Abstract
Context
The impact of the COVID-19 pandemic on individuals with type 1 diabetes remains poorly defined.
Objective
We examined United States trends in diabetic ketoacidosis (DKA) among individuals with type 1 diabetes (T1D) during the COVID-19 pandemic at 7 large US medical centers and factors associated with these trends.
Methods
We compared DKA events among children and adults with T1D during COVID-19 surge 1 (March-May 2020) and COVID-19 surge 2 (August-October 2020) to the same periods in 2019. Analysis was performed using descriptive statistics and chi-square tests.
Results
We found no difference in the absolute number of T1D patients experiencing DKA in 2019 vs 2020. However, a higher proportion of non-Hispanic Black (NHB) individuals experienced DKA in 2019 than non-Hispanic White (NHW) individuals (44.6% vs 16.0%; P < .001), and this disparity persisted during the COVID-19 pandemic (48.6% vs 18.6%; P < .001). DKA was less common among patients on continuous glucose monitor (CGM) or insulin pump in 2020 compared to 2019 (CGM: 13.2% vs 15.0%, P < .001; insulin pump: 8.0% vs 10.6%, P < .001). In contrast to annual DKA totals, a higher proportion of patients had DKA during COVID-19 surges 1 and 2 compared to the same months in 2019 (surge 1: 7.1% vs 5.4%, P < .001; surge 2: 6.6% vs 5.7%, P = .001).
Conclusion
DKA frequency increased among T1D patients during COVID-19 surges with highest frequency among NHB patients. DKA was less common among patients using CGM or insulin pumps. These findings highlight the urgent need for improved strategies to prevent DKA among patients with T1D—not only under pandemic conditions, but under all conditions—especially among populations most affected by health inequities.
Keywords: type 1 diabetes, diabetic ketoacidosis, COVID-19 pandemic, inequities
The COVID-19 pandemic has had a far-reaching effect on 21st century health care. For type 1 diabetes (T1D), multiple reports indicate that presentations of newly diagnosed T1D have been more severe during the COVID-19 pandemic than in previous years (1-9). The current pandemic has also exposed inequities present in our health care system (10-14). Among individuals with T1D and concomitant COVID-19 infection, patients from minority races/ethnicities have been more likely to present to hospitals with diabetic ketoacidosis (DKA), a common, life-threatening, yet generally preventable diabetes complication that results from insulin deficiency and is characterized by production of excess acids in the form of ketones (15-17). Despite the disruptive effect of COVID-19 on health care delivery and the risks such disruptions pose to those with T1D, studies assessing trends in DKA during the COVID-19 pandemic and the populations most likely to be affected have been limited, with conflicting results (2, 5, 18-20).
Within this context, the T1D Exchange Quality Improvement Collaborative (T1DX-QI) developed a multicenter population health study to examine trends in DKA across the United States and across the lifespan during the COVID-19 pandemic relative to a prepandemic comparison. T1DX-QI is a growing community with clinics across the United States engaged in sharing data and best practices to improve diabetes health care delivery and outcomes (21). Our aim was to understand the factors associated with observed trends in DKA during the pandemic to drive clinical quality improvement activities during the pandemic and thereafter.
Materials and Methods
This retrospective cohort study was conducted by T1DX-QI and comprised aggregate longitudinal data from 7 large medical centers in the United States: SUNY Upstate Medical University, New York; Barbara Davis Center for Diabetes, Colorado; NYU Langone Pediatrics, New York; Rady Children’s Hospital, California; University of Michigan C. S. Mott Children’s Hospital, Michigan; Johns Hopkins Children’s Center, Maryland; and Cincinnati Children’s Hospital Medical Center, Ohio.
Data were collected on DKA events among children and adults with T1D during the 2020 COVID-19 pandemic period (defined as January 1, 2020-December 31, 2020) in comparison with the same period in the previous year (January 1, 2019-December 31, 2019). The timelines for COVID-19 surge 1 (March-May 2020) and surge 2 (August-October 2020) were determined from the increased proportion of new COVID-19 case submissions to the T1DX-QI registry during the 2 time periods and correspond to national trends in new COVID-19 cases (22). Patients with established T1D who developed DKA as well as patients with newly diagnosed T1D who presented in DKA at T1D diagnosis were included. Month-to-month DKA trend calculation was performed on monthly aggregate data using T1D patients with DKA as numerator and established T1D patients with DKA seen via in-person/telemedicine visit by each month as denominator. DKA was defined per 2018 International Society for Pediatric and Adolescent Diabetes DKA guidelines as pH less than 7.3 and/or bicarbonate less than 15 mmol/L (17) and subcategorized as mild DKA (pH 7.2-< 7.3 or bicarbonate 10-< 15 mmol/L), moderate DKA (pH 7.1-< 7.2 or bicarbonate 5-< 10 mmol/L), or severe DKA (pH < 7.1 or bicarbonate < 5 mmol/L). Data and demographics for the number of unique patients presenting in DKA during each time period were collected as well as the total number of T1D patients followed at each participating institution per year. Data on patient age and glycated hemoglobin A1c (HbA1c) at time of DKA, sex, self-reported race/ethnicity, insurance type, and continuous glucose monitor (CGM) or insulin pump use were collected from participating institutions in monthly aggregates. Patients were categorized based on information on these variables recorded at the time of DKA admission.
