Abstract
DNA-encoded library (DEL) is an efficient high-throughput screening technology platform in drug discovery and is also gaining momentum in academic research. Today, the majority of DELs are assembled and encoded with double-stranded DNA tags (dsDELs) and has been selected against numerous biological targets; however, dsDELs are not amendable to some of the recently developed selection methods, such as the cross-linking-based selection against immobilized targets and live-cell-based selections, which require DELs encoded with single-stranded DNAs (ssDELs). Herein, we present a simple method to convert dsDELs to ssDELs using exonuclease digestion without library redesign and resynthesis. We show that dsDELs could be efficiently converted to ssDELs and used for affinity-based selections either with purified proteins or on live cells.
Introduction
Identification of high-quality binders that can modulate the function of biological targets is important for the advancement of drug discovery as well as chemical biology research. The advent of DNA-encoded chemical libraries (DELs), originally proposed by Brenner and Lerner in 1992,1 as well as Gallop and co-workers in 1993,2 has made the expensive and complex high-throughput screening (HTS) accessible to many researchers. In a DEL, each library compound is coupled with a unique DNA tag, which encodes its chemical structure and also acts as an amplifiable template for structural elucidation. Since the spatial encoding is replaced with DNA encoding in DELs, it allows the synthesis, processing, and selection of hundreds of millions to many billions of compounds in a single mixture at the minute scale (Figure 1a), and it does not require sophisticated equipment and logistics for compound handling and the selection can be done in a short time frame.3,4 In addition, DELs can access greater chemical space than biological display libraries, especially with the recent development of DEL-compatible reactions.5−14 In the past decades, this technology has been widely adopted by the pharmaceutical industry and also showed potential as a powerful tool in academic research.3,9,10,15−30
Figure 1.
(a) Scheme for DEL selection against immobilized protein targets. (b) HP-based encoding method for dsDELs. (c) Converting dsDELs to ssDELs may enable wider target scope and more versatile applications of DELs.
In 2009, GSK published a seminal work, in which they demonstrated the application of DELs on an industrial scale.31 In this report, a short, covalently linked double-stranded DNA (dsDNA), named the “headpiece (HP)”, was designed as the starting point of library synthesis (Figure 1b). In each chemical synthesis step, the compounds are encoded by enzymatically ligating the respective DNA codes, also in the form of dsDNA, to the HP. Finally, a closing primer containing the distal primer-binding site for PCR amplification is added to complete the library synthesis/encoding. In addition, polymerase extension could be used for library encoding, which also resulted in dsDNA-encoded DELs (dsDELs).32−34 The dsDNA tags may better protect the nucleobases from chemical modifications during library synthesis and are less likely to bind to proteins due to the more rigid structure.31 In fact, today, the majority of DELs are encoded with dsDNA tags. Although having been used in early studies,2,35 DELs encoded with single-stranded DNAs (ssDELs) are less abundant and they are often prepared with special encoding methods, such as DNA-templated synthesis (DTS),36 DNA routing,37 and DNA-directed assembly.38,39 ssDNAs can also be used to assemble the encoded self-assembling chemical (ESAC) libraries40−42 and DNA-encoded dynamic libraries.43−51 Moreover, ssDELs are amendable to a number of selection methods suitable for nonimmobilized proteins, such as the interaction determination using unpurified proteins (IDUP),52,53 the cross-linking-based methods,54−60 and the selections against membrane proteins on/inside live cells (Figure 1c).61,62 In these methods, the ssDNA tag provides a site for hybridization with an additional DNA to covalently connect with the target upon binding. These methods may expand the target scope of DELs to more complex targets in a more biologically relevant environment.63,64 In addition, a recent report showed that ssDELs had higher enrichment folds than dsDELs, especially for low- to moderate-affinity binders.65 Collectively, it is desirable to convert dsDELs, which are more widely available, to ssDELs that can be used for more diverse applications. Here, we show that dsDELs could be converted to ssDELs without library redesign and resynthesis using nuclease digestion. The resulting ssDELs can be used in affinity-based selections with purified proteins in solution or on live cells.
Results and Discussion
Previously, several strategies have been developed for converting dsDNAs to ssDNAs, including affinity separation,66 asymmetric PCR,67,68 denaturing electrophoresis,69 and selective enzymatic digestion.70,71 For converting dsDELs, ideally the method should (a) be compatible with the architecture of the majority of dsDELs, (b) not degrade ssDNAs that contain the DNA codes and primer-binding sites, (c) have a mild condition without damaging/modifying the library compounds, and (d) be technically simple with minimal loss of the precious library material. Thus, we chose to use DNA exonuclease digestion to selectively remove one strand of the dsDNA tag (Figure 1c), and the resulting ssDELs could be purified with a simple ethanol precipitation step. Initially, we tested T7 exonuclease and exonuclease III, as they can progressively digest one strand of DNA duplexes.72,73 Unfortunately, we observed that both enzymes overcame the unnatural linkage and digested both of the DNA strands of HP (Figure S1). Next, we switched to Lamdba exonuclease (λ-exo), which does not have ssDNA activity but usually requires a 5′-phosphate group for digestion (Figure 2a).74 As shown in Figure 2b, an 18-base pair HP (HP-1; 5 μM), which contains an amino group as the starting point of library synthesis and a 2-nt sticky end for adding the encoding tags, was treated with λ-exo (5 u/μL; 37 °C). The digestion mixture was ethanol-precipitated and resolved with denaturing electrophoresis, which showed the digestion of one DNA strand of HP-1 (Figure 2b). The DNA bands were excised, extracted, and characterized with mass spectrometry (MS). The results confirmed that the lower band was mostly the single-stranded digestion product of HP-1 with one phosphorylated nucleobase (an adenosine) remaining; a small amount of the product with all of the nucleobases before the unnatural linker digested was also observed (Figure 2c). Based on our previous studies, the remaining base will not interfere with DNA hybridization and DNA-templated cross-linking;75,76 thus, we reason that there is no need to push for complete base removal and the mixed products could be used for ssDEL-based selections and other applications.63 In addition, the processive digestion pattern of λ-exo77 was clearly observed and a near-complete digestion was achieved after 16 h.
Figure 2.
(a) Structure and DNA sequence of HP-1; Lamdba exonuclease (λ-exo) selectively digests the 5′-phosphorylated strand in HP-1 to yield ssDNA products. (b) Denaturing electrophoresis (20% TBE-urea) analysis of the digestion reactions. Conditions: 5 μM HP-1 in λ-exo buffer (5 μL); 37 °C at varied reaction times; λ-exo: 5 units/μL. (c) MS analysis of the digestion products extracted from each lane in (b). The product peaks are highlighted with an asterisk, and the expected/observed masses (m/z) and the structures/sequences are shown as marked.
