Abstract
Background
The role of inhaled corticosteroids (ICS) in chronic obstructive pulmonary disease (COPD) has been the subject of much controversy. Major international guidelines recommend selective use of ICS. Recently published meta‐analyses have reported conflicting findings on the effects of inhaled steroid therapy in COPD.
Objectives
To determine the efficacy and safety of inhaled corticosteroids in stable patients with COPD, in terms of objective and subjective outcomes.
Search methods
A pre‐defined search strategy was used to search the Cochrane Airways Group Specialised Register for relevant literature. Searches are current as of July 2011.
Selection criteria
We included randomised trials comparing any dose of any type of inhaled steroid with a placebo control in patients with COPD. Acute bronchodilator reversibility to short‐term beta2‐agonists and bronchial hyper‐responsiveness were not exclusion criteria. The a priori primary outcome was change in lung function. We also analysed data on mortality, exacerbations, quality of life and symptoms, rescue bronchodilator use, exercise capacity, biomarkers and safety.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We contacted study authors for additional information. We collected adverse effects information from the trials.
Main results
Fifty‐five primary studies with 16,154 participants met the inclusion criteria. Long‐term use of ICS (more than six months) did not consistently reduce the rate of decline in forced expiratory volume in one second (FEV1) in COPD patients (generic inverse variance analysis: mean difference (MD) 5.80 mL/year with ICS over placebo, 95% confidence interval (CI) ‐0.28 to 11.88, 2333 participants; pooled means analysis: 6.88 mL/year, 95% CI 1.80 to 11.96, 4823 participants), although one major trial demonstrated a statistically significant difference. There was no statistically significant effect on mortality in COPD patients (odds ratio (OR) 0.98, 95% CI 0.83 to 1.16, 8390 participants). Long‐term use of ICS reduced the mean rate of exacerbations in those studies where pooling of data was possible (generic inverse variance analysis: MD ‐0.26 exacerbations per patient per year, 95% CI ‐0.37 to ‐0.14, 2586 participants; pooled means analysis: MD ‐0.19 exacerbations per patient per year, 95% CI ‐0.30 to ‐0.08, 2253 participants). ICS slowed the rate of decline in quality of life, as measured by the St George's Respiratory Questionnaire (MD ‐1.22 units/year, 95% CI ‐1.83 to ‐0.60, 2507 participants). Response to ICS was not predicted by oral steroid response, bronchodilator reversibility or bronchial hyper‐responsiveness in COPD patients. There was an increased risk of oropharyngeal candidiasis (OR 2.65, 95% CI 2.03 to 3.46, 5586 participants) and hoarseness. In the long‐term studies, the rate of pneumonia was increased in the ICS group compared to placebo, in studies that reported pneumonia as an adverse event (OR 1.56, 95% CI 1.30 to 1.86, 6235 participants). The long‐term studies that measured bone effects generally showed no major effect on fractures and bone mineral density over three years.
Authors' conclusions
Patients and clinicians should balance the potential benefits of inhaled steroids in COPD (reduced rate of exacerbations, reduced rate of decline in quality of life and possibly reduced rate of decline in FEV1) against the potential side effects (oropharyngeal candidiasis and hoarseness, and risk of pneumonia).
Plain language summary
Inhaled steroids for stable chronic obstructive pulmonary disease
Steroid preventer medications given by inhaler ('inhaled steroids') help to reduce inflammation in the air passages of people with asthma. However, it is uncertain whether these medications are beneficial in people with chronic obstructive pulmonary disease (COPD, i.e. chronic bronchitis or emphysema or both).
We undertook a systematic review of the benefits and safety of inhaled steroids for people with COPD. Our review analysed the effects on breathing capacity, death rates, frequency of flare‐ups ('exacerbations'), quality of life and side effects.
Pooling of the data from the 55 trials with 16,154 people showed that there was no consistent long‐term benefit in the rate of decline in breathing capacity. Death rates were unchanged. Inhaled steroids were beneficial in slowing down the rate of decline in quality of life and reducing the frequency of exacerbations. Inhaled steroids increased the risk of side effects including thrush (candida) infection in the mouth and hoarseness, and the rate of pneumonia.
In deciding whether to use this treatment, consumers and health professionals should weigh up the benefits (reduced rate of exacerbations, reduced decline in quality of life and possible reduction in the rate of decline of breathing capacity) against the side effects (mouth thrush, hoarseness and increased risk of developing pneumonia).
Background
Inhaled corticosteroids (ICS) have proven benefit in the treatment of airway inflammation in asthma, but there are still questions about their use in patients with chronic obstructive pulmonary disease (COPD). The Global Initiative for Obstructive Lung Disease (GOLD) guidelines for COPD recommend adding ICS to long‐acting beta2‐agonists for symptomatic COPD patients with forced expiratory volume in one second (FEV1) less than 50% predicted and repeated exacerbations (GOLD COPD guidelines, www.goldcopd.org). The rationale for use of ICS in COPD has been discussed extensively in editorials (van Schayck 1996; Calverley 1999; Mapp 2000; Burge 2003b; Epstein 2003; Woodhead 2007; Welte 2009; Sin 2010), pro/con debates (Barnes 2000; Calverley 2000), narrative reviews (Hudson 1990; Postma 1999; Sapey 2000; Whittaker 2000; Burge 2001;; Bonay 2002; Highland 2004; Selroos 2004; Bonay 2005; Calverley 2005; Man 2005b) and systematic reviews (van Grunsven 1999; Alsaeedi 2002; Highland 2003; Sin 2003b; Sin 2003c; Sutherland 2003; Gan 2005; Sin 2005; Gartlehner 2006; Soriano 2007; Drummond 2008; Sin 2009; Singh 2009; Agarwal 2010; Loke 2011). As the effectiveness and safety of ICS in COPD patients are still contentious, we undertook this updated Cochrane systematic review of ICS for COPD.
Objectives
To determine the efficacy and safety of inhaled corticosteroids in stable patients with COPD, in terms of objective and subjective outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We considered all published and unpublished randomised controlled trials (RCTs) of regular ICS in COPD. Placebo‐controlled trials with random allocation and double‐blinding were included. We preferred trials analysed on an intention‐to‐treat basis. We considered parallel‐group and cross‐over studies.
Types of participants
We reviewed studies of adults of either gender, regardless of smoking history, with COPD defined as progressive chronic airflow limitation. Patients were in a clinically stable state at the start of the study, without recent exacerbation, hospitalisation or need for antibiotics or systemic steroids. Patients did not have clinical features of asthma. Studies recruiting patients with acute bronchodilator reversibility to short‐acting beta2‐agonists or patients with bronchial hyper‐responsiveness (BHR) were included. We analysed these BHR studies separately from studies of COPD patients in which BHR was not an inclusion criteria or in which BHR was excluded.
Types of interventions
We included studies of regular ICS administered by inhalation devices including metered‐dose inhaler, dry powder inhaler or spacer devices. We excluded studies delivering ICS by nebuliser. We did not include ICS versus placebo with long‐acting beta2‐agonists as a co‐intervention in each group.
Types of outcome measures
Primary outcomes
Lung function
Secondary outcomes
Mortality
Exacerbations of COPD
Quality of life and symptoms
Use of rescue bronchodilators
Exercise capacity
Biomarkers
Predictors of response
Side effects: oropharyngeal side effects (throat irritation, oral candidiasis), skin bruising, hypothalamic‐pituitary‐adrenal (HPA) axis function, fractures, pneumonia
Search methods for identification of studies
Electronic searches
In the original review, we examined and combined randomised controlled trials of inhaled corticosteroids in adults with COPD from 1966 to October 2006. The 2011 update includes trials from October 2006 to July 2011. Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (see Appendix 1 for more details). We searched all records in the Specialised Register coded as 'COPD' using the following terms:
(corticosteroid* or cortico‐steroid* or beclomethasone or beclazone or becotide or becloforte or budesonide or pulmicort* or fluticasone or flixotide or qvar or zonivent or filair or aerobec or asmabec or becodisk* or triamcinolone or mometasone or flunisolide)
Searching other resources
We searched the bibliographies of each included study for additional relevant studies. We undertook additional searches of manufacturers' websites in order to identify unpublished data (http://ctr.gsk.co.uk; http://www.astrazenecaclinicaltrials.com/; http://www.clinicalstudyresults.org).
Data collection and analysis
Selection of studies
Two review authors independently assessed for relevance the titles and, where available, abstracts of all trials retrieved by the search strategy. We then retrieved all relevant or potentially relevant articles in full. We categorised these articles as relevant (met the inclusion criteria for considering studies) or not relevant (did not meet the inclusion criteria for considering studies). We resolved disagreements about relevance by consensus.
Data extraction and management
Three review authors (IY, ES and TL) extracted data from included studies for the original review, and two review authors (IY, MC) extracted data from included studies for the 2011 update. Wherever possible, we sought missing data in the publication from the authors by correspondence (email, fax or letter).
Assessment of risk of bias in included studies
Two review authors independently assessed the quality of all relevant trials, using the Cochrane approach, to assess risk of bias.
Measures of treatment effect
If appropriate data for mean, standard deviation (SD) and number of participants in each treatment and placebo arm were available, we combined data from trials using Review Manager 5 (RevMan 2011), generating a mean difference and 95% confidence interval. We used a mean difference (MD) for continuous variables. We summarised proportional outcomes, such as proportion who improved, using an odds ratio. We used the Mantel‐Haenszel method to combine estimates of the odds ratios.
Assessment of heterogeneity
We performed tests for heterogeneity using Review Manager 5. We also applied a random‐effects model as part of sensitivity analysis.
Assessment of reporting biases
A funnel plot of studies will be created if 10 or more trials are included in any single meta‐analysis comparison.
Data synthesis
We used a fixed‐effect mean difference (MD) for continuous variables. If data were reported on different metrics, we planned to use a standardised mean difference (SMD), which expresses differences as standard deviation units.
Subgroup analysis and investigation of heterogeneity
The treatment periods were separated into short‐term (less than two months), medium‐term (greater than two months to six months) and long‐term (greater than six months). We stratified data by equivalent beclomethasone dosage.
Results
Description of studies
Results of the search
We retrieved and assessed a total of 2205 abstracts in the original review. In the 2011 update, we retrieved and assessed 339 additional abstracts (see Table 1: 'Search history detail'), with 36 studies meeting the inclusion criteria; of these, eight were new studies not previously included. A total of 55 studies (compared to 47 studies in the original review) met the inclusion criteria for the systematic review. For full details on individual studies, please see 'Characteristics of included studies'
1. Search history detail.
| Year | Abstracts retrieved |
| Up to and including 1999 | 1340 |
| 2000 | 464 |
| 2001 | 131 |
| 2002 | 34 |
| 2003 | 72 |
| 2004 | 116 |
| 2005 | 48 |
| 2006 | 40 |
| 2007 | 62 |
| 2008 | 100 |
| 2009‐10 | 60 |
| 2011 | 77 |
Included studies
Study design
All studies were randomised, placebo‐controlled trials. All studies were described as either double‐blind or double‐dummy. Thirteen studies were of a cross‐over design (Robertson 1986; Weir 1990a; Wempe 1992; Weiner 1995; Boothman‐Burrell 1997; Culpitt 1999; Nishimura 1999; Weiner 1999; Ferreira 2001; Loppow 2001; Thompson 2002; Brightling 2005; Guenette 2011). The remaining studies were conducted with a parallel‐group design.
Participants
A total of 16,154 participants (13,139 in the original review) with COPD were recruited in the studies. More recent trials tended to use international criteria for the definition of COPD, and the remaining studies based their definition of COPD on lung function and smoking history (see table: 'Characteristics of included studies'). The entry criteria differed between the studies in terms of permissible bronchial hyper‐responsiveness (BHR) or bronchodilator reversibility; hence we stratified studies by whether COPD patients with these features were included. The majority of studies excluded participants who had an exacerbation within six to eight weeks prior to recruitment.
Interventions
All studies were placebo‐controlled. There were five types of inhaled steroid used in the trials: BUD (budesonide), BDP (beclomethasone dipropionate), FP (fluticasone propionate), TAA (triamcinolone acetonide) and mometasone furoate (MF). Study durations were as follows.
Up to two months in 18 studies (Robertson 1986; Weir 1990a; Auffarth 1991; Thompson 1992; Wempe 1992; Weiner 1995; Llewellyn‐Jones 1996; Rutgers 1998; Culpitt 1999; Nishimura 1999; Weiner 1999; Ferreira 2001; Loppow 2001; Ferreira 2003; Sin 2004; Brightling 2005; Sin 2008; Guenette 2011).
Longer than two months and up to six months in 17 studies (Boothman‐Burrell 1997; Bourbeau 1998; Paggiaro 1998; Senderovitz 1999; Mirici 2001; Hattotuwa 2002; Laptseva 2002; Mahler 2002; Thompson 2002; Verhoeven 2002; Hanania 2003; Yildiz 2004; John 2005; Ozol 2005; GSK 2005 (FCO30002); GSK 2005 (FLTA3025); Bourbeau 2007).
Longer than six months in 20 studies (Kerstjens 1992; Derenne 1995; Renkema 1996; Pauwels 1999; Vestbo 1999; Weir 1999; Burge 2000; LHS 2000; Calverley 2003a; Calverley 2003b; Calverley 2003c; Szafranski 2003; van Grunsven 2003; SCO30002 2005; Calverley 2007; Calverley 2008; Tashkin 2008; Lapperre 2009; Schermer 2009; Shaker 2009).
Outcomes
Various outcomes were measured in the studies (see tables 'Characteristics of included studies'). The long‐term studies (more than six months) reported FEV1 in terms of rate of decline, and short to medium‐term studies tended to report change in FEV1 from baseline. Exacerbations were variously reported as dichotomous data (e.g. patients with one or more exacerbations), exacerbation episodes per treatment arm, or mean rate per patient per year. Some studies measured quality of life, symptoms and rescue bronchodilator usage. A group of studies specifically focused on changes in biomarkers (for example, sputum analysis). Long‐term studies also analysed adverse effects.
Excluded studies
Risk of bias in included studies
The quality of published studies was generally good, although many studies had unclear risk of bias in relation to randomisation method and allocation concealment. Unpublished abstracts generally has greater risk of bias, due to lack of details in reporting. See Figure 1.
1.
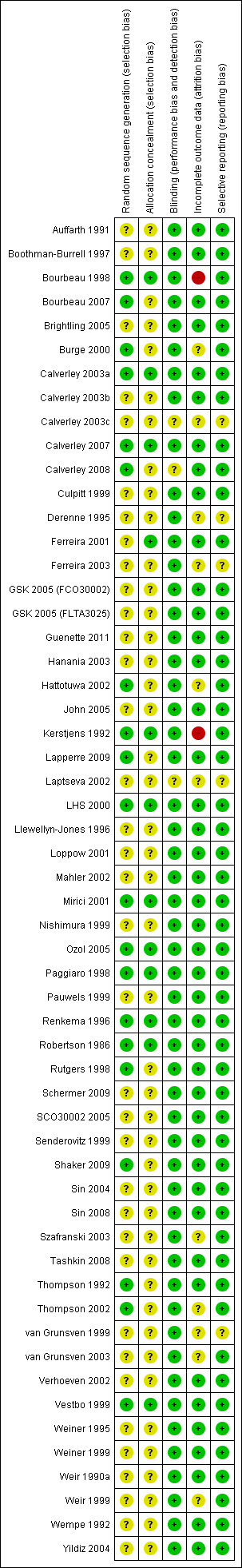
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Studies used random allocation. However, many studies did not specifically state the randomisation method, or whether allocation was concealed.
Blinding
All published studies were double‐blind. Several studies presented in abstract form did not specifically state whether the study was double‐blind.
Incomplete outcome data
The attrition rate was acceptable in the majority of studies. For studies with higher attrition rates, the studies provided adequate detail about the rates of withdrawal in the ICS and placebo arms.
Selective reporting
The published included studies reported the outcomes listed a priori in their methods. This was difficult to ascertain for studies presented in abstract form.
Effects of interventions
Studies in people with COPD (without bronchial hyper‐responsiveness or bronchodilator reversibility)
Long‐term studies (longer than six months)
Three‐year studies
Six large, long‐term trials of ICS versus placebo were reported in COPD participants without bronchial hyper‐responsiveness or bronchodilator reversibility. All were parallel studies. In the European Respiratory Society Study on COPD (EUROSCOP) study, Pauwels et al studied 1277 participants with BUD 800 µg/day versus placebo for three years (Pauwels 1999). The participants were current smokers with mild COPD, with mean FEV1 77% predicted. In the initial six months of the study, BUD resulted in an increase in FEV1 of 17 mL/year compared to a decline of 81 mL/year in the placebo group. After the initial six months, the rates of decline in FEV1 were similar.
