ABSTRACT
Novel coronavirus disease infection (coronavirus disease 2019, COVID-19) was declared a global pandemic in March 2020 and since then has become a major public health problem. The prevalence of COVID-19 infection and acute kidney injury (AKI) is variable depending on several factors such as race/ethnicity and severity of illness. The pathophysiology of renal involvement in COVID-19 infection is not entirely clear, but it could be in part explained by the viral tropism in the kidney parenchyma. AKI in COVID-19 infection can be either by direct invasion of the virus or as a consequence of immunologic response. Diverse studies have focused on the effect of COVID-19 on glomerulonephritis (GN) patients or the ‘novo’ GN; however, the effect of COVID-19 in acute tubulointerstitial nephritis (ATIN) has been scarcely studied. In this article, we present five cases with different spectrums of COVID-19 infection and ATIN that may suggest that recent diagnosis of ATIN is accompanied by a worse clinical prognosis in comparison with long-term diagnosed ATIN.
Keywords: acute kidney injury, acute tubulointerstitial nephritis, COVID-19, kidney biopsy, SARS-CoV-2
Graphical Abstract
INTRODUCTION
Novel coronavirus disease infection (coronavirus disease 2019, COVID-19) was declared a global pandemic in March 2020 and since then has become a major public health problem to both out- and inpatients [1]. COVID-19 infection presents from its asymptomatic form to pneumonia associated with multiple organ failure and death. The prevalence of COVID-19 infection and acute kidney injury (AKI) varies between 20 and 80% of cases, and this may be in part ascribed to the clinical characteristics of patients and their geographical location [2, 3].
Several publications have focused on the pathogenesis of kidney injury in the setting of COVID-19 [2]. AKI appears to have different aetiologies such as prerenal azotemia, tubular injury, cytokine storm, microangiopathy and virus-mediated injury [4] (Figure 1). The pathophysiology of renal involvement in COVID-19 infection is not entirely clear, but it seems that kidney injury could result from direct damage to the parenchyma, sepsis, thrombotic phenomena and treatment complications [1, 2, 4]. In addition, other authors consider that kidney disease is more likely related to the systemic and proinflammatory effect of sepsis instead of direct damage from viral-like particles in the kidney parenchyma [5]. Taking this into consideration, in our opinion, the mechanisms of AKI secondary to COVID-19 are mainly multifactorial, involving both direct and indirect viral damage. Histologically, COVID-19 infection develops a wide spectrum of glomerular and tubular diseases, with acute tubular necrosis being the most frequent kidney lesion, and focal and segmental collapsing glomerulonephritis (GN) the most common glomerulopathy [6, 7]. Whereas some authors have identified viral-like particles by electron microscopy or by in situ hybridization of RNA severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the kidney with unknown clinical significance, other authors have not been able to identify SARS-CoV-2 viral particles in COVID-19 patients with associated kidney injury [7, 8, 9].
Figure 1:
Spectrum of kidney damage in COVID-19 infection. The kidney damage lesions in the different compartments namely glomeruli, tubuli and renal vasculature associated with COVID-19 infection are shown in this picture. Interestingly, COVID-19 infection in the kidney has been associated with several diseases such as focal and segmental glomerulosclerosis, acute interstitial injury and thrombotic microangiopathy.
Acute tubulointerstitial nephritis (ATIN) is a common cause of AKI and presents with inflammatory infiltrates and oedema in histological samples. Pharmacological exposure is the most frequent aetiology; however, bacterial and viral infections are also important, accounting for 5–10% of cases [10]. High clinical suspicion is needed to diagnose ATIN since the initial phase of ATIN does not typically manifest with oliguric AKI and haematuria. A proper and fast diagnosis of ATIN is required because the disease is reversible when early corticosteroid treatment is started [2, 10]. ATIN patients started kidney function recovery within a few weeks and complete improvement of baseline kidney function after 5–8 weeks of steroid treatment. Maintenance of steroid treatment should be shortened to 2–3 months, avoiding the secondary effects related to steroids [11].
Currently, there is limited scientific literature on COVID-19 and ATIN in terms of its clinical course and short- and long-term prognosis. In this article, we present five cases of COVID-19 infection and ATIN, and review current evidence on AKI and COVID-19 infection.
