Abstract
Coronavirus disease 2019 (COVID-19) vaccines have yielded definitive prevention and major reductions in morbidity and mortality from severe acute respiratory syndrome coronavirus 2 infection, even in the context of emerging and persistent variants of concern. Newer variants have revealed less vaccine protection against infection and attenuation of vaccine effects on transmission. COVID-19 vaccines still likely reduce transmission compared with not being vaccinated at all, even with variants of concern; however, determining the magnitude of transmission reduction is constrained by the challenges of performing these studies, requiring accurate linkage of infections to vaccine status and timing thereof, particularly within households. In this review, we synthesize the currently available data on the impact of COVID-19 vaccines on infection, serious illness, and transmission; we also identify the challenges and opportunities associated with policy development based on this data.
Keywords: asymptomatic infection, COVID-19, SARS-CoV-2, viral shedding, transmission, variants
In just over 1 year, >10 billion doses of coronavirus disease 2019 (COVID-19) vaccines have been administered globally [1], with 10 vaccines granted emergency use listing by the World Health Organization so far, including mRNA, adenovirus-vectored, soluble protein, and inactivated virus vaccines [2]. These vaccines have demonstrated efficacy in preventing symptomatic COVID-19 in randomized controlled trials, in the context of both the original severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) strains (D614 and D614G) and real-world effectiveness against variants of concern [3]. Economists at Indiana University and the RAND Corporation estimated that COVID-19 vaccination prevented at least 140 000 deaths in the United States alone by May 2021 [4].
While COVID-19 vaccines have yielded definitive prevention and major reductions in morbidity and mortality from SARS-CoV-2 infection, the impact of these vaccines against asymptomatic infection, viral shedding, and secondary SARS-CoV-2 transmission with emerging variants is more nuanced [5]. Real-world effectiveness studies in the context of the Alpha variant suggest that multiple vaccines do reduce infection and onward transmission [6–8], but the coordinated, robust surveillance systems needed to track this in real time for emerging variants of concern are lacking. This dearth of real-time data stymies policy-makers, who are navigating public policy decisions around primary and booster vaccination, the rollout of monoclonal antibodies and small-molecule antivirals, and guidance on nonpharmaceutical interventions like masking. Many misinterpreted reports of increased breakthrough infections as evidence of wholesale loss of vaccine protection against transmission have resulted in public confusion on the topic, and these questions are even more pronounced given emergence of the most recent variant of concern as of November 2021, Omicron (B.1.1.529) [9].
In this review, we outline the literature on the impact of COVID-19 vaccines on SARS-CoV-2 infection, peak and duration of viral shedding, and transmission of SARS-CoV-2 following vaccination, with an emphasis on COVID-19 vaccine effects against variants of concern. COVID-19 vaccines remain a critical tool to in the path to ending the pandemic, even in the context of newer variants and waning immunity. We argue that surveillance of transmission among both vaccinated and unvaccinated populations is woefully inadequate, leaving policy-makers uninformed and vulnerable to poor decision-making in the face of potentially more transmissible variants on the horizon. Prospective studies evaluating vaccine efficacy in the context of evolving variants are necessary for providing definitive answers about the magnitude of reduction in infection and transmission of SARS-CoV-2 variants.
POLICY IMPLICATIONS OF UNDERSTANDING SARS-COV-2 TRANSMISSION
Our understanding of transmission of SARS-CoV-2 and the impact that vaccines have on reducing risk have significant policy implications. As of January 2022, the Centers for Disease Control and Prevention (CDC) continues to recommend that individuals in areas of substantial or high transmission should wear masks indoors in public regardless of vaccination status, due to the increased transmissibility of SARS-CoV-2 [10, 11], although by February 2022 many states have begun to reverse mask mandates [12]. Given the general overwhelmed state of hospital systems and overworked state of health care workers, ultraconservative isolation and return-to-work policies for infected workers may result in both absenteeism (further overwhelming the system with possible delays in care due to understaffing) or presenteeism (placing patients at risk for being infected when workers come to work sick) [13–15]. In December 2021, the CDC recommended a 5-day isolation after a positive SARS-CoV-2 test for vaccinated, asymptomatic individuals while keeping the 10-day isolation for unvaccinated individuals [16]. Understanding SARS-COV-2 transmission and when individuals can safely return to work, school, and public venues also has economic implications including more businesses remaining open, fewer days off from work for vaccinated sick workers, and less negative employment change overall, which may lead to improved psychological distress and more equitable racial distribution of negative impacts [17].
VACCINE EFFECTS ON TRANSMISSION—A 2-STEP PROCESS
Step 1: Vaccine Protection Against SARS-CoV-2 Infection
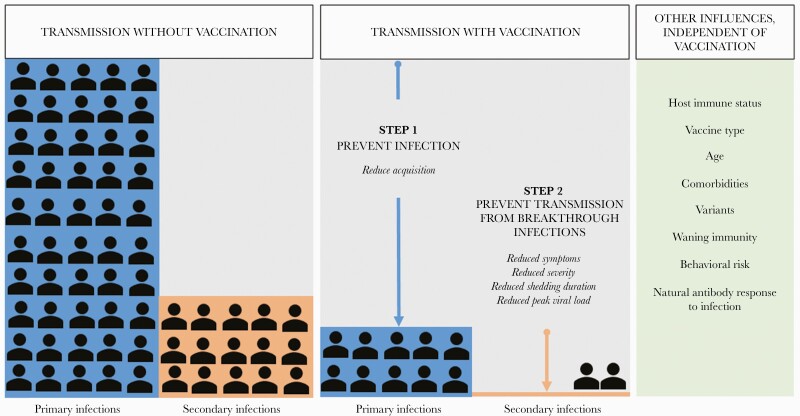
The first step in preventing transmission is preventing infection. If an individual is not infected, they cannot transmit virus to someone else. Therefore, when considering how well a vaccine protects against transmission, we must first determine how well it protects against infections, including asymptomatic infections. If a vaccine can prevent an overwhelming majority of infections, it will have a major impact on curtailing the epidemic, protecting the vaccinated as well as their close contacts (Figure 1). Total vaccine protection against infection is sometimes referred to as sterilizing immunity, and while it can be achieved in individual cases, it is exceedingly rare for any vaccine to achieve this across a population of vaccinated individuals [18]. The mechanism of sterilizing immunity is typically attributed to the presence of neutralizing antibodies that can bind to surface structures on an infectious particle, such as the spike (S) protein on the surface of SARS-CoV-2, and inhibit entry into cells to block replication before it begins [18]. Neutralizing antibody to mRNA-1273 vaccine has also been shown to be a direct correlate of protection against symptomatic SARS-CoV-2 infection among mRNA-1273 recipients, though whether vaccine-induced neutralizing antibodies are a correlate of protection against all infections and for other vaccine platforms remains unknown [19]. Vaccinated individuals can experience breakthrough infections, which can be either symptomatic or asymptomatic, people who are immunocompromised may have less robust responses to vaccination and thereby less protection against disease [20–22], and evolution of SARS-CoV-2 variants may interfere with development of neutralizing antibodies, thereby leading to these breakthrough infections or rendering monoclonal antibodies obsolete [23].
