We used the World Health Organization growth standards to calculate the z-scores for BMI-for-age. Parental education was calculated as the average number of years of education of both parents. Household income is a component of socio-economic status and has been found to be independently associated with mental health outcomes.29,30 Awakening time, saliva collection time were also included in the model to account for circadian effects. Tanner staging is an indicator of pubertal status, and has been correlated with hormonal markers of puberty stage.31 All analyses were stratified by gender. Because hormone concentrations were not normally distributed, log-transformed hormone concentrations were used in the models. Model estimates were then multiplied by log(1.10) to assess whether a 10% change in hormone concentration was associated with a β-unit difference in depression or anxiety score
We assessed effect modification by cortisol, testosterone, DHEA and estradiol of the hormone-mental health associations through testing a multiplicative term (e.g. cortisol*hormone), and by stratification by median concentrations of testosterone and cortisol calculated separately for boys and girls. Curvilinear associations were assessed by testing quadratic and cubic terms for all hormones. Interaction by cortisol, was considered since stress and cortisol have inhibitory effects on estrogen and testosterone secretion,18 and stress can affect the mood of individuals. Interaction by testosterone was considered as it can modulate the stress response and HPA axis.18
We used logistic regression to calculate the odds ratios (OR) of elevated (T-score ≥60) depression and anxiety scores if participants had (age- and sex-specific) low (<10th percentile) or elevated hormone concentrations (≥90th percentile), compared to those who had normal levels (10th – 89th percentile). Percentile cut-offs were determined within quartiles of age, ranked separately for boys and girls then pooled. Logistic regression models included all participants and were also stratified by gender.
Median levels of hormone concentrations were calculated across cortisol and testosterone tertiles to assess hormone-hormone relationships (Table S2, S3). Also, linear regression models assessed the relationship between log-transformed cortisol concentration with the three-remaining log-transformed hormone concentrations, independently (i.e. cortisol-estradiol association), adjusting for age, z-score for body max index (BMI)-for-age, parental education, household monthly income, hemoglobin concentration, awakening time, saliva collection time, and Tanner staging. This was repeated using testosterone as the independent variable. As for the main analyses, the estimate was back transformed (anti-log) from their log-transformed values to identify whether a 10% increase in cortisol or testosterone concentration (independent variable) was associated with a percent change in hormone concentration (dependent variable). This was done by multiplying the estimate by log(1.10) All statistical analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Figures.
Forest plots for boys, girls, and pooled analyses were created showing the OR of low (<10th percentile) and elevated (≥90th percentile) hormone concentrations, when compared to individuals with normal concentrations (Figures 2). The linear association of estradiol with depression and anxiety scores were depicted by plotting the adjusted least squares means of depression and anxiety scores for 150 ranks of estradiol concentrations. We then used locally weighted polynomial regression (LOESS) to graph the adjusted relationships between the ranks of estradiol concentration and the anxiety and depression symptom scores.
