Abstract
Background
The immunologic features of children with coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are not clearly delineated. This study was conducted to evaluate SARS-CoV-2-specific antibody responses in children with COVID-19.
Methods
The levels of anti-spike (S) IgG, anti-SARS-CoV-2 IgG, and neutralizing antibody (NAb) were measured during various time points in children <19 years of age with COVID-19 in South Korea from February 2020 to September 2020.
Results
One hundred sixty-five blood samples from 114 children with COVID-19 (43.9% asymptomatic and 56.1% mildly symptomatic) were analyzed. In both asymptomatic and mildly symptomatic children, the positive rates of anti-S IgG, anti-SARS-CoV-2 IgG, and NAb were low within 7 days after onset, but they soon reached 100% 14 to <28 days after onset. In symptomatic children, the geometric mean titers (GMTs) of antibodies were all below the positive cutoff during the first 2 weeks from onset and peaked at 28 to <56 days (5.6 for anti-S IgG, 383.6 for anti-SARS-CoV-2 IgG, and 55.0 for NAb, P < .001, respectively). Antibody levels remained detectable up to 3 months after infection. The antibody GMTs during the period 14 to <56 days after symptom onset were highest in children aged 0-4 years.
Conclusions
These results collectively present the humoral immune responses during SARS-CoV-2 infection in children. A further longitudinal study is needed to thoroughly understand the immune system and for effective vaccine development in children during the COVID-19 pandemic.
Keywords: antibody response, children, COVID-19, SARS-CoV-2
SARS-CoV-2 antibodies were detected from 7 days after onset in children with asymptomatic and mild COVID-19 and peaked at 28 to <56 days from onset. The antibodies remained detectable for at least 3 months with slight decreasing titers.
Numerous studies on coronavirus disease 2019 (COVID-19) have hitherto observed that the prevalence and severity of COVID-19 are both lower in children compared to adults [1]. Children seem to comprise approximately 17.4% of all COVID-19 cases in the United States [2]. In South Korea, where large-scale virus testing is performed, 120 712 cases of COVID-19 in children aged ≤19 years have been confirmed, comprising 18.2% of the total cases, with no deaths reported, as of January 9, 2022 [3]. Most children with COVID-19 are asymptomatic or have only mild symptoms [4, 5].
The background of this difference in clinical manifestation in children remains unknown, and several hypotheses have been proposed. The expression and affinity of angiotensin-converting enzyme 2 receptor are shown to be reduced in children [6]. Children might also have distinct immune responses that modulate clinical severity [7]. Preexisting antibodies to seasonal human coronaviruses may also contribute to protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) children at some level [8]. Many aspects of the innate and adaptive immune responses in children have not yet been characterized fully due to limited studies in this population.
Vaccination against COVID-19 is currently being conducted not only in adults, but also in children in many countries. At the same time, a highly transmissible variant, named Omicron, is recently causing the vast majority of new COVID-19 cases. In order to face the ongoing pandemic, the basic characteristics of immunity in children with COVID-19 need to be understood. This study thus aimed to evaluate the magnitude and persistence of SARS-CoV-2-specific IgG, IgA, and neutralizing antibody (NAb) in asymptomatic and mildly symptomatic children confirmed with COVID-19.
MATERIALS AND METHODS
Study Population
This study investigated children <19 years of age who had COVID-19 confirmed by SARS-CoV-2 real-time reverse transcription-polymerase chain reaction (RT-PCR) in nasopharyngeal swabs, and who were hospitalized at 9 hospitals across 5 provinces in South Korea from February 2020 to September 2020. Children were tested for SARS-CoV-2 infection when they had symptoms of COVID-19 or have come into close contact with confirmed cases. During the study period, all patients with COVID-19 in South Korea had to be isolated in hospitals or in residential treatment centers regardless of age or symptoms. Clinical data, including age, sex, date of diagnosis, symptoms, and radiographic findings were collected. Patients who did not develop any symptoms until lift of isolation were defined as asymptomatic and those showing any symptoms with or without mild infiltrates on chest imaging were defined as mild COVID-19. Moderate case was defined when a child required oxygen supplementation, whereas severe case was defined when mechanical ventilation was required. The onset of COVID-19 was defined as the onset of symptom development in symptomatic children and the time of first positive SARS-CoV-2 PCR in asymptomatic children. This study was approved by the institutional review board at each hospital and informed consent was obtained.
