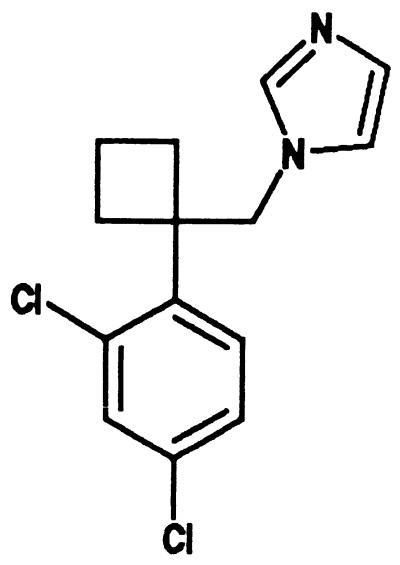
Abstract
Pneumocystis carinii synthesizes sterols with a double bond at C-7 of the sterol nucleus and an alkyl group with one or two carbons at C-24 of the side chain. Also, some human-derived Pneumocystis carinii f. sp. hominis strains contain lanosterol derivatives with an alkyl group at C-24. These unique sterols have not been found in other pathogens of mammalian lungs. Thus, P. carinii may have important differences in its susceptibility to drugs known to block reactions in ergosterol biosynthesis in other fungi. In the present study, inhibitors of 3-hydroxy-3-methyglutaryl coenzyme A reductase, squalene synthase, squalene epoxidase, squalene epoxide-lanosterol cyclase, lanosterol demethylase, Δ8 to Δ7 isomerase, and S-adenosylmethionine:sterol methyltransferase were tested for their effects on P. carinii viability as determined by quantitation of cellular ATP levels in a population of organisms. Compounds within each category varied in inhibitory effect; the most effective included drugs targeted at squalene synthase, squalene epoxide-lanosterol cyclase, and Δ8 to Δ7 isomerase. Some drugs that are potent against ergosterol-synthesizing fungi had little effect against P. carinii, suggesting that substrates and/or enzymes in P. carinii sterol biosynthetic reactions are distinct. Amphotericin B is ineffective in clearing P. carinii infections at clinical doses; however, this drug apparently binds to sterols and causes permeability changes in P. carinii membranes, since it reduced cellular ATP levels in a dose-dependent fashion.
Organisms with parasitic lifestyles commonly scavenge host sterols for the formation of new membranes and for other cellular functions. Some parasites and other organisms can survive solely on the sterols taken up, or scavenged, from their environments, such as mammalian hosts and culture media. Cholesterol is abundant in lung surfactant (11), which is secreted by the epithelial type II cells into the alveolar lumen, in which Pneumocystis spp. proliferate extracellularly. The pathogen apparently forms the bulk of its membrane bilayers by scavenging host cholesterol, which accounts for about 75% of the organism's free sterols (17). Some microdomains within membranes contain complexes where sterol molecules with a specific and precise three-dimensional conformation must be present in order to function optimally. Thus, if the array of available host sterols does not include those that fulfill the stereochemical requirements for proper functioning of the parasite's membrane components, the organism must synthesize those particular sterols to remain viable. These parasite-synthesized sterols, distinct from those available from the host, have been described as metabolic sterols (12) because the organism needs them and continues expending energy to synthesize them.
Antiergosterol agents have proved particularly useful for deeply invasive or systemic mycosis, but Pneumocystis carinii does not contain ergosterol, which is consistent with the unusual nature of this pathogen and its unique phylogenetic status (24). The susceptibility of enzymes in P. carinii sterol biosynthesis to inhibitors may differ from those in ergosterol-producing fungi. In that regard, Bartlett et al. (2) demonstrated that six imidazole antifungal agents (fluconazole, ketoconazole, miconazole, itraconazole, etanidazole, and Sch39304) that target sterol nucleus demethylation in ergosterol biosynthesis had little or no activity in clearing P. carinii pneumonia (PCP) in experimental animals or in reducing P. carinii proliferation in primary culture. On the other hand, terbinafine (see Table 1), an inhibitor of squalene epoxidase in the sterol biosynthetic pathway, was shown by Contini et al. (6) to be effective in clearing PCP in experimental animals. Terbafine also suppressed organism proliferation in short-term cultures (5, 6). Furthermore, inhibitors of sterol C-24 alkylation were shown by Urbina et al. (25) to inhibit the biosynthesis of P. carinii-specific sterols and the proliferation of P. carinii in short-term cultures. These observations are consistent with the suggestion that P. carinii synthesizes its own distinct metabolic sterols in order to remain viable and proliferate in an environment rich in cholesterol.
TABLE 1.
Drugs tested for their effects on P. carinii f. sp. carinii
The sterols found in most fungi and plants differ from cholesterol (the dominant C27 sterol of mammals) in important structural features. These nonmammalian sterols have an alkyl group at C-24 of the sterol side chain. Most fungal sterols have a methyl or methylene group at this site (e.g., C28 ergosterol), whereas sterols found in higher plants include both C28 (e.g., campesterol and brassicasterol) and C29 (e.g., β-sitosterol and stigmasterol) 24-alkylsterols. A double bond at C-5 of the sterol nucleus is a feature of the sterols commonly found in most organisms. In contrast, structural analyses of rat-derived Pneumocystis carinii f. sp. carinii sterols demonstrated that the dominant organism-specific sterols have a double bond at C-7 and an alkyl group consisting of one or two carbons at C-24 of the side chain (10, 14, 17, 25). 24-Alkylsterols with a double bond at C-7 of the sterol nucleus are also found in some plants and fungi (e.g., rust fungi) but are not major components of cellular membranes of vertebrates or of pathogens known to infect the lungs of vertebrates.
