Abstract
This article provides an overview of the recommendations of the Aviation and Occupational Cardiology Task Force of the European Association of Preventive Cardiology on returning individuals to work in high-hazard occupations (such as flying, diving, and workplaces that are remote from healthcare facilities) following symptomatic Coronavirus Disease 2019 (COVID-19) infection. This process requires exclusion of significant underlying cardiopulmonary disease and this consensus statement (from experts across the field) outlines the appropriate screening and investigative processes that should be undertaken. The recommended response is based on simple screening in primary healthcare to determine those at risk, followed by first line investigations, including an exercise capacity assessment, to identify the small proportion of individuals who may have circulatory, pulmonary, or mixed disease. These individuals can then receive more advanced, targeted investigations. This statement provides a pragmatic, evidence-based approach for those (in all occupations) to assess employee health and capacity prior to a return to work following severe disease, or while continuing to experience significant post-COVID-19 symptoms (so-called ‘long-COVID’ or post-COVID-19 syndrome).
Keywords: SARS-CoV-2, COVID-19, Post-COVID-19 syndrome, Long COVID, High-hazard occupations, Cardiopulmonary disease
Introduction
A novel strain of coronavirus [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] emerged in Wuhan, China at the end of 2019 spreading rapidly across the globe with widespread transmission. The novelty of this virus means that knowledge of transmission, clinical features, treatment, and long-term consequences of infection is incomplete and continues to develop.
SARS-CoV-2 infection [more commonly described as Coronavirus Disease 2019 (COVID-19)] usually manifests as a non-specific viral respiratory tract infection, with fever, cough, breathlessness, dysgeusia, and anosmia being the predominant reported symptoms. The presentation is variable however, and headache, fatigue, sore throat, loss of appetite, and gastrointestinal symptoms may all also represent the predominant presenting features.1
An estimated 20% of COVID-19 infections are asymptomatic, and most symptomatic COVID-19 infections are not severe. Early studies identified consistently that of those symptomatic cases admitted to hospital, 10–20% of cases were admitted to critical care, 3–10% were intubated, and 2–5% died.2 Morbidity and mortality from COVID-19 increase with age, increased body mass index (BMI),3 and chronic comorbidity, including cardiopulmonary disease, diabetes, and hypertension.4–6
COVID-19 infection is associated with multisystem pathology, including acute cardiopulmonary, renal, and neurological sequelae.2 From a cardiorespiratory perspective, hospitalized cases are commonly associated with breathlessness and hypoxia, with bilateral infiltrates on chest X-ray and bilateral ground glass shadowing on chest computed tomography (CT) with a predilection for the periphery and inferior lobes. Chest X-ray can be normal on admission.
Direct cardiovascular effects of COVID-19 include myocardial injury or myocarditis, and early reports suggested the incidence of myocarditis was as high as to 15–60%.7,8 More recent data suggest that this figure is likely a significant overestimate.8–10 In individuals who work in high-hazard occupations exclusion of significant cardiopulmonary pathology remains a critical component of occupational risk assessment, exercise rehabilitation,11 and return to work.
Most individuals who suffer from symptomatic COVID-19 recover within a few weeks, with no ongoing clinical disease manifestations. However, ∼10% of individuals have residual symptoms beyond this.12 Post-COVID-19 syndrome, sometimes referred to as ‘long-COVID’ is a poorly defined entity, which encapsulates a myriad of physical and neurocognitive symptoms. A PubMed review of the literature was undertaken and highlighted the paucity of specific manuscripts that relate to the recovery period for SARS-CoV-2 which is understandably dominated by an older cohort of (usually hospitalized, and most ventilated) patients.13 Much of this data are not applicable to the younger working age cohort we address in this article and evidence in this area remains understandably limited, hence this position statement.
The UK National Institute for Healthcare Excellence (NICE) describes patients with ongoing signs and symptoms between 4 and 12 weeks following initial symptoms as ‘ongoing symptomatic COVID-19’ and those, with ongoing symptoms beyond 12 weeks (with no alternative explanation) as ‘post-COVID-19 syndrome’.14 This is the terminology used throughout this position statement, which will focus on cardiopulmonary assessment but highlight relevant additional clinical manifestations.