MATERIALS AND METHODS
We performed a retrospective study of medical records of 60 patients with histological diagnosis of ATIN from January 2013 to November 2020. In these patients, we studied the prevalence of COVID-19 infection, clinical characteristics and prognosis from March to December 2020. In addition, we reviewed the published literature in PubMed up to 19 July 2021. The search strategy included the terms ‘acute tubulointerstitial nephritis,’ ‘acute kidney injury and COVID,’ ‘COVID-19,’ ‘coronavirus’ and ‘SARS-CoV2’ without language or type of article restrictions.
RESULTS
Of 60 patients with ATIN, 5 (8.3%) patients were diagnosed with COVID-19 (Table 1]. The characteristics of the five patients are detailed below.
Table 1.
Clinical characteristics of our five patients with COVID-19 infection and ATIN
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age (years) | 85 | 64 | 82 | 75 | 86 |
| Gender | Female | Male | Female | Male | Male |
| ATIN cause | Nivolumab AntiLAG3 |
COVID-19 infection | Ciprofloxacin | MEK/Braf inhibitors | Proton-binding inhibitors |
| ATIN treatment: steroids (pulses) | Yes (Yes) | Yes (Yes) | Yes (Yes) | Yes (Yes) | Yes (Yes) |
| Cumulative dose of prednisone (mg/kg) | 82.4 | 48.4 | 5.7 | 61.8 | 61.8 |
| Date of positive COVID-19 swab test | 23 April 2020 | 23 September 2020 | 10 October 2020 | 24 April 2020 | 11 December 2020 |
| Time from ATIN to COVID-19 diagnosis from ATIN to COVID-19 diagnosis (months) | 19 | 0 | 0 | 13 | 0 |
| Basal serum creatinine (mg/dL) | 1.6 | 0.9 | 0.8 | 1.7 | 1.6 |
| Serum creatinine (mg/dL) in COVID-19 diagnosis | Unknown | 0.9 | 0.9 | 2.4 | 4.9 |
| Serum creatinine (mg/dL) 7 days after COVID-19 infection | Unknown | 2.4 | 0.5 | 2.4 | 3.7 |
| Serum creatinine (mg/dL) 3 months after COVID-19 infection | 1.4 | 0.8 | Unknown | 1.8 | 1.7 |
| Death (date) related to COVID-19 infection | NA | NA | 30/10/2020 | NA | NA |
Abbreviations: ATIN, allergic tubulointerstitial nephritis; NA, not applicable.
First case
An 85-year-old woman with a history of scalp melanoma BRAF wild-type gene mutation with pulmonary metastasis that was successfully treated with nivolumab and LAG-3 antibody, who developed ATIN secondary to immunobiological agents in 2018. She was diagnosed with mild COVID-19 in April 2020 with a positive swab test performed in a hospice as screening without indication for hospital admission. Her renal function was stable and maintained after 3 and 6 months of follow-up.
Second case
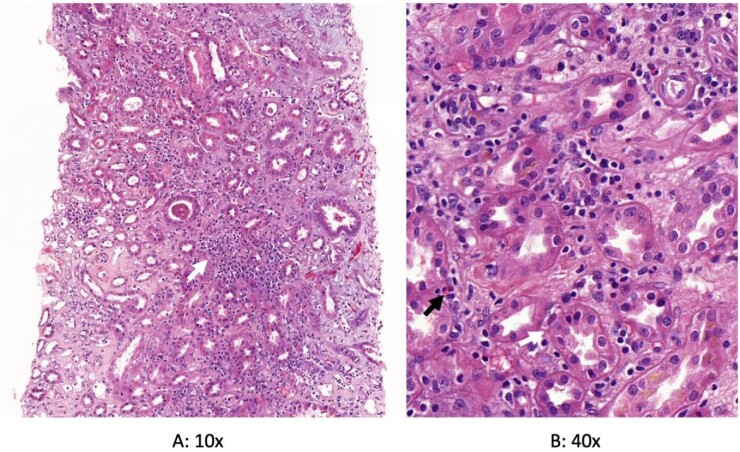
A 64-year-old man without prior renal impairment presented with acute shortness of breath and fever. He was admitted and diagnosed with COVID-19 infection. Twenty-four hours after admission, he developed severe COVID-19 pneumonia and AKI Akin stage 3 with proteinuria/creatinine ratio of 1.7 g/g (albumin/creatinine 0.13 g/g) without haematuria that required orotracheal intubation and continuous veno-venous haemodiafiltration. During his admission, antibiotic therapy with ceftriaxone, nebulized tobramycin and linezolid was started due to pneumonia secondary to methicillin-resistant Staphylococcus aureus (MRSA). Kidney biopsy was performed and ATIN probably associated with COVID-19 infection was diagnosed. Steroid treatment was initiated with methylprednisolone pulses for 3 days followed by maintenance steroid therapy (mg/kg) with complete recovery of renal function and improvement of proteinuria at 3 months of follow-up (see Figure 2).