Sample Collection
Blood samples were obtained during clinical care and at the time of outpatient visits. The optimal time of the second blood sample draw for each patient was at least 1 month from COVID-19 onset; yet, the number and timing of blood sample draw varied from institution to institution. Serum samples were separated from whole blood by centrifugation and kept frozen in aliquots at −70°C until analysis. All antibody tests were performed in duplicate.
SARS-CoV-2 S Protein ELISA
IgG and IgA antibodies to the spike protein of SARS-CoV-2 (anti-S IgG and anti-S IgA) were measured using the EUROIMMUN Anti-SARS-CoV-2 enzyme-linked immunosorbent assay (ELISA) (Euroimmune, Lübeck, Germany), an ELISA, according to the manufacturer’s instruction [9, 10]. Optical density (OD) ratios of ≥1.1 were interpreted as positive.
Indirect Immunofluorescence Assay (IFA)
An in-house, indirect, immunofluorescence assay (IFA) was performed to detect anti-SARS-CoV-2 IgG antibodies. Initially, Vero cells were infected with NMC-nCoV02 and attached to a glass slide, which was then cultured for 18 hours in a 37°C CO2 incubator, and fixed with 80% acetone. A 1:8- to 2-fold serial dilution of patient serum was added to the antigen slide and was incubated for 45 minutes at 37°C. After washing, Alexa-488 conjugated goat anti-human IgG (Jackson, Pennsylvania, USA) was added. The slide was further incubated and was observed at ×400 magnification under a fluorescence microscope. For each experiment, anti-nucleocapsid monoclonal antibody was included as a positive control. Two researchers who were unaware of the clinical information for the samples tested read the assay results independently. The applicability of the IFA method was validated by correlation analysis with the EUROIMMUN Anti-SARS-CoV-2 ELISA results (Spearman’s correlation coefficient ρ was 0.869). From the receiver operating characteristics (ROC) analysis, based on the ELISA OD ratio of 1.1, the cutoff was selected at ≥1:32, where the IFA indicated a specificity of 95.1% and a sensitivity of 93.5%.
Neutralizing Antibody (NAb) Assay
The NAb assay to NMC-nCoV02 was performed to evaluate the neutralization titer of the patients’ serum samples. Equal amounts of 100 TCID50 NMC-nCoV02 were combined with heat-inactivated serum which was serially diluted 2-fold from 1:2.5 to 1:640, and then placed in a 37°C 5% CO2 incubator to react for 1 hour. After the neutralization reaction, the viral solution was infected with Vero cells and cultured for 4-5 days to observe the cytopathic effect. The virus was neutralized by fixing and staining with 10% formalin solution containing crystal violet, and the surviving cell titer was determined to be the NAb titer. Titers of ≥1:10 were interpreted as positive neutralizing activity.
Statistical Analysis
Geometric mean titer (GMT) was determined by calculating the mean and the standard deviation of the log-transformed data points, and then back-transforming to the original scale. The positive rate of SARS-CoV-2 antibody at a given point was calculated when 5 or more samples were collected. The differences between continuous variables were analyzed by the 2-sample t test or the Mann-Whitney U test. The differences were statistically significant when P < .05. Statistical analysis was performed using SPSS, version 26.0 (IBM).
RESULTS
Patient Characteristics
A total of 114 children with COVID-19 were included in this study. The median age of the children was 9 years (range, 0-18 years), and males comprised 63.2% (Table 1). Among the children, 50 (43.9%) were asymptomatic and 64 (56.1%) had mild symptoms. Of those, 3 had pneumonia. No children had moderate-to-severe clinical manifestation including multisystem inflammatory syndrome in children during the study period. The median period to the last blood sample draw was 28 days (range, 0-191 days) from onset for symptomatic children and 16 days (range, 0-94 days) for asymptomatic children. A total of 165 blood samples were collected for analysis, where blood was drawn once in 73 patients, twice in 32 patients, 3 times in 8 patients, and 4 times in 1 child. Fifty-seven blood samples were drawn within 7 days from onset, 38 samples were collected after 7 to <14 days, 12 samples after 14 to <28 days, 41 samples after 28 to <56 days, 11 samples after 56 to <84 days, and 6 samples after 84 days from onset.