Figure 2.
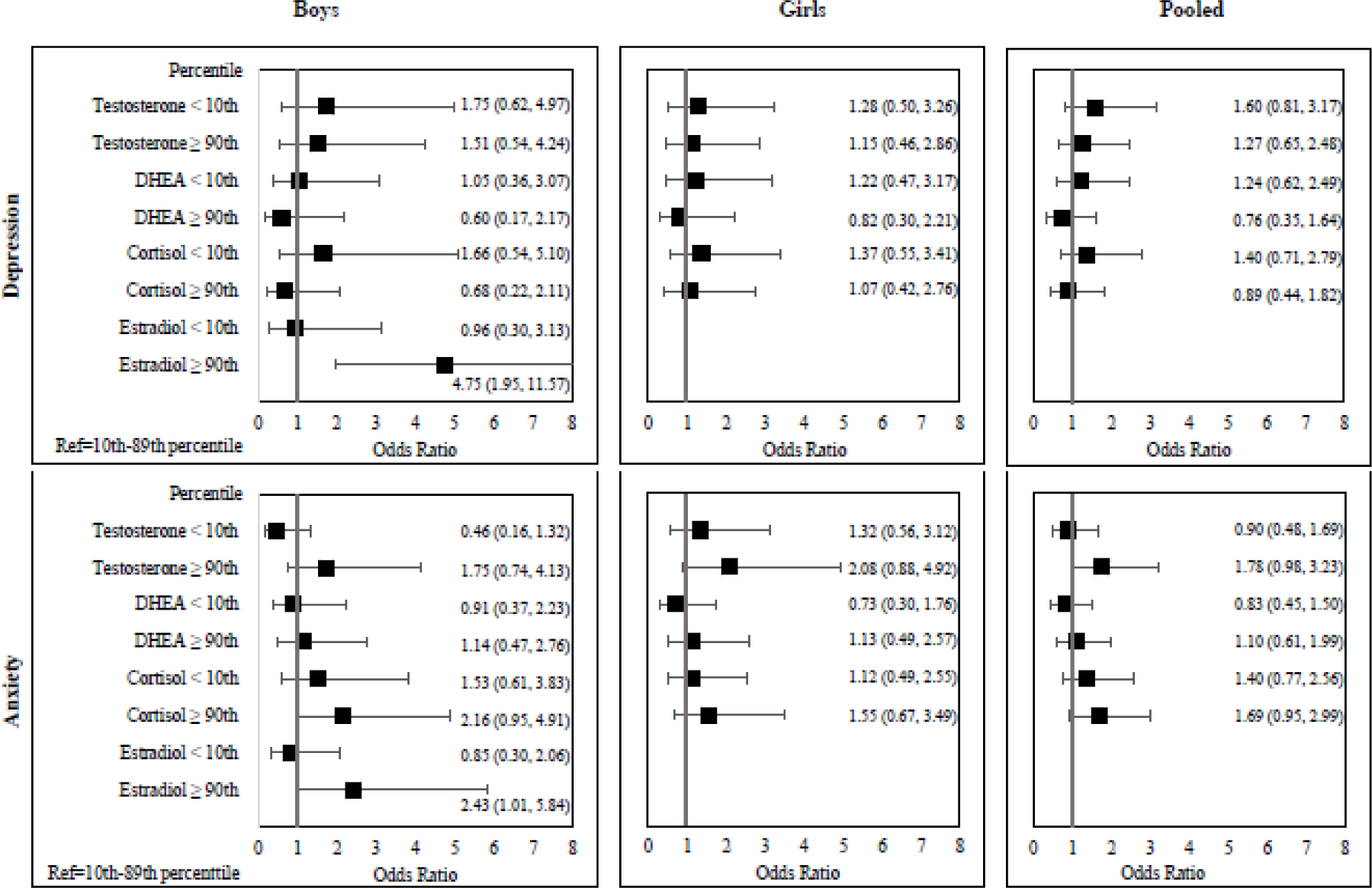
Odds ratios for elevated (T-score ≥60) depression and anxiety scores associated with elevated (≥90th percentile) or low (<10th percentile) hormone levels compared to those with hormone levels between the 10th–89th percentile.
Models adjusted for age, BMI-for-age z-score, parental education, household income, hemoglobin concentration, awakening time, saliva collection time, and sexual maturation.
2.4. Imputation of missing variables
To maximize the study’s sample, we imputed missing information of maternal education for 18 children and paternal education for 32 children as follows: for 10 children we imputed maternal education using maternal education reported in 2008, and for 27 children using paternal education reported in 2008. For the remaining eight children with missing maternal education, we conducted a random imputation based on a normal distribution of maternal education. For five of those eight children, we also imputed paternal education using random imputation based on a normal distribution of paternal education. Parental education was calculated as the average number of years of education of both parents. We also imputed household income for 13 children. Income values were imputed using 2008 information for five children. In 2008, income was collected as a 7-category variable. We calculated the group’s mean and SD of income in 2016 for each of the seven categories of income in 2008. We then imputed the 2016 income using a random normal distribution based on the mean (SD) appropriate for the reported 2008 income category. For the remaining eight children we imputed income based on the average of the mother’s and father’s education. We calculated the group’s mean (SD) income in 2016 for each year of education and imputed the 2016 income using a random normal distribution based on the mean (SD) appropriate for the corresponding parental years of education.
2. Results
3.1. Participant characteristics
The mean age of adolescents at the time of assessment was 14.5 years (SD=1.76) and 50.1% of the cohort were females. The average (SD) household monthly income was $581 ($400), and the average number of years of parental education was 8.1 years (3.5). The median [25th – 75th percentile] for sex and adrenal hormones and the mean (SD) depression and anxiety T-scores and other characteristics are listed in Table 1. Compared to males, female participants had higher BMI-for-age z-score, scores of anxiety and depression, and DHEA levels. Male participants had higher hemoglobin [13. mg/dL (1.3) vs 12.6 mg/dL (0.9), p<0.001] and testosterone concentrations [58.5 pg/mL vs 32.1 pg/mL, p<0.001] compared to females. These trends were expected, as hemoglobin and testosterone levels are naturally greater in males than females.32,33 A total of 49 males and 65 females had elevated depression scores (CDI-2 score ≥60). While 97 males and 128 females had elevated anxiety scores (MASC-2 scores ≥60). All hormones were significantly correlated with one another, except cortisol and estradiol in boys (Table S1).
3.2. Associations of hormones with depression and anxiety scores
The associations of sex and adrenal hormones with depression and anxiety scores in linear regression models are presented in Table 2 for boys and Table 3 for girls. Overall, only estradiol was significantly associated with both depression and anxiety scores in boys; For every 10% increase in estradiol concentration (β) there was an increase in the depression score by 0.45 units (95% CI: 0.15, 0.75) and anxiety score by 0.42 (95% CI: 0.13, 0.72) units (Table 2). The positive association of estradiol with depression and anxiety is shown in Figure 1.
Figure 1.