The challenge in assessing patients with ongoing symptoms following COVID-19 infection is the requirement to discriminate symptoms caused by organ pathology, from those caused by a more typical post-viral syndrome, documented in many well-characterized viral diseases.15 This challenge is often compounded by health anxiety, which is particularly pertinent to employees undertaking high-hazard work due to additional concerns regarding future employment.16 Whilst the overall likelihood of significant pathology in this cohort maybe low, employers need to be able to sign off an employee to undertake their high-hazard (and high risk) employment, and to ensure risk is mitigated. Even those who are asymptomatic, may need investigation, given that subclinical disease may be occupationally significant.
For employees undertaking high-hazard work and presenting with cardiopulmonary symptoms (such as dyspnoea and chest pain), occupational health physicians should be provided with occupationally contextualized clinical data. After consultation with specialist clinicians, this will enable them to reassurance the patient and to give appropriate risk advice to the employer and to ensure that employees are fit for exercise rehabilitation and graduated or managed return to work. High-hazard work applies to persons who engage in physically demanding (military), or safety critical occupations (commercial transportation), particularly in environments that expose individuals to hypoxia (flying), barometric or environmental extremes (including diving, flying, mountaineering, polar, mountain and cold-storage workers etc.), isolated working (offshore and polar workers etc.), or those who may be re-exposed to COVID-19 (healthcare workers).
Classification of high-hazard employment
High-hazard employment encompasses specific roles in which distraction, incapacitation, or physical impairment may lead to morbidity or mortality of the individual, or others, either directly (e.g. aircraft accident) or indirectly (e.g. due to damage to the environment). Risk may be compounded further by the physical environment in which individuals work. This includes, but is not limited to, acceleration forces in fast jet pilots, thermal loads (firefighters), hypo-/hyperbaric environments (aircrew and divers), or remoteness from healthcare (military, polar workers, miners, mariners etc.).17 Many workers in these environments may be required to undergo periodic maximal fitness tests to continue their employment.
Detailed guidance on the assessment of high-hazard workers is beyond the scope of this article but information has been published previously (mainly in aviation cardiology) on general principles, nuanced risk assessment, and the approach to sub-specialty cardiology in this cohort.18 Specific hazards pertinent to various ‘high risk’ professions are provided in Table 1, highlighting why these cases need a differential approach from standard clinical assessment.
Table 1.
Specific hazards pertinent to various ‘high risk’ professions, highlighting why these cases need a differential approach from standard clinical assessment
| Occupation | Environmental/occupational risks | Additional clinical cardiovascular considerations |
|---|---|---|
Commercial transportation:
|
|
|
Emergency services
|
|
Effect on environment on occult cardiovascular disease |
| Divers |
|
Effect on environment on occult cardiovascular disease |
| Military |
|
Serious event may become life-threatening without timely access to appropriate specialist care |
Isolated/Remote workers
|
|
|
Such as navigators and weapons systems operators of high-performance aircraft, or others who have a mission critical role.
General evaluation of high-hazard employees post-COVID-19 infection
The effects of COVID-19 infection will vary amongst employees.19 The majority who are symptomatic will develop only mild symptoms and will have no clinical sequelae. However, some will be hospitalized and a proportion of those may receive high-dependency or critical care [high-flow oxygen/CPAP/non-invasive ventilation (NIV)/invasive ventilation]. Additionally, some may develop severe symptoms in the community, but not present to healthcare services.
The longer-term effects of COVID-19 infection (post-COVID-19 syndrome) remain poorly understood, but based on evidence to date, are more likely to be significant in those with more fulminant presentations (hospitalized or not), and with known risk factors for more severe disease (increased age, elevated BMI, pre-existing cardiopulmonary disease, diabetes, and cancer).3–6
It is essential that individuals who work in high-hazard occupations who have had previous confirmed, or presumed, symptomatic COVID-19 infection are appropriately medically risk assessed prior to return to work or training. This is to ensure exclusion of significant pathology and support appropriate rehabilitation,12 to optimize return to work, and, where required, to ensure appropriate work restrictions/graduated-return-to-work programmes are implemented.
