Abstract
Background
Few therapies are approved for hospitalized patients with severe coronavirus disease 2019 (COVID-19). Ibrutinib, a once-daily Bruton tyrosine kinase inhibitor, may mitigate COVID-19–induced lung damage by reducing inflammatory cytokines. The multicenter, randomized, double-blind phase 2 iNSPIRE study evaluated ibrutinib for prevention of respiratory failure in hospitalized patients with severe COVID-19.
Methods
Adult patients with severe COVID-19 requiring hospitalization and supplemental oxygen but without respiratory failure were randomized 1:1 (stratified by remdesivir prescription) to ibrutinib 420 mg or placebo once daily for up to 28 days plus standard of care (SOC), including remdesivir and/or dexamethasone.
Results
Forty-six patients were randomized to ibrutinib plus SOC (n = 22) or placebo plus SOC (n = 24). The primary endpoint (proportion of patients alive and without respiratory failure through day 28) was not met, with no statistically significant difference adjusting for remdesivir prescription (86% with ibrutinib plus SOC vs 79% with placebo plus SOC; adjusted difference, 5.8% [80% confidence interval, –9.2% to 20.4%]; P = .599). Secondary endpoints also showed no statistically significant improvement with ibrutinib plus SOC. Median treatment duration was 14 days for ibrutinib and placebo. Adverse events were similar with ibrutinib plus SOC vs placebo plus SOC (overall: 55% vs 50%; serious: 18% vs 13%) and were consistent with the known safety profile of ibrutinib.
Conclusions
Addition of ibrutinib to SOC did not improve the proportion of patients alive and without respiratory failure through day 28 in hospitalized patients with severe COVID-19. Ibrutinib had a manageable safety profile, with similar safety to placebo.
Clinical Trials Registration
Keywords: COVID-19, ibrutinib, lung injury, respiratory failure, SARS-CoV-2
Ibrutinib had a manageable safety profile but did not improve the proportion of patients alive and without respiratory failure through day 28 vs placebo in hospitalized patients with severe COVID-19 also receiving supportive standard of care, including remdesivir and/or dexamethasone.
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Severe COVID-19 occurs in approximately 20% of cases, with respiratory failure being the leading cause of death [1, 2]. The etiology of pulmonary injury and respiratory failure associated with COVID-19 involves an exaggerated cytokine response resembling macrophage activation syndrome [3]. Elevated inflammatory cytokines have been reported in hospitalized patients with COVID-19, with even higher levels reported in patients requiring intensive care [1, 4].
Ibrutinib is a once-daily Bruton tyrosine kinase inhibitor approved in the United States for various B-cell malignancies and for chronic graft-vs-host disease, but uncertainty remains regarding the benefit of continued ibrutinib treatment in patients who develop COVID-19. Ibrutinib decreased inflammatory cytokines and prevented lung injury and death in a mouse influenza model [5]. In the phase 3 iLLUMINATE study, ibrutinib suppressed obinutuzumab-induced increases in multiple inflammatory cytokines associated with infusion-related reactions [6]. In a case series of 6 patients with COVID-19 receiving ibrutinib for Waldenström macroglobulinemia, no dyspnea or hypoxia was observed in 5 patients on full-dose ibrutinib (420 mg/day), whereas 1 patient on reduced-dose ibrutinib (140 mg/day) experienced progressive dyspnea and hypoxia requiring hospitalization that improved rapidly after increasing the ibrutinib dose to 420 mg/day [7]. These findings suggest that ibrutinib may mitigate the hyperinflammatory state associated with COVID-19–induced lung damage. We conducted the phase 2 iNSPIRE study to evaluate the efficacy and safety of ibrutinib for prevention of respiratory failure in hospitalized patients with severe COVID-19.
METHODS
Study Design and Patients
iNSPIRE (ClinicalTrials.gov: NCT04375397) was a multicenter, randomized, double-blind, placebo-controlled phase 2 study conducted at 7 hospitals in the United States (Supplementary Table 1). The study was conducted between 6 June 2020 and 8 June 2021, with the majority of patients (65%) enrolled and treated before authorization of any COVID-19 vaccines in the United States.