Table 1.
Clinical Characteristics of Children With COVID-19 Tested for Serology, South Korea
| Characteristics | No. (%) |
|---|---|
| Total No. | 114 |
| Sex | |
| Male | 72 (63.2) |
| Female | 42 (36.8) |
| Age, yr | |
| Median (range) | 9 (0-18) |
| <1 | 5 (4.4) |
| 1-5 | 41 (36.0) |
| 6-10 | 22 (19.3) |
| 11-15 | 36 (31.6) |
| 16-18 | 10 (8.8) |
| Clinical manifestation | |
| Asymptomatic | 50 (43.9) |
| Symptomatica | 64 (56.1) |
| Mild | 64 (56.1) |
| Moderate | 0 (0.0) |
| Severe | 0 (0.0) |
| Blood collection | |
| 1 time | 73 (64.0) |
| 2 times | 32 (28.1) |
| 3 times | 8 (7.0) |
| 4 times | 1 (0.9) |
Abbreviations: COVID-19, coronavirus disease 2019.
Mild cases were defined by the presence of any symptoms with or without mild infiltrates on chest imaging, moderate cases were defined by the requirement of oxygen supplementation, and severe cases were defined when a child was on mechanical ventilation.
Positive Rate of Antibody to SARS-CoV-2 in Children With COVID-19
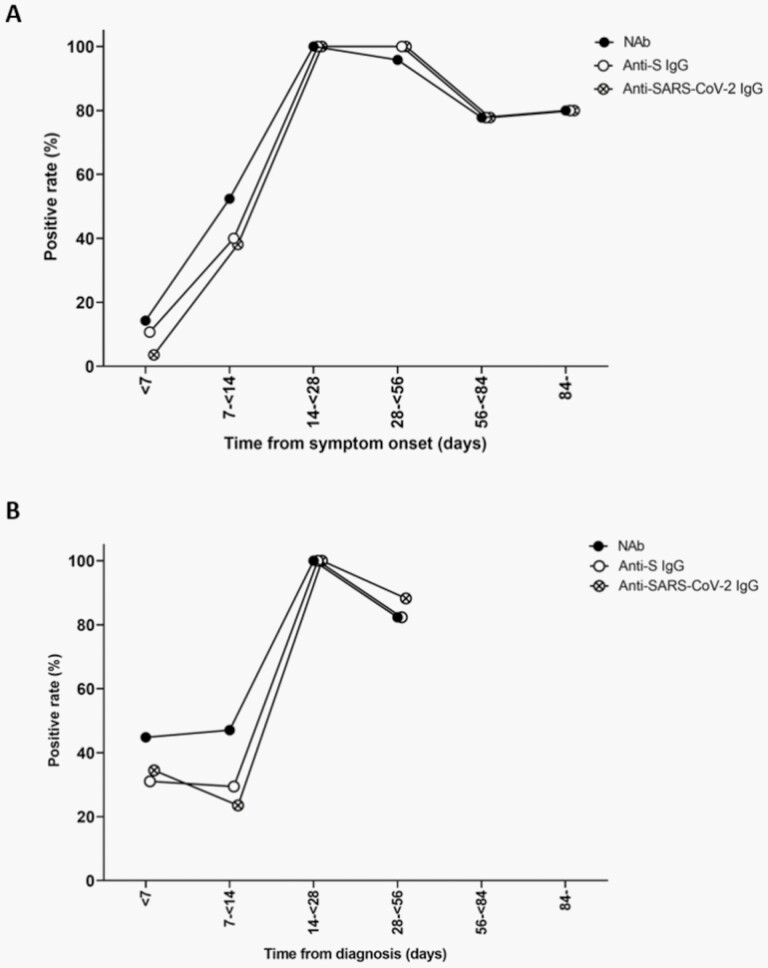
In symptomatic children, the positive rates of anti-S IgG, anti-SARS-CoV-2 IgG, and NAb were as low as 10.7%, 3.6%, and 14.3%, respectively, within 7 days after onset (Figure 1A; Table 2). The positive rates gradually increased and reached 100% 14 to <28 days after onset. After reaching plateau until 56 days from onset, the positive rates slightly declined to 77.8%-80.0%. The positive rates of anti-S IgG, anti-SARS-CoV-2 IgG, and NAb within 7 days after onset in asymptomatic children were 29.4%, 34.5%, and 47.1%, respectively (Figure 1B; Table 2). The antibodies were detected in all asymptomatic cases at 14 to <28 days and were detected in 82.4%-88.2% after 28 to <56 days from onset.
