Abstract

Glycolipids with diacetylene functional groups are fascinating compounds with many practical uses. Among these, diacetylene-containing gelators are especially important because they can form photopolymerizable gels, which are useful stimuli-responsive materials. Inspired by the unique properties of diacetylene-containing gelators and to understand the structural influences especially the location of the diacetylene functional groups on the self-assembling properties, a series of 15 novel N-acetyl-d-glucosamine derivatives with the diacetylene functional group introduced at the anomeric position were designed and synthesized. The diacetylene function is attached to the sugar through α-glycosylation with the distance from the anomeric oxygen being varied from one, two, and three methylene groups, and the other side contains hydroxyl, carboxyl, phenyl, and alkyl substituents. Remarkably, all compounds can form self-assembled gels in one or more selected solvents. A majority of these synthesized diacetylene glycosides are effective gelators for ethanol/water (v/v 1:1), dimethyl sulfoxide/water (v/v 1:1), and toluene, and one compound also formed a hydrogel at 1.0 wt %. Typically, these glycosides form gels that are photopolymerizable to afford red-colored gels. Scanning electronic microscopy indicated that the gelators formed helices, fibers, and planar sheet-like morphologies. The chemical structures of the derivatives affected their gelation properties and responses to UV treatment. The carboxylic acid-functionalized derivative 17 was able to immobilize basic solutions and form transparent gels. We expect that these diacetylene glycosides especially the hydroxyl and carboxylic acid derivatives will be useful as stimuli-responsive glycolipids for biomedical research.
Introduction
Designing smart materials through the formation of self-assemblies with small molecules containing built-in functionality is a useful bottom-up strategy. Among these, the molecular assemblies formed using an interesting class of small molecules, the low-molecular weight gelators (LMWGs), have drawn much attention due to their numerous applications. These include environmental remediation, drug delivery, dye absorption, tissue engineering, and as therapeutic agents.1,2 The LMWGs containing stimuli-responsive functional groups have also been extensively studied and have been utilized in various other research fields.3−7 Carbohydrates particularly monosaccharide derivatives have been utilized frequently as a template for the design and synthesis of molecular gelators.8−10 LMWGs containing diacetylene functional groups are fascinating due to the possible photochemical reactions among the gelators, which typically form cross-linked ene–yne conjugated systems through intermolecular 1,4-addition while the rest of the molecules are unaffected, thus retaining the functionalities of the corresponding monomers. The resulting polydiacetylenes (PDAs) are of special interest as they typically exhibit a blue to red color transition upon change in environmental stimuli.11−14 Diacetylene-containing gelators possess interesting properties and unique applications, especially if they can form gels that are photopolymerizable, which in turn result in novel stimuli-responsive functional materials.15−17 The properties of the PDA derivatives have a strong dependance on their structures. The distance of the diacetylene group from the terminal functional group seems to strongly influence the molecular self-assembly and gel formation. The commercially available 10,12-pentacosadiynoic acid and 10,12-tricosadiynoic acid have been used for the formation of two-component supramolecular gels with imidazole derivatives.18,19 Diacetylene acids have been utilized for the preparation of PDA-based co-gels using a chaperone gelator.20,21 Diacetylene-containing fatty acids have also been used in the synthesis of amide-linked gelators, which are photopolymerizable.22,23 Many symmetrical diacetylene-containing compounds with diacetylene in the center and different functional groups at the terminal have been synthesized, and their photo-responsive properties have been studied. These include symmetrical dimers containing terminal amides,24−29 ureas,30 and hydroxy groups,31 diarylbutadiyne derivatives,32 and a dimeric diacetylene amido derivative functionalized with a diketopyrrolopyrrole.33 Several 4, 6-O-benzylidene acetal-protected sugar-based diacetylene glycolipids have been designed and synthesized; they were studied for their optical properties and utilized for lectin binding and other applications.34−39
We have incorporated diacetylene-containing fatty acids into glucose and glucosamine headgroups and obtained effective LMWGs; the topochemical polymerization requires the diacetylene functional groups to be aligned at a suitable distance and orientation for the cross-linking reaction to take place. The diacetylene-containing acids were introduced at the C-2 and C-3 positions of d-glucose and d-glucosamine as the corresponding esters, amides, and ureas.34−36 Studies have shown that different functional groups can affect the UV response significantly; as reported previously, different sugar anomeric substitutions and acyl functions give very different properties when they form assemblies.34−36 In an effort to expand the structural diversity and availability of different chemical derivatives, we are interested in structure-based design of non-symmetrical diacetylene derivatives for the formation of self-assemblies and photopolymerization tendencies.
Using the 4,6-O-benzylidene acetal-protected glycosamides I as a template, it is possible to rationally design a novel series of organogelators by introducing diacetylene-containing functional groups at the anomeric position, which gives compounds II as shown in Figure 1. From a structural point of view, the diacetylene-containing derivatives have not been extensively or systematically studied at the anomeric glycoside position for N-acetyl d-glucosamine (NAG). The general structure II contains an acetamide function at the C-2 position but a diacetylene function at anomeric position; this can lead to an interesting new class of organogelators. In order to extend the scope of carbohydrate-based functional LMWGs, in this research, we designed and synthesized a series of 15 glycosides, in which the diyne functional groups are at a distance of one to three carbons from the anomeric oxygen and different substituents are on the other side of the diyne. This enables us to probe the structural requirement for successful gelation and polymerization of resulting gels. The availability of these classes of materials will be useful for the preparation of other advanced materials, and the effective stimuli-response gelators can be used to immobilize enzymes or other biological molecules.
Figure 1.
Design rationale for a new class of diacetylene-based gelators by adding diacetylene chains at the anomeric position.
Results and Discussion
To probe the impact of anomeric structures on the gelation properties and to discover effective polymerizable diacetylene-containing glycolipids as molecular gelators, we have designed and synthesized a series of glycoside derivatives. These are 4,6-O-benzylidene-protected N-acetyl glucosamine derivatives (6–20) as shown in Schemes 1–3 and Figure 2. The library of diacetylene-containing glycolipids was prepared by coupling with alkynyl glycosides 3A–C through CuI-catalyzed Cadiot–Chodkiewicz alkyne coupling reactions;40 these compounds contain terminal alcohol, acid, phenyl groups, and alkyl groups. As shown in Scheme 1, the starting material NAG 1 was converted to the corresponding glycosides 2A–C, and the glycosides were purified to obtain the alpha isomer or directly converted to the 4,6-O-benzylidene acetal-protected headgroups 3A–C and purified at this step. For the compounds with one methylene spacer from the anomeric center (n = 1), eight compounds 6–13 with different substituents were synthesized (Scheme 2).
Scheme 1. Synthesis of α-Alkynyl Glycosides.

Scheme 3. Alternative Method of Synthesis of Diacetylene Glycoside.
Figure 2.
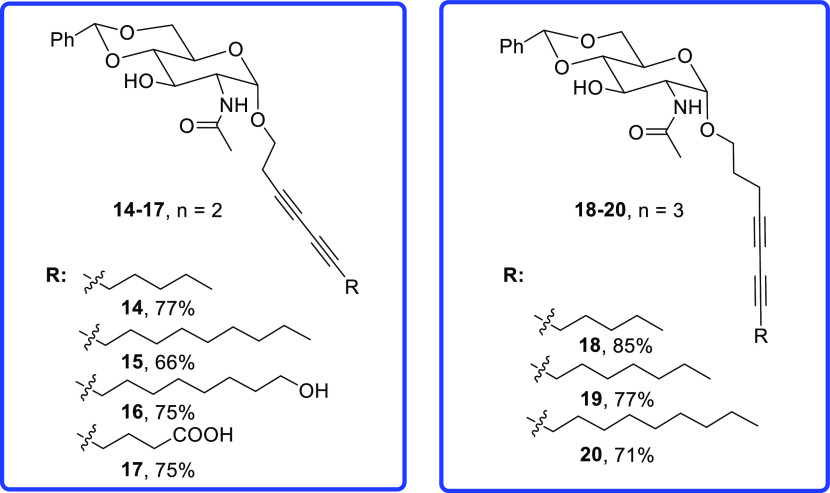
Structures of diacetylene-containing glycosides 14–20.
Scheme 2. Synthesis of Diacetylene-Containing Glycosides 6–13.
Due to the higher cost of the longer-chain alkynyl alcohols in comparison to propargyl alcohol, we only synthesized a few derivatives for the glycoside using 3-butyn-1-ol and 4-pentyn-1-ol, which are typically 20 and 30 times more expensive than propargyl alcohol, respectively. For the compounds with two carbons (n = 2) from the anomeric center, four derivatives 14–17 were synthesized (Figure 2). For the compounds with three methylene groups (n = 3) from the anomeric center, three compounds 18–20 with 6, 8, and 10 carbons from the diacetylene functional group were synthesized.
For the diyne coupling reaction, besides using the method shown in Scheme 2 by coupling the alkynyl bromides with the sugar-headgroup glycosides, as a proof of principle, we also carried out the synthesis by converting the sugar-headgroup alkyne to the corresponding bromide derivative 3A-Br and coupling with the corresponding alkyne; this afforded the same desired product. As shown in Scheme 3, we synthesized compound 6 using this method. This reversed sequence would be more useful for low-molecular weight alkynes that are volatile because the sugar alkynyl bromide is a solid and can be weighed easily for coupling reactions.