Eligible patients were aged ≥18 years with confirmed SARS-CoV-2 by reverse-transcription polymerase chain reaction who required hospitalization and supplemental oxygen (oxygen saturation ≤94% on breathing room air), had been on supplemental oxygen for ≤5 days, and had radiographic evidence of pulmonary infiltrates. Patients with respiratory failure (defined as clinical diagnosis of respiratory failure requiring treatment with 1 of the following: endotracheal intubation and mechanical ventilation, extracorporeal membrane oxygenation, high-flow nasal cannula oxygen delivery, or noninvasive positive pressure ventilation) were excluded.
Patients were randomized 1:1 (stratified by remdesivir prescription) to receive oral ibrutinib 420 mg once daily or placebo plus standard of care (SOC); for patients unable to take oral medications, capsule contents could be mixed with water and administered via feeding tube (Supplementary Methods). Treatment continued for up to 28 days in the absence of unacceptable toxicity or intercurrent illness. Treatment could be stopped after 14 days if the patient was clinically stable and off supplemental oxygen for >48 hours at the discretion of the treating physician. Patients were followed for 58 days after start of therapy or until death, whichever occurred first.
Outcomes
The primary endpoint was the proportion of patients alive and without respiratory failure (as defined above) through study day 28. Secondary endpoints included change in World Health Organization (WHO) 8-point ordinal scale [8] (Supplementary Table 2) from baseline to day 14; duration of supplemental oxygen; all-cause mortality rate by days 7, 14, 21, and 28; respiratory failure or death rate by days 7, 14, 21, and 28; mechanical ventilation–free survival; duration of mechanical ventilation; duration of hospitalization; time to discharge; and safety and tolerability.
Statistical Analysis
A sample size of 46 patients would provide ≥80% power to detect a between-group response rate difference of 30% for the primary endpoint at a 2-sided α = .2. For binary endpoints, between-group differences in proportions of patients were analyzed using the Miettinen-Nurminen method adjusting for the stratification factor of remdesivir prescription. For time-to-event endpoints, distribution of time to the specified event was estimated using the Kaplan-Meier method. Efficacy and safety were assessed in all randomized patients who received ≥1 dose of study treatment.
Patient Consent Statement
The study was conducted in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice and principles of the Declaration of Helsinki. The protocol was approved by institutional review boards or independent ethics committees of all participating institutions. All patients provided written informed consent.
RESULTS
Patients
Forty-six patients were enrolled and received ibrutinib plus SOC (n = 22) or placebo plus SOC (n = 24). Study treatment was discontinued before day 28 in 18 (82%) patients in the ibrutinib plus SOC arm and 18 (75%) in the placebo plus SOC arm; most patients who discontinued treatment did so due to being clinically stable and off supplemental oxygen for >48 hours on day 14 (7 [32%] and 12 [50%], respectively), followed by adverse events (AEs; 6 [27%] and 4 [17%], respectively). Median duration of treatment was 14 days for both ibrutinib plus SOC (range, 5–33 days) and placebo plus SOC (range, 2–40 days). Overall, 13 (59%) patients in the ibrutinib plus SOC arm and 18 (75%) in the placebo plus SOC arm completed the study.
Baseline characteristics were generally balanced between arms (Table 1). In the ibrutinib plus SOC and placebo plus SOC arms, 11 (50%) patients and 13 (54%) patients, respectively, were Hispanic/Latino, and 4 (18%) and 4 (17%) were Black/African American. There were no major differences between the ibrutinib plus SOC and placebo plus SOC arms in the prevalence of comorbid conditions associated with risk for severe COVID-19 (Table 1; Supplementary Methods). Thirteen (59%) patients in the ibrutinib plus SOC arm and 16 (67%) in the placebo plus SOC arm had a prescription for remdesivir at randomization. During the study period, 15 (68%) patients in the ibrutinib plus SOC arm and 18 (75%) in the placebo plus SOC arm received concomitant remdesivir, and 13 (59%) and 16 (67%), respectively, received concomitant dexamethasone. No patients received concomitant tocilizumab during the study period.
Table 1.
