Abstract
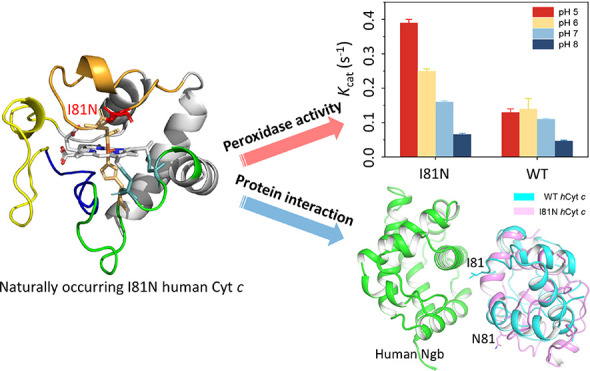
Human cytochrome c (hCyt c) is a crucial heme protein and plays an indispensable role in energy conversion and intrinsic apoptosis pathways. The sequence and structure of Cyt c were evolutionarily conserved and only a few naturally occurring mutants were detected in humans. Among those variable sites, position 81 was proposed to act as a peroxidase switch in the initiation stages of apoptosis. In this study, we show that Ile81 not only suppresses the intrinsic peroxidase activity but also is essential for Cyt c to interact with neuroglobin (Ngb), a potential protein partner. The kinetic assays showed that the peroxidase activity of the naturally occurring variant I81N was enhanced up to threefold under pH 5. The local stability of the Ω-loop D (residues 70–85) in the I81N variant was decreased. Moreover, the Alphafold2 program predicted that Ile81 forms stable contact with human Ngb. Meanwhile, the Ile81 to Asn81 missense mutation abolishes the interaction interface, resulting in a ∼40-fold decrease in binding affinity. These observations provide an insight into the structure–function relationship of the conserved Ile81 in vertebrate Cyt c.
Introduction
Human cytochrome c (hCyt c) is one member of a large distributed group of heme proteins, which contains a hexa-coordinated heme c with His18 and Met80 as axial ligands in the native state.1−6 The porphyrin is covalently linked to the peptide chain via two thioether bonds through a conserved CXXCH motif. The protein is composed of five α-helices connected by several loop regions, namely Ω-loops (Figure 1, PDB code 3ZCF).7 Cyt c is located on the outer surface of the inner mitochondrial membrane and has widely been known as an electron shuttle in the mitochondrial respiratory chain.1 The electrons are transported from respiratory complex III to the terminal oxygen reductase via Cyt c, which is essential to cellular life throughout the electron–proton energy supplement process.
Figure 1.
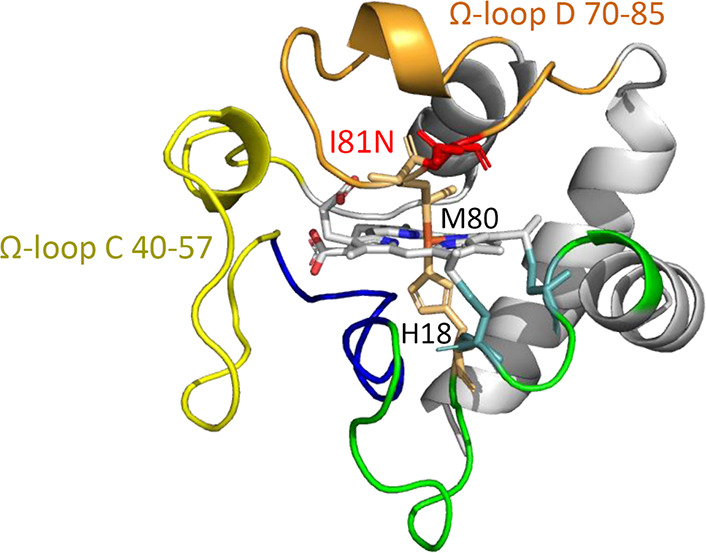
X-ray structure of hCyt c (PDB code 3ZCF)7 showing the overall structure, Ω-loops, and the heme coordination site. The mutation of I81N was modeled using PyMol.
