Abstract
Background
We determined circulating anti-S severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) antibody titers in a vaccinated healthcare workers (HCWs) cohort from Northern Israel in the 11 months following primary vaccination according to age, ethnicity, and previous infection status.
Methods
All consenting HCWs were invited to have their IgG levels measured before vaccination and at 6 subsequent timepoints using a quantitative S1/S2 IgG assay. All HCWs with suspected coronavirus disease 2019 (COVID-19) were polymerase chain reaction (PCR) tested. We described trends in circulating IgG geometric mean concentration (GMC) by age, ethnicity, timing of boosting, and previous infection status and compared strata using Kruskall-Wallis tests.
Results
Among 985 vaccinated HCWs, IgG titers between 1 month post 2nd dose to pre-boosting gradually decreased in all age groups. Younger or previously infected individuals had higher initial post-vaccination IgG levels (P < .001 in both cases); differences substantially decreased or disappeared at 7–9 months, before boosting. The proportion of individuals infected prior to initiating vaccination and re-infected after dose 1 was comparable to the proportion of breakthrough infection post-dose 2 in those not previously infected (4.2 vs 4.7%). Pre-infection IgG levels in the 40 participants with breakthrough infection after dose 2 were similar to levels measured at the same timepoint in vaccinated HCWs who remained uninfected (P > .3). Post-dose3 IgG levels were more than 10-fold those 1 month post-dose 2.
Conclusions
Immunity waned in all age groups and previously infected individuals, reversed by boosting. IgG titers decrease and reinfections in individuals with hybrid immunity (infection + vaccination) suggests they may also require further doses. Our study also highlights the difficulty in determining protective IgG levels.
Keywords: COVID-19, vaccines, vaccine immunogenicity, Israel, SARS-CoV-2
In a group of 985 vaccinated Israeli healthcare workers Anti-SARS-CoV-2 IgG titres waned 6 months post-priming regardless of age and previous infection, reversed by boosting. Determining protective IgG levels remains a challenge.
Ten months after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a pandemic, mass vaccination campaigns commenced with vaccines showing trial efficacy of over 90% against symptomatic illness [1–3]. Post-introduction empirical observational studies confirmed vaccine effectiveness against severe disease and death [4], and initially apparent effectiveness against infection [4] raised hopes of control and perhaps elimination. However, bottlenecks in production, supply, and delivery and challenges in regulatory capacity meant many low- and middle-income countries remain at very low vaccination coverage [5], and vaccine hesitancy led to gaps in coverage even in countries with ready access to vaccine doses. In addition, viral variants emerged with relative immune evasion (eg, Beta) or increased transmissibility (e.g., Delta) [6, 7] that together with waning of humoral immunity [8, 9] left 2-dose recipients sub-optimally protected.
In Israel, mass vaccination started in December 2020 using 2 doses of BNT162b2 messenger RNA (mRNA) vaccine scheduled 21 days apart as per CE% for those aged between 16 and 29 years [10]. In June 2021 coronavirus disease 2019 (COVID-19) community transmission ceased briefly, following which importation of the Delta variant caused the largest epidemic yet experienced in the country. Israel rapidly initiated booster doses. Experimental and observational data comparing 3 vs 2 doses, demonstrated the effectiveness of boosters against symptomatic infection with the Delta variant [11, 12]. However, given the low rates of severe disease outcomes among 2 dose recipients, the absolute risk reduction in severe disease is more modest, and inversely the number needed to vaccinate to avert one severe outcome is high. Thus, the appetite to introduce boosters was initially variable, and by November 2021 no other country offered universal boosting. In September 2021 the World Health Organization called for a moratorium on boosting until the end of 2021 [13]. In the United Kingdom, in September 2021, the Joint Committee for Vaccination and Immunization, the independent body advising the government on vaccine policy, recommended boosting to vulnerable individuals only [14]. The duration of clinical protection conferred by the booster remains unknown, nor do we yet have a clear-cut humoral correlate of protection.
