Abstract
Background
Multidisciplinary (MD) rehabilitation is an important component of symptomatic and supportive treatment for Multiple Sclerosis (MS), but evidence base for its effectiveness is yet to be established.
Objectives
To assess the effectiveness of organized MD rehabilitation in adults with MS. To explore rehabilitation approaches that are effective in different settings and the outcomes that are affected.
Search methods
We searched the Cochrane Multiple Sclerosis Group's Trials Register (25 February 2011), PeDRO (1990 ‐ 2011), the Cochrane Rehabilitation and Related Therapies Field trials Register, the National Health Service National Research Register (NRR) and relevant journals were handsearched. No language restrictions were applied.
Selection criteria
Randomized controlled trials (RCT) and controlled clinical trials (CCT) that compared MD rehabilitation with routinely available local services or lower levels of intervention; or trials comparing interventions in different settings or at different levels of intensity.
Data collection and analysis
Three reviewers selected trials and rated their methodological quality independently. A 'best evidence' synthesis based on methodological quality was performed. Trials were grouped in terms of setting and type of rehabilitation and duration of patient follow up.
Main results
Ten trials (9 RCTs and 1 CCT) (954 participants and 73 caregivers) met the inclusion criteria. Eight RCTs scored well; while one RCT and one CCT scored poorly on the methodological quality assessment. Despite no change in the level of impairment, there was ’strong evidence’ to support inpatient MD rehabilitation in producing short‐term gains at the levels of activity (disability) and participation in patients with MS. There is ‘moderate evidence’ to support inpatient or outpatient rehabilitation programmes (compared with control wait‐list groups) in improving disability; and bladder related activity and participation outcomes up to 12 months following MD rehabilitation intervention. For outpatient and home‐based rehabilitation programmes there was 'limited evidence' for short‐term improvements in symptoms and disability with high intensity programmes, which translated into improvement in participation and quality of life. For low intensity programmes conducted over a longer period there was 'strong evidence' for longer‐term gains in quality of life; and also 'limited evidence' for benefits to carers. Although some studies reported potential for cost‐savings, there is no convincing evidence regarding the long‐term cost‐effectiveness of these programmes. It was not possible to suggest best 'dose' of therapy or supremacy of one therapy over another. This review highlights the limitations of RCTs in rehabilitation settings and need for better designed randomized and multiple centre trials.
Authors' conclusions
MD rehabilitation programmes do not change the level of impairment, but can improve the experience of people with MS in terms of activity and participation. Regular evaluation and assessment of these persons for rehabilitation is recommended. Further research into appropriate outcome measures, optimal intensity, frequency, cost and effectiveness of rehabilitation therapy over a longer time period is needed. Future research in rehabilitation should focus on improving methodological and scientific rigour of clinical trials.
Plain language summary
Multidisciplinary rehabilitation as supportive treatment for adults with multiple sclerosis
Multiple sclerosis is a chronic neurological condition, which can cause multiple disabilities and limit participation in young adults. This review looked for evidence of MD rehabilitation in adults with multiple sclerosis. The authors concluded there was strong evidence that inpatient or outpatient rehabilitation can lead to improvement in activity (disability) and in overall ability to participate in society, even though there is no reduction in actual impairment. There was limited evidence for short‐term improvements in symptoms and disability, and in participation and quality of life with the high intensity outpatient and home‐based rehabilitation programmes. For low intensity programmes conducted over a longer period there were longer term gains in quality of life; and for benefits to carers in terms of general health and engagement in social activities. The evidence available for other aspects of MD rehabilitation, including outpatient and home based therapy is not yet sufficient to allow many conclusions to be drawn.
Background
Description of the condition
Multiple sclerosis (MS) is a chronic neurological disease that is characterized by patchy inflammation, gliosis and demyelination within the central nervous system. It is the third most common cause of neurological disability in adults between 18‐50 years of age after trauma and arthritis (Dombovy 1998) affecting approximately 2.5 million worldwide and 20,000 persons in Australia (MS Society 2011). Prevalence of MS (per 100,000 people) in Australia has been estimated at 40‐80 in Victoria and Hobart, 30‐35 in Perth and Newcastle (Hammond 1988) compared with a much higher prevalence in Europe of 30‐150 (Compston 1998), and in the US 40‐220 (Kraft 2005).
The exact etiology of MS is unclear but is associated with an abnormal immune response within the central nervous system. An infectious agent has been implicated in its etiology (Kurtzke 1983). MS has a latitudinal discrepancy and occurs more frequently in temperate regions away from the equator. The likelihood of a person getting the disease is predicted by a person's residence for the first 15 years of their life (Detels 1978). MS is more common in females‐ 2.5:1 ratio.
The patterns of presentation in MS can vary.
Relapsing remitting (RR) MS (80%) ‐ This pattern is marked by exacerbations and remissions. The attacks may be followed by complete or near‐complete recovery and the patient remains well until the next exacerbation.
Secondary progressive (SP) MS: Over time person with RR MS may convert to a SP form of MS (Kraft 2005), with progressive disability acquired between attacks.
Primary progressive (PP) MS (15%). A smaller group of persons do not have acute attacks, but develop progressive disability from the onset. This form is less responsive to immunotherapy, has a more equal gender distribution and tends to have an older age of onset (Kraft 2005; Yorkston 2002).
Progressive relapsing MS (5%) ‐ These patients begin worsening gradually and subsequently start to experience discrete attacks.
The Expanded Disability Status Scale (EDSS) (Kurtzke 1983) is commonly used to rate the neurological status of MS patients. It is a 10‐point scale divided into half steps (0, 1, 1.5 etc. with 0= normal, 10= death due to MS). Patients with EDSS score of 3 have minimal disability, at 6 require a gait aid to ambulate and at 7 are wheelchair bound.
The prognosis in MS is variable, the rate of progression depends on type, severity and location of MS. Cognitive and behavioural problems can be subtle and often precede overt physical disability, but up to 50% will require a gait aid within 15 years of onset (Weinshenker 1989). Immune modulatory drugs have been shown to decrease relapse rates and produce a trend towards slowed accrual of impairment (Langdon 1999). However a clinically meaningful effect of drug treatment in minimizing disability (activity) has not yet been demonstrated.
MS is a costly disease due to early onset, long disease duration, detrimental impact on functional status and issues of job retention/employment (Battaglia 2000). In 2001‐2002, in Australia, the average annual direct and indirect costs per patient were estimated to be AU$20,396 and AU$15,085, respectively (Taylor 2007). In United States, currently MS is estimated to cost $28 billion annually in medical costs and lost productivity (Society of Neuroscience 2011). One study estimated the total lifetime cost per patient of MS in 1994 at $A 2.5 million (Whetten‐Gol 1998). There is a positive correlation between EDSS score categories and rising cost of care (Patwardhan 2005).
The World Health Organization has developed an International Classification of Functioning, Disability and Health (ICF) (WHO 2001) which defines a common language for describing the impact of disease at different levels:
Impairments are problems with body (anatomical) structures or (physiological) function‐ the symptoms and signs of disease such as paresis, pain etc
Activity limitation (previously known as 'disability') (WHO 2001) describes the difficulties that a person may have in executing everyday tasks such as self care
Restriction in participation (previously known as handicap) (WHO 2001) relates to problems experienced by a person with involvement in societal participation and life situations such as employment, social activities.
Contextual factors include: ‐Environmental factors which make up the physical, social and attitudinal environment in which people live their lives.‐Personal factors include gender, race, self‐efficacy, coping style, social and educational background which may affect the person's experience of living with their condition.
Person with MS (pwMS) can present to rehabilitation with various combinations of deficits, such as physical, cognitive, psychosocial, behavioural and environmental problems. Classified according to the WHO ICF, these include impairments (strength, coordination, balance, spasticity, memory, urinary urgency), which result in activity limitation (mobility, self care, incontinence, pain, cognitive deficits) and restriction in societal participation (impact on work, driving, family, finances). The issues of progressive physical disability, psychosocial adjustment, social reintegration progress over time (Frankel 2001).
Figure 1 ICF diagram (Figure 1)
1.
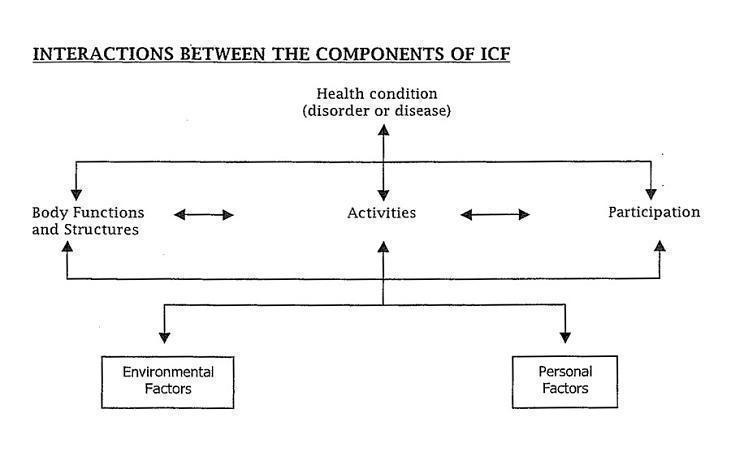
This diagram has been reprinted with the permission of the World Health Organization (WHO), and all rights are reserved by the organization. World Health Organization (WHO) ‐ International Classification of Functioning, Disability and Health (ICF) Geneva. WHO 2001.
Description of the intervention
Rehabilitation may be defined as 'a problem‐solving educational process aimed at reducing impairment (disability) and participation (handicap) experienced by someone as a result of disease or injury' (Wade 1992). Although it is sometimes effective in reducing impairment, its principal focus is on reducing symptoms and limitations at the level of activity and participation, through holistic interventions which include personal and environmental factors (NICE 2003).
For the purpose of this review, multidisciplinary (MD) (or interdisciplinary) rehabilitation was defined as 'an inpatient, outpatient, home or community‐based coordinated intervention, delivered by two or more disciplines in conjunction with physician consultation (neurologist or rehabilitation medicine physician), which aims to limit patient symptoms, and enhance functional independence and maximise participation, as defined by ICF (WHO 2001).
The multiple disciplines include nursing, physiotherapy, occupational therapy, speech pathology, orthotics, dietetics, social work, psychology or neuropsychology. Settings were either inpatient (hospital ward or specialist rehabilitation unit), outpatient (hospital or community), or home‐based settings. Rehabilitation multidisciplinary care is usually tailored to suit an individual’s specific needs and therefore varies in content and intensity.
We included studies with interventions that satisfy the definition of MD care and which compared to some form of ‘control’ condition, these include:
lower level or different types of intervention such as 'routinely available local services’ (for example, medical and nursing care);
'minimal intervention’ (such as 'information only’);
wait‐list conditions;
intervention given in different settings;
lower intensity of intervention.
We excluded studies with interventions that assessed the effect of therapy from a single discipline (for example, physiotherapy), or any unidisciplinary intervention or modality (for example, physical exercise).
How the intervention might work
Systematic reviews have demonstrated that holistic MD rehabilitation is effective in other neurological conditions such as Stroke (Greener 2002; Langhorne 1995; Langhorne 2002), and Acquired Brain Injury (Turner‐Stokes 2005), but the evidence base for the effectiveness in patients with MS is not yet established. A number of Cochrane reviews have addressed the effects of unidisciplinary or limited interventions.
Steultjens et al (Steultjens 2003) identified three trials with a total of 271 patients, which addressed the effects of energy conservation techniques and counselling, but were unable to identify clear benefits from these limited studies. They highlighted lack of properly controlled efficacy studies for most intervention categories of occupational therapy (OT). Reitberg et al (Reitberg 2004) examined the effectiveness of exercise therapy (alone) in MS, identifying nine trials with a total of 260 patients. They found evidence for benefits in terms of power and mobility‐related activities, and to a lesser extent in mood. However, these did not carry over into functional benefits in terms of activities of daily living or quality of life (QoL).
Why it is important to do this review
There are no systematic reviews of MD rehabilitation care in person with MS. Other reasons to do this review include the following:
There are inherent difficulties in demonstrating evidence‐based effectiveness of rehabilitation through experimental trials due to heterogeneity of types of MS patients and disease manifestations, unpredictable disease course, the variety of rehabilitation services and inconsistent use of appropriate outcome measures (Thompson 2000; Whyte 2002).
The MD rehabilitation intervention vary in different settings (inpatient, ambulatory), intensities and modalities of therapies, these need further investigation as there are associated cost implications.
Long‐term MS related disability results in a significant burden of disease both for the patient and society as a whole.
Advances in medical care and increased life expectancy among persons with disabilities (including pwMS), the ongoing health and well being become increasingly important and require long‐term planning for health service delivery. (Campbell 1999; Turk 2001). From the rehabilitation perspective, the challenge is not just helping the pwMS become as independent as possible, but helping them stay independent in the community in the face of changes brought with aging (Kemp 2005).
This review is un update of the review first published in 2008.
This review analysed published (and unpublished) controlled trials relating to MS and rehabilitation, and specifically at MD rehabilitation in MS to identify the existing evidence base for MD rehabilitation in adults with MS, and to discuss issues for future expansion of the evidence base by traditional research and other methods.
Objectives
To assess the effectiveness of organized MD rehabilitation in adults with Multiple Sclerosis (MS), aged 18 years and above. To explore approaches that are effective in different settings, and the outcomes that are affected. Specific questions addressed by this review are:
Does organized MD rehabilitation achieve better outcomes than the absence of such services in pwMS?
Does organized MD rehabilitation achieve better outcomes than the absence of such services in caregivers of pwMS?
Which type of programmes are effective and in which setting?
Which specific outcomes are influenced (dependency, mood, social reintegration, return to work)?
Does a greater intensity (time and /or expertise) of rehabilitation lead to greater gains?
Are there demonstrable cost‐ benefits for multi disciplinary rehabilitation in MS?
Methods
Criteria for considering studies for this review
Types of studies
All trials that stated or implied use of MD or interdisciplinary rehabilitation intervention were included. All randomized controlled trials (RCTs) and controlled clinical trials (CCTs) that were included compared MD rehabilitation with either routinely available local services; or lower or minimal levels of intervention; or wait list conditions; or intervention in different settings or at different levels of intensity.
In this review, definition of a clinical trial used was 'a prospective experimental evaluation of a clinical intervention that assesses its outcome against those of a control group' (Fuhrer 2005).
Types of participants
Trials were included if the study population had the diagnosis of MS based on the validated criteria (McDonald 2001; Poser 1983; Schumaker 1965), above 18 years of age and of either gender. These participants included all diagnostic subgroups of MS and all stages of disease such as relapsing remitting (RR), primary progressive (PP) and secondary progressive (SP) MS.
Types of interventions
All trials included MD or interdisciplinary rehabilitation programme. These comprise elements of physical therapy (PT), occupational therapy (OT), speech pathology (SP), psychology and or neuropsychology (NP), cognitive therapy and or behaviour management, social work (SW), nutrition, orthotics, counselling input, recreation and vocational therapy.
The setting of rehabilitation programmes included:
Inpatient rehabilitation settings that provide 24 hour care, such as a specialist medical rehabilitation unit or a hospital ward unit (general medical unit, neurology)
Outpatient or day treatment settings, located within the hospital, a community centre/ day centre or a specialist rehabilitation environment.
Home‐based setting in the patients' own home and local community.
For the purpose of this review, intensity of MD rehabilitation programme was subdivided into 'high' or 'low' intensity.
High intensity therapy involved input from at least two disciplines, a minimum of thirty minutes per session and total duration of at least 2‐3 hours of interrupted therapy per day for at least 4 days per week. This is usually provided in inpatient settings and some outpatient programmes.
Low intensity programmes varied, the intensity and duration of therapy was lesser than that provided in inpatient rehabilitation settings and was dependent upon the type of rehabilitation setting and available resources
All studies that involved a MD or an interdisciplinary rehabilitation programme were included, provided they compared the named intervention with some form of control condition. For the purpose of this review the control conditions considered were:
A lower level or different type of intervention such as 'routinely available local services' or 'minimal intervention such as' information only' or 'single session treatment'
MD Interventions given in different settings, such as inpatient versus community rehabilitation
Lower intensity of treatment programmes
Wait list conditions
Studies assessing the effect of the following were excluded:
Therapy from a single discipline (physiotherapy), including studies on intensity of treatment within that discipline
A single uni‐disciplinary intervention or modality (e.g., physical exercise)
Efficacy of occupational therapy programmes ‐ already dealt with Cochrane review (Steultjens 2003) ‐ except where it formed part of a coordinated multidisciplinary approach.
Programmes that included complementary medicine (yoga, meditation) in the absence of rehabilitation.
Types of outcome measures
Studies were included if outcomes were reported at the level of activity or participation according to the WHO ICF (WHO 2001). Studies where outcomes were reported only at the level of impairment were excluded.