Ethics approval was obtained by a central review board (Western Institutional Review Board) as an exempt study. Each participating institution obtained local institutional review board approval as needed. Participating institutions shared deidentified aggregate data by month through existing data use agreements using the Smartsheet online data platform (www.smartsheet.com).
Statistical Analysis
Descriptive statistics were used to summarize data. Categorical data were presented with frequency and percentage of patients in each group. Bar graphs and line charts were used to visualize categorical data and trends over time. The midpoint coding method was used to calculate mean and SD from categorical age data. Chi-square tests were used to compare differences in patient characteristics during the pandemic period compared to the prepandemic comparison group. Loess smoothing was performed to visualize DKA trends across years. All analyses were performed using R version 2.4.1.
Results
Year-to-Year Trends in Diabetic Ketoacidosis
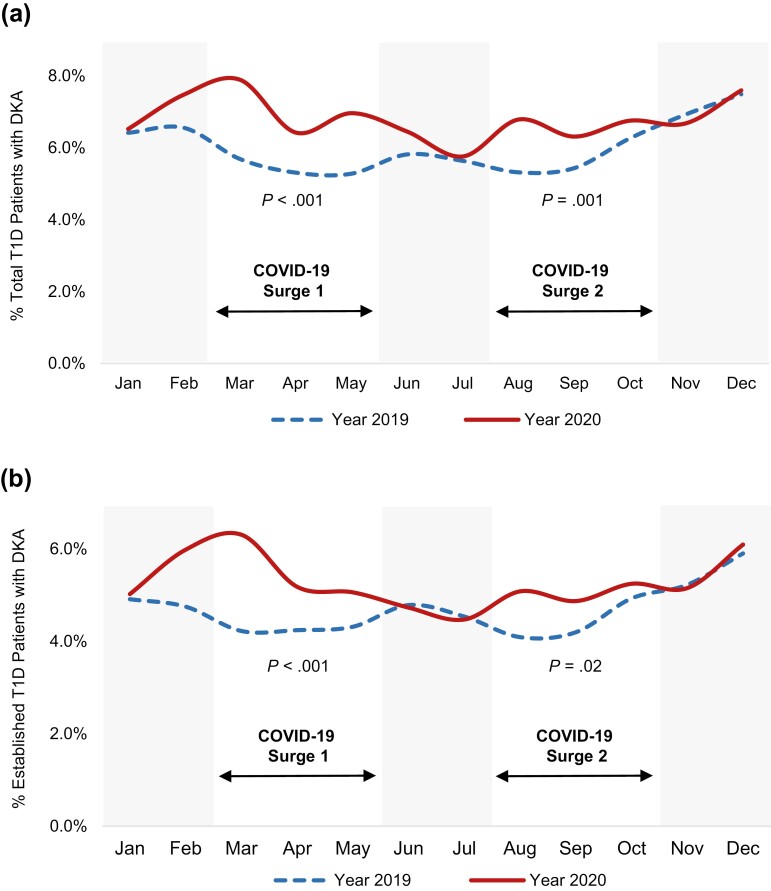
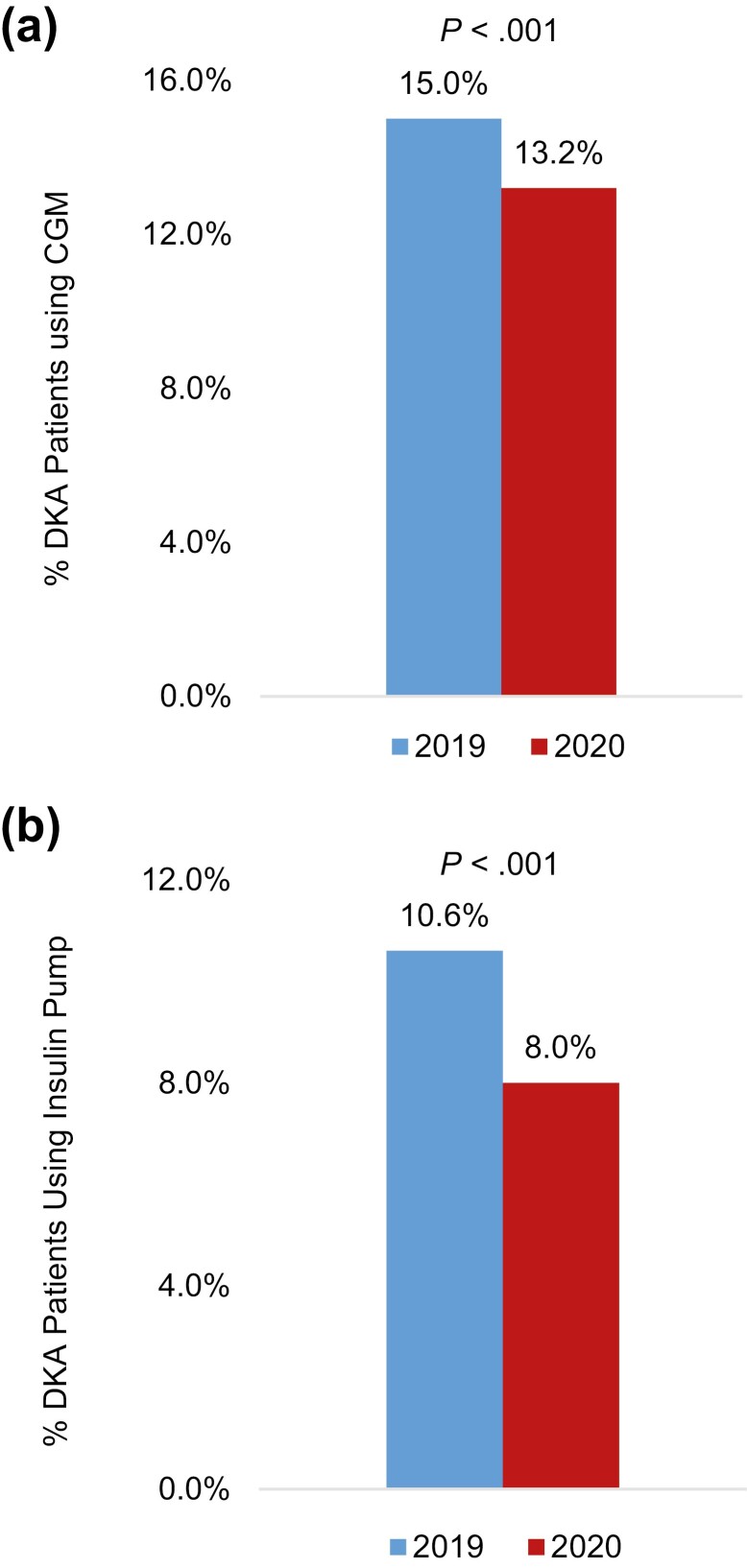
Seven large medical centers located across the United States contributed to this study. Together, the total T1D population from these centers was 15 267 in 2019 and 15 176 in 2020 (Table 1). There were no year-to-year differences in the total number of patients with DKA or within-race differences in DKA by race/ethnicity (see Table 1 and Fig. 1). There were also no differences in the proportion of patients with DKA by sex (see Table 1), insurance type, or severity of DKA (not shown). However, the proportion of patients with a DKA event was higher in 2020 among patients aged 60 years or older (32.2% vs 29.4%, P = .02) and among patients with HbA1c less than 7% (19.1% vs 16.4%, P = .008; see Table 1). Conversely, the proportion of patients with a DKA event was lower in 2020 among patients using a CGM (13.2% vs 15.0%, P < .001; see Table 1 and Fig. 2) or an insulin pump (8.0% vs 10.6%, P < .001; see Table 1 and Fig. 2).
Table 1.
Distribution of patient characteristics presenting with diabetic ketoacidosis in 2019 vs 2020