After these compounds had been synthesized, their gelation performance in organic solvents and polar solvent mixtures with water was analyzed and is shown in Table 1. We were delighted to observe that all 15 diacetylene-functionalized glycosides formed gels in at least one of the selected test solvents. Among the eight derivatives (6–13) with n = 1, compounds 6–9 contain linear alkyl chains of 4, 6, 9, and 10 carbons and compounds 10–13 contain terminal non-methyl functional groups. The compounds with linear alkyl chains showed similar self-assembling and gelation properties, and they tended to form gels in dimethyl sulfoxide (DMSO) water mixtures and EtOH water mixtures. The 10-carbon derivative 9 was the most efficient among compounds 6–9; it also formed clear gels in toluene at 6.7 mg/mL, while the other three did not. The compounds with terminal non-methyl functional groups, including a terminal phenyl group (10), hydroxyl (11), and carboxyl groups (12 and 13), formed gels in a broader range of solvents. The four derivatives with two carbons from the anomeric center include two with linear alkyl chains (14 and 15) and one with terminal hydroxyl (16) and one terminal-carboxyl derivative (17). Compound 14 with a six-carbon straight alkyl chain formed gels in five solvents, and compound 15 with a 10-carbon linear alkyl chain formed gels in three solvents, and they both formed gels in ethanol. In contrast, the terminal-hydroxyl derivatives with long-alkyl chain linker compound 16 also formed gels in three solvents but only in DMSO/water and ethanol/water mixtures. The short-chain carboxylate derivative compound 17 only formed gels in EtOH/H2O (v/v 1:1) solvent, much less effective in comparison to compound 12, which has one less methylene group in total. Three derivatives (18–20) with three methylene spacers from the anomeric position (n = 3) and linear alkyl substituents on the opposite side were also prepared. Compound 18 is a highly efficient gelator, forming gels in six of the selected solvents and at relatively low MGCs in DMSO/water mixtures. Compound 19 formed gels in five solvents, and the long-chain derivative compound 20 also formed gels in toluene, isopropanol, and ethanol. Compounds 7, 14, and 18 contain the same terminal substituents but at different distance from anomeric carbon. Their gelation properties showed an interesting trend that with increasing distance from the anomeric center, the gelation properties enhanced. This indicated that the increased hydrophobic alkyl chain was beneficial for the gelation and self-assembly.
Table 1. Gelation Properties of the Synthesized Diacetylene Lipids 6–20a.
| no. | Tol | IPA | EtOH | DMSO/H2O (1:1) | DMSO/H2O (1:2) | EtOH/H2O (1:1) | EtOH/H2O (1:2) | H2O |
|---|---|---|---|---|---|---|---|---|
| 6 | G* 20.0 | P | G 20.0 | G 10.0 | G 10.0 | G 10.0 | P | I |
| 7 | P | S | S | G 10.0 | G 10.0 | G 20.0 | G 10.0 | P |
| 8 | S | P | P | G 10.0 | G 10.0 | G 10.0 | P | P |
| 9 | GC 6.7 | P | P | G 6.7 | G 20.0 | G 5.0 | G 20.0 | I |
| 10 | G 10.0 | G 20.0 | G 10.0 | G 10.0 | G 20.0 | G 10.0 | P | I |
| 11 | GT 10.0 | S | S | G 20.0 | G 6.7 | GT 6.7 | G 6.7 | G 10.0 |
| 12 | G 20.0 | G 20.0 | S | G 20.0 | G 20.0 | G 20.0 | G 20.0 | P |
| 13 | G 10.0 | G 10.0 | P | G 10.0 | G 10.0 | G 20.0 | G 10.0 | I |
| 14 | GC 20.0 | P | G 10.0 | G 5.0 | G 6.7 | G 20.0 | P | I |
| 15 | P | P | G 10.0 | G 10.0 | P | G 3.3 | P | I |
| 16 | P | P | P | P | G 5.0 | G 20.0 | G 5.0 | I |
| 17 | P | S | S | P | P | G 10.0 | P | I |
| 18 | G 10.0 | GT* 20.0 | S | G 4.0 | G 5.0 | G 2.5 | GT* 20.0 | P |
| 19 | GC 10.0 | GT* 20.0 | P | G 6.7 | G 10.0 | G* 20.0 | P | I |
| 20 | G 5.0 | G 20.0 | G 20.0 | G 20.0 | I | P | I | I |
All compounds were tested starting from 20 mg/mL. G, stable gel at rt and G* partial gel or unstable gel at 20 mg/mL; the numbers next to “G” are MGCs in mg/mL; P, precipitation; S, soluble; and I, insoluble. All gels were opaque except those labeled. The subscribed letters denote the gel appearances: C, clear or transparent and T, translucent; all compounds were insoluble in hexane, except compound 15, which formed a precipitate.
The gelation mechanism for compounds 7 and 18 was studied utilizing 1H NMR spectroscopy at various temperatures; the spectra are shown in Figure 3 and Figures S49–S51 in Supporting Information. Using CDCl3 as a solvent from 30 to 55 °C, most signals did not change upon moderate heating and only the 3-hydroxyl and 2-amide NH showed certain upfield shifts. The spectra for compound 18 showed a 0.06 ppm upfield shift of the amide peak, which indicated that the intermolecular hydrogen bonding from the NH group is essential for the molecular assembly; the 3-OH group also shifted upfield and more significantly with 0.14 ppm change, which indicated the 3-hydroxyl group’s participation in intermolecular hydrogen bonding being important for the gelation process. The NMR experiment helps to elucidate the importance of hydrogen bonds using CDCl3 as a solvent, which will not participate in hydrogen bonding.
Figure 3.
1H NMR spectra (2.5–6.5 ppm) of compound 18 from 30 to 55 °C in CDCl3 (10.0 mg/mL).
The morphologies of the gels formed by several gelators were then characterized using an optical microscope (Figure S52) and a field emission scanning electronic microscope (Figure 4). Figure S52 shows the optical micrographs of a few gels before and after UV irradiation. The gel formed by compound 14 in DMSO/H2O (v/v 1:1) showed fibrous morphology together with tubular sheet-like aggregates (Figure S52a); upon irradiation using a 6 W TLC-illuminating UV lamp at 254 nm for 5 min, the gel turned pink. The optical micrograph of the UV-treated xerogels (on the slide) showed similar morphology as that before the UV treatment, but the tubular ribbons turned red for most parts with some areas unchanged (Figure S52b). This indicated that the gel was partially polymerized. The gel formed by compound 15 in EtOH/H2O (v/v 1:1) after UV treatment by irradiating the slides directly for 5 min (254 nm) is shown in Figure S53c, which exhibited red-colored ribbons densely packed together. Figure S53d shows the UV-treated gels formed by compound 16 in EtOH/H2O (v/v 1:2) after UV irradiation (254 nm for 20 min), which displayed round ball-shaped aggregates composed of a fibrous network. The gel formed by compound 17 in EtOH/H2O (v/v 1:1) is shown in Figure S53e, which showed bundled fibers, and after UV treatment, it formed orange-colored fibrous assemblies (Figure S54f).
Figure 4.
(a,b) Compound 8 in EtOH/H2O (v/v 1:1) at 10.0 mg/mL, (c) compound 9 in EtOH/H2O (v/v 1:1) at 3.3 mg/mL, (d) compound 11 in EtOH/H2O (v/v 1:2) at 5.0 mg/mL, (e) compound 15 in EtOH/H2O (v/v 1:1) at 3.3 mg/mL, (f,g) compound 15 in EtOH at 10.0 mg/mL, and (h,i) compound 17 in EtOH/H2O (v/v 1:1) at 10.0 mg/mL.
The scanning electronic micrographs of several dried gels were also obtained, and these are shown in Figure 4. They showed diverse arrays of morphology that ranges from strands to planar sheets and tubules. As shown in Figure 4a,b, the gel of compound 8 in EtOH/H2O (v/v 1:1) showed a long fibrous network with some helices and more bundled connected fibers, and the helices had diameters less than 1 μm. The compound 9 has one extra methylene group compared to compound 8; the gel formed by compound 9 showed mostly cylindrical tubes and planar sheet-like morphology, with a width of 10 μm and length over 200 μm (Figure 4c). The gel of compound 11 in EtOH/H2O (1:1) formed a smaller fibrous network and flower-like fibers and ribbons, with diameters less than 4 μm (Figure 4d). The gel formed by compound 15, which contains two carbon spacer at the anomeric position, in EtOH/H2O (v/v 1:1) formed interesting uniform planar flower-like assemblies (Figure 4e), but in EtOH, the morphology of the gel showed a uniform fibrous network, which contains twisted or circular bundled fibers of diameters less than 1 μm, but the fibers are long and uniform (Figure 4f,g). The gel formed from gelator 17 exhibited the typical bundled fibrous assemblies and tubules of 0.5 μm in diameter (Figure 4h,i).
The photo-responses of these diacetylene-containing gelators are also analyzed in order to obtain useful stimuli-responsive materials. We found that these compounds are typically more resistant to photopolymerization, and they required longer UV irradiation time (3–30 min) and formed red-colored cross-linked conjugates. The stability of the UV-treated gel was analyzed to evaluate whether the photopolymerization can enhance the gel’s stability. Using compound 14 as a representative compound, the gel melting points were measured before and after UV treatment. The gel formed by compound 14 in ethanol (10.0 mg/mL) was prepared in a quartz NMR tube, and it was irradiated under a UV lamp at 254 nm for 30 min (Figure S53). The white opaque gel turned red after UV irradiation; the melting points of the gels before and after UV treatment were measured and are shown in the Table S1. After UV treatment, the polymerized gel showed improved thermal stability from 33 to 39 °C before UV irradiation to 35–73 °C after UV treatment. Besides ethanol, a DMSO/water gel prepared in a 1 dram vial was also irradiated with UV light, and the sample turned red after half an hour (Figure S54).
The photo-polymerization of the diacetylenes was also characterized using Raman spectroscopy (Figure S55). The xerogels of compound 15 (EtOH, 10.0 mg/mL) were prepared on a glass slide and were treated with a 6 W UV lamp at 254 nm at a distance of 5 cm. The color changed to red under UV irradiation for 30 min. The Raman spectrum before irradiation showed a signal at 2265 cm–1, which corresponds to the diacetylene moiety (−C≡C−), and after photopolymerization, the peak at 2265 cm–1 shifted to 2135 cm–1 (−C≡C−) and 1519 cm–1 (−C=C−), which corresponds to the alkyne and alkene functions of the cross-linked enyne moiety.
