Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2, has currently affected >220 million individuals worldwide. The complex interplay of immune dysfunction, active malignancy, the effect of cancer treatment on the immune system and additional comorbidities associated with cancer and COVID-19 all affect the outcomes of COVID-19 in patients with cancer. We have discussed the published findings (through the end of September 2021) on the effects of cancer on the morbidity and mortality of COVID-19, common factors between cancer and COVID-19, the interaction of cancer and COVID-19 treatments, the impact of COVID-19 on cancer clinical services, immune test findings in cancer patients with COVID-19 and the long-term effects of COVID-19 on cancer survivors.
Keywords: cancer, COVID-19, haematologic malignancies, immunology, outcomes, solid malignancies
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has currently affected >220 million individuals worldwide.1 COVID-19 has been shown to cause more severe disease and increased mortality in patients with active cancer.2–5 The complex interplay of immune dysfunction, active malignancy, the effects of cancer treatment on the immune system and additional comorbidities associated with cancer and COVID-19 all affect the outcomes of COVID-19 in patients with cancer. The emergence of the Delta variant, which has been shown to have higher transmissibility, cause more severe disease among the unvaccinated and have somewhat less responsiveness to current COVID-19 vaccines6 may further alter the current landscape with regard to COVID-19 infections in patients with cancer. The effect of cancer on COVID-19 has also been shown to vary according to the type of cancer.7 The emergence of variants of SARS-CoV-2 has also led to changes in clinical presentations in cancer patients. In this article we discuss what is known about the effect of cancer on the morbidity and mortality of COVID-19, common factors that are shared between cancer and COVID-19, the interaction of cancer and COVID-19 treatments, the impact of COVID-19 on cancer clinical services, immune test findings in cancer patients with COVID-19 and the long-term effects of COVID-19 on cancer survivors.
Literature search
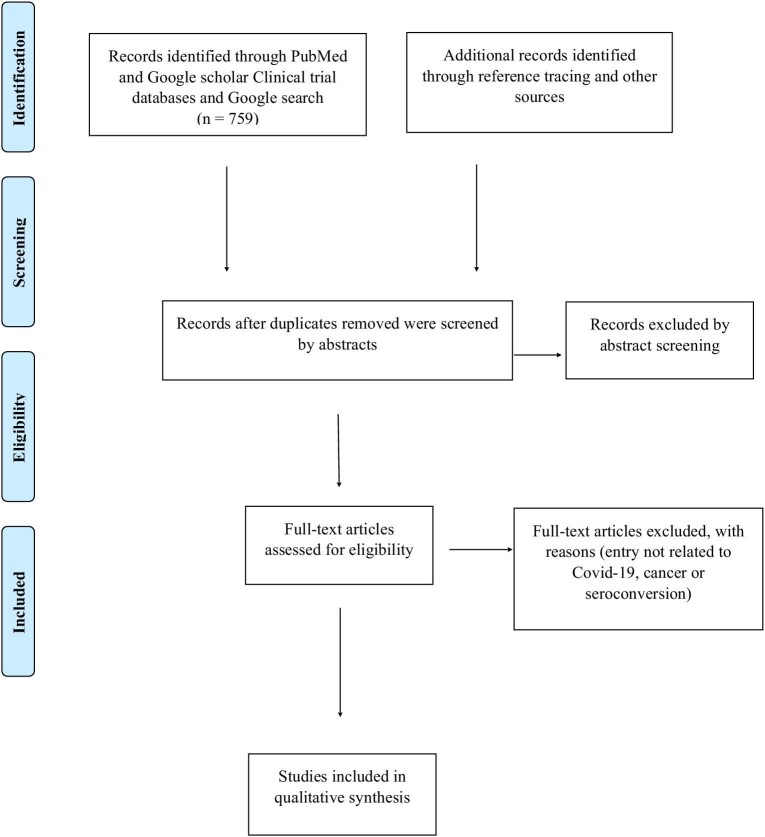
The PubMed and Google Scholar databases were searched from 1 January 2020 to 30 September 2021 using the search terms ‘COVID AND cancer screening OR cancer diagnosis OR cancer management’, ‘COVID AND cancer AND seroconversion OR immunity OR antibodies’ and ‘COVID AND cancer AND mortality OR outcomes OR clinical characteristics’. Retrospective and prospective observational and cohort studies describing clinical outcomes and changes in the immunity of cancer patients with COVID-19 and the impact on cancer services due to COVID-19 were included in the review after eliminating duplicates and irrelevant articles (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart.
The immune system in cancer
When the immune system does not function properly one may get autoimmunity, allergy or cancer. Cancer occurs when the immune system fails to get rid of cancerous cells. When the immune system detects cancerous cells, it secretes several chemicals, including cytotoxic granules, cytokines and chemokines, and activates various immune cells that together act to eliminate the cancerous cells.8 At times, the cancerous cells escape such immune processes and start multiplying, leading to growth and spread of the cancer. The immune system applies a selective pressure to tumour development and growth and tumours are forced to edit the immune components that surround it, which is known as cancer immunoediting. There are three phases of cancer immunoediting: tumour dormancy/equilibrium, where the immune system fights against the cancerous cells and both are at an equilibrium; tumour immunity/elimination, where the immune system eliminates the cancerous cells; and tumour progression/escape, where the tumour overtakes the immune system and evades the immune surveillance.9 Tumour cells evade immune attack by avoiding immune recognition and instigating an immunosuppressive tumour microenvironment.8 Tumour-induced immune defects may weaken the host immune response, and this is a common feature in patients with haematologic malignancies such as leukaemia.10
In certain haematologic malignancies, such as multiple myeloma and chronic lymphocytic leukaemia (CLL), the tumour cells need to share the same developmental space with normal immune cells and this may lead to immunoparesis. Some haematologic malignancies are characterised by systemic involvement of secondary lymphoid organs. The bone marrow represents a distinct immune tissue and haematopoietic stem cell involvement in leukaemia leads to cytopenia.11 These distinct biological characteristics of haematologic malignancies reflect the immunosuppression associated with these malignancies. Cancer treatments such as chemotherapy, immunotherapy, targeted therapy and haematopoietic stem cell transplantation (HSCT) may also lead to myelosuppression and immunosuppression in cancer patients.
The viral envelope of SARS-CoV-2 is typically made up of three proteins that include the membrane, envelope and spike proteins. The latter helps the virus to bind to and enter host cells.12,13 Here it multiplies and then spreads from cell to cell and person to person. The immune system is activated in different ways, including increased or decreased proliferation of various cells and secretion of cytokines and downstream effects due to this. The immune system produces different CD4 and CD8 T cells against different parts of the virus and the process of vaccination tries to mimic this before the infection occurs.12,13 Thus a person with cancer, in whom the immune system is weak due to the cancer and the various treatments received, may be more prone to getting COVID-19 and getting severe disease with adverse clinical outcomes including death.
Coronavirus infections in cancer patients
Studies have assessed the effects of SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV) infections in cancer patients. Information on the mortality and morbidity of cancer patients following SARS-CoV-1 infection are sparse. A patient with acute myeloid leukaemia (AML) and SARS-CoV-1 infection developed bilateral infiltrates on chest radiography but did not require intubation and was discharged uneventfully from the hospital.14 On the other hand, there is high morbidity and mortality among cancer patients infected with MERS-CoV, especially in those with haematologic malignancies. Atypical presentations of MERS-CoV infections were seen in those with a current or recent history of blood cancers. These included delayed symptom development, 20-d incubation period and persistent viral shedding without clinical deterioration.15 Multiple studies have found higher mortality rates among blood cancer patients who developed MERS-CoV infection. One study found MERS-CoV infection in blood cancer patients to have a very poor prognosis regardless of the status of the underlying disease.16 Other studies found the disease to be more severe among patients with cancers,17 with a 100% case fatality rate among patients with haematologic malignancies and advanced solid cancers. The major causes of death were multi-organ failure and septic shock.18
Common factors in cancer and COVID-19
Cancer is a genetic disease that is influenced by the tissue microenvironment and COVID-19 is an infectious disease.19 There are several similarities between cancer and COVID-19 with regards to risk factors for severe disease and immune changes, such as overproduction of some cytokines (e.g. interleukin-6 [IL-6] and type I interferon [IFN-I]), the involvement of androgen receptors and immune checkpoint signalling.20 Studies have clearly shown gender, age, ethnicity, comorbidities (including cardiovascular and respiratory), obesity, smoking and various medical conditions significantly impact the morbidity and mortality rates in COVID-19.3,21,22 The same factors are known to have a significant adverse impact on cancer patients as well. Socio-economic factors, including the gross domestic product, may also affect the association between cancer and COVID-19.20,23 Further similarities between cancer and COVID-19 have been elucidated at a molecular level by Zong et al.20 Four major signalling pathways—androgen receptor (AR), cytokine, IFN-I and immune checkpoint signalling—have been shown to govern the incidence and severity of both diseases.19
Angiotensin-converting enzyme 2 (ACE2) expression in lung tissue has been found to increase with age.24,25 Since most cancers occur in older individuals, high ACE expression has been suggested as a mechanism mediating adverse clinical outcomes in older cancer patients.26 Furthermore, smoking increases ACE2 expression in the lung tissue. There is a dose–response relationship between the number of pack-years of smoking and ACE2 expression.27,28 Smoking also correlates with adverse outcomes in patients with COVID-19.29
The S protein of coronavirus consists of two components, S1 and S2. S1 is involved in recognizing and binding the virus to the host cell surface, while S2 is involved in fusing the viral and cell membranes, allowing viral entry into the cell. TMPRSS2 is the protease that mediates S protein cleavage, setting S1 and S2 apart. This event is called S protein priming and is believed to be essential for the interaction of SARS-CoV-2 with ACE2 and cell entry.30 Co-expression of ACE2 and TMPRSS2 in such cells as type II pneumocytes, absorptive enterocytes of the small intestine and nasal goblet secretory cells reflects the high susceptibility of these cells to SARS-CoV-2.31 ACE2 is also highly expressed in males, especially in urogenital organs such as the prostate. It was demonstrated in a recent study that patients with chronic urinary diseases are highly prone to SARS-CoV-2 infection.32
Further, expression of TMPRSS2, one of the target genes of ARs, has an impact on both cancer and COVID-19. TMPRSS2 expression is modulated by the ARs in normal prostate secretory epithelium and prostate cancer. TMPRSS2 has also been found to be expressed in lung tissue.33 Recent studies suggest that patients with prostate cancer treated by androgen deprivation therapy (ADT) were less likely to be infected by SARS-CoV-2 compared with those not on ADT or those with other tumour types.34 They are also less likely to develop adverse clinical outcomes (hospitalization and oxygen supplementation).35 Patients with pre-existing primary or secondary immune dysregulation are at an increased risk of adverse or severe outcomes following COVID-19.36
Severe COVID-19 is associated with increased levels of cytokines such as IL-6, leading to a cytokine storm.26,37–40 IL-6 is also overexpressed in almost all types of tumours and is one of the major cytokines in the tumour microenvironment and a driver of tumour progression.41 On the other hand, one study showed that IFN-I levels are reduced in COVID-19 infection.42 IFN-I is implicated in inhibiting tumour growth and promoting tumour cell senescence and death.43 Decreased levels of IFN-I and IFN-stimulated genes along with increased levels of IL-6 has been demonstrated in peripheral blood samples from critical COVID-19 patients.44 In contrast, a few studies have demonstrated elevated IFN-I levels along with high IFN-stimulated genes in bronchoalveolar lavage or peripheral blood mononuclear cells of COVID-19 patients.45,46 Immune checkpoint molecules are expressed on a considerable proportion of tumour cells in many different cancers, including some haematologic malignancies such as Hodgkin lymphoma and primary mediastinal B cell lymphoma.47 Several studies have shown an upregulation of checkpoint receptors in association with T cell exhaustion and lymphopenia in severe COVID-19 cases.48–51
Both cancer and COVID-19 have an increased risk of venous thromboembolic events (or, more rarely, arterial thromboses).52 The mechanisms involved in COVID-19 coagulopathy include inflammation and activation of the innate immune system that in turn activates the systemic coagulation pathways.52–54 Viral endotheliopathy and microcirculatory dysfunction may further contribute to the thrombotic complications seen in COVID-19.53–55 In cancer patients, local or systemic therapy, multiple comorbidities and cancer cells expressing coagulation factors have been implicated in the pro-coagulant state.56
Morbidity and mortality patterns of COVID-19 in cancer patients
Both cancer and COVID-19 may have some common predisposing factors, including increased age, obesity and immunosuppression, and these may also increase the chance of a person getting severe COVID-19. In cancer, there may be a greater chance of the immune system becoming further dysregulated by COVID-19 as compared with a healthy person. The outcomes in patients with cancer who develop COVID-19 can be divided into three facets: clinical, laboratory and general management outcomes. Table 1 outlines the significant findings with regard to changes in outcomes of patients with solid and/or haematologic malignancies following COVID-19 infection.3,5,7,21,22,29,57–108
Table 1.