| 2019 | 2020 | P | |
|---|---|---|---|
| DKA patient characteristics | No. (%) | No. (%) | |
| Total T1D patients | 15 267 | 15176 | |
| No. of patients with DKA | 2774 (18.17) | 2812 (18.53) | .41 |
| Established T1D patients with DKA | 2306 (17.10) | 2268 (16.90) | .67 |
| Newly diagnosed T1D patients with DKA | 468 (3.06) | 544 (3.58) | .02 |
| Mean age (SD) | 37.7 (23.70) | 38.5 (23.90) | .87 |
| DKA by age, y | |||
| 0-19 | 1041 (37.53) | 1035 (36.81) | .59 |
| 20-60 | 918 (33.09) | 871 (30.97) | .09 |
| ≥ 60 | 815 (29.38) | 906 (32.22) | .02 |
| DKA by sex | |||
| Male | 1384 (49.89) | 1467 (52.17) | .09 |
| DKA by race/ethnicity | |||
| NHW | 1886 (67.99) | 1930 (68.63) | .60 |
| NHB | 434 (15.65) | 466 (16.57) | .36 |
| Hispanic | 230 (8.29) | 198 (7.04) | .09 |
| Asian | 67 (2.42) | 70 (2.49) | .90 |
| Others/Not reported | 157 (5.66) | 148 (5.26) | .22 |
| DKA by HbA 1c a , % | |||
| < 7 | 454 (16.37) | 537 (19.10) | .008 |
| 7-9 | 401 (14.46) | 405 (14.40) | .45 |
| > 9 | 1127 (40.63) | 1114 (39.62) | .45 |
| Patients with DKA using CGM | 416 (15.00) | 372 (13.23) | < .001 |
| Patient with DKA using insulin pump | 294 (10.60) | 225 (8.00) | < .001 |
Abbreviations: CGM, continuous glucose monitor; DKA, diabetic ketoacidosis; HbA1c, glycated hemoglobin A1c; NHB, non-Hispanic Black; NHW, non-Hispanic White; T1D, type 1 diabetes.
a Where data available.
Figure 1.
Increased frequency of diabetic ketoacidosis (DKA) among patients with type 1 diabetes (T1D) during COVID-19 surges 1 and 2. A, Percentage of total patients with T1D (newly diagnosed T1D and established T1D) presenting in DKA month over month in 2019 vs 2020. B, Percentage of patients with established T1D presenting in DKA month over month in 2019 vs 2020. P values correspond to differences in frequencies of DKA during the COVID-19 surges in 2019 vs 2020.
Figure 2.
Diabetic ketoacidosis (DKA) was less common among patients using A, a continuous glucose monitor (CGM) or B, an insulin pump during 2020 compared to 2019. A, CGM use among patients presenting in DKA. B, Insulin pump use among patients presenting in DKA.