The study from Copenhagen reported by Vestbo et al used BUD 1200 µg/day for six months then 800 µg/day for 30 months (total three years) versus placebo in 290 participants (Vestbo 1999). The sample was population‐based and participants were recruited if they had a FEV1/VC ratio of 0.7 or less without bronchodilator reversibility or oral steroid response. Forty per cent of participants stated that they had no breathing problems and 4% were never smokers. Mean post‐bronchodilator FEV1 was 86% to 87% predicted. There was no statistically significant effect of BUD on rate of decline in FEV1, rate of exacerbations or symptoms.
In the ICS in Obstructive Lung Disease in Europe (ISOLDE) study, Burge et al randomised 751 participants to FP 1000 µg/day versus placebo for three years (Burge 2000). This was a moderate to severe group of participants, with mean FEV1 50% of predicted. All were current or ex‐smokers. The majority of participants received a two‐week oral prednisolone course during the run‐in. FP did not alter the rate of overall rate of decline of FEV1, although the mean FEV1 of the FP group remained about 70 mL higher than the placebo group throughout the study. FP reduced the median exacerbation rate (Burge 2000), particularly in the moderate‐severe group of participants (Jones 2003). FP also slowed the decline in health status as determined by the St George's Respiratory Questionnaire (SGRQ) (Spencer 2001).
The Lung Health Study II enrolled 1116 participants and randomised them to inhaled triamcinolone (TAA) 1200 µg/day versus placebo for a mean duration of follow‐up of 40 months (Lung Health Study Research Group 2000). Mean FEV1 was 64% predicted and all were current smokers or ex‐smokers. The rate of decline in FEV1 was similar in the TAA and placebo groups. TAA reduced respiratory symptoms and visits to doctors for respiratory illnesses. TAA also lowered the airway reactivity to methacholine over the course of treatment.
The large TOwards a Revolution in COPD Health (TORCH) study recruited 6115 participants and randomised them to salmeterol/fluticasone, salmeterol, FP 1000 µg/day (1534 participants) and placebo (1524 participants). In the FP versus placebo comparison, there was a reduction in COPD exacerbation rate, with odds ratio (OR) 0.823 (95% confidence interval (CI) 0.758 to 0.894) (Calverley 2007). No mortality benefit was observed with FP alone compared to placebo, with hazard ratio 1.060 (95% CI 0.886 to 1.268) (Ferguson 2006). There was a benefit in quality of life measured by the SGRQ, with a difference of ‐2.0 units (95% CI ‐2.9 to ‐1.0) with FP, compared to placebo. The rate of FEV1 decline was slower in the FP group compared to placebo (difference 13 mL/year, 95% CI 5 to 22) (Celli 2008).
The COOPT trial recruited 286 participants (78% COPD, 22% chronic bronchitis) from 44 general practices and randomised them to FP 500 µg twice daily or placebo for three years (N‐acetylcysteine was used in a separate arm) (Schermer 2009). Exacerbation rate was 1.3 times higher for the FP group compared with the placebo group, although this did not reach statistical significance. Annual decline in post‐bronchodilator FEV1 was similar between groups.
Two‐year studies
Four parallel studies of two years duration have been performed in COPD participants without bronchial hyper‐responsiveness or bronchodilator reversibility (Derenne 1995; Renkema 1996; Weir 1999; Lapperre 2009). The study by Derenne was reported in abstract form (Derenne 1995) and summarised in the meta‐analysis by van Grunsven et al (van Grunsven 1999). Renkema et al studied 39 participants with BUD 1500 µg/day versus placebo for two years (Renkema 1996). They observed a reduced rate of decline in FEV1 (although this was not statistically significant) and reduced symptoms with BUD alone versus placebo. There was no change in rate of exacerbations (Renkema 1996). Weir et al studied 98 participants using BDP 1500 µg/day versus placebo for two years (Weir 1999). There were trends to benefits with BDP in terms of decline in FEV1 and exacerbation rates but these did not reach statistical significance. There was no change in BHR to histamine or dyspnoea as measured by the Mahler dyspnoea index. The data from COPD subgroups of the study by Kerstjens et al, which included COPD participants with BHR (Kerstjens 1992), and Derenne (Derenne 1995) were combined with the data from Renkema (Renkema 1996) in the meta‐analysis by van Grunsven et al (van Grunsven 1999) (see 'Discussion' for details). Lapperre et al randomised 114 participants with moderate to severe COPD to FP 500 µg twice daily for six months or 30 months, or placebo twice daily (salmeterol/fluticasone was used in a separate arm) (Lapperre 2009). FP for 30 months was found to slow the rate of FEV1 decline, and improve dyspnoea and quality of life. A small four‐year trial studied the effect of inhaled corticosteroids on lung density in COPD (Shaker 2009). Shaker et al demonstrated that inhaled BUD 800 µg daily over two to four years showed a non‐significant trend towards reducing the progression of emphysema as determined by the CT‐derived 15th percentile lung density, without any statistically significant effect on decline in lung function (Shaker 2009).
One‐year studies
Four parallel studies of combined ICS/long‐acting beta2‐agonist (LABA) in COPD included ICS versus placebo arms. In the one‐year TRISTAN study of salmeterol/FP, salmeterol, FP or placebo by Calverley et al in 1465 participants, data were available for FP 1000 µg/day versus placebo in one of the comparisons (Calverley 2003a). FP increased pre‐bronchodilator FEV1 by 2%, compared to a fall of 3% with placebo at one year, and reduced the mean exacerbation rate of 1.3 in the placebo group to 1.05 in the FP group. There was no significant change in SGRQ total score or symptom scores with FP compared to placebo, although FP reduced the use of relief medications and awakenings per week (Calverley 2003a). Szafranski et al studied BUD/formoterol, BUD, formoterol or placebo in 812 COPD participants for one year (Szafranski 2003). Small but statistically significant benefits were observed with BUD 800 µg/day compared to placebo for lung function changes and exacerbation rates. Calverley et al similarly studied these medications in 1022 COPD participants for one year, and found fewer exacerbations with BUD compared to placebo, and no significant difference in FEV1 (Calverley 2003b). An unpublished study of salmeterol/FP in COPD (GlaxoSmithKline trial SCO30002 2005) included a comparison of FP 1000 µg/day versus placebo in 256 COPD participants (SCO30002 2005). There was no statistically significant difference in time to first moderate or severe exacerbation with FP or change in post‐bronchodilator FEV1.
Two parallel studies of MF for one year duration have been reported. A study of MF 800 µg/day versus placebo in 631 COPD participants was reported in abstract form (Calverley 2003c). MF was associated with a benefit in post‐bronchodilator FEV1 of 40 mL, compared to placebo, reduced COPD symptoms and increased time to first exacerbation. Calverley et al randomised 911 participants with moderate to severe COPD to MF‐DPI 800 µg once daily, MF‐DPI 400 µg twice daily or placebo (Calverley 2008). MF‐DPI significantly increased post‐bronchodilator FEV1 from baseline and reduced exacerbations. The twice daily MF‐DPI group reported a statistically significant reduction (19%) in COPD symptoms scores compared with placebo. SGRQ improved significantly in all domains in the pooled MF‐DPI groups versus placebo.
Pooled results
Lung function
In the studies of two years or longer, we analysed the main treatment effect of change in rate of FEV1 decline. Two approaches to analysing rate of decline of post‐bronchodilator FEV1 were used, due to the various ways the data were presented in the studies.
When analysing data using the generic inverse variance function of RevMan 5 (RevMan 2011), the pooled difference in rate of decline in post‐bronchodilator FEV1 in four studies (Vestbo 1999; Weir 1999; Burge 2000; LHS 2000) and one combined result (van Grunsven 1999) was 5.80 mL/year with ICS (95% CI ‐0.28 to 11.88; 2333 participants) (Figure 2). In the study by Pauwels et al (1277 participants), there was no significant difference between the median decline of FEV1 of ‐57 mL/year in the budesonide group, compared to the ‐69 mL/year in the placebo group (Pauwels 1999).
2.
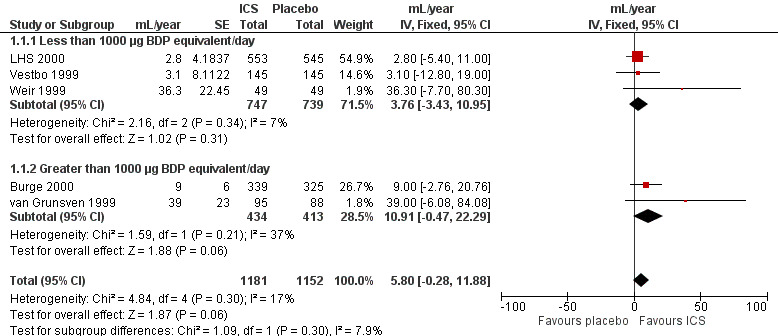
Forest plot of rate of decline of post‐bronchodilator FEV1 (mL/yr), using generic inverse variance analysis
When analysing means for the ICS versus placebo groups, the pooled difference in rate of decline in post‐bronchodilator FEV1 in five studies (Burge 2000; LHS 2000; Celli 2008; Schermer 2009; Shaker 2009) was 6.88 mL/year (95% CI 1.80 to 11.96, 4823 participants) (Figure 3). The main contributor to this statistically significant difference was the TORCH study, which showed a difference for the ICS alone (fluticasone 1000 µg/day, 42 mL/year decline) versus placebo (55 mL/year decline) (Celli 2008). In the TORCH trial, salmeterol (42 mL/year decline) and salmeterol/fluticasone (39 mL/year decline) also had similar benefits in rate of decline in FEV1 (Celli 2008). The study of Lapperre 2009 demonstrated a statistically significant difference in rate of decline of FEV1 (mean difference 86.30 mL/year, 95% CI 43.02 to 129.58); however, this result was not pooled because the rate of decline measured was from six months to 30 months of treatment, instead of from 0 months.
3.
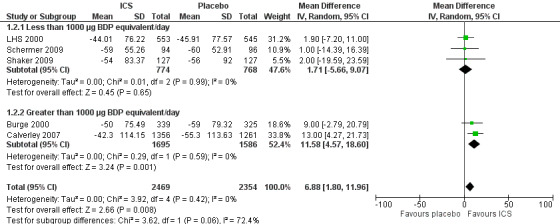
Forest plot of rate of decline in post‐bronchodilator FEV1 (mL/yr), using pooled means analysis
In the studies of one year duration, improvements with ICS were reported for pre‐bronchodilator FEV1 (Szafranski 2003) and post‐bronchodilator FEV1 (Calverley 2003b; Calverley 2003c; Calverley 2008). In one study, there was no significant difference (SCO30002 2005) and one study did not report the spirometry results specifically for the inhaled steroid versus placebo comparison (Calverley 2003a).
Mortality
Mortality was reported in nine long‐term studies (Figure 4). The overall OR for mortality for all nine studies was 0.98 (95% CI 0.83 to 1.16, 8390 participants). In studies of one‐year duration (Calverley 2003a; Calverley 2003b; Szafranski 2003; SCO30002 2005) pooling showed an OR of 0.66 for death with ICS compared to placebo (95% CI 0.33 to 1.31, 1907 participants). In studies of two or more years duration, pooling showed an OR of 1.01 for death with ICS compared to placebo (95% CI 0.85 to 1.20, 6483 participants) (Pauwels 1999; Vestbo 1999; Burge 2000; LHS 2000; Calverley 2007).
4.
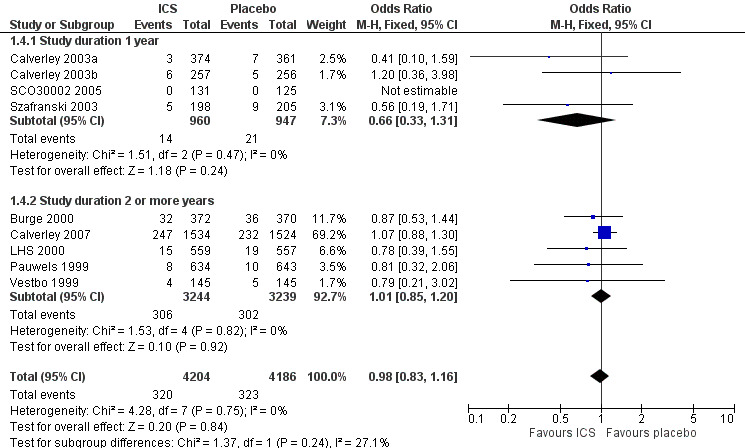
Forest plot of mortality in long‐term studies
Exacerbations
Using the generic inverse variance function, pooling was possible for four long‐term studies (Burge 2000; Calverley 2003a; Calverley 2003b; Szafranski 2003) and the meta‐analysis of three long‐term studies (van Grunsven 1999). The mean difference (MD) for this analysis was ‐0.26 exacerbations per patient per year with ICS (95% CI ‐0.37 to ‐0.14; 2586 participants) (Comparison 1.5) (Figure 5). We also pooled mean rate of exacerbation per patient per year using data from treatment and control groups from four long‐term studies (Burge 2000; Calverley 2003a; Szafranski 2003; Schermer 2009) and a combined rate from the van Grunsven et al meta‐analysis of three long‐term studies (van Grunsven 1999). The MD was ‐0.19 exacerbations per patient per year with ICS (95% CI ‐0.30 to ‐0.08, 2253 participants) (Comparison 1.6) (Figure 6). The study of Schermer 2009 found an increased exacerbation rate with ICS, whereas the other studies had reduced exacerbation rates with ICS.
5.
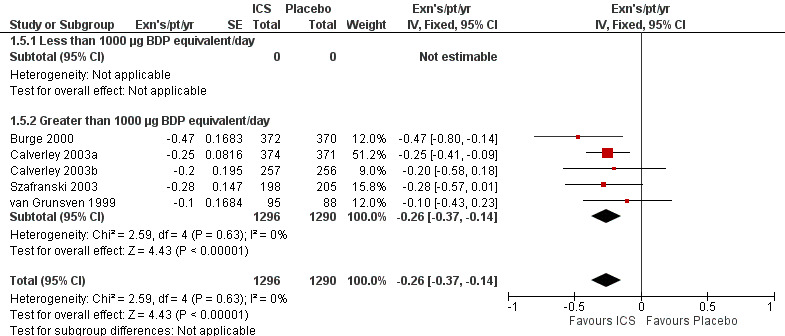
Forest plot of exacerbations per patient per year, using generic inverse variance analysis
6.
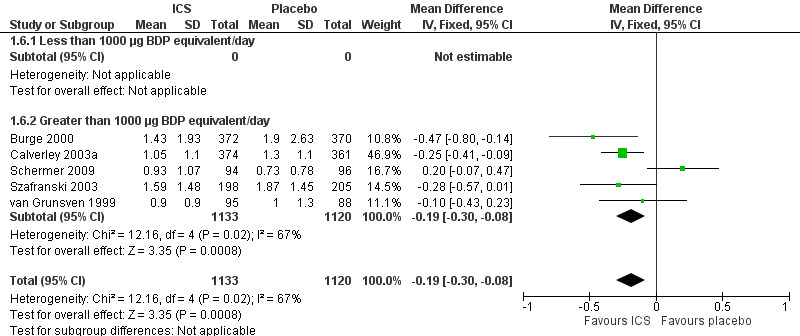
Forest plot of rate of exacerbations per patient per year, using pooled means analysis
In other long‐term studies, exacerbation events were not reported in sufficient detail to pool as mean rate of exacerbation per patient per year. The results of these studies were: no significant difference in mean exacerbation rates per year between BDP (0.36/year) and placebo (0.57/year) (Weir 1999), no significant difference in total number of exacerbations between BUD (155 exacerbations) and placebo (161 exacerbations) (Vestbo 1999), and reduced number of unscheduled physician visits and hospitalisation for respiratory conditions (data not stated) (LHS 2000). In one unpublished study, the total number of exacerbations was reported without analysis being performed, with 123 exacerbations in the FP group and 127 exacerbations in the placebo group (SCO30002 2005). In the TORCH study, the mean number of exacerbations per year was 0.93 in the FP group, compared to 1.13 in the placebo group, giving a statistically significant rate ratio of 0.82 (95% CI 0.76 to 0.89) (Calverley 2007). The EUROSCOP study did not report exacerbation rates (Pauwels 1999).
Four studies reported percentage of participants with at least one exacerbation (Comparison 1.8). Pooling of these results showed an OR of 0.83 in favour of ICS (95% CI 0.7 to 0.98, 2347 participants). Studies of less than 1000 µg BDP equivalent/day (Calverley 2008; Shaker 2009) did not show a statistically significant difference. However, studies of greater than 1000 µg/day (Calverley 2003c; SCO30002 2005; Calverley 2008) did show a statistically significant reduced percentage of patients with exacerbations (OR 0.8, 95% CI 0.65 to 0.98) with low heterogeneity (I2 = 0%), although none of these studies were statistically significant individually.
