Abstract
Background
Pericarditis, along with myocarditis, is being increasingly reported after the coronavirus disease 2019 (COVID-19) vaccine, but the best treatment strategy in this specific setting is still unclear.
Case summary
We report a case of acute pericarditis after the second dose of mRNA COVID-19 vaccine with recurrence of large pericardial effusion after a previous pericardiocentesis and anti-inflammatory drugs tapering. The patient was successfully treated with the recombinant interleukin-1 receptor antagonist anakinra, with full reabsorption of the pericardial effusion and an abrupt drop of the inflammatory markers within 72 h. The patient was discharged a few days later, with a further decrease of the inflammatory markers and no residual symptoms.
Discussion
Anakinra is being increasingly used in the treatment of recurrent pericarditis due to its capability to interrupt the autoinflammatory response leading to deleterious cytokine storms. On account of its high efficacy and rapid onset, it has been reported to rapidly reverse large inflammatory pericardial effusions. Pericarditis and myocarditis have been reported after the COVID-19 vaccine, but this is the first case of COVID-19 vaccine-related pericarditis and pericardial effusion successfully treated with anakinra, avoiding a second pericardiocentesis.
Keywords: Pericarditis, Anakinra, COVID-19 vaccine, Pericardial effusion, Pericardiocentesis, Case report
Learning points.
Anakinra, an interleukin-1 receptor inhibitor that can rapidly interrupt the autoinflammatory process leading to cytokine storm, is increasingly used in recurrent pericarditis.
Treatment with anakinra may be considered in pericarditis with large pericardial effusions, even in the setting of coronavirus disease 2019 vaccine-related pericarditis, due to its rapid onset and high efficacy.
Primary specialities involved other than cardiology
Rheumatology, internal Medicine.
Introduction
The management of recurrent pericarditis can be challenging because of frequent recurrences with severely impaired quality of life and requires close co-operation between cardiologists, rheumatologists, and internists.1,2 Acute pericarditis has been described as a rare complication of the coronavirus disease 2019 (COVID-19) vaccine, showing an overall incidence of 1.8/million vaccine doses in the European Economic Area and the USA, with a slightly higher incidence for Adenovirus-vectored vaccines as compared with mRNA vaccines.3 The incidence of recurrent pericarditis after the COVID-19 vaccine is currently unknown. Treatment options for COVID-19 vaccine-related pericarditis have not been thoroughly investigated.3 We report a case of COVID-19 vaccine-related recurrent pericarditis with pericardial effusion successfully treated with anakinra, avoiding a second pericardiocentesis.
Timeline
| Day 1 | Second dose of mRNA SARS-CoV-2 vaccine. |
| Day 2 | Fever; pleuritic chest pain, which decreases in intensity in the next 4 days but never ceases. |
| Day 30 | Hospital admission because of worsening of chest pain, diagnosis of acute pericarditis with cardiac tamponade; pericardiocentesis is performed. Non-steroid anti-inflammatory drugs and colchicine are started |