Figure 1.
Changes in the positive rate of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in symptomatic (A) and asymptomatic (B) children with coronavirus disease 2019 over time, South Korea. Abbreviations: NAb, neutralizing antibody; S, spike protein.
Table 2.
Detection of Antibodies to SARS-CoV-2 in Children With COVID-19, South Korea
| Days From Onseta | Anti-S IgG | Anti-SARS-CoV-2 IgG | Anti-S IgA | NAb | ||||
|---|---|---|---|---|---|---|---|---|
| No./Total No. | (%) | No./Total No. | (%) | No./Total No. | (%) | No./Total No. | (%) | |
| Symptomatic children | 52/92 | (56.5) | 51/93 | (54.8) | 32/48 | (66.7) | 55/93 | (59.1) |
| <7 | 3/28 | (10.7) | 1/28 | (3.6) | 2/10 | (20.0) | 4/28 | (14.3) |
| 7 to <14 | 8/20 | (40.0) | 8/21 | (38.1) | 6/8 | (75.0) | 11/21 | (52.4) |
| 14 to <28 | 6/6 | (100) | 6/6 | (100) | 4/4 | (100) | 6/6 | (100) |
| 28 to <56 | 24/24 | (100) | 24/24 | (100) | 15/17 | (88.2) | 23/24 | (95.8) |
| 56 to <84 | 7/9 | (77.8) | 7/9 | (77.8) | 5/6 | (83.3) | 7/9 | (77.8) |
| 84- | 4/5 | (80.0) | 4/5 | (80.0) | 0/3 | 4/5 | (80.0) | |
| Asymptomatic children | 37/72 | (51.4) | 42/72 | (58.3) | 22/36 | (61.1) | 44/72 | (61.1) |
| <7 | 9/29 | (31.0) | 10/29 | (34.5) | 6/13 | (46.2) | 13/29 | (44.8) |
| 7 to <14 | 5/17 | (29.4) | 4/17 | (23.5) | 3/5 | (60.0) | 8/17 | (47.1) |
| 14 to <28 | 6/6 | (100) | 6/6 | (100) | 3/5 | (60.0) | 6/6 | (100) |
| 28 to <56 | 14/17 | (82.4) | 15/17 | (88.2) | 8/10 | (80.0) | 14/17 | (82.4) |
| 56 to <84 | 2/2 | 2/2 | 1/2 | 2/2 | ||||
| 84- | 1/1 | 1/1 | 1/1 | 1/1 | ||||
Abbreviations: COVID-19, coronavirus disease 2019; NAb, neutralizing antibody; S, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Days from onset indicate days from symptom onset for symptomatic children and days from diagnosis for asymptomatic children.
Compared to IgG antibodies and NAb, the positive rate of anti-S IgA within 14 days from onset was higher in both symptomatic and asymptomatic children (Table 2). Specifically, 75.0% and 60.0% of the symptomatic and asymptomatic children, respectively, were already positive for anti-S IgA after 7 to <14 days from onset.