Quality of life and symptoms
Pooling of rate of change in SGRQ in units/year was analysed with the generic inverse variance function in five long‐term studies (Burge 2000; Calverley 2003a; Calverley 2003b; Szafranski 2003; SCO30002 2005). The MD was ‐1.22 units/year (95% CI ‐1.83 to ‐0.60, 2507 participants), indicating a slowing in the rate of decline of quality of life in the ICS group, compared to placebo (Comparison 1.10). There was no improvement in SF‐36 with ICS (LHS 2000). The TORCH study reported a mean benefit in SGRQ of ‐2.0 units averaged over three years (95% CI ‐2.9 to ‐1.0) with FP compared to placebo (Calverley 2007).
Data for symptoms were mostly not presented in sufficient detail to pool. Symptom scores in general decreased with ICS (Renkema 1996; Calverley 2003c;), and night awakenings were reduced (Calverley 2003b). In one study, there was no change in dyspnoea score measured by the Mahler dyspnoea index (Weir 1999).
Use of rescue bronchodilators
Only one long‐term study analysed rescue bronchodilator use. In this study, there was no significant difference in use of reliever medication with ICS (Calverley 2003b).
Exercise capacity
Shuttle walking test was measured in one long‐term study (SCO30002 2005) but there was insufficient detail provided in order to analyse for statistical significance.
Medium‐term studies (longer than two months and up to six months)
Three‐month studies
Parallel studies
Mirici et al studied 40 participants using BUD 800 µg/day versus placebo for 12 weeks (Mirici 2001). FEV1 and forced vital capacity (FVC) increased significantly with BUD treatment compared to placebo, despite no change in sputum inflammatory neutrophil or eosinophil counts. Hattotuwa et al randomised 31 participants to FP 1000 µg/day versus placebo for three months in a biopsy study (Hattotuwa 2002). Although no significant differences in lung function or dyspnoea were found, FP improved cough and sputum, and reduced reliever medication use, and there was a reduction in exacerbation rate. Yildiz et al studied 38 participants using BUD 800 µg/day versus placebo (Yildiz 2004). Total and activity scores of the SGRQ improved with BUD, without changes in spirometry or arterial blood gases. In a biopsy study, Bourbeau et al randomised 60 participants to a combination of 50 µg salmeterol and 500 µg FP twice daily, or 500 µg FP twice daily, or placebo (Bourbeau 2007). There was no difference in lung function or health‐related quality of life at three months. One unpublished study (GSK 2005 (FCO30002)) randomised 217 participants from multiple centres to placebo tablets (two weeks) followed by FP 500 µg twice daily (12 weeks), or prednisolone 20 to 40 mg daily plus placebo inhaler (two weeks) followed by FP 500 µg twice daily (10 weeks), or placebo tablets (two weeks) followed by placebo inhaler (12 weeks). There was no statistically significant difference in change in FEV1 in the ICS versus placebo groups.
Cross‐over studies
John et al performed a cross‐over study of 11 participants using HFA‐BDP 800 µg/day versus placebo (John 2005). With HFA‐BDP, spirometry remained unchanged, hyperinflation was reduced (RV/TLC%), and quality of life improved (SGRQ).
Six‐month studies
Parallel studies
Bourbeau et al used BUD 1600 µg/day versus placebo in 79 COPD participants who were non‐responders to oral steroids (Bourbeau 1998). They found no significant differences in lung function, six‐minute walk test, symptoms or quality of life (Chronic Respiratory Questionnaire) with BUD compared to placebo. Paggiaro et al studied 281 participants using FP 1000 µg/day versus placebo. FP treatment was associated with a reduced rate of moderate‐severe exacerbations, improved peak expiratory flow rate (PEFR) and FEV1, increased six‐minute walk distance, and improvement in diary card symptoms (Paggiaro 1998). Senderovitz et al studied BUD 800 µg/day versus placebo in 40 participants and observed no significant differences in median post‐bronchodilator FEV1, exacerbations or symptom scores (Senderovitz 1999). In a study published in abstract form, Laptseva et al treated 49 participants with BUD 800 µg/day versus placebo, and found reduction in moderate‐severe exacerbation rate and improvement in FEV1 (Laptseva 2002). Mahler et al studied 691 participants using FP 1000 µg/day or placebo for 24 weeks (Mahler 2002). FP alone improved FEV1, PEFR, dyspnoea, salbutamol use, night awakenings and quality of life (Chronic Respiratory Disease Questionnaire, Chronic Bronchitis Symptoms Questionnaire (CBSQ)) compared to placebo (Mahler 2002). In a similar study design using half the FP dose (500 µg/day), Hanania et al showed similar results (Hanania 2003). In a biomarker study of BUD 800 µg/day versus placebo in 26 participants, Ozol et al found no improvement in post‐bronchodilator FEV1 or FVC (Ozol 2005). In an unpublished study of 640 participants, there was a statistically significant improvement in pre‐bronchodilator FEV1 for FP 500 µg twice daily versus placebo (GSK 2005 (FLTA3025)), however, this was not shown in the FP 250 µg twice daily group. Tashkin 2008 studied BUD 640 µg/day versus placebo for six months (and included other arms), and found that BUD did not significantly change FEV1 but reduced exacerbations, compared to placebo.
Pooled results
Lung function
Using the generic inverse variance function, we performed pooling for change in pre‐bronchodilator FEV1 in seven medium‐term studies (Bourbeau 1998; Hattotuwa 2002; Mahler 2002; Hanania 2003; GSK 2005 (FCO30002); GSK 2005 (FLTA3025); Tashkin 2008). The mean change in FEV1 was MD 0.04 L in favour of ICS (95% CI 0.03 to 0.06) (Comparison 2.1). Pooling of change in post‐bronchodilator FEV1 from four medium‐term studies (Paggiaro 1998; Mahler 2002; Hanania 2003; Tashkin 2008) showed MD 0.11 L in favour of ICS (95% CI 0.07 to 0.16) (Analysis 2.4). Other studies could not be pooled due to presentation of data as per cent increase (Mirici 2001), pre‐treatment and post‐treatment (Yildiz 2004; John 2005; Ozol 2005), medians (Senderovitz 1999) or summary statement without data (Laptseva 2002).
2.4. Analysis.
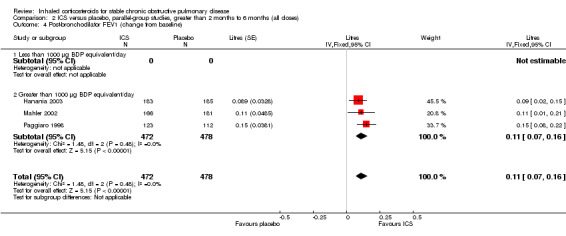
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 4 Post‐bronchodilator FEV1 (change from baseline).
Mortality
Mortality was reported in five medium‐term studies (Hattotuwa 2002; Mahler 2002; Hanania 2003; GSK 2005 (FCO30002); GSK 2005 (FLTA3025)). Pooling of the total number of deaths showed an OR of 0.26 with ICS compared to placebo (95% CI 0.05 to 1.28; 1308 participants) (Comparison 2.8).
Exacerbations
We pooled results for number of participants with at least one exacerbation for five medium‐term studies (Paggiaro 1998; Laptseva 2002; Mahler 2002; Hanania 2003; GSK 2005 (FLTA3025)). One of these studies reported only moderate/severe exacerbations (Laptseva 2002) and the remaining four studies analysed all severities of exacerbations. The pooled OR for having at least one exacerbation during the study period was 0.90 for ICS compared to placebo (95% CI 0.75 to 1.08) (Comparison 2.9). Change in number of exacerbations was reported in several studies (Bourbeau 1998; Senderovitz 1999; Hattotuwa 2002) although not in sufficient detail to pool.
Quality of life and symptoms
Quality of life improved significantly within ICS, as measured by the SGRQ total and activity scores (Yildiz 2004) and symptoms score (John 2005), and by the Chronic Respiratory Questionnaire (Mahler 2002; Hanania 2003). There were no changes in health‐related quality of life as measured by the Chronic Respiratory Questionnaire in one study (Bourbeau 1998).
Results of symptom scores were reported in several medium‐term studies, but numerical data were generally not given in sufficient detail to pool. Cough improved in two studies (Paggiaro 1998; Hattotuwa 2002). Dyspnoea improved in one study (Mahler 2002) and was unchanged in three studies (Paggiaro 1998; Hattotuwa 2002; Hanania 2003). Sputum symptom score improved in two studies (Paggiaro 1998; Hattotuwa 2002) whereas chronic bronchitis symptoms were unchanged in two studies (Mahler 2002; Hanania 2003). Symptoms in general did not change in two studies (Bourbeau 1998; Senderovitz 1999).
Use of rescue bronchodilators
Rescue bronchodilator usage was reduced with ICS in two studies (Hattotuwa 2002; Mahler 2002) but not in the study by Tashkin 2008.
Exercise capacity
There was significant heterogeneity in change in six‐minute walk distance between the two medium‐term studies that measured this outcome (Bourbeau 1998; Paggiaro 1998). When these data were pooled, there was no statistically significant difference found with ICS compared to placebo (MD ‐4 metres, 95% CI ‐50 to 42) (Analysis 2.11).
2.11. Analysis.
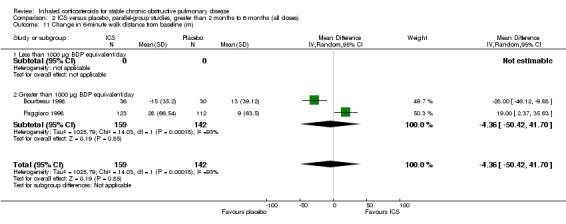
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 11 Change in 6‐minute walk distance from baseline (m).
Short‐term studies (up to two months)
Cross‐over studies
Two‐week studies: Robertson et al studied 83 COPD participants in a cross‐over study of BDP 1500 µg/day versus placebo, and also versus oral prednisolone for two weeks (Robertson 1986). Eighteen per cent of participants (15/83) showed an increase of at least 20% in FEV1, FVC or PEFR over placebo or baseline when taking BDP. In a similar study design, Weir et al studied 127 participants using BDP 1500 µg/day versus placebo (and also a prednisolone arm) for two weeks (Weir 1990a). A few participants had bronchodilator reversibility, and some were non‐smokers. Twenty‐four per cent (8/34) of participants in the first of the cross‐over periods showed at least 20% increased in FEV1, FVC or PEFR from baseline. The effect of BDP on PEFR was still increasing at 14 days, and when withdrawn, the BDP effect was sustained above baseline for at least 14 days (Weir 1990b). Ferreira et al used BDP 1000 µg/day versus placebo in a two‐week cross‐over study of 20 participants (Ferreira 2001). There was no significant difference in FEV1, FVC, bronchodilator response or diffusion capacity (DLCO) with BDP. In a study reported in abstract form, Ferreira et al studied 40 participants with FP 1000 µg/day versus placebo, and observed no significant differences in FEV1, quality of life (Chronic Respiratory Questionnaire (CRQ)) or six‐minute walk test (Ferreira 2003). Guenette et al studied 17 patients using FP 1000 µg/day versus placebo for two weeks, showing improvements in FEV1 and reductions in lung volumes, as well as increased exercise endurance (Guenette 2011).
Four‐week studies: Nishimura et al performed a cross‐over study of BDP 3000 µg/day versus placebo for four weeks in 34 participants (Nishimura 1999). Overall, BDP significantly increased FEV1, FVC and PEFR over placebo. BDP also improved scores of daily symptoms, wheeze and dyspnoea. Culpitt et al studied 13 participants with FP 1000 µg/day versus placebo in a four‐week cross‐over study (Culpitt 1999). There was no significant difference between FP and placebo in terms of FEV1, PEFR, dyspnoea score, cough, sputum production or colour, or days free of relief medication. Brightling et al studied the effect of 400 µg/day of inhaled MF on 49 participants. There was no significant difference in FEV1 between MF and placebo over six weeks (Brightling 2005).
Parallel studies
Two‐week studies: in a study designed to test the effect of FP on systemic inflammation, Sin et al firstly withdrew participants from ICS then used FP 1000 µg/day versus placebo for two weeks, before continuing open‐label FP (Sin 2004). Pre‐bronchodilator FEV1 did not change significantly in the first two weeks, although the authors noted that the study was not primarily designed for this.
Four‐week studies: in a biomarker study, Sin et al studied FP 1000 µg/day versus placebo in 132 patients, as well as a salmeterol/fluticasone arm (Sin 2008). FP improved health status but not FEV1.
Six‐week studies: Thompson et al (Thompson 1992) studied BDP 2000 µg/day versus placebo for six weeks in 30 participants, and found that BDP increased FEV1 by 10%, compared to 3% with placebo, although there was no change in rescue bronchodilator usage.
Eight‐week studies: Llewellyn‐Jones et al (Llewellyn‐Jones 1996) found no significant difference in spirometry or PEFR, when using FP 1500 µg/day versus placebo for eight weeks.
Pooled results
The short‐term studies focused mainly on lung function as an outcome. Pooling of lung function data was not possible, due to different spirometric outcomes measured or missing data. Taken together, these short‐term studies of up to two months ICS in non‐reversible COPD participants were generally of small sample size. The high dose of ICS used in these studies improved FEV1 over the short term in a proportion of participants in some studies (Robertson 1986; Weir 1990a; Thompson 1992; Nishimura 1999; Guenette 2011) but there was no significant difference found in other studies (Llewellyn‐Jones 1996; Culpitt 1999; Ferreira 2001; Ferreira 2003; Sin 2004; Brightling 2005). Symptoms or health status were generally not measured in these short‐term studies; in the studies that did, symptoms or health status were improved (Nishimura 1999; Sin 2008) or unchanged (Culpitt 1999; Ferreira 2003).
Studies in people with COPD with bronchial hyper‐responsiveness or bronchodilator reversibility
Long‐term studies (longer than six months)
Kerstjens et al used BDP 800 µg/day, ipratropium, terbutaline or placebo for 30 months in 274 participants with obstructive airways disease (asthma, asthmatic bronchitis, COPD or undefined diagnosis) (Kerstjens 1992). The COPD subgroup data were analysed in the meta‐analysis by van Grunsven et al (van Grunsven 1999). Data were included from the subgroup of 12 COPD participants (with BHR) who had BDP versus placebo in the Kerstjens study, who met criteria of absence of acute bronchodilator reversibility and other criteria (see 'Discussion').
The Detection, Intervention and Monitoring of COPD and Asthma (DIMCA) trial by van Grunsven et al studied 48 participants with COPD, of whom 27% had BHR (van Grunsven 2003). Participants received FP 500 µg/day or placebo for two years. In the initial three months, there was a benefit in post‐bronchodilator FEV1 of 125 mL. From three months to two years, there were no statistically significant differences in FEV1 decline, symptoms or exacerbations.
Medium‐term studies (longer than two months and up to six months)
Cross‐over studies
Three month studies: Boothman‐Burrell et al studied 18 COPD participants with salbutamol reversibility of less than 25%, in a cross‐over study of BDP 2000 µg/day for three months each treatment period (Boothman‐Burrell 1997). No significant differences in lung function tests were observed with BDP versus placebo. Thompson et al studied 52 participants using FP 880 µg/day versus placebo in a three‐month cross‐over study (Thompson 2002). Sixteen out of 36 participants had bronchodilator reversibility. Pre‐bronchodilator FEV1 improved with FP, as did RV/TLC ratio. PaO2 increased with FP but there was no change in PaCO2 or pH in the arterial blood. A small improvement in dyspnoea was observed in the CRQ quality of life questionnaire. There was no significant difference in the rate of exacerbations or symptoms such as sputum, wheezing or cough (Thompson 2002).
Parallel studies
Six‐month studies: in a study of FP 1000 µg/day versus placebo over six months in 23 COPD participants with BHR, Verhoeven et al found that FP prevented the decline in FEV1 but had no effect on BHR or inflammatory cell indices on bronchial biopsy (Verhoeven 2002).
Short‐term studies (up to two months)
Cross‐over studies
Four‐week studies: Loppow et al investigated FP 1000 µg/day versus placebo in a four‐week cross‐over study in 19 participants with chronic bronchitis (Loppow 2001). Fourteen out of the 19 participants had BHR. No significant differences were found in lung function between the two treatment groups.
Six‐week studies: Weiner et al recruited 30 participants, of whom eight had bronchodilator reversibility. Participants were treated with BUD 800 µg/day or placebo in a six‐week cross‐over study (Weiner 1995). BUD increased FEV1 by at least 20% in six out of eight participants with bronchodilator reversibility, whereas there was no significant increase in FEV1 in participants without bronchodilator reversibility. Rescue bronchodilator usage decreased in those participants who had bronchodilator reversibility and who were taking BUD (Weiner 1995). Weiner et al replicated and extended the study in 168 participants, of whom 44 had bronchodilator reversibility (Weiner 1999). Six‐week cross‐over comparisons were BUD 800 µg/day versus placebo, then BUD 1600 µg/day versus BUD 800 µg/day, then oral prednisone 40 mg/day versus placebo. In the participants with bronchodilator reversibility, there was a significant increase in FEV1 with BUD 800 µg/day, and a decrease in the use of rescue bronchodilators. The higher dose of BUD or prednisone use did not improve the response. Participants without bronchodilator reversibility had no response to any of the active treatments (Weiner 1999).
