Abstract

In this study, a novel heterocyclic amide derivative, N-(3-cyanothiophen-2-yl)-2-(thiophen-2-yl)acetamide (I), was obtained by reacting 2-aminothiophene-3-carbonitrile with activated 2-(thiophen-2-yl)acetic acid in a N-acylation reaction and characterized by elemental analyses, FT-IR, 1H and 13C NMR spectroscopic studies, and single crystal X-ray crystallography. The crystal packing of I is stabilized by C–H···N and N–H···N hydrogen bonds. In addition, I was investigated computationally using the density functional theory (DFT) method with the B3LYP exchange and correlation functions in conjunction with the 6311++G(d,p) basis set in the gas phase. Fukui function (FF) analysis was also carried out. Electrophilicity-based charge transfer (ECT) method and charge transfer (ΔN) were computed to examine the interactions between I and DNA bases (such as guanine, thymine, adenine, and cytosine). The most important contributions to the Hirshfeld surface are H···H (21%), C···H (20%), S···H (19%), N···H (14%), and O···H (12%). An ABTS antioxidant assay was used to evaluate the in vitro antioxidant activity of I. The compound exhibited moderate antioxidant activity. The antimicrobial activity of the title molecule was investigated under aseptic conditions, using the microdilution method, against Gram-positive and Gram-negative bacterial strains, and it also demonstrated significant activity against yeasts (Candida glabrata ATCC 90030, Candida krusei ATCC 34135). The findings revealed that the molecule possesses significant antioxidant and antimicrobial properties.
Introduction
In organic chemistry, amides that contain the R–CO–NHR functional group are typically formed when a carboxylic acid reacts with an amine.1,2 Furthermore, the amide bond exists in the structures of proteins, peptides, and other important biological molecules.3 It is also an important functional group and essential component of many pharmaceuticals, natural products, agrochemicals, and polymers.4,5 Recently, the chemistry of amide compounds has recently been a fascinating field of research. Some of these compounds have been shown to have significant antifungal, antibacterial,6,7 antioxidant,7−11 insecticide,12 anticonvulsant, analgesic, antitumor, anti-inflammatory, and anti-HSV activity.13−17 Additionally, amides also have a broad range of different applications in polymers, dyes, and liquid crystals.6,9,17
Herein, we designed a novel amide compound containing a heteroatom and synthesized by N-acylation of 2-aminothiophene-3-carbonitrile with activated 2-(thiophen-2-yl)acetic acid. The structure of I was confirmed by IR, 1H NMR, 13C NMR, elemental analysis, and X-ray diffraction. The ABTS antioxidant assay was used to measure in vitro antioxidant activity. In addition, the antimicrobial activity of I in vitro was also performed to test its antimicrobial effects against various microbial species. In recent years, the direct comparison of experimental and theoretical results for the characterization of a compound has been of great importance.18,19 Therefore, in this study, we applied density functional theory (DFT) to understand the detailed electronic properties of I at the B3LYP/6-311++G(d,p) level of theory. Fukui function for electrophilic fk+(r) and nucleophilic attack fk(r) were used to assess the chemical reactivity. Electron affinity (EA), ionization potential (IA), hardness (η), and chemical potential (μ) were also investigated. Finally, the geometry optimizations were obtained in the same level of theory to analyze the interactions between compound I and the DNA bases (such as guanine, thymine, adenine, and cytosine). The single point energy of the optimized structure was computed for its neutral, cation, and anion states to understand the electrophilicity-based charge transfer (ECT) method and charge transfer (ΔN) phenomena. Hirshfeld surface analysis was used to specify the close intermolecular interactions in the molecule.
Experimental Part
Instruments
Reagents have been bought from Sigma-Aldrich, Merck, or ABCR, and solvents used are of analytical purity. 1H and 13C NMR spectra were taken on Bruker/Biospin (400 MHz) in CDCl3 spectrometer. FT-IR spectrum was recorded on a Bruker Vertex 80 V spectrophotometer. Melting points were recorded with a Stuart SMP 30. Elemental analyses were performed with a Costech, ECS 4010 elemental analyzer.
Synthesis of N-(3-Cyanothiophen-2-yl)-2-(thiophen-2-yl)acetamide, I, C11H8N2OS2
Synthesis of N-(3-cyanothiophen-2-yl)-2-(thiophen-2-yl)acetamide is a two-step process. In this two-step process, 2-(thiophen-2-yl)acetic acid was first activated by converting it into 2-(thiophen-2-yl)acetyl chloride and then reacted with 2-aminothiophene-3-carbonitrile. We have described the activation of the first step, carboxylic acid, with thionyl chloride in our previous study.20 The acylation step was carried out in the second step, which involved dissolving 2-aminothiophene-3-carbonitrile (10 mmol) in 12 mL of THF and the addition of 0.95 mL of triethylamine (10 mmol) into it. 2-(Thiophen-2-yl)acetyl chloride (1.19 g, 11 mmol) dissolved in 10 mL of THF was slowly added to this reaction mixture. After 15 h of stirring at room temperature, the reaction mixture was filtered and the white salt residue was removed. The solid product was washed with water several times before being filtered, dried, and crystallized from acetonitrile.
Yield: 1.68 g, mp 163–166 °C, yield 58%; Anal. Calcd for C11H8N2S2O: C, 53.17; H, 3.22; N, 11.28; S, 25.78. Found: C, 53.19; H, 3.20; N, 11.06; S, 24.20.21
The N-(3-cyanothiophen-2-yl)-2-(thiophen-2-yl)acetamide molecule was synthesized through a two-step reaction shown in Scheme 1. The acid chloride intermediate was formed in the first step, activation, and then the key intermediates reacted with the heterocyclamine in the second step. We preferred the amide synthesis method from acyl chlorides, which are widely used in the literature. Due to this method, we were able to easily separate the product from the reaction medium.
Scheme 1. Synthesis of N-(3-Cyanothiophen-2-yl)-2-(thiophen-2-yl)acetamide.
Crystal Structure Determination
A Bruker diffractometer equipped with graphite-monochromatic Mo Kα radiation at 296 K (λ = 0.71073 Å) was used to collect the data of I. A Bruker APEX222 was used during the data collection. The structure of the cobalt complex was solved using SHELXT,23 and refinement was made on Olex2 with SHELXL-2018 with least-squares minimization versus F2.24 Also, Mercury for Windows,25 PLATON,26 WinGX,27 and publCIF28 were used for the process. Table 1 summarizes the experimental details for I.