Changes in Antibody Titer in Children With COVID-19
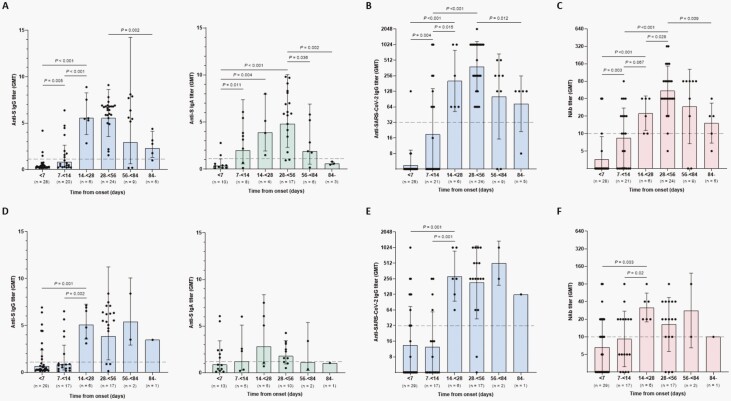
The antibody titers analyzed from 165 blood samples of 114 individual children were plotted according to the time of onset. In symptomatic children, the GMTs of anti-S IgG, anti-SARS-CoV-2, and NAb were all below the positive cutoff during the first 2 weeks from onset (Figure 2A–C). Anti-S IgG GMT titer significantly increased and peaked at 14 to <56 days from onset (GMT 5.6 [SD 1.5], P < .001) (Figure 2A; Supplementary Table). The titer subsequently decreased to 3.0 (SD 4.8, P = .592) and 2.3 (SD 1.8, P = .002) after 56 to <84 days and ≥84 days, respectively, but was still above the positive cutoff. The anti-SARS-CoV-2 IgG and NAb GMTs also steadily increased and peaked at 28 to <56 days from onset (GMT 383.6 [SD 3.1], P < .001 and GMT 55.0 [SD 2.7], P < .001, respectively) (Figure 2B and C). The titers significantly decreased thereafter, but remained above the positive limit beyond 84 days from onset. In asymptomatic children, the anti-S IgG, anti-SARS-CoV-2 IgG, and NAb GMTs were also below the positive cutoff during the first 2 weeks after onset (Figure 2D–F). The titers significantly increased and peaked at 14 to <28 days from onset, where the GMT was 5.1 (SD 1.4, P = .002) for anti-S IgG (Figure 2D; Supplementary Table), 287.4 (SD 3.0, P = .001) for anti-SARS-CoV-2 IgG (Figure 2E), and 31.7 (SD 1.8, P = .002) for NAb (Figure 2F). There were no significant changes in GMTs thereafter.
Figure 2.
Changes of titers of antibodies specific for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in children with coronavirus disease 2019, South Korea. Antibody titers were analyzed in both symptomatic (A, B, and C) and asymptomatic children (D, E, and F). SARS-CoV-2 S protein (S)-specific IgG and IgA titers were analyzed by enzyme-linked immunosorbent assay (A and D). SARS-CoV-2-specific IgG titers were analyzed by the in-house, indirect, immunofluorescence assay (B and E). Neutralizing antibody (NAb) was analyzed by a neutralizing antibody assay using live SARS-CoV-2, NMC-nCoV02 (C and F). Bars indicate geometric mean titers (GMTs) and solid lines indicate geometric standard deviations. Dashed lines indicate the positive cutoff.
Anti-S IgA was analyzed in 84 samples of 68 children. The anti-S IgA in both symptomatic and asymptomatic children was already detectable from 7 to <14 days after onset (Figure 2A and D). Anti-S IgA increased and reached peak GMT of 4.8 (SD 2.1) after 28 to <56 days in symptomatic children (P < .001) and of 2.8 (SD 3.0) after 14 to <28 days in asymptomatic children (P = .124) (see Supplementary Table). The GMTs subsequently decreased and were undetectable beyond 84 days from onset.
The antibody titers of 41 children whose blood was collected twice or more were further evaluated to analyze the change of titers over time. Similar to the results of the whole cohort, the antibody titers increased rapidly and gradually decreased with time in symptomatic children (see Supplementary Figure).