Eight‐week studies: Wempe et al studied 10 COPD participants with BHR in a cross‐over study of BUD 1600 µg/day versus placebo for eight weeks (Wempe 1992). Oral prednisolone was also included as a separate treatment arm. No change in FEV1 or PC20 was found with BUD in this study.
Parallel studies
Six‐week studies: Rutgers et al examined BUD 1600 µg/day versus placebo for six weeks in 44 moderate‐severe COPD participants with BHR (Rutgers 1998). They found no significant differences in FEV1, PC20 to methacholine or PC20 to adenosine‐monophosphate with BUD compared to placebo. Eight‐week studies: Auffarth et al studied 23 COPD participants with BHR using BUD 1600 µg/day versus placebo for eight weeks in a parallel study (Auffarth 1991). BUD reduced dyspnoea, but there was no change in spirometry, PEFR, PC20 histamine or citric acid cough threshold when compared to placebo.
Pooled results
In these studies of COPD participants with bronchial hyper‐responsiveness or bronchodilator reversibility, pooling was not possible due to the small number of studies and various outcomes measured. Even in this subgroup of COPD participants who could be expected to have a greater benefit from ICS, there was no major effect on lung function. Mortality and exacerbations were generally not reported. There were minor improvements in quality of life and symptoms in a few studies. In general, these studies did not measure use of rescue bronchodilators or exercise capacity.
Predictors of response
Long‐term studies (longer than six months)
Response to BUD was not predicted by gender, smoking or bronchodilator reversibility (Szafranski 2003). In the Copenhagen study, no significant difference in FEV1 decline was noted with gender, smoking status or baseline FEV1 (using a threshold of 70% predicted), although the authors commented that the study was not primarily powered for these subgroup analyses (Vestbo 1999). In the EUROSCOP study, BUD had a more beneficial effect in those participants who had smoked less than the median of 36 pack‐years (Pauwels 1999). No association with response was found with age, gender, baseline FEV1, atopy or bronchodilator reversibility. In the ISOLDE study, the decline in FEV1 with FP versus placebo was not affected by age, smoking status, gender or FEV1 response to oral steroids (Burge 2000; Burge 2003a). Current smokers had a reduced response to oral steroids, compared to ex‐smokers, in COPD participants screened for the ISOLDE study (Burge 2003a).
Medium‐term studies (longer than two months and up to six months)
Senderovitz et al employed response to oral steroids as a predictor of response to ICS (Senderovitz 1999). However, there were too few oral steroid‐reversible participants for analysis. In the remaining participants who were non‐reversible to oral steroids, there was no significant response to BUD 800 µg/day. Bourbeau et al measured response to oral steroids in potential participants then studied only those who had no response to oral steroids (Bourbeau 1998). In these oral steroid non‐responders, there was no significant difference in FEV1 or other secondary measures with BUD 1600 µg/day versus placebo. Paggiaro et al found no baseline predictors of response to FP, except for history of COPD of greater than 10 years (Paggiaro 1998). Mahler et al found that bronchodilator reversibility was associated with slightly better improvements in FEV1 and dyspnoea (Mahler 2002).
Short‐term studies (up to two months)
Some participants responded to either BDP or prednisolone in the cross‐over study by Robertson et al (Robertson 1986), and only a minority of participants responded to both. Weir et al similarly showed that there were some responders to either BDP or prednisolone, with some full or partial responders to each (Weir 1990a). The presence of bronchodilator reversibility did not predict the presence of response to BDP or prednisolone (Weir 1990a). Smoking history and the presence of emphysema had no influence on being a responder (Weir 1990b; Weir 1991). There was a weak correlation (r = 0.38) between peripheral eosinophilia and response to high dose BDP in the study by Nishimura et al, whereas there was no correlation with other factors such as bronchodilator reversibility, total serum IgE or smoking history (Nishimura 1999).
Bronchodilator reversibility was found to be a predictive factor for response to ICS in the study by Weiner et al (Weiner 1995). They found that 25% of non‐reversible COPD participants increased their FEV1 significantly with BUD, and this response rate increased to 75% if bronchodilator reversibility was present. These results were replicated in a later study by the same group (Weiner 1999). A moderate correlation (r = 0.53) was observed between FEV1 response to FP and bronchodilator reversibility (Thompson 2002). However, some participants with a substantial response to FP had no bronchodilator reversibility, which therefore did not exclude the possibility of a spirometric response to FP. Brightling et al observed that higher sputum eosinophilia was associated with a greater mean change in post‐bronchodilator FEV1 with inhaled MF, although there was no fall in sputum eosinophil count with MF (Brightling 2005).
Biomarker studies
Biopsy studies
Hattotuwa et al studied the effect of FP 1000 µg/day versus placebo on bronchial inflammation in 37 participants (Hattotuwa 2002). At three months, FP reduced mast cell numbers in the subepithelium and reduced the CD8:CD4 ratio in the epithelium. There was some improvement in symptoms but lung function was unchanged. Reduction in mucosal mast cell numbers was also shown by transmission electron microscopy in biopsies from the same study (Gizycki 2002). There was no change in eosinophil numbers in the biopsies (Gizycki 2002). It is unclear how the reduction in mast cell numbers relates to changes in symptoms, although it has been postulated that mast cells may be involved in mucus hypersecretion, and that reduction of mast cell numbers could contribute to the short‐term improvements that are seen initially with ICS (Gizycki 2002). FP also apparently increased the number of neutrophils in the biopsies (Gizycki 2002). In a study of FP 1000 µg/day versus placebo over six months in 23 COPD participants with BHR, Verhoeven et al found no effect on inflammatory cell indices on bronchial biopsy (Verhoeven 2002). There were also no detectable effects on reactive oxygen species production from inflammatory cells in the bronchoalveolar lavage (BAL) (Verhoeven 2000), although some reduction in arachidonic acid metabolites was observed (Verhoeven 2001). FP 1000 µg/day for three months did not significantly change counts of CD8+ lymphocytes or CD68+ macrophages in bronchial biopsies, compared to placebo (Bourbeau 2007). However, a biopsy study at 30 months of FP 1000 µg/day showed reductions in CD4+ and CD8+ lymphocytes, reduction in mast cells, increase in eosinophils and increase in intact bronchial epithelium, as well as reduced sputum neutrophils, macrophages and lymphocytes (Lapperre 2009).
Induced sputum
Llewellyn‐Jones et al measured sputum markers of inflammation (Llewellyn‐Jones 1996). FP reduced the chemotactic activity of the sputum sol phase, and increased the capacity of the sputum to inhibit neutrophil elastase. There were no significant differences in sputum/serum albumin ratio, sputum myeloperoxidase concentration or peripheral blood neutrophil function (Llewellyn‐Jones 1996). Culpitt et al measured inflammatory indices in induced sputum in a cross‐over study of FP 1000 µg/day for four weeks (Culpitt 1999). FP did not alter sputum total cell count, neutrophil count or eosinophil count. There were no changes in sputum IL‐8, MMP‐1, MMP‐9, TIMP‐1, SLPI or elastase activity (Culpitt 1999). The authors concluded that FP had no anti‐inflammatory effect in stable COPD. Mirici et al performed a 12‐week study of BUD 800 µg/day versus placebo in 50 participants (Mirici 2001). They showed an improvement in FEV1 of 7.4% predicted with BUD, compared to 0.7% predicted in the placebo group (P < 0.01). There was an increase in sputum macrophages but no change in sputum neutrophils with BUD, compared to placebo (Mirici 2001). Brightling et al examined the short‐term response to six weeks of inhaled MF 800 µg/day (Brightling 2005). There were no treatment associated changes in sputum characteristics including eosinophil counts, histamine, IL‐8 and ECP.
Exhaled nitric oxide (NO)
In a cross‐over study of 20 participants, Ferreira et al found that BDP 1000 µg/day for two weeks resulted in a fall in median exhaled nitric oxide concentration, compared to placebo (Ferreira 2001). There were no changes in hydrogen peroxide in the exhaled breath condensate or lung function. The authors suggested that exhaled nitric oxide could be useful in predicting which participants would have an FEV1 response to ICS.
Bronchoalveolar lavage (BAL)
Thompson et al performed bronchoscopy before and after six weeks of BDP or placebo in 30 participants with chronic bronchitis (Thompson 1992). In the BAL‐, BDP reduced cellularity, decreased levels of albumin (indicating reduced epithelial permeability), and decreased levels of lactoferrin and lysozyme (indicating reduced airway epithelial secretion). These results suggested the BDP was having an anti‐inflammatory effect in these participants with chronic bronchitis. Ozol et al studied the effect of BUD 800 µg/day versus placebo for six weeks on BAL IL‐8 and cell counts (Ozol 2005). BUD treated participants were found to have a statistically significant effect on markers on BAL‐ neutrophil counts and IL‐8. These findings did not correlate with reported symptoms as only borderline improvements in sputum production and lung function were reported.
Systemic inflammation
Sin et al studied systemic inflammation in 41 mild to moderate COPD participants (Sin 2004). Withdrawal of ICS from COPD participants resulted in an increase in C‐reactive protein (CRP), a marker of systemic inflammation. Addition of FP 1000 µg/day for two weeks decreased CRP by 50%, and a further eight weeks of FP reduced the CRP to below the baseline levels. In another study, Sin et al found that FP 1000 µg daily did not significantly effect the generalised biomarkers of C‐reactive protein and IL‐6, but did significantly reduce the lung‐specific biomarker, surfactant protein D (Sin 2008). John et al studied three months treatment with HFA‐BDP 800 µg/day, compared to placebo, in 11 participants. The HFA‐BDP did not alter cytokine production from peripheral blood mononuclear cells (no change in IL‐10, IFN‐ , GM‐CSF and MIP‐1) (John 2005). A systematic review has been performed for changes in sputum cell counts with ICS (Gan 2005) (see 'Discussion').
Side effects
Local steroid side effects
Long‐term studies (longer than six months)
Pooling of available data in the long‐term studies showed an increased risk of oropharyngeal candidiasis with ICS (OR 2.65, 95% CI 2.03 to 3.46, 5586 participants) (Analysis 1.15). For participants randomised to less than 1000 µg/day BDP equivalent this gave a number needed to treat to harm NNT(h) of 37. In studies assessing more than 1000 µg/day BDP equivalent, there was some variation in baseline risk. In participants from the control group of Burge 2000 risk was around 7%, and NNT(h) for participants randomised to steroid was 13 (95% CI 7 to 34), whereas in Calverley 2003a the control group event rate was 1.4%, giving a NNT(h) of 57 (95% CI 29 to 156). In Calverley 2008, the event rate was 11% amongst those randomised to ICS giving a NNT(h) of 13. There was also an increased risk of hoarseness or dysphonia (OR 1.95, 95% CI 1.41 to 2.70, 3267 participants) (Comparison 1.16). There was minimal heterogeneity, implying a consistent effect across the studies.
1.15. Analysis.
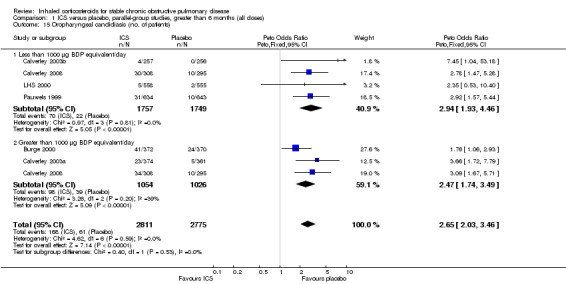
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 15 Oropharyngeal candidiasis (no. of patients).
Medium‐term studies (longer than two months and up to six months)
Pooling of the medium‐term studies showed an increased risk of oropharyngeal candidiasis (OR 5.59, 95% CI 3.58 to 8.74, 2109 participants) (Comparison 2.18). Similarly there was an increase in hoarseness or dysphonia (OR 4.21, 95% CI 2.17 to 8.17, 1520 participants) (Comparison 2.19). There was a milder increase in throat irritation (OR 1.61, 95% CI 1.09 to 2.37, 1572 participants), although there was some heterogeneity between studies (Comparison 2.17).
Short‐term studies (up to two months)
Hoarseness and sore throat were more common with very high‐dose BDP (3000 µg/day) over four weeks (Nishimura 1999). FP 880 µg/day increased the risk of hoarseness (Thompson 2002).
Bone turnover and fractures
Long‐term studies (longer than six months)
In the EUROSCOP study, there was no significant increased risk of vertebral fractures or osteoporosis in the participants treated with BUD (Pauwels 1999; Johnell 2002). In the ISOLDE study, there was no significant increase in the rate of fractures of any type (Burge 2003a). In the LHS II, a significant reduction in bone mineral density in the lumbar spine and femoral neck was measured in the group taking TAA, compared to placebo (LHS 2000; Scanlon 2004). In the TORCH study, there was no statistically significant difference in rate of fractures between FP and placebo over three years, and in a sub‐study there was no statistically significant difference in bone mineral density (Calverley 2007). Pooling of available data on fractures from studies of a duration of one year or longer found no increase in the risk of fractures (OR 1.00, 95% CI 0.75 to 1.32, 5226 participants) (Comparison 1.21).
Short‐term studies (up to two months)
Very high‐dose BDP (3000 µg/day) reduced serum osteocalcin, compared to placebo (Nishimura 1999).
Cortisol
Long‐term studies (longer than six months)
Serum cortisol did not differ at the end of two years of therapy with BUD 1600 µg/day versus placebo (Renkema 1996). The number of participants whose serum cortisol changed from normal to below normal did not differ between FP 1000 µg/day versus placebo (Calverley 2003a). In the ISOLDE study, there was a small decrease in mean serum cortisol with FP, compared to placebo (Burge 2003a). In the Lung Health Study II, TAA 1200 µg/day over three years did not significantly suppress baseline cortisol levels function or diminish adrenal responsiveness to cosyntropin stimulation (Eichenhorn 2003).
Medium‐term studies (longer than two months and up to six months)
Serum cortisol was lower with six months of FP 1000 µg/day compared to placebo (Paggiaro 1998).
Short‐term studies (up to two months)
The use of very high‐dose BDP (3000 µg/day) over four weeks reduced serum cortisol levels in the study by Nishimura et al, but serum cortisol also decreased during the placebo period (Nishimura 1999). FP 880 µg/day over three months reduced pre‐ and post‐ adrenocorticotropic hormone (ACTH) cortisol levels, but there was no significant difference in the number who passed the ACTH stimulation test (Thompson 2002).
Pneumonia
In the long‐term studies (longer than six months), the rate of pneumonia was increased in the ICS group compared to placebo, in six studies that reported pneumonia as an adverse event (OR 1.56, 95% CI 1.30 to 1.86, 6235 participants) (Comparison 1.25). The statistically significant association was in the studies using ICS > 1000 µg BDP equivalent/day, whereas there was no statistically significant association in the ICS < 1000 µg BDP equivalent/day group.
Other effects
Skin bruising was increased with BUD in the EUROSCOP study (Pauwels 1999) and there were trends to increased skin bruising in other long‐term studies (Burge 2000; LHS 2000; Calverley 2003a; Calverley 2008). Overall, the pooled OR for skin bruising with ICS was 1.63 (95% CI 1.31 to 2.03, 5073 participants). In the LHS, there was no overall difference in bruising or cataracts with TAA (LHS 2000). However, Tashkin et al, as part of the LHS II, found that amongst those participants who were adherent to ICS, a significantly higher proportion of participants reported easy bruising and slow healing of cuts or sores (LHS 2000). There was no increase in the rate of cataract formation (Burge 2003a; Calverley 2007).
Discussion
This systematic review of inhaled corticosteroids (ICS) for chronic obstructive pulmonary disease (COPD) has analysed the following outcomes:
Lung function: Long‐term use of ICS (more than six months) did not consistently reduce the rate of decline in forced expiratory volume in one second (FEV1) in COPD patients (generic inverse variance analysis: mean difference (MD) 5.80 mL/year with ICS, 95% confidence interval (CI) ‐0.28 to 11.88, 2333 participants; pooled means analysis: 6.88 mL/year, 95% CI 1.80 to 11.96, 4823 participants).
Mortality: Long‐term use of ICS had no statistically significant effect on mortality in COPD patients (odds ratio (OR) 0.98, 95% CI 0.83 to 1.16, 8390 participants).
