Abstract
Background
Several underlying medical conditions have been reported to be associated with an increased risk of coronavirus disease 2019 (COVID-19) and related hospitalization and death. Population attributable fractions (PAFs) describing the proportion of disease burden attributable to underlying medical conditions for COVID-19 diagnosis and outcomes have not been reported.
Methods
A retrospective population-based cohort study was conducted using Optum’s de-identified Clinformatics Data Mart database. Individuals were followed up from 20 January 2020 to 31 December 2020 for diagnosis and clinical progression, including hospitalization, intensive care unit admission, intubation and mechanical ventilation or extracorporeal membrane oxygenation, and death. Adjusted rate ratios and PAFs of underlying medical conditions for COVID-19 diagnosis and disease progression outcomes were estimated by age (18–49, 50–64, 65–74, or ≥75 years), sex, and race/ethnicity.
Results
Of 10 679 566 cohort members, 391 964 (3.7%) were diagnosed with COVID-19, of whom 87 526 (22.3%) were hospitalized. Of those hospitalized, 26 640 (30.4%) died. Overall, cardiovascular disease and diabetes had the highest PAFs for COVID-19 diagnosis and outcomes of increasing severity across age groups (up to 0.49 and 0.35, respectively). Among adults ≥75 years of age, neurologic disease had the second-highest PAFs (0.05‒0.27) after cardiovascular disease (0.26‒0.44). PAFs were generally higher in Black persons than in other race/ethnicity groups for the same conditions, particularly in the 2 younger age groups.
Conclusions
A substantial fraction of the COVID-19 disease burden in the United States is attributable to cardiovascular disease and diabetes, highlighting the continued importance of COVID-19 prevention ( eg, vaccination, mask wearing, social distancing) and disease management of patients with certain underlying medical conditions.
Keywords: population attributable fraction, progression outcomes, SARS-CoV-2, COVID-19
Cardiovascular disease and diabetes account for a substantial portion of the public health burden of COVID-19 in the United States. COVID-19 mitigation efforts should promote continued prevention as well as close clinical management of patients with certain medical conditions.
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in >396 million reported cases and >5.7 million deaths worldwide, including >75 million cases and >895 000 deaths in the United States (US), as of 8 February 2022 [1].
Older persons, males, and persons with underlying medical conditions are at increased risk of COVID-19 diagnosis and COVID-19 disease progression including medical complications and death [2–6]. Underlying medical conditions associated with an increased risk of severe COVID-19 outcomes include cardiovascular disease, chronic obstructive pulmonary disease (COPD), hypertension, diabetes, chronic kidney disease, obesity, and cancer [3, 4, 7, 8]. Among patients with COVID-19, the presence of cardiovascular disease, diabetes, or hypertension increases the relative risk of progression to severe disease by approximately 1.5, and the relative risk of death by 1.5–2.1 [4].
Describing the COVID-19 disease burden attributable to underlying medical conditions can help focus COVID-19 prevention, treatment, and management of persons with underlying medical conditions [9, 10]. The population attributable fraction (PAF) is an epidemiologic tool that estimates the proportion of disease burden that is attributable to a particular risk factor [11, 12]. PAFs of underlying medical conditions for COVID-19 diagnosis and disease outcomes have not been reported. The aim of this study was to quantify and compare the burden of COVID-19 attributable to selected underlying medical conditions in the US.
METHODS
Data Source and Population
A population-based study was conducted using Optum’s de-identified Clinformatics Data Mart (CDM) database. CDM is derived from a database of administrative health claims for members of large commercial and Medicare Advantage health plans from all 50 US states. Administrative claims submitted for payment by providers and pharmacies are verified, adjudicated, and de-identified before inclusion in CDM. These data, including patient-level enrollment information, are derived from claims submitted for healthcare services at outpatient medical clinics, pharmacies, hospital emergency departments, and inpatient hospital settings. Medical conditions are identified using diagnosis and procedure codes attached to individual claims. Death information is available as year and month of death only.
A retrospective cohort was created that included all adults aged ≥18 years in the CDM database during the period of 20 January 2019–19 January 2020 provided they did not have any gaps in insurance coverage of >45 days during this 1-year baseline period, as recommended in the US Food and Drug Administration (FDA) Sentinel Initiative COVID-19 Natural History Master Protocol [13]. This cohort was followed up from 20 January 2020 (ie, the date of the first laboratory-confirmed SARS-CoV-2 infection in the US) to 31 December 2020 for COVID-19 diagnosis and COVID-19 disease progression outcomes.