Studies on COVID-19 in cancer patients
| First author, year and month (country) | Type of cancer | Objective | Cancer cohort size | Methodology and duration of study | Significant findings |
|---|---|---|---|---|---|
| Alpert, 2020 November59 (USA) | S, H | Compared clinical characteristics of COVID-19 patients with and without cancer | 421 (S 325, H 96) | Retrospective. In-patient or emergency care at Mount Sinai Health System (March–May 2020) | Cancer patients – higher risk for adverse outcomes (thromboembolism, sepsis). No difference in AKI, ICU admissions and mortality |
| Bange, 2020 May86 (USA) | H | Compared mortality in haematologic and solid cancer patients with COVID-19 | 100 | Prospective, multicentre, observational (April–September 2020) | Haematologic cancer – higher mortality than solid cancer. Those with high CD8 T cell responses had better outcomes |
| Bernard, 2021 March7 (France) | S, H | Compare clinical findings in hospitalised patients | 6201 | Retrospective study. Assessed French national database of hospitalized COVID-19 patients (March–April 2020) | COVID-19 and cancer – twofold higher risk of death vs COVID-19 without cancer. Blood cancer – admission to ICU significantly more frequent (24.8% vs 16.4%). Lung, digestive (excluding colorectal cancer) and metastatic solid cancers – higher risk of death |
| Cai, 2021 January87 (China) | S, H | COVID-19 with cancer vs those without cancer | 93 | Multicentre, retrospective, cohort study. Compared the immunological characteristics of both cancer and non-cancer patients (February–March 2020) | COVID-19 patients with cancer – poorer prognosis vs non-cancer patients due to dysregulated immune responses. Higher mortality in patients with cancer compared with healthy controls |
| Calles, 2020 September60 (Spain) | S (lung) | Assess outcomes of lung cancer patients affected by COVID-19 | 23 | Prospective study. Evaluated patients with lung cancer treated at a medical centre in Spain and diagnosed with COVID-19 (March–May 2020) | Lung cancer patients – high rate of hospitalization, onset of ARDS and high mortality rate |
| Cattaneo, 2020 September61 (Italy) | H | Determine clinical characteristics and risk factors for mortality in haematologic patients affected by COVID-19 | 102 | Retrospective cohort study. (March 2020) | Chronic myeloproliferative neoplasms – lower risk of acquiring COVID-19. Immune-mediated anaemia and on immunosuppressive-related treatments – higher risk of acquiring COVID-19. Haematologic patients – higher mortality than non-haematologic patients with COVID-19 and uninfected haematologic patients. Worst prognosis among patients on active haematologic treatment |
| Chai, 2021 July57 (China) | S, H | Assess mortality and consequences of COVID-19 in cancer patients | 166 | Multicentre comparative cohort study. Compared COVID-19 patients with cancer and non-cancer COVID-19 patients and non-COVID cancer patients (January–March 2020) | If cancer patients with COVID-19 survive acute COVID-19 infection, long-term mortality appears similar to the cancer patients without COVID-19 and their long-term clinical sequelae are similar to COVID-19 patients without cancer. High risk – haematologic, nasopharyngeal, brain, digestive system and lung tumours. Moderate risk – genitourinary, female genital, breast and thyroid tumours |
| Costa, 2021 August58 (Brazil) | S, H | Evaluate risk factors of in-hospital mortality among COVID-19 patients with and without cancer | Cancer: 7406; no Cancer: 315 410 | Retrospective study (March–December 2020) | COVID-19 patients with cancer – higher disease severity, higher risk of mortality, longer hospital stay, higher ICU admissions, receive more invasive mechanical ventilation than patients without cancer. Haematologic neoplasia – higher risk of mortality than solid tumours |
| Crolley, 2020 October62 (UK) | S, H | Assess whether cancer patients (on systemic anticancer therapies) are at greater risk of contracting COVID-19 or having more severe outcomes | 68 | Retrospective study (March–May 2020) | Patients on SACT – higher mortality if they contract COVID-19. Receiving chemotherapy – increased risk of developing COVID-19 (high-dose chemotherapy – significantly high risk). Respiratory or intrathoracic neoplasm – increased risk of developing COVID-19. Receiving targeted treatment – protective effect. Solid vs haematologic cancer – no significant effect on risk |
| Dai, 2020 January63 (China) | S, H | Determine clinical characteristics and outcomes of COVID-19 patients with cancer | 105 | Multicentre retrospective study (January–February 2020) | COVID-19 patients with cancer – higher risks of severe outcomes. Haematologic cancer, lung cancer or with metastatic cancer (stage 4) – highest frequency of severe events. Non-metastatic cancer – frequency of severe conditions similar to patients without cancer. Patients who underwent surgery – had higher risks of severe events. Only radiotherapy – no significant differences in severe events compared to patients without cancer |
| De Azambuja, 2020 August64 (Belgium) | S | Determine the in-hospital mortality within 30 d of COVID-19 diagnosis among patients with solid cancer compared with patients without cancer | Cancer: 1187; no cancer: 12 407 | Retrospective study. Data from adult patients registered until 24 May 2020 in the Belgian nationwide database of Sciensano were used (April–May 2020) | Solid cancer – adverse prognostic factor for in-hospital mortality. Younger patients and without other comorbidities – adverse effect more pronounced |
| De Melo, 2020 October21 (Brazil) | S, H | Describe the outcomes, demographics and clinical characteristics of cancer in patients with COVID-19 | 181 | Retrospective cohort study. Cancer patients admitted to the Brazilian National Cancer Institute were assessed (April–May 2020) | Cancer – important prognostic factor for patients with COVID-19. Higher rates of complications (respiratory failure, septic shock, AKI) and COVID-19-specific death. COVID-19-specific mortality associated with age >75 y, metastatic cancer, ≥2 sites of metastases, presence of lung/bone metastases, non-curative treatment or best supportive care intent, higher CRP levels, admission due to COVID-19 and antibiotics use |
| Elkrief, 2020 November65 (Canada) | S, H | Determine the incidence and impact of hospital-acquired COVID-19 in cancer patients | 252 | Prospective observational cohort study. Used provincial registries and hospital databases (March–May 2020) | Hospital-acquired COVID-19-increased mortality in the cancer population. Age and advanced stage of cancer were negative predictive factors for COVID-19 severity |
| Erdal, 2021 January22 (Turkey) | S, H | Investigate clinical course and the factors affecting mortality in cancer patients affected by COVID-19 | 77 | Single-centre retrospective study. Compared cancer patients with non-cancer patients who were admitted to a hospital in Istanbul with COVID-19 infection (March–May 2020) | Cancer patients – severe disease and mortality higher vs non-cancer patients. Associations with severe disease and mortality – stage of the disease, receiving chemotherapy in the last 30 d, lymphopenia, elevated troponin I, D-dimer and CRP. Most important factors influencing survival – severe lung involvement and lymphopenia |
| Fernandes, 2021 February88 (Brazil) | S, H | To analyse COVID-19 mortality in cancer patients and associated factors | 51 | Cross-sectional retrospective study (April–August 2020) | Higher fatality – lung and haematologic cancers, age >60 y, currently undergoing cancer treatment |
| Ferrari, 2021 January89 (Brazil) | S, H | Investigate the predictors of death after COVID-19 diagnosis in patients with cancer | S: 167; H: 31 | Longitudinal multicentre cohort study (March–July 2020) | Factors associated with death – age ≥60 y, current/former smoking, coexisting comorbidities, respiratory tract cancer. COVID-19 rates similar to general population if otherwise healthy and have curable malignancies |
| Fillmore, 2020 October66 (USA) | S, H | Evaluate prevalence and outcome of COVID-19 infection in cancer patients and identify risk factors associated with infection and outcomes | 1794 | Retrospective study. Data from the Veterans Affairs Corporate Data Warehouse (January–May 2020) | Cancer – high susceptibility to COVID-19 infection and severe eventual outcome. Determinants of mortality – age, comorbidity and specific cancer types. COVID-19-attributable mortality similar in patients recently treated for cancer vs treated >6 months ago or never treated with systemic therapy. Mortality in patients using ICI within the last 6 months significantly lower (7%) vs patients receiving chemotherapy (14.0%), hormone therapy (16.2%) or targeted therapy (14.1%) |
| Ganatra, 2020 November67 (USA) | S, H | Evaluate outcomes in patients with COVID-19 with both cancer and comorbid CVD | Total: 2476; cancer: 195 | Retrospective study (March–May 2020) | Cancer was an independent predictor of severe disease. Patients with both cancer and CVD had a higher risk of severe disease and death vs only cancer or only CVD. Arrhythmias and encephalopathy more frequent with both cancer and CVD vs cancer alone |
| Grivas, 2021 June3 (USA) | S, H | Identify prognostic clinical factors, including laboratory measurements and anticancer therapies | 4966 | Retrospective, observational cohort study (March–November 2020) | Associations with higher COVID-19 severity – older age, male sex, obesity, cardiovascular and pulmonary comorbidities, renal disease, diabetes mellitus, non-Hispanic black race, Hispanic ethnicity, performance status, recent cytotoxic chemotherapy and haematologic malignancy. Among hospitalized patients, associations with higher COVID-19 severity – low/high absolute lymphocyte count, high absolute neutrophil count, low platelet count, abnormal creatinine, troponin, lactate dehydrogenase, and CRP. Higher mortality with anticancer therapies like R-CHOP, platinum combined with etoposide and DNA methyltransferase inhibitors |
| Höllein, 2021 January90 (Germany) | S, H | Determine the immune competence of individual patients by assessing absolute numbers of CD4+ T-cells, CD8+ T-cells, CD19+ B cells and CD16+/CD56+ NK cells in peripheral blood | S: 5; H: 12 | Retrospective study. Patients with cancer and COVID-19 treated in haematology and oncology centres from the greater Munich area (March–May 2020) | Haematologic cancers – higher risk for a severe course of COVID-19 compared with solid tumours. Cancer patients – higher risk of dying from COVID-19 than patients without cancer |
| Jee, 2020 August68 (USA) | S, H | Analyse the outcomes of cancer patients with COVID-19 and determine the impact of recent cytotoxic chemotherapy | 309 | Retrospective study. Patients with cancer and concurrent COVID-19 at Memorial Sloan Kettering Cancer Centre (March–April 2020) | Recent cytotoxic chemotherapy treatment not associated with adverse COVID-19 outcomes. Associations with worse outcomes – active haematologic or lung malignancies, peri-COVID-19 lymphopenia or baseline neutropenia. Rate of adverse events lower in cancer patients without COVID-19 |
| Kabarriti, 2020 August69 (USA) | S (lung) | Assess impact of prior lung irradiation on death as a result of COVID-19 infection | 107 | Retrospective study (March–April 2020) | History of radiation therapy (between 1 month and 1 y before COVID-19 testing) for cancer – poor prognosis. Mortality risk associated with the extent of lung irradiation |
| Khusid, 2021 February91 (USA) | S, H | Evaluate COVID-19 patients with a history of malignancy with regard to adverse clinical outcomes | 374 | Multicentre retrospective cohort study (March 2020–August 2020) | History of cancer – no greater risk for AKI or ICU admissions but higher risk for mortality vs without a history of cancer. Pulmonary neoplasm – higher mortality. History of genitourinary malignancies – not at higher risk for AKI or for mortality compared with the general population |
| Kuderer, 2020 June29 (USA, Canada and Spain) | S, H | Characterise the outcomes of patients with cancer and COVID-19 and identify potential prognostic factors for mortality and severe illness | 928 | Retrospective cohort study. Data on cancer patients ≥18 y of age with COVID-19 infection from the USA, Canada and Spain from the COVID-19 and Cancer Consortium database (March–April 2020) | 30-d all-cause mortality high in cancer patients. Associated with general risk factors and risk factors unique to patients with cancer (cancer status –remission vs active disease, responding to treatment vs progressing, active anticancer therapy). No associations with mortality – race and ethnicity, obesity status, cancer type, type of anticancer therapy and recent surgery |
| Lara Álvarez, 2020 September70 (Spain) | S | Determine the mortality due to COVID-19 in cancer patients during the first 3 weeks of the epidemic | 36 cancer (15 deaths), 1033 non-cancer (117 deaths) | Retrospective study. Cancer patients who died of COVID-19 were reviewed (March 2020) | COVID-19 mortality in cancer patients four times higher than that of the general population |
| Lara, 2020 July71 (USA) | S (gynaecologic cancer) | Determine the clinical characteristics and outcomes of patients with gynaecologic cancer with COVID-19 infection | 121 | Retrospective, observational study (March–April 2020) | Mortality 14% (risk of death similar to the age-specific mortality risk). Associations with increased risk of mortality – recent immunotherapy use. Associations with hospitalization – age ≥64 y, African American race and ≥3 comorbidities. Chemotherapy and recent major surgery not predictive of COVID-19 severity or mortality |