Exacerbations: Long‐term use of ICS reduced the mean rate of exacerbations in those studies where pooling of data was possible (generic inverse variance analysis: MD ‐0.26 exacerbations per patient per year, 95% CI ‐0.37 to ‐0.14, 2586 participants; pooled means analysis: MD ‐0.19 exacerbations per patient per year, 95% CI ‐0.30 to ‐0.08, 2253 participants).
Quality of life and symptoms: ICS slowed the rate of decline in quality of life, as measured by the St George's Respiratory Questionnaire (SGRQ) (MD ‐1.22 units/year, 95% CI ‐1.83 to ‐0.60, 2507 participants).
Rescue bronchodilator use: There was a reduction in rescue bronchodilator use in some medium‐term studies.
Exercise capacity: This outcome was generally not measured.
Biomarkers: The relatively few studies that measured airway biomarkers showed a mixed response to ICS, with only some studies demonstrating an anti‐inflammatory effect of ICS.
Predictors of response: Response to ICS was not predicted by oral steroid response, bronchodilator reversibility or bronchial hyper‐responsiveness in COPD patients.
Side effects: ICS increased the risk of oropharyngeal candidiasis (OR 2.65, 95% CI 2.03 to 3.46, 5656 participants) and hoarseness. The few long‐term studies that measured bone effects showed generally showed no major effect on fractures and bone mineral density over three years. In long‐term studies that reported pneumonia as an adverse event, the rate of pneumonia was increased in the ICS group (OR 1.56, 95% CI 1.30 to 1.86, 6235 participants).
Lung function
Change in lung function was the primary outcome of the majority of long‐term studies; hence, this was the a priori primary outcome of this systematic review. The question of effect of ICS on progression of airflow limitation has been addressed in a number of systematic reviews. The first systematic review was performed by van Grunsven et al (van Grunsven 1999). This meta‐analysis combined data from two‐year studies by Renkema et al (Renkema 1996) and Derenne (Derenne 1995), and a subgroup of the study by Kerstjens et al (Kerstjens 1992). They included individual patient data from these studies, and applied stricter criteria for COPD, consisting of pulmonary symptoms compatible with COPD, age 40 and over, persisting airflow obstruction post‐bronchodilator, lack of reversibility to bronchodilator, and presence of smoking history. From the two‐year Renkema et al study of 39 patients having budesonide (BUD) 1600 µg/day versus placebo (Renkema 1996), 30 were eligible for the meta‐analysis. From the 30‐month Kerstjens et al study of 51 COPD patients with bronchial hyper‐responsiveness (BHR) having 800 µg/day versus placebo (or ipratropium, which was counted as "placebo") (Kerstjens 1992), 15 were eligible. The previously unpublished details of the study by Derenne 1995 were reported in the meta‐analysis. Beclomethasone dipropionate (BDP) 1500 µg/day versus placebo was assessed over two years in 194 patients with moderate to severe COPD. Of these patients, 152 were eligible. Overall, the meta‐analysis of these three studies found no benefit for change in post‐bronchodilator FEV1 with ICS, although there was a small benefit for change in pre‐bronchodilator FEV1 (van Grunsven 1999). The benefit was significant for higher doses of ICS; however, there were few patients receiving the lower dose. A review by Riancho 2002, published in the Spanish language, pooled short‐term studies and found a small increase of 96 mL FEV1 over one to six months. They observed that there was a small difference in FEV1 of 51 mL in favour of ICS after one to three years of continued treatment. They concluded that ICS were probably not of benefit in patients with non‐asthmatic COPD. In a systematic review of studies published up to 2001, Alsaeedi et al (Alsaeedi 2002) were unable to pool data for decline in FEV1 due to lack of standard deviations for some of the studies.
Highland et al (Highland 2003) reviewed the long‐term effects on FEV1 in studies published up to 2002. These reviewers pooled data for rate of decline in FEV1 for six long‐term studies, and found a non‐statistically significant difference of 5.0 mL/year with ICS (P = 0.11; 3571 participants). They concluded that there was no effect of ICS on long‐term decline in FEV1. The Highland et al meta‐analysis was subsequently corrected by the authors, giving a MD of 5.31 mL/year (95% CI ‐0.64 to 11.2) (P = 0.08) (erratum in Ann Intern Med 2003;139(10):873). The accompanying editorial suggested that heterogeneity in inflammatory responses may explain some of the discordance between short‐term clinical and long‐term FEV1 responses (Epstein 2003). In a similar analysis, Sutherland et al (Sutherland 2003) pooled data for rate of decline in FEV1 for long‐term studies published up to early 2003. In contrast to the Highland et al meta‐analysis, the Sutherland et al meta‐analysis showed that ICS reduced the rate of FEV1 decline by 7.7 mL/year (P = 0.02; 3715 participants), and the effect was greater for higher doses of ICS. They concluded that ICS may potentially have important long‐term effects in COPD. The accompanying editorial (Burge 2003b) elucidated the possible differences between the meta‐analyses of Highland et al and Sutherland et al. With hypothetical adjustments to achieve more concordance in the data, the editorial by Burge and Lewis (Burge 2003b) showed that the Highland et al effect size would have been 5.5 mL/year (P = 0.07), compared to the Sutherland et al effect size of 7.7 mL/ year (P = 0.02). They pointed out that these mean effect sizes and P values were not too dissimilar. In our meta‐analysis, the effect size of 5.8 mL/year using the generic inverse variance analysis was between the two effect sizes found by Highland et al and Sutherland et al, as was our P value of 0.06 for this analysis. The main factors that explain the small differences between these effect sizes include (I) interpretation of numerical data, i.e. whether the direction of improvement in the Vestbo et al study (Vestbo 1999) was positive or negative (Burge 2003b), (ii) inclusion/exclusion of the EUROSCOP study which presented median values (Pauwels 1999), (iii) inclusion/exclusion of the van Grunsven et al meta‐analysis, and (iv) calculation of missing standard deviations.
In the Inhaled Steroids Effect Evaluation in COPD (ISEEC) study, Soriano et al (Soriano 2007) pooled data from seven randomised controlled trials of ICS (3911 participants) versus placebo lasting ≥ 12 months in patients with moderate to severe COPD. Studies included were LHS‐2, CCLS (Vestbo 1999), ISOLDE (Burge 2000), EUROSCOP (Pauwels 1999), TRISTAN (Calverley 2003a), Szafranski 2003 and Calverley 2003b. These authors found that in the first six months, ICS was associated with a significant mean increase in FEV1 (mean change in FEV1 2.42%, SE 0.19%, P < 0.01), and was more effective in ex‐smokers (compared to current smokers) and women. However, for use of ICS in studies longer than six months, their systematic review found that ICS did not significantly improve FEV1 decline (mean change in FEV1 ‐0.01%, standard error (SE) 0.09%, P = 0.86).
In our updated systematic review, we pooled data for rate of FEV1 decline in the long‐term studies (> six months duration) using two statistical approaches, depending on reporting of data in the studies. The generic inverse variance analysis did not show a statistically significant difference in rate of FEV1 decline calculated from baseline to study completion; however, the pooled means analysis of 4781 participants, which included the large TORCH study (Calverley 2007; Celli 2008), found a relatively small but statistically significant difference of 6.88 mL/year benefit, albeit with a wide confidence interval. In some of the medium‐term studies (greater than two months and up to six months), there were small improvements in pre‐ and post‐bronchodilator FEV1 in favour of ICS.
Whether objective physiological measures are the best outcomes in COPD studies is still contentious. Furthermore, even if physiology is the optimal outcome, other measures such as inspiratory capacity may correlate better with subjective outcomes, compared to FEV1. However, FEV1 has been shown to be a prognostic factor in COPD, and remains the defining criterion for the diagnosis and severity of COPD. Hence lung function was the primary outcome of interest in the majority of the long‐term trials. The clinically important difference in change in rates of FEV1 decline is not yet clearly known. As discussed by others, a difference in rate of decline in FEV1 of magnitude ˜6 mL/year could be considered clinically unimportant when compared to a current smoker rate of 60 mL/year, and clinically important when compared to a non‐smoker rate of 30 mL/year (Burge 2003b). Another issue is that excessive dropouts from the placebo group who have rapid decline may mean that the effect size of active treatment is underestimated, because the remaining participants in the placebo group have less rapid decline (Calverley 2003d), although attrition bias could affect decline in the opposite direction (Suissa 2008). It has also been debated as to whether the small improvement on FEV1 observed in some short and medium‐term studies is of clinical importance (Burge 2003b).
Taking these considerations into account, our systematic review has found that use of ICS alone in COPD patients results in a small, initial improvement in FEV1, and then no consistent improvement in the long‐term rate of decline in FEV1, although long‐term use of ICS > 1000 µg BDP daily equivalent may be associated with a small improvement in the rate of decline in post‐bronchodilator FEV1.
Mortality
Mortality is a major health outcome in COPD. Of the long‐term studies, only Calverley 2007 was designed to study the effect of ICS on mortality as a primary outcome; hence, we analysed mortality as a secondary outcome. In this current review, the available mortality data from nine long‐term studies involving long‐term use of ICS had no statistically significant effect on mortality (OR 0.98 for mortality, 95% CI 0.83 to 1.16, P = 0.84, 8390 participants). The data from five medium‐term studies also showed no statistically significant effect on mortality.
Observational studies have found reduced mortality with the use of ICS in COPD patients (Sin 2001; Sin 2003a; Soriano 2003; Mapel 2006), including reduction in cardiovascular deaths (Macie 2006). Various epidemiological issues arising from these observational studies have been discussed, including immortal time bias, which is the issue of unaccounted‐for survival time in the 'treatment' group before they actually received treatment (Suissa 2003; Suissa 2004).
The effect of ICS on mortality in COPD patients has been addressed by recent meta‐analyses. Alsaeedi et al (Alsaeedi 2002) found a non‐significant relative risk of 0.84 (95% CI 0.60 to 1.18, 3473 participants) in five long‐term studies published up to 2001. The systematic review by Gartlehner et al of 12 studies published up to early 2005 observed a non‐significant relative risk of 0.81 (95% CI 0.60 to 1.08, 4370 participants) (Gartlehner 2006).
The systematic review by Sin et al, using individual patient data from seven studies up to 2005 involving 5085 patients (Sin 2005), found a mortality benefit with ICS in COPD. The adjusted hazard ratio for all‐cause mortality from their review was 0.73 (95% CI 0.57 to 0.99, P = 0.03, 5085 participants). Their review found that the mortality benefit with ICS was stronger in specific subgroups: females, former smokers and patients with baseline post‐bronchodilator FEV1 less than 60% predicted. The systematic review of Sin et al had the methodological strength of access to individual patient data, in order to adjust for age, sex, baseline lung function, smoking status and body mass index (Wedzicha 2005). Hence they were able to present adjusted hazard ratios across the individual trials. As discussed in the editorial accompanying the Sin et al meta‐analysis (Wedzicha 2005), the effect sizes of ICS in various meta‐analyses appeared to be similar across several major outcomes, e.g. ˜25% reduction in exacerbations, 25% improvement in rate of decline of FEV1 (compared to the non‐smoker rate) and 27% reduction in mortality from the Sin et al review.
Our current review has found no significant difference in mortality rate with the use of ICS as a mono‐component versus placebo. This lack of effect was found both in the long‐term studies published prior to the TORCH study, and in all long‐term studies pooled including TORCH. The TORCH study itself, which was the largest of the long‐term studies, found no mortality benefit with fluticasone propionate (FP) as a mono‐component, although the combination of salmeterol/fluticasone potentially reduced mortality (Calverley 2007). Limitations of pooling data from the long‐term studies have been discussed in detail by others, including the use of intention‐to‐treat analysis versus completed participants in some studies, different run‐in protocols and differential effect of dropouts (Wedzicha 2005). Even given these limitations, the pooled data indicate no statistically significant mortality benefit for ICS given as a mono‐component. The meta‐analysis by Drummond 2008 of 11 studies (14,426 participants), including studies of combination inhaler versus long‐acting beta2‐agonist (LABA), found no difference in mortality rate at one year.
Exacerbations
Acute exacerbations are an important cause of morbidity and mortality in COPD patients (Donaldson 2006). In our current review, data were available for pooling in some of the long‐term and medium‐term studies. Where pooling of data was possible in the long‐term studies, there was a statistically significant benefit of ICS in reducing the mean rate of exacerbations (generic inverse variance analysis: MD ‐0.26 exacerbations per patient per year, P < 0.0001, 2586 participants; pooled means analysis: MD ‐0.19 exacerbations per patient per year, 95% CI ‐0.30 to ‐0.08, 2253 participants).
In a systematic review of studies up to 2001, Alsaeedi et al pooled the total COPD exacerbation rates, by calculating the frequency of COPD exacerbations per patient‐month of treatment (Alsaeedi 2002). Their review found a significant benefit of ICS for reducing exacerbations (risk ratio (RR) 0.70, 95% CI 0.58 to 0.84, 2615 participants). In the systematic review of studies up to early 2005, Gartlehner et al found a reduction in the rate of COPD exacerbations with ICS (RR 0.67, 95% CI 0.59 to 0.77, 4300 participants) (Gartlehner 2006). This effect size was based on data pooled from both medium and long‐term studies. In their review, the benefit was mainly in the moderate to severe COPD subgroup. Variations in the approach to analysis are seen in the systematic reviews of Alsaeedi et al and Gartlehner et al, compared to our review. Their data were primarily analysed in terms of relative risk, rather than differences in mean rates of exacerbations. Furthermore, there were some minor differences with our review in terms of the selection of studies for inclusion and the particular exacerbation outcome extracted. The meta‐analysis by Agarwal 2010 found a small but statistically significant reduction in the risk of exacerbations (RR 0.82, 95% CI 0.73 to 0.92, 8164 participants), when analysing risk of exacerbations, as opposed to exacerbations per patient per year. This was similar to our result of OR 0.83 when analysing risk of exacerbations (Comparison 1.8).
Methodological issues have recently been discussed in detail in relation to analysis of exacerbation rates. It has been suggested that accounting for the length of follow‐up time of each participant (weighted approach) is a potentially less biased method of analysis than not accounting for this (unweighted approach) (Suissa 2006). To explore this, we stratified the long‐term studies by whether they used the weighted or unweighted approach (Comparison 1.7). In both approaches, a statistically significant reduction in exacerbations was observed. Variation in the definition of exacerbations and in study design (for example, run‐in periods) may also account for differences between study outcomes in relation to exacerbation rates (Scott 2006). Cohort studies have observed populations of frequent and non‐frequent exacerbators (Vestbo 2011). The presence of these distinct patient groups may lead to a bimodal, non‐normal distribution of exacerbation rates, making analysis more complex (Scott 2006). Therefore, it may be that ICS can reduce the number of exacerbations, particularly in frequent exacerbators (that is, frequent exacerbators have a reduction in the number of exacerbations) yet may not reduce the percentage of patients with one or more exacerbations (i.e. the frequent exacerbators do not become non‐frequent exacerbators). Other methodological issues raised included the discontinuation of ICS in patients who are already taking these prior to commencement of a trial, and lack of complete follow‐up of exacerbations on an intention‐to‐treat basis in those participants who have withdrawn from the study (Suissa 2008a), which could both overestimate the observed benefits of ICS.
Even taking into account these issues, and also the lack of available data to pool in some of the medium and long‐term studies, our systematic review has observed a statistically significant reduction in the mean exacerbation rate per year with ICS. The magnitude of the effect is relatively small yet potentially important, given that frequent exacerbations worsen lung function decline (Kanner 2001; Donaldson 2002; Vestbo 2011) and reduce quality of life.
Quality of life and symptoms
Improving patient‐centred, subjective outcomes is an important goal in the management of COPD. This systematic review found that, in those long‐term studies which measured quality of life and where data could be pooled, ICS slowed the rate of decline in quality of life, when measured with the SGRQ. The magnitude of this benefit was relatively small (MD ‐1.22 units/year), compared to the minimum clinically significant difference of 4 units with the SGRQ. The effect on quality of life appeared linear, based on graphical analysis of change in quality of life scores in each study (data not shown). Medium‐term use of ICS tended to improve quality of life, although pooling of data was not possible. Some medium‐term studies showed that an improvement in respiratory symptoms, but not all studies were able to demonstrate this. In the systematic review by Gartlehner et al, quality of life was examined qualitatively and was not pooled due to heterogeneity (Gartlehner 2006). Overall our review showed a small yet statistically significant benefit for quality of life using ICS. It is not clear whether this benefit is related to other benefits such as reduced frequency of exacerbations.
Rescue bronchodilator use
There was reduction of rescue bronchodilator use found in some medium‐term studies, although the data could not be pooled. Rescue bronchodilator use was generally not measured in the long‐term studies.
Exercise capacity
This outcome measure was only infrequently measured, and overall no significant difference was found with ICS.