Table 1. Data Collection and Structure Refinement for I.
| CCDC | 2132193 |
| chemical formula | C11H8N2OS2 |
| temperature (K) | 296 |
| space group | P21/c |
| crystal system | monoclinic |
| Mr | 448.31 |
| a, b, c (Å) | 4.4439 (2), 22.6851 (13), 11.1242 (7) |
| α, β, γ (deg) | 90, 96.530 (2), 90 |
| volume, V (Å3) | 1114.16 (11) |
| crystal size (mm) | 0.30 × 0.25 × 0.22 |
| calculated density (Mg/m3) | 1.480 |
| F000 | 512 |
| μ (mm–1) | 0.46 |
| Z | 4 |
| diffractometer | Bruker APEX3 CCD |
| θ range (deg) | 2.6 ≤ θ ≤ 27.3 |
| wavelength (Å) | 0.71073 |
| measurement method | ω scan |
| absorption correction | Multiscan |
| hmin, hmax | –5, 5 |
| kmin, kmax | –30, 30 |
| lmin, lmax | –14, 14 |
| Rint | 0.046 |
| reflections collected | 31325 |
| independent reflections | 2751 |
| observed reflections [I > 2σ(I)] | 1966 |
| refinement method | SHELXL18/3 |
| parameters | 148 |
| R[F2 > 2σ(F2)] | 0.060 |
| wR(F2) | 0.204 |
| GooF = S | 1.06 |
| Δρmin, Δρmax (e/Å3) | –0.48, 0.84 |
Computational Details
Gaussian 09W29 package was used to fully relax all the geometries (Figure 1b) at the B3LYP/6-311++G(d,p) level of theory,30,31 which is considered to be an effective and low-cost approach to obtain reliable molecular structures and for the analyses of Fukui functions. To confirm the local minima of the studied systems, vibrational frequency calculations were also performed. The CrystalExplorer21 program32 was used to analyze the Hirshfeld surfaces.
Figure 1.

(a) Crystal structure and (b) optimized geometry of I.
Antioxidant Activity Using ABTS Radical Scavenging Assay
ABTS radical cation decolorization assay was performed for free radical scavenging activity of I. ABTS•+ cation radical was generated by reacting 7 mM ABTS in water with 2.45 mM potassium persulfate (1:1) and leaving it at room temperature in the dark for 12–16 h before use. ABTS•+ solution was solubilzed with methanol and then an absorbance of 0.700 at 734 nm was obtained. A serial solubilization of 0.5–0.0078 mM concentration of I was mixed with ABTS•+ solution, and absorbance was measured 30 min after first stirring. Percent inhibition of absorbance at 734 nm was computed using the following formula:
Here, AB stands for the absorbance of ABTS radical + methanol; AA represents the absorbance of ABTS radical + sample extract/standard.
Antimicrobial Activity
Minimal Inhibitory Concentration (MIC) Method
Antimicrobial activities were applied against standard Gram-positive bacterial strains (Staphylococcus aureus ATCC 6538P, Listeria monocytogenes ATCC 19111, Micrococcus luteus NRRL B-4375) and gram strain negative (Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, Salmonella typhimurium ATCC 14028), yeasts (Candida glabrata ATCC 90030, Candida krusei ATCC 34135) using the Minimal Inhibitory Concentration Method (MIC). The title molecule used in this study was dissolved in dimethyl sulfoxide (DMSO) at a suitable concentration. To incubate all cultures, they were maintained in broth at 37 °C for 24 h. Then, 50 mL nutrient broth was used to suspend the bacterial and yeast cells. The turbidity of bacterial and yeast suspensions was adjusted to a concentration of nearly 106 cells/mL by pairing with 0.5 McFarland turbidity standards. Microorganisms were transferred as 1 mL aliquots into test tubes and added to mixtures. The incubation time of all test cultures is 24 h in an incubator at 37 °C. The MIC value (μg/mL) (Table 2) was used for the minimum inhibitory concentration at which no growth was observed.
Table 2. Minimum Inhibition Concentrations (MIC’s) of the Tested Compound.
Results and Discussion
Crystal Structure of I, C11H8N2OS2
The title compound (Figure 1a), N-(3-cyanothiophen-2-yl)-2-(thiophen-2-yl)acetamide (I), is a heterocyclic amide derivative formed from 2-aminothiophene-3-carbonitrile with activated 2-(thiophen-2-yl)acetic acid in a N-acylation reaction. The crystal structure of I crystallized in a monoclinic space group P21/c, and Z = 4 (Table 1). There is one independent molecule in the asymmetric unit. The dihedral angle causing the molecule to be nonplanar between thiophene and thiophene-3-carbonitrile ring is 74.27(10)° dihedral angle. The thiophene ring (C1–C4/S1) and the C1/C5/C6/N1/C7 acetamide bridge are twisted with a dihedral angle of 60.38(22)°. The angle between the planes of the C1/C5/C6/N1/C7 acetamide bridge and the thiophene-3-carbonitrile ring is 75.70(25)°. The molecules are linked by C–H···N and N–H···N hydrogen bonds in the crystal (Table 3). The N1 and C5 atoms act as hydrogen-bond donors, via atoms H1 and H5B, to atom N2 in the molecule at (−x, 1 – y, 1 – z), forming centrosymmetric R22(12) and R22(16) rings centered at (0, 1/2, 1/2). The combination of these hydrogen bonds produces the R21(6) ring (Figure 2). The nitrile group is typical of the C11≡N2 triple bond [1.142(5) Å], while the C6–O1 bond distance shows a typical double bond character at 1.217(4) Å (Table 4). The S1–C1, S1–C4, S2–C7, and S2–C8 bond lengths are 1.702(3), 1.688(5), 1.723(3), and 1.718(4) Å, which are within the range of values in the previously reported thiophene ring-containing compounds.33−35
Table 3. Hydrogen Bonds of I (Å, deg).
| D–H···A | D–H | H···A | D···A | D–H···A | symmetry code |
|---|---|---|---|---|---|
| N1–H1···N2i | 0.84(2) | 2.21(2) | 3.049(4) | 172(4) | (i) −x, −y + 1, −z + 1 |
| C5–H5B···N2i | 0.97 | 2.57 | 3.409(5) | 145 |
Figure 2.
