Abstract
Current bone defect treatment strategies are associated with several risks and have major limitations. Therefore, it is necessary to develop an inexpensive growth factor delivery system that can be easily produced in large quantities and can promote long-term bone regeneration. An osteogenic growth peptide (OGP) is a 14 amino acid peptide with a short peptide sequence active fragment. In this study, we developed two OGP-based self-assembling supramolecular hydrogels (F- and G-sequence hydrogels) and investigated the in vitro and in vivo effects on proliferation and osteogenesis, including the mechanism of hydrogel-mediated bone defect repair. The hydrogels presented excellent biocompatibility and cell proliferation-promoting properties (1.5–1.7-fold increase). The hydrogels could effectively upregulate the expression of osteogenic factors, including RUNX2, BMP2, OCN, and OPN, to promote osteogenesis differentiation. Interestingly, 353 differentially expressed genes were identified in hBMSCs treated with hydrogels. The hydrogels were proved to be involved in the inflammatory pathways and folate-related pathways to mediate the osteogenesis differentiation. Furthermore, the therapeutic efficiency (bone volume/total volume, trabecular number, and bone mineral density) of hydrogels on bone regeneration in vivo was evaluated. The results showed that the hydrogels promoted bone formation in the early stage of bone defect healing. Taken together, this study was the first to develop and evaluate the properties of OGP-based self-assembling supramolecular hydrogels. Our study will provide inspiration for the development of delivering OGP for bone regeneration.
Introduction
Bone defects are usually caused by trauma, surgery (for tumor removal, reconstruction, or other indications), congenital malformations, or infections. Despite the ability of bones to self-repair, pathological fractures and massive bone defects are associated with repair failure.1 For example, in the United States, approximately 6 million fractures occur each year, and almost 300,000–600,000 fractures exhibit delayed healing or nonhealing, which imposes a heavy burden on the public healthcare system.2 Autografts and allografts are considered the gold standard treatments for bone defects.3 However, repair of bone defects is associated with risks of infection, long-term pain, immunogenicity, donor site morbidity, and weak osteoinductive effects, as well as increased operative duration and cost.4
Biomaterial-based tissue engineering has recently emerged as a promising treatment option for bone defects. A hydrogel, a three-dimensional polymeric network material with a high water content, is a potential biomaterial used in regenerative medicine that has excellent biocompatibility and degradation properties, as well as controllable mechanical properties.5 Recently, supramolecular hydrogels have gained remarkable interest in the bone tissue engineering field due to their unique properties. The injectable supramolecular hydrogels could facilitate cell adhesion and proliferation, with the resemble structure and function of an extracellular matrix and good compatibility.6 By means of supramolecular self-assembly technology, the active hydrogels obtained by controllable folding of active fragments not only have biological activity comparable to that of proteins but also can effectively prolong the action time by resisting protease hydrolysis.7 Mesenchymal stem cells (MSC) are multipotent stromal cells that can undergo self-renewal and multilineage differentiation,8 which are considered as one of the most suitable stem cell populations for bone regeneration.9 The osteogenic differentiation and mineralization of MSCs may be regulated by an endogenous peptide, the osteogenic growth peptide (OGP).10−12 OGP is a 14 amino acid peptide with the active fragment OGP10-14 (Tyr–Gly–Phe–Gly–Gly, YGFGG), which increases bone mass, promotes fracture healing, and stimulates the formation of blood and bone marrow cells.13 Recent studies have demonstrated the role of OGP in promoting bone regeneration with direct injection or incorporation with other biomaterials such as PLGA scaffolds.14,15 Our study is the first to develop the OGP-based self-assemble hydrogels, which improve the limitation including excessively rapid degradation and poor bioavailability of OGP.
In this study, we aim to design two self-assembling hydrogels (F- and G-sequence hydrogels), consisting of OGP active fragments, to enhance bone regeneration. We illustrated the potential of these hydrogels to promote osteogenesis differentiation in vitro and bone regeneration in vivo. The designed peptides could self-assemble into stable hydrogels after heating and cooling. The micromorphology, chemical structure, pro-proliferation, and osteogenesis capacity of the hydrogels were systemically evaluated. In addition, we applied the RNA-sequencing analysis to further explore the molecular mechanism of the osteogenesis effect of OGP-based hydrogels. These injectable hydrogels showed stable physical and chemical properties, excellent biocompatibility, and osteogenesis-promoting capacity. The hydrogels were involved in the inflammatory-related pathway to mediate the osteogenic process. Moreover, microcomputed tomography (micro-CT) and quantitative analysis were assessed in vivo to investigate the therapeutic effect of the hydrogels in the bone defect model (Figure 1).
Figure 1.
Schematic depiction of the mechanisms of OGP-based self-assembly supramolecular hydrogels on promoting bone regeneration.
Experimental Section
Materials
Fmoc-amino acids and O-benzotriazole-N,N,N′,N′-tetramethyl-uronium-hexafluorophosphate (HBTU) were obtained from GL Biochem (Shanghai) Ltd. (Shanghai, China). Diisopropylethylamine (DIEA), N,N′-dimethyl formamide (DMF), piperidine, absolute ethyl ether, and biotin were purchased from Alfa Aesar(Shenzhen, China). Carbinol and trifluoroacetic acid were purchased from J&K Scientific (Beijing, China). Dichloromethane and dimethyl sulfoxide (DMSO) were purchased from Kaitong (Tianjin KaitongCo., Ltd., Tianjin, China); 2-chlorotrityl resin was purchased from Hecheng (Tianjin Nankai Hecheng S&T Co., Ltd., Tianjin, China). Sodium carbonate was purchased from Yuanli Chemical Group Co., Ltd. (Tianjin, China). The Cell Counting Kit-8 (CCK-8) and the BCA protein assay kit were purchased from Beyotime Biotechnology (Shanghai, China). Primary antibodies against RUNX2, OPN, BMP2, and GAPDH, as well as UNlQ-10 column TRIzol total RNA isolation kit, were purchased from BBI Life Sciences Corporation (Shanghai, China).