Lanosterol (with a double bond at C-8 of the sterol nucleus and another between C-24 and C-25 of the side chain) is a key intermediate in the biosynthesis of cholesterol and ergosterol. This sterol intermediate has two methyl groups at C-4 and one at C-14 of the sterol nucleus (C30). In some organisms, sterol nucleus demethylation is requisite for optimal S-adenosylmethionine:sterol Δ24(25) methyltransferase (SAM:SMT) activity, which results in the addition of alkyl groups at C-24 of the sterol side chain. Thus, demethylated intermediates such as zymosterol (C27; cholesta-8,24(25)-diene-3β-ol) are preferred over lanosterol as substrates for SAM:SMT in these organisms. A drug could therefore reduce the formation of 24-alkylsterols in response to a block in sterol nucleus demethylation rather than by inhibiting SAM:SMT activity per se. Furthermore, sterols derived from lanosterol have been identified in Pneumocystis carinii f. sp. hominis. Recently, the lanosterol derivatives pneumocysterol, (24Z)-ethylidenelanost-8-en-3β-ol (C32) (15, 16), and 24-methylenelanost-8-en-3β-ol (C31) (J.-L. Giner, E. J. Parish, D. H. Beach, K. Jayasimhulu, and E. S. Kaneshiro, unpublished data) were described in P. carinii f. sp. hominis organisms isolated from the lungs of humans who died with PCP (14–16). The presence of these sterols in P. carinii suggests that lanosterol can serve as a substrate for SAM:SMT in this organism, although it is not necessarily the preferred substrate for the methyltransferase activity.
In the present study, a number of different classes of inhibitors with known targets in sterol biosynthesis were tested for their effects on P. carinii f. sp. carinii viability and for the purpose of determining whether P. carinii and ergosterol-producing fungi differ in their responses to these drugs. Viability was assessed by quantifying cellular ATP levels in populations of the pathogen.
MATERIALS AND METHODS
Organisms.
Mixed-life-cycle preparations of P. carinii f. sp. carinii were isolated from the lungs of corticosteroid-immunosuppressed rats and purified from host contaminants by a series of gravity sedimentation steps and microfiltration as described previously (4, 7). The organism preparation, comprising approximately 95% trophic forms and 5% cystic forms, was suspended in a modified RPMI 1640 medium (7) containing 10% calf serum and 7.5% dimethyl sulfoxide (DMSO) and was cryopreserved in liquid nitrogen (3). Aliquots from the organism preparations in the RPMI-based medium were incubated at 35°C for 72 h to test for contamination by other microbes; contaminant-free specimens were used for ATP assays.
ATP assay.
The cryopreserved organisms were thawed rapidly by placing the container in a 37°C water bath, and then the suspension was centrifuged and washed free of DMSO by using the supplemented RPMI 1640 medium. Organisms (108 as total nuclei) were distributed into multiwell plates with or without test compounds. The incubation medium consisted of 1 to 2 ml of the modified RPMI-based medium (7) supplemented with 20% calf serum (Summit Biotechnology, Fort Collins, Colo.) (pH 7.5 to 8.0; 380 mOsm) (4, 7). Drugs were added to the culture medium in DMSO (final concentration, <0.2%). In each experiment, triplicate assays were performed at each drug concentration. The cultures were incubated at 35°C under a 10% CO2 humidified atmosphere. Each day, the cultures were centrifuged at 2,400 × g, and the medium in each well was removed and replaced with fresh medium, with or without the test compound.
After 24, 48, and 72 h of incubation, the ATP content of the population of organisms was quantified by the luciferin-luciferase assay (Wallac, Inc., Gaithersburg, Md.) modified for use with P. carinii (4). Fluorescence emission was quantified with an AutoLumat LB 953 luminometer (Wallac) and recorded in relative light units.
Freshly isolated P. carinii organisms that were not treated with experimental compounds increased in number under these incubation conditions. A total of 108 cells (nuclei) increased to 1.9 × 108, 1.7 × 108, and 5.1 × 108 cells, containing 44.1, 37.3, and 24.7 fmol of ATP/108 cells, after 24, 48, and 72 h, respectively (7). In contrast, untreated control cryopreserved organisms placed under equivalent incubation conditions doubled in number during the initial 24 h but maintained the same number of organisms and total ATP (measured in relative light units) of the population from 24 to 72 h (M. S. Collins and M. T. Cushion, unpublished data).
The effects of the drugs tested in this study were expressed as percentages of ATP contents in controls. In each experiment, the final ATP content was expressed as the mean of nine values (triplicate assays; three readings per well). Values were expressed as the averages from two separate experiments performed in triplicate.
Controls consisted of untreated organisms and of organisms treated with 10.0 μg of ampicillin/ml (negative control), 0.1 and 10.0 μg of pentamidine isethionate/ml (positive controls), 0.1% DMSO (vehicle control), or the treatment for opening the lactone ring of the lovastatin prodrug to produce the active form. A quench control was included for each compound, wherein an aliquot of the highest concentration of drug used was spiked directly into the luciferin-luciferase driven by ATP at 10−8 M to evaluate inhibitory effects. No compounds exhibited quenching activity. The ATP contents of P. carinii populations were not affected by the vehicle control or ampicillin, whereas the organisms treated with pentamidine exhibited time- and dose-dependent reductions in ATP as previously reported (4, 7).