| Larfors, 2020 December92 (Sweden) | S, H | Evaluate COVID-19 intensive care admissions and mortality among patients with cancer | 2278 cancer and non-cancer COVID patients admitted to the ICU (104 cancer patients); 5027 total deaths (461 cancer patients) | Retrospective, observational study. Data extracted from the Swedish Intensive Care Registry (March–June 2020) | Patients with cancer had a higher risk of intensive care need and death |
| Lee, 2020 June108 (UK) | S, H | Describe the clinical and demographic characteristics and COVID-19 outcomes in patients with cancer | 800 | Prospective cohort study (March–April 2020) | Associations with risk of death – advanced age, male gender, presence of other comorbidities such as hypertension and cardiovascular disease. Chemotherapy in the past 4 weeks had no significant effect on mortality vs patients with cancer who had not received recent chemotherapy. Immunotherapy, hormone therapy, targeted therapy or radiotherapy use within the past 4 weeks had no significant effect on mortality |
| Lee, 2020 October107 (UK) | S, H | Determine COVID-19 risk according to tumour subtype and patient demographics in patients with cancer | 1044 | Prospective cohort study (March–May 2020) | Associations with risk of death – increasing age. Haematologic malignancies had a more severe COVID-19 clinical course compared with solid organ tumours. Patients with leukaemia – significantly increased case fatality rate. Haematologic malignancies with recent chemotherapy – increased risk of death during COVID-19-associated hospital admission |
| Liang, 2021 March93 (China) | S, H | Assess the clinical characteristics and risk factors for mortality in cancer patients with COVID-19 | 109 | Retrospective study (January–March 2020) | Cancer patients – higher risk of COVID-19 infection and poorer prognosis vs patients without cancer. Higher risk of mortality in advanced tumour stage or recent adjuvant therapy (<1 month) |
| Lunski, 2020 October94 (USA) | No specific type of cancer mentioned | Evaluate the difference in mortality from COVID-19 between patients with cancer and patients without cancer | Cancer: 312; without cancer: 4833 | Retrospective study (March–April 2020) | Patients with cancer – higher mortality vs controls. Greatest risk – patients with cancer age ≥65 y and those with certain comorbidities, male sex, history of CKD, obesity or recent therapy. Laboratory measures – decreased absolute lymphocyte counts, thrombocytopenia, elevated creatinine, lactic acidosis and elevated procalcitonin increased the risk of death |
| Mangone, 2021 April95 (Italy) | S, H | Evaluate the impact of having had cancer on COVID-19 risk and prognosis during the first wave of the pandemic | 407 | Population-based cohort study. Used registry data from the Reggio Emilia province (January–May 2020) | Cancer patients – greater risk of hospitalization and death, especially if <70 y of age or recent diagnosis. Greatest risk of hospitalization – cancers of the GI tract, lymphoma or other haematologic neoplasms. Strongest excess mortality – cancer of the urinary tract, other haematologic neoplasms, melanoma and female genital organs |
| Mehta, 2020 July72 (USA) | S, H | Evaluate case fatality rate of different cancer types who acquired with COVID-19 | 218 | Retrospective study (March–April 2020) | Cancer patients – significant increase in mortality. Highest susceptibility in haematologic or lung malignancies |
| Meng, 2020 June73 (China) | S, H | Evaluate the risk of cancer history and mortality in COVID-19 patients | 109 | Retrospective study (January–March 2020) | Cancer patients with COVID-19 – higher risk of mortality. Cancer history only independent risk factor for COVID-19. Haematologic malignancies – worse clinical outcomes (mortality rate twice that of patients with solid tumours [50% vs 26.1%]). Elevations in ferritin, high-sensitivity CRP, ESR, procalcitonin, prothrombin time, IL-2 receptor and IL-6 seen in cancer patients |
| Mohamed, 2021 January96 (UK) | S (renal transplant patients) | Compare waitlisted and transplant patients who got COVID-19 and assess clinical outcomes | 60 (32 active waitlisted patients and 28 functioning renal transplants) | Single-centre prospective study. COVID-19-positive waitlisted and transplant patients were compared and assess clinical outcomes were assessed (beginning of the pandemic–end of April 2020) | Both groups – CRP at 48 h and peak CRP associated with mortality. Incidence of COVID-19 higher in the waitlisted population. Transplant patients have more severe disease and higher mortality. CRP at 48 h can be used as a predictive tool |
| Mushtaq, 2021 September97 (USA) | H (HSCT) | Evaluate the impact of SARS-CoV-2 in HSCT and CAR T cell therapy recipients | 58 HCT/CAR T cell therapy patients (32 allogeneic HSCT, 23 autologous HSCT) | Single-centre prospective study (March–May 2021) | COVID-19 caused significant mortality in patients undergoing HCT and CAR T cell therapy, especially among allogenic HCT recipients |
| Nakamura, 2020 November98 (Japan) | S, H | Evaluate the association between clinical outcomes and potential prognostic factors | 32 | Retrospective study (January–May 2020) | Associations with mortality – lymphocyte count, albumin, LDH, serum ferritin and CRP on admission. Patients who received cytotoxic chemotherapy recently or were treated with chemotherapy had a poor prognosis and prolonged periods of viral shedding |
| Nie, 2020 November99 (China) | S (lung) | Determine the clinical characteristics and risk factors for in-hospital mortality of lung cancer patients with COVID-19 | 45 | Multicentre retrospective study (January–May 2020) | Lung cancer patients – atypical presentation of COVID-19. Associations with increased risk of in-hospital mortality – prolonged PT and elevated high-sensitivity cardiac troponin I |
| Pinato, 2020 July74 (UK) | S, H | Describe the outcomes in cancer patients during the initial outbreak of COVID-19 in Europe | 204 | Retrospective, multicentre observational study (February–April 2020) | Associations with mortality – age ≥65 y, multiple comorbidities. No associations – tumour stage, disease activity, provision of active anticancer therapy at COVID-19 diagnosis |
| Ramachandran, 2020 October75 (USA) | S, H | Compare clinical characteristics and outcomes of patients with and without a cancer history who were infected with COVID-19 | Cancer 53; non-cancer 135 | Retrospective observational study (March–April 2020) | Cancer patients – higher levels of lactic acidosis, CRP, LDH and ALP vs non-cancer patients. Age ≥60 y – rapid clinical deterioration. Higher mortality – age ≥60 y, especially when they had concomitant hypertension and elevated levels of CRP and LDH |
| Rogado, 2020 August77 (Spain) | S (lung) | Determine whether there is a difference in incidence and severity of COVID-19 infection in lung cancer patients | 17 | Retrospective study (March–April 2020) | Lung cancer patients had a higher mortality rate than the general population |
| Rogado, 2020 May76 (Spain) | S | Describe COVID-19 cumulative incidence, treatment outcome, mortality and associated risk factors | 45 | Retrospective study. Reviewed medical records of cancer patients admitted at an oncology department between 1 February and 7 April 2020 (February–April 2020) | Cancer patients – vulnerable to COVID-19, increased complications. Indicators of mortality – severity of the infection at admission, elderly patients |
| Rogiers Aljosja, 2021 January100 (multiple countries) | S (mainly melanoma, non-small cell lung cancer, renal cell carcinoma) | Determine clinical impact of COVID-19 on patients with cancer treated with ICIs | 110 | Multicentre, retrospective, cohort study. 19 centres across 9 countries (Australia, Canada, France, Germany, Italy, Switzerland, The Netherlands, UK and USA) (March–May 2020) | Cancer patients – higher risk of severe COVID-19 infection vs individuals without comorbidities. Treatment with ICI not associated with severe COVID-19 infection in patients with cancer. Increased risk for hospital admission – treatment with combination ICI and the presence of COVID-19 symptoms |
| Russell, 2020 July78 (UK) | S, H | Determine factors affecting COVID-19 outcomes in cancer patients | 156 | Retrospective study (February–May 2020) | Associations with severe COVID-19 – initial cancer diagnosis >24 months before COVID-19, presenting with fever, dyspnoea, GI symptoms and higher levels of CRP. Associations with COVID-19 death – Asian ethnicity, receiving palliative treatment, having an initial cancer diagnosis >24 months before, dyspnoea and increased CRP levels. Inverse association observed with increased levels of albumin |
| Rüthrich, 2020 November101 (Europe) | S, H | Determine the clinical characteristics and outcomes | Cancer: 435; non-cancer 2636 | Retrospective study (March–August 2020) | Associations of higher mortality – male sex, advanced age and active malignancy. Outcome of COVID-19 comparable after adjusting for age, sex and comorbidities for cancer vs non-cancer patients |
| Safari, 2021 August102 (Iran) | S, H | Investigate the epidemiology of cancer patients and identify risk factors affecting their mortality | 66 | Retrospective cohort study of hospitalized patients with cancer and COVID-19 in Hamadan (2020) | Factors influencing death – nausea, mechanical ventilation, admission to the ICU and length of hospital stay in the ward |
| Sharma, 2021 March103 (USA) | H (allogeneic HSCT recipients) | Describe the characteristics and outcomes of HSCT recipients after developing COVID-19 | 318 | Observational cohort study (March–August 2020) | Recipients of autologous and allogeneic HSCT – poor survival. Associations with higher risk of mortality – age ≥50 y, male sex, development of COVID-19 ≤12 months after transplantation. Disease indication of lymphoma associated with a higher risk of mortality vs plasma cell disorder/myeloma |
| Sorouri, 2020 December79 (Iran) | S, H | Determine the clinical characteristics, outcomes and risk factors for mortality in hospitalized patients with COVID-19 and cancer | Cancer 53; non-cancer 106 | Case–control study (February–April 2020) | Associations with mortality – dyspnoea, history of cancer, impaired consciousness level, tachypnoea, tachycardia, leucocytosis and thrombocytopenia. Haematologic cancer had a higher mortality than solid tumours (63% vs 37%) |
| Sun, 2021 January104 (USA) | S, H | Outcomes in cancer compared with non-cancer patients who got COVID-19 | Cancer 67; non-cancer 256 | Retrospective study. Cancer patients and non-cancer patients were compared (June 2020) | Associations with higher hospitalization and mortality – smoking status, comorbidities and a diagnosis of cancer |
| Tian, 2020 July5 (China) | S, H (malignant solid tumours and haematologic malignancy) | Determine clinical features and determine risk factors of COVID-19 disease severity for patients with cancer and COVID-19 | 232 | Multicentre, retrospective, cohort study (January–March 2020) | Patients with cancer – severe COVID. Risk factors – elevated TNF-α and NT-proBNP and decreased CD4+ T cells or albumin:globulin ratio, advanced tumour stage (previously reported risk factors of older age; elevated IL-6, procalcitonin and D-dimer; and decreased lymphocytes) |
| Trapani, 2020 December105 (Italy) | S (solid organ transplantation recipients) | Compare risk of infection, hospitalization and admission in the ICU and mortality among solid organ transplant recipients and non-solid organ transplant recipients who got COVID-19 | 450 | Nationwide population-based study. Assessed the cumulative incidence and outcome of COVID-19 in solid organ transplant recipients and compared the results with those of COVID-19 non-transplanted patients (January–June 2020) | Higher mortality (30.6%) in transplant patients than in non-solid organ transplant recipients (15.4%). Solid organ transplant recipients – higher risk of infection, hospitalization and admission in the ICU and mortality vs non-solid organ transplant recipients |
| Wang, 2020 May80 (China) | S, H | Assess the clinical characteristics and outcomes of COVID-19-infected cancer patients | 283 | Retrospective study. Data of cancer patients admitted to 33 designated hospitals for COVID-19 in Hubei province, China (December 2019–March 2020) | Associations with worse outcome – current cancer patients (vs former cancer patients), lymphohaematopoietic malignancies. Mortality higher among patients receiving recent chemotherapy (33%), surgery (26%), other anti-tumour treatments (19%) and no anti-tumour treatment (15%) |
| Wang, 2020 December106 (USA) | S, H | Investigate how patients with specific types of cancer are at risk for COVID-19 infection and its adverse outcomes and to study cancer-specific race disparities for COVID-19 infection | 1200 | Retrospective case–control study (August 2020) | Patients with cancer had a high risk for COVID-19 infection and worse outcomes. This was further exacerbated among African Americans |
| Yang, 2020 May81 (China) | S | Determine clinical characteristics and outcomes of cancer patients with COVID‐19 | 52 | Retrospective study (January–April 2020) | Cancer patients had a higher risk of COVID-19 vs the general population. Complications – liver injury, ARDS, sepsis, myocardial injury, renal insufficiency and MODS are common in cancer patients |
| Yang, 2020 July82 (China) | S, H | Describe clinical characteristics and outcomes of patients with cancer and COVID-19 and examine risk factors for mortality | 205 | Retrospective, multicentre, cohort study (January–March 2020) | Poor prognosis (death during admission to hospital) – haematologic malignancies (vs solid tumours), receiving chemotherapy within 4 weeks before symptom onset and male sex were risk factors |