Preparation and Characterization of Hydrogels
Peptides were prepared with the standard solid-phase peptide synthesis protocol, which used 2-chlorotrityl chloride resin and the corresponding N-Fmoc-protected amino acids. First, the C-terminal of the first amino acid was conjugated to the resin. Anhydrous DMF containing 20% piperidine was used to remove the Fmoc-protected group. Next, the next amino acid was coupled to the free amino group using the coupling reagent HBTU. DIEPA was used as a catalytic reagent. Biotin was used for capping in the final step. Finally, 95% trifluoroacetic acid (TFA) containing 2.5% H2O and 2.5% TIS was used to cleave the peptide derivatives from the resin, and the mixture was then filtered. TFA was removed by vacuum evaporation. Anhydrous ether was added to the concentrated liquid to create a crude peptide gel precursor product. After letting the product rest, the anhydrous ether was discarded and the crude peptide product was dried using a vacuum. High-performance liquid chromatography (HPLC) was used to purify the peptides, and the products were obtained by lyophilization. Nuclear magnetic resonance spectroscopy (1H NMR; ARX-400 MHz spectrometer; Bruker BioSpin AG, Fallanden, Switzerland) was applied to evaluate the chemical structure. The peptide powder was dissolved in phosphate-buffered saline (PBS, pH = 7.4) and pH was adjusted to 7.4 with Na2CO3 to prepare the peptide solutions. After the solutions had been boiled, self-assembly proceeded during cooling to room temperature.
Transmission Electron Microscopy (TEM)
The nanostructures were characterized using a transmission electron microscope (Tecnai G20 F20; FEI Company, Hillsboro, OR) operating at 200 kV. The samples were prepared as follows: 10 μL of both F-sequence and G-sequence hydrogels were dropped onto a carbon-coated copper grid and allowed to rest for 1 min. The excess hydrogel was absorbed by a filter paper. The sample plate was stained with a saturated uranyl acetate solution and left to dry for observation.
Cell Proliferation Assay
The hBMSCs were seeded onto a 96-well plate at a density of 2000 cells/well and cultured for 6 h in the presence of diluted F-sequence (0, 1, 10, 100, or 500 nM) and G-sequence (0, 1, 10, 100, or 500 nM) hydrogels in an α-MEM medium. The number of cells was quantified using the CCK-8 kit at 24, 48, and 72 h.
Western Blotting
The hBMSCs were cultured for 7 days with diluted F-sequence (0, 1, 10, or 100 nM) and G-sequence (0, 1, 10, or 100 nM) hydrogels in an α-MEM medium, followed by protein extraction and measurement according to the manufacturer’s instructions. Briefly, the total protein from hBMSCs was extracted using radioimmunoprecipitation assay lysis buffer (Beyotime Biotechnology). Protein concentrations were measured using a BCA protein kit (Beyotime Biotechnology). Western blotting analysis was performed using standard methods. Proteins were separated by electrophoresis using 5–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to a poly(vinylidene fluoride) (PVDF) membrane (Millipore, Billerica, MA). The PVDF membrane was blocked by soaking it in 5% skimmed milk and incubated overnight at 4 °C in the primary antibodies diluted with the blocking solution. Monoclonal rabbit anti-GAPDH antibody was used as a control. The PVDF membranes were washed three times with Tris-buffered saline and incubated with horseradish peroxidase-conjugated secondary antibody at room temperature. The enhanced chemiluminescence solution was mixed with the stable peroxidase solution (ratio of 1:1), followed by dropping onto the PVDF membrane. After allowing the reaction to proceed for several minutes, the film was developed, fixed, and printed.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
After the hBMSCs were cultured for 72 h in diluted YGFGG (0, 1, 10, or 100 nM) and YGGGF (0, 1, 10, or 100 nM) hydrogels in an α-MEM medium, total RNA was extracted from the cells using the UNlQ-10 Column TRIzol total RNA isolation kit according to the manufacturer’s instructions. The RNA samples were stored in a refrigerator at −70 °C. The sequences of the primers are listed in Table 1. After reverse transcription, amplification, and fluorescence reaction, the obtained ΔCt values were compared to those of GAPDH.
Table 1. Quantitative Reverse Transcription Polymerase Chain Reaction Sequences.