Outcomes were categorized as indicated in Table 1 into:
1. List of Outcome Measures focusing on goals at the levels of impairment, disability.
| Outcome at the level | Outcome Measures |
| Impairment and symptoms |
Motor
Amended Motor Club Assessment
(AMCA)
Expanded Disability status scale (EDSS) Functional Assessment in Multiple Sclerosis (FAMS) Multiple Sclerosis Impairment Scale (MSIS) Cognitive Hopkins Verbal Learning Test (HVL) Mini‐mental state examination (MMSE) Symptoms Beck Depression Inventory (BDI) Clinical depression questionnaire (CDQ) Fatigue Frequency MS symptom checklist composite score Self‐reported relapses State trait anxiety inventory (STAI) State trait anger expression inventory(STAXI) Bladder American Urological Association Symptom Index (AUA) |
| Activity limitation | Barthel index (BI)
Fatigue severity scale (FSS) Functional Independence Measure (FIM) Functional Assessment Scale (RIC FAS) Guy's Neurological Disability Scale (GNDS) General Health Questionnaire (GHQ28) Incapacity Status Scale (ISS) Nine‐Hole Peg Test (9HPT) Rehabilitation Institute of Chicago Timed 10‐metre Walking Test (TW10) Visual Analogue Scales (VAS) Bladder Neurological Disability Scale (NDS) Urogenital Distress Inventory (UDI6) |
| Participation |
Quality of Life
Fatigue impact scale (FIS) General Health Questionnaire (GHQ28) Multiple Sclerosis Quality‐of‐Life Questionnaire (MS QOL) Multiple Sclerosis Impact Scale (MSIS29) Life Appreciation and Satisfaction Questionnaire (LASQ) Perceived Deficits Questionnaire (PDQ) 36 item Short Form Health Survey Questionnaire (SF36) Social Barriers to Health Promoting Activities for Disabled Persons Scale (BHPADPS) Employment Health Promoting Lifestyle Profile II (HPLP‐II) Human Activity Profile (HAP) London Handicap Scale (LHS) Personal Resource Questionnaire (PRQ) Questionnaire on Resources and Stress (QRS) Revised UCLA Loneliness Scale Sickness Impact Profile (SIP) Self‐Rated Abilities for Health Practices scale (SRAHP) Tempelar Social Experience Checklist (SET) Bladder Incontinence Impact Questionnaire (IIQ7) |
| Other | Discharge destination (home/ institution) Drug Management Cost of care Hospital length of stay or treatment Hours of home assistance Qualitative final programme evaluation Satisfaction with care/services Timeliness of health care |
Those that focus on impairment (muscle tone and strength);
Those that focus on goals at the level of limitation in activities (disabilities), such as the Functional Independence Measure (Granger 1990) with domains for self care, mobility, communication and cognition;
Those that focus on goals at the level of participation, environmental and/or personal context. These include QoL, psychosocial adjustment, social integration in pwMS and caregiver mood.
Those reflecting other outcomes such as associated costs, service utilization, caregiver burden.
It should be noted however that some outcome scales crossed boundaries between these ICF concepts,for example include items relating to both impairment (symptoms) and activity.
Primary outcomes
The primary outcome was the minimisation of disability (limitation in activity) using validated measures.
In this review all patients were assessed at the time of admission and discharge from the rehabilitation programme, irrespective of its length:
Short‐term studies' referred to trials where patients were followed up within 6 months of completion of programme.
Long‐term studies' referred to follow up any time over six months (usually one year and longer) after the intervention.
Secondary outcomes
The secondary outcomes included:
Participatory issues, such as QoL, psychological adjustment assessed at less than 12 months and at 12 months or more, using validated measures.
Outcomes that reflect cost, service utilization and care burden
Search methods for identification of studies
No language restrictions were applied to the search.
Electronic searches
The Trials Search Co‐ordinator searched the Cochrane Multiple Sclerosis Group's Specialised Register (25 February 2011).
The Cochrane Multiple Sclerosis Trials Register is updated regularly and contains trials identified from:
The Cochrane Central Register of Controlled Trials (CENTRAL) (recent issue);
MEDLINE (PubMed) (1966 to date);
EMBASE (Embase.com) (1974 to date);
CINAHL (Ebsco host) (1981 to Feb 2011);
LILACS (Bireme) (1982 to date);
Clinical trials registries.
Information on the Cochrane Multiple Sclerosis Group's Trials Register and details of search strategies used to identify trials can be found in the 'Specialised Register' section within the Cochrane Multiple Sclerosis Group's module.
The keywords used to search for this review are listed in (Appendix 1).
For search methods used in the previous version, please see (Appendix 2).
Additional databases searched by the authors:
PeDRO (1990 ‐ 2011)(Appendix 3)
Searching other resources
Reference lists from published reviews on MD rehabilitation in MS and identified RCTs and CCTs;
Personal communication with first authors of relevant trials or reviews and other multiple sclerosis experts;
The Cochrane Rehabilitation and Related Therapies Field trials Register;
National Health Service National Research Register (NRR) including Medical Research Council Clinical Trials Directory;
Handsearch of relevant journals: "Multiple Sclerosis" (January 1998 ‐ February 2011) with the search engine Proquest, "Archives of Physical Medicine and Rehabilitation (January 1996 ‐ February 2011), "Clinical Rehabilitation" (1998 ‐ February 2011) and "International Journal of MS Care" (1999 ‐ 2011).
Unpublished trials were identified using strategies 2, 3 and 4.
Authors and well‐known experts in this field were contacted if further information about the trials was needed.
Data collection and analysis
Selection of studies
The reviewers (FK, BA, LN, LTS) independently screened all abstracts and titles of studies that were identified by the search strategy for inclusion and appropriateness based on the selection criteria. Once all potentially appropriate studies had been obtained, each study was independently evaluated by the three reviewers for inclusion. If necessary, further information was obtained to determine if the trial met the criteria. If no consensus was met about the possible inclusion/exclusion of any individual study, a final consensus decision was made by discussion amongst all the reviewers. If there was still no consensus agreement regarding inclusion/exclusion of the study, then the full article was to be submitted to the editorial board for arbitration. Reviewers were not masked to the name(s) of the author(s), institution(s) or publication source at any level of the review.
Data extraction and management
Three reviewers independently extracted the data from each study that met the inclusion criteria. If insufficient data were available, then authors were contacted to provide data and clarification. If the data were unavailable or insufficient, the study was reported but not included in the final analysis. All studies that met the inclusion criteria were summarized in the 'Included Studies' table provided in the Review Manager software developed by the Cochrane Collaboration (RevMan 5.1.1) to include details on design, participants, interventions, and outcomes.
Assessment of risk of bias in included studies
The methodological quality of studies included in this review were assessed independently by the reviewers (FK, LN, BA, LTS). The methodological quality criteria proposed by Van Tulder (van Tulder 1997, van Tulder 2003) were used for assessing internal validity, descriptive and statistical criteria (Table 2: Assessment of Methodological Quality). This criteria list draws from the previous Cochrane Musculoskeletal Group list (van Tulder 1997), which incorporated the three Jadad criteria (Jadad 1996): randomization (subsequently developed to include concealment of allocation), double blinding (participant and outcome assessor) and description of withdrawals (subsequently developed to include intention to treat analysis).
2. Methodological criteria list (van Tulder 1997/2003).
| Internal validity A. Was the method of randomization adequate? B. Was the treatment allocation concealed? C. Were the groups similar at baseline regarding the most important prognostic indicators? D. Was the patient blinded to the intervention? E. Was the care provider blinded to the intervention? F. Was the outcome assessor blinded to the intervention? G. Were co‐interventions avoided or similar? H. Was compliance acceptable in all groups? I. Was the drop out rate described and acceptable? J. Was the timing of the outcome assessment in all groups similar? K. Did the analysis include an intention to treat analysis? Descriptive criteria (external validity) L. Were eligibility criteria for entry clearly mentioned? M. Were the index and control interventions explicitly described? N. Were adverse effects described? O. Was the timing follow up measurements (short term/long term) described? Statistical criteria P. Was the sample size for each group described? Q. Were point estimates and measures for variability presented for the primary outcome measures? * Criteria A‐K constitute the internal validity criteria recommended by van Tulder (2003). Criteria L‐Q are the remaining descriptive and statistical criteria from their earlier list van Tulder (1997) |
The van Tulder et al (van Tulder 2003) criteria consist of 11 methodological criteria for internal validity. In addition, for improved sensitivity and discrimination between high and poor quality trials, we included the four remaining descriptive and two statistical criteria from the earlier list (van Tulder 1997) giving a total of 17 criteria. All items on the methodological criteria list had equal weight. Each item was scored at 2 points for 'Yes', 1 for 'Don't know', and 0 for 'No', and item scores were summated to a single total score. Any disagreements regarding scoring were resolved by consensus between reviewers. Studies were considered to be of high methodological quality if the score was at least 50% ‐ i.e., 11 out of 22 for the internal validity criteria, 4 out of 8 for descriptive criteria and 2 out of 4 for statistical criteria. Studies scoring 50% out of a total maximum score of 34 were considered of high methodological quality. Studies were rated low methodological quality if they achieved less than these scores.
Measures of treatment effect
A quantitative analysis was not possible due to the use of diverse outcome measures and other clinical heterogeneity. Therefore, qualitative synthesis of "best evidence" was presented based on the levels of evidence proposed by Van Tulder et al (van Tulder 2003) as detailed in Table 3. This includes the statistical analyses in the included studies.
3. Method for synthesis of best evidence (based on van Tulder 2003).
| Strong evidence: provided by consistent, statistically significant findings in outcome measures in at least two high quality RCTs. |
| Moderate evidence: provided by consistent, statistically significant findings in outcome measures in at least one high quality RCT and at least one low quality RCT or a high quality CCT. |
| Limited evidence: provided by statistically significant findings in outcome measures in at least one high quality RCT; or provided by consistent, statistically significant findings in outcome measures in at least two high quality CCTs (in the absence of high quality RCTs). |
| Indicative evidence: provided by statistically significant findings in outcome and or process measures in at least one high quality CCT or low quality RCT (in the absence of high quality RCTs). |
| No/ insufficient evidence: Results of eligible studies do not meet the criteria for one of the above stated levels of evidence; or no eligible studies. |
| Conflicting evidence: (statistically significant positive and statistically significant negative) results among RCTs and CCTs; or no eligible studies |
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was completed by the type, setting and intensity of rehabilitation intervention, and by duration of participant follow‐up. We discussed the trial strengths and limitations, and identified gaps in our current knowledge and suggested future research directions.
Sensitivity analysis
Due to the small number of studies included we were unable to perform a sensitivity analysis to determine whether the overall results would be the same if studies above different methodologic cut‐off points were analysed.
Details of the operational definitions of the criteria list and scoring used in methodological quality assessment are given in Table 4.
4. Operational definitions and Scoring of the Methodological criteria list.
| (Score: Yes = 2, don't know =1, no = 0) |
| A. Method of randomization was positively scored if a random assignment sequence was used (computer generated random table number and/or use of opaque sealed envelopes). |
| B. Concealment of treatment allocation was scored positively where an independent person generated the assignment and was not responsible for determining eligibility of the patients. A central randomization scheme involved numbered or coded containers such as on‐site computer systems that provided allocation in locked unreadable files accessible only after inputting the characteristics of an enrolled participant and sequentially numbered sealed opaque envelopes. If concealment of treatment allocation was described only as randomized, it was considered unclear. |
| C. To get a positive score the groups had to be similar at baseline (regarding demographic factors, duration and severity of complaints, percentage of patients with neurologic symptoms and value of main outcome measures). |
| D. The reviewer determines if enough information about blinding is given to score a 'yes'. |
| E. The reviewer determines if enough information about blinding is given to score a 'yes' |
| F. The reviewer determines if enough information about blinding is given to score a 'yes'. |
| G. Co‐interventions should either be avoided in the trial design or should be similar between index and control groups to score a 'yes'. |
| H. The reviewer determines if the compliance to the interventions is acceptable, based on reported intensity, duration and number of sessions for both index intervention and control intervention, to score a 'yes'. |
| I. The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and drop outs does not exceed 20% for short term follow‐up and 30% for long term follow‐up and does not led to a substantial bias, a 'yes' is scored. |
| J. Timing of outcome assessment should be identical for all intervention groups and for all important outcome assessments to score a 'yes'. |
| K. All randomized patients are analysed in the group they were allocated to by randomization, for the most important moment of effect measurement (minus missing values) irrespective of non‐ compliance and co‐interventions to score a 'yes' |
| L. The reviewer determines whether the participant inclusion and exclusion criteria for entry were clearly defined to score a 'yes'. |
| M. The reviewer determines whether details of the index and control interventions were explicitly described ‐ including disciplines involved and treatment duration. For example, whether a clearly defined treatment protocol was present. |
| N. Each adverse event should be described and attributed to the allocated treatment. If it is explicably reported that no adverse effects have occurred, a 'yes' should be scored. |
| O. The outcomes measured within 6 months of intervention period (short term) or after 6 months of randomization (long term) should be scored 'yes' if mentioned. |
| P. To be presented for each group at randomization and for most important outcome assessments to score a 'yes'. Therefore in contrast to previous lists there is no preset cut‐off point to determine whether sample size is sufficient. |
| Q. Both point estimates and measures of variability should be presented (to be scored for each important outcome parameter separately) to score a 'yes'. Point estimates include means, medians and modes. Measures of variability include standard deviations and 95% confidence intervals. |
| Other procedures used included: |
| Blinding: Single blinding of outcome assessors was scored positive only if assessors were blinded regarding treatment allocation and when standardized assessment measures or procedures were used to structure interviews. |
| Patient blinded to intervention is unlikely in rehabilitation settings if consent procedures were applied. |
| Intention to treat (ITT): was scored positive if all patients randomized to the intervention group were included in the analysis regardless of non‐ compliance and co‐interventions. If loss to follow‐up was substantial (20% or greater), an ITT analysis, as well as an alternative analysis, which accounts for missing values (eg. worst case analysis) should have been performed. |
| Eligibility criteria: scored positive if a list of explicit inclusion/ exclusion criteria was provided. |
| Outcome measures relevant: outcome measures reflecting limitation in impairment, activity (disability) and participation as relevant to the intervention. |
| Adverse effects can occur with rehabilitation intervention. We looked for evidence on report of adverse effects in the included trials for this review. |
| Fatal flaws in studies included: withdrawals of more than 40% of patients, non‐adherence to the protocol and poor or non‐adjusted comparability in the baseline criteria. These studies were considered inadequate for inclusion in the review. |
Results
Description of studies
Results of the search
Previously (Khan 2007) this review identified a total of 1516 titles and abstracts. Of these, 44 studies passed the first screening and 7 RCTs (one with 2 reports) and 1 CCT (with 2 reports) were included (see below). This review search update yielded a further 287 additional titles, of these 59 were scrutinised further and 2 additional RCTs (1 with 2 reports) were included.
Included studies
Ten trials fulfilled the inclusion criteria for this review (Freeman 1997; Craig 2003; Francabandera 1988; Pozzilli 2002; Patti 2002; Patti 2003; Stuifbergen 2003; Guagenti‐Tax 2000; DiFabio 1998; DiFabio 1997; Storr 2006; Khan 2008; Khan 2010). Of these, eight ((7 RCTs (one with 2 reports ) and one CCT with 2 reports (DiFabio 1998; DiFabio 1997)) were included in our previous review (Khan 2007). In this update two new trials (one with 2 reports) were identified (Storr 2006; Khan 2008; Khan 2010; ). See: Characteristics of Included Studies.
The included studies were conducted in five different countries: four in the US, two in Italy, two in the UK, one each in Denmark and Australia. All trials were published between 1988 and 2010, and written in English language. These trials involved a total of 954 participants and 73 caregivers. Eight trials included between 70 and 201 patients (Francabandera 1988; Patti 2002; Patti 2003; Pozzilli 2002; Stuifbergen 2003; Freeman 1997; Guagenti‐Tax 2000; Khan 2008; Khan 2010; Storr 2006), while two had between 40‐50 participants (Craig 2003; DiFabio 1997; AcknowledgementsDiFabio 1998).
All included trials compared MD rehabilitation in the treatment group with a control group of some sort.
Three studies with a total of 217 participants compared inpatient rehabilitation with a control wait list or lower intensity group (Freeman 1997; Craig 2003; Storr 2006). One study with 101 participants compared inpatient or outpatient rehabilitation with a control wait‐list group (Khan 2008; Khan 2010). One study with 84 participants compared inpatient rehabilitation with an outpatient rehabilitation programme (Francabandera 1988). One study with 201 participants compared home rehabilitation with a control hospital group (Pozzilli 2002). Four studies with a total of 351 participants compared outpatient rehabilitation with a control group (Patti 2002; Patti 2003; Stuifbergen 2003; Guagenti‐Tax 2000; DiFabio 1997; DiFabio 1998). One of these studies (Guagenti‐Tax 2000) included paired participants with MS and their carers (n=73).
Excluded studies
Fourty‐nine studies (and abstracts) (36 previously identified and 13 newly identified) were excluded for the reasons shown in the table Characteristics of Excluded Studies.
The main reasons for exclusion were:
Not an RCT or CCT (n=29)
Variable was not multi‐disciplinary rehabilitation (n=10)
Not MS patients or details of MS subgroup not provided (n=2)
Abstract only and details insufficient or results not available from authors (n=6)
Fatal flaws, including excessive attrition (n=1), different co‐interventions (n=1)
Risk of bias in included studies
The methodological quality scores of the ten included studies are provided in Table 5. The study scores ranged between 15 and 26 out of a total possible score of 34.