Comparison of Antibody Titers According to Age Groups
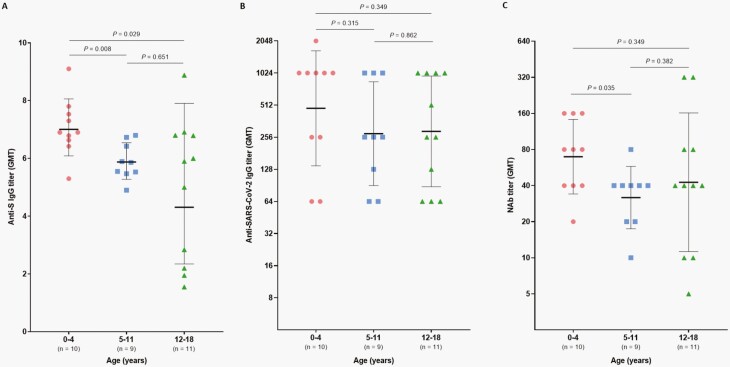
Symptomatic children with COVID-19 were divided into 3 age groups (0-4 years, 5-11 years, and 12-18 years) in order to analyze the difference of antibody titers according to age during the period 14 to <56 days after onset. The median length of time for blood draws was comparable between the 3 groups (37 days [range, 23-53 days] for 0-4 years, 32 days [range, 24-51 days] for 5-11 years, and 34 days [range, 26-47 days] for 12-18 years). Anti-S IgG GMT was significantly higher in children aged 0-4 years (GMT 7.0 [SD 1.2]) compared to children aged 5-11 years and 12-18 years (GMT 5.9 [SD 1.1], P = .008 and GMT 3.7 [SD 1.8], P = .029, respectively) (Figure 3A). NAb GMT also was higher in 0- to 4-year-olds (GMT 65.8 [SD 2.0]) compared to 5- to 11-year-olds (GMT 30.2 [SD 1.8], P = .077) (Figure 3C). Anti-SARS-CoV-2 IgG GMT tended to be higher in children aged 0-4 years compared to those aged 5-11 years and 12-18 years but was not statistically significant.
Figure 3.
Comparison of antibodies specific for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during 14 to <56 days after symptom onset in symptomatic children (aged 0-4 years and 5-11 years) and adolescents (aged 12-18 years) with confirmed coronavirus disease 2019. SARS-CoV-2 S protein (S)-specific IgG titers were analyzed by enzyme-linked immunosorbent assay (A). SARS-CoV-2-specific IgG titer was analyzed by the in-house, indirect, immunofluorescence assay (B). Neutralizing antibody (NAb) was analyzed by a neutralizing antibody assay using live SARS-CoV-2, NMC-nCoV02 (C). Center horizontal lines indicate geometric mean titer (GMT) with whiskers indicating geometric standard deviation.
DISCUSSION
In this multicenter study of asymptomatic and mildly symptomatic children with COVID-19, anti-S IgG, anti-SARS-CoV-2 IgG, and NAbs were detectable in all children after 14 to <28 days from onset. The antibody GMTs peaked at 28 to <56 days in symptomatic children and at around 14 to <28 days in asymptomatic children. A previous short report of 6 children with COVID-19 similarly presented that antigen-specific antibody was produced within 2-3 weeks after symptom onset [11]. A more recent study on 69 children demonstrated robust antibody responses against SARS-CoV-2 during the acute phase of infection [12]. Production of SARS-CoV-2-specific IgG and NAbs in most children with COVID-19 is reassuring. It has recently been shown that SARS-CoV-2-infected children with humoral responses have lower viral load than those with negative serologic response, and higher levels of antibody titers correlate with faster virus clearance [13]. Children with neutralizing activity have more frequent antigen-specific B cells, with more switched memory B cells, along with SARS-CoV-2-specific T-cell production. As with adults, children are able to control viral replication via their own protective immune system [14].
The GMTs of SARS-CoV-2-specific IgG and NAb remained above the positive cutoff for the observed 3 months in children in this study. Both the positive rate and antibody GMTs, however, started to decrease 56 to <84 days from symptom onset in symptomatic children. Similar observation has been described in a long-term analysis of 11 adults with SARS-CoV-2 infection, which showed that anti-S and anti-nucleocapsid antibodies peaked around 4-7 weeks after diagnosis and declined thereafter [15]. NAbs targeting the receptor-binding domain of SARS-CoV-2 also decayed by 18-30 weeks after diagnosis, implying that humoral immunity may not be long-lasting. This finding was also supported by a study of 65 patients where neutralizing activity waned after 1 month from symptom onset, regardless of disease severity [16].
