Lay Summary
This brief report investigated the impact of clinical, biochemical, and endoscopic activity of IBD on the severity and long-term outcomes of COVID-19 in a prospective population-based cohort. The study did not identify any association between IBD activity and COVID-19 outcomes.
Keywords: inflammatory bowel diseases, disease activity, COVID-19 outcomes, sequelae, population-based
Introduction
It is well known that coronavirus disease 2019 (COVID-19) is a pandemic that resulted in a global health crisis in 2020. Emerging population-based data indicate that inflammatory bowel diseases (IBD) is a risk factor for adverse outcomes of COVID-19 when compared with the background population.1 However, there is a paucity of data regarding the potential association between IBD activity and the severity of COVID-19.1 Using physician global assessment (PGA) scores, a study from The Surveillance Epidemiology Under Research Exclusion for IBD (SECURE-IBD) cohort reported that increasing IBD activity was found to be associated with adverse outcomes of COVID-19 in younger patients,2 which was also found in an earlier Italian cohort of 79 patients3; however, other cohorts could not confirm this association.4,5 In addition, any association between IBD activity and long-term health effects, including the development of sequelae, of COVID-19 remains uninvestigated.
In this study, we aimed to investigate the impact of clinical, biochemical, and endoscopic disease activity of IBD and the severity and sequelae of COVID-19 in the Danish COVID-19 IBD Cohort, which is a homogenous, generalizable, and population-based cohort.
Methods
The methodology of the Danish COVID-19 IBD Cohort and the collection and categorization of data have been reported previously.1 Briefly, all health care in Denmark is provided by 5 regions, and access to their registers enables population-based analysis covering the whole geographical area of Denmark. Accordingly, we used these registers to identify patients with a reverse transcription-polymerase chain reaction (RT-PCR) analysis for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Subsequently, we combined these data with the medical records of each patient using the unique personal identification number (CPR number) to identify patients with confirmed IBD and COVID-19. The inclusion period was from the first test for SARS-CoV-2 in Denmark, which took place on January 28th, 2020, until April 1st, 2021.
Severity of COVID-19 was categorized as follows: adverse, defined as a need for COVID-19-related hospitalization; severe, defined as a composite of intensive care unit (ICU) admission, mechanical ventilation, or death; and mild, defined as an absence of these outcomes. Sequelae following COVID-19 were defined as symptoms that (1) develop during or after an infection consistent with COVID-19, (2) are present for more than 12 weeks, (3) and are not attributable to alternative diagnoses.6 This outcome was primarily patient-reported through secure surveys, which were sent to the whole population irrespective of hospital contact.
The most recent clinical assessment of IBD prior to COVID-19 was used for the analysis with no cutoff date. Disease activity was evaluated using either the Simple Clinical Colitis Activity Index (SCCAI) in UC and unclassified IBD or the Harvey-Bradshaw Index (HBI) for CD. Biochemical disease activity was defined as having C-reactive protein (CRP) values >5 mg/L or fecal calprotectin values >250 μg/g. Endoscopic disease activity was defined as a Mayo Endoscopic subscore of at least 2 in UC and a Simple Endoscopic Score Crohn’s Disease (SES-CD) of at least 3 for CD.
Results
Cohort Description
During the inclusion period, a total of 516 patients with IBD developed COVID-19, and all were included in this study and stratified according to the level of disease activity of IBD at the time of COVID-19. The clinical assessment of IBD was performed a median of 1.4 months (interquartile range [IQR], 0-6.4) prior to COVID-19 infection. The baseline demographic and clinical characteristics are reported in Supplementary Tables 1-2.
