Abstract
Rhythmic gene expression is found throughout the central nervous system. This harmonized regulation can be dependent on- and independent of- the master regulator of biological clocks, the suprachiasmatic nucleus (SCN). Substantial oscillatory activity in the brain’s reward system is regulated by dopamine. While light serves as a primary time-giver (zeitgeber) of physiological clocks and synchronizes biological rhythms in 24-h cycles, nonphotic stimuli have a profound influence over circadian biology. Indeed, reward-related activities (e.g., feeding, exercise, sex, substance use, and social interactions), which lead to an elevated level of dopamine, alters rhythms in the SCN and the brain’s reward system. In this chapter, we will discuss the influence of the dopaminergic reward pathways on circadian system and the implication of this interplay on human health.
Keywords: Dopamine, Ventral tegmental area, Mesolimbic system, Striatum, Reward
4.1. The Dopaminergic Mesolimbic System and Reward
The mesolimbic system, also known as the reward system, is composed of brain structures that are responsible for mediating the physiological and cognitive processing of reward. Reward is a natural process during which the brain associates diverse stimuli (substances, situations, events, or activities) with a positive or desirable outcome. This results in adjustments of an individual’s behavior, ultimately leading them to search for that particular positive stimulus. Reward requires the coordinated release of heterogenous neurotransmitters. However, of the brain substrates implicated in reward, dopamine has a central position. Dopamine plays a critical role in mediating the reward value of food, drink, sex, social interaction, and substance abuse (Hernandez and Hoebel 1988; Everitt 1990; Robbins and Everitt 1996; Bardo 1998; Beninger and Miller 1998).
The dopaminergic pathway mostly involved in reward is the so-called mesolimbic system, which is formed by projections of midbrain dopamine neurons of the ventral tegmental area (VTA) to the striatum, prefrontal cortex, amygdala, hippocampus, and many other structures of the limbic system. When rewarding stimuli are experienced, the dopaminergic mesolimbic system is activated which causes the release of dopamine to the targeted nuclei (Small et al. 2003; Cameron et al. 2014). The ventral striatum, including the nucleus accumbens (NAcc), is a major substrate involved in reward (Marche et al. 2017). The dorsal striatum is critically involved in action selection and initiation components of decision making and also seems to mediate feedback properties such as valiance and magnitude in addition to controlling habitual behavior (Balleine et al. 2007; Burton et al. 2015; Lipton et al. 2019). Therefore, both dorsal and ventral regions have collaborative roles in mediating reward. Nevertheless, the NAcc is most appreciated for its involvement in reward processing and its role in evaluation and incentive-based learning (Schultz et al. 1992; Daniel and Pollmann 2014).
The most prominent striatal neurons are the γ-aminobutyric acid (GABA) producing medium spiny neurons (MSNs). These cells make up to 90–95% of the neuronal population and serve as the sole output from the striatum (Kemp and Powell 1971; Graveland and DiFiglia 1985). MSNs outputs generate two pathways: the direct pathway formed by dopamine D1 receptor (D1R) expressing medium spiny neurons (dMSNs) and the indirect pathway by dopamine D2 receptor (D2R) expressing medium spiny neurons (iMSNs). Coordinated dopamine signaling to dMSNs and iMSNs within the striatum is critical for integrating and responding to rewarding stimuli.
The other 5–10% of striatal neurons are interneurons, which serve as intrastriatal regulators of MSNs activity (Oorschot 2013). The majority of interneurons are inhibitory GABAergic interneurons which modulate reward through their signaling to MSNs and expression of a variety of modulatory peptides (Gittis et al. 2010). About 1–2% are formed by the tonically active cholinergic interneurons which, despite their low abundance, critically regulate MSNs (Kharkwal et al. 2016a; Lewis et al. 2020). Indeed, activation of cholinergic interneurons has been linked to the salience of events (Gittis and Kreitzer 2012). Thus, inter- and intra-striatal connections modulate striatal circuits and play a critical role in reward processing.
Natural rewards, such as eating, drinking, and mating are necessary for survival and maintenance of a species. At its core, the reward system determines the valence of a stimulus and signals whether it is to be avoided or approached, as well as assigning the priority of one stimulus over another. Substances of abuse, whether illicit (e.g. cocaine, heroin, etc.) or licit (e.g. alcohol, nicotine, etc.), hijack the mesolimbic system by offering a reward without an obvious biological function. However, the pleasure and reward linked to initial substance use are then lost by their abuse, which leads to a vicious circle of addiction (Volkow et al. 2016).
Recent studies have shown that reward is subjective and is highly influenced by the chemistry of the individual, homeostatic state (Paulus 2007; Keramati and Gutkin 2014) and genetics (Comings and Blum 2000; Jia et al. 2016), as well as by the environment and epigenetics (Xu et al. 2007; Solinas et al. 2009; De Decker et al. 2017). Indeed, how, when, and where rewarding stimuli are experienced can have a profound influence on reward-related behaviors, as a result of activation of several circuits located in the striatum as well as in other brain regions responsible for encoding and storing memory of events. Importantly, the mesolimbic system is connected to the suprachiasmatic nucleus (SCN)—the master regulator of circadian rhythms (Grippo et al. 2017). The SCN is known to influence reward-related behavior and reciprocally, rewarding stimuli can serve as time-givers (zeitgebers) to entrain the SCN as well as peripheral clocks through the release of dopamine (Honma and Honma 1995; Davidson et al. 2005; Baba et al. 2017).
4.2. Rhythmic Variation in Dopamine-Related Activity
Rhythmic control of an organism’s behavior is a critical part of adapting and anticipating environmental changes in light, temperature, and resources. Though time-keeping mechanisms are more complex and developed in mammals, diurnal control is conserved throughout nature (Edgar et al. 2012). In mammals, the SCN organizes behavior and its correlated cellular activity through hormone and neurotransmitter release, in a 24-hour cycle based on daily light and dark phases (Dunlap 1999).
Support of a circadian regulation of reward was initially highlighted by admittance of patients experiencing substance overdose into the emergency room predominantly in the evening (Morris 1987; Raymond et al. 1992). Thus, the night spikes in overdose are likely related to differences in the metabolism of drugs of abuse during different times of day (Baird and Gauvin 2000; Abarca et al. 2002). Importantly, a variety of medications have been shown to have better clinical efficacy at precise times during day (Musiek and FitzGerald 2013; Nobis et al. 2019; Samir et al. 2020). Timing effects of rewarding stimuli also extend to natural rewards where time of day influences physiological responses as well as anticipatory rhythms (Castro 2004; Landry et al. 2012; Johnston 2014).