A systematic photopolymerization study for all gelators that were able to form gels in EtOH/H2O (1:1) was also carried out. These include four one-carbon series, four two-carbon series, and two three-carbon series. The gels after 3 min of UV irradiation are shown in Figure 5; additional images are included in Figure S56. The UV–vis spectra of several samples are shown in Figures 6 and S57, and the UV absorption peaks are included in Table S2. The absorption intensity increased with prolonged irradiation, but the peak positions (λmax) typically stayed the same. The gels formed by the n = 1 series compounds are polymerizable to give red-colored gels with typically two absorption signals λmax at about 490 and 530 nm. Compound 10 contains a terminal phenyl substituent, and it showed weak absorption when treated with UV for 7 min and absorbed broadly in the range of 520–580 nm and appeared purple red. This sample needs longer irradiation time or a different method for polymerization; the gel formed by compound 12 exhibited a major absorption λmax at 550 nm and a weaker peak at 512 nm. The gelators of n = 2 series also showed structural dependence on the side opposite to the sugar; the gel formed by 14 and 15 changed to pink or dark red color upon UV treatment; 14 showed absorption signals at 500 and 546 nm, and compound 15 showed absorption at mainly 567 nm and a broad signal from 500 to 610 nm. The gel formed by 16 and 17 showed weak absorptions at 488 and 532 nm; the gel appeared light pink, and the intensities were weak, which indicated that the gels need longer irradiation time to polymerize. Moreover, under 7 min of irradiation, the gels formed by compounds 18 and 19 did not change color, which indicated that stronger UV irradiation and longer time are required to cross-link the gelators.
Figure 5.

Gels of EtOH/H2O (1:1) after 3 min of UV irradiation at 254 nm; the gelator concentrations are 10.0 mg/mL for gelators 6, 9, 10, 15, 17, and 18 and 20.0 mg/mL for compounds 12, 14, 16, and 19.
Figure 6.
UV–vis spectra of the gels of compounds 9 and 17 in EtOH/H2O (v/v 1:1, 10 mg/mL) for different duration of UV irradiation.
Among these glycolipids, compound 17 with a terminal carboxylic acid functional group was the least versatile gelator, which only formed a gel in EtOH/water (v/v 1:1) at 10.0 mg/mL. This compound was further analyzed by forming the corresponding salt under alkaline conditions. It is possible that upon conversion to the carboxylate anion, the intermolecular interactions become more favorable in forming supramolecular gels. To test this hypothesis, three identical gels were prepared using 0.3 mg of compound 17 and 0.3 mL of EtOH/H2O (1/1). To these vials, 0.3 mL of pH 7, 10, and 12 solutions were added, respectively; the samples were left standing at rt for 2 h (Figure S58). The gel at pH 12 showed much degradation while the other two remained mostly intact. The liquid was removed from the vials (a) and (b), and the residue gels were irradiated with UV light at 254 nm for 20 min, after which the removed liquids were added back to the corresponding vials. The gels at pH 10 and 12 showed swelling properties and upon standing, the mixtures turned to gels. As shown in Figure 7, the compound became a more efficient gelator under basic conditions. This result indicated that it is possible to adjust the properties of the glycolipid by changing the pH.
Figure 7.

(a) Gel was formed by compound 17 in EtOH/H2O (v/v 1:1) at 10 mg/mL first, and then, equal volume of pH 10 solution was added and treated with UV irradiation; the final gelator concentration was 5.0 mg/mL in EtOH/H2O (v/v 1:3), 0.15 mL of EtOH and 0.45 mL of water. (b) Gel of compound 17 in pH 12 solution at 4.3 mg/mL; the final concentration was in EtOH/water (v/v 1:3.7).
Conclusions
We have designed and synthesized a library of 15 diacetylene-containing glycosides and evaluated their gelation performances in a few solvents. The diacetylene functional groups are attached to the anomeric position through alpha-glycosidic linkage with one, two, and three methylene groups from the anomeric oxygen. The influence of the diacetylene positions on molecular self-assembly and gelation was analyzed. All compounds synthesized were found to be effective gelators for at least one of the tested solvents. The diacetylene glycosides with one and three carbon spacers from the anomeric center formed gels in toluene as well as in an ethanol water (v/v 1:1) mixture and a DMSO water (v/v 1:1) mixture. Interestingly, the hydroxyl-substituted derivative 11 also formed a hydrogel at 1.0 wt %. The derivatives with two carbon spacers formed gels in fewer solvents. The gels were photopolymerizable to afford red- and purple-colored gels. These new diacetylene-containing gelators may have potential applications as stimuli-responsive materials that respond to photoirradiation and therefore can be used as sensors for UV lights. Interestingly, the carboxylic-functionalized derivative 17, which only formed gels in ethanol water (v/v 1:1) mixtures, under alkaline conditions was able to form gels at lower concentrations, resulting in a pH-responsive gelator. The hydrogelators and carboxylic acid derivatives can have applications as stimuli-responsive materials in biomedical research. They can be used as co-gelators with other glycolipids or drugs for the exploration in enzyme immobilization and drug delivery studies.
Experimental Section
Materials and Methods
N-Acetyl-d-glucosamine and some alkynes were purchased from AKSci; other general chemicals were obtained from general chemical companies including Acros, TCI, and so forth. Flash chromatography was carried out using 230–400-mesh silica gel with mixed solvents. 1H NMR and 13C NMR and some 2D NMR spectra were obtained using a Bruker 400 MHz NMR spectrometer in CDCl3. Melting points of solids or crystals were measured using a Fisher Jones melting point apparatus. The LCMS data were obtained using an Agilent 6120B Single Quad mass spectrometer and a LC1260 system; the high-resolution MS data were obtained on a Bruker 10 Tesla APEX-Qe Fourier-transform ion cyclotron resonance mass spectrometer with an Apollo II ion source using positive electrospray ionization.
Optical micrographs were obtained using an Olympus BC60M optical microscope following the literature procedure.41 Gelation tests were performed similarly to the procedure described before.42 Field emission scanning electron microscopy (SEM) studies were carried out using a SU8010 Hitachi field emission scanning electron microscope using the same method described.43 The melting points for the gels were measured using a similar method reported before.41
Photopolymerization Studies
UV spectra for the study of the series of gelators were recorded by treating the gels formed in EtOH/H2O (v/v 1:1) for the samples. The gels were prepared for the compounds, and then, 0.1 mL of the gels was transferred to a 96-well microplate; then, the plate was placed under UV lamp irradiation with 254 nm UV light for 1, 2, 3, and 7 min. The UV absorption for each gel sample indicated that longer irradiation time gives stronger UV signals, which indicates that the degree of polymerization increases as the time of irradiation increases. To observe the optical images, for the slides with UV treatment, the wet gel was first transferred from the vials to clean slides, and then, they were treated with a TLC lamp with UV of 254 nm for 3 min at approximately 5 cm distance between the lamp and slide.
Preparation of Bromoalkynes 5b–5i
Alkynyl bromide was synthesized following literature procedures31 by treating the corresponding alkynes with NBS in acetone and catalytic amount of AgNO3. The detailed synthesis and characterization of the acetylene bromides used in this study are included in the Supporting Information.
Synthesis of Propargyl Glycoside Intermediate 3A
To a 100 mL round-bottomed flask, compound 1 (N-acetyl-d-glucosamine, 3.00 g, 13.57 mmol, 1 equiv), propargyl alcohol (12 mL, 203 mmol, 15 equiv), and BF3·Et2O (0.43 mL, 3.30 mmol, 0.25 equiv) were added in the given order. The reaction mixture was stirred at 75 °C for 10 h, and 1H NMR spectrum showed completion of the reaction. The heating was stopped, and the reaction mixture was diluted with EtOAc (10 mL ×3) and extracted with water (20 mL ×3). The combined aqueous phase was concentrated to obtain thick oil, which was re-dissolved in EtOH (10 mL) and concentrated to ensure the removal of water. The resulting oil was dried using a high-vacuum pump to obtain a yellow solid (3.53 g, quantitative) as the desired product 2A. The prop-2-ynyl-2-acetamido-2-deoxy-α-d-glucoside 2A has been reported in the literature using CD3OD as NMR solvent.441H NMR (400 MHz, D2O): δ 5.02 (d, J = 3.6 Hz, 1H), 4.34–4.22 (m, 2H), 3.94–3.69 (m, 5H), 3.51–3.44 (m, 1H), 2.89–2.84 (m, 1H), 2.00 (s, 3H); 13C NMR (100 MHz, D2O + 1 drop of CH3OH): δ 174.5, 96.0 79.1, 75.9, 72.3, 70.9, 69.9, 60.4, 55.2, 53.4, 21.9. LCMS (ESI+) m/z: calcd for C11H18NO6 [M + H]+ 260; found, 260. The crude product 2A (3.00 g, 11.6 mmol, 1 equiv) prepared and PTSA (219.7 mg, 1.16 mmol, 0.1 equiv) were dissolved in 10 mL of anhydrous dimethylformamide (DMF), followed by addition of benzaldehyde dimethyl acetal (2.61 mL, 17.4 mmol, 1.5 equiv) under nitrogen, The reaction mixture was stirred at 70 °C for 10 h under nitrogen. Then, a small amount of NaHCO3 was added to the rection mixture, and the mixture was stirred for 30 min at rt. The reaction mixture was diluted with EtOAc (10 mL) followed by water (10 mL); during phase separation, insoluble white solid was obtained which was filtered and recrystallized using ethanol to give the pure product as an off-white solid. The separated water layer was then extracted with EtOAc (20 mL ×3) again, and the combined organic phases were dried over Na2SO4, filtered, and concentrated. The residue was purified by column chromatography using DCM to 5% MeOH/DCM. The product 3A(45) was obtained as an off-white solid in a total amount of 2.04 g and 51% yield. mp 149.0–151.0 °C. 1H NMR (400 MHz, CDCl3): 7.53–7.51 (m, 2H), 7.39–7.38 (m, 3H), 5.89 (d, J = 8.7 Hz, 1H), 5.59 (s, 1H), 5.06 (d, J = 3.9 Hz, 1H), 4.35–4.26 (m, 4H), 3.95 (t, J = 9.6 Hz, 1H), 3.91–3.85 (m, 1H), 3.78 (t, J = 10.0 Hz, 1H), 3.63 (t, J = 9.2 Hz, 1H), 2.51 (t, J = 2.4 Hz, 1H), 2.09 (s, 3H); 13C NMR (100 MHz, CDCl3): 171.5, 137.0, 129.2, 128.3, 126.3, 102.0, 96.7, 82.0, 78.4, 75.3, 70.5, 68.7, 63.0, 55.1, 53.8, 23.3. LCMS (ESI+) m/z: calcd for C18H22NO6 [M + H]+, 348; found, 348.