Biomarkers
There have been relatively few randomised, controlled trials of the effects of inhaled steroid on biomarkers in COPD. Due to the heterogeneity of outcomes used, we performed a narrative review of these biomarker studies. Some studies observed reductions in airway neutrophil counts and other anti‐inflammatory effects such as reduced exhaled nitric oxide. Other studies found no significant difference in inflammatory cells in the airways (as measured in biopsies, induced sputum and BAL fluid). Overall, this qualitative review showed a mixed response of airway biomarkers to ICS in COPD patients. This could partially be explained by the relative steroid resistance due to reduced histone deacetylase activity in COPD lungs (Barnes 2004; Ito 2005).
A systematic review was performed by Gan et al of the effects of ICS on sputum cell counts (Gan 2005). Gan et al found that longer treatment duration (more than six weeks) or higher dose (> 60 mg cumulative dose) resulted in greater reductions in sputum total cell count, neutrophil count and lymphocyte count, with no significant differences in macrophages or eosinophil counts (Gan 2005). Due to differences in reporting study outcomes, they analysed standardised mean differences. Some of their nine included studies were double‐blinded and other studies were single‐blinded or open‐label studies, which differs from our review in that only double‐blinded studies were included in our review. Another report has suggested that ICS may have an important effect on systemic inflammation in COPD (Man 2005a).
Predictors of response
Predicting the response to ICS in COPD patients is a clinically useful goal, in order to individualise treatment. Clinical indicators such as oral steroid responsiveness or current smoking did not adequately predict responders to ICS. Some studies included COPD patients with bronchodilator reversibility or bronchial hyper‐responsiveness, although the number of studies and their sample sizes were small. The presence of bronchodilator reversibility and bronchial hyper‐responsiveness in COPD generally did not predict responsiveness to ICS. Therefore, the challenge remains to identify clinical or biological factors that predict those COPD patients who are more likely to benefit from long term ICS.
Side effects
The safety aspects of ICS are important in the long term, especially in older COPD patients with co‐morbidities. As expected, our review found an increased risk of local side effects such as oropharyngeal candidiasis. The systemic effects of prolonged use were less clear, with several studies showing no change in fracture rate or bone mineral density (Pauwels 1999; Johnell 2002; Burge 2003a; Calverley 2007), whereas one study using a smaller dose of ICS showed a reduction in bone mineral density (LHS 2000). The systematic review by Alsaeedi et al noted that there was an increase in oropharyngeal candidiasis and skin bruising, and variable effects on bone mineral density (Alsaeedi 2002). The systemic review by Gartlehner et al observed that there were variable results on bone mineral density and risk of fractures, including in some case‐control studies (Gartlehner 2006). The meta‐analysis by Loke 2011 pooled results from randomised controlled trials (RCTs) of ICS or combination inhalers, and observational studies, and found a small but statistically significant increase in risk of fractures with ICS use in COPD. Our review found no statistically significant increase in fracture risk in studies of ICS as a mono‐component versus placebo for one year or longer.
In the long‐term studies, the rate of pneumonia as an adverse event was increased in the ICS group, in the six studies that reported this outcome (OR 1.56, 95% CI 1.30 to 1.86, 6235 participants). The TORCH study observed a reduction in rate of exacerbations, but also an increase in self reported pneumonia in the adverse events and serious adverse events (Calverley 2007; Crim 2009), although radiological or microbiological confirmation was not required. In a meta‐analysis using patient‐level data and adjusting for clinical confounders, Sin et al found no increased risk of pneumonia at one year of budesonide (or budesonide/formoterol) use in COPD (adjusted hazard ratio 1.05, 95% CI 0.81 to 1.37, 7042 participants) (Sin 2009). In contrast, two meta‐analysis found similar results to our pooled result of increased risk of pneumonia. The meta‐analysis by Drummond 2008 of any ICS (including in combination inhalers) found an increase in risk of pneumonia (RR 1.34, 95% CI 1.03 to 1.75). Similarly the meta‐analysis by Singh 2009 found an increased risk of pneumonia with any ICS use (including in combination inhalers) for at least 24 weeks in COPD, with RR 1.60 (95% CI 1.33 to 1.92), but no increase in pneumonia‐related mortality or overall mortality.
The mechanisms for this potential increase in pneumonia are unclear. In the two‐year INSPIRE study of salmeterol/fluticasone versus tiotropium, the number of de novo pneumonias not preceded by symptoms of exacerbations were similar between the two treatment groups (Calverley 2011). However, unresolved exacerbations preceding pneumonia were more common in the salmeterol/fluticasone‐treated patients (32 exacerbations in 658 patients), compared to the tiotropium‐treated group (seven exacerbations in 665 patients). Future studies should prospectively confirm the diagnosis of pneumonia using clinical and radiological evidence (Welte 2009). Until the risk of pneumonia is confirmed, clinicians should be vigilant to the development of pneumonia in COPD patients, particularly those with prolonged exacerbations.
Interpretation of results
In interpreting the results of this systematic review, a number of clinical and epidemiological issues should be considered. There was wide variability in study characteristics, including dose and duration of ICS, severity of COPD, inclusion criteria (e.g. current or ex‐smokers, bronchial hyper‐responsiveness, bronchodilator reversibility) and outcomes studied. Furthermore, results for outcomes were sometimes either missing or could not be pooled (e.g. non‐parametric data; continuous versus categorical classification of similar outcomes such as change in FEV1). Subgroup analysis, whilst potentially useful, was not possible due to lack of individual data. With the use of the generic inverse variance function, it was possible to pool data for some of the medium‐term and long‐term studies. Side effects were measured in most of the studies, although even longer studies would probably be required to determine the rates of adverse effects such as vertebral fractures and cataracts. The ICS only studies reviewed here should be interpreted in the light of the newer studies using combination inhalers of LABA/ICS, which may be more effective than ICS alone in COPD. Finally, participants who withdrew from the placebo arm of the long‐term ICS studies may have been those patients who were deteriorating the most rapidly, which may underestimate the effect of active treatment (Calverley 2003d).
Conclusions
This systematic review has analysed all relevant published and unpublished RCTs of inhaled steroids used as a mono‐component, in patients with stable COPD. Despite variability in study design, interventions and outcomes used, pooling of data was possible for important outcomes, particularly in the long‐term studies. Inhaled steroids had a beneficial effect on frequency of COPD exacerbations and rate of decline of quality of life, whereas they did not appear to have consistent effects on lung function decline or mortality in COPD. Local side effects (oropharyngeal candidiasis and hoarseness) were increased, and the risk of pneumonia was possibly increased. Therefore clinicians and patients should balance the potential benefits of inhaled steroids in COPD (possible reduction in rate of lung function decline, reduced exacerbations, reduced rate of decline in quality of life) against the potential side effects (local oropharyngeal effects and increase in risk of pneumonia).
Authors' conclusions
Implications for practice.
We are able to make several statements on the efficacy and safety of long‐term use of inhaled steroids in people with COPD:
Long‐term use of inhaled steroids as a mono‐component has not been shown to consistently reduce the rate of decline in FEV1 in COPD patients.
Long‐term use of inhaled steroids as a mono‐component does not significantly reduce mortality in COPD.
Long‐term use of inhaled steroids reduces the mean rate of COPD exacerbations per patient per year.
Inhaled steroids slow the rate of decline in quality of life in COPD.
No factors adequately predict response to inhaled steroids in COPD.
Local side effects are increased with use of inhaled steroids in COPD patients, whereas the long‐term effects may include increased risk of pneumonia.
Clinicians and patients should balance the potential benefits of inhaled steroids in COPD (possibly reduced rate of decline in FEV1, reduced exacerbations, reduced rate of decline in quality of life) against the potential side effects (oropharyngeal candidiasis and hoarseness, and pneumonia).
Implications for research.
This review has raised several questions which merit further research:
Which COPD patients should be commenced on inhaled steroids?
What are the clinical and biological factors that predict response to inhaled steroids in COPD patients?
What is the dose‐response for treatment effects of inhaled steroids in COPD patients?
What are the long‐term side effects of inhaled steroids in COPD patients, especially risk of pneumonia?
What are the benefits of adding inhaled steroids to long‐acting beta2‐agonists, anticholinergics and other anti‐inflammatory agents such as roflumilast?
What are the mechanisms for the variable response to inhaled steroids in COPD patients and what are the potential strategies for reversing steroid resistance?
Feedback
Feedback regarding TORCH study, 23 June 2016
Summary
We read your review with great interest, and thank the authors for clarifying and updating the evidence for inhaled corticosteroids (ICS) in COPD patients. Our group have some concerns regarding the evaluation of the TOwards A Revolution in COPD Health (TORCH) trial, which we feel may benefit from some clarification by the authorship team. We feel the TORCH trial may have a higher risk of bias than its attributed overall 'low' rating which may impact upon the review findings considering its large size. This is due to the following observations:
The TORCH protocol indicates that an intention‐to‐treat (ITT) analysis was to be conducted (3), however, following randomization 72 (1.2%) participants were excluded from five independent study sites because of unblinding caused by: fraudulent data, falsified signatures, failure to follow GCP and International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceutical use for Human Use guidelines, and compromised patient safety (1). 38.3% participant withdrawal occurred prior to study completion. The TORCH authors state that “adverse events included death during the study period but may not have included deaths occurring after patients withdrew from the study” (2) which appears to contradict the protocol that indicated outcome data would be collected for the duration of the study for all enrolled and randomized participants (3). It is unclear whether mortality outcomes were collected for the duration of the study in participants who withdrew early. This analysis may not therefore constitute an ITT, and may have underestimated the incidence of mortality in all treatment arms.
The TORCH study authors did not provide clear criteria related to symptoms for the definition of an acute exacerbation which may make it difficult to conclude significant benefit of inhaled corticosteroids on moderate to severe exacerbations. Their analysis of exacerbations per patient per year may be affected by a small portion of patients who experience a large number of exacerbations per year when reported as a mean. And it is unclear whether exacerbations were recorded once participants withdrew or were excluded from the trial, contrary to their pre‐specified protocol (2).
As study participants were screened for adverse effects and changes in FEV1 by physicians approximately every 12 and 24 weeks, we feel there is potential for both study participants and personnel to have become unblinded to treatments posing risks of performance and/or detection bias. The decrease in number of exacerbations in participants receiving fluticasone when compared to placebo may therefore not necessarily yield the same results once this is taken into account.
In their primary analysis of St. George's Respiratory Questionnaire (SGRQ) data, the TORCH study authors only included data from “linguistically valid translations” (2). 36% of participants were not included as part of the author's primary analyses, with 19% initially reported as having not completed a validated questionnaire. A further 21% were censored despite having competed a linguistically valid SGRQ. The precise reasons for these observations are unclear, however the significant attrition, potentially caused by compromised randomization, may affect conclusions about the efficacy of fluticasone on patient‐reported quality of life. We believe your statement that fluticasone confers a mean “benefit” of ‐2.0 units over 3 years when compared to placebo as derived from the TORCH trial may be better expressed in the context of the margin being below the threshold difference of four points for clinical significance, and the data are likely incomplete (4).
Our independent analysis of the risk of bias for the TORCH trial is as follows: Selection bias (low), performance bias (unclear), detection bias (unclear), attrition bias (high), reporting bias (low).
References
Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. NEJM. 2007; 356: 775‐89. DOI: 10.1056/NEJMoa063070 Supplementary Appendix 2,Online Figure 1, p11
Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. NEJM. 2007; 356: 775‐89. DOI: 10.1056/NEJMoa063070
Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. NEJM. 2007; 356: 775‐89. DOI: 10.1056/NEJMoa063070 Supplementary Appendix 1
Aaron SD, Fergusson D, Marks GB, Suissa S, Vandemheen KL, Doucette S, Maltais F, Bourbeau JF, Goldstein RS, Balter M, O’Donnell D, FitzGerald M.Counting, analysing and reporting exacerbations of COPD in randomised controlled trials. Thorax 2008;63:122–128. DOI:10.1136/thx.2007.082636
Submitter declaration: I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment.
Reply
We greatly appreciate the interest in our review, and would like to address the helpful feedback about potential limitations of the TORCH study.
Whilst 72 of the 6184 participants were excluded due to five sites that failed audits, the Supplemental Appendix 2 indicates that ‘post‐hoc analyses in which the 72 participants who were removed from the study were included did not materially change the outcome measures.’ As these were exclusions, rather than participant withdrawals, it is appropriate that the data from these participants not be included in the mortality analysis, and that the study remains as intention to treat. For participants who were withdrawn, Appendix 2 states that ‘For any subject who withdrew prematurely from the study, all available data up to the time of discontinuation were included in the analyses. Mortality data continued to be collected for subjects who withdrew early, up to 3 years after the start of study treatment.’ As indicated in the feedback, the legend of Figure 1 of the paper states ‘Adverse events included death during the study period but may not have included deaths occurring after patients withdrew from the study.’ This reflects the reality of any clinical trial, in which data are available and included for analysis whilst participants are enrolled, and are often more difficult to collect once participants are withdrawn, despite the best efforts of the investigators. This is still within the scope of an intention to treat analysis. Imputation analysis gives wide confidence intervals for possible effects.
In their paper, the investigators provide a satisfactory and complete definition of exacerbation, as ‘a symptomatic deterioration requiring treatment with antibiotic agents, systemic corticosteroids, hospitalization, or a combination of these.’ The protocol and paper do not describe that exacerbation data were collected after participant withdrawal.
Potential unblinding is a possibility in any study. However, this is not likely to substantially affect the performance of objective outcomes such as mortality and FEV1, or health‐related outcomes such as exacerbations treated by antibiotics and/or steroids, or hospitalisation.
The investigators stated in advance that linguistically valid SGRQ versions would be analysed, and that the opportunity would be taken to prospectively validate SGRQ in countries without an existing validated version. The power calculation for the study was based on mortality, and therefore did not impact on the SGRQ analysis.
Our discussion interprets the pooled quality of life results as ‘the magnitude of this benefit was relatively small (MD ‐1.22 units/year), compared to the minimum clinically significant difference of 4 units with the SGRQ.’
We have rated the attrition bias as low risk, as both of the ICS and placebo groups had similar rates of attrition. Given this and the other discussions above, we have maintained the current assessment of risk bias for the TORCH study in our review. Once again, we appreciate the detailed feedback that we have received for this review topic in an area of importance for COPD management.
Contributors
Feedback submitter lead: Alex Sykelyk, Pharmacy Resident, Lower Mainland Pharmacy Services, BC, Canada alexander.sykelyk@fraserhealth.ca Other contributors to feedback: Tanveer Brar, Natalie Baclawska, Kieran Shah, Aaron Tejani.
Ian Yang, the lead author of the review, responded to the feedback with support from the Cochrane Airways Feedback Editor, Christian Osadnik.
What's new
| Date | Event | Description |
|---|---|---|
| 25 August 2016 | Feedback has been incorporated | Feedback received regarding evaluation of the TORCH study. |
History
Protocol first published: Issue 1, 2001 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 29 July 2011 | New search has been performed | Updated literature searches 2006 to 2011. |
| 29 July 2011 | New citation required and conclusions have changed | We added eight studies involving 3015 participants, strengthening the lung function result. 'Risk of bias' has been updated to the latest tool. |
| 22 June 2008 | New search has been performed | Converted to new review format. |
| 6 January 2007 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We are very grateful for the invaluable assistance and helpful advice from Chris Cates, Karen Blackhall, Liz Stovold, John White, Toby Lasserson and Emma Welsh in the Cochrane Airways Group. We acknowledge the helpful contribution of the many authors who provided additional information about their studies.
Ian Yang was supported by a National Health and Medical Research Council (NHMRC) Career Development Award, an NHMRC project grant and The Prince Charles Hospital Foundation project grants. Kwun Fong was supported by an NHMRC Practitioner Fellowship, NHMRC project grants and The Prince Charles Hospital Foundation project grants. Esther Sim was supported by a Cochrane Airways Group Scholarship. The funding bodies had no role in the preparation of this systematic review.