Figure 1.
Vaccine effects on reducing SARS-CoV-2 transmission. The left panel demonstrates SARS-CoV-2 transmission with primary infections leading to secondary infections. The middle panel demonstrates 2 steps of transmission reduction through vaccination: reduction of infection acquisition and reduction of breakthrough infections. The right panel describes other factors independent of vaccination status that influence transmission likelihood. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Step 2: Vaccine Protection Against Transmission of Breakthrough Infections
In the setting of breakthrough infections, however, vaccines can also reduce the likelihood of onward transmission [24] by decreasing the magnitude and duration of viral shedding, reducing the degree of symptoms, and possibly rendering breakthrough viruses less infectious (Figure 1). Perhaps most significant among these effects is a vaccine-induced reduction in viral shedding. Among those infected with SARS-CoV-2, nasopharyngeal viral load may be a strong direct correlate for human-to-human transmission [25]. Evidence for this is nuanced and based on models [26, 27] or nonhuman primate studies that have shown that COVID-19 vaccines reduce viral load in the lower and upper respiratory tracts [28, 29]. One study found no link between viral load cycle threshold and transmission in a college student cohort [30], and while a large prospective cohort showed no significant difference between cycle threshold peaks of symptomatic vs asymptomatic individuals, vaccinated individuals had faster viral load clearance and therefore lower overall duration of infection [31, 32]. Unfortunately, we have not yet identified the best laboratory method for reliable prediction of infectiousness [33]; therefore, these inferences of the vaccine effect on infectiousness remain indirect and imperfect. From a mechanistic standpoint, vaccines eliciting neutralizing antibodies block the SARS-CoV-2 spike protein from interaction with the ACE2 receptor at the mucosal surface and obstruct viral entry into cells, but not all cells may be protected [34]. While some cells may not escape infection, resulting in brief bursts of viral replication, the total magnitude of viral replication will be reduced. Moreover, vaccine-induced T-cell immunity and other non-neutralizing immune responses can further limit the spread of small pockets of mucosal infection, reducing the duration of infection. In addition to reducing viral shedding, vaccine-induced immune responses may also limit symptoms during breakthrough infections by preventing progression of disease from the mucosal compartment to the lower respiratory tract and the rest of the body. Furthermore, a vaccine may have a “sieve effect,” whereby vaccination preferentially blocks infection with viruses that are more transmissible [35, 36].
DRIVERS OF CONTINUED SARS-COV-2 TRANSMISSION
Despite the fact that >60% of world’s population has received at least 1 dose of a COVID-19 vaccine [1], nearly all countries have experienced a surge of infections from April to December 2021. The drivers of the continued population transmission of SARS-CoV-2 are multifactorial (Figure 2). The emergence of new, highly transmissible viral variants in January/February of 2021 [37], complete saturation by the Delta variant by July 2021, and subsequent dominance of the Omicron variant toward the end of 2021/early 2022 with concern for even greater vaccine escape [38] are important factors. Potential waning of vaccine-induced immunity, immune evasion by new variants, and increased pathogenicity of variants are others; evasion of the immune response, whether from natural infection or vaccine resistance, has been observed with other infectious diseases [39–41]. A root cause of much of the ongoing transmission is the inequitable distribution of COVID-19 vaccines globally, leaving low-income countries particularly vulnerable to unabated infections and attendant onward transmission, as well as the risk for development of new variants of concern [42]. Importantly, in addition to the virus and vaccine effects, several other factors may influence transmission, including individual immune system function and comorbidities, community behaviors (eg, masking, isolation, travel, and large gatherings), seasonal effects (eg, cold weather forcing people indoors), and the impact of natural or hybrid immune response to infection (Figure 1).
Figure 2.
Potential drivers of continued SARS-CoV-2 transmission in the context of vaccines. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
VACCINE IMPACT ON TRANSMISSION OF SARS-COV-2 ALPHA VARIANT INFECTIONS
In late 2020, the United Kingdom reported a new SARS-CoV-2 variant, designated Alpha (B.1.1.7) [43]. This variant was found to be 50%–75% more transmissible than previously circulating strains and had a higher secondary attack rate [44, 45]. The Alpha variant contained several mutations in the spike protein, which did not confer significant immune evasion of vaccine-induced neutralizing antibody responses as tested in vitro [46, 47]. Data from both observational cohort studies and randomized controlled clinical trials during the period of time in which Alpha was the predominant circulating strain in the study location (Alpha period) confirmed that COVID-19 vaccines maintained activity against the Alpha variant, including partial protection against infection regardless of symptoms (Table 1) [3, 48–59]. A meta-analysis of 12 studies found that the effectiveness of the adenovirus-vectored vaccines against infection was 73% (95% CI, 69%–77%), and for mRNA vaccines it was 85% (95% CI, 82%–88%) in participants ≥18 years [3]. Some of these data provide evidence of effectiveness against Beta and Gamma variant strains as well [48, 55]. Furthermore, evidence suggested that individuals with vaccine breakthrough SARS-CoV-2 infection during the Alpha period were less likely to transmit to household contacts than unvaccinated individuals with SARS-CoV-2 infection. For example, Prunas et al. analyzed a database of >2 million individuals in Israel who had received 2 doses of BNT162b2 between June 2020 and March 2021, in which positive SARS-CoV-2 tests were linked by addresses, allowing the tracking of index cases and household contacts [8]. Modeling estimated that vaccination reduced susceptibility to infection by ~80% and reduced infectiousness by 41%, resulting in an overall inferred reduction in transmission risk of 88.5% [60, 61].
Table 1.