An activity scale based on the concentration of a compound required to decrease the ATP levels by 50% (50% inhibitory concentration [IC50]) compared to the untreated control organisms' ATP levels was used to compare the effects of the different drugs (7). The effects of the drugs were categorized as a marked effect (IC50, <0.1 μg/ml), a moderate effect (IC50, 1.0 to 9.9 μg/ml), and no effect (IC50, >10 μg/ml). Statistical analysis was performed using GraphPad InSTAT 3.0, and results were graphed with GraphPad Prism 3.0.
Experimental drugs.
Twenty-five drugs reported to have inhibitory activity in sterol biosynthesis and the polyene antibiotic amphotericin B were tested for their effects in reducing the cellular ATP pools of P. carinii organism populations. The sterol biosynthesis inhibitors included those with targets that had been previously identified in other organisms (e.g., Candida albicans) (Table 1). Sources of inhibitors tested in the present study are identified in Table 1. Lovastatin was tested both as the inactive lactone prodrug (which is normally converted by intracellular enzymes to its active dihydroxy-open acid form) and in its active form by pretreatment under alkaline conditions (A. Alberts, personal communication).
RESULTS
HMG-CoA reductase.
The conversion of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) to mevalonic acid is catalyzed by a reductase activity, which plays a key regulatory role in isoprenoid biosynthesis. This reaction represents the first committed step in the acetate-mevalonate pathway for de novo isoprenoid biosynthesis. Lovastatin, tested both as a prodrug and in its active free-acid form, as well as L-647,318 and L-654,164, had no adverse effects on the P. carinii ATP pool (Table 2). Simvastatin, an analog of lovastatin with an additional methyl group, is known to be more potent than its parent compound. This HMG-CoA inhibitor had only a slight effect in reducing P. carinii ATP levels, and only after 3 days of exposure. By comparison, trimethoprim-sulfamethoxazole and pentamidine exhibited marked activity in the same ATP assay, while the respiration inhibitor potassium cyanide produced very marked activity and sulfadoxine produced moderate effects (7).
TABLE 2.
IC50s for inhibitors at three exposure times as determined by the reduction in cellular ATP contents of P. carinii f. sp. carinii populations
| Drug | IC50 (μg/ml [μM]) at the
following treatment time (h):
|
||
|---|---|---|---|
| 24 | 48 | 72 | |
| HMG-CoA reductase inhibitors | |||
| Simvastatin | —a | — | 101.39 (2,422) |
| Lovastatin | — | — | — |
| L-647,318 | — | — | — |
| L-654,164 | — | — | — |
| Squalene synthase inhibitors | |||
| CCI 14993 | 1,244.51 (2,832) | 7.89 (18) | 3.91 (8) |
| CCI 16543 | 138.68 (434) | 21.83 (68) | 10.45 (33) |
| Squalestatin (GR 105155X) | — | — | — |
| Squalene epoxidase inhibitors | |||
| Terbinafine | 1,706.08 (5,863) | 36.98 (130) | 3.67 (13) |
| Tolnaftate | — | 1,221.80 (3,980) | 49.90 (1,625) |
| Squalene epoxide-lanosterol cyclase inhibitors | |||
| GR 90525A | 1.89 (7) | 1.06 (4) | 0.93 (3) |
| GR 193018A | 8.07 (30) | 2.82 (11) | 2.23 (8) |
| GR 54985A | 40.27 (121) | 5.44 (16) | 2.85 (9) |
| UI 8666A | 30.76 (212) | 8.79 (60) | 9.71 (67) |
| GR 31149A | 1,419.10 (4,464) | 14.80 (47) | 1.90 (6) |
| Lanosterol demethylase inhibitors | |||
| GR 40317A | 12.33 (33) | 2.90 (8) | 2.02 (5) |
| GR 42539X | 23.71 (81) | 2.33 (8) | 1.98 (7) |
| GR 40665X | 194.53 (692) | 127.35 (453) | 104.47 (372) |
| Fluconazole | — | — | — |
| GR 71539X | — | — | — |
| GR 77303X | — | — | — |
| Δ8 to Δ7 isomerase inhibitor, AY 9944 | 25.06 (51) | 17.82 (36) | 3.68 (7) |
| SAM:SMT inhibitors | |||
| 24(25)-Epiminolanosterol | — | 2,084.49 (4,737) | — |
| 24-Bromolanosterol | — | — | — |
| 24-Iodolanosterol | — | — | — |
| Sinefungin | — | — | — |
| Polyene antimycotic, amphotericin B | 1.84 (2) | 1.06 (1) | 0.94 (1) |
—, inhibition was always less than 50%.
Squalene synthase.
Mevalonic acid is converted to a five-carbon isopentenyl unit, and after several of these isoprene units condense, a long polyprenyl chain, farnesyl diphosphate (C15), containing three isoprene units, is formed. The condensation of two farnesyl diphosphate molecules to squalene (C30) is catalyzed by squalene synthase. Two drugs that inhibit this reaction, CCI 16543 and CCI 14993, caused reductions in P. carinii cellular ATP levels after 24 h at IC50s considered to indicate moderate activity (Table 2) (7). The other compound tested, GR 105155X, had no effect on ATP pools (Table 2).