Moreover, Cyt c has received interest for its multifunctionality involving the apoptosis pathway, gene regulation, and redox signaling.1,4,5,8 The discovery of the pro-apoptosis activity of Cyt c was a breakthrough in the biological field. Cyt c can bind to apoptotic protease-activating factor 1 (apaf-1), which is essential for the successful assembly of an activated apoptosome. This process was related to the inherent peroxidase activity of Cyt c, which was further connected to missense mutation, post-translational modification, pH-dependent conformational change, and lipid binding.9 In contrast to the inaccessible hexa-coordinated heme site in the native state, Cyt c can transform into alternative conformations under these perturbations with a more accessible heme site and enhanced peroxidase activity. Thus, Cyt c can oxidize the lipids such as cardiolipin on the mitochondrial membrane to enhance the membrane permeability, which promotes the release of Cyt c to the cytoplasm and subsequently facilitates the assembly of the apoptosome.10,11
This view was supported by experimental results that can be classified as follows: (i) the modification of distal ligand Met80 or the structure change near Met80,12−15 (ii) the structure changes aim to destroy the hydrogen bond networks constituted by Asn52, Tyr67, Thr78, and water,16−18 (iii) the substitution of Lys relating to an alkaline conformation in different pH values,19 and (iv) the losing of Met80 ligation and the formation of a well-defined pocket for binding hydrocarbons when Cyt c interacts with detergents,20 a conformation rearrangement mimic for Cyt c-mediated cardiolipin oxidation.21,22hCyt c was also found to interact with partner proteins to regulate the intrinsic apoptotic process. For example, Cyt c can bind to neuroglobin (Ngb), a heme protein highly expressed in the vertebrate nervous systems.23 This interaction inhibits the binding between apaf-1 and hCyt c, which was proposed as a crucial mechanism to protect mammalian neurons against the apoptotic stimulus.24−26
Because of the important and diverse functions of hCyt c, a few naturally occurring variants were detected.27 Although most of the 19 missense mutations are still lacking studies, 4 mutations (G41S, Y48H, A51V, and Lys100del) were found to be associated with autosomal dominant thrombocytopenia 4.28−31 Our group also showed that the N52S variant exhibited a small fraction of high-spin species and 3–8-fold enhanced peroxidase activity at neutral pH.18 Among other variants, we noticed that position 81 was only allowed a unique Ile-to-Asn missense mutation.5 The appearance of the I81N hCyt c variant inspired us to investigate the structural and functional consequences of the I81N substitution (Figure 1). Position 81 evolves from Ala in yeast to a conserved Ile residue in vertebrates.11 Recently, Bowler and co-workers showed that the I81A mutation had a significant influence on the thermodynamics and kinetics of the access to alternate conformers of hCyt c.32 The substitution may perturb the heme environment close to distal ligand Met80 and Lys79/Lys72. Moreover, I81A hCyt c exhibited a substantial enhancement in peroxidase activity, particularly below pH 7. In addition, Ile81 is suggested to interact with Lys67, Leu70, Val71, Ala74, Leu85, Tyr88, and heme of Ngb, whereas it lacks experimental studies.26
In this study, we combined experimental technologies and the protein structure prediction program Alphafold233,34 to investigate the properties of I81N hCyt c, including the inherent peroxidase activity and the interaction with human Ngb (hNgb). Notably, the results showed that I81N hCyt c exhibited enhanced inherent peroxidase activity compared to that of wild-type (WT) protein, which was pH dependent and raised at low pH values. Moreover, it was found to interact much weakly with hNgb, as determined by isothermal titration calorimetry (ITC), suggesting the unique role of Ile81 in supporting the structure and function of hCyt c and its interaction with Ngb.
Results and Discussion
Spectroscopic Studies of I81N hCyt c
The I81N hCyt c variant was purified from Escherichia coli with high purity (Figure S1A) and was further confirmed by mass spectroscopy (Figure S1B). The ultraviolet–visible (UV–vis) spectra of the I81N variant were similar to those of the WT hCyt c in both the ferric and ferrous forms (Figure S2), with similar maximum absorptions of the Soret band and Q bands. The circular dichroism (CD) spectroscopy showed that the protein retained a typical α-helical structure with negative peaks at 208 and 222 nm and a positive peak at 190 nm in the far-UV region (Figure S3). Although the ellipticity (θ) value at 222 nm was slightly decreased compared to that of the WT hCyt c, the proportion of the α-helical structure (∼36%) and β-strand the structure (∼10%) was almost the same for both proteins, as calculated by K2D2,35 which suggests that the overall secondary structure was not perturbed by the I81N mutation.
To investigate whether the Ile-to-Asn mutation affects the local structural stability of Cyt c, we performed pH perturbation studies in both acidic and alkaline conditions. The Cyt c undergoes the acidic unfolding and the alkaline conformational transition process as described by Theorell and Åkesson.36 These conformational transitions are related to the inherent peroxidase activities of Cyt c and are routinely performed to study the local structural dynamics around the heme moiety.37 The specific peak of the Fe(III)–S bond at 695 nm was chosen to monitor the alkaline transition process, in which the Met-heme moiety transforms to a Lys-heme moiety.38 It also induced a 7 nm blue shift at the Soret peak due to the structural change adjacent to the heme moiety (Figure S4). As shown in Figure 2A, the pH midpoint (pH1/2) of the alkaline transition decreased from 9.23 ± 0.04 of the WT hCyt c to 8.75 ± 0.03 of the I81N variant, with the proton linkage number (n) varying from 0.85 to 1 (Table 1). This result suggests that Ile-to-Asn substitution altered the Met80-heme ligation of hCyt c in alkaline pHs.
Figure 2.
Alkaline conformational transition and acidic unfolding studies on the WT and I81N hCyt c. (A) Normalized absorbance at 695 nm in the alkaline pH range. (B) pH-dependent UV–vis spectra changes of the I81N hCyt c in acidic unfolding studies. (C) Normalized absorbance of the Soret band in the acidic pH range. (D) Absorbance at 622 nm in the acidic pH range.
Table 1. Experimental Parameters Related to the Local Structural Stability of I81N Cyt c.
| I81N | WT | ||
|---|---|---|---|
| alkaline transition | pH1/2 695nm | 8.75 ± 0.03 | 9.23 ± 0.04 |
| n | 0.85 ± 0.07 | 0.99 ± 0.08 | |
| acidic unfolding | pH1/2 soret | 2.55 ± 0.07 | 2.03 ± 0.03 |
| n | 0.48 ± 0.06 | 0.88 ± 0.05 | |
| pH1/2 622nm | 2.92 ± 0.01 | 2.32 ± 0.03 | |
| n | 1.07 ± 0.03 | 1.09 ± 0.06 | |
| azide binding | kobs (s–1) | 0.0085 ± 0.002 | 0.0014 ± 0.0002 |
The alteration of the local structure of the I81N hCyt c was also observed under acidic conditions. The acidic unfolding process was monitored at both the Soret band and the Q band at 622 nm (Figures 2B and S5). The Q band is contributed by the Fe(III)–O bond of the high spin H2O-heme, as alternative conformers under acidic conditions.39 The acid titration data show that the I81N variant exhibits a 0.5–0.6 units higher pH1/2 than that of the WT protein (Figure 2C,D and Table 1). The pH1/2 soret increases from 2.03 ± 0.03 to 2.55 ± 0.07 and the pH1/2 622nm increases from 2.32 ± 0.03 to 2.92 ± 0.01, respectively. Together with the alkaline titration data, the results show that the I81N variant unfolds for ∼0.5 units of pH early in both acidic and alkaline conditions, which indicates that the Met80-heme moiety of the I81N variant might be less stable than that of the WT Cyt c.