IBD Activity and Severity of COVID-19
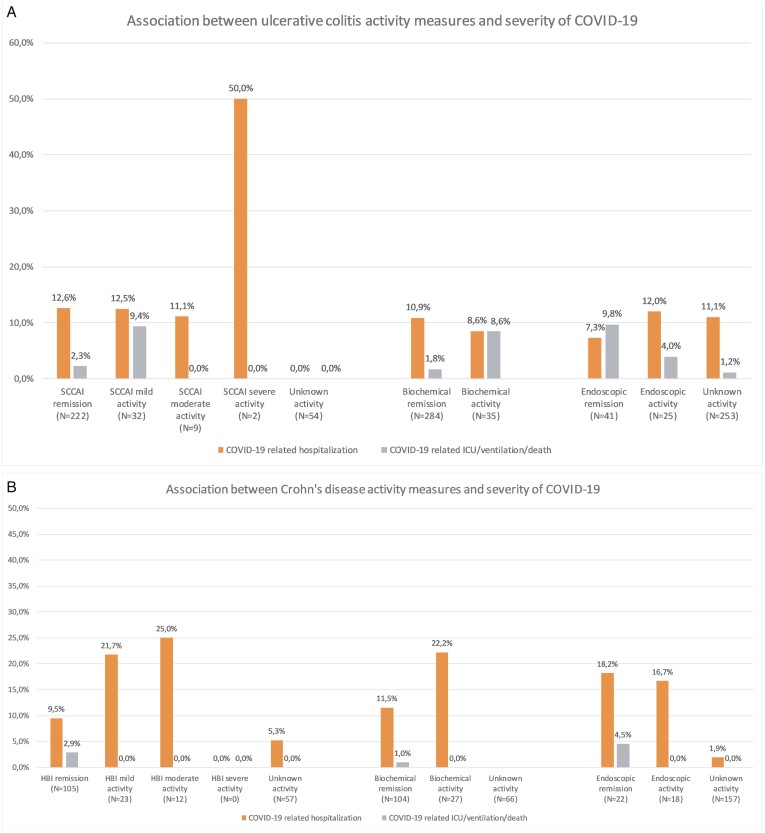
Outcomes of COVID-19 according to activity of UC or CD are depicted in Figure 1. Using patients in either clinical, biochemical, or endoscopic remission as a reference as appropriate, we did not observe any overall trend between IBD activity and risk of COVID-19-related hospitalization in unadjusted analysis (Table 1). Subsequently, we conducted an adjusted analysis that implemented a priori covariates based on findings from our previous report and included age, gender, smoking at the diagnosis of COVID-19, timing of clinical assessment of IBD within 3 months prior to COVID-19 infection, and IBD-related medications.1 Again, no statistically significant association was observed between clinical (adjusted odds ratio [aOR], 0.45; 95% confidence interval [CI], 0.01-2.79), biochemical (aOR, 1.30; 95% CI, 0.04-51.82), nor endoscopic disease activity (aOR, 5.91; 95% CI, 0.53-68.01) of UC and the risk of COVID-19-related hospitalization (Table 1). Similar findings were made in patients with CD (Table 1). With regard to the risk of severe COVID-19, no association was found with clinical activity of UC in unadjusted analysis (odds ratio [OR], 1.28; 95% CI, 0.28-4.19) or adjusted analysis (aOR, 1.35; 95% CI, 0.10-9.62), but this could not be assessed in patients with CD.
Figure 1.
Proportion of patients experiencing adverse and severe COVID-19 outcomes by (A) UC and (B) CD disease activity measures. Data are presented as unadjusted proportions. None of the patients with unknown clinical activity of UC experienced adverse or severe COVID-19.
Table 1.
Unadjusted and adjusted associations between disease activity of ulcerative colitis or Crohn’s disease and severity and long-term outcomes of COVID-19.