Contradicting larger studies, however, reported that antibodies to SARS-CoV-2 persist for at least 4-6 months following SARS-CoV-2 infection [17, 18]. Recent studies conducted in Spain and Italy demonstrated persistent SARS-CoV-2 IgG and NAbs for more than 6 months in children with asymptomatic or mildly symptomatic COVID-19 [19, 20]. Although antibody levels gradually decrease over time, their responses are still robust 4 months after acute infection [12]. More encouragingly, recent in-depth studies on adults revealed that SARS-CoV-2-specific memory B cells were detectable and maintained for at least 6 months after infection, and these memory B cells could generate monoclonal antibodies with neutralizing function [21, 22]. Cytokine-secreting memory T cells likewise persist for at least 3 months and expand when encountered with antigens [23]. If the responses of memory B cells and T cells are also robust in children and cells react actively upon reinfection, declining NAb responses over time might count little. Further long-term follow-up studies on children with SARS-CoV-2 infection are needed to observe whether the neutralizing activity will continue to decline or, plateau. In addition, comparable assays are needed to compare the results of immunity. More importantly, the activity of memory cells in children needs to be analyzed.
In this study, the GMTs of anti-S IgG and NAb of symptomatic children aged 0-4 years were higher than that of children aged 5-11 years and adolescents aged 12-18 years. The more robust humoral immune responses in younger children has been similarly observed in another study, where compared to adolescent and young adults, young children exhibited higher SARS-CoV-2 IgG, total receptor-binding domain-binding antibody, and NAb levels [24]. A recent study further demonstrated that SARS-CoV-2-specific IgG responses declined more slowly in asymptomatic or mild symptomatic children and adolescents compared to adults with COVID-19 [12]. Conversely, in the studies of Weisberg et al and Pierce et al, adults produced higher NAb titers and more vigorous T-cell responses than children [7, 25]. The same study by Pierce et al observed that children had higher IL-17A and IFN-γ within 7 days of presentation [25]. There is also an increase in the activation of neutrophils, and more recruitment of monocytes, dendritic cells, and natural killer cells in the infection site in children [26, 27]. The combination of active innate and adaptive immune responses might account for the mild clinical symptoms in children with COVID-19 [6].
We additionally observed that there was an early rise in anti-S IgA in children with COVID-19. Data suggest that IgA contributes more than IgG in enhanced SARS-CoV-2 neutralization in the first week after symptom onset [28, 29], hence IgA-mediated immunity might also be an important defense mechanism in children with COVID-19. It has been previously proposed that the early potent activity of IgA in SARS-CoV-2 infection might have a negative influence on the clinical course through enhanced inflammation [27, 30]. However, our results of higher IgA response in young children with mild COVID-19 do not support this hypothesis, warranting further investigations on the functional significance of IgA in COVID-19.
A partial analysis of anti-SARS-CoV-2 IgM by IFA was conducted on 24 children in this study, and none had positive results during their course of infection. This contrasts with the data of adult COVID-19 patients who display broader antibody responses with higher titers of SARS-CoV-2-specific IgM [8, 15]. This most likely suggests that in children, most of the SARS-CoV-2-specific B cells undergo class switching within 1 week after virus exposure [11, 12]. Early induction of protective antibodies in children might contribute to the milder clinical manifestation in children compared to adults.
There are several limitations to this study. Few children had their blood drawn >56 days after onset, and paired samples were not obtained from all children, limiting long-term analysis and interpretation of the antibody kinetics. The antibody titers obtained in this study more reflect the titers at different time points in a heterogenous pediatric population with COVID-19. Moreover, we did not compare the antibody responses between asymptomatic and symptomatic children because determining the start of SARS-CoV-2 infection in asymptomatic children was not possible. Severe cases of COVID-19 were also not included in this study, thus investigating immunological differences according to severity was not possible. Despite these limitations, however, we were able to present a comprehensive characterization of humoral immune responses and antibody kinetics of children with asymptomatic or mild COVID-19. The results of this study add information for vaccine development and application against SARS-CoV-2 infection in children and provide implications for public health responses, including vaccination of children.
In conclusion, SARS-CoV-2-specific IgG and NAb are produced by children with asymptomatic or mild COVID-19 from 14 days of illness and for at least 3 months with statistically significantly decreasing antibody titers. A further longitudinal study in children with COVID-19 with individuals followed over time is needed to thoroughly understand the response of the immune system for safe and effective vaccine development in children during the era of the COVID-19 pandemic.