Studies Describing COVID-19 Vaccine Protection Against Infection in the Period Before Delta Variant Predominance
| Time Period of Enrollment and Follow-up | SARS-CoV-2 Variant(s) | Population | Design | Location | Outcome | Vaccine | VE (95% CI) 1 Dose |
VE (95% CI) 2 Dose |
Citation |
|---|---|---|---|---|---|---|---|---|---|
| 12/2020–2/2021 | Alphaa | Health care workers | Prospective biweekly PCR screening | England | All infection | BNT162b2 | 70 (55 to 85) | 85 (74 to 96) | Hall et al. [52] |
| 12/2020–1/2021 | Original/wild-typeb | PCR + health care workers | Retrospective medical record review | Israel | All infection | BNT162b2 | 30 (2 to 50) | 75 (72 to 84) | Amit et al. [49] |
| 12/2020–3/2021 | Alphab | Health care workers | Prospective weekly PCR screening | USA | All infection | Mix: BNT162b mRNA-1273 |
80 (59 to 90) | 90 (68 to 97) | Thompson et al. [58] |
| 12/2020–4/2021 | Alphab | Individuals in health care system | Retrospective medical record review | USA | All infection | BNT162b2 mRNA-1273 |
61 (51 to 59) 67 (52 to 77) |
88 (84 to 91) 92 (82 to 97) |
Pawloski et al. [54] |
| 12/2020–2/2021 | Alphaa | Individuals in health care system | Prospective medical record review | Israel | All infection | BNT162b2 | 60 (53 to 66) | 92 (88 to 95) | Dagan et al. [50] |
| 12/2020–2/2021 | Alphab | Individuals in health care system | Retrospective medical record review of preprocedural PCR screening | USA | Asymptomatic infection | BNT162b2 | 79 (62 to 89) | 80 (56 to 91) | Tande et al. [57] |
| 4/2021–8/2021 | Alphac Delta |
Individuals in national registry | Retrospective medical record review, with sequencing of positive samples | Norway | All infection | Mix: BNT162b2 mRNA-1273 ChAdOx1 |
Alpha: 55 (50 to 58) Delta: 22 (17 to 27) |
Alpha: 84 (82 to 87) Delta: 65 (61 to 68) |
Seppala et al. [56] |
| 1/2021–4/2021 | Gammaa | Health care workers | Retrospective medical record review | Brazil | All infection | CoronaVac | 35 (–7 to 61) | 38 (–46 to 74) | Hitchings et al. [53] |
| 5/2020–11/2020 | Original/wild-typeb | Clinical trial participants | RCT, weekly PCR screening | UK | Asymptomatic infection | ChAdOx1 | N/A | 27 (–17 to 55) | Voysey et al. [59] |
| 9/2020–1/2021 | Betac Zeta |
Clinical trial participants | RCT, N-immunoassay seroconversion | USA, Latin America, South Africa | Asymptomatic infection | Ad26.COV2.S | 66 (40 to 81) | N/A | Sadoff et al. [55] |
| 7/2020–3/2021 | Epsilonb Beta Alpha |
Clinical trial participants | RCT, PCR screening at visits, and N-assay seroconversion | USA | Asymptomatic infection | mRNA-1273 | N/A | 63 (57 to 69) | El Sahly et al. [51] |
| 7/2020–12/2020 | Original/wild-typea | Clinical trial participants | RCT, any PCR test in study | UAE Bahrain |
All infection | WIV04 HB02 |
N/A N/A |
64 (49 to 75) 74 (61 to 82) |
Al Kaabi et al. [48] |
Abbreviations: COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; RCT; randomized controlled trial; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VE, vaccine effectiveness/efficacy; VOC, variant of concern.
SARS-CoV-2 variant(s) as indicated by the authors of this manuscript.
SARS-CoV-2 variant(s) not indicated in the manuscript. Variant(s) listed in the table is the dominant sequence at the time and location of the study, as per Nextstrain.org.
SARS-CoV-2 variant(s) as per in-study sequencing.
Data from the United Kingdom also suggested that vaccination led to a 40%–50% reduction in transmission of breakthrough infections following only a single dose of either ChAdOx1 or BNT162b2 in a large centralized database study of households with positive SARS-CoV-2 tests in England during January to February 2021 [62]. Following full vaccination, investigators in the Netherlands calculated that vaccination led to a 70% reduction in transmission of breakthrough infections between February and May 2021 in a study utilizing detailed contact-tracing strategies [63]. Interestingly, when evaluating transmission pairs, the estimated attack rate was 10% if either member of the pair was vaccinated, vs 30% for unvaccinated–unvaccinated pairs [60, 61], supporting benefit of vaccination of some household members even when others remain unvaccinated. Investigators also demonstrated reductions in the development of COVID-19 among household members of vaccinated health care workers in Scotland [64] and among nonimmune household members of individuals who demonstrated either vaccine-induced or natural infection-induced immunity to COVID-19 in Sweden [65].
The likely mechanisms behind this reduced transmission of breakthrough infections were decreases in viral shedding and symptoms. In the United States, Thompson et al. looked at a prospective cohort of US health care workers between December 2020 and April 2021 who received weekly nasal SARS-CoV-2 polymerase chain reaction (PCR) [61]. Investigators found that vaccination led to a reduction of mean peak viral load from 3.8 log10 copies/μL to 2.3 log10 copies/μL and a reduction of duration of RNA detection from 9 days to 3 days. Moreover, infected vaccinated participants had a shorter duration of symptoms, and only 25% had fever compared with 63% of unvaccinated participants. Pouwels et al. also showed that vaccination with multiple different platforms significantly reduced viral load in breakthrough infections compared with nonbreakthrough infections in the Alpha period [60].
REDUCED VACCINE IMPACT ON TRANSMISSION OF SARS-COV-2 DELTA VARIANT INFECTIONS
The Delta variant (B.1.617.2 lineage) was first identified in India in December 2020 and became the prevalent variant worldwide by July 2021. The Delta variant is highly transmissible compared with both Alpha and ancestral strains, potentially due to the L452R mutation in the spike protein leading to better binding avidity with the ACE2 receptor [60, 66–68]. Unlike the Alpha variant, the Delta variant also appears to partially evade immune responses in vitro [69], though very high D164 S-specific neutralizing antibody titers can overcome this evasion in laboratory studies [70, 71]. The vast majority of reports regarding protection against Delta infection focus on symptomatic infection.
Real-world data during the period of time in which Delta was the predominant circulating strain in the study location (Delta period) suggest that vaccine effectiveness against symptomatic SARS-CoV-2 infection is attenuated compared with the pre-Delta period, but that protection against moderate to severe disease remains very strong [72]. Observational studies of mRNA-1273 have shown a reduction in protection against symptomatic illness from >93% for Alpha to as low as 84% for Delta, but mRNA-1273 remained >90% effective against moderate to severe disease [51, 73–76]. For ChAdOx1, vaccine effectiveness also decreased after 2 doses from 75% for Alpha to 67% for Delta infections, but protection against moderate to severe disease remained much higher at 82% [77, 78]. Preliminary data from South Africa show that Ad26.COV2.S maintains strong protection against moderate to severe COVID-19 [79], as do inactivated COVID-19 vaccines studied in Guangdong, China [80]. BNT162b2 also decreased in effectiveness against symptomatic illness from 94% for Alpha to 88% for Delta infections but maintained effectiveness of 93% against hospitalizations for infections with the Delta variant [77, 81]. It is unclear if these reductions in effectiveness against disease are due to Delta immune evasion or waning immunity, as evidenced by studies that show that attenuation is focused on older age groups or people more remotely vaccinated [75, 82].