| gene | sequence |
|---|---|
| H-GAPDH-F | 5′TGGGTGTGAACCATGAGAAGT 3′ |
| H-GAPDH-R | 5′TGAGTCCTTCCACGATACCAA 3′ |
| H-RUNX2-F | 5′ CCCCGTCCATCCACTCTAC 3′ |
| H-RUNX2-R | 5′ATGAAATGCTTGGGAACTGC 3′ |
| H-OCN-F | 5′ GAGGGCAGCGAGGTAGTGA 3′ |
| H-OCN-R | 5′ TCCTGAAAGCCGATGTGGT 3′ |
| H-OPN-F | 5′AGGACTCCATTGACTCGAACG 3′ |
| H-OPN-R | 5′ TGGATGTCAGGTCTGCGAAA 3′ |
| H-BMP2-F | 5′GTCACAGATAAGGCCATTGCTA 3′ |
| H-BMP2-R | 5′ GTGGCAGTAAAAGGCGTGAT 3′ |
RNA-Sequencing Analysis
When the concentration of hBMSCs reached 50–60%, various concentrations of supramolecular hydrogels (F- [0, 1, 10, or 100 nM] or G- [0, 1, 10, or 100 nM] sequence) and a blank medium were added for comparison. After 72 h, the cells were washed with Hanks’ buffer, digested, beaten, and centrifuged at 1000 rpm for 5 min to obtain the cell precipitates. The Trizol reagent was added to create a cell suspension, which was sent to BGI Genomics (Shenzhen, China). The mRNA was screened using Oligo magnetic beads. The samples were sequenced using the BigSeq-500 platform, and an enrichment analysis was performed to determine the biological functions of differentially expressed genes. Gene ontology (GO) fictional enrichment analysis was used to calculate p-values with the following formula
where N is the number of genes with GO annotations, n is the number of candidate genes in N, M is the number of genes annotated with a specific GO term, and m is the number of differentially expressed genes (DEGs) annotated with a specific GO term.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) is the main public database of signaling pathways. We used hypergeometric tests to identify signaling pathways that were significantly enriched in candidate genes relative to the overall genomic background using the following formula
where N is the number of genes with KEGG-annotated genes, n is the number of candidate genes in N, M is the number of genes annotated with a specific pathway, and m is the number of candidate genes annotated with a specific signaling pathway.
Animal Models
All animal experiments were approved by the Animal Ethics Committee of Tianjin Hospital, China, and followed the guidelines of the Tianjin Committee of Use and Care of Laboratory Animals. Male Sprague-Dawley rats (330–350 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The in vivo experiments were grouped into three cages for the blank control group (taken at 2, 4, and 8 weeks), three cages for the F-sequence hydrogel group (taken at 2, 4, and 8 weeks), and three cages for the G-sequence hydrogel group (taken at 2, 4, and 8 weeks), with six rats per cage. Briefly, the rats were anesthetized by intraperitoneal injection of 10% chloral hydrate. Hair overlying the right dorsal knee was shaved up to the hip joint, the limbs were fixed in the prone position, and the operation site was disinfected with iodophor. Surgical scissors were then used to make a 1.5 cm vertical incision above the knee joint of each rat; the subcutaneous fascia and muscle were separated, cortical bone was exposed, and a 1 mm full-thickness bone defect was drilled. The muscle and skin were sutured, followed by the rearing of the rats. Starting at 3 days after the operation, 250,000 units of penicillin were intramuscularly injected daily into each animal. Similar to previous studies,16 rats with femur defects were randomly assigned to receive intramuscular injections of saline (100 μL; n = 18), F-sequence hydrogel (1 μM, 100 μL; n = 18), or G-sequence hydrogel (1 μM, 100 μL; n = 18) on alternate days. Right femur samples were obtained at 2, 4, and 8 weeks after surgery for micro-CT and histology analysis.
Statistical Analysis
SPSS software (version 19.0; IBM Corp., Armonk, NY) was used for the statistical analysis. All data are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to analyze the optical density values obtained from the cell proliferation assay, relative gray values obtained from western blotting, relative expression levels obtained from reverse transcription polymerase chain reaction, and GO and KEGG enrichment analysis results. p-values <0.05 were considered statistically significant
Results and Discussion
Characterization of Peptides and Hydrogels
The chemical structure of the F-sequence peptide and the G-sequence peptide are illustrated in Figure 2A,B. The 1H NMR results (Figure 2C,D) for the two gelator compounds were consistent with the designed molecular structures, indicating that a homogeneous gelator compound could be synthesized. Therefore, the preparation reaction was relatively stable, and the molecular structures of the obtained compounds were uniform. The self-assembled peptides were synthesized via standard Fmoc solid-phase peptide synthesis and formed the stable transparent hydrogels, F-sequence and G-sequence hydrogels, through the heating–cooling process (Figure 2E,F). Transmission electron microscopy (TEM) images revealed a three-dimensional network of nanofibers for both hydrogels. The nanofibers in the F-group (diameter: 30–70 nm) were denser compared to those in the G-group (Figure 2E). The nanofibers in the G-group were sparser with about an 80–120 nm diameter (Figure 2F). Subsequently, rheological experiments were carried out to test the mechanical properties of hydrogels. The elastic modulus (G′) of the two hydrogels always occupies a dominant position compared with the viscous modulus (G″), indicating that they have good viscoelastic properties and are promising biological scaffold materials (Figure 2G,H). Currently, OGP is either injected or applied to the surface of the biological scaffold for bone repair. The need for frequent OGP administration (due to rapid degradation) is a vital disadvantage of in vivo injections.15 Incorporating peptides into biomaterial scaffolds could prolong their action and enhance osteogenesis but the poor bioavailability of the incorporated peptides needs to be addressed.17 Better methods of OGP delivery are needed to enhance the osteogenic effects of biomaterials. Our study introduced a kind of supramolecular hydrogel consisting of the pentapeptide YGFGG from the OPG, which effectively improved the therapeutic effect of bone defect repair.
Figure 2.
Characteristics of self-assembled hydrogels. (A) Chemical structure of the F-sequence self-assembling peptide, (B) chemical structure of the G-sequence self-assembling peptide, (C) H-nuclear magnetic resonance (1H NMR) spectrum of the F-sequence self-assembling peptide, (D) 1H NMR spectrum of the G-sequence self-assembling peptide, (E) optical and TEM images of the F-sequence hydrogel, (F) optical and TEM images of the G-sequence hydrogel, (G) dynamic frequency sweep of the F hydrogel, and (H) dynamic frequency sweep of the G hydrogel.