Supplementary Material
Notes
Acknowledgments. We thank Sun Jung Kim and Seong Yeon Lee at the Department of Pediatrics, Seoul National University Children’s Hospital, Seoul, South Korea, for their technical assistance.
Financial support. This work was supported by the Korea Disease Control and Prevention Agency [2020-ER5326-00].
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020; 109:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Academy of Pediatrics. Children and COVID-19: state-level data report. Accessed January 10, 2022. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report
- 3. Korea Disease Control and Prevention Agency. The updates of COVID1-9 in Republic of Korea. 2021. Accessed January 10, 2022. http://kdca.go.kr/board/board.es?mid=a20501010000&bid=0015
- 4. Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr 2021; 175:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med 2020; 382:1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmermann P, Curtis N.. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections [manuscript published online ahead of print December 1, 2020]. Arch Dis Child 2020; 106:429–39. [DOI] [PubMed] [Google Scholar]
- 7. Weisberg SP, Connors TJ, Zhu Y, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol 2021; 22:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ng K, Faulkner N, Cornish GH, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020; 370:1339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. EUROIMMUN. Anti-SARS-CoV-2 ELISA IgG, Package Insert. EI_2606G_A_US_C02.docx Version: 2020-05-04 . Germany: EUROIMMUN; 2020. [Google Scholar]
- 10. EUROIMMUN. Anti-SARS-CoV-2 ELISA IgA, Package Insert. EI_2606G_A_US_C01.docx Version: 2020-03-24. Germany: EUROIMMUN; 2020. [Google Scholar]
- 11. Zhang Y, Xu J, Jia R, et al. Protective humoral immunity in SARS-CoV-2 infected pediatric patients. Cell Mol Immunol 2020; 17:768–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garrido C, Hurst JH, Lorang CG, et al. Asymptomatic or mild symptomatic SARS-CoV-2 infection elicits durable neutralizing antibody responses in children and adolescents. JCI Insight 2021; 6:e150909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cotugno N, Ruggiero A, Bonfante F, et al. Virological and immunological features of SARS-CoV-2-infected children who develop neutralizing antibodies. Cell Rep 2021; 34:108852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020; 26:845–8. [DOI] [PubMed] [Google Scholar]
- 15. Mai HK, Trieu NB, Long TH, et al. Long-term humoral immune response in persons with asymptomatic or mild SARS-CoV-2 infection, Vietnam. Emerg Infect Dis 2021; 27:663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 2020; 5:1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 2020; 383:1724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. L’Huillier AG, Meyer B, Andrey DO, et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study [manuscript published online ahead of print January 20, 2021]. Clin Microbiol Infect 2021; 784:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonfante F, Costenaro P, Cantarutti A, et al. Mild SARS-CoV-2 infections and neutralizing antibody titers. Pediatrics 2021; 148:e2021052173. [DOI] [PubMed] [Google Scholar]
- 20. Méndez-Echevarría A, Talía S, Falces-Romero I, et al. Long-term persistence of anti-SARS-CoV-2 antibodies in a pediatric population. Pathogens 2021; 10:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abayasingam A, Balachandran H, Agapiou D, et al. Long-term persistence of RBD-positive memory B cells encoding neutralising antibodies in SARS-CoV-2 infection. Cell Rep Med 2021; 2:100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wheatley AK, Juno JA, Wang JJ, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun 2021; 12:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodda LB, Netland J, Shehata L, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell 2021; 184:169–83.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang HS, Costa V, Racine-Brzostek SE, et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw Open 2021; 4:e214302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pierce CA, Preston-Hurlburt P, Dai Y, et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med 2020; 12:eabd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neeland MR, Bannister S, Clifford V, et al. Innate cell profiles during the acute and convalescent phase of SARS-CoV-2 infection in children. Nat Commun 2021; 12:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bartsch YC, Wang C, Zohar T, et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat Med 2021; 27:454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 2021; 13:eabd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ejemel M, Li Q, Hou S, et al. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat Commun 2020; 11:4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu HQ, Sun BQ, Fang ZF, et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J 2020; 56:2001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.