| Day 48 | Access to the Emergency Room because of relapse of pleuritic chest pain; in the ER, elevated inflammatory markers and moderate pericardial effusion are detected. Admitted to our Cardiology ward. |
| Day 50 | Admitted to cardiac intensive care unit because of worsening pericardial effusion with mild signs of impaired ventricular filling. C-reactive protein (CRP) 306 mg/L. Anakinra is started 100 mg twice daily on the first day, then 100 mg daily. |
| Day 51 | No chest pain; CRP 287 mg/L |
| Day 52 | No chest pain; CRP 179 mg/L |
| Day 53 | Complete reabsorption of pericardial effusion; CRP 104 mg/L |
| Day 55 | CRP 34 mg/L |
| Day 56 | Discharge. The patient is asymptomatic and in good clinical conditions. |
Case presentation
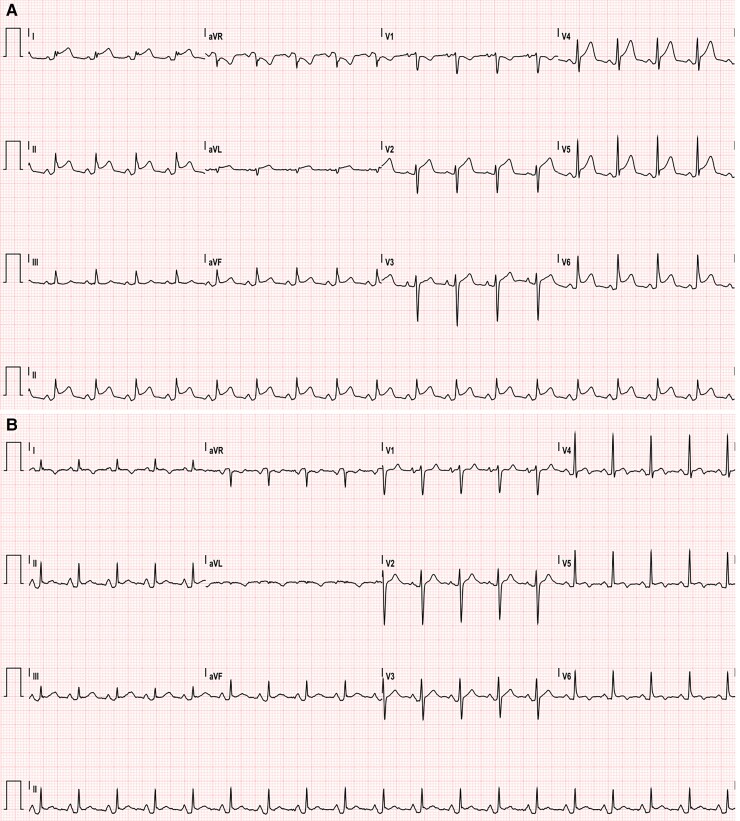
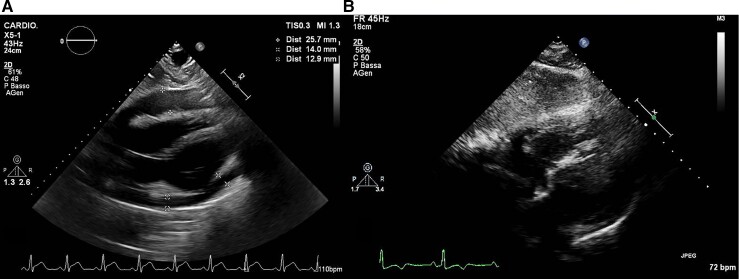
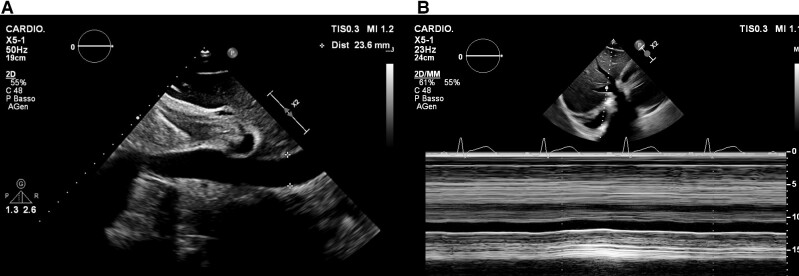
A 30-year-old man with no past medical history (in particular, he had no prior evidence of pericarditis or any inflammatory/autoimmune disease) was admitted to our cardiac intensive care unit (CICU) because of cardiac tamponade with large circumferential (mainly anterior) pericardial effusion, in the context of acute pericarditis, with diffuse ST elevation and PR depression on the electrocardiogram (ECG) (Figure 1A). The cardiovascular examination showed increased heart rate (100 b.p.m.), muffled heart sounds, jugular vein distension, low blood pressure (90/55 mmHg). He had received his second dose of mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech) 40 days before and had complained of fever and typical pleuritic chest pain for the next 5 days, with subsequent reduction of the symptoms (which had never completely disappeared though), followed by presyncope about 30 days later. The patient’s anti-spike SARS-CoV-2 IgGs and IgMs were found positive. Laboratory tests for Epstein–Barr virus (serology and genoma), Echovirus, Coxsackevirus, Adenovirus, Cytomegalovirus (serology), as well as Quantiferon and autoimmunity tests (ANA, ENA SSB/La, anti-dsDNA antibodies, pANCA-MPO, cANCA-PR3) were negative. High-sensitivity Troponin I was also negative in three samples over 4 days. The emergency echocardiogram showed normal biventricular global and regional function, right ventricular diastolic collapse, transmitral E wave peak velocity respiratory variation by 30%, distended inferior vena cava without respiratory variation. He underwent emergency pericardiocentesis, and ibuprofen 600 mg t.i.d. and colchicine 0.5 mg b.i.d. were started, with symptom remission and a downward trend of inflammatory markers at discharge. Pericardial fluid analysis was negative for bacteria, mycobacteria, fungi, yeasts, moulds, and Enterovirus genome. After 1 week, ibuprofen tapering was started, and the patient had a disease flare with fever, pleuritic chest pain, increased inflammatory markers [C-reactive protein (CRP): 306 mg/L; cut-off <0.5 mg/L], neutrophil leucocytosis, ECG signs of acute pericarditis consisting again in PR depression and diffuse ST elevation with inverted T waves (Figure 1B), sinus tachycardia. The echocardiogram showed large pericardial effusion, up to 25 mm anteriorly (Figure 2A), with no clear signs of tamponade but incipient 2D signs of impaired ventricular filling (Figure 3). He was readmitted to the CICU for pericardiocentesis and strict multiparameter monitoring. However, we decided to defer the procedure and to start