Proliferation Assessment of F- and G-Sequence Hydrogels
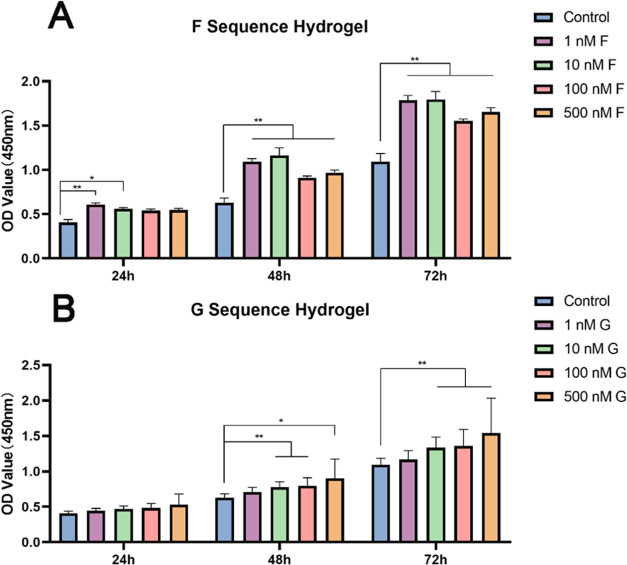
Cell proliferation is the first stage of bone regeneration. During this stage, hBMSCs could proliferate while maintaining pluripotency.18 Biomaterials and their degradation products should be biocompatible and safe to ensure the proliferation of seed cells and bone formation. To assess the cytotoxicity of hydrogels, we used a CCK-8 assay to evaluate the proliferation of hBMSCs (Figure 3). The number and viability of hBMSCs increased in both hydrogel groups over time, indicating nontoxicity and biocompatibility of the hydrogels. Compared to the control group, all concentrations of the F-sequence hydrogel significantly enhanced cell proliferation at 48 and 72 h (all p < 0.01) (Figure 3A). In particular, the cell numbers in the 10 nM F groups were as twice as the control group at 48 h; 1 and 10 nM F groups promoted cell proliferation by 1.7 times at 72 h. The pro-proliferative effect of low-concentration F-sequence hydrogels on hBMSCs was more substantial, indicating that hBMSCs have a higher sensitivity to OGP. For the G-sequence hydrogel, higher concentrations promoted cell proliferation more effectively. At 72 h, the number in the 100 nM G-group increased 1.5-fold compared to the control group (Figure 3B). Overall, the proliferation assessment indicates that F- and G-sequence hydrogels present excellent biocompatibility.
Figure 3.
F- and G-sequence hydrogel cytotoxicity and proliferation assessment. (A) Cell Counting Kit-8 (CCK-8) data for the F hydrogel and (B) CCK-8 data for the G hydrogel; *p < 0.05 and **p < 0.01.
Osteogenic Differentiation of F- and G-Sequence Hydrogels on hBMSCs
Several osteogenic-related factors, including runt-related transcription factor 2 (Runx2), bone morphogenetic protein 2 (BMP2), osteocalcin (OCN), and osteopontin (OPN), were applied to illustrate the effects of different concentrations of F- and G-sequence hydrogels on the osteogenic differentiation of hBMSCs. Western blotting results clearly demonstrated that RUNX2, BMP2, OCN, and OPN bands were obvious in both hydrogels at low concentrations (1 and 10 nM). In contrast, the expression of these proteins was appropriately reduced at higher concentrations (100 nM). This accords with the concentration-dependent principle that growth factors generally follow, that is, low concentration promotes and high concentration inhibits. In general, the F-sequence hydrogel promoted the expression of RUNX2, OPN, and OCN more strongly than the G-sequence hydrogel at the same concentration, while BMP2 protein expression was weaker (Figure 4A). It should be noted that OPN protein showed the most obvious band in the 1 nM F-sequence hydrogel, while BMP2 protein showed the strongest expression in the 10 nM G-sequence hydrogel, suggesting that both hydrogels can promote osteogenic differentiation but the emphasis is slightly different. To this end, we further detected mRNA expressions of RUNX2, BMP2, OPN, and OCN in hBMSCs for analysis. The results of qRT-PCR were basically consistent with western blotting (Figure 4B), except for differences in the expression levels of RUNX2 and OCN, which might be related to the instability of mRNA variation. Generally speaking, scholars believe that the protein expression level is more directly related to ″phenotype″.19 Based on the results of the obvious osteogenic effects of 1 and 10 nM in the F-sequence hydrogel obtained by western blotting, we found that both of them could significantly promote the expression of four mRNAs compared with the control group, and 1 nM was better than 10 nM. Therefore, we determined that the 1 nM F-sequence hydrogel is the most suitable for osteogenesis. On the contrary, in the G-sequence hydrogel, the mRNA expressions of RUNX2 and OCN at 1 nM were higher than those at 10 nM, while the other two mRNA expressions (BMP2 and OPN) were slightly lower than 10 nM, suggesting that the trend of the bone-promoting concentration in the G-sequence hydrogel was not obvious. To facilitate comparison with the F-sequence hydrogel, 1 nM was selected for subsequent experiments on related signaling pathways promoting bone regeneration in vivo.
Figure 4.