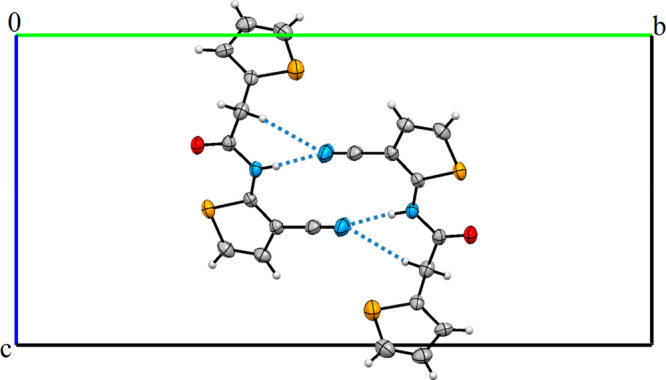
View of crystal packing of I forming R21(6), R22(12), and R22(16) rings.
Table 4. Some Geometric Parameters of I (Å, deg).
| geometric parameters | X-ray | geometric parameters | X-ray |
|---|---|---|---|
| bond lengths (Å) | bond angles (deg) | ||
| C1–S1 | 1.702 (3) | C1–S1–C4 | 92.5 (2) |
| C4–S1 | 1.688 (5) | C7–S2–C8 | 91.61 (17) |
| C7–S2 | 1.723 (3) | C6–N1–C7 | 124.2 (3) |
| C8–S2 | 1.718 (4) | N1–C6–O1 | 121.4 (3) |
| C6–O1 | 1.217 (4) | C5–C6–O1 | 122.8 (3) |
| C11–N2 | 1.142 (5) | C7–C10–C11 | 122.7 (3) |
| C9–C10 | 1.429 (5) | C9–C10–C11 | 124.4 (3) |
| C6–N1 | 1.370 (4) | torsion angles (deg) | |
| C7–N1 | 1.380 (4) | S2–C7–C10–C11 | –177.6 (3) |
| C10–C11 | 1.421 (5) | S1–C1–C5–C6 | –92.7 (3) |
| C1–C5 | 1.504 (5) | N1–C7–C10–C9 | 179.7 (3) |
| C5–C6 | 1.511 (5) | C1–C5–C6–O1 | –79.8 (4) |
IR Spectroscopy
Figure 3 shows the experimental IR spectrum of I. The absorption band at around 3262 cm–1 shows the existence of N–H stretching vibration. The characteristic absorption C=O (amide I) band of the amide compound appeared at 1688 cm–1, whereas another band was observed ∼1433 cm–1 corresponding to the C–N stretching vibration with the N–H bending vibration (amide II). The other significant band appearing at 2222 cm–1 belongs to the C≡N stretching vibration. These experimental data support the structure of similar molecules in the literature.36−42
Figure 3.
IR spectrum of N-(3-cyanothiophen-2-yl)-2-(thiophen-2-yl) acetamide.
NMR Spectroscopy
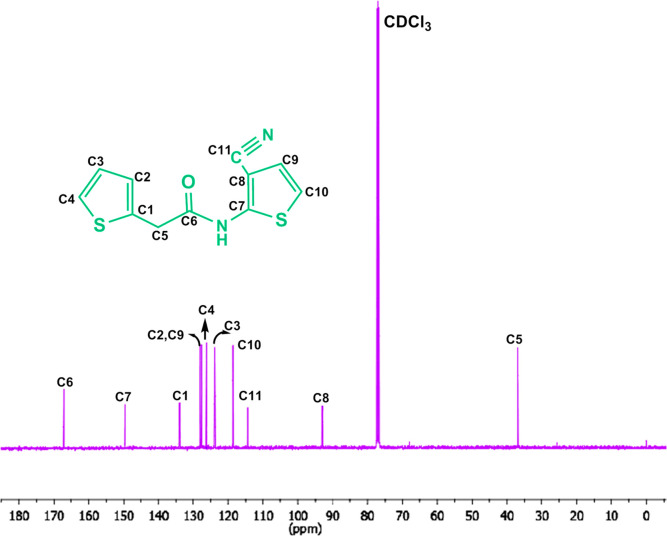
The 1H NMR spectrum of I was taken in CDCl3 (Figure 4). The singlet signal at 9.32 ppm (s, 1H, NH–C=O) was attributed to the amide group proton in the structure, while the signal at 4.10 ppm (s, 2H, −CH2−) belongs to methylene protons. The signals of the thiophene ring protons H1, H2, and H3 were observed in the region of δ = 7.06–7.33 ppm as a multiplet, excepting the H1 proton, observed at 7.33 ppm as a doublet. The other thiophene ring protons H4 and H5 interacted with each other and appeared as doublets at 6.98 and 6.89 ppm, respectively.
Figure 4.
1H NMR spectrum of I.
Figure 5 shows the 13C NMR spectrum of I taken in CDCl3. The 13C NMR spectrum of the title molecule displayed 11 different resonances compatible with the proposed structure. Eight aromatic carbons are belonging to two thiophene rings. The C5 and C4 carbons which gave the signal at 149.6 and 134.0 ppm, respectively, were the most downfield in comparison with the other carbons of the thiophene rings. The other thiophene ring carbons appeared at 28.02–93.04 ppm.
Figure 5.
13C NMR spectrum of I.
The signal for the methylene group (−CH2) between the carbonyl group and the thiophene ring was observed at 36.82 ppm while the signal at 167.16 ppm was assigned to the amide carbonyl group. The presence of the acetoxy group on the phenyl ring causes the carbon of the nitrile group to shift to the lower domain. The nitrile group (−C≡N) carbon attached to the thiophene ring was observed at 114.45 ppm. The nitrile group (−C≡N) (C6) on the thiophene ring was observed at 114.45 ppm.
Consequently, these experimental data also support the structure of the title compound and previously reported values for similar structures.39−42
Fukui Function Analysis
The Fukui function (FF) provides information about the local reactivity of the whole molecule and is one of the most commonly used determinants of local reactivity.43,44 The nucleophilic, electrophilic, and radically prone positions in I were assessed with FF. The compact Fukui functions45 are represented as fk+(r) for the nucleophilic attack, fk(r) for an electrophilic attack, and fk0(r) for a free radical attack.
| 1 |
| 2 |
| 3 |
The difference between nucleophilic and electrophilic FF is defined as the binary identifier [Δfk(r)] given below:
| 4 |
If Δfk(r) > 0, the region is preferred for a nucleophilic attack, whereas if Δfk(r) < 0, the site is preferred for an electrophilic attack. The binary identifiers with their sign provide a clear distinction between the nucleophilic and electrophilic attack in a given region by providing a positive value for the region susceptible to nucleophilic attack and a negative value for the electrophilic attack. The binary identifier condition shows that the nucleophilic region for I involves C1, C3, C4, C8, C9, and C10 (positive value, i.e., Δfk (r) > 0) atoms [Table 5]. Similarly, the electrophilic regions are found on C2, C5, C6, C7, C11, O1, N1, N2, S1, and S2 atoms (negative value, i.e. Δfk(r) < 0). The results of FF show that compound I was more prone to electrophilic attack than nucleophilic attack and radical attack.
