Abstract
We conducted 3 successive seroprevalence surveys, 3 months apart, using multistage cluster sampling to measure the extent and dynamics of the severe acute respiratory syndrome coronavirus 2 epidemic in Conakry, the capital city of Guinea. Seroprevalence increased from 17.3% (95% CI, 12.4%–23.8%) in December 2020 during the first survey (S1) to 28.9% (95% CI, 25.6%–32.4%) in March/April 2021 (S2), then to 42.4% (95% CI, 39.5%–45.3%) in June 2021 (S3). This significant overall trend of increasing seroprevalence (P < .0001) was also significant in every age class, illustrating a sustained transmission within the whole community. These data may contribute to defining cost-effective response strategies.
Keywords: COVID-19, Guinea, population-based survey, SARS-CoV-2, seroprevalence
The first cases of coronavirus disease 2019 (COVID-19) were reported in late December 2019 in Wuhan (China), and 2 years later, >280 million cases have been reported worldwide, with almost 5.5 million deaths [1]. In Africa, as of January 21, 2021, ~8 million cases and 162 000 deaths have been reported [1], representing <3% of cumulative cases and deaths worldwide, making the African continent apparently the least affected, in contrast to the bleak scenario initially predicted for Africa at the onset of the COVID-19 pandemic [2]. Limited access to care and diagnostics, weak surveillance systems, and a high proportion of the young population often associated with asymptomatic infections [3] may have masked the extent of the epidemic in Africa. Therefore, the World Health Organization (WHO) recommends conducting repeated population seroprevalence surveys in order to measure the extent of the epidemic, to monitor its spread over time, and to provide reasonable estimates of the cumulative incidence of infection to guide the public health response to COVID-19 [4].
Seroprevalence studies around the world have established that a small proportion of the population had been infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after the first epidemic wave in Spring 2020, with most seroprevalence estimates ranging from <0.1% to >20%, with a pooled estimate of 3.38% (95% CI, 3.05%–3.72%) in the general population [5]. Most of these studies have estimated infection levels several times higher than previously reported by surveillance systems based on confirmed cases [5]. In Africa, however, most seroprevalence surveys have focused on specific risk groups (such as health care workers, blood donors, etc.) rather than on the general population, and mostly with cross-sectional instead of repeated surveys, which can better capture the dynamics of the epidemic [6–12]. Nevertheless, these studies showed that SARS-CoV-2 infections in Africa are much higher than observed by cases confirmed by polymerase chain reaction (PCR).
In Guinea, the first SARS-CoV-2 infection was detected on March 12, 2020. As in many African countries, the capital city, Conakry, accounts for nearly 80% of reported COVID-19 cases in the country. In this study, we evaluated the extent and dynamics of the COVID-19 epidemic and its dynamics in Conakry through 3 successive population-based surveys measuring the prevalence of immunoglobulin (IgG) antibodies to SARS-CoV-2.
METHODS
Study Design and Population
Based on the WHO population-based sero-epidemiological investigation protocol for COVID-19 [4], 3 repeated cross-sectional household-based and age-stratified seroprevalence surveys were conducted in Conakry, Guinea, just before the peak (December 2–26, 2020; first survey: S1), during the peak (March 19–April 3, 2021; second survey: S2), and after the second wave of the COVID-19 epidemic (June 4–19, 2021; third survey: S3) (Supplementary Figure 1).
For each survey, a stratified 2-stage random cluster sampling design was used to select households with a first-stage probability to be selected proportional to the population number in each enumeration area. At the second stage, all residents age >40 years were invited to participate, while residents age <40 years were invited to participate in half of the selected households. The samples for the 3 surveys were independent and representative of the general population of Conakry, stratified by age.
After consent, household and individual questionnaires were used to collect general demographic characteristics (sex, age, education, marital status, occupation), symptoms related to SARS-CoV-2 infection, previous testing and COVID-19 vaccination, other health-promoting behavior, and previous contact with a confirmed or probable COVID-19 patient. PCR testing was offered to all individuals with suspicion of COVID-19 infection. All staff involved in the study were tested by PCR before the survey and followed infection prevention and control recommendations.