Any individual who has acute cardiovascular symptoms, such as arrhythmia, heart failure, or chest pain should be treated accordingly and noting that acute COVID-19 has been associated with heart muscle disease, increased acute coronary syndromes and arrhythmias. In this instance, extensive investigation is likely in those who undertake high-hazard occupations, and depending on the acute cardiovascular manifestations thorough imaging [likely echocardiography, cardiac magnetic resonance imaging (MRI) and potentially CT coronary angiography] and electrophysiological testing [exercise electrocardiogram (ECG), Holter etc.] may be required both in the acute phase and prior to consideration of return to work.
Out with those individuals with an acute cardiac presentation, any individuals who have had a symptomatic COVID-19 infection should be initially reviewed by occupational health departments or primary healthcare providers to triage their clinical or rehabilitation needs, and to assess fitness to return to unrestricted work or training. The employer has a responsibility to identify workers that might require this review and to refer them to the appropriate primary/occupational facility for initial assessment.
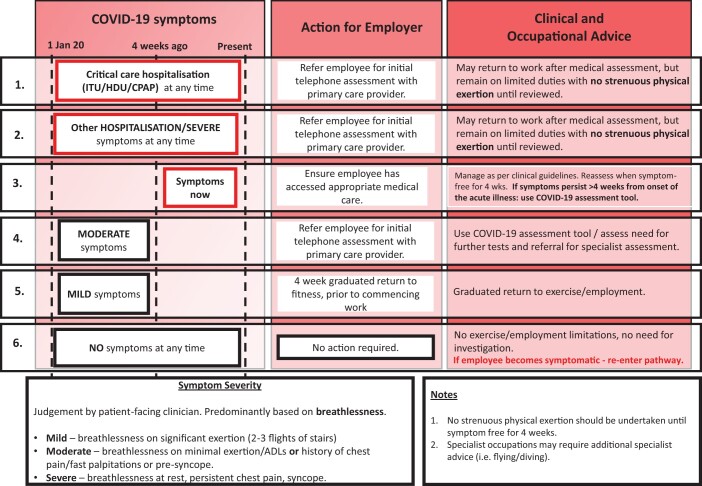
All workers that have had a confirmed or a presumed symptomatic COVID-19 infection (i.e. have had COVID-19-like symptoms) should undergo triage (as outlined in Figure 1). Where individuals have had no symptoms at any time, no action (beyond initial isolation in accordance with infection control guidance) is required.
Figure 1.
An example flow chart for post-COVID-19 risk assessment for high-hazard employers to use for their employees.
Workers who are currently unwell with COVID-19 symptoms or are <4 weeks into their recovery since the onset of symptoms, should continue to be managed in accordance with local clinical guidelines for acute COVID-19 infection. The employer should consider establishing procedures to check that symptomatic employees have accessed appropriate acute medical care. Both clinical and rehabilitation pathways must be in place for high-hazard employees that have had COVID-19.
The threshold for referral to clinical assessment will be determined by the employer taking into account the employee’s specific role, their working environment and the organizational risk threshold of the employer, or statutory body [e.g. European Aviation Safety Agency (EASA) standards for aircrew]. This enables the employer (who has a duty of care to their employees) to sign off an employee to undertake their employment, even if asymptomatic, given that subclinical disease may be occupationally significant. Organizations whose primary role is to deliver outputs that rely on high-hazard activities should consider dedicated services to support their employees.20–22
An example flow chart for post-COVID-19 risk-assessment for high-hazard employees is shown in Figure 1.