Diabetic Ketoacidosis Trends During US 2020 COVID-19 Surges 1 and 2
The early COVID-19 pandemic in the United States consisted of an initial rise in COVID-19 cases in spring 2020 and a second rise in mid-to-late summer 2020 (22). Corresponding to these national trends, we observed an increased proportion of new COVID-19 case submissions to the T1DX-QI registry during spring and late summer 2020 and therefore focused on the time periods March through May 2020 (COVID-19 surge 1) and August through October 2020 (COVID-19 surge 2) for further study. Among all patients with T1D, a higher proportion had DKA during COVID-19 surge 1 or 2 compared to the same periods in 2019 (surge 1: 7.1% vs 5.4%, P < .001; surge 2: 6.6% vs 5.7%, P = .001; Table 2 and Fig. 1A). Similarly, among patients with established T1D, a higher proportion had DKA during COVID-19 surge 1 or 2 compared to the same periods in 2019 (surge 1: 6.2% vs 4.7%, P < .001; surge 2: 5.6% vs 4.9%, P = .02; see Table 2 and Fig. 1B). There were no differences in DKA events by age or sex during COVID-19 surges 1 or 2 compared to the same periods in 2019, nor were there within-race differences in DKA (see Table 2). As with year-to-year trends, the proportion of patients with a DKA event was lower among individuals using insulin pumps during both COVID-19 surges compared to the same periods in 2019 (surge 1: 6.4% vs 10.3%, P = .008; surge 2: 8.1% vs 11.2%, P = .03) (Table 2B).
Table 2.
Patients with diabetic ketoacidosis in COVID-19 surges 1 and 2 vs same periods in 2019
| A, Patients with DKA in COVID-19 surges 1 and 2 vs same periods in 2019, No. (% of T1D patients) | ||||||
|---|---|---|---|---|---|---|
| March-May (corresponding to COVID-19 surge 1) | August-October (corresponding to COVID-19 surge 2) | <?Char=Decimal?> | ||||
| 2019 | 2020 | P | 2019 | 2020 | P | |
| Total T1D patients with encounters a in reporting period | 13 852 | 11 178 | 13 703 | 13 557 | ||
| Total No. of patients with DKA | 751 (5.42) | 793 (7.09) | < .001 | 776 (5.66) | 897 (6.61) | .001 |
| No. of established patients with DKA | 643 (4.71) | 676 (6.15) | < .001 | 661 (4.90) | 738 (5.55) | .02 |
| B, Distribution of patient characteristics with DKA in COVID-19 surges 1 and 2 vs same periods in 2019, No. (% of T1D patients) | ||||||
| 2019 | 2020 | P | 2019 | 2020 | P | |
| (N = 751) | (N = 793) | (N = 776) | (N = 897) | |||
| DKA by age, y | ||||||
| 0-19 | 253 (33.69) | 245 (30.90) | .26 | 294 (37.89) | 311 (34.67) | .1 |
| 20-60 | 273 (36.35) | 282 (35.56) | .78 | 274 (35.31) | 305 (34.00) | .5 |
| ≥ 60 | 225 (29.96) | 266 (33.54) | .14 | 208 (26.80) | 281 (31.33) | .07 |
| DKA with COVID-19 | – | 23 (2.96) | – | 6 (0.69) | ||
| DKA by sex | ||||||
| Male | 362 (48.20) | 406 (51.20) | .26 | 397 (51.16) | 480 (53.51) | .5 |
| DKA by race/ethnicity | ||||||
| NHW | 521 (69.37) | 557 (70.24) | .75 | 506 (65.21) | 601 (67.00) | .7 |
| NHB | 120 (15.98) | 147 (18.54) | .2 | 128 (16.49) | 170 (18.95) | .2 |
| Hispanic | 53 (7.06) | 49 (6.18) | .5 | 67 (8.63) | 66 (7.36) | .3 |
| Asian | 21 (2.80) | 22 (2.77) | ≥ .999 | 15 (1.93) | 14 (1.56) | .6 |
| Others/Not reported | 36 (4.80) | 18 (2.26) | .01 | 42 (5.41) | 46 (5.12) | .98 |
| DKA by HbA 1c b , % | ||||||
| < 7 | 120 (15.98) | 117 (14.75) | .5 | 98 (12.63) | 147 (16.39) | .04 |
| 07-Sep | 93 (12.38) | 81 (10.21) | .2 | 104 (13.40) | 117 (13.04) | .8 |
| > 9 | 250 (33.29) | 224 (28.25) | .03 | 263 (33.89) | 281 (31.33) | .2 |
| Patients with DKA using CGM | 105 (13.98) | 90 (11.35) | .1 | 123 (15.85) | 139 (15.50) | .8 |
| Patients with DKA using insulin pump | 77 (10.25) | 51 (6.43) | .008 | 87 (11.21) | 73 (8.14) | .03 |
Abbreviations: CGM, continuous glucose monitor; DKA, diabetic ketoacidosis; HbA1c, glycated hemoglobin A1c; NHB, non-Hispanic Black; NHW, non-Hispanic White; T1D, type 1 diabetes.
a In-person or telemedicine.
b Where data available.