While COVID-19 vaccines (particularly mRNA) have maintained robust protection against symptomatic illness and moderate to severe disease from recent variants, there appears to be a more marked attenuation in protection against infection (Table 2) [56, 60, 74, 78, 81, 83–85]. For example, estimates of mRNA-1273 effectiveness against infections among nursing home residents decreased from 74.7% against Alpha to 50.6% against Delta [83], with other studies also estimating mRNA and Ad-vectored vaccine effectiveness in the 50%–60% range against Delta infection, particularly for BNT162b2 [74, 78, 81, 84, 85]. Estimates of Ad26.COV2.S against Delta-predominant COVID-19 disease suggest vaccine effectiveness of 60%–65% for preventing emergency department/urgent care encounters or hospitalizations for COVID-19 [75]. However, not all studies have estimated such sharp reductions in effectiveness against Delta, with other data suggesting rates closer to the 70%–80% range for mRNA vaccines [60, 84, 85].
Table 2.
Studies Describing COVID-19 Vaccine Effectiveness Against Delta, Omicron, and Other Variants of Concern
| Time Period of Enrollment and Follow-up | SARS-CoV-2 Variant(s) | Population | Design | Location | Outcome | Vaccine | VE (95% CI) 1 Dose |
VE (95% CI) 2 Dose |
Citation |
|---|---|---|---|---|---|---|---|---|---|
| 4/2021–8/2021 | Alphac Delta |
Individuals in national registry | Retrospective medical record review, with sequencing of positive samples | Norway | All infection | Mix: BNT162b2 mRNA-1273 ChAdOx1 |
Alpha: 55 (50 to 58) Delta: 22 (17 to 27) |
Alpha: 84 (82 to 87) Delta: 65 (61 to 68) |
Seppala et al. 2021 [56] |
| 3/2021–9/2021 | Betac Delta |
Individuals in national registry | Retrospective medical record review, with sequencing of positive samples | Qatar | All infection | BNT162b2 mRNA-1273 BNT162b2 mRNA-1273 |
Beta: 19 (–2 to 35) 66 (56 to 74) Delta: 45 (22 to 62) 74 (58 to 84) |
Beta: 74 (70 to 78) 81 (69 to 88) Delta: 52 (47 to 56) 73 (68 to 78) |
Tang et al. 2021 [85] |
| 4/2021–5/2021 | Deltac | Individuals in health care system | Retrospective medical record review, with sequencing of positive samples | India | All infection | ChAdOx1 | 46 (32 to 58) | 63 (52 to 72) | Thiruvengadam et al. 2021 [78] |
| 12/2020–8/2021 | Epsilonc Alpha Delta |
Individuals in health care system | Retrospective medical record review, with sequencing of positive samples | USA | All infection | BNT162b2 1 mo 5 mo BNT162b2 1 mo 5 mo |
N/A | Non-Delta: 97 (95 to 99) 67 (45 to 80) Delta: 93 (85 to 97) 53 (39 to 65) |
Tartof et al. 2021 [81] |
| 7/2021–8/2021 | Deltac | Male prisoners | Prospective weekly PCR screening | USA | All infection | mRNA-1273 | N/A | 57 (42 to 68) | Chin et al. 2021 [74] |
| 4/2021–6/2021 | Deltac | Individuals in national registry | Retrospective medical record review, with sequencing of positive samples | Scotland | All infection | BNT162b2 ChAdOx1 |
N/A N/A |
79 (75 to 82) 60 (53 to 66) |
Sheikh et al. 2021 [84] |
| 12/2020–8/2021 | Alphaa Delta |
Individuals in community-based survey across Alpha and Delta periods | Prospective monthly PCR screening | UK | All infection | BNT162b2 ChAdOx1 BNT162b2 ChAdOx1 |
Alpha: 59 (62 to 65) 63 (55 to 69) Delta: 57 (50 to 63) 46 (55 to 69) |
Alpha: 78 (68 to 84) 80 (77 to 83) Delta: 79 (56 to 90) 67 (62 to 71) |
Pouwels et al. 2021 [60] |
| 3/2021–8/2021 | Alphaa Delta |
Nursing home residents | Retrospective medical record review | USA | All infection | BNT162b2 mRNA-1273 BNT162b2 mRNA-1273 |
N/A | Pre-Delta: 74 (69 to 79) 75 (66 to 81) Delta: 52 (48 to 56) 51 (45 to 56) |
Nanduri et al. 2021 [83] |
| 11/2021–12/2021 | Deltac Omicron |
Individuals in national registry | Retrospective medical record review, with sequencing of positive samples | Denmark | All infection | BNT162b2 mRNA-1273 BNT162b2 mRNA-1273 |
N/A | Delta: 87 (85 to 89) 88 (83 to 92) Omicron: 55 (24 to 74) 37 (–70 to 76) |
Hansen et al. 2022 [96] |
| 12/2021 | Deltac Omicron |
Individuals in health care system | Retrospective medical record review, with sequencing of positive samples | USA | All infection | mRNA-1273 +Boost mRNA-1273 +Boost |
Delta: 57 (41 to 68) N/A Omicron: 20 (10 to 30) N/A |
Delta: 80 (68 to 88) 94 (92 to 95) Omicron: 44 (35 to 52) 72 (70 to 73) |
Tseng et al. 2022 [97] |
Abbreviations: COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; RCT; randomized controlled trial; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VE, vaccine effectiveness/efficacy; VOC, variant of concern.
SARS-CoV-2 variant(s) as indicated by the authors of the manuscript.
SARS-CoV-2 variant(s) not indicated in manuscript. Variant(s) listed in table is the dominant sequence at the time and location of the study, as per Nextstrain.org.
SARS-CoV-2 variant(s) as per in-study sequencing.
Because the Delta surge occurred simultaneously with waning immunity for most vaccinated individuals globally, it is difficult to disentangle the precise mechanism for the attenuation of vaccine effectiveness observed during the Delta period. Nevertheless, the evidence suggests a reduced impact of vaccines against transmission by virtue of the increased rate of breakthrough infections (Step 1 of vaccine effects on transmission) (Figure 1). There also appears to be a reduction in vaccine effect on the infectiousness of breakthrough infections (Step 2 of vaccine effects on transmission) (Figure 1), as suggested by data from Singanayagam et al., who performed a prospective, longitudinal study of ambulatory close contacts of confirmed COVID-19 cases [86]. In this study, the secondary attack rate among household contacts exposed to fully vaccinated index cases was no different than the rate among those exposed to unvaccinated index cases (25% vs 23%, respectively). In contrast, Harris et al. and de Gier et al. estimated reductions of 40%–70% in secondary attack rates during the Alpha period [62, 63].