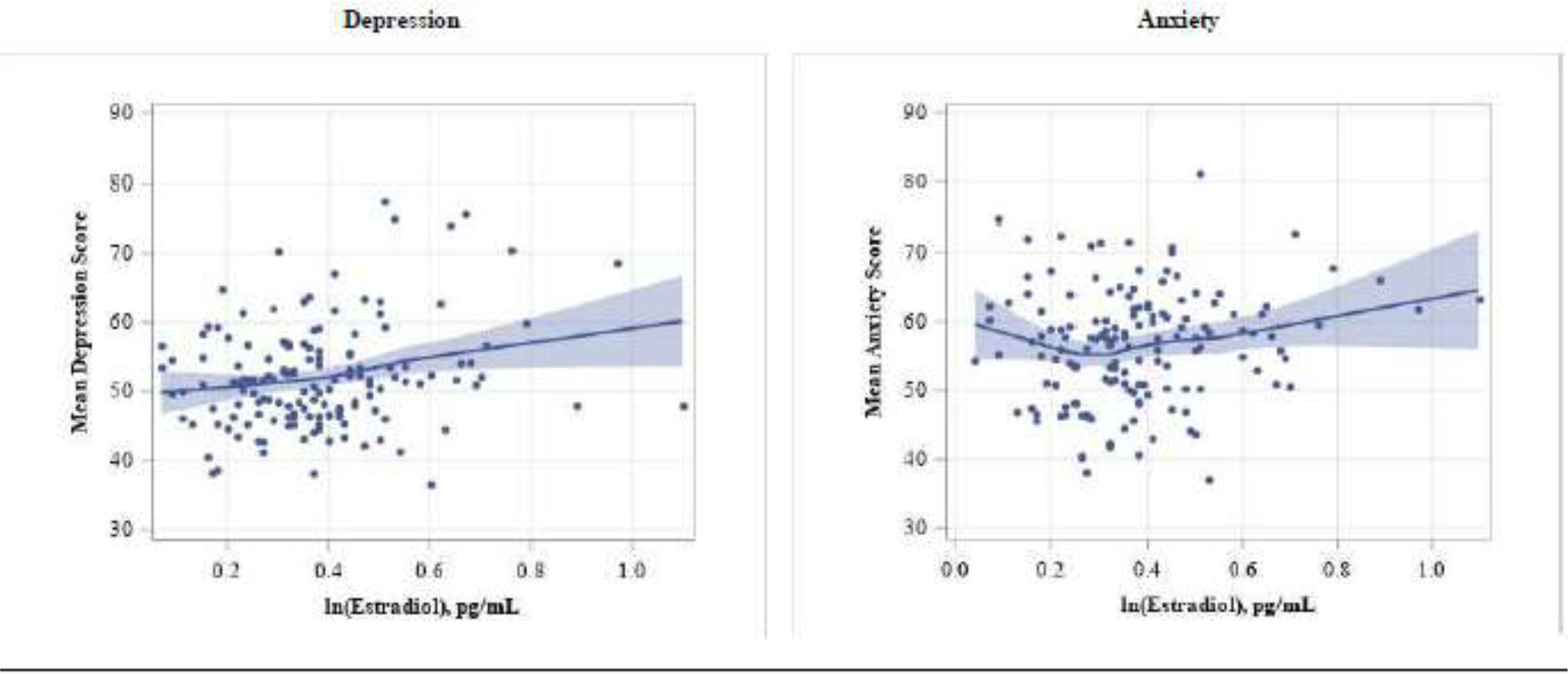
Locally weighted polynomial regression (LOESS) of adjusted associations of estradiol concentrations with depression and anxiety scores among boys.
Adjusted for age, BMI-for-age z-score, parental education, household income, hemoglobin concentration, awakening time, saliva collection time, and sexual maturation. Each data point represents the adjusted least squares of anxiety or depression score for 150 ranks of ln(estradiol) concentrations. The line represents the locally weighted fitted value, while the blue-grey shaded bands are the 95% confidence intervals across each value of the x axis.
Using logistic regression models, boys with elevated estradiol concentrations had increased odds of elevated depression (OR: 4.75 [95% CI: 1.95, 11.57]) and anxiety (OR=2.43 [1.01, 5.84]) scores, compared to boys with normal estradiol concentrations (Figure 2). Elevated cortisol in boys was marginally associated with increased odds of elevated anxiety (OR=2.16 [0.95, 4.91]) score, but not depression (OR=0.68 [0.22, 2.11]). There were no significant associations between any hormones with depression nor anxiety for girls. However, the association between elevated testosterone concentration and anxiety in girls did trend towards significance (OR=2.08 [0.88, 4.92]). After pooling boys and girls, elevated testosterone concentrations (OR=1.78 [0.98, 3.23]) and elevated cortisol concentrations (OR=1.69 [0.95, 2.99]) had borderline significant associations with elevated anxiety scores. Like the linear regression results, there were no associations between any of the hormones with depression nor anxiety for girls.
3.3. Effect modification by cortisol
There was effect modification by cortisol, and it affected the associations differently for males and females. Boys above the median of cortisol (AMC) had a stronger (more positive) estradiol-depression association (βAMC=0.54 [95% CI: 0.12, 0.96]) than those below the median of cortisol (BMC) (βBMC 0.28 [−0.16, 0.72], Table 2). The DHEA-depression association was also stronger, although not statistically significant in boys AMC. The testosterone -anxiety association was stronger in boys in below vs. above median of cortisol (β BMC= 0.11 [95% CI: −0.001, 0.22], βAMC= 0.01 [95% CI: −0.15, 0.13]).