To further confirm the lability of the Met80-heme ligation, we performed kinetic studies using a small ligand, the azide ion (N3–), to compete with the axial ligand Met80 in the protein matrix (Figures 3 and S6). The reaction is assumed to follow an SN1 mechanism, in which the hexa-coordinated heme is in equilibrium with a penta-coordinated state and thus leaves an open proximal site for ligand binding (Figure 3C). The observed rate constant (kobs) was obtained under pseudo first-order conditions. As shown in Table 1, I81N hCyt c (kobs = 8.53 ± 2.41 × 10–3 s–1) reacts with azide ∼6 times faster than the WT protein (kobs = 1.44 ± 0.2 × 10–3 s–1). The faster rate of the azide binding to the heme of I81N hCyt c may suggest the faster dissociation of the Met80-heme ligation. Previous studies have shown that mutations in the Ω-loop D (70–85) such as K72A, P76C, K79G/M80X, I81A, F82K, and V83G may alter the heme crevice dynamics and ligand-binding properties.19,32,40−42
Figure 3.
Azide-binding studies on I81N hCyt c. (A) Time-dependent UV–vis spectra upon mixing 200 mM NaN3 with 10 mM protein sample in 50 mM potassium phosphate buffer at pH 7.0, 25 °C. (B) Difference spectra obtained by subtracting the spectrum at 0 s. (C) Proposed SN1 mechanism for the azide-binding reaction. (D) Absorbance changes at 420 nm upon NaN3 titration.
Peroxidase Activity of I81N hCyt c
The structural perturbations by I81N substitution may affect the inherent peroxidase activity of Cyt c because a tight and stable conformation is essential to keep it at a low peroxidase activity level in physiological conditions.28 As recently reported, Cyt c samples the high-spin conformers more frequently under acidic conditions.43 Meanwhile, the intermembrane space of mitochondria is an acidic pH environment (6.88 ± 0.09) and the intracellular pH decreases during apoptosis.10,38,44 Thus, it is physiologically relevant to investigate the relationship between the peroxidase activity of I81N hCyt c and the acidic pH change.
To confirm this speculation, we performed the stopped-flow assay under pH 5–8 conditions using guaiacol as a substrate (Figures 4 and S7). The kinetic parameters (kcat and Km) were obtained by fitting the data into the Michaelis–Menten model. As shown in Table 2, the difference in Km was small for those of the WT and I81N Cyt c variant, which was comparable to the data of the I81A variant reported by Bowler and co-workers.32 However, the kcat value of the I81N variant exhibited a strong relationship with pH values, increasing from 0.16 s–1 at pH 7 to 0.39 s–1 at pH 5, whereas the difference was subtle in the case of the WT protein at pH 5–7 (Figure 4B). The enhancement of the peroxidase activity of I81N Cyt c suggests that the variant samples the high-spin conformation easier than the WT under acidic conditions, which agrees with the acidic unfolding experiments. Notably, the kcat value for the I81N variant is also up to threefold higher than that of the WT at pH 5. The strong correlation between the peroxidase activity and acidic pH indicates that Ile81 is a peroxidase trigger in hCyt c. It is worth mentioning that the peroxidase activity of the I81A variant (kcat = 0.96 ± 0.01 s–1)32 is 6-fold higher than that of the I81N (kcat = 0.16 ± 0.01 s–1) and 8.7-fold higher than that of the WT (kcat = 0.11 ± 0.01 s–1) at neutral pH, and thus the I81A mutation may not be allowed naturally.
Figure 4.

Peroxidase activity assay using guaiacol as a substrate. (A) Michaelis–Menten plots vs the concentrations of guaiacol for I81N hCyt c at pH 5–8. (B) kcat vs pH values for I81N and WT hCyt c. The error bars are based on the standard deviation of six independent experiments.
Table 2. Kinetic Parameters of the Peroxidase Activity of hCyt c Variants at Different pH Values with Guaiacol as a Substrate.
|
kcat (s–1) |
Km (μM) |
|||
|---|---|---|---|---|
| pH | I81N | WT | I81N | WT |
| 5.0 | 0.39 ± 0.01 | 0.13 ± 0.01 | 36.9 ± 3 | 50.2 ± 9 |
| 6.0 | 0.25 ± 0.006 | 0.14 ± 0.03 | 21.2 ± 2 | 20.0 ± 1 |
| 7.0 | 0.16 ± 0.003 | 0.11 ± 0.002 | 30.5 ± 1 | 27.5 ± 1 |
| 8.0 | 0.066 ± 0.003 | 0.047 ± 0.002 | 28.1 ± 3 | 12.9 ± 3 |
In addition to the guaiacol assay, the heme degradation by H2O2 was also performed to gain more information about the enzymatic kinetics. The partial oxidation of Cyt c is a crucial step to initiating the peroxidase activity.45 In the absence of a substrate, the activation of H2O2 in the heme center results in heme degradation by self-oxidation. This process was monitored at the Soret band, which decreased over time during the degradation process (Figures 5 and S8). The observed rate constants (kobs) were obtained at various concentrations of H2O2. It showed the I81N variant degraded about 1.5–3-fold faster than the WT protein depending on the concentration of H2O2 (Figure 5B). It should be noted that the reaction between I81N Cyt c and H2O2 did not follow a pseudo first-order mechanism, with a positive intercept (∼0.005 s–1) representing the dissociation rate constant (kd) of the Cyt c–H2O2 complex.46 This observation is similar to those reported for H2O2 binding to other heme proteins such as bacterial peroxidase and myoglobin mutants with altered heme active sites,47,48 which suggests that the I81N mutation facilitates not only H2O2 activation but also its dissociation from the heme center.
