Abstract
Background
Detailed characteristics of rheumatic symptoms of coronavirus disease 2019 (COVID-19) were still unknown. We aim to investigate the proportions, characteristics, and risk factors of this condition.
Methods
In this prospective, longitudinal cohort study, discharged patients with COVID-19 were interviewed face-to-face at 12 months after symptom onset. Rheumatic symptoms following COVID-19 included newly occurring joint pain and/or joint swelling. The risk factors of developing rheumatic symptoms were identified by multivariable logistic regression analysis.
Results
In total, 1296 of 2469 discharged patients with COVID-19 were enrolled in this study. Among them, 160 (12.3% [95% confidence interval {CI}, 10.6%–14.3%]) suffered from rheumatic symptoms following COVID-19 at 12-month follow-up. The most frequently involved joints were the knee joints (38%), followed by hand (25%) and shoulder (19%). Rheumatic symptoms were independent of the severity of illness and corticosteroid treatment during the acute phase, while elderly age (odds ratio [OR], 1.22 [95% CI, 1.06–1.40]) and female sex (OR, 1.58 [95% CI, 1.12–2.23]) were identified as the risk factors for this condition.
Conclusions
Our investigation showed a considerable proportion of rheumatic symptoms following COVID-19 in discharged patients, which highlights the need for continuing attention. Notably, rheumatic symptoms following COVID-19 were independent of the severity of illness and corticosteroid treatment during the acute phase.
Keywords: COVID-19, post-COVID-19 condition, rheumatic symptoms
As of 18 March 2022, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been diagnosed in >462 million people worldwide, causing the death of >6 million [1]. A collection of long-term manifestations of COVID-19, including fatigue, shortness of breath, and loss of taste or smell, were identified by various follow-up studies from different countries and have been termed “postacute COVID-19 syndrome” [2]. Rheumatic symptoms as a long-term manifestation of COVID-19 have been frequently mentioned in published follow-up studies [3–11].
It was known that viral infection, such as parvovirus B19, hepatitis B and C, human immunodeficiency virus, and the alphaviruses, was recognized as an important cause of virally mediated arthritis, with a wide spectrum of rheumatic symptoms ranging from arthralgia to chronic arthritis [12]. Rheumatic symptoms after other coronavirus infection, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome, were also reported with an incidence of 10% and 32%, respectively. It is worth noting that, the incidence may be underestimated under the scenarios of high doses of glucocorticoids (cumulative doses during hospitalization of >1 g) [13–15].
Although the condition of rheumatic symptoms following COVID-19 has been noticed in some studies, the details of proportions and clinical characteristics have not yet been well described, and the risk factors of the presence of rheumatic symptoms following COVID-19 are unknown. Thus, the objective of this study was to investigate the proportion, describe the characteristics, and identify the potential factors of rheumatic symptoms following COVID-19 among discharged patients.
METHODS
Study Population and Patient Consent Statement
This prospective, single-center cohort study was conducted at the designated hospital at 12 months after COVID-19 symptom onset. All laboratory-confirmed patients admitted to the designated hospital met the diagnostic criteria for COVID-19 [16]. A total of 2469 patients were discharged between 7 January and 29 May 2020 as previously reported [9].
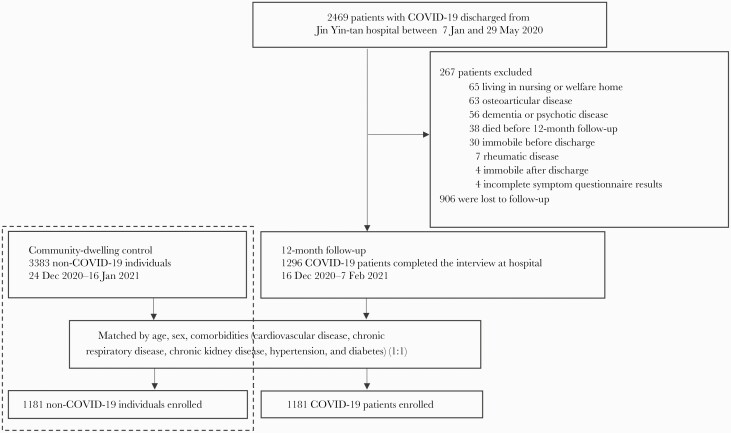
All 2469 patients were eligible for inclusion. We contacted the patients in the order of the symptom onset date documented in their medical record via telephone. Patients were excluded if they met the following criterion: (1) those who died before the follow-up visit; (2) those living in a nursing or welfare home; (3) those with a history of dementia or psychotic disease; or (4) those who had a history of rheumatic disease (including osteoarticular disease) or were immobile before or after discharge. Eligible patients were invited to participate in the face-to-face interview at the outpatient clinic of the hospital, and 906 patients were lost to follow-up. A total of 1296 patients who completed the interview and symptom questionnaire at the hospital from 16 December 2020 to 7 February 2021 were enrolled in this study (Figure 1). We evaluated the proportions of self-reported rheumatic symptoms following COVID-19 through symptom questionnaire, and obtained detailed characteristics.