Synthesis of 3-Butynyl Glycoside Intermediate 3B
Compound 1 (5.00 g, 22.6 mmol, 1 equiv), 3-butyn-1-ol (8.6 mL, 113 mmol, 5 equiv), and BF3·Et2O (800.2 mg, 5.65 mmol, 0.25 equiv) were added in the given order. The reaction mixture was heated at 80 °C for 24 h. The reaction mixture was cooled to rt followed by addition of 20 mL of water. The mixture was transferred to a separating funnel, and the aqueous layer was washed with EtOAc (20 mL ×3). About 6 mL of unreacted 3-butyn-1-ol was recovered by concentrating the EtOAc layer. The aqueous layer was concentrated using a rotary evaporator to afford the crude product, which was purified by column chromatography using 5% MeOH/DCM to 15% MeOH/DCM to afford a brown solid (3.61 g, 59%) as the desired product 2B (Rf = 0.55 in 15% MeOH/DCM). mp 149.0–151.0 °C. 1H NMR (400 MHz, D2O): δ 4.92 (d, J = 3.6 Hz, 1H), 3.94–3.67 (m, 6H), 3.67–3.55 (m, 1H), 3.53–3.41 (m, 1H), 2.56–2.43 (m, 2H), 2.40 (t, J = 2.6 Hz, 1H), 2.02 (s, 3H); 13C NMR (100 MHz, D2O): δ 174.4, 97.0 82.9, 72.0, 71.0, 70.3, 70.0, 66.0, 60.6, 53.7, 22.0, 19.0. LCMS (ESI+) m/z: calcd for C12H20NO6 [M + H]+, 274; found, 274. To a 100 mL round-bottomed flask compound 2B (2.50 g, 9.15 mmol, 1 equiv) and toluene (3 mL ×3) were added and co-evaporated to remove residual moisture. The dried compound 2B was dissolved in anhydrous DMF (5 mL) followed by addition of benzaldehyde dimethyl acetal (1.79 mL, 11.90 mmol, 1.3 equiv) and PTSA·H2O (0.174 g, 0.915 mmol, 0.1 equiv) under a N2 atmosphere. The reaction mixture was stirred at 80 °C for 17 h. The reaction mixture was cooled to rt, and NaHCO3 (85 mg, 1.019 mmol, 0.12 equiv) was added and stirred at rt for 15–30 min. After removing DMF under reduced pressure, the crude product was recrystallized in EtOH to give a white solid (2.61 g, 79%) as the desired product 3B. Compound 3B was reported previously without characterization data.46 mp 213.2–215.1 °C. 1H NMR (400 MHz, CDCl3): δ 7.54–7.47 (m, 2H), 7.40–7.33 (m, 3H), 6.06 (d, J = 8.5 Hz, 1H), 5.59 (s, 1H), 4.91 (d, J = 3.9 Hz, 1H), 4.31–4.20 (m, 2H), 3.91–3.81 (m, 3H), 3.76 (t, J = 10.3 Hz, 1H), 3.62–3.54 (m, 2H), 2.56–2.49 (m, 2H), 2.05 (s, 3H), 2.04 (t, J = 2.6 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 171.5, 137.1, 129.2, 128.3, 126.3, 102.0, 98.0, 82.1, 81.3, 70.8, 69.8, 68.8, 66.1, 62.7, 54.2, 23.2, 19.8. LCMS (ESI+) m/z: calcd for C19H24NO6 [M + H]+, 362; found, 362.
Synthesis of 4-Pentynyl Glycoside Intermediate 3C
Compound 1 (5.00 g, 22.6 mmol, 1 equiv), 4-pentyn-1-ol (10.5 mL, 113 mmol, 5 equiv), and BF3·Et2O (800.2 mg, 5.65 mmol, 0.25 equiv) were added in the given order. The reaction mixture was heated at 80 °C for 36 h. The reaction mixture was cooled to rt followed by addition of 20 mL of water. The mixture was transferred to a separating funnel, and the aqueous layer was washed with EtOAc (20 mL ×3). The aqueous layer was concentrated under reduced pressure to afford the crude product, which was purified by column chromatography using 5% MeOH/DCM to 15% MeOH/DCM to afford brown solid (3.41 g, 53%) as the desired product 2C. (Rf = 0.45 in 15% MeOH/DCM). mp 137.0–141.1 °C. 1H NMR (400 MHz, D2O): δ 4.85 (d, J = 3.6 Hz, 1H), 3.89–3.70 (m, 6H), 3.55–3.42 (m, 2H), 2.36–2.29 (m, 2H), 2.20 (s, 1H), 2.02 (s, 3H), 1.80–1.77 (m, 2H); 13C NMR (100 MHz, D2O): δ 174.5, 97.0, 85.1, 71.8, 71.0, 70.0, 69.7, 66.4, 60.5, 53.8, 27.3, 21.9, 14.4. LCMS (ESI+) m/z: calcd for C13H22NO6 [M + H]+, 288; found, 288. Compound 2C (500 mg, 1.74 mmol, 1 equiv) and toluene (2 mL ×3) were co-evaporated to remove residue water. To a 100 mL round-bottomed flask, the dried compound 2C was dissolved in anhydrous DMF (5 mL) followed by addition of benzaldehyde dimethyl acetal (0.34 mL, 2.26 mmol, 1.3 equiv) and PTSA·H2O (33.1 mg, 0.174 mmol, 0.1 equiv) under a N2 atmosphere. The reaction mixture was stirred at 75 °C for 13 h. The reaction mixture was cooled to rt, and NaHCO3 (17.6 mg, 0.209 mmol, 0.12 equiv) was added and stirred at rt for 15 min. After removing DMF under reduced pressure, the crude product was purified by column chromatography using eluents from pure DCM to 5% MeOH/DCM to give a light-yellow solid (402 mg, 62%) as the desired product 3C. (Rf = 0.45 in 5% MeOH/DCM). mp 205.3–208.5 °C. 1H NMR (400 MHz, CDCl3): δ 7.52–7.49 (m, 2H), 7.37–7.35 (m, 3H), 5.94 (d, J = 8.4 Hz, 1H), 5.56 (s, 1H), 4.84 (d, J = 3.7 Hz, 1H), 4.29–4.20 (m, 2H), 3.94–3.72 (m, 4H), 3.62–3.50 (m, 2H), 2.37–2.31 (m, 2H), 2.06 (s, 3H), 2.01 (t, J = 2.6 Hz, 1H), 1.88–1.82 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 171.5, 137.1, 129.2, 128.3, 126.3, 101.9, 97.9, 83.3, 82.1, 70.7, 69.2, 68.8, 66.6, 62.6, 54.2, 27.8, 23.3, 15.4. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C20H26NO6, 376.1755; found, 376.1752.
General Procedure of the Synthesis of Sugar Anomeric Diacetylene Compounds 6–20
To a 50 or 100 mL round-bottomed flask, CuCl (0.08–0.1 equiv) was added to a 30% solution of aqueous n-BuNH2 at rt, which resulted in a blue solution. A few crystals of hydroxylamine hydrochloride were added next to discharge the color. The alkynyl glycoside intermediate (1 equiv) in THF was then added, and the mixture was cooled with an ice-water bath and stirred for 20 min. A solution of bromoalkyne (1.4–1.5 equiv) in THF was added dropwise to the above solution, and the ice bath was removed. The reaction mixture was stirred for 4–28 h at rt (more crystals of hydroxylamine hydrochloride were occasionally added to discharge the blue color to obtain a light-yellow solution). The stirring was then stopped, and the pH of the solution was adjusted to ∼2 with 2 N HCl, and the product was extracted with EtOAc (3 ×20 mL). The combined organic layers were washed with water followed by brine, dried (Na2SO4), filtered, and concentrated to give the crude, which was purified by column chromatography to afford the desired sugar anomeric diacetylene coupling product. Precaution needs to be taken to protect the product from light during entire experiment including work up and column chromatography to avoid polymerization.
Synthesis of Compound 6
CuCl (2.9 mg, 0.0288 mmol, 0.1 equiv), 30% solution of aqueous n-BuNH2 (5 mL), alkynyl glycoside 3A (100 mg, 0.288 mmol, 1 equiv) in THF (3 mL), and bromoalkyne 5a (69.1 mg, 0.432 mmol, 1.5 equiv) in THF (1 mL) were mixed. The reaction mixture was stirred for 6 h at rt. The crude product was purified by column chromatography using a solvent system from pure DCM to 2.2% MeOH/DCM to afford a pink solid (92.9 mg, 76%) as the desired product (Rf = 0.40 in 5% MeOH/DCM). mp 213.0–215.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.51–7.48 (m, 2H), 7.37–7.35 (m, 3H), 5.90 (d, J = 8.8 Hz, 1H), 5.55 (s, 1H), 4.98 (d, J = 3.8 Hz, 1H), 4.32–4.24 (m, 4H), 3.92–3.81 (m, 2H), 3.75 (t, J = 10.1 Hz, 1H), 3.59 (t, J = 9.2 Hz, 1H), 2.75 (br s, 1H), 2.29 (t, J = 7.0 Hz, 2H), 2.06 (s, 3H), 1.54–1.49 (m, 2H), 1.44–1.38 (m, 2H), 0.91 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 171.5, 137.1, 129.2, 128.3, 126.3, 102.0, 96.9, 82.3, 82.0, 72.2, 70.5, 70.0, 68.7, 64.1, 63.0, 56.0, 53.8, 30.1, 23.3, 21.9, 18.9, 13.4. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C24H30NO6, 428.2068; found, 428.2062.