Baseline Demographics and Disease Characteristics
| Characteristic | Placebo + SOC (n = 24) |
Ibrutinib + SOC (n = 22) |
All Patients (N = 46) |
|---|---|---|---|
| Age, y, median (range) | 54.5 (37–75) | 48.5 (35–71) | 51.5 (35–75) |
| Sex | |||
| Male | 16 (67) | 16 (73) | 32 (70) |
| Female | 8 (33) | 6 (27) | 14 (30) |
| Race | |||
| White | 12 (50) | 14 (64) | 26 (57) |
| Black/African American | 4 (17) | 4 (18) | 8 (17) |
| Native Hawaiian or other Pacific Islander | 1 (4) | 0 | 1 (2) |
| Asian | 1 (4) | 0 | 1 (2) |
| Missing | 6 (25) | 4 (18) | 10 (22) |
| Ethnicity | |||
| Hispanic/Latino | 13 (54) | 11 (50) | 24 (52) |
| Non-Hispanic/Latino | 11 (46) | 11 (50) | 22 (48) |
| Body mass index, kg/m2 | |||
| Mean (SD) | 33.2 (7.1) | 31.9 (6.0) | 32.6 (6.5) |
| Median (range) | 33.4 (20.0–53.8) | 31.9 (24.0–42.5) | 32.3 (20.0–53.8) |
| Stratification factor of prescription for remdesivir | 16 (67) | 13 (59) | 29 (63) |
| Concomitant use of remdesivira | 18 (75) | 15 (68) | 33 (72) |
| Concomitant use of dexamethasonea | 16 (67) | 13 (59) | 29 (63) |
| WHO 8-point ordinal scale at baseline | |||
| 4 | 22 (92) | 22 (100) | 44 (96) |
| 5 | 2 (8) | 0 | 2 (4)b |
| CRP, mg/L, median (range) | 98.9 (26.0–309.0) |
69.0 (8.0–1668.0) |
83.5 (8.0–1668.0) |
| Oxygen flow rate at baseline, L/min | |||
| Mean (SD) | 7.0 (12.0) | 4.5 (5.8) | 5.8 (9.5) |
| Median (range) | 3.0 (1.0–50.0) | 3.0 (2.0–30.0) | 3.0 (1.0–50.0) |
| COVID-19 symptom onset duration before baseline, d, median (range) | 9.0 (5.0–16.0) | 9.0 (4.0–14.0) | 9.0 (4.0–16.0) |
| COVID-19 diagnosis duration before baseline, d, median (range) | 3.0 (2.0–10.0) | 4.0 (2.0–9.0) | 3.0 (2.0–10.0) |
| Comorbidities at baseline in ≥1 patient | |||
| Asthma | 2 (8) | 2 (9) | 4 (9) |
| Chronic kidney disease | 2 (8) | 0 | 2 (4) |
| Chronic liver diseasec | 0 | 1 (5) | 1 (2) |
| Chronic lung diseasec | 0 | 1 (5) | 1 (2) |
| Diabetes mellitus | 11 (46) | 9 (41) | 20 (43) |
| Heart conditionsc | 1 (4) | 1 (5) | 2 (2) |
| Hypertension | 11 (46) | 7 (32) | 18 (39) |
| Obesity | 6 (25) | 5 (23) | 11 (24) |
| Smoking (current or former smoker) | 8 (33) | 4 (18) | 12 (26) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; SD, standard deviation; SOC, standard of care; WHO, World Health Organization.
At any time during the study period.
Both patients had a score of 4 at screening.
Combined terms; see Supplementary Methods for full list of terms.
Primary Outcome
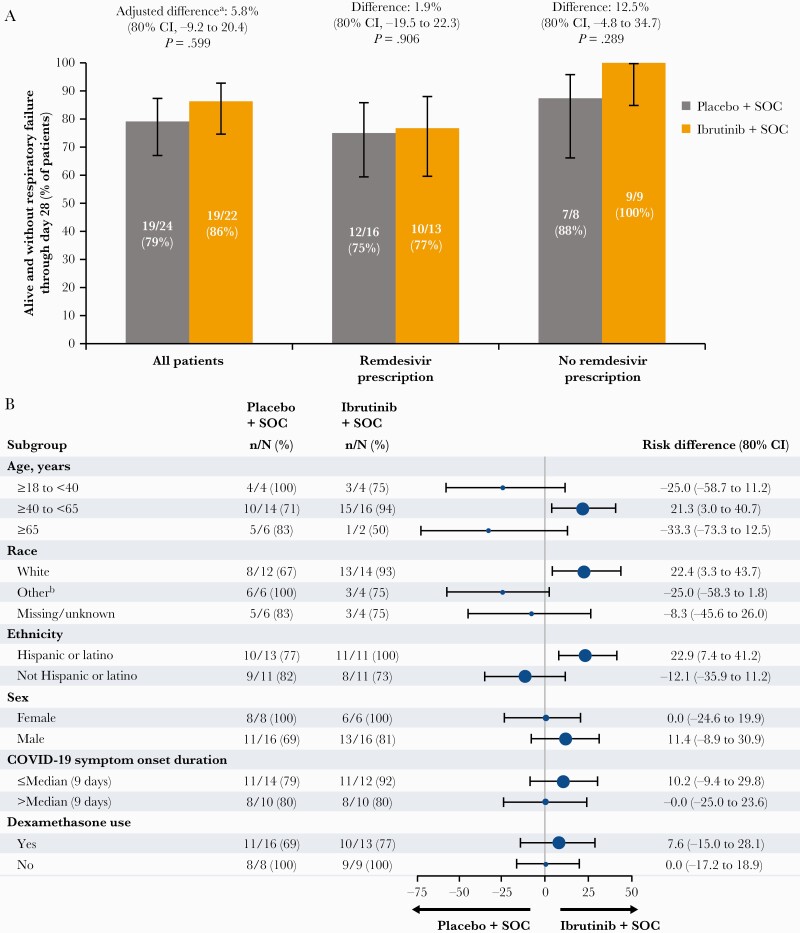