Ziv Medical Center (ZMC) is a 300-bed government regional referral hospital located in Safed, Northern Israel. Like all hospitals in the country it started offering vaccination to its healthcare workers (HCW) in December 2020, achieving over 90% coverage by late January 2021, followed by boosting from July 2021. We conducted prospective serosurveillance of HCWs to evaluate trends over time in SARS-CoV-2 humoral immunity by age, vaccination, infection status, and time elapsed between priming and boosting, and other predictors. Using the same cohort, we have previously published findings of vigorous anamnestic responses among previously infected single-dose recipients, and the need for second dose among individuals experiencing breakthrough primary SARS-CoV-2 infection shortly after their first dose [15, 16].
Here we describe trends in antibody-mediated immunity over 11 months following vaccination by age, ethnicity, infection status, and time elapsed between priming and boosting, and compare anamnestic responses resulting from 3rd dose receipt to those resulting from breakthrough infection.
METHODS
All ZMC employees were invited to participate. We verified prior infection status among consenting participants by measuring the presence of anti-Nucleocapsid (N) immunoglobulin G (IgG) antibodies using a highly sensitive and specific SARS-CoV-2 IgG qualitative assay (Abbott, Abbot Park, USA) [17]. Workers with detectable anti-N IgG antibodies and/or documented past positive SARS-CoV-2 polymerase chain reaction (PCR) were considered previously infected. Thereafter quantitative anti-SARS-CoV-2 Spike (S) IgG levels were measured using the LIAISON Diasorin SARS-CoV-2 S1/S2 IgG assay [17] at six time points from dose 1; t1: 21 days (range 15-35 days), t2: 51 days (range 41-65 days), t3: 100-150 days, t4: 151-210 days, t5: 211-270 days and t6: 271-310 days. Where the IgG level reading reached the maximum, serial dilutions were performed in order to obtain a precise quantitative value. HCWs were asked to report any arising symptoms. Those whose symptoms were consistent with the standard clinical case definition of COVID-19 were tested by reverse transcription polymerase chain reaction (RT-PCR). Individuals with a positive PCR test were classified as infected post-vaccination (breakthrough infection). Antibody levels were reported using geometric mean concentration (GMC) in arbitrary units/mL (AU/mL) alongside 95% confidence intervals (95% CI). We used log-GMC when reporting trends graphically. anti-S IgG GMCs were reported by strata defined by number of vaccine doses received, infection status (never infected, infected prior to vaccination, infected after full vaccination), age (according to age at recruitment), ethnicity and timing of boosting. We tested to reject the null hypothesis of no difference in GMC across strata using Kruskall-Wallis tests. To determine any differences in immunogenicity by age and ethnicity we restricted analysis to never infected individuals who had received at least 2 doses of vaccine. We restricted the ethnicity analysis to individuals aged 35–54 years because of the higher proportion of older HCWs in the Jewish group compared with others. It is worth noting that the number of individuals providing a blood sample at each time point varied (range: 324–646) and therefore the GMC at each time point is based on a different number of individuals. The study was approved by ZMC’s ethics committee (0133–20-ZIV).
RESULTS
Of 1500 employees, 985 consented to take part in the study, received at least 1 dose of vaccine and had at least 1 serological test post vaccination. Of these, 86 received only a single dose, 141 received 2 doses, and 758 received 3 doses (Table 1). The median time between doses 1 and 2 was 21 days, and 223 days between doses 2 and 3. HCWs who received a single priming dose (generally because of previous infection) and a second dose more than 6 months after the first were considered boosted. One hundred and eighteen HCWs were infected prior to vaccination, of which 5 (4.2%) were reinfected after vaccination. Of the 856 participants who received at least 2 doses and were seronegative at the initiation of vaccination, 82 participants (9.6%) were infected after initiating their vaccine course, of which 40 (4.7%) were infected 30 days or more after receipt of dose 2. The proportion of individuals not infected prior to vaccination initiation who had a breakthrough infection following the beginning of their vaccination course ranged from 7.1% in the >55 years group (16/208) to 12.7% in the <35 years group (26/178). There was no statistically significant association between age group and the incidence of breakthrough infection (P = .26). Participants of all ages, genders, and ethnicities represented in the general adult population of Israel were represented in the sample (Table 1).
Table 1.