We tested whether the ssDNA product could be further digested by λ-exo under prolonged reaction times. As shown in Figure 3a, gel analysis showed a >95% digestion yield after 6 h and no overdigestion was observed after 72 h. Moreover, we used quantitative PCR (qPCR) to quantify the DNA copy numbers in the reaction mixture at different time points; the results showed a 90.7% recovery yield after 48 hours of digestion (Figures 3b and S2). In the synthesis of dsDELs, after the encoding DNA tags are ligated to the headpiece, a closing primer will be added to install the distal primer-binding site and complete the library encoding (Figure 1b). In most cases, the 5′-end of the closing primer is a hydroxyl group without phosphorylation; thus, it is more desirable if the 5′-unphosphorylated headpiece DNAs could also be digested. Previously, the Zhao group showed that DNA duplexes with 5′-hydroxy or other modifications could be digested by λ exo, suggesting that a 5′-phosphate may not be absolutely required for λ exo.77 To test this, a series of unphosphorylated headpiece DNAs (duplex region: 16–20-bp), containing 5′-overhang (2-, 10-, 20-, and 30-nt), blunt end, or 3′-overhang (2-, 10-, 20-, and 30-nt), were prepared and subjected to λ exo digestion (Figure 4a). The results showed that λ exo efficiently digested the unphosphorylated DNAs with 3′-overhangs or blunt end (Figures 4b,c), but it could only digest the duplexes with a short 2-nt 5′-overhang (Figure 4d), which is consistent with the known activities of the enzyme.74 In practice, both 3′- and 5′-overhang are being used in dsDELs, depending on the closing primer;78−85 therefore, the λ-exo-mediated digestion may be directly applied to 3′-overhang dsDELs, whereas a primer extension step may be needed for dsDELs with 5′-overhang to fill the single-stranded region.
Figure 3.
(a) HP-1 was digested with λ-exo at different time durations (up to 72 h); the reaction mixtures were analyzed with electrophoresis (20% TBE-urea), and the digestion yields were calculated (details in Figure S2). (b) qPCR quantitation of the DNA copy numbers of the reaction mixtures. See qPCR details and the standard curves in Figure S2; all data are based on biological triplicate. Conditions: HP-1; 4 nM; λ-exo (5 u/μL, 20 μL) in lambda nuclease buffer at 37 °C.
Figure 4.

(a) Series of unphosphorylated headpieces (duplex region: 16–20 bp) were subjected to λ-exo digestion; n indicates the number of overhanging bases. See the Supporting Information for details. (b–d) Denaturing gel analysis of the digestion reactions. The conditions are the same as in Figure 3, and the duplex substrates used are as marked on each lane.
Next, to verify the feasibility of λ exo digestion in a library format, we prepared a model library containing five compounds on an 18-bp headpiece duplex with a 2-nt 3′-overhang (dsDEL-1; Figure 5a). The mixture was treated with λ exo at 37 °C for 20 h and analyzed with UPLC-MS. As shown in Figure 5c, the LC trace showed three major product peaks and the MS analysis of the peaks have identified the ssDNA products of all five compounds. Similar to Figure 2c, both the products with one nucleobase remaining and with all bases digested (prior to the unnatural linker) were identified (Figure 5c). Furthermore, to mimic the DNA tag length of typical dsDELs, another model library was prepared by conjugating the five compounds to a 70-bp, unphosphorylated headpiece (dsDEL-2; Figure 5a). After λ exo digestion, UPLC-MS analysis again showed the ssDNA products of all five compounds (Figure 5d). Collectively, these results demonstrated that lambda exonuclease could be used to convert dsDELs to ssDELs.
Figure 5.

(a, b) Two model libraries (dsDEL-1 and deDEL-2) with different tag lengths were prepared and subjected to λ exo digestion. (c) Digestion reaction of dsDEL-1 was analyzed with UPLC-MS; the LC trace showed three major peaks, and the respective MS data of each peak are shown below. (d) Digestion reaction of dsDEL-2 was also analyzed with UPLC-MS; MS results are shown. The ssDNA digestion products are as marked; dAp: with an adenosine phosphate remaining.
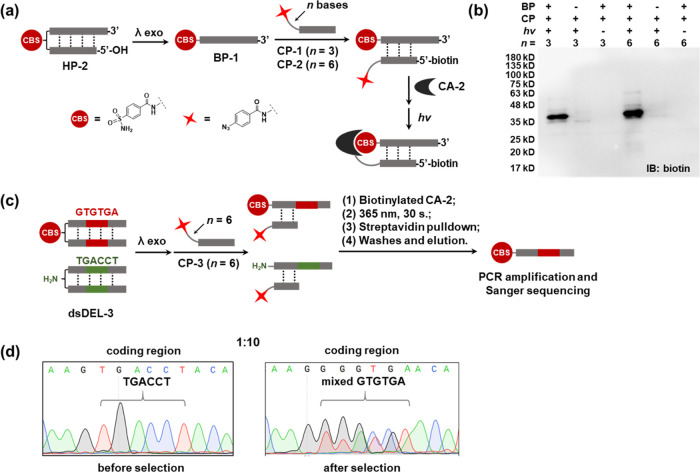
Recently, cross-linking-based approaches have been developed to perform DEL selections against nonimmobilized targets in solution, in cell lysates, or with live cells.54−60 These methods require an ssDNA region close to the library compound for hybridization with a reactive DNA. Upon ligand binding, the reactive DNA covalently captures the protein, thus establishing a stable connection between the ligand and the target. We further tested whether the ssDNA product generated by λ exo digestion could be used in ligand-directed target cross-linking.75 As shown in Figure 6a, we prepared an 18-bp headpiece DNA (HP-2) conjugated with a 4-carboxybenzene sulfonamide (CBS), a moderate-affinity ligand for carbonic anhydrase II (CA-II, Kd = 3.2 μM).86HP-2 was digested with λ exo to generate an ssDNA (binding probe; BP-1). Next, two complementary DNA strands carrying a 5′-biotin and a 3′-photo-reactive group phenylazide (capture probes; CP-1 and CP-2) were hybridized with BP-1. CP-1 and CP-2 have a 3 and 6-nt spacer, respectively, to extend the photo-cross-linker to reach the protein target. The BP-1/CP duplexes were incubated with CA-II (2 μM). After brief UV irradiation (365 nm, 30 s.), the mixtures were analyzed with Western blotting. The results clearly showed the capture of CA-II with the detectable bands matching the molecular weight of the DNA-CA-II conjugate (lane1 and lane 4; Figure 6b). No capture was observed without BP-1 or UV irradiation, indicating that the cross-linking was specific.