Table 5. Results of the Natural Population Analysis (NPA) for I.
| atom | qk0 | qk+ | qk– | fk– | fk+ |
|---|---|---|---|---|---|
| C1 | –0.23911 | –0.19579 | –0.25123 | 0.01212 | 0.04332 |
| C2 | –0.25265 | –0.24466 | –0.30465 | 0.05200 | 0.00799 |
| C3 | –0.24474 | –0.19263 | –0.25004 | 0.00530 | 0.05211 |
| C4 | –0.37493 | –0.30184 | –0.42038 | 0.04545 | 0.07309 |
| C5 | –0.49603 | –0.51053 | –0.49164 | –0.00439 | –0.01450 |
| C6 | 0.69587 | 0.70071 | 0.61084 | 0.08503 | 0.00484 |
| C7 | 0.05599 | 0.11139 | –0.02532 | 0.08131 | 0.05540 |
| C8 | –0.39458 | –0.25352 | –0.46233 | 0.06775 | 0.14106 |
| C9 | –0.20415 | –0.17736 | –0.21147 | 0.00732 | 0.02679 |
| C10 | –0.27316 | –0.17606 | –0.33920 | 0.06604 | 0.09710 |
| C11 | 0.28127 | 0.24577 | 0.27757 | 0.00370 | –0.03550 |
| O1 | –0.60117 | –0.52799 | –0.67673 | 0.07556 | 0.07318 |
| N1 | –0.62357 | –0.55701 | –0.54771 | 0.07586 | 0.06656 |
| N2 | –0.32644 | –0.20934 | –0.44469 | 0.11825 | 0.1171 |
| S1 | 0.43262 | 0.48268 | 0.37975 | 0.05287 | 0.05006 |
| S2 | 0.50290 | 0.56078 | 0.38703 | 0.11587 | 0.05788 |
Electrophilicity-Based Charge Transfer (ECT) Method
The ECT calculation is applied to determine the direction of charge transfer. The ECT method is of great importance to search for molecules and DNA bases that are electron acceptors or donors (electrophilic or nucleophilic behavior). In the case where ECT is greater than zero, charges are transferred from the base to the functional group, while if ECT is less than zero, charges migrate from the functional group to the base compound.46 The electron affinity (EA) and ionization potential (IP) obtained from the energy of anionic, cationic, and neutral types are expressed as given below.
| 5 |
| 6 |
The stabilization value in energy is measured with the new reactivity index when the system reserves the additional electronic load (ΔN). The electronic chemical potential of the molecule is effective in determining the direction of charge transfer. After an electrophile receives an electronic charge, its energy decreases, and thus the electronic chemical potential becomes negative. The species may behave like a nucleophile with a lower electrophilic index in a reaction between two molecules. From the electrophilicity index results, it appears that DNA-bases such as cytosine, adenine, thymine, and guanine are good enough nucleophiles to attack C11H8N2OS2 (I). ECT is the difference between ΔNmax values of interacting molecules. Two molecules, A (I) and B (guanine, cytosine, thymine, and adenine) can be considered to be approaching each other; here, two situations arise: (i) ECT < 0, charge flow from A to B, and (ii) ECT > 0, charge flow from B to A. ECT is computed with the following expressions:
| 7 |
| 8 |
where μA, μB and ηA, ηB are the chemical potentials and chemical hardness of systems A and B, respectively.47 The ECT values for the guanine, adenine, thymine, and cytosine, were obtained as 0.11589, 0.07436, 0.05139, and −0.49282, respectively. In Table 6 are summarized the IP, EA, μ, η, ΔNmax, and ECT values for I and DNA bases. From these results, it is seen that electrons were transfused from the DNA bases (guanine, adenine, and cytosine) to I. Here, the cytosine, adenine, and guanine bases are the electron donor, while I is an electron acceptor. In addition, while the DNA bases of adenine, cytosine, and guanine showed nucleophilic nature, I exhibited an electrophilic nature. According to the ECT results, it is seen that I interacts more with thymine than other DNA bases. Also, only the thymine base is treated as the electron acceptor.
Table 6. IP, EA, μ, η, ΔNmax, and ECT values for I.
| compound and DNA bases | IP (au) | EA (au) | μ (au) | η (au) | ΔNmax |
|---|---|---|---|---|---|
| I (C11H8N2OS2) | 5.4 × 10–3 | 0.2989 | –0.15215 | –0.14675 | 1.03670 |
| adenine | –0.01258 | 0.30519 | –0.14630 | –0.158886 | 0.92081 |
| ECT = 0.11589 | |||||
| cytosine | –0.00616 | 0.321254 | –0.15754 | –0.163709 | 0.96234 |
| ECT = 0.07436 | |||||
| guanine | –0.00214 | 0.290467 | –0.14415 | –0.146308 | 0.98531 |
| ECT = 0.05139 | |||||
| thymine | 0.076022 | 0.3631556 | –0.21958 | –0.143566 | 1.52952 |
| ECT = −0.49282 |
Hirshfeld Surface Analysis
Using Hirshfeld surface (HS) analysis and fingerprint plots (FPs) are frequently used methods to analyze the contribution and percentage of distinct intermolecular interactions to the crystal packing.48,49 This method has received special attention in recent years, as it allows an accurate and complete understanding of intermolecular interactions.50−56 The Hirshfeld surface is defined as based on the normalized contact distance (dnorm) given by eq 9. To recognize the interatomic contacts, a red, blue, and white color scheme is used, representing the Hirshfeld surface calculated from the dnorm. In here, while a red spot shows the shortest contacts, the blue spot shows longer contacts.
| 9 |
Figure 6 illustrates the Hirshfeld surface mapped with dnorm, di, de, shape index, and curvedness; the indexes are −0.4512 to 1.0427, 0.8369 to 2.5676, 0.8346 to 2.5514, – 1 to 1, and −4 to 4 Å for I, respectively. Figure 7 shows the important interactions visible on the HSs as well as in the 2D fingerprints. H···H, C···H/H···C, S···H/H···S, N···H/H···N, and O···H/H···O interactions exist in the fingerprint plots of I. With a value of 21%, the contribution of H···H interactions has the largest share in the crystal packing of I. N···H/H···N interactions have a smaller share with a 14% contribution, while the heteronuclear interactions appear as in two long sharp. O···H interactions are related to C5–H5B···N2i and N1–H1···N2i intermolecular hydrogen bonds. S···H/H···S (19%) and C···H/H···C (20%) interactions form with two broad short spikes (Figure 7). The full intermolecular interactions and their percentages in I are shown in Figure 8. The HS and its 2D-FP are very useful tools to get information about the contributions of various intercontact that provide the stabilizing the molecular structure.