The proportion of patients alive and without respiratory failure through day 28, adjusting for remdesivir prescription, was not statistically significant (86% [19/22] with ibrutinib plus SOC and 79% [19/24] with placebo plus SOC; adjusted difference, 5.8% [80% confidence interval {CI}, –9.2% to 20.4%]; P = .599) (Figure 1A). In patients with remdesivir prescription at randomization, the proportion alive and without respiratory failure through day 28 was 77% (10/13) with ibrutinib plus SOC and 75% (12/16) with placebo plus SOC (difference, 1.9% [80% CI, –19.5% to 22.3%]; P = .906); in patients without remdesivir prescription, corresponding proportions were 100% (9/9) and 88% (7/8), respectively (difference, 12.5% [80% CI, –4.8% to 34.7%]; P = .289) (Figure 1A). Differences in risk of respiratory failure or death were observed among some subgroups (Figure 1B).
Figure 1.
A, Proportion of patients alive and without respiratory failure through day 28 by treatment arm in all patients and in patients with and without remdesivir prescription. B, Forest plot of between-arm difference in the proportion of patients with respiratory failure or death through day 28 across patient subgroups. Error bars represent 80% confidence intervals. aAdjusted for randomization stratification factor of remdesivir prescription. b“Other” includes Black (n = 8), Asian (n = 1), and Native Hawaiian or Pacific Islander (n = 1). Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; SOC, standard of care.
Secondary Outcomes
A ≥2-point improvement in WHO 8-point ordinal scale from baseline to day 14 was observed in 19 (86%) vs 19 (79%) patients with ibrutinib plus SOC vs placebo plus SOC (Supplementary Figure 1A); a ≥3-point improvement was observed in 14 (64%) vs 12 (50%), respectively (Supplementary Figure 1B). No secondary endpoints reached statistical significance (Supplementary Table 3).
Safety
Overall, AEs occurred in 12 (55%) patients with ibrutinib plus SOC and 12 (50%) with placebo plus SOC. The most frequent AEs (>2 patients overall) were elevated alanine aminotransferase, anemia, acute respiratory failure, hypertension, elevated aspartate aminotransferase, nausea, and sepsis (Table 2). All hypertension events in the ibrutinib plus SOC arm were grade 1 and considered unrelated to study treatment as assessed by investigators. Serious AEs occurred in 4 (18%) patients with ibrutinib plus SOC and 3 (13%) with placebo plus SOC (Table 2). One patient on the ibrutinib plus SOC arm died of acute respiratory failure due to COVID-19 pneumonia (unrelated to study treatment) on day 21; 1 patient on the placebo plus SOC arm died of COVID-19 pneumonia (unrelated to study treatment) on day 43. Overall, AEs leading to discontinuation of study treatment occurred in 10 (22%) patients, including 6 patients (27%) in the ibrutinib plus SOC arm and 4 patients (17%) in the placebo plus SOC arm. The only AE leading to discontinuation in 2 or more patients overall was acute respiratory failure, which was reported by 1 patient in each arm (Supplementary Table 4).
Table 2.
