Abstract
Stenotrophomonas maltophilia is an underappreciated source of morbidity and mortality among gram-negative pathogens. Effective treatment options with acceptable toxicity profiles are limited. Phenotypic susceptibility testing via commercial automated test systems is problematic and no Food and Drug Administration breakpoints are approved for any of the first-line treatment options for S maltophilia. The lack of modern pharmacokinetic/pharmacodynamic data for many agents impedes dose optimization, and the lack of robust efficacy and safety data limits their clinical utility. Levofloxacin has demonstrated similar efficacy to trimethoprim-sulfamethoxazole, although rapid development of resistance is a concern. Minocycline demonstrates the highest rate of in vitro susceptibility, however, evidence to support its clinical use are scant. Novel agents such as cefiderocol have exhibited promising activity in preclinical investigations, though additional outcomes data are needed to determine its place in therapy for S maltophilia. Combination therapy is often employed despite the dearth of adequate supporting data.
Keywords: cefiderocol, levofloxacin, minocycline, outcomes, pharmacodynamics, pharmacokinetics, resistance, sulfamethoxazole, Stenotrophomonas maltophilia, treatment, trimethoprim
Stenotrophomonas maltophiliais an underappreciated source of morbidity and mortality among gram-negative pathogens. The prevalence of S maltophiliais increasing. Unfortunately, effective treatment options with acceptable toxicity profiles are limited.
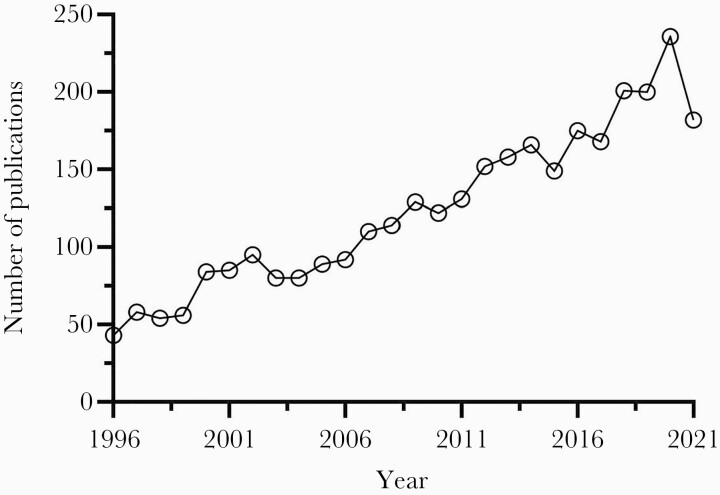
The nonfermenting gram-negative bacillus now known as Stenotrophomonas maltophilia has been problematic since its initial identification as Bacterium bookeri in 1943 [1]. After numerous taxonomic reassignments from Pseudomonas spp to Xanthomonas spp, the novel genus Stenotrophomonas was proposed to describe its narrow nutritional spectrum and S maltophilia officially became the only known pathogenic species in 1993. While well-recognized for its ability to colonize and thrive across a wide range of biotopes including plant and marine environments, the pathogenicity and associated morbidity and mortality of S maltophilia in humans has been a source of contention [2]. Consequently, it was largely ignored and upstaged by more commonly encountered pathogens until the early 2000s when the prevalence and recognition of S maltophilia infections began to increase dramatically. This resurgence was driven in large part by the report of its genome being sequenced in 2008, which revealed the full scale of its resistome for the first time [3]. Since then, the interest has surged as evidenced by the roughly 200% increase in PubMed-indexed papers mentioning S maltophilia (Figure 1). In the United States (US), S maltophilia is now the most prevalent carbapenem-resistant gram-negative bloodstream pathogen, above Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae and one of the most common causes of respiratory infections, particularly in the intensive care unit (ICU) [4–7]. Stenotrophomonas maltophilia also plagues the immunocompromised, especially hematopoietic stem cell transplant recipients, due in part to alterations of the microbiome secondary to chemotherapy and antimicrobial exposure [8, 9]. The rapid rise in prevalence is likely multifactorial and related to its extensive antimicrobial resistance and the improvements in medical management leading to an increase in the number of vulnerable patients and their greater life expectancy [10]. Unfortunately, this rising prevalence has not been consistently matched with the necessary contemporary data to inform the optimal detection or therapeutic approach to this problematic pathogen.
Figure 1.
Number of PubMed-indexed papers mentioning Stenotrophomonas maltophilia, 1996–2021.
Stenotrophomonas maltophilia possesses several traits that contribute to its versatility and complexity, challenging our ability to appropriately care for patients with serious infections. It is ubiquitous in the environment, can survive on almost any humid surface, and demonstrates a strong predilection for catheters, endoscopes, sink drains, and hemodialysis and ventilator circuits in the hospital setting [11]. Beyond surviving, its propensity for adhering to and forming biofilms on both biotic and abiotic surfaces confers protection from host defenses, antimicrobial treatment, and infection control measures [12]. Although demonstrated to be weakly invasive in vivo, S maltophilia is highly immunostimulatory and elicits significant airway inflammation [13]. In addition, the presence of 2 intrinsic, inducible β-lactamase enzymes (L1 and L2) from Ambler class B and A, respectively, eliminates virtually all β-lactam agents as treatment options [14]. As such, non-β-lactam drugs with intracellular targets are the primary therapeutic agents, although their activity is also limited by the vast array of efflux pumps present in S maltophilia, notably those from the resistance-nodulation-cell division (RND) family [15]. While the extensive phenotypic resistance of S maltophilia is well described, the underlying genotypic mechanisms leading to this resistance are not well understood [16]. The optimal management of S maltophilia infections is further hindered by unreliable susceptibility information, the lack of clinical breakpoints, limited treatment options, and paucity of robust outcomes data [17]. Taken together, these factors are devastating to patients infected with this pathogen, clinical microbiologists trying to provide accurate susceptibility information, and clinicians attempting to treat these infections.
Advances in our ability to identify pathogenic bacteria, understand resistance mechanisms, leverage antimicrobial pharmacokinetic (PK) and pharmacodynamic (PD) principles, develop novel antibiotics, and perform clinical outcomes studies will help formulate the optimal evidence-based approach for the treatment of S maltophilia and lead to reductions in the unacceptably high attributable mortality rates of up to 38% [18–20]. Thus, the objective of this scoping review is to collate recent literature regarding the management of S maltophilia infections including microbiological approaches, PK/PD considerations, and therapeutic strategies to provide clinicians and scientists with the most up-to-date compendium to inform their current and future practice.
THERAPEUTIC STRATEGIES
PK/PD Considerations
The lack of sufficient PK/PD data combined with diagnostic hurdles specific to S maltophilia impedes the optimization of antimicrobial therapy and makes it difficult for clinical laboratories to provide useful actionable information to clinicians [21]. As most S maltophilia infections occur in the respiratory tract and human epithelial lining fluid is known to have an acidic pH [22], drug concentrations and antibacterial activity at the site of infection must be considered. This creates additional challenges given the considerable PK variability among the limited number of available treatment options for S maltophilia, such as between-class differences in pulmonary penetration (~24% for cefiderocol vs >100% for levofloxacin) and within-class differences in activity at acidic pH among the fluoroquinolones [23–25]. From a PD perspective, there are 1 or more published studies to inform the exposure-response relationship of trimethoprim-sulfamethoxazole (TMP-SMX), levofloxacin, or minocycline against S maltophilia, impeding the assessment of optimal dosing or of the appropriateness of current susceptibility breakpoints. Furthermore, available PK/PD data from other gram-negative pathogens including Enterobacterales and P aeruginosa cannot be extrapolated to S maltophilia due to significant differences in PD activity even at optimized PK exposures against similar minimum inhibitory concentrations (MICs) [26–28]. Finally, discrepancies between in vitro and in vivo activity and clinical efficacy are common owing in part to its slow growth rate and high mutation frequency [29]. Together these issues challenge the ability to maximize drug efficacy and minimize toxicity, leading to the low cure and high mortality rates observed in S maltophilia infections [22]. Although TMP-SMX is typically considered a preferred agent for S maltophilia, there is no established PK/PD index or target threshold for efficacy or toxicity, severely impairing the ability to optimize its clinical use. TMP-SMX is further limited by its side effect profile of being a major cause of myelosuppression, which largely precludes its use in neutropenic cancer patients, a major target host population for S maltophilia infections. Thus, cancer centers widely utilize alternative agents such as minocycline, levofloxacin, ceftazidime, or agents that lack Clinical and Laboratory Standards Institute (CLSI) breakpoints such as ciprofloxacin, tigecycline, or colistin. This emphasizes the need to bolster the expansion of clinical breakpoints for agents other than TMP-SMX and further studies needed for other agents. Although the MIC50/90 values against S maltophilia are low at 0.5/1 mg/L, TMP-SMX is bacteriostatic in vitro and cannot be reliably tested in vivo due to the increased systemic thymidine concentrations in rodents compared to humans [30, 31]. The majority of dosing recommendations for TMP-SMX are based on achieving target maximum concentrations (Cmax) of 100–200 mg/L of SMX and 5–8 mg/L of TMP against Pneumocystis [32]. Oral (PO) and intravenous (IV) doses of 15 mg/kg/day of TMP should achieve these targets while maintaining trough concentrations above the MIC90 for S maltophilia. Doses of 20 mg/kg/day are associated with unacceptably high rates of gastrointestinal and central nervous system toxicities [33]. Among 106 patients with S maltophilia infections treated with TMP-SMX who underwent therapeutic drug monitoring (TDM), there was no association between SMX levels and efficacy or toxicity despite TDM-based dose adjustments [34]. Despite the lack of PK/PD-supported dosing, TMP-SMX remains a first-line agent per the recent Infectious Diseases Society of America (IDSA) guidance document for mild infections due to S maltophilia whereas combination therapy with minocycline is preferred for serious infections [35]. The recommended dose in this guidance document is 10–15 mg/kg/day of TMP IV/PO divided in 3 doses and given every 8 hours with a maximum daily dose of TMP of 960 mg. Pooled time-kill analyses data of TMP-SMX alone against 25 S maltophilia isolates with TMP MICs of 0.25–8 mg/L reveal a lack of bactericidal activity and moderate correlation between MIC and reduction in bacterial density (R2 = 0.80) regardless of concentration tested [26, 36, 37]. In one of the only available dynamic in vitro PK/PD studies, TMP-SMX was tested against 4 clinical isolates of S maltophilia [38]. Despite susceptibility and drug concentrations above the MIC, TMP-SMX (5 mg/kg every 12 hours [Q12h]) alone again had no activity against any isolate even when retested at doses equivalent to 15 mg/kg TMP Q12h.
The fluoroquinolones maintain in vitro susceptibility against S maltophilia and their use as first-line and alternative therapy continues to increase based on clinical outcomes data. As discussed later in this review, the clinical efficacy of the fluoroquinolones may be tempered by the potential for development of resistance while on therapy. The justification for this concern is often attributed to a 1-compartment in vitro PK/PD analysis from 1996 that evaluated ciprofloxacin (area under the curve [AUC] ~11 mg × hour/L) against a resistant clinical isolate (MIC ≥8 mg/L) of S maltophilia [29]. As the target AUC/MIC for maximal antibacterial activity and clinical efficacy of the fluoroquinolones is at least 87 [39], ciprofloxacin unsurprisingly demonstrated no bacterial kill, and resistant mutants (MIC ≥16 and ≥128 mg/L) emerged after 12 hours. A subsequent 2-compartment, 48-hour in vitro PK/PD model evaluated ciprofloxacin (750 mg PO Q12h) and moxifloxacin (400 mg PO every 24 hours [Q24h]) against 3 strains of S maltophilia with MICs to ciprofloxacin of 0.5, 1, and 2 mg/L and to moxifloxacin of 0.06, 0.25, and 0.5 mg/L [40]. As the AUC/MIC ratios ranged from only 12 to 50 for ciprofloxacin compared to 35 to 287 for moxifloxacin, moxifloxacin achieved a more rapid kill and slower bacterial regrowth than ciprofloxacin, although there were no significant differences in activity at 48 hours. Levofloxacin is the only fluoroquinolone with established CLSI breakpoints and MIC50/90 values are 1/>4 mg/L overall and >4/>4 mg/L against TMP-SMX–resistant strains [30]. A study evaluated the PD of a human-simulated regimen of levofloxacin 750 mg Q24h against 26 S maltophilia isolates via a neutropenic mouse thigh infection model. Mean ± standard deviation (SD) changes in log10 colony-forming units (CFU)/thigh at 24 hours were –1.66 ± 0.89, 0.13 ± 0.97, and 1.54 ± 0.43 for isolates with an MIC of ≤1, 2, and ≥4 mg/L, respectively. Only 1 of 6 isolates with an MIC of ≤2 mg/L achieved at least a 1 log10 CFU/thigh reduction. A strong positive correlation (R2 = 0.82) was demonstrated between change in log10 CFU/thigh and the fAUC/MIC in efficacy studies, with observed thresholds for stasis and 1-log kill of 39.9 and 54.9, respectively. These thresholds were then used in a 5000-subject Monte Carlo simulation to evaluate the probability of target attainment (PTA) of a 750 mg Q24h as a 1.5-hour IV infusion dosing regimen across MICs from 0.5 to 8 mg/L based on PK parameters derived from 58 patients with nosocomial pneumonia [39]. Probability of target attainment for stasis and 1-log kill was maintained above 90% at MIC values ≤0.5 mg/L, but was <50% at the current CLSI MIC breakpoint of 2 mg/L. These results suggest a potential need to lower the current CLSI breakpoint for levofloxacin, although no data evaluating clinical outcomes based on levofloxacin MIC are available. Of note, the CLSI levofloxacin breakpoint against Enterobacterales was revised in 2019 from ≤2 mg/L to ≤0.5 mg/L based on available PK/PD and clinical outcomes data [41]. If this breakpoint were applied to S maltophilia, overall susceptibility to levofloxacin would be reduced to approximately 21%. Levofloxacin monotherapy is recommended at a dose of 750 mg IV/PO Q24h per the aforementioned IDSA guidance document for mild infections while combination therapy with a second active agent is recommended for serious infections [35].