Demographic characteristics of interest included age (in 2019), sex, and race/ethnicity. Age was stratified into 4 categories: 18–49 years, 50–64 years, 65–74 years, and ≥75 years.
Patient Consent Statement
As stated above, administrative claims submitted for payment by providers and pharmacies are verified, adjudicated, and de-identified before inclusion in CDM. Per the Health Insurance Portability and Accountability Act of 1996 (HIPAA), 45 Code of Federal Regulations §§ 164.502(d)(2), 164.514(a) and (b), “There are no restrictions on the use or disclosure of de-identified health information.” Accordingly, this study did not include factors necessitating patient consent.
Identification of Study Outcomes and Underlying Medical Conditions
COVID-19 diagnosis was identified using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code U07.1 (confirmed COVID-19 due to SARS-CoV-2), which became effective 1 April 2020 [14], and could not have code Z03.818 occurring between 7 days before and 30 days after code U07.1. Code Z03.818 indicates that SARS-CoV-2 exposure has been ruled out [14]. Clinical progression outcomes among persons diagnosed with COVID-19 included hospitalization, intensive care unit (ICU) admission, intubation and mechanical ventilation or extracorporeal membrane oxygenation (ECMO) with COVID-19, and death (either in or out of hospital). For persons with multiple health encounters with a COVID-19 diagnosis, the first encounter (inpatient or outpatient) was retained for analysis and individuals contributed person-time starting from 20 January 2020 until the date of first COVID-19 diagnosis or end of the follow-up period. For patients having multiple hospitalizations with COVID-19, the first hospitalization was retained for analysis and individuals contributed person-time starting from 20 January 2020 until, as applicable, the date of in-hospital COVID-19 diagnosis, ICU admission, ventilation, death, or end of the follow-up period.
Cohort members were classified as having ≥1 of the following underlying medical conditions if they had ≥2 reports of that condition on separate health encounters during 20 January 2019–19 January 2020: asthma, cardiovascular disease, cerebrovascular disease, chronic kidney disease, chronic liver disease, COPD, diabetes, hypertension, malignancy (including melanoma but no other skin cancers), neurologic disease, obesity, rheumatic disease, or smoking history.
All diagnosis and procedure codes used to identify underlying medical conditions and COVID-19 outcomes were drawn from the FDA Sentinel Initiative COVID-19 Natural History Code List [13].
Statistical Analysis
Categorical variables were reported as counts and percentages. The prevalence of underlying medical conditions on 20 January 2020 was calculated within age-, sex-, and race/ethnicity-specific subgroups. Rate ratios for a specific underlying medical condition were obtained from Poisson regression models for a COVID-19 outcome (ie, diagnosis, hospitalization, or COVID-19 disease progression outcomes while hospitalized [ICU admission, intubation and mechanical ventilation/ECMO], and death [either in or out of hospital]) within each specific subgroup, adjusting for each of the other medical conditions listed above as well as for emphysema, human immunodeficiency virus, and alcohol history. PAFs for a specific underlying medical condition were calculated as follows:
where pc is the prevalence of the underlying medical condition among cases during the baseline period and RR is the adjusted rate ratio for the condition in the specific age, sex, and race/ethnicity strata [11, 12]. PAFs, incidence rates, and adjusted rate ratios with associated 95% confidence intervals were calculated. All analyses were conducted with SAS version 9.4 software (SAS Institute, Cary, North Carolina).
RESULTS
Demographics and Underlying Medical Conditions
Demographic characteristics and prevalence of underlying medical conditions stratified by age group among persons in the cohort are shown in Table 1. The proportion of the cohort that was male decreased with increasing age, from 51.6% among the age group 18–49 years to 41.0% among those aged ≥75 years. The overall population was 56.7% White, 11.0% Hispanic, 8.5% Black, and 4.2% Asian; race/ethnicity was unknown for 19.6%.
Table 1.