| Zhang, 2020 October83 (China) | S (breast) | Evaluate characteristics and outcomes of breast cancer patients infected with COVID-19 | 35 | Retrospective study. Five designated tertiary hospitals for the treatment of COVID-19 in Wuhan, China (January–May 2020) | Breast cancer patients had less severe disease compared with other cancer patients when infected with COVID-19 |
| Zhang, 2020 July84 (China) | S | Determine clinical characteristics of COVID-19-infected cancer patients and assess the risk factors associated with severe events | 28 | Retrospective cohort study (January–February 2020) | Cancer patients – poor outcomes. Risk factors for severe disease – last anti-tumour treatment within 14 d, patchy consolidation on CT on admission |
| Zhou, 2020 July85 (China) | H | Determine the clinical characteristics of haematologic patients who were infected with COVID-19 | 9 | Retrospective study (February 2020) | Patients had atypical clinical features, defective viral clearance and a lower level of SARS-CoV-2-specific antibodies |
AKI: acute kidney injury; ALP: alkaline phosphatase; ARDS: acute respiratory distress syndrome; CAR: chimeric antigen receptor; CKD: chronic kidney disease; CRP: C-reactive protein; CT: computed tomography; CVD: cardiovascular disease; ESR: erythrocyte sedimentation rate; GI: gastrointestinal; H: haematologic cancer; HCT: haematocrit; HSCT: haematopoietic stem cell transplant; ICI: immune checkpoint inhibitor; IL: interleukin; LDH: lactate dehydrogenase; MODS: multiorgan dysfunction syndrome; NK: natural killer; NT-proBNP: N-terminal brain natriuretic peptide; PT: prothrombin time; R-CHOP: rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine sulphate and prednisone; S: solid cancer; SACT: systemic anticancer therapy; TNF: tumour necrosis factor.
Solid malignancies
In studies that included solid cancer patients, those who got COVID-19 had a higher probability of death compared with patients without cancer.7,57,60 Several risk factors for increased mortality have been identified, including advanced age, male sex, smoking history, metastatic cancer, the presence of lung or bone metastases, higher C-reactive protein levels, the number of comorbidities and poor performance status.7,21 Some studies found the age cut-off for worse prognosis to be 65 y,96 while others found it to be 75 y.21 Severe lung involvement due to cancer is an important determinant,22 and those from ethnic minorities have a worse prognosis.3
Those with lung, digestive (excluding colorectal), nasopharyngeal, brain and skin cancers had high mortality, with lung cancer as the highest.7,57,101 Moderately higher mortality was noted with genitourinary, female genital tract, breast and thyroid cancers.57 One study found genitourinary malignancies to not have a higher risk for mortality compared with the general population91 and another found those with gynaecologic cancer to have a similar age-specific mortality risk.71 Non-metastatic cancer patients experience similar frequencies of severe conditions as patients without cancer.63 Another study reported gastrointestinal tract cancer (together with lymphoma or other haematologic neoplasms) had the highest excess of hospitalization, while cancers of the urinary tract, female genital organs and melanoma had the highest excess mortality.97 Those with solid cancers had a higher risk of thromboembolism and sepsis,59 acute respiratory and kidney failure, venous thrombosis, atrial fibrillation,7 acute respiratory distress syndrome (ARDS),60 liver injury, myocardial injury, renal insufficiency and multi-organ dysfunction syndrome (MODS).81
Haematologic malignancies
Owing to high immunosuppression as a result of underlying disease, patients with haematologic malignancies are at significantly higher risk of a variety of severe infections, including COVID-19. They often receive myelosuppressive and immunosuppressive treatment, which further reduces their immune responses and further increases their risk of infections.
Increased mortality from COVID-19 infection has been reported in patients with immunosuppression (including those taking methotrexate). In particular, those with haematologic malignancies had a 2.5-fold increased risk.109 A retrospective study found that patients with both COVID-19 infection and progressive cancer had a fivefold increase in the risk of 30-d mortality compared with COVID-19-positive cancer patients who were in remission or had no evidence of cancer.29 A retrospective Italian study early in the pandemic also showed higher mortality in haemato-oncology patients with COVID-19 infection on the order of 39.2%, which was significantly higher when compared with rates for non-haematologic patients with COVID-19 (23.5%) and uninfected haematologic controls (3%).61 A large retrospective study by Passamonti et al.110 from Italy revealed increased mortality of patients with haematologic malignancies infected with COVID-19 when compared with a non-COVID-19 cohort with haematologic malignancies. Older age, progressive disease status, diagnosis of AML, indolent non-Hodgkin lymphoma, aggressive non-Hodgkin lymphoma or plasma cell neoplasms and severe or critical COVID-19 were associated with worse overall survival in this study. A meta-analysis of 34 adult and 5 paediatric studies from Asia, Europe and North America that included 3377 predominantly hospitalised haemato-oncology patients reported a comparable risk of death on the order of 34%.111 Patients >60 y of age had a significantly higher relative risk of death compared with those <60 y of age. Several more studies have demonstrated higher mortality in patients with haematologic malignancies following COVID-19 infection compared with solid cancer patients.73,79,82,90 Children with blood cancers seem to have a low risk of death and the risk is higher in those with blood compared with solid organ cancers.111
A multicentre audit in six National Health Service (NHS) centres revealed that patients with haematologic malignancies treated with chemotherapy in the preceding 28 d to COVID-19 diagnosis and nosocomial COVID-19 infection had a significantly higher mortality rate than those with solid malignancies.112 In contrast, the aforementioned Italian study by Passamonti et al.110 found that recent therapy had no association with mortality. This finding is consistent with the studies of patients with cancer in general and provides reassurance of the general safety of cancer treatment during the pandemic, although it is not a guarantee for every specific treatment in every clinical scenario.27 Several studies have shown haematologic malignancies adversely affect COVID-19 severity. A study conducted at two centres in Wuhan, China among haematologic malignancy patients found no differences in baseline characteristics between those who did or did not develop COVID-19. However, the haematologic malignancy patients with COVID-19 had a higher mortality rate than those without COVID-19.113 Patients with haematologic malignancies have significantly more admissions to the intensive care unit (ICU) than those without cancer.7 Factors that adversely affected prognosis were advanced age, male sex, presence of lung or bone metastases, higher C-reactive protein levels, lymphopenia, non-Hispanic black race and Hispanic ethnicity, the number of comorbidities and poor performance status.3,7,21
Immune test findings in cancer patients with COVID-19
Table 2 outlines the significant immune test findings in cancer patients with COVID-19.86,114–124 Laboratory studies have found weaker SARS-CoV-2 antibody responses in patients with cancer compared with those without.119 A study found seroconversion following COVID-19 to be lower in patients with haematologic malignancies and those who had received rituximab or a stem cell transplant.124 Thus a differential rate of seroconversion was seen in different cancer patient groups. Another study found survival to be lower in patients with blood cancers compared with those with solid organ cancers. In this study there was greater impairment of B cell and SARS-CoV-2-specific antibody responses in those with blood cancers compared with solid cancers.86 Significantly reduced immunoglobulin G (IgG) and IgM antibodies to SARS-CoV-2 are seen among blood cancer patients compared with solid cancer patients following COVID-19.86 However, a subset of patients developed good CD4+ and CD8+ T cell responses and those with good CD8+ T cell responses were able to compensate for the weak antibody responses.86
Table 2.
Studies on SARS-CoV-2-related immune responses in cancer patients
| First author, year and month (country) | Type of cancer | Objective | Cancer cohort size | Methodology and duration of study | Significant findings |
|---|---|---|---|---|---|
| Abdul-Jawad, 2021 January117 (UK) | S, H | Determine how the immune system is affected by SARS-CoV-2 infection of cancer patients | 41 (23 S, 18 H) | Prospective study (March–May 2020) | Haematologic malignancies – heterogeneous humoral responses, exhausted T cell phenotype and high prevalence of prolonged virus shedding. Recovered haematologic cancer patients – lingering immunological legacies. Recovered solid cancer patients’ immunophenotypes almost similar to those of non-virus-exposed cancer patients |
| Annika, 2021 September118 (UK) | S, H | Compare humoral and cellular immunity against SARS-CoV-2 in solid and haematologic cancer patients | 357 | Prospective, longitudinal cohort study (May 2020–March 2021) | Solid malignancies – capable of developing humoral and cellular immunity against SARS-CoV-2. Haematologic malignancies – impaired humoral response associated with malignancy type and anti-CD20 treatments |
| Bange, 2021 May86 (USA) | H | Determine immune parameters that lead to clinical outcomes | 100 | Prospective observational cohort study of patients with cancer who were hospitalized with COVID-19 (April–September 2020) | Patients with cancer – significantly reduced SARS-CoV-2-specific IgG and IgM antibodies than patients without cancer; most prominent in haematologic cancer patients; solid cancer patients had IgG and IgM antibody responses that were more similar to patients without cancer |
| Esperança, 2021 May119 (Portugal) | S, H | Determine the humoral immune response of patients with cancer against SARS-CoV-2 | 72 (19 cancer, 53 controls) | Single-centre, retrospective study (March–June 2020) | Patients with cancer – weaker SARS-CoV-2 antibody response compared with those without cancer. Associations with persistently weak serological responses – treatment with chemotherapy within 14 d before positivity |
| Hempel, 2020 January120 (Germany) | S, H | Analyse development of antibodies in patients with cancer following SARS-CoV-2 | 77 | Multicentre, prospective study (April 2020) | Patients with cancer had poorly developed antibodies following infection |
| Huang, 2021 February121 (USA) | H | Study underlying immune mechanisms leading to increased mortality in cancer patients with COVID-19 | Cancer 106, non-cancer controls 113 | Prospective study (April–September 2020) | Haematologic cancer – significant impairment of B cells and SARS-CoV-2-specific antibody responses. Solid cancer – immune phenotype similar to non-cancer patients. Even in haematologic cancers if preserved CD8 T cells they had a lower viral load and mortality. Even if depletion of B cells with anti-CD20 therapy resulted in complete impairment of SARS-CoV-2-specific IgG and IgM antibodies, mortality was not increased if adequate CD8 T cells were present |
| Huang, 2020 September114 (China) | S, H | Describe the characteristics, screening methods and outcomes of cancer patients with asymptomatic COVID-19 infection | 16 | Retrospective study (February–April 2020) | Lymphocyte counts were normal in all asymptomatic carriers (host immunity of asymptomatic carriers is not significantly disrupted by COVID-19) |
| Liu, 2020 June115 (China) | S, H | Compare the antibody response to SARS-CoV-2 in cancer patients and non-cancer patients | Cancer 40, non-cancer 1430 | Prospective study (February–April 2020) | Prevalence of IgG antibodies to SARS-CoV-2 in cancer patients with COVID-19 was much lower than in patients without cancer |
| Marra, 2020 January122 (France) | S, H | Determine seroconversion rates of cancer patients and COVID-19 | Cancer 61, non-cancer 105 (86 PCR positive) | Multicentre, observational, prospective study (March–May 2020) | No difference in SARS-CoV-2-specific IgG antibody detection in cancer patients and healthy subjects |
| Paschold, 2020 October123 (Germany) | S, H | Determine SARS-CoV-2-specific antibody rearrangements of immune systems of cancer patients in order to explain the outcome | Cancer 500, healthy controls 200 | Prospective study (July 2020) | B cell response restriction in aging and cancer |
| Solodky, 2020 May116 (France) | S, H | Compare the SARS-CoV-2 antibodies in cancer patients versus healthcare workers after symptomatic COVID-19 | 85 | Retrospective study (March–April 2020) | Seroconversion rate significantly lower in cancer patients. Antibodies almost undetectable in patients receiving cancer treatments in the month before antibody testing |
| Thakkar, 2021 March124 (USA) | S, H | Evaluate the rate of seroconversion for SARS-CoV-2 IgG for patients with cancer and its association with the type of malignancy and type of anticancer therapy | 261 | Observational, retrospective exploratory cohort study (March–September 2020) | Significantly reduced (absent) seroconversion observed in haematologic malignancies, patients receiving anti-CD20 therapy, CAR T cell therapy and stem cell transplant |
CAR: chimeric antigen receptor; H: haematologic cancer; PCR: polymerase chain reaction; S: solid cancer.