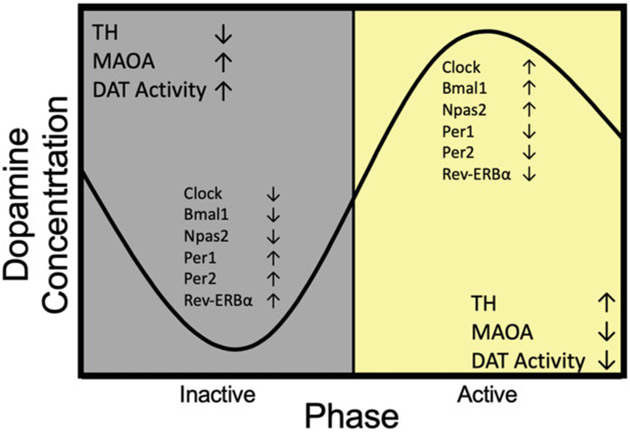
Dopamine levels in SN and VTA follow circadian oscillations, rising in the active phase and falling in the resting phase of the day (Smith et al. 1992; Hood et al. 2010; Ferris et al. 2014), as does its precursor and metabolites (Paulson and Robinson 1996; Castañeda et al. 2004) (Fig. 4.1). Rhythmic expressions of clock genes including Clock, Rev-ERBα, Per, Npas2, and Bmal1 are involved in dopamine metabolism (McClung et al. 2005; Chung et al. 2014). Indeed, Clock and Rev-ERBα negatively regulate the expression of tyrosine hydroxylase (TH), the rate limiting enzyme in dopamine synthesis (Musacchio 1975). Levels of TH increase during the active phase, which is opposite to that of Clock and Rev-ERBα; loss of either circadian gene results in disrupted rhythmic TH expression (McClung et al. 2005; Chung et al. 2014). Transcription of monoamine oxidase A (MAOA), the enzyme responsible for dopamine breakdown, is regulated by the expression of NPAS2, BMAL1, and PER2 (Hampp et al. 2008). Deletion of Per2 causes a lack of MAOA expression during the resting phase, which leads to elevated basal levels of dopamine in the NAcc (Hampp et al. 2008).
Fig. 4.1.
Overview of dopamine-related activity in the reward system. Dopamine levels peak in the active phase when tyrosine hydroxylase (TH) levels are high while monoamine oxidase A (MAOA) levels are low and dopamine transporter (DAT) activity is decreased. This corresponds to core clock gene expression in the striatum, which regulates the expression of these dopamine metabolism-related gene expressions
Psychostimulants increase extracellular dopamine levels and alter the expression of clock genes in the striatum (Nikaido et al. 2001; Uz et al. 2003; Lynch et al. 2008). Though drugs like cocaine and methamphetamine simultaneously alter levels of other neurotransmitters such as serotonin (Haughey et al. 2000; Andrews and Lucki 2001), their impact on clock gene expression is largely dependent on dopamine signaling. Indeed, administration of the D1R agonist, SKF-38393, increases mRNA levels of Per1, Clock, Bmal1, and Npas2 while the D2R agonist, quinpirole, decreases Clock and Per1 expression (Imbesi et al. 2009). D1R signaling plays a critical role in Per2 expression, as D1R-null mice have reduced Per2 in the striatum (Gallardo et al. 2014). Interestingly, depletion of dopamine by 6-hydroxydopamine lesions of dopaminergic neurons results into suppression of PER2 oscillations which can be rescued by D2R agonists (Hood et al. 2010). These results imply that the simultaneous activation of both D1R and D2R is necessary for the normal Per2 oscillations in the striatum. The effect of D2R on Per2 expression might not be direct, but mediated by the inhibitory regulation of iMSNs on dMSNs through collaterals (Lemos et al. 2016; Kharkwal et al. 2016b).
Dopamine’s influence on the SCN was inferred by expression of both D1R and D5R on its neurons (Weiner et al. 1990; Rivkees and Lachowicz 1997; Doyle et al. 2002). In neonatal hamsters, light pulses mirror the effects of D1R agonists suggesting that the maternal levels of DA correspond to the active phase in the fetal SCN (Viswanathan and Davis 1997). Dopamine has been reported to play a critical role in entraining fetal development through the SCN and that after this period the SCN’s responsiveness to dopamine declines (Weaver and Reppert 1995; Mendoza and Challet 2014). Nevertheless, D1R activation in the SCN shifts the phase of circadian rhythms and a direct connection between the VTA and the SCN has been described (Grippo and Güler 2019). Furthermore, D2R seems to be absolutely required for the light-induced suppression of locomotor activity (masking), whereas other visual or nonvisual photic responses seem to be D2R independent (Doi et al. 2006). These results showed a yet unappreciated function of D2R-mediated signaling in regulating the proper organization of daily locomotor activity in light-dark cycles.
Thus, the daily fluctuation in VTA dopamine neuron activity has a substantial role in SCN entrainment and other circadian activities.
4.3. Food and its Relationship to the Circadian Control of the Mesolimbic System
It is a complex process that both the type of food we consume and how much is consumed integrates the homeostatic and reward systems. Controlled food intake relies on balanced responses between orexigenic and anorexigenic neurons of the hypothalamus, which respond to circulating hormones and nutrients (Kalra et al. 1999; Meister 2007). The hypothalamus regulates the production of neuropeptides like ghrelin, leptin, and neuropeptide Y (NPY) in a diurnal manner, which contributes to appetite regulation (Kalra et al. 1999). Genetically engineered mice with deletions of genes encoding either ghrelin, leptin, or NPY have aberrant feeding behaviors or metabolic fuel preference (Bannon et al. 2000; Wortley et al. 2004; Cristino et al. 2013; Schéle et al. 2016). In a simplistic model, low levels of nutrients such as glucose, fats, and amino acids increase levels of ghrelin and decrease leptin (Weigle et al. 1997; Tschöp et al. 2000; Klok et al. 2007). Ghrelin acts on NPY-producing neurons in the hypothalamus which cause the release of NPY (Kohno et al. 2003). Food intake restores deficits in nutrients, decreasing ghrelin and causes the release of leptin from adipose tissue (Izadi et al. 2014). Leptin acts on NPY-producing neurons in the hypothalamus, reducing the amount of NPY released (Baver et al. 2014). An intact control of homeostatic regulation through integration of these signals and the subsequent response is necessary for the maintenance of a stable body weight. Dysregulation of this system leads to obesity and its associated comorbidities including heart disease and diabetes (Turek et al. 2005; Depner et al. 2014; Reutrakul and Knutson 2015).