One potential reason for the reduced vaccine effect on infectiousness may be a loss of vaccine effects on peak viral load in breakthrough infections. Brown et al. studied peak viral loads in 469 individuals identified in an outbreak in Massachusetts wherein 74% of cases were in fully vaccinated individuals, and >90% of the viruses were sequenced as Delta variant [87]. Investigators found no difference in viral load (as measured by cycle threshold) between breakthrough infections and nonbreakthrough infections. Pouwels et al. also demonstrated no difference in peak viral load during the Delta period in a prospective study of vaccine recipients in the United Kingdom including individuals vaccinated with ChAdOx1, Pfizer BNT162b2, and Moderna mRNA-1273 vaccines from December 2020 to August 2021 [60]. Singanayagam et al. also reported no difference in peak viral load between vaccinated and unvaccinated individuals [86].
While peak viral load may be the same between breakthrough and nonbreakthrough infections, the total duration of viral shedding may not be the same. Investigators in Singapore found that the viral kinetics of breakthrough infections were significantly different than in unvaccinated infections, with a steeper decline in viral load and accelerated clearance [88]. Singanayagam also found that vaccinated individuals had a faster mean rate of viral load decline than did unvaccinated individuals, as did Kissler et al. [32, 86]. Moreover, Shamier et al. found that breakthrough infections were much less likely to be culture positive (ie, live virus) compared with nonbreakthrough infections, even when samples had the same viral load on PCR) [89]. In contrast, in a study of a subset of 70 symptomatic persons who provided swabs for serial testing in a Texas prison, no significant difference was found in the median interval between reported symptom onset and last positive reverse transcription PCR result in vaccinated vs unvaccinated persons (9 vs 11 days) [90]. Virus was cultured from 42% of unvaccinated samples compared with 38% of fully vaccinated samples (with the limitation that these data may not reflect the viral kinetics of asymptomatic infections).
These data suggest that there may be vaccine effects on the total duration of shedding of infectious virus that may not necessarily translate to meaningful reductions in household transmission, perhaps because most transmission may occur during early, peak shedding periods and during so-called “superspreader events” [91, 92]. A retrospective study in the UK found a more pronounced effect of reduction of onward transmission with vaccination against the Alpha variant compared with Delta; however, although vaccination was associated with higher cycle threshold (Ct) values (aka lower viral loads), differences in Ct values alone did not fully explain the effect of vaccination [93]. Furthermore, breakthrough Delta infections could still be less transmittable at the population level. Phylogenetic evaluation of the Massachusetts outbreak in a highly vaccinated population suggested an overdispersion phenomenon in which a few individuals propagated most transmission events, suggesting that not all cases with high viral load detected by PCR ended up as the source of secondary transmission [92, 94].
OMICRON AND OTHER VARIANTS OF CONCERN
There is comparatively little data on COVID-19 vaccine effects on transmission of other variants of concern such as Beta and Gamma. Hitchings et al. evaluated CoronaVac (an inactivated vaccine) during the Gamma variant wave in Manaus, Brazil, and found that after 2 doses, the vaccine was 37.9% effective against all SARS-CoV-2 infection [53]. Sadoff et al. found that Ad26.COV2.S was 66% effective against asymptomatic infections that included a large proportion of Beta, given that 15% of the study population was from South Africa during their Beta surge [55]. There are no additional published data on viral dynamics and secondary transmission of breakthrough infections for these variants.
In November 2021, a new variant was reported in South Africa that was noted to overtake Delta sequences in surveillance data and was subsequently designated the variant of concern Omicron (B.1.1.529); as of February 2022, it has been identified in almost every country worldwide and is the predominant circulating strain in the United States since January 2022 [9]. The BA.1 Omicron variant has >30 mutations in the spike protein, including changes associated with increased transmissibility and immune evasion [38, 95]. Available data demonstrate reduced mRNA vaccine effectiveness against Omicron compared with Delta (Table 2) [96, 97]. The full impact of COVID-19 vaccines on transmission of Omicron is still being evaluated; however, data suggest increased transmission with higher secondary attack rates in households compared with rates during the Delta predominant period [98]. Evolving data regarding the clinical impact of Omicron suggest that despite increased infections and hospitalization rates, there is a reduced risk of severe disease and death compared with Delta [99–102]. However, the rates of infection and hospitalization in children and adolescents increased rapidly during the Omicron wave, a feature of Delta but not other variants [103].
The waning effects of vaccines against the Omicron variant are concerning [96]. Preliminary reports suggest that prior infection- and vaccine-induced antibody neutralization response against Omicron is significantly reduced [38, 104–106]—possibly explaining increased infection rates, while vaccine-induced cellular immune responses may remain robust in response to Omicron, possibly explaining protection against severe disease [107]. There is evidence that 3 doses may be needed to maintain a similar level of effectiveness against Omicron as was achieved with 2 doses against prior variants [97, 105, 108, 109], and vaccine effectiveness may be significantly reduced in immunocompromised people even with 3 doses [97]. Vaccinated individuals (and even those boosted) are still at risk of acquisition of SARS-CoV-2; however, unvaccinated people had rates of infection and hospitalization with Omicron that were 3.8 and 23.0 times higher compared with vaccinated people who had received boosters [110]. As such, the CDC still recommends booster doses for people older than 12 years [111], and a fourth dose for people with immunocompromising conditions [112].
The BA.2 sublineage of Omicron has emerged with additional mutations, distinguishing it from the predominant BA.1 lineage [113]. Preliminary data suggest that BA.2 may be more transmissible than BA.1, even in individuals who have been fully vaccinated; however, the largest secondary attack rates occurred among unvaccinated household individuals [98]. Like BA.1, BA.2 appears to evade neutralizing antibody responses in vaccinated individuals, a finding remedied by introduction of a vaccine boost/third dose, and, importantly, infection with BA.1 in vaccinated individuals may result in hybrid immunity with development of robust neutralizing antibodies against BA.2 [114]. Evolution of mutations leading to future variants of concern continues to be a possibility and should embolden worldwide vaccination efforts to reduce the proportion of individuals susceptible to future infections.
GAPS IN THE DATA: GETTING WHAT WE NEED WITH THE RESOURCES AVAILABLE
This review highlights several limitations to understanding SARS-CoV-2 transmission patterns effectively. First, retrospective observational studies have a delay in providing information, leading to a situation in which the literature applies to a variant that is already out of circulation. However, given the progression of this pandemic with rapidly evolving variants, even prospective studies may find themselves engaging in a race against time to the “next variant,” or collecting data prospectively that spans the course of more than 1 variant wave.
Second, many studies rely predominantly on proxy markers of asymptomatic infection (such as seroconversion) or are based on relatively infrequent PCR testing (eg, less than twice weekly). Both incorporate “length bias,” with individuals with short-duration infections or who never seroconvert misclassified as uninfected. If missed infections occur more often among vaccinated individuals, due to shortened duration of shedding or reduced seroconversion, this will inflate estimates of vaccine effects against SARS-CoV-2 infection. Furthermore, it will inflate estimates of vaccine effects on secondary transmission to the extent that testing of contacts/household members is triggered by diagnosis of the index case.