Demographics and Prevalence of Underlying Medical Conditions by Age Group
| Variable | All Adults (N = 10 679 566) |
18–49 y (n = 3 922 320) |
50–64 y (n = 2 214 490) |
65–74 y (n = 2 446 888) |
≥75 y (n = 2 095 868) |
|---|---|---|---|---|---|
| Male, No. (%) | 5 064 018 (47.4) | 2 023 066 (51.6) | 1 106 165 (50.0) | 1 075 516 (44.0) | 859 271 (41.0) |
| Race/ethnicity, No. (%) | |||||
| Asian | 451 943 (4.2) | 231 066 (5.9) | 73 731 (3.3) | 79 471 (3.2) | 67 675 (3.2) |
| Black | 903 450 (8.5) | 324 547 (8.3) | 195 522 (8.8) | 206 373 (8.4) | 177 008 (8.4) |
| Hispanic | 1 177 678 (11.0) | 516 025 (13.2) | 228 236 (10.3) | 222 376 (9.1) | 211 041 (10.1) |
| White | 6 053 817 (56.7) | 2 102 240 (53.6) | 1 350 883 (61.0) | 1 290 418 (52.7) | 1 310 276 (62.5) |
| Unknown | 2 092 678 (19.6) | 748 442 (19.1) | 366 118 (16.5) | 648 250 (26.5) | 329 868 (15.7) |
| Underlying medical condition, No. (%) | |||||
| Asthma | 331 849 (3.1) | 77 603 (2.0) | 77 373 (3.5) | 98 339 (4.0) | 78 534 (3.7) |
| Cerebrovascular disease | 319 155 (3.0) | 8369 (0.2) | 39 880 (1.8) | 101 554 (4.2) | 169 352 (8.1) |
| Cardiovascular disease | 4 005 853 (37.5) | 309 735 (7.9) | 786 127 (35.5) | 1 415 327 (57.8) | 1 494 664 (71.3) |
| Chronic kidney disease | 874 032 (8.2) | 27 655 (0.7) | 100 337 (4.5) | 282 112 (11.5) | 463 928 (22.1) |
| Chronic liver disease | 208 343 (2.0) | 31 640 (0.8) | 59 589 (2.7) | 74 762 (3.1) | 42 352 (2.0) |
| COPD | 565 015 (5.3) | 15 922 (0.4) | 99 884 (4.5) | 202 385 (8.3) | 246 824 (11.8) |
| Diabetes | 1 560 803 (14.6) | 110 866 (2.8) | 323 734 (14.6) | 593 094 (24.2) | 533 109 (25.4) |
| Hypertension | 3 528 192 (33.0) | 247 976 (6.3) | 688 268 (31.1) | 1 260 468 (51.5) | 1 331 480 (63.5) |
| Malignancy | 823 902 (7.7) | 40 474 (1.0) | 135 473 (6.1) | 291 063 (11.9) | 356 892 (17.0) |
| Neurologic disease | 365 094 (3.4) | 28 969 (0.7) | 44 678 (2.0) | 75 200 (3.1) | 216 247 (10.3) |
| Obesity | 1 033 588 (9.7) | 226 294 (5.8) | 281 309 (12.7) | 336 429 (13.7) | 189 556 (9.0) |
| Rheumatic disease | 245 576 (2.3) | 39 295 (1.0) | 65 193 (2.9) | 77 890 (3.2) | 63 198 (3.0) |
| Smoking history | 741 474 (6.9) | 87 314 (2.2) | 173 726 (7.8) | 251 871 (10.3) | 228 563 (10.9) |
| SARS-CoV-2 outcome, No. (%) | |||||
| COVID-19 diagnosis | 391 964 (3.7) | 132 094 (3.4) | 82 921 (3.7) | 81 421 (3.3) | 95 528 (4.6) |
| Hospitalization | 87 526 (0.8) | 5589 (0.1) | 12 565 (0.6) | 24 904 (1.0) | 44 468 (2.1) |
| Length of stay, d, median (Q1, Q3) | 7 (4, 15) | 5 (3, 8) | 6 (4, 12) | 7 (4, 15) | 8 (5, 16) |
| ICU admission | 41 533 (0.4) | 2653 (0.1) | 6757 (0.3) | 13 065 (0.5) | 19 058 (0.9) |
| Intubation and MV or ECMO | 12 758 (0.1) | 612 (0.0) | 2292 (0.1) | 4624 (0.2) | 5230 (0.2) |
| Diagnosed and died | 35 388 (0.3) | 419 (0.0) | 2359 (0.1) | 7891 (0.3) | 24 719 (1.2) |
| Hospitalized and died | 26 640 (0.2) | 308 (0.0) | 1915 (0.1) | 6359 (0.3) | 18 058 (0.9) |
| Died the same month as discharge month | 17 899 (0.2) | 222 (0.0) | 1349 (0.1) | 4492 (0.2) | 11 836 (0.6) |
| Died the month after discharge month | 4204 (0.0) | 28 (0.0) | 266 (0.0) | 838 (0.0) | 3072 (0.1) |
| Died ≥2 months after discharge month | 4537 (0.0) | 58 (0.0) | 300 (0.0) | 1029 (0.0) | 3150 (0.2) |
Only the month of death was available.