Adverse Events in the Safety Population
| AEs | Placebo + SOC (n = 24) |
Ibrutinib + SOC (n = 22) |
All Patients (N = 46) |
|---|---|---|---|
| Any AE | 12 (50) | 12 (55) | 24 (52) |
| Most common AEs (≥5% of all patients) | |||
| ALT increased | 3 (13) | 2 (9) | 5 (11) |
| Anemia | 2 (8) | 3 (14) | 5 (11) |
| Acute respiratory failure | 3 (13) | 1 (5) | 4 (9) |
| Hypertension | 1 (4)a | 3 (14)b | 4 (9) |
| AST increased | 2 (8) | 1 (5) | 3 (7) |
| Nausea | 1 (4) | 2 (9) | 3 (7) |
| Sepsis | 2 (8) | 1 (5) | 3 (7) |
| Grade ≥3 AE | 4 (17) | 3 (14) | 7 (15) |
| Serious AE | 3 (13) | 4 (18) | 7 (15) |
| Fatal AE | 1 (4)c | 1 (5)d | 2 (4) |
| AE leading to discontinuation | 4 (17) | 6 (27) | 10 (22) |
| AE leading to dose reduction | 0 | 0 | 0 |
Data are presented as No. (%).
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SOC, standard of care.
Grade 2 hypertension in 1 patient.
Grade 1 hypertension in 3 patients.
Death due to coronavirus disease 2019 (COVID-19) pneumonia (unrelated to study treatment) on study day 43.
Death due to acute respiratory failure due to COVID-19 pneumonia (unrelated to study treatment) on study day 21.
DISCUSSION
The primary endpoint of proportion of patients alive and without respiratory failure through day 28 was not met, with no statistically significant difference observed between ibrutinib plus SOC and placebo plus SOC. Small sample size precluded detection of statistically significant differences in outcomes, and the possibility of a modest treatment effect cannot be excluded. Despite small sample size, the study population was diverse with regard to race and ethnicity; a substantial proportion of Hispanic/Latino and Black/African American patients were included, representing populations that are disproportionately affected by COVID-19 but typically underrepresented in clinical trials.
Most patients received concomitant remdesivir and/or dexamethasone, which may have obscured any additional treatment benefit from ibrutinib. Remdesivir has been shown to shorten time to recovery in COVID-19–infected patients requiring hospitalization but does not reduce mortality [9]. Until recently, dexamethasone was the only therapy shown to reduce mortality in patients with severe COVID-19, and it was approved for the treatment of severe COVID-19 shortly after initiation of the current study [10]. Subsequently, both tocilizumab and baricitinib have also demonstrated modest reductions in mortality in hospitalized patients with severe COVID-19 [11, 12]. Ibrutinib did not appear to provide any added clinical benefit in the context of effective SOC, including dexamethasone and/or remdesivir. It remains unclear whether ibrutinib may provide benefit in the absence of the confounding effect of these concomitant medications.
Importantly, ibrutinib demonstrated a manageable safety profile among hospitalized patients with severe COVID-19 receiving SOC, with no new safety signals identified. Although ibrutinib exposure was limited to 28 days in this study, these findings may be considered when assessing benefit-risk of continued ibrutinib in patients who develop COVID-19 during ibrutinib therapy.
In summary, ibrutinib had a manageable safety profile but did not improve the proportion of patients alive and without respiratory failure through day 28 vs placebo in this population of hospitalized patients with severe COVID-19 also receiving supportive standard of care.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
In Memoriam
Steven Edward Coutre MD

On November 9, 2021, our co-investigator, colleague and friend, Steven Edward Coutre, M.D. succumbed to complications of chronic lymphocytic leukemia (CLL) and COVID-19. Steve was a Professor of Medicine (Hematology) at Stanford University and head of Stanford Health Care’s Hematology Clinic. He was an exceptional and caring physician, a gifted teacher, and a tireless investigator who devoted his life to advancing treatments for patients with CLL, and other hematological malignancies. He helped organize and spearhead this study, allowing us to understand the role of BTK-inhibitors as anti-inflammatory agents to treat respiratory complications of COVID-19. We dedicate this work to his memory, with immense gratitude for his inspiration, dedication, and hard work. May his memory be eternal.
Steve Treon MD, PhD
Harvard Medical School, Boston MA, USA
Notes
Acknowledgments. We thank the patients who participated in the study, as well as the investigators and clinical research staff from the study centers. iNSPIRE was sponsored by Pharmacyclics LLC, an AbbVie Company. Medical writing support was provided by Melanie Sweetlove, MSc, and funded by Pharmacyclics LLC, an AbbVie Company.