Characteristics of Participants
| n | % | |
|---|---|---|
| No. of priming doses | ||
| 1 priming dose | 86 | 9 |
| 2 priming doses | 899 | 93 |
| Booster | ||
| Yes | 758 | 77 |
| No | 227 | 23 |
| Infection status | ||
| Previously infected | 118 | 12 |
| Infected post vaccination | 93 | 9 |
| Never infected | 779 | 79 |
| Age | ||
| <35 years old | 258 | 26 |
| 35–44 years old | 238 | 24 |
| 45–54 years old | 224 | 23 |
| ≥55 | 243 | 25 |
| Unknown | 22 | 2 |
| Ethnicity | ||
| Jewish | 437 | 44 |
| Christian | 77 | 8 |
| Muslim | 110 | 11 |
| Druze | 76 | 8 |
| Circassian | 6 | 1 |
| Unknown | 279 | 28 |
| Gender | ||
| Female | 613 | 62 |
| Male | 372 | 38 |
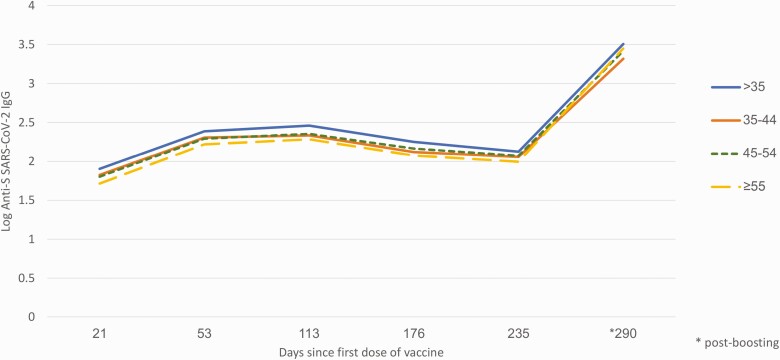
We observed a decrease in circulating IgG levels in all subgroups from after receipt of the second dose (T2) until boosting or infection (T4, T5, or T6 depending on the subgroup, Table 2). At T1, compared with never infected individuals, those previously infected (referred to in the literature as having hybrid immunity or “superimmunity” [18]) had 13-fold higher GMC (876.6 vs 63.9 AU/mL, P < .0001). Among the same individuals the fold-difference at T4 (5–7 months post dose 1) was 1.9 (268.4 vs 139.1 AU/mL, P < .001). At T5 there was no statistically significant difference in GMC between the 2 groups although the number of previously infected individuals with available data at this time point was very small (n = 4). Among never-infected participants, younger age was associated with higher GMC post dose 1 (T1) (Table 2, P < .001) but the difference in GMC was barely significant by T5 (7–9 months post dose 1 but prior to dose 3, Table 2 and Figure 1, P = .05). There was no association between GMC and ethnicity among never infected, fully vaccinated individuals at any time point.
Table 2.
Geometric Mean Concentration of Anti-SARS-CoV-2 Spike IgG Antibodies Among Healthcare Workers in the 11 months Following Vaccination Against COVID-19, Israel, December 2020 to October 2021
| Total Number in Category | T1 | T 2 | T3 | T4 | T5 | T6 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | n | GMC | CI | n | GMC | CI | n | GMC | CI | n | GMC | CI | n | GMC | CI | N | GMC | CI | |
| Previously infected, received boosterb | 18 | 35 | 876.6 | 566.4–1356.7 | 28 | 583.9 | 411–829.4 | 25 | 471 | 331.2–670 | 21 | 268.4 | 185.6–388.2 | 4 | 121.5 | 41.8–353.1 | 7a | 2016.3 | 1010.1–4024.7 |
| Previously infected, not boostedb | 100 | 2 | 120..9 | 22.6–646.4 | |||||||||||||||
| Infected between tests 4 and 5 (not boosted) | 22 | 12 | 64.1 | 43.8–93.6 | 14 | 246..6 | 176.1–345.5 | 11 | 305.1 | 189.1–492.3 | 11 | 184.4 | 115.4–294.5 | 17 | 2704.3 | 1353.5–5403.3 | 11 | 3699.9 | 2759.6–4960.6 |
| Infected between tests 5 and 6 (not boosted) | 17 | 10 | 44.8 | 25.6–78.4 | 11 | 205.2 | 170.2–247.4 | 15 | 209.4 | 161.4–271.5 | 12 | 128.2 | 86.4–190.3 | 10 | 165.1 | 65.9–414 | 8 | 6805.3 | 3791.1–12215.7 |