Taste, smell, texture, and temperature all contribute to the subjective pleasantness of food and rely on the mesolimbic dopamine system. The taste of saccharin sweetened water, for example, is chosen over intravenous cocaine administration in mice (Lenoir et al. 2007). Food that is more palatable, and as a result more rewarding, is expected to cause increased release of dopamine in the NAcc (Volkow et al. 2010, 2012). Indeed, palatable foods containing high levels of sugars (Rada et al. 2005) and fats (Rada et al. 2012; Cone et al. 2013) are known to stimulate the release of dopamine into the NAcc. Dopamine has an essential role in mediating appetite which goes above the homeostatic system. Dopamine-deficient mice with inactive TH in VTA neurons (Szczypka et al. 2001) as well as mice with constitutive deletions of both D1R and D2R (Kobayashi et al. 2004) develop early fatal hypophagia. Dopaminergic pathways have been found to be altered in obese subjects. Striatal D2R expression is reduced by a palatable food diet in mice (Johnson and Kenny 2010) and in humans striatal D2R availability is significantly lower in obese patients compared to control individuals (Wang et al. 2001).
Repeated exposure to food with high fat and sugar content results in compulsive food consumption, poor control of food intake, and food stimulus conditioning (Jauch-Chara and Oltmanns 2014). These results suggest that palatable food can disrupt endogenous homeostatic regulation of food intake through activation of the reward system. Interestingly, leptin receptors have been found in the VTA and SNpc, and a putative role in regulating dopamine release has been proposed (Figlewicz et al. 2003). Moreover, ghrelin is known to stimulate VTA dopamine neurons to release dopamine into the NAcc (Abizaid et al. 2006). Thus, endogenous and exogenous signals control appetite through important interactions between the physiological need for food and the reward system.
Food consumption follows circadian rhythms. Through regulation of complex networks involving the homeostatic and reward systems, food intake sets time. One hypothesis posits that orexigenic pathways, which increase feeding behavior, become gradually activated during fasting while sleeping. However, this hypothesis contrasts evidence in humans showing that hunger has an endogenous circadian rhythm with lowest levels in the morning (8am) and greatest in the evening (8pm) regardless of the type of food intake (Scheer et al. 2013). Moreover, in the absence of external time cues individuals seek 2–3 meals during their active phase; however, the timing when these meals occur shows massive subject variability and is influenced by differences in circadian period and wakefulness (Aschoff et al. 1986).
A number of clocks in the brain can be reset by peripheral metabolic signals, which may contribute to food anticipation. Palatable foods can trigger anticipatory bouts of locomotor activity and arousal indicating an activation of the mesolimbic dopamine system (Mistlberger 1994). Despite this insight, the anatomical locations and molecular mechanisms for the food clock remain elusive. Mice with genetic deletions of Bmal1, Per1, and Per2 have normal food anticipatory behavior as do SCN-lesioned mice (Storch and Weitz 2009). This information indicates that the central clock is not required for food anticipation. However, mutations in Per1 have been shown to shift food intake to the resting phase, which leads to obesity in mice (Liu et al. 2014). Additionally, mice carrying deletions of Bmal1 and Per2 become obese from eating food equally during day and night as do ClockΔ19 mutant mice (Turek et al. 2005; Guo et al. 2012). The most likely candidates for the food clock lie in other regions of hypothalamus as well as in the striatum (Gallardo et al. 2014).
Nutrition, metabolism, and circadian rhythms are intricately linked with each other (Fig. 4.2). Timing of food intake can alter the circadian system positively or negatively. Indeed, meal timing can affect sleep/wake cycles, body temperature, performance, and alertness (Hotz et al. 1987; Hawley and Burke 1997; GRANT et al. 2017; Hou et al. 2019). These effects are enhanced by calorie restriction, high-fat and high-sugar, among others. Rhythmic dopamine levels from the VTA to the NAcc underlie motivation, food craving, and anticipation (Parekh et al. 2015). The SCN indirectly projects to the VTA through the medial preoptic nucleus of the hypothalamus (Luo and Aston-Jones 2009); this connection might conceivably allow for food-seeking directed movement through modulation of dopamine signaling in the striatum. This connection may also affect reinforcement and conditioned learning associated with food intake. Thus, the control of food intake is dependent on a balanced interaction between metabolic and hedonic circadian brain circuits.
Fig. 4.2.
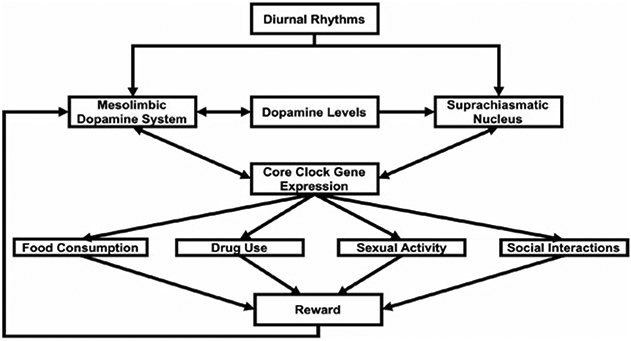
A schematic representation of the brain's reward system in relation to circadian rhythms. Diurnal rhythms of the mesolimbic dopamine system and suprachiasmatic nucleus directly influence the activity of these brain regions. Rhythmic dopamine levels influence the activity of the mesolimbic dopamine system and suprachiasmatic nucleus. The activation of dopamine receptors in these brain regions alters core clock gene expression. The expression of core clock gene affects rewarding behaviors including food consumption, drug use, sexual activity, and social interactions, which activate the mesolimbic dopamine system
4.4. Rhythmicity of Mating Behavior and Sex-Driven Reward
To facilitate necessity for species survival, mating activity highly engages the reward system and principally involves dopamine (Balfour et al. 2004). Dopamine release critically affects mating at the motor, arousal, motivation, and reward levels. In rats, systemic pharmacological treatments, which increase or decrease dopamine signaling, improve or worsen parameters of copulatory activity, respectively (Melis and Argiolas 1995). Dopamine signaling in the striatum has been postulated to mediate the reinforcing properties of sexual reproductive activity (Becker et al. 2001; Sanna et al. 2020). Regardless, dopamine signaling appears to play a critical role in sex-driven reward.