Squalene epoxidase.
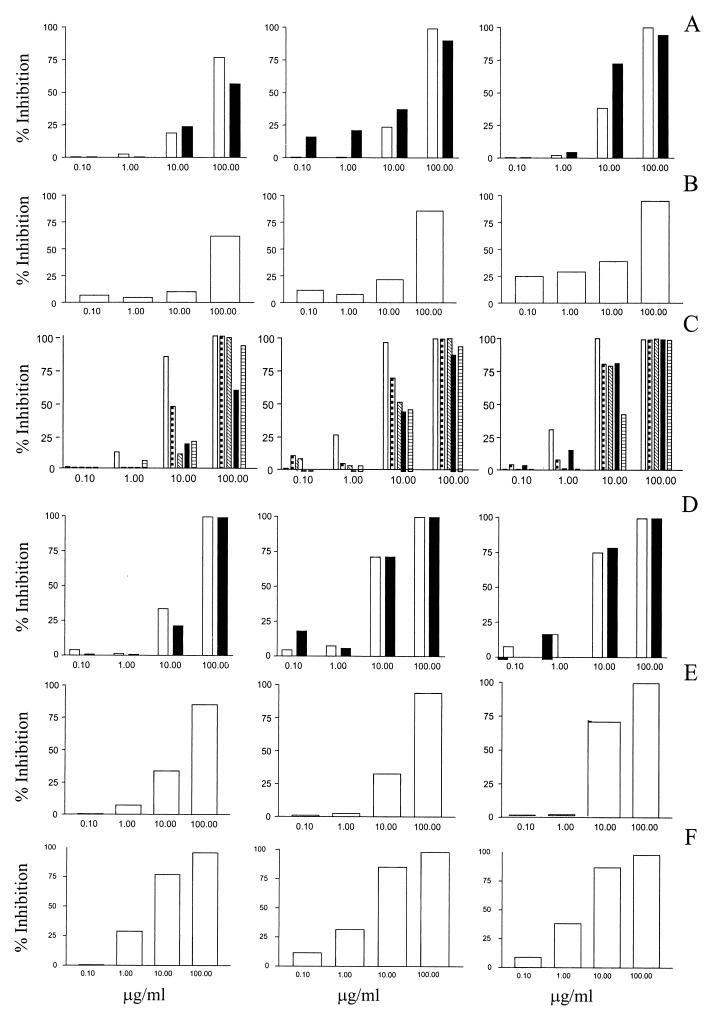
Squalene epoxidase catalyzes the addition of an oxygen atom into the squalene molecule, producing the reactive intermediate squalene epoxide (C30). Both terbinafine and tolnaftate, inhibitors of this reaction, were effective in reducing P. carinii cellular ATP levels. Both drugs reduced the viability of P. carinii populations in a dose- and time-dependent manner. The IC50s reached levels indicating moderate activity after 24 h of exposure for terbinafine (Fig. 1; Table 2) and after 48 h with tolnaftate (Table 2). The results for terbinafine are consistent with the earlier report that this drug was effective both in clearing PCP in experimental animals and in inhibiting proliferation of short-term primary cultures of human-derived P. carinii f. sp. hominis (6).
FIG. 1.
Effects of drugs on P. carinii f. sp. carinii ATP levels after 24 (left), 48 (center), and 72 (right) h. (A) Inhibitors of squalene synthase CCI 16543 (□) and CCI 14993 (▄). (B) Terbinafine, an inhibitor of squalene epoxidase. (C) Inhibitors of squalene epoxide-lanosterol cyclase GR 90525A (□), GR 193018A (⬒), GR 54985A (▧), UI 8666A (▄), and GR 31149 (▤). (D) Inhibitors of lanosterol demethylase GR 40317A (□) and GR 42539X (▄). (E) AY 9944, an inhibitor of Δ8 to Δ7 isomerase activity. (F) Amphotericin B, a polyene drug that binds to membrane sterols.
Squalene epoxide-lanosterol cyclase.
Squalene epoxide is converted to lanosterol (C30) by a cyclase enzyme and the migration of two methyl groups during the closure of rings in the molecule. Drugs tested within this category of inhibitors were among the most effective in reducing ATP levels of P. carinii populations. All five inhibitors tested showed mostly moderate activity in reducing the cellular ATP contents of P. carinii populations (Fig. 1; Table 2). After 3 days of exposure to GR 90525A, marked activity was observed, as indicated by the low IC50.
Lanosterol demethylase.
In most organisms, three methyl groups are removed from the lanosterol nucleus (two at C-4 and one at C-14), the double bond at C-8 is removed, and a double bond at C-5 is added. In some organisms, the double bond at C-24 of lanosterol is also reduced (e.g., cholesterol synthesis in mammals). In most organisms, lanosterol is the preferred substrate for enzymes, including the C-14 cytochrome P-540-dependent lanosterol demethylase activity. However, the methyl groups at C-4 and C-14 of sterol molecules other than lanosterol can be removed. Interestingly, the activity of lanosterol demethylase inhibitors tested in this study can be placed in two general groups. Two imidazoles (GR 40317A and GR 42539X) were moderately effective against P. carinii (Fig. 1; Table 2), whereas the triazoles, including fluconazole, were not effective. The results for fluconazole are consistent with the report that this drug was ineffective in clearing PCP in experimental animals and in inhibiting proliferation of short-term primary cultures of the organism (2).