Detection of Antibodies to SARS-CoV-2/COVID-19
Blood samples were collected as dried blood spots (DBS) on a Whatman 903 filter paper. DBS were eluted, and 100 μL of diluted eluate, adjusted at a final plasma dilution of 1/200, taking into account the hematocrit, was used to test for the presence of antibodies to SARS-CoV-2 with a previously developed, highly sensitive and specific Luminex-based assay (Luminex Corp, Austin, TX, USA) using recombinant nucleocapsid (NC) and spike (SP) SARS-CoV-2 proteins [13]. Samples were considered positive for SARS-CoV-2 IgG antibodies when they reacted simultaneously to NC and SP proteins. Samples reacting to only 1 antigen were considered “indeterminate” because this could be related either to antibody decline or lower specificity of single-antigen reaction, especially samples from Africa [14]. The test has been previously evaluated on a panel of 1197 samples from Africa before the COVID-19 pandemic from Guinea, Cameroon, and the DRC, with 99.7% specificity [13].
Statistical Analysis
Sociodemographic characteristics were described as proportion or mean and were weighted to take into account the probability of selection from cluster sampling. For the calculation of seroprevalence (SP + NC), the denominator was composed of indeterminates and negatives. We used χ² tests to compare proportions. Weighted and age-standardized serological results were stratified by the other characteristics of the study population. Data were collected with the Research Electronic Data Capture (REDCap) platform and analyzed using Stata 16 software (StataCorp, College Station, TX, USA).
Ethical Considerations
The study was approved by the National Ethics Committee for Health Research (CNERS) of Guinea (No. 114/CNERS/20). The consent of each participant was required before inclusion in the study.
RESULTS
Study Population
A total of 596 individuals from 174 households were included in the first survey (S1), 1207 from 227 households in the second survey (S2) and 1082 from 193 households in the third survey (S3) (Table 1). Of these, 535/596 (89.7%) participants of S1 were tested for anti-SARS-CoV-2 IgG antibodies, 1195/1207 (99.0%) for S2, and 1073/1082 (99.2%) for S3. Age, sex distribution, and other demographic characteristics are shown in Table 1. With respect to the clinical characteristics, 17.8% (95% CI, 10.3%–36.2%), 30.7% (95% CI, 10.3%–36.2%), and 43.6% (95% CI, 10.3%–36.2%) of participants declared no symptoms of COVID-19 during S1, S2, and S3, respectively (Table 1). Few participants reported having been tested previously for SARS-CoV-2: 69 (12.9%) for S1, 65 (5.4%) for S2, and 70 (6.5%) for S3, with 0, 2, and 4 positives in each survey, respectively (Supplementary Table 1). Regarding vaccination, none of the participants received a vaccine in S1, and 29 (2.4%%) participants were vaccinated in S2, although the majority received only the first dose. In the third survey, 130 (12.1%) participants were vaccinated, and half of them received 2 doses (Supplementary Table 2).
Table 1.