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; MV, mechanical ventilation; Q, quartile; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Cardiovascular disease and hypertension were the most prevalent underlying medical conditions across age groups, and with increasing prevalence by age. Cardiovascular disease was reported in 8%, 35%, 58%, and 71% in the age groups 18–49, 50–64, 65–74, and ≥75 years, respectively. Hypertension was reported in 6%, 31%, 52%, and 64%, respectively. Diabetes was the third-most prevalent condition in each age group except 18- to 49-year-olds, where obesity was more common.
Demographic characteristics and baseline prevalence of underlying medical conditions of the cohort stratified by age group and sex are shown in Supplementary Table 1. The prevalence of cardiovascular disease, hypertension, and diabetes was similar in males and females in the cohort, with increasing prevalence across age groups. Supplementary Table 2 shows demographic characteristics and the prevalence of underlying medical conditions stratified by age group and race/ethnicity. Prevalence of cardiovascular disease and hypertension increased with increasing age, with Black persons reporting the highest rates across all age groups compared with White or Asian races or Hispanic ethnicity. In 50- to 64-year-olds, for example, cardiovascular disease and hypertension were reported in 49% and 46% of Black individuals compared with 25% and 22% of Asian, 33% and 30% of Hispanic, and 34% and 29% of White individuals, respectively.
COVID-19 Diagnosis and Hospitalization Outcomes
Of the 10 679 566 persons in the cohort, 391 964 (3.7%) were diagnosed with COVID-19, of whom 87 526 (22.3%) were hospitalized. Among the hospitalized COVID-19 patients, 41 533 (47.5%) were admitted to ICU, 12 758 (14.6%) had intubation and mechanical ventilation or ECMO, and 26 640 (30.4%) died (Table 1). Persons of Black race and Hispanic ethnicity were more likely to be diagnosed with COVID-19 (4.3% and 4.9%, respectively) compared with White and Asian persons (3.5% and 2.1%, respectively), and also more likely to be hospitalized due to COVID-19. Overall, 0.4% of Black and 0.3% of Hispanic persons were hospitalized and died with COVID-19, compared with 0.2% for White and 0.1% for Asian persons. Rates of COVID-19 diagnosis were slightly lower for the youngest vs the oldest groups: 1.8% vs 2.7% for Asian individuals; 3.4 vs 5.8% for Black individuals; 4.6% vs 5.1% for Hispanic individuals; and 3.3% vs 4.4% for White individuals (Supplementary Table 2).
Population Attributable Fraction
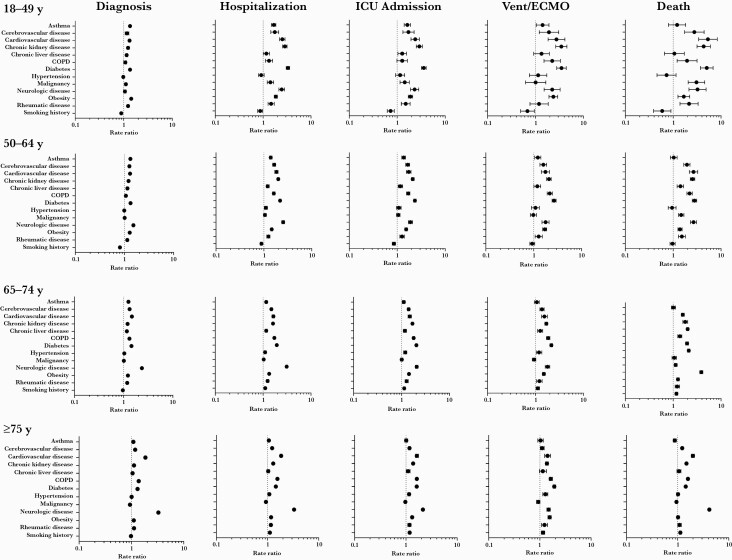
Across all age groups, several underlying medical conditions were associated with an increased risk for COVID-19 diagnosis and clinical outcomes of increasing severity (Figure 1 and Supplementary Table 3).