Like almost all physiological parameters in animals and humans, mating also shows some rhythmicity. In humans, most sexual encounters occur around midnight (Refinetti 2005). Environmental factors, namely partner availability, is the predominant factor important for human sexual activity. Peripheral tissues in the reproductive axis have been shown to have rhythmic clock gene expression, which might influence or synchronize with sexual behavior (Sen and Hoffmann 2020).
In animals, mating rhythmicity is important for avoiding predation and is also important for finding the right mating partner. Strong seasonal rhythms, which are linked to the amount of light, are apparent in males of many species including rodents and sheep, which are better suited models in this area of chronobiology (Reiter et al. 1980). As an example, rams are sensitive to daily changes in light across the year which induce hormonal variations and modulate gonadal function as well as libido without changes in hormone secretion (Lincoln et al. 2003). This provides evidence that, unlike other natural rewards like food, reproductive behavior is not under homeostatic regulation.
Anticipation rhythms have been observed in rodents in response to schedules; thus, suggesting that anticipatory rhythms may be located within the reward system or could be entrained by stimuli, which also engage the reward system. Indeed, circadian clock genes in the dopaminergic pathways can be shifted by natural rewards as well as dopaminergic compounds. These findings imply that copulation could also induce robust circadian anticipatory rhythms. Male rodents can anticipate daily opportunities to mate (Landry et al. 2012). Interestingly, rats can anticipate scheduled mating toward the end of their daily active phase and in the middle of their resting phase. Reproductive behavior also shows diurnal variation as does sex-related reward, which peaks in the daily active phase and corresponds with dopamine levels in the striatum (Webb et al. 2009). These results suggest that sexual anticipation and reward are linked with diurnal rhythms in the dopaminergic mesolimbic system (Melis and Argiolas 1995) though the molecular mechanisms remain elusive.
4.5. Drugs of Abuse
Although our understanding of the specific actions of drugs on the reward system has been growing, the complexity of the fundamental mechanisms underlying drug abuse and dependence increases. Drugs of abuse share one common mechanism: they raise dopamine levels in the brain, which elicits reward, driving vulnerable (Swendsen and Moal 2011) substance users to seek for more drugs leading to addiction. At the cellular level, the drug-induced dopamine increase alters neuronal plasticity at the molecular level leading to alterations of gene expression and the consequent modification of neuronal circuits.
A growing body of evidence connects perturbations of circadian rhythms and clock genes to the development and progression of addictive disorders (Webb 2017). People with addiction have highly disrupted rhythms which could be a result of genetic and/or epigenetic factors like sleep deprivation (Logan et al. 2018). Indeed, those with an evening chronotype (night owls) have been linked to disorders of the mesolimbic dopamine system including depression, insomnia, and substance abuse (Merikanto et al. 2013; Kivelä et al. 2018). Many behaviors that depend on the mesolimbic system, such as psychomotor sensitization and drug-seeking, show rhythmic patterns and are under the control of circadian genes (Abarca et al. 2002). Surprisingly, substance abuse leads to lasting changes in circadian rhythms, which can persist even after cessation of the drug intake (Jones et al. 2003).
Like for natural rewards, there are diurnal variations in the behavioral response to substances of abuse. Addictive drugs are known to influence behavioral rhythms, through modifications of the expression of clock genes such as Clock, Per1, and Per2. Clock is expressed in the VTA and NAcc and has been implicated in modulating reward processing. Mice with Clock null mutations show enhanced sensitivity to cocaine which has been demonstrated by conditioned place preference (CPP) (McClung et al. 2005) and self-administration (Ozburn et al. 2012) models of substance abuse. Similarly, Per1 and Per2 seem to have roles in cocaine sensitization (Uz et al. 2003), which is thought to be a critical component of drug craving that leads to dependence (Robinson and Berridge 2008). Per1 and Per2 expressions appear modulated by D1R and D2R. Interestingly, Per1 and Per2 mutants show increased alcohol CPP compared to WT controls (Gamsby et al. 2013). Per1 null mice show decreased morphine CPP (Perreau-Lenz et al. 2017) and absence of cocaine CPP (Abarca et al. 2002). In contrasts Per2 mutants show no difference from WT littermates when tested for cocaine CPP (Abarca et al. 2002).
4.6. Social Reward, Electronics, and the Clock
The developed world has a long-held fascination for technologies with entertainment purposes, which continues to grow. Adults in the United States spend 2–4 h per day using electronic devices, making technology a deeply engrained part of our lives (Dyck et al. 2011). The aberrant and persistent usage of these devices has called into question whether one could become addicted to them. Indeed, research focusing on television (Horvath 2004), internet (Caplan 2010), and smartphone (van Deursen et al. 2015) use has sought to understand these behaviors in terms of addiction. As previously discussed, natural rewards release dopamine through activation of the mesolimbic system to promote survival and maintenance of the species. Like for substance use disorders, could technology equally hijack the reward system? Social media platforms leverage the reward system in ways similar to what gambling does to promote usage as much as possible through activation of the dopaminergic pathways (Izuma et al. 2008). Evidence has recently been presented which connects successful social interactions and the dopaminergic mesolimbic system (Torquet et al. 2018).
Growing evidence suggests that electronic devices can negatively impact circadian rhythms. Studies recently emerged have linked smartphone usage to increased anxiety and depression as well as poor sleep quality (Demirci et al. 2015). Indeed, lights from backlit screens can delay and advance circadian timing causing asynchronization (Blume et al. 2019). Associations between loss of sleep and electronic media exposure have been extensively reported in adolescents and adults (Suganuma et al. 2007; Fossum et al. 2014; Lemola et al. 2015). The alerting effects of night time use of electronics could be due to the suppression of melatonin by blue light exposure from the device in the retina (West et al. 2010). This emphasizes the utility of using “night shifting” modes, which switch displays to decreased blue light. Beyond the effects light has on sleep disruption, nighttime use of electronics and media engagement likely has profound effects on circadian rhythms. This effect can in part be ascribed to the release of dopamine in the striatum from the rewarding nature of social interactions. It is clear that more studies are required to trace the links between dopamine-mediated reward and circadian rhythms. While the connection is warranted, the molecular mechanisms that define dopamine-circadian interactions and their consequences on our health are still in their infancy.