Figure 6.
(a) Ligand-directed labeling of the target CA-2 using BP-1, generated by λ exo digestion, and CP-1/CP-2. (b) Western blots of the labeling experiments. Conditions: DNA probes: 2 μM each; CA-II: 1 μM; reaction buffer: 1× phosphate-buffered saline (PBS) and 0.1 M NaCl; UV: 365 nm, 30 s, 0 °C; λ exo digestion, 20 h at 37 °C. IB: immunoblotting. (c) Selection scheme of dsDEL-3 against CA-2.55,56 (d) Sanger sequencing results before and after the selection. The coding regions are highlighted. See the Supporting Information for experimental details.
Next, we proceeded to model library selections. Two model libraries were prepared, in which the CBS ligand was encoded with a “GTGTGA” codon and mixed with the nonbinding background (with an amino group) encoded with a “TGACCT” codon at a ratio of 1:10 (dsDEL-3) (Figure 6c). dsDEL-3 was digested by λ exo, hybridized with a capture probe with a 6-nt spacer, and selected against a biotinylated CA-II following previously reported procedures.55,56 In brief, after the library was incubated with the target and UV irradiation, the CA-2/DNA complexes were affinity-purified with streptavidin beads. After washes, the selected library was eluted, PCR-amplified, and analyzed with Sanger sequencing. As shown in Figure 6d, although the postselection library still had mixed sequences at the coding region, enrichment of the CBS-encoding GTGTGA sequence could be observed, indicating that λ-exo-digested dsDELs are amenable to cross-linking-based selections.
Finally, we conducted live-cell-based selections using a λ-exo-converted DEL. Carbonic anhydrase 12 (CA-12) is a membrane carbonic anhydrase implicated in malignant cancers, and it can be inhibited by arylsulfonamide ligands.87 Previously, we used ligand-directed photo-cross-linking to enable the selections of both DELs and regular, non-DNA-encoded chemical libraries against CA-12 on live cells.88,89 Neri and co-workers also reported cell-based selections against CA-9, another membrane carbonic anhydrase isoform using dual-pharmacophore DELs.90 CBS is a known ligand for CA-12 (Kd = 0.97 μM)88 and can be used as a positive control in the selection.88,89 We first tested whether the CBS-conjugated ssDNA, obtained from λ exo digestion, could selectively capture the CA-12 protein on live cells. As shown in Figure 7a, A549 cells were cultured under hypoxia condition to increase CA-12 expression (Figure S3).91,92HP-2 was converted to the ssDNA BP-1 (Figure 6a) and then hybridized with CP-2. The BP-1/CP-2 duplex was directly incubated with A549 cells (4 °C, 1.5 h). After UV irradiation (365 nm, 30 s) and washes, the biotinylated proteins were affinity-purified using streptavidin beads and analyzed with Western bolt, and the results showed the specific capture of CA-12 (Figure 7b). Next, we prepared a model library containing a CBS-conjugated headpiece and 200-fold excess of the nonbinding background (dsDEL-4; Figure 7c). The CBS-conjugated headpiece and the background headpiece have orthogonal primer-binding sites.54 The library was digested with λ exo, hybridized with the photo-reactive CP-4 (6-nt spacer), and then incubated with A549 cells overexpressing CA-12. After washes to remove the nonbinders, the binders were eluted under strong denaturing condition (90 °C, 10 min.).88 The selected library was PCR-amplified and the enrichment fold of CBS was quantified by qPCR following previous reports.54,55 The results showed a 13.7-fold enrichment of CBS (average of two biological replicates; Figure 7d). In contrast, a control selection with MEF cells with a low CA-12 expression level did not show significant enrichment of CBS. Collectively, these results demonstrated that λ-exo-converted dsDEL is compatible with cross-linking-based selections on live cells.
Figure 7.
(a) Ligand-directed labeling of CA-12 on live A549 cells using BP-1, generated by λ exo digestion of HP-2, hybridized with CP-2 (with a 6-nt spacer). (b) Western blot analysis of the affinity pulldown experiments. Conditions: DNA, 5 μM; cell number, 100 million; incubation buffer, 1× PBS with 0.05 M NaCl; UV, 365 nm, 30 s, 0 °C; λ exo digestion, 20 h at 37 °C. Lane 1: BP-1/CP-2 with hv; lane 2: no BP-1; lane 3 no hv. (c) dsDEL-4 (a 1:200 mixture of CBS-conjugated headpiece and an amino-headpiece) was digested with λ exo, hybridized with CP-4, and used for selections against A549 and MEF cells, respectively. (d) After washes and elution, the selected library was analyzed with qPCR to determine the enrichment fold of the binders.54 See the Supporting Information for detailed procedures.
Conclusions
In summary, we have described a method to convert dsDELs to ssDELs using lambda exonuclease, therefore making the library amendable to the applications and selection methods that require an ssDNA region, including ligand-directed target capture and cross-linking-based selection on live cells. Typically, λ exo preferably digests the 5′-phosphorylated strand in a DNA duplex; however, the results from Zhao and co-worker77 and in Figure 4 showed that λ exo also had reactivity toward 5′-unphosphorylated DNA strands, although the reactivity might be relatively lower.77 The unnatural PEG linker in the headpiece DNA impeded λ exo digestion and the ssDNA strand that contains the compound, and the DNA codes remained intact. A relatively strong condition is needed to drive the digestion to completion, but again the unnatural linker appeared to be quite stable and prevented overdigestion. Moreover, this method avoids the need for library redesign and resynthesis, and the existing dsDELs may be straightforwardly converted to ssDELs for various applications. Considering that nearly all commercial and open-source DELs are encoded with dsDNA tags, we anticipated that this method will facilitate the applications of large-scale DELs to a wider range of biological targets.