| Never infected, fully vaccinated (3 doses)b | 740 | 500 | 63.9 | 60.3–67.9 | 529 | 197.3 | 189.9–205.1 | 526 | 223.4 | 211.1–236.5 | 506 | 139.1 | 130.4–148.4 | 419 | 114.9 | 106.6–123.9 | 302a | 2618.4 | 2411.4–2843 |
| Never infected, 2 doses (no booster)b | 116 | 5 | 150.9 | 25.6–890.4 | |||||||||||||||
| <35, never infected, fully vaccinated (3 doses) | 167 | 112 | 79.7 | 70.3–90.2 | 110 | 243.1 | 219–269.9 | 96 | 288.3 | 253.8–327.5 | 96 | 177.8 | 158.3–199.6 | 76 | 132.8 | 117.1–150.6 | 46a | 3208.2 | 2689–3827.7 |
| 35–44 never infected, fully vaccinated (3 doses) | 186 | 130 | 66.7 | 60–74.3 | 133 | 202.4 | 185.8–220.5 | 127 | 216.3 | 195.5–239.3 | 118 | 131.8 | 115.9–149.7 | 107 | 113.7 | 101.9–126.9 | 76a | 2079.3 | 1768.2–2445.3 |
| 45–54 never infected, fully vaccinated (3 doses) | 185 | 119 | 63.5 | 56.7–71 | 135 | 196.3 | 185.1–208.1 | 147 | 225.3 | 202.4–250.7 | 130 | 146.3 | 129.2–165.5 | 129 | 118 | 103.3–136.6 | 84a | 2605.7 | 2252.2–3014.7 |
| ≥55+ never infected, fully vaccinated (3 doses) | 204 | 130 | 51.8 | 45.5–59.1 | 142 | 165.3 | 156.5–174.7 | 146 | 192.1 | 170.6–216.2 | 153 | 118.7 | 103.7–135.8 | 96 | 98.7 | 79.4–122.7 | 91a | 2806.2 | 2370.4–3322.4 |
| 35–54 never infected, Jewish fully vaccinated (3 doses) | 228 | 189 | 64.9 | 59–71.3 | 205 | 197.6 | 187.5–208.2 | 216 | 217.4 | 199.3–237.1 | 194 | 144.9 | 131.1–160.2 | 199 | 122.3 | 108.9–137.4 | 122a | 2409.2 | 2146.6–2703.9 |
| 35–54 never infected, Arab (incl Druze) fully vaccinated (3 doses) | 234 | 179 | 68.2 | 61–76.2 | 214 | 201.3 | 188.6–214.9 | 218 | 234.5 | 214.7–256.2 | 194 | 149.9 | 134.3–167.2 | 195 | 134.3 | 118.6–152.1 | 117a | 2640.3 | 2313–3013.8 |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; GMC, geometric mean concentration; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Indicates groups and timepoints where IgG levels were measured post boosting.
Where groups are similar except for boosting, timepoints prior to the booster are reported together.
Figure 1.
Anti-SARS-CoV-2 S IgG geometric mean concentration (log) among never infected healthcare workers according to age, Israel, January–October 2021. Abbreviations: IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Of the 899 HCW who received ≥ 2 doses (including those infected prior to vaccination), 44 (4.9%) were confirmed positive on PCR between 30 days after dose 2 and before dose 3, including 4 reinfections among those infected prior to vaccination. Of those infected for the first time, 4 tested PCR positive prior to T4, 20 had a positive PCR test between T4 and T5 and 16 between T5 and T6. Among those first infected after vaccination, IgG GMC just prior to infection was not different than among those who remained uninfected at the same time point (184 vs 139 AU/mL, P = .3 for those infected between tests 4 and 5, 165 vs 114 AU/mL, P = .9 for those infected between tests 5 and 6). The 40 previously uninfected individuals experiencing breakthrough infections were younger than never infected HCWs (mean age 39 vs 45 years old, P < .002).