5. Methodological Quality scores for included studies.
| Description | Freeman 1997 | Craig 2003 | Storr 2006 | Khan 2008 | Khan 2010 | Francabandera 1988 | Pozilli 2002 | Patti 2002,2003 | Stuifbergen 2003 | Guagenti‐Tax 2000 | DiFabio 1997, 1998 |
| Randomized | yes | yes | Yes | Yes | Yes | yes | yes | yes | yes | yes | no |
| Internal Validity +abcdefghijk | 2, 1, 2, 0, 0, 0, 2, 2, 2, 2, 0 | 2,1,2,0,0,0,2,2,2,2,0, | 0,0,2,0,1,2,1,0,0,1,0 | 2,1,1,0,1,2,1,2,2,2,0 | 2,2,1,0,1,2,1,1,2,2,0 | 2, 0, 1, 0, 0, 0, 2, 2, 2, 2, 0 | 2, 0, 2, 0, 0, 0, 2, 2, 2, 2, 0 | 2, 2, 2, 0, 0, 2, 2, 2, 2, 2, 2. | 2, 0, 2, 0, 0, 0, 1, 2, 2, 2, 0 | 2, 0, 1, 0, 0, 0, 1, 2, 2, 2, 1. | 0, 0, 2, 0, 0, 0, 1, 1,1, 2, 0 |
| Sum Score (max 22) | 13 | 13 | 7 | 14 | 14 | 11 | 12 | 18 | 11 | 11 | 7 |
| External Validity +l,m,n,o, | 2, 2,1, 2 | 1,2,0,2, | 2,2,0,2 | 2,2,2,2 | 2,2,2,2 | 2,2,.0,2 | 1,1, 0, 2 | 2,2,0,2 | 2,2,0,2 | 1,2,0,2 | 2,2,0,2 |
| Sum Score (max 8) | 7 | 5 | 6 | 8 | 8 | 6 | 4 | 6 | 6 | 5 | 6 |
| Statistical Criteria +p,q | 0,2 | 0,2 | 0,1 | 2,1 | 2,1 | 0.2 | 2.2 | 0.2 | 2.2 | 0.2 | 0.2 |
| Sum Score (max 4) | 2 | 2 | 1 | 3 | 3 | 2 | 4 | 2 | 4 | 2 | 2 |
| Total Study score (Max 34) | 22 | 20 | 14 | 25 | 25 | 19 | 20 | 26 | 21 | 18 | 15 |
| Total score % | 64.7 | 58,8 | 41.1 | 73.5 | 73.5 | 55.8 | 58.8 | 76.4 | 61.7 | 52.9 | 44.1 |
| Quality | high | high | low | high | high | high | high | high | high | high | low |
The summary of key indicators for randomization, concealed allocation, intention to treat and blinding of outcome assessor are included in the descriptive tables of included studies. Table 6 shows the Results, Description and Characteristics of Included Studies. (See Characteristics of included studies for further details)
6. Description of results of included studies.
| Author | Description |
| Three studies addressing Inpatient Rehabilitation | |
| Freeman, 1997 | |
| Participants | N = 70. Inclusion criteria ‐ definite progressive MS Exclusion criteria ‐ patients in relapse or within 1 month of receiving steroids |
| Interventions | Treatment group (N=34) ‐ inpatient individualized, MD rehabilitation programme for an average of 20 days (SD 3) including two 45‐minute PT sessions and one OT session per day and other disciplines as required. Control group (N=36) ‐ no rehabilitation intervention. |
| Outcomes | Impairment: EDSS, self‐reported relapses; Activity: FIM; Participation: London Handicap Scale (LHS) Other: drug management |
| Assessment points | Baseline and 6 weeks |
| Summary of results | Compared with the control group, the treatment group showed a statistically significant improvement in the FIM domains (as listed below) and in LHS. However, the magnitude of differences was small (for FIM, ES +0.21;mean +3.9; 95% CI +1.76, +6.12 and for LHS, ES +0.23; mean +2.76; 95% CI ‐0.44, +5.96). The Confidence Intervals were given only for total disability. There was no change in EDSS in either group (p=0.4202), nor in drug management. Two patients in the treatment group reported a relapse. Statistical test: Wilcoxon rank sum test, unpaired student t test, C2 statistics |
| Results ‐ outcomes in favour of intervention group | FIM ‐ motor p<0.001 FIM ‐ self care p<0.0001 FIM ‐ transfers p<0.001 FIM ‐ sphincter control p<0.001 FIM ‐ locomotion (wheelchair) p=0.0315 LHS p<0.01 |
| Author's conclusions | Inpatient rehabilitation was effective at reducing disability and handicap in persons with progressive MS despite unchanging levels of impairment |
| Craig, 2003 | |
| Participants | N = 41. Inclusion criteria: RR MS, relapse requiring admission as either day case or inpatient, for treatment of 3 days of IVMP. Exclusion criteria ‐ not specified |
| Interventions | Treatment group (N=20)‐ received IVMP and MD care as inpatient (3‐8 days) or day case. Control group (N=21) ‐ 0.26 hours (mean) PT and 0.075 hours (mean) OT. |
| Outcomes | Impairment: AMCA; Activity: GDNS, BI; Participation: HAP, SF‐36 |
| Assessment points | 1 and 3 months after 1st day of IVMP |
| Summary of results | Compared with the control group, the treatment group showed statistically significant differences in mean change from baseline in GNDS, AMCA, HAPM, HAPA and BI. Effect size for GNDS was ‐1.12 for treatment group and ‐0.24 for the control group. The differences in SF36 parameters did not reach statistical differences. Statistical tests: ANOVA, Mann‐Whitney |
| Results ‐ mean change from baseline in favour of treatment group | GNDS P=0.03 AMCA P=0.03 HAPM p<0.01 HAPA p<0.02 BI p=0.02 SF36 NS |
| Author's conclusions | Inpatient MD care combined with steroids is superior to treatment in a standard neurology or day ward setting for the disability and handicap measures. |
| Storr 2006 | |
| Participants | N = 106. Inclusion criteria: definite MS; age 18‐70 years; Expanded Disability Status Scale (EDSS) score < 9.0; ability to co‐operate for a 90‐min session. Exclusion criteria: age <18 years, no inform consent, relapse within past 3 months, concurrent disease interfering with assessment, significant cognitive impairments |
| Interventions | Intervention group (N=41): an individualised inpatient multidisciplinary (MD) rehabilitation programme: 45‐minutes physiotherapy sessions 4‐5 times/week; 30 minutes occupational therapy 3 times/week. self training in the gymnasium 30 minntes‐1hour/day. Control group (N=65): no treatment. |
| Outcomes | Impairment: MSIS, EDSS, GNDS; Activity: FAMS, 9HPT, TW10; Participation: LASQ; Others: VAS for patients’ own perception of bodily pain, bladder symptoms, spasticity, fatigue, impaired walking and transfers |
| Assessment points | Baseline and 2 follow‐ups with an interval of 10 weeks (exact period not specified) |
| Summary of results | There were no statistically significant differences between the two groups in any of the outcome measures. There was a trend in benefit of intervention for the 9HPT(right hand) and EDSS, while there was a trend in benefit of the control group for bodily pain. The primary outcome, FAMS, was in favour of the control arm. Stastical test: Fisher's exact test, Chi2 test |
| Results ‐ change from baseline in favour of intervention group | None |
| Author's conclusions | Although the study was underpowered, the negative outcome exposes the difficulties in quantitative analyses of the efficacy of multidisciplinary rehabilitation. The confounding factors include variation in the indication for treatment, in the placebo effect, and in the reliability and responsiveness of the outcome measures used. |
| One study with two reports addressing Outpatient or Inpatient Rehabilitation | |
| Khan 2008 | |
| Participants | N = 101. Inclusion criteria: definite MS; mobile and living in the community (EDSS mobility score 2‐8); cognitively intact (Kurtzke Functional Systems range 0–2), age ≥18 years |
| Interventions | Intervention group (N = 49): an comprehensive individualised MD inpatient or outpatient rehabilitation programme up to 6 weeks followed by maintenance programmes (stretching, home exercises). Control group (N=52): usual care (regular reviews by general practitioners and neurologists in the community) |
| Outcomes | Activity: FIM; Participation: MSIS29, GHQ28 |
| Assessment points | Baseline and 12 months |
| Summary of results | Data for 98 patients (treatment N=48, control N=50) showed reduced disability in the treatment group, with statistically significant differences in post‐treatment FIM motor scores for the two groups (p<0.001) and the FIM motor domains of: transfer (p<0.001), locomotion (p=0.001), self‐care (p<0.001) and the FIM cognitive subscale (p<0.035). Compared with of controls more participants in the treated group improved (13% vs. 70.8%) and significantly more patients in the control group deteriorated over the study period (58.7% vs 16.7%; p<0.001). There were no differences between the control and treatment group scores on the MSIS29 or GHQ28 subscales. An additional analyses comparing those patients that received treatment (n=61) and those that did not receive treatment (N=40) showed consistent results, with significant differences between the groups being detected in favour of treatment group for the FIM motor (p=0.001), the FIM domains of self‐care (p=0.001), sphincter (p=0.01), transfers (p=0.003), locomotion (p=0.007), but not for the MSIS29 or GHQ28 subscales. |
| Results ‐ change from baseline in favour of intervention group | FIM motor total ‐ p<0.001, ES = 1.13 FIM locomotion ‐ p=0.001, ES = 0.69 FIM selfcare ‐ p<0.001, ES = 0.95 FIM transfers ‐ p<0.001, ES = 1.04 FIM cognition ‐ p<0.035, ES = 0.44 |
| Author's conclusions | An individualised rehabilitation programme reduces disability in persons with MS compared with no intervention. The impact of rehabilitation on QoL needs further evaluation. |
| Khan 2010 | |
| Participants | N = 74. Inclusion criteria: definite MS, who reported bladder symptoms/issues; mobile and living in the community (EDSS mobility score 2‐8), age ≥18 years |
| Interventions | Intervention group (N=40): individualised inpatient or outpatient MD bladder rehabilitation programme comprising: assessment of bladder type, pattern and function (3 day voiding charts, strict fluid balance, post void residual volume measurement, renal and lower urinary ultrasounds, baseline urodynamic study, urine culture and microscopy, urinary creatinine clearance); bladder re‐education, behaviour management, pelvic floor exercises, strategies for timed and double voiding, intermittent catheterisation techniques, use of prophylactic medication (cranberry capsules); and strict bowel programme). Control group (N=34): usual care (regular reviews by general practitioners and neurologists). . |
| Outcomes | Impairment and Activity: UDI6, NDS and AUA; Participation: AUA QoL, IIQ7 |
| Assessment points | Baseline and 12 months |
| Summary of results | Analysis of per protocol data from 58 patients (treatment N=24, control N=34) showed reduced disability in the treatment group, with significant differences (p<0.001) and large effect sizes (>0.5) in post‐treatment UDI6, NDS, AUA total, AUA QoL and IIQ7 scores for the two groups. Compared with the control group, the treatment group showed improvement: 78% versus 27% for UDI6 and 59% versus 17% improved for IIQ7. More patients in the control group deteriorated over the study period on the UDI6 (30% vs 0%; p<0.001) and IIQ7 (39 vs 0%; p=0.001). During the course of the study 10 patients from the control group required and received the treatment. An additional analyses to compare those patients that received treatment (n=34) and those that did not receive treatment (N=24) found a significant differences in the change scores between the treated and not treated groups for the UDI6 (p=0.007), AUA total (p<0.001), AUA QoL (p=0.003), NDS (p=0.03) and the IIQ7 (p=0.001). |
| Results ‐ change from baseline in favour of intervention group | UDI6 ‐ p<0.001, ES = 0.51 NDS ‐ p<0.001, ES = 0.58 AUA total ‐ p<0.001, ES = 0.77 AUA QoL ‐ p<0.001, ES = 0.72 IIQ7 scores ‐ p<0.001, ES = 0.58 |
| Author's conclusions | A multifaceted, individualised bladder rehabilitation programme reduces disability and improves QoL in person with MS compared with no intervention after 12 months of follow‐up. Information on specific interventions in different bladder types in MS and the impact on QoL need further evaluation. |
| One study comparing inpatient with outpatient rehabilitation | |
| Francabandera, 1988 | |
| Participants | N = 84. Inclusion criteria: definite and severe MS Exclusion criteria: institutionalized and unable to return home following treatment |
| Interventions | Inpatient group (N=42): individualised care plan, average of 2x 45 minute sessions of PT and 1x OT session /day .Outpatient group (N=42): PT and OT, bladder management, speech therapy and social services when applicable. |
| Outcomes | Activity: ISS; Other: Hours of home assistance required (self‐care). |
| Assessment points | Baseline and 3 monthly for 2 years (phone assessments) |
| Summary of results | Compared with the control group, the treatment group showed statistically significant improvements in adjusted ISS (analysis of covariance) at three months. No significant differences were detected for need in home assistance (t(71) = ‐0.70 NS). Statistical test: ANCOVA |
| Results ‐ change from baseline in favour of intervention group | Inpatient Outpatient Significance Baseline ISS mean 28.2 (SD 9.0), 3 month ISS mean 26.0 (SD 9.4); Baseline ISS mean 24.0 (SD 7.2), 3 month ISS mean 25.5 (SD 8.5) F (1, 70) =4.3 p<0.05 |
| Author's conclusions | ISS scores improved in the inpatient rehabilitation group compared with the outpatient group |
| One study addressing home rehabilitation | |
| Pozzilli, 2002 | |
| Participants | N = 201. Inclusion criteria ‐ clinically definite MS. Exclusion criteria ‐ not specified. |
| Interventions | Treatment group (N=133) ‐ individualised clinical care and coordinated home services Control group (N=68) ‐ routine hospital care at their MS referral centres as required ‐ details not specified. |
| Outcomes | Impairment: EDSS, MMSE, CDQ, STAI, STAXI; Activity: FIM, FSS; Participation: SF36; Other: resource use and cost |
| Assessment Points | Baseline and at 12 months |
| Summary of results | Compared with the control group, the treatment group showed statistically significant improvements in SF36 domains (as listed below). The cost of home‐ based care was less (E822 euros/patient/year) than hospital care, mainly due to decreased hospital admissions. Increased need for resources (medical care and nursing, social and psychological support) in the home‐based group (p = 0.0002 and 0.0067 respectively). No significant differences between intervention and control groups were detected for the following outcome measures: EDSS FIM, MMSE, CDQ, FSS, STAI and STAXI. Statistical test: T‐test, Wilcoxon rank sum test, C2 statistics |
| Results ‐ change from baseline in favour of intervention group | SF36 ‐ general health p=0.0001 SF36 ‐ bodily pain p=0.0001 SF36 ‐ role emotional p=0.0001 SF36 ‐ social functioning p=0.001 |
| Author's conclusion | MD home‐based rehabilitation may provide a cost effective approach to management of persons with MS and improve their QOL. |
| Four studies (including two studies with two reports each) addressing outpatient rehabilitation | |
| Patti, 2002 | |
| Participants | N = 111. Inclusion criteria ‐definite MS.Exclusion criteria ‐exacerbation in the preceding 3 months, cognitive impairment, history of other systemic or psychiatric conditions precluding participation, pregnancy, treatment with immunosuppressive and chemotherapy, rehabilitation in the 3 months before admission. |
| Interventions | Treatment group (N=58) ‐ comprehensive outpatient rehabilitation programme followed by a home exercise programme for 6 weeks. Control group (N=53)‐ Home exercise programme for 12 weeks. |
| Outcomes | Impairment: EDSS, BDI; Participation: SF36, FIS, SET |
| Assessment points | Baseline, 6 and 12 weeks |
| Summary of results | Compared with the control group, the treatment group showed statistically significant improvements in SF36 (except role emotional domain), FIS, SET, BDI. The Kazis effect value for SF36 ranged from 0.29 to 0.70. The ES for differences in FIS, SET and BDI was ‐0.77, ‐0.46 and ‐0.50 respectively. Statistical test: ANOVA, Mann Whitney U test |
| Results ‐ outcomes in favour of intervention group | SF36 ‐ physical functioning p<0.001 SF36 ‐ role physical p<0.001 SF36 ‐ bodily pain p<0.001 SF36 ‐ general health p<0.001 SF36 ‐ vitality p<0.001 SF 36 ‐ social functioning p<0.001 SF36 ‐ mental health p<0.001 SF36 ‐ role emotional p<0.005 FIS ‐ p<0.001 SET ‐ p<0.001 BDI ‐ p<0.001 |
| Author's conclusions | Outpatient rehabilitation is effective in improving quality of life, mood, fatigue and social function. |
| Patti, 2003 | |
| Participants | N = 111. Inclusion criteria ‐definite MS. Exclusion criteria ‐exacerbation in the preceding 3 months, cognitive impairment, history of other systemic or psychiatric conditions precluding participation, pregnancy, treatment with immunosuppressive and chemotherapy, rehabilitation in the 3 months before admission. |
| Interventions | Treatment group (N=58)‐ comprehensive outpatient rehabilitation programme followed by a home exercise programme for 6 weeks. Control group (N=53)‐ Home exercise programme for 12 weeks. |
| Outcomes | Impairment: EDSS; Activity: FIM |
| Assessment points | Baseline and 12 weeks |
| Summary of results | Compared with the control group, the treatment group showed statistically significant improvements in the FIM domains (as listed below). There was a moderate to large effect size of treatment on locomotion (0.76; 95% CI ‐0.4 to +6.9, self‐care (0.73; 95% CI ‐0.1 to +6.8) and transfers (0.65; 95% CI ‐0.1 to +5.9) and a small to moderate effect size on sphincter function (0.40; 95% CI ‐0.4 to +3.8). There were no differences between the groups in cognitive function. The EDSS score did not change over time in either group. Statistical test: ANOVA, Mann‐Whitney U test, Fisher's exact test |
| Results ‐ outcomes in favour of intervention group | FIM motor scores p<0.001 |
| Author's conclusions | A short outpatient rehabilitation programme improves disability in motor and sphincter parameters in MS patients. |
| Three studies (including one with two reports) addressing low intensity outpatient rehabilitation | |
| Stuifbergen, 2003 | |
| Participants | N = 121. Inclusion criteria ‐ female, definite MS diagnosed for at least 6 months. Exclusion criteria ‐ pregnancy, concurrent medical conditions for which changes in exercise or diet would be contraindicated. |
| Interventions | Treatment group (N=61) ‐ 2‐phased MD wellness programme consisting of an educational and skill‐building lifestyle change phase, followed by supportive bimonthly telephone calls for 3 months post completion of programme. Control group (N=60)‐ monthly phone call. |
| Outcomes | Activity: ISS; Participation: SF36, SRAHP, HPLP‐II, BHPADPS, PRQ85, employment |
| Assessment points | Baseline, 8 weeks, 3 and 8 months |
| Summary of results | Compared with the control group, the treatment group showed statistically significant improvements in SRAHP, HPLP‐II and SF36 mental health and bodily pain scales. The treatment group were more likely to be employed (C2 = 3.91) than the control group at follow up. There were no differences between the groups in the Barriers Scale or the PRQ85 .Statistical test: T‐test, Chi‐square, linear regression analysis |
| Results ‐ outcomes in favour of intervention group | SRAHP ‐ p<0.001 HPLP‐II ‐ p<0.01 SF36 ‐ mental health p<0.001 SF36 ‐ bodily pain p<0.001 Employment ‐ p<0.05 |
| Author's conclusions | Low intensity therapy and education improves health behaviours and some dimensions of QOL for women with MS |
| Guagenti‐Tax, 2000 | |
| Participants | N = 73 patient‐caregiver units. Inclusion criteria: Definite MS requiring assistance with basic life activities and who lived with a caregiver. Exclusion criteria ‐ not specified. |
| Interventions | Treatment group (43 units)‐ Twice‐monthly MD day‐care programme for 12 months with group‐based therapy. In addition, both the carers and patients in the treatment group attended ten workshops on coping with MS. Control group (30 units) ‐ standard care mentioned but not specified. |
| Outcomes | Impairment: EDSS, HVLActivity: ISS; Participation: PDQ, MHI, SIP, Revised UCLA Loneliness‐Companionship Scale, QRS, SF36; Other: Satisfaction with care (including timeliness), Cost of health care and home assistance, length of stay and reason for nursing home placement, qualitative final programme evaluation. |
| Assessment points | Baseline, 12 and 24 months |
| Summary of results | Compared with the control group, the treatment group caregivers showed statistically significant improvements in SF36 (social function and physical role domains). There was a decline in both groups in EDSS and ISS scores. The intervention group reported an increase in PDQ. The control group (both patients and caregivers) had significantly greater decline in SF36 (perceived general health). Statistical test: ANOVA |
| Results ‐ outcomes in favour of intervention group (caregiver) | SF 36 ‐ social function p = 0.004 SF36 ‐ role physical p = 0.002 |
| Author's conclusions | Outpatient therapy and carer/patient education improves patient perceived health and anxiety. Caregivers in the control group deteriorated in terms of socialization and perceived health. |
| Di Fabio, 1997 | |
| Participants | N = 44. Inclusion criteria ‐definite MS Exclusion criteria ‐ not specified. |
| Interventions | Treatment group (N=19)‐ 5 hours one day a week in an outpatient setting for 1 year with integrated physical and occupational therapy and supportive services Control group (N=25) ‐ medical wait‐list. |
| Outcomes | Activity: RIC‐FAS; Participation: SF36, MSQOL‐54 |
| Assessment points | Baseline and 12 months |
| Summary of results | Compared with the control group, the treatment group showed small improvements in some SF36 domains (as listed below) Statistical test: Effect size calculation, multiple regression analysis |
| Results ‐ outcomes in favour of intervention group | SF36 ‐ Physical Health Partial R2 = 0.30 p<0.05 SF36 ‐ Energy/fatigue Partial R2 = 0.43 p<0.05 SF36 ‐ Bodily pain Partial R2 = 0.33 p<0.05 SF36 ‐ general health Partial R2 = 0.17 p<0.05 SF36 ‐ social function Partial R2 = 0.21 p<0.05 MSQOL ‐ social support Partial R2 = 0.26 p<0.05 |
| Author's conclusions | Extended outpatient rehabilitation may have a small effect on some quality of life parameters, energy and fatigue. |
| Di Fabio, 1998 | |
| Participants | N = 46. Inclusion criteria ‐ definite MS Exclusion criteria ‐ not specified. |
| Interventions | Treatment group (N=20) ‐ 5 hours one day a week in a multidisciplinary outpatient setting for 1 year with integrated PT and OT and supportive services Control group (N=26) ‐ medical (pharmacologic) management only. |
| Outcomes | Impairment: Fatigue frequency, MS symptom checklist composite score; Activity: selected items from RIC‐FAS |
| Assessment points | Baseline and 12 months |
| Summary of results | Compared with the control group, the treatment group showed statistically significant reduction in frequency of symptoms (treatment group ES = 0.27 vs control group ES = ‐0.32) and fatigue (treatment group ES = 0.46 vs control group ES = 0.20). There was no change in RIC‐FAS items for both groups. Statistical test: ANCOVA, Effect size calculation, multiple regression analysis |
| Results ‐ outcomes in favour of intervention group | Frequency of symptoms F12,17 = 2.60 p= 0.035 Fatigue F1,30 = 9.68 p= 0.004 |
| Author's conclusions | Outpatient rehabilitation in MS is effective in reducing fatigue and severity of symptoms. |
| Abbreviations (excluding outcome measures) | |
| ANOVA = Aalysis of Variance | ANCOVA = Aalysis of Covariance |
| CI = confidence interval | N = number |
| ES = effect size | OT = occupational therapy |
| IVMP = intravenous methylprednisolone | PT = physiotherapy |
| MD = multidisciplinary | RR = relapsing remitting |
| MS = multiple sclerosis | SD = standard deviation |
Effects of interventions
Participant characteristics
The participants of studies considered in this review included 954 (920 completers) persons with MS and 73 caregivers. This included the 207 persons with MS from the two new identified studies (including one study with two reports) (Khan 2008; Khan 2010; Storr 2006). Details are presented in the Table 6 showing the results of Included Studies. Table 7 highlights the Comparative Characteristics of Included Studies and the participant characteristics, details of interventions and outcomes. Most included studies describe a neurologist's assessment of patient eligibility for inclusion. Criteria for exacerbation or relapse of MS however were not always defined.