Figure 1.
Flowchart of participants’ enrollment in this study. Abbreviation: COVID-19, coronavirus disease 2019.
The rheumatic symptoms following COVID-19 were defined as self-reported newly occurring joint symptoms, including joint pain and/or joint swelling. We list 10 joint groups (including hand, foot, wrist, ankle, jaw, elbow, shoulder, neck, hip, and knee) for patients to choose the location of the symptomatic joint site (any other if yes, specify additionally). The hand joint group included the metacarpophalangeal joints (MCP), the distal interphalangeal joints (DIP), the proximal interphalangeal joints (PIP), and the interphalangeal joint of the thumb. Symptom localizing to the chest, abdomen, or back was not considered for further analysis. In addition, we conducted the EQ-5D-5L questionnaire and 6-minute walking test to evaluate the quality of life (QoL) of patients.
As control, 3383 community-dwelling individuals without SARS-CoV-2 infection were recruited. Methods, inclusion, and exclusion criteria of control have been described previously [17]. For every SARS-CoV-2 survivor, we randomly sampled up to 1 individual with control, without replacement, matching age, sex, and comorbidities including cardiovascular disease, chronic respiratory disease, chronic kidney disease, hypertension, and diabetes (Figure 1).
The study was approved by the Research Ethics Commission of the designated hospital that enrolled the participants (KY-2020-78.01, KY-2020-78.03). Written informed consent was obtained from all patients and controls.
Data Collection of COVID-19 Patients
Data collection consisted of 3 parts, including demographic data, acute phase data, and follow-up data. The patients’ demographic data were retrieved from electronic medical records, which included age, sex, education, and cigarette smoking.
We collected clinical data during hospital stay for acute phase, such as symptoms, self-reported comorbidities, medication history, disease severity, and treatment record from the REDCap electronic database. The severity of disease was characterized by the highest 7-category scale during the hospital stay [18], which consisted of the following categories: scale 1, not hospitalized with resumption of normal activities; scale 2, not hospitalized, but unable to resume normal activities; scale 3, hospitalized, not requiring supplemental oxygen; scale 4, hospitalized, requiring supplemental oxygen; scale 5, hospitalized, requiring nasal high-flow oxygen therapy, noninvasive mechanical ventilation, or both; scale 6, hospitalized, requiring extracorporeal membrane oxygenation, invasive mechanical ventilation, or both; and scale 7, death.
Follow-up data were collected via face-to-face interviews at the outpatient clinic of the hospital. Physicians were arranged for the patients to complete a structured symptom questionnaire to ask if the patients had any obvious discomfort after discharge. Patients with self-reported newly occurring and persistent rheumatic symptoms after COVID-19 were further asked for more details about their joint complaints. We provided 10 joint groups aforementioned for patients to choose from. For each pain joint location, an 11-point number of pain score ranging from 0 to 10 (0 no pain, 10 intolerable pain) was applied to evaluate the intensity [19]; scores were categorized as mild (1–3), moderate (4–6), and severe (7–10). Joint swelling was recorded at the same time.
Data Collection of Community-Dwelling Individuals Without COVID-19
Data of community-dwelling individuals without COVID-19 were collected via face-to-face interviews at their community center by medical staff from the same hospital. Demographic characteristics, personal medical history, and lifestyle information were collected via standard questionnaires. Then, individuals without COVID-19 underwent physical examination as well as prevalent symptoms and EQ-5D-5L questionnaires.
Statistical Analysis
Descriptive analyses of the continuous variables were expressed as median (IQR), and categorical variables were expressed as number (%). Demographic characteristics and clinical data for acute phase between patients with or without rheumatic symptoms following COVID-19 were shown. For the comparison of health-related QoL and exercise capacity between the 2 groups, we used the Mann-Whitney U test, χ2 test, or Fisher exact test where appropriate. Comparison of symptoms and health-related QoL between COVID-19 patients and controls was done with Mann-Whitney U test, χ2 test, or Fisher exact test where appropriate, as well.
The rheumatic symptoms following COVID-19 were included as the dependent variable in a univariable logistic regression model (crude association) and were then corrected for several covariables. Variables included in the multivariable adjusted logistic regression models are as follows: age; sex; comorbidity including hypertension, diabetes, and cardiovascular disease; disease severity; medical treatment including corticosteroids, antivirals, thymosin, and intravenous immunoglobulin; length of hospital stay; and admission to intensive care unit (ICU). For association of sex, corticosteroids, antivirals, thymosin, and intravenous immunoglobulin, length of hospital stay, and admission to ICU with rheumatic symptoms, these variables were included in the models together with age, disease severity, and comorbidity including hypertension, diabetes, and cardiovascular disease. For association of disease severity and rheumatic symptoms, the aforementioned variables were all adjusted in the model, except for length of hospital stay and ICU admission. Disease severity was not included in the model when exploring the association of comorbidity with rheumatic symptoms. In the model for association of age and rheumatic symptoms, only age and sex were adjusted.