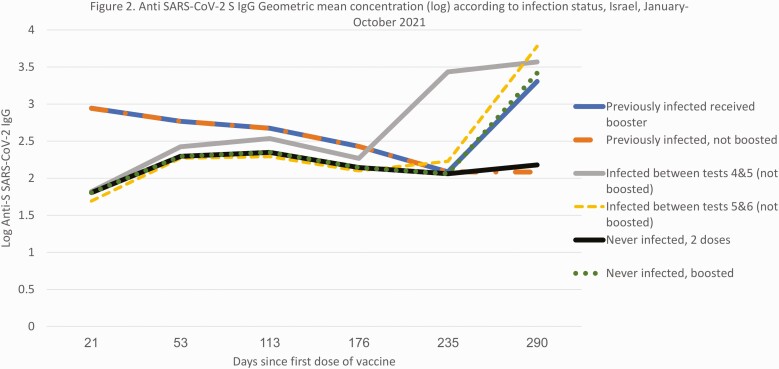
Of the 302 never infected HCWs who received dose 3 and were tested 1-2 months afterwards, t6 GMC (1-2 months post dose 3) was 2618 (95% CI 2411–2843 AU/mL), although among the 21 nonboosted individuals infected after dose 2 for whom data were available, GMC was significantly higher (4213 AU/mL, P < .001, Figure 2). Among those never-infected, all age groups saw an increase in IgG levels 18-fold or more post boosting. Post-boosting GMC in the 36 individuals boosted 6–7 months after dose 2 was lower compared with those 266 boosted 8–9 months after dose 2 (2012 vs 2713, P = .03). However, individuals boosted earlier were older (mean age 50 vs 45, P < .01).
Figure 2.
Anti-SARS-CoV-2 S IgG geometric mean concentration (log) according to infection status, Israel, January–October 2021. Abbreviations: IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
Our convenience cohort provided a well-representative setting in which to monitor serologic responses over time. Consistent with other observational data [8], we found that 6 months post dose 2 IgG titers had waned in all age groups, and initial differences in IgG levels had reduced. This phenomenon occurs irrespective of previous infection status. We also found that despite initially higher GMCs in younger individuals, after 6–7 months differences were much smaller or no longer apparent, suggesting that all age groups might require boosting to achieve optimal protection. Previously infected individuals, who had IgG levels 1 order of magnitude higher than those never infected after 1 dose [15] also saw their circulating IgG levels drop at 6–7 months, with levels less than twice as high as those never infected. The proportion of reinfections among individuals infected prevaccination was comparable to the proportion of breakthrough infections among never infected individuals who received 2 doses. These findings suggest that, in line with other observational studies [19], hybrid or super-immunity (natural immunity boosted by vaccination), wanes and may eventually need boosting as well, at least if decision making is based on circulating IgG levels. Observational data have shown a high effectiveness of boosting against infection and severe disease [11, 12] including against the recently emerged Omicron variant [20]. The IgG levels achieved after boosting were 1 order of magnitude higher than after the priming course and close to levels achieved in those infected after the priming course. Our data do not allow to estimate duration of protection, and no robust real world or modeling studies that estimate duration of protection are available yet. The large fold increase in circulating IgG following infection among vaccinated individuals may also have diagnostic value where it is not possible or practical to swab individuals for PCR tests during the narrow window of opportunity that the PCR modality offers.
We have demonstrated previously no difference in GMC by ethnicity following a single dose of vaccine. In the present study we found that this remains consistent after subsequent doses. This findings matter because risk of infection and disease was indeed associated with ethnicity in Israel and elsewhere, both before and after national introduction of COVID-19 vaccine [21–23].
Our study also highlights the limits of using circulating IgG to determine immunogenicity. Anti-S IgG GMCs measured just prior to infection among individuals who became infected after dose 2 were not significantly different than uninfected individuals at the same time point. Infected individuals had high circulating IgG levels just prior to infection (>100 AU/mL on average, much higher in some individuals) and would have been considered strongly positive on any routine serology test. These elements suggest circulating IgG levels are not a robust predictor of protection against infection or disease, and it is not currently possible to easily determine correlates of protection for COVID-19. Evidence demonstrates the persistence and importance of cellular immunity, both B and T cell [24–26]. Confirming protection following vaccination or infection cannot solely rely on circulating IgG titers and requires other measures of immunity such as functional assays, or B-cell and T-cell assays, none of which are routinely available for diagnostic purposes. Our study also suggests that the timeframe in which the booster is offered in Israel- 6 months after the second dose triggers a large anamnestic response. Later boosting was associated with higher IgG levels, although this could be explained by older individuals being boosted earlier. In any case is unclear at this stage to what extent these differences would be clinically relevant in terms of effectiveness or duration of protection. A better understanding of how IgG levels correlate with protection followed by head-to-head studies of different boosting schedules to optimize protection longevity are required, especially where new variants continue to emerge and calls for further doses beyond a single booster are beginning to be made.