Similar to TMP-SMX, there are a dearth of in vitro or in vivo PK/PD or clinical outcomes data to support the promising in vitro potency of minocycline (MIC50/90 0.5/2 mg/L) [30]. Pooled time-kill analysis data of minocycline alone against 21 S maltophilia isolates with MICs of 0.125–16 mg/L demonstrate a lack of bactericidal activity and poor correlation between MIC and reduction in bacterial density (R2 = 0.44) regardless of concentration tested [36, 37]. Only 1 in vivo PK/PD study has investigated the PK/PD index of efficacy and exposure targets for stasis and 1-log kill of minocycline against S maltophilia via the neutropenic murine infection model [42]. Against the 4 isolates used in dose fractionation experiments with MICs of 0.25–1 mg/L, fAUC/MIC best correlated with antibacterial activity (R2 = 0.57), and composite targets for stasis and 1-log kill were 9.6 and 23.6, respectively. In efficacy experiments utilizing a human simulated minocycline regimen of 100 mg Q12h as a 1-hour IV infusion against all 17 isolates with MICs of 0.25–8 mg/L, the mean (± SD) change in bacterial density against isolates with MICs ≤0.5 mg/L was –1.44 ± 1.37 log10 CFU/thigh vs 1.18 ± 0.79 log10 CFU/thigh for those with MICs ≥1 mg/L. A reduction of at least 1 log10 CFU/thigh was achieved at 24 hours against only 6 of 17 isolates (all with MICs ≤0.5 mg/L) while all 10 isolates with MICs ≥1 mg/L demonstrated regrowth similar to untreated controls. These targets were further explored in a 5000-subject Monte Carlo simulation to evaluate the PTA of minocycline regimens of 100 mg and 200 mg Q12h as 1-hour infusions across MICs from 0.125 to 8 mg/L based on the exposure thresholds of 9.6 and 23.6 for stasis and 1-log kill identified in dose fractionation studies. At the 100 mg Q12h IV dose, PTA was >90% for stasis and 1-log kill at MICs ≤0.5 and ≤0.25 mg/L, respectively, compared to ≤1 and ≤0.5 mg/L for the 200 mg Q12h IV dose. At the current CLSI breakpoint of 4 mg/L, PTA was ≤15% regardless of dose or target assessed. Of note, the PK parameter estimates used for these Monte Carlo simulations were taken from a population model derived from a single-dose study of minocycline 200 mg IV in 50 critically ill patients with suspected or documented gram-negative infection [43]. Importantly, the mean clearance value (5.2 L/hour) in this study was almost 5-fold higher than that observed in a 1975 study of health volunteers (1.2 L/hour), but roughly 1.5-fold lower than that observed in a 2018 study of healthy volunteers given a single 200 mg dose (8.2 L/hour) [44, 45]. Similar to levofloxacin, these results indicate that a breakpoint of ≤0.5 mg/L based on a “high-dose” minocycline regimen of 200 mg IV Q12h may be more appropriate than the current breakpoint of ≤4 mg/L, although clinical outcomes data according to MIC are similarly lacking. Contrary to levofloxacin, roughly 64% of S maltophilia would remain susceptible to minocycline at a breakpoint of ≤0.5 mg/L, and doses as high as 600 mg have been tolerated in healthy volunteers [46] The “high-dose” minocycline regimen is also recommended as the preferred monotherapy agent for mild infections in the IDSA guidance document [35].
Cefiderocol is a novel siderophore cephalosporin with similarly favorable in vitro activity against S maltophilia, demonstrating MIC90 values as low as 0.12–0.5 mg/L, including against isolates resistant to TMP-SMX and/or levofloxacin. In an in vitro time kill analysis against 9 clinical isolates, cefiderocol was bactericidal against only 2 strains at 4 times the MIC or fCmax [37]. In an in vitro 1-compartment PK/PD study, a human-simulated cefiderocol regimen of 2 g every 8 hours (Q8h) IV over 3 hours was evaluated against 3 clinical S maltophilia isolates [47]. Although all 3 isolates had cefiderocol MICs of 0.25 mg/L, bactericidal activity achieved by 8 hours and sustained through 24 hours was subsequently lost as regrowth and the development of resistant mutants (MIC >64 mg/L) was observed by 72 hours against all strains. Despite this relatively poor in vitro activity in static and dynamic PK/PD models, cefiderocol has demonstrated excellent in vivo activity against S maltophilia in neutropenic and immunocompetent mouse and rat thigh and lung infection models [28, 48, 49]. Importantly, the efficacy of cefiderocol was equal to or better than that of comparator agents such as ceftazidime, levofloxacin, and minocycline and was not impacted by resistance to these agents. The current CLSI breakpoint for cefiderocol against S maltophilia is ≤4 mg/L, despite the lack of in vitro or in vivo data against isolates with MICs >0.5 mg/L and very limited clinical data as is discussed later. Similarly to TMP-SMX, levofloxacin, and minocycline, cefiderocol is recommended as monotherapy for mild infections and in combination with a second active agent for serious infections per the IDSA guidance document.
As a result of the suboptimal antibacterial activity observed with monotherapy in vitro, exploring combination therapy against S maltophilia has been of interest as early as 1979. Initial in vitro checkerboard assays demonstrated relatively modest and variable rates of synergy overall (40%–80%), although the combination of TMP-SMX and ticarcillin-clavulanate consistently showed significantly better synergy than TMP-SMX with a fluoroquinolone or minocycline [50–52]. An early in vitro synergy study evaluated 31 isolates of S maltophilia against ticarcillin-clavulanate combined with ciprofloxacin or TMP-SMX via checkerboard assay [53]. All 31 isolates were resistant to ticarcillin-clavulanate and TMP-SMX and 52% were ciprofloxacin resistant. Ticarcillin-clavulanate and TMP-SMX were synergistic against all 31 (100%) isolates and ticarcillin-clavulanate plus ciprofloxacin was synergistic against 24 (77%). Twenty of these 31 isolates were selected for subsequent time-kill analysis based on the ciprofloxacin MIC, which ranged from 2 to 64 mg/L. Ceftazidime was also included in time-kill experiments. The combination of ticarcillin-clavulanate and TMP-SMX was again synergistic against all 20 (100%) strains, despite in vitro resistance to TMP-SMX. Synergy of the combination of ciprofloxacin with either ticarcillin-clavulanate or ceftazidime was dependent on the ciprofloxacin MIC as the 15 strains with ciprofloxacin MIC <32 mg/L demonstrated synergy whereas the 5 strains with MIC ≥32 mg/L did not. A subsequent time-kill study of TMP-SMX in combination with ceftazidime, minocycline, moxifloxacin, and tigecycline against 12 strains also demonstrated the highest rate of synergy between TMP-SMX and moxifloxacin at 50% and all 6 strains had a moxifloxacin MIC ≤4 mg/L [36]. Finally, in combination at 0.5 times the MIC, cefiderocol was synergistic with minocycline and TMP-SMX against 6 of 9 isolates, with polymyxin B against 5 and with levofloxacin against 4, which coincided with the susceptibility agent used in combination with cefiderocol [37]. Overall, cefiderocol was synergistic with another agent in 58% of experiments but bactericidal in combination in just 11%. While considerably more work is needed to identify optimal combination regimens against S maltophilia, these findings, together with previous data, indicate that synergy is most often achieved when β-lactams are combined with an intracellular agent like TMP-SMX [54, 55], a fluoroquinolone [56], or minocycline [37] and that it likely can be predicted by the MIC of the agent used in combination.
Antimicrobial Treatment
The optimal treatment for S maltophilia infections has been the subject of numerous reviews, especially with the continued emergence of its resistance to commonly used agents. Similar to other pathogens with significant intrinsic and acquired resistance, antimicrobial treatment regimens are often selected almost solely based on microbiology and AST results as clinical outcomes data capable of guiding therapy are scarce at best. Moreover, the limited clinical data available almost always exist in the form of uncontrolled observational studies rather than reliable controlled trials. In fact, no prospective trials comparing treatment options for S maltophilia have ever been conducted. As such, reviews such as this one can be especially helpful to clinicians by collating, synthesizing, and translating the available pieces of data into as coherent and evidence-based recommendations as possible. To achieve this goal in the current work, we searched Medline via PubMed from its inception in 1996 until April 2021 using the terms [(Stenotrophomonas) AND (treatment OR outcome OR clinical)] or [(Stenotrophomonas) AND (sulfamethoxazole OR trimethoprim OR fluoroquinolone OR levofloxacin OR tetracycline OR minocycline OR tigecycline OR β-lactam OR ceftazidime OR cefiderocol OR ticarcillin)]. All clinical studies were screened and considered for review with no specific exclusion criteria other than being published in English. The references of included studies were reviewed to identify other relevant works. As noncomparative and/or single group studies detailing the clinical manifestations, risk factors, and outcomes related to S maltophilia infection have been reviewed and meta-analyzed thoroughly previously, we chose to focus on studies directly comparing the clinical outcomes between 2 or more treatment groups (either different agents or monotherapy vs combination therapy). We felt this was the primary area of clinical uncertainty and would be most beneficial to clinicians, especially since there has been a surge of these comparative studies published within the last 3–5 years that have yet to be comprehensively analyzed and summarized. Consequently, our initial search strategy resulted in 1246 articles, of which 17 were comparative studies [51, 57–72] and were therefore included along with 1 systematic review and 1 meta-analysis focused on evaluating treatment regimens [73, 74]. These were then grouped into strata based on the treatments compared, year of publication, and the type of study. Select characteristics of included studies are summarized in Table 1.
Table 1.
Select Characteristics of the 19 Publications Included in This Review
| Author (Publication Year) |
Study Design, Period, Region | Treatment Arms, No. of Patients | Dosing and Duration | Patient Demographics | Diagnosis and Source of Infection | Mortality | Clinical Outcomes | Microbiologic Outcomes |
|---|---|---|---|---|---|---|---|---|
| Clinical studies comparing TMP-SMX to fluoroquinolones | ||||||||
| Czosnowski (2011) [57] | Retrospective, single-center, 1997–2007, USA | 101 pts; 77 TMP-SMX, 14 CIP | TMP-SMX 11.2 mg/kg/d, 11 d | Adult trauma pts; age 40 y, 76% male, APACHE II 17; 0% IC | VAP | ACM 13%; VAP-related 7% | Clinical success 87%, clinical + microbiological success 82% | NR |
| Cho (2014) [58] | Retrospective, single-center, 2000–2012, Korea | 86 pts; 53 TMP-SMX, 35 LVX | NR | Adults; age 58 y; MV 16.3%; IC 18.6%; septic shock 23.3% | BSI | 30 d ACM; 27.5% TMP-SMX; 20% LVX (P = .43) | LOS: 25 d TMP-SMX, 27 d LVX (P = .82); recurrent bacteremia: 11.9% TMP-SMX, 5.7% LVX (P = .46) | 50% of pts with recurrence developed LVX-R isolates |
| Wang (2014) [59] | Retrospective, single-center, 2008–2011, USA | 98 pts; 35 TMP-SMX, 63 FQ (48 LVX, 15 CIP) | TMP-SMX 8 d (IQR, 2–28); FQ 9 d (IQR, 2–38 d) (P = .265) Median daily dose: TMP-SMX 7.8 mg/kg/d; LVX 500 mg/d; CIP 1000 mg/d (PO) |
Adults; age 73 y; 24% ICU; 39% cancer, 43% recent surgery | PNA 56% (TMP-SMX 49%; FQ 60%); 77% polymicrobial infection (TMP-SMX 63%, FQ 84%) | 30 d mortality: FQ 31%, TMP-SMX 22% (P = .42) In-hospital mortality: FQ 25%; TMP-SMX 20% (P = .55) In-hospital mortality: PNA, 31%; BSI 33% |
Overall, clinical success 55% (FQ 52%; TMP-SMX 61%) (P = .451) PNA: clinical success 50%; BSI: clinical success 40% |
Cure at EOT: TMP-SMX 65% (13/20); FQ 62% (23/37) (P = .832) PNA: cure, 50%; BSI: cure, 40% Resistance on repeat culture within 30 d: FQ 30%; TMP-SMX 20% (P = .426) Reduced % S: TMP-SMX 96%→ 71%; LVX 82%→ 29% |
| Watson (2018) [60] | Retrospective, single-center, 2004–2014, USA | 54 pts; 32 TMP-SMX, 22 FQ | NR | Adults; age 50–53 y; APACHE II 12–16; MV 27%–37%; IC 36%–50% | BSI | Inpatient mortality: 13.6% FQ; 31.3% TMP-SMX (P = .20) | LOS: 9 d FQ; 15 d TMP-SMX (P = .12) | Baseline: TMP-SMX 0%; FQ 9% |
| Samonis (2012) [61] | Retrospective, single-center, 2005–2010, Greece | 68 pts; 5 TMP-SMX, 23 CIP | NR | Adults; age 71 y, 21% ICU; 66% IC | PNA 54% BSI 16% |
ACM 14.7% IRM 4.4% |
78% clinical cure, LOS 17 d | NR |
| Nys (2019) [62] | Retrospective, single-center, 2012–2016, USA | 76 pts; 45 TMP-SMX, 31 LVX | TMP-SMX 10.3 mg/kg/d, LVX 500 mg/d 13 d |
Adults; age 63 y; APACHE II 16; MV 47%; ICU 55%, IC 17% | PNA 92% | 28 d ACM 14.5%; NR for treatment groups | Clinical cure: 82.2% TMP-SMX, 74.2% LVX (P = .40); ME: 84.4% TMP-SMX, 77.4% LVX (P = .55) | 19.3% developed R in LVX, 6.7% TMP-SMX AEs: 4% TMP-SMX, 0% LVX (P = .26) |
| Clinical studies comparing TMP-SMX to fluoroquinolones and/or tetracyclines | ||||||||
| Tekçe (2012) [63] | Retrospective, single-center, 2008–2010, Turkey | 45 pts; 26 TMP-SMX, 19 TGC | TMP-SMX, 800/160 mg Q8h; TGC, 50 mg Q12h 14–21d | Adults; age 65.4 y, 87% ICU, 62.2% APACHE II >20, 60% MV | PNA 51%, surgical site 29%, 11% BSI | 30 d ACM: 31% TMP-SMX, 21% TGC (P = .52) |
14 d clinical improvement: 69.2% TMP-SMX, 68.4 TGC (P = .954) |
NR |
| Hand (2016) [64] | Retrospective, single-center, 2006–2012, USA | 45 pts; 22 TMP-SMX, 23 MIN | Mean daily dose (PO/IV): TMP-SMX, 8.5 mg/kg/d; MIN, 200 mg Median (range): TMP-SMX, 7 d (3–15); MIN, 13 d (4–32) |
Adults, age: TMP-SMX 49 y, MIN 54 y MV: MIN 57% (13/23); TMP-SMX 45% (10/22) Chronic lung disease: MIN 52% (12/23); TMP-SMX 9% (2/22) |
PNA: MIN 69% (16/23); TMP-SMX 59% (13/22); polymicrobial 65%–82% | TMP-SMX 9% (2/22); MIN 8.7% (2/23) | Clinical failure: TMP-SMX 41% (9/22); MIN 30% (7/23) (P = .67) | Positive repeat cultures: MIN 21.7% (5/23); TMP-SMX 31.8% (7/22) No resistance in follow-up cultures within 30 d posttherapy |
| Ebara (2017) [65] | Retrospective, 2 centers, 2007–2013, Japan | 44 adults; 3 TMP-SMX, 15 FQ, 10 MIN, 16 other | NR | Adults; age 48.9 y; 52.3% ICU, 54.5% MV, 36.5% malignancy, 31.5% neutropenia | BSI | 30 d mortality 37.5%; 90 d mortality 62.5%; no difference between treated (42%) and untreated (70%) | NR | NR |
| Junco (2021) [66] | Retrospective, single-center, 2010–2016, USA | 284 pts; 217 TMP-SMX, 39 MIN, 28 FQ | TMP-SMX 9.7 mg/kg/d, MIN 200 mg/d, CIP 800 mg/d LVX 750 mg/d, MOX 400 mg/d Median, 12 d (all) (P = .22) |
Adults; age 59.6 y; APACHE II 19; MV 55.6%; HAI 63.4% | PNA 68.3%; BSI 10.2%; UTI 8.5%; skin 11.3%; other 1.8% | ACM (30 d): TMP-SMX 14.7%; MIN 5.1%; FQ, 7.1% (P = .16) |
Clinical failure: TMP-SMX 35.5%; MIN 30.8%; FQ 28.6% (P = .69) |