Figure 1.
Adjusted rate ratios (95% confidence intervals) of underlying medical conditions for coronavirus disease 2019 (COVID-19) diagnosis and COVID-19 disease progression outcomes. Rate ratios are adjusted for all other conditions plus emphysema, human immunodeficiency virus, and alcohol history. Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; Vent/ECMO, intubation and mechanical ventilation or extracorporeal membrane oxygenation.
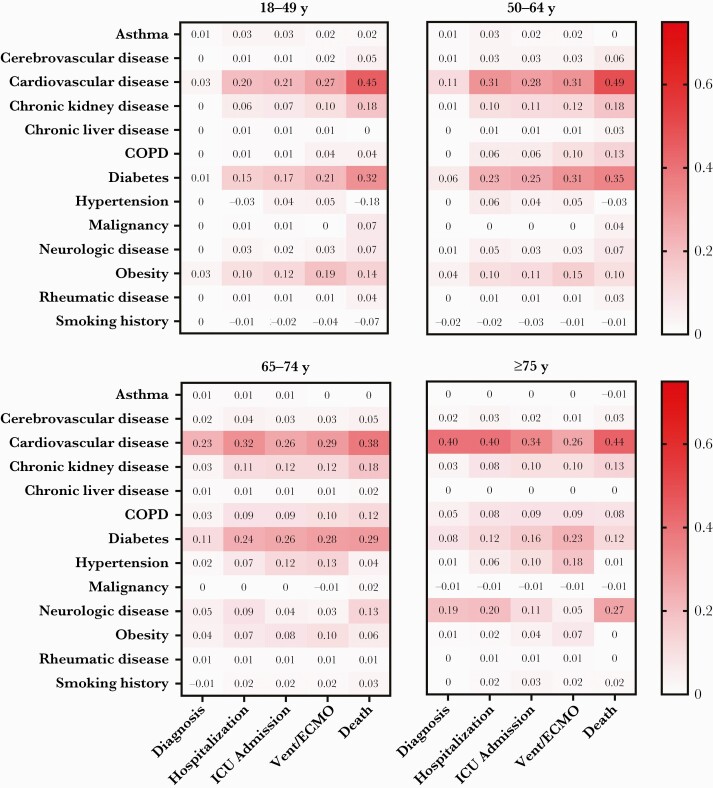
In all but the oldest age group, cardiovascular disease and diabetes had the highest PAFs for COVID-19 diagnosis and all COVID-19 disease progression outcomes (Figure 2). In persons ≥75 years old, neurologic disease had the second-highest PAF after cardiovascular disease for COVID-19 diagnosis and outcomes of hospitalization and death. The PAF for cardiovascular disease generally increased with increasing severity of clinical outcomes for COVID-19. For 18- to 49-year-olds, the PAF for cardiovascular disease was 0.03 for COVID-19 diagnosis and 0.45 for death. However, for persons ≥75 years old, the PAF for cardiovascular disease was similar for COVID-19 diagnosis (0.40) and for COVID-19 disease progression outcomes of increasing severity, including death (0.44). The PAF for diabetes generally increased by increasing severity of COVID-19 outcomes, especially for the 2 younger age groups. For persons 18–49 years and 50–64 years of age, the PAFs for diabetes were 0.01 and 0.06, respectively, for diagnosis, and 0.32 and 0.35, respectively, for death. The PAFs for diagnosis and death for diabetes were 0.11 and 0.29, respectively, for persons aged 65–74 years and 0.08 and 0.12, respectively, for those aged ≥75 years.
Figure 2.
Population attributable fractions of underlying medical conditions for coronavirus disease 2019 (COVID-19) diagnosis and COVID-19 disease progression outcomes by age. Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; Vent/ECMO, intubation and mechanical ventilation or extracorporeal membrane oxygenation.
When stratified by sex, cardiovascular disease had the highest PAF for most COVID-19 disease progression outcomes in all age groups for both sexes (Supplementary Figure 1). Increasing PAFs were generally observed with increasing age for worsening COVID-19 disease progression outcomes overall and for both females and males. Females of all age groups who had cardiovascular disease, diabetes, obesity, and neurologic disease had generally higher PAFs for COVID-19 diagnosis and disease progression outcomes compared with males. The discrepancies between males and females were most apparent in the age group 50–64 years. In this group, PAFs for cardiovascular disease were 0.38 and 0.24 for hospitalization for females and males, respectively; 0.39 and 0.19, respectively, for ICU admission; and 0.64 and 0.35, respectively, for progression to death.
