Abstract
We found no significant difference in cycle threshold values between vaccinated and unvaccinated persons infected with severe acute respiratory syndrome coronavirus 2 Delta, overall or stratified by symptoms. Given the substantial proportion of asymptomatic vaccine breakthrough cases with high viral levels, interventions, including masking and testing, should be considered in settings with elevated coronavirus disease 2019 transmission.
Keywords: asymptomatic testing, COVID, 19, Ct value, SARS-CoV-2, Delta variant
Vaccines reduce infection, severe disease, and death from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Yet breakthrough cases occur, and this risk increases over time [2]. Reports predominantly from non-US settings suggest that viral loads from nasal swabs are similar among unvaccinated and vaccinated individuals; other reports suggest that virus levels are lower in unvaccinated persons [3–6]. Here, we report on cycle threshold (Ct) values among 869 fully vaccinated and unvaccinated individuals, asymptomatic and symptomatic at the time of testing, during a period of high transmission of the Delta variant in 2 distinct populations: Unidos en Salud (UeS) community-based testing in the Mission District of San Francisco and Healthy Yolo Together (HYT) asymptomatic testing through the University of California (UC), Davis.
METHODS
Study Populations
Data were collected on individuals who voluntarily sought testing for SARS-CoV-2 from 2 demographically distinct populations in California during a 2-month period (June 17–August 31, 2021), during which Delta was the predominant variant.
HYT
As part of the response to coronavirus disease 2019 (COVID-19), UC Davis deployed an extensive free asymptomatic testing program that included the City of Davis and Yolo County (Healthy Yolo Together). Asymptomatic individuals over the age of 2 were eligible for testing. Asymptomatic cases were classified as individuals not reporting symptoms for at least 24 hours before testing. Under supervision, samples were collected by individuals transferring their saliva into barcoded tubes (COVID-19 Testing | Campus Ready). Smaller numbers of symptomatic individuals were processed using a different workflow and an antigen test; therefore, they were not included in this study.
UeS
The study population included individuals who sought SARS-CoV-2 testing at the UeS walk-up site, an ongoing academic (UC San Francisco, CZ Biohub, and UC Berkeley), community organization (Latino Task Force), and government (SFDPH) partnership. The outdoor, free BinaxNOW testing site was located at a public transport and commercial hub in the Mission District, a setting of ongoing transmission in San Francisco [7, 8]. Individuals 1 year of age and older, with or without symptoms, were eligible for testing. Anterior-nasal swab samples (iClean, Chenyang Global) were collected by certified lab technicians.
Measurements
Infections were classified as breakthrough infections if the individual was fully vaccinated (2 weeks following receipt of all vaccine doses). Individuals who had had only 1 dose or were tested within 2 weeks of the second dose, in the case of the Pfizer and Moderna vaccines, were not included in the analysis.
HYT
Demographic information was collected from individuals at the time of registration. Vaccination status was obtained at the time of contact tracing and confirmed in the California Vaccine Registry. Only confirmed, fully vaccinated individuals were used in the analysis; discordant samples, self-reported as vaccinated but unconfirmed, were treated as status unknown. Saliva samples from asymptomatic individuals were tested for the presence of the N1 and N2 regions of the viral nucleocapsid (N) gene using primers and probes described in the CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel using IntelliQube high-throughput quantitative polymerase chain reaction (PCR) instruments (LGC Biosearch Technologies). Ct values were calculated with FastFinder software (UgenTec | FastFinder). Samples were routinely run multiple times for quality control; replicates had very similar Ct values.
Genotypes of all N1/N2-positive samples were determined using reverse transcription PCR (RT-PCR) single-nucleotide polymorphism analysis at 11 loci diagnostic for variants of concern (SARS-CoV-2 Variant ValuPanel assays | LGC Biosearch Technologies). A subset of samples (39%) were also sequenced using the Illumina MiSeq sequencing platform. Consensus genomes were generated with Viralrecon2 and variants called in Pangolin, version 3.1.11, and PLEARN, version 1.2.66. Sequencing confirmed the variants called by genotyping.