The decrease in IgG levels in the cohort described in this study occurred during a time of increase in the incidence of reported COVID-19 infection in Israel [8] but also at a time of a shift in the dominant circulating strain in Israel from Alpha to Delta. It is therefore a challenge to distinguish the effects of declining immunity from those of higher infectivity attributable to novel variants. In addition, although waning immunity has caused vaccine effectiveness against infection to decrease from over 90% to 50–60% [27], the decrease in effectiveness against severe outcomes such hospitalization and death is much less pronounced [28]. Although our study supports widespread boosting in all age groups from the immunogenicity perspective, the public health benefit of boosting should be balanced against priming previously unvaccinated individuals, both at the national and global levels, when formulating boosting policies.
Repeated blood sampling in the cohort was challenging. The number of latter tests was small, particularly within strata. We caution against drawing inference from later subgroup comparisons. Secondly, PCR testing only occurred upon report of symptoms, which likely under-ascertained true infection incidence with potential misclassification of infected asymptomatic participants as never infected. However, we did not observe increases in titers unexplained by either vaccination or reported symptoms. It is also possible that individuals who were infected early during the pandemic were not detected and classified as such at the beginning of the study due to decreasing sensitivity 4–5 months post-infection of the anti-N IgG assay used in this study [29]. Finally, although we compared titers, we did not measure neutralizing ability.
Our study demonstrates antibody waning and high post-boosting IgG levels in all age groups, suggesting widespread boosting policies may be beneficial, although this needs to be substantiated by effectiveness studies going forward. The need for such policy becomes more urgent with the emergence of strains such as Omicron that likely requires much higher antibody titers for neutralization in order to achieve protection [30, 31]. Our data suggest that immunological waning occurs in vaccinated, naturally infected, and infected-then-vaccinated groups, regardless of age and ethnicity. Ongoing detailed large observational cohorts that measure antibody function and have sufficient clinical outcome incidence will help clarify to what extent, after how long and in terms of which variants, these individuals are again at risk. We continue to monitor anti-S titers in order to determine the durability of boosted immune responses by age, infection history, and interval between priming and boosting.
Notes
Financial support. This work was supported by internal funds from Ziv medical Centre. No external funds were received.
Potential conflicts of interest . The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Michael Edelstein, Ziv Medical Centre, Safed, Israel; Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel.
Karine Wiegler Beiruti, Ziv Medical Centre, Safed, Israel.
Hila Ben-Amram, Ziv Medical Centre, Safed, Israel.
Naor Bar-Zeev, International Vaccine Access Center, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Christian Sussan, Ziv Medical Centre, Safed, Israel.
Hani Asulin, Ziv Medical Centre, Safed, Israel.
David Strauss, Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel.
Younes Bathish, Ziv Medical Centre, Safed, Israel; Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel.
Salman Zarka, Ziv Medical Centre, Safed, Israel; Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel.
Kamal Abu Jabal, Ziv Medical Centre, Safed, Israel; Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel.
References
- 1. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021; 396:1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dagan, N, Barda N, Kept E, et al. BNT162b2 167 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 1423; 384:1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghebreyesus TA. Five steps to solving the vaccine inequity crisis. PLOS Glob Public Health 2021; 1:e0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowlkes A, Gaglani M, Groover K, Thiese MS, Tyner H, Ellingson K.. HEROES-RECOVER cohorts. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance - eight US locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seppälä E, Veneti L, Starrfelt J, Danielsen A, et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveil. 2021; 26:2100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldberg Y, Mandel M, Bar-On YM, et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis 2021; 21:939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Israel Ministry of Health. COVID-19 dashboard. Available at: https://datadashboard.health.gov.il/COVID-19/general. Accessed 11 November 2021.