Osteogenic differentiation of hBMSCs. (A) Western blotting showing the protein levels of RUNX2, BMP2, OCN, and OPN and (B) mRNA levels of RUNX2, BMP2, OCN, and OPN; *p < 0.05 and **p < 0.01.
The early osteogenic marker RUNX2 is expressed when hBMSCs begin to undergo osteogenic differentiation.20 RUNX2 is an essential transcription factor for osteogenesis and endochondral ossification and induces the expression of OCN, OPN, and osteosalivary proteins in nonosteoblastic cells.21 BMP2 is upstream from RUNX2 signaling and effectively induces osteogenesis and chondrogenesis in MSCs.22 Sox9 is a transcription factor of the Sry-associated high mobility group box (Sox) protein family and is thought to be a crucial transcription factor in BMP2-induced chondrogenesis.23 SOX9 also inhibits the transactivation of RUNX2.24 The overexpression of Sox9 is related to deficient levels of Runx2 expression, which leads to delayed osteogenic differentiation and endochondral ossification.25 Therefore, the opposite trend in the expression levels of BMP2, RUNX2, and other osteogenic-related proteins in the G-sequence hydrogel group could be that Sox9 enhanced BMP2-induced chondrogenic differentiation and marker expression, whereas BMP2 inhibited osteogenic differentiation and marker expression.
Related Signaling Pathways of F- and G-Sequence Hydrogels Promoting Bone Regeneration In Vivo
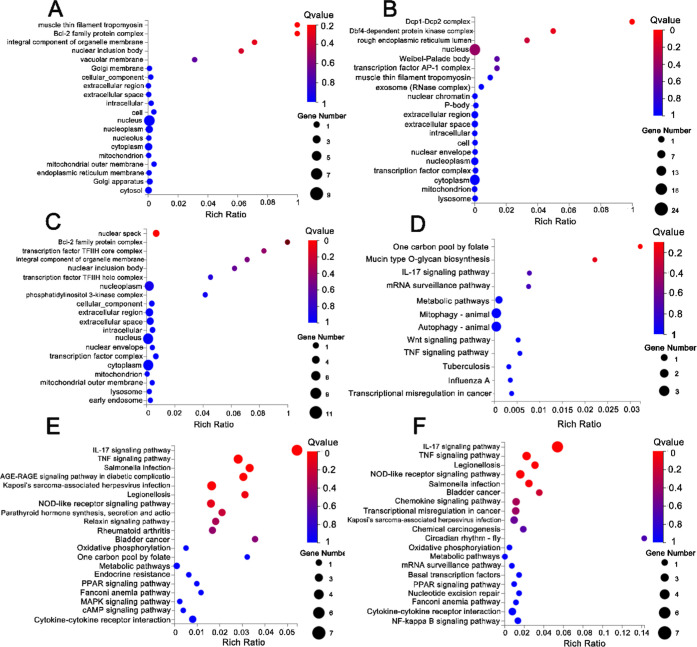
To explore gene expression and signaling pathway activity during hydrogel activation, an RNA-sequencing analysis was performed to investigate the differential gene expression between the experimental groups (1 nM F- and 1 nM G-sequence hydrogels, respectively) and the control group (UT). The clean read ratios of these samples were all greater than 90% and accounted for the majority of the raw data, suggesting the excellent reliability of RNA-seq. We identified a subset of 353 DEGs (102 significantly upregulated and 119 significantly downregulated genes) and presented them in the form of a volcano plot and a heat map (Figure 5A,D). GO and KEGG enrichment analyses of the DEGs were performed using the cluster Profiler R package. The GO functional enrichment analysis showed that DEGs of the action in the F-sequence hydrogel group were involved in tropomyosin and apoptosis (Figure 6A). Corresponding to the above-mentioned results, the KEGG pathway enrichment illustrated that DEGs in the F-sequence group were mainly involved in the folate-related pathway, which exerts an influence on bone metabolism (Figure 6E). Moreover, the DEGs in the G-sequence group were associated with biological functions related to DNA binding (Figure 6B). Furthermore, the G-sequence hydrogel differentially expressed genes were enriched in the IL-17 signaling pathway and the TNF signaling pathway (Figure 6F).
Figure 5.
Effect of F- and G-hydrogels on the expression of different genes in hBMSCs. (A) Volcano plot showing the upregulation and downregulation of differentially expressed genes (DEGs) between the 1 nM F-treated group and the untreated group (UT), (B) volcano plot showing the upregulation and downregulation of DEGs between the 1 nM G-treated group and UT, (C) volcano plot showing upregulation and downregulation of DEGs between the 1 nM G-treated and 1 nM F-treated groups., and (D) heat map showing unsupervised clustering of DEGs within different groups.
Figure 6.
GO and KEGG pathway analysis results. (A) GO analyses of DEGs in 1 nM F compared to UT, (B) GO analyses of DEGs in 1 nM G compared to UT, (C) GO analyses of DEGs in 1 nM G compared to 1 nM F, (D) KEGG enrichment analyses of DEGs in 1 nM F compared to UT, (E) KEGG enrichment analyses of DEGs in 1 nM G compared to UT, and (F) KEGG enrichment analyses of DEGs in 1 nM G compared to 1 nM F.