PAFs for COVID-19 disease progression outcomes stratified by race/ethnicity were similar to that seen in the overall cohort, with cardiovascular disease and diabetes having higher PAFs among Black persons and White persons. Among Hispanic patients, hypertension also had high PAFs in addition to cardiovascular disease and diabetes, depending on the age group (Supplementary Figure 2). Across many underlying conditions and progression outcomes, Black race was often associated with slightly higher PAFs compared with Asian or White race or Hispanic ethnicity, particularly among those 18–49 years of age. In 18- to 49-year-olds, the PAF for cardiovascular disease for death was 0.57 for Black race vs 0.32 for White race. In 18- to 49-year-olds, the PAF for diabetes for death was 0.49 for Black race vs 0.28 for White race. PAFs could not be calculated for some Asian and Hispanic age groups for some progression outcomes due to low numbers of patients.
DISCUSSION
In the largest study to date of the population-level contribution of underlying medical conditions to COVID-19 diagnosis and COVID-19 disease progression outcomes in the US, a large proportion of the COVID-19 disease burden was attributed to several underlying medical conditions. To our knowledge, this study is the first to evaluate the population burden of COVID-19 attributable to these underlying conditions.
Overall, cardiovascular disease and diabetes were associated with the highest PAFs in the study, of up to 0.49 and 0.35, respectively, depending on the outcome and age group of interest. Other studies have reported that cardiovascular disease and diabetes are common underlying medical conditions among persons diagnosed with COVID-19 and are associated with increased risk of severe disease and death [2, 5, 6, 9]. Neurologic disease also accounted for a large proportion of the COVID-19 disease burden in persons aged ≥75 years, ranging from 0.05 to 0.27 depending on the outcome, consistent with previous reports that neurologic comorbidity may be an independent predictor of COVID-19 disease severity and death [15, 16].
Older age was associated with higher PAFs in this study, which is consistent with data showing poor outcomes in older patients [5, 6, 17]. PAFs for cardiovascular disease and diabetes were generally higher for female sex compared with male sex, especially among patients aged 50–64 years; the reasons for this are unclear.
Smoking history, adjusting for medical conditions, appears to be protective for COVID-19 outcomes for <65-year-olds in this study, in contrast with results of other studies reporting worse COVID-19 outcomes for smokers [18, 19], although it should be noted that differences in analysis (ie, adjustment for comorbidities, age stratification) may explain the mixed findings. It has not been established whether smokers are more susceptible to SARS-CoV-2 infection; further research is needed on the role of smoking in SARS-CoV-2 infection and COVID-19 outcomes [19] and whether the relationship varies by age. Similarly, low PAFs observed for other conditions, such as hypertension, may be due to adjusting for other conditions.
In the US, cardiovascular disease is more prevalent in Black individuals, while diabetes is more common among both Black and Hispanic compared with White individuals [20]. In patients infected with COVID-19, the risk of hospitalization and mortality is higher in Black and Hispanic patients vs White and non-Hispanic patients, respectively [21–23]. The fractions of COVID-19 disease burden attributable to underlying medical conditions in this study were generally higher in Black persons than in Asian, White, and Hispanic persons for the same conditions, particularly in the 2 younger age groups. Among Black persons and White persons across age groups, cardiovascular disease and diabetes generally accounted for the largest share of the burden, while for Hispanic patients, diabetes accounted for the largest share, followed by cardiovascular disease for older age groups and hypertension for persons aged 50–64 years. For both Black and Hispanic populations, evidence pointing to ongoing racial disparities in receiving adequate access to medical care [24] could mean that these populations are routinely underdiagnosed with underlying medical conditions associated with more severe COVID-19, which may result in biased estimates and/or underestimates of the prevalence and PAF of certain medical conditions for these groups.
More than 10 billion COVID-19 vaccine doses have been administered to date, and real-world evidence indicates that immunization is effective against COVID-19, including asymptomatic infection and severe disease [1, 25–27]. Studies have shown that Black, Hispanic, and Asian individuals are more likely to be vaccine hesitant, and Black individuals are less likely to be vaccinated against COVID-19 compared with White individuals [28, 29]. It is essential, therefore, that racial and ethnic disparities are addressed to minimize unequal vaccine uptake among vulnerable groups [30].