Figure 5.
Heme degradation of hCyt c by H2O2. (A) Time-dependent UV–vis spectra of I81N hCyt c in a reaction with 100 mM H2O2. The spectral change of the Soret band was shown as an inset. (B) Linear fitting of kobs as a function of H2O2 concentrations.
Interactions between I81N hCyt c and Ngb
It is believed that the enhanced peroxidase activity of Cyt c is related to the instinct apoptosis pathway. However, it must be strictly regulated, especially in neurons. The interaction between Ngb and Cyt c was proposed as a crucial mechanism to protect neurons in vertebrates.24 Previous ITC studies showed the dissociation constant between hNgb and horse heart Cyt c was Kd = 22.0 ± 1.7 μM in 10 mM phosphate buffer.49 Several acidic residues in Ngb were also found to participate in the interaction with Cyt c.26,49−51 Meanwhile, depicting the precise binding interface is still a great challenge.
To gain more insight into the structure–function relationship of the conserved Ile81, we applied the most accurate protein structure prediction program Alphafold2 to predict the potential interactions between hNgb and hCyt c. The five WT hNgb–hCyt c models generated using Alphafold2 displayed a convergent interaction interface with a high predicted local distance difference test (pLDDT) score and a low predicted align error (Figures 6A,B). As the program identified, the interface hotspots include four pairs of hydrogen bonds (K72-D73, K86-D63, Q16-S91, and Q16-K95, Figure 6C) and several hydrophobic contacts (Figure 6D). Notably, the Ile81 forms a hydrophobic core, which interacts with the strictly conserved residues Leu70, Val71, Ala74, Lys85, and Tyr88 in Ngb (Figure S9). This is a reasonable prediction because these two proteins are coevolutionary in vertebrates and the Ile81 is conserved among vertebrate Cyt c. Other interface hotspots in hCyt c include Gln16, Thr28, Lys72, Lys79, Val83, and Lys86 in both oxidation states (Figure S10). It should be noted that some of these residues are also located at the contacting interface with apaf-1, such as Ile81, Lys72, and Lys86 (Figure S11),52 which suggests that the interaction between hCyt c and apaf-1 might be blocked by the binding of Ngb.
Figure 6.
WT hNgb–hCyt c protein complex model predicted by the Alphafold2 multimer. (A) Cartoon representation of the five predicted models. These models were colored according to the pLDDT score. (B) Predicted alignment error of the model with the highest confidence. (C) Hydrogen bond networks in the predicted protein–protein interaction interface formed by the αE and αF helices of hNgb (green) and the Ω loop D and the α1 helix of hCyt c (cyan). The distance between the heme b and heme c groups was indicated as black dash arrows. (D) Hydrophobic core of the interaction interface. The Ile81 of hCyt c was colored red.
To confirm that the Ile81 residue of hCyt c plays a critical role in interaction with hNgb, we performed ITC studies. To make a comparison of the binding affinity, we performed the titrations in 1 mM potassium phosphate solution at pH 7 (Figure 7) because the binding affinity was lower with the increase in the concentration of phosphate (Figure S12). The binding constant was determined to be Ka = (5.30 ± 0.26) × 103 M–1 (Kd = 189 μM) for I81N hCyt c (the error was produced from the data fitting), which decreases almost 40-folds compared to that for the WT hCyt c, Ka = (2.14 ± 0.53) × 105 M–1 (Kd = 4.67 μM). Moreover, the reaction enthalpy (ΔH) and entropy (ΔS) changes for I81N hCyt c binding to Ngb were ∼6-fold and ∼2-fold higher than that of hCyt c, respectively. The positive ΔH and ΔS suggest a predominant entropy-driven formation of the Cyt c–Ngb complex, resulting in negative Gibbs free energy (ΔG). The increased entropy may be related to the release of water molecules from the binding interface during the formation of the protein–protein complex.49
Figure 7.
Representative ITC thermogram of WT (A) and I81N (B) hCyt c titrated into hNgb. Both samples were prepared in a 1 mM potassium phosphate solution at pH 7.0. The data were fitted to the OneSites binding model.
The Alphafold2 program suggests that the binding interface mediated by position 81 was abolished due to the Ile-to-Asn mutation in the hydrophobic core, resulting in different orientations for residue 81 (Figure 8A). The new contact interface includes Lys8, Ile11, Met12, Lys13, and Gln16 in I81N hCyt c and Asp73, Leu70, Ala74, Leu85, Tyr88, and Ser91 in Ngb, where two hydrogen bonds (Q16-S91 and K8-D73) were formed in addition to hydrophobic interactions among other residues (Figure 8B). The molecular dynamics simulations further suggest that this interaction is less stable than that of the WT protein, with a large root-mean-square deviation (rmsd) (∼6.5 Å) of the protein backbone (Figure S13). These experimental and simulation results thus confirm that the residue Ile81 is important for hCyt c to interact with hNgb.