Figure 2:
Histopathology of the kidney biopsy performed in the second case. Acute tubulointerstitial nephritis and acute tubular damage are shown in haematoxylin eosin staining. (A) White arrow shows interstitial inflammatory infiltrates in low power light micrograph. (B) White and black arrows show tubulitis and eosinophils, respectively. SARS-CoV-2 immunohistochemistry was negative.
Third case
An 82-year-old woman with a past medical history of hypertension, diabetes mellitus and mixed anxiety-depressive disorder was admitted with AKI Akin stage 3 due to ATIN secondary to ciprofloxacin. After 8 days of her admission, she developed hospital-acquired pneumonia associated with severe COVID-19 infection. The patient died 20 days after the COVID-19 diagnosis despite ventilatory support delivered by warmed and humidified oxygen at high flows through nasal cannula at 90%, 60 L/min, high-dose steroids therapy, remdesivir and tocilizumab.
Fourth case
A 75-year-old man with a past medical history of metastatic lung adenocarcinoma successfully treated with dabrafenib/trametinib complicated with AKI Akin stage 3 secondary to ATIN in 2018. He was admitted in March 2020 with mild COVID-19 pneumonia. A deterioration in kidney function was observed and oxygen support with low flows oxygen (2 L/min) was needed. Dexamethasone and hydroxychloroquine were administered for 5 days with complete recovery of basal renal and pulmonary function. The patient was discharged one week after the admission.
Fifth case
An 86-year-old man with a past medical history of hypertension, diabetes mellitus, atrial fibrillation and chronic kidney disease stage 3b A3 was admitted with deterioration of kidney function due to ATIN secondary to proton-binding inhibitors on December 2020. Eight days after his admission, he developed a nosocomial COVID-19 infection without oxygen requirement or respiratory involvement. After 2 weeks of steroids initiation, kidney function improved and reached basal serum creatinine levels at 3 months.
DISCUSSION
In this article, we described five cases of ATIN and COVID-19 infection in different scenarios: (i) COVID-19 infection diagnosed 1 year after ATIN diagnosis (n = 2), (ii) recently diagnosed ATIN patients who presented nosocomial COVID-19 infection (n = 2) and (iii) ATIN related to COVID-19 infection (n = 1). We defined nosocomial COVID-19 infection in patients with a positive nasopharyngeal swab test and/or development of pneumonia more than 5 days after admission in accordance with the Swissnoso guidelines [12]. Only one patient from the nosocomial COVID-19 group died 20 days after nosocomial infection despite empirical treatment with corticosteroids, remdesivir and tocilizumab. These results may suggest that nosocomial COVID-19 in recently ATIN diagnosed patients may have worse clinical prognosis when compared with longtime ATIN diagnosis. Perhaps this can be explained by the high immunosuppression therapy at the moment of COVID-19 infection. However, further studies are needed to confirm this hypothesis.
During the pandemic, many patients with severe COVID-19 infection presented AKI requiring renal replacement therapy and extracorporeal membrane oxygenation (ECMO) secondary to multiorgan failure. Kidney biopsy in many patients could not be performed due to high hemorrhagic risk; therefore, the real prevalence of ATIN may be underestimated.