All tests were 2-sided, and P < .05 was considered statistically significant. Data were analyzed with the use of SAS version 9.4 software. Statistical graphs were drawn with GraphPad Prism8, BioRender.com, and Adobe Illustrator 2020.
RESULTS
Demographic and Clinical Characteristics of Study Patients
Of 2469 discharged COVID-19 patients, 1296 who completed the face-to face follow-up visit at 12 months after illness onset (from 16 December 2020 to 7 February 2021) were included in the final analysis. Median follow-up time was 349.0 days (IQR, 337.0–361.0 days) from symptom onset to follow-up visit. The median age of the 1296 patients was 57.0 years (IQR, 48.0–65.0), and 609 (47%) of them were women (Table 1). The most common comorbidity was hypertension (28%), followed by diabetes (12%) and cardiovascular disease (9%).
Table 1.
Characteristics of Patients According to the Presence or Absence of Rheumatic Symptoms Following Coronavirus Disease 2019 at 12-Month Follow-up
| Characteristic | Total (n = 1296) | Without Rheumatic Symptoms Following COVID-19 (n = 1136) | With Rheumatic Symptoms Following COVID-19 (n = 160) |
|---|---|---|---|
| Age, y, median (IQR) | 57.0 (48.0–65.0) | 57.0 (47.0–65.0) | 60.0 (53.0–65.0) |
| Sex | |||
| Male | 687 (53) | 617 (54) | 70 (44) |
| Female | 609 (47) | 519 (46) | 90 (56) |
| Education | |||
| College or higher | 346/1138 (30) | 306/997 (31) | 40/141 (28) |
| Middle school or lower | 792/1138 (70) | 691/997 (69) | 101/141 (72) |
| Cigarette smoking | |||
| Never-smoker | 1147/1263 (91) | 1001/1106 (91) | 146/157 (93) |
| Current smoker | 84/1263 (7) | 76/1106 (7) | 8/157 (5) |
| Former smoker | 32/1263 (3) | 29/1106 (3) | 3/157 (2) |
| Comorbidity | |||
| Hypertension | 354/1264 (28) | 308/1107 (28) | 46/157 (29) |
| Diabetes | 150/1263 (12) | 131/1106 (12) | 19/157 (12) |
| Cardiovascular diseases | 116/1262 (9) | 95/1105 (9) | 21/157 (13) |
| Malignancy | 35/1265 (3) | 27/1108 (2) | 8/157 (5) |
| COPD | 19/1264 (2) | 16/1107 (1) | 3/157 (2) |
| Chronic kidney disease | 15/1265 (1) | 14/1108 (1) | 1/157 (1) |
| Acute phase symptoms | |||
| Fever | 1041/1265 (82) | 907/1108 (82) | 134/157 (85) |
| Cough | 929/1264 (73) | 810/1107 (73) | 119/157 (76) |
| Productive sputum | 359/1264 (28) | 310/1107 (28) | 49/157 (31) |
| Hemoptysis | 22/1265 (2) | 18/1108 (2) | 4/157 (3) |
| Stuffy nose | 13/1262 (1) | 10/1106 (1) | 3/156 (2) |
| Headache | 65/1264 (5) | 54/1108 (5) | 11/156 (7) |
| Tachypnea | 419/1264 (33) | 363/1107 (33) | 56/157 (36) |
| Myalgia/arthralgia | 138/1262 (11) | 123/1106 (11) | 15/156 (10) |
| Fatigue | 528/1264 (42) | 455/1108 (41) | 73/156 (47) |
| Gastrointestinal symptom | 212/1264 (17) | 185/1107 (17) | 27/157 (17) |
| Highest 7-category scale during hospitalization | |||
| 3: Admitted to hospital, not requiring supplemental oxygen | 315/1265 (25) | 278/1108 (25) | 37/157 (24) |
| 4: Admitted to hospital, requiring supplemental oxygen | 856/1265 (68) | 753/1108 (68) | 103/157 (66) |
| 5–6: Admitted to hospital, requiring HFNC, MV, ECMO, or combination | 94/1256 (7) | 77/1108 (7) | 17/157 (11) |
| Treatment received during hospitalization | |||
| Corticosteroids | 305/1265 (24) | 262/1108 (24) | 43/157 (27) |
| Antivirals | 697/1265 (55) | 608/1108 (55) | 89/157 (57) |
| Thymosin | 203/1265 (16) | 180/1108 (16) | 23/157 (15) |
| IVIG | 248/1265 (20) | 212/1108 (19) | 36/157 (23) |
| Length of hospital stay, d, median (IQR) | 13.8 (9.9–19.7) | 13.8 (9.9–19.2) | 14.5 (9.9–22.7) |
| Time from symptom onset to 12-mo follow-up, d, median (IQR) | 349.0 (337.0–361.0) | 349.0 (337.0–361.0) | 352.0 (342.0–362.0) |
| ICU admission | 54/1265 (4) | 44/1108 (4) | 10/157 (6) |
Data are No. (%) or no./No. (%) unless otherwise indicated. The differing denominators used indicate missing data.