Initial clinical assessment of high-hazard employees post-COVID-19 infection
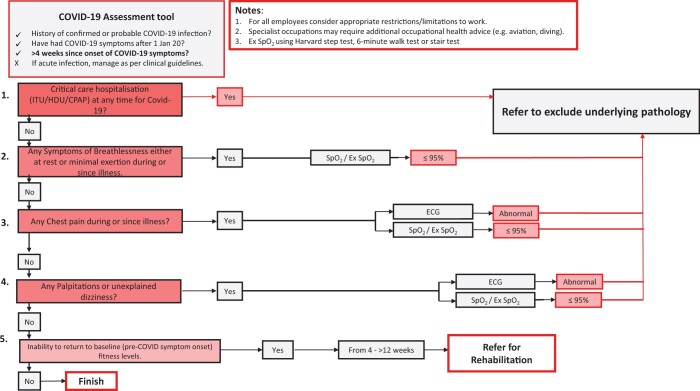
Initial clinical assessment is designed to be simple and to capture the severity of COVID-19 infection amongst high-hazard employees whilst identifying those with persistent symptoms or signs that may impact on occupational function (as outlined in Figure 2). These employees can be directed to the appropriate clinical and/or rehabilitation pathway, as required. Prior to any assessment, it is necessary to exclude active COVID-19 with appropriate molecular testing.
Figure 2.
A flow chart for clinical risk triage based on symptoms, evidence of hypoxia, or electrocardiogram abnormalities.
SARS-CoV-2 is associated with autonomic dysregulation. This is characterized by a resting tachycardia, brisk heart rate response (both to the orthostatic challenge of standing and to light exercise), and blunted chronotropic recovery following exertion. This may lead to, or compound the sensation of, fatigue, breathlessness, and physical debility.23,24
High-hazard employees identified as needing further evaluation should be assessed with simple clinical investigations, performed in primary/occupational healthcare settings. This includes assessment of oxygen saturations (SpO2) at rest and with a simple, standardized exercise stress [e.g. Harvard step test, 6-min walk test (6-MWT), three flights of stairs etc.] and a 12-lead ECG.
Employees with high-risk characteristics (including high-flow oxygen or ventilatory support, pulmonary embolus during admission, elevated cardiac enzymes, pathological ECG changes, left ventricular dysfunction) in the acute phase of COVID-19 illness, and those with ongoing peripheral oxyhaemoglobin desaturation should be referred for further assessment.
Individuals who have not been hospitalized and are free of high-risk characteristics appear to have a low likelihood of cardiopulmonary pathology, including those who meet the criteria for post-COVID-19 syndrome. Additionally, also in individuals who have required hospitalization for COVID-19, exercise limitation and persisting symptoms at discharge might not reflect residual cardiovascular or lung involvement but rather muscle disuse or deconditioning.25 However, even in the absence of definitive cardiopulmonary pathology, symptoms of breathlessness, fatigue, and cognitive impairment (often termed‘brain-fog’) may well require support from rehabilitation services and could impact on high-hazard employees’ ability to safely undertake their usual role, without restrictions or limitations.
A flow chart for clinical risk triage based on symptoms, evidence of hypoxia, or ECG abnormalities, is shown in Figure 2.
Specialist cardiopulmonary evaluation of high-hazard employees posts-COVID-19 infection
High-hazard employees with physiological deficits [reduced oxyhaemoglobin saturation (SpO2) at rest or on exercise] or an abnormal 12-lead ECG who are 4 weeks or more post-acute COVID-19 infection require further investigations to exclude underlying pathology (usually cardiopulmonary, but also more rarely neurological) and should be referred to a clinical service that can confirm or exclude significant underlying cardiopulmonary disease.20
Clinical assessment based on the objective measurement of peak exercise capacity, is central to the assessment of individuals undertaking high-hazard occupations,26 and in addition to a standard 12-lead ECG, is safe, cheap, and reproducible. Importantly, it is also readily scalable and prognostically powerful.27–31
Evidence from post-COVID-19 clinics20 also suggests that established principles of risk assessment and subsequent investigation guided by history, physical examination, ECG, and functional capacity should be adhered to. For example, echocardiography is not a useful ‘screening’ investigation in this setting (several months post-infection, in those with no evidence of heart failure), with a diagnostic yield, which does not differ from the ‘background rate’ expected in a healthy population. Finding unrelated abnormalities in otherwise healthy individuals has the potential to do more harm than good.32
For high-hazard employees formal cardiopulmonary exercise testing (CPET) with SpO2 measurement should be regarded as the gold-standard measure of cardiorespiratory fitness.27,28 Where peak capacity is limited, CPET can identify the physiological component responsible (circulatory, ventilatory, or peripheral) and in most cases will refine the differential diagnosis. Cardiopulmonary exercise testing is well established for the evaluation of unexplained breathlessness33–35 and can also reveal disordered breathing (e.g. inappropriate hyperventilation), which is associated with breathlessness in the cohort of post-COVID 19 employees troubled by fatigue and anxiety36 or might reflect chemoreflex activation.25 Where resources permit, the ability to detect an elevated alveolar-arterial oxygen gradient with a capillary blood gas in addition to CPET has shown the potential to enhance sensitivity to detect significant lung disease.25
Six-minute walk tests, whilst easy to conduct and well-standardized, are a sub-maximal test and do not appear to provide sensitive discrimination between individuals with, vs. without, significant clinical pathology. If formal CPET is unavailable, alternative maximal exercise assessment, such as a ramped bike protocol is a robust alternative, with the achievement of >85% age-predicted maximal workload being a reliable means to exclude significant underlying pathology. Maximal testing also affords the additional benefit of being able to measure the peak heart rate and heart rate recovery over 60 s following peak exertion, which combined with the resting heart rate can aid in the discrimination of autonomic dysfunction.