7. Comparative characteristics of included studies ‐ participant characteristics,
| Reference | Number | Type MS | Disease duration | Mean EDSS score | Age mean | % female | Intervention | Dose and Type | Outcome |
| Freeman 1997 | 70 (34/36) | P | 9.6/11.4 | 6.5/6.5 | 43.2/44.6 | 66/62 | Rehabilitation vs no rehabilitation intervention | Disciplines: medical, nursing, OT, PT, SP, SW, neuropsychology Intensity: 2 45 minute PT sessions and 1 OT session dailyDuration: 20 days | Impairment: EDSS, self‐reported relapses Activity (Disability) : FIM Participation:LHSOher: drug management |
| Craig 2003 | 41 (20/21) | RR | 7.42/5.69 | 5.4/5.1 | 38/42 | 55/80 | Inpatient or day case MD care vs control | Disciplines: OT, PT, SP, nursingIntensity: 2.62 hours (mean) PT, 1.49 hours (mean) OT Duration: 3‐8 days | Impairment: AMCA Activity (Disability): GDNS, BI Participation: HAP, SF‐36 |
| Storr 2006 | 90 (38/52) | All type | 9.0 for both groups | 6.5 for both groups | 53.0/50.1 | 57.9/69.2 | MD rehabilitation (IP) vs. usual care | Disciplines: PT, OT, Psychologist, SW, nurses, neurologists. Intensity: 45 minutes 4‐5 times/week Duration: 3‐5 weeks |
Impairment: MSIS, EDSS, GND Activity (disability): 9HPT, TW10 Participation: FAMS, VAS LASQ |
| Khan 2008 | 101 (49/52) | All type | 10.96/9.73 | 49.5/51.1 | 63.3/78.8 | MD rehabilitation (IP or OP) vs. usual care | Disciplines: medical, PT, OT, SP, SW. Intensity: 3 hour/daily for 5 days; and half hour sessions with SP, neuropsychology, SW 3 times/week |
Activity: FIM Participation:MSIS29; GHQ28 |
|
| Khan 2010 | 74 (40/34) | All type | 12.2/10.0 | 49.9/51.1 | 62.5/85.3 | MD rehabilitation (IP or OP) vs. usual care | Disciplines: medical, PT, OT, SP, SW. Intensity: 3 hour/daily for 5 days; and half hour sessions with SP, neuropsychology, SW 3 times/week; Plus individualised bladder management programme |
Impairment and activity: UDI6, NDS, AUA Participation: AUA QoL, IIQ7 | |
| Francabandera 1988 | 84 (42/42) | ? | ? | 6.0‐9.0 | "comparable" (not specified) | ? | Inpatient rehabilitation vs outpatient rehabilitation | Disciplines: medical, nursing (continence care), PT, OT, SW, SP Intensity (inpatient): 2 45‐minute PT sessions, 1 OT session daily Duration: ? | Impairment: not assessedActivity (Disability): ISSParticipation: Not assessedOther: hours of home assistance required(self‐care) |
| Pozzilli 2002 | 201 (133/68) | RR P | 18.4/18.6 | 6.0/5.8 | 47/46.7 | 65/69 | MD individualised clinical care and coordinated home services vs routine hospital care | Disciplines: medical, nursing, psychologist, PT, SW Intensity: ?Duration: ? | Impairment: EDSS, MMSE, CDQ, STAI, STAXI Activity (Disability): FIM, FSS Participation: SF36 Other: resource use and cost |
| Patti 2002 | 111 (58/53) | P | 17.2/17.2 | 6.2/6.1 | 45.2/46.1 | 58.6/56.6 | Outpatient rehabilitation with individualized goal‐oriented programme vs home‐exercise programme | Disciplines: PT, OT, SP Intensity: 6 days a week. PT ‐ 1 hr sessions, 5 days a week. OT ‐ ½ hr sessions biweekly.Duration: 6 weeks Followed by home exercise programme for 6 weeks | Impairment: EDSS, BDI Activity (Disability): not assessed Participation: SF36, FIS, SET |
| Patti 2003 | 111 (58/53) | P | 17.2/17.2 | 6.2/6.1 | 45.2/46.1 | 58.6/56.6 | Outpatient rehabilitation with individualized goal‐oriented programme vs home exercise programme | Disciplines: PT, OT, SPIntensity: 6 days a week. PT ‐ 1 hr sessions, 5 days a week. OT ‐ ½ hr sessions biweekly.Duration: 6 weeks Followed by home exercise programme for 6 weeks | Impairment: EDSS Activity (Disability): FIM Participation: not assessed |
| Stuifbergen 2003 | 121 (61/60) | MostlyRR | 10.76 | ? | 45.79 (all participants) | 100 | 2‐phased wellness programme on education and skill‐building lifestyle changes vs monthly phone call | Disciplines: nursing, dietician, counsellor, fitness instructorIntensity: 90 minute sessions weekly or 3 hour fortnightlyDuration: 8 weeks | Impairment: not assessedActivity (Disability): ISSParticipation: SF36, SRAHP, HPLP‐II, BHPADPS, PRQ‐85 (part 2), employment |
| Guagenti‐Tax 2000 | 73 paired units (i.e.146)86/60 | Mostly P | Client group: 8.9/14.2 | Client group: 7.06/7.24 | Client group: 44/49Carer group:44.9/51.8 | Client group:86.7/69Carer group: 56.7/51.7 | Day‐care programme vs routine care | Disciplines: PT, OT, SW, RT, nursingIntensity: Twice‐monthly groups, 10 workshops and monthly home visits.Duration: 12 months | Impairment: EDSS, HVL Activity (Disability): ISS Participation: PDQ, MHI, SIP, Revised UCLA Loneliness Companionship Scale, QRS, SF36 Other: Satisfaction with care, (cost and timeliness), home assistance, length of stay, reason for residential care, qualitative programme evaluation. |
| Di Fabio 1997 | 44 (19/25) | P | 17.6/14.2 | 5‐8 (inclusion criteria) | 44.5/49.2 | 83/79 | Outpatient rehabilitation vs control (wait‐list) | Disciplines: medical, OT, SW, RT, PT, nursing (wounds/falls), dietetics Intensity: 5 hrs for 1 day weeklyDuration: 1 year | Impairment: Not assessed Activity (Disability): RIC‐FAS Participation: SF36, MSQOL‐54 |
| Di Fabio 1998 | 46 (20/26) | P | 17/15 | 5‐8 (inclusion criteria) | 49/50 | 75/73 | Outpatient rehabilitation vs medical management only | Disciplines: medical, OT, SW, RT, PT, nurse (wounds/falls), dietetics Intensity: 5 hrs for 1 day weeklyDuration: 1 year | Impairment: fatigue frequency, MS symptom checklist composite score Activity (Disability): RIC‐FAS Participation: Not assessed |
| Abbreviations for Table 9 (excluding outcome measures) | |||||||||
| Hr = hour | PT = physiotherapist | ||||||||
| MD = multidisciplinary | RR = relapsing remitting | ||||||||
| MS = multiple sclerosis | RT = recreational therapist | ||||||||
| OT = occupational therapist | SW = social work | ||||||||
| P = progressive | SP = speech therapist | ||||||||
In the three trials that compared 'Inpatient rehabilitation with controls', the mean EDSS scores were between 5 (Craig 2003) and 6.5 (Freeman 1997; Storr 2006). Craig 2003 included only patients with relapsing‐remitting MS, Freeman 1997 considered progressive MS (primary and secondary) while Storr 2006 included participants with all types of MS (63% secondary progressive). The disease duration range of the participants was from 0 to 37 years. Approximately two‐thirds of the participants were women (range 64 ‐ 67%) with a mean age ranged from 40 to 51.5 years.
The preliminary trial by Francabandera 1988 was the only study comparing 'Inpatient rehabilitation with outpatient therapy'. Although originally targeted at 24 months, the analysis in this report was limited to 3 months. However despite contacting the centre we were unable to trace any further report of the study. The participant EDSS scores ranged from 6 to 9, but the types of MS, mean disease duration and mean age of participants were not specified, and could not be provided by the author.
One trial with two reports (Khan 2008; Khan 2010) compared ‘Inpatient or outpatient rehabilitation with controls’. The primary patient cohort in this trial included all types of MS (more than half were secondary progressive). The participant EDSS score ranged from 2 ‐ 8, with majority (58.4%) in EDSS score range of 3.5 to 6.0. The mean disease duration was 10.2 years. Approximately two‐thirds were women (71%) with a mean age of 50.3 years (range 29‐65 years).
The trial by Pozzilli 2002 comparing 'Home rehabilitation with a control group' included patients with a slightly wider range of EDSS scores (3.6 to 8.0). It included both relapsing‐remitting, and progressive MS (primary and secondary). Again two‐thirds of the sample were women, with a mean age of 47 years and disease duration 18.5 years.
For the four trials (DiFabio 1997; DiFabio 1998; Guagenti‐Tax 2000; Patti 2002; Patti 2003; Stuifbergen 2003) comparing 'Outpatient rehabilitation with controls', the severity of disease (EDSS scores) ranged from 4 to 8.5, and crossed the range of MS types. The proportion of women ranged from 56‐100%, mean age 44‐49 years and mean disease duration of the subjects was wide ranging (1 to 40 years). Study characteristics
See Table 6 for Description of results of Included Studies and Table 7 comparative characteristics of Included Studies. All details of outcome measures based on between‐group assessments are presented in Table 8 (Between Group Effects of included Trials (BGE)).
8. Between group effects of included studies (BGE).
| Description | Reference | BGE Impairment | BGE Activity | BGE participation | Other |
| Inpatients vs control | Freeman 1997 | EDSS, | FIM+ | LHS+ | Drug Management |
| Self‐reported | |||||
| Relapses | |||||
| Craig 2003 | AMCA + | GNDS+ | SF36‐ | ||
| BI+ | HAP+ | ||||
| Storr 2010 | MSIS‐ |
9HPT‐ | FAMS‐ | VAS‐ (bladder, pain, spasticity, fatigue, mobility, transfer) | |
| EDSS‐ | TW10‐ | LASQ‐ | |||
| GND‐ | |||||
| Inpatient/outpatient vs control | |||||
| Khan 2008 | FIM motor+ | MSIS29‐ | |||
| FIM cognitive+ | GHQ28‐ | ||||
| Khan 2010 | UDI6+ | AUA+ | AUA QoL+ | ||
| NDS+ | IIQ7+ | ||||
| Inpatient rehabilitation vs. outpatient rehabilitation | Francabandera 1988 | not assessed | ISS+ | Not assessed | Hours of home assistance required (self‐care) ‐ |
| Home rehabilitation vs control | Pozzilli 2002 | EDSS‐ | FIM‐ | SF36+ | Resource use ‐ |
| MMSE‐ | FSS‐ | Cost + | |||
| CDQ‐ | |||||
| STAI‐ | |||||
| STAXI‐ | |||||
| Outpatient rehabilitation vs control | Patti 2002 | EDSS‐ | Not assessed | SF36+ | |
| BDI+ | FIS+ | ||||
| SET+ | |||||
| Patti 2003 | EDSS ‐ | FIM total+ | Not assessed | ||
| FIM | |||||
| * motor+ | |||||
| *cognitive‐ | |||||
| Stuifbergen 2003 | Not assessed | ISS nr | SF36+ | ||
| SRAHP+ | |||||
| HPLPII+ | |||||
| BHPADPS ‐ | |||||
| PRQ85‐ | |||||
| Employment+ | |||||
| Guagenti‐Tax 2000 | EDSS‐ | ISS‐ | PDQ‐ | Satisfaction with care (including timeliness) ‐ | |
| HVL ‐ | MHI‐ | Cost of health care and home assistance nr | |||
| SIP nr | Qualitative final programme evaluation+ | ||||
| Revised UCLA | Length of stay and reason for nursing home placement nr | ||||
| Loneliness Scale‐ | |||||
| QRS‐ | |||||
| SF36+ | |||||
| Caregiver SF36+ | |||||
| Abbreviations (excluding outcome measures) | |||||
| + = significant between groups effect in favour of rehabilitation intervention | ES= Effect Size | ||||
| ‐ = non‐significant between groups effect | *=results unclear | ||||
| nr = no results reported |
A) Effectiveness of Inpatient MD therapy versus control
The three trials addressing the efficacy of inpatient MD rehabilitation (Freeman 1997; Craig 2003; Storr 2006) recruited a total of 217 patients.
Freeman 1997 and Storr 2006 compared individualized inpatient MD rehabilitation (for approximately 3‐5 weeks) with a wait‐list control group that received no intervention. The study by Craig 2003, focused specifically on patients admitted following an acute relapse of MS. Both groups received intravenous methylprednisolone, but in addition, the treatment group received a programme of MD rehabilitation (either on an in‐patient or day‐patient basis) while the control group received only standard care (which included therapy intervention according to local availability (quantity unspecified).