The limitations of retrospective administrative data must be considered when interpreting these results. Administrative databases rely on the accuracy and coding of diagnoses and procedures, which can underestimate disease prevalence for conditions known to be underdiagnosed, such as obesity [31]. In this cohort, 10% of individuals were diagnosed with obesity, which is much lower than the estimated percentage of obesity in US adults of >40% [32]. A limitation of this study therefore is that it is not possible to quantify the proportion of individuals who were obese but not diagnosed with obesity, and how much this affected comorbidities such as diabetes and heart disease. Though the models were adjusted for several underlying medical conditions and stratified by age, sex, and race/ethnicity, confounding such as from other medical conditions and socioeconomic status is possible. Although low socioeconomic status is generally associated with poor COVID-19 outcomes, study results vary [33, 34]. It is not possible to predict how analysis of socioeconomic status would affect the results of this study.
Results from this study are from an insurance database and therefore may only be generalizable to the population with commercial insurance coverage, who may be different from the general population in terms of healthcare access, appropriate use of healthcare services, health outcomes, and other characteristics [35]. The percentage of White individuals in this study (57%) is lower than that estimated by the US Census Bureau (76%), while the percentages of Black and Hispanic individuals in this study (9% and 11%, respectively) were lower than those estimated in the US (13% and 19%) [36]. Notably, 20% of the current cohort studied had unknown race/ethnicity. The prevalence of cardiovascular disease (38%) in our cohort is lower than that estimated among the general US population by the American Heart Association (48%) [37]; the reason for this is not clear. Nevertheless, we found that a large portion of COVID-19 disease burden in our cohort was attributable to cardiovascular disease. The prevalence of diabetes in this study (15%) is higher than that estimated for the total population (10% diagnosed and 13% overall) [38].
The first COVID-19 vaccine was granted emergency use authorization on 11 December 2020 [39]. We do not have data on how many individuals, if any, in the cohort received a COVID-19 vaccine before the data cutoff of 31 December 2020.
CONCLUSIONS
In this large retrospective cohort study, a substantial portion of the population burden of COVID-19 in the United States was attributable to 2 high-prevalence medical conditions, cardiovascular disease and diabetes, both of which may result from obesity [40]. Overall, 20%‒40% of COVID-19 hospitalizations and approximately 40% of COVID-19 deaths among US adults can be attributed to cardiovascular disease. Diabetes accounted for 12%‒24% of COVID-19 hospitalizations among US adults and approximately 30% of COVID-19 deaths among adults aged 18‒74 years. COVID-19 mitigation efforts should continue to promote prevention (ie, vaccination, mask wearing, social distancing, handwashing) as well as close clinical management of patients with certain medical conditions known to increase the risk of severe illness from COVID-19 [17].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Editorial/medical writing support was provided by Sheena Hunt, PhD, and Srividya Ramachandran, PhD, of ICON (Blue Bell, Pennsylvania), and was funded by Pfizer Inc.
Financial support. This work was supported by Pfizer Inc.
Potential conflicts of interest. J. L. N., T. A., M. R., D. M., F. K., D. S., and F. J. A. are or were employees of Pfizer Inc and may hold stock or stock options.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1. World Health Organization. Coronavirus disease (COVID-19) dashboard. https://covid19.who.int/. Accessed 9 February 2022.
- 2. Liang WH, Guan WJ, Li CC, et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): a nationwide analysis of China. Eur Respir J 2020; 55:2000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jordan RE, Adab P, Cheng KK.. COVID-19: risk factors for severe disease and death. BMJ 2020; 368:m1198. [DOI] [PubMed] [Google Scholar]
- 4. Dorjee K, Kim H, Bonomo E, Dolma R.. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One 2020; 15:e0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 2020; 81:e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020; 12:12493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM.. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One 2020; 15:e0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang B, Li R, Lu Z, Huang Y.. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020; 12:6049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang T, Du Z, Zhu F, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 2020; 395:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Frequently asked questions on estimations of attributable burden of disease due to a risk factor. https://www.who.int/news-room/questions-and-answers/item/environmental-health-estimations-of-attributable-burden-of-disease-due-to-a-risk-factor. Accessed 25 March 2022.