Figure 8.
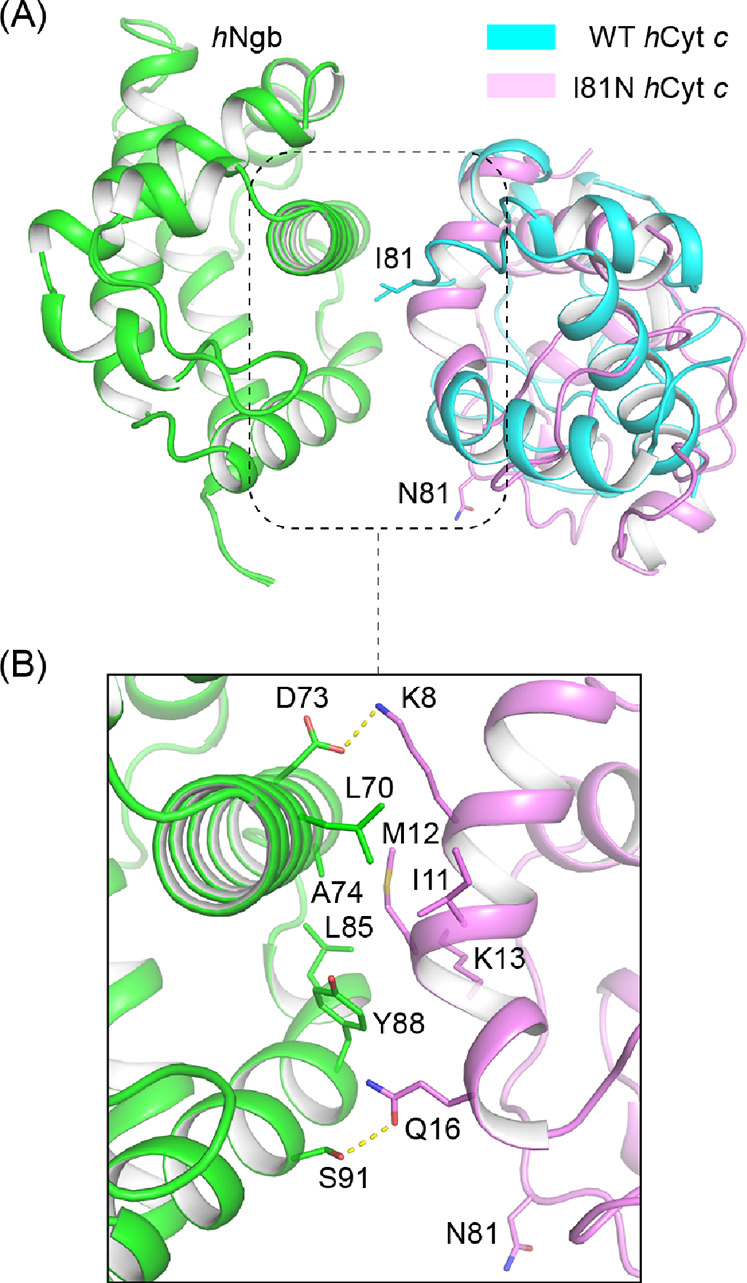
Complex models of hNgb-WT/I81N hCyt c predicted using Alphafold2. (A) Structural alignment of WT and I81N hCyt c, showing the different orientations of residue 81. (B) Protein–protein interface of hNgb-I81N hCyt c in the model with the highest confidence.
Conclusions
In summary, we have shown that the naturally occurring I81N mutation in hCyt c regulates both the inherent peroxidase activity and the interaction with human Ngb. The Ile-to-Asn mutation perturbs the local structural stability under both alkaline and acidic pH conditions. The Met80-Fe(III) bond reacts with azide sixfold faster than that of the WT protein, which confirms the lability of Met80-heme ligation in the I81N variant. Therefore, the inherent peroxide activity was increased due to a more accessible penta-coordinated heme site. High-accuracy structure prediction by deep neural networks, the Alphafold2 program, suggests that Ile81 is located at the hNgb–hCyt c interaction interface, serving as the hydrophobic core. The mutation of the hydrophobic Ile to the hydrophilic Asn may abolish the specific interaction between hCyt c and hNgb. As further shown by the ITC studies, the apparent binding affinity decreased by ∼40-fold upon I81N mutation. Taken together, these results illustrate that the residue Ile81 of hCyt c plays a critical role in protein function regulation, which provides an insight into the coevolution of Cyt c and Ngb in vertebrates.
Acknowledgments
We gratefully thank Prof. T. Burmester of the Gutenberg University of Mainz, Germany, for graciously providing the gene of human Ngb. The pBTR1 plasmid containing the coding sequence of the WT hcyt c gene was obtained from Addgene (no. 22468). This work was supported by the National Natural Science Foundation of China (21977042 and 32171270).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01256.