Figure 6.

Hirshfeld surface mapped with dnorm, di, de, shape index, and curvedness of I.
Figure 7.
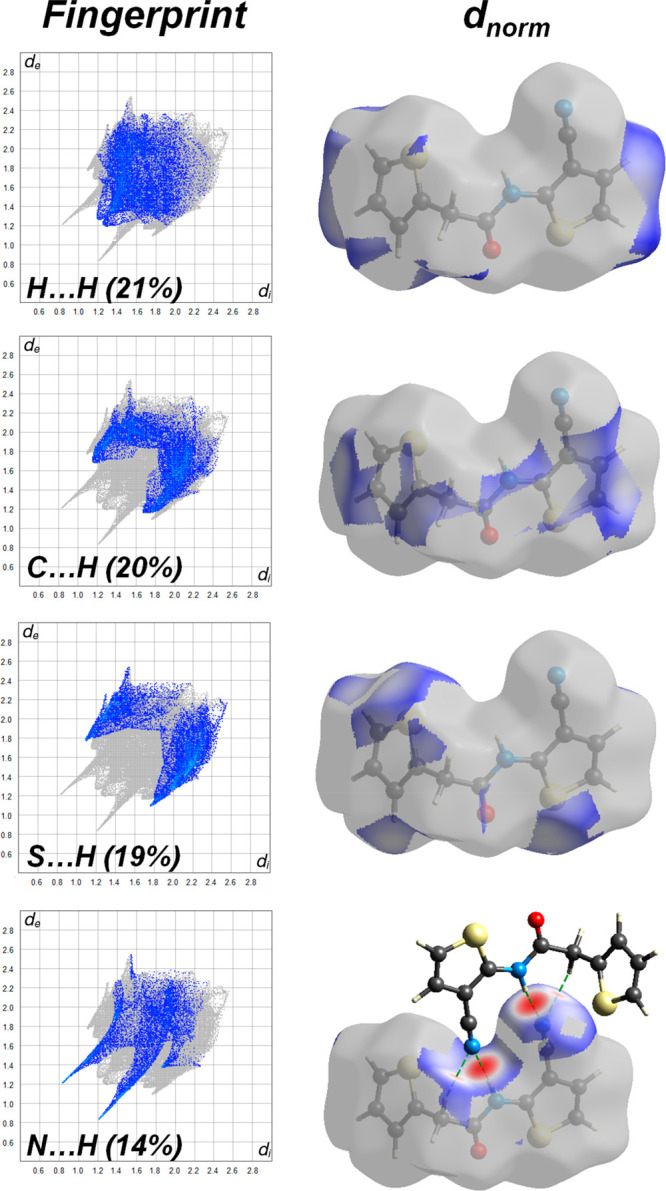
Two-dimensional fingerprint plots and HS for I.
Figure 8.

Intermolecular interactions and their percentages in I.
Evaluation of Antioxidant Activity
Antioxidants can avoid oxidation of oxidizable substrates when they exist in lower concentrations than the substrate.57 Therefore, the discovery of substances with antioxidant properties has become important for human health. In here, the antioxidant activity of the newly prepared title molecule has been investigated to evaluate the radical scavenging activity of I according to the ABTS assay. Figure 9 gives the results of the ABTS radical scavenging activity. Various concentrations of (0.5–0.0078 mM) compound were mixed, and ABTS radical and dose-dependent radical scavenging activities were observed. The IC50 value of the compound and BHA were calculated as 0.18 mM and 0.019 mg/mL, respectively. As a result, it has been determined that the antioxidant property of the newly synthesized compound is of medium level.
Figure 9.

ABTS radical scavenging activity of the title compound.
Evaluation of Antimicrobial Activity
The antimicrobial effect of the title compound in this study was done according to the MIC method. Considering the study results, it was seen that DMSO used as a control did not have a significant antimicrobial effect (>4000 μg/mL). However, the synthesized title compound has been found to have a significant antimicrobial effect on some microorganisms. When MIC values for gram-negative microorganisms are tested, it is observed that these data are 1000 μg/mL for E. coli and 2000 μg/mL for S. typhimurium and K. pneumoniae. As shown in Table 2, the MIC values were 250 μg/mL for M. luteus and S. aureus, which are among the gram-positive microorganisms, besides this, the value was 125 μg/mL for L. monocytogenes. Further to this, the MIC values were determined to be 2000 μg/mL for two yeast Candida species (i.e., C. krusei and C. glabrata).
As a result of the study, it is seen that the synthesized substance affects E. coli most among gram-negative bacteria. In addition, among gram–positive bacteria, it was determined that it affects L. monocytogenes more than others. When gram-positive and gram-negative bacteria were compared, it was tested that it affects gram-positive microorganisms more than gram-negative. It has been determined that it affects the gram-positive L. monocytogenes more than any other microorganism.
L. monocytogenes causes meningitis, encephalitis, and septicemia in nonpregnant adults.58 It is known that especially elderly people or those who have cellular immunity weakened, such as organ transplantation and lymphoma. It is also an important bacterium in terms of the high mortality rate in the elderly and in immunocompromised patients.59 Increasing resistance to antibiotics in recent years leads people to new alternatives. Therefore, the new chemical synthesized in this study is considered to be important in the fight against such bacteria (especially for L. monocytogenes), which are considered to be a risk to human health.