Impairment of B cells has been found in other studies as well.121,123 In one study there were heterogeneous humoral responses and an exhausted T cell phenotype in blood cancer patients. There is compelling evidence from case reports and series that immunosuppressed patients, including those with cancers, are also at increased risk of prolonged, persistent SARS-CoV-2 infection and viral shedding, accelerated viral evolution during infection and treatment, emergence of viral variants that show evidence of reduced susceptibility to neutralizing antibodies and low antibody titres to SARS-CoV-2 variants.125–135 The immunophenotypes of recovered solid cancer patients were similar to non-virus-exposed cancer individuals, while recovered blood cancer patients showed a weak immune response.117 Anti-cancer therapy has been reported to be associated with impaired seroconversion. Patients receiving anti-CD20 therapy and chimeric antigen receptor (CAR) T cell therapy have absent seroconversion.124
However, some studies reported SARS-CoV-2-specific IgG antibody detection did not differ between cancer patients and healthy individuals.122,136 Furthermore, immune disruption is higher in symptomatic COVID-19 patients, while asymptomatic carriers have normal immunity and lymphocyte counts.114 Finally, the resolution of any immune dysfunction that follows COVID-19 occurs faster and better in those with a solid cancer. There is delayed or absent antibody responses in patients with haematologic malignancies and these patients will take longer time to clear the virus.137
Interaction of cancer and COVID-19 treatments
The treatment of cancer patients with COVID-19 may be complicated by changes in immunity when using certain medications and drug interactions. In Hodgkin lymphoma, bleomycin needs to be used with caution, owing to its lung toxicity.138 Immune checkpoint inhibitor (ICI)-induced pneumonitis has made clinicians cautious about using this class of medicines. Concomitant ICI-induced pneumonitis and COVID-19 pneumonia may potentially increase the risk of lung damage.139 Among those with cancer that develop COVID-19, those receiving ICI therapy had worse outcomes in some studies40 but not in others.102 Several cancer treatment protocols or regimens do not affect the outcomes of COVID-19 and have thus been recommended for use in cancer patients during the pandemic.140 Immunotherapy, hormone therapy or radiotherapy in the month prior to SARS-CoV-2 infection is not associated with an increased risk of mortality among cancer patients with COVID-19.34 Androgen therapy reduces the risk of contracting COVID-19 among patients with prostate cancer.
The Bruton tyrosine kinase inhibitors (BTKis) are used to treat CLL, Waldenström macroglobulinaemia and chronic graft-vs-host disease and have been shown to have potent anti‐inflammatory effects resulting in decreased levels of pro‐inflammatory cytokines that are commonly elevated in severe COVID-19.141 Thus some authors have suggested a protective role of BTKis from severe COVID-19 morbidity due to an attenuated inflammatory response.142,143
It is recommended that granulocyte colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) be stopped in patients with cancer and COVID-19 who do not have bacterial or fungal infections, to avoid the potential risk of increasing inflammatory cytokine levels and pulmonary inflammation.144,145 On the other hand, it is recommended that G-CSF be given with chemotherapy regimens to reduce the risk of febrile neutropenia in patients with COVID-19.146 Thus, further trials on this aspect are urgently needed.
With CAR T cell and bispecific T cell engineer therapy, cytokine release syndrome is a known complication and shares many similarities with the COVID-19-associated cytokine storm.147 The optimal time interval between resolution of infection and initiating or restarting cancer-directed therapy is still to be determined. Currently it has been recommended that treatment be withheld until COVID-19 symptoms have resolved.140 With radiation therapy, hypofractionation has been suggested to minimize the number of hospital visits during the pandemic.148
The overall recommendations for treating COVID-19 in cancer patients are similar to those for the general population.140 Dexamethasone has been found to reduce mortality rates in patients with COVID-19 who require supplemental oxygen or invasive mechanical ventilation.149 In cancer patients, dexamethasone is used for several reasons, including preventing chemotherapy-induced nausea and to treat inflammation associated with brain metastasis or spinal cord compression and as part of treatment protocols in acute lymphoblastic leukaemia and multiple myeloma. The side effects of dexamethasone are expected to be similar in patients with cancer to those without.140 However, as dexamethasone is a weak to moderate cytochrome P450 (CYP3A4) inducer, possible interactions do need to be considered.140
Interactions are seen between medications that are used to treat cancer and COVID-19.150 Tocilizumab is known to interact with vincristine and doxorubicin. Some COVID-19 treatments may cause QT interval prolongation and thus need to be used with caution in patients receiving a bcl-2 inhibitor (venetoclax) or tyrosine kinase inhibitors (such as gilteritinib or nilotinib). Lopinavir/ritonavir are CYP3A4 inhibitors and may increase methotrexate, vincristine or ruxolitinib concentrations.151,152
Impact of COVID-19 on cancer clinical services
Taking into account the impact of COVID-19 on cancer clinical services, clinicians have had to rethink and remodel the provision of curative care for such patients. Table 3 outlines important findings with regard to the impact on clinical services and psychological aspects of cancer patients due to the COVID-19 pandemic.137,153–167 One study suggested that while appropriate therapy is continuing, physicians should take steps to reduce the risk from COVID-19, such as the selection of oral over intravenous treatment regimens wherever there is equipoise, the more judicious use of growth factor support and reducing surveillance laboratory and radiologic evaluations when possible.144,145,148 Another study discussed the use of tele-oncology services to reduce the risk of cancer patients being exposed to SARS-CoV-2.168 There is a need for clear communication and education about hand hygiene, infection control measures, high-risk exposure and awareness of the signs and symptoms of COVID-19. Some have suggested the need to individually evaluate the necessity of active intervention, postponing elective surgery or adjuvant chemotherapy for patients with a low risk of progression and minimizing outpatient visits to mitigate exposure and transmission.169
Table 3.
Studies on the effect of COVID-19 on cancer services
| First author, year and month (country) | Type of cancer | Objective | Cancer cohort size | Methodology and duration of study | Significant findings |
|---|---|---|---|---|---|
| Blay, 2021 April162 (France) | S | Determine impact of COVID-19 on cancer patient management | N/A | Retrospective study. The number of patients diagnosed and treated within 17 of the 18 Uni cancer centres was collected in 2020 and compared with the same periods between 2016 and 2019 (January–July 2020) | Delays in cancer patient management – only for newly diagnosed patients, more frequently in women, for breast cancer, prostate cancer, and non-metastatic cancers |
| Dinmohamed, 2020 April153 (The Netherlands) | N/A | Evaluate the impact of the COVID-19 pandemic on cancer diagnosis | N/A | Retrospective observational study (February–April 2020) | Significant decrease in cancer diagnoses mainly in skin cancers. Probable causes for barriers in cancer diagnosis – barriers to consulting a GP, GP consultations for non-acute issues transitioned to telehealth, hospitals postponed diagnostic evaluation and temporary discontinuation of national screening programmes for breast, colorectal, and cervical cancer |
| Gathani, 2020 November163 (UK) | S (breast) | Analyse the impact on diagnosis due to COVID-19 by comparing activity for breast cancer in the first 6 months of 2020 compared with the same time period in 2019 | N/A | Retrospective observational study (January–June 2020) | Number of breast cancers diagnosed during the first half of 2020 is not significantly low compared with the first half of 2019 |
| Guven, 2020 July154 (Turkey) | S, H | Evaluate the early changes in the inpatient and outpatient oncology clinics | N/A | Retrospective study. Patients admitted to four medical centres in Massachusetts were recruited (March–May 2020) | Significant decrease in newly diagnosed patients, patients having elective interventional procedures and patients in palliative care services. The frequency of hospitalisations for chemotherapy was higher than in previous years |
| Jazieh, 2020 September155 (Global) | N/A | Evaluate the impact of the COVID-19 pandemic on cancer care worldwide | N/A | Cross-sectional study (April–May 2020) | Cancer care affected worldwide. Main reasons for restricted cancer care – overwhelmed system, lack of personal protective equipment, staff shortage and restricted access to medications |
| Juanjuan, 2020 October156 (China) | S (breast) | Analyse the psychological status of patients with breast cancer during the pandemic | 658 | Retrospective study. Evaluated patients with breast cancer recruited from multiple breast cancer centres in Hubei Province and assessed the psychological impact by assessing anxiety, depression, distress and insomnia (February 2020) | High rates of anxiety, depression, distress and insomnia were observed. Independent factors associated with anxiety – poor general condition, shorter duration after breast cancer diagnosis, aggressive breast cancer molecular subtypes and close contact with patients with COVID-19. Factors independently associated with depression – poor general condition and central venous catheter flushing delay. Independent factors associated with insomnia – poor general condition. Independent factors associated with distress – poor physical condition and treatment discontinuation |
| Lai, 2020 June137 (UK) | N/A | Evaluate the impact of the COVID-19 pandemic on cancer services | 3 862 012 | Multicentre retrospective observational study (through April 2020) | Decreases in admissions for chemotherapy and urgent referrals for early cancer diagnosis |
| Maringe, 2020 August157 (UK) | S (breast, colorectal and oesophageal cancer and lung cancer) | Estimate the impact of delays in diagnosis on cancer survival outcomes in four major tumour types | 32 583 patients with breast cancer, 24 975 with colorectal cancer, 6744 with oesophageal cancer and 29 305 with lung cancer | National population-based modelling study (retrospective observational and modelling) (March 2020–March 2021) | An increase in the number of avoidable cancer deaths is expected as a result of diagnostic delays due to the pandemic in the UK |
| Morris, 2021 March164 (UK) | S (colorectal) | Investigate the impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England | N/A | Retrospective observational study. Used data from four population-based datasets spanning NHS England (National Cancer Waiting Time Monitoring, Monthly Diagnostic, Secondary Uses Service Admitted Patient Care and the National Radiotherapy datasets) (January 2020–October 2020) | Reduction in the number of people referred, diagnosed and treated for colorectal cancer. Reduced number of colonoscopies performed. Reduction in patients receiving surgery. Lower proportion of laparoscopic and greater proportion of stoma-forming procedures. For rectal cancer, increase in the use of neoadjuvant radiotherapy due to a greater use of short-course regimens |
| Patt, 2020 November158 (USA) | N/A | Evaluate the impact of COVID-19 on the US cancer population, including identification of new patients, access to care, and disruption of treatment | N/A | Retrospective observational study (March–July 2020) | Decreases and delays in identifying new cancers, delivery of treatment, cancer screenings, visits, therapy and surgeries. Mastectomies, colectomies and prostatectomies mainly affected |
| Rutter, 2020 July165 (UK) | S (GI cancer) | Analyse the impact of the COVID-19 pandemic on endoscopy services and endoscopic cancer diagnosis | N/A | Retrospective study. The average weekly number of cancers, proportion of missing cancers and cancer detection rates were calculated (January–May 2020) | Reduction in endoscopic services and a resultant reduction in cancer detection |
| Sozutek, 2021 February166 (Turkey) | S (GI cancer surgery) | Evaluate the feasibility of performing elective GI cancer surgery during the COVID-19 pandemic | 176 patients | Observational unicentric cohort study. Conducted to evaluate whether surgery could be safely performed during the COVID-19 pandemic (March–November 2020) | GI cancer surgery can be safely performed even within a pandemic hospital if proper isolation measures can be achieved for both patients and health workers |
| Van Haren, 2021 April167 (USA) | S (lung) | Examine the impact of COVID-19 on lung cancer screening and subsequent cancer diagnosis | N/A | Prospective study. Analysis of the prospective institutional low-dose CT screening database (March–July 2020) | Significant disruption in lung cancer screening with a resultant decrease in the number of new patients screened and an increase in the proportion of nodules suspicious for malignancy once screening was resumed |
| Vanni, 2020 June159 (Italy) | S (breast) | Estimate the impact of anxiety among breast cancer patients due to COVID-19 | 160 | Retrospective study (January–March 2020) | Higher rates of procedure refusal and surgical refusal. Infection risk was the primary reason for refusal |
| Wang, 2020 July160 (China) | S, H | Explore mental health problems in patients diagnosed with cancer during the COVID-19 pandemic | 6213 | Cross-sectional study (April 2020) | High prevalence of depression, anxiety, PTSD and hostility. Risk factors for mental health problems – history of mental disorder, excessive alcohol consumption, higher frequency of worrying about cancer management due to COVID-19, higher frequency of a feeling of overwhelming psychological pressure from COVID-19 and a higher level of fatigue and pain. Only 1.6% sought psychological counselling during COVID-19. Protective factors associated with a lower risk of mental health problems among cancer patients – better quality of life, good relationships with family members |
| Zadnik, 2020 July161 (Slovenia) | N/A | Analyse whether cancer diagnosis and management were affected during the COVID-19 epidemic in Slovenia | N/A | Retrospective observational study. Used data from the Slovenian Cancer Registry (November 2019–May 2020) | Significant decrease in first referrals for oncological services, first visits, imaging studies and cancer notifications |
CT: computed tomography; GI: gastrointestinal; GP: general practitioner; H: haematologic cancer; N/A: not applicable; PTSD: post-traumatic stress disorder; S: solid cancer.