Abbreviations: COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; HFNC, high-flow nasal cannula for oxygen therapy; ICU, intensive care unit; IVIG, intravenous immunoglobulin; MV, mechanical ventilation.
In total, 12.3% (95% confidence interval [CI], 10.6%–14.3%) of COVID-19 patients (160/1296) complained they were suffering from at least 1 newly occurring or persistent rheumatic symptom after COVID-19. Of 160 patients, the median age was 60.0 years (IQR, 53.0–65.0 years), 90 (56%) of them were women, and 40 (28%) had college or higher education. Hypertension (29%), cardiovascular disease (13%), and diabetes (12%) were the most common comorbidities.
Factors Associated With Rheumatic Symptoms Following COVID-19
Supplementary Table 1 showed the relative risk of variables to presence of rheumatic symptoms following COVID-19. Older age, women, and prolonged hospital stay increased the risk of rheumatic symptoms, whereas rheumatic symptoms following COVID-19 were independent of the severity of illness and corticosteroid treatment during acute phase (Supplementary Table 2).
For exploring the risk factors associated with rheumatic symptoms, data were available from 1296 patients (1136 without and 160 with rheumatic symptoms), and 4 logistic regression models were performed. After multivariable adjustment, patients with older age had a higher risk of rheumatic symptoms (odds ratio [OR], 1.22 [95% CI, 1.06–1.40], per 10-year age increase). Compared with men, women had an OR of 1.58 (95% CI, 1.12–2.23) for presenting rheumatic symptoms following COVID-19 (Table 2).
Table 2.
Multivariable Logistic Regression Analysis for Risk Factors of Rheumatic Symptoms Following Coronavirus Disease 2019 at 12-Month Follow-up
| Variable | Odds Ratio (95% CI) |
|---|---|
| Age (per 10-year age increase) | 1.22 (1.06–1.40) |
| Sex | |
| Men | Ref |
| Women | 1.58 (1.12–2.23) |
| Hypertension | .90 (.60–1.34) |
| Diabetes | .93 (.54–1.58) |
| Cardiovascular diseases | 1.42 (.83–2.42) |
| Highest 7-category scale during hospitalization | |
| 3: Admitted to hospital, not requiring supplemental oxygen | Ref |
| 4: Admitted to hospital, requiring supplemental oxygen | 1.01 (.67–1.51) |
| 5–6: Admitted to hospital, requiring HFNC, MV, ECMO, or combination | 1.55 (.77–3.12) |
| Treatment during hospitalization | |
| Corticosteroids | 1.05 (.66–1.68) |
| Antivirals | .95 (.67–1.35) |
| Thymosin | .81 (.49–1.32) |
| IVIG | 1.19 (.75–1.89) |
| Length of hospital stay, d | 1.01 (1.00–1.03) |
| ICU admission | 1.15 (.50–2.65) |
For association of sex, corticosteroids, antivirals, thymosin, IVIG, length of hospital stay, and admission of ICU with rheumatic symptoms, these variables were included in the models together with age, disease severity, and comorbidity including hypertension, diabetes, and cardiovascular disease. For association of disease severity and rheumatic symptoms, the aforementioned variables were all adjusted for in the model, except for length of hospital stay and admission of ICU. Disease severity was not included in the model when exploring the association of comorbidity with rheumatic symptoms. In the model for association of age and rheumatic symptoms, only age and sex were adjusted.
Abbreviations: CI, confidence interval; ECMO, extracorporeal membrane oxygenation; HFNC, high-flow nasal cannula for oxygen therapy; ICU, intensive care unit; IVIG, intravenous immunoglobulin; MV, mechanical ventilation.