Specialist cardiopulmonary imaging of high-hazard employees post-COVID-19 infection
In cases where CPET is abnormal, or gross ECG changes (such as deep T-wave inversion, ST changes, or arrhythmia on 12-lead) are present, further sub-specialty imaging (usually with HRCT, cardiac MRI, and occasionally CT coronary angiography) may be required, to assess the lungs or heart, or both. More recent published evidence amongst healthcare workers and sportsmen suggests that the incidence of significant myocardial injury is small (low single %)9,10,27,37 which reflects findings in the UK military population, and cardiovascular magnetic resonance should be used judiciously.38
Equally, those who are found to have pulmonary fibrosis, often have little or no abnormality on spirometry and the limited scarring seen in many individuals is likely to have little, if any, physiological impact, unless extensive. A high volume of fibrosis is relatively uncommon, even in individuals who were treated with prolonged mechanical ventilation and the physiological effect of minor scarring/fibrosis is unlikely to have major occupational ramifications, especially in the absence of exercise hypoxaemia or limited functional capacity on a maximal test.
In individuals who undertake high-hazard employment in extreme environments (such as high-performance aircrew or divers), further environmental assessment may be deemed necessary to ensure employees meet their employers risk tolerance in these climates. Further testing to ensure sub-clinical pathology of the heart, or lung, do not have occupational consequences (e.g. via centrifuge, reduced oxygen breathing devices and/or pressure chamber assessment) may need to be considered. Additionally, the effect of subtle neurological or cognitive impairment, anxiety and psychological/psychiatric effects of disease may also require close assessment (with functional tests, such as in the cockpit, or for weapon handling) with initial (and hopefully temporary) occupational restrictions being required to ensure safety of both the individual and those they work with.
Summary
Emerging evidence suggests a relatively low likelihood of significant cardiopulmonary pathology in those with symptomatic COVID-19 infection.
Following office-based assessment with simple first line investigations, employees with high-risk characteristics and those with peripheral oxyhaemoglobin desaturation should be referred for further assessment.
Further assessment should be based around assessment of peak exercise capacity, ideally CPET. A normal CPET is highly reassuring. It should be noted that echocardiography, spirometry and 6-MWTs do not appear to add value and should only be performed if there is a usual clinical indication to do so. They are not recommended as routine screening tools for those with post-COVID-19 symptoms.
The incidence of significant cardiopulmonary pathology (such as myocarditis and lung fibrosis) appears to be low post-COVID-19. Even in those with abnormal cardiovascular MRI and thoracic CT results the severity of pathology appears mild in most cases and is likely to be of limited long-term consequence for most.
However, careful discussion between occupationally informed clinicians is required to put the imaging, clinical and physiological data, and occupational risk into context and to determine whether an organization’s risk threshold has been exceeded. It is likely that non cardiopulmonary symptoms, such as fatigue and cognitive impairment may have a greater impact on return to work for high-hazard employees than those due to underlying cardiopulmonary pathology.
Conflict of interest: none declared.