Materials and methods; SDS-PAGE of the purified WT and I81N Cyt c; ESI-MS spectra of the purified I81N hCyt c; UV–vis spectra of I81N and WT hCyt c; CD spectra of the ferric WT and I81N hCyt c; alkaline conformational transition of WT hCyt c; acidic titration of WT hCyt c; azide-binding experiment for WT hCyt c; stopped-flow spectra of guaiacol oxidation by H2O2 catalyzed by WT or I81N hCyt c; Michaelis–Menten plots for WT hCyt c at pH 5–8; time-dependent UV–vis spectra of WT hCyt c in a reaction with H2O2; multisequence alignment of vertebrate Ngb; 20 lowest energy solution structures of the reduced (PDB code 2N9I) and oxidized (PDB code 2N9J) hCyt c solved by NMR; cryo-EM structure of the apoptosome complex (PDB codes 3JBT and 5JUY); representative ITC thermogram of WT hCyt c titrated into hNgb; and rmsd of the Cα atoms in a 200 ns molecular dynamics simulation trajectory (PDF)
Author Contributions
∥ Y.F. and X.-C.L. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Bertini I.; Cavallaro G.; Rosato A. Cytochrome c: occurrence and functions. Chem. Rev. 2006, 106, 90–115. 10.1021/cr050241v. [DOI] [PubMed] [Google Scholar]
- Smith L. J.; Kahraman A.; Thornton J. M. Heme proteins--diversity in structural characteristics, function, and folding. Proteins 2010, 78, 2349–2368. 10.1002/prot.22747. [DOI] [PubMed] [Google Scholar]
- Liu J.; Chakraborty S.; Hosseinzadeh P.; Yu Y.; Tian S.; Petrik I.; Bhagi A.; Lu Y. Metalloproteins Containing Cytochrome, Iron–Sulfur, or Copper Redox Centers. Chem. Rev. 2014, 114, 4366–4469. 10.1021/cr400479b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal L.; Tomasina F.; Capdevila D. A.; Demicheli V.; Tórtora V.; Alvarez-Paggi D.; Jemmerson R.; Murgida D. H.; Radi R. Alternative Conformations of Cytochrome c: Structure, Function, and Detection. Biochemistry 2016, 55, 407–428. 10.1021/acs.biochem.5b01385. [DOI] [PubMed] [Google Scholar]
- Alvarez-Paggi D.; Hannibal L.; Castro M. A.; Oviedo-Rouco S.; Demicheli V.; Tórtora V.; Tomasina F.; Radi R.; Murgida D. H. Multifunctional Cytochrome c: Learning New Tricks from an Old Dog. Chem. Rev. 2017, 117, 13382–13460. 10.1021/acs.chemrev.7b00257. [DOI] [PubMed] [Google Scholar]
- Lin Y.-W. Structure and function of heme proteins regulated by diverse post-translational modifications. Arch. Biochem. Biophys. 2018, 641, 1–30. 10.1016/j.abb.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Rajagopal B. S.; Edzuma A. N.; Hough M. A.; Blundell K. L. I. M.; Kagan V. E.; Kapralov A. A.; Fraser L. A.; Butt J. N.; Silkstone G. G.; Wilson M. T.; et al. The hydrogen-peroxide-induced radical behaviour in human cytochrome c-phospholipid complexes: implications for the enhanced pro-apoptotic activity of the G41S mutant. Biochem. J. 2013, 456, 441–452. 10.1042/BJ20130758. [DOI] [PubMed] [Google Scholar]
- Santucci R.; Sinibaldi F.; Cozza P.; Polticelli F.; Fiorucci L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. 10.1016/j.ijbiomac.2019.06.180. [DOI] [PubMed] [Google Scholar]
- McClelland L. J.; Mou T.-C.; Jeakins-Cooley M. E.; Sprang S. R.; Bowler B. E. Structure of a mitochondrial cytochrome c conformer competent for peroxidase activity. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 6648–6653. 10.1073/pnas.1323828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S.; Llopis J.; Deveraux Q. L.; Tsien R. Y.; Reed J. C. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2000, 2, 318–325. 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- Bandi S.; Bowler B. E. Effect of an Ala81His mutation on the Met80 loop dynamics of iso-1-cytochrome c. Biochemistry 2015, 54, 1729–1742. 10.1021/bi501252z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.-H.; Lin Y.-W.; Rosell F. I.; Ni F.-Y.; Lu H.-J.; Yang P.-Y.; Tan X.-S.; Li X.-Y.; Huang Z.-X.; Mauk A. G. Converting cytochrome c into a peroxidase-like metalloenzyme by molecular design. Chembiochem 2007, 8, 607–609. 10.1002/cbic.200600547. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Matsuo T.; Nagao S.; Hirota S. Peroxidase activity enhancement of horse cytochrome c by dimerization. Org. Biomol. Chem. 2011, 9, 4766–4769. 10.1039/c1ob05552f. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Ando Y.; Nugraheni A. D.; Ren C.; Nagao S.; Hirota S. Self-oxidation of cytochrome c at methionine80 with molecular oxygen induced by cleavage of the Met-heme iron bond. Mol. Biosyst. 2014, 10, 3130–3137. 10.1039/c4mb00285g. [DOI] [PubMed] [Google Scholar]
- Nugraheni A. D.; Ren C.; Matsumoto Y.; Nagao S.; Yamanaka M.; Hirota S. Oxidative modification of methionine80 in cytochrome c by reaction with peroxides. J. Inorg. Biochem. 2018, 182, 200–207. 10.1016/j.jinorgbio.2018.02.017. [DOI] [PubMed] [Google Scholar]