Weighted and Age-Standardized Prevalence of SARS-CoV-2 Antibodies by Gender, Sociodemographic Characteristics, and Medical History in 3 Consecutive Population-Based Surveys at 3-Month Intervals, Conakry, Guinea, 2020–2021
| Sociodemographic Characteristics | First Survey | Second Survey | Third Survey |
P Values for Comparisons Over Time |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants, No. | Seropositive Participants | Participants, No. | Seropositive Participants | Participants, No. | Seropositive Participants | ||||||||||
| No. (%) | 95% CI | P Value | No. (%) | 95% CI | P Value | No. (%) | 95% CI | P Value | S1 vs S2 | S1 vs S3 | S2 vs S3 | ||||
| Age, y | .096 | <.0001 | <.001 | ||||||||||||
| 0–19 | 190 (35.5) | 35 (18.9) | 12.1–28.4 | 597 (50.0) | 138 (23.1) | 19.3–27.5 | 516 (48.1) | 183 (35.5) | 31.3–39.8 | .224 | <.0001 | <.0001 | |||
| 20–39 | 133 (24.9) | 15 (11.3) | 6.6–18.7 | 309 (25.9) | 98 (31.7) | 25.8–38.3 | 298 (27.8) | 138 (46.3) | 40.8–51.9 | <.0001 | <.0001 | <.0001 | |||
| ≥40 | 212 (39.6) | 48 (22.6) | 16.3–30.6 | 289 (24.2) | 126 (43.6) | 36.5–51.0 | 258 (24.1) | 152 (58.9) | 52.6–64.9 | <.0001 | <.0001 | <.0001 | |||
| Gender | .072 | .830 | .621 | ||||||||||||
| Female | 304 (56.8) | 64 (20.1) | 13.5–28.9 | 657 (55.0) | 201 (29.2) | 25.1–33.6 | 634 (59.1) | 274 (41.8) | 37.9–45.9 | .003 | <.0001 | <.0001 | |||
| Male | 231 (43.2) | 35 (13.6) | 9.0–20.0 | 538 (45.0) | 161 (28.7) | 24.9–32.9 | 438 (40.9) | 199 (43.3) | 38.5–48.2 | <.0001 | <.0001 | <.0001 | |||
| Marital status | .636 | <.0001 | <.001 | ||||||||||||
| Single | 223 (46.8) | 32 (12.9) | 8.7–18.7 | 768 (64.4) | 195 (29.3) | 24.2–35.1 | 651 (60.7) | 253 (43.8) | 38.9–48.9 | <.0001 | <.0001 | <.0001 | |||
| Married/as a couple | 194 (40.8) | 38 (6.6) | 4.0–10.9 | 354 (29.7) | 136 (43.2) | 19.4–70.6 | 369 (34.4) | 196 (46.2) | 26.5–67.2 | <.0001 | <.0001 | .417 | |||
| Divorced/separated/widow(er) | 59 (12.4) | 15 (4.3) | 2.5–7.3 | 70 (5.9) | 31 (21.5) | 10.2–39.9 | 52 (4.9) | 24 (22.1) | 12.1–36.9 | .005 | .005 | .937 | |||
| Education | .370 | .072 | .003 | ||||||||||||
| Primary school | 144 (27.2) | 22 (13.5) | 7.1–24.1 | 434 (36.5) | 124 (29.7) | 23.3–37.0 | 402 (37.7) | 151 (38.6) | 32.6–45.0 | <.0001 | <.0001 | .007 | |||
| Secondary school | 197 (37.2) | 35 (17.9) | 12.0–26.0 | 366 (30.8) | 115 (28.8) | 23.8–34.4 | 287 (26.9) | 145 (50.1) | 42.5–57.7 | .004 | <.0001 | <.0001 | |||
| University | 90 (17.0) | 13 (5.6) | 3.8–8.1 | 157 (13.2) | 58 (34.9) | 29.0–41.4 | 135 (12.7) | 71 (23.3) | 19.3–27.9 | <.001 | <.0001 | .030 | |||
| None | 98 (18.5) | 27 (21.0) | 8.7–42.7 | 233 (19.6) | 64 (22.4) | 18.1–27.5 | 241 (22.6) | 101 (31.0) | 29.3–41.7 | .779 | .063 | .035 | |||
| Households size | .315 | .746 | .226 | ||||||||||||
| ≤6 | 192 (35.9) | 29 (12.6) | 6.2–23.8 | 286 (24.8) | 93 (26.8) | 20.5–34.1 | 215 (20.0) | 101 (42.9) | 35.0–51.2 | <.0001 | <.0001 | <.0001 | |||