Among girls, higher concentrations of cortisol also led to stronger (more negative) associations of depression with DHEA (βAMC =−0.12 [95% CI: −0.22, −0.02]) and testosterone (βAMC= −0.19 [95% CI: −0.35, −0.03]) compared to girls BMC. The relationship of testosterone with anxiety was also stronger, although non-significant, in girls AMC, whereas the DHEA-anxiety association did not differ by cortisol concentration (Table 3).
3.4. Effect modification by testosterone
Testosterone modified the associations differently among boys (Table 2) and girls (Table 3). For boys with levels above the median of testosterone (AMT) concentration, we observed stronger positive associations between estradiol and depression (βAMT= 0.80 [95% CI: 0.39, 1.22]) compared to the unstratified and AMC estimates. Stronger (positive) associations of cortisol with depression (βAMT= 0.45 [−0.19, 1.08]) and anxiety (βAMT= 0.59, [95% CI: −0.06, 1.23]) were also observed in AMT participants. Similarly, lower testosterone (below the median of testosterone [BMT]) concentrations in boys resulted in negative associations of cortisol (βBMT= −0.49 [−1.27, 0.29]) and DHEA (βBMT= −0.12 [−0.21, −0.03]) with anxiety. The only significant interaction term found was between DHEA and testosterone for boys expressing anxiety.
Contrary to findings in boys, lower testosterone concentrations in girls resulted in stronger positive associations between cortisol and depression (βBMT= 0.94, [95% CI: 0.22, 1.66]) and cortisol and anxiety (βBMT= 0.50, [95% CI: −0.30, 1.32]); however, the latter association was not statistically significant. The associations of DHEA with depression and anxiety did not differ by testosterone level in girls.
3.5. Associations of hormones with cortisol and testosterone concentrations
All hormones were significantly correlated with one another except the relationship between cortisol and estradiol in boys (r=0.09, Table S1). DHEA and testosterone in girls had the strongest relationship seen between all pairs (r=0.66, p<0.05). Cortisol concentrations were strongly positively associated with testosterone and DHEA in boys and girls (Table S2): in boys, there was a 15.7% (95%CI: 8.8%, 23.2%) increase in testosterone concentration and 16.3% (95%CI: 4.8%, 29.1%) increase in DHEA concentration for every 10% increase in cortisol. However, there was a minimal percent change observed for estradiol (β=1.73 [−1.41, 3.63]). In girls, there was a 17.2% (95%CI: 12.1%, 22.5%) increase in testosterone concentration and a 26.1% (95%CI: 17.4%, 35.5%) increase in DHEA concentration related to a 10% increase in cortisol concentration.
Estrogen, cortisol, and DHEA were also positively associated with testosterone concentration in boys and girls (Table S3). With a 10% increase in testosterone concentration, there was a small percent change in estradiol (β=0.90% [0.57, 1.23%]) and cortisol concentration (β=0.50% [0.29%, 0.71%]) in boys, and in cortisol for girls (β=0.95% [0.68%, 1.20%]). There was a larger percent change in DHEA levels for both boys (β=8.96% [3.97%, 10.98%]) and girls (β=10.1% [8.5%, 11.7%]) with a 10% increase in testosterone concentration.
4. Discussion
We found that adolescents with levels at or above the 90th percentile of testosterone, cortisol, or estradiol had increased odds of elevated depression and anxiety symptomology. The associations were strongest for estradiol, which also resulted in significant linear associations with both depression and anxiety in boys. For girls, there were borderline non-significant associations between elevated testosterone and anxiety, although there were no linear associations between any hormones with anxiety. Both testosterone and cortisol were considerable effect modifiers of the hormone and mood score associations for both boys and girls. This study is among the first to characterize the associations of various sex and adrenal hormones with mood in adolescents and one of the first to test hormone interactions in such associations.
These findings partially align with the three existing studies assessing associations of estradiol and mood in men. Similar to our findings, elevated estradiol has been positively associated with depression in young men,13 and has been found to limit men’s responsiveness to antidepressants.34 However, comparing estradiol levels of depressed adult men and demographic-matched controls, no differences in estradiol concentrations were identified.35 Animal studies have indirectly examined this; in gonadectomized rats, supplementation with estradiol pellets resulted in antidepressant-like effects,36 similar to those achieved through testosterone supplementation. As estradiol supplementation returns hormone levels of gonadectomized rats to normal concentrations, these results are not applicable to our cohort of predominantly healthy adolescents.
The association we observed between elevated cortisol and elevated anxiety symptomology has been previously reported in the literature. In a study of 8- to 17-year-old adolescents, cortisol levels exhibited positive linear relationships with general and social anxiety in teenagers, both in boys and girls.37 While in another study done in 9- to-14-year-old girls, higher baseline cortisol levels predicted the onset of depression at the age of 18 years compared to those with lower baseline cortisol levels. This suggests that elevated cortisol production in daily life introduces susceptibility to social and physical environment adversity and greater potential for mood alterations.38 These studies, however, did not identify that both low and high cortisol levels were positively associated with feelings of anxiety, as our study suggests.