A related issue occurs when infection is exclusively or predominantly triggered by an event such as onset of COVID-19 symptoms or potential SARS-CoV-2 exposure. If the frequency of the trigger is not balanced across vaccinated and unvaccinated groups, as in the case of symptom-prompted testing, infections will be differentially misclassified, leading to bias. Symptom-prompted testing is likely to miss more infections among vaccinated individuals and therefore to inflate estimates of vaccine effects on SARS-CoV-2 infection and secondary transmission. Symptom-prompted testing will also miss characterizing the effect of vaccination on shedding of asymptomatic infections.
Evaluating vaccine effects on secondary transmission requires distinguishing primary and secondary infection events within transmission clusters, for example, households. However, even prospective household transmission studies may have challenges. First, it may be difficult to accurately account for possible shared (eg, siblings at daycare or adults with similar friend circles) or other community-acquired exposures and challenging to determine directionality of transmission in a household when community spread is high. Additionally, while frequent testing would provide the most complete viral load trajectory, perfect adherence to daily procedures is difficult, even with incentives, because sometimes participants forget, or perhaps lack motivation to test. Furthermore, it is possible that individuals with more personal concern about COVID-19 may have greater incentive to adhere to testing procedures, which could potentially result in systematic bias when evaluating viral load trajectories. However, even with frequent, systematic SARS-CoV-2 testing of all members of transmission units, inferring transmission chains is challenging, given that individuals are likely infectious only for a few days early in the course of infection and just on or before the onset of any symptoms that develop [31, 115]. When testing is infrequent or triggered by symptoms or potential SARS-CoV-2 exposure, it is considerably more challenging, and misclassification of transmission events is very likely. This misclassification will bias estimates of vaccine effects on secondary transmission.
Finally, uncontrolled studies are subject to confounding, possibly due to differential likelihood of exposure to SARS-CoV-2 status or different behavior influencing transmission conditional on vaccination status. The driving forces of these differences may not be captured in the collected data or adequately controlled for in the design. Confounding is a major concern, especially in settings such as the United States where there has been politicization of vaccines and other prevention measures and in settings where vaccine access is limited by prioritization guidance. Retrospective observational studies are especially subject to uncontrolled confounding given limited capability to retrospectively capture exposure/transmission variables.
Despite the challenges outlined above in accurately measuring vaccine effects on transmission, it is nevertheless critical to push forward and invest in such research. In particular, we recommend increased support for prospective studies that (1) follow individuals with routine, frequent PCR testing for viral quantification, infectiousness testing, and sequencing; (2) simultaneously collect risk behavior information on these individuals, as well as vaccination history, comorbidities, demographic data, and symptomatology; (3) prospectively follow secondary contacts of these individuals (eg, their households) to accurately infer transmission events and calculate secondary attack rates, as well as capture full viral load trajectories on both asymptomatic and symptomatic individuals; and (4) provide rapid, transparent sharing of data to inform real-time evidence-based public health decision-making. While no study is perfect, this type of prospective “household transmission study” would provide invaluable data on vaccine effects on SARS-CoV-2 transmission that are more robust than most retrospective studies can provide.
With evolving recommendations for booster vaccinations, understanding what the optimal boosting frequency and schedule will be now and into the future to prevent illness, infection, and reduce onward transmission will be an important question to keep future SARS-CoV-2 outbreaks under control. These efforts require considerable support and willingness by federal and local agencies, as well as vaccine developers. As new data emerge and influence policy, transparent communication of these data requires refinement that recognizes the interplay between waning humoral immunity and changing individual/community risk behaviors regarding gathering, masking, and attitudes toward vaccination; this communication will be paramount in framing future public health initiatives.
CONCLUSIONS
Over the last 2 years, we have seen unparalleled scientific collaboration and innovation including rapid sequencing of SARS-CoV-2, vaccine development, new and repurposed treatment agents, and evolving understanding of the epidemiology of transmission. Looking to the future, funding for intensive surveillance for infection and transmission in sentinel cohorts, with real-time reporting of data, would provide opportunities to understand how to best adapt policy and vaccine development to new variants that emerge. Recognition of ongoing transmission possibilities in the face of effective vaccines should not weaken confidence in vaccines, but rather strengthen confidence in a multipronged public health response that include vaccines paired with additional interventions such as masks, physical distancing, and high-quality ventilation, among others, in areas of high community transmission. As we head into the third year of this pandemic with the unfortunate realization that complete eradication of SARS-CoV-2 may be out of reach, the ongoing evolution and inevitable emergence of new variants will continue to present challenges for halting transmission. Enhancing our understanding of transmission dynamics will be pivotal in developing flexible, nimble public health systems operating within the nuance of how and when prevention strategies can be turned on and off to prevent unnecessary illness and death.
Acknowledgments
Financial support. None.
Potential conflicts of interest. All authors are members of protocol leadership for the NIH/NIAID/CoVPN vaccine study CoVPN 3006/Prevent COVID U and receive salary support for this activity, not related to this manuscript. J.R.M. received a consultative honorarium in 2021 for serving on a Pfizer Global Medical Grants/Mayo Clinic Global Bridges Antimicrobial Stewardship Grant review panel. E.R.B. reports grants from the NIH and the Bill and Melinda Gates Foundation and serves as a consultant on a data safety monitoring board for Merck. There are no other relevant conflicts of interest to declare for any other authors. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This work does not include factors necessitating patient consent.
References
- 1. Our World in Data. Statistics and research: coronavirus (COVID-19) vaccinations. Available at: https://ourworldindata.org/covid-vaccinations. Accessed 16 December 2021.
- 2. World Health Organization. COVID-19 vaccine tracker. Available at: https://covid19.trackvaccines.org/agency/who/. Accessed 16 December 2021.
- 3. Fan YJ, Chan KH, Hung IF.. Safety and efficacy of COVID-19 vaccines: a systematic review and meta-analysis of different vaccines at phase 3. Vaccines (Basel) 2021; 9:989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta S, Cantor J, Simon KI, Bento AI, Wing C, Whaley CM.. Vaccinations against COVID-19 may have averted up to 140,000 deaths in the United States. Health Aff. 2021; 40(9):1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richterman A, Meyerowitz EA, Cevik M.. Indirect protection by reducing transmission: ending the pandemic with SARS-CoV-2 vaccination. Open Forum Infect Dis 2021; XXX:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Layan M, Gilboa M, Gonen T, et al. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. Am J Epidemiol 2022: kwac042. doi: 10.1093/aje/kwac042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prunas O, Warren JL, Crawford FW, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. Science 2022; 375(6585):1151–54. doi: 10.1126/science.abl4292. Epub 2022 Jan 27. PMID: 35084937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. 2021. [Google Scholar]
- 10. Centers for Disease Control and Prevention. When you’ve been fully vaccinated. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html. Accessed 16 December 2021.