ATIN is a common and reversible cause of AKI that sometimes may be underdiagnosed due to the fact that most of the patients present only a mild alteration in kidney function and urinalysis [13]. The following causes of ATIN have been described: (i) use of immunobiological therapies for cancer, (ii) use of antibiotics, (iii) proton-pump inhibitors and (iv) COVID-19 damage. To our knowledge, few cases of ATIN and COVID-19 disease have been published. Recently, May et al. in an international collaborative study where a total of 284 biopsies from COVID-19 patients were evaluated, including 240 native biopsies and 44 allografts, identified 4 (1.7%) patients with histological diagnosis of acute interstitial nephritis in COVID-19 patients [14]. Szajek et al. also described a 62-year-old Caucasian man with cough, fever, myalgia and chills who was admitted for severe acute respiratory distress syndrome and multiorgan failure secondary to COVID-19 infection and AKI with renal replacement therapy [15]. Kidney biopsy was performed and revealed granulomatous tubulointerstitial nephritis thus steroids treatment was initiated with partial recovery of kidney function at 2 months of follow-up. In concordance, our patient with ATIN-related COVID-19 presented a good prognosis with complete kidney function recovery.
There has been a wide spectrum of histological findings in COVID-19 infection. Viral involvement of kidney parenchyma has been shown by Westhoff et al. [16], who presented a 69-year-old male with pancreas–kidney transplantation 13 years ago admitted with COVID-19 pneumonia, and impaired pancreas and kidney allograft function. Tubular damage and an interstitial mononuclear cell infiltrate were present in the kidney biopsy. Reverse transcriptase polymerase chain reaction detected positive SARS-CoV-2 RNA in tubular cells and interstitial parenchyma [16, 17]. Another study that analysed kidney biopsies in 26 autopsies of patients with COVID-19 also identified direct evidence of the invasion of SARS-CoV-2 into kidney tissue with coronavirus-like particles in the tubular epithelium and podocytes. This was associated with upregulation of the angiotensin-converting enzyme (ACE) 2 receptor and positive SARS-CoV nucleoprotein antibody in tubules [18]. In contrast, in our patient with ATIN related-COVID-19, we were not able to detect viral SARS-CoV-2 particles by electron microscope.
Acute tubular injury has been identified as the main cause of AKI in patients with COVID-19 infection secondary to prolonged volume depletion and haemodynamic changes with renal hypoperfusion [19, 20]. The hyperinflammatory state with release of proinflammatory cytokines, especially interleukin-6, is also an exacerbating factor for poor prognosis at the renal tubule and lung since its high concentration is associated with greater respiratory distress [20]. Werion et al. described that COVID-19 infection showed prominent tubular injury with brush border loss, acute tubular necrosis, intraluminal debris and decrease in the expression of megalin; proximal tubule dysfunction was independent of comorbidities, adverse drugs reactions and viral load [21]. Hypouricemia with uricosuria has also been identified as a risk factor for severity and respiratory failure [21].
Collapsing glomerulopathy is the most common glomerular disease, especially in Afro-American people with high-risk genotypes of apolipoprotein L1 (APOL1) [22]. To date, 24 cases of AKI with nephrotic syndrome and COVID-19 infection associated with APOL1 have been described [20, 22]. The pathophysiology still remains uncertain, although it is believed that viral involvement resembles other infections such as HIV-associated nephropathy, Epstein–Barr virus, cytomegalovirus and Parvovirus B19 ascribed to cytokine storm release and podocyte involvement due to upregulated viral infection that results in an inflammatory ‘second hit’ [23, 24]. Furthermore, León-Román et al. described a case of non-collapsing focal and segmental glomerulosclerosis in a 56-year-old patient with nephrotic syndrome and mild COVID-19 infection in which treatment with corticosteroids was started and cyclosporine was added after 12 weeks due to persistent proteinuria [8].
The incidence of disseminated intravascular coagulation reaches 75% in patients with severe COVID-19 infection. In contrast, there are limited clinical cases with the development of thrombotic microangiopathy (TMA) in which cortical necrosis and glomerular microthrombosis are seen in the biopsy [20]. The development of coagulopathy in COVID-19 infection is not fully clarified despite the fact that many studies associate it with sepsis [25]. TMA secondary to COVID-19 infection is similar to systemic diseases such as systemic lupus erythematosus, antiphospholipid syndrome, typical and atypical haemolytic uremic syndrome and thrombotic thrombocytopenic purpura [26]. COVID-19 infection seems to involve complement and platelet activation without observing platelet consumption. One of the suggested mechanisms for complement activation has been the binding of the nucleocapsid protein to a protease of the lectin-dependent complement pathway [26].