Two trials (Craig 2003; Freeman 1997) were rated as good quality RCTs on all assessment criteria, although both were unblinded and intention to treat was unspecified. The trial by Storr 2006 was of low quality due to a number of methodological flaws ‐ it was quasi‐randomised and despite reporting double‐blinding, the method for patient blinding was unspecified. The follow up periods were at 6 weeks (Freeman 1997), 12 weeks (Craig 2003) and two further follow ups with an interval of 10 weeks (Storr 2006) (Table 6).
Pooling of data from these three studies was confounded by the design differences high‐lighted above, and in addition by the use of different outcome measures.
For assessment at the level of impairment, Freeman 1997 used EDSS, Craig 2003 used the Amended Motor Club Assessment (AMCA) whilst Storr 2006 used EDSS and the MS Impairment Scale (MSIS). At the level of activity, Freeman 1997 used the Functional Independence Measure (FIM) as the primary outcome measure, Craig 2003 used the Guy's Neurological Disability Scale (GNDS) and Barthel Index (BI) whilst Storr 2006 used GNDS, Nine‐Hole Peg Test (9HPT) and Timed 10‐meter walking test (TW10). At the level of participation and quality of life, Freeman 1997 used the London Handicap Scale (LHS), Craig 2003 used the Human Activities Profile (HAP) and SF‐36 whilst Storr 2006 used Life Appreciation and Satisfaction Questionnaire (LASQ) and the Functional Assessment in MS (FAMS) scales.
Despite no change at the level of impairment, two studies (Craig 2003; Freeman 1997) showed modest benefits at the level of activities and participation, but these were not reflected in quality of life scores (Craig 2003). Storr 2006, however, did not demonstrate any benefits at the level of impairment, disability (activities) or participation.
The best evidence synthesis from these three RCTs (217 patients) suggests:
Strong evidence that organised MD rehabilitation can improve overall disability as measured by the total FIM (Freeman 1997) and the BI (Craig 2003); and overall level of participation in favour of the treatment group as measured by LHS (Freeman 1997) and HAP (Craig 2003).
Moderate evidence that inpatient rehabilitation can improve symptoms as measured by the GNDS (Craig 2003); and self care and sphincter control as measured by FIM (Freeman 1997); mobility and locomotion (wheelchair users only) in the study by (Freeman 1997), measured by the AMCA (Craig 2003) and FIM (Freeman 1997).
There is conflicting evidence that inpatient rehabilitation can improve symptoms/disability as measured by the GNDS (Craig 2003; Storr 2006).
No evidence was offered at the level of service costs or utility.
B) Effectiveness of Inpatient rehabilitation versus Outpatient rehabilitation:
One study with 84 participants (Francabandera 1988) compared the effectiveness of inpatient rehabilitation with outpatient rehabilitation in persons with severe MS. The follow up was completed either by a nurse via phone or when the patient visited the MS Center. Although this RCT reached the requirements for high methodological quality on the assessment criteria, it was unblinded and intention to treat was not specified. Details of participants and interventions such as length of rehabilitation and contents of the outpatient rehabilitation programme were not given and could not be obtained from authors. The participant attrition rate was high at 13%. In addition, the groups were poorly matched for incapacity status (ISS) at baseline (t=2.18 p<0.05), so that analysis of covariance was used to adjust for this at the outcome assessment point. Originally planned with a 2 year follow up period, this report presents only the 3 month data, and we were unable to find any further published reports of this trial or to obtain further data from the author.
According to this report, the ambulatory status and independence in self care (ISS) improved by two points for the in‐patient group, whilst deteriorating slightly (1.5 points) for the outpatients, resulting in a statistically significant effect in favour of in‐patient treatment group at 3 months. However, there was no difference between the groups in their need for aids and home care ‐ indeed both showed a trend towards increased need for assistance.
The best evidence synthesis of this study (84 patients) suggests:
Limited evidence that inpatient rehabilitation in persons with MS can result in greater short‐term gains at the level of activity (ambulatory status and independence in self‐care (ISS) compared with the outpatient care.
No evidence was offered at the level of participation
In addition, there was no evidence that these benefits result in reduction in the need for aids or assistance at home, which might have translated into cost‐effectiveness, and the longer term benefits are unknown.
C) Effectiveness of Inpatient or Outpatient MD therapy versus control
One trial with two reports (Khan 2008; Khan 2010) addressed efficacy of inpatient or outpatient MD rehabilitation in a total of 101 patients with MS. Khan 2008 compared individualized MD inpatient or outpatient rehabilitation (for approximately 3‐6 weeks) with a wait‐list control group. Although breakdown of the number of inpatient versus outpatient participants was provided, further subgroup analysis was not presented. Khan 2010, specifically included only patients with bladder issues (n = 74) from the original cohort and focused on individualised MD inpatient or outpatient bladder management compared with control wait‐list group (managed in the community by their usual treating doctors) in a MD rehabilitation programme.
At the level of activity, Khan 2008 used the Functional Independence Measure (FIM) as the primary outcome measure; and the Multiple sclerosis Impact Scale (MSIS29) and General Health Questionnaire (GHQ28) for measuring participation. Khan 2010 used the Guy’s Neurological Disability Scale (NDS) (bladder subscale only), Urogenital Distress Inventory (UDI6), American Urological Association Symptom Index (AUA) scale for symptoms and activity (disability). While measures for participation included the Incontinence Impact Questionnaire (IIQ7), and a single QoL item in the AUA scale.
The trial was rated as good quality on all assessment criteria. The follow up period was 12 months.
The synthesis of best evidence from this study (101 patients) suggests:
Moderate evidence to support the effectiveness of the inpatient or outpatient MD rehabilitation programme in reducing disability (FIM) (Khan 2008); and bladder impairment and ‘activity limitation’ outcomes (UDI6, NDS, AUA) (Khan 2010).
Moderate evidence that inpatient or outpatient MD bladder rehabilitation improves continence‐related QoL and participation outcomes (AUA, IIQ7) (Khan 2010).
Insufficient evidence to support the effectiveness of the inpatient or outpatient MD rehabilitation programme for improvement in participation and patient QoL (MSIS29, GHQ28) (Khan 2008).
D) Effectiveness of MD Home rehabilitation versus control
Only one study addressed the effectiveness of home‐based rehabilitation in MS compared with a control group (Pozzilli 2002) reported a reasonably high quality study for 201 participants with definite MS, although once again, the study was unblinded and intention to treat was unspecified, as was the amount of therapy received. The study aimed to assess the benefits and cost effectiveness of a coordinated multi‐disciplinary home‐based rehabilitation programme, compared with a control group receiving standard out‐patient hospital care at their usual MS referral centre.
The outcome point was at 12 months and 188 patients reached this point (6.5% attrition). No difference was found in activity measures (both FIM and FSS) between the home‐based programme group and standard outpatient hospital care group. There was however significant difference in four SF‐36 health dimensions (bodily pain, general health, social functioning and emotional role) favouring home‐based management. The cost of home‐based care was slightly less (€ 822 euros/patient/year) than hospital care, mainly due to decreased hospital admissions. The authors concluded from this study that MD home‐based rehabilitation may provide a cost‐effective approach to management of persons with MS and improve their quality of life.
Based on this one reasonably high quality study (201 patients), the best evidence synthesis suggests:
Limited evidence that MD home based rehabilitation can produce significant advantage over standard hospital care in some domains of patient quality of life‐ (SF‐36) for up to 12 months, despite no change at the level of activity (disability).
Whilst there may be potential for reducing service costs if hospital admissions are avoided, actual evidence for cost effectiveness has yet to be demonstrated.
E) Effectiveness of MD Outpatient rehabilitation versus control
Four studies examined the effects of out‐ patient rehabilitation programmes for MS. Two reports of the same study by Patti et al (Patti 2002; Patti 2003) examined the short‐term effects of a high intensity out‐ patient rehabilitation programme in 111 patients. The other three studies (one with 2 reports) (Stuifbergen 2003; Guagenti‐Tax 2000; DiFabio 1997; DiFabio 1998) (total 240 patients) addressed the effects of a low intensity outpatient rehabilitation programme compared with control group with MS with a longer‐term follow up (more than 6 months). Attrition rates were unfortunately high: 24% in DiFabio 1998, 30% in DiFabio 1997, and 38% in Guagenti‐Tax 2000; although not sufficient to cause exclusion of the study.
Patti 2002; Patti 2003 assessed the effect of a high intensity outpatient rehabilitation programme (six days per week) for 6 weeks, followed by a home self exercise programme for 6 weeks in a randomized, single blind controlled trial. The control group received only home exercises. The study was of high methodological quality on all criteria assessed. The first report (Patti 2002) addressed outcomes at the level of participation, mood and quality of life, and the second (Patti 2003) at the level of activities (disability). Despite no change in impairment (EDSS scores) or cognitive function (FIM cognitive score), the treatment group showed significant improvements in self‐care, mobility, transfer skills and continence (as measured by the FIM motor scale) compared with controls; and also in quality of life (SF‐36), mood (Beck Depression Inventory), fatigue (FIS) and social function (SET). Of the three studies addressing lower intensity programmes, two were unblinded RCTs (Stuifbergen 2003, Guagenti‐Tax 2000) of reasonably high methodological quality on assessment criteria. The third (DiFabio 1997; DiFabio 1998) was a non‐randomised controlled trial (CCT) which therefore scored poorly on many of the items of internal validity and achieved an overall 'low quality' grading, although it fared better on descriptive and statistical criteria.
Stuifbergen 2003 examined the effects of an 8‐week MD group‐based outpatient "wellness intervention programme" for women with MS on health behaviours and quality of life, compared with a wait‐list control group who received their programme after the last data collection point (n=121). Data collection points were at the beginning and end of the programme and at 3 and 6 months post rehabilitation. Data at the level of impairment and activity (ISS) were provided only at baseline and 6 month follow‐up to confirm group comparability, and they did not change in either group. Measures of participation such as barriers to health promoting activities and personal support were also similar for the two groups. A hierarchical linear modelling regression technique was used to avoid bias from repeated measures data and showed a statistically significant 'group by time' effect for self‐efficacy and health‐promoting behaviours and for the 'mental health' and 'pain' scales of the SF‐36. The treatment group were also more likely to be employed than the control group at follow‐up. The authors concluded that low intensity therapy and education improves health behaviours and some dimensions of QOL for women with MS.
Guagenti‐Tax 2000 compared low intensity MD group based therapy for patients and caregivers for 12 months (twice‐monthly groups and 10 workshops) compared with standard care (which was not specified) (total 73 patient‐caregiver units). Outcomes were measured at 12 and 24 months. This complex study included a large number of parameters, with no apparent attempt to correct for multiple statistical tests, and the analysis was sometimes hard to follow. Both groups declined significantly over the two year period in terms of impairment and physical functioning (EDSS and ISS) and in cognitive deficits; and both groups reported increased satisfaction with caregiver help. However, in terms of participation a significant interaction was seen on the SF‐36 general health subscale, for both patients and carers with the control group reporting a greater decline in perceived health. Caregivers in the control group also reported greater interference with social activities over time. The authors concluded that the outpatient therapy and carer/patient education improved patient perceived health and anxiety.
DiFabio 1997; DiFabio 1998, examined extended low intensity outpatient therapy (1 day per week for 1 year) compared with a wait‐list control group in a total of 46 subjects. Outcomes were measured at 12 months. DiFabio 1998 reports outcomes at the level of impairment and functional status (Symptom checklist, fatigue and the Rehabilitation Institute of Chicago Functional Assessment scale). DiFabio 1997 reports outcomes at the level of participation and quality of life (SF‐36 and the Multiple Sclerosis Quality of Life Questionnaire). The principal findings from this study were that patients receiving out‐patient rehabilitation had significantly reduced frequency of symptoms and fatigue (F 1, 30 =9.68, p=0.004) at one year compared to the control group. The treatment group also showed small improvements in six quality of life measures (fatigue, general health, social function and support). These did not improve in the control group. No data were presented on cost benefit or other utility measures.
The synthesis of best evidence from these 4 studies (351 patients) of out‐patient rehabilitation suggests:
For short, high‐intensity outpatient programmes (111 patients) (Patti 2002; Patti 2003) despite no change at the level of impairment (EDSS), there is:
Limited evidence that MD rehabilitation can provide short‐term benefits at the level of activity (FIM), in particular in motor disability (locomotor and transfer function), self‐care and sphincter function.
Limited evidence for improvement in patient quality of life (SF 36), fatigue (FIS), mood and social function (SET).
No evidence is as yet available regarding the longer‐term benefits or cost‐effectiveness.
For lower intensity out‐patient programmes over longer term follow‐up (at 6‐24 months) there was no evidence for improvement at the level of activity (disability) in any of the studies (ISS, RIC FAS) (DiFabio 1997; DiFabio 1998; Guagenti‐Tax 2000). However, there was:
Insufficient evidence from one study (44 patients) (DiFabio 1997; DiFabio 1998) (that outpatient MD therapy can produce benefits at the level of impairment to reduce symptom frequency and fatigue (MS Symptom checklist composite score).
Strong evidence from 3 studies (238 patients) for improvement in quality of life particularly in the domains of bodily pain and mental health domains (SF 36).
Limited evidence from one study (121 patients) for improved self‐efficacy and engagement in health‐promoting behaviours (SRAHP, HPLP‐II), as well as employment status (Stuifbergen 2003).
Limited evidence from one study (73 carers) (Guagenti‐Tax 2000) at the level of participation for better general health and less interference with social activities for carers (SF36).
Discussion
This review investigated the effectiveness of organized MD rehabilitation in adults with MS based on measures of activities and participation in the ICF (WHO 2001), and also of utility and service costs. Unfortunately, there was a large degree of variation in patient baseline characteristics and severity of MS, stages of MS, measurement tools used (even for identical outcomes), treatment and control protocols and length of follow up in these studies. Because of this heterogeneity, it was not possible to pool data statistically. Instead best evidence synthesis was performed using a qualitative analysis.
We found ’strong evidence’ that inpatient MD can produce short‐term gains at the levels of activity impairment (disability) and participation for patients with MS. There is ‘moderate evidence’ to support inpatient or outpatient rehabilitation programmes (compared with control wait‐list groups) in improving disability; and bladder related activity and participation outcomes up to 12 months following rehabilitation intervention. This updated review increases the body of evidence for centre‐based MD rehabilitation programmes. For outpatient and home‐based rehabilitation programmes there was 'limited evidence' for short‐term improvements in symptoms and disability with high intensity programmes, which translated into improvement in participation and quality of life. For low intensity programmes conducted over a longer period there was 'strong evidence' for longer term gains in quality of life (albeit to a modest degree and not in all domains); and also 'limited evidence' for benefits to carers in terms of general health and engagement in social activities. Although some studies reported potential for cost‐savings, at the current time there is no convincing evidence regarding the long‐term cost‐effectiveness of these programmes.
The strength of findings was limited by the small number of studies and in some cases by methodological weaknesses. All of the studies were single centre trials, with fairly small participant numbers, with a risk of type I and II errors. Many of the trials, particularly in outpatient settings used a larger number of statistical tests without adjustment of the level of probability. Details of the participants and the interventions were frequently missing and were not obtainable from the authors despite attempts to contact them.
Local practices tended to vary in different countries (US, UK, Italy, Denmarkand Australia) making it harder to interpret and compare outcomes. The issues of design in MD health services intervention are complex. The ‘real life’ clinical settings may have operational issues such as relating to rehabilitation ‘slots’ availability within health service, appropriate staffing levels for timely intervention etc. In several of the studies (Freeman 1997; Khan 2008; Khan 2010; Patti 2002; Patti 2003; Storr 2006), rehabilitation programmes were individually tailored for MS participants and therefore not possible to standardize across the care spectrum. This heterogeneity may reflect the wide variety of the rehabilitation programmes in different settings and under different healthcare system. From this review, it has not been possible to suggest best 'dose' of therapy, further studies are needed to suggest optimum number, duration and intensity of treatment sessions.
Adverse effects of rehabilitation are possible, but rarely seen in practice. Only one trial with two reports (Khan 2008; Khan 2010), reported adverse effects attributable to the rehabilitation. Fatigue is a major issue in MS, and many of the studies measured fatigue, but none showed an increase in fatigue as a result of rehabilitation.
This review has served to highlight some of the limitations and challenges for randomised controlled trial methodologies in complex interventions such as rehabilitation for chronic degenerative conditions. Previous authors (Khan 2010c; Ng 2009; Turner‐Stokes 2005; Whyte 2002; Thompson 2000) have highlighted some of these limitations which include the following:
Rehabilitation is a complex form of treatment which is difficult to quantify, and may include multiple interventions, and depends on the interaction between the patient and the clinician. Programmes frequently involve dynamic interplay in behaviour between patient and therapist, which are dependent upon patient response and potentially confound simple division into 'treatment' and 'control' conditions.
The target population is characterised by relatively small numbers of patients with marked heterogeneity within participants in terms of disease type, clinical presentation, goals for treatment. A large pool of patients or 'pure participants' would be required to minimize confounding impairments, and this is often beyond the capacity of a single institution. Multi‐centre trials may provide larger numbers but often at the expense of increased heterogeneity, with little net benefit.
In longer term conditions, intervention must be evaluated over a significant period of time and this may lead to difficulties with recruitment and retention, especially in the control groups. There may also be ethical and consent issues, particularly where patients have cognitive deficits arising from their condition.
True 'blinding' of participants and care providers is rarely possible in the context of rehabilitation, and attempts to blind outcome assessor may be confounded if patients unwittingly volunteer information about their treatment during the course of assessment.