Proportion and Characteristics of Rheumatic Symptoms Following COVID-19
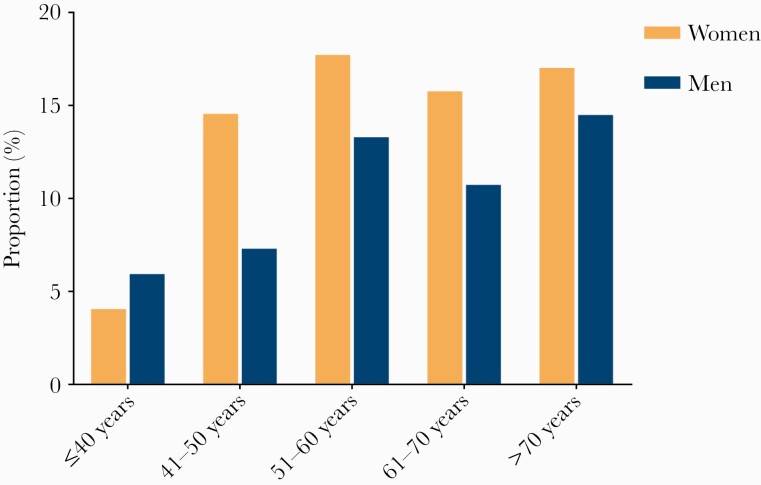
Of 160 patients reporting rheumatic symptoms following COVID-19 at 12-month follow-up, the proportions in men and women were 10% (70/687) and 15% (90/609), respectively. The age distributions of rheumatic symptoms following COVID-19 were shown in Figure 2: COVID-19 patients aged 51–60 years (15.6%) were the most affected group, followed by those aged >70 years (15.5%) and aged 61–70 years (13.2%). According to the illness severity of COVID-19 during hospital stay, the proportions in patients with scale 3, scale 4, and scale 5–6 were 11.7% (37/315), 12.0% (103/856), and 18% (17/94), respectively.
Figure 2.
Proportions of rheumatic symptoms following coronavirus disease 2019 at 12-month follow-up according to age and sex.
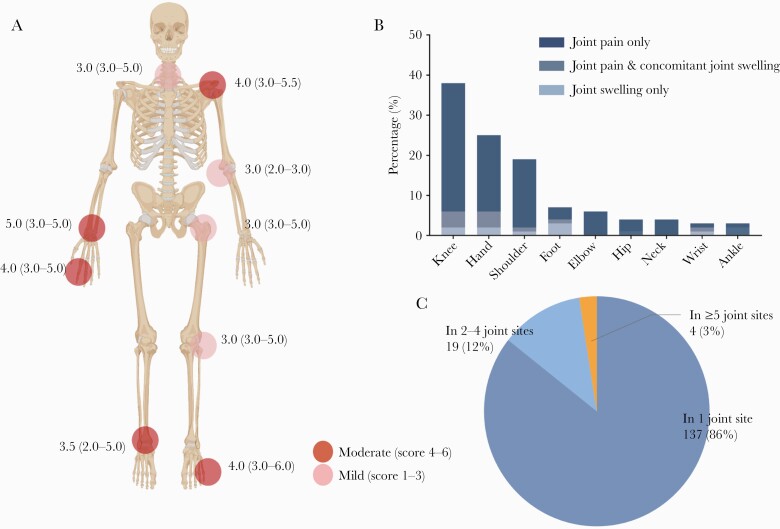
Figure 3B showed the frequencies of joint sites among patients with rheumatic symptoms following COVID-19. The most common joint was the knee joint (38%), followed by hand (25%, including MCP, PIP, or DIP, etc) and shoulder (19%). For all rheumatic symptoms, joint pain only (86%) was mainly represented; joint pain concomitant with joint swelling (8%) and joint swelling only (5%) were relatively rare. The intensity of joint pain is detailed in Figure 3A and graded as mild to moderate. Rheumatic symptoms reported in 1 joint site (86%) was the most common type, followed by in 2–4 joint sites (12%) and in ≥5 joint sites (3%; Figure 3C). There was no significant improvement in the proportions of COVID-19 patients with joint pain between 6-month and 12-month follow-up visits (P = .092; Supplementary Table 3).
Figure 3.
Proportions and severity of rheumatic symptoms following coronavirus disease 2019 (COVID-19) at 12-month follow-up. A, Joint pain intensity. The darker the color, the higher the number of pain score of the pain sites. Data are median (interquartile range). B, Proportions of joint pain sites, joint swelling sites, and joint pain with concomitant joint swelling sites among COVID-19 patients. C, Proportions (number and %) of pain styles according to the number of joint groups involved.
Quality of Life and Six-Minute Walk Test in Patients With or Without Rheumatic Symptoms Following COVID-19
The EQ-5D-5L questionnaire was used to evaluate QoL. At 12-month follow-up, all dimensions of health-related QoL were impaired in patients with rheumatic symptoms following COVID-19 compared with those without, including mobility, self-care, usual activity capabilities, pain/discomfort, and anxiety/depression (all P < .05; Supplementary Table 4). Patients with rheumatic symptoms had less distance walked in 6 minutes (480.0 vs 497.0 meters, P = .018) compared to those without. There were no significant differences in the proportion of lower limit of the normal range between 2 COVID-19 patient groups (Supplementary Table 4).
Prevalence of Rheumatic Symptoms Among COVID-19 and Non–COVID-19 Adults
To compare the prevalence of rheumatic symptoms between COVID-19 and non–COVID-19 adults, 3383 community-dwelling individuals without SARS-CoV-2 infection were recruited. A total of 1181 COVID-19 patients included in this study were matched to 1181 non–COVID-19 individuals. The differences in age, sex, and comorbidities between COVID-19 and non–COVID-19 cohorts were eliminated after propensity score match (Supplementary Table 5). Of 1181 COVID-19 patients, 301 (25%) had at least 1 rheumatic symptom, which was significantly higher than for controls (89/1181 [8%], P < .0001). Pain joint sites and swelling joint sites are detailed in Table 3.
