Abstract
Background
Humoral immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may wane rapidly in persons recovered from mild coronavirus disease 2019 (COVID-19), but little is known about the longevity.
Methods
Serum samples were obtained 8, 12, and 18 months after infection from 20 patients with mild COVID-19. The binding activities of serum antibodies (IgA, IgG, and IgM) against SARS-CoV-2 antigens of the Wuhan-1 reference strain (wild-type) and the B.1.1.7, P.1, B.1.167.2, and B.1.1.529 variants were measured by enzyme-linked immunosorbent assays. Neutralizing antibody titers were measured using a cytopathic effect-based live virus neutralization assay.
Results
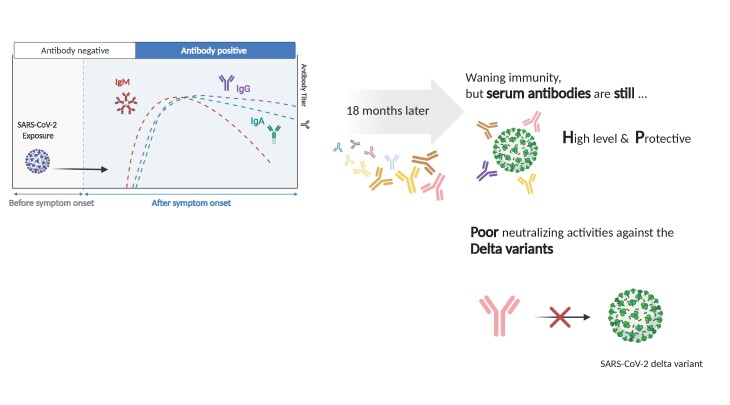
Serum IgA and IgG antibodies against spike or receptor-binding domain (RBD) protein of wild-type SARS-CoV-2 were detected for up to 18 months, and neutralizing antibodies persisted for 8 to 18 months after infection. However, any significant antibody responses against RBD proteins of SARS-CoV-2 variants were not observed, and median neutralizing antibody titers against the Delta variant at 8, 12, and 18 months were 8–11 fold lower than against wild-type viruses (P < .001).
Conclusions
Humoral immunity persisted for up to 18 months after SARS-CoV-2 infection in patients with mild COVID-19. Humoral immune activity against more recently circulating variants, however, was reduced in this population.
Keywords: SARS-CoV-2, COVID-19, neutralizing antibodies, ELISA, serological response
Graphical Abstract
Graphical Abstract.
Contributor Information
Pyoeng Gyun Choe, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea.
Jisu Hong, Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Republic of Korea; Department of Pharmacology, Seoul National University College of Medicine, Seoul, Republic of Korea.
Jiyoung Park, Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Republic of Korea; Department of Pharmacology, Seoul National University College of Medicine, Seoul, Republic of Korea.
Euijin Chang, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea.
Chang Kyung Kang, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea.
Nam Joong Kim, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea.
Chang-Han Lee, Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Republic of Korea; Department of Pharmacology, Seoul National University College of Medicine, Seoul, Republic of Korea; BK21 FOUR Biomedical Science Project, Seoul National University College of Medicine, Seoul, Republic of Korea; Wide River Institute of Immunology, Seoul National University, Hongcheon, Republic of Korea.
Wan Beom Park, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea.
Myoung-don Oh, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea.
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.