More recently an alternative approach to gathering evidence was trial led through the use of ‘clinical practice trials’ that acquire prospective and retrospective data without disrupting the natural milieu of treatment (Gassaway 2005). This routine data collection can provide additional information about the nature of services provided, the outcomes and models of care for rehabilitation and implications for clinicians. This approach was used recently in a pilot study in an inpatient MS population (n=24) to quantify the intensity and frequency of rehabilitation to understand patient complexity and need for therapy (Khan 2010a). More work is needed to build evidence base in this context and identify components comprising the ‘black box’ of rehabilitation.
Persons with MS often have complex issues. Clinicians may not always agree with one another or incorporate the perspective of the person with MS and/or caregivers. The clinical decision making process can be subjective and biased (Elstein 2002). The use of only standardized instruments for functional assessments can also cause bias (Brown 2004). One patient centred outcome process is the use of ‘goal attainment scaling’ (GAS) procedure (Kiresuk 1968). In one MS inpatient cohort, GAS was found to be more meaningful compared with standard outcomes measures (FIM, BI), as it evaluated outcomes considered important by patients (Khan 2008a). Incorporation of the perspectives of patients with MS (and their carers) can facilitate clinical decision making processes and approaches. In one study of persons with MS (n=101), their self reported problems were linked with the ICF (Khan 2007a) for incorporation in MD care programmes. Further, an expert consensus opinion (used a Delphi exercise) about issues that should be addressed in MD programmes for pwMS was also reported (Khan 2007b). Lists of ICF categories or ‘core set’ was selected by experts to facilitate clinical care and agreement, and these in the future may assist in outcome development in MS using the ICF item banking and scale development techniques (Cieza 2009; Grill 2009).
Assimilation of data from different trial also poses challenges because of marked heterogeneity in intervention type and setting; and also with respect to the outcome measures used and time points for assessment. Floor/ceiling effects may limit sensitivity to treatment effects, and even the most carefully standardised measures may behave differently in different cultures and settings (Tennant 2002). In this review we have attempted to subdivide outcomes into the levels of impairment, activity (disability) and participation as described by the WHO ICF. However, many of the measures straddle these concepts and include items at different levels. For example, the EDSS includes a mixture of impairment and disability, and whilst it may provide useful categorisation of disease severity, it is not a sensitive outcome measure for rehabilitation which generally does not alter impairment.
Many of the outcomes measures used in MS trials were primarily designed for other conditions (for example the London Handicap Scale was developed for use in stroke patients and the SF‐36 for general populations). These scales may not be sensitive to changes peculiar to fluctuating conditions such as MS (Freeman 1999; Thompson 2000). Although specific measures, such as the Multiple Sclerosis Impact Scale (MSIS) (Hobart 2001) have since been developed for use in this context, these have yet to be widely taken up and most of the trials included in this review pre‐dated their development. Newer techniques of data analyses such as application of Rasch analyses can convert ordinal data to interval estimates, which allow improved measurement (Ramp 2009). Rasch analyses can demonstrate significant difference in responsiveness at the ‘individual person’ level rather than ‘group level’ estimates. This technique is recommended for future rehabilitation trials.
Finally, more research is needed (at national and international level) to study the variability and outcomes of MS rehabilitation programmes, to improve data collection and identify health service planning needs. One recent report (Khan 2009) used the Australian national rehabilitation dataset to review the rehabilitation outcomes of 1100 pwMS (over a 5 year period) from 162 accredited rehabilitation facilities across Australia. Analysis of outcome measures showed that following inpatient rehabilitation, there was reduction in disability (FIM) and in hospital length of stay, and increased discharge of these persons back into the community. This study highlighted need for further research to improve standardized data collection in the national datasets for the information to be more clinically meaningful in the future.
Limitations of this review
The assimilation of available evidence was challenging due to the diversity of trials in this review. The authors accept that there may have been a degree of:
selection bias from the literature search (van Tulder 2003)
publication bias (Egger 1998) if trials have not been published due to they have small patient numbers and negative results
reference bias (Goetzsche 1987) for published studies included in this review.
The review has taken an inclusive approach to a broad area of clinical practice and this approach has posed significant challenges for the assessment and assimilation of the available evidence. It may be contended that we have adopted too low threshold for inclusion of studies of low quality. On the other hand, we believe that the presented synthesis of 'best evidence' based on assessment of methodological quality, has facilitated a helpful comparison of the various studies available. It also allows open acknowledgement of the 'limited evidence' which comes from these poorer (or single ) studies which is nevertheless the best available at the current time.
Our attempt to categorise evidence according to the WHO ICF posed some methodological problems, since many of the outcome measures used in trials crossed the boundaries between the different levels of the model. However we still believe that this model is helpful to clarify the experience of people who live with long term neurological conditions.
Summary and future research
Based on a qualitative synthesis of best evidence, this review suggests that, despite no change at the level of impairment, multi‐disciplinary rehabilitation can improve the experience of people living with multiple impairment in terms of both activity and participation. However, the evidence for cost‐effectiveness is as yet 'suggestive' and further direct evidence is required. No adverse effects of rehabilitation intervention were reported, and it was not possible to suggest best 'dose' of therapy or supremacy of one therapy over another.
With spiraling health care costs and the increased demand for rehabilitation services, it has become increasingly important to justify expense of rehabilitation services. This review highlights the limitations of RCTs in rehabilitation settings and the need for better designed randomized and multiple centre trials; also the use of complimentary research methods to build a rounded evidence‐base in this area. The perspective of the person with MS should be incorporated; and participation issues relevant to MS (such as return to work, driving, community reintegration, leisure, parenting and psychosocial issues) need further evaluation.
Authors' conclusions
Implications for practice.
The evidence presented in this review supports the recommendation that patients with all types of MS should undergo regular specialist evaluation and follow up to assess their need for appropriate rehabilitation intervention as well as maintenance therapy in order to maximise their capacity for independent living and societal participation. The type and setting of the rehabilitation treatment (inpatient, community) should be individualized based on patients' specific needs. Inclusion or exclusion of patients for rehabilitation shortly after relapse is available only for some studies (Craig 2003; Freeman 1997) and could be a significant confounding factor when it is not reported or where there is a significant imbalance between treatment and control groups. Gaps in this knowledge could then suggest directions for future research. The needs of their caregivers should also be addressed.
Implications for research.
This review highlights the need for: High quality RCTs and other designs where appropriate, which assess
the effectiveness of specific rehabilitation interventions (and components),
the appropriate intensity and settings of therapy,
the cost effectiveness of comprehensive MD rehabilitation programmes
the impact of therapy on patients and their families.
·Development of appropriate outcome measures including
Reliable and valid outcome measures which reflect domains of the ICF.
Application of improved data analyses techniques e.g. Rasch for improved measurement.
Measurement of the effects of rehabilitation over longer periods (over 12 months) in terms of effects on persons with MS and on social costs (associated with care costs, inability to drive, work).
A consensus on a core set of measurement of outcomes in MS trials.
What's new
| Date | Event | Description |
|---|---|---|
| 6 April 2011 | New search has been performed | The review has been updated 2011. |
| 25 February 2011 | New search has been performed | Search updated |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 13 May 2008 | Amended | The text has been amended throughout. |
| 12 May 2008 | Feedback has been incorporated | We have replied to feedback, and as a result of which have edited the Background section. |
| 15 April 2008 | Amended | Converted to new review format. |
Acknowledgements
We are grateful to Ms L Coco and Dr G Fillippini of the MS Review Group Editorial Board for their support and assistance. We also wish to thank Mr S McDonald of the Australasian Cochrane Group, Ms V Delafosse and Ms K Greig for initial literature searches in 2007 and Ms Deirdre Beecher of the Cochrane Multiple Sclerosis Review Group for the updated literature search.
Appendices
Appendix 1. Keywords used to search the MS Group Specialisd Register
{ambulatory care} OR {rehabilitation} OR {hospitalization} OR {hospitalisation} OR {physical therapy modalit\*} OR {home care service\*} OR {hospital‐based home care service\*} OR {inpatient\*} OR {outpatient\*} OR {cognitive therap\*} OR {behavior therap\*} OR {behaviour therap\*} OR {social work} OR {dietetic\*} OR {dietary service\*} OR {neurologic gait disorder\*} OR {counseling} OR {counselling} OR {home health care} OR {physiotherap\*} OR {physical therap\*} OR {speech} OR {nutrition} OR {diet} OR {food} OR {home} OR {wellness} OR {occupation\*} OR {health behavior\*} OR {health behaviour\*}
Appendix 2. Search methods used in previous version
Search methods for identification of studies This review drew on the search strategy developed in consultation with search coordinators for the Cochrane Multiple Sclerosis Group to avoid unnecessary duplication. We used Cochrane handbook search strategy for optimal sensitivity in identifying randomized clinical trials (Handbook 2000) (11a 15 Appendix B. Handbook 4.1 June 2000). A systematic search without language restrictions was conducted to identify all relevant published and unpublished randomised controlled trials, with translation available if required. Electronic searches Relevant trials were identified from the following sources: 1) Electronic searches of a) Cochrane Multiple Sclerosis Group's Specialised Trials Register, b) Cochrane Central Register of Controlled Trials "CENTRAL" (Present Issue‐2006), c) MEDLINE (1966 ‐September 2005), d) CINAHL (1982 to September 2005), e) PEDro (from 1990 ‐ September 2005), f) EMBASE (1988‐2005) The review search strategy for MEDLINE, CINAHL, EMBASE and Cochrane databases are shown in Appendix 1 (Appendix 1) (Ovid MEDLINE:1966‐September 2005) Appendix 2( Appendix 2) (CINAHL:1982‐September 2005), Appendix 3 (Appendix 3) (EMBASE: 1988 to September 2005) and Appendix 4 (Appendix 4) (COCHRANE Database (September 2005) Searching other resources 2) Reference lists from published reviews on multidisciplinary rehabilitation in multiple sclerosis and identified RCTs and CCTs. 3) Personal communication with first authors of relevant trials or reviews and other multiple sclerosis experts. 4) The Cochrane Rehabilitation and Related Therapies Field trials Register 5) National Health Service National Research Register (NRR) including Medical Research Council Clinical Trials Directory. 6) Handsearch of relevant journals: "Multiple Sclerosis" (January 1998 ‐ October 2005) with the search engine Proquest, "Archives of Physical Medicine and Rehabilitation (January 1996 ‐ October 2005), "Clinical Rehabilitation" (1998 ‐ October 2005) and "International Journal of MS Care" (1999 ‐ 2005). Unpublished trials were identified using strategies 3, 4 and 5. Authors and well‐known experts in this field were contacted if further information about the trials was needed.
Appendix 3. PEDro search strategy
Abstract + Title = (Multiple Sclerosis) AND (rehabilitation) + Method = Clinical Trial
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Craig 2003.
| Methods | RCT. Block randomisation. | |
| Participants | U.K., N = 41 (1 drop out), treatment group = 20, control = 21 (1 drop out). Patients randomised to brief (3‐8 days) inpatient or day case therapy or usual hospital care while receiving IVMP. | |
| Interventions | Treatment group ‐ received IVMP and MD care as inpatient or day case. Control group ‐ limited PT and OT. | |
| Outcomes | GDNS, AMCA, HAP, BI, SF‐36 | |
| Notes | Length of follow up 3 months. Breakdown of those treated as inpatients versus outpatients not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly allocated to treatment or control group according to the randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | No information provided for the method of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 dropout in the control group, no other missing outcome data, |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. |
| Other bias | High risk | Analyses not performed according to the ITT; inclusion and exclusion criteria not clearly specified; more females and older participants with shorter disease duration in the control group. |
DiFabio 1997.
| Methods | CCT. | |
| Participants | USA, N = 44, (13 drop outs), treatment = 19 (7 drop outs) and control (wait‐list) = 25 (6 drop outs) | |
| Interventions | Treatment group ‐ MD outpatient rehabilitation for 1 year Control group ‐ medical wait‐list. | |
| Outcomes | RIC‐FAS, SF36, MSQOL‐54 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall 13 participants (30%) dropout and missing data not provided. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported |
| Other bias | High risk | Analyses were not performed according to ITT. Bias related to study design. |
DiFabio 1998.
| Methods | CCT. | |
| Participants | USA, N = 46, Treatment = 20 (7 drop outs) and control (wait‐list) = 26 (6 drop outs) | |
| Interventions | Treatment group ‐ MD outpatient rehabilitation for 1 year Control group ‐ medical (pharmacologic) management only. | |
| Outcomes | Selected items from RIC‐FAS, MS related symptom checklist composite score, Fatigue frequency | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised. |
| Allocation concealment (selection bias) | High risk | Allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall 28% dropout. Participants who dropout in the control group had lower functional status than those who remained in the study. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. |
| Other bias | High risk | Analyses were not performed according to ITT. Bias related to study design. |
Francabandera 1988.
| Methods | RCT. Block randomization. | |
| Participants | USA, N = 84 (11 drop outs), Treatment (inpatient group) = 42, control (outpatient) = 42 | |
| Interventions | Inpatient group: individualised MD care Outpatient group: individualised MD care as required | |
| Outcomes | ISS, Hours of home assistance required (self‐care). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Particpants randomly assigned to treatment and control group, detail procedure not provided. |
| Allocation concealment (selection bias) | High risk | Allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 13% dropout. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. |
| Other bias | High risk | Analyses were not performed according to ITT. |
Freeman 1997.
| Methods | RCT. Randomization, stratified based on EDSS scores. | |
| Participants | U.K. N = 70 (4 drop outs), treatment = 34 (2 dropouts) and control = 36 (2 dropouts) | |
| Interventions | Treatment group ‐ inpatient individualized, MD rehabilitation programme Control group ‐ no rehabilitation intervention. | |
| Outcomes | EDSS, self‐reported relapses, FIM, LHS, drug management | |
| Notes | Follow up was limited (6 weeks only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants stratified based on the entry EDSS scores and randomly assigned to treatment and control group (wait‐list). |
| Allocation concealment (selection bias) | High risk | Allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors not totally blinded, justification to minimise the detection bias was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 6% dropout. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. |
| Other bias | High risk | Analyses were not performed according to ITT. |
Guagenti‐Tax 2000.
| Methods | RCT. | |
| Participants | USA, N = 73 patient‐caregiver units. Dropout of 14 units prior to programme start (group not specified). Treatment group 43 and control 30 units. | |
| Interventions | Treatment group ‐ Twice‐monthly MD day‐care group programme for 12 months and 10 workshops.Control group ‐ standard care mentioned but not specified. | |
| Outcomes | EDSS, ISS, PDQ, HVL, MHI, SIP, Revised UCLA Loneliness‐Companionship Scale, QRS, SF36, Satisfaction with care (including timeliness), Cost of health care and home assistance, length of stay and reason for nursing home placement, qualitative final programme evaluation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization followed the Zelen "randomised consent" procedure initially but was changed to a conventional randomization in year 3. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment procedure not provided. Participants were allocated to treatment and control group, without their knowledge or consent. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 19% dropout. |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified primary and secondary outcomes reported. |
| Other bias | Low risk | Analyses were performed according to ITT. |
Khan 2008.
| Methods | RCT. | |
| Participants | Australia N = 101 Intervention =49 (1 drop out), control =52 (2 drop outs) | |
| Interventions | Treatment group ‐ individualised inpatient or outpatient MD rehabilitation programme. Control group: wait list (no treatment) | |
| Outcomes | FIM, MSIS29, GHQ28 | |
| Notes | Length of follow up 12 months. 12 participants from control group also required and received the intervention. Additional analysis for those who received treatment was performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation sequence stratified by EDSS score, with wait list control group. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment mentioned in acknowledgement section only, detail information not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treating personnel blinded but participants became blinded 8 months into the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 3% dropout. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. Additional analysis performed comparing those who received and those who did not received the intervention, irrespective of the randomisation. |
| Other bias | High risk | Analyses were not performed according to ITT. |
Khan 2010.
| Methods | RCT. | |
| Participants | Australia, N = 74 Intervention =40 (16 drop outs), control =34 ( 0 drop out) | |
| Interventions | Treatment group ‐ individualised MD bladder rehabilitation programme. Control group: usual care. | |
| Outcomes | UDI6, NDS,AUA, IIQ7 | |
| Notes | Same group of patients as Khan 2008 with bladder issues. Length of follow up 12 months. 10 participants from control group received the intervention. Additional analysis for those who received treatment was performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation sequence stratified by EDSS score, with wait list control group. |
| Allocation concealment (selection bias) | Low risk | Numbered opaque sealed envelopes used to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treating personnel blinded but participants became blinded 8 months into the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall there was 22% dropout and all were from the treatment group. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. Additional analysis was performed comparing those who received and those who did not received the intervention, irrespective of the randomisation. |
| Other bias | High risk | Analyses were not performed according to ITT. |
Patti 2002.
| Methods | RCT. | |
| Participants | Italy N = 111 (5 drop outs), Treatment = 58 (4 drop outs) control = 53 (1 drop out) | |
| Interventions | Treatment group ‐ comprehensive outpatient rehabilitation programme followed by a home exercise programme for 6 weeks.Control group ‐ Home exercise programme for 12 weeks. | |
| Outcomes | EDSS, SF36, FIS, SET, BDI | |
| Notes | Same group of patients as Patti 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation sequence stratified by EDSS score, with wait list control group. |
| Allocation concealment (selection bias) | Low risk | Numbered opaque sealed envelope used to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 5% dropout. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. |
| Other bias | Low risk | Analyses were performed according to ITT. |
Patti 2003.
| Methods | RCT. | |
| Participants | Italy N = 111 (5 drop outs), Treatment = 58 (4 drop outs), control = 53 (1 drop out) | |
| Interventions | Treatment group ‐ comprehensive outpatient rehabilitation programme followed by a home exercise programme for 6 weeks.Control group ‐ Home exercise programme for 12 weeks. | |
| Outcomes | EDSS, FIM | |
| Notes | Same group of patients as Patti 2002 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation sequence stratified by EDSS score, with wait list control group. |
| Allocation concealment (selection bias) | Low risk | Numbered opaque sealed envelopes used to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 5% dropout. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. |
| Other bias | Low risk | Analyses were performed according to ITT. |
Pozzilli 2002.