Table 3.
Rheumatic Symptoms According to Matched Coronavirus Disease 2019 (COVID-19) Patients at 12-Month Follow-up and Participants Without COVID-19
| Variable | Matched Non–COVID-19 Participants (n = 1181) | Matched COVID-19 Patients at 12-Month Follow-up Visit (n = 1181) | P Value |
|---|---|---|---|
| Rheumatic symptoms | 89 (8) | 301 (25) | <.001 |
| Joint pain | |||
| Knee | 45 (4) | 166 (14) | <.001 |
| Shoulder | 17 (1) | 59 (5) | <.001 |
| Hand | 8 (1) | 49 (4) | <.001 |
| Elbow | 2 (0) | 21 (2) | <.001 |
| Neck | 11 (1) | 12 (1) | .83 |
| Foot | 4 (0) | 10 (1) | .11 |
| Hip | 3 (0) | 11 (1) | .032 |
| Ankle | 1 (0) | 8 (1) | .013 |
| Wrist | 2 (0) | 4 (0) | .41 |
| Joint swelling | |||
| Knee | 6 (1) | 11 (1) | .22 |
| Shoulder | 1 (0) | 1 (0) | 1.00 |
| Hand | 2 (0) | 8 (1) | .06 |
| Elbow | 1 (0) | 0 (0) | 1.00 |
| Neck | 0 (0) | 0 (0) | |
| Foot | 0 (0) | 5 (0) | .0084 |
| Hip | 0 (0) | 0 (0) | |
| Ankle | 0 (0) | 2 (0) | .10 |
| Wrist | 1 (0) | 2 (0) | .56 |
| Nonrheumatic symptoms | |||
| Tired | 63 (5) | 239 (20) | <.0001 |
| Muscle weak | 22 (2) | 159 (13) | <.0001 |
| Myalgia | 6 (1) | 64 (5) | <.0001 |
| Headache | 34 (3) | 94 (8) | <.0001 |
Data are presented as No. (%). For every severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) survivor, 1 community-dwelling individual without SARS-CoV-2 infection was randomly sampled without replacement, matching age, sex, and comorbidities including cardiovascular disease, chronic respiratory disease, chronic kidney disease, hypertension, and diabetes.
Abbreviation: COVID-19, coronavirus disease 2019.
DISCUSSION
Arthralgia/arthritis has been increasingly reported in the setting of COVID-19, which has aroused the attention of clinicians [20, 21], but several important issues remain unsettled. In this prospective cohort study, 12.3% of discharged patients developed rheumatic symptoms following COVID-19 at 12-month follow-up. Of the 160 patients presenting rheumatic symptoms, the most involved joint was the knee, followed by hand and shoulder. Joint pain was the most common presentation, with the pain intensity graded mild to moderate. Notably, rheumatic symptoms following COVID-19 were independent of the severity of illness and corticosteroid treatment during acute phase.
In comparison to community-dwelling controls, COVID-19 patients reported significantly more rheumatic symptoms, indicating that COVID-19 patients did not recover to general status even 12 months after symptom onset. Previous studies suggest that viral infection is one of the well-recognized causes of arthralgia. Many kinds of viruses, including coronaviruses, can cause varying degrees of joint disease [12]. A large retrospective study from South Korea showed that ambient respiratory viral infections by coronavirus, parainfluenza virus, and metapneumovirus were associated with an increased number of incident rheumatoid arthritis [22].
On the basis of the published follow-up studies of COVID-19 thus far, the proportion of self-reported rheumatic symptoms following COVID-19 ranged from 4.5% to 27.3% [3–9]. The differences in illness severity of the study populations, the differences in trial designs, and even the differences in virus strains might be the explanations for the varied proportions. The prevalence of joint symptoms caused by other viral infections continues to evolve. Rubella, for instance, becomes less common due to vaccination, while some alphaviruses have become more widespread [12]. Although these viral infectious diseases in humans are usually self-limiting, from the limited experience, rheumatic symptoms caused by these diseases usually lasts weeks to months and can be protracted [23].