Inequities in Diabetic Ketoacidosis During COVID-19
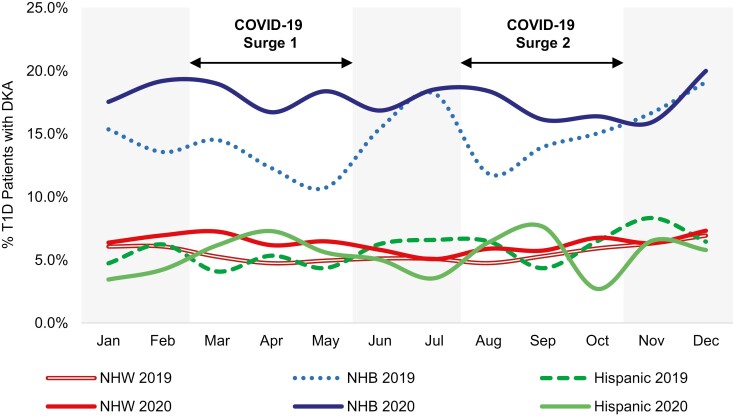
Of the 15 267 individuals in the 2019 cohort, 71.4% (n = 10,904) were non-Hispanic White (NHW), 6.4% (n = 973) were non-Hispanic Black (NHB), 8.7% (n = 1,334) were Hispanic, 0.8% were Asian (n = 127), 6.9% (n = 1054) were listed as “Other,” and 5.7% (n = 863) had no race/ethnicity reported. Similarly, of the 15 176 individuals in the 2020 cohort, 69.6% (n = 10,570) were NHW, 6.3% (n = 959) were NHB, 9.5% (n = 1447) were Hispanic, 0.9% were Asian (n = 135), 7.8% (n = 1186) were listed as “Other,” and 5.6% (n = 850) had no race/ethnicity reported. These percentages are consistent with national data of race/ethnicity among individuals with T1D (23). Month-to-month DKA frequencies were higher among NHB patients with T1D compared to NHW (P < .001) or Hispanic patients (P = .01) both in 2019 and 2020 (Fig. 3). There were no within-race differences in monthly DKA frequencies in 2019 vs 2020. Moreover, a higher proportion of NHB patients experienced DKA in 2019 compared to NHW or Hispanic patients (44.6% among NHB vs 16.0% among NHW [P < .001] and 17.2% among Hispanic patients [P = .001]), and this disparity persisted in 2020 during the COVID-19 pandemic (48.6% among NHB vs 18.4% among NHW [P < .001] and 13.7% among Hispanic patients [P = .001]).
Figure 3.
Racial/ethnic inequities in diabetic ketoacidosis (DKA) persisted during the first year of the COVID-19 pandemic. Month-over-month percentage of patients with DKA by race/ethnicity in 2019 and 2020. The percentage of non-Hispanic Black (NHB) patients presenting in DKA in 2019 and 2020 were statistically significantly higher than those of non-Hispanic White (NHW) patients (P < .001 for 2019 and 2020 comparisons) or Hispanic patients (P = .01 for 2019 and 2020 comparisons). There were no within-race differences in monthly DKA frequencies in 2019 vs 2020.
Discussion
We report here the results of a large US study examining trends in DKA in a diverse cohort of children and adults with T1D during the first year of the COVID-19 pandemic. We found that the proportion of patients with a DKA event was higher during both COVID-19 surge 1 (March-May 2020) and surge 2 (August-October 2020) compared to the same periods in the year prior. Compared to other racial/ethnic groups, the proportion of patients with DKA was highest by far among NHB patients before the pandemic and during both COVID-19 surges. Conversely, DKA was less common for individuals using CGMs and insulin pumps during the COVID-19 pandemic compared to the year prior. These findings underscore the critical need for innovative efforts not only to prevent DKA in patients with T1D under pandemic conditions but to do so especially for those most affected by health inequities.
To our knowledge, this is the largest study to date to report on trends in DKA in the United States during the COVID-19 pandemic. Comprising month-by-month DKA admission data from 7 large medical institutions located around the United States and corresponding to more than 15 000 children and adults with T1D, our observation that DKA frequencies were higher during COVID-19 surges 1 and 2 compared to the same periods in the year prior illustrates the disruptive nature of the COVID-19 pandemic on individuals with T1D as the virus swept through the country (see Fig. 1A and 1B). Many investigations into the effect COVID-19 has on T1D care have focused on the pandemic’s effect on incidence and severity of newly diagnosed diabetes (1-9). However, the present study includes not only individuals with newly diagnosed T1D but also the much larger population with established T1D (see Fig. 1B). Among other reports that included patients with established T1D, a study of 11 Israeli emergency departments found that DKA rates increased among pediatric patients with established T1D from March through May 2020 compared to the same period in 2019 (18). Similarly, a study of 6 pediatric diabetes centers in Saudi Arabia including children aged 1 to 14 years with newly diagnosed or established T1D found an increase in overall DKA frequency from March through May 2020, though no difference in DKA frequency among patients with established T1D (2). In the United States, a study of 5 emergency departments in New York City reported a rise in DKA diagnosis from March through May 2020, but a distinction between newly diagnosed vs established T1D among patients was not made (19). In contrast, a cross-sectional study involving 53 Italian pediatric diabetes centers found no difference in DKA among children with established T1D in the first 40 days of the COVID-19 pandemic compared to the same period the previous year, though this finding was limited by small sample size (5). Also, a population-based study using a national data set from England found that emergency hospital admissions with diagnosis code DKA regardless of diabetes type were higher from March 1 to June 30, 2020, and from November 1, 2020 to February 28, 2021, compared to equivalent periods in 2017 to 2020, but lower specifically for individuals with established T1D (20). The present study is distinct from these prior reports given its large and geographically diverse US patient population, the inclusion both of children and adults with T1D, the requirement of laboratory-confirmed diagnosis of DKA, and its longitudinal nature, which allowed us to characterize the effect of 2 distinct US COVID-19 surges on individuals with T1D. Of note, multiple studies have also investigated DKA frequency among T1D patients infected with COVID-19 (16, 24-28). This study was not designed to evaluate this question, and COVID-19 virus testing or infection was not a criterion for inclusion, though COVID-19 testing results were collected where available. In our study, 23 patients diagnosed with DKA tested positive for COVID-19 during COVID-19 surge 1, and 6 patients with DKA tested positive for COVID-19 during COVID-19 surge 2 (see Table 2B).