| COVID-19-related Hospitalization OR (95% CI) | Long-term Sequelae Following COVID-19 OR (95% CI) | |||
|---|---|---|---|---|
| Unadjusted analysis | Adjusted analysis | Unadjusted analysis | Adjusted analysis | |
| Ulcerative colitis | ||||
| SCCAI: 3-5 (mild activity) | 1.37 (0.51–3.28) | 2.60 (0.60–11.38) | 1.22 (0.41–3.78) | 0.92 (0.26–1.80) |
| SCCAI: 6-11 (moderate activity) | 0.61 (0.03–3.49) | -0.45 (0.01–2.79) | NA | |
| SCCAI: ≥12 (severe activity) | 4.88 (0.19–12.51) | NA | NA | |
| SCCAI: ≥6 (moderate or severe activity) | 1.09 (0.16–4.45) | 0.37 (0.02–2.87) | NA | |
| Biochemical activity (C-reactive protein ≥5 mg/L or Fecal calprotectin ≥250 μg/g) |
0.79 (0.04–7.33) | 1.30 (0.04–51.82) | 1.33 (0.13–11.80) | NA |
| Endoscopic activity (Mayo Endoscopic Subscore >1) |
0.79 (0.22–2.67) | 5.91 (0.53–68.01) | 0.71 (0.17–2.83) | 0.83 (0.20–5.31) |
| Crohn’s disease | ||||
| HBI: 5-7 (mild activity) | 1.29 (0.19–5.53) | 0.61 (0.12–4.57) | 1.46 (0.29–8.15) | 0.92 (0.08–11.20) |
| HBI: 8-16 (moderate activity) | NA | 4.38 (0.59–89.42) | 4.49 (0.68–98.13) | |
| HBI: >16 (severe activity) | NA | NA | ||
| HBI: ≥8 (moderate or severe activity) | NA | 4.38 (0.59–89.42) | ||
| Biochemical activity (C-reactive protein ≥5 mg/L or Fecal calprotectin ≥250 μg/g) |
2.67 (0.10–72.44) | 1.19 (0.07–4.79) | 0.73 (0.16–3.11) | 0.97 (0.21–4.59) |
| Endoscopic activity (SES-CD >2) | 0.76 (0.14–3.62) | 0.30 (0.10–2.71) | 1.75 (0.22–16.98) | 1.83 (0.15–17.90) |
Abbreviations: OR, odds ratio; CI, confidence interval; SCCAI, simple clinical colitis index; HBI, Harvey-Bradshaw Index; SES-CD, Simple Endoscopic Score- Crohn’s disease. Adjusted analysis implemented a priori covariates including age, gender, smoking at the diagnosis of COVID-19, and IBD-related medications.
IBD Activity and Long-term Outcomes Following COVID-19
The median time to the assessment of long-term outcomes of COVID-19 was 5.1 months (IQR 4.5-7.9) after infection. In the overall cohort, 58 (42.3%) and 39 (45.9%, P = .60) of patients with UC or CD, respectively, reported symptoms consistent with the development of post-COVID-19 syndrome. The sequelae have been extensively described recently.1 Although a higher proportion of patients with UC with either clinical (8 of 15, 53.3%) or biochemical activity (6 of 12, 50.0%) experienced post-COVID-19 syndrome compared with patients in clinical (38 of 82, 46.3%) or biochemical remission (52 of 125, 41.6%), this did not reach statistical significance in the unadjusted or adjusted analysis; the same pattern was observed concerning endoscopic disease activity (Supplementary Figure 1 and Table 1). Accordingly, patients with CD did not differ in the COVID-19 sequelae based on clinical, biochemical, or endoscopic disease activity of CD (Supplementary Figure 1 and Table 1).
Discussion
The current work is the first prospective population-based report on the association between activity of UC and CD and long-term health consequences of COVID-19. We observed that disease activity of UC and CD was not associated with the outcomes of COVID-19 in neither unadjusted nor adjusted analysis. This finding was consistent regardless of whether the disease activity was measured clinically, biochemically, or endoscopically. Importantly, we found no association between IBD activity and the risk of long-term health effects of COVID-19.
The current literature on the association between IBD activity and severity of COVID-19 remains limited to selected cohorts with conflicting findings.2–5 An important methodological difference between our study and the report from SECURE-IBD is that the latter was based on voluntary referring of patients worldwide, increasing the risk of selection and reporting biases considerably. Second, we provided a more sensitive analysis of disease activity utilizing both disease-specific symptom measures along with biochemical and endoscopic indices, whereas the report of SECURE-IBD was based solely on PGA, which is prone to heterogeneous assessment across physicians in the international registry.