Emergence of resistance: TMP-SMX 3.7%; MIN 7.7%; FQ 3.6% (P = .45) |
| Zha (2021) [67] | Retrospective, multicenter (3 centers), 2017–2020, China | 82 pts; 46 TGC, 36 FQ | TGC 50 mg Q12h, LVX 500 mg Q12h, MOX 400 mg/d 9 d |
Adults; age 76 y, APACHE II 21, MV 100% | VAP | 28 d ACM: 47.8% TGC, 27.8% FQ (P = .105) | Clinical cure: 32.6% TGC, 63.9% FQ (P = .009) | Microbiological cure: 28.6% TGC, 59.1% FQ (P = .045) |
| Meta-analysis comparing TMP-SMX to fluoroquinolones | ||||||||
| Ko (2019) [74] | 14 publications; 7 retrospective cohort studies, 7 case-control through Mar 2018 | 663 pts; 332 TMP-SMX, 331 FQ (187 LVX, 114 CIP, 15 other) | NR | All | Any | Overall mortality: 29.6% (30 d ACM or in-hospital mortality) Mortality: FQ 25.7% (85/331); TMP-SMX 33.4% (111/332) Mortality in FQ cohort: OR, 0.62 (95% CI, .39–.99) but LVX, CIP separately showed no mortality benefit vs TMP-SMX (CIP: OR, 0.44 [95% CI, .17–1.12]; LVX: OR, 0.78 [95% CI, .48–1.26]) |
NR | NR |
| Systematic reviews evaluating monotherapy vs combination therapy | ||||||||
| Falagas (2008) [20] | 34 publications through Feb 2008 | 49 pts (29 case reports, 5 case series with 18 pts) | NR | Avg age, 52 y (0–80y) | Any | IRM 5/49 (10.2%) | Cure/improvement: CIP MT or CT, 90%; CRO or CAZ MT or CT, 75%; T/C MT or CT, 66.7% | NR |
| Clinical studies comparing monotherapy to combination therapy | ||||||||
| Jacobson (2016) [68] | Retrospective, single-center; 2010–2014, USA | 93 adults; 45 MIN, 48 MIN combination | MIN 200 mg/d | Adults, 53% ICU; APACHE II 15 ± 6.6 | PNA (63%), BSI (15%) | 30 d mortality: MIN 16.0% (15/94) | Clinical failure: MIN MT and CT, 18% (17/94). MIN MT, 9% (4/45) Failure related to APACHE II, or MIC = 4 mg/L (29.4%) vs MIC <4 mg/L (2.6%) (P = .004) |
NR |
| Araoka (2017) [51] | Retrospective, single-center, 2012–2014, Japan | 20 pts; 14 TMP-SMX + FQ, 6 TMP-SMX or FQ | NR | Adults; ages, 60.5–65 y; Pitt scores 1–2.5; neutropenia 43%–50% | BSI | 30 d mortality: TMP-SMX + FQ, 50% (7/14); TMP-SMX alone, 33% (2/6) | NR | NR |
| Shah (2019) [69] | Retrospective, single-center, 2011–2017, USA | 252 adult pts; 218 monotherapy, 38 combination (various) | NR | Age 62 y; MV 69.4%; ICU 76.2%; 54.4% polymicrobial pneumonia; median APACHE II score 16 | PNA | 30 d ACM: CT 39.5% (15/38); MT 22.9% (49/214); (P = .03) 30 d IRM: CT 15.8% (6/38); MT 8.9% (19/214); (P = .19) |
7 d clinical response: CT 47.4%; MT 39.7% (P = .38) controlling for immune status, APACHE II score, and polymicrobial pneumonia | Emergence of resistance during or after treatment (n = 33 pts): CT 15.8% (6/38); MT 12.6% (27/214) 30 d infection recurrence: CT 10.5%; MT 7.9% Emergence of resistance during therapy (n = 54): CT 37.5% (3/8); MT 32.6% (15/46) Emergence of resistance after therapy (n = 21): CT 75% (3/4); MT 70.6% (12/17) |
| Tokatly Latzer (2019) [70] | Retrospective, multicenter (4 sites), 2012–2017, Israel | 61 pts; 22 TMP-SMX, 13 CIP, 6 CAZ, 11 TMP-SMX + CIP, 9 TMP-SMX + CIP + MIN, 7 none | TMP-SMX 20 mg/kg/d, MIN 8 mg/kg/d, CIP 30 mg/kg/d, CAZ 150 mg/kg/d (IV) |
Pediatric; age 2.1 y; prior MV 72%; recent chemotherapy 27% | BSI | 42% ACM; attributable mortality within 30 d, 25%; CIP + TMP-SMX + MIN (n = 9) resulted in longest survival (mean, 54 d [range, 44–65 d]) |
NR | NR |
| Guerci (2019) [71] | Retrospective, multicenter (25 ICUs), 2012–2017, France | 282 pts; 82 TMP-SMX, 71 CIP, 68 T/C | NR Median duration of effective therapy, 11 d (7–15) |
Adults; age 65 y, 81% VAP, 84% intubated; 100% ICU, IC <15% | 100% nosocomial PNA; 81% VAP | In-hospital mortality 49.7%; attributable mortality 24.3% | Treatment failure 23.1%; combination therapy and DOT >7 d did not impact mortality | NR |
| Sierra-Hoffman (2020) [72] | Retrospective, multicenter registry (6 sites), 2015–2018; USA | 29 pts; 9 MIN, 20 MIN combination | 25 MIN 100 mg BID, 4 MIN 200 mg BID Median 9 d (IQR, 5–15) |
Adults; age 57.6 y; MV 53.5% | PNA 71.4%; BSI 14.3%; skin 8.6%; UTI 5.7% | 30.0% (in-hospital) | Clinical response: PNA + BSI, 79.3% (23/29) | 27.6% (PNA + BSI); 1 emergence of R in MIN |
Abbreviations: ACM, all-cause mortality; AE, adverse event; APACHE, Acute Physiology and Chronic Health Evaluation; BID, twice-daily dose; BSI, bloodstream infection/bacteremia; CAZ, ceftazidime; CI, confidence interval; CIP, ciprofloxacin; CRO, ceftriaxone; CT, combination therapy; DOT, days of therapy; EOT, end of therapy; FQ, fluoroquinolone; HAI, hospital-acquired infection; IC, inhibitory concentration; ICU, intensive care unit; IQR, interquartile range; IRM, infection-related mortality; IV, intravenous; LOS, length of stay; LVX, levofloxacin; ME, microbiological eradication; MIC, minimum inhibitory concentration; MIN, minocycline; MOX, moxifloxacin; MT, monotherapy; MV, mechanical ventilation; NR, not reported; OR, odds ratio; PNA, pneumonia; PO, oral; pts, patients; Q8h, every 8 hours; Q12h, every 12 hours; R, resistant; S, susceptible; T/C, ticarcillin-clavulanate; TGC, tigecycline; TMP-SMX, trimethoprim-sulfamethoxazole; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
TMP-SMX Versus Fluoroquinolones
As discussed, the use of TMP-SMX as the primary therapy for S maltophilia infections has been and continues to be almost exclusively based on reliable in vitro susceptibility coupled with observational data rather than robust PK/PD or clinical outcomes studies. While there are no clear signals of clinical failure in the literature regarding TMP-SMX for S maltophilia infections, it continues to fall out of favor due primarily to the lack of information to guide optimal dosing and the significant associated adverse drug events (ADEs) [58]. This was evident in a recent retrospective analysis (2010–2015) of adult patients with S maltophilia bacteremia from the Premier Health Database in the US, which demonstrated that although TMP-SMX demonstrated 95% susceptibility, only 38.3% of 444 patients received it as definitive therapy whereas 65% received a fluoroquinolone [75]. Fluoroquinolones, primarily levofloxacin, have been the most thoroughly explored alternative agents to TMP-SMX given their susceptibility rates and efficacy and safety profile.
One of the first clinical studies to evaluate fluoroquinolones for the treatment of S maltophilia included 101 critically ill trauma patients with ventilator-associated pneumonia (VAP) due to S maltophilia from 1997 to 2007 [57]. The majority (66%) of patients had polymicrobial VAP, most commonly (31%) with P aeruginosa. Almost all (86%) patients received TMP-SMX either as monotherapy (77%) or combination therapy (9%) while 14% received ciprofloxacin monotherapy (6%) or in combination (8%). The most common (5%) combination regimen was TMP-SMX plus ciprofloxacin. The average dose of TMP was 11.2 mg/kg/day and led to hyperkalemia in 42% of patients vs 13% on non–TMP-SMX regimens (P < .04). The average duration of treatment was 11 days, and no significant differences were observed in success rates between patients treated for <10 days or ≥10 days. The overall clinical and clinical plus microbiological success rates were 87% and 82%, respectively, and were similar between monomicrobial and polymicrobial infection. The clinical success rate was 86% in patients receiving a regimen containing TMP-SMX vs 93% without (P = .68). All-cause and infection-related mortality were low at 13% and 7%, respectively, likely due in part to the inclusion of young, otherwise healthy trauma patients and the exclusion of immunocompromised and medically ill patients.
Bacteremia is the second most common infection due to S maltophilia after pneumonia, and mortality is high regardless of treatment [76, 77]. Cho et al were the first to publish on the clinical efficacy of levofloxacin compared to TMP-SMX for the treatment of S maltophilia bacteremia [58]. They identified 203 adult patients with bacteremia from 2000 to 2012 and included 86 who received IV monotherapy for at least 48 hours with either TMP-SMX (n = 51) or levofloxacin (n = 35). The 2 groups were well balanced on baseline clinical characteristics including almost half (44%) who were neutropenic and 23% who had septic shock at baseline. Overall, there was no significant difference in 30-day mortality between the TMP-SMX and levofloxacin groups (27.5% vs 20%, P = .43), which persisted after excluding those with polymicrobial bacteremia (19.8%) and those who received combination therapy (14%). After univariate and multivariate analyses, only pneumonia and septic shock were associated with 30-day mortality whereas treatment agent was not. Adverse events were significantly more common in the TMP-SMX group (23.5% vs 0%, P = .001), with 10 AEs in TMP-SMX patients necessitating discontinuation. Eight levofloxacin patients were switched to TMP-SMX for unknown reasons, although the authors state that treatment failure was not observed. There were no significant differences in length of stay (LOS) or recurrent bacteremia, although in the 8 patients with recurrence, 4 developed a levofloxacin-resistant isolate (2 from each treatment group). No information is provided on actual MICs, doses received, or duration of therapy. Although efficacy appeared similar with fewer ADEs in the levofloxacin group, the emergence of resistance observed even in patients who did not receive levofloxacin is concerning although not well described.
One of the earliest studies to include multiple infection types started in 2008 and ran through 2011, during which Wang et al performed a retrospective evaluation of 98 patients who received TMP-SMX (n = 35) or a fluoroquinolone (n = 63 [48 levofloxacin, 15 ciprofloxacin]) monotherapy for S maltophilia infection from any site [59]. There were no clinically significant differences in baseline characteristics that were likely to impact the exposure or outcome. Pulmonary infections accounted for 56% of all infections, followed by skin and soft tissue (19%), urinary tract infection (UTI) (9%), complicated intra-abdominal infection (9%), and bacteremia (6%) and 24% of patients were in the ICU at the time of index culture. As is common with S maltophilia, 77% of patients had a polymicrobial infection (most commonly P aeruginosa) and this was significantly more frequent in the fluoroquinolone group (84% vs 63%, P = .017). Levofloxacin demonstrated 82% susceptibility vs 96% with TMP-SMX and the median daily dose was 500 mg/day for levofloxacin, 1000 mg/day for ciprofloxacin, and 7.8 mg/kg/day of TMP. Overall clinical success was roughly 55% in each group and 30-day mortality was 31% for levofloxacin and 22% for TMP-SMX (P = .42). Separating those patients who had pneumonia or bacteremia demonstrated worse outcomes, with microbiological cure and clinical success at 50% and 40% for pneumonia and bacteremia, respectively, and in-hospital mortality at 31% and 33%, although no difference was observed between treatment arms in these subgroups. Factors associated with increased mortality upon multivariable analysis were ICU admission and receipt of chemotherapy, but treatment arm was not included as a covariate as there was no difference on univariate analysis. Microbiological cure in patients with repeat cultures was similar at 62% and 65% (P = .832) for levofloxacin and TMP-SMX, respectively, although 14% (2 of 7) of the isolates in the TMP-SMX group recultured at the end of therapy developed resistance to TMP-SMX compared to 71% (10 of 14) of isolates in the levofloxacin group. This development of resistance occurred after a median duration of therapy of just 8–9 days in each group. The authors do report MIC data in this study, which demonstrated a 2- to 8-fold log2 dilution increase in median MIC from baseline to end of therapy for TMP-SMX (20–40 mg/L), levofloxacin (1–8 mg/L), and minocycline (1–4 mg/L). Although clinical outcomes were similar, the emergence of resistance is again worrisome, especially for levofloxacin, although the high baseline MIC values in relation to the relatively low doses used may have contributed.
Another retrospective study of S maltophilia bacteremia included 54 patients and compared outcomes between those who received TMP-SMX (n = 32) or fluoroquinolone (n = 22 [11 ciprofloxacin, 5 levofloxacin, and 6 moxifloxacin]) monotherapy for ≥48 hours [60]. The groups were well matched on baseline characteristics and the mortality rate was 31.3% in the TMP-SMX group and 13.6% in the fluoroquinolone group (P = .20). On multivariable analysis, treatment group was not identified as a significant independent predictor of mortality, whereas Acute Physiology and Chronic Health Evaluation II (APACHE II) score and the use of broad-spectrum antibiotics prior to index blood culture were. There were no significant differences in any secondary outcomes, and the rate of discontinuation due to an adverse event was similar between groups. Unfortunately, microbiological outcomes were not assessed and no information was provided regarding MICs or dosing in either group.
A 6-year retrospective study (2005–2010) in Greece evaluated the characteristics, susceptibilities, and treatment outcomes of 68 patients with S maltophilia infections [61]. Roughly two-thirds (66%) were immunocompromised, 21% were in the ICU, and 54% had pneumonia while 16% were bacteremic. As expected, most infections were polymicrobial (66%), most commonly with K pneumoniae (10%) or P aeruginosa (9%). Susceptibility results were similar for TMP-SMX (85%) and ciprofloxacin (82%). Of the 55 patients with available treatment data, only 35 received effective targeted antimicrobial therapy, most commonly with a regimen including ciprofloxacin (42%). Interestingly, <10% of patients received monotherapy with any agent and only 9% received TMP-SMX at all (none received monotherapy). Although specific combination regimens were not reported, colistin (26%) and tigecycline (13%) use was higher than in similar studies from other geographic areas. All-cause in-hospital mortality was 14.7% while death due to S maltophilia was 4.4%, all of which occurred in the ICU. The median LOS was 17 days and 78% of patients achieved a clinical cure. The authors evaluated risk factors for mortality and found numerous significant covariates upon univariate analysis including the use of tigecycline, colistin, or TMP-SMX as targeted therapy associated with increased mortality while the use of ciprofloxacin as empiric therapy was protective. After multivariate analysis, though, only ICU admission was significantly associated with mortality. The interpretation of this study is impeded by the low rates of effective targeted therapy, which may argue toward colonization vs true infection in these patients and would coincide with the low mortality rate observed, especially in relation to the predominantly immunosuppressed population and infrequent use of TMP-SMX.