| 7–13 | 192 (35.9) | 40 (17.8) | 12.6–24.5 | 373 (32.4) | 108 (28.0) | 23.8–32.8 | 334 (31.1) | 160 (46.0) | 39.5–52.7 | .008 | <.0001 | <.0001 | |||
| ≥13 | 151 (28.2) | 30 (20.5) | 11.7–33.4 | 492 (42.7) | 150 (30.3) | 25.7–35.4 | 524 (48.8) | 213 (41.1) | 36.7–45.5 | .019 | <.0001 | <.0001 | |||
| No. of symptoms | .636 | .203 | .098 | ||||||||||||
| No | 95 (17.8) | 20 (19.9) | 10.0–35.7 | 367 (30.7) | 120 (32.7) | 27.5–38.4 | 468 (43.6) | 190 (40.5) | 36.9–44.2 | .015 | <.0001 | .021 | |||
| 1–2 symptoms | 178 (33.3) | 32 (15.0) | 10.0–21.8 | 390 (32.6) | 109 (26.5) | 22.7–30.6 | 289 (26.9) | 141 (45.6) | 39.0–52.4 | .002 | <.0001 | <.0001 | |||
| 3–5 symptoms | 168 (31.4) | 29 (18.4) | 11.6–27.9 | 344 (28.8) | 105 (27.2) | 23.0–31.8 | 261 (24.3) | 120 (44.0) | 38.1–50.0 | .029 | <.0001 | <.0001 | |||
| ≥5 symptoms | 94 (17.6) | 18 (18.4) | 10.8–29.5 | 94 (7.9) | 28 (28.1) | 20.0–37.8 | 55 (5.1) | 23 (37.3) | 26.8–49.2 | .115 | .011 | .243 | |||
| Hospitalization | .141 | .912 | .160 | ||||||||||||
| Yes | 18 (4.0) | 4 (27.7) | 12.1–51.5 | 16 (2.0) | 5 (25.3) | 10.8–48.7 | 8 (1.3) | 5 (70.9) | 34.0–92.0 | .874 | .039 | .032 | |||
| No | 427 (96.0) | 75 (15.7) | 12.4–20.8 | 797 (98.0) | 234 (27.1) | 24.1–30.3 | 592 (98.7) | 276 (43.9) | 39.4–48.5 | <.0001 | <.0001 | <.0001 | |||
| Overall | 535 | 99 (17.3) | 12.4–23.8 | 1195 | 362 (28.9) | 25.6–32.4 | 1073 | 474 (42.4) | 39.5–45.3 | <.0001 | <.0001 | <.0001 | |||
S1: first survey (December 2020); S2: second survey (March–April 2021); S3: third survey (June 2021).
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Seroprevalence
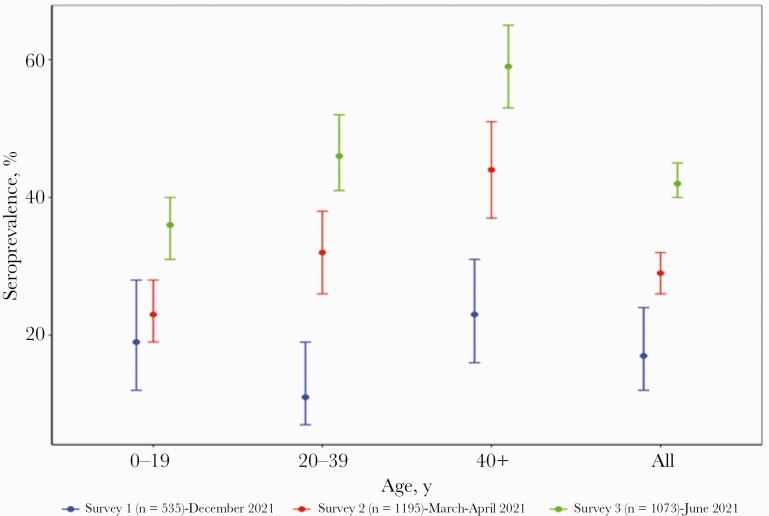
The overall weighted and age-standardized SARS-CoV-2 IgG seroprevalence was 17.3% (95% CI, 12.4%–23.8%) in S1 (December 2020). Seroprevalence increased to 28.9% (95% CI, 25.6%–32.4%) in S2 participants 3 months later and to 42.4% (95% CI, 39.5%–45.3%) 6 months after S1, with a significant increase throughout the 3 surveys (P < .0001) (Table 1, Figure 1). Seroprevalence differed by age in S2 and S3 (P < .0001) and was constantly higher in individuals aged >40 years for the 3 surveys: 22.6% (95% CI, 16.3%–30.6%) in S1, 43.6% (95% CI, 36.5%–51.0%) in S2, and 58.9% (95% CI, 52.6%–64.9%) in S3. Across the 3 consecutive surveys, seroprevalence increased significantly in each age category. In S2 and S3, seroprevalence differed significantly by marital status (P < .0001), with the highest prevalence observed in single individuals. Seroprevalence did not differ by sex or by reported symptoms in the 3 surveys. In general, whatever the sociodemographic characteristics considered, a significant increase in seroprevalence was observed between S1 and S3 (Table 1). Among seropositive participants, 19/362 (5.2%) in S2 and 86/474 (18.1%) in S3 were vaccinated. The proportion of individuals with SP antibodies only was 17.9% in S1, 36.1% in S2, and 27.2% in S3, whereas the proportion of individuals with NC antibodies only was 6.6% in S1, 2.7% in S2, and 3.2% in S3 (Supplementary Tables 3 and 4).