References
- 1. Elliott J, Whitaker M, Bodinier B, Riley S, Ward H, Cooke G, Darzi A, Chadeau-Hyam M, Elliott P.. Predictive symptoms for COVID-19 in the community: REACT-1 study of over 1 million people. PLoS Med 2021;18:e1003777. 10.1371/journal.pmed.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 3. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, Alsukait RF, Alluhidan M, Alazemi N, Shekar M.. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev 2020;21:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC.. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020;17:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lim S, Bae JH, Kwon HS, Nauck MA.. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol 2021;17:11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muhamad SA, Ugusman A, Kumar J, Skiba D, Hamid AA, Aminuddin A.. COVID-19 and hypertension: the what, the why, and the how. Front Physiol 2021;12:665064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ.. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol 2021;6:116–118. Erratum in: JAMA Cardiol 2021 Jan 1;6(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E.. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–1273. Erratum in: JAMA Cardiol 2020 Nov 1;5(11):1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hendrickson BS, Stephens RE, Chang JV, Amburn JM, Pierotti LL, Johnson JL, Hyden JC, Johnson JN, Philip RR.. Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: results of an algorithm-guided screening. Circulation 2021;143:1926–1928. [DOI] [PubMed] [Google Scholar]
- 10. Berry C, Mangion K.. Cardiovascular complications are very uncommon in healthcare workers with mild or asymptomatic COVID-19 infection. JACC Cardiovasc Imaging 2021;14:2167–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halle M, Bloch W, Niess AM, Predel H-G, Reinsberger C, Scharhag J, Steinacker J, Wolfarth B, Scherr J, Niebauer J.. Exercise and sports after COVID-19—guidance from a clinical perspective. Transl Sports Med 2021;4:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021 (24 May 2021).
- 13. Demeco A, Marotta N, Barletta M, Pino I, Marinaro C, Petraroli A, Moggio L, Ammendolia A.. Rehabilitation of patients post-COVID-19 infection: a literature review. J Int Med Res 2020;48:300060520948382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Institute for Healthcare Excellence. Guideline COVID-19 rapid guideline: managing the long-term effects of COVID-19NICE guideline [NG188]. 2020. https://www.nice.org.uk/guidance/ng188(24 May 2021).
- 15. Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, Reeves WC, Lloyd A; Dubbo Infection Outcomes Study Group. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 2006;333:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pan K-Y, Kok AAL, Eikelenboom M, Horsfall M, Jörg F, Luteijn RA, Rhebergen D, Oppen P. V, Giltay EJ, Penninx BWJH.. The mental health impact of the COVID-19 pandemic on people with and without depressive, anxiety, or obsessive-compulsive disorders: a longitudinal study of three Dutch case-control cohorts. Lancet Psychiatry 2021;8:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chamley RR, Holdsworth DA, D'arcy JL, Nicol ED.. An Introduction to Occupational Cardiology. Eur Heart J 2019;40:2389–2392. [DOI] [PubMed] [Google Scholar]
- 18. Holdsworth DA, Eveson LJ, Manen O, Nicol ED.. Assessment of clinical and occupational cardiovascular risk. Eur Heart J 2019;40:2392–2395. [DOI] [PubMed] [Google Scholar]
- 19. O'Sullivan O. Long-term sequelae following previous coronavirus epidemics. Clin Med (Lond) 2021;21:e68–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Sullivan O, Barker-Davies R, Chamley R, Sellon E, Jenkins D, Burley R, Holden L, Nicol AM, Phillip R, Bennett AN, Nicol E, Holdsworth DA.. Defence medical rehabilitation centre (DMRC) COVID-19 recovery service. BMJ Mil Health 2021;e001681. [DOI] [PubMed] [Google Scholar]
- 21. Panait CI, Peco-Arregui C; on behalf of the Medical Expert Group. European Aviation Safety Agency (EASA). Guidelines for Aero-Medical Centres and Aeromedical Examiners regarding the examination and assessment of applicants: guidelines in relation to the COVID-19 pandemic. 2021. https://www.easa.europa.eu/sites/default/files/dfu/guidelines_for_aemcs_and_ames_regarding_the_examination_and_assessment_of_applicants.pdf (15 June 2021).