The possible causes of rheumatic symptoms following COVID-19 may be musculoskeletal disorders before COVID-19, gout, or rheumatic symptoms specific following the infection with SARS-CoV-2, which need to be further demonstrated by an orthopedist or rheumatologist through complete examinations [24]. So far, the specific mechanism of rheumatic symptoms following COVID-19 remains unclear, but several hypotheses have been proposed. First, excessive or uncontrolled cytokine responses in COVID-19 patients, including interleukin (IL) 17 and tumor necrosis factor, as important therapeutic targets in spondyloarthritis and psoriatic arthritis, also play an important role in the progress of COVID-19 [15]. Several studies showed that the elevated level of IL-4, IL-9, and IL-13 in COVID-19 patients also played differently in the joints [15, 25–27]. Additionally, some antirheumatic drugs also appear to have potential efficacy in COVID-19, such as hydroxychloroquine, colchicine, baricitinib, and tocilizumab [28]. Second, by means of immune disorders and immune complex deposition, viral arthritis such as hepatitis B and parvovirus [29, 30] have been proposed to contribute to the rheumatic symptoms following COVID-19. Third, SARS-CoV-2 antigens activate cross-reactive T cells through molecular mimicry, or host self-antigens released from damaged tissue allow the activation of autoreactive T cells, which induce the pathological process [31].
Factors independently associated with rheumatic symptoms following COVID-19 at 12-month follow-up were being elderly and female. Similar conclusions have been reached by other virus infection studies. The result of a systematic review of chikungunya virus studies showed that older age and female sex were the major risk factors for persistent joint pain [32]. Recently, several studies reported that older age played an important role in adverse outcomes during acute and postacute COVID-19 [33]. Male sex was associated with an increased risk of severe COVID-19; but more women were experiencing “long COVID.” Notably, we found that rheumatic symptoms following COVID-19 were not associated with the severity of illness and corticosteroid treatment during acute phase. A cross-sectional study from Anaya et al has corroborated this finding [10].
When assessing the QoL in patients with rheumatic symptoms following COVID-19, all 5 aspects of EQ-5D-5L questionnaire were found to be impaired, reflecting the physical and mental impact of this condition. A prospective longitudinal study of chikungunya virus–infected French military policemen showed that individuals presented with rheumatic morbidity, and impaired QoL persisted 6 years later [34]. In addition, SARS can also directly or indirectly affect multiple organ systems, including the musculoskeletal system. Incapacitating arthralgia, muscle dysfunction, osteoporosis, and osteonecrosis are common sequelae of SARS, causing a serious decline of the QoL and even disability [35]. In this study, we found that of all joint pains, the intensity was graded mild to moderate. Severe impairment of mobility, self-care, and usual activity capabilities were also rarely reported.
This study has several limitations. First, this is a single-center study in a designated hospital for COVID-19 and specific to the virus strain that caused the Wuhan pandemic. Second, due to lack of mechanism exploration, the observed rheumatic symptoms following COVID-19 cannot be directly attributed to COVID-19. Third, there is a lack of more detailed investigation of symptoms, such as whether joint involvement is symmetrical, and further examination methods, such as joint imaging and serologic markers. In the future, longer follow-up studies are needed to dynamically observe the changes of rheumatic symptoms following COVID-19, together with surveys of related medical expenses. Further studies are needed to uncover mechanisms of arthralgia following COVID-19.
CONCLUSIONS
We found a considerable proportion of rheumatic symptoms following COVID-19 in discharged patients at 12 months after symptom onset, which highlights the need for continuing attention. Notably, rheumatic symptoms following COVID-19 were independent of the severity of illness and corticosteroid treatment during the acute phase. Our data may provide reference and evidence for future clinical research and related pathological mechanism hypothesis of COVID-19.
Supplementary Material
Notes
Author contributions. C. W., B. C., Y. W., L. H., and D. C. conceived and designed the study. Z. H., S. M., L. H., Y. W., and D. C. collected the data. D. C. and X. G. analyzed and interpreted data. D. C. drafted the manuscript, and B. C., Y. W., and X. G. revised the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial support. This work was supported by the Natural Science Foundation of China (82041011/H0104); the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1-003); the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2020-I2M-CoV19-005); the National Key Research and Development Program of China (2018YFC1200102); and Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis (2020ZX09201001). This work was also supported by the China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical Ltd, Ping An Insurance (Group), and New Sunshine Charity Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Dan Cui, Department of Pulmonary and Critical Care Medicine, The Second Affiliated Hospital of Harbin Medical University, Harbin, China; Department of Pulmonary and Critical Care Medicine, National Center for Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, China.
Yeming Wang, Department of Pulmonary and Critical Care Medicine, National Center for Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, China.
Lixue Huang, Department of Pulmonary and Critical Care Medicine, National Center for Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, China; Department of Pulmonary and Critical Care Medicine, Capital Medical University, Beijing, China.
Xiaoying Gu, Department of Pulmonary and Critical Care Medicine, National Center for Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, China; Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, Beijing, China.
Zhisheng Huang, Department of Pulmonary and Critical Care Medicine, National Center for Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, China; Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China.
Shengrui Mu, Department of Pulmonary and Critical Care Medicine, National Center for Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, China; Department of Pulmonary and Critical Care Medicine, Capital Medical University, Beijing, China.
Chen Wang, Department of Pulmonary and Critical Care Medicine, The Second Affiliated Hospital of Harbin Medical University, Harbin, China; Department of Pulmonary and Critical Care Medicine, National Center for Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, China; Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China; Key Laboratory of Respiratory Disease Pathogenomics, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China; Tsinghua University-Peking University Joint Center for Life Sciences, Beijing, China.