References
- Abarca C, Albrecht U, Spanagel R (2002) Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A 99:9026–9030. 10.1073/pnas.142039099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abizaid A, Liu Z-W, Andrews ZB et al. (2006) Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116:3229–3239. 10.1172/JCI29867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews CM, Lucki I (2001) Effects of cocaine on extracellular dopamine and serotonin levels in the nucleus accumbens. Psychopharmacology 155:221–229. 10.1007/s002130100704 [DOI] [PubMed] [Google Scholar]
- Aschoff J, Von Goetz C, Wildgruber C, Wever RA (1986) Meal timing in humans during isolation without time cues. J Biol Rhythm 1:151–162. 10.1177/074873048600100206 [DOI] [PubMed] [Google Scholar]
- Baba K, DeBruyne JP, Tosini G (2017) Dopamine 2 receptor activation entrains circadian clocks in mouse retinal pigment epithelium. Sci Rep 7:5103. 10.1038/s41598-017-05394-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TJ, Gauvin D (2000) Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacol Biochem Behav 65:289–299. 10.1016/s0091-3057(99)00207-5 [DOI] [PubMed] [Google Scholar]
- Balfour ME, Yu L, Coolen LM (2004) Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology 29:718–730. 10.1038/sj.npp.1300350 [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O (2007) The role of the dorsal striatum in reward and decision-making. J Neurosci 27:8161–8165. 10.1523/JNEUROSCI.1554-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon AW, Seda J, Carmouche M et al. (2000) Behavioral characterization of neuropeptide Y knockout mice. Brain Res 868:79–87. 10.1016/S0006-8993(00)02285-X [DOI] [PubMed] [Google Scholar]
- Bardo MT (1998) Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus Accumbens. CRN 12:37–67. 10.1615/CritRevNeurobiol.v12.i1-2.30 [DOI] [PubMed] [Google Scholar]
- Baver SB, Hope K, Guyot S et al. (2014) Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J Neurosci 34:5486–5496. 10.1523/JNEUROSCI.4861-12.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, Jenkins WJ (2001) The role of dopamine in the nucleus Accumbens and striatum during sexual behavior in the female rat. J Neurosci 21:3236–3241. 10.1523/JNEUROSCI.21-09-03236.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ, Miller R (1998) Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev 22:335–345. 10.1016/S0149-7634(97)00019-5 [DOI] [PubMed] [Google Scholar]
- Blume C, Garbazza C, Spitschan M (2019) Effects of light on human circadian rhythms, sleep and mood. Somnologie (Berl) 23:147–156. 10.1007/s11818-019-00215-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AC, Nakamura K, Roesch MR (2015) From ventral-medial to dorsal-lateral striatum:neural correlates of reward-guided decision-making. Neurobiol Learn Mem 0:51–59. 10.1016/j.nlm.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CM, Wightman RM, Carelli RM (2014) Dynamics of rapid dopamine release in the nucleus accumbens during goal-directed behaviors for cocaine versus natural rewards. Neuropharmacology 86:319–328. 10.1016/j.neuropharm.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan SE (2010) Theory and measurement of generalized problematic internet use: a two-step approach. Comput Hum Behav 26:1089–1097. 10.1016/j.chb.2010.03.012 [DOI] [Google Scholar]
- Castaneda TR, de Prado BM, Prieto D, Mora F (2004) Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res 36:177–185. 10.1046/j.1600-079x.2003.00114.x [DOI] [PubMed] [Google Scholar]
- de MJC (2004) The time of day of food intake influences overall intake in humans. J Nutr 134:104–111. 10.1093/jn/134.1.104 [DOI] [PubMed] [Google Scholar]
- Chung S, Lee EJ, Yun S et al. (2014) Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell 157:858–868. 10.1016/j.cell.2014.03.039 [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K (2000) Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res 126:325–341. 10.1016/S0079-6123(00)26022-6 [DOI] [PubMed] [Google Scholar]
- Cone JJ, Chartoff EH, Potter DN et al. (2013) Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS One 8:e58251. 10.1371/journal.pone.0058251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, Busetto G, Imperatore R et al. (2013) Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc Natl Acad Sci U S A 110(24):E2229–E2238. 10.1073/pnas.1219485110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R, Pollmann S (2014) A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiol Learn Mem 0:90–100. 10.1016/j.nlm.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Tataroglu Ö, Menaker M (2005) Circadian effects of timed meals (and other rewards). In: Young MW (ed) Methods in enzymology. Academic Press, San Diego, CA, pp 509–523 [DOI] [PubMed] [Google Scholar]
- De Decker A, Verbeken S, Sioen I et al. (2017) Palatable food consumption in children: interplay between (food) reward motivation and the home food environment. Eur J Pediatr 176:465–474. 10.1007/s00431-017-2857-4 [DOI] [PubMed] [Google Scholar]
- Demirci K, Akgönül M, Akpinar A (2015) Relationship of smartphone use severity with sleep quality, depression, and anxiety in university students. J Behav Addict 4:85–92. 10.1556/2006.4.2015.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depner CM, Stothard ER, Wright KP (2014) Metabolic consequences of sleep and circadian disorders. Curr Diab Rep 14:507. 10.1007/s11892-014-0507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Yujnovsky I, Hirayama J et al. (2006) Impaired light masking in dopamine D2 receptor-null mice. Nat Neurosci 9:732–734. 10.1038/nn1711 [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M (2002) Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci 19:593–601. 10.1017/s0952523802195058 [DOI] [PubMed] [Google Scholar]
- Dunlap JC (1999) Molecular bases for circadian clocks. Cell 96:271–290. 10.1016/S0092-8674(00)80566-8 [DOI] [PubMed] [Google Scholar]
- Dyck DV, Cardon G, Deforche B et al. (2011) Sociodemographic, psychosocial and home-environmental attributes associated with adults’ domestic screen time. BMC Public Health 11:1–10. 10.1186/1471-2458-11-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RS, Green EW, Zhao Y et al. (2012) Peroxiredoxins are conserved markers of circadian rhythms. Nature 485:459–464. 10.1038/nature11088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ (1990) Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev 14:217–232. 10.1016/S0149-7634(05)80222-2 [DOI] [PubMed] [Google Scholar]
- Ferris MJ, España RA, Locke JL et al. (2014) Dopamine transporters govern diurnal variation in extracellular dopamine tone. PNAS 111:E2751–E2759. 10.1073/pnas.1407935111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J et al. (2003) Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964:107–115. 10.1016/S0006-8993(02)04087-8 [DOI] [PubMed] [Google Scholar]