A concerning finding of this study is that DKA was most common by far among NHB patients with T1D during both COVID-19 surges (see Fig. 3). The finding that health disparities exist in T1D between racial/ethnic groups is consistent with a growing body of literature (23, 29-34). As the pandemic spread, racial inequities relating to COVID-19 were identified and widely reported, including among individuals with T1D and COVID-19 (12-15, 26). However, despite this heightened awareness of racial health inequities during COVID-19 and efforts made to address them, we observed that DKA frequency did not improve among NHB patients as the pandemic progressed (see Fig. 3). These findings underscore the critical need to redouble efforts to target the root causes of health disparities in T1D, both during the COVID-19 pandemic and beyond. To this end, the T1DX-QI has recently provided a framework to facilitate equitable post-COVID-19 diabetes care (35).
We observed that Hispanic patients did not suffer from the same inequity in DKA frequency as NHB patients. Our findings are similar to those of Ebekozien and colleagues (15), who found NHB patients with T1D had increased odds of presenting with DKA with COVID-19 infection compared to Hispanic patients. These findings are similar to inequities in T1D outcomes before the COVID-19 pandemic, with Hispanic patients faring better than NHB patients (23). There are several potential explanations for this difference, including the role of the Hispanic culture, which is considerably different from that of NHB patients, as described by LaVeist-Ramos and colleagues (36). Furthermore, the contributors to inequities for Hispanic T1D patients are different from those of NHB as both groups are not homogeneous (37).
Another key finding of this study is that the proportion of patients with a DKA event was lower among individuals on CGMs or insulin pumps during the COVID-19 pandemic compared to the year prior (see Fig. 2A and 2B). It is well known that CGM and insulin pump use improves management of T1D and is associated with decreased rates of acute T1D complications (38-40). However, the unique observation here is that CGM and insulin pump use were each associated with lower frequency of DKA in 2020 during the COVID-19 pandemic compared to the year prior. This finding suggests that CGM and insulin pump use may help protect individuals with T1D from DKA in the midst of the COVID-19 pandemic, perhaps by increasing automation of diabetes care so disruptions in care resulting in DKA are less frequent. Our study did not adjust for the effect of diabetes technology use and other potential confounders on DKA frequency. However, an earlier manuscript from our group using a different data set demonstrated that CGM, insulin pump, and combined CGM and insulin pump use is associated with a reduced risk of DKA and hospitalization during COVID-19 infection (41). Likewise, rates of hospitalization are higher among pediatric and adult patients both with T1D and COVID-19 who do not use CGM or insulin pumps compared to those who use these devices (16, 26). Despite the benefits of CGM and insulin pump use during COVID-19, differential access to diabetes technologies remains a major and growing health disparity in T1D care today (23, 41, 42). Rates of diabetes technology use are lowest by far among NHB patients, in part due to social inequities and systemic racism (41, 42). Owing to the aggregate nature by which data were collected for the present study, the demographic associations of diabetes technology users and nonusers cannot be determined. However, it is revealing in this data set that CGM and insulin pump use were each associated with decreased frequency of DKA during the COVID-19 pandemic, whereas NHB patients, who historically have the lowest rates of diabetes technology use, had the highest frequency of DKA.
While this study has several strengths, we also acknowledge several limitations. Though data from 7 centers from across the United States were included in this study, these may not be representative of all diabetes centers across the country, thereby limiting the generalizability of our findings. Further, it is unlikely that all patients with DKA presented to their home diabetes centers for care; therefore, additional DKA events likely occurred that were not included in the totals presented in this study. Also, because this was a large, multicenter analysis, data were submitted in monthly aggregates, and not patient-level data, which restricted the analyses we were able to perform. Owing to the manner by which aggregate data were collected, we were restricted in separating patient-level characteristics of patients presenting in DKA at T1D diagnosis from those with established T1D presenting in DKA. As a result, we could assess month-to-month trends in DKA among patients with established T1D but not individual factors for these patients. Further, since data were collected from electronic medical records of 7 independent hospital systems, there was inherently some variation as to how data elements were tracked and tabulated at each institution.