Conclusions
The studied compound I, C11H8N2OS2, which was synthesized in a two-step procedure, was examined using IR, 1H NMR, and 13C NMR spectroscopic studies. In this two-step reaction, 2-(thiophen-2-yl)acetic acid was first activated by conversion to 2-(thiophen-2-yl)acetyl chloride and then reacted with 2-aminothiophene-3-carbonitrile. Density functional theory (DFT) at the B3LYP/6-311++G(d,p) level of theory was used to determine the ground-state optimized geometry of I. Appropriate regions for the electrophilic and nucleophilic attack were searched using location-based identifiers in the form of fused Fukui function. The DNA/ECT predicted charge transfer from I to the DNA bases (such as cytosine, adenine, guanine, and thymine). ECT values showed that the molecule can interact more with thymine than with other DNA bases. Also, only the thymine base is treated as an electron acceptor.
Acknowledgments
The authors acknowledge the financial support through Researchers Supporting Project Number (RSP-2021/147), King Saud University, Riyadh, Saudi Arabia. This study was supported by Sinop University as a Scientific Research Project (Project No.: SHMYO-1901-17-25). The authors acknowledge the Scientific and Technological Research Application and Research Center, Sinop University, Turkey, for the use of the Bruker D8 QUEST diffractometer.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00318.
The authors declare no competing financial interest.
Supplementary Material
References
- Chan W. K.; Ho C. M.; Wong M. K.; Che C. M. Oxidative amide synthesis and N-terminal α-Amino group ligation of peptides in aqueous medium. J. Am. Chem. Soc. 2006, 128, 14796–14797. 10.1021/ja064479s. [DOI] [PubMed] [Google Scholar]
- Gawande M. B.; Branco P. S. An efficient and expeditious Fmoc protection of amines and amino acids in aqueous media. Green Chem. 2011, 13, 3355–3359. 10.1039/c1gc15868f. [DOI] [Google Scholar]
- Humphrey J. M.; Chamberlin A. R. Chemical synthesis of natural product peptides: Coupling methods for the incorporation of noncoded amino acids into peptides. Chem. Rev. 1997, 97, 2243–2266. 10.1021/cr950005s. [DOI] [PubMed] [Google Scholar]
- Carey J. S.; Laffan D.; Thomson C.; Williams M. T. Analysis of the reactions used for the preparation of drug candidate molecules. Biomol. Chem. 2006, 4, 2337–2347. 10.1039/b602413k. [DOI] [PubMed] [Google Scholar]
- Roughley S. D.; Jordan A. M. The medicinal chemist’s toolbox: An analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 2011, 54, 3451–3479. 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- Şener E. A.; Bingöl K. K.; Ören İ.; Arpacı Ö. T.; Yalçın İ.; Altanlar N. Synthesis and microbiological activity of some N-(o-hydroxyphenyl)benzamides and phenylacetamides as the possible metabolites of antimicrobial active benzoxazoles: part II. Il Farmaco 2000, 55 (6-7), 469–476. 10.1016/S0014-827X(00)00070-7. [DOI] [PubMed] [Google Scholar]
- Fu J.; Cheng K.; Zhang Z.-M.; Fang R.-Q.; Zhu H.-L. Synthesis, structure and structure-activity relationship analysis of caffeic acid amides as potential antimicrobials. Eur. J. Med. Chem. 2010, 45 (6), 2638–2643. 10.1016/j.ejmech.2010.01.066. [DOI] [PubMed] [Google Scholar]
- Carbonnelle D.; Ebstein F.; Rabu C.; Petit J. Y.; Gregoire M.; Lang F. A new carboxamide compound exerts immuno-suppressive activity by inhibiting dendritic cell maturation. Eur. J. Immunol. 2005, 35, 546–556. 10.1002/eji.200425007. [DOI] [PubMed] [Google Scholar]
- Kushwaha N.; Saini R. K.; Kushwaha S. K. Synthesis of some amide derivatives and their biological activity. Int. J. Chem. Technol. Res. 2011, 3 (1), 203–209. [Google Scholar]
- Li X.; Li Z.; Deng H.; Zhou X. An efficient protocol for the preparation of amides by copper-catalyzed reactions between nitriles and amines in water. Tetrahedron Lett. 2013, 54 (18), 2212–2216. 10.1016/j.tetlet.2013.02.058. [DOI] [Google Scholar]
- Chandra Shekhar A.; Ravi Kumar A.; Sathaiah G.; Luke Paul V.; Sridhar M.; Shanthan Rao P. Facile N-formylation of amines using Lewis acids as novel catalysts. Tetrahedron Lett. 2009, 50 (50), 7099–7101. 10.1016/j.tetlet.2009.10.006. [DOI] [Google Scholar]
- Suzuki K.; Nagasawa H.; Uto Y.; Sugimoto Y.; Noguchi K.; Wakida M.; Wierzba K.; Terada T.; Asao T.; Yamada Y.; et al. Napthalimidobenzamide DB-51630: A novel DNA binding agent inducing p300 gene expression and exerting a potent anti-cancer activity. Bioorg. Med. Chem. 2005, 13 (12), 4014–4021. 10.1016/j.bmc.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Yu B.; Tang L.; Li Y.; Song S.; Ji X.; Lin M.; Wu C.-F. Design, synthesis and evaluation of clinafloxacin triazole hybrids as a new type of antibacterial and antifungal agents. Bioorg. Med. Chem. Lett. 2012, 22 (1), 110–114. 10.1016/j.bmcl.2011.11.061. [DOI] [PubMed] [Google Scholar]
- Karanth S. N.; Badiadka N.; Balladka Kunhanna S.; Shashidhara K. S.; Peralam Yegneswaran P. Synthesis of novel Schiff base benzamides via ring opening of thienylidene azlactones for potential antimicrobial activities. Res. Chem. Intermed. 2018, 44, 4179–4194. 10.1007/s11164-018-3362-8. [DOI] [Google Scholar]
- Rehse K.; Kotthaus J.; Khadembashi L. New 1H-pyrazole-4-carboxamides with antiplatelet activity. Arch. der Pharm. 2009, 342, 27–33. 10.1002/ardp.200800181. [DOI] [PubMed] [Google Scholar]