To date, few cases of acute interstitial nephritis have been described as a result of SARS-CoV-2 viral damage and/or pharmacological treatment [20–27]. For this reason, the prognosis of COVID-19 in ATIN patients is unknown in accordance with the scarcity of literature data.
Our study has some limitations. First, the retrospective character of the study, and second the small sample size (n = 5) of ATIN patients with COVID-19 infection. However, we would like to highlight the importance that this study was performed in one of the centres with highest COVID-19 incidence in Spain and which is the reference centre for ECMO in Barcelona [28].
Our data expand the current knowledge of ATIN and COVID-19 in three different scenarios: (i) long-term ATIN patients that presented SARS-CoV-2 infection, (ii) recent ATIN diagnosis that developed nosocomial SARS-CoV-2 infection and (iii) ATIN diagnosed in patients with severe SARS-CoV-2 infection. In our study, the recent ATIN diagnosis patient that presented with nosocomial COVID-19 disease died. This may suggest a poor prognosis in recently diagnosed older patients undergoing intensive steroids treatment. However, further studies are needed to assess the effect of COVID-19 on newly diagnosed ATIN.
CONCLUSIONS
COVID-19 infection affects different sites of the kidney parenchyma (Figure 1]. Our five patients with ATIN and COVID-19 infection presented different spectrums of the disease. In our opinion, it seems that nosocomial COVID-19 infection in patients admitted with recent diagnosis of ATIN presented a worse clinical prognosis when compared with long-term diagnosed ATIN. Further multicentric studies including a larger sample size would be of great interest.
ACKNOWLEDGEMENTS
The article is dedicated to all health care personnel who have contributed to the investigation and treatment of COVID-19 infection.
Contributor Information
Juan León-Román, Vall d'Hebron University Hospital, Department of Nephrology, Barcelona, Spain.
Irene Agraz, Vall d'Hebron University Hospital, Department of Nephrology, Barcelona, Spain.
Ander Vergara, Vall d'Hebron University Hospital, Department of Nephrology, Barcelona, Spain.
Natalia Ramos, Vall d'Hebron University Hospital, Department of Nephrology, Barcelona, Spain.
Nestor Toapanta, Vall d'Hebron University Hospital, Department of Nephrology, Barcelona, Spain.
Clara García-Carro, Clinico San Carlos University Hospital, Department of Nephrology, Madrid, Spain.
Alejandra Gabaldón, Vall d'Hebron University Hospital, Department of Pathology, Barcelona, Spain.
Roxana Bury, Vall d'Hebron University Hospital, Department of Nephrology, Barcelona, Spain.
Sheila Bermejo, Vall d'Hebron University Hospital, Department of Nephrology, Barcelona, Spain.
Oriol Bestard, Vall d'Hebron University Hospital, Department of Nephrology, Barcelona, Spain; Nephrology Research Group, Vall d'Hebron Research Institute (VHIR), Nephrology.
María José Soler, Vall d'Hebron University Hospital, Department of Nephrology, Barcelona, Spain; Nephrology Research Group, Vall d'Hebron Research Institute (VHIR), Nephrology; Red de Investigación Renal (REDINREN), Instituto Carlos IIIFEDER, Madrid, Spain.
Funding
This research was funded by ISCIIII-FEDER and ISCIII-RETICS REDinREN, grant number PI17/00257, PI21/01292, RD16/0009/0030, and RICORS RD21/0005/0016. Enfermedad Glomerular Compleja del Sistema Nacional de Salud (CSUR), enfermedades glomerulares complejas.