Bin Cao, Department of Pulmonary and Critical Care Medicine, National Center for Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, China; Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China; Tsinghua University-Peking University Joint Center for Life Sciences, Beijing, China.
REFERENCES
- 1. World Health Organization. Coronavirus (COVID-19). https://covid19.who.int/. Accessed 18 March 2022.
- 2. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27:601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carfi A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 2021; 27:258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2020; 76:399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moreno-Pérez O, Merino E, Leon-Ramirez J-M, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect 2021; 82:378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petersen M, Kristiansen M, Hanusson K, et al. . Long COVID in the Faroe Islands—a longitudinal study among non-hospitalized patients. Clin Infect Dis 2021; 73:e4058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stavem K, Ghanima W, Olsen M, Gilboe H, Einvik GJT.. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2020; 76:405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaolin H, Lixue H, Yeming W, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anaya J-M, Rojas M, Salinas ML, et al. Post-COVID syndrome. A case series and comprehensive review. Autoimmun Rev 2021; 20:102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmed S, Gasparyan AY, Zimba O.. Comorbidities in rheumatic diseases need special consideration during the COVID-19 pandemic. Rheumatol Int 2021; 41:243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marks M, Marks JJC.. Viral arthritis. Clin Med (Lond) 2016; 16:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christian MD, Poutanen SM, Loutfy MR, Muller MP, Low DE.. Severe acute respiratory syndrome. Clin Infect Dis 2004; 38:1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Memish ZA, Perlman S, Van Kerkhove MD, Zumla A.. Middle East respiratory syndrome. Lancet 2020; 395:1063–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schett G, Manger B, Simon D, Caporali R.. COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol 2020; 16:465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. China National Health Commission . Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment,7th ed. 2020. http://kjfy.meetingchina.org/msite/news/show/cn/3337.html. Accessed 18 March 2022. [Google Scholar]
- 17. Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021; 398:747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382:1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galer B, Jensen MJN.. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology 1997; 48:332–8. [DOI] [PubMed] [Google Scholar]
- 20. Hoong C, Amin M, Tan T.. Viral arthralgia a new manifestation of COVID-19 infection? A cohort study of COVID-19-associated musculoskeletal symptoms. Int J Infect Dis 2021; 104:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parisi S, Borrelli R, Bianchi S, Fusaro E.. Viral arthritis and COVID-19. Lancet Rheumatol 2020; 2:e655–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joo YB, Lim YH, Kim KJ, Park KS, Park YJ.. Respiratory viral infections and the risk of rheumatoid arthritis. Arthritis Res Ther 2019; 21:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suhrbier A. Rheumatic manifestations of chikungunya: emerging concepts and interventions. Nat Rev Rheumatol 2019; 15:597–611. [DOI] [PubMed] [Google Scholar]
- 24. Javelle E, Ribera A, Degasne I, Gaüzère B-A, Marimoutou C, Simon F.. Specific management of post-chikungunya rheumatic disorders: a retrospective study of 159 cases in Reunion Island from 2006 to 2012. PLoS Negl Trop Dis 2015; 9:e0003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Z, Andreev D, Oeser K, et al. Th2 and eosinophil responses suppress inflammatory arthritis. Nat Commun 2016; 7:11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rauber S, Luber M, Weber S, et al. Resolution of inflammation by interleukin-9–producing type 2 innate lymphoid cells. Nat Med 2017; 23:938–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahmed S, Zimba O, Gasparyan AY.. Thrombosis in coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clin Rheumatol 2020; 39:2529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wands JR, Mann E, Alpert E, Isselbacher KJ.. The pathogenesis of arthritis associated with acute hepatitis-B surface antigen-positive hepatitis. Complement activation and characterization of circulating immune complexes. J Clin Invest 1975; 55:930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ono K, Kishimoto M, Shimasaki T, et al. Reactive arthritis after COVID-19 infection. RMD Open 2020; 6:e001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rojas M, Restrepo-Jiménez P, Monsalve D, et al. . Molecular mimicry and autoimmunity. J Autoimmun 2018; 95:100–23. [DOI] [PubMed] [Google Scholar]
- 32. van Aalst M, Nelen CM, Goorhuis A, Stijnis C, Grobusch MP.. Long-term sequelae of chikungunya virus disease: a systematic review. Travel Med Infect Dis 2017; 15:8–22. [DOI] [PubMed] [Google Scholar]
- 33. Fei Z, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marimoutou C, Ferraro J, Javelle E, Deparis X, Simon F.. Chikungunya infection: self-reported rheumatic morbidity and impaired quality of life persist 6 years later. Clin Microbiol Infect 2015; 21:688–93. [DOI] [PubMed] [Google Scholar]
- 35. Disser N, De Micheli A, Schonk M, et al. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am 2020; 102:1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.