the interleukin-1 (IL-1) receptor inhibitor anakinra subcutaneously at a dose of 100 mg b.i.d. on the first day, thereafter 100 mg/day. Few hours after the first administration, the patient reported complete resolution of his symptoms and remained afebrile and asymptomatic throughout his hospital stay. After 24 h, his heart rate got back to normal. Within 72 h, complete reabsorption of the pericardial effusion was observed (Figure 2B), and the inflammatory markers showed an abrupt reduction in the next 6 days (CRP from 306 to 34 mg/L). After reduction of the CRP, the patient was discharged uneventfully on anakinra 100 mg/day for 6 months (then it will be gradually tapered), ibuprofen 600 mg t.i.d. (tapering as per current guidelines), colchicine 0.5 mg b.i.d. for 6 months, and a proton pump inhibitor. At 1-month follow-up visit (28 September) and on a further telephonic contact (1 December), he was still asymptomatic.
Figure 1.
(A) Electrocardiogram on first admission showing sinus tachycardia, typical ST segment elevation, and PR depression, consistent with acute pericarditis. (B) Electrocardiogram on second admission, showing sinus tachycardia, PR depression, and ST elevation; the latter is less pronounced as compared with the first admission. Inverted T waves are also present, as an evolution of the first pericarditis episode.
Figure 2.
Echocardiographic still images of sub-xiphoidal view showing large pericardial effusion at the second intensive care admission (A) and complete reabsorption of the pericardial fluid 72 h after starting treatment with anakinra (B).
Figure 3.
Echocardiographic still images of sub-xiphoidal view showing dilated inferior vena cava (A) with impaired inspiratory collapse (B).
Discussion
We report a case of acute pericarditis occurring after the COVID-19 vaccine, presenting with recurrent large pericardial effusion and cardiac tamponade after a first pericardiocentesis, despite ongoing non-steroid anti-inflammatory drugs (NSAIDs) and colchicine therapy. To avoid repeating the pericardiocentesis, we started the recombinant IL-1 receptor antagonist anakinra achieving the resolution of the pericardial effusion and clinical improvement.
In Western countries, most cases of recurrent pericarditis are considered idiopathic, although they may follow a previous viral infection. The pathogenic mechanism is considered auto-inflammatory starting from an acute injury to the pericardial mesothelial cells, which activates the Nod-like receptor 3 inflammasome, with a subsequent release of IL-1β and amplification of the inflammatory response.4 The first-line treatment is based on NSAIDs and colchicine. However, relapses are quite common during tapering or after cessation of treatment.
Anakinra is a recombinant IL-1 receptor antagonist with good efficacy and safety profile that has been studied extensively in cryopyrin-associated periodic syndromes, rheumatoid arthritis, and sepsis.4–6 It has been recently introduced in the treatment of inflammatory cardiac diseases, particularly pericarditis.7 According to the current evidence and guidelines,1,2 Anakinra is recommended as third-line therapy in patients with recurrent idiopathic pericarditis after NSAIDs, colchicine, and corticosteroids (corticosteroid-dependent and colchicine-resistant recurrent pericarditis).
