Abstract
Objectives
Humoral response to vaccines in RA patients treated with rituximab (RTX) in standard dosages (≥1000 mg) is decreased. Ultra-low dosages (500 or 200 mg) may have better response. Also, timing after latest RTX infusion may be an important variable. We aimed to investigate the influence of RTX dosage and timing on response to COVID-19 vaccination in RA patients.
Methods
A single-centre observational study (n = 196) investigated the humoral response, measured by total Ig anti-COVID-19 assay (positive response ≥1.1), 2–6 weeks after complete COVID-19 vaccination. A multivariable logistic regression model was built to study the effect of RTX dosage and time between latest rituximab and vaccination on response, adjusting for age and methotrexate use.
Results
After two-dose vaccination, the response rate was significantly better for patients receiving 200 mg (n = 31, 45%) rituximab compared with 1000 mg (n = 98, 26%; odds ratio 3.07, 95% CI 1.14–8.27) and for each additional month between latest rituximab and vaccination (OR 1.67, 1.39–2.01).
Conclusion
Both increased time between latest rituximab infusion and complete vaccination, and 200 mg as latest dose were associated with a better response to COVID-19 vaccination and should be considered when trying to increase vaccine response after rituximab in RA patients.
Trial registration
Netherlands Trial Register, https://www.trialregister.nl/, NL9342.
Keywords: RA, rituximab, biologic therapies, COVID-19, vaccination
Rheumatology key messages.
Overall response to COVID-19 vaccine was low in rheumatoid arthritis patients treated with rituximab.
Response was significantly better in patients treated with 200 mg than with higher doses of rituximab.
Increased time between latest rituximab and vaccination was also associated with a significantly better response.
Introduction
Since the beginning of 2020, SARS CoV-2 virus rapidly spread around the world, causing widespread coronavirus disease-19 (COVID-19) infections. Although the risk of severe SARS-CoV-2 is not increased for patients with RA in general [1], RA patients treated with rituximab (RTX) do have an increased risk [2].
Treatment with rituximab also impairs humoral response to both COVID-19 [3–6] and non-COVID-19 vaccines [7]. Thus, for optimal prevention of COVID-19 in this patient group, increasing vaccine response is of utmost importance.
Two factors could conceptually influence vaccine response in patients treated with RTX: RTX dose and vaccination timing. The effect of RTX on severity of infection and vaccination response has been shown for standard doses only (1000–2000 mg per cycle). However, data from a randomized controlled study shows that ultra-low-dose rituximab, 500 or 200 mg per cycle, has similar efficacy, and halves infection risk [8]. Therefore, it would be of interest to investigate humoral response to COVID-19 vaccines after these ultra-low doses.
Recent studies on COVID-19 vaccination with small RTX populations suggest an association between longer time since latest RTX infusion at first vaccination and better humoral response [3, 4, 9]. Again, these studies only described patients receiving full dose RTX, indicating the importance of data on ultra-low-dosed rituximab.
The Dutch nationwide COVID-19 vaccination effort started in the spring of 2021. This provided us with the opportunity to study the effects of RTX dose and relative timing of vaccination on humoral response to COVID-19 vaccination in our large cohort of RA patients using regular and ultra-low dose RTX.
Patients and methods
Patients
All RA patients aged ≥16 years of the Sint Maartenskliniek (Nijmegen, the Netherlands) were invited to participate in the cohort, if (i) they received at least one dose of rituximab (200 mg, 500 mg or 1000 mg) in the year prior to their first dose of COVID-19 vaccination and (ii) COVID-19 vaccination was performed according to the registered dose and interval. The RTX dose was based on the treating physician’s discretion. At the time of the study, the Dutch national vaccine programme included four vaccines against COVID-19, of which three were two-dose regimens: BNT162b2 (Comirnaty; Pfizer-BioNtech), ChAdOx1 nCoV-19 (Vaxzevria; AstraZeneca) and CX-024414 (Spikevax; Moderna), and one was single-dose: Ad.26.COV2.S (COVID-19 vaccine Janssen) [10]. If a COVID-19 infection had occurred in the six months prior to first vaccination, the Dutch government also approved one dose of a two-dose vaccine as fully vaccinated [11].