- Graul A.; Castaner J. Atorvastatin calcium. Drugs Future. 1997, 22, 956–968. 10.1358/dof.1997.022.09.423212. [DOI] [Google Scholar]
- Humphrey J. M.; Chamberlin A. R. Chemical synthesis of natural product peptides: Coupling methods for the incorporation of noncoded amino acids into peptides. Chem. Rev. 1997, 97 (6), 2243–2266. 10.1021/cr950005s. [DOI] [PubMed] [Google Scholar]
- Milenković D.; Avdović E.; Dimić D.; Sudha S.; Ramarajan D.; Milanović Z.; Trifunović S.; Marković Z. S. Vibrational and Hirshfeld surface analyses, quantum chemical calculations, and molecular docking studies of coumarin derivative 3-(1-m-toluidinoethylidene)-chromane-2,4-dione and its corresponding palladium(II) complex. J. Mol. Struct. 2020, 1209, 127935. 10.1016/j.molstruc.2020.127935. [DOI] [Google Scholar]
- Milenković D.; Dimitrić Marković J. M.; Dimić D.; Jeremić S.; Amić D.; Stanojević Pirković M.; Marković Z. S. Structural characterization of kaempferol: A spectroscopic and computational study. Maced. J. Chem. Chem. Eng. 2019, 38, 49–62. 10.20450/mjcce.2019.1333. [DOI] [Google Scholar]
- Cakmak S.; Kutuk H.; Odabasoglu M.; Yakan H.; Buyukgungor O. Spectroscopic properties and preparation of some 2,3-dimethoxybenzamide derivatives. Lett. Org. Chem. 2016, 13 (3), 181–194. 10.2174/1570178613666151230210931. [DOI] [Google Scholar]
- Kırca B. K.; Çakmak Ş.; Kütük H.; Odabaşoğlu M.; Büyükgüngör O. Synthesis and characterization of 3-acetoxy-2-methyl-N-(phenyl)benzamide and 3-acetoxy-2-methyl-N-(4- methylphenyl)benzamide. J. Mol. Struct. 2018, 1151, 191–197. 10.1016/j.molstruc.2017.09.034. [DOI] [Google Scholar]
- APEX2; Bruker AXS Inc.: Madison WI, 2013. [Google Scholar]
- Sheldrick G. M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. A: Found. Adv. 2015, 71, 3–8. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C: Struct. Chem. 2015, 71, 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae C. F.; Edgington P. R.; McCabe P.; Pidcock P. E.; Shields G. P.; Taylor R.; Towler M.; van de Streek J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. 10.1107/S002188980600731X. [DOI] [Google Scholar]
- Spek A. L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. 10.1107/S0021889802022112. [DOI] [Google Scholar]
- Farrugia L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854. 10.1107/S0021889812029111. [DOI] [Google Scholar]
- Westrip S. P. publCIF: Software for editing, validating and formatting crystallographic information files. J. Appl. Crystallogr. 2010, 43, 920–925. 10.1107/S0021889810022120. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Montgomery J. A. Jr.; Vreven T.; Kudin K. N.; Burant J. C.; Millam J. M.; Iyengar S. S.; Tomasi J.; Barone V.; Mennucci B.; Cossi M.; Scalmani G.; Rega N.; Petersson G. A.; Nakatsuji H.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Klene M.; Li X.; Knox J. E.; Hratchian H. P.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Ayala P. Y.; Morokuma K.; Voth G. A.; Salvador P.; Dannenberg J. J.; Zakrzewski V. G.; Dapprich S.; Daniels A. D.; Strain M. C.; Farkas O.; Malick D. K.; Rabuck A. D.; Raghavachari K.; Foresman J. B.; Ortiz J. V.; Cui Q.; Baboul A. G.; Clifford S.; Cioslowski J.; Stefanov B. B.; Liu G.; Liashenko A.; Piskorz P.; Komaromi I.; Martin R. L.; Fox D. J.; Keith T.; Al-Laham M. A.; Peng C. Y.; Nanayakkara A.; Challacombe M.; Gill P. M. W.; Johnson B.; Chen W.; Wong M. W.; Gonzalez C.; Pople J. A.. Gaussian 03, Rev. E.01; Gaussian, Inc., Wallingford, CT, 2004.
- Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Turner M. J.; MacKinnon J. J.; Wolff S. K.; Grimwood D. J.; Spackman P. R.; Jayatilaka D.; Spackman M. A.. Crystal Explorer, Ver. 17.5; University of Western Australia: 2017. https://wiki.crystalexplorer.net/features/hirshfeldsurface. [Google Scholar]
- Hasan Tanak; Karataş Ş.; Meral S.; Ağar A. A. Crystal structure, spectroscopic and DFT computational studies of N-(4-fluorophenyl)-1-(5-nitrothiophen-2-yl)methanimine. Crystallogr. Rep. 2020, 65 (7), 1221–1225. 10.1134/S1063774520070263. [DOI] [Google Scholar]
- Evecen M.; Tanak H.; Ağar A. A.; Meral S.; Özdemir N. Comparative structural, spectroscopic and nonlinear optical analysis of a Schiff base compound with experimental and theoretical methods (HF, B3LYP and WB97X-D). Optik 2021, 228, 166133. 10.1016/j.ijleo.2020.166133. [DOI] [Google Scholar]
- Hasan Tanak; Karataş Ş.; Meral S.; Ağar A. A. Synthesis, molecular structure and quantum chemical studies of N-(2-fluorophenyl)-1-(5-nitrothiophen-2-yl)methanimine. Crystallogr. Rep. 2020, 65 (7), 1212–1216. 10.1134/S106377452007024X. [DOI] [Google Scholar]
- Watanabe Y.; Mukaiyama T. A convenient method for the synthesis of carboxamides and peptides by the use of tetrabutylammonium salts. Chem. Lett. 1981, 10 (3), 285–288. 10.1246/cl.1981.285. [DOI] [Google Scholar]
- Hoegberg T.; Stroem P.; Ebner M.; Raemsby S. Cyanide as an efficient and mild catalyst in the aminolysis of esters. J. Org. Chem. 1987, 52 (10), 2033–2036. 10.1021/jo00386a025. [DOI] [Google Scholar]
- Barrett A. G.; Lana J. C. A. Photoluminescence from copper(I) complexes with low-lying metal-to-ligand charge transfer excited states. J. Chem. Soc. Chem. Commun. 1978, 11, 471–472. 10.1039/c39780000471. [DOI] [Google Scholar]
- Cakmak S.; Kutuk H.; Odabasoglu M.; Yakan H.; Buyukgungor O. Spectroscopic properties and preparation of some 2,3-dimethoxybenzamide derivatives. Lett. Org. Chem. 2016, 13 (3), 181–194. 10.2174/1570178613666151230210931. [DOI] [Google Scholar]