Owing to its rapid onset, high efficacy in blocking IL-1β and IL-1α, it has been used in some instances to avoid pericardiocentesis in the setting of overwhelming pericarditis, with excellent results. Sicignano et al.8 reported a case of pericardial effusion reabsorption right after starting treatment with anakinra in the setting of Staphylococcus aureus sepsis and acute pericarditis. Another group described the efficacy of anakinra in rapidly reducing pericardial effusion in a patient with Erdheim–Chester disease-related pericarditis.9
Anakinra has been investigated in retrospective studies among patients affected by COVID-19 with cytokine storm, hyperinflammation, and acute respiratory failure.10 The COVID-19-related cytokine storm syndrome is characterized by excessive activation of proinflammatory cytokines from both the IL-1β and tumour necrosis factor (TNF) families, which can eventually cause myocarditis. In the setting of severe COVID-19, myocardial injury has been identified as a predictor of adverse outcomes.11 Proinflammatory cytokines, such as IL-1, IL-6, and TNF-α, lead to activation of T-lymphocytes, which cause myocyte damage, alarmin production, and further cytokine release that sustain the auto-inflammatory response. Immunomodulatory therapy has emerged as the main strategy to break such a vicious circle. Trpkov et al.12 described a case of fulminant myocarditis successfully treated with anakinra and dexamethasone; the patient showed rapid clinical benefit, as well as an improvement in cardiac magnetic resonance imaging parameters (ejection fraction, extracellular volume, tissue oedema) after only 72 h, and she had markedly recovered upon discharge.
Acute pericarditis and myocarditis after vaccination is quite rare but has been reported after some types of vaccines, such as influenza, smallpox, meningococcal, and hepatitis B.13,14
In the past few months, several cases of myocarditis and/or pericarditis have been reported to occur, more often in young males, after receiving the second dose of mRNA (Pfizer-BioNTech BNT162b2/Comirnaty and Moderna mRNA-1273/Spikevax) and viral vector (Astra Zeneca/Vaxzevria) COVID-19 vaccines.15–18 The median onset of symptoms was 3–5 days, but it could be as late as 179 days after the injection. It also occurred after the first dose in a few cases. Our patient was admitted to the Emergency Department 40 days after the completion of his mRNA Comirnaty vaccine schedule; however, he had already complained about the onset of symptoms 1 day after the second dose, with persistence of mild pleuritic chest pain throughout the next month.
The mechanisms underlying the occurrence of myocarditis after the COVID-19 vaccine are still unclear, and a different mechanism causing myocardial injury, other than the traditional lymphocytic or eosinophilic myocarditis or myonecrosis, have been proposed. Molecular mimicry between the spike glycoprotein and self-antigens, triggering of pre-existing dysregulated immune pathways, immunogenicity of mRNA molecules in predisposed individuals, and disproportionate cytokine expression have been postulated.14
Vaccine-related myocarditis or pericarditis is usually rapidly resolvable with careful medical care,19 whereas COVID-19-related myocarditis often has a more severe clinical course with extensive myocardial involvement.12 Despite close vigilance of rare serious adverse events, including myocarditis and pericarditis, is mandatory, it should not discourage the population adherence to the vaccination campaign, since the benefits of COVID-19 vaccination clearly outweigh the risks in all age groups.20
A recently published case series included one patient treated with anakinra for post-COVID-19 vaccine pericarditis.3 To the best of our knowledge, this is the first reported case of large pericardial effusion effectively treated with the IL-1 inhibitor anakinra, avoiding the pericardiocentesis, in the context of acute pericarditis following COVID-19 vaccination. Our case report may add to the current literature to warrant further research about early introduction of anakinra in the treatment of rapidly worsening COVID-19 vaccine-associated pericarditis.
Lead author biography

Dr Francesco Perna is an attending physician working in the Cardiac Arrhythmia Unit at Policlinico Universitario A. Gemelli in Rome, Italy. He graduated from Medical School and Cardiology Fellowship and earned a PhD in Pathophysiology of Heart Failure at Catholic University of the Sacred Heart in Rome. He worked as a Research Fellow at Massachusetts General Hospital, Harvard Medical School, in Boston MA (USA). His clinical work and research interest mainly focuses on cardiac arrhythmias.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: Elena Verrecchia has received speaker honoraria by Sanofi Genzyme, Amicus Therapeutics, Novartis and Chiesi, and invitation to a conference by SOBI. Antonio Brucato has received unrestricted research grants by ACARPIA and SOBI. Raffaele Manna has received speaker honoraria by Novartis and SOBI. The remaining authors have no conflicts of interest to declare.
Funding: None declared.
Contributor Information
Francesco Perna, Cardiac Arrhythmia Unit, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Elena Verecchia, Department of Internal Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Periodic Fevers Research Center, Catholic University of the Sacred Heart, Rome, Italy.