Our analysis builds on the initial report from the Danish COVID-19 IBD Cohort7 and supports the findings of a multicenter retrospective cohort study of 100 patients with IBD from the Netherlands, which also used the PGA but did not demonstrate an association.4
The current study provides the first comprehensive assessment of the association between activity of IBD and long-term health effects of COVID-19 in a population-based setting. In the previous report of the Danish COVID-19 IBD cohort, we found that fatigue and anosmia were the most frequent durable symptoms, whereas gastrointestinal symptoms did not sustain after infection.1 Furthermore, the analysis found that only discontinuation of immunosuppressive therapies for UC during COVID-19 and the severity of COVID-19 among patients with CD were independently associated with the long-term effects of COVID-19.1 Given the paucity of data and the majority of patients surviving COVID-19, these positive and negative findings related to IBD activity need to be confirmed in other population-based cohorts.
The strengths of this study include the population-based design covering a homogenous population. Second, analyses were adjusted for relevant covariates including age, presence of comorbid diseases, timing of IBD follow-up, and corticosteroid exposure. However, we acknowledge several limitations to our study, which first and foremost include the limited sample size of patients with severe COVID-19 or severe activity of IBD. Furthermore, lack of data regarding vaccination status and the imprecise timing of clinical follow-up of IBD prior to COVID-19 implicates caution in the interpretation of these findings. Finally, missing data affected several analyses; however, the patients were systematically included in the data presentation with no appearance of unbalanced bias.
Conclusion
In conclusion, we found that in this population-based cohort, the severity and long-term health effects of COVID-19 did not appear to differ according to clinical, biochemical, or endoscopic disease activity of UC and CD. The findings might have implications in the risk stratifications and vaccine prioritization in the COVID-19 era.
Supplementary Material
Contributor Information
Mohamed Attauabi, Department of Gastroenterology and Hepatology, Herlev Hospital, University of Copenhagen, Herlev, Denmark; Gastrounit, Medical Section, Hvidovre University Hospital, Hvidovre, Denmark; Copenhagen Center for Inflammatory Bowel Disease in Children, Adolescents and Adults, University of Copenhagen, Hvidovre Hospital, Denmark.
Jens Frederik Dahlerup, Department of Hepatology and Gastroenterology, Aarhus University Hospital, Aarhus, Denmark.
Anja Poulsen, Digestive Disease Center, Bispebjerg University Hospital, Copenhagen, Denmark.
Malte Rosager Hansen, Department of Gastroenterology, North Zealand University Hospital, Frederikssund, Denmark.
Marianne Kajbæk Vester-Andersen, Department of Internal Medicine, Zealand University Hospital, Koege, Denmark.
August Pilegaard Prahm, Digestive Disease Center, Bispebjerg University Hospital, Copenhagen, Denmark.
Natalia Pedersen, Department of Gastroenterology, Slagelse Hospital, Slagelse, Denmark.
Lone Larsen, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark.
Tine Jess, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark; National Center of Excellence for Molecular Prediction of Inflammatory Bowel Disease PREDICT, Department of Clinical Medicine, Aalborg University, Copenhagen, Denmark.
Anders Neumann, Department of Internal Medicine, Region Hospital Viborg, Viborg, Denmark.
Kent V Haderslev, Department of Gastroenterology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
Akbar Molazahi, Department of Internal Medicine, Holbaek Hospital, Holbaek, Denmark.
Anders Berg Lødrup, Department of Internal Medicine, Region Hospital West Jutland, Herning, Denmark.
Henning Glerup, Department of Internal Medicine, Region Hospital Silkeborg, Silkeborg, Denmark.
Asser Mathiassen Oppfeldt, Department of Internal Medicine, Region Hospital Horsens, Horsens, Denmark.
Michael Dam Jensen, Department of Internal Medicine, Section of Gastroenterology, Lillebaelt Hospital, Vejle, Denmark.
Klaus Theede, Gastrounit, Medical Section, Hvidovre University Hospital, Hvidovre, Denmark; Copenhagen Center for Inflammatory Bowel Disease in Children, Adolescents and Adults, University of Copenhagen, Hvidovre Hospital, Denmark.