The most recent study was conducted by Nys et al (2012–2016) and included 76 adult patients with monomicrobial infections due to S maltophilia treated with either TMP-SMX (n = 45) or levofloxacin (n = 31) monotherapy for at least 48 hours [62]. Almost all (92%) patients had pneumonia and 50% were in the ICU at the time of infection. Patients receiving TMP-SMX had a higher median APACHE II score (18 vs 14, P = .004) and twice as many were in the ICU and on mechanical ventilation than those who received levofloxacin. Clinical cure, microbiological eradication, and 28-day mortality were 78.9%, 81.6%, and 14.5% overall. Despite higher severity of illness in the TMP-SMX group, outcomes were similar between the TMP-SMX and levofloxacin groups (clinical cure: 82.2% vs 74.2%, P = .40; microbiological eradication: 84.4% vs 77.4%, P = .55). Mortality in each treatment group was not reported. Logistic regression analysis included treatment group but only identified APACHE II score being related to clinical cure. Of the 14 isolates cultured following treatment, resistance developed in 3 receiving TMP-SMX (6.7%) vs 6 who received levofloxacin (19.3%) after a median duration of 13 days of therapy. Median doses of TMP and levofloxacin were 10.3 mg/kg/day and 750 mg/day, respectively, although MICs were not reported. Adverse events occurred in 4% of TMP-SMX patients vs 0% of levofloxacin (P = .26). Despite the observed selection bias in the TMP-SMX group and the suboptimal TMP-SMX dosing, outcomes were similar to levofloxacin while resistance development was again more common after levofloxacin treatment.
TMP-SMX Versus Fluoroquinolones and/or Tetracyclines
Tigecycline was US Food and Drug Administration (FDA) approved in 2005 for complicated skin and soft structure infections, complicated intra-abdominal infections, and community-acquired bacterial pneumonia. It does not have an FDA indication for S maltophilia nor CLSI breakpoints, but its package insert does include S maltophilia in the group 2 organisms for which it demonstrates adequate in vitro activity but lacks supporting clinical data from its clinical trials. Given its documented in vitro activity and the challenges with other alternative therapies, Tekçe et al compared the efficacy of tigecycline to that of TMP-SMX in 45 adult patients who had received at least 3 days of therapy for a nosocomial S maltophilia infections (19 in the tigecycline group and 26 in the TMP-SMX group) [63]. Patients with a polymicrobial culture, those who were colonized with S maltophilia, and those who received combination therapy were excluded. Standard doses of each agent (tigecycline 50 mg Q12h, TMP-SMX 800/160 mg Q8h) were given for 14–21 days and sites of infection included 51% pneumonia, 29% surgical site infection, and 11% bacteremia secondary to pneumonia. Patients were critically ill at baseline with 62.2% having an APACHE II score >20. Concomitant infections were common as 36% of patients had A baumannii, 11% P aeruginosa, and 9% Enterobacter spp. Using a susceptibility breakpoint of ≤2 mg/L for tigecycline, 100% of isolates were susceptible vs 80% to levofloxacin and 98% to TMP-SMX. At day 14 of therapy, clinical improvement was 69.2% in the TMP-SMX group vs 68.4% in the tigecycline group (P = .954), and the mortality rate at 30 days was 31% for TMP-SMX vs 21% for tigecycline (P = .52).
In a study comparing the outcomes between alternative therapies, Zha et al conducted a multicenter retrospective study of 82 adult inpatients with VAP due to S maltophilia from 2017 to 2020 in China who received monotherapy with either standard-dose tigecycline (n = 46) or a fluoroquinolone (levofloxacin or moxifloxacin) (n = 36) [67]. Patients who had combination or sequential therapy with tigecycline and a fluoroquinolone, had polymicrobial infections with a pathogen resistant to tigecycline and fluoroquinolones, or received ineffective definitive therapy for ≥48 hours were excluded. The median age overall was 76 years and median APACHE II score was 21. VAP occurred late, as expected, with a median duration of 15 days from intubation to onset of infection. More than two-thirds (71%) of VAP was polymicrobial with A baumannii (45%), Enterobacterales (38%), and P aeruginosa (17%). The primary outcomes of interest demonstrated that patients who received tigecycline had significantly lower clinical cure (32.6% vs 63.9%, P = .009) and microbiological cure (28.6% vs 59.1%, P = .045), whereas no significant difference was observed in 28-day mortality (47.8% vs 27.8%, P = .105). These findings remained consistent after regression modeling incorporating inverse probability of treatment weighting and excluding patients coinfected with P aeruginosa. Although patients who received tigecycline had a higher severity of illness at baseline, logistic regression found that therapy with tigecycline was significantly associated with an approximate 70% reduction in the odds of clinical cure along with older age, malignancy, and higher APACHE II score. Although MIC values were not reported, suboptimal dosing was utilized for tigecycline (50 mg Q12h) while high dosing was used for levofloxacin (500 mg Q12h), which may have contributed to the differences in clinical outcomes. Particularly in patients with VAP, previous studies suggest that high-dose tigecycline (100 mg Q12h) is needed to ensure optimal outcomes [78, 79].
Given the well-known shortcomings associated with tigecycline for serious infections along with the promising in vitro activity of minocycline, several studies have evaluated its potential as alternative therapy to TMP-SMX. Hand et al retrospectively analyzed laboratory data from 2006 to 2012 of patients with at least 1 positive culture for S maltophilia and cross-referenced it with pharmacy data for patients treated with either TMP-SMX (n = 22) or minocycline (n = 23) monotherapy for >48 hours [64]. Polymicrobial infection was present in 73% of patients overall, 65% in the minocycline group and 82% in the TMP-SMX group, with the most common concomitant pathogen being P aeruginosa. The primary site of infection was the respiratory tract in 69.5% of minocycline patients and 59% of TMP-SMX–treated patients. All S maltophilia isolates were susceptible to both drugs but MICs were not reported. Mean duration of treatment was twice as long in the minocycline group vs the TMP-SMX group (13 days vs 7 days, P = .009) for unclear reasons but may be indicative of clinical imbalances between the groups and/or selection bias. The dosing was suboptimal in both groups, with minocycline patients receiving 100 mg Q12h and the TMP-SMX group receiving just 8.5 mg/kg/day of TMP. Although 65% and 86% of minocycline and TMP-SMX patients, respectively, received the drug orally and outcomes were good, adverse events were not reported so tolerability could not be assessed. The authors’ primary outcome was treatment failure, defined as receipt of alternative antibiotics with in vitro activity against S maltophilia, isolation of S maltophilia on repeat culture, or death within 30 days of treatment. Unfortunately, follow-up respiratory cultures were collected within 15 days regardless of clinical signs and symptoms, which likely inflated the clinical failure rates to 41% and 30% in the TMP-SMX and minocycline groups, respectively. Encouragingly, the mortality was relatively low at 9% in each group.
Two studies have evaluated multiple alternative agents concomitantly. In a case series of S maltophilia bacteremia, Ebara et al retrospectively reviewed the clinical characteristics, outcomes, and risk factors for mortality in 44 patients across 2 study sites in Japan from 2007 to 2013 [65]. The median age was 49 years, half (52.3%) were in the ICU at enrollment, 54.5% were intubated, and 71% were immunocompromised. Roughly one-third of bacteremias were catheter-related while 43% were from an unknown source. Susceptibility to levofloxacin was 82%, minocycline was 100%, and TMP-SMX was just 80%. Interestingly, there were 16 patients who never received effective antimicrobial therapy (all were treated with a carbapenem) and 11 (69%) survived, potentially due to the high rate of catheter-related bacteremias. Among the 28 patients who were treated, 15 received a fluoroquinolone, 10 received minocycline, and 3 received TMP-SMX with no differences in 90-day survival noted between the treatment arms. Overall survival across the 3 treatment groups was only approximately 42% compared to approximately 70% in the untreated arm, likely indicating colonization in those patients although this was not significantly different (log-rank P = .391). Multivariable analysis did not include treatment regimen as a covariate but revealed that underlying comorbidities and intubation were the only significant predictors of mortality. The lack of information regarding source control, MICs, doses administered, and clinical and/or microbiological outcomes other than mortality in this work make these outcomes difficult to interpret, although no clear difference in mortality between antimicrobial treatment agents was observed.
In the largest and most robust retrospective comparative study to date, 284 patients (2010–2016) received TMP-SMX (n = 217), minocycline (n = 39), or a fluoroquinolone (n = 28; 9 ciprofloxacin, 2 levofloxacin, and 17 moxifloxacin) monotherapy for S maltophilia infection from any site [66]. More than 60% of patients in each group had pulmonary infections and approximately 10% had bacteremia. The median doses of each agent were 9.7 mg/kg/day of TMP, 800 mg/day of ciprofloxacin, 750 mg/day of levofloxacin, 400 mg/day of moxifloxacin, and 200 mg/day of minocycline, and median duration of therapy was 12 days. As expected, there was clear evidence of a selection bias as patients who received TMP-SMX had a higher severity of illness at baseline compared to those who received a fluoroquinolone. The authors employed propensity score weighting and inverse probability–weighted regression to account for this bias. After adjustment for confounding factors, treatment was not associated with the primary outcome of clinical failure while lower mortality was observed in patients receiving minocycline (adjusted odds ratio [aOR], 0.2 [95% confidence interval {CI}, .1–.7], P = .02) compared to TMP-SMX (5.5% vs 15%, P = .011). Mortality was similar between the fluoroquinolone and TMP-SMX groups (9.9% vs 15%, P = .41; aOR, 0.3 [95% CI, .1–2.1], P = .23). Other significant independent predictors included age, APACHE II, vasopressor use, and LOS prior to index culture. There were no significant differences in any other secondary outcomes including LOS and development of resistance. Although documented susceptibility was part of the eligibility criteria, microbiological cure was not explored and MIC values were not reported.
Notwithstanding the inherent limitations related to the retrospective study designs and small patient populations, these 11 studies taken together indicate that levofloxacin or minocycline may be reasonable alternatives for treatment of serious infections due to S maltophilia. None of the available data suggest that levofloxacin or minocycline are associated with worse clinical outcomes compared to TMP-SMX including mortality, clinical cure, or microbiological eradication. Despite being underdosed in the majority of studies, TMP-SMX may pose a higher risk of ADEs than levofloxacin or minocycline, while levofloxacin seemed to increase the risk of resistance development compared to TMP-SMX or minocycline. There was a clear selection bias problem in many of the included studies as TMP-SMX is still perceived as the optimal therapy. In the only study that did attempt to adjust for this, minocycline was associated with decreased odds of mortality compared to TMP-SMX. Unfortunately, none of the studies reported outcomes by MIC, which may help discern antimicrobial failure from other covariates in these complex patients with high severity of illness and frequent polymicrobial infections.
Given the limited number of observations and low statistical power of the individual studies available, Ko et al sought to compare the clinical efficacy of TMP-SMX and fluoroquinolones in hospitalized patients with S maltophilia infections via systematic review and meta-analysis [74]. Seven retrospective cohort and 7 case-control studies published between 2014 and 2018 were identified, including a total of 633 patients: 332 in the TMP-SMX group (50%) and 331 in the fluoroquinolone group (50%) (187 levofloxacin and 114 ciprofloxacin). Combination therapy was excluded and the primary outcome was 30 day all-cause mortality. Only 3 of the 14 included studies were designed to compare 2 or more treatment groups, and all 3 demonstrated no differences in outcomes. The overall mortality rate in the meta-analysis was 29%, and the authors report finding a survival benefit associated with the fluoroquinolones over TMP-SMX (OR, 0.62 [95% CI, .39–.99], I2 = 18%). Importantly, the ORs for morality in this meta-analysis were pooled and not adjusted for covariates that are well known to confound and/or modify the relationship between the exposure (treatment) and outcome (mortality), especially selection bias. As TMP-SMX has long been the standard-of-care agent for serious S maltophilia infections and fluoroquinolones have only recently started to become acceptable alternatives, selection bias is a virtual certainty and must be accounted for. To illustrate the impact of this, the e-value for the point estimate (1.92) and upper confidence limit (1.11) of the 95% CI were calculated from the risk ratio (RR) of the meta-analysis by Ko et al (0.75 [95% CI, .58–.99]). This analysis reveals that an unmeasured confounder with an RR of just 1.92 could explain away the observed association between fluoroquinolones and reduced mortality, and an even weaker association with an RR of just 1.11 could move the CI to include an RR of 1 and therefore no longer be statistically significant [80]. Examining the RRs from Cho et al [58] and Junco et al [66] (after converting from ORs) clearly shows that virtually any covariate included in their multivariable analysis would be capable of nullifying the effect reported by Ko et al. Regrettably, the impact of selection bias and pooling unadjusted ORs is familiar to the infectious diseases community after 3 separate meta-analyses published in 2013 and 2014 evaluating daptomycin vs linezolid for the treatment of vancomycin-resistant enterococcal bacteremia incorrectly concluded that treatment with daptomycin was associated with higher mortality, when in fact the exact opposite was true [81–84]. In addition to selection bias, variable case definitions, limited sample sizes, heterogenous patient populations, variation in outcome measures, and differences in dosing and PK/PD can cause misleading conclusions if the appropriate methods are not employed [85]. Until further data are available and/or appropriate meta-analysis methodology is employed, the value of the current work is its reminder of the dangers of selection bias and reporting pooled, unadjusted outcomes.
Monotherapy Versus Combination Therapy
Although combination therapy is a common therapeutic strategy especially against resistant and difficult-to-treat gram-negative pathogens [86], exceedingly few studies exist exploring this approach against S maltophilia. In the first systematic review of antimicrobial treatment options for S maltophilia in 2008, Falagas et al sought to specifically evaluate the treatment outcomes related to agents other than TMP-SMX [73]. The PubMed and Scopus databases were searched through February 2008 and identified 34 publications from 1975 to 2007 comprised of 29 case reports and 5 case series including just 49 patients. Types of infections ranged widely, although many of them included pneumonia as expected and most patients had 1 or more significant comorbidities. In 20 (41%) of the cases, ciprofloxacin was used as monotherapy (8 cases) or in combination (12 cases), resulting in a cure rate of 85%. Twelve patients received ceftriaxone or ceftazidime (6 as monotherapy) and 6 (50%) of these were cured while 5 patients received ticarcillin-clavulanate (3 as monotherapy) and 3 (60%) were cured. One case each of a patient treated with levofloxacin and another with minocycline were included and both achieved cure. Given the limited number of cases included and the varying treatment regimens used, no statistical comparisons between monotherapy and combination therapy are possible, although there did not appear to be an obvious signal for efficacy or failure either way. As a whole, this early analysis suggests that alternative agents other than TMP-SMX may be viable treatment options alone or as part of combination therapy.