Gaetano Pinnacchio, Cardiac Arrhythmia Unit, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Laura Gerardino, Department of Internal Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Periodic Fevers Research Center, Catholic University of the Sacred Heart, Rome, Italy.
Antonio Brucato, Department of Medicine, Azienda Socio Sanitaria Territoriale (ASST) Fatebenefratelli-Sacco and Department of Biomedical and Clinical Sciences Luigi Sacco, University of Milan, Milan, Italy.
Raffaele Manna, Department of Internal Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Periodic Fevers Research Center, Catholic University of the Sacred Heart, Rome, Italy.
References
- 1. Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W; ESC Scientific Document Group . 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC)endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Imazio M, Andreis A, De Ferrari GM, Cremer PC, Mardigyan V, Maestroni S, Luis SA, Lopalco G, Emmi G, Lotan D, Marcolongo R, Lazaros G, De Biasio M, Cantarini L, Dagna L, Cercek AC, Pivetta E, Varma B, Berkson L, Tombetti E, Iannone F, Prisco D, Caforio ALP, Vassilopoulos D, Tousoulis D, De Luca G, Giustetto C, Rinaldi M, Oh JK, Klein AL, Brucato A, Adler Y. Anakinra for corticosteroid-dependent and colchicine-resistant pericarditis: the IRAP (International Registry of Anakinra for Pericarditis) study. Eur J Prev Cardiol 2020;27:956–964. [DOI] [PubMed] [Google Scholar]
- 3. Lazaros G, Anastassopoulou C, Hatziantoniou S, Kalos T, Soulaidopoulos S, Lazarou E, Vlachopoulos C, Vassilopoulos D, Tsakris A, Tsioufis C. A case series of acute pericarditis following COVID-19 vaccination in the context of recent reports from Europe and the United States. Vaccine 2021;39:6585–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011;117:3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD005121. doi: 10.1002/14651858.CD005121.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Opal SM, Fisher CJ, Dhainaut J-FA, Vincent J-L, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, Pribble JP, LaBrecque JF, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, doubleblind, placebo-controlled, multicenter trial. Crit Care Med 1997;25:1115–1124. [DOI] [PubMed] [Google Scholar]
- 7. Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin-1 in heart disease. Circulation 2013;128:1910–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sicignano LL, Massaro MG, Savino M, Rigante D, Gerardino L, Manna R. Early introduction of anakinra improves acute pericarditis and prevents tamponade in Staphylococcal sepsis. Intern Emerg Med 2021;16:1391–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tomelleri A, Cavalli G, De Luca G, Campochiaro C, D’Aliberti T, Tresoldi M, Dagna L. Treating heart inflammation with Interleukin-1 blockade in a case of Erdheim–Chester disease. Front Immunol 2018;9:1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Tassan Din C, Boffini N, Tomelleri A, Farina N, Ruggeri A, Rovere-Querini P, Di Lucca G, Martinenghi S, Scotti R, Tresoldi M, Ciceri F, Landoni G, Zangrillo A, Scarpellini P, Dagna L. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020;2:e325–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trpkov C, MacMullan P, Feuchter P, Kachra R, Heydari B, Merchant N, Bristow MS, White JA. Rapid response to cytokine storm inhibition using anakinra in a patient with COVID-19 myocarditis. CJC Open 2021;3:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hajjo R, Sabbah DA, Bardaweel SK, Tropsha A. Shedding the light on post-vaccine myocarditis and pericarditis in COVID-19 and non-COVID-19 vaccine recipients. Vaccines (Basel) 2021;9:1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Government of Israel, Ministry of Health . June 02, 2021 Surveillance of Myocarditis (Inflammation of the Heart Muscle) Cases Between December 2020 and May 2021 (Including)
- 16. Medicines and. Healthcare Products Regulatory Agency (MHRA) . Coronavirus vaccine-weekly summary of Yellow Card reporting [Internet]. London (United Kingdom): Department of Health and Social Care 2021 September 15. [Google Scholar]
- 17. European Medicines Agency Pharmacovigilance Risk Assessment Committee . Summary 30 August-2 September, 2021 [Google Scholar]
- 18. Center for Disease Control. United States of America Department of Health and Human Services. COVID-19 Vaccine Safety Technical (VaST) Work Group Report, June 28, 2021
- 19. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 2021;6:1202–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pepe S, Gregory AT, Myocarditis DA. Pericarditis and Cardiomyopathy after COVID-19 vaccination. Heart Lung Circ 2021;30:1425–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]