In summary, this study identified that DKA was more frequent among patients with T1D during COVID-19 surges 1 and 2 compared to the same periods in the year prior. Moreover, DKA frequency was highest by far among NHB patients, both during the COVID-19 pandemic and prior. More encouragingly, we provide evidence that DKA was less common among patients on CGM or insulin pump during the first year of the COVID-19 pandemic. Further work is needed to improve strategies to prevent DKA in patients with T1D under pandemic conditions as well as post pandemic, especially among those most affected by inequities in health care.
Glossary
Abbreviations
- CGM
continuous glucose monitor
- DKA
diabetic ketoacidosis
- HbA1c
glycated hemoglobin A1c
- NHB
non-Hispanic Black
- NHW
non-Hispanic White
- T1D
type 1 diabetes
- T1DX-QI
T1D Exchange Quality Improvement Collaborative
Contributor Information
Andrew R Lavik, Division of Endocrinology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, Ohio 45229, USA.
Osagie Ebekozien, T1D Exchange, Boston, Massachusetts 02111, USA.
Nudrat Noor, T1D Exchange, Boston, Massachusetts 02111, USA.
G Todd Alonso, University of Colorado, Barbara Davis Center for Diabetes, Aurora, Colorado 80045, USA.
Sarit Polsky, University of Colorado, Barbara Davis Center for Diabetes, Aurora, Colorado 80045, USA.
Scott M Blackman, Division of Pediatric Endocrinology and Diabetes, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287, USA.
Justin Chen, SUNY Upstate Medical University, Syracuse, New York 13210, USA.
Sarah D Corathers, Division of Endocrinology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, Ohio 45229, USA.
Carla Demeterco-Berggren, Rady Children’s Hospital, University of California, San Diego, San Diego, California 92123, USA.
Mary Pat Gallagher, Hassenfeld Children’s Hospital at NYU Langone, New York, New York 10016, USA.
Margaret Greenfield, SUNY Upstate Medical University, Syracuse, New York 13210, USA.
Ashley Garrity, Division of Pediatric Endocrinology, C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, Michigan 48109, USA.
Saketh Rompicherla, T1D Exchange, Boston, Massachusetts 02111, USA.
Robert Rapaport, T1D Exchange, Boston, Massachusetts 02111, USA.
Nana-Hawa Yayah Jones, Division of Endocrinology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, Ohio 45229, USA.
Financial Support
The T1DX-QI is supported by the Leona M. and Harry B. Helmsley Charitable Trust. The funding organization had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Disclosures
A.R.L., N.N., G.T.A., S.M.B., J.C., S.D.C., C.D.B., M.P.G., M.G., A.G., S.R., and R.R. have nothing to disclose. N.H.Y.J. consults for Medtronic, Inc. O.E. is on the Medtronic Diabetes Health Equity Advisory Board and has received financial support from Medtronic, Dexcom, and Eli Lilly through his organization. S.P. was a contributing writer for diaTribe, consulted for the JAEB Center, was on the medical advisory board for Medtronic MiniMed, Inc, and receives support from Dexcom, Inc, Eli Lilly, JDRF, Leona M. and Harry B. Helmsley Charitable Trust, the National Institute of Diabetes and Digestive and Kidney Diseases, and Sanofi US Services through her organization.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Ho J, Rosolowsky E, Pacaud D, et al. . Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID-19 pandemic. Pediatr Diabetes. 2021;22(4):552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alaqeel AF, Aljuraibah M, Alsuhaibani M, et al. . The impact of COVID-19 pandemic lockdown on the incidence of new-onset type 1 diabetes and ketoacidosis among Saudi children. Front Endocrinol (Lausanne). 2021;12:669302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dżygało K, Nowaczyk J, Szwilling A, Kowalska A. Increased frequency of severe diabetic ketoacidosis at type 1 diabetes onset among children during COVID-19 pandemic lockdown: an observational cohort study. Pediatr Endocrinol Diabetes Metab. 2020;26(4):167-175. [DOI] [PubMed] [Google Scholar]
- 4. Lawrence C, Seckold R, Smart C, et al. . Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary centre during the COVID-19 pandemic. Diabet Med. 2021;38(1):e14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A;. Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes. Has COVID-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care. 2020;43(11):2870-2872. [DOI] [PubMed] [Google Scholar]
- 6. Kamrath C., Mönkemöller K, Biester T, et al. . Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA. 2020;324(8):801-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ng SM, Woodger K, Regan F, et al. . Presentation of newly diagnosed type 1 diabetes in children and young people during COVID-19: a national UK survey. BMJ Paediatr Open. 2020;4(1):e000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Unsworth RS, Wallace NS, Oliver S, et al. . New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43(11):e170-e171. [DOI] [PubMed] [Google Scholar]
- 9. Alonso GT, Murphy C, Pyle L, Thomas S, Ohman-Hanson R, Rewers A. Increased prevalence of diabetic ketoacidosis among Colorado children at diagnosis of type 1 diabetes during the COVID-19 pandemic lockdown resolves after reopening. Diabetes Technol Ther. 2021;23(9):663-664. [DOI] [PubMed] [Google Scholar]
- 10. Evans NG, Berger ZD, Phelan AL, Silverman RD. Covid-19, equity, and inclusiveness. BMJ. 2021;373:n1631. https://www.bmj.com/content/373/bmj.n1631. [DOI] [PubMed] [Google Scholar]
- 11. Baker DW. Breaking links in the chain of racial disparities for COVID-19. JAMA Netw Open. 2021;4(6):e2112879. [DOI] [PubMed] [Google Scholar]