Experimental Procedures
DNA Modification and Purification
Modified oligonucleotides with amine and biotin were purchased from Hitgen, Inc. or BGI Genomics Co. Ltd and used after HPLC purification. For other modifications, most small molecules were coupled with amine-modified oligonucleotides through amidation reactions and then purified by reversed-phase HPLC using a gradient of acetonitrile (5–80%) in 100 mM TEAA (pH 7.0), followed by lyophilization. Oligonucleotides were quantitated by a BioTek Epoch UV–vis spectrometer based on the calculated extinction coefficient at 260 nm. Oligonucleotides were characterized by an Agilent 1290 Infinity II UPLC coupled with ESI-MS.
Oligonucleotide Purification by Ethanol Precipitation
To an aqueous DNA solution (ideally the sample volume is less than 400 μL), 0.1 volume of 3 M NaOAc (pH 5.0) was added to adjust the sample pH to 5.0, followed by the addition of 1/40 volume of 10 mg/mL glycogen (Aldrich, final glycogen concentration: 133.33 μg/mL) and 2.5 volume of absolute ethanol. The solution was maintained at −80 °C for at least 1 h and then centrifuged at 14,000g for 30 min at 4 °C. The supernatant was discarded, and the pellet was dried by a SpeedVac. The recovered sample was dissolved in an appropriate buffer for subsequent analysis or experiments.
Lamdba Exonuclease Digestion
5 μM library or the DNA probes, 5 μL of 10× Lamdba exonuclease buffer, 20 μL (5 units/μL) of Lamdba exonuclease were mixed. The mixture was supplemented with water to a final volume of 50 μL. Digestion reactions were maintained at 37 °C for the specified time durations, before subjected to ethanol precipitation. The samples after ethanol precipitation were directly analyzed with Western blot or mass spectrometry.
Cell Culture
A549 cells with elevated CA12 expression were obtained with hypoxia cultivation93 (AnaeroPack; Mitsubishi Gas Chemical) at 37 °C for 36 h in DMEM (10% FBS, 100 unit/mL penicillin, and 100 g/mL streptomycin). MEF cells were cultured in DMEM supplemented with 10% FBS, in a 5% CO2 humidified incubator at 37 °C.
Acknowledgments
This work was supported by grants from the Shenzhen Bay Laboratory, Shenzhen, China (SZBL2020090501008), the Research Grants Council of Hong Kong SAR, China (AoE/P-705/16, 17301118, 17111319, 17303220, 17300321, and C7005-20G), NSFC of China (21572014, 21877093, 21907011, and 91953119), and the Fundamental Research Funds for the Central Universities (2020CQJQY-Z002, 2021CDJYGRH-002). The authors acknowledge the support from “Laboratory for Synthetic Chemistry and Chemical Biology” under the Health@InnoHK Program and State Key Laboratory of Synthetic Chemistry by Innovation and Technology Commission, Hong Kong SAR, China. Correspondence should be addressed to X.L.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01152.
More details on ESI-MS characterization of DNA-small-molecule conjugates, DNA sequences, selection protocols, full Sanger sequencing data, and other experimental details (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Brenner S.; Lerner R. A. Encoded Combinatorial Chemistry. Proc. Natl. Acad. Sci. U.S.A. 1992, 89, 5381–5383. 10.1073/pnas.89.12.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needels M. C.; Jones D. G.; Tate E. H.; Heinkel G. L.; Kochersperger L. M.; Dower W. J.; Barrett R. W.; Gallop M. A. Generation and screening of an oligonucleotide-encoded synthetic peptide library. Proc. Nat. Acad. Sci. U.S.A. 1993, 90, 10700–10704. 10.1073/pnas.90.22.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri D.; Lerner R. A. DNA-Encoded Chemical Libraries: A Selection System Based On Endowing Organic Compounds with Amplifiable Information. Annu. Rev. Biochem. 2018, 87, 479–502. 10.1146/annurev-biochem-062917-012550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow R. A., A Handbook for DNA-Encoded Chemistry: Theory and Applications for Exploring Chemical Space and Drug Discovery. John Wiley & Sons, Inc.: Hoboken, New Jersey, 2014. [Google Scholar]
- Fitzgerald P. R.; Paegel B. M. DNA-Encoded Chemistry: Drug Discovery from a Few Good Reactions. Chem. Rev. 2021, 121, 7155–7177. 10.1021/acs.chemrev.0c00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götte K.; Chines S.; Brunschweiger A. Reaction development for DNA-encoded library technology: From evolution to revolution?. Tetrahedron Lett. 2020, 61, 151889 10.1016/j.tetlet.2020.151889. [DOI] [Google Scholar]
- Patel S.; Badir S. O.; Molander G. A. Developments in Photoredox-Mediated Alkylation for DNA-Encoded Libraries. Trends in Chem. 2021, 3, 161–175. 10.1016/j.trechm.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson P.; Kodadek T. Chemical composition of DNA-encoded libraries, past present and future. Org. Biomol. Chem. 2019, 17, 4676–4688. 10.1039/C9OB00581A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conole D.; Hunter J. H.; Waring M. J. The maturation of DNA encoded libraries: opportunities for new users. Future Med. Chem. 2021, 13, 173–191. 10.4155/fmc-2020-0285. [DOI] [PubMed] [Google Scholar]
- Flood D. T.; Kingston C.; Vantourout J. C.; Dawson P. E.; Baran P. S. DNA Encoded Libraries: A Visitor’s Guide. Isr. J. Chem. 2020, 60, 268–280. 10.1002/ijch.201900133. [DOI] [Google Scholar]
- Lenci E.; Baldini L.; Trabocchi A. Diversity-oriented synthesis as a tool to expand the chemical space of DNA-encoded libraries. Biorg. Med. Chem. 2021, 41, 116218 10.1016/j.bmc.2021.116218. [DOI] [PubMed] [Google Scholar]