Low testosterone levels have been associated with greater depressive symptoms in elderly men,10 and testosterone supplementation has been found to improve depression and anxiety symptoms among hypogonadal men.39 These findings contrast with the associations found, and may be explained by the substantial age and health status differences between the study populations. Based on the potential for a testosterone threshold effect, it is possible that testosterone replacement therapy in hypogonadal and elderly men may return testosterone concentrations to normal levels but not to a level which increases the risk of anxiety.
4.1. Effect modification by Cortisol
Our analyses suggest the presence of effect modification by cortisol on the hormone-mood associations, with varying effects by gender. For all associations observed, they were present in boys or girls, but never in both. For example, boys in the BMC category exhibited a positive association between testosterone and anxiety, whereas girls had and null associations. Similarly, in the AMC category, we observed negative associations of testosterone and DHEA with depression in girls, and null associations for boys. We expected to see differences in hormone-mood associations between boys and girls, as differential mood responses to adrenal and gonadal hormones by gender have been observed. 32,33 Yet, it was surprising to find that, unlike what was observed in the threshold effect results, the associations seen in stratified analyses did not trend in the same direction when comparing boys and girls within the same cortisol category.
Our results provide evidence that the interplay between the HPA and HPG axes may affect mood. The interactions of cortisol on the relationships of estradiol and testosterone with depression and anxiety suggest that the HPA axis can modulate the associations between the HPG axis and mood. The interaction observed between testosterone and cortisol support the dual-hormone hypothesis, which considers that the HPA axis moderates the relationship between the HPG axis and human behavior; more specifically, that low cortisol concentrations may increase sensitivity to testosterone’s effects. 40,41 The dual-hormone hypothesis parallels the relationship observed with testosterone and depression, as testosterone concentration was solely positively associated with anxiety symptoms in the BMC category for boys. Tackett et al. was the first to extend the dual-hormone hypothesis outside of the testosterone and cortisol interaction, to find that high estradiol concentrations are linked with externalizing behaviors only when cortisol concentrations are low among adolescents (ages 13–18).42 We also observed interactions of cortisol on the estradiol-internalizing symptom (depression) association; however the association was only present in higher (as opposed to lower) cortisol concentrations. The directionality of the effect modification by cortisol between these studies may not be comparable since the outcomes assessed were different (externalizing vs internalizing).
The interactions by cortisol on the DHEA-depression association in girls observed in our study suggest that there is effect modification within the HPA axis as well. Although the DHEA and cortisol interaction is excluded from the dual-hormone hypothesis since they are both HPA axis hormones, cortisol/DHEA ratio measures have been associated with treatment resistant depression and other psychological conditions, as depressed patients exhibit increased cortisol/DHEA ratios.43–45 The magnitude of change observed in DHEA under psychosocial stress has been found to be positively associated with the magnitude of changes in cortisol.45 Although these studies do not directly assess the role of effect modification between these two adrenal hormones, they suggest that DHEA and cortisol jointly affect depression symptom severity and should be further characterized.
4.2. Effect modification by Testosterone
Similar to stratification across cortisol medians, testosterone was a significant effect modifier of the hormone-mood relationships, and all associations observed either were present in boys or girls, but never both. For boys, we observed the strongest relationships between estradiol and depression in the AMT, demonstrating the strength of effect modification between sex hormones themselves. Others have also reported positive associations between estradiol and depression in obese men; however, contrary to our findings, such associations were strongest among participants with low testosterone levels.46 There has been scant research on the effect of estradiol in males, as most research has focused on the testosterone-mood relationship.
Unlike the dual-hormone hypothesis which focuses on the influence of the HPA axis on the HPG axis, the DHEA-anxiety relationship in the BMT demonstrates that the HPG axis can also influence effects of the HPA axis. There is conflicting evidence on the relationship between DHEA and mood disorders. Some evidence indicates that DHEA antagonizes the effects of cortisol on the body, which helps to modulate negative consequences of stress.47,48 As we only identified a negative association between DHEA concentration and depression symptomology when assessing effect modification by testosterone, it is possible that the sensitivity to the effects of DHEA increase when there are lower concentrations of testosterone.
4.3. Strengths, Limitations, and Future Directions
Strengths of this analysis includes our use of a large cohort to assess both the role of adrenal and sex hormones independently, but also examining the interplay between them. Given that adrenal and sex hormone regulation both independently but co-dependently regulate their secretion, our findings help contribute to the understanding of how their relationships effect mood disorders. Likewise, this study works to expand mental health research of Latin American populations, a population that is generally under-represented in the field.
A limitation of this study includes not measuring more HPA and HPG signaling hormones. Further study into corticotropin releasing hormones, (CRH) gonadotropin releasing hormones, (GnRH) luteinizing hormone, (LH) follicle stimulating hormone, (FSH) and others would provide greater clarity regarding the relationship between the axes on depression and anxiety. Although we were unable to confirm adolescent stage with LH and FSH concentrations, self-reported tanner staging has been found to be an appropriate research method to obtain puberty stage approximations,31,49 which is why we adjust for Tanner staging in our models. Another limitation was the quantification of salivary hormone levels using enzymatic methods, which are known to have lower accuracy compared to measurements using mass-spectrometry. Nonetheless, the enzymatic salivary kits used have high correlations with serum measured levels.50–53 This study did not capture information regarding traumatic life events nor adverse childhood events (ACEs). Therefore, we were unable to identify whether these events influenced hormone levels or mental health.