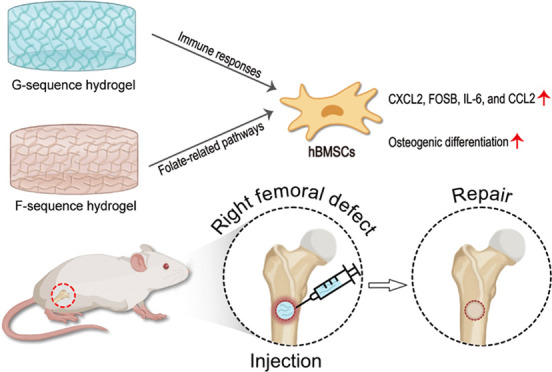
In this study, we observed that the novel hydrogels promoted the production of CXCL2, FOSB, IL-6, and CCL2 in hydrogel-treated hBMSCs (Figure 5D). CXCL2 is a member of the CXC family involved in various immune and inflammatory processes.26 Lin et al. observed that CXCL2 is upregulated in the early stages of healing and downregulated in the later stages, suggesting its importance in host defense during inflammation.27 FOSB belongs to the AP-1 family of transcription factors involved in the regulation of osteoblast differentiation and bone formation.28 CCL2 is a chemokine involved with the regulation of monocytes and macrophages and influences the progression of wound healing.29 Furthermore, signaling pathways enriched in the G-sequence hydrogel group are mainly involved in immune responses, such as the IL-17 signaling pathway and the TNF signaling pathway (Figure 6E,G). These data implied that G-sequence hydrogels could affect osteogenic differentiation by modulating tissue inflammatory response. In addition, the mechanism of osteogenic effect of F-sequence hydrogels is related to folic acid-related pathways. Folate has already been proved to influence bone health. Folate could affect the bone structure and bone mass mainly by regulating the balance of osteoblast and osteoclast activity.30
In Vivo Bone Regeneration Effect of F- and G-Sequence Hydrogels
Since peptide sequences within peptide self-assembled hydrogels rely on intermolecular forces to form nanofibrous networks, they are more suitable for repairing small nonstressed areas of bone defects in vivo. Due to the weak mechanical properties of supramolecular hydrogels, they are mechanically insufficient to retain bone defects in areas of high stress for a long time. Therefore, in this study, we referred to the 1 mm borehole rat femoral shaft defect model used in Tripathi et al.31 and Khedgikar et al.32 to evaluate the fracture healing characteristics of the two hydrogels. All rats were euthanatized to collect tissue samples at 2, 4, and 8 weeks after surgery. As shown in the micro-CT images (Figure 7A), at 2 and 4 weeks after surgery, the control and F-sequence hydrogel group reduced the bone cortex’s continuity and a larger callus (Figure 7A). The G-sequence group had greater continuity of the bone cortex compared to the other two groups. New bone formation was observed in the defect areas in all groups by 8 weeks. The compact bone substance in the F- and G-sequence groups was significantly thicker than the control group. The statistical results of the quantitative analysis showed no significant differences in the parameter values of bone volume/total volume (BV/TV), trabecular number (Tb.N), and bone mineral density (BMD) between three groups at 2 weeks. At 4 weeks, the BMD and Tb.N parameters in the G- group were significantly higher than the other two groups (p < 0.05) (Figure 7B).
Figure 7.
Effect of bone regeneration of F- and G-sequence hydrogels in vivo. (A) Microtomography images of a rat with a right femoral defect and (B) quantitative analysis of bone morphological parameter values in the femoral defect area. Abbreviations: BV/TV: bone volume/total volume, Tb.N: trabecular number, and BMD: bone mineral density.
Several studies have reported good results using OGP in the treatment of bone defects. Bab et al. intravenously administered OGP to adult rats for 9 consecutive days and reported an increase in the trabecular bone mass in the mandibular condyle, thereby supporting a role of OGP in vivo bone formation.10 Brager et al. subcutaneously injected OGP into rats with femur fractures over 2 weeks and reported a remarkable increase in the mitogenicity and osteogenicity of marrow-derived cultures.33 These studies suggest that OGP could be used for bone regeneration. Furthermore, OGP has been combined with tissue-engineered scaffold carriers to prolong the duration of action of OGP. Maia et al. developed an alginate hydrogel that delivered OGP and hBMSCs to minor bone defects for minimal invasive healing in an animal model.34 In this study, no inflammatory reaction was observed at the hydrogel implantation site, indicating good biocompatibility of F- and G-sequence hydrogel. After 2 weeks of the bone defect, the defect area would be filled with hematoma and granulation tissue, fibroblasts gradually forming fibrous bone scabs, chondrocytes appearing within the granulation tissue, and internalization of bone occurs. The BMD and Tb.N were higher in the G-sequence hydrogel group, suggesting that the thickness of trabeculae and bone density in the femoral defect area were more incredible in this group than in the other two groups. According to the KEGG pathway analysis results, the G-sequence hydrogel was enriched in IL-17 pathways and TNF pathways, suggesting that the G-sequence hydrogel may regulate the expression of inflammatory factors in the early stage of bone repair to influence the tissue inflammatory response. It indicates that the G-sequence hydrogel may have accelerated bone matrix calcification. There were no significant differences in the values of bone morphological parameters among the specimens in each group at 8 weeks. Since most of the rats’ femur fractures had completed calcification of the bone scab at 8 weeks, the bony joints were more robust and the fractures had reached clinical healing, so all three groups of rats’ femurs may have reached clinical healing.
Conclusions
We successfully developed the self-assembled supramolecular hydrogels based on OGP10-14. In vitro analyses demonstrated that the hydrogels significantly promoted hBMSC proliferation and osteogenic differentiation. The hydrogels are involved in the inflammatory-related pathways and folate-related pathways to regulate cell viability and osteogenesis process. In vivo experimental results illustrated the remarkable therapeutic effect of hydrogels to induced bone formation and promote bone regeneration in a rat bone defect model. Moreover, compared to other reports on OGP,35 F- and G-sequence hydrogels could prolong the duration of action of OGP and have the potential for novel bone regeneration strategies.