This study has been approved by the ethics committee (CMO Arnhem-Nijmegen, 2021–7406) and the competent authority (CCMO, NL76709.091.21). The study protocol was registered in the Netherlands Trial Register (NL9342) before start. All participants provided written informed consent.
Study design
Relevant demographics and RA disease characteristics were obtained at study inclusion (Supplementary Data S1, available at Rheumatology online). Also, we recorded relevant treatment characteristics including concomitant csDMARD use, prednisolone use, current b/tsDMARD, cumulative RTX dose, and dosage and date of the latest RTX administration. Details on a previous COVID-19 infection (including date of positive test) and COVID-19 vaccination (type and dates) were provided by the participant. For humoral response assessment, blood samples were drawn two to six weeks after the second COVID-19 vaccination [3, 12]. Total immunoglobulin levels (IgT, including IgA, IgG and IgM) against COVID-19 were measured in serum using a CE-marked diagnostic ELISA assay (Wantai SARS-CoV-2 Ab assay®, Beijing Wantai Biological BV, Beijing, China) [13]. Test results were reported by the laboratory as negative (index number <1), borderline (0.9–1.1) or positive (≥1.1), in accordance with the cut-off points by the manufacturer [13, 14].
Statistical analysis
Descriptive statistics were appropriately used to assess group characteristics. Fisher’s Exact Test was used to univariately assess difference between the three dosage groups, and the association between previous COVID-19 infection and humoral response. Variables were used in the multivariate model with highest associations in the univariate analysis or with prognostic value according to previous literature (see Supplementary Table S1, available at Rheumatology online). We used a rule of thumb of 10 events per variable included in the multivariable model. A multivariable logistic regression model was built using humoral response 2–6 weeks after last vaccination as dependent variable, dose of latest RTX and time between latest RTX and first vaccination as central determinants and corrected for age, methotrexate use and prednisolone use. A cut-off point of ≥1.1 of the IgT index number was used to dichotomize the outcome. All data were entered in an electronic data capture database (Castor EDC, Amsterdam, Netherlands) and subsequently exported to StataIC (version 13, StataCorp LLC, TX, USA) for statistical analyses.
Results
Patients
Between 7 April and 15 July 2021, 376 patients were asked to participate. A total of 259 (69%) provided written informed consent. Post-vaccination serology was taken in 196 (52%) patients. Of these 196 participants, 31 (16%) received 200 mg rituximab as latest dose, 67 (34%) 500 mg and 98 (50%) 1000 mg, including one participant with 2× 1000 mg.
Baseline characteristics
Baseline demographic and clinical data were similar in the three groups (200 mg, 500 mg and 1000 mg) (Table 1). The median time between latest RTX and first vaccination was 128 days (IQR 90–165). Most patients received the BNT162b2 vaccine (n = 153, 78%), followed by the ChAdOx1 nCoV-19 (n = 30, 16%) and CX-024414 (n = 13, 6%). No patients in the 200 mg group received the CX-024414 vaccine. A total of 20 (10%) reported a previous COVID-19 infection, of which five patients (25%) only received one vaccine dose.
Table 1.