- Ying T.; Wang Z.-H.; Lin Y.-W.; Xie J.; Tan X.; Huang Z.-X. Tyrosine-67 in cytochrome c is a possible apoptotic trigger controlled by hydrogen bonds via a conformational transition. Chem. Commun. 2009, 4512–4514. 10.1039/b904347k. [DOI] [PubMed] [Google Scholar]
- Lan W.; Wang Z.; Yang Z.; Ying T.; Zhang X.; Tan X.; Liu M.; Cao C.; Huang Z.-X. Structural basis for cytochrome c Y67H mutant to function as a peroxidase. PLoS One 2014, 9, e107305 10.1371/journal.pone.0107305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou D.; Liu X.-C.; Wang X.-J.; Gao S.-Q.; Wen G.-B.; Lin Y.-W. The importance of Asn52 in the structure–function relationship of human cytochrome c. RSC Adv. 2020, 10, 44768–44772. 10.1039/d0ra09961a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold S. M.; Lei H.; Mou T.-C.; Bowler B. E. Effect of a K72A Mutation on the Structure, Stability, Dynamics, and Peroxidase Activity of Human Cytochrome c. Biochemistry 2017, 56, 3358–3368. 10.1021/acs.biochem.7b00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland L. J.; Steele H. B. B.; Whitby F. G.; Mou T.-C.; Holley D.; Ross J. B. A.; Sprang S. R.; Bowler B. E. Cytochrome c Can Form a Well-Defined Binding Pocket for Hydrocarbons. J. Am. Chem. Soc. 2016, 138, 16770–16778. 10.1021/jacs.6b10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A.; Hoop C. L.; DeLucia M.; Kodali R.; Kagan V. E.; Ahn J.; van der Wel P. C. A. Structural Changes and Proapoptotic Peroxidase Activity of Cardiolipin-Bound Mitochondrial Cytochrome c. Biophys. J. 2015, 109, 1873–1884. 10.1016/j.bpj.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parui P. P.; Sarakar Y.; Majumder R.; Das S.; Yang H.; Yasuhara K.; Hirota S. Determination of proton concentration at cardiolipin-containing membrane interfaces and its relation with the peroxidase activity of cytochrome c. Chem. Sci. 2019, 10, 9140–9151. 10.1039/c9sc02993a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester T.; Weich B.; Reinhardt S.; Hankeln T. A vertebrate globin expressed in the brain. Nature 2000, 407, 520–523. 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- Fago A.; Mathews A. J.; Moens L.; Dewilde S.; Brittain T. The reaction of neuroglobin with potential redox protein partners cytochrome b5 and cytochrome c. FEBS Lett. 2006, 580, 4884–4888. 10.1016/j.febslet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Basu S.; Keszler A.; Azarova N. A.; Nwanze N.; Perlegas A.; Shiva S.; Broniowska K. A.; Hogg N.; Kim-Shapiro D. B. A novel role for cytochrome c: Efficient catalysis of S-nitrosothiol formation. Free Radic. Biol. Med. 2010, 48, 255–263. 10.1016/j.freeradbiomed.2009.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari P. B.; Chapagain P. P.; Üren A. Investigating molecular interactions between oxidized neuroglobin and cytochrome c. Sci. Rep. 2018, 8, 10557. 10.1038/s41598-018-28836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek M.; Karczewski K. J.; Minikel E. V.; Samocha K. E.; Banks E.; Fennell T.; O’Donnell-Luria A. H.; Ware J. S.; Hill A. J.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsisiotis A. I.; Deacon O. M.; Wilson M. T.; Macdonald C.; Blumenschein T. M. A.; Moore G. R.; Worrall J. A. R. Increased dynamics in the 40-57 Omega-loop of the G41S variant of human cytochrome c promote its pro-apoptotic conformation. Sci. Rep. 2016, 6, 30447. 10.1038/srep30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon O. M.; Svistunenko D. A.; Moore G. R.; Wilson M. T.; Worrall J. A. R. Naturally Occurring Disease-Related Mutations in the 40-57 Omega-Loop of Human Cytochrome c Control Triggering of the Alkaline Isomerization. Biochemistry 2018, 57, 4276–4288. 10.1021/acs.biochem.8b00520. [DOI] [PubMed] [Google Scholar]
- Lei H.; Bowler B. E. Naturally Occurring A51V Variant of Human Cytochrome c Destabilizes the Native State and Enhances Peroxidase Activity. J. Phys. Chem. B 2019, 123, 8939–8953. 10.1021/acs.jpcb.9b05869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon O. M.; White R. W.; Moore G. R.; Wilson M. T.; Worrall J. A. R. Comparison of the structural dynamic and mitochondrial electron-transfer properties of the proapoptotic human cytochrome c variants, G41S, Y48H and A51V. J. Inorg. Biochem. 2020, 203, 110924. 10.1016/j.jinorgbio.2019.110924. [DOI] [PubMed] [Google Scholar]
- Lei H.; Nold S. M.; Motta L. J.; Bowler B. E. Effect of V83G and I81A Substitutions to Human Cytochrome c on Acid Unfolding and Peroxidase Activity below a Neutral pH. Biochemistry 2019, 58, 2921–2933. 10.1021/acs.biochem.9b00295. [DOI] [PubMed] [Google Scholar]
- Jumper J.; Evans R.; Pritzel A.; Green T.; Figurnov M.; Ronneberger O.; Tunyasuvunakool K.; Bates R.; Žídek A.; Potapenko A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R.; O’Neill M.; Pritzel A.; Antropova N.; Senior A.; Green T.; Žídek A.; Bates R.; Blackwell S.; Yim J.; Ronneberger O.; Bodenstein S.; Zielinski M.; Bridgland A.; Potapenko A.; Cowie A.; Tunyasuvunakool K.; Jain R.; Clancy E.; Kohli P.; Jumper J.; Hassabis D.. Protein complex prediction with AlphaFold-Multimer. 2021, Biorxiv: 10.1101/2021.10.04.463034. [Google Scholar]