Acknowledgments
This work was supported by the National Science Fund for National Key Research and Development Program of China (2020YFC1107402) and the National Natural Science Foundation of China (81871777).
Author Contributions
∥ Y.Z. and Y.X. contributed equally to this work.
The authors declare no competing financial interest.
References
- Wan Z.; Zhang P.; Lv L.; Zhou Y. NIR light-assisted phototherapies for bone-related diseases and bone tissue regeneration: A systematic review. Theranostics 2020, 10, 11837–11861. 10.7150/thno.49784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novicoff W. M.; Manaswi A.; Hogan M. V.; Brubaker S. M.; Mihalko W. M.; Saleh K. J. Critical analysis of the evidence for current technologies in bone-healing and repair. J. Bone Jt. Surg. 2008, 90, 85–91. 10.2106/JBJS.G.01521. [DOI] [PubMed] [Google Scholar]
- Khan S. N.; Cammisa F. P. Jr.; Sandhu H. S.; Diwan A. D.; Girardi F. P.; Lane J. M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. 10.5435/00124635-200501000-00010. [DOI] [PubMed] [Google Scholar]
- Finkemeier C. G. Bone-grafting and bone-graft substitutes. J. Bone Jt. Surg. 2002, 84, 454–464. 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- Lee J.; Byun H.; Madhurakkat Perikamana S. K.; Lee S.; Shin H. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv. Healthcare Mater. 2019, 8, e1801106. [DOI] [PubMed] [Google Scholar]
- Dou X.; Mehwish N.; Zhao C.; Liu J.; Xing C.; Feng C. Supramolecular Hydrogels with Tunable Chirality for Promising Biomedical Applications. Acc. Chem. Res. 2020, 53, 852–862. 10.1021/acs.accounts.0c00012. [DOI] [PubMed] [Google Scholar]
- Shang Y.; Zhi D.; Feng G.; Wang Z.; Mao D.; Guo S.; Liu R.; Liu L.; Zhang S.; Sun S.; et al. Supramolecular Nanofibers with Superior Bioactivity to Insulin-Like Growth Factor-I. Nano Lett. 2019, 19, 1560–1569. 10.1021/acs.nanolett.8b04406. [DOI] [PubMed] [Google Scholar]
- Xu L.; Liu Y.; Sun Y.; Wang B.; Xiong Y.; Lin W.; Wei Q.; Wang H.; He W.; Wang B.; et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275 10.1186/s13287-017-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janko M.; Sahm J.; Schaible A.; Brune J. C.; Bellen M.; Schroder K.; Seebach C.; Marzi I.; Henrich D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regener. Med. 2018, 12, 653–666. 10.1002/term.2484. [DOI] [PubMed] [Google Scholar]
- Bab I.; Gazit D.; Chorev M.; Muhlrad A.; Shteyer A.; Greenberg Z.; Namdar M.; Kahn A. Histone H4-related osteogenic growth peptide (OGP): a novel circulating stimulator of osteoblastic activity. EMBO J. 1992, 11, 1867–1873. 10.1002/j.1460-2075.1992.tb05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C.; Bab I.; Mansur N.; Muhlrad A.; Shteyer A.; Namdar-Attar M.; Gavish H.; Vidson M.; Chorev M. Structure-bioactivity of C-terminal pentapeptide of osteogenic growth peptide [OGP(10-14)]. J. Pept. Res. 2000, 56, 147–156. 10.1034/j.1399-3011.2000.00763.x. [DOI] [PubMed] [Google Scholar]
- Li S.; Xu Y.; Yu J.; Becker M. L. Enhanced osteogenic activity of poly(ester urea) scaffolds using facile post-3D printing peptide functionalization strategies. Biomaterials 2017, 141, 176–187. 10.1016/j.biomaterials.2017.06.038. [DOI] [PubMed] [Google Scholar]
- Robinson D.; Bab I.; Nevo Z. Osteogenic growth peptide regulates proliferation and osteogenic maturation of human and rabbit bone marrow stromal cells. J. Bone Miner. Res. 1995, 10, 690–696. 10.1002/jbmr.5650100504. [DOI] [PubMed] [Google Scholar]
- Shuqiang M.; Kunzheng W.; Xiaoqiang D.; Wei W.; Mingyu Z.; Daocheng W. Osteogenic growth peptide incorporated into PLGA scaffolds accelerates healing of segmental long bone defects in rabbits. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 1558–1560. 10.1016/j.bjps.2008.03.040. [DOI] [PubMed] [Google Scholar]
- Zhao Z. Y.; Shao L.; Zhao H. M.; Zhong Z. H.; Liu J. Y.; Hao C. G. Osteogenic growth peptide accelerates bone healing during distraction osteogenesis in rabbit tibia. J. Int. Med. Res. 2011, 39, 456–463. 10.1177/147323001103900213. [DOI] [PubMed] [Google Scholar]
- Gabet Y.; Müller R.; Regev E.; Sela J.; Shteyer A.; Salisbury K.; Chorev M.; Bab I. Osteogenic growth peptide modulates fracture callus structural and mechanical properties. Bone 2004, 35, 65–73. 10.1016/j.bone.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Pigossi S. C.; de Oliveira G. J.; Finoti L. S.; Nepomuceno R.; Spolidorio L. C.; Rossa C. Jr; Ribeiro S. J.; Saska S.; Scarel-Caminaga R. M. Bacterial cellulose-hydroxyapatite composites with osteogenic growth peptide (OGP) or pentapeptide OGP on bone regeneration in critical-size calvarial defect model. J. Biomed. Mater. Res., Part A 2015, 103, 3397–3406. 10.1002/jbm.a.35472. [DOI] [PubMed] [Google Scholar]