In a letter to the editor, Jacobson et al retrospectively reviewed records of 93 patients who received minocycline therapy for >48 hours (alone or in combination) for S maltophilia infections [68]. The overall mean APACHE II score was 15 ± 6.6 and 53% were in the ICU. Pneumonia was diagnosed in 63% and bacteremia in 15%. All isolates were susceptible to minocycline and MIC results demonstrated a modal MIC of 1 mg/L. All patients except 1 received the suboptimal 100 mg Q12h dose (unknown if IV or PO) and 46% initially received monotherapy. Unfortunately, combination therapy regimens were not described and a comparator arm was not included, making it difficult to associate outcomes with either treatment modality. The authors report an 18% clinical failure rate, primarily due to death within 30 days (15 of 17 patients). Patients who failed therapy were significantly more likely to have a pathogen with a minocycline MIC of 4 mg/L (29.4% vs 2.6%, P = .004) and a higher APACHE II score (18.1 vs 14.3, P < .05).
The first study to report TMP-SMX–based combination therapy retrospectively analyzed 20 cases of cancer patients, 17 of whom had hematological malignancies and monobacterial bacteremia with S maltophilia. TMP-SMX plus a fluoroquinolone (n = 14) and TMP-SMX alone (n = 6) showed no difference in 30-day survival [51]. The largest study to date and the only one to compare multiple dual combination regimens included 252 patients with pneumonia due to S maltophilia who received treatment as monotherapy (n = 214) or combination therapy (n = 38) for at least 48 hours [69]. Most (66%) monotherapy patients received TMP-SMX followed by levofloxacin (22%), ciprofloxacin (6%), moxifloxacin (3%), minocycline (3.3%), or ceftazidime (0.5%). The combination group regimens were highly variable based on in vitro susceptibility, but the 3 most common regimens were TMP-SMX plus ciprofloxacin (26.3%), TMP-SMX plus levofloxacin (21%), and TMP-SMX plus minocycline (15.8%). The groups were fairly well balanced except that more patients in the combination therapy arm had received antibiotics active against S maltophilia in the previous 30 days and more were managed by an infectious diseases specialist. The primary outcome was clinical response at day 7 and secondary outcomes included resistance emergence, recurrence, and mortality. There was no difference in clinical response at day 7 between the combination and monotherapy groups (47.4% vs 39.7%, P = .38). This persisted after controlling for immune status, APACHE II, and polymicrobial infection. There were also no significant differences in any secondary outcome.
In a multicenter retrospective, observational study (2012–2017), Tokatly Latzer et al reviewed the treatment outcomes of 68 critically ill children with bacteremia and found a crude mortality rate of 42% and an attributable mortality rate of 18% [70]. Antibiotic treatments were based on “standard institutional pharmacy protocols.” Survival time was longest when patients were treated with a combination of TMP-SMX, ciprofloxacin, and minocycline (P < .01) compared to monotherapy with TMP-SMX or ciprofloxacin and the combination of ciprofloxacin plus TMP-SMX.
A 2016 retrospective, single-center study published in abstract form sought to compare monotherapy and combination therapy comprised of regimens with and without TMP-SMX [87]. One hundred six patients were included, 61 who received monotherapy (38 included TMP-SMX) and 45 who received combination therapy (27 included TMP-SMX). Patients who received combination therapy were more critically ill as evidenced by higher APACHE II scores (14 vs 13, P < .01) and prevalence of sepsis/septic shock (62% vs 38%, P = .02). Mortality, clinical response at the end of therapy, and emergence of resistance were 16%, 72%, and 26%, respectively, with monotherapy and 40%, 49%, and 65% with combination therapy. After adjustment for severity of illness, combination therapy was not associated with mortality. No significant differences were observed between regimens containing TMP-SMX and those that did not. No information regarding alternative agents used or susceptibility is provided.
A retrospective study conducted from 2012 to 2017 in 25 French ICUs described the epidemiology and prognostic factors of 282 adult patients with nosocomial pneumonia (81% VAP) due to S maltophilia with a focus on antimicrobial therapies [71]. Patients were critically ill at baseline as 44% were in septic shock, 84% were intubated, and the median Sequential Organ Failure Assessment score was 8, although <15% were immunocompromised. The most commonly used treatment agents were TMP-SMX (29%), which was used in combination 80% of the time, ciprofloxacin (25%), and ticarcillin-clavulanate (24%). Treatment failure was 23.1% and in-hospital mortality was 49.7%. Attributable mortality was determined to be 24.3%. More than half (59%) of patients received combination therapy, and time to mortality was not affected by combination therapy (59% of patients) (hazard ratio [HR], 1.27 [95% CI, .88–1.83], P = .20) or by treatment for >7 days (median duration, 11 days) (HR, 1.06 [95% CI, .6–1.86], P = .84). Dosing, MICs, ADEs, and development of resistance were not reported and specific treatment agents were not compared.
Finally, Sierra-Hoffman et al analyzed an observational cohort of cases from 6 geographically diverse sites from 2015 to 2018 where intravenous minocycline was used for suspected or documented gram-negative infection. They included 71 patients, of whom 35 had primary S maltophilia infections (25 pneumonia, 5 bacteremia, 3 acute bacterial skin and skin structure infections, and 2 UTIs) [72]. The included patients were severely ill as 54% received mechanical ventilation and 52% received vasopressors. The vast majority of patients received minocycline 100 mg IV Q12h along with a second agent as combination therapy although no information is provided regarding the second agent. Susceptibility testing was performed but only on 63% of the available gram-negative isolates and MICs were not reported, although 100% of S maltophilia isolates tested were susceptible to minocycline at baseline. All together, there were 29 patients with a documented clinical and microbiologic response, of whom 24 had pneumonia, and their overall clinical and microbiologic response rates were 75% and 66.7%, respectively, with an in-hospital mortality rate of 36% despite the use of the lower 100 mg Q12h dose. The other 5 patients had bacteremia; all 5 achieved a clinical and microbiological cure and none died. There were 9 patients who received monotherapy and 20 who received combination therapy with no differences in response rates observed. Interestingly, of 11 posttreatment isolates available for repeat susceptibility testing, 1 isolate of S maltophilia demonstrated a minocycline MIC increase from 0.75 mg/L (susceptible) to 8 mg/L (intermediate) after just 12 days of therapy, though the lower 100 mg Q12h dose was used as monotherapy and resulted in clinical and microbiologic failure in this patient.
Other Alternative Agents
Cefiderocol.
Although comparative data are not yet available for these agents, cefiderocol and aztreonam-avibactam are welcome β-lactam additions to the therapeutic armamentarium against S maltophilia. Cefiderocol was FDA approved on 14 November 2019 for complicated UTIs and more recently for nosocomial pneumonia. In the cefiderocol versus high-dose extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia study of cefiderocol in the treatment of gram-negative pneumonia, 1 patient in the cefiderocol group and 3 patients in the meropenem group had pneumonia due to S maltophilia [88]. The patient receiving cefiderocol successfully achieved a clinical cure and microbiological eradication. The cefiderocol or best available therapy for the treatment of severe infections caused by carbapenem-resistant gram-negative pathogens study was a phase 3, randomized, open-label trial of serious infections caused by carbapenem-resistant gram-negative pathogens that included 5 patients (6%) with S maltophilia treated with cefiderocol vs 0% in the best available therapy arm [89]. At the test-of-cure visit in the carbapenem-resistant modified intent-to-treat population, none of the 5 patients with S maltophilia demonstrated a clinical cure or microbiological eradication. One patient’s cefiderocol MIC was ≤0.03 mg/L, 3 subjects were 0.06 mg/L, and 1 was 0.25 mg/L [90]. Of the 17 subjects in the cefiderocol arm who had a 4-fold increase in MIC during the study, 3 had hospital-acquired pneumonia/VAP due to S maltophilia. Although each of their MIC values increased 4-fold, they were still well within the range of susceptible with the highest being 0.25 mg/L. Two of these subjects’ microbiological and clinical outcomes were considered “indeterminate” at the test-of-cure visit whereas 1 was considered a clinical failure [91]. The expanded-access, compassionate use program of cefiderocol for serious gram-negative infections without alternative therapy enrolled 3 patients with S maltophilia infections as of October 2019. One patient had a history of acute myeloid leukemia and developed bacteremia from S maltophilia. He was treated with combination therapy including TMP-SMX and tigecycline, and received 2 doses of cefiderocol before he died. Another patient had a history of necrotizing pneumonia and developed a new pneumonia due to S maltophilia and P aeruginosa. She was treated with TMP-SMX, tobramycin, piperacillin-tazobactam, and ciprofloxacin along with a 14-day course of cefiderocol. She recovered and was discharged home. The last patient had a history of cystic fibrosis and a lung transplant and relapsed/refractory diffuse large B-cell lymphoma. He developed pneumonia due to a resistant S maltophilia and received cefiderocol for 21 days and recovered.
Aztreonam + ceftazidime-avibactam.
Although aztreonam is stable to the hydrolysis of the L1 metallo-β-lactamase constitutively expressed in S maltophilia, it is easily hydrolyzed by the class A serine L2 enzyme and therefore is not effective as monotherapy. Ceftazidime has no activity against metallo-β-lactamases and therapy with ceftazidime-avibactam alone is ineffective. When combined, the avibactam is able to inhibit the L2 enzyme and protect aztreonam so it can exert its activity. There are currently only a few case reports published on the use of aztreonam plus ceftazidime-avibactam for S maltophilia in patients. An immunocompromised patient with idiopathic medullary aplasia complicated by multidrug-resistant S maltophilia bacteremia was treated with aztreonam and ceftazidime avibactam for 25 days with successful microbiological eradication [92]. Emeraud et al reported a single case of pulmonary infection due to S maltophilia treated successfully with aztreonam pus ceftazidime-avibactam [93]. Similarly, Mojica et al reported the successful use of the same combination in a renal transplant recipient with prolonged bacteremia that failed colistin [94]. Cowart and Ferguson also successfully treated a pneumonia in a patient with cystic fibrosis [95].
MICROBIOLOGICAL APPROACHES
Mechanisms of Resistance
Similar to other gram-negative non–glucose fermenters, S maltophilia often harbors multiple intrinsic resistance mechanisms that are inducible by environmental factors and/or antimicrobial agents [96]. These various chromosomally mediated mechanisms are responsible for its common resistant phenotype, which is not easily or accurately described by available nomenclature. Although the unique chromosomally mediated resistance mechanisms of S maltophilia are not detected by molecular rapid diagnostic tests (mRDTs), antimicrobial therapy can be tailored from the rapid identification alone given the known intrinsic resistance profile. Together this vast resistance and lack of routine genotypic or phenotypic susceptibility testing impedes epidemiologic surveillance and delays time to effective therapy and therapeutic optimization, contributing to the poor outcomes associated with this pathogen.
Only 3 antimicrobial agents (TMP-SMX, levofloxacin, and minocycline) are included in group A and are therefore recommended for routine testing and reporting per CLSI, although only TMP-SMX and levofloxacin are tested >50% of the time in US clinical microbiology laboratories [75, 97]. Furthermore, many laboratories not only do not test clinically valuable agents, but many also test agents that S maltophilia is intrinsically resistant to, which risks overuse of futile agents. Similarly, S maltophilia has not traditionally been included on mRDTs, which are becoming ever more frequently used as they have been shown to significantly improve patient outcomes [98]. Fortunately, it is now included on 2 blood culture identification panels (ePlex BCID-GN and FilmArray BCID2) and 1 lower respiratory tract infection panel (Unyvero pneumonia panel).
Based on the presence of multiple β-lactamases, S maltophilia is resistant to first-line agents with high efficacy and low toxicity such as the β-lactams, due to the presence of 2 intrinsic β-lactamases: a class B zinc-dependent metallo-β-lactamase (blaL1) that, seen from a therapeutic perspective, hydrolyzes all β-lactams except for aztreonam and is not inhibited by clinically in-use β-lactamase inhibitors such as vaborbactam, avibactam, or relebactam; and a class A serine-β-lactamase (blaL2) that may be inhibited by current β-lactamase inhibitors and may hydrolyze β-lactams including cephalosporins and carbapenems. The presence of these chromosomally encoded β-lactamases is involved in the resistance toward virtually all available cephalosporins, monobactams, and carbapenems, including β-lactams/β-lactamase inhibitors, and the pattern of resistance varies according to the level of expression and the enzymatic affinity to individual β-lactams [16], a pattern that is observed on ceftazidime-susceptible strains on isolates with low level of expression of blaL1 and blaL2 β-lactamases. A large-scale global genome-based analysis of 2389 S maltophilia isolates from 22 countries detected blaL1 and blaL2 in 83% and 63% of strains, respectively [99]. These β-lactamases confer significant cross-resistance within this class and are responsible for the lack of activity for agents to which the isolate has not been previously exposed [100]. Modification of penicillin-binding proteins seems to have a less direct impact on the activity of β-lactams. In fact, in some isolates of S maltophilia, the loss of penicillin-binding protein activity secondary to β-lactam binding can induce β-lactamase expression, resulting in a normally bactericidal β-lactam agent becoming a potent β-lactamase inducer leading to high-level β-lactam resistance [101].
In addition to the complex β-lactamase activity within S maltophilia, the presence of broad-range efflux pumps such as those from the RND family further reduce the already limited number of treatment options. These nonspecific efflux pumps have a wide range of substrates and can target specific agents, entire drug classes, and/or multiple agents from different classes simultaneously. Stenotrophomonas maltophilia has been shown to harbor 8 putative RND-type efflux systems, 6 of which have been characterized for their role in antimicrobial extrusion (SmeABC, SmeEF, SmeIJK, SmeOP, SmeVWX, and SmeYZ) [76]. The presence and overexpression of the genes encoding these pumps can lead to resistance to aminoglycosides, β-lactams, chloramphenicol, fluoroquinolones, macrolides, and tetracyclines. The MacABCsm pump of the ATP-binding cassette family and the SmrA pump of the major facilitator superfamily are also common in S maltophilia and responsible for broad-spectrum antimicrobial resistance similar to that of the RND family but are also involved in biofilm formation and protection against oxidative stress [11]. Conversely, resistance mechanisms frequently found in other gram-negative pathogens, including class-specific target site mutations like gyrA and parC against the ciprofloxacin and levofloxacin and/or Tet(A) and Tet(B) efflux pumps against the tetracyclines, are not common in S maltophilia [102, 103].
Although reports of increasing phenotypic resistance of S maltophilia to TMP-SMX are sporadic and conflicting, advances in molecular techniques have improved resistance surveillance and identified known and novel genotypic mechanisms such as sul1, sul2, and insertion element common regions [104]. These genes codify for dihydropteroate synthase, a key enzyme of the nucleic acid synthesis pathway that are resistant to inhibition by TMP-SMX. Of particular concern is the fact that these mechanisms appear to be mobile, increasing the probability of genetic transference among organisms, which may impact in proportion with continued use of TMP-SMX. Fortunately, sul1 and sul2 genes remain rare among clinical isolates (<2%), and multiple mutations are typically necessary to induce high-level phenotypic resistance to sul1 and sul2 [99, 105]. The aforementioned efflux pumps SmeDEF and SmeYZ have also been implicated in resistance to TMP-SMX [106]. Levofloxacin is currently the recommended second-line therapy for S maltophilia, although resistance is common (up to 30%) and increasing (17% in 2003–2008 to 29% in 2019) [107]. Consistent with the inimitable resistance of S maltophilia discussed thus far, it is the only known pathogen in which fluoroquinolone resistance is not primarily mediated by mutations in the target site (gyrA and parC) [108]. Instead, S maltophilia possess a putative chromosomal qnr gene, which likely protects the topoisomerase target sites from the activity of quinolones [109]. Overexpression of the efflux pumps smeDEF, smeIJK, smeABC, and smeVWX also contributes to intrinsic and acquired resistance [110].