| Methods | RCT. Block randomisation. | |
| Participants | Italy N = 201 (13 drop outs) Treatment = 133 (10 drop outs), control = 68 (3 drop outs). | |
| Interventions | Treatment group ‐individualised clinical care and coordinated home services Control group ‐ routine hospital care at their MS referral centres as required | |
| Outcomes | EDSS, MMSE, FIM, FSS, CDQ, STAI, STAXI, SF36, resource use and cost | |
| Notes | The amount of therapy was not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated block randomisation, stratified by age and EDSS. |
| Allocation concealment (selection bias) | High risk | Allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 7% dropout. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. |
| Other bias | High risk | Analyses were not performed according to ITT. |
Storr 2006.
| Methods | RCT. | |
| Participants | Denmark N = 106, Intervention =41 (3 drop out), control =65 ( 13 drop out) | |
| Interventions | Treatment group ‐ individualised inpatient MD rehabilitation programme. Control group: no treatment waitlist. | |
| Outcomes | MSIS, EDSS, GND, 9HPT, TW10, FAMS, LASQ, VAS | |
| Notes | Length of follow‐up of 10 week. Unequal distribution of participants in two groups, and 20% drop outs in the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random allocation to treatment and control groups by odd and even numbers. |
| Allocation concealment (selection bias) | High risk | Allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The investigators state that patients were unaware of treatment, but likely patient knew they received treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 15% dropout. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. |
| Other bias | High risk | Analyses were not performed according to ITT. |
Stuifbergen 2003.
| Methods | RCT. | |
| Participants | USA N = 121 (8 dropouts) treatment = 61 (5 drop outs), control = 60 (3 drop outs) | |
| Interventions | Treatment group ‐ 2‐phased MD wellness programme consisting of a lifestyle change phase, followed by telephone calls post completion of programme. Control group ‐ monthly phone call. | |
| Outcomes | ISS, SF36, SRAHP, HPLP‐II, BHPADPS, PRQ85 (part 2), employment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed by random number table method. |
| Allocation concealment (selection bias) | High risk | Allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 75 dropout. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes reported. |
| Other bias | Unclear risk | ITT was specified but figures were not provided. |
ITT = Intention to Treat IVMP = intravenous methylprednisolone MD = Multidisciplinary MS = Multiple sclerosis N = Number OT = Occupational therapy PT = Physiotherapy RCT = Randomised Controlled Trial
CCT Clinical Controlled Trial UK = United Kingdom USA = United States of America
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aisen 1996 | Not a RCT/CCT |
| Barrett 2009 | Randomised variable was physical modalities not MD therapy. |
| Bethoux 2003 | Abstract only |
| Bethoux 2005 | Not a RCT/CCT |
| Bethoux 2005 A | Poster only |
| Bourdette 1991 | Poster only |
| Brissart 2010 | Not a RCT/CCT |
| Carey 1988 | Not a RCT/CCT |
| Carton 2000 | Not a RCT/CCT |
| Cendrowski 1999a | Not a RCT/CCT |
| Cendrowski 1999b | Not a RCT/CCT |
| Coote 2009 | Randomised variable was physical modalities not MD therapy. |
| Edwards 2002 | Not a RCT/CCT |
| Feigenson 1981 | Not a RCT/CCT |
| Fink 2010 | Randomised variable was executive function intervention not MD therapy. |
| Flavia 2010 | Randomised variable was cognitive intervention not MD therapy. |
| Freeman 1996a | Abstract only |
| Freeman 1996b | Not a RCT/CCT |
| Freeman 1999 | Not a RCT/CCT |
| Freeman 2005 | Not a RCT/CCT |
| Grasso 2005 | Not a RCT/CCT |
| Greenspun 1987 | Not a RCT/CCT |
| Grossman 2010 | Randomised variable was cognitive intervention not MD therapy. |
| Hebert 2009 | Randomised variable was vestibular intervention not MD therapy. |
| Hinrichs 2003 | Not a RCT/CCT |
| Jones 1996 | Fatal flaw (attrition rate 40%) |
| Jonsson 1996 | Not a RCT/CCT |
| Jorger 2001 | Not a RCT/CCT |
| Kalina 2009 | Not a RCT/CCT |
| Khan 2010b | Not a RCT/CCT |
| Kidd 1995 | Not a RCT/CCT |
| Kidd 1997 | Not a RCT/CCT |
| Liu 2003 | Not a RCT/CCT |
| Mostert 2005 | Randomised variable was pulsed magnetic field therapy not MD therapy. |
| Palmisano 1999 | Results published in Pozzilli 2002 |
| Plow 2009 | Randomised variable was physical modalities not MD therapy. |
| Portillo 2009 | Not a RCT/CCT |
| Pozzilli 2004 | Not a RCT/CCT |
| Reding 1987 | Not a RCT/CCT |
| Riazi 2004 | Co‐interventions were different |
| Roush 1995 | Not a RCT/CCT |
| Sitzia 1998 | Not a RCT/CCT |
| Slade 2002 | Primarily stroke and TBI patients. The number of MS participants not specified. |
| Storr 1999 | Poster only |
| Van der Putten 1999 | Not a RCT/CCT |
| Vaney 1996 | Not a RCT/CCT |
| Wahls 2010 | Randomised variable was neuromuscular electric stimulation not MD therapy. |
| Ward 2004 | Results of the MS subgroup not provided |
CCT = Controlled clinical trial RCT = Randomised controlled trial MS = Multiple sclerosis TBI = Traumatic brain injury
Contributions of authors
F Khan is the author of the review. L Turner‐Stokes provided valuable input into design of the review. F Khan, B Amatya, L Turner‐Stokes, L Ng were responsible for all literature searches and methodological quality of included studies. T Kilpatrick provided valuable assistance with the discussion. All authors critically reviewed the manuscript and discussed the protocol, data collection, results and conclusions.
Sources of support
Internal sources
Department of Rehabilitation Medicine, Royal Park Campus, Royal Melbourne Hospital, Australia.
External sources
No sources of support supplied
Declarations of interest
None Sources of support:
External sources of support: None Internal sources of support: Rehabilitation Department ‐ Royal Melbourne Hospital, Royal Park Campus Melbourne Australia. Prof Turner‐Stokes received financial support from the Luff Foundation and the Dunhill Medical Trust.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Craig 2003 {published data only}
- Craig J, Young C A, Ennis M, Baker G, Boggild M. A randomised controlled trial comparing rehabilitation against standard therapy in multiple sclerosis patients. Journal of Neurology, Neurosurgery and Psychiatry 2003;74(9):1225‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
DiFabio 1997 {published data only}
- DiFabio RP, Choi T, Soderberg J, Hansen CR. Health related quality of life for patients with progressive multiple sclerosis: influence of rehabilitation. Physical Therapy 1997;77(12):1704‐16. [DOI] [PubMed] [Google Scholar]
DiFabio 1998 {published data only}
- DiFabio RP, Soderberg J, Choi T, Hansen CR, Schapiro RT. Extended outpatient rehabilitation : its influence on symptom frequency, fatigue, and functional status for persons with progressive multiple sclerosis. Archives of Physical Medicine and Rehabilitation 1998;79(2):141‐6. [DOI] [PubMed] [Google Scholar]
Francabandera 1988 {published data only}
- Francabandera F L, Holland N J, Wiesel‐Levison P, Scheinberg L C. Multiple sclerosis rehabilitation: inpatient versus outpatients. Rehabilitation Nursing 1988;13(5):251‐3. [DOI] [PubMed] [Google Scholar]
Freeman 1997 {published data only}
- Freeman J A, Langdon D W, Hobart J C, Thompson A J. The impact of inpatient rehabilitation on progressive multiple sclerosis. Annals of Neurology 1997;42(2):236‐44. [DOI] [PubMed] [Google Scholar]
Guagenti‐Tax 2000 {published data only}
- Guagenti‐Tax EM, DiLorenzo TA, Tenteromano L, LaRocca NG, Smith CR. Impact of a comprehensive long‐term care program on caregivers and persons with multiple sclerosis. International Journal of Multiple Sclerosis Care 2000;2(1):5‐18. [Google Scholar]
Khan 2008 {published data only}
- Khan F, Pallant JF, Brand C, Kilpatrick T. Effectiveness of rehabilitation intervention in person with multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry 2008;79:1230‐5. [DOI] [PubMed] [Google Scholar]
Khan 2010 {published data only}
- Khan F, Pallant JF, Pallant JI, Brand C, Kilpatrick TJ. A randomised controlled trial: outcomes of bladder rehabilitation in persons with multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry 2010;81(9):1033‐8. [DOI] [PubMed] [Google Scholar]
Patti 2002 {published data only}
- Patti F, Ciancio MR, Reggio E, Lopes R, Palermo F, Cacopardo M, Reggio A. The impact of outpatient rehabilitation on quality of life in multiple sclerosis. Journal of Neurology 2002;249(8):1027‐33. [DOI] [PubMed] [Google Scholar]
Patti 2003 {published data only}
- Patti F, Ciancio MR, Cacopardo M, Reggio E, Fiorilla T, Palermo F, et al. Effects of a short outpatient rehabilitation treatment on disability of multiple sclerosis patients: a randomised controlled trial. Journal of Neurology 2003;250(7):861‐6. [DOI] [PubMed] [Google Scholar]
Pozzilli 2002 {published data only}
- Pozzilli C, Brunetti M, Amicosante AMW, Gasperini C, Ristori G, Palmisano L, et al. Home based management in multiple sclerosis: results of a randomised controlled trial. Journal of Neurology, Neurosurgery and Psychiatry 2002;73:250‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Storr 2006 {published data only}
- Storr LK, Sorensen PS, Ravnborg M. The efficacy of multidisciplinary rehabilitation in stable multiple sclerosis patients. Multiple sclerosis 2006;12:235‐42. [DOI] [PubMed] [Google Scholar]
Stuifbergen 2003 {published data only}
- Stuifbergen AK, Becker H, Blozis S, Timmerman G, Kulberg V. A randomized clinical trial of a wellness intervention for women with multiple sclerosis. Archives of Physical Medicine and Rehabilitation 2003;84(4):467‐76. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aisen 1996 {published data only}
- Aisen ML, Sevilla D, Fox N. Inpatient rehabilitation for multiple sclerosis. Journal of Neurological Rehabilitation 1996;10(1):43‐6. [Google Scholar]
Barrett 2009 {published data only}
- Barrett C, Mann G, Taylor P, Strike P. A randomized trial to investigate the effects of functional electrical stimulation and therapeutic exercise on walking performance for people with multiple sclerosis. Multiple sclerosis 2009;15:493‐504. [DOI] [PubMed] [Google Scholar]
Bethoux 2003 {published data only}
- Bethoux FA, Miller DM, Stough D, Kinkel P. Trials of Rehabilitation after exacerbation of multiple sclerosis: recovery prior to randomisation. The Mellen Centre for Multiple Sclerosis Treatment and Research, Cleveland Clinic CMSC. 2003.
Bethoux 2005 {published data only}
- Bethoux FA, Miller DM, Stough D. Efficacy of outpatient rehabilitation after exacerbation of multiple sclerosis. Archives of Physical Medicine and Rehabilitation 2005;86:e27. [Google Scholar]
Bethoux 2005 A {published data only}
- Bethoux FA, Stough G, Sutliff M. Treatment of severe spasticity with intrathecal baclofen therapy in ambulatory multiple sclerosis patients: six month follow up. ACRM/ASNR Annual Meeting Abstracts. 2005.
Bourdette 1991 {published data only}
- Bourdette D, Prochazka AV, Mitchell W, Burks J, Licari P. Evidence that multidisciplinary team care clinic is more effective than standard medical care in treating veterans with multiple sclerosis. Neurology 1991;41:253. [Google Scholar]
Brissart 2010 {published data only}
- Brissart H, Leroy M, Debouverie M. Cognitive rehabilitation in Multiple Sclerosis: preliminary results and presentation of a new program, PROCOG‐SEP. Revue Neurologique 2010;166:406‐11. [DOI] [PubMed] [Google Scholar]
Carey 1988 {published data only}
- Carey RG, Seibert J H, Posavac EJ. Who makes the most progress in inpatient rehabilitation? An analysis of functional gain. Archives of Physical Medicine and Rehabilitation 1988;69(5):337‐43. [PubMed] [Google Scholar]
Carton 2000 {published data only}
- Carton H, Loos R, Pacolet J, Versieck K, Vlietinck R. A quantitative study of unpaid care giving in multiple sclerosis. Multiple Sclerosis 2000;6:274‐79. [DOI] [PubMed] [Google Scholar]
Cendrowski 1999a {published data only}
- Cendrowski WS, Kwolek A, Wielczko E. Quality of life stabilised by a 3 year rehabilitation in secondary progressive multiple sclerosis. Multiple Sclerosis ECTRIMS/ACTRIMS. 1999.
Cendrowski 1999b {published data only}
- Cendrowski WS, Kwolek A, Wieliczko E. Relationship between disability and quality of life: rehabilitation outcomes in multiple sclerosis. Multiple Sclerosis ECTRIMS/ACTRIMS. 1999.
Coote 2009 {published data only}
- Coote S, Garrett M, Hogan N, Larkin A, Saunders J. Getting the balance right: a randomised controlled trial of physiotherapy and exercise interventions for ambulatory people with multiple sclerosis. BMC Neurology 2009;9:34‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2002 {published data only}
- Edwards SG, Playford ED, Hobart JC, Thompson AJ. Comparison of physician outcome measures and patients perception of benefits of inpatient neurorehabilitation. British Medical Journal 2002;324:1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feigenson 1981 {published data only}
- Feigenson JS, Scheinberg L, Catalano M, Polkow L, Mantegazza PM, Feigenson WD, et al. The cost effectiveness of multiple sclerosis rehabilitation: a model. Neurology 1981;31(10):1316‐22. [DOI] [PubMed] [Google Scholar]
Fink 2010 {published data only}
- Fink F, Rischkau E, Butt M, Klein J, Eling P, Hildebrandt H. Efficacy of an executive function intervention program in MS: a placebo‐controlled and pseudo‐randomised trial. Multiple Sclerosis 2010;16:1148‐51. [DOI] [PubMed] [Google Scholar]
Flavia 2010 {published data only}
- Flavia M, Stampatori C, Zanotti D, Parrinello G, Capra R. Efficacy and specificity of intensive cognitive rehabilitation of attention and executive functions in Multiple Sclerosis. Journal of Neurology and Science 2010;288:101‐5. [DOI] [PubMed] [Google Scholar]
Freeman 1996a {published data only}
- Freeman JA, Langdon DW, Hobart JC, Thompson AJ. The impact of rehab on disability and handicap in progressive MS: a randomised controlled trial. Neurology 1996;43(2):517. [Google Scholar]
Freeman 1996b {published data only}
- Freeman Ja, Langdon DW, Hobart JC, Thompson AJ. Health related quality of life in people with multiple sclerosis undergoing inpatient rehab. Neurology 1996;10:185‐94. [Google Scholar]
Freeman 1999 {published data only}
- Freeman JA, Langdon DW, Hobart JC, Thompson AJ. Inpatient rehab in multiple sclerosis : do the benefits carry over into the community?. Neurology 1999;52(1):50‐6. [DOI] [PubMed] [Google Scholar]
Freeman 2005 {published data only}
- Freeman JA, Hobart JC, Playford ED, Undy B, Thompson AJ. Evaluating neurorehabilitation : lessons from routine data collection. Neurology, Neurosurgery Psychiatry 2005;76:723‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grasso 2005 {published data only}
- Grasso MG, Troisi E, Rizzi F, Morelli D, Paolucci S. Prognostic factors in multidisciplinary rehab treatment in multiple sclerosis : an outcome study. MS 2005;11:719‐24. [DOI] [PubMed] [Google Scholar]
Greenspun 1987 {published data only}
- Greenspun B, Stineman M, Agri R. Multiple sclerosis and rehab outcome. Archives of Physical Medicine & Rehab 1987;68(7):434‐7. [PubMed] [Google Scholar]
Grossman 2010 {published data only}
- Grossman P, Kappos L, Gensicke H, D'Souza M, Mohr DC, Penner IK, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology 2010;75:1141‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hebert 2009 {published data only}
- Hebert JR. Effects of vestibular rehabilitation on MS‐related fatigue: randomized control trial. PhD defence and award, Clinical Sciences Program. Denver: Anschutz Medical Campus, University of Colorado, 2009. [Google Scholar]
Hinrichs 2003 {published data only}
- Hinrichs J, Archibald C, Pain K, Metz L. A pilot study measuring the utility of the MSQLI within a short term rehab program. CMSC Abstracts‐Posters. 2003.