Future directions include conducting longitudinal analysis and assessing potential social and environmental influences on hormone expression and mood disorders. Specifically, assessing the association of pesticide exposure with hormonal changes and mental health is warranted given our previous findings of associations between lower acetylcholinesterase activity, a biomarker of greater pesticide exposure, with elevated depression scores (Suarez-Lopez 2020).Additionally, identifying the influence of traumatic life events and ACEs on the hormone-mental health associations would be important to address in future studies. Lastly, measuring additional signaling hormones, like CRH, GnRH, LH, and FSH, would provide further provide clarity regarding the interplay of the HPA and HPG, and its subsequent effect on depression and anxiety.
4.5. Conclusion
Our study identified that elevated cortisol, estradiol, and testosterone levels increased the odds of having elevated depression and anxiety symptomology, and that there is significant effect modification by testosterone and cortisol on the hormone-mood associations. As the hormone-mood associations varied by gender, it highlights the importance of understanding how the relationship between hormone concentration and mood differs between boys and girls. The estradiol-mood association in boys was the most consistent and strongest relationship observed. Further characterization of this association in both males and females is necessary. These cross-sectional findings among adolescents support our hypothesis that both adrenal and gonadal hormones are associated with depression and anxiety, and that there exists an interplay between the HPA and HPG axes on their relationships with mood. Future longitudinal studies are warranted.
Supplementary Material
Highlights.
In 2016, we examined 545 boys and girls (ages 11–17y) in rural Ecuador.
Elevated testosterone, cortisol and estradiol were associated with altered mood.
Estradiol had the strongest associations with depression and anxiety scores.
Cortisol and Testosterone were effect modifiers to hormone-mood associations.
There was effect modification between and within the HPA and HPG with mood.
Acknowledgements:
We thank Fundación Cimas del Ecuador, the Parish Governments of Pedro Moncayo County, community members of Pedro Moncayo and the Education District of Pichincha-Cayambe-Pedro Moncayo counties for their support on this project.
Funding:
The ESPINA study received funding from the National Institute of Occupational Safety and Health (1R36OH009402) and the National Institute of Environmental Health Sciences (R01ES025792, R21ES026084).
Role of Funder/Sponsor (if any):
The NIH had no role in the design and conduct in the study.
Footnotes
Conflict of interest Disclosures (Includes financial disclosures): None of the authors have any conflict of interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66 Suppl 7:5–13. http://europepmc.org/abstract/MED/16124836. [PubMed] [Google Scholar]
- 2.Rehm J, Shield KD. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr Psychiatry Rep 2019;21(2):10. doi: 10.1007/s11920-019-0997-0 [DOI] [PubMed] [Google Scholar]
- 3.Perou R, Bitsko RH, Blumberg SJ, et al. Mental health surveillance among children--United States, 2005–2011. MMWR Surveill Summ. 2013;62 Suppl 2(2):1–35. [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- 5.Naicker K, Galambos NL, Zeng Y, Senthilselvan A, Colman I. Social, demographic, and health outcomes in the 10 years following adolescent Depression. J Adolesc Heal. 2013;52(5):533–538. doi: 10.1016/j.jadohealth.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 6.Olivera-Figueroa LA, Juster R-P, Morin-Major JK, Marin M-F, Lupien SJ. A time to be stressed? Time perspectives and cortisol dynamics among healthy adults. Biol Psychol. 2015;111:90–99. doi: 10.1016/j.biopsycho.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 8.Morita T, Senzaki K, Ishihara R, et al. Plasma dehydroepiandrosterone sulfate levels in patients with major depressive disorder correlate with remission during treatment with antidepressants. Hum Psychopharmacol. 2014;29(February):280–286. doi: 10.1002/hup.2400 [DOI] [PubMed] [Google Scholar]
- 9.Erdinçler D, Bugay G, Ertan T, Eker E. Depression and sex hormones in elderly women. Arch Gerontol Geriatr. 2004;39:239–244. doi: 10.1016/j.archger.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 10.Morsink LFJ, Vogelzangs N, Nicklas BJ, et al. Associations between sex steroid hormone levels and depressive symptoms in elderly men and women : Results from the Health ABC study. Psychoneuroendocrinology. 2007;32:874–883. doi: 10.1016/j.psyneuen.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 11.Baischer W, Koinig G, Hartmann B, Huber J, Langer G. Hypothalamic-pituitary-gonadal axis in depressed premenopausal women: Elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology. 1995;20(5):553–559. doi: 10.1016/0306-4530(94)00081-K [DOI] [PubMed] [Google Scholar]