Figure 1.
Age-standardized seroprevalence of SARS-CoV-2 in 3 consecutive population based surveys at 3 months interval in Conakry, Guinea. The graphs show the prevalence of SARS-CoV-2 IgG antibodies per age category and total for survey 1 (blue), survey 2 (red), survey 3 (green). Dots represent the estimated prevalence and bars represent 95% CIs. Abbreviations: IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
These 3 consecutive cross-sectional surveys showed that the proportion of the population in Conakry with antibodies to SARS-CoV-2 increased sharply from 17.3% to 42.4% between December 2020 and June 2021, just before and after the second wave of the COVID-19 epidemic, respectively. This 2.4-fold increase in seroprevalence over 6 months shows intense community transmission during the second wave, and the increase concerns all age categories and all sociodemographic parameters studied. Like almost all previous studies in Africa [7–10, 12], we showed that seroprevalence is significantly associated with age but does not differ by sex or reported symptoms associated with COVID-19. More precisely, seroprevalence was constantly higher in individuals age >40 years, similar to studies in Kenya [7] and Sudan [8], which have shown that participants age ≥50 years were among the most affected. A study using a similar population-based approach in Kinshasa, DRC, showed the same trend [10].
An increase in seroprevalence after the second wave has been reported in other African countries, for example, in Mali [11], Zimbabwe [12], and Kenya [7]. Our results also suggest that the virus has spread widely in the community during the second wave of COVID-19 in Conakry. Indeed, at the end of the third survey, the cumulative number of positive cases reported in Guinea was 23 543, but seroprevalence suggests that by June 2021, around 42% of the population of Conakry had already been in contact with the SARS-CoV-2 virus, which corresponds to at least 700 000 individuals from the total population of Conakry, which is estimated to be ~1.7 million inhabitants. Similar to other reports from Africa, our data clearly show that the vast majority of cases went underreported, with only 1 case detected by the surveillance system, based on PCR confirmation, for 30 infections in the community based on serology. Interestingly, this infection-to-case ratio was the same at the end of S1 and S2 (30 and 33, respectively), implying that diagnostic capacities were similar over time. Another estimation from a systematic review reported that the seroprevalence (interquartile range) was on average 18.1 (5.9–38.7) times higher than the corresponding cumulative incidence of PCR-confirmed SARS-CoV-2 infection [15]. These results suggest that most of the SARS-CoV-2 infections were pauci- or asymptomatic, had limited access to diagnosis, and that serological surveys are the best way to monitor the true extent of the spread of the pandemic.
These repeated surveys have the advantage of having included a large number of people using a rigorous sampling methodology. Moreover, while many studies have used rapid diagnostic tests or enzyme-linked immunosorbent assay, this study used the same serological test throughout the 3 surveys with strict interpretation criteria, and therefore reported seroprevalences were likely to be underestimated. It cannot be excluded that some participants seroconverted during the survey and that the antibody profile was not complete yet to both antigens. On the other hand, presence of antibodies to a single antigen can also be due to waning of antibodies over time. Given the retrospective reporting of COVID-19-related symptoms, a nondifferential recall bias is likely, resulting in a dilution of the association between symptoms and seropositivity.
It is important to note that in S1 vaccination had not yet started, and it had just started during S2. Among seropositive participants, 5% in S2 and 18% in S3 were vaccinated.