AUTHORS’ CONTRIBUTIONS
J.L.R. and M.J.S. collaborated on the original idea and study design. I.A., A.V., N.R., N.T., C.G.C., R.B., S.B., and O.B. contributed to the inclusion of patients. J.L.R. and M.J.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST STATEMENT
M.J.S. reports conflicts of interest with fees from NovoNordisk, personal fees from Janssen, grants and non-financial support from Boehringer, non-financial support from Eli Lilly, personal fees from AstraZeneca, non-financial support from Esteve, personal fees from Fresenius, personal fees from Mundipharma, personal fees from Pfizer, personal fees from Bayer and personal fees from Vifor, outside the submitted work. M.J.S. is member of the CKJ editorial board. The results presented in this article have not been published previously in whole or part, except in abstract format.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Farouk S, Fiaccadori E, Cravedi Pet al. COVID-19 and the kidney: what we think we know so far and what we don't. J Nephrol 2020; 33: 1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ng JH, Hirsch JS, Hazzan Aet al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis 2021; 77: 204–215.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirsch J, Mersema A, Ross D, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batlle D, Soler MJ, Sparks MAet al. COVID-19 and ACE2 in cardiovascular, lung, and kidney working group. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 2020; 31: 1380–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golmai P, Larsen CP, DeVita MVet al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol 2020; 31: 1944–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kudose S, Batal I, Santoriello Det al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 2020; 31: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nasr SH, Alexander MP, Cornell LDet al. Kidney biopsy findings in patients with COVID-19, kidney injury, and proteinuria. Am J Kidney Dis 2021; 77: 465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. León-Román J, Vergara A, Agraz Iet al. Glomeruloesclerosis focal y segmentaria asociada a infección por COVID-19. Nefrología 2021; 41: 706–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma P, Uppal NN, Wanchoo Ret al. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol 2020; 31: 1948–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Praga M, González E. Acute interstitial nephritis. Kidney Int 2010; 77: 956–961 [DOI] [PubMed] [Google Scholar]
- 11. Fernandez-Juarez G, Perez JV, Caravaca-Fontán Fet al. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol 2018; 13: 1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abbas M, Robalo Nunes T, Cori Aet al. Explosive nosocomial outbreak of SARS-CoV-2 in a rehabilitation clinic: the limits of genomics for outbreak reconstruction. J Hosp Infect 2021; 117: 124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng J, Zaidan M, Jhaveri Ket al. Acute tubulointerstitial nephritis and coronavirus. Clin Kidney J 2021; 14: 2151–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. May RM, Cassol C, Hannoudi Aet al. A multi-center retrospective cohort study defines the spectrum of kidney pathology in coronavirus 2019 disease (COVID-19). Kidney Int 2021; 100: 1303–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Szajek K, Kajdi ME, Luyckx VAet al. , Granulomatous interstitial nephritis in a patient with SARS-CoV-2 infection. BMC Nephrol 2021; 22: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westhoff TH, Seibert FS, Bauer Fet al. Allograft infiltration and meningoencephalitis by SARS-CoV-2 in a pancreas-kidney transplant recipient. Am J Transplant 2020; 20: 3216–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hassler L, Reyes, F, Sparks MAet al. Evidence for and against direct kidney infection by SARS-CoV-2 in patients with COVID-19 Clin J Am Soc Nephrol 2021; 16: 1755–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Su H, Yang M, Wan Cet al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xia P, Wen Y, Duan Yet al. Clinicopathological features and outcomes of acute kidney injury in critically ill COVID-19 with prolonged disease course: a retrospective cohort. J Am Soc Nephrol 2020; 31: 2205–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ng JH, Bijol V, Sparks MAet al. Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis 2020; 27: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Werion A, Belkhir L, Perrot Met al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int 2020; 98: 1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kissling S, Rotman S, Gerber Cet al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int 2020; 98: 228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santoriello D, Khairallah P, Bomback ASet al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol 2020; 31: 2158–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wyatt CM, Klotman PE, D'Agati VD. HIV-associated nephropathy: clinical presentation, pathology, and epidemiology in the era of antiretroviral therapy. Semin Nephrol 2008; 28: 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sweeney JM, Barouqa M, Krause GJ, Gonzalez-Lugo JD, Rahman S, Gil MR. Evidence for secondary thrombotic microangiopathy in COVID-19. medRxiv : the preprint server for health sciences2020; 2020.10.20.20215608. 10.1101/2020.10.20.20215608medRxiv medRxiv [DOI] [Google Scholar]
- 26. Merrill JT, Erkan D, Winakur J, James JA. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol 2020; 16: 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Portolés J, Marques M, López-Sánchez Pet al. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dialysis Transplant 2020; 35: 1353–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrer R, Báguena M, Balcells Jet al. Planning for the assistance of critically ill patients in a pandemic situation: the experience of Vall d'Hebron university hospital. Enfermedades Infecciosas y Microbiología Clínica 2022; 40: 71–77(Engl Ed). S0213-005X(20)30272-X. English, Spanish. https://doi:10.1016/j.eimc.2020.08.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.