- 12.Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: A preliminary report. Am J Obstet Gynecol. 2000;183(2):414–420. doi: 10.1067/mob.2000.106004 [DOI] [PubMed] [Google Scholar]
- 13.Stanikova D, Luck T, Bae YJ, et al. Increased estrogen level can be associated with depression in males. Psychoneuroendocrinology. 2018;87:196–203. doi: 10.1016/j.psyneuen.2017.10.025 [DOI] [PubMed] [Google Scholar]
- 14.Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas. 2002;41(SUPPL. 1):25–46. doi: 10.1016/s0378-5122(02)00013-0 [DOI] [PubMed] [Google Scholar]
- 15.Mchenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression : Role of testosterone. Front Neuroendocrinol. 2014;35(1):42–57. doi: 10.1016/j.yfrne.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and Depression: Systematic Review and Meta-Analysis. J Psychiatr Pract 2009;15(4):289–305. doi: 10.1097/01.pra.0000358315.88931.fc [DOI] [PubMed] [Google Scholar]
- 17.Vigil P, del Río JP, Carrera B, Aránguiz FC, Rioseco H, Cortés ME. Influence of sex steroid hormones on the adolescent brain and behavior: An update. Linacre Q. 2016;83(3):308–329. doi: 10.1080/00243639.2016.1211863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toufexis D, Rivarola MA, Lara H, Viau V. Stress and the reproductive axis. J Neuroendocrinol. 2014;26(9):573–586. doi: 10.1111/jne.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyola MG, Handa RJ. Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: sex differences in regulation of stress responsivity. 2018;20(5):476–494. doi: 10.1080/10253890.2017.1369523.Hypothalamic [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suarez-Lopez JR, Jacobs DRJ, Himes JH, Alexander BH, Lazovich D, Gunnar M. Lower acetylcholinesterase activity among children living with flower plantation workers. Environ Res. 2012;114:53–59. doi: 10.1016/j.envres.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suarez-Lopez JR, Hood N, Suárez-Torres J, Gahagan S, Gunnar MR, López-Paredes D. Associations of acetylcholinesterase activity with depression and anxiety symptoms among adolescents growing up near pesticide spray sites. Int J Hyg Environ Health. 2019;222(7):981–990. doi: 10.1016/j.ijheh.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricardo J, Lopez S, Nguyen A, et al. Associations of Acetylcholinesterase Inhibition Between Pesticide Spray Seasons with Depression and Anxiety Symptoms in Adolescents, and the Role of Sex and Adrenal Hormones on Gender Moderation. Expo Heal. 2020;(0123456789). doi: 10.1007/s12403-020-00361-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Training Course on Child Growth Assessment. Vol WS 103. Geneva: World Health Organization; 2008. [Google Scholar]
- 24.Kovacs M, MHS staff. CDI 2: Children’s Depression Inventory 2nd Edition, Technical Manual. 2nd ed. North Tonawanda, NY: MHS Inc; 2011. [Google Scholar]
- 25.Bae Y Test Review: Children’s Depression Inventory 2 (CDI 2)KovacsM.Children’s Depression Inventory 2 (CDI 2) (2nd ed.). North Tonawanda, NY: Multi-Health Systems Inc, 2011. J Psychoeduc Assess. 2012;30(3):304–308. doi: 10.1177/0734282911426407 [DOI] [Google Scholar]
- 26.Fraccaro RL, Stelnicki AM, Nordstokke DW. Test Review: Multidimensional Anxiety Scale for Children by MarchMarch JSJS (2013). Multidimensional Anxiety Scale for Children (2nd ed.). Toronto, Ontario, Canada: Multi-Health Systems. Can J Sch Psychol. 2015;30(1):70–77. doi: 10.1177/0829573514542924 [DOI] [Google Scholar]
- 27.Isasi CR, Carnethon MR, Ayala GX, et al. The Hispanic Community Children’s Health Study/Study of Latino Youth (SOL Youth): design, objectives, and procedures. Ann Epidemiol. 2014;24(1):29–35. doi: 10.1016/j.annepidem.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marmorstein NR, Iacono WG, Legrand L. Obesity and depression in adolescence and beyond: reciprocal risks. Int J Obes. 2014;38(7):906–911. doi: 10.1038/ijo.2014.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization Multicentre Growth Reference Study Group. World Health Organization Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;450:76–85. [Google Scholar]
- 30.Brooks BBL, Sherman EMS, Iverson GL. Healthy children get low scores too: prevalence of low scores on the NEPSY-II in preschoolers, children, and adolescents. Arch Clin Neuropsychol. 2010;25(3):182–190. doi: 10.1093/arclin/acq005 [DOI] [PubMed] [Google Scholar]
- 31.Rapkin AJ, Tsao JCI, Turk N, Anderson M, Zeltzer LK. Relationships among Self-Rated Tanner Staging, Hormones, and Psychosocial Factors in Healthy Female Adolescents. J Pediatr Adolesc Gynecol. 2006;19(3):181–187. doi: 10.1016/j.jpag.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 32.Murphy WG. Blood Reviews The sex difference in haemoglobin levels in adults — Mechanisms, causes, and consequences. YBLRE. 2014. doi: 10.1016/j.blre.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 33.Handelsman DJ, Hirschberg AL, Bermon S. Circulating testosterone as the hormonal basis of sex differences in athletic performance. Endocr Rev. 2018;39(5):803–829. doi: 10.1210/er.2018-00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel W, Klaiber EL, Broverman DM. Roles of the gonadal steroid hormones in psychiatric depression in men and women. Prog Neuropsychopharmacol. 1978;2(4):487–503. doi: 10.1016/0364-7722(78)90107-8 [DOI] [Google Scholar]