This review is dedicated to the memory of Professor Peter Black FRACP who passed away on 10 January 2010. Peter made significant and broad contributions to asthma and COPD research, including as a reviewer and editor for the Cochrane Airways Group, and a reviewer in the original version of this review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (the Cochrane Library) | Quarterly |
| PSYCINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Data and analyses
Comparison 1. ICS versus placebo, parallel‐group studies, greater than 6 months (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐bronchodilator FEV1 ‐ Rate of decline | 5 | 2333 | mL/year (Fixed, 95% CI) | 5.80 [‐0.28, 11.88] |
| 1.1 Less than 1000 µg BDP equivalent/day | 3 | 1486 | mL/year (Fixed, 95% CI) | 3.76 [‐3.43, 10.95] |
| 1.2 Greater than 1000 µg BDP equivalent/day | 2 | 847 | mL/year (Fixed, 95% CI) | 10.91 [‐0.47, 22.29] |
| 2 Change in post‐bronchodilator FEV1 (mL/yr) | 5 | 4823 | Mean Difference (IV, Random, 95% CI) | 6.88 [1.80, 11.96] |
| 2.1 Less than 1000 µg BDP equivalent/day | 3 | 1542 | Mean Difference (IV, Random, 95% CI) | 1.71 [‐5.66, 9.07] |
| 2.2 Greater than 1000 µg BDP equivalent/day | 2 | 3281 | Mean Difference (IV, Random, 95% CI) | 11.58 [4.57, 18.60] |
| 3 FEV1 (% change from baseline) | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than 1000 µg BDP equivalent/day | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Greater than 1000 µg BDP equivalent/day | 1 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Total number of deaths | 9 | 8390 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.16] |
| 4.1 Study duration 1 year | 4 | 1907 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.33, 1.31] |
| 4.2 Study duration 2 or more years | 5 | 6483 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.20] |
| 5 Exacerbation rate | 5 | 2586 | Exn's/pt/yr (Fixed, 95% CI) | ‐0.26 [‐0.37, ‐0.14] |
| 5.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Exn's/pt/yr (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Greater than 1000 µg BDP equivalent/day | 5 | 2586 | Exn's/pt/yr (Fixed, 95% CI) | ‐0.26 [‐0.37, ‐0.14] |
| 6 Exacerbation rate (no. per patient per yr) | 5 | 2253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.30, ‐0.08] |
| 6.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Greater than 1000 µg BDP equivalent/day | 5 | 2253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.30, ‐0.08] |
| 7 Exacerbation rate ‐ stratified by analysis approach | 5 | 2586 | Exn's/pt/yr (Fixed, 95% CI) | ‐0.26 [‐0.37, ‐0.14] |
| 7.1 Unweighted analysis | 2 | 925 | Exn's/pt/yr (Fixed, 95% CI) | ‐0.29 [‐0.52, ‐0.05] |
| 7.2 Weighted analysis | 3 | 1661 | Exn's/pt/yr (Fixed, 95% CI) | ‐0.25 [‐0.38, ‐0.12] |
| 8 No. of patients with at least one exacerbation | 4 | 2347 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.70, 0.98] |
| 8.1 Less than 1000 µg BDP equivalent/day | 2 | 857 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.67, 1.16] |
| 8.2 Greater than 1000 µg BDP equivalent/day | 3 | 1490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.65, 0.98] |
| 9 Change in SGRQ total score (units/yr) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Greater than 1000 µg BDP equivalent/day | 2 | 1335 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐2.00, ‐0.34] |
| 10 Mean change in SGRQ ‐ total scores | 5 | 2507 | Units on SGRQ scale (Fixed, 95% CI) | ‐1.22 [‐1.83, ‐0.60] |
| 10.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Units on SGRQ scale (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Greater than 1000 µg BDP equivalent/day | 5 | 2507 | Units on SGRQ scale (Fixed, 95% CI) | ‐1.22 [‐1.83, ‐0.60] |
| 11 Total SGRQ score (units) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Cough score | 3 | 739 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.14, 0.02] |
| 12.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Greater than 1000 µg BDP equivalent/day | 3 | 739 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.14, 0.02] |
| 13 Breathlessness score | 3 | 739 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.16, 0.00] |
| 13.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Greater than 1000 µg BDP equivalent/day | 3 | 739 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.16, 0.00] |
| 14 Throat irritation (no. of patients) | 2 | 1855 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.68, 1.41] |
| 14.1 Less than 1000 µg BDP equivalent/day | 1 | 1113 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.30, 0.89] |
| 14.2 Greater than 1000 µg BDP equivalent/day | 1 | 742 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.01, 2.69] |
| 15 Oropharyngeal candidiasis (no. of patients) | 6 | 5586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.65 [2.03, 3.46] |
| 15.1 Less than 1000 µg BDP equivalent/day | 4 | 3506 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.94 [1.93, 4.46] |
| 15.2 Greater than 1000 µg BDP equivalent/day | 3 | 2080 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.47 [1.74, 3.49] |
| 16 Hoarseness or dysphonia (no. of patients) | 4 | 3267 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [1.41, 2.70] |
| 16.1 Less than 1000 µg BDP equivalent/day | 2 | 1790 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.81 [1.16, 2.84] |
| 16.2 Greater than 1000 µg BDP equivalent/day | 2 | 1477 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [1.32, 3.36] |
| 17 Bruising (no. of patients) | 5 | 5073 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.63 [1.31, 2.03] |
| 17.1 Less than 1000 µg BDP equivalent/day | 3 | 2993 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.87 [1.38, 2.52] |
| 17.2 Greater than 1000 µg BDP equivalent/day | 3 | 2080 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [1.03, 1.93] |
| 18 Vertebral fractures (no. of patients) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 18.1 Less than 1000 µg BDP equivalent/day | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Greater than 1000 µg BDP equivalent/day | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Cataracts (no. of patients) | 3 | 1949 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 19.1 Less than 1000 µg BDP equivalent/day | 1 | 1113 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.81, 1.44] |
| 19.2 Greater than 1000 µg BDP equivalent/day | 2 | 836 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.34, 1.58] |
| 20 No. of patients with serum cortisol below normal range at any time | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 20.1 Less than 1000 µg BDP equivalent/day | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Greater than 1000 µg BDP equivalent/day | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 Any fractures (no. of patients) | 4 | 5226 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.75, 1.32] |
| 21.1 Less than 1000 µg BDP equivalent/day | 1 | 653 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.41, 7.28] |
| 21.2 Greater than 1000 µg BDP equivalent/day | 3 | 4573 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.30] |
| 22 Sputum production score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Sputum colour score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 No. of patients with change from within to below normal for serum cortisol | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 24.1 Less than 1000 µg BDP equivalent/day | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 Greater than 1000 µg BDP equivalent/day | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 Pneumonia | 7 | 6235 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.30, 1.86] |
| 25.1 Less than 1000 µg BDP equivalent/day | 2 | 803 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.45] |
| 25.2 Greater than 1000 µg BDP equivalent/day | 5 | 5432 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.38, 2.00] |
1.1. Analysis.
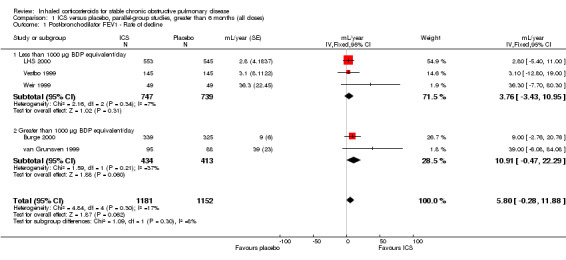
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 1 Post‐bronchodilator FEV1 ‐ Rate of decline.
1.2. Analysis.
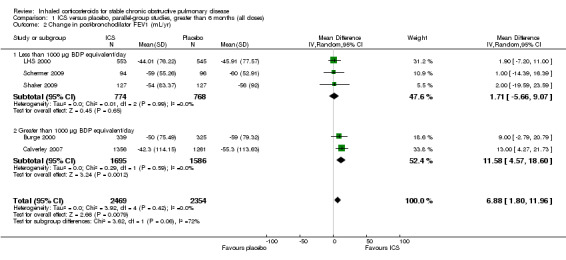
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 2 Change in post‐bronchodilator FEV1 (mL/yr).
1.3. Analysis.
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 3 FEV1 (% change from baseline).
1.4. Analysis.
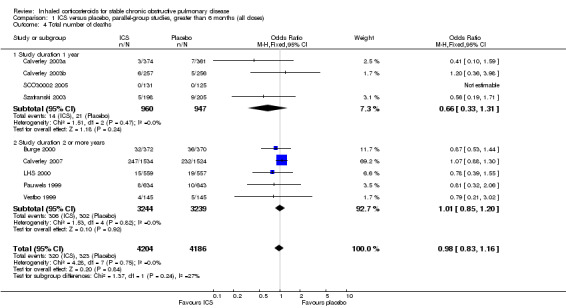
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 4 Total number of deaths.
1.5. Analysis.
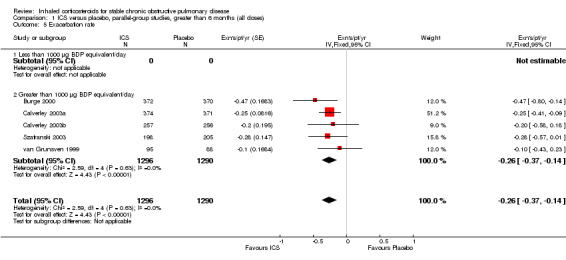
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 5 Exacerbation rate.
1.6. Analysis.
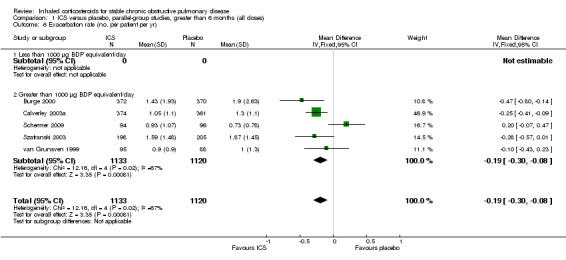
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 6 Exacerbation rate (no. per patient per yr).
1.7. Analysis.
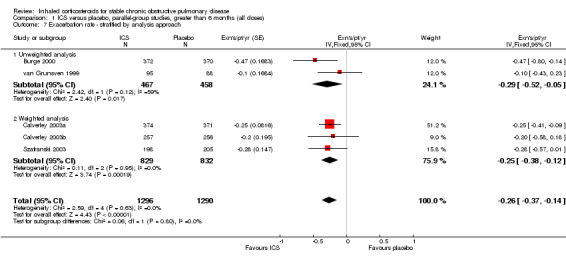
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 7 Exacerbation rate ‐ stratified by analysis approach.
1.8. Analysis.
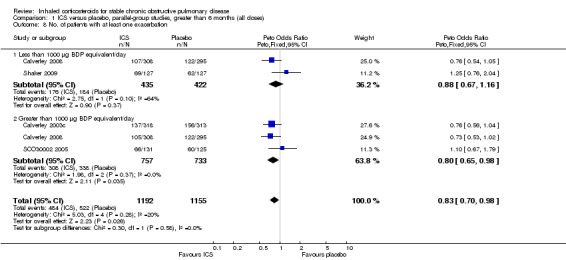
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 8 No. of patients with at least one exacerbation.
1.9. Analysis.
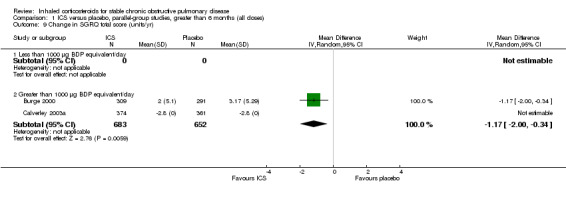
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 9 Change in SGRQ total score (units/yr).
1.10. Analysis.
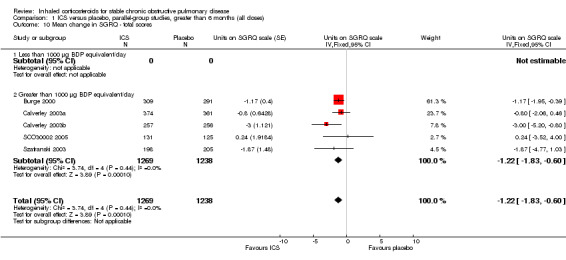
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 10 Mean change in SGRQ ‐ total scores.
1.11. Analysis.
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 11 Total SGRQ score (units).
1.12. Analysis.
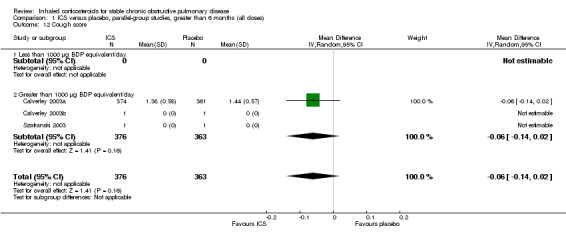
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 12 Cough score.
1.13. Analysis.
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 13 Breathlessness score.
1.14. Analysis.
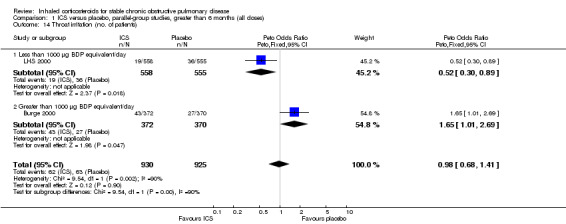
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 14 Throat irritation (no. of patients).
1.16. Analysis.
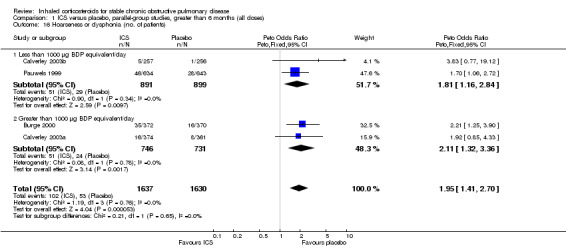
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 16 Hoarseness or dysphonia (no. of patients).
1.17. Analysis.
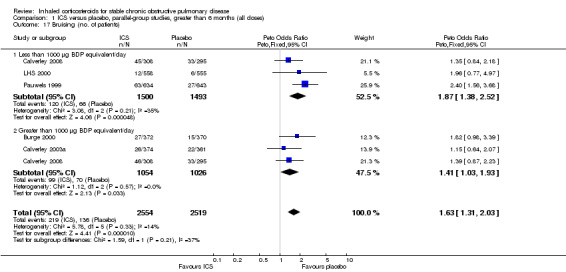
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 17 Bruising (no. of patients).
1.18. Analysis.
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 18 Vertebral fractures (no. of patients).
1.19. Analysis.
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 19 Cataracts (no. of patients).
1.20. Analysis.
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 20 No. of patients with serum cortisol below normal range at any time.
1.21. Analysis.
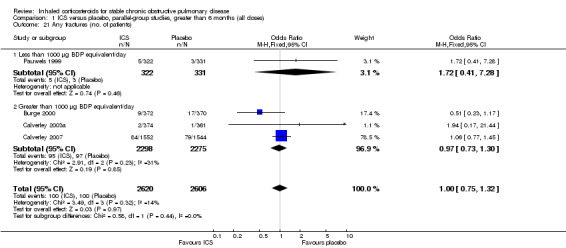
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 21 Any fractures (no. of patients).
1.22. Analysis.
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 22 Sputum production score.
1.23. Analysis.
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 23 Sputum colour score.
1.24. Analysis.
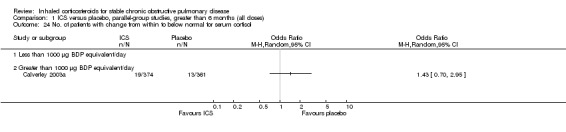
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 24 No. of patients with change from within to below normal for serum cortisol.
1.25. Analysis.
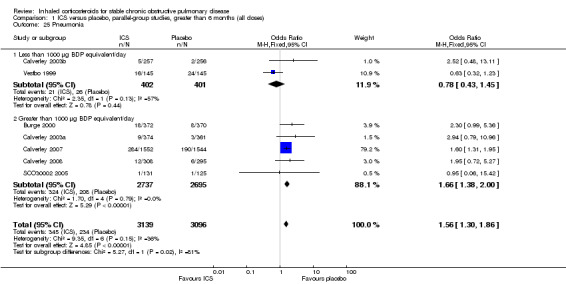
Comparison 1 ICS versus placebo, parallel‐group studies, greater than 6 months (all doses), Outcome 25 Pneumonia.