Δ8 to Δ7 Isomerase.
In most organisms, the double bond in the lanosterol nucleus at C-8 is reduced and the bond at C-7 is desaturated. However, the sterols that eventually accumulate in fungi, plants, and animals are dominated by those with a double bond at C-5. Pneumocystis is unusual in that the sterols that accumulate in the organism are mainly those with a C-7 instead of a C-5 double bond. The only Δ8 to Δ7 isomerase inhibitor tested in this study was AY 9944, which exhibited dose- and time-dependent activity and had moderate activity against P. carinii ATP pools (Fig. 1; Table 2).
SAM:SMT.
In organisms that produce 24-alkylsterols, the addition of alkyl groups to the C-24 position of the sterol side chain is catalyzed by one or two methyltransferase reactions, which commonly occur after demethylation of the lanosterol nucleus. Of the putative SAM:SMT inhibitors tested, only 24(25)-epiminolanosterol exhibited any ability to reduce P. carinii ATP levels, and only at the highest concentration used (Table 2). This compound also exhibited moderate inhibition of 24-alkylsterol biosynthesis in P. carinii and of proliferation of the organism in short-term cultures (25).
Polyene antimycotics.
Polyene antibiotics do not inhibit sterol biosynthesis but bind to sterols within biomembranes. The sterol-drug complexes aggregate and form large pores in the membranes, leading to collapse of chemical gradients and cell death. Amphotericin B, which is used clinically for deeply invasive mycosis, has a higher affinity for ergosterol (in fungal membranes) than for cholesterol (in mammalian-cell membranes). However, at sufficient concentrations, it will bind cholesterol and other membrane sterols as well. The effects on P. carinii ATP levels demonstrate a dose-dependent adverse effect of amphotericin B on the viability of the pathogen (Fig. 1; Table 2) at moderate levels from 24 to 48 h of exposure and at marked levels after 72 h.
DISCUSSION
Reduction in cellular ATP levels of P. carinii by inhibitors of sterol biosynthesis.
Cholesterol is present in calf serum added to the culture medium and is also abundant in the lung surfactant that lines the mammalian alveolus. Although P. carinii can scavenge cholesterol from its environment and incorporates it into new membranes, this sterol alone does not appear to be sufficient for maintaining cell viability. The experiments performed in this study demonstrate that the specific steps in sterol biosynthesis are necessary to maintain the integrity of the organisms and that their disruption leads to the loss of organism viability. The distinct 24-alkylsterols identified in the organism (10, 14, 17, 25) apparently fulfill the precise structural requirements for proper functioning of specific dynamic membrane microdomains. The cholesterol structure evidently cannot substitute at these sites. Thus, despite the presence of abundant cholesterol, which it can and does scavenge from the host or culture medium, the pathogen must synthesize its own distinctive sterol components. Hence, the sterols that are synthesized de novo by P. carinii can be considered the organism's metabolic sterols (12). The results of this study indicate that reactions in sterol biosynthesis are attractive drug targets for treatment of P. carinii infections. Cotreatment with compounds targeting other metabolic pathways would seem a rational approach for PCP therapy.
HMG-CoA reductase.
The inhibitors of the reaction producing mevalonate had little or no adverse effect on P. carinii cellular ATP levels. In light of the effectiveness of other inhibitors of sterol biosynthesis in reducing organism viability, these results raise the question of whether P. carinii may have an operational Rohmer-Arigoni (pyruvate–glyceraldehyde 3-phosphate or 1-deoxy-d-xylulose 5-phosphate [DOXP]) pathway by which isopentenyl diphosphate is synthesized (13, 22). This suggestion is consistent with the observation that lovastatin failed to enhance the incorporation of exogenous radiolabeled mevalonate into P. carinii isoprenoid products (D. Sul and E. S. Kaneshiro, unpublished data), unlike its effect in many other cell types with only the acetate-mevalonate pathway (19). Also, it has been reported that the P. carinii HMG-CoA reductase activity is sensitive to lovastatin (IC50 = 4 nM) (18). However, the enzyme activity in this organism is only 100 pmol/mg of total P. carinii protein/min (18), which is about a 1,000 times less than that found in other cell types such as rat liver (130 nmol/mg of total liver homogenate protein/min) (23). The other drugs tested in the present study that are known to inhibit sterol biosynthesis are targeted to reactions subsequent to the formation of isopentenyl diphosphate. Thus, if isopentenyl units are synthesized via the alternative DOXP pathway in P. carinii, this could explain how the organism can continue synthesizing sterols and maintaining its cellular ATP pool with a block in the acetate-mevalonate pathway. The DOXP pathway may coexist with the acetate-mevalonate pathway as found in Scenedesmus obliquus (22). Such a mevalonate-independent pathway was recently identified in Plasmodium falciparum (13) and was shown to provide a novel drug target for these parasites. Treatment of malarial parasites in vitro with fosmidomycin and FR-900098 showed reductions in viability as well as inhibition of the target enzyme, DOXP reductase. Fosmidomycin was well tolerated by human volunteers and patients with bacterial infections. The putative existence of the nonmevalonate DOXP pathway opens new potential chemotherapeutic strategies against P. carinii infections (13).
Squalene synthase.