Optimal protection of healthcare staff is important during the pandemic. Rapid response by institutions, adjustments to organizational structure, strategic planning, developing and implementing effective guidelines and providing effective and alternative ways to protect and support clinical staff, employees and patients are key to achieving good care during the pandemic.
Many patients with haematologic malignancies rely on clinical trials to receive their care, but during the pandemic the conduct of several clinical trials has been affected. The risk of adverse events from cancer therapies is unlikely to be increased.170 Haematologic malignancy treatment units should be SARS-CoV-2 free zones, dedicated solely to haematologic treatment. Patients should strictly comply with social distancing and hospital outpatient visits should be reduced.
During the pandemic, cancer patients may experience adverse impacts from a screening, treatment or psychological perspective. Two-thirds of new cancer patients have presented at a more advanced stage. Nearly 75% missed their cancer screening appointment and two-thirds of patients who are already on cancer treatment have had their treatment interrupted.171 A significant proportion of screening and diagnostic investigations have had to be postponed due to the pandemic. There has been a large effect on endoscopic services, leading to reductions in cancer detection.165 Significant disruption has been seen in lung cancer screening167 and imaging studies.161 Urgent referrals for early cancer diagnosis were significantly reduced.137 National screening programmes for breast, colorectal and cervical cancer were temporarily stopped or reduced due to the pandemic.153
An Israeli national survey among haemato-oncology patients reported a delay in treatment among 9.1% of patients.172 Interruptions to treatment at any stage have been reported by 77.5% of breast cancer patients during the pandemic.156 A systematic review of 62 descriptive studies showed that interruptions and disruptions largely affected facilities up to 77.5%, the supply chain up to 79% and personnel availability up to 60%.173 Despite repeated reassurances from officials that the UK's NHS remained open for urgent care, it was estimated that 45% of those with potential cancer symptoms did not contact their doctor during the UK's first wave of the pandemic from March to August 2020. The main reasons cited were fear of contracting COVID-19 and avoiding placing extra strain on the NHS. Consequently, suspected cancer referrals fell by 350 000 compared with the same period in 2019.174 By April 2021, the UK NHS had >4.6 million cases on the waiting list for surgery and >300 000 have a waiting time of >12 months, a considerable proportion of which are for cancer.174
There have been disruptions to interventional procedures, palliative care services154 and therapeutic surgeries,158,164,175 with decreases in admissions for chemotherapy.137 One study found the delay occurred among newly diagnosed patients with breast, prostate and non-metastatic cancers.162 Some of the reasons for delayed and disrupted care included an overwhelmed health system, lack of personal protective equipment, staff shortages, restricted access to medications155 and barriers to consulting a general practitioner.153 A 20% increase of cancer deaths has been predicted in England due to COVID-19 because of delays in patients seeking care, getting referred or receiving their chemotherapy.137
During the COVID-19 pandemic, adverse psychological effects have been noted among cancer patients. A high prevalence of mental health problems among cancer patients has been observed.160 Patients with breast cancer were reported to have high rates of anxiety, depression, distress and insomnia.156 COVID-19-related anxiety among breast cancer patients may affect their decision-making process, such as attending diagnostic and therapeutic procedures.159 The aforementioned Israeli national survey including >400 haemato-oncology patients recorded high rates of depression, and it was more evident among patients with chronic myeloid leukaemia.172 A prospective study that included 77 lymphoma patients from Italy reported anxiety in 36% of patients, depression in 31% when the Hospital and Anxiety Depression Scale was administered and post-traumatic stress disorder (PTSD) among 36%.176 Some may develop issues with a range of addictions.177
Long-term effects of COVID-19 on cancer
Cancer patients are at an increased risk of developing post-COVID complications,178 mainly affecting their heart, lung, kidney, skin and brain.179 At least 15% of patients with cancer who have recovered from COVID-19 experience at least one post-infection sequelae,180 such as respiratory symptoms, fatigue, neurocognitive dysfunction and weight loss.181 Those at higher risk of long-term sequalae include men, those ≥65 y of age, having two or more comorbidities and a history of smoking.178 One study found that if cancer patients with COVID-19 survive the immediate sequelae, their long-term sequalae and mortality are similar to those of cancer patients without COVID-19.57
Interestingly, due to mounting evidence that SARS-CoV-2 is able to modulate certain oncogenic pathways, promote chronic low-grade inflammation and cause tissue damage, it has been hypothesised that long COVID-19 may predispose recovered patients to cancer development and accelerate cancer progression.182 Comprehensive studies are urgently required to elucidate the effects of long COVID-19 on cancer susceptibility.
Conclusions
COVID-19 has significant adverse clinical, laboratory, socio-economic and psychological effects among patients with malignancies. This is more prominent in those with haematologic malignancies compared with solid malignancies and the magnitude of the risk has implications for clinical decision making. Those with lung cancer have the highest mortality among solid cancers from COVID-19. COVID-19 and cancer intersect at a molecular level, which may have therapeutic implications in cancer patients with COVID-19. Certain oncogenic molecular pathways commonly involved by cancer are also affected by COVID-19. Autoantibodies against IFN-I have been found in patients with life-threatening COVID-19.183 Comprehensive studies are required to elucidate long-term sequelae of COVID-19 on cancer survivors. The COVID-19 pandemic has caused major disruptions to clinical services for cancer patients worldwide. Delayed presentations and diagnosis due to strained health systems and patients’ reluctance to seek medical care during the pandemic have caused a considerable impact on cancer survival. In order to mitigate the catastrophic effects of the pandemic on cancer care, a thorough and collaborative effort is required at the national and international level. Findings from various studies have important policy implications, including, but not limited to, the need for increased surveillance and testing for SARS-CoV-2, minimising healthcare system exposure and reconsideration of procedures and treatments in patients with cancer in order to minimise the morbidity and mortality caused by COVID-19. Vaccination is an important preventive measure for patients with solid and haematologic malignancies against getting severe COVID-19.
Acknowledgements
None.
Contributor Information
Suranjith L Seneviratne, Institute of Immunity and Transplantation, Royal Free Hospital and University College London, UK; Nawaloka Hospital Research and Education Foundation, Nawaloka Hospitals, Colombo, Sri Lanka.
Widuranga Wijerathne, Faculty of Medicine, University of Colombo, Sri Lanka.
Pamodh Yasawardene, Faculty of Medicine, University of Colombo, Sri Lanka.
Buddhika Somawardana, National Cancer Institute, Maharagama, Sri Lanka.
Authors’ contributions
SLS, WW, PY and BS designed the study. WW and PY conducted the search. WW, PY, SLS and BS assessed the article titles, abstracts and full texts. SLS, WW, PY, and BS wrote the first version of the manuscript and SLS and BS performed the final editing. All the authors reviewed and approved the final version. All authors had full access to the data in the study and final responsibility for the decision to submit for publication.
Funding
None.
Competing interests
None declared.
Ethical approval
Not required.
Data availability
The data underlying this article are available in the article.