Comparison 2. ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in pre‐bronchodilator FEV1 compared with baseline | 7 | 2325 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [0.03, 0.06] |
| 1.1 Less than 1000 µg BDP equivalent/day | 2 | 985 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.04] |
| 1.2 Greater than 1000 µg BDP equivalent/day | 6 | 1340 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [0.05, 0.10] |
| 2 Change in post bronchodilator FEV1 compared to baseline (L) | 4 | 1527 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.01, 0.14] |
| 2.1 Less than 1000 µg BDP equivalent/day | 1 | 575 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 2.2 Greater than 1000 µg BDP equivalent/day | 3 | 952 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.06, 0.13] |
| 3 Morning PEFR (L/min) | 2 | 577 | Mean Difference (IV, Random, 95% CI) | 5.42 [0.59, 10.25] |
| 3.1 Less than 1000 µg BDP equivalent/day | 1 | 575 | Mean Difference (IV, Random, 95% CI) | 5.42 [0.59, 10.25] |
| 3.2 Greater than 1000 µg BDP equivalent/day | 1 | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Post‐bronchodilator FEV1 (change from baseline) | 3 | 950 | Litres (Fixed, 95% CI) | 0.11 [0.07, 0.16] |
| 4.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Greater than 1000 µg BDP equivalent/day | 3 | 950 | Litres (Fixed, 95% CI) | 0.11 [0.07, 0.16] |
| 5 Change in pre‐bronchodilator FEV1 compared with baseline | 4 | 814 | Litres (Fixed, 95% CI) | 0.06 [0.02, 0.11] |
| 5.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Greater than 1000 µg BDP equivalent/day | 4 | 814 | Litres (Fixed, 95% CI) | 0.06 [0.02, 0.11] |
| 6 PEF (change scores) | 2 | Litres/min (Fixed, 95% CI) | Totals not selected | |
| 6.1 Less than 1000 µg BDP equivalent/day | 1 | Litres/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Greater than 1000 µg BDP equivalent/day | 2 | Litres/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 FVC (change from baseline) | 1 | Litres (Fixed, 95% CI) | Totals not selected | |
| 7.1 Less than 1000 µg BDP equivalent/day | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Greater than 1000 µg BDP equivalent/day | 1 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Total number of deaths | 5 | 1730 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.05, 1.28] |
| 8.1 Less than 1000 µg BDP equivalent/day | 1 | 422 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Greater than 1000 µg BDP equivalent/day | 5 | 1308 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.05, 1.28] |
| 9 No. of patients with at least one exacerbation | 5 | 1893 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.75, 1.08] |
| 9.1 Less than 1000 µg BDP equivalent/day | 3 | 839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.71, 1.23] |
| 9.2 Greater than 1000 µg BDP equivalent/day | 3 | 1054 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 10 6‐minute walk (change scores) | 1 | Metres (Fixed, 95% CI) | Totals not selected | |
| 10.1 Less than 1000 µg BDP equivalent/day | 0 | Metres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Greater than 1000 µg BDP equivalent/day | 1 | Metres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Change in 6‐minute walk distance from baseline (m) | 2 | 301 | Mean Difference (IV, Random, 95% CI) | ‐4.36 [‐50.42, 41.70] |
| 11.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Greater than 1000 µg BDP equivalent/day | 2 | 301 | Mean Difference (IV, Random, 95% CI) | ‐4.36 [‐50.42, 41.70] |
| 12 Change from baseline in dyspnoea on CRQ (units) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Change from baseline in emotion on CRQ (units) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Change from baseline in fatigue on CRQ | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Change from baseline in mastery on CRQ (units) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Rescue beta‐agonist use (puffs/day) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16.1 Less than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Throat irritation (no. of patients) | 3 | 1572 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.09, 2.37] |
| 17.1 Less than 1000 µg BDP equivalent/day | 2 | 790 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.92, 2.79] |
| 17.2 Greater than 1000 µg BDP equivalent/day | 2 | 782 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.94, 2.79] |
| 18 Oropharyngeal candidiasis (no. of patients) | 5 | 2109 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.59 [3.58, 8.74] |
| 18.1 Less than 1000 µg BDP equivalent/day | 2 | 790 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.80 [2.20, 10.48] |
| 18.2 Greater than 1000 µg BDP equivalent/day | 4 | 1319 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.02 [3.50, 10.38] |
| 19 Hoarseness or dysphonia (no. of patients) | 4 | 1520 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.21 [2.17, 8.17] |
| 19.1 Less than 1000 µg BDP equivalent/day | 1 | 422 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.34 [1.55, 12.17] |
| 19.2 Greater than 1000 µg BDP equivalent/day | 4 | 1098 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.13 [1.74, 9.80] |
| 20 Pneumonia (no. of patients) | 1 | 846 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.35, 10.47] |
| 20.1 Less than 1000 µg BDP equivalent/day | 1 | 422 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.17, 21.29] |
| 20.2 Greater than 1000 µg BDP equivalent/day | 1 | 424 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.17, 21.09] |
| 21 No. of patients with serum cortisol below normal range at any time | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 21.1 Less than 1000 µg BDP equivalent/day | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Greater than 1000 µg BDP equivalent/day | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
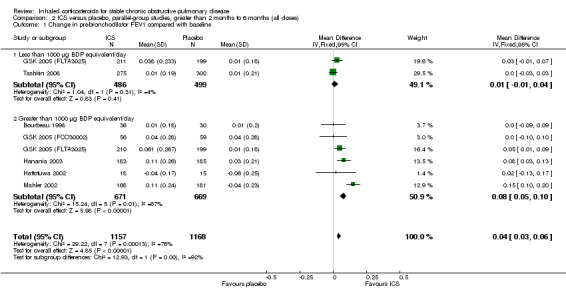
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 1 Change in pre‐bronchodilator FEV1 compared with baseline.
2.2. Analysis.
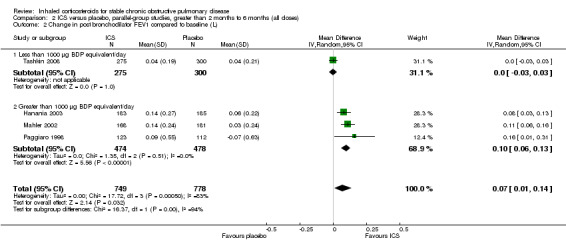
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 2 Change in post bronchodilator FEV1 compared to baseline (L).
2.3. Analysis.
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 3 Morning PEFR (L/min).
2.5. Analysis.
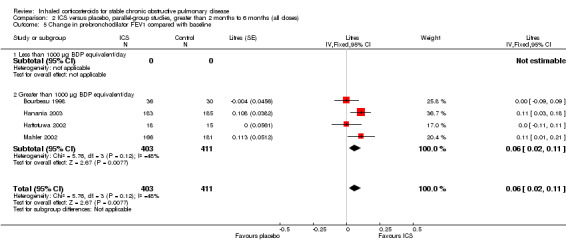
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 5 Change in pre‐bronchodilator FEV1 compared with baseline.
2.6. Analysis.
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 6 PEF (change scores).
2.7. Analysis.
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 7 FVC (change from baseline).
2.8. Analysis.
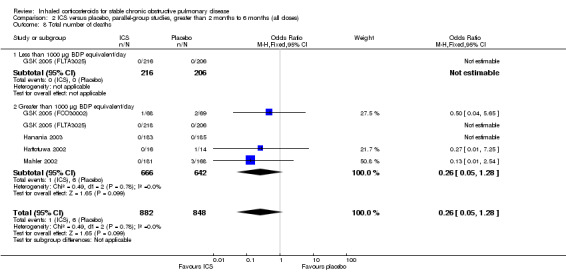
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 8 Total number of deaths.
2.9. Analysis.
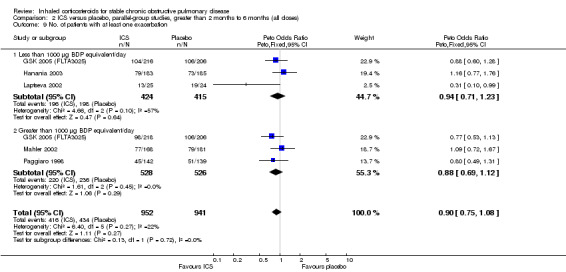
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 9 No. of patients with at least one exacerbation.
2.10. Analysis.

Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 10 6‐minute walk (change scores).
2.12. Analysis.
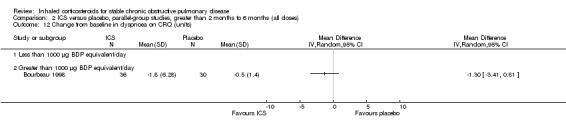
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 12 Change from baseline in dyspnoea on CRQ (units).
2.13. Analysis.
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 13 Change from baseline in emotion on CRQ (units).
2.14. Analysis.
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 14 Change from baseline in fatigue on CRQ.
2.15. Analysis.
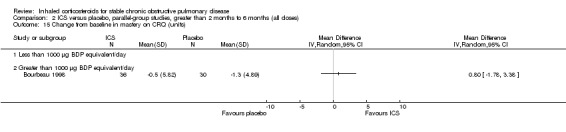
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 15 Change from baseline in mastery on CRQ (units).
2.16. Analysis.
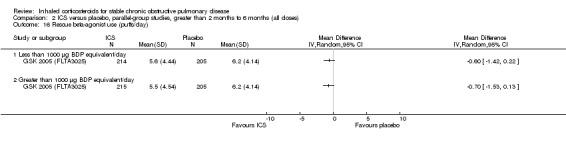
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 16 Rescue beta‐agonist use (puffs/day).
2.17. Analysis.
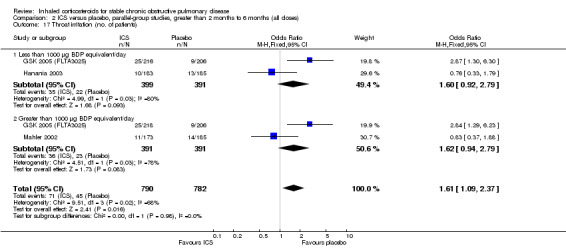
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 17 Throat irritation (no. of patients).
2.18. Analysis.
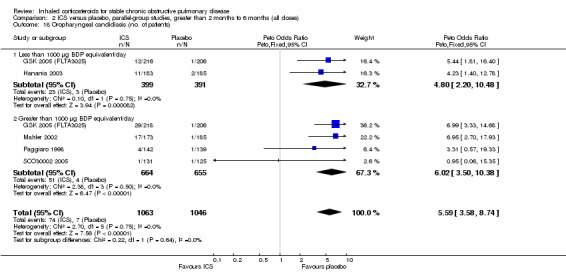
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 18 Oropharyngeal candidiasis (no. of patients).
2.19. Analysis.
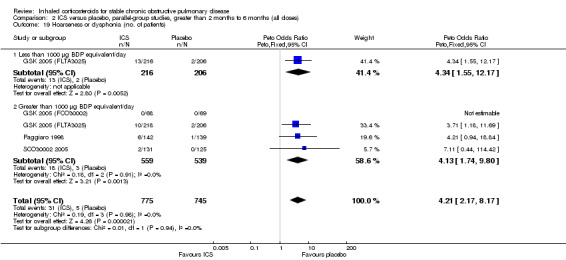
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 19 Hoarseness or dysphonia (no. of patients).
2.20. Analysis.
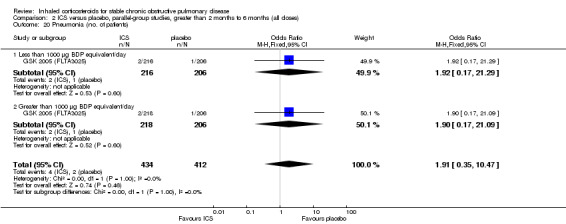
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 20 Pneumonia (no. of patients).
2.21. Analysis.
Comparison 2 ICS versus placebo, parallel‐group studies, greater than 2 months to 6 months (all doses), Outcome 21 No. of patients with serum cortisol below normal range at any time.
Comparison 3. ICS versus placebo, parallel‐group studies, 2 months or less (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline (% increase) | 2 | 57 | Mean Difference (IV, Random, 95% CI) | 3.84 [‐4.82, 12.50] |
| 1.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Greater than 1000 µg BDP equivalent/day | 2 | 57 | Mean Difference (IV, Random, 95% CI) | 3.84 [‐4.82, 12.50] |
| 2 Change in FVC compared to baseline (% increase) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in MMEFR compared to baseline (% increase) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Morning PEFR (L/min) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Evening PEFR (L/min) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in PEFR compared to baseline (% increase) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 No. of patients with at least one exacerbation | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Less than 1000 µg BDP equivalent/day | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Greater than 1000 µg BDP equivalent/day | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Rescue beta‐agonist use (puffs/day) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Oropharyngeal candidiasis (no. of patients) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Less than 1000 µg BDP equivalent/day | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Greater than 1000 µg BDP equivalent/day | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
Comparison 3 ICS versus placebo, parallel‐group studies, 2 months or less (all doses), Outcome 1 Change in FEV1 compared to baseline (% increase).
3.2. Analysis.
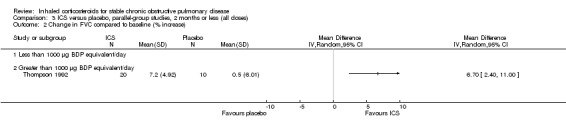
Comparison 3 ICS versus placebo, parallel‐group studies, 2 months or less (all doses), Outcome 2 Change in FVC compared to baseline (% increase).
3.3. Analysis.
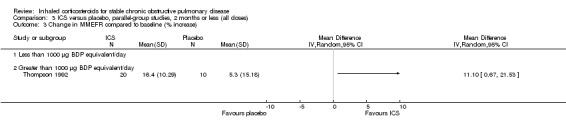
Comparison 3 ICS versus placebo, parallel‐group studies, 2 months or less (all doses), Outcome 3 Change in MMEFR compared to baseline (% increase).
3.4. Analysis.
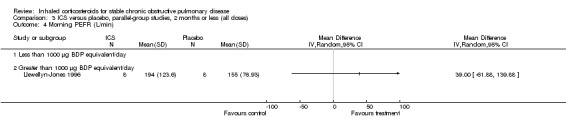
Comparison 3 ICS versus placebo, parallel‐group studies, 2 months or less (all doses), Outcome 4 Morning PEFR (L/min).
3.5. Analysis.
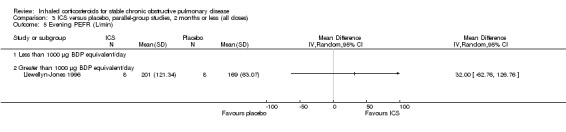
Comparison 3 ICS versus placebo, parallel‐group studies, 2 months or less (all doses), Outcome 5 Evening PEFR (L/min).
3.6. Analysis.
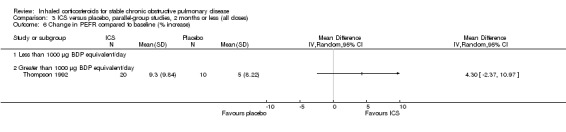
Comparison 3 ICS versus placebo, parallel‐group studies, 2 months or less (all doses), Outcome 6 Change in PEFR compared to baseline (% increase).
3.7. Analysis.
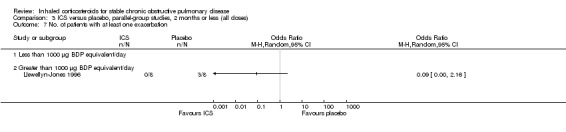
Comparison 3 ICS versus placebo, parallel‐group studies, 2 months or less (all doses), Outcome 7 No. of patients with at least one exacerbation.
3.8. Analysis.
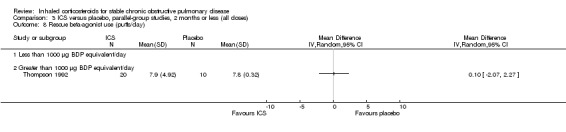
Comparison 3 ICS versus placebo, parallel‐group studies, 2 months or less (all doses), Outcome 8 Rescue beta‐agonist use (puffs/day).
3.9. Analysis.
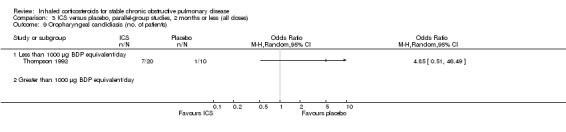
Comparison 3 ICS versus placebo, parallel‐group studies, 2 months or less (all doses), Outcome 9 Oropharyngeal candidiasis (no. of patients).
Comparison 4. ICS versus placebo, cross‐over studies, 2 months or less (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 2 | 396 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.05, 0.32] |
| 1.1 Less than 1000 µg BDP equivalent/day | 1 | 336 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.16, 0.74] |
| 1.2 Greater than 1000 µg BDP equivalent/day | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.10, 0.30] |
| 2 Daily PEFR (L/min) | 2 | 86 | Mean Difference (IV, Fixed, 95% CI) | 18.78 [‐20.17, 57.72] |
| 2.1 Less than 1000 µg BDP equivalent/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Greater than 1000 µg BDP equivalent/day | 2 | 86 | Mean Difference (IV, Fixed, 95% CI) | 18.78 [‐20.17, 57.72] |
| 3 FEV1 % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Rescue beta‐agonist use (puffs/day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Less than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Greater than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in post‐bronchodilator FEV1 | 1 | L (Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
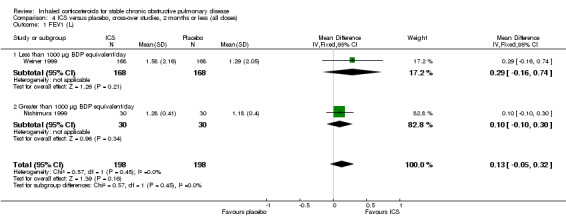
Comparison 4 ICS versus placebo, cross‐over studies, 2 months or less (all doses), Outcome 1 FEV1 (L).
4.2. Analysis.
Comparison 4 ICS versus placebo, cross‐over studies, 2 months or less (all doses), Outcome 2 Daily PEFR (L/min).
4.3. Analysis.
Comparison 4 ICS versus placebo, cross‐over studies, 2 