Marianne Kiszka-Kanowitz, Gastrounit, Medical Section, Hvidovre University Hospital, Hvidovre, Denmark; Copenhagen Center for Inflammatory Bowel Disease in Children, Adolescents and Adults, University of Copenhagen, Hvidovre Hospital, Denmark.
Jakob Benedict Seidelin, Department of Gastroenterology and Hepatology, Herlev Hospital, University of Copenhagen, Herlev, Denmark.
Johan Burisch, Gastrounit, Medical Section, Hvidovre University Hospital, Hvidovre, Denmark; Copenhagen Center for Inflammatory Bowel Disease in Children, Adolescents and Adults, University of Copenhagen, Hvidovre Hospital, Denmark.
Author Contributions
M.A. and J.B.: Verification of the underlying data.
M.A.: Study concept design, patient inclusion, data extraction, analysis and interpretation of data, and article drafting.
J.F.D., A.P., M.R.H., M.K.V.A., A.P.P., N.P., L.L., T.J., A.N., K.V.H., A.M., A.B.L., H.G., A.M.O., M.D.J., K.T., M.K.K.: Patient inclusion, data extraction, and critical revision of the article.
J.B.S., J.B.: Study concept design, critical revision of the article, and supervision.
All authors approved the final version of the article, including the authorship list.
The authors are grateful to all Danish COVID-IBD Study Group members who have screened the outpatient lists for COVID-19. The group members are listed in the Supplementary Data.
Transparency declaration: The article’s guarantor (J.B.) affirms that this article is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Funding
None.
Conflicts of Interest
J.B.S.: research grants from Takeda and the Capital Region Denmark, national coordinator of studies from AbbVie, Arena Pharmaceuticals, Ely Lilly, and Boehringer Ingelheim. None of these pertain to the research submitted here.
J.B.: personal fees from AbbVie, Janssen-Cilag, Celgene, Samsung Bioepis, and Pfizer; grants and personal fees from Takeda, MSD, and Tillots Pharma; grants from Novo Nordisk Foundation, and Bristol Meyers Squibb. None of these pertain to the research submitted here.
M.A., J.F.D., A.P., M.R.H., M.K.V.A., A.P.P., N.P., L.L., T.J., A.N., K.V.H., A.M., A.B.L., H.G., A.M.O., M.D.J., K.Z., and M.K.K. declare no conflicts of interest.
Data Availability
All data are incorporated into the article and its online Supplementary Data.
References
- 1. Attauabi M, Dahlerup JF, Poulsen A, et al. Outcomes and long-term effects of COVID-19 in patients with inflammatory bowel diseases – a Danish prospective population-based cohort study with individual-level data. J Crohn’s Colitis. 2021;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ricciuto A, Lamb CA, Benchimol EI, et al. Inflammatory bowel disease clinical activity is associated with COVID-19 severity especially in younger patients. J Crohns Colitis. 2021:jjab172. doi: 10.1093/ecco-jcc/jjab172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69(7):1213–1217. doi: 10.1136/gutjnl-2020-321411 [DOI] [PubMed] [Google Scholar]
- 4. Derikx LAAP, Lantinga MA, De Jong DJ, et al. Clinical outcomes of Covid-19 in patients with inflammatory bowel disease: a nationwide cohort study. J Crohn’s Colitis. 2021;15(4):529–539. doi: 10.1093/ecco-jcc/jjaa215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lukin DJ, Kumar A, Hajifathalian K, Sharaiha RZ, Scherl EJ, Longman RS. Baseline Disease Activity and Steroid Therapy Stratify Risk of COVID-19 in Patients With Inflammatory Bowel Disease. Gastroenterology. 2020;159(4):1541–1544.e2. doi: 10.1053/j.gastro.2020.05.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:n136. doi: 10.1136/bmj.n136 [DOI] [PubMed] [Google Scholar]
- 7. Attauabi M, Poulsen A, Theede K, et al. Prevalence and outcomes of COVID-19 among patients with inflammatory bowel disease—a Danish Prospective Population-based Cohort Study. J Crohn’s Colitis. 2021;15(4):540–550. doi: 10.1093/ecco-jcc/jjaa205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online Supplementary Data.