Synthesis of Compound 6 by an Alternative Method
The synthesis and NMR data for intermediate 3A-Br are included in the ESI. The coupling reaction followed similar procedure as described above: CuCl (1.7 mg, 0.015 mmol, 0.08 equiv), 30% aqueous nBuNH2 solution (3 mL), hydroxylamine hydrochloride crystals, alkynyl glycoside bromide 3A-Br (80.0 mg, 0.19 mmol, 1 equiv), and THF (3 mL) were mixed. The mixture was stirred at 0 °C for about 30 min. Then, a solution of hex-1-yne (23.1 mg, 0.28 mmol, 1.5 equiv) in anhydrous THF (1.5 mL) was added dropwise to the above solution, and the ice bath was removed. The reaction mixture was stirred at rt, and small amount of hydroxylamine hydrochloride was occasionally added to obtain a light-yellow solution. After 10 h at room temperature, 6 mL of water was added, and aqueous phase was extracted with EtOAc (5 mL ×3). The combined organic phases were dried with Na2SO4 (anhydrous), filtered, and concentrated. The product was purified by column chromatography on silica gel from 0.5% MeOH/DCM to 2% MeOH/DCM to afford compound 6 (59.2 mg, 0.14 mmol, 73%).
Synthesis of Compound 7
CuCl (2.9 mg, 0.0288 mmol, 0.1 equiv), 30% solution of aqueous n-BuNH2 (5 mL), alkynyl glycoside 3A (100 mg, 0.288 mmol, 1 equiv) in THF (3 mL), and bromoalkyne 5b (85.3 mg, 0.432 mmol, 1.5 equiv) in THF (2 mL) were used. The reaction mixture was stirred for 8 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 2.2% MeOH/DCM to afford a pink solid (117 mg, 90%) as the desired product (Rf = 0.4 in 5% MeOH/DCM). mp 179.1–181.5 °C; 1H NMR (400 MHz, CDCl3): δ 7.51–7.48 (m, 2H), 7.38–7.35 (m, 3H), 5.90 (d, J = 7.9 Hz, 1H), 5.55 (s, 1H), 4.98 (d, J = 3.4 Hz, 1H), 4.36–4.25 (m, 4H), 3.93–3.81 (m, 3H), 3.59 (t, J = 9.1 Hz, 1H), 3.15 (br s, 1H), 2.28 (t, J = 7.0 Hz, 2H), 2.06 (s, 3H), 1.55–1.50 (m, 2H), 1.40–1.33 (m, 2H), 1.32–1.25 (m, 4H), 0.89 (t, J = 6.9 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 171.6, 137.1, 129.2, 128.3, 126.3, 102.0, 96.9, 82.4, 82.0, 72.2, 70.5, 70.0, 68.7, 64.1, 63.0, 56.0, 53.8, 31.2, 28.5, 28.0, 23.3, 22.4, 19.2, 14.0. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C26H34NO6, 456.2381; found, 456.2377.
Synthesis of Compound 8
CuCl (2.9 mg, 0.0288 mmol, 0.1 equiv), 30% solution of aqueous n-BuNH2 (5 mL), alkynyl glycoside 3A (100 mg, 0.288 mmol, 1 equiv) in THF (3 mL), and bromoalkyne 5d (99.8 mg, 0.432 mmol, 1.5 equiv) in THF (2 mL) were used. The reaction mixture was stirred for 8 h at rt. The combined organic phase was dried over sodium sulphate and concentrated. The crude product was purified by column chromatography using eluents from pure DCM to 2.2% MeOH/DCM to afford a pink solid (110 mg, 77%) as the desired product (Rf = 0.28 in 5% MeOH/DCM). mp 219.0–222.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.51–7.48 (m, 2H), 7.38–7.35 (m, 3H), 5.87 (d, J = 7.9 Hz, 1H), 5.56 (s, 1H), 5.00 (d, J = 3.4 Hz, 1H), 4.37–4.26 (m, 4H), 3.93–3.72 (m, 3H), 3.60 (t, J = 9.2 Hz, 1H), 2.28 (t, J = 7.0 Hz, 2H), 2.07 (s, 3H), 1.55–1.50 (m, 2H), 1.39–1.32 (m, 2H), 1.27 (br s, 10H), 0.88 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 171.5, 137.1, 129.2, 128.3, 126.4, 102.0, 97.0, 82.4, 82.0, 72.2, 70.4, 70.0, 68.7, 64.1, 63.1, 56.0, 53.8, 31.8, 29.4, 29.2, 29.0, 28.8, 28.1, 23.3, 22.6, 19.2, 14.1. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C29H40NO6, 498.2850; found, 498.2843.
Synthesis of Compound 9
CuCl (11.5 mg, 0.12 mmol, 0.08 equiv), 30% solution of aqueous n-BuNH2 (25 mL), alkynyl glycoside 3A (500 mg, 1.44 mmol, 1 equiv) in THF (15 mL), and bromoalkyne 5e (501 mg, 2.0 mmol, 1.42 equiv) in THF (5 mL) were used. The reaction mixture was stirred for 4 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 1.5% MeOH/DCM to afford a pink solid (585 mg, 82%) as the desired product (Rf = 0.46 in 5% MeOH/DCM). mp 176.5–179.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.53–7.51 (m, 2H), 7.39–7.36 (m, 3H), 5.90 (d, J = 8.6 Hz, 1H), 5.58 (s, 1H), 5.01 (d, J = 3.7 Hz, 1H), 4.39–4.28 (m, 4H), 3.93 (t, J = 9.6 Hz, 1H), 3.89–3.83 (m, 1H), 3.77 (t, J = 10.0 Hz, 1H), 3.62 (t, J = 9.6 Hz, 1H), 2.30 (t, J = 7.0 Hz, 2H), 2.09 (s, 3H), 1.56 (quint, J = 7.0 Hz, 2H), 1.52–1.29 (m, 14H), 0.98 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 171.5, 137.1, 129.2, 128.3, 126.3, 102.0, 96.9, 82.4, 82.0, 72.2, 70.5, 69.9, 68.7, 64.1, 63.0, 56.0, 53.8, 31.9, 29.5, 29.4, 29.3, 29.0, 28.8, 28.1, 23.3, 22.7, 19.2, 14.1. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C30H42NO6, 512.3007; found, 512.3000.
Synthesis of Compound 10
CuCl (2.9 mg, 0.0288 mmol, 0.1 equiv), 30% solution of aqueous n-BuNH2 (5 mL), alkynyl glycoside 3A (100 mg, 0.288 mmol, 1 equiv) in THF (3 mL), and bromoalkyne 5f (96.3 mg, 0.432 mmol, 1.5 equiv) in THF (1 mL) were used. The reaction mixture was stirred for 10 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 2.2% MeOH/DCM to afford a pink solid (115 mg, 81%) as the desired product (Rf = 0.38 in 5% MeOH/DCM). mp 215.0–217.6 °C; 1H NMR (400 MHz, CDCl3): δ 7.51–7.48 (m, 2H), 7.37–7.35 (m, 3H), 7.30–7.26 (m, 2H), 7.21–7.17 (m, 3H), 5.89 (d, J = 8.3 Hz, 1H), 5.56 (s, 1H), 5.00 (d, J = 3.6 Hz, 1H), 4.34–4.25 (m, 4H), 3.94–3.82 (m, 2H), 3.76 (t, J = 10.1 Hz, 1H), 3.60 (t, J = 9.2 Hz, 1H), 2.72 (t, J = 7.8 Hz, 2H), 2.30 (t, J = 7.8 Hz, 2H), 2.07 (s, 3H), 1.90–1.82 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 171.6, 141.0, 137.0, 129.2, 128.5, 128.4, 128.3, 126.3, 126.1, 102.0, 97.0, 82.0, 81.7, 72.1, 70.5, 70.2, 68.7, 64.6, 63.0, 55.9, 53.8, 34.7, 29.6, 23.3, 18.6. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C29H32NO6, 490.2224; found, 490.2220.
Synthesis of Compound 11
CuCl (2.1 mg, 0.023 mmol, 0.08 equiv), 30% solution of aqueous n-BuNH2 (3 mL), alkynyl glycoside 3A (100.0 mg, 0.288 mmol, 1 equiv) in THF (3 mL), and bromoalkyne 5g (99 mg, 0.404 mmol, 1.4 equiv) in THF (1 mL) were used. The reaction mixture was stirred for 10 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 3% MeOH/DCM to afford a white solid (121 mg, 82%) as the desired product (Rf = 0.3 in 5% MeOH/DCM). mp 172.0–174.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.52–7.46 (m, 2H), 7.40–7.33 (m, 3H), 5.93 (d, J = 8.8 Hz, 1H), 5.56 (s, 1H), 4.99 (d, J = 3.9 Hz, 1H), 4.39–4.24 (m, 4H), 3.95–3.72 (m, 3H), 3.66–3.57 (m, 3H), 2.28 (t, J = 7.0 Hz, 2H), 2.08 (s, 3H), 1.60–1.49 (m, 4H), 1.43–1.24 (m, 10H); 13C NMR (100 MHz, CDCl3): δ 171.5, 137.1, 129.2, 128.3, 126.4, 102.0, 96.9, 82.4, 82.0, 72.2, 70.5, 70.0, 68.7, 64.2, 63.04, 62.99, 56.0, 53.8, 32.8, 29.30, 29.25, 28.9, 28.7, 28.0, 25.7, 23.3, 19.2. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C29H40NO7, 514.2800; found, 514.2791.