Baseline characteristics
| Total (n = 196) | 200 mg (n = 31) | 500 mg (n = 67) | 1000 mga (n = 98) | |
|---|---|---|---|---|
| Age (years)b | 68 ± 11 | 66 ± 11 | 68 ± 12 | 64 ± 11 |
| Female sex | 138 (70) | 16 (52) | 55 (82) | 67 (68) |
| Disease duration (years)c | 15 (8-23) | 17 (9-25) | 16 (8-23) | 13 (5-21) |
| RF and/or ACPA positive | 165 (84) | 29 (94) | 58 (87) | 78 (80) |
| Concomitant csDMARD use | 112 (57) | 18 (58) | 35 (52) | 59 (60) |
| MTX | 65 (33) | 10 (32) | 21 (31) | 34 (35) |
| HCQ | 20 (10) | 4 (13) | 5 (7) | 11 (11) |
| SSZ | 10 (5) | 1 (3) | 3 (4) | 6 (6) |
| AZA | 7 (4) | 2 (6) | 3 (4) | 2 (2) |
| LEF | 6 (3) | 1 (3) | 1 (1) | 4 (4) |
| Multiple | 4 (2) | 0 0 | 2 (3) | 2 (2) |
| Concomitant prednisolone use | 36 (18) | 2 (6) | 7 (10) | 27 (28) |
| Duration of rituximab use (years)b | 4.7 ± 3.4 | 6.2 ± 3.2 | 4.7 ± 2.9 | 4.3 ± 3.7 |
| Days between rituximab & 1st vaccinec | 128 (90–165) | 126 (86–161) | 131 (86–171) | 128 (93–162) |
| Vaccine type | ||||
| BNT162b2 (BioNTech/Pfizer) | 153 (78) | 26 (84) | 56 (84) | 71 (72) |
| ChAdOx1 nCoV-19 (AstraZeneca) | 30 (16) | 5 (16) | 7 (10) | 18 (18) |
| CX-024414 (Moderna) | 13 (6) | 0 0 | 4 (6) | 9 (9) |
| Prior documented COVID infection | 20 (10) | 2 (6) | 8 (12) | 10 (10) |
Either displayed as number (percentage),
Includes 1 patient treated with 2× 1000 mg.
mean (s.d.).
median (interquartile range) or
Factors associated with vaccination response
Fifty-five patients (28%) had a vaccination response positive antibody test (IgT≥1.1). In the univariate analysis, lower dosage and later timing were associated with vaccinations response (P < 0.05) (Supplementary Table S1, available at Rheumatology online). Compared with the 1000 mg group, a positive vaccination response was significantly more frequent in the 200 mg group [26% vs 45% (P = 0.045)] but not for 500 mg (26% vs 24%, P = 0.856). Response rate was 29% (45/153) for the BNT162b2 vaccine, 20% (6/30) for the ChAdOx1 nCoV-19 vaccine and 31% (4/13) for the CX-024414 vaccine. A previous COVID-19 infection had a nonsignificant higher chance of a positive vaccination response (9/20, 45% vs 46/175, 26%, P = 0.11). In the participants with a previous COVID-19 infection, response rate was 46% (7/15) for participants who received two-dose vaccination, and 40% for one-dose (2/5).
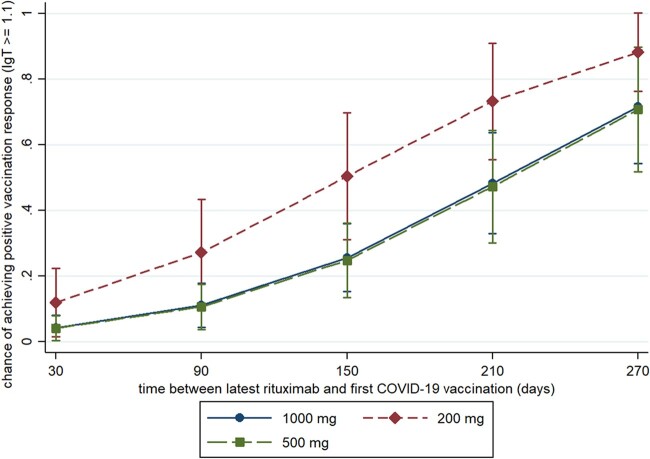
The multivariable model including time between latest rituximab infusion and first vaccination, age, concomitant csDMARD use and prednisolone use confirmed the association between vaccination response and low dose RTX {200 mg group vs 1000 mg [OR 3.07 (95% CI 1.55, 8.27, P = 0.03)]}. The time between most recent infusion and first vaccination was positively associated with higher chance of vaccination response in the multivariable model (per month OR 1.67, 95% CI 1.39, 2.01, P < 0.0001, see Fig. 1).