- 12. Asch DA, Islam MN, Sheils NE, et al. . Patient and hospital factors associated with differences in mortality rates among Black and White US Medicare beneficiaries hospitalized with COVID-19 infection. JAMA Netw Open. 2021;4(6):e2112842. [DOI] [PubMed] [Google Scholar]
- 13. Bambra C, Riordan R, Ford J, Matthews F. The COVID-19 pandemic and health inequalities. J Epidemiol Community Health. 2020;74(11):964-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maani N, Abdalla SM, Galea S. Avoiding a legacy of unequal non-communicable disease burden after the COVID-19 pandemic. Lancet Diabetes Endocrinol. 2021;9(3):133-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebekozien O, Agarwal S, Noor N, et al. . Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alonso GT, Ebekozien O, Gallagher MP, et al. . Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolfsdorf JI, Glaser N, Agus M, et al. . ISPAD Clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19 (Suppl 27):155-177. [DOI] [PubMed] [Google Scholar]
- 18. Jacob R, Weiser G, Krupik D, et al. . Diabetic ketoacidosis at emergency department presentation during the first months of the SARS-CoV-2 pandemic in Israel: a multicenter cross-sectional study. Diabetes Ther. 2021;12(5):1569-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ditkowsky J, Lieber AC, Leibner ES, Genes N. SARS-CoV-2 infection and associated rates of diabetic ketoacidosis in a New York City emergency department. West J Emerg Med. 2021;22(3):599-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Misra S, Barron E, Vamos E, et al. . Temporal trends in emergency admissions for diabetic ketoacidosis in people with diabetes in England before and during the COVID-19 pandemic: a population-based study. Lancet Diabetes Endocrinol. 2021;9(10):671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alonso GT, Corathers S, Shah A, et al. . Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2021. https://covid.cdc.gov/covid-data-tracker. Accessed September 21, 2021.
- 23. Majidi S, Ebekozien O, Noor N, et al. . Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. . Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. [DOI] [PubMed] [Google Scholar]
- 26. O’Malley G, Ebekozien O, Desimone M, et al. . COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pasquel FJ, Messler J, Booth R, et al. . Characteristics of and mortality associated with diabetic ketoacidosis among US patients hospitalized with or without COVID-19. JAMA Netw Open. 2021;4(3):e211091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barrett CE, Park J, Kompaniyets L, et al. . Intensive care unit admission, mechanical ventilation, and mortality among patients with type 1 diabetes hospitalized for COVID-19 in the U.S. Diabetes Care. 2021;44(8):1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kahkoska AR, Shay CM, Crandell J, et al. . Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1(5):e181851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Redondo MJ, Libman I, Cheng P, et al. . Pediatric Diabetes Consortium. Racial/Ethnic minority youth with recent-onset type 1 diabetes have poor prognostic factors. Diabetes Care. 2018;41(5):1017-1024. [DOI] [PubMed] [Google Scholar]
- 31. Petitti DB, Klingensmith GJ, Bell RA, et al. . SEARCH for Diabetes in Youth Study Group. Glycemic control in youth with diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155(5):668-672.e1-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Willi SM, Miller KM, DiMeglio LA, et al. . T1D Exchange Clinic Network. Network, racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lipman TH, Hawkes CP. Racial and socioeconomic disparities in pediatric type 1 diabetes: time for a paradigm shift in approach. Diabetes Care. 2021;44(1):14-16. [DOI] [PubMed] [Google Scholar]
- 34. Weinstock RS, Xing D, Maahs DM, et al. . T1D Exchange Clinic Network. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange Clinic registry. J Clin Endocrinol Metab. 2013;98(8):3411-3419. [DOI] [PubMed] [Google Scholar]
- 35. Ebekozien O, Odugbesan O, Rioles N, Majidi S, Yayah Jones N, Kamboj M. Equitable post-COVID-19 care: a practical framework to integrate health equity in diabetes management. J Clin Outcomes Manag. 2020;27(6):256-259. [Google Scholar]
- 36. LaVeist-Ramos TA, Galarraga J, Thorpe RJ Jr, Bell CN, Austin CJ. Are black Hispanics black or Hispanic? exploring disparities at the intersection of race and ethnicity. J Epidemiol Community Health. 2012;66(7):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tremblay ES, Ruiz J, Dykeman B, Maldonado M, Garvey K. Hispanic caregivers’ experience of pediatric type 1 diabetes: a qualitative study. Pediatr Diabetes. 2021;22(7):1040-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beck RW, Riddlesworth T, Ruedy K, et al. . DIAMOND Study Group. Group, effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371-378. [DOI] [PubMed] [Google Scholar]
- 39. Bergenstal RM, Tamborlane WV, Ahmann A, et al. . STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320. [DOI] [PubMed] [Google Scholar]
- 40. Karges B, Schwandt A, Heidtmann B, et al. . Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318(14):1358-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noor N, Ebekozien O, Levin L, et al. . Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 surveillance registry. Diabetes Care. 2021;44(8): e160-e162. [DOI] [PubMed] [Google Scholar]
- 42. Addala A, Auzanneau M, Miller K, et al. . A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44(1): 133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.