Author contributions. S. E. C., R. Q., L. S., and S. P. T. designed the study. S. E. C., C. B., O. O., D. H., M. R., A. C. F., and S. P. T. collected data. Y. H., J. N., N. N. A., and L. S. performed data analysis. All authors contributed to interpretation of the data and to editing, revising, and finalizing the article before submission. All authors approved the final article and are accountable for all aspects of the work.
Financial support. This work was supported by Pharmacyclics LLC, an AbbVie Company.
Potential conflicts of interest. S. E. C. reports honoraria from AbbVie, Janssen, and Pharmacyclics LLC, an AbbVie Company; consulting or advisory role for AbbVie, Adaptive, AstraZeneca, Celgene, Genentech, Novartis, BeiGene, Janssen, and Pharmacyclics LLC, an AbbVie Company; research funding from AbbVie, AstraZeneca, Janssen, and Pharmacyclics LLC, an AbbVie Company; and expert testimony for Genentech and Janssen. C. B. reports stock or other ownership in Biogen; and institutional research funding from AbbVie, BeiGene, and United Therapeutics. O. O. reports stock or other ownership in Gilead and Pfizer; honoraria from Merck and Gilead; consulting or advisory role for Merck, Gilead, and ViiV Healthcare; research funding from Allergan; and speakers bureau for Gilead and ViiV Healthcare. D. H. reports stock or other ownership in Aprea Therapeutics and Provention Bio; consulting or advisory role for Amgen and AbbVie; and research funding from AbbVie, Juno, and Regeneron. M. R. reports employment with Associates in Infectious Diseases and Midway Immunology and Research Center; stock or other ownership with CRC Pharma, CytoDyn, Gilead, and Merck; honoraria from and consulting or advisory role for Allergan, Gilead, Janssen, Merck, and ViiV Healthcare; research funding from Janssen; and speakers bureau for Allergan, Gilead, Janssen, and ViiV Healthcare. A. C. F. reports stock or other ownership in AbbVie, Boston Scientific, Johnson & Johnson, McKesson, Procter & Gamble, Regeneron, and Stryker; and research funding from AbbVie. R. Q. reports former employment with AbbVie and current employment with Nektar Therapeutics; and stock or other ownership, travel, accommodations, expenses, and other relationships with AbbVie. Y. H. and N. N. A. report research funding from and employment, leadership role, and stock or other ownership with AbbVie. J. N. reports former employment with Pharmacyclics LLC, an AbbVie Company, and current employment with Summit Therapeutics; and stock or other ownership with AbbVie. L. S. reports former employment with Pharmacyclics LLC, an AbbVie Company, and current employment with Summit Therapeutics; and stock or other ownership with AbbVie. S. P. T. reports honoraria from Janssen; consulting or advisory role with AbbVie, BeiGene, and Janssen; research funding from the Jon and Mary Orszag Fund at the Dana-Farber Cancer Institute, AbbVie, BeiGene, Bristol Myers Squibb, Eli Lilly, and Pharmacyclics LLC, an AbbVie Company; and travel, accommodations, or expenses from AbbVie, BeiGene, Janssen, and Pharmacyclics LLC, an AbbVie Company.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 3. McGonagle D, Sharif K, O’Regan A, Bridgewood C.. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome–like disease. Autoimmun Rev 2020; 19:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McElvaney OJ, McEvoy NL, McElvaney OF, Carroll TP, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med 2020; 202:812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Florence JM, Krupa A, Booshehri LM, Davis SA, et al. Inhibiting Bruton’s tyrosine kinase rescues mice from lethal influenza-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2018; 315:L52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greil R, Tedeschi A, Moreno C, Anz B, et al. Pretreatment with ibrutinib reduces cytokine secretion and limits the risk of obinutuzumab-induced infusion-related reactions in patients with CLL: analysis from the iLLUMINATE study. Ann Hematol 2021; 100:1733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Treon SP, Castillo JJ, Skarbnik AP, Soumerai JD, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood 2020; 135:1912–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. WHO R&D blueprint: novel coronavirus: COVID-19 therapeutic trial synopsis. 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf. Accessed 1 September 2021.
- 9. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horby P, Lim WS, Emberson JR, Mafham M, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021; 9:1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.