- Fair R. J.; Walsh R. T.; Hupp C. D. The expanding reaction toolkit for DNA-encoded libraries. Bioorg. Med. Chem. Lett. 2021, 51, 128339 10.1016/j.bmcl.2021.128339. [DOI] [PubMed] [Google Scholar]
- Gerry C. J.; Schreiber S. L. Recent achievements and current trajectories of diversity-oriented synthesis. Curr. Opin. Chem. Biol. 2020, 56, 1–9. 10.1016/j.cbpa.2019.08.008. [DOI] [PubMed] [Google Scholar]
- Kölmel D. K.; Zhu H.; Flanagan M. E.; Sakata S. K.; Harris A. R.; Wan J.; Morgan B. A. Employing Photocatalysis for the Design and Preparation of DNA-encoded Libraries: A Case Study. Chem. Rec. 2021, 21, 616–630. 10.1002/tcr.202000148. [DOI] [PubMed] [Google Scholar]
- Satz A. L.; Kuai L.; Peng X. Selections and screenings of DNA-encoded chemical libraries against enzyme and cellular targets. Bioorg. Med. Chem. Lett. 2021, 39, 127851 10.1016/j.bmcl.2021.127851. [DOI] [PubMed] [Google Scholar]
- Song M.; Hwang G. T. DNA-Encoded Library Screening as a Core Platform Technology in Drug Discovery. Its Synthetic Method Development and Applications in DEL Synthesis. J. Med. Chem. 2020, 63, 6578–6599. 10.1021/acs.jmedchem.9b01782. [DOI] [PubMed] [Google Scholar]
- Kunig V. B. K.; Potowski M.; Klika Skopic M.; Brunschweiger A. Scanning Protein Surfaces with DNA-Encoded Libraries. ChemMedChem 2021, 16, 1048–1062. 10.1002/cmdc.202000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Li X. Recent Advances on the Selection Methods of DNA-encoded Libraries. ChemBioChem 2021, 22, 2384–2397. 10.1002/cbic.202100144. [DOI] [PubMed] [Google Scholar]
- Kodadek T.; Paciaroni N. G.; Balzarini M.; Dickson P. Beyond protein binding: recent advances in screening DNA-encoded libraries. Chem. Commun. 2019, 55, 13330–13341. 10.1039/C9CC06256D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satz A. L.; Brunschweiger A.; Flanagan M. E.; Gloger A.; Hansen N. J. V.; Kuai L.; Kunig V. B. K.; Lu X.; Madsen D.; Marcaurelle L. A.; Mulrooney C.; O’Donovan G.; Sakata S.; Scheuermann J. DNA-encoded chemical libraries. Nat. Rev. Dis. Primers 2022, 2, 3 10.1038/s43586-021-00084-5. [DOI] [Google Scholar]
- Ottl J.; Leder L.; Schaefer J. V.; Dumelin C. E. Encoded Library Technologies as Integrated Lead Finding Platforms for Drug Discovery. Molecules 2019, 24, 1629 10.3390/molecules24081629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plais L.; Scheuermann J. Macrocyclic DNA-encoded chemical libraries: a historical perspective. RSC Chem. Biol. 2022, 3, 7–17. 10.1039/D1CB00161B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gironda-Martínez A.; Donckele E. J.; Samain F.; Neri D. DNA-Encoded Chemical Libraries: A Comprehensive Review with Succesful Stories and Future Challenges. ACS Pharmacol. Transl. Sci. 2021, 4, 1265–1279. 10.1021/acsptsci.1c00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L. H.; Franzini R. M. Achievements, Challenges, and Opportunities in DNA-Encoded Library Research: An Academic Point of View. ChemBioChem 2017, 18, 829–836. 10.1002/cbic.201600567. [DOI] [PubMed] [Google Scholar]
- Goodnow R. A. Jr.; Dumelin C. E.; Keefe A. D. DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nat. Rev. Drug Discov. 2017, 16, 131–147. 10.1038/nrd.2016.213. [DOI] [PubMed] [Google Scholar]
- Kunig V.; Potowski M.; Gohla A.; Brunschweiger A. DNA-encoded libraries - an efficient small molecule discovery technology for the biomedical sciences. Biol. Chem. 2018, 399, 691–710. 10.1515/hsz-2018-0119. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Savych O.; Moroz Y.; Chen Y. Y.; Goodnow R. A. DNA-Encoded Library Chemistry: Amplification of Chemical Reaction Diversity for the Exploration of Chemical Space. Aldrichimica Acta 2019, 52, 75–87. [Google Scholar]
- Reddavide F. V.; Thompson M.; Mannocci L.; Zhang Y. X. DNA-Encoded Fragment Libraries: Dynamic Assembly, Single-Molecule Detection, and High-Throughput Hit Validation. Aldrichimica Acta 2019, 52, 63–74. [Google Scholar]
- Huang Y. R.; Li Y. Z.; Li X. Y. Strategies for developing DNA-encoded libraries beyond binding assays. Nat. Chem. 2022, 14, 129–140. 10.1038/s41557-021-00877-x. [DOI] [PubMed] [Google Scholar]
- Goodnow R. A.; Davie C. P. DNA-Encoded Library Technology: A Brief Guide to Its Evolution and Impact on Drug Discovery. Annu. Rep. Med. Chem. 2017, 50, 1–15. 10.1016/bs.armc.2017.09.002. [DOI] [Google Scholar]
- Clark M. A.; Acharya R. A.; Arico-Muendel C. C.; Belyanskaya S. L.; Benjamin D. R.; Carlson N. R.; Centrella P. A.; Chiu C. H.; Creaser S. P.; Cuozzo J. W.; Davie C. P.; Ding Y.; Franklin G. J.; Franzen K. D.; Gefter M. L.; Hale S. P.; Hansen N. J. V.; Israel D. I.; Jiang J. W.; Kavarana M. J.; Kelley M. S.; Kollmann C. S.; Li F.; Lind K.; Mataruse S.; Medeiros P. F.; Messer J. A.; Myers P.; O’Keefe H.; Oliff M. C.; Rise C. E.; Satz A. L.; Skinner S. R.; Svendsen J. L.; Tang L. J.; van Vloten K.; Wagner R. W.; Yao G.; Zhao B. G.; Morgan B. A. Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat. Chem. Biol. 2009, 5, 647–654. 10.1038/nchembio.211. [DOI] [PubMed] [Google Scholar]
- Buller F.; Mannocci L.; Zhang Y.; Dumelin C. E.; Scheuermann J.; Neri D. Design and synthesis of a novel DNA-encoded chemical library using Diels-Alder cycloadditions. Bioorg. Med. Chem. Lett. 2008, 18, 5926–5931. 10.1016/j.bmcl.2008.07.038. [DOI] [PubMed] [Google Scholar]