Susceptibility Testing
The challenges surrounding antimicrobial susceptibility testing (AST) of S maltophilia are well described, especially when utilizing commercial automated antimicrobial test (cAST) systems. The accuracy of AST against S maltophilia can be impacted significantly by the testing conditions including temperature, duration of incubation, and the type of media used [111, 112]. Furthermore, manufacturer variability in methods such as disks and gradient strips greatly impact accuracy and reproducibility. Since there are no approved FDA breakpoints recognized for S maltophilia other than for ceftazidime, there are no cASTs approved for performing AST against any of the group A agents. Moreover, despite substantial changes in the susceptibility rates since 2008, no cAST system or manual method has been reviewed by the FDA for its accuracy against S maltophilia since before 2009 [113]. It is unlikely that FDA breakpoints will ever be available given the agency’s requirement that S maltophilia be listed in the antimicrobial’s prescribing information as having activity both in vitro and for clinical infections, which is not even in the label for the drug of choice, TMP-SMX [113]. Therefore, clinical microbiology laboratories are forced to apply CLSI breakpoints to MIC values generated by these cAST systems in order to provide usable information to clinicians. Table 2 displays the antimicrobial agents with available CLSI breakpoints against S maltophilia along with the MIC50, MIC90, and percentage susceptible for each agent. These agents are recommended for testing and reporting in groups that cascade from one to another based on the presence of resistance in the previous group. TMP-SMX, levofloxacin, and minocycline are included in group A and therefore are recommended for routine testing and reporting of results. Ceftazidime is included in group B and thus should only be tested if resistance is observed to group A agents [97]. Chloramphenicol is listed in group C and as such should not be routinely tested. Although ticarcillin-clavulanate is included in group O, it has not been available in the US since 2015 and therefore should not be tested [114]. Investigational CLSI breakpoints are provided for the novel agent cefiderocol and thus it is not included in any testing/reporting groups at the time of this publication. Of note, CLSI voted to revise the cefiderocol breakpoints against S maltophilia from susceptible at ≤4 mg/L to ≤1 mg/L and nonsusceptible at ≥8 mg/L to >1 mg/L in January 2021 based on available preclinical and clinical data, although these proposed revised breakpoints have not been approved at the time of this review. There are also no FDA breakpoints for cefiderocol as it is not included in the approved prescribing information.
Table 2.
Clinical and Laboratory Standards Institute Minimum Inhibitory Concentration (mg/L) Breakpoints and In Vitro Activity of Select Agents Against Stenotrophomonas maltophiliaa
| Antimicrobial | Susceptible | Intermediate | Resistant | Count | MIC50 | MIC90 | % Susceptible | % Resistant |
|---|---|---|---|---|---|---|---|---|
| TMP-SMXb | ≤2/38 | … | ≥4/76 | 2095 | ≤0.5 | 1 | 95 | 5 |
| Levofloxacinb | ≤2 | 4 | ≥8 | 2099 | 1 | >4 | 75 | 15 |
| Minocyclineb | ≤4 | 8 | ≥16 | 1977 | 0.5 | 2 | 99 | 0.2 |
| Ceftazidimeb | ≤8 | 16 | ≥32 | 2098 | 32 | >32 | 26 | 64 |
| T/Cc | ≤16/2 | 32/2 to 64/2 | ≥128/2 | 130 | 32 | 128 | 43d | 57 |
| Chloramphenicole | ≤8 | 16 | ≥32 | 66 | 4 | 16 | 80 | NR |
| Cefiderocolf | ≤4 | 8 | ≥16 | 217 | 0.06 | 0.25 | 100 | 0 |
Abbreviations: MIC50, minimum inhibitory concentration at which ≥50% isolates inhibited; MIC90, minimum inhibitory concentration at which ≥90% of isolates inhibited; NR, not reported; T/C, ticarcillin-clavulanate; TMP-SMX, trimethoprim-sulfamethoxazole.
According to Clinical and Laboratory Standards Institute M100-S30.
Data from United States (US) medical centers as part of the SENTRY surveillance program (JMI Laboratories, North Liberty, Iowa).
No longer commercially available in the US. Breakpoints extrapolated from Pseudomonas aeruginosa. Data from Vartivarian S, Anaissie E, Bodey G, et al. A changing pattern of susceptibility of Xanthomonas maltophilia to antimicrobial agents: implications for therapy. Antimicrob Agents Chemother 1994; 38:624–7.
% susceptible includes intermediate isolates as breakpoint was ≤64 mg/L at the time.
Limited availability in the US. Data from Nicodemo AC, Araujo MRE, Ruiz AS, Gales AC. In vitro susceptibility of Stenotrophomonas maltophilia isolates: Comparison of disc diffusion, Etest and agar dilution methods. J Antimicrob Chemother 2004; 53:604–8.
Investigational breakpoints. Data from Hackel MA, Tsuji M, Yamano Y, et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 2018; 62:e01968-17.
TMP-SMX remains the mainstay of therapy for S maltophilia, primarily due to the stably high rates of in vitro susceptibility. Surveillance MIC testing via broth microdilution (BMD) of a worldwide collection of >6000 isolates demonstrated 96% susceptibility to TMP-SMX that was consistent from 1997 to 2016 [115]. Two recent studies of 109 S maltophilia bloodstream isolates demonstrated ≥90% susceptibility to TMP-SMX and acceptable performance of the MicroScan and Phoenix cASTs along with manual gradient diffusion strips from bioMérieux and Liofilchem [116, 117]. The Vitek 2 system did not demonstrate acceptable performance and misclassified isolates as resistant >20% of the time; however, only 9 TMP-SMX–resistant isolates were included, so these results should be interpreted with caution. Two previous studies evaluating the performance of TMP-SMX on Vitek 2 against S maltophilia included 11 and 27 isolates, respectively, and demonstrated 100% essential agreement with BMD, although only 2 resistant isolates were included [118, 119]. Nevertheless, it is recommended to use a second method such as a gradient diffusion strip to confirm results if TMP-SMX resistance is reported by Vitek 2 [17].
Levofloxacin is often used as a second-line agent in the setting of resistance, contraindication, or intolerability to TMP-SMX. Although susceptibility rates are considerably lower (75.8%) than TMP-SMX at the current CLSI breakpoint of ≤2 mg/L [30], available clinical data suggest comparable outcomes between the agents [59, 60, 66, 74]. In the aforementioned study of cAST performance against 109 bloodstream isolates of S maltophilia, levofloxacin susceptibility was 70%–72% depending on the method used. Levofloxacin demonstrated lower error rates than TMP-SMX (Vitek 2 major error rate, 25.3% vs 2.6%) and errors were primarily minor. Notably, utilizing a PK/PD breakpoint of ≤0.5 mg/L resulted in worse performance than the current CLSI breakpoint of 2 mg/L. Although CLSI breakpoints exist only for levofloxacin, the activity of other fluoroquinolones has been the subject of exploration for >20 years. Against 326 clinical strains of S maltophilia, Weiss et al evaluated and compared the in vitro activity of 7 different fluoroquinolones and found that sparfloxacin, moxifloxacin, trovafloxacin, and clinafloxacin had the lowest MIC50/90 values and the highest rate of susceptibility, whereas ciprofloxacin and levofloxacin had the lowest at 40% and 73%, respectively [120]. Biagi et al included 41 clinical isolates of S maltophilia, of which 29 were nonsusceptible to levofloxacin, 26 were resistant to TMP-SMX, and 14 were nonsusceptible to both levofloxacin and TMP-SMX [121]. Against all 41 isolates, the MICs to delafloxacin and moxifloxacin ranged from 0.5 to 32 mg/L and 0.125 to 16 mg/L, respectively, while levofloxacin MICs were 0.25 to >16 mg/L. These results were identical when tested against the 26 TMP-SMX–resistant isolates. Moxifloxacin MICs were ≥1 log2 dilution less than that of levofloxacin and consistent with previous reports detailing the improved potency of moxifloxacin over levofloxacin against S maltophilia [27, 122]. The fluoroquinolones have also demonstrated improved anti-biofilm activity against S maltophilia compared to TMP-SMX and therefore may be preferred in certain clinical scenarios [123].
Minocycline susceptibility and agreement between methods was also evaluated in the studies by Khan et al [116, 117]. Consistent with previous data [115, 121], minocycline was the most active agent with 100% of the 109 isolates testing susceptible by BMD at the current CLSI breakpoint of ≤4 mg/L. The lack of inclusion of minocycline-resistant isolates did not allow for assessment of the cAST systems to detect resistance. In addition to demonstrating the highest rate of susceptibility, minocycline was also the only agent to demonstrate acceptable performance across the cAST systems tested. Global resistance rates to minocycline are very low in S maltophilia clinical strains. Unfortunately, minocycline cannot be tested via Vitek 2 as it is not included on the gram-negative susceptibility cards. This likely significantly influences its clinical use as only 10% of clinical microbiology laboratories who perform AST for TMP-SMX on S maltophilia also test minocycline, despite it being included in group A [124]. In the aforementioned study by Biagi et al, minocycline demonstrated the highest susceptibility (97.2%) and lowest MIC90 value (4 mg/L) of any of the 12 agents tested [121]. Three novel tetracycline analogues were also included in this study with tigecycline and eravacycline demonstrating similar activity to that of minocycline, whereas omadacycline MICs were 2- to 8-fold higher. Interestingly, there appeared to be a correlation between levofloxacin activity and that of the tetracyclines (other than minocycline). The MIC50/90 values for eravacycline, omadacycline, and tigecycline were consistently higher against levofloxacin-nonsusceptible isolates vs those that were levofloxacin susceptible (Figure 2). This correlation may be due to the SmeDEF efflux pump from the RND family, which is known to effect tetracyclines and fluoroquinolones, and a similar cross-resistance pattern has been demonstrated in patients with previous exposure to fluoroquinolones or TMP-SMX [125].
Figure 2.
Minimum inhibitory concentration (MIC) values for eravacycline, omadacycline, minocycline, and tigecycline.
Although ceftazidime is included in group B per CLSI, the activity against S maltophilia is simply too poor to recommend it be tested for susceptibility. In the studies by Khan et al, susceptibility rates to ceftazidime were ≤50% and none of the automated methods demonstrated acceptable performance [116, 117]. It was also the only agent that did not meet performance criteria for any manual method tested. Results from Flamm et al and Biagi et al were similar with ceftazidime and ceftazidime-avibactam susceptibilities ranging from 17% to 27% overall and as low as 7% against strains that were TMP-SMX resistant [30, 121]. Other β-lactam agents that have demonstrated promising in vitro data against S maltophilia include aztreonam in combination with a β-lactamase inhibitor (avibactam) and cefiderocol. In a study by Biagi et al, the activity of aztreonam was tested in combination with avibactam, clavulanate, relebactam, and vaborbactam against 47 clinical S maltophilia isolates [16]. Avibactam restored putative aztreonam susceptibility in 98% of aztreonam-resistant isolates compared to 61%, 71%, and 15% with clavulanate, relebactam, and vaborbactam. After an aztreonam-avibactam–resistant (MIC 16 mg/L) isolate was discovered for the first time in this study, the underlying mechanisms were investigated via whole genome sequencing and quantitative reverse-transcription polymerase chain reaction. Significantly higher expression levels of SmeA and L1 were demonstrated in this strain compared to the strains with MICs of 2 mg/L and 0.5 mg/L as displayed in Figure 3. Conversely, the isolate with an MIC of 2 mg/L showed elevated expression of L2 compared to the other 2 strains. These results underscore the contribution of efflux pumps to β-lactamase expression and antimicrobial resistance among S maltophilia, even for cell wall–active agents. Cefiderocol is a novel siderophore cephalosporin agent with potent activity against S maltophilia. In a third study by Biagi et al, cefiderocol MICs were tested against 37 isolates of S maltophilia along with ceftazidime and minocycline [37]. Cefiderocol demonstrated the lowest MIC values (<0.03–1 mg/L) and the highest susceptibility (100%) even compared to minocycline (0.125–8 mg/L, 97.3%). These results are comparable to previous studies of cefiderocol including roughly 1000 isolates of S maltophilia demonstrating MIC90 values of 0.12–0.5 mg/L and 100% susceptibility regardless of resistance to other agents [126, 127].
Figure 3.
Expression levels of SmeA and L1. Bars represent mean ± SD values from 3 independent experiments in triplicate.
Until more data are available and/or additional breakpoints are revised, manual testing via gradient diffusion strips is the optimal testing strategy for S maltophilia [117]. The limitations of MIC testing by cASTs should be discussed with institutional antimicrobial stewardship programs and infectious diseases clinicians so that awareness of the limitations of such testing is incorporated into treatment decisions. Moreover, improved coordination between standards agencies is critical including FDA recognition of clinical breakpoints to allow for improvements to be made to cASTs so clinical laboratories can better guide treatment decisions. Given the unreliability of current MIC testing methods, the antiquated or absent clinical breakpoints, and dearth of high-quality clinical data available, treatment decisions should rely heavily on our knowledge of patient-specific PK/PD parameters and precision dosing.
DISCUSSION
Stenotrophomonas maltophilia is an enigmatic pathogen that presents challenges to all facets of the diagnostic, microbiological, and therapeutic processes. The increasing prevalence of S maltophilia over the last 2 decades has forced clinicians and scientists to reexamine its true pathogenicity along with the methods used to identify, perform susceptibility testing, and treat this formidable organism. The ability of S maltophilia to survive for long periods on fomites and form biofilms on abiotic and biotic surfaces contributes to its nosocomial prevalence and challenges infection control programs. Moreover, the ambiguity between colonization and infection and the common polymicrobial presentation of S maltophilia, especially among immunocompromised hosts, leads to delays in appropriate antimicrobial therapy in those with true infection and contributes to the overuse and misuse of antibiotics in those without. In addition, its vast amount of intrinsic and acquired mechanisms of resistance severely limits the number of available treatment options.
Currently, gradient diffusion methods are recommended for susceptibility testing against S maltophilia up front (for agents like minocycline that are not included on Vitek 2 panels) or to confirm results provided by cAST systems. Among agents with available CLSI breakpoints, minocycline consistently demonstrates the highest rates of in vitro susceptibility, followed by TMP-SMX then levofloxacin. The novel cephalosporin cefiderocol has demonstrated potent in vitro and in vivo activity and approved breakpoints are expected from CLSI in January 2022. Similarly, aztreonam in combination with ceftazidime-avibactam demonstrates reliable in vitro activity, although no breakpoints exist and clinical data are scarce. In vitro PK/PD demonstrate that although monotherapy is rarely bactericidal against S maltophilia despite susceptible MICs, combination therapy does not appear to significantly improve the antibacterial activity. If combination therapy is desired, the most synergistic regimens are TMP-SMX or a fluoroquinolone (selected based on MIC) with a β-lactam. Limited PK/PD data available suggest that minocycline, especially at a dose of 200 mg twice daily, provides the highest probability of target attainment across its MIC distribution. The majority of emerging clinical data suggest that monotherapy with TMP-SMX, levofloxacin, or minocycline results in comparable outcomes, although TMP-SMX may lead to more ADEs and levofloxacin may be associated with resistance development. While combination therapy with 2 or more of the aforementioned agents is reasonable due to the complex resistance of S maltophilia and the suboptimal mortality rates observed with monotherapy, not enough data currently exist to routinely recommend this approach nor a specific combination regimen. Despite the dearth of supporting data, combination therapy with TMP-SMX and minocycline is endorsed in the recent IDSA guidance document for moderate to serious S maltophilia infections, while the preferred monotherapy regimens for mild infections are TMP-SMX and high-dose minocycline.