Synthesis of Compound 12
CuCl (3.4 mg, 0.034 mmol, 0.08 equiv), 30% solution of aqueous n-BuNH2 (5 mL), alkynyl glycoside 3A (150 mg, 0.413 mmol, 1 equiv) in THF (3 mL), and bromoalkyne 5h (150.4 mg, 0.613 mmol, 1.42 equiv) in THF (5.0 mL) were used. The reaction mixture was stirred for 6 h at rt. The combined organic layers were then basified with NaHCO3, making the sodium salt of the carboxylic acid product, which was then extracted twice with water. To this aqueous layer was then added 2 M HCl dropwise to obtain a white precipitate, which was filtered to obtain a white solid (138 mg, 71%) as the desired product. mp 175.2–179.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.51–7.48 (m, 2H), 7.37–7.35 (m, 3H), 5.92 (d, J = 8.6 Hz, 1H), 5.55 (s, 1H), 5.02 (d, J = 3.6 Hz, 1H), 4.32–4.26 (m, 4H), 3.94–3.81 (m, 2H), 3.75 (t, J = 10.0 Hz, 1H), 3.60 (t, J = 9.2 Hz, 1H), 2.43–2.34 (m, 4H), 2.08 (s, 3H), 1.80–1.73 (m, 2H), 1.65–1.57 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 176.8, 137.0, 129.2, 128.3, 126.3, 102.0, 96.8, 82.0, 81.4, 72.1, 70.4, 70.3, 68.7, 64.7, 63.1, 55.9, 53.9, 33.1, 27.3, 23.8, 23.3, 19.0. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C25H30NO8, 472.1966; found, 472.1959.
Synthesis of Compound 13
CuCl (2.9 mg, 0.0288 mmol, 0.1 equiv), 30% solution of aqueous n-BuNH2 (3 mL), alkynyl glycoside 3A (100 mg, 0.288 mmol, 1.0 equiv) in THF (3 mL), and bromoalkyne 5i (113 mg, 0.432 mmol, 1.5 equiv) in THF (2 mL) were used. The reaction mixture was stirred for 10 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 6% MeOH/DCM to afford a white solid (111.4 mg, 73%) as the desired product (Rf = 0.36 in 5% MeOH/DCM). mp 151.0–153.5 °C; 1H NMR (400 MHz, CDCl3): δ 7.50–7.48 (m, 2H), 7.38–7.34 (m, 3H), 5.96 (d, J = 8.9 Hz, 1H), 5.55 (s, 1H), 5.00 (d, J = 3.8 Hz, 1H), 4.36–4.25 (m, 4H), 3.93–3.80 (m, 3H), 3.60 (t, J = 9.2 Hz, 1H), 2.33 (t, J = 7.4 Hz, 2H), 2.28 (t, J = 6.9 Hz, 2H), 2.08 (s, 3H), 1.64–1.60 (m, 2H), 1.54–1.49 (m, 2H), 1.45–1.23 (m, 8H); 13C NMR (100 MHz, CDCl3): δ 177.6, 171.8, 137.0, 129.2, 128.3, 126.3, 102.0, 96.9, 82.3, 81.9, 72.3, 70.4, 70.0, 68.7, 64.2, 63.1, 56.0, 53.8, 33.8, 28.9, 28.8, 28.7, 28.6, 27.9, 24.7, 23.3, 19.2. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C29H38NO8, 528.2591; found, 528.2584.
Synthesis of Compound 14
CuCl (4.4 mg, 0.044 mmol, 0.08 equiv), 30% solution of aqueous n-BuNH2 (5 mL), alkynyl glycoside 3B (200 mg, 0.55 mmol, 1 equiv) in THF (5 mL), and bromoalkyne 5b (157 mg, 0.83 mmol, 1.5 equiv) in THF (1.5 mL) were used. The reaction mixture was stirred for 28 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 2% MeOH/DCM to afford a white solid (198 mg, 77%) as the desired product (Rf = 0.45 in 5% MeOH/DCM). mp 118.0–121.1 °C; 1H NMR (400 MHz, CDCl3): δ 7.52–7.46 (m, 2H), 7.39–7.33 (m, 3H), 6.08 (d, J = 8.4 Hz, 1H), 5.59 (s, 1H), 4.88 (d, J = 3.9 Hz, 1H), 4.32–4.22 (m, 2H), 3.97 (t, J = 9.6 Hz, 1H), 3.91–3.83 (m, 2H), 3.78 (t, J = 10.2 Hz, 1H), 3.65–3.53 (m, 2H), 3.32 (br s, 1H), 2.63–2.58 (m, 2H), 2.26 (t, J = 7.1 Hz, 2H), 2.12 (s, 3H), 1.58–1.47 (m, 2H), 1.43–1.21 (m, 6H), 0.91 (t, J = 6.7 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 171.8, 137.1, 129.2, 128.3, 126.4, 102.0, 98.0, 82.1, 78.9, 73.5, 71.0, 68.8, 67.0, 65.9, 64.8, 62.7, 54.2, 31.2, 29.7, 28.5, 28.2, 23.3, 22.5, 20.5, 19.1, 14.0. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C27H36NO6, 470.2537; found, 470.2534.
Synthesis of Compound 15
CuCl (2.2 mg, 0.022 mmol, 0.08 equiv), 30% solution of aqueous n-BuNH2 (3 mL), alkynyl glycoside 3B (100 mg, 0.277 mmol, 1 equiv) in THF (2 mL), and bromoalkyne 5e (95.1 mg, 0.3878 mmol, 1.4 equiv) in THF (0.8 mL) were used. The reaction mixture was stirred for 24 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 2% MeOH/DCM to afford a white solid (96.5 mg, 66%) as the desired product (Rf = 0.4 in 5% MeOH/DCM). mp 107.0–111.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.57–7.49 (m, 2H), 7.43–7.34 (m, 3H), 6.11 (d, J = 8.0 Hz, 1H), 5.58 (s, 1H), 4.88 (d, J = 3.5 Hz, 1H), 4.37–4.20 (m, 2H), 3.97 (t, J = 9.5 Hz, 1H), 3.92–3.82 (m, 2H), 3.77 (t, J = 10.2 Hz, 1H), 3.65–3.53 (m, 2H), 2.66–2.52 (m, 2H), 2.25 (t, J = 7.0 Hz, 2H), 2.11 (s, 3H), 1.59–1.47 (m, 2H), 1.43–1.21 (m, 14H), 0.90 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 171.8, 137.1, 129.2, 128.2, 126.4, 102.0, 98.0, 82.0, 78.9, 73.5, 70.9, 68.8, 67.0, 65.9, 64.8, 62.8, 54.2, 31.9, 29.6, 29.5, 29.3, 29.1, 28.9, 28.2, 23.3, 22.7, 20.5, 19.1, 14.1. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C31H44NO6, 526.3163; found, 526.3157.
Synthesis of Compound 16
CuCl (2.2 mg, 0.022 mmol, 0.08 equiv), 30% solution of aqueous n-BuNH2 (3 mL), alkynyl glycoside 3B (100 mg, 0.277 mmol, 1 equiv) in THF (2 mL), and bromoalkyne 5g (102.6 mg, 0.415 mmol, 1.5 equiv.) in THF (0.8 mL) were used. The reaction mixture was stirred for 28 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 2% MeOH/DCM to afford a white solid (110 mg, 75%) as the desired product (Rf = 0.25 in 5% MeOH/DCM). mp 165.0–168.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.53–7.48 (m, 2H), 7.40–7.33 (m, 3H), 6.09 (d, J = 8.6 Hz, 1H), 5.56 (s, 1H), 4.87 (d, J = 3.9 Hz, 1H), 4.31–4.20 (m, 2H), 3.95 (t, J = 9.6 Hz, 1H), 3.90–3.82 (m, 2H), 3.77 (t, J = 10.2 Hz, 1H), 3.66–3.52 (m, 4H), 2.64–2.54 (m, 2H), 2.24 (t, J = 7.0 Hz, 2H), 2.10 (s, 3H), 1.63–1.45 (m, 4H), 1.40–1.24 (m, 10H); 13C NMR (100 MHz, CDCl3): δ 171.8, 137.1, 129.2, 128.3, 126.4, 102.0, 98.0, 82.1, 78.8, 73.6, 70.9, 68.8, 67.0, 65.9, 64.9, 63.0, 62.8, 54.2, 32.7, 29.33, 29.26, 28.9, 28.8, 28.1, 25.7, 23.3, 20.5, 19.1. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C30H42NO7, 528.2956; found, 528.2953.
Synthesis of Compound 17
CuCl (4.4 mg, 0.044 mmol, 0.08 equiv), 30% solution of aqueous n-BuNH2 (4 mL), alkynyl glycoside 3B (200 mg, 0.55 mmol, 1 equiv) in THF (4 mL), and bromoalkyne 5h (170 mg, 0.83 mmol, 1.5 equiv) in THF (2 mL) were used. The combined organic layers were then basified with NaHCO3, making the sodium salt of the carboxylic acid product, which was then extracted twice with water. To this aqueous layer was then added 2 M HCl dropwise to obtain a white precipitate, which was filtered to obtain a white solid (200 mg, 75%) as the desired product. mp 163.0–165.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.56–7.50 (m, 2H), 7.41–7.35 (m, 3H), 6.26 (d, J = 8.7 Hz, 1H), 5.58 (s, 1H), 4.90 (d, J = 3.8 Hz, 1H), 4.32–4.20 (m, 2H), 3.97 (t, J = 9.6 Hz, 1H), 3.92–3.71 (m, 3H), 3.66–3.51 (m, 2H), 2.60 (t, J = 5.7 Hz, 2H), 2.35 (t, J = 7.3 Hz, 2H), 2.29 (t, J = 6.8 Hz, 2H), 2.10 (s, 3H), 1.80–1.69 (m, 2H), 1.63–1.51 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 177.2, 172.1, 137.2, 129.2, 128.2, 126.4, 101.9, 97.9, 81.9, 77.9, 74.0, 70.3, 68.8, 66.8, 65.8, 65.5, 62.9, 54.2, 33.3, 27.5, 23.9, 23.2, 20.5, 18.9. HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C26H32NO8, 486.2122; found, 486.2123.