References
- 1. World Health Organization . Coronavirus disease (COVID-2019) situation reports. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. [Google Scholar]
- 2. Dai M, Liu D, Liu M et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grivas P, Khaki AR, Wise-Draper TM et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32(6):787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pinato DJ, Zambelli A, Aguilar-Company J et al. Clinical portrait of the SARS-CoV-2 epidemic in European patients with cancer. Cancer Discov. 2020;10(10):1465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tian J, Yuan X, Xiao J et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seneviratne S, Yasawardene P, Hettiarachchi D et al. The Delta variant of SARS-CoV-2: the current global scourge. Sri Lankan Fam Physician. 2021;36(1):17–25. [Google Scholar]
- 7. Bernard A, Cottenet J, Bonniaud P et al. Comparison of cancer patients to non-cancer patients among COVID-19 inpatients at a national level. Cancers. 2021;13(6):1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kunimasa K, Goto T. Immunosurveillance and immunoediting of lung cancer: current perspectives and challenges. Int J Mol Sci. 2020;21(2):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y. T-cell immune suppression in patients with hematologic malignancies: clinical implications. Int J Hematol Oncol. 2014;3(4):289–97. [Google Scholar]
- 11. Dhodapkar MV, Dhodapkar KM. Immune modulation in hematologic malignancies. Semin Oncol. 2015;42(4):617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Astuti I, Ysrafil. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr. 2020;14(4):407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seneviratne S, Jayarajah U, Abeysuriya V et al. COVID-19 vaccine landscape. J Ceylon Coll Physicians. 2020;51(2):120–31. [Google Scholar]
- 14. Lam M-F, Ooi GC, Lam B et al. An indolent case of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2004;169(1):125–8. [DOI] [PubMed] [Google Scholar]
- 15. Kim S-H, Ko J-H, Park GE et al. Atypical presentations of MERS-CoV infection in immunocompromised hosts. J Infect Chemother. 2017;23(11):769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alaskar A, Bosaeed M, Rehan H et al. Middle East respiratory syndrome (MERS) infection in hematological and oncological patients: a case series from a tertiary care center in Saudi Arabia. Blood. 2019;134(Suppl 1):5871. [Google Scholar]
- 17. Motabi IH, Zaidi SZA, Ibrahim MH et al. Report of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in four patients with hematological malignancies treated at King Fahad Medical City, Riyadh, Saudi Arabia. Blood. 2016;128(22):4903. [Google Scholar]
- 18. Jazieh A-R, Alenazi TH, Alhejazi A et al. Outcome of oncology patients infected with coronavirus. JCO Glob Oncol. 2020(6):471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newman LA, Winn RA, Carethers JM. Similarities in risk for COVID-19 and cancer disparities. Clin Cancer Res. 2021;27(1):24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zong Z, Wei Y, Ren J et al. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer. 2021;20(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Melo AC, Thuler LC, Da Silva JL et al. Cancer inpatients with COVID-19: a report from the Brazilian National Cancer Institute. PLoS One. 2020;15(10):e0241261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erdal GS, Polat O, Erdem GU et al. The mortality rate of COVID-19 was high in cancer patients: a retrospective single-center study. Int J Clin Oncol. 2021;26(5):826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller LE, Bhattacharyya R, Miller AL. Data regarding country-specific variability in Covid-19 prevalence, incidence, and case fatality rate. Data Brief. 2020;32:106276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Shan K, Qian W. Asians do not exhibit elevated expression or unique genetic polymorphisms for ACE2, the cell-entry receptor of SARS-CoV-2. PrePrints 2020;doi: 10.20944/preprints202002.0258.v2. [Google Scholar]
- 25. Barbry P, Muus C, Luecken M et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv 2020; 10.1101/2020.04.19.049254. [DOI] [Google Scholar]
- 26. Bakouny Z, Hawley JE, Choueiri TK et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38(5):629–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith JC, Sausville EL, Girish V et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev. 2020;53(5):514–29.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siegel RL, Miller KD, Goding Sauer A et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–64. [DOI] [PubMed] [Google Scholar]
- 29. Kuderer NM, Choueiri TK, Shah DP et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertram S, Dijkman R, Habjan M et al. TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium. J Virol. 2013;87(11):6150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ziegler CGK, Allon SJ, Nyquist SK et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–35.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu Z-s, Zhang Z-q, Wu S. Focus on the crosstalk between COVID-19 and urogenital systems. J Urol. 2020;204:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stopsack KH, Mucci LA, Antonarakis ES et al. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov. 2020;10(6):779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montopoli M, Zumerle S, Vettor R et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020;31(8):1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel VG, Zhong X, Liaw B et al. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann Oncol. 2020;31(10):1419–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2021;72(2):340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mangalmurti N, Hunter CA. Cytokine storms: understanding COVID-19. Immunity. 2020;53(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lucas C, Wong P, Klein J et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qin C, Zhou L, Hu Z et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robilotti EV, Babady NE, Mead PA et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumari N, Dwarakanath BS, Das A et al. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016;37(9):11553–72. [DOI] [PubMed] [Google Scholar]
- 42. Blanco-Melo D, Nilsson-Payant BE, Liu W-C et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–45.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. [DOI] [PubMed] [Google Scholar]
- 44. Hadjadj J, Yatim N, Barnabei L et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou Z, Ren L, Zhang L et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–90.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wilk AJ, Rustagi A, Zhao NQ et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26(7):1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marcucci F, Rumio C, Corti A. Tumor cell-associated immune checkpoint molecules – drivers of malignancy and stemness. Biochim Biophys Acta Rev Cancer. 2017;1868(2):571–83. [DOI] [PubMed] [Google Scholar]
- 48. Thibaudin M, Fumet J-D, Bon M et al. Immunological features of coronavirus disease 2019 in patients with cancer. Eur J Cancer. 2020;139:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hou H, Zhang B, Huang H et al. Using IL-2R/lymphocytes for predicting the clinical progression of patients with COVID-19. Clin Exp Immunol. 2020;201(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang W, Berube J, McNamara M et al. Lymphocyte subset counts in COVID-19 patients: a meta-analysis. Cytometry A. 2020;97(8):772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Diao B, Wang C, Tan Y et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jayasekara D, Seneviratne SL, Jayasekara A et al. Atypical presentations of COVID-19. Adv Infect Dis. 2020;10(3):136–42. [Google Scholar]
- 54. Rahman A, Niloofa R, Jayarajah U et al. Hematological abnormalities in COVID-19: a narrative review. Am J Trop Med Hyg. 2021;104(4):1188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Varga Z, Flammer AJ, Steiger P et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11(2):223–33. [DOI] [PubMed] [Google Scholar]
- 57. Chai C, Feng X, Lu M et al. One-year mortality and consequences of COVID-19 in cancer patients: a cohort study. IUBMB Life. 2021;73(10):1244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Costa GJ, de Azevedo CRAS, Júnior JIC et al. Higher severity and risk of in-hospital mortality for COVID-19 patients with cancer during the year 2020 in Brazil: A countrywide analysis of secondary data. Cancer. 2021;127(22):4240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Alpert N, Rapp JL, Marcellino B et al. Clinical course of cancer patients with COVID-19: a retrospective cohort study. JNCI Cancer Spectr. 2020;5(1):Pkaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Calles A, Aparicio MI, Alva M et al. Outcomes of COVID-19 in patients with lung cancer treated in a tertiary hospital in Madrid. Front Oncol. 2020;10:1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cattaneo C, Daffini R, Pagani C et al. Clinical characteristics and risk factors for mortality in hematologic patients affected by COVID-19. Cancer. 2020;126(23):5069–76. [DOI] [PubMed] [Google Scholar]
- 62. Crolley VE, Hanna D, Joharatnam-Hogan N et al. COVID-19 in cancer patients on systemic anti-cancer therapies: outcomes from the CAPITOL (COVID-19 Cancer PatIenT Outcomes in North London) cohort study. Ther Adv Med Oncol. 2020;12:1758835920971147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dai M, Liu D, Liu M et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Azambuja E, Brandão M, Wildiers H et al. Impact of solid cancer on in-hospital mortality overall and among different subgroups of patients with COVID-19: a nationwide, population-based analysis. ESMO Open. 2020;5(5):e000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Elkrief A, Desilets A, Papneja N et al. High mortality among hospital-acquired COVID-19 infection in patients with cancer: a multicentre observational cohort study. Eur J Cancer. 2020;139:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fillmore NR, La J, Szalat RE et al. Prevalence and outcome of COVID-19 infection in cancer patients: a national Veterans Affairs study. J Natl Cancer Inst. 2020;113(6):691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ganatra S, Dani SS, Redd R et al. Outcomes of COVID-19 in patients with a history of cancer and comorbid cardiovascular disease. J Natl Compr Canc Netw. 2020;doi: 10.6004/jnccn.2020.7658. [DOI] [PubMed] [Google Scholar]
- 68. Jee J, Foote MB, Lumish M et al. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol. 2020;38(30):3538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kabarriti R, Brodin NP, Maron MI et al. Extent of prior lung irradiation and mortality in COVID-19 patients with a cancer history. Adv Radiat Oncol. 2020;5(4):707–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lara Álvarez MÁ, Rogado Revuelta J, Obispo Portero B et al. COVID-19 mortality in cancer patients in a Madrid hospital during the first 3 weeks of the epidemic. Med Clin (Barc). 2020;155(5):202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lara OD, O'Cearbhaill RE, Smith MJ et al. COVID-19 outcomes of patients with gynecologic cancer in New York City. Cancer. 2020;126(19):4294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mehta V, Goel S, Kabarriti R et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Meng Y, Lu W, Guo E et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J Hematol Oncol. 2020;13(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pinato DJ, Lee AJX, Biello F et al. Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers. 2020;12(7):1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ramachandran P, Kathirvelu B, Chakraborti A et al. COVID-19 in cancer patients from New York City: a comparative single center retrospective analysis. Cancer Control. 2020;27(1):1073274820960457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rogado J, Obispo B, Pangua C et al. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. 2020;22(12):2364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rogado J, Pangua C, Serrano-Montero G et al. Covid-19 and lung cancer: a greater fatality rate? Lung Cancer. 2020;146:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Russell B, Moss C, Papa S et al. Factors affecting COVID-19 outcomes in cancer patients: a first report from Guy's Cancer Center in London. Front Oncol. 2020;10:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sorouri M, Kasaeian A, Mojtabavi H et al. Clinical characteristics, outcomes, and risk factors for mortality in hospitalized patients with COVID-19 and cancer history: a propensity score-matched study. Infect Agents Cancer. 2020;15(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang J, Song Q, Chen Y et al. Systematic investigations of COVID-19 in 283 cancer patients. medRxiv. 2020; 10.1101/2020.04.28.20083246. [DOI] [Google Scholar]
- 81. Yang F, Shi S, Zhu J et al. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020;92(10):2067–73. [DOI] [PubMed] [Google Scholar]
- 82. Yang K, Sheng Y, Huang C et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang B, Xie R, Hubert SM et al. Characteristics and outcomes of 35 breast cancer patients infected with COVID-19. Front Oncol. 2020;10:570130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang L, Zhu F, Xie L et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhou X, Wang G, Chen L et al. Clinical characteristics of hematological patients concomitant with COVID-19. Cancer Sci. 2020;111(9):3379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bange EM, Han NA, Wileyto P et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27(7):1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cai G, Gao Y, Zeng S et al. Immunological alternation in COVID-19 patients with cancer and its implications on mortality. Oncoimmunology. 2021;10(1):1854424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fernandes GA, Feriani D, ILA França e Silva et al. Differences in mortality of cancer patients with COVID-19 in a Brazilian cancer center. Semin Oncol. 2021;48(2):171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ferrari BL, Ferreira CG, Menezes M et al. Determinants of COVID-19 mortality in patients with cancer from a community oncology practice in Brazil. JCO Glob Oncol. 2021;7:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Höllein A, Bojko P, Schulz S et al. Characteristics and outcomes of patients with cancer and COVID-19: results from a cohort study. Acta Oncol. 2021;60(1):24–7. [DOI] [PubMed] [Google Scholar]
- 91. Khusid JA, Becerra AZ, Gallante B et al. Cancer, mortality, and acute kidney injury among hospitalized patients with SARS-CoV-2 infection. Asian Pac J Cancer Prev. 2021;22(2):517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Larfors G, Pahnke S, State M et al. Covid-19 intensive care admissions and mortality among Swedish patients with cancer. Acta Oncol. 2021;60(1):32–4. [DOI] [PubMed] [Google Scholar]
- 93. Lee AJX, Purshouse K. COVID-19 and cancer registries: learning from the first peak of the SARS-CoV-2 pandemic. Br J Cancer. 2021;124(11):1777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lee KA, Ma W, Sikavi DR et al. Cancer and risk of COVID-19 through a general community survey. Oncologist. 2021;26(1):e182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liang J, Jin G, Liu T et al. Clinical characteristics and risk factors for mortality in cancer patients with COVID-19. Front Med. 2021;15(2):264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lunski MJ, Burton J, Tawagi K et al. Multivariate mortality analyses in COVID-19: comparing patients with cancer and patients without cancer in Louisiana. Cancer. 2021;127(2):266–74. [DOI] [PubMed] [Google Scholar]
- 97. Mangone L, Gioia F, Mancuso P et al. Cumulative COVID-19 incidence, mortality and prognosis in cancer survivors: a population-based study in Reggio Emilia, Northern Italy. Int J Cancer. 2021;149(4):820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mohamed IH, Chowdary PB, Shetty S et al. Outcomes of renal transplant recipients with SARS-CoV-2 infection in the eye of the storm: a comparative study with waitlisted patients. Transplantation. 2021;105(1):115–20. [DOI] [PubMed] [Google Scholar]