Future directions necessary to adequately combat this pathogen including a deeper understanding of the underlying genotypic resistome responsible for its resistant phenotype. An increased number of rapid diagnostic platforms able to identify S maltophilia are desperately needed to ensure timely effective antimicrobial therapy along with improved microbiological methods capable of providing reliable susceptibility information. Additional PK/PD data utilizing modern, advanced in vitro and in vivo methods will be crucial to setting and revising clinical breakpoints, associating MICs with clinical outcomes, and defining the therapeutic window of current and future treatment options, especially those with significant toxicodynamic concerns. Critically, more rigorous clinical outcomes studies will be essential to identifying the optimal therapeutic approach for S maltophilia infections and hopefully lead to an established standard of care for this pathogen. Finally, a greater focus on S maltophilia from antimicrobial stewardship programs will improve patient outcomes and reduce the healthcare burden associated with this pathogen.
Notes
Author contributions. R. K., E. W., J. A., and E. J. C. G. were responsible for conceptualization, data curation, and writing/reviewing/editing the manuscript. All authors read and approved the final manuscript.
Acknowledgments. The authors would like to thank Mark Redell, PharmD, for his critical review of this manuscript.
Potential conflicts of interest. E. W. serves on the speaker’s bureaus for Melinta Therapeutics, Astellas Pharma, Allergan Plc, and Tetraphase Pharmaceuticals, and serves on the advisory boards for GenMark Diagnostics and Shionogi & Co, Ltd. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Palleroni NJ, Bradbury JF.. Stenotrophomonas, a new bacterial genus for Xanthomonas maltophilia (Hugh 1980) Swings et al. 1983. Int J Syst Bacteriol 1993; 43:606–9. [DOI] [PubMed] [Google Scholar]
- 2. Ryan RP, Monchy S, Cardinale M, et al. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat Rev Microbiol 2009; 7:514–25. [DOI] [PubMed] [Google Scholar]
- 3. Crossman LC, Gould VC, Dow JM, et al. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol 2008; 9:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lockhart SR, Abramson MA, Beekmann SE, et al. Antimicrobial resistance among gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol 2007; 45:3352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 2010; 51:S81–7. [DOI] [PubMed] [Google Scholar]
- 6. Hoban DJ, Biedenbach DJ, Mutnick AH, Jones RN.. Pathogen of occurrence and susceptibility patterns associated with pneumonia in hospitalized patients in North America: results of the SENTRY antimicrobial surveillance study (2000). Diagn Microbiol Infect Dis 2003; 45:279–85. [DOI] [PubMed] [Google Scholar]
- 7. Sader HS, Farrell DJ, Flamm RK, Jones RN.. Antimicrobial susceptibility of gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY antimicrobial surveillance program, 2009-2012. Int J Antimicrob Agents 2014; 43:328–34. [DOI] [PubMed] [Google Scholar]
- 8. Karaba SM, Goodman KE, Amoah J, et al. StenoSCORE: predicting Stenotrophomonas maltophilia bloodstream infections in the hematologic malignancy population. Antimicrob Agents Chemother 2021; 65:e0079321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aitken SL, Sahasrabhojane PV, Kontoyiannis DP, et al. Alterations of the oral microbiome and cumulative carbapenem exposure are associated with Stenotrophomonas maltophilia infection in patients with acute myeloid leukemia receiving chemotherapy. Clin Infect Dis 2021; 72:1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harpaz R, Dahl RM, Dooling KL.. Prevalence of immunosuppression among US adults, 2013. JAMA 2016; 316:2547–8. [DOI] [PubMed] [Google Scholar]
- 11. Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 2012; 25:2–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Oliveira-Garcia D, Dall’Agnol M, et al. Fimbriae and adherence of Stenotrophomonas maltophilia to epithelial cells and to abiotic surfaces. Cell Microbiol 2003; 5:625–36. [DOI] [PubMed] [Google Scholar]
- 13. Waters VJ, Gómez MI, Soong G, et al. Immunostimulatory properties of the emerging pathogen Stenotrophomonas maltophilia. Infect Immun 2007; 75:1698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walsh TR, MacGowan AP, Bennett PM.. Sequence analysis and enzyme kinetics of the L2 serine beta-lactamase from Stenotrophomonas maltophilia. Antimicrob Agents Chemother 1997; 41:1460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alonso A, Martínez JL.. Cloning and characterization of SmeDEF, a novel multidrug efflux pump from Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2000; 44:3079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biagi M, Lamm D, Meyer K, et al. Activity of aztreonam in combination with avibactam, clavulanate, relebactam, and vaborbactam against multidrug-resistant Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2020; 64:e00297-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhoads DD. Stenotrophomonas maltophilia susceptibility testing challenges and strategies. J Clin Microbiol 2021; 59:e0109421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aisenberg G, Rolston KV, Dickey BF, et al. Stenotrophomonas maltophilia pneumonia in cancer patients without traditional risk factors for infection, 1997–2004. Eur J Clin Microbiol Infect Dis 2007; 26:13–20. [DOI] [PubMed] [Google Scholar]
- 19. Senol E, DesJardin J, Stark PC, et al. Attributable mortality of Stenotrophomonas maltophilia bacteremia. Clin Infect Dis 2002; 34:1653–6. [DOI] [PubMed] [Google Scholar]
- 20. Falagas ME, Kastoris AC, Vouloumanou EK, et al. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol 2009; 4:1103–9. [DOI] [PubMed] [Google Scholar]
- 21. Brooke JS. Advances in the microbiology of Stenotrophomonas maltophilia. Clin Microbiol Rev 2021; 34:e0003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarzynski SH, Warner S, Sun J, et al. Trimethoprim-sulfamethoxazole versus levofloxacin for Stenotrophomonas maltophilia infections: a retrospective comparative effectiveness study of electronic health records from 154 US hospitals. Open Forum Infect Dis 2022; 9:ofab644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng AW, Bidani A, Heming TA.. Innate host defense of the lung: effects of lung-lining fluid pH. Lung 2004; 182:297–317. [DOI] [PubMed] [Google Scholar]
- 24. Lemaire S, Tulkens PM, Van Bambeke F.. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drwiega EN, Rodvold KA.. Penetration of antibacterial agents into pulmonary epithelial lining fluid: an update. Clin Pharmacokinet 2022; 61:17–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lasko MJ, Gethers ML, Tabor-Rennie JL, Nicolau DP, Kuti JL.. In vitro time kill of trimethoprim/sulfamethoxazole against Stenotrophomonas maltophilia versus Escherichia coli using cation adjusted Muller Hinton broth and ISO-Sensitest broth [manuscript published online ahead of print 10 January 2022]. Antimicrob Agents Chemother 2022. doi:10.1128/aac.02167-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grillon A, Schramm F, Kleinberg M, Jehl F.. Comparative activity of ciprofloxacin, levofloxacin and moxifloxacin against Klebsiella pneumoniae, Pseudomonas aeruginosa inhibitory concentrations and time-kill studies. PLoS One 2016; 11:e0156690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamura R, Ito-Horiyama T, Takemura M, et al. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother 2019; 63:e02031-02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garrison MW, Anderson DE, Campbell DM, et al. Stenotrophomonas maltophilia: emergence of multidrug-resistant strains during therapy and in an in vitro pharmacodynamic chamber model. Antimicrob Agents Chemother 1996; 40:2859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flamm RK, Shortridge D, Castanheira M, et al. In vitro activity of minocycline against U.S. isolates of Acinetobacter baumannii–Acinetobacter calcoaceticus species complex, Stenotrophomonas maltophilia, and Burkholderia cepacia complex: results from the SENTRY antimicrobial surveillance program, 2014 to 2018. Antimicrob Agents Chemother 2019; 63:e01154-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tokunaga T, Oka K, Takemoto A, et al. Efficacy of trimethoprim in murine experimental infection with a thymidine kinase-deficient mutant of Escherichia coli. Antimicrob Agents Chemother 1997; 41:1042–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown GR. Cotrimoxazole—optimal dosing in the critically ill. Ann Intens Care 2014; 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stevens RC, Laizure SC, Williams CL, Stein DS.. Pharmacokinetics and adverse effects of 20-mg/kg/day trimethoprim and 100-mg/kg/day sulfamethoxazole in healthy adult subjects. Antimicrob Agents Chemother 1991; 35:1884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ice LL, Barreto JN, Dao BD, et al. Relationship of sulfamethoxazole therapeutic drug monitoring to clinical efficacy and toxicity: a retrospective cohort study. Ther Drug Monit 2016; 38:319–26. [DOI] [PubMed] [Google Scholar]
- 35. Tamma PD, Aitken SL, Bonomo RA, et al. Infectious Diseases Society of America guidance on the treatment of AmpC β-lactamase producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections [manuscript published online ahead of print 5 December 2021]. Clin Infect Dis 2021. doi:10.1093/cid/ciab1013. [DOI] [PubMed] [Google Scholar]
- 36. Wei C, Ni W, Cai X, Zhao J, Cui J.. Evaluation of trimethoprim/sulfamethoxazole (SXT), minocycline, tigecycline, moxifloxacin, and ceftazidime alone and in combinations for SXT-susceptible and SXT-resistant Stenotrophomonas maltophilia by in vitro time-kill experiments. PLoS One 2016; 11:e0152132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Biagi M, Vialichka A, Jurkovic M, et al. Activity of cefiderocol alone and in combination with levofloxacin, minocycline, polymyxin b, or trimethoprim-sulfamethoxazole against multidrug-resistant Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2020; 64:e00559-00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zelenitsky SA, Iacovides H, Ariano RE, Harding GK.. Antibiotic combinations significantly more active than monotherapy in an in vitro infection model of Stenotrophomonas maltophilia. Diagn Microbiol Infect Dis 2005; 51:39–43. [DOI] [PubMed] [Google Scholar]
- 39. Drusano GL, Preston SL, Fowler C, Corrado M, Weisinger B, Kahn J.. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J Infect Dis 2004; 189:1590–7. [DOI] [PubMed] [Google Scholar]
- 40. Ba BB, Feghali H, Arpin C, Saux MC, Quentin C.. Activities of ciprofloxacin and moxifloxacin against Stenotrophomonas maltophilia and emergence of resistant mutants in an in vitro pharmacokinetic-pharmacodynamic model. Antimicrob Agents Chemother 2004; 48:946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Charlson RJ, Schwartz SE, Hales JM, et al. Response. Science 1992; 256:598–9. [DOI] [PubMed] [Google Scholar]
- 42. Fratoni AJ, Nicolau DP, Kuti JL.. Minocycline pharmacodynamics against Stenotrophomonas maltophilia in the neutropenic murine infection model: implications for susceptibility breakpoints [manuscript published online ahead of print 2 February 2022]. J Antimicrob Chemother 2022. doi:10.1093/jac/dkac018. [DOI] [PubMed] [Google Scholar]
- 43. Lodise TP, Van Wart S, Sund ZM, et al. Pharmacokinetic and pharmacodynamic profiling of minocycline for injection following a single infusion in critically ill adults in a phase IV open-label multicenter study (ACUMIN). Antimicrob Agents Chemother 2021; 65:e01809-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Welling PG, Shaw WR, Uman SJ, Tse FL, Craig WA.. Pharmacokinetics of minocycline in renal failure. Antimicrob Agents Chemother 1975; 8:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cornely O, Arenz D, Barraud O. Phase I study to evaluate the safety and pharmacokinetics of single and multiple ascending doses of intravenous minocycline in healthy adult subjects [P1387]. In: IDWeek, San Francisco, CA, October 3–7, 2018.