Squalestatin, GR 105155X, belongs to a group of complex fungal metabolites named squalestatins by Glaxo and zaragosic acids by Merck (21). Squalestatins inhibit squalene synthase activity at nanomolar concentrations and display broad-spectrum antifungal activity (21), but even at 100 μg/ml GR 105155X had no effect on P. carinii viability. There are now a number of squalestatin and zaragosic acid derivatives, and it is known that compounds with similar enzyme-inhibitory activities can have very different effects in lowering sterol biosynthesis. Thus, it is possible that some derivatives of squalestatin not tested in this survey may be effective in reducing P. carinii viability.
In contrast to GR 105155X, both the biphenyl and naphthalene alkylamine derivatives CCI 16543 and CCI 14993 exhibited time- and dose-dependent effects in reducing cellular ATP levels in this organism. These results indicate that the inhibition of sterol biosynthesis by blocking squalene synthase activity leads to a loss of P. carinii viability.
Squalene epoxidase activity.
The more-promising candidates for anti-P. carinii therapy tested in the present study were those compounds that target the squalene epoxidase step in sterol biosynthesis. The squalene epoxidase inhibitors terbinafine and tolnaftate exerted moderate effects on the organism's ATP pool after 48 h of exposure.
Squalene epoxide-lanosterol cyclase activity.
Inhibitors targeted to the step in which lanosterol is produced by ring closure of a linear molecule included compounds that were also promising anti-P. carinii agents. GR 90525A was even more efficacious than terbinafine or tolnaftate, with a marked effect after 72 h of exposure.
Lanosterol demethylase activity.
The drugs targeted against lanosterol demethylation that were tested in the present study fell into two groups with respect to efficacy in reducing P. carinii ATP pools. The imidazole drugs were effective, but the triazoles were not. The triazole drugs tested are more specific for lanosterol demethylation, whereas the imidazoles are known to have additional inhibitory activity. It remains to be tested whether the efficacy of the imidazoles in reducing P. carinii ATP levels results from the specific blocking of lanosterol demethylation or is due to other effects, e.g., proton ATPase activity.
Since the sequences of steps in the biosynthesis of the various sterols identified in P. carinii have yet to be elucidated, it is not clear if lanosterol is demethylated prior to other reactions, e.g., Δ8 to Δ7 isomerization or C-24 alkylation. However, the identification of pneumocysterol, (24Z)-ethylidenelanost-8-en-3β-ol (15), and 24-methylenelanost-8-en-3β-ol (Giner et al., unpublished data) in some human-derived P. carinii f. sp. hominis preparations suggests that 24-alkylation in this organism can precede sterol nucleus demethylation. Nonetheless, fluconazole was shown to be ineffective in clearing P. carinii infections and in inhibiting short-term culture growth (2), and fluconazole and the other two triazoles also were ineffective in reducing P. carinii ATP levels. Together, these observations suggest that sterol nucleus demethylation in P. carinii differs significantly from that which occurs in fungi that synthesize ergosterol as their major sterol product. The substrate(s) and/or enzyme(s) for sterol nucleus demethylation in P. carinii may have important structural differences from those of ergosterol-synthesizing fungi. It would be worthwhile to screen other sterol demethylase inhibitors with low toxicity; some may prove to have greater efficacy against P. carinii than against other fungal pathogens.
Δ8 to Δ7 Isomerase.
The cationic amphiphilic herbicide AY 9944 appears attractive as a potential anti-P. carinii agent. At high concentrations, it can have teratogenic effects on human development, but it is considered nontoxic at millimolar levels (20). Because most of the P. carinii-specific sterols have a double bond at C-7, it is likely that the organism requires sterols with this specific configuration. Blocking the isomerization of the C-8 double bond to the C-7 position may lead to an inability of the organism to form new functional membranes. Although ergosterol contains a double bond at C-7, growth of the yeast Saccharomyces cerevisiae by treatment with millimolar concentrations of AY 9944 (20), suggesting that the Δ7 double bond may not be as critical for membrane function in this fungus. Hence, P. carinii appears to differ from other fungi in its response to this drug and in the requirement of sterols with a Δ7 double bond, since AY 9944 at micromolar concentrations reduced cellular ATP levels in P. carinii.
Interestingly, Achour et al. (1) reported that AY 9944, at a nontoxic concentration of 3 μM, improved mitogenic responses and cytokine profiles of AIDS patients' peripheral mononuclear cells. These workers suggested that the drug might indirectly enhance the patients' ability to resist AIDS and opportunistic infections. The results of the present study demonstrating a direct deleterious effect on P. carinii organisms suggest that AY 9944 may have dual, complementary benefits for PCP patients among the AIDS population. Other inhibitors of this isomerase activity should be examined for their effects on P. carinii viability as well as their efficacy in blocking the formation of Δ7 sterols in P. carinii.
SAM:SMT.
Urbina et al. (25) previously reported that 24(25)-epiminolanosterol inhibited sterol C-24 alklylation and P. carinii growth; the 22,26-azasterol, 20-piperidine-2-yl-5α-pregnan-3β-20(R)-diol was more potent. The results of that study and our data on the effects of 24(25)-epiminolanosterol on P. carinii ATP levels suggest that this inhibitor has a fungistatic effect, but little fungicidal effect, on the organism.
The 24-bromo- and 24-iodolanosterol compounds tested were designed as potential substrate analogs that act as mechanism-based suicide inhibitors and/or closely resemble transition-state structures (E. J. Parish, unpublished data). It is likely that the atomic radii of the halogen substituents are too large to enable the SAM:SMT enzyme to interact with the drugs. It would be interesting to see if the chloro-derivative would have activity, since it would have a smaller substituent.