UeS
Individuals provided demographic data and information on symptoms immediately before testing using BinaxNOW kits. COVID-19 vaccine status, including date of final shot, was obtained through the California Vaccine Registry. Swabs from BinaxNOW-positive individuals were placed in DNA/RNA Shield (Zymo, Inc.) and processed for quantitative RT-PCR (qRT-PCR), genome recovery, and variant/lineage determination, as previously described [8, 9]. Ct values for the detection of the N and E genes [9] were determined via the single threshold Cq determination mode using Bio-Rad CFX Maestro, version 4.1 (Bio-Rad Inc). SARS-CoV-2 genomes were sequenced using the Illumina NovaSeq platform. Consensus genomes were generated via the COVID module of the IDseq pipeline (https://idseq.net), as previously described [9].
Analysis
Ct values were plotted, stratified by site, fully vaccinated vs not vaccinated, and symptom status. For persons in the UES with symptoms, Ct values were plotted by time from symptom onset. Ct values were compared by vaccine status, overall, and stratified by symptom status using a 2-sided t test.
Patient Consent
All participants provided informed consent for testing.
HYT
The Genome Center laboratory that conducted COVID-19 testing was Clinical Laboratory Improvement Amendments approved as an extension to the Student Health Center’s laboratory. The UC Davis Institutional Review Board (IRB) Administration determined that the study met criteria for public health reporting and was exempt from IRB review and approval.
UeS
The UC San Francisco Committee on Human Research determined that the study met criteria for public health surveillance.
RESULTS
A total of 869 samples, 500 from HYT and 369 from UeS, were included in the analysis. All analyzed samples from HYT were asymptomatic at the time of collection, and 75% of the positive samples were from unvaccinated individuals (n = 375). Positive samples from UeS were from both symptomatic (n = 237) and asymptomatic individuals (n = 132). The frequency of vaccine breakthroughs among the UeS samples (171 fully vaccinated, 198 unvaccinated) was greater than among the HYT samples, reflecting the different populations sampled. The Delta variant was the predominant variant detected in both populations (95.1% and 96.4% of identified variants for HYT and UeS, respectively) (Supplementary Table 1).
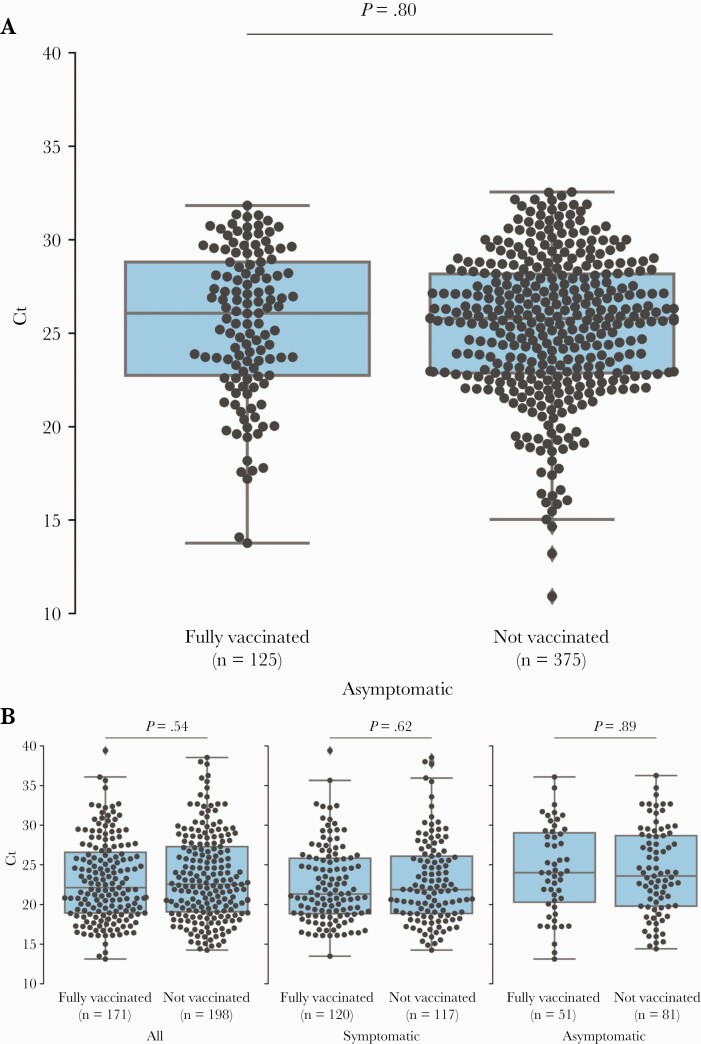
There were no statistically significant differences in mean Ct values of vaccinated vs unvaccinated samples in either HYT (vaccinated 25.5 vs unvaccinated 25.4; P = .80) (Figure 1A) or UeS (vaccinated 23.1, unvaccinated 23.4; P = .54) (Figure 1B). In UeS, this remained true after stratifying by symptoms at the time of testing (Supplementary Figure 1). In both vaccinated and unvaccinated, there was great variation among individuals, with Ct values of <15 to >30 in both UeS and HYT data (Figure 1A, B). Mean Ct values of asymptomatic samples were higher (UeS 24.3) than those of symptomatic samples (UeS 22.7; P = .006). Among symptomatic persons in the UeS study, mean Ct values increased over time since symptom onset (Supplementary Figure 1).