The vaccines deployed in Guinea were Sputnik or Sinopharm, and the majority received only the first dose. Although 12% of participants in S3 received at least the first vaccine dose, part of the antibody responses can thus be due to the recent introduction of vaccines. Lastly, given the sociopolitical situation in December 2020, several people refused to participate in S1, and certain enumeration areas could not be visited, which may have had an impact on the representativeness of S1, compared with S2 and S3.
Taken together, these population-based studies provide an estimation of the extent of the epidemic and its dynamics. This study in Guinea is, to our knowledge, the first to combine 3 successive population-based surveys in order to evaluate the level of SARS-CoV-2 dissemination in the general population in a large Sub-Saharan capital, illustrating sustained community transmission. These results also contribute to guiding cost-effective public health responses to the COVID-19 epidemic.
Supplementary Material
Acknowledgments
We thank the study participants, the investigation teams, the community health staff, the logistical support of Guinea and the national institute of statistics of Guinea, the health D-department of Conakry, Caroline Coulon, and René Ecochard.
Financial support. This work was supported by the French Development Agency (AFD) and the French National Agency for Research on AIDS and Emerging Infectious Diseases (ANRS-MIE, ANRS-COV16 grant).
Potential conflicts of interest. All authors: no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 25 January 2022.
- 2. Massinga Loembe M, Tshangela A, Salyer SJ, et al. COVID-19 in Africa: the spread and response. Nat Med 2020; 26:999–1003. [DOI] [PubMed] [Google Scholar]
- 3. Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med 2020; 17:e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection. 2020. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Seroepidemiology-2020.2/. Accessed 25 January 2022.
- 5. Rostami A, Sepidarkish M, Leeflang MMG, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect 2021; 27:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chisale MRO, Ramazanu S, Mwale SE, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in Africa: a systematic review and meta-analysis. Rev Med Virol 2021; 32:e2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gignoux E, Atthanassiadis F, Garat Yarrow A, et al. Seroprevalence of SARS-CoV-2 antibodies and retrospective mortality in a refugee camp, Dagahaley, Kenya. PLoS One 2021; 16:e0260989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moser W, Fahal MAH, Abualas E, et al. Retrospective mortality and prevalence of SARS-CoV-2 antibodies in greater Omdurman, Sudan: a population-based cross-sectional survey. medRxiv 2021.08.22.21262294 [Preprint]. 26 August 2021. Available at: 10.1101/2021.08.22.21262294. Accessed 21 January 2022. [DOI] [Google Scholar]
- 9. Mulenga LB, Hines JZ, Fwoloshi S, et al. Prevalence of SARS-CoV-2 in six districts in Zambia in July, 2020: a cross-sectional cluster sample survey. Lancet Glob Health 2021; 9:e773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nkuba AN, Makiala SM, Guichet E, et al. High prevalence of anti-severe acute respiratory syndrome coronavirus 2 (anti-SARS-CoV-2) antibodies after the first wave of coronavirus disease 2019 (COVID-19) in Kinshasa, Democratic Republic of the Congo: results of a cross-sectional household-based survey. Clin Infect Dis. 2022; 74:882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sagara I, Woodford J, Kone M, et al. Rapidly increasing SARS-CoV-2 seroprevalence and limited clinical disease in three Malian communities: a prospective cohort study. medRxiv 2021.04.26.21256016 [Preprint]. 29 April 2021. Available at: 10.1101/2021.04.26.21256016. Accessed 21 January 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fryatt A, Simms V, Bandason T, et al. Community SARS-CoV-2 seroprevalence before and after the second wave of SARS-CoV-2 infection in Harare, Zimbabwe. EClinicalMedicine 2021; 41:101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ayouba A, Thaurignac G, Morquin D, et al. Multiplex detection and dynamics of IgG antibodies to SARS-CoV2 and the highly pathogenic human coronaviruses SARS-CoV and MERS-CoV. J Clin Virol 2020; 129:104521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lumley SF, Wei J, O’Donnell D, et al. The duration, dynamics, and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin Infect Dis 2021; 73:e699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bobrovitz N, Arora RK, Cao C, et al. Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. PLoS One 2021; 16:e0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.