Sinefungin is not specific for SAM:SMT, but it was tested as an analog of SAM. It had no activity in reducing P. carinii viability. It is unlikely that other methyltransferase reactions inhibited by this drug are not vital to the organism; the possibility that the lack of activity observed resulted from the inability of the cells to transport the compound cannot be ruled out. In general, P. carinii takes up water-soluble compounds much more slowly than lipid-soluble compounds (14).
Amphotericin B.
The information presented in this report should prove useful for investigators performing in vitro culture studies. The polyene antibiotic amphotericin B has only a relatively higher affinity for binding ergosterol within membranes, but in sufficiently high concentrations, it will also bind cholesterol in mammalian-cell membranes and hence can be toxic. Amphotericin B clearly exhibited concentration-dependent effects on P. carinii ATP levels, consistent with its known mode of action in reducing the permeability barrier of cell membranes. Although some investigators assume that the organism is refractory to this drug, the data shown herein indicate that at concentrations between 0.25 and 2.5 μg/ml (the recommended final concentration for routine tissue cultures), the integrity of the organism's membranes is altered; deleterious effects become evident between 0.1 and 1 μg/ml. Thus, the interpretation of results obtained in experiments performed on organisms exposed to amphotericin B should taken into account the effects that the compound could have on cultured P. carinii organisms.
In contrast to the efficacy of amphotericin B in the present study, Cushion et al. (7) found no effect on the ATP pools of freshly isolated organisms treated with as much as 10 μg of the agent/ml. One important difference in the present study may help to explain the difference in results. In the present study, organisms were taken out of cryopreservation and placed in medium containing the compound. In the previous study, organisms freshly isolated from infected rat lungs were used immediately. There is a more dramatic increase in the ATP levels of organisms taken out of liquid nitrogen at the 24-h time point than in those of organisms freshly isolated from lungs, suggesting higher metabolic activity as the organisms acclimate to the culture conditions (Collins and Cushion, unpublished data). Thus, cryopreserved organisms may be more susceptible to polyene antibiotics than organisms freshly isolated from rat lungs. Longer incubation times may be necessary to detect susceptibilities in organisms purified directly from animal lungs.
De novo sterol biosynthesis in P. carinii.
The results of testing the effects on P. carinii cellular ATP levels of compounds known to inhibit sterol biosynthesis in other organisms are consistent with the growing evidence that this pathogen is fully capable of synthesizing its unique metabolic sterols de novo (10, 14, 15, 17, 18, 25). Lovastatin-inhibitable HMG-CoA reductase activity (18), incorporation of radiolabeled mevalonate and squalene into sterols (9), and incorporation of mevalonate into squalene, squalene epoxides, and C30 sterols (presumably lanosterol) (8) have been demonstrated in P. carinii organisms. Also, incorporation of isopentenyl, geranyl, and farnesyl diphosphates into sterols have been demonstrated using a cell-free system (Sul and Kaneshiro, unpublished data). Furthermore, expressed sequence tag analyses of P. carinii have identified nucleotide sequences homologous to the C. albicans gene sequences encoding squalene epoxidase (Erg1), oxidosqualene-lanosterol cyclase (Erg7), and SAM:SMT (Erg6), (http://www.uky.edu/Projects/Pneumocystis). The observations made in the present study provide strong evidence that inhibition of sterol biosynthesis in P. carinii offers an alternative means of controlling the replication of the organism.
ACKNOWLEDGMENTS
We thank Edward Parish, Helen Jackson, Frederico Gomez De Las Heras, Alfred Alberts, Leslie Koch, David H. Beach, Cyrus Bacchi, and Burt Goldberg for providing inhibitors tested in this study and information about the drugs. We thank Michael A. Wyder for technical assistance.
This work was supported by Public Health Service grants RO1 AI38758 and RO1 AI29316 (E.S.K.) and RO1 AI32436 and NO1 AI75319 (M.T.C.) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Achour A, Landureau J-C, Salerno-Concalves R, Mazière J-C, Zagury D. Restoration of immune response by a cationic amphiphilic drug (AY 9944) in vitro: a new approach to chemotherapy against human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1998;42:2482–2491. doi: 10.1128/aac.42.10.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett M S, Queener S F, Shaw M M, Richardson J D, Smith J W. Pneumocystis cariniiis resistant to imidazole antifungal agents. Antimicrob Agents Chemother. 1994;38:1859–1861. doi: 10.1128/aac.38.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boylan C J, Current W L. Improved model of Pneumocystis carinii pneumonia: induced laboratory infection in Pneumocystis-free animals. Infect Immun. 1992;60:1589–1597. doi: 10.1128/iai.60.4.1589-1597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen F, Cushion M T. Use of an ATP bioluminescent assay to evaluate viability of Pneumocystis cariniifrom rats. J Clin Microbiol. 1994;32:2791–2800. doi: 10.1128/jcm.32.11.2791-2800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirioni O, Giacometti A, Balducci M, Burzacchini F, Scalise G. In-vitro activity of terbinafine, atovaquone and co-trimoxazole against Pneumocystis carinii. J Antimicrob Chemother. 1995;36:740–742. doi: 10.1093/jac/36.4.740. [DOI] [PubMed] [Google Scholar]