- Buller F.; Zhang Y.; Scheuermann J.; Schafer J.; Buhlmann P.; Neri D. Discovery of TNF inhibitors from a DNA-encoded chemical library based on diels-alder cycloaddition. Chem. Biol. 2009, 16, 1075–1086. 10.1016/j.chembiol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Litovchick A.; Clark M. A.; Keefe A. D. Universal strategies for the DNA-encoding of libraries of small molecules using the chemical ligation of oligonucleotide tags. Artif. DNA PNA XNA 2014, 5, e27896 10.4161/adna.27896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J.; Brenner S.; Janda K. D. Synthetic Methods for the Implementation of Encoded Combinatorial Chemistry. J. Am. Chem. Soc. 1993, 115, 9812–9813. 10.1021/ja00074a063. [DOI] [Google Scholar]
- Gartner Z. J.; Brian N. T.; Grubina R.; Doyon J. B.; Snyder T. M.; Liu D. R. DNA-templated organic synthesis and selection of a library of macrocycles. Science 2004, 305, 1601–1605. 10.1126/science.1102629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin D. R.; Harbury P. B. DNA display I. Sequence-encoded routing of DNA populations. PLoS Biol. 2004, 2, e173 10.1371/journal.pbio.0020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaene F.; Mejias L.; Harris J. L.; Winssinger N. Synthesis of a PNA-encoded cysteine protease inhibitor library. Tetrahedron 2004, 60, 8677–8690. 10.1016/j.tet.2004.05.107. [DOI] [Google Scholar]
- Vummidi B. R.; Farrera-Soler L.; Daguer J. P.; Dockerill M.; Barluenga S.; Winssinger N. A mating mechanism to generate diversity for the Darwinian selection of DNA-encoded synthetic molecules. Nat. Chem. 2022, 14, 141–152. 10.1038/s41557-021-00829-5. [DOI] [PubMed] [Google Scholar]
- Melkko S.; Scheuermann J.; Dumelin C. E.; Neri D. Encoded self-assembling chemical libraries. Nat. Biotechnol. 2004, 22, 568–574. 10.1038/nbt961. [DOI] [PubMed] [Google Scholar]
- Wichert M.; Krall N.; Decurtins W.; Franzini R. M.; Pretto F.; Schneider P.; Neri D.; Scheuermann J. Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation. Nat. Chem. 2015, 7, 241–249. 10.1038/nchem.2158. [DOI] [PubMed] [Google Scholar]
- Plais L.; Lessing A.; Keller M.; Martinelli A.; Oehler S.; Bassi G.; Neri D.; Scheuermann J. Universal encoding of next generation DNA-encoded chemical libraries. Chem. Sci. 2022, 13, 967–974. 10.1039/D1SC05721A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddavide F. V.; Lin W.; Lehnert S.; Zhang Y. DNA-Encoded Dynamic Combinatorial Chemical Libraries. Angew. Chem., Int. Ed. 2015, 7924–7928. 10.1002/anie.201501775. [DOI] [PubMed] [Google Scholar]
- Farrera-Soler L.; Daguer J. P.; Raunft P.; Barluenga S.; Imberty A.; Winssinger N. PNA-Based Dynamic Combinatorial Libraries (PDCL) and screening of lectins. Bioorg. Med. Chem. 2020, 28, 115458 10.1016/j.bmc.2020.115458. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Li C.; Peng J.; Xie L.; Meng L.; Li Q.; Zhang J.; Li X. D.; Li X.; Huang X.; Li X. DNA-Encoded Dynamic Chemical Library and Its Applications in Ligand Discovery. J. Am. Chem. Soc. 2018, 140, 15859–15867. 10.1021/jacs.8b09277. [DOI] [PubMed] [Google Scholar]
- Reddavide F. V.; Cui M.; Lin W.; Fu N.; Heiden S.; Andrade H.; Thompson M.; Zhang Y. Second generation DNA-encoded dynamic combinatorial chemical libraries. Chem. Commun. 2019, 55, 3753–3756. 10.1039/C9CC01429B. [DOI] [PubMed] [Google Scholar]
- Grunzner S.; Reddavide F. V.; Steinfelder C.; Cui M.; Busek M.; Klotzbach U.; Zhang Y.; Sonntag F., Lab-on-a-chip platform for high throughput drug discovery with DNA-encoded chemical libraries. Proc. Spie. 2017, 10061. [Google Scholar]
- Machida T.; Novoa A.; Gillon E.; Zheng S.; Claudinon J.; Eierhoff T.; Imberty A.; Romer W.; Winssinger N. Dynamic Cooperative Glycan Assembly Blocks the Binding of Bacterial Lectins to Epithelial Cells. Angew. Chem., Int. Ed. 2017, 56, 6762–6766. 10.1002/anie.201700813. [DOI] [PubMed] [Google Scholar]
- Li G.; Zheng W.; Chen Z.; Zhou Y.; Liu Y.; Yang J.; Huang Y.; Li X. Design, preparation, and selection of DNA-encoded dynamic libraries. Chem. Sci. 2015, 6, 7097–7104. 10.1039/C5SC02467F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Shen W.; Peng J.; Deng Y.; Li X. Identification of isoform/domain-selective fragments from the selection of DNA-encoded dynamic library. Bioorg. Med. Chem. 2021, 45, 116328 10.1016/j.bmc.2021.116328. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Peng J.; Shen W.; Li X. Psoralen as an interstrand DNA crosslinker in the selection of DNA-Encoded dynamic chemical library. Biochem. Biophys. Res. Commun. 2020, 533, 215–222. 10.1016/j.bbrc.2020.04.033. [DOI] [PubMed] [Google Scholar]
- McGregor L. M.; Jain T.; Liu D. R. Identification of ligand-target pairs from combined libraries of small molecules and unpurified protein targets in cell lysates. J. Am. Chem. Soc. 2014, 136, 3264–3270. 10.1021/ja412934t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. I.; McGregor L. M.; Jain T.; Liu D. R. Discovery of a Covalent Kinase Inhibitor from a DNA-Encoded Small-Molecule Library × Protein Library Selection. J. Am. Chem. Soc. 2017, 139, 10192–10195. 10.1021/jacs.7b04880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P.; Chen Z.; Li Y.; Sun D.; Gao Y.; Huang Y.; Li X. Selection of DNA-encoded small molecule libraries against unmodified and non-immobilized protein targets. Angew. Chem., Int. Ed. 2014, 53, 10056–10059. 10.1002/anie.201404830. [DOI] [PubMed] [Google Scholar]