- Perez-Iratxeta C.; Andrade-Navarro M. A. K2D2: estimation of protein secondary structure from circular dichroism spectra. BMC Struct. Biol. 2008, 8, 25. 10.1186/1472-6807-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theorell H.; Åkesson Å. Studies on cytochrome c. III. Titration curves. J. Am. Chem. Soc. 1941, 63, 1818–1820. 10.1021/ja01852a007. [DOI] [Google Scholar]
- Rosell F. I.; Ferrer J. C.; Mauk A. G. Proton-linked protein conformational switching: Definition of the alkaline conformational transition of yeast iso-1-ferricytochrome c. J. Am. Chem. Soc. 1998, 120, 11234–11245. 10.1021/ja971756+. [DOI] [Google Scholar]
- Nilsson C.; Kågedal K.; Johansson U.; Öllinger K. Analysis of cytosolic and lysosomal pH in apoptotic cells by flow cytometry. Methods Cell Sci. 2004, 25, 185–194. 10.1007/s11022-004-8228-3. [DOI] [PubMed] [Google Scholar]
- Rosell F. I.; Harris T. R.; Hildebrand D. P.; Döpner S.; Hildebrandt P.; Mauk A. G. Characterization of an alkaline transition intermediate stabilized in the Phe82Trp variant of yeast iso-1-cytochrome c. J. Biochem. 2000, 39, 9047–9054. 10.1021/bi001095k. [DOI] [PubMed] [Google Scholar]
- Lalli D.; Rosa C.; Allegrozzi M.; Turano P. Distal Unfolding of Ferricytochrome c Induced by the F82K Mutation. Int. J. Mol. Sci. 2020, 21, 2134. 10.3390/ijms21062134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsri S.; Prasertsuk P.; Nutho B.; Pornsuwan S. Molecular insights on the conformational dynamics of a P76C mutant of human cytochrome c and the enhancement on its peroxidase activity. Arch. Biochem. Biophys. 2022, 716, 109112. 10.1016/j.abb.2021.109112. [DOI] [PubMed] [Google Scholar]
- Zhong F.; Alden S. L.; Hughes R. P.; Pletneva E. V. Comparing Properties of Common Bioinorganic Ligands with Switchable Variants of Cytochrome c. Inorg. Chem. 2022, 61, 1207–1227. 10.1021/acs.inorgchem.1c02322. [DOI] [PubMed] [Google Scholar]
- Samsri S.; Pornsuwan S. Influence of cysteine-directed mutations at the Omega-loops on peroxidase activity of human cytochrome c. Arch. Biochem. Biophys. 2021, 709, 108980. 10.1016/j.abb.2021.108980. [DOI] [PubMed] [Google Scholar]
- Porcelli A. M.; Ghelli A.; Zanna C.; Pinton P.; Rizzuto R.; Rugolo M. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem. Biophys. Res. Commun. 2005, 326, 799–804. 10.1016/j.bbrc.2004.11.105. [DOI] [PubMed] [Google Scholar]
- Matsui T.; Nakajima A.; Fujii H.; Matera K. M.; Migita C. T.; Yoshida T.; Ikeda-Saito M. O2-and H2O2-dependent verdoheme degradation by heme oxygenase: Reaction mechanisms and potential physiological roles of the dual pathway degradation. Biol. Chem. 2005, 280, 36833–36840. 10.1074/jbc.m503529200. [DOI] [PubMed] [Google Scholar]
- Tomášková N.; Varinska L.; Sedlak E. Rate of oxidative modification of cytochrome c by hydrogen peroxide is modulated by Hofmeister anions. Gen. Physiol. Biophys. 2010, 29, 255–265. 10.4149/gpb.2010.03.255. [DOI] [PubMed] [Google Scholar]
- Auer M.; Nicolussi A.; Schütz G.; Furtmüller P. G.; Obinger C. How covalent heme to protein bonds influence the formation and reactivity of redox intermediates of a bacterial peroxidase. J. Biol. Chem. 2014, 289, 31480–31491. 10.1074/jbc.M114.595157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D.-J.; Yuan H.; Li W.; Xiang Y.; He B.; Nie C.-M.; Wen G.-B.; Lin Y.-W.; Tan X. How a novel tyrosine-heme cross-link fine-tunes the structure and functions of heme proteins: a direct comparitive study of L29H/F43Y myoglobin. Dalton Trans. 2015, 44, 18815–18822. 10.1039/c5dt03040d. [DOI] [PubMed] [Google Scholar]
- Tiwari P. B.; Astudillo L.; Pham K.; Wang X.; He J.; Bernad S.; Derrien V.; Sebban P.; Miksovska J.; Darici Y. Characterization of molecular mechanism of neuroglobin binding to cytochrome c: A surface plasmon resonance and isothermal titration calorimetry study. Inorg. Chem. Commun. 2015, 62, 37–41. 10.1016/j.inoche.2015.10.010. [DOI] [Google Scholar]
- Bønding S. H.; Henty K.; Dingley A. J.; Brittain T. The binding of cytochrome c to neuroglobin: a docking and surface plasmon resonance study. Int. J. Biol. Macromol. 2008, 43, 295–299. 10.1016/j.ijbiomac.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Tejero J. Negative surface charges in neuroglobin modulate the interaction with cytochrome c. Biochem. Biophys. Res. Commun. 2020, 523, 567–572. 10.1016/j.bbrc.2019.12.089. [DOI] [PubMed] [Google Scholar]
- Yu T.; Wang X.; Purring-Koch C.; Wei Y.; McLendon G. L. A mutational epitope for cytochrome C binding to the apoptosis protease activation factor-1. J. Biol. Chem. 2001, 276, 13034–13038. 10.1074/jbc.M009773200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