- Fossum IN, Nordnes LT, Storemark SS et al. (2014) The association between use of electronic Media in bed before Going to sleep and insomnia symptoms, day-time sleepiness, Morningness, and Chronotype. Behav Sleep Med 12:343–357. 10.1080/15402002.2013.819468 [DOI] [PubMed] [Google Scholar]
- Gallardo CM, Darvas M, Oviatt M et al. (2014) Dopamine receptor 1 neurons in the dorsal striatum regulate food anticipatory circadian activity rhythms in mice. eLife 3:e03781. 10.7554/eLife.03781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamsby J, Templeton E, Bonvini L et al. (2013) The circadian Per1 and Per2 genes influence alcohol intake, reinforcement, and blood alcohol levels. Behav Brain Res 249:15–21. 10.1016/j.bbr.2013.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Kreitzer AC (2012) Striatal microcircuitry and movement disorders. Trends Neurosci 35:557–564. 10.1016/j.tins.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT et al. (2010) Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci 30:2223–2234. 10.1523/JNEUROSCI.4870-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CL, Dorrian J, Coates AM et al. (2017) The impact of meal timing on performance, sleepiness, gastric upset, and hunger during simulated night shift. Ind Health 55:423–436. 10.2486/indhealth.2017-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland GA, DiFiglia M (1985) The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res 327:307–311. 10.1016/0006-8993(85)91524-0 [DOI] [PubMed] [Google Scholar]
- Grippo RM, Güler AD (2019) Dopamine signaling in circadian Photoentrainment:consequences of Desynchrony. Yale J Biol Med 92:271–281 [PMC free article] [PubMed] [Google Scholar]
- Grippo RM, Purohit AM, Zhang Q et al. (2017) Direct midbrain dopamine input to the suprachiasmatic nucleus accelerates circadian entrainment. Curr Biol 27:2465–2475.e3. 10.1016/j.cub.2017.06.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Chatterjee S, Li L et al. (2012) The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J 26:3453–3463. 10.1096/fj.12-205781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T et al. (2008) Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol 18:678–683. 10.1016/j.cub.2008.04.012 [DOI] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Metzger RR, Hanson GR (2000) The effects of methamphetamine on serotonin transporter activity: role of dopamine and hyperthermia. J Neurochem 75:1608–1617. 10.1046/j.1471-4159.2000.0751608.x [DOI] [PubMed] [Google Scholar]
- Hawley JA, Burke LM (1997) Effect of meal frequency and timing on physical performance. Br J Nutr 77:S91–S103. 10.1079/BJN19970107 [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG (1988) Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci 42:1705–1712. 10.1016/0024-3205(88)90036-7 [DOI] [PubMed] [Google Scholar]
- Honma S, Honma K (1995) Phase-dependent phase shift of methamphetamine-induced circadian rhythm by haloperidol in SCN-lesioned rats. Brain Res 674:283–290. 10.1016/0006-8993(95)00027-N [DOI] [PubMed] [Google Scholar]
- Hood S, Cassidy P, Cossette M-P et al. (2010) Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci 30:14046–14058. 10.1523/JNEUROSCI.2128-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath CW (2004) Measuring television addiction. J Broadcast Electron Media 48:378–398. 10.1207/s15506878jobem4803_3 [DOI] [Google Scholar]
- Hotz MM, Connolly MS, Lynch CB (1987) Adaptation to daily meal-timing and its effect on circadian temperature rhythms in two inbred strains of mice. Behav Genet 17:37–51. 10.1007/BF01066009 [DOI] [PubMed] [Google Scholar]
- Hou T, Wang C, Joshi S et al. (2019) Active time-restricted feeding improved sleep-wake cycle in db/db Mice. Front Neurosci 13:969. 10.3389/fnins.2019.00969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbesi M, Yildiz S, Arslan AD et al. (2009) Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience 158:537–544. 10.1016/j.neuroscience.2008.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadi V, Saraf-Bank S, Azadbakht L (2014) Dietary intakes and leptin concentrations. ARYA Atheroscler 10:266–272 [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N (2008) Processing of social and monetary rewards in the human striatum. Neuron 58:284–294. 10.1016/j.neuron.2008.03.020 [DOI] [PubMed] [Google Scholar]
- Jauch-Chara K, Oltmanns KM (2014) Obesity – a neuropsychological disease? Systematic review and neuropsychological model. Prog Neurobiol 114:84–101. 10.1016/j.pneurobio.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Jia T, Macare C, Desrivières S et al. (2016) Neural basis of reward anticipation and its genetic determinants. Proc Natl Acad Sci U S A 113:3879–3884. 10.1073/pnas.1503252113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ (2010) Addiction-like reward dys-function and compulsive eating in obese rats: role for dopamine D2 receptors. Nat Neurosci 13:635–641. 10.1038/nn.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JD (2014) Physiological responses to food intake throughout the day. Nutr Res Rev 27:107–118. 10.1017/S0954422414000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EM, Knutson D, Haines D (2003) Common problems in patients recovering from chemical dependency. Am Fam Physician 68:1971–1978 [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S et al. (1999) Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20:68–100. 10.1210/edrv.20.1.0357 [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP (1971) The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond Ser B Biol Sci 262:383–401. 10.1098/rstb.1971.0102 [DOI] [PubMed] [Google Scholar]
- Keramati M, Gutkin B (2014) Homeostatic reinforcement learning for integrating reward collection and physiological stability. eLife 3:e04811. 10.7554/eLife.04811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkwal G, Brami-Cherrier K, Lizardi-Ortiz JE et al. (2016a) Parkinsonism driven by antipsychotics originates from dopaminergic control of striatal cholinergic interneurons. Neuron 91:67–78. 10.1016/j.neuron.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkwal G, Radl D, Lewis RG, Borrelli E (2016b) Dopamine D2 receptors in striatal output neurons enable the psychomotor effects of cocaine. PNAS 113:11609–11614. 10.1073/pnas.1608362113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivelä L, Papadopoulos MR, Antypa N (2018) Chronotype and psychiatric disorders. Curr Sleep Med Rep 4:94–103. 10.1007/s40675-018-0113-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok MD, Jakobsdottir S, Drent ML (2007) The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev 8:21–34. 10.1111/j.1467-789X.2006.00270.x [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Iaccarino C, Saiardi A et al. (2004) Simultaneous absence of dopamine D1 and D2 receptor-mediated signaling is lethal in mice. Proc Natl Acad Sci U S A 101:11465–11470. 10.1073/pnas.0402028101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno D, Gao H-Z, Muroya S et al. (2003) Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 52:948–956. 10.2337/diabetes.52.4.948 [DOI] [PubMed] [Google Scholar]