Synthesis of Compound 18
CuCl (1.0 mg, 0.011 mmol, 0.08 equiv), 30% solution of aqueous n-BuNH2 (4 mL), alkynyl glycoside 3C (50.0 mg, 0.133 mmol, 1 equiv) in THF (2 mL), and bromoalkyne 5b (35.2 mg, 0.186 mmol, 1.4 equiv) in THF (5 mL) were used. The reaction mixture was stirred for 6 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 2% MeOH/DCM to afford a white solid (54 mg, 85%) as the desired product (Rf = 0.6 in 5% MeOH/DCM). mp 169.0–171.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.54–7.48 (m, 2H), 7.40–7.33 (m, 3H), 5.93 (d, J = 8.6 Hz, 1H), 5.57 (s, 1H), 4.83 (d, J = 3.8 Hz, 1H), 4.32–4.20 (m, 2H), 3.97–3.92 (m, 4H), 3.62–3.49 (m, 2H), 2.50–2.34 (m, 2H), 2.34 (t, J = 7.1 Hz, 2H), 2.08 (s, 3H), 1.90–1.79 (m, 2H), 1.56–1.45 (m, 2H), 1.42–1.21 (m, 6H), 0.88 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 171.5, 137.1, 129.2, 128.3, 126.4, 102.0, 97.9, 82.1, 78.5, 75.8, 70.7, 68.9, 66.7, 66.4, 64.9, 62.7, 54.2, 31.3, 28.5, 28.2, 27.7, 23.3, 22.5, 19.2, 16.3, 14.0; HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C28H38NO6, 484.2694; found, 484.2687.
Synthesis of Compound 19
CuCl (4.3 mg, 0.042 mmol, 0.08 equiv), 30% solution of aqueous n-BuNH2 (5 mL), alkynyl glycoside 3C (200 mg, 0.53 mmol, 1 equiv) in THF (4.0 mL), and bromoalkyne 5c (173 mg, 0.80 mmol, 1.5 equiv) in THF (1.5 mL) were used. The reaction mixture was stirred for 24 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 2% MeOH/DCM to afford a white solid (208 mg, 77%) as the desired product (Rf = 0.3 in 5% MeOH/DCM). mp 158.0–161.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.54–7.52 (m, 2H), 7.39–7.38 (m, 3H), 5.92 (d, J = 8.6 Hz, 1H), 5.59 (s, 1H), 4.86 (d, J = 3.8 Hz, 1H), 4.33–4.21 (m, 2H), 3.98–3.76 (m, 4H), 3.63–3.52 (m, 2H), 3.13 (s, 1H), 2.51–2.37 (m, 2H), 2.25 (t, J = 7.0 Hz, 2H), 2.11 (s, 3H), 1.91–1.83 (m, 2H), 1.55–1.50 (m, 2H), 1.40–1.29 (m, 10H), 0.90 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 171.5, 137.1, 129.2, 128.3, 126.4, 101.9, 97.9, 82.1, 78.5, 75.8, 70.7, 68.8, 66.7, 66.4, 64.9, 62.7, 54.2, 31.8, 29.1, 29.0, 28.9, 28.3, 27.8, 23.3, 22.6, 19.2, 16.3, 14.1; HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C30H42NO6, 512.3007; found, 512.3003.
Synthesis of Compound 20
CuCl (1.3 mg, 0.0134 mmol, 0.08 equiv), 30% solution of aqueous n-BuNH2 (3 mL), alkynyl glycoside 3C (63 mg, 0.168 mmol, 1 equiv) in THF (1.5 mL), and bromoalkyne 5e (58.44 mg, 0.238 mmol, 1.42 equiv) in THF (0.8 mL) were used. The reaction mixture was stirred for 17 h at rt. The crude product was purified by column chromatography using eluents from pure DCM to 2% MeOH/DCM to afford a white solid (64.5 mg, 71%) as the desired product (Rf = 0.55 in 5% MeOH/DCM). mp 150.2–153.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.57–7.47 (m, 2H), 7.40–7.32 (m, 3H), 5.95 (d, J = 8.2 Hz, 1H), 5.58 (s, 1H), 4.85 (d, J = 3.8 Hz, 1H), 4.36–4.20 (m, 2H), 3.99–3.73 (m, 4H), 3.64–3.49 (m, 2H), 3.09 (d, J = 3.1 Hz, 1H), 2.51–2.34 (m, 2H), 2.25 (t, J = 7.1 Hz, 2H), 2.09 (s, 3H), 1.92–1.81 (m, 2H), 1.57–1.48 (m, 2H), 1.44–1.23 (m, 14H), 0.90 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 171.5, 137.1, 129.2, 128.3, 126.3, 101.9, 97.9, 82.1, 78.5, 75.8, 70.7, 68.8, 66.7, 66.4, 64.9, 62.7, 54.2, 31.9, 29.7, 29.6, 29.5, 29.3, 29.1, 28.9, 28.3, 27.7, 23.3, 22.7, 19.2, 16.3, 14.1; HRMS (ESI/ICR) ([M + H]+) m/z: calcd for C32H46NO6, 540.3320; found, 540.3315.
Acknowledgments
We thank the financial support from the National Science Foundation grant CHE#1808609. We also thank Dr. Harold O. Lee III for his assistance with using the scanning electron microscope at Norfolk State University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00403.
1H and 13C NMR spectra for compounds 6–20, 2D-COSY and HSQC NMR spectra for selected diacetylene glycosides, HRMS data for all final compounds, stacked 1H NMR spectra at increased temperatures, UV–vis spectra after treatment with UV light, and images of gelator 17 under basic conditions (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Du X.; Zhou J.; Xu B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. 10.1021/acs.chemrev.5b00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Song S.; Ren X.; Zhang J.; Lin Q.; Zhao Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 10.1021/acs.chemrev.1c00815. [DOI] [PubMed] [Google Scholar]; Ahead of Print
- Díaz Díaz D.; Kühbeck D.; Koopmans R. J. Stimuli-responsive gels as reaction vessels and reusable catalysts. Chem. Soc. Rev. 2011, 40, 427–448. 10.1039/c005401c. [DOI] [PubMed] [Google Scholar]
- Jones C. D.; Steed J. W. Gels with sense: supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. 10.1039/c6cs00435k. [DOI] [PubMed] [Google Scholar]
- Draper E. R.; Adams D. J. Photoresponsive gelators. Chem. Commun. 2016, 52, 8196–8206. 10.1039/c6cc03485c. [DOI] [PubMed] [Google Scholar]
- Panja S.; Adams D. J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200. 10.1039/d0cs01166e. [DOI] [PubMed] [Google Scholar]
- Liu M.; Ouyang G.; Niu D.; Sang Y. Supramolecular gelatons: towards the design of molecular gels. Org. Chem. Front. 2018, 5, 2885–2900. 10.1039/c8qo00620b. [DOI] [Google Scholar]
- Datta S.; Bhattacharya S. Multifarious facets of sugar-derived molecular gels: molecular features, mechanisms of self-assembly and emerging applications. Chem. Soc. Rev. 2015, 44, 5596–5637. 10.1039/c5cs00093a. [DOI] [PubMed] [Google Scholar]
- Basu N.; Chakraborty A.; Ghosh R. Carbohydrate derived organogelators and the corresponding functional gels developed in recent time. Gels 2018, 4, 52. 10.3390/gels4020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.; Bietsch J.; Bashaw K.; Wang G. Recently Developed Carbohydrate Based Gelators and Their Applications. Gels 2021, 7, 24. 10.3390/gels7010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Kim J.-Y.; Chen X.; Yoon J. Recent progress in stimuli-induced polydiacetylenes for sensing temperature, chemical and biological targets. Chem. Commun. 2016, 52, 9178–9196. 10.1039/c6cc03584a. [DOI] [PubMed] [Google Scholar]
- Tahir M. N.; Nyayachavadi A.; Morin J.-F.; Rondeau-Gagné S. Recent progress in the stabilization of supramolecular assemblies with functional polydiacetylenes. Polym. Chem. 2018, 9, 3019–3028. 10.1039/c8py00536b. [DOI] [Google Scholar]
- Qian X.; Städler B. Recent Developments in Polydiacetylene-Based Sensors. Chem. Mater. 2019, 31, 1196–1222. 10.1021/acs.chemmater.8b05185. [DOI] [Google Scholar]
- Rao V. K.; Shauloff N.; Sui X.; Wagner H. D.; Jelinek R. Polydiacetylene hydrogel self-healing capacitive strain sensor. J. Mater. Chem. C 2020, 8, 6034–6041. 10.1039/d0tc00576b. [DOI] [Google Scholar]
- Nagasawa J. i.; Kudo M.; Hayashi S.; Tamaoki N. Organogelation of Diacetylene Cholesteryl Esters Having Two Urethane Linkages and Their Photopolymerization in the Gel State. Langmuir 2004, 20, 7907–7916. 10.1021/la049459n. [DOI] [PubMed] [Google Scholar]
- Stone D. A.; Hsu L.; Wheeler N. R.; Wilusz E.; Zukas W.; Wnek G. E.; Korley L. T. J. Mechanical enhancement via self-assembled nanostructures in polymer nanocomposites. Soft Matter 2011, 7, 2449–2455. 10.1039/c0sm01262a. [DOI] [Google Scholar]
- Heo J.-M.; Son Y.; Han S.; Ro H.-J.; Jun S.; Kundapur U.; Noh J.; Kim J.-M. Thermochromic polydiacetylene nanotube from amphiphilic macrocyclic diacetylene in aqueous solution. Macromolecules 2019, 52, 4405–4411. 10.1021/acs.macromol.9b00635. [DOI] [Google Scholar]