Fig. 1.
Cumulative humoral response on COVID-19 vaccination per dosage group, corrected for relevant confounders
Discussion
This study is the first to show that 200 mg RTX in RA patients is associated with a significantly better humoral response to COVID-19 vaccines than higher dosages (500 mg and 1000 mg) of RTX. Also, timing the vaccination longer after the RTX infusion yields significantly better vaccination response, confirming data in the literature [3, 4, 9].
Although this study is the largest cohort investigating humoral response to COVID-19 vaccines in RA patients using (ultra-low dose) RTX, this study has some limitations. First, a relatively small number of patients received 200 mg. Nonetheless, statistically significant factors were identified. Second, we did not include a comparison group of RA patients with other DMARDs. However, COVID-19 vaccination response in patients with other DMARDs and has been extensively investigated in other studies and the focus of the study was different RTX dosages, not co-DMARDs [3, 5]. Third, total IgG levels were not measured during this study and therefore the relationship between these levels and humoral response could not be investigated. Fourth, this study was only conducted in RA patients so cannot easily be extrapolated to other populations treated with rituximab. Last, because of study feasibility, only one surrogate vaccination response outcome was investigated, namely humoral response, and not T-cell response nor clinical vaccine efficacy. A clear cut-off in amount of protective antibodies measured by commercial assays is yet unclear; however, the assay used in this study has been clinically validated [13, 15]. Besides, T-cell response is associated with humoral response [9]. Therefore, we think that the differences in vaccination response could indeed translate to differences in clinical vaccine efficacy.
Based on our findings, two recommendations can be formulated. First, COVID-19 vaccination should be timed as late as possible after the latest rituximab infusion, preferably more than one rituximab cycle (6 months). Second, RTX should be dosed as low as possible, preferably 200 mg. The safety and feasibility of this dosage is supported by high-quality evidence [8, 16].
Some important questions remain, including the effects of a third booster vaccination in patients who did not show response to the first vaccination, and the optimal timing for RTX retreatment after the vaccination. Also, it would be important to see whether these differences between dose and timing on vaccination response can be extrapolated to COVID-19 infection risk and infection outcome. Last, it would be of interest to investigate how long vaccination response lasts.
In conclusion, COVID-19 vaccination response can be improved in RTX-treated RA patients by adjusting the dose and time between rituximab treatment and vaccination.
Supplementary Material
Acknowledgements
We thank Kasper Jolink and the staff of the rheumatology outpatient clinic of the Sint Maartenskliniek for performing additional blood sampling for this study, and Paul Daemen for performing the assays.
Author contributions: C.J.T.vdT., D.F.T.C., N.dB., B.J.F.vdB. and A.A.dB. designed the study. C.J.T.vdT., D.F.T.C. and A.A.dB. informed and included patients. J.R.-L. selected the assay, supervised and interpreted the antibody measurements. C.J.T.vdT. and N.dB. had access to all the data and performed the statistical analyses. C.J.T.vdT., D.F.T.C. and A.A.dB. drafted the manuscript, and all other authors critically revised the final version of the manuscript.
Ethics statements: The study was approved by the Ethics Committee of the Radboudumc ‘CMO Arnhem-Nijmegen’ (protocol number 2021–7406) and the National Ethics Committee of the Netherlands ‘CCMO’ (protocol number NL76709.091.21). The study was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice guidelines.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: A.A.dB. reports personal fees from Boehringer, Fresenius, Amgen, Merck, Abbvie and Novartis, congress invitations from Galapagos and Sanofi, and grants from Abbvie, Pfizer, Lilly, Novartis and Sanofi, outside of the submitted work. The other authors declare no competing interests.
Data availability statement
Data are available upon request. Researchers that are interested in doing additional analyses using these data can contact the Dr. Alfons den Broeder, via a.denbroeder@maartenskliniek.nl. Data can only be used for scientific research without conflict of interests.