- 11. Barda N, Dagan N, Cohen Cet al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;S0140-6736(21)02249–2. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bar-On Y, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021; 385:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 8 September 2021. Available at: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---8-september-2021 Accessed 18 November 2021.
- 14. Joint Committee on Vaccination and Immunisation. JCVI statement regarding a COVID-19 booster vaccine programme for winter 2021 to 2022 - GOV.UK (www.gov.uk). Available at: https://www.gov.uk/government/publications/jcvi-statement-september-2021-covid-19-booster-vaccine-programme-for-winter-2021-to-2022/jcvi-statement-regarding-a-covid-19-booster-vaccine-programme-for-winter-2021-to-2022 Accessed 14 December 2021.
- 15. Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill 2021; 26:2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abu Jabal K, Ben-Amram H, Beiruti K, et al. SARS-CoV-2 immunogenicity in individuals infected before and after COVID-19 vaccination: Israel, January-March 2021. . Epidemiol Infect 2021; 149:e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turbett SE, Anahtar M, Dighe AS, et al. Evaluation of three commercial SARS-CoV-2 serologic assays and their performance in two-test algorithms. J Clin Microbiol 2020; 59:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Callaway E. COVID super-immunity: one of the pandemic’s great puzzles. Nature 2021; 598:393–4. [DOI] [PubMed] [Google Scholar]
- 19. Goldberg Y, Mandel M, Bar-On Yet al. Protection and waning of natural and hybrid COVID-19 immunity. Medrxiv. [Preprint]. 10 December 2021. Available at: https://www.medrxiv.org/content/10.1101/2021.12.04.21267114v1. Accessed 14 December 2021. [Google Scholar]
- 20. Andrews N, Stowe J, Kirsebom Fet al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. KHub. [Preprint]. 10 December 2021. Available at: https://khub.net/documents/135939561/430986542/Effectiveness+of+COVID-19+vaccines+against+Omicron+variant+of+concern.pdf/f423c9f4-91cb-0274-c8c5-70e8fad50074. Accessed 14 December 2021. [Google Scholar]
- 21. Aldridge RW, Lewer D, Katikireddi SV, et al. Black, Asian and Minority Ethnic groups in England are at increased risk of death from COVID-19: indirect standardisation of NHS mortality data. Wellcome Open Res 2020; 5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaskell K, Johnson M, Gould V, et al. SARS-CoV-2 seroprevalence in a strictly-Orthodox Jewish community in the UK: a retrospective cohort study. Lancet Reg Health Eur. 2021; 6:100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muhsen K, Na’aminh W, Lapidot Y, et al. A nationwide analysis of population group differences in the COVID-19 epidemic in Israel, February 2020–February 2021. Lancet Reg Health Eur. 2021; 7:100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feng C, Shi J, Fan Q, et al. Protective humoral and cellular immune responses to SARS-CoV-2 persist up to 1 year after recovery. Nat Commun 2021; 12:4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Bert N, Chia W, Wan W, et al. Widely heterogeneous humoral and cellular immunity after mild SARS-CoV-2 infection in a homogeneous population of healthy young men. Emerg Microbes Infect 2021; 10:2141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaebler, C., Wang, Z., Lorenzi, JCC.et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021; 591, 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tartof S, Slezak J, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021; 398:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andrews N, Tessier E, Stowe Jet al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. medRxiv 2021; 09.15.21263583. [preprint]. 21 September 2021 Available at: 10.1101/2021.09.15.21263583. Accessed 14 December 2021. [DOI] [Google Scholar]
- 29. Allen N, Brady M, Carrion Martin A.. SARS-CoV-2 antibody testing in health care workers: a comparison of the clinical performance of three commercially available antibody assays. Microbiol Spectr 2021; 9:e0039121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilhelm A, Widera M, Grikscheit Ket al. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. Medrxiv [preprint]. 8 December 2021 Available at: https://www.medrxiv.org/content/10.1101/2021.12.07.21267432v4. [Google Scholar]
- 31. Sheward D, Kim C, Pankow A, et al. Preliminary report. Early release, subject to modification: quantification of the neutralization resistance of the Omicron variant of concern. [preprint]. December 2021. Available at: https://drive.google.com/file/d/1CuxmNYj5cpIuxWXhjjVmuDqntxXwlfXQ/view. Accessed 14 December 2021.