- 46. Charlson ME. Clinical indices of disease progression. Experientia Suppl 1982; 41:86–116. [PubMed] [Google Scholar]
- 47. Gill CM, Abdelraouf K, Oota M, et al. Discrepancy in sustained efficacy and resistance emergence under human-simulated exposure of cefiderocol against Stenotrophomonas maltophilia between in vitro chemostat and in vivo murine infection models. J Antimicrob Chemother 2021; 76:2615–21. [DOI] [PubMed] [Google Scholar]
- 48. Nakamura R, Oota M, Matsumoto S, Sato T, Yamano Y.. In vitro activity and in vivo efficacy of cefiderocol against Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2021; 65:e01436-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen IH, Kidd JM, Abdelraouf K, et al. Comparative in vivo antibacterial activity of human-simulated exposures of cefiderocol and ceftazidime against Stenotrophomonas maltophilia in the murine thigh model. Antimicrob Agents Chemother 2019; 63:e01558-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. San Gabriel P, Zhou J, Tabibi S, et al. Antimicrobial susceptibility and synergy studies of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis. Antimicrob Agents Chemother 2004; 48:168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Araoka H, Baba M, Okada C, et al. Evaluation of trimethoprim-sulfamethoxazole based combination therapy against Stenotrophomonas maltophilia: in vitro effects and clinical efficacy in cancer patients. Int J Infect Dis 2017; 58:18–21. [DOI] [PubMed] [Google Scholar]
- 52. Cho S-Y, Lee D-G, Choi S-M, et al. Stenotrophomonas maltophilia bloodstream infection in patients with hematologic malignancies: a retrospective study and in vitro activities of antimicrobial combinations. BMC Infect Dis 2015; 15:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Poulos CD, Matsumura SO, Willey BM, et al. In vitro activities of antimicrobial combinations against Stenotrophomonas (xanthomonas) maltophilia. Antimicrob Agents Chemother 1995; 39:2220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Felegie TP, Yu VL, Rumans LW, Yee RB.. Susceptibility of Pseudomonas maltophilia to antimicrobial agents, singly and in combination. Antimicrob Agents Chemother 1979; 16:833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu VL, Felegie TP, Vee RB, Pasculle AW, et al. Synergistic interaction in vitro with use of three antibiotics simultaneously against Pseudomonas maltophilia. J Infect Dis 1980; 142:602–7. [DOI] [PubMed] [Google Scholar]
- 56. Chow AW, Wong J, Bartlett KH.. Synergistic interactions of ciprofloxacin and extended-spectrum beta-lactams or aminoglycosides against multiply drug-resistant Pseudomonas maltophilia. Antimicrob Agents Chemother 1988; 32:782–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Czosnowski QA, Wood GC, Magnotti LJ, et al. Clinical and microbiologic outcomes in trauma patients treated for Stenotrophomonas maltophilia ventilator-associated pneumonia. Pharmacotherapy 2011; 31:338–45. [DOI] [PubMed] [Google Scholar]
- 58. Cho SY, Kang C-I, Kim J, et al. Can levofloxacin be a useful alternative to trimethoprim-sulfamethoxazole for treating Stenotrophomonas maltophilia bacteremia? Antimicrob Agents Chemother 2014; 58:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang YL, Scipione MR, Dubrovskaya Y, Papadopoulos J.. Monotherapy with fluoroquinolone or trimethoprim-sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother 2014; 58:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Watson L, Esterly J, Jensen AO, et al. Sulfamethoxazole/trimethoprim versus fluoroquinolones for the treatment of Stenotrophomonas maltophilia bloodstream infections. J Glob Antimicrob Resist 2018; 12:104–6. [DOI] [PubMed] [Google Scholar]
- 61. Samonis G, Karageorgopoulos DE, Maraki S, et al. Stenotrophomonas maltophilia infections in a general hospital: patient characteristics, antimicrobial susceptibility, and treatment outcome. PLoS One 2012; 7:e37375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nys C, Cherabuddi K, Venugopalan V, Klinker KP.. Clinical and microbiologic outcomes in patients with monomicrobial Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother 2019; 63:e00788-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tekçe YT, Erbay A, Cabadak H, Sen S.. Tigecycline as a therapeutic option in Stenotrophomonas maltophilia infections. J Chemother 2012; 24:150–4. [DOI] [PubMed] [Google Scholar]
- 64. Hand E, Davis H, Kim T, Duhon B.. Monotherapy with minocycline or trimethoprim/sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. J Antimicrob Chemother 2016; 71:1071–5. [DOI] [PubMed] [Google Scholar]
- 65. Ebara H, Hagiya H, Haruki Y, et al. Clinical characteristics of Stenotrophomonas maltophilia bacteremia: a regional report and a review of a Japanese case series. Intern Med 2017; 56:137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Junco SJ, Bowman MC, Turner RB.. Clinical outcomes of Stenotrophomonas maltophilia infection treated with trimethoprim/sulfamethoxazole, minocycline, or fluoroquinolone monotherapy. Int J Antimicrob Agents 2021; 58:106367. [DOI] [PubMed] [Google Scholar]
- 67. Zha L, Zhang D, Pan L, et al. Tigecycline in the treatment of ventilator-associated pneumonia due to Stenotrophomonas maltophilia: a multicenter retrospective cohort study. Infect Dis Ther 2021; 10: 2415–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jacobson S, Junco Noa L, Wallace MR, Bowman MC.. Clinical outcomes using minocycline for Stenotrophomonas maltophilia infections. J Antimicrob Chemother 2016; 71:3620. [DOI] [PubMed] [Google Scholar]
- 69. Shah MD, Coe KE, El Boghdadly Z, et al. Efficacy of combination therapy versus monotherapy in the treatment of Stenotrophomonas maltophilia pneumonia. J Antimicrob Chemother 2019; 74:2055–9. [DOI] [PubMed] [Google Scholar]
- 70. Tokatly Latzer I, Nahum E, Cavari Y, et al. Treatment outcomes of Stenotrophomonas maltophilia bacteremia in critically ill children: a multicenter experience. Pediatr Crit Care Med 2019; 20:e231–9. [DOI] [PubMed] [Google Scholar]
- 71. Guerci P, Bellut H, Mokhtari M, et al. Outcomes of Stenotrophomonas maltophilia hospital-acquired pneumonia in intensive care unit: a nationwide retrospective study. Crit Care 2019; 23:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sierra-Hoffman M, Redell M, Benefield R, et al. Minocycline intravenous for the treatment of serious infections due to gram-negative nonpseudomonal bacteria, including Stenotrophomonas maltophilia, Acinetobacter baumannii, and Burkholderia cepacia. Infect Dis Clin Pract 2020; 28:209–15. [Google Scholar]
- 73. Falagas ME, Valkimadi PE, Huang YT, et al. Therapeutic options for Stenotrophomonas maltophilia infections beyond co-trimoxazole: a systematic review. J Antimicrob Chemother 2008; 62:889–94. [DOI] [PubMed] [Google Scholar]
- 74. Ko JH, Kang CI, Cornejo-Juárez P, et al. Fluoroquinolones versus trimethoprim-sulfamethoxazole for the treatment of Stenotrophomonas maltophilia infections: a systematic review and meta-analysis. Clin Microbiol Infect 2019; 25:546–54. [DOI] [PubMed] [Google Scholar]
- 75. Cai B, Tillotson G, Benjumea D, et al. The burden of bloodstream infections due to Stenotrophomonas maltophilia in the United States: a large, retrospective database study. Open Forum Infect Dis 2020; 7:ofaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chang YT, Lin CY, Chen YH, Hsueh P-R.. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 2015; 6:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Muder RR, Harris AP, Muller S, et al. Bacteremia due to Stenotrophomonas (Xanthomonas) maltophilia: a prospective, multicenter study of 91 episodes. Clin Infect Dis 1996; 22:508–12. [DOI] [PubMed] [Google Scholar]
- 78. De Pascale G, Montini L, Pennisi M, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 2014; 18:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ramirez J, Dartois N, Gandjini H, Yan JL, et al. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 2013; 57:1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. VanderWeele TJ, Ding P.. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167:268–74. [DOI] [PubMed] [Google Scholar]
- 81. Balli EP, Venetis CA, Miyakis S.. Systematic review and meta-analysis of linezolid versus daptomycin for treatment of vancomycin-resistant enterococcal bacteremia. Antimicrob Agents Chemother 2014; 58:734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Whang DW, Miller LG, Partain NM, McKinnell JA.. Systematic review and meta-analysis of linezolid and daptomycin for treatment of vancomycin-resistant enterococcal bloodstream infections. Antimicrob Agents Chemother 2013; 57:5013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chuang YC, Wang JT, Lin HY, Chang SC.. Daptomycin versus linezolid for treatment of vancomycin-resistant enterococcal bacteremia: systematic review and meta-analysis. BMC Infect Dis 2014; 14:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McKinnell JA, Arias CA.. Editorial commentary: linezolid vs daptomycin for vancomycin-resistant enterococci: the evidence gap between trials and clinical experience. Clin Infect Dis 2015; 61:879–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Britt NS, Potter EM, Patel N, Steed ME.. Comparison of the effectiveness and safety of linezolid and daptomycin in vancomycin-resistant enterococcal bloodstream infection: a national cohort study of Veterans Affairs patients. Clin Infect Dis 2015; 61:871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tamma PD, Cosgrove SE, Maragakis LL.. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 2012; 25:450–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lake P, Furuya EY, Kubin CJ.. Comparison of monotherapy versus combination therapy for Stenotrophomonas maltophilia pneumonia including trimethoprim-sulfamethoxazole-containing and -sparing regimens. Open Forum Infect Dis 2016; 3:2029. [Google Scholar]
- 88. Wunderink RG, Matsunaga Y, Ariyasu M, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2021; 21:213–25. [DOI] [PubMed] [Google Scholar]
- 89. Bassetti M, Echols R, Matsunaga Y, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21:226–40. [DOI] [PubMed] [Google Scholar]
- 90. Pompei P, Charlson ME, Douglas RG Jr. Clinical assessments as predictors of one year survival after hospitalization: implications for prognostic stratification. J Clin Epidemiol 1988; 41:275–84. [DOI] [PubMed] [Google Scholar]
- 91. Thielke TS, Charlson JT, Heckethorn D.. Determining drug dispensing costs for use in cost-accounting systems. Am J Hosp Pharm 1988; 45:844–7. [PubMed] [Google Scholar]
- 92. Diarra A, Pascal L, Carpentier B, et al. Successful use of avibactam and aztreonam combination for a multiresistant Stenotrophomonas maltophilia bloodstream infection in a patient with idiopathic medullary aplasia. Infect Dis Now 2021; 51:637–8. [DOI] [PubMed] [Google Scholar]
- 93. Emeraud C, Escaut L, Boucly A, et al. Aztreonam plus clavulanate, tazobactam, or avibactam for treatment of infections caused by metallo-β-lactamase-producing gram-negative bacteria. Antimicrob Agents Chemother 2019; 63:e00010-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mojica MF, Ouellette CP, Leber A, et al. Successful treatment of bloodstream infection due to metallo-β-lactamase-producing Stenotrophomonas maltophilia in a renal transplant patient. Antimicrob Agents Chemother 2016; 60:5130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cowart MC, Ferguson CL.. Optimization of aztreonam in combination with ceftazidime/avibactam in a cystic fibrosis patient with chronic Stenotrophomonas maltophilia pneumonia using therapeutic drug monitoring: a case study. Ther Drug Monit 2021; 43:146–9. [DOI] [PubMed] [Google Scholar]
- 96. Gil-Gil T, Martínez JL, Blanco P.. Mechanisms of antimicrobial resistance in Stenotrophomonas maltophilia: a review of current knowledge. Expert Rev Anti Infect Ther 2020; 18:335–47. [DOI] [PubMed] [Google Scholar]
- 97. Boutin-Foster C, Euster S, Rolon Y, et al. Social work admission assessment tool for identifying patients in need of comprehensive social work evaluation. Health Soc Work 2005; 30:117–25. [DOI] [PubMed] [Google Scholar]
- 98. Wenzler E, Timbrook TT, Wong JR, et al. Implementation and optimization of molecular rapid diagnostic tests for bloodstream infections. Am J Health Syst Pharm 2018; 75:1191–202. [DOI] [PubMed] [Google Scholar]
- 99. Gröschel MI, Meehan CJ, Barilar I, et al. The phylogenetic landscape and nosocomial spread of the multidrug-resistant opportunist Stenotrophomonas maltophilia. Nat Commun 2020; 11:2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mojica MF, Rutter JD, Taracila M, et al. Population structure, molecular epidemiology, and β-lactamase diversity among Stenotrophomonas maltophilia isolates in the United States. mBio 2019; 10:e00405-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Huang YW, Wang Y, Lin Y, et al. Impacts of penicillin binding protein 2 inactivation on β-lactamase expression and muropeptide profile in Stenotrophomonas maltophilia. mSystems 2017; 2:e00077-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Poole K. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob Agents Chemother 2000; 44:2233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Med 2016; 6:a025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Charlson ME. Delay in the treatment of carcinoma of the breast. Surg Gynecol Obstet 1985; 160:393–9. [PubMed] [Google Scholar]
- 105. Hu LF, Chang X, Ye Y, et al. Stenotrophomonas maltophilia resistance to trimethoprim/sulfamethoxazole mediated by acquisition of sul and dfrA genes in a plasmid-mediated class 1 integron. Int J Antimicrob Agents 2011; 37:230–4. [DOI] [PubMed] [Google Scholar]
- 106. Sánchez MB, Martínez JL.. The efflux pump SmeDEF contributes to trimethoprim-sulfamethoxazole resistance in Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2015; 59:4347–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chang Y-T, Lin C-Y, Chen Y-H, Hsueh P-R.. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 2015; 6:893–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ribera A, Doménech-Sanchez A, Ruiz J, et al. Mutations in gyrA and parC QRDRs are not relevant for quinolone resistance in epidemiological unrelated Stenotrophomonas maltophilia clinical isolates. Microb Drug Resist 2002; 8:245–51. [DOI] [PubMed] [Google Scholar]
- 109. Sánchez MB, Hernández A, Rodríguez-Martínez JM, et al. Predictive analysis of transmissible quinolone resistance indicates Stenotrophomonas maltophilia as a potential source of a novel family of Qnr determinants. BMC Microbiol 2008; 8:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. García-León G, Salgado F, Oliveros JC, et al. Interplay between intrinsic and acquired resistance to quinolones in Stenotrophomonas maltophilia. Environ Microbiol 2014; 16:1282–96. [DOI] [PubMed] [Google Scholar]
- 111. Bonfiglio G, Livermore DM.. Effect of media composition on the susceptibility of Xanthomonas maltophilia to beta-lactam antibiotics. J Antimicrob Chemother 1991; 28:837–42. [DOI] [PubMed] [Google Scholar]
- 112. Wheat PF, Winstanley TG, Spencer RC.. Effect of temperature on antimicrobial susceptibilities of Pseudomonas maltophilia. J Clin Pathol 1985; 38:1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Humphries RM, Hindler JA.. Emerging resistance, new antimicrobial agents … but no tests! The challenge of antimicrobial susceptibility testing in the current us regulatory landscape. Clin Infect Dis 2016; 63:83–8. [DOI] [PubMed] [Google Scholar]
- 114. Kovar PA, Allegrante JP, MacKenzie CR, et al. Supervised fitness walking in patients with osteoarthritis of the knee. a randomized, controlled trial. Ann Intern Med 1992; 116:529–34. [DOI] [PubMed] [Google Scholar]
- 115. Gales AC, Seifert H, Gur D, et al. Antimicrobial susceptibility of Acinetobacter calcoaceticus–Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY antimicrobial surveillance program (1997–2016). Open Forum Infect Dis 2019; 6(Suppl 1):S34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Khan A, Arias CA, Abbott A, et al. Evaluation of the Vitek 2, Phoenix, and Microscan for antimicrobial susceptibility testing of Stenotrophomonas maltophilia. J Clin Microbiol 2021; 59:e0065421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Khan A, Pettaway C, Dien Bard J, et al. Evaluation of the performance of manual antimicrobial susceptibility testing methods and disk breakpoints for Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2021; 65:e02631-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bobenchik AM, Deak E, Hindler JA, et al. Performance of Vitek 2 for antimicrobial susceptibility testing of Acinetobacter baumannii, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia with Vitek 2 (2009 FDA) and CLSI M100s 26th edition breakpoints. J Clin Microbiol 2017; 55:450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Joyanes P, del Carmen Conejo M, Martínez-Martínez L, Perea EJ.. Evaluation of the VITEK 2 system for the identification and susceptibility testing of three species of nonfermenting gram-negative rods frequently isolated from clinical samples. J Clin Microbiol 2001; 39:3247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Weiss K, Restieri C, De Carolis E, et al. Comparative activity of new quinolones against 326 clinical isolates of Stenotrophomonas maltophilia. J Antimicrob Chemother 2000; 45:363–5. [DOI] [PubMed] [Google Scholar]
- 121. Biagi M, Tan X, Wu T, et al. Activity of potential alternative treatment agents for Stenotrophomonas maltophilia isolates nonsusceptible to levofloxacin and/or trimethoprim-sulfamethoxazole. J Clin Microbiol 2020; 58:e01603-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wei C, Ni W, Cai X, Cui JA.. Monte Carlo pharmacokinetic/pharmacodynamic simulation to evaluate the efficacy of minocycline, tigecycline, moxifloxacin, and levofloxacin in the treatment of hospital-acquired pneumonia caused by Stenotrophomonas maltophilia. Infect Dis (Lond) 2015; 47:846–51. [DOI] [PubMed] [Google Scholar]
- 123. Santos Carvalhais BE, Souza ESC, Dos Santos KV, et al. Effect of antimicrobials on Stenotrophomonas maltophilia biofilm. Future Microbiol 2021; 16:83–93. [DOI] [PubMed] [Google Scholar]
- 124. College of American Pathologists. Surveys and Anatomic Pathology Education Programs: Bacteriology Final Critique D-A 2021. Northfield, IL: College of American Pathologists; 2019. [Google Scholar]
- 125. Wu RX, Yu CM, Hsu ST, Wang CH.. Emergence of concurrent levofloxacin- and trimethoprim/sulfamethoxazole-resistant Stenotrophomonas maltophilia: risk factors and antimicrobial sensitivity pattern analysis from a single medical center in Taiwan. J Microbiol Immunol Infect 2022; 55:107–13. [DOI] [PubMed] [Google Scholar]
- 126. Hackel MA, Tsuji M, Yamano Y, et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 2018; 62:e01968-01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Delgado-Valverde M, Conejo MDC, Serrano L, et al. Activity of cefiderocol against high-risk clones of multidrug-resistant Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J Antimicrob Chemother 2020; 75:1840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]