Jones 1996 {published data only}
- Jones L, Lewis Y, Harrison J, Wiles CM. The effectiveness of occupational therapy and physiotherapy in MS patients with ataxia of the upper limb and trunk. Clinical Rehababilitation 1996;10(4):277‐82. [Google Scholar]
Jonsson 1996 {published data only}
- Jonsson A, Dock J, Ravnborg MH. Quality of life as a measure of rehab outcome in patients with multiple sclerosis. Acta Neurologica Scandinavica 1996;93(4):229‐235. [DOI] [PubMed] [Google Scholar]
Jorger 2001 {published data only}
- Jorger M, Beer S, Kesselring J. Impact of Neurorehab on disability in patients with acutely and chronically disabling diseases of the nervous system measured by the extended Barthel Index. Neurorehab and Neural Repair 2001;15:15‐22. [DOI] [PubMed] [Google Scholar]
Kalina 2009 {published data only}
- Kalina JT. The Role of occupational therapy in a Multiple Sclerosis center: a multidisciplinary team, including occupational therapy practitioners, ensures an individualized, holistic approach to intervention. Occupational Therapy Practice 2009;14:9‐13. [Google Scholar]
Khan 2010b {published data only}
- Khan F, Pallant JF, Zhang N, Turner‐Stokes L. Clinical practice improvement approach in multiple sclerosis rehabilitation: a pilot study . International Journal of Rehabilitation Research 2010;33:238‐47. [DOI] [PubMed] [Google Scholar]
Kidd 1995 {published data only}
- Kidd D, Howard RS, Losseff NA, Thompson AJ. The benefits of inpatient neurorehabilitation in multiple sclerosis. Clinical Rehab 1995;9(3):198‐203. [Google Scholar]
Kidd 1997 {published data only}
- Kidd D, Thompson AJ. Prospective study of neurorehabilitation in multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry 1997;62(4):423‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2003 {published data only}
- Liu C, Playford ED, Thompson AJ. Does neurorehabilitation have a role in relapsing‐remitting multiple sclerosis?. Neurology 2003;250(10):1214‐8. [DOI] [PubMed] [Google Scholar]
Mostert 2005 {published data only}
- Mostert S, Kesselring J. Effect of pulsed magnetic field therapy on the level of fatigue in patients with Multiple Sclerosis ‐ a randomised controlled trial. Multiple Sclrerosis 2005;11:302‐5. [DOI] [PubMed] [Google Scholar]
Palmisano 1999 {published data only}
- Palmisano L, Pozzilli C, Battaglia MA, Fleschi C. A randomised trial on home care in patients with Multiple Sclerosis. Conference proceeding. 1999.
Plow 2009 {published data only}
- Plow MA, Mathiowetz V, Lowe DA. Comparing individualized rehabilitation to a group wellness intervention for persons with Multiple Sclerosis. American Journal of Health Promotion 2009;24:23‐6. [DOI] [PubMed] [Google Scholar]
Portillo 2009 {published data only}
- Portillo MC, Corchn S, Lopez‐Dicastillo O, Cowley S. Evaluation of a nurse‐led social rehabilitation programme for neurological patients and carers: an action research study. International Journal of Nursing Studies 2009;46:204‐19. [DOI] [PubMed] [Google Scholar]
Pozzilli 2004 {published data only}
- Pozzilli C, Palmisano L, Mainero C, Tomassini V, Marinelli F, Ristory G, et al. Multiple sclerosis. Multiple Sclerosis 2004;10:442‐226. [DOI] [PubMed] [Google Scholar]
Reding 1987 {published data only}
- Reding MJ, Larocca NG, Madonna M. Acute hospital care versus rehab hospitalisation for management of non‐emergent complications in multiple sclerosis. Neurorehabilitation 1987;1(1):13‐7. [Google Scholar]
Riazi 2004 {published data only}
- Riazi A, Thompson AJ, Hobart JC. Self efficacy predicts self reported health status in multiple sclerosis. Multiple Sclerosis 2004;10:61‐6. [DOI] [PubMed] [Google Scholar]
Roush 1995 {published data only}
- Roush SE. The satisfaction of patients with multiple sclerosis regarding services received from physical and occupational therapists. International Journal of Rehabilitation and Health 1995;1(3):155‐66. [Google Scholar]
Sitzia 1998 {published data only}
- Sitzia J, Haddrell V, Rice‐Oxley M. Evaluation of a nurse led multidisciplinary neurological rehab programme using the Nottingham Health Profile. Clinical Rehabilitation 1998;12:389‐94. [DOI] [PubMed] [Google Scholar]
Slade 2002 {published data only}
- Slade A, Tennant A, Chamberlain MA. A randomised controlled trial to determine the effect of intensity of therapy upon length of stay in a neurological rehabilitation setting. Journal of Rehabilitation Medicine 2002;34(6):260‐6. [DOI] [PubMed] [Google Scholar]
Storr 1999 {published data only}
Van der Putten 1999 {published data only}
- Putten JJM, Hobart JC, Freeman JA, Thompson AJ. Measuring change in disability after inpatient rehabilitation : comparison of the responsiveness of the Barthel Index and the Functional INdependence Measure. Journal of Neurology, Neurosurgery and Psychiatry 1999;66(4):480‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaney 1996 {published data only}
- Vaney C, Blavroch H, Gattlen B, Melsela C. Assessing mobility in multiple sclerosis using Rivermead Mobility Index and gait speed. Clinical Rehabilitation 1996;10:216‐26. [Google Scholar]
Wahls 2010 {published data only}
- Wahls TL, Reese D, Kaplan D, Darling WG. Rehabilitation with neuromuscular electrical stimulation leads to functional gains in ambulation in patients with secondary progressive and primary progressive multiple sclerosis: a case series report. Journal of Alternative and Complementary Medicine 2010;16:1343‐9. [DOI] [PubMed] [Google Scholar]
Ward 2004 {published data only}
- Ward CD, Turpin G, Dewey ME, Fleming S, Fleming S, Hurwitz B, et al. Education for people with progressive neurological conditions can have negative effects: evidence from a randomised controlled trial. Clinical Rehabilitation 2004;18(7):717‐25. [DOI] [PubMed] [Google Scholar]
Additional references
Battaglia 2000
- Battaglia MA, Zagami, Uccelli MM. A cost evaluation of multiple sclerosis. Journal of Neurovirology 2000;6(Suppl 2):S191‐93. [PubMed] [Google Scholar]
Brown 2004
- Brown M, Gordon WA. Empowerment in measurement: ’muscle’,’voice’ and subjective quality of life as a gold standard. Archives of Physical Medicine and Rehabilitation 2004;85(suppl 4):S13‐20. [DOI] [PubMed] [Google Scholar]
Campbell 1999
- Campbell ML, Sheets D, Strong PS. Secondary health conditions among middle‐aged individuals with chronic physical disabilities:implications for unmet needs for services. Assistive Technology 1999;11(2):105‐22. [DOI] [PubMed] [Google Scholar]
Cieza 2009
- Cieza A, Hilfiker R, Boonen A, Chatterji S, Kostanjsek N, Ustün BT, et al. Items from patient‐oriented instruments can be integrated into interval scales to operationalize categories of the International Classification of Functioning, Disability and Health. Journal ofClinical Epidemiology 2009;62(9):912‐21. [DOI] [PubMed] [Google Scholar]
Compston 1998
- Compston D, Ebers G, Lassman H, McDonald W, Matthews W, Wekerie H. The genetic epidemiology of multiple sclerosis. McAlpine's Multiple Sclerosis. W B Saunders London, 1998:45‐142. [Google Scholar]
Detels 1978
- Detels R, Visscher BR, Haile RW, Malmgren RM, Dudley JP, Coulson AH. Multiple sclerosis and age at migration. American Journal of Epidemiology 1978;108(5):386‐93. [DOI] [PubMed] [Google Scholar]
Dombovy 1998
- Dombovy ML. Multiple sclerosis and Parkinson's disease rehabilitation. In: Lazar R editor(s). Principles of Neurological Rehabiliation. McGraw Hill New York, 1998:173‐97. [Google Scholar]
Egger 1998
- Egger M, Smith GD. Bias in location and selection of studies. British Medical Journal 1998;316(7124):61‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elstein 2002
- Elstein AS, Schwarz A. Clinical problem solving and diagnostic decision making: selective review of the cognitive literature. BMJ 2002;324(7339):729‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frankel 2001
- Frankel D. MS ‐ Neurological Rehabilitation. In: Umphred DA editor(s). Neurological Rehabilitation. 4th Edition. St Louis Mosby, 2001. [Google Scholar]
Fuhrer 2005
- Fuhrer M J. Conducting multiple site clinical trials in medical rehabilitation research. American Journal of Physical Medical Rehabilitation 2005;84:823‐31. [DOI] [PubMed] [Google Scholar]
Gassaway 2005
- Gassaway J, Horn SD, DeJong G, Smout RJ, Clark C, James R. Applying the clinical practice improvement approach to stroke rehabilitation: methods used and baseline results. Archives of Physical Medicine and Rehabilitation 2005;86(12 Suppl 2):S16‐33. [DOI] [PubMed] [Google Scholar]
Goetzsche 1987
- Goetzsche PC. Reference bias in reports of drug trials. British Medical Journal 1987;295:654‐6. [PMC free article] [PubMed] [Google Scholar]
Granger 1990
- Granger CV, Cotter AC, Hamilton BB, Fiedler RC, Hens MM. Functional assessment scales: a study of persons with multiple sclerosis. Archives of Physical Medicine and Rehabilitation 1990;71(11):870‐5. [PubMed] [Google Scholar]
Greener 2002
- Greener J, Langhorne P. Systematic reviews in rehabilitation for stroke: issues and approaches to addressing them. Clinical Rehababilitation 2002;16(1):69‐74. [DOI] [PubMed] [Google Scholar]
Grill 2009
- Grill E, Stucki G. Scales should be developed based on simple clinical ratings of International Classification of Functioning,Disability and Health Core Set categories. Journal of Clinical Epidemiology 2009;62(9):891‐98. [DOI] [PubMed] [Google Scholar]
Hammond 1988
- Hammond SR, McLeod JG, Millingens KS, Stewart‐Wynne EG, English D, Holland JT, et al. The epidemiology of multiple sclerosis in 3 Australian cities: Perth, Newcastle & Hobart. Brain 1988;111(Pt 1):1‐25. [DOI] [PubMed] [Google Scholar]
Hobart 2001
- Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The multiple sclerosis impact scale: a new patient based outcome measure. Brain 2001;124:962‐73. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad A R, Moore R A, Carroll D, Jenkinson C, Reynolds D J, Gavagahn D J. Assessing the Quality of Reports of Randomised Clinical Trials: Is Blinding Necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Kemp 2005
- Kemp BG. What the rehabilitation professional and the consumer need to know. Physical Medicine Rehabilitation Clinics of NorthAmerica 2005;16:1‐18 vii. [DOI] [PubMed] [Google Scholar]
Khan 2007
- Khan F, Turner‐Stokes L, Ng L, Kilpatrick T. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006036.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2007a
- Khan F, Pallant JF. Use of International Classification of Functioning, Disability and Health (ICF) to describe patient‐reported disability in multiple sclerosis and identification of relevant environmental factors. Journal of Rehabilitation Medicine 2007;39(1):63‐70. [DOI] [PubMed] [Google Scholar]
Khan 2007b
- Khan F, Pallant JF. Use of the International Classification of Functioning, Disability and Health (ICF) to identify preliminary comprehensive and brief core sets for multiple sclerosis. Disability and Rehabilitation 2007;29(3):205‐13. [DOI] [PubMed] [Google Scholar]
Khan 2008a
- Khan F, Pallant JF, Turner‐Stokes L. Use of Goal attainment Scaling in inpatient rehabilitation for persons With multiple sclerosis. Archives Of Physical Medicine And Rehabilitation 2008;89(4):652‐9. [DOI] [PubMed] [Google Scholar]
Khan 2009
- Khan F, Turner Stokes L, Stevermuer T, Simmonds F. Multiple Sclerosis rehabilitation outcomes: analysis of a National casemix dataset from Australia. Multiple Sclerosis 2009;15:869‐75. [DOI] [PubMed] [Google Scholar]
Khan 2010a
- Khan F, Pallant JF, Zhang N, Turner‐Stokes L. Clinical practice improvement approach in multiple sclerosis rehabilitation: a pilot study. International Journal Of Rehabilitation Research 2010;33(3):238‐47. [DOI] [PubMed] [Google Scholar]
Khan 2010c
- Khan F, Ng L, Amatya B, Brand C, Turner‐Stokes L. Multidisciplinary care for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008505.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiresuk 1968
- Kiresuk TJ, Sherman RE. Goal attainment scaling: a general method of evaluating comprehensive mental health programmes. Community Mental Health Journal 1968;4(6):443‐53. [DOI] [PubMed] [Google Scholar]
Kraft 2005
- Kraft G H, Cui J Y. Multiple Sclerosis. In: DeLisa J editor(s). Physical Medicine and Rehabilitation: Principles and Practice. 4th Edition. Lippincott Williams & Wilkins, 2005:1753‐69. [Google Scholar]
Kurtzke 1983
- Jurtzke J F, Gundmunsson K R, Bergmann S. Mulptiple sclerosis in Iceland: evidence of post war epidemic. Neurology 1982;32(2):143‐50. [DOI] [PubMed] [Google Scholar]
Langdon 1999
- Langdon D W, Thompson A J. MS: A preliminary study of selected variables affecting rehabilitation outcome. MS 1999;5:94‐100. [DOI] [PubMed] [Google Scholar]
Langhorne 1995
- Langhorne P. Developing new stroke services: an evidence based approach. Post Graduate Medical Journal 1995;71:733‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langhorne 2002
- Langhorne P, Pollock A, in conjunction with the Stroke Unit Trialists Collaboration. What are the components of stroke unit care?. Age and Ageing 2002;31:365‐71. [DOI] [PubMed] [Google Scholar]
McDonald 2001
- McDonald WI, Compston A, Edan G, Goodkin D, Goodkin D, Hartung HP, et al. Recommended diagnostic criteria for MS: guidelines from the international panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. [DOI] [PubMed] [Google Scholar]
MS Society 2011
- MS Society Australia. Multiple Sclerosis in Australia. Available: www.msaustralia.org.au [Accessed March 2011].
Ng 2009
- Ng L, Khan F, Mathers S. Multidisciplinary care for adults with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007425.pub2] [DOI] [PubMed] [Google Scholar]
NICE 2003
- National Institute for Clinical Excellence. Management of multiple sclerosis in primary and secondary care. Clinical guidelines B 2003 November.
Patwardhan 2005
- Patwardhan MB, Matacher DB, Samsa GP, McRory DC, Williams R G, Li TT. Cost of MS by level of disability : a review of literature. Multiple Sclerosis 2005;11:232‐39. [DOI] [PubMed] [Google Scholar]
Poser 1983
- Poser CM, Paty DW, SCheiberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;3:227‐31. [DOI] [PubMed] [Google Scholar]
Ramp 2009
- Ramp M, Khan F, Misajon RA, Pallant JF. Rasch analysis of the Multiple Sclerosis Impact Scale MSIS‐29. Health and Quality of Life Outcomes 2009;7:58‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitberg 2004
- Reitberg MB, Brooks D, Utidehaag BMJ, Kwakkel G. Exercise therapy for MS. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003980.pub2] [DOI] [Google Scholar]
Schumaker 1965
- Schumaker GA, Beebe GW, Kibler RF, Kurland LT, Kurtzke JF, McDowell F, et al. Problems of experimental trials of therapy in multiple sclerosis. Annals of New York Academy of Sciences 1965;122:552‐68. [DOI] [PubMed] [Google Scholar]
Society of Neuroscience 2011
- Society of Neuroscience. Multiple sclerosis: making a difference tomorrow. Brain Research Success Stories 2011; Vol. from: http://www.sfn.org/skins/main/pdf/brss/BRSS_Multiple_Sclerosis.pdf [Accessed May 2011].
Steultjens 2003
- Steultjens EMJ, Dekker J, Bouter LM, Cardol M, Nes JCM, Ende CHM. Occupational therapy for multiple sclerosis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003608.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2007
- Taylor B, McDonald E, Fantino B, Sedal L, MacDonell R, Pittas F, et al. The cost of multiple sclerosis in Australia. Journal of Clinical Neuroscience 2007;14(6):532‐39. [DOI] [PubMed] [Google Scholar]
Tennant 2002
- Tennant A. The Rasch model and standardisation outcomes in rehabilitation: the PRO‐ESOR project. European Congress in Physical and Rehabilitation Medicine ‐ Brighton. 2002.
Thompson 2000
- Thompson AJ. The effectiveness of neurological rehab in MS. Journal of Rehabilitation Research Development 2000;37(4):455‐61. [PubMed] [Google Scholar]
Turk 2001
- Turk MA, Scandale J, Rosenbaum PF, Weber RJ. The health of women with cerebral palsy. Physical Medicine and RehabilitationClinics of North America 2001;12(1):153‐68. [PubMed] [Google Scholar]
Turner‐Stokes 2005
- Turner‐Stokes L, Disler P, Nair A, Wade D T. Mutlidisciplinary rehabilitation for acquired brain injury in adults of working age. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004170.pub2] [DOI] [PubMed] [Google Scholar]
van Tulder 1997
- Tulder M W, Assendelft W J, Koes B W, Bouter L M. Method guidelines for systematic reviews in the Cochrane Collaborations Back Review Group for Spinal Disorders. Spine 1997;22:2323‐30. [DOI] [PubMed] [Google Scholar]
van Tulder 2003
- Tulder MW, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collabvoration back review group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Wade 1992
- Wade DT. Measurement in neurological rehabilitation. Oxford University Press, 1992. [PubMed] [Google Scholar]
Weinshenker 1989
- Weinshenker BG, Bass B, RIce GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. Predictive value of early clinical course. Brain 1989;112:1419‐28. [DOI] [PubMed] [Google Scholar]
Whetten‐Gol 1998
- Whetten‐Goldstein K, Sloan F A, Goldstein L B, Kulas E D. A comprehensive assessment of the cost of multiple sclerosis in the USA. Multiple Sclerosis 1998;4:419‐25. [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organisation (WHO). International Classification of Functioning, Disability and Health (ICF). WHO Geneva 2001.
Whyte 2002
- Whyte J. Traumatic brain injury rehab: are there alternatives to randomised clinical trials. Archves of Physical medicine and Rehabilitation 2002;83(9):1320‐2. [DOI] [PubMed] [Google Scholar]
Yorkston 2002
- Yorkston K, Johnson K, Juehn C, Klasner E, Kraft G. Age and gender issues related to multiple sclerosis: a survey study. International Journal of Multiple Sclerosis Care 2002;4(2):94. [Google Scholar]