- 6.Contini C, Manganaro M, Romani R, Tzantzoglou S, Poggesi I, Vullo V, Delia S, De Simone C. Activity of terbinafine against Pneumocystis cariniiin vitro and its efficacy in the treatment of experimental pneumonia. J Antimicrob Chemother. 1994;34:727–735. doi: 10.1093/jac/34.5.727. [DOI] [PubMed] [Google Scholar]
- 7.Cushion M T, Chen F, Kloepfer N. A cytotoxicity assay for evaluation of candidate anti-Pneumocystisagents. Antimicrob Agents Chemother. 1997;41:379–384. doi: 10.1128/aac.41.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis J E, Wyder M A, Zhou L, Gupta A, Rudney H, Kaneshiro E S. Composition of Pneumocystis carinii neutral lipids and identification of coenzyme Q10as the major ubiquinone homolog. J Eukaryot Microbiol. 1996;43:165–170. doi: 10.1111/j.1550-7408.1996.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 9.Florin-Christensen M, Florin-Christensen J, Wu Y-P, Zhou L, Gupta A, Rudney H, Kaneshiro E S. Occurrence of specific sterols in Pneumocystis carinii. Biochem Biophys Res Commun. 1994;198:236–242. doi: 10.1006/bbrc.1994.1033. [DOI] [PubMed] [Google Scholar]
- 10.Furlong S T, Samia J A, Rose R M, Fishman J A. Phytosterols are present in Pneumocystis carinii. Antimicrob Agents Chemother. 1994;38:2534–2540. doi: 10.1128/aac.38.11.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harwood J L. Lung surfactant. Prog Lipid Res. 1987;26:211–256. doi: 10.1016/0163-7827(87)90004-x. [DOI] [PubMed] [Google Scholar]
- 12.Haughan P A, Goad L J. Lipid biochemistry of trypanosomatids, P. 312–328. In: Coombs G H, North M D, editors. Biochemical protozoology. London, United Kingdom: Taylor & Frances; 1991. [Google Scholar]
- 13.Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Türbachova I, Eberl M, Zeidler J, Lichtenthaler H K, Soldati D, Beck E. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 14.Kaneshiro E S. The lipids of Pneumocystis carinii. Clin Microbiol Rev. 1998;11:27–41. doi: 10.1128/cmr.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneshiro E S, Amit Z, Swonger M M, Kreishman G P, Brooks E E, Kreishman M, Jayasimhulu K, Parish E J, Sun H, Kizito S A, Beach D H. Pneumocysterol [(24Z)-ethylidenelanost-8-en-3β-ol]), a rare sterol detected in the opportunistic pathogen Pneumocystis carinii f. sp. hominis: structural identity and chemical synthesis. Proc Natl Acad Sci USA. 1999;96:97–102. doi: 10.1073/pnas.96.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneshiro E S, Amit Z, Chandra J, Baughman R P, Contini C, Lundgren B. The sterol composition of Pneumocystis carinii hominisorganisms isolated from human lungs. Clin Diagn Lab Immunol. 1999;6:970–976. doi: 10.1128/cdli.6.6.970-976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneshiro E S, Ellis J E, Jayasimhulu K, Beach D H. Evidence for the presence of “metabolic sterols” in Pneumocystis: identification and initial characterization of Pneumocystis cariniisterols. J Eukaryot Microbiol. 1994;41:78–85. doi: 10.1111/j.1550-7408.1994.tb05938.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaneshiro E S, Ellis J E, Zhou L H, Rudney H, Gupta A, Jayasimhulu K, Setchell K D, Beach D H. Isoprenoid metabolism in Pneumocystis carinii. J Eukaryot Microbiol. 1994;41:93S. [PubMed] [Google Scholar]
- 19.Lujan H D, Mowatt M R, Chen G Z, Nash T E. Isoprenylation of proteins in the protozoan Giardia lamblia. Mol Biochem Parasitol. 1995;72:121–127. doi: 10.1016/0166-6851(94)00070-4. [DOI] [PubMed] [Google Scholar]
- 20.Pereira R, Holmlund C E, Whittaker N. The effect of AY-9944 on yeast sterol and sterol ester metabolism. Lipids. 1983;18:545–552. doi: 10.1007/BF02535395. [DOI] [PubMed] [Google Scholar]
- 21.Procopiou P A, Cox B, Kirk B E, Lester M G, McCarthy A D, Sareen M, Sharratt P J, Snowden M A, Spooner S J, Watson N S, Widdowson J. The squalestatins: inhibitors of squalene synthase. Enzyme inhibitory activities and in vivo evaluation of C3-modified analogues. J Med Chem. 1996;39:1413–1422. doi: 10.1021/jm950893j. [DOI] [PubMed] [Google Scholar]
- 22.Schwender J, Seemann M, Lichtenthaler H K, Rohmer M. Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green alga Scenedesmus obliquus. Biochem J. 1996;316:73–80. doi: 10.1042/bj3160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro D J, Rodwell V W. Regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and cholesterol synthesis. J Biol Chem. 1971;246:3210–3216. [PubMed] [Google Scholar]
- 24.Stringer J R. Pneumocystis carinii: what is it exactly? Clin Microbiol Rev. 1996;9:489–498. doi: 10.1128/cmr.9.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbina J A, Visbal G, Contreras L M, McLaughlin G, Docampo R. Inhibitors of Δ24(25) sterol methyltransferase block sterol synthesis and cell proliferation in Pneumocystis carinii. Antimicrob Agents Chemother. 1997;41:1428–1432. doi: 10.1128/aac.41.7.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]