Figure 1.
SARS-CoV-2 cycle threshold values in asymptomatic, symptomatic, vaccinated, and unvaccinated individuals in California. SARS-CoV-2 reverse transcription polymerase chain reaction cycle threshold values for specimens from patients by vaccine status from Healthy Yolo Together (City of Davis and Yolo County, CA, USA) (A) and from specimens by vaccine and symptom status from Unidos en Salud (Mission District, San Francisco, CA, USA) (B). Box plots show first quartile, median, and third quartiles in the shaded region; diamonds indicate outliers beyond 1.5 times the interquartile range; P values were calculated with 2-sided t tests. Abbreviations: Ct, cycle threshold; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In all groups, there were individuals with low Ct values indicative of high viral loads. A total of 69 fully vaccinated individuals had Ct values <20. Of these, 24 were asymptomatic at the time of testing. Ct values in some children under 12, who were not yet eligible for vaccination, were also low. Twenty out of 109 (18.3%) children under 12 years of age had Ct values <20, of whom 14 were asymptomatic at the time of testing.
DISCUSSION
In our study, mean viral loads as measured by Ct value were similar for large numbers of vaccinated and unvaccinated individuals infected with SARS-CoV-2 during the Delta variant surge, regardless of symptom status, at two distinct California testing sites. These results are in contrast to a large ongoing UK community cohort, in which the median Ct value was higher for vaccinated individuals (27.6) than for unvaccinated individuals (23.1) [3], but are consistent with multiple other reports from the United Kingdom, Singapore, and the United States [4–6, 10, 11]. Our study contributes data from a large number of persons, including children, who were asymptomatic at the time of testing to inform this topic; most importantly, we demonstrate that 20% of positive, vaccinated individuals had low Ct values (<20), a third of whom were asymptomatic when tested.
Given that low Ct values are indicative of high levels of virus, culture positivity (including among vaccine breakthrough infections), and increased transmission [6, 12], our detection of low Ct values in asymptomatic, fully vaccinated individuals is consistent with the potential for, although it does not prove, transmission from breakthrough infections before any emergence of symptoms. Two recent studies document that vaccinated individuals can transmit infection to vaccinated or unvaccinated persons even though they may show faster decay of viral loads and remain infectious for shorter periods of time than unvaccinated individuals [5, 12]. These viral dynamics may explain epidemiologic studies showing reduced transmission from vaccinated individuals [11, 13]. One important unanswered question is what proportion of infections are due to transmission from asymptomatic vaccine breakthroughs.