- 11. Rockhill B, Newman B, Weinberg C.. Use and misuse of population attributable fractions. Am J Public Health 1998; 88:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mansournia MA, Altman DG.. Population attributable fraction. BMJ 2018; 360:k757. [DOI] [PubMed] [Google Scholar]
- 13. Yih WK, Hua W, Draper C, et al. Master Protocol Development: COVID-19 Natural History. https://www.sentinelinitiative.org/methods-data-tools/methods/master-protocol-development-covid-19-natural-history. Accessed 25 March 2022.
- 14. Centers for Disease Control and Prevention. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf. Accessed 25 March 2022.
- 15. García-Azorín D, Martínez-Pías E, Trigo J, et al. Neurological comorbidity is a predictor of death in COVID-19 disease: a cohort study on 576 patients. Front Neurol 2020; 11:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romagnolo A, Balestrino R, Imbalzano G, et al. Neurological comorbidity and severity of COVID-19. J Neurol 2020; 268:762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. People with certain medical conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed 23 August 2021.
- 18. Reddy RK, Charles WN, Sklavounos A, Dutt A, Seed PT, Khajuria A.. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J Med Virol 2021; 93:1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shastri MD, Shukla SD, Chong WC, et al. Smoking and COVID-19: what we know so far. Respir Med 2021; 176:106237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev 2015; 11:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parpia AS, Martinez I, El-Sayed AM, et al. Racial disparities in COVID-19 mortality across Michigan, United States. EClinicalMedicine 2021; 33:100761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia E, Eckel SP, Chen Z, Li K, Gilliland FD.. COVID-19 mortality in California based on death certificates: disproportionate impacts across racial/ethnic groups and nativity. Ann Epidemiol 2021; 58:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among Black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health 2021; 21:1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Academies of Sciences, Engineering, and Medicine. Health-Care Utilization as a Proxy in Disability Determination. Washington, DC: National Academies Press; 2018. [PubMed] [Google Scholar]
- 25. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397:1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernal JL, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021; 385:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vasileiou E, Simpson CR, Robertson C, et al. Effectiveness of first dose of COVID-19 vaccines against hospital admissions in Scotland: national prospective cohort study of 5.4 million people. SSRN [Preprint]. Posted online 19 February 2021. doi: 10.2139/ssrn.3789264. [DOI] [Google Scholar]
- 28. Willis DE, Andersen JA, Bryant-Moore K, et al. COVID-19 vaccine hesitancy: race/ethnicity, trust, and fear. Clin Transl Sci 2021; 14:2200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen LH, Joshi AD, Drew DA, et al. Racial and ethnic differences in COVID-19 vaccine hesitancy and uptake. medRxiv [Preprint]. Posted online 28 February 2021. doi: 10.1101/2021.02.25.21252402. [DOI] [Google Scholar]
- 30. Burger AE, Reither EN, Mamelund SE, Lim S.. Black-White disparities in 2009 H1N1 vaccination among adults in the United States: a cautionary tale for the COVID-19 pandemic. Vaccine 2021; 39:943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kapoor A, Kim J, Zeng X, Harris ST, Anderson A.. Weighing the odds: assessing underdiagnosis of adult obesity via electronic medical record problem list omissions. Digit Health 2020; 6:2055207620918715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Center for Health Statistics. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. https://www.cdc.gov/nchs/data/databriefs/db360-h.pdf. Accessed 25 March 2022.
- 33. Khanijahani A, Iezadi S, Gholipour K, Azami-Aghdash S, Naghibi DA.. Systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health 2021; 20:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic review and meta-analysis. JAMA Netw Open 2021; 4:e2134147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Institute of Medicine Committee on the Consequences of Uninsurance. Care Without Coverage: Too Little, Too Late. Chapter 3. Effects of Health Insurance on Health. Washington, DC: National Academies Press;2002. [Google Scholar]
- 36. United States Census Bureau. QuickFacts. 2021. https://www.census.gov/quickfacts/fact/table/US/PST045221. Accessed 25 March 2022.
- 37. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020; 141:e139–596. [DOI] [PubMed] [Google Scholar]
- 38. National Diabetes Statistics Report. Estimates of diabetes and its burden in the United States. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 25 March 2022.
- 39. US Food and Drug Administration. FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine. 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19. Accessed 25 March 2022.
- 40. Scherer PE, Hill JA.. Obesity, diabetes, and cardiovascular diseases: a compendium. Circ Res 2016; 118:1703–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.