- 99. Mushtaq MU, Shahzad M, Chaudhary SG et al. Impact of SARS-CoV-2 in hematopoietic stem cell transplantation and chimeric antigen receptor T cell therapy recipients. Transplant Cell Ther. 2021;27(9):796.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nakamura S, Kanemasa Y, Atsuta Y et al. Characteristics and outcomes of coronavirus disease 2019 (COVID-19) patients with cancer: a single-center retrospective observational study in Tokyo, Japan. Int J Clin Oncol. 2021;26(3):485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nie L, Dai K, Wu J et al. Clinical characteristics and risk factors for in-hospital mortality of lung cancer patients with COVID-19: a multicenter, retrospective, cohort study. Thorac Cancer. 2021;12(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rogiers A, Pires da Silva I, Tentori C et al. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J Immunother Cancer. 2021;9(1):e001931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rüthrich MM, Giessen-Jung C, Borgmann S et al. COVID-19 in cancer patients: clinical characteristics and outcome—an analysis of the LEOSS registry. Ann Hematol. 2021;100(2):383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Safari M, Faradmal J, Bashirian S et al. Identifying the risk factors for mortality in patients with cancer and COVID-19 in Hamadan, the West of Iran. J Gastrointest Cancer. 2021;Aug 4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sharma A, Bhatt NS, St Martin A et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sun L, Surya S, Le AN et al. Rates of COVID-19–Related Outcomes in Cancer Compared With Noncancer Patients. JNCI Cancer Spectr. 2021;5(1):pkaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Trapani S, Masiero L, Puoti F et al. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: a nationwide population-based study. Am J Transplant. 2021;21(7):2509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Williamson EJ, Walker AJ, Bhaskaran K et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Passamonti F, Cattaneo C, Arcaini L et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vijenthira A, Gong IY, Fox TA et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bhogal T, Khan UT, Lee R et al. Haematological malignancy and nosocomial transmission are associated with an increased risk of death from COVID-19: results of a multi-center UK cohort. Leuk Lymphoma. 2021;62(7):1682–91. [DOI] [PubMed] [Google Scholar]
- 113. He W, Chen L, Chen L et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Huang Q, Hu S, Ran F et al. Asymptomatic COVID-19 infection in patients with cancer at a cancer-specialized hospital in Wuhan, China – preliminary results. Eur Rev Med Pharmacol Sci. 2020;24(18):9760–4. [DOI] [PubMed] [Google Scholar]
- 115. Liu T, Zeng G, Tao H et al. Low prevalence of IgG antibodies to SARS-CoV-2 in cancer patients with COVID-19. Int J Cancer. 2020;147(11):3267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Solodky ML, Galvez C, Russias B et al. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol. 2020;31(8):1087–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Abdul-Jawad S, Baù L, Alaguthurai T et al. Acute immune signatures and their legacies in severe acute respiratory syndrome coronavirus-2 infected cancer patients. Cancer Cell. 2021;39(2):257–75.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Au L, Fendler A, Shepherd STC et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med. 2021;27(8):1362–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Esperança-Martins M, Gonçalves L, Soares-Pinho I et al. Humoral immune response of SARS-CoV-2–infected patients with cancer: influencing factors and mechanisms. Oncologist. 2021;26(9):e1619–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hempel L, Molnar J, Robert S et al. Rare SARS-CoV-2 antibody development in cancer patients. Semin Oncol. 2021;48(2):160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Huang A, Bange E, Han N et al. CD8 T cells compensate for impaired humoral immunity in COVID-19 patients with hematologic cancer. Res Sq. 2021;doi: 10.21203/rs.3.rs-162289/v1. [Google Scholar]
- 122. Marra A, Generali D, Zagami P et al. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Ann Oncol. 2021;32(1):113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Paschold L, Simnica D, Willscher E et al. SARS-CoV-2–specific antibody rearrangements in prepandemic immune repertoires of risk cohorts and patients with COVID-19. J Clin Invest. 2021;131(1):e142966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Thakkar A, Pradhan K, Jindal S et al. Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer. 2021;2(4):392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Truong TT, Ryutov A, Pandey U et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. medRxiv. 2021;doi: 10.1101/2021.02.27.21252099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hensley MK, Bain WG, Jacobs J et al. Intractable coronavirus disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-cell therapy recipient: a case study. Clin Infect Dis. 2021;73(3):e815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Baang JH, Smith C, Mirabelli C et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis. 2020;223(1):23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Choi B, Choudhary MC, Regan J et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383(23):2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Clark SA, Clark LE, Pan J et al. SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms. Cell. 2021;184(10):2605–17.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kemp SA, Collier DA, Datir RP et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592(7853):277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Khatamzas E, Rehn A, Muenchhoff M et al. Emergence of multiple SARS-CoV-2 mutations in an immunocompromised host. medRxiv. 2021; 10.1101/2021.01.10.20248871. [DOI] [Google Scholar]
- 132. Strengert M, Becker M, Ramos GM et al. Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA vaccine in patients on hemodialysis. EBioMedicine. 2021;70:103524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Avanzato VA, Matson MJ, Seifert SN et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183(7):1901–12.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Nakajima Y, Ogai A, Furukawa K et al. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother. 2021;27(2):387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tarhini H, Recoing A, Bridier-Nahmias A et al. Long-term severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis. 2021;223(9):1522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yip C-H, Newman LA. American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology guideline for management of hereditary breast cancer. JAMA Surg. 2021;156(3):284–5. [DOI] [PubMed] [Google Scholar]
- 137. Lai AG, Pasea L, Banerjee A et al. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. medRxiv. 2020; 10.1101/2020.05.27.20083287. [DOI] [Google Scholar]
- 138. American Society of Hematology . COVID-19 and Hodgkin lymphoma: frequently asked questions. Available from: https://www.hematology.org:443/covid-19/covid-19-and-hodgkin-lymphoma [accessed 27 September 2021]. [Google Scholar]
- 139. Reuss JE, Suresh K, Naidoo J. Checkpoint inhibitor pneumonitis: mechanisms, characteristics, management strategies, and beyond. Curr Oncol Rep. 2020;22(6):56. [DOI] [PubMed] [Google Scholar]
- 140. National Institutes of Health . Special populations. Cancer. Special considerations in adults and children with cancer. Available from: https://www.covid19treatmentguidelines.nih.gov/special-populations/cancer/ [accessed 24 February 2022]. [Google Scholar]
- 141. Niemann CU, Herman SEM, Maric I et al. Disruption of in vivo chronic lymphocytic leukemia tumor–microenvironment interactions by ibrutinib – findings from an investigator-initiated phase II study. Clin Cancer Res. 2016;22(7):1572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Treon SP, Castillo JJ, Skarbnik AP et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19–infected patients. Blood. 2020;135(21):1912–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Thibaud S, Tremblay D, Bhalla S. Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID-19. Br J Haematol. 2020;190(2):e73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. National Comprehensive Cancer Network . NCCN hematopoietic growth factors: short-term recommendations specific to issues with COVID-19 (SARS-CoV-2). Available from: https://www.iononline.com/-/media/assets/ion/pdf/covid19-resources/nccn_hgf_covid-19_19may20.pdf?la=en&hash=9FB98741E9E83D354F8FEFF66AC5F5188E139471 [accessed 24 February 2022]. [Google Scholar]
- 145. Nawar T, Morjaria S, Kaltsas A et al. Granulocyte-colony stimulating factor in COVID-19: is it stimulating more than just the bone marrow? Am J Hematol. 2020;95(8):e210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Becker PS, Griffiths EA, Alwan LM et al. NCCN Guidelines Insights: Hematopoietic Growth Factors, Version 1.2020. J Natl Compr Canc Netw. 2020;18(1):12–22. [DOI] [PubMed] [Google Scholar]
- 147. Shimabukuro-Vornhagen A, Gödel P, Subklewe M et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yahalom J, Dabaja BS, Ricardi U et al. ILROG emergency guidelines for radiation therapy of hematological malignancies during the COVID-19 pandemic. Blood. 2020;135(21):1829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. American Society of Hematology . COVID-19 resources. Available from: https://www.hematology.org/covid-19 [accessed 24 February 2022]. [Google Scholar]
- 151. Seneviratne SL, Abeysuriya V, De Mel S et al. Favipiravir in Covid-19. Int J Prog Sci Technol. 2020;19(2):143–5. [Google Scholar]
- 152. Seneviratne SL, Niloofa R, De Zoysa I et al. Remdesivir and Covid-19. Int J Adv Res. 2020;8(4):565–7. [Google Scholar]
- 153. Dinmohamed AG, Visser O, Verhoeven RH et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Guven DC, Aktas BY, Aksun MS et al. COVID-19 pandemic: changes in cancer admissions. BMJ Support Palliat Care. 2020;doi: 10.1136/bmjspcare-2020-002468. [DOI] [PubMed] [Google Scholar]
- 155. Jazieh AR, Akbulut H, Curigliano G et al. Impact of the COVID-19 pandemic on cancer care: a global collaborative study. JCO Glob Oncol. 2020; Sep 6:1428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Juanjuan L, Santa-Maria CA, Hongfang F et al. Patient-reported outcomes of patients with breast cancer during the COVID-19 outbreak in the epicenter of China: a cross-sectional survey study. Clin Breast Cancer. 2020;20(5):e651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Maringe C, Spicer J, Morris M et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Patt D, Gordan L, Diaz M et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Vanni G, Materazzo M, Pellicciaro M et al. Breast cancer and COVID-19: the effect of fear on patients' decision-making process. In Vivo. 2020;34(3 Suppl):1651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wang Y, Duan Z, Ma Z et al. Epidemiology of mental health problems among patients with cancer during COVID-19 pandemic. Transl Psychiatry. 2020;10(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zadnik V, Mihor A, Tomsic S et al. Impact of COVID-19 on cancer diagnosis and management in Slovenia – preliminary results. Radiol Oncol. 2020;54(3):329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Blay JY, Boucher S, Le Vu B et al. Delayed care for patients with newly diagnosed cancer due to COVID-19 and estimated impact on cancer mortality in France. ESMO Open. 2021;6(3):100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Gathani T, Clayton G, MacInnes E et al. The COVID-19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020. Br J Cancer. 2021;124(4):710–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Morris EJA, Goldacre R, Spata E et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021;6(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rutter MD, Brookes M, Lee TJ et al. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis. Gut. 2021;70(3):537–43. [DOI] [PubMed] [Google Scholar]
- 166. Sozutek A, Seker A, Kuvvetli A et al. Evaluating the feasibility of performing elective gastrointestinal cancer surgery during the COVID-19 pandemic: an observational study with 60 days follow-up results of a tertiary referral pandemic hospital. J Surg Oncol. 2021;123(4):834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Van Haren RM, Delman AM, Turner KM et al. Impact of the COVID-19 pandemic on lung cancer screening program and subsequent lung cancer. J Am Coll Surg. 2021;232(4):600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Centers for Disease Control and Prevention . Managing healthcare operations during COVID-19. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/facility-planning-operations.html [accessed 24 February 2022] . [Google Scholar]
- 169. Al-Shamsi HO, Alhazzani W, Alhuraiji A et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25(6):e936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Marcum M, Kurtzweil N, Vollmer C et al. COVID-19 pandemic and impact on cancer clinical trials: an academic medical center perspective. Cancer Med. 2020;9(17):6141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wise J. Covid-19: cancer mortality could rise at least 20% because of pandemic, study finds. BMJ. 2020;369:m1735. [DOI] [PubMed] [Google Scholar]
- 172. Levy I, Sharf G, Norman S et al. The impact of COVID-19 on patients with hematological malignancies: the mixed-method analysis of an Israeli national survey. Support Care Cancer. 2021;29(12):7591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Riera R, Bagattini ÂM, Pacheco RL et al. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. JCO Glob Oncol. 2021;7:311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. COVID-19 and cancer: 1 year on. Lancet Oncol. 2021;22(4):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kariyawasam JC, Jayarajah U, Riza R et al. Gastrointestinal manifestations in COVID-19. Trans R Soc Trop Med Hyg. 2021;115(12):1362–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Romito F, Dellino M, Loseto G et al. Psychological distress in outpatients with lymphoma during the COVID-19 pandemic. Front Oncol. 2020;10;1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Jemberie WB, Stewart Williams J, Eriksson M et al. Substance use disorders and COVID-19: multi-faceted problems which require multi-pronged solutions. Front Psychiatry. 2020;11:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Pace Hospitals. Post COVID complications, long term effects of coronavirus after recovery 2021. Available from: https://www.pacehospital.com/post-covid-complications-long-term-effects-of-coronavirus-after-recovery [accessed 24 February 2022] [Google Scholar]
- 179. Healio . Long-term effects of COVID-19 and cancer create population of ‘dual survivors’. Available from: https://www.healio.com/news/hematology-oncology/20210816/longterm-effects-of-covid19-and-cancer-create-population-of-dual-survivors [accessed 24 February 2022]. [Google Scholar]
- 180. Healio . Long-term COVID-19 effects impact 15% of patients with cancer, may impair outcomes. Available from: https://www.healio.com/news/hematology-oncology/20210909/longterm-covid19-effects-impact-15-of-patients-with-cancer-may-impair-outcomes [accessed 24 February 2022]. [Google Scholar]
- 181. Rahman A, Niloofa R, De Zoysa IM et al. Neurological manifestations in COVID-19: a narrative review. SAGE Open Med. 2020;8:2050312120957925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Saini G, Aneja R. Cancer as a prospective sequela of long COVID-19. BioEssays. 2021;43(6):2000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Bastard P, Rosen LB, Zhang Q et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.