- Denton K. E.; Krusemark C. J. Crosslinking of DNA-linked ligands to target proteins for enrichment from DNA-encoded libraries. Medchemcomm 2016, 7, 2020–2027. 10.1039/C6MD00288A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannino A.; Gironda-Martinez A.; Gorre E. M. D.; Prati L.; Piazzi J.; Scheuermann J.; Neri D.; Donckele E. J.; Samain F. Critical Evaluation of Photo-cross-linking Parameters for the Implementation of Efficient DNA-Encoded Chemical Library Selections. ACS Comb. Sci. 2020, 22, 204–212. 10.1021/acscombsci.0c00023. [DOI] [PubMed] [Google Scholar]
- Shi B.; Deng Y.; Li X. Polymerase-Extension-Based Selection Method for DNA-Encoded Chemical Libraries against Nonimmobilized Protein Targets. ACS Comb. Sci. 2019, 21, 345–349. 10.1021/acscombsci.9b00011. [DOI] [PubMed] [Google Scholar]
- Shi B.; Deng Y.; Zhao P.; Li X. Selecting a DNA-Encoded Chemical Library against Non-immobilized Proteins Using a “Ligate-Cross-Link-Purify” Strategy. Bioconjug Chem. 2017, 28, 2293–2301. 10.1021/acs.bioconjchem.7b00343. [DOI] [PubMed] [Google Scholar]
- Winssinger N.; Harris J. L. Microarray-based functional protein profiling using peptide nucleic acid-encoded libraries. Expert Rev. Proteom. 2005, 2, 937–947. 10.1586/14789450.2.6.937. [DOI] [PubMed] [Google Scholar]
- Harris J. L.; Winssinger N. PNA encoding (PNA = peptide nucleic acid): from solution-based libraries to organized microarrays. Chemistry 2005, 11, 6792–6801. 10.1002/chem.200500305. [DOI] [PubMed] [Google Scholar]
- Cai B.; Kim D.; Akhand S.; Sun Y.; Cassell R. J.; Alpsoy A.; Dykhuizen E. C.; Van Rijn R. M.; Wendt M. K.; Krusemark C. J. Selection of DNA-Encoded Libraries to Protein Targets within and on Living Cells. J. Am. Chem. Soc. 2019, 141, 17057–17061. 10.1021/jacs.9b08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Meng L.; Nie Q.; Zhou Y.; Chen L.; Yang S.; Fung Y. M. E.; Li X.; Huang C.; Cao Y.; Li Y.; Li X. Selection of DNA-encoded chemical libraries against endogenous membrane proteins on live cells. Nat. Chem. 2021, 13, 77–88. 10.1038/s41557-020-00605-x. [DOI] [PubMed] [Google Scholar]
- Song Y.; Li X. Evolution of the Selection Methods of DNA-Encoded Chemical Libraries. Acc. Chem. Res. 2021, 54, 3491–3503. 10.1021/acs.accounts.1c00375. [DOI] [PubMed] [Google Scholar]
- Kodadek T.; Paciaroni N. G.; Balzarini M.; Dickson P. Beyond protein binding: recent advances in screening DNA-encoded libraries. Chem. Commun. 2019, 55, 13330–13341. 10.1039/C9CC06256D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi G.; Favalli N.; Oehler S.; Martinelli A.; Catalano M.; Scheuermann J.; Neri D. Comparative evaluation of DNA-encoded chemical selections performed using DNA in single-stranded or double-stranded format. Biochem. Biophys. Res. Commun. 2020, 533, 223–229. 10.1016/j.bbrc.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.; Avci-Adali M.; Ziemer G.; Wendel H. P. Streptavidin-coated magnetic beads for DNA strand separation implicate a multitude of problems during cell-SELEX. Oligonucleotides 2009, 19, 243–254. 10.1089/oli.2009.0194. [DOI] [PubMed] [Google Scholar]
- Nehdi A.; Samman N.; Aguilar-Sanchez V.; Farah A.; Yurdusev E.; Boudjelal M.; Perreault J. Novel Strategies to Optimize the Amplification of Single-Stranded DNA. Front. Bioeng. Biotechnol. 2020, 8, 401 10.3389/fbioe.2020.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiat M.; Ranjbar R.; Latifi A. M.; Rasaee M. J.; Farnoosh G. Essential strategies to optimize asymmetric PCR conditions as a reliable method to generate large amount of ssDNA aptamers. Biotechnol. Appl. Biochem. 2017, 64, 541–548. 10.1002/bab.1507. [DOI] [PubMed] [Google Scholar]
- Liang C.; Li D.; Zhang G.; Li H.; Shao N.; Liang Z.; Zhang L.; Lu A.; Zhang G. Comparison of the methods for generating single-stranded DNA in SELEX. Analyst 2015, 140, 3439–3444. 10.1039/C5AN00244C. [DOI] [PubMed] [Google Scholar]
- Citartan M.; Tang T.-H.; Tan S.-C.; Gopinath S. C. B. Conditions optimized for the preparation of single-stranded DNA (ssDNA) employing lambda exonuclease digestion in generating DNA aptamer. World J. Microbiol. Biotechnol. 2011, 27, 1167–1173. 10.1007/s11274-010-0563-8. [DOI] [Google Scholar]
- Murgha Y. E.; Rouillard J. M.; Gulari E. Methods for the preparation of large quantities of complex single-stranded oligonucleotide libraries. PLoS One 2014, 9, e94752 10.1371/journal.pone.0094752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr C.; Sadowski P. D. Gene 6 exonuclease of bacteriophage T7. I. Purification and properties of the enzyme. J. Biol. Chem. 1972, 247, 305–310. 10.1016/S0021-9258(19)45790-6. [DOI] [PubMed] [Google Scholar]
- Richardson C. C.; Lehman I. R.; Kornberg A. A Deoxyribonucleic Acid Phosphatase-Exonuclease from Escherichia Coli. Ii. Characterization of the Exonuclease Activity. J. Biol. Chem. 1964, 239, 251–258. 10.1016/S0021-9258(18)51775-0. [DOI] [PubMed] [Google Scholar]
- Little J. W. Lambda exonuclease. Gene Amplif Anal 1981, 2, 135–145. [PubMed] [Google Scholar]
- Li G.; Liu Y.; Chen L.; Wu S.; Li X.; et al. Photoaffinity Labeling of Small-Molecule-Binding Proteins by DNA-Templated Chemistry. Angew. Chem., Int. Ed. 2013, 52, 9544–9549. 10.1002/anie.201302161. [DOI] [PubMed] [Google Scholar]
- Li G.; Liu Y.; Yu X.; Li X. Multivalent photoaffinity probe for labeling small molecule binding proteins. Bioconjug. Chem. 2014, 25, 1172–1180. 10.1021/bc500195w. [DOI] [PubMed] [Google Scholar]