- Aubin J. E.; Triffitt J. T.. Chapter 4 - Mesenchymal Stem Cells and Osteoblast Differentiation. In Principles of Bone Biology, 2nd ed.; Academic Press, 2002; pp 59–81. [Google Scholar]
- Buccitelli C.; Selbach M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. 10.1038/s41576-020-0258-4. [DOI] [PubMed] [Google Scholar]
- Bedair T. M.; Lee C. K.; Kim D. A.-O.; Baek S. W.; Bedair H. M.; Joshi H. A.-O.; Choi U. Y.; Park K. H.; Park W.; Han I.; et al. Magnesium hydroxide-incorporated PLGA composite attenuates inflammation and promotes BMP2-induced bone formation in spinal fusion. J. Tissue Eng. 2020, 11, 2041731420967591 10.1177/2041731420967591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersbach C. A.; Le Doux J. M.; Guldberg R. E.; GarcÃa A. J. Inducible regulation of Runx2-stimulated osteogenesis. Gene Ther. 2006, 13, 873–882. 10.1038/sj.gt.3302725. [DOI] [PubMed] [Google Scholar]
- Lee S. J.; Kang S. W.; Do H. J.; Han I.; Shin D. A.; Kim J. H.; Lee S. H. Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials 2010, 31, 5652–5659. 10.1016/j.biomaterials.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod. Rheumatol. 2008, 18, 213–219. 10.3109/s10165-008-0048-x. [DOI] [PubMed] [Google Scholar]
- Cheng A.; Genever P. G. SOX9 determines RUNX2 transactivity by directing intracellular degradation. J. Bone Miner. Res. 2010, 25, 2680–2689. 10.1002/jbmr.174. [DOI] [PubMed] [Google Scholar]
- Liao J.; Hu N.; Zhou N.; Lin L.; Zhao C.; Yi S.; Fan T.; Bao W.; Liang X.; Chen H.; et al. Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation. PLoS One 2014, 9, e89025 10.1371/journal.pone.0089025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A.; Bandow K.; Kusuyama J.; Kakimoto K.; Ohnishi T.; Miyawaki S.; Matsuguchi T. Induction of CXCL2 and CCL2 by pressure force requires IL-1Î2-MyD88 axis in osteoblasts. Bone 2015, 74, 76–82. 10.1016/j.bone.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Lin Z.; Rios H. F.; Volk S. L.; Sugai J. V.; Jin Q.; Giannobile W. V. Gene expression dynamics during bone healing and osseointegration. J. Periodontol. 2011, 82, 1007–1017. 10.1902/jop.2010.100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasper C.; Jagodzinski M.; Drescher M.; Meller R.; Wehmeier M.; Krettek C.; Hesse E. Cyclic strain induces FosB and initiates osteogenic differentiation of mesenchymal cells. Exp. Toxicol. Pathol. 2008, 59, 355–363. 10.1016/j.etp.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Whelan D. S.; Caplice N. M.; Clover A. J. P. Mesenchymal stromal cell derived CCL2 is required for accelerated wound healing. Sci. Rep. 2020, 10, 2642 10.1038/s41598-020-59174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacek T. P.; Kalani A.; Voor M. J.; Tyagi S. C.; Tyagi N. The role of homocysteine in bone remodeling. Clin. Chem. Lab. Med. 2013, 51, 579–590. 10.1515/cclm-2012-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi J. K.; Pal S.; Awasthi B.; Kumar A.; Tandon A.; Mitra K.; Chattopadhyay N.; Ghosh J. K. Variants of self-assembling peptide, KLD-12 that show both rapid fracture healing and antimicrobial properties. Biomaterials 2015, 56, 92–103. 10.1016/j.biomaterials.2015.03.046. [DOI] [PubMed] [Google Scholar]
- Khedgikar V.; Kushwaha P.; Ahmad N.; Gautam J.; Kumar P.; Maurya R.; Trivedi R. Ethanolic extract of Dalbergia sissoo promotes rapid regeneration of cortical bone in drill-hole defect model of rat. Biomed. Pharmacother. 2017, 86, 16–22. 10.1016/j.biopha.2016.11.140. [DOI] [PubMed] [Google Scholar]
- Brager M. A.; Patterson M. J.; Connolly J. F.; Nevo Z. Osteogenic growth peptide normally stimulated by blood loss and marrow ablation has local and systemic effects on fracture healing in rats. J. Orthop. Res. 2000, 18, 133–139. 10.1002/jor.1100180119. [DOI] [PubMed] [Google Scholar]
- Maia F. R.; Barbosa M.; Gomes D. B.; Vale N.; Gomes P.; Granja P. L.; Barrias C. C. Hydrogel depots for local co-delivery of osteoinductive peptides and mesenchymal stem cells. J. Controlled Release 2014, 189, 158–168. 10.1016/j.jconrel.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Policastro G. M.; Lin F.; Smith Callahan L. A.; Esterle A.; Graham M.; Sloan Stakleff K.; Becker M. L. OGP functionalized phenylalanine-based poly(ester urea) for enhancing osteoinductive potential of human mesenchymal stem cells. Biomacromolecules 2015, 16, 1358–1371. 10.1021/acs.biomac.5b00153. [DOI] [PubMed] [Google Scholar]