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
Céleste J T van der Togt, Radboud Institute for Health Sciences, Department of Rheumatology, Radboud University Medical Center, Nijmegen; Department of Rheumatology, Sint Maartenskliniek, Ubbergen.
David F Ten Cate, Department of Rheumatology, Sint Maartenskliniek, Ubbergen.
Nathan den Broeder, Radboud Institute for Health Sciences, Department of Rheumatology, Radboud University Medical Center, Nijmegen; Department of Rheumatology, Sint Maartenskliniek, Ubbergen.
Janette Rahamat-Langendoen, Department of Viroscience, ErasmusMC, Rotterdam.
Bart J F van den Bemt, Department of Pharmacy, Sint Maartenskliniek, Ubbergen; Department of Clinical Pharmacy.
Alfons A den Broeder, Department of Rheumatology, Sint Maartenskliniek, Ubbergen; Department of Rheumatic Diseases, Radboudumc, Nijmegen, The Netherlands.
References
- 1. Wang Q, Liu J, Shao R. et al. Risk and clinical outcomes of COVID-19 in patients with rheumatic diseases compared with the general population: a systematic review and meta-analysis. Rheumatol Int 2021;41:851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avouac J, Drumez E, Hachulla E. et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol 2021;3:e419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Furer V, Eviatar T, Zisman D. et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 4. Spiera R, Jinich S, Jannat-Khah D.. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis 2021;80:1357–9. [DOI] [PubMed] [Google Scholar]
- 5. Boekel L, Steenhuis M, Hooijberg F. et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol 2021;3:e778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benucci M, Damiani A, Infantino M. et al. Correspondence on “SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response” by Bonelli et al. Ann Rheum Dis 2021;80:e166. [DOI] [PubMed] [Google Scholar]
- 7. Hua C, Barnetche T, Combe B. et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2014;66:1016–26. [DOI] [PubMed] [Google Scholar]
- 8. Verhoef LM, den Broeder N, Thurlings RM. et al. Ultra-low doses of rituximab for continued treatment of rheumatoid arthritis (REDO study): a randomised controlled non-inferiority trial. Lancet Rheumatol 2019;1:e145–53. [DOI] [PubMed] [Google Scholar]
- 9. Moor MB, Suter-Riniker F, Horn MP. et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol 2021;3:e789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RIVM (Dutch National Institute for Public Health and the Environment). The COVID-19 vaccines. https://www.rivm.nl/en/covid-19-vaccination/covid-19-vaccines (7 October 2021, date last accessed).
- 11.Government of the Netherlands. Using proof of vaccination to generate a COVID Certificate. https://www.government.nl/topics/coronavirus-covid-19/covid-certificate/proof-of-vaccination (7 October 2021, date last accessed).
- 12. Walsh EE, Frenck RW Jr, Falsey AR. et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020;383:2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beijing Wantai Biological Pharmacy Enterprise Co. L. WANTAI SARS-CoV-2 Ab ELISA. https://www.fda.gov/media/140929/download (7 October 2021, date last accessed).
- 14. Trabaud MA, Icard V, Milon MP. et al. Comparison of eight commercial, high-throughput, automated or ELISA assays detecting SARS-CoV-2 IgG or total antibody. J Clin Virol 2020;132:104613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geurts van Kessel CH, Okba NMA, Igloi Z. et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun 2020;11:3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. den Broeder N, Verhoef LM, De Man Y. et al. Long-term effectiveness of ultra-low doses of rituximab in rheumatoid arthritis [abstract]. Arthritis Rheumatol 2021;73. https://acrabstracts.org/abstract/long-term-effectiveness-of-ultra-low-doses-of-rituximab-in-rheumatoid-arthritis/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request. Researchers that are interested in doing additional analyses using these data can contact the Dr. Alfons den Broeder, via a.denbroeder@maartenskliniek.nl. Data can only be used for scientific research without conflict of interests.