- Wu T.; Yang Y.; Chen W.; Wang J.; Yang Z.; Wang S.; Xiao X.; Li M.; Zhao M. Noncanonical substrate preference of lambda exonuclease for 5′-nonphosphate-ended dsDNA and a mismatch-induced acceleration effect on the enzymatic reaction. Nucleic Acids Res. 2018, 46, 3119–3129. 10.1093/nar/gky154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölmel D. K.; Meng J.; Tsai M. H.; Que J.; Loach R. P.; Knauber T.; Wan J.; Flanagan M. E. On-DNA Decarboxylative Arylation: Merging Photoredox with Nickel Catalysis in Water. ACS Comb. Sci. 2019, 21, 588–597. 10.1021/acscombsci.9b00076. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Graybill T. L.; Zeng X.; Platchek M.; Zhang J.; Bodmer V. Q.; Wisnoski D. D.; Deng J.; Coppo F. T.; Yao G.; Tamburino A.; Scavello G.; Franklin G. J.; Mataruse S.; Bedard K. L.; Ding Y.; Chai J.; Summerfield J.; Centrella P. A.; Messer J. A.; Pope A. J.; Israel D. I. Cell-Based Selection Expands the Utility of DNA-Encoded Small-Molecule Library Technology to Cell Surface Drug Targets: Identification of Novel Antagonists of the NK3 Tachykinin Receptor. ACS Comb. Sci. 2015, 17, 722–731. 10.1021/acscombsci.5b00124. [DOI] [PubMed] [Google Scholar]
- Deng H.; O’Keefe H.; Davie C. P.; Lind K. E.; Acharya R. A.; Franklin G. J.; Larkin J.; Matico R.; Neeb M.; Thompson M. M.; Lohr T.; Gross J. W.; Centrella P. A.; O’Donovan G. K.; Bedard K. L.; van Vloten K.; Mataruse S.; Skinner S. R.; Belyanskaya S. L.; Carpenter T. Y.; Shearer T. W.; Clark M. A.; Cuozzo J. W.; Arico-Muendel C. C.; Morgan B. A. Discovery of highly potent and selective small molecule ADAMTS-5 inhibitors that inhibit human cartilage degradation via encoded library technology (ELT). J. Med. Chem. 2012, 55, 7061–7079. 10.1021/jm300449x. [DOI] [PubMed] [Google Scholar]
- Shin M. H.; Lee K. J.; Lim H. S. DNA-Encoded Combinatorial Library of Macrocyclic Peptoids. Bioconjug. Chem. 2019, 30, 2931–2938. 10.1021/acs.bioconjchem.9b00628. [DOI] [PubMed] [Google Scholar]
- Ling X.; Lu W.; Miao L.; Marcaurelle L. A.; Wang X.; Ding Y.; Lu X. Divergent On-DNA Transformations from DNA-Linked Piperidones. J. Org. Chem. 2022, 87, 1971–1976. 10.1021/acs.joc.1c00670. [DOI] [PubMed] [Google Scholar]
- Du H. C.; Simmons N.; Faver J. C.; Yu Z.; Palaniappan M.; Riehle K.; Matzuk M. M. A Mild, DNA-Compatible Nitro Reduction Using B2(OH)4. Org. Lett. 2019, 21, 2194–2199. 10.1021/acs.orglett.9b00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacchi S.; Fabrizi G.; Goggiamani A. Palladium-catalyzed hydroxycarbonylation of aryl and vinyl halides or triflates by acetic anhydride and formate anions. Org. Lett. 2003, 5, 4269–4272. 10.1021/ol0354371. [DOI] [PubMed] [Google Scholar]
- Dawadi S.; Simmons N.; Miklossy G.; Bohren K. M.; Faver J. C.; Ucisik M. N.; Nyshadham P.; Yu Z.; Matzuk M. M. Discovery of potent thrombin inhibitors from a protease-focused DNA-encoded chemical library. Proc. Nat. Acad. Sci. U.S.A. 2020, 117, 16782–16789. 10.1073/pnas.2005447117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West G. M.; Tang L.; Fitzgerald M. C. Thermodynamic analysis of protein stability and ligand binding using a chemical modification- and mass spectrometry-based strategy. Anal. Chem. 2008, 80, 4175–4185. 10.1021/ac702610a. [DOI] [PubMed] [Google Scholar]
- Vullo D.; Innocenti A.; Nishimori I.; Pastorek J.; Scozzafava A.; Pastorekova S.; Supuran C. T. Carbonic anhydrase inhibitors. Inhibition of the transmembrane isozyme XII with sulfonamides-a new target for the design of antitumor and antiglaucoma drugs?. Bioorg. Med. Chem. Lett. 2005, 15, 963–969. 10.1016/j.bmcl.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Meng L.; Nie Q.; Zhou Y.; Chen L.; Yang S.; Fung Y. M. E.; Li X.; Huang C.; Cao Y.; Li Y.; Li X. Selection of DNA-encoded chemical libraries against endogenous membrane proteins on live cells. Nat. Chem. 2021, 13, 77–88. 10.1038/s41557-020-00605-x. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Deng Y.; Zhang J.; Meng L.; Li X. Direct ligand screening against membrane proteins on live cells enabled by DNA-programmed affinity labelling. Chem. Commun. 2021, 57, 3769–3772. 10.1039/D1CC00961C. [DOI] [PubMed] [Google Scholar]
- Oehler S.; Catalano M.; Scapozza I.; Bigatti M.; Bassi G.; Favalli N.; Mortensen M. R.; Samain F.; Scheuermann J.; Neri D. Affinity Selections of DNA-Encoded Chemical Libraries on Carbonic Anhydrase IX-Expressing Tumor Cells Reveal a Dependence on Ligand Valence. Chem. - Eur. J. 2021, 27, 8985–8993. 10.1002/chem.202100816. [DOI] [PubMed] [Google Scholar]
- Ivanov S.; Liao S. Y.; Ivanova A.; Stanbridge E. J.; et al. Expression of hypoxia inducible cell surface transmembrane carbonic anhydrases in human cancer. Am. J. Clin. Pathol. 2001, 158, 905–919. 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T.; Fujishima S. H.; Komatsu K.; Kuwata K.; Kiyonaka S.; Hamachi I. LDAI-based chemical labeling of intact membrane proteins and its pulse-chase analysis under live cell conditions. Chem. Biol. 2014, 21, 1013–1022. 10.1016/j.chembiol.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Song Y.; Xiong F.; Peng J.; Fung Y. M. E.; Huang Y.; Li X. Introducing aldehyde functionality to proteins using ligand-directed affinity labeling. Chem. Commun. 2020, 56, 6134–6137. 10.1039/D0CC01982H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.