- 11. Centers for Disease Control and Prevention. When to wear a mask. Available at: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/about-face-coverings.html. Accessed 22 February 2021.
- 12. The New York Times. The U.S. states that are ending mask mandates. The New York Times. 1 March 2022. Available at: https://www.nytimes.com/article/mask-mandates-us.html. Accessed 22 February 2021. [Google Scholar]
- 13. Jo HJ, Kim JS, Choe PG, et al. Work restrictions for healthcare personnel with potential in-hospital exposure to SARS-CoV-2: experience at a tertiary hospital. J Korean Med Sci 2021; 36:e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maltezou HC, Giannouchos TV, Pavli A, et al. Costs associated with COVID-19 in healthcare personnel in Greece: a cost-of-illness analysis. J Hosp Infect 2021; 114:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yaghoubi M, Salimi M, Meskarpour-Amiri M.. Systematic review of productivity loss among healthcare workers due to Covid-19. Int J Health Plann Manage 2022; 37:94–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. Quarantine and isolation. Available at: https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fif-you-are-sick%2Fquarantine.html. Accessed 16 December 2021.
- 17. Matthews TA, Chen L, Chen Z, et al. Negative employment changes during the COVID-19 pandemic and psychological distress: evidence from a nationally representative survey in the U.S. J Occup Environ Med 2021; 63:931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pollard AJ, Bijker EM.. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 2021; 21:83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of two-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults—nine states, January-September 2021. Am J Transplant 2022; 22:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haidar G, Agha M, Bilderback A, et al. Prospective evaluation of COVID-19 vaccine responses across a broad spectrum of immunocompromising conditions: the COVICS study. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tenforde MW, Patel MM, Gaglani M, et al. Effectiveness of a third dose of Pfizer-BioNTech and Moderna vaccines in preventing COVID-19 hospitalization among immunocompetent and immunocompromised adults - United States, August-December 2021. MMWR Morb Mortal Wkly Rep 2022; 71:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. In press. [DOI] [PubMed] [Google Scholar]
- 24. Mostaghimi D, Valdez CN, Larson HT, Kalinich CC, Iwasaki A.. Prevention of host-to-host transmission by SARS-CoV-2 vaccines. Lancet Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawasuji H, Takegoshi Y, Kaneda M, et al. Transmissibility of COVID-19 depends on the viral load around onset in adult and symptomatic patients. PLoS One 2020; 15:e0243597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goyal A, Reeves DB, Cardozo-Ojeda EF, Schiffer JT, Mayer BT.. Viral load and contact heterogeneity predict SARS-CoV-2 transmission and super-spreading events. Elife. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marc A, Kerioui M, Blanquart F, et al. Quantifying the relationship between SARS-CoV-2 viral load and infectiousness. Elife. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vogel AB, Kanevsky I, Che Y, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021; 592:283–9. [DOI] [PubMed] [Google Scholar]
- 29. Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020; 586:567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian D, Lin Z, Kriner EM, et al. Ct values do not predict severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmissibility in college students. J Mol Diagn 2021; 23:1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kissler SM, Fauver JR, Mack C, et al. Viral dynamics of acute SARS-CoV-2 infection and applications to diagnostic and public health strategies. PLoS Biol 2021; 19:e3001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kissler SM, Fauver JR, Mack C, et al. Viral dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated persons. N Engl J Med 2021; 385:2489–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Binnicker MJ. Can testing predict SARS-CoV-2 Infectivity? The potential for certain methods to be surrogates for replication-competent virus. J Clin Microbiol 2021; 59:e0046921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 2020; 11:2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edlefsen PT, Gilbert PB, Rolland M.. Sieve analysis in HIV-1 vaccine efficacy trials. Curr Opin HIV AIDS 2013; 8:432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rolland M, Gilbert PB.. Sieve analysis to understand how SARS-CoV-2 diversity can impact vaccine protection. PLoS Pathog 2021; 17:e1009406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 2021; 19:409–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carman WF, Zanetti AR, Karayiannis P, et al. Vaccine-induced escape mutant of hepatitis B virus. Lancet 1990; 336:325–9. [DOI] [PubMed] [Google Scholar]
- 40. Hegerle N, Guiso N.. Bordetella pertussis and pertactin-deficient clinical isolates: lessons for pertussis vaccines. Expert Rev Vaccines 2014; 13:1135–46. [DOI] [PubMed] [Google Scholar]
- 41. Weinberger DM, Malley R, Lipsitch M.. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rouw A, Kates J, Wexler A, Michaud J.. Tracking global COVID-19 vaccine equity: an update. Available at: https://www.kff.org/coronavirus-covid-19/issue-brief/tracking-global-covid-19-vaccine-equity-an-update/. Accessed 16 December 2021.
- 43. European Centre for Disease Prevention and Control. Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom, December 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf. Accessed 16 December 2021.
- 44. Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021; 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Volz E, Mishra S, Chand M, et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 2021; 593:266–9. [DOI] [PubMed] [Google Scholar]
- 46. Emary KRW, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet 2021; 397:1351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Muik A, Wallisch AK, Sanger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science 2021; 371:1152–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 2021; 326:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E.. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021; 397:875–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 2021; 397:P1725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hitchings MDT, Ranzani OT, Torres MSS, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 gamma variant transmission in Manaus, Brazil: a test-negative case-control study. Lancet Reg Health Am 2021; 1:100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pawlowski C, Lenehan P, Puranik A, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y) 2021; 2:979–92.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seppala E, Veneti L, Starrfelt J, et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tande AJ, Pollock BD, Shah ND, et al. Impact of the COVID-19 vaccine on asymptomatic infection among patients undergoing pre-procedural COVID-19 molecular screening. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep 2021; 70:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pouwels KB, Pritchard E, Matthews PC, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med 2021; 27:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med 2021; 385:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G.. Effect of vaccination on household transmission of SARS-CoV-2 in England. N Engl J Med 2021; 385:759–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de Gier B, Andeweg S, Joosten R, et al. Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands, February to May 2021. Euro Surveill. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shah ASV, Gribben C, Bishop J, et al. Effect of vaccination on transmission of SARS-CoV-2. N Engl J Med 2021; 385:1718–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nordström P, Ballin M, Nordströöm A.. Association between risk of COVID-19 infection in nonimmune individuals and COVID-19 immunity in their family members. JAMA Internal Medicine 2021; 181:1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Despres HW, Mills MG, Shirley DJ, et al. Quantitative measurement of infectious virus in SARS-CoV-2 Alpha, Delta and Epsilon variants reveals higher infectivity (viral titer:RNA ratio) in clinical samples containing the Delta and Epsilon variants. medRxiv 2021.09.07.21263229 [Preprint]. 20 September 2021. Available at: 10.1101/2021.09.07.21263229. Accessed 14 April 2022. [DOI] [Google Scholar]
- 67. Hendy M, Kaufman S, Ponga M.. Molecular strategies for antibody binding and escape of SARS-CoV-2 and its mutations. Sci Rep 2021; 11:21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu Y, Rocklov J.. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021; 596:276–80. [DOI] [PubMed] [Google Scholar]
- 70. Alter G, Yu J, Liu J, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021; 596:268–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barouch DH, Stephenson KE, Sadoff J, et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med 2021; 385:951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Keehner J, Horton LE, Binkin NJ, et al. Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. N Engl J Med 2021; 385:1330–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chin ET, Leidner D, Zhang Y, et al. Effectiveness of the mRNA-1273 vaccine during a SARS-CoV-2 Delta outbreak in a Prison. N Engl J Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Grannis SJ, Rowley EA, Ong TC, et al. Interim estimates of COVID-19 vaccine effectiveness against COVID-19-associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance - nine states, June-August 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tenforde MW, Self WH, Naioti EA, et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults - United States, March-July 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021; 385:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thiruvengadam R, Awasthi A, Medigeshi G, et al. Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the Delta (B.1.617.2) variant surge in India: a test-negative, case-control study and a mechanistic study of post-vaccination immune responses. Lancet Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Foley KE. J&J shot effective against Delta variant in large South Africa study. Available at: https://www.politico.eu/article/johnson-johnson-coronavirus-vaccine-delta-variant/. Accessed 14 April 2022.