- 35.Rubin RT, Poland RE, Lesser IM. Neuroendocrine aspects of primary endogenous depression VIII. Pituitary-gonadal axis activity in male patients and matched control subjects. Psychoneuroendocrinology. 1989;14(3):217–229. doi: 10.1016/0306-4530(89)90020-6 [DOI] [PubMed] [Google Scholar]
- 36.Carrier N, Saland SK, Duclot F, He H, Mercer R, Kabbaj M. The Anxiolytic and Antidepressant-like Effects of Testosterone and Estrogen in Gonadectomized Male Rats. Biol Psychiatry. 2015;78(4):259–269. doi: 10.1016/j.biopsych.2014.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiefelbein VL, Susman EJ. Cortisol levels and longitudinal cortisol change as predictors of anxiety in adolescents. J Early Adolesc. 2006;26(4):397–413. doi: 10.1177/0272431606291943 [DOI] [Google Scholar]
- 38.LeMoult J, Ordaz SJ, Kircanski K, Singh MK, Gotlib IH. Predicting first onset of depression in young girls: Interaction of diurnal cortisol and negative life events. J Abnorm Psychol. 2015;124(4):850–859. doi: 10.1037/abn0000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Alexander G, Berman N, et al. Testosterone replacement therapy improves mood in hypogonadal men - A clinical research center study. J Clin Endocrinol Metab. 1996;81(10):3578–3583. doi: 10.1210/jc.81.10.3578 [DOI] [PubMed] [Google Scholar]
- 40.Mehta PH, Josephs RA. Testosterone and cortisol jointly regulate dominance: Evidence for a dual-hormone hypothesis. Horm Behav. 2010;58(5):898–906. doi: 10.1016/j.yhbeh.2010.08.020 [DOI] [PubMed] [Google Scholar]
- 41.Mehta PH, Prasad S. The dual-hormone hypothesis: A brief review and future research agenda. Curr Opin Behav Sci. 2015;3:163–168. doi: 10.1016/j.cobeha.2015.04.008 [DOI] [Google Scholar]
- 42.Tackett JL, Reardon KW, Herzhoff K, Page-Gould E, Harden KP, Josephs RA. Estradiol and cortisol interactions in youth externalizing psychopathology. Psychoneuroendocrinology. 2015;55:146–153. doi: 10.1016/j.psyneuen.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 43.Markopoulou K, Papadopoulos A, Juruena MF, Poon L, Pariante CM, Cleare AJ. The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology. 2009;34(1):19–26. doi: 10.1016/j.psyneuen.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 44.Mouthaan J, Sijbrandij M, Luitse JSK, Goslings JC, Gersons BPR, Olff M. The role of acute cortisol and DHEAS in predicting acute and chronic PTSD symptoms. Psychoneuroendocrinology. 2014;45:179–186. doi: 10.1016/j.psyneuen.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 45.Lennartsson AK, Kushnir MM, Bergquist J, Jonsdottir IH. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol Psychol. 2012;90(2):143–149. doi: 10.1016/j.biopsycho.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 46.Monteagudo PT, Falcão AA, Verreschi ITN, Zanella MT. The imbalance of sex-hormones related to depressive symptoms in obese men. Aging Male. 2016;19(1):20–26. doi: 10.3109/13685538.2015.1084500 [DOI] [PubMed] [Google Scholar]
- 47.Maninger N, Capitanio JP, Mason WA, Ruys JD, Mendoza SP. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology. 2010;35(7):1055–1062. doi: 10.1016/j.psyneuen.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan CA, Rasmusson A, Pietrzak RH, Coric V, Southwick SM. Relationships Among Plasma Dehydroepiandrosterone and Dehydroepiandrosterone Sulfate, Cortisol, Symptoms of Dissociation, and Objective Performance in Humans Exposed to Underwater Navigation Stress. Biol Psychiatry. 2009;66(4):334–340. doi: 10.1016/j.biopsych.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 49.Schlossberger NM, Turner RA, Irwin CE. Validity of self-report of pubertal maturation in early adolescents. J Adolesc Heal. 1992;13(2):109–113. doi: 10.1016/1054-139X(92)90075-M [DOI] [PubMed] [Google Scholar]
- 50.Salimetrics. Expanded Range Salivary Testosterone Enzyme Immunoassay Kit. 101 Innovation Boulevard, Suit 302, State College, PA 16803; 2019. [Google Scholar]
- 51.Salimetrics. High Sensitivity Salivary 17β -Estradiol Enzyme Immunoassay Kit. 101 Innovation Boulevard, Suit 302, State College, PA 16803; 2019. [Google Scholar]
- 52.Salimetrics. Salivary Dhea Enzyme Immunoassay Kit. 101 Innovation Boulevard, Suit 302, State College, PA 16803; 2019. [Google Scholar]
- 53.Salimetrics. Expanded Range High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit. 101 Innovation Boulevard, Suit 302, State College, PA 16803; 2019. https://www.salimetrics.com/assets/documents/1-3002.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