- Liu F.; Ding Z.; Xu Y.; Gao J.; Lalevée J. Polydiacetylene (PDA) based supramolecular gel upon coassembly with a bolaamphiphilic cogelator. Polym. Adv. Technol. 2020, 31, 2640–2646. 10.1002/pat.4990. [DOI] [Google Scholar]
- Xu Y.; Fu S.; Liu F.; Yu H.; Gao J. Multi-stimuli-responsiveness of a novel polydiacetylene-based supramolecular gel. Soft Matter 2018, 14, 8044–8050. 10.1039/c8sm01515e. [DOI] [PubMed] [Google Scholar]
- Chen C.; Chen J.; Wang T.; Liu M. Fabrication of Helical Nanoribbon Polydiacetylene via Supramolecular Gelation: Circularly Polarized Luminescence and Novel Diagnostic Chiroptical Signals for Sensing. ACS Appl. Mater. Interfaces 2016, 8, 30608–30615. 10.1021/acsami.6b10392. [DOI] [PubMed] [Google Scholar]
- Fan H.; Jiang H.; Zhu X.; Zhu M.; Zhang L.; Liu M. Homo- and heterochirality regulated blue and red phase polymerization of diacetylene with enantiomeric and racemic gelators. Eur. Polym. J. 2019, 118, 146–152. 10.1016/j.eurpolymj.2019.05.056. [DOI] [Google Scholar]
- Kim J. H.; Seo M.; Kim S. Y. Lithographically Patterned Breath Figure of Photoresponsive Small Molecules: Dual-Patterned Honeycomb Lines from a Combination of Bottom-Up and Top-Down Lithography. Adv. Mater. 2009, 21, 4130–4133. 10.1002/adma.200900868. [DOI] [Google Scholar]
- Choi Y.-J.; Jung D.; Lim S.-I.; Yoon W.-J.; Kim D.-Y.; Jeong K.-U. Diacetylene-Functionalized Dendrons: Self-Assembled and Photopolymerized Three-Dimensional Networks for Advanced Self-Healing and Wringing Soft Materials. ACS Appl. Mater. Interfaces 2020, 12, 33239–33245. 10.1021/acsami.0c08137. [DOI] [PubMed] [Google Scholar]
- Fujita N.; Sakamoto Y.; Shirakawa M.; Ojima M.; Fujii A.; Ozaki M.; Shinkai S. Polydiacetylene Nanofibers Created in Low-Molecular-Weight Gels by Post Modification: Control of Blue and Red Phases by the Odd-Even Effect in Alkyl Chains. J. Am. Chem. Soc. 2007, 129, 4134–4135. 10.1021/ja069307+. [DOI] [PubMed] [Google Scholar]
- Aoki K. i.; Kudo M.; Tamaoki N. Novel Odd/Even Effect of Alkylene Chain Length on the Photopolymerizability of Organogelators. Org. Lett. 2004, 6, 4009–4012. 10.1021/ol048364+. [DOI] [PubMed] [Google Scholar]
- Kim D.-Y.; Lee S.-A.; Jung D.; Koo J.; Soo Kim J.; Yu Y.-T.; Lee C.-R.; Jeong K.-U. Topochemical polymerization of dumbbell-shaped diacetylene monomers: relationship between chemical structure, molecular packing structure, and gelation property. Soft Matter 2017, 13, 5759–5766. 10.1039/c7sm01166k. [DOI] [PubMed] [Google Scholar]
- Meng Y.; Jiang J.; Liu M. Self-assembled nanohelix from a bolaamphiphilic diacetylene via hydrogelation and selective responsiveness towards amino acids and nucleobases. Nanoscale 2017, 9, 7199–7206. 10.1039/c7nr02126g. [DOI] [PubMed] [Google Scholar]
- Haridas V.; Sharma Y. K.; Creasey R.; Sahu S.; Gibson C. T.; Voelcker N. H. Gelation and topochemical polymerization of peptide dendrimers. New J. Chem. 2011, 35, 303–309. 10.1039/c0nj00544d. [DOI] [Google Scholar]
- Bhowmik S.; Jadhav R. G.; Das A. K. Nanoporous Conducting Covalent Organic Polymer (COP) Nanostructures as Metal-Free High Performance Visible-Light Photocatalyst for Water Treatment and Enhanced CO2 Capture. J. Phys. Chem. C 2018, 122, 274–284. 10.1021/acs.jpcc.7b07709. [DOI] [Google Scholar]
- Dautel O. J.; Robitzer M.; Lère-Porte J.-P.; Serein-Spirau F.; Moreau J. J. E. Self-Organized Ureido Substituted Diacetylenic Organogel. Photopolymerization of One-Dimensional Supramolecular Assemblies to Give Conjugated Nanofibers. J. Am. Chem. Soc. 2006, 128, 16213–16223. 10.1021/ja065434u. [DOI] [PubMed] [Google Scholar]
- Fan J.; Xu X.; Yu W.; Wei Z.; Zhang D. Hydrogen-bond-driven supramolecular self-assembly of diacetylene derivatives for topochemical polymerization in solution. Polym. Chem. 2020, 11, 1947–1953. 10.1039/c9py01745c. [DOI] [Google Scholar]
- Néabo J. R.; Tohoundjona K. I. S.; Morin J.-F. Topochemical Polymerization of a Diarylbutadiyne Derivative in the Gel and Solid States. Org. Lett. 2011, 13, 1358–1361. 10.1021/ol200051m. [DOI] [PubMed] [Google Scholar]
- Nyayachavadi A.; Mason G. T.; Nazir Tahir M.; Ocheje M. U.; Rondeau-Gagné S. Covalent Cross-Linking of Diketopyrrolopyrrole-Based Organogels with Polydiacetylenes. Langmuir 2018, 34, 12126–12136. 10.1021/acs.langmuir.8b02807. [DOI] [PubMed] [Google Scholar]
- Nie X.; Wang G. Synthesis and self-assembling properties of diacetylene-containing glycolipids. J. Org. Chem. 2006, 71, 4734–4741. 10.1021/jo052317t. [DOI] [PubMed] [Google Scholar]
- Wang G.; Yang H.; Cheuk S.; Coleman S. Synthesis and self-assembly of 1-deoxyglucose derivatives as low molecular weight organogelators. Beilstein J. Org. Chem. 2011, 7, 234–242. No. 231 10.3762/bjoc.7.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.; Goyal N.; Mangunuru H. P. R.; Yang H.; Cheuk S.; Reddy P. V. N. Preparation and self-assembly study of amphiphilic and bispolar diacetylene-containing glycolipids. J. Org. Chem. 2015, 80, 733–743. 10.1021/jo501568u. [DOI] [PubMed] [Google Scholar]
- Krishnan B. P.; Raghu S.; Mukherjee S.; Sureshan K. M. Organogel-assisted topochemical synthesis of multivalent glyco-polymer for high-affinity lectin binding. Chem. Commun. 2016, 52, 14089–14092. 10.1039/c6cc07993h. [DOI] [PubMed] [Google Scholar]
- Prathap A.; Sureshan K. M. Sugar-Based Organogelators for Various Applications. Langmuir 2019, 35, 6005–6014. 10.1021/acs.langmuir.9b00506. [DOI] [PubMed] [Google Scholar]
- Krishnan B. P.; Mukherjee S.; Aneesh P. M.; Namboothiry M. A. G.; Sureshan K. M. Semiconducting Fabrics by In Situ Topochemical Synthesis of Polydiacetylene: A New Dimension to the Use of Organogels. Angew. Chem., Int. Ed. 2016, 55, 2345–2349. 10.1002/anie.201507475. [DOI] [PubMed] [Google Scholar]
- Siemsen P.; Livingston R. C.; Diederich F. Acetylenic coupling: a powerful tool in molecular construction. Angew. Chem., Int. Ed. 2000, 39, 2632–2657. . [DOI] [PubMed] [Google Scholar]
- Chen A.; Samankumara L. P.; Garcia C.; Bashaw K.; Wang G. Synthesis and characterization of 3-O-esters of N-acetyl-D-glucosamine derivatives as organogelators. New J. Chem. 2019, 43, 7950–7961. 10.1039/c9nj00630c. [DOI] [Google Scholar]
- Chen A.; Okafor I. S.; Garcia C.; Wang G. Synthesis and self-assembling properties of 4,6-O-benzylidene acetal protected D-glucose and D-glucosamine β-1,2,3-triazole derivatives. Carbohydr. Res. 2018, 461, 60–75. 10.1016/j.carres.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Wang D.; Chen A.; Morris J.; Wang G. Stimuli-responsive gelators from carbamoyl sugar derivatives and their responses to metal ions and tetrabutylammonium salts. RSC Adv. 2020, 10, 40068–40083. 10.1039/d0ra07587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A. S.; Nicely N. I.; Cochrane N.; Wlassoff W. A.; Claiborne A.; Hamilton C. J. Nucleoside triphosphate mimicry: a sugar triazolyl nucleoside as an ATP-competitive inhibitor of B. anthracis pantothenate kinase. Org. Biomol. Chem. 2009, 7, 4029–4036. 10.1039/b909729e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M.; Fukase K.; Kusumoto S. TMSCl as a mild and effective source of acidic catalysis in Fischer glycosidation and use of propargyl glycoside for anomeric protection. Biosci., Biotechnol., Biochem. 2002, 66, 211–214. 10.1271/bbb.66.211. [DOI] [PubMed] [Google Scholar]
- Izumi M.; Shibakami M. Preparation of monosaccharide having (mercaptomethyl)diacetylene compounds using solid-support as a thiol source. Synlett 2003, 1395–1398. 10.1055/s-2003-40853. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.