A limitation of our study is the lack of longitudinal data or information on the time of exposure for each person because these data were gathered as part of public health screening programs rather than in a clinical setting. Also, we did not collect data on immunodeficiency status. Vaccinated asymptomatic persons may have been tested either earlier or later in the course of infection than unvaccinated persons, complicating comparisons of Ct values between these groups. It is also possible that some persons who were asymptomatic at the time of testing later developed symptoms. However, our analysis shows that a vaccinated person may harbor high levels of virus during a period in which they are asymptomatic or presymptomatic.
The data gathered in this study during the surge of the Delta variant strongly support the notion that neither vaccine status nor the presence or absence of symptoms should, by itself, influence the recommendation and implementation of public health practices including mask wearing, testing, social distancing, and other measures designed to mitigate the spread of SARS-CoV-2 during periods of high transmission.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Many people were responsible for collecting the samples, running the tests, performing the genotyping and sequencing, and processing the data, as listed in Supplementary Table 2.
Financial support. This work was supported by the Chan Zuckerberg Biohub, Healthy Yolo Together, the University of California, San Francisco, the Chan Zuckerberg Initiative, and the University of California, Davis.
Potential conflicts of interest. Dr. DeRisi reports being a scientific advisor to the Public Health Co. and a scientific advisor to Allen & Co. Dr. Havlir reports nonfinancial support from Abbott outside of the submitted work. The other authors declare no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. J.D., R.W.M., D.H., and M.P. conceived the project. D.C., C.M., S.R., D.H., S.B., and G.P. helped collect the data. C.A., A.M., C.Y.W., and J.L. helped perform the tests, genotyping, and sequencing. C.A., J.H., L.S., J.D., A.M., C.Y.W., J.S., and J.L. prepared the data for publication. R.M., E.G., D.H., M.P., D.C., J.S., and J.D. contributed to the writing of the manuscript. All authors read and approved the final manuscript.
References
- 1. Abu-Raddad LJ, Chemaitelly H, Butt AA; National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021; 385:187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tartoff SY, Slezak Jeff M, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancent 2021; 398:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elliott P, Haw D, Wang H, et al. Exponential growth, high prevalence of SARS-CoV-2, and vaccine effectiveness associated with the Delta variant. Science 2021; 374(6574). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pouwels KB, Pritchard E, Matthews PC, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med 2021; 27:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chia PY, Ong SWX, Chiew CJ, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: a multicentre cohort study. Clin Microbiol Infect. 2021; 28(4). doi: 10.1016/j.cmi.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riemersma KK, Grogan BE, Kita-Yarbro A, et al. Shedding of infectious SARS-CoV-2 despite vaccination. medRxiv 2021.07.31.21261387 [Preprint]. 6 November 2021. https://www.medrxiv.org/content/10.1101/2021.07.31.21261387v4. Accessed 24 August 2021.
- 7. Pilarowski G, Lebel P, Sunshine S, et al. Performance characteristics of a rapid severe acute respiratory syndrome coronavirus 2 antigen detection assay at a public plaza testing site in San Francisco. J Infect Dis 2021; 223:1139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peng J, Liu J, Mann SA, et al. Estimation of secondary household attack rates for emergent spike L452R severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants detected by genomic surveillance at a community-based testing site in San Francisco. Clin Infect Dis. 2022; 74(1):32–39. doi: 10.1093/cid/ciab283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crawford E, Acosta I, Ahyong V, et al. Rapid deployment of SARS-CoV-2 testing: the CLIAHUB. PLoS Pathog 2020; 16:e1008966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings-Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1059–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singanyagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020; 25:2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jefferson T, Spencer EA, Brassey J, Heneghan C.. Viral cultures for COVID-19 infectious potential assessment – a systematic review. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eyre DW, Taylor D, Purver M, et al. Effect of Covid-19 vaccination on transmission of alpha and delta variants. N Engl J Med 2022; 386:744–756. doi: 10.1056/NEJMoa2116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.