- Landry GJ, Opiol H, Marchant EG et al. (2012) Scheduled daily mating induces circadian anticipatory activity rhythms in the Male Rat. PLoS One 7(7):e40895. 10.1371/journal.pone.0040895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemola S, Perkinson-Gloor N, Brand S et al. (2015) Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolescence 44:405–418. 10.1007/s10964-014-0176-x [DOI] [PubMed] [Google Scholar]
- Lemos JC, Friend DM, Kaplan AR et al. (2016) Enhanced GABA transmission drives bradykinesia following loss of dopamine D2 receptor signaling. Neuron 90:824–838. 10.1016/j.neuron.2016.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH (2007) Intense sweetness surpasses cocaine reward. PLoS One 2: e698. 10.1371/journal.pone.0000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RG, Serra M, Radl D et al. (2020) Dopaminergic control of striatal cholinergic interneurons underlies cocaine-induced psychostimulation. Cell Rep 31 (3):107527. 10.1016/j.celrep.2020.107527 [DOI] [PubMed] [Google Scholar]
- Lincoln GA, Andersson H, Hazlerigg D (2003) Clock genes and the long-term regulation of prolactin secretion: evidence for a photoperiod/circannual timer in the pars Tuberalis. J Neuroendocrinol 15:390–397. 10.1046/j.1365-2826.2003.00990.x [DOI] [PubMed] [Google Scholar]
- Lipton DM, Gonzales BJ, Citri A (2019) Dorsal striatal circuits for habits, compulsions and addictions. Front Syst Neurosci 13:28. 10.3389/fnsys.2019.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Huang M, Wu X et al. (2014) PER1 phosphorylation specifies feeding rhythm in mice. Cell Rep 7:1509–1520. 10.1016/j.celrep.2014.04.032 [DOI] [PubMed] [Google Scholar]
- Logan RW, Hasler BP, Forbes EE et al. (2018) Impact of sleep and circadian rhythms on addiction vulnerability in adolescents. Biol Psychiatry 83:987–996. 10.1016/j.biopsych.2017.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Aston-Jones G (2009) Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. Eur J Neurosci 29:748–760. 10.1111/j.1460-9568.2008.06606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Girgenti MJ, Breslin FJ et al. (2008) Gene profiling the response to repeated cocaine self-administration in dorsal striatum: a focus on circadian genes. Brain Res 1213:166–177. 10.1016/j.brainres.2008.02.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marche K, Martel A-C, Apicella P (2017) Differences between dorsal and ventral striatum in the sensitivity of Tonically active neurons to rewarding events. Front Syst Neurosci 11:52. 10.3389/fnsys.2017.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M et al. (2005) Regulation of dopaminergic transmission and cocaine reward by the clock gene. Proc Natl Acad Sci U S A 102:9377–9381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B (2007) Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav 92:263–271. 10.1016/j.physbeh.2007.05.021 [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A (1995) Dopamine and sexual behavior. Neurosci Biobehav Rev 19:19–38. 10.1016/0149-7634(94)00020-2 [DOI] [PubMed] [Google Scholar]
- Mendoza J, Challet E (2014) Circadian insights into dopamine mechanisms. Neuroscience 282:230–242. 10.1016/j.neuroscience.2014.07.081 [DOI] [PubMed] [Google Scholar]
- Merikanto I, Lahti T, Kronholm E et al. (2013) Evening types are prone to depression. Chronobiol Int 30:719–725. 10.3109/07420528.2013.784770 [DOI] [PubMed] [Google Scholar]
- Mistlberger RE (1994) Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev 18:171–195. 10.1016/0149-7634(94)90023-X [DOI] [PubMed] [Google Scholar]
- Morris RW (1987) Circadian and circannual rhythms of emergency room drug-overdose admissions. Prog Clin Biol Res 227B:451–457 [PubMed] [Google Scholar]
- Musacchio JM (1975) Enzymes involved in the biosynthesis and degradation of Catecholamines. In: Iversen LL, Iversen SD, Snyder SH (eds) Biochemistry of biogenic amines. Springer US, Boston, MA, pp 1–35 [Google Scholar]
- Musiek ES, FitzGerald GA (2013) Molecular clocks in pharmacology. Handb Exp Pharmacol 217:243–260. 10.1007/978-3-642-25950-0_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T, Akiyama M, Moriya T, Shibata S (2001) Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol 59:894–900. 10.1124/mol.59.4.894 [DOI] [PubMed] [Google Scholar]
- Nobis CC, Laramée GD, Kervezee L et al. (2019) The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. PNAS 116:20077–20086. 10.1073/pnas.1905080116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oorschot DE (2013) The percentage of interneurons in the dorsal striatum of the rat, cat, monkey and human: a critique of the evidence. Basal Ganglia 3:19–24. 10.1016/j.baga.2012.11.001 [DOI] [Google Scholar]
- Ozburn AR, Larson EB, Self DW, McClung CA (2012) Cocaine self-administration behaviors in ClockΔ19 mice. Psychopharmacology 223:169–177. 10.1007/s00213-012-2704-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh PK, Ozburn AR, McClung CA (2015) Circadian clock genes: effects on dopamine, reward and addiction. Alcohol 49:341–349. 10.1016/j.alcohol.2014.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE (1996) Regional differences in the effects of amphetamine withdrawal on dopamine dynamics in the striatum. Neuropsychopharmacology 14:325–337. 10.1016/0893-133X(95)00141-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP (2007) Neural basis of reward and craving--a homeostatic point of view. Dialogues Clin Neurosci 9:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau-Lenz S, Hoelters L-S, Leixner S et al. (2017) mPer1 promotes morphine-induced locomotor sensitization and conditioned place preference via histone deacetylase activity. Psychopharmacology 234:1713–1724. 10.1007/s00213-017-4574-0 [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG (2005) Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 134:737–744. 10.1016/j.neuroscience.2005.04.043 [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Barson JR et al. (2012) A high-fat meal, or intraperitoneal Administration of a fat Emulsion, increases extracellular dopamine in the nucleus Accumbens. Brain Sci 2:242–253. 10.3390/brainsci2020242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond RC, Warren M, Morris RW, Leikin JB (1992) Periodicity of presentations of drugs of abuse and overdose in an emergency department. J Toxicol Clin Toxicol 30:467–478. 10.3109/15563659209021561 [DOI] [PubMed] [Google Scholar]
- Refinetti R (2005) Time for sex: nycthemeral distribution of human sexual behavior. J Circad Rhythms 3:art.4. 10.1186/1740-3391-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Richardson BA, Johnson LY et al. (1980) Pineal melatonin rhythm: reduction in aging Syrian hamsters. Science 210:1372–1373. 10.1126/science.7434032 [DOI] [PubMed] [Google Scholar]