- Demir S.; Cakmak S.; Dege N.; Kutuk H.; Odabasoglu M.; Kepekci R. A. A novel 3-acetoxy-2-methyl-N-(4-methoxyphenyl)benzamide: Molecular structural describe, antioxidant activity with use X-ray diffractions and DFT calculations. J. Mol. Struct. 2015, 1100, 582–591. 10.1016/j.molstruc.2015.08.014. [DOI] [Google Scholar]
- Iriarte A. G.; Erben M. F.; Gholivand K.; Jios J. L.; Ulic S. E.; Della Védova C. O. [Chloro(difluoro)acetyl]phosphoramidic acid dichloride ClF2CC(O)NHP(O)Cl2, synthesis, vibrational and NMR spectra and theoretical calculations. J. Mol. Struct. 2008, 886 (1–3), 66–71. 10.1016/j.molstruc.2007.10.036. [DOI] [Google Scholar]
- Kırca B. K.; Çakmak Ş.; Kütük H.; Odabaşoğlu M.; Büyükgüngör O. Synthesis and characterization of 3-acetoxy-2-methyl-N-(phenyl)benzamide and 3-acetoxy-2-methyl-N-(4- methylphenyl)benzamide. J. Mol. Struct. 2018, 1151, 191–197. 10.1016/j.molstruc.2017.09.034. [DOI] [Google Scholar]
- Demircioğlu Z.; Kaştaş Ç. A.; Kaştaş G.; Ersanlı C. C. Synthesis, crystal structure, computational chemistry studies and Hirshfeld surface analysis of two Schiff bases, (E)-2-[(4-bromo-2-methylphenylimino)methyl]-4-methylphenol and (E)-2-[(4-bromo-2-methylphenylimino)methyl]-6-methylphenol. Mol. Cryst. Liq. Cryst. 2021, 723 (1), 45–61. 10.1080/15421406.2020.1871178. [DOI] [Google Scholar]
- Demircioğlu Z. Synthesis, crystal structure, spectroscopic characterization, chemical activity and molecular docking studies of (E)-2-(((3-chloro-4-methylphenyl)imino)methyl)-6-ethoxyphenol. J. Mol. Struct. 2021, 1246, 131114. 10.1016/j.molstruc.2021.131114. [DOI] [Google Scholar]
- Ayers P. W.; Parr R. G. Variational principles for describing chemical reactions: The Fukui function and chemical hardness revisited. J. Am. Chem. Soc. 2000, 122 (9), 2010–2018. 10.1021/ja9924039. [DOI] [PubMed] [Google Scholar]
- Parr R. G.; Szentpály L. v.; Liu S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. 10.1021/ja983494x. [DOI] [Google Scholar]
- Padmanabhan J.; Parthasarathi R.; Subramanian V.; Chattaraj P. K. Electrophilicity-based charge transfer descriptor. J. Phys. Chem. A 2007, 111, 1358–1361. 10.1021/jp0649549. [DOI] [PubMed] [Google Scholar]
- McKinnon J. J.; Jayatilaka D.; Spackman M. A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. 10.1039/b704980c. [DOI] [PubMed] [Google Scholar]
- Spackman M. A.; Jayatilaka D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. 10.1039/B818330A. [DOI] [Google Scholar]
- Al-thamili D. M.; Almansour A. I.; Arumugam N.; Kansız S.; Dege N.; Soliman S. M.; Azam M.; Kumar R. S. Highly functionalized N-1-(2-pyridinylmethyl)-3,5-bis[(E)-arylmethylidene]tetrahydro-4(1H)-pyridinones: Synthesis, characterization, crystal structure and DFT studies. J. Mol. Struct. 2020, 1222, 128940. 10.1016/j.molstruc.2020.128940. [DOI] [Google Scholar]
- Ramalingam A.; Kansız S.; Dege N.; Sambandam S. Synthesis, crystal structure, DFT calculations and Hirshfeld surface analysis of 3-chloro-2,6-bis(4-chlorophenyl)-3-methylpiperidin-4-one. J. Chem. Crystallogr. 2021, 51, 273–287. 10.1007/s10870-020-00852-3. [DOI] [Google Scholar]
- Kansız S.; Qadir A.; Dege N.; Faizi S. H. Two new copper (II) carboxylate complexes based on N,N,N′,N′-tetramethylethyleneamine: Synthesis, crystal structures, spectral properties, dft studies and hirshfeld surface analysis. J. Mol. Struct. 2021, 1230, 129916. 10.1016/j.molstruc.2021.129916. [DOI] [Google Scholar]
- Al-Resayes S. I.; Azam M.; Trzesowska-Kruszynska A.; Kruszynski R.; Soliman S. M.; Mohapatra R. K.; Khan Z. Structural and theoretical investigations, Hirshfeld surface analyses, and cytotoxicity of a naphthalene-based chiral compound. ACS Omega 2020, 5, 27227–27234. 10.1021/acsomega.0c03376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albayati M. R.; Kansız S.; Dege N.; Kaya S.; Marzouki R.; Lgaz H.; Salghi R.; Ali I. H.; Alghamdi M. M.; Chung I.-M. Synthesis, crystal structure, Hirshfeld surface analysis and DFT calculations of 2-[(2,3-dimethylphenyl)amino]-N′-[(E)-thiophen-2-ylmethylidene]benzohydrazide. J. Mol. Struct. 2020, 1205, 127654. 10.1016/j.molstruc.2019.127654. [DOI] [Google Scholar]
- Al-Karawi A. J. M.; OmarAli A.-A. B.; Dege N.; Kansız S. Formation of a new CuII–triazole ester complex from 1,2-cyclohexanedione-bis(p-bromobenzohydrazone) compound as a consequence of copper(II)-catalyzed click reaction. Chem. Pap. 2021, 75, 3901–3914. 10.1007/s11696-021-01614-x. [DOI] [Google Scholar]
- Yağcı N. K.; Kansız S.; Özcandan E. Synthesis, crystal structure, DFT studies, Hirshfeld surface analysis and drug delivery performance of bis(2-chloro-4,6-diaminopyrimidine)copper(II)-dichloride. J. Mol. Struct. 2021, 1246, 131142. 10.1016/j.molstruc.2021.131142. [DOI] [Google Scholar]
- Halliwell B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35 (5), 1147–1150. 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- Murray P. R.; Baron E. J.; Jorgensen J. H.; Landry M. L.; Pfaller M. A.. Manual of Clinical Microbiology, 9th ed.; ASM Press: Washington, DC, 2007, 474–479. [Google Scholar]
- Evirgen Ö. Listeria monocytogenes infeksiyonu; kliniği, tanı ve tedavi özellikleri. Van Tıp Dergisi 2005, 12 (1), 32–35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.