- 22. The Diving Medical Advisory Committee. Return to diving after Covid-19. 2020. http://www.dmac-diving.org/guidance/DMAC33.pdf (15 June 2021).
- 23. Goodman BP, Khoury JA, Blair JE, Grill MF.. COVID-19 dysautonomia. Front Neurol 2021;12:624968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barker-Davies RM, O'Sullivan O, Senaratne KPP, Baker P, Cranley M, Dharm-Datta S, Ellis H, Goodall D, Gough M, Lewis S, Norman J, Papadopoulou T, Roscoe D, Sherwood D, Turner P, Walker T, Mistlin A, Phillip R, Nicol AM, Bennett AN, Bahadur S.. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med 2020;54:949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chilazi M, Duffy EY, Thakkar A, Michos ED.. COVID and cardiovascular disease: what we know in 2021. Curr Atheroscler Rep 2021;23:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holdsworth DA, Chamley RR, Rider OJ, Nicol ED.. The importance of exercise testing in occupational cardiovascular assessment for high-hazard professions. Eur Heart J 2019;40:3078–3080. [DOI] [PubMed] [Google Scholar]
- 27. Baratto C, Caravita S, Faini A, Perego GB, Senni M, Badano LP, Parati G.. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J ApplPhysiol (1985) 2021;130:1470–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE.. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
- 29. Kannel WB. Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am Heart J 1985;110:1100–1107. [DOI] [PubMed] [Google Scholar]
- 30. Leon AS, Connett J, Jacobs DR, Rauramaa R.. Leisure-time physical activity levels and risk of coronary heart disease and death. The Multiple Risk Factor Intervention Trial. JAMA 1987;258:2388–2395. [PubMed] [Google Scholar]
- 31. Paffenbarger RS Jr, Hyde RT, Hsieh CC, Wing AL.. Physical activity, other life-style patterns, cardiovascular disease and longevity. Acta Med Scand Suppl 1986;711:85–91. [DOI] [PubMed] [Google Scholar]
- 32. Shaikh U, Lewis-Jones H.. VOMIT—victim of medical investigative technology. BMJ 2008;336:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palange P, Ward SA, Carlsen K-H, Casaburi R, Gallagher CG, Gosselink R, O'Donnell DE, Puente-Maestu L, Schols AM, Singh S, Whipp BJ; ERS Task Force. Recommendations on the use of exercise testing in clinical practice. Eur Respir J 2007;29:185–209. [DOI] [PubMed] [Google Scholar]
- 34. Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J; EACPR; AHA. EACPR/AHA Joint Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J 2012;33:2917–2927. [DOI] [PubMed] [Google Scholar]
- 35. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 36. Brat K, Stastna N, Merta Z, Olson LJ, Johnson BD, Cundrle I.. Cardiopulmonary exercise testing for identification of patients with hyperventilation syndrome. PLoS One 2019;14:e0215997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, Jeudy J, Mattson SE, Law IH, Borchers J, Kovacs R, Kovan J, Rifat SF, Albrecht J, Bento AI, Albers L, Bernhardt D, Day C, Hecht S, Hipskind A, Mjaanes J, Olson D, Rooks YL, Somers EC, Tong MS, Wisinski J, Womack J, Esopenko C, Kratochvil CJ, Rink LD, Simonetti O, Zareba K, Bhatti S, Addison D, Obarski T, Daoud E, Granger M, Smart S, Mayercin-Johnson J, Subramanian P, Glitt J, Mitchell D, Chumita R, Mumford A, Garcia A, Garris L, Liu H, Hatfield B, Zhang Y, Boersma D, Schlader Z, Goodwin S, Port N, Zuidema T, Maldonado J, Eckhardt L, Reeder S, Baker M, Sebastianelli W, Wadlinger R, Millard R, Bosha P, Sunday H, Steele D, Chaudhry A, Smith S, Pfeiffer M, Kellerman J, Billy G, Krystofiak J, Eimer M; Big Ten COVID-19 Cardiac Registry Investigators. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection. Results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol 2021;6:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Udelson JE, Rowin EJ, Maron BJ.. Return to play for athletes after COVID-19 infection: the fog begins to clear. JAMA Cardiol 2021;6:997. [DOI] [PubMed] [Google Scholar]