- Reutrakul S, Knutson KL (2015) Consequences of circadian disruption on Cardiometabolic health. Sleep Med Clin 10:455–468. 10.1016/j.jsmc.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkees SA, Lachowicz JE (1997) Functional D1 and D5 dopamine receptors are expressed in the suprachiasmatic, supraoptic, and paraventricular nuclei of primates. Synapse 26:1–10. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ (1996) Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 6:228–236. 10.1016/S0959-4388(96)80077-8 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2008) The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond Ser B Biol Sci 363:3137–3146. 10.1098/rstb.2008.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samir R, Prabhu SD, Young ME (2020) Chronobiological influence over cardiovascular function. Circ Res 126:258–279. 10.1161/CIRCRESAHA.119.313349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna F, Bratzu J, Serra MP et al. (2020) Altered sexual behavior in dopamine transporter (DAT) knockout male rats: a behavioral, neurochemical and intracerebral microdialysis study. Front Behav Neurosci 14:58. 10.3389/fnbeh.2020.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FAJL, Morris CJ, Shea SA (2013) The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 21:421–423. 10.1002/oby.20351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schéle E, Bake T, Rabasa C, Dickson SL (2016) Centrally administered ghrelin acutely influences food choice in rodents. PLoS One 11(2):e0149456. 10.1371/journal.pone.0149456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T (1992) Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci 12:4595–4610. 10.1523/JNEUROSCI.12-12-04595.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Hoffmann HM (2020) Role of core circadian clock genes in hormone release and target tissue sensitivity in the reproductive axis. Mol Cell Endocrinol 501:110655. 10.1016/j.mce.2019.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A (2003) Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 19:1709–1715. 10.1016/S1053-8119(03)00253-2 [DOI] [PubMed] [Google Scholar]
- Smith AD, Olson RJ, Justice JB (1992) Quantitative microdialysis of dopamine in the striatum: effect of circadian variation. J Neurosci Methods 44:33–41. 10.1016/0165-0270(92)90111-P [DOI] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Rawas RE et al. (2009) Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology 34:1102–1111. 10.1038/npp.2008.51 [DOI] [PubMed] [Google Scholar]
- Storch K-F, Weitz CJ (2009) Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. PNAS 106:6808–6813. 10.1073/pnas.0902063106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma N, Kikuchi T, Yanagi K et al. (2007) Using electronic media before sleep can curtail sleep time and result in self-perceived insufficient sleep. Sleep Biol Rhythms 5:204–214. 10.1111/j.1479-8425.2007.00276.x [DOI] [Google Scholar]
- Swendsen J, Moal ML (2011) Individual vulnerability to addiction. Ann N Y Acad Sci 1216:73–85. 10.1111/j.1749-6632.2010.05894.x [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Kwok K, Brot MD et al. (2001) Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron 30:819–828. 10.1016/s0896-6273(01)00319-1 [DOI] [PubMed] [Google Scholar]
- Torquet N, Marti F, Campart C et al. (2018) Social interactions impact on the dopaminergic system and drive individuality. Nat Commun 9(1):3081. 10.1038/s41467-018-05526-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML (2000) Ghrelin induces adiposity in rodents. Nature 407:908–913. 10.1038/35038090 [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A et al. (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Science 308:1043–1045. 10.1126/science.1108750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz T, Akhisaroglu M, Ahmed R, Manev H (2003) The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology 28:2117–2123. 10.1038/sj.npp.1300254 [DOI] [PubMed] [Google Scholar]
- van Deursen AJAM, Bolle CL, Hegner SM, Kommers PAM (2015) Modeling habitual and addictive smartphone behavior: the role of smartphone usage types, emotional intelligence, social stress, self-regulation, age, and gender. Comput Hum Behav 45:411–420. 10.1016/j.chb.2014.12.039 [DOI] [Google Scholar]
- Viswanathan N, Davis FC (1997) Single prenatal injections of melatonin or the D1-dopamine receptor agonist SKF 38393 to pregnant hamsters sets the offsprings’ circadian rhythms to phases 180° apart. J Comp Physiol A 180:339–346. 10.1007/s003590050053 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS et al. (2010) Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. BioEssays 32:748–755. 10.1002/bies.201000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS et al. (2012) Food and drug reward: overlapping circuits in human obesity and addiction. In: Carter CS, Dalley JW (eds) Brain imaging in behavioral neuroscience. Springer, Berlin, Heidelberg, pp 1–24 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT (2016) Neurobiologic advances from the brain disease model of addiction. N Engl J Med 374:363–371. 10.1056/NEJMra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Logan J et al. (2001) Brain dopamine and obesity. Lancet 357:354–357. 10.1016/S0140-6736(00)03643-6 [DOI] [PubMed] [Google Scholar]
- Weaver DR, Reppert SM (1995) Definition of the developmental transition from dopaminergic to photic regulation of c-fos gene expression in the rat suprachiasmatic nucleus. Mol Brain Res 33:136–148. 10.1016/0169-328X(95)00117-B [DOI] [PubMed] [Google Scholar]
- Webb IC (2017) Circadian rhythms and substance abuse: Chronobiological considerations for the treatment of addiction. Curr Psychiatry Rep 19:12. 10.1007/s11920-017-0764-z [DOI] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Wang X et al. (2009) Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythm 24:465–476. 10.1177/0748730409346657 [DOI] [PubMed] [Google Scholar]
- Weigle DS, Duell PB, Connor WE et al. (1997) Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab 82:561–565. 10.1210/jcem.82.2.3757 [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR (1990) Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A 87:7050–7054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KE, Jablonski MR, Warfield B et al. (2010) Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J Appl Physiol 110:619–626. 10.1152/japplphysiol.01413.2009 [DOI] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Garcia K et al. (2004) Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. PNAS 101:8227–8232. 10.1073/pnas.0402763101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Hou B, Gao Y et al. (2007) Effects of enriched environment on morphine-induced reward in mice. Exp Neurol 204:714–719. 10.1016/j.expneurol.2006.12.027 [DOI] [PubMed] [Google Scholar]