- 80. Kang M, Yi Y, Li Y, et al. Effectiveness of inactivated COVID-19 vaccines against illness caused by the B.1.617.2 (Delta) variant during an outbreak in Guangdong, China: a cohort study. Ann Intern Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021; 398:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Baden LR, El Sahly HM, Essink B, et al. Phase 3 trial of mRNA-1273 during the Delta-variant surge. NEJM. 2021; 385:2485–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant - National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sheikh A, McMenamin J, Taylor B, Robertson C; Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021; 397:2461–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. In press. [DOI] [PubMed] [Google Scholar]
- 86. Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 2022; 22:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brown CM, Vostock J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings — Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1059–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chia PY, Ong SWX, Chiew CJ, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: a multicentre cohort study. Clin Microbiol Infect. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shamier MC, Tostmann A, Bogers S, et al. Virological characteristics of SARS-CoV-2 vaccine breakthrough infections in health care workers. medRxiv 2021.08.20.21262158 [Preprint]. 21 August 2021. Available at: 10.1101/2021.08.20.21262158. Accessed 14 April 2022. [DOI] [Google Scholar]
- 90. Hagan LM, McCormick DW, Lee C, Sleweon S, Nicolae L, Dixon T.. Outbreak of SARS-CoV-2 B.1.617.2 (Delta) variant infections among incarcerated persons in a federal prison — Texas, July–August 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Althouse BM, Wenger EA, Miller JC, et al. Superspreading events in the transmission dynamics of SARS-CoV-2: opportunities for interventions and control. PLoS Biol 2020; 18:e3000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Endo A, Abbott S, Kucharski AJ, Funk S.. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Res 2020; 5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Eyre DW, Taylor D, Purver M, et al. Effect of Covid-19 vaccination on transmission of Alpha and Delta variants. N Engl J Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Siddle KJ, Krasilnikova LA, Moreno GK, et al. Transmission from vaccinated individuals in a large SARS-CoV-2 Delta variant outbreak. Cell 2022; 185:485–92.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. South Africa National Institute for Communicable Diseases N. Frequently asked questions for the B.1.1.529 mutated SARS-CoV-2 lineage in South Africa. Available at: https://www.nicd.ac.za/frequently-asked-questions-for-the-b-1-1-529-mutated-sars-cov-2-lineage-in-south-africa/. Accessed 16 December 2021.
- 96. Hansen CH, Schelde AB, Moustsen-Helm IR, et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. medRxiv 2021.12.20.21267966 [Preprint]. 23 December 2021. Available at: 10.1101/2021.12.20.21267966. Accessed 14 April 2022. [DOI] [Google Scholar]
- 97. Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lyngse FP, Mortensen LH, Denwood MJ, et al. SARS-CoV-2 Omicron VOC transmission in Danish households. medRxiv 2021.12.27.21268278 [Preprint]. 30 January 2021. Available at: 10.1101/2022.01.28.22270044. Accessed 14 April 2022. [DOI] [Google Scholar]
- 99. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Modes ME, Directo MP, Melgar M, et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance - one hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ulloa AC, Buchan SA, Daneman N, Brown KA.. Estimates of SARS-CoV-2 Omicron variant severity in Ontario, Canada. JAMA. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa: a data linkage study. Lancet 2022; 399:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Marks KJ, Whitaker M, Anglin O, et al. Hospitalizations of children and adolescents with laboratory-confirmed COVID-19 - COVID-NET, 14 states, July 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Doria-Rose N, Shen X, Schmidt SD, et al. Booster of mRNA-1273 vaccine reduces SARS-CoV-2 Omicron escape from neutralizing antibodies. medRxiv 2021.12.15.21267805 [Preprint]. 20 December 2021. Available at: 10.1101/2021.12.15.21267805. Accessed 14 April 2022. [DOI] [Google Scholar]
- 105. Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. In press. [DOI] [PubMed] [Google Scholar]
- 106. Lu L, Mok BWY, Chen L-L, et al. Neutralization of severe acute respiratory syndrome coronavirus 2 Omicron variant by sera from BNT162b2 or CoronaVac vaccine recipients. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA 2022; 327:639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity and mRNA vaccine effectiveness for Omicron, Delta, and Alpha SARS-CoV-2 variants in the United States: a prospective observational study. medRxiv 2022.02.06.22270558 [Preprint]. 7 February 2022. Available at: 10.1101/2022.02.06.22270558. Accessed 14 April 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Danza P, Koo TH, Haddix M, et al. SARS-CoV-2 infection and hospitalization among adults aged ≥18 years, by vaccination status, before and during SARS-CoV-2 B.1.1.529 (Omicron) variant predominance - Los Angeles County, California, November 7, 2021-January 8, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Centers for Disease Control and Prevention. COVID-19 vaccine booster shots. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html. Accessed 14 April 2022.
- 112. Centers for Disease Control and Prevention. COVID-19 vaccines for moderately or severely immunocompromised people. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. Accessed 14 April 2022.
- 113. World Health Organization. Tracking SARS-CoV-2 variants. Available at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed 14 April 2022. [PubMed]
- 114. Yu J, Collier AY, Rowe M, et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 variants. N Engl J Med 2022. doi: 10.1056/NEJMc2201849. Epub ahead of print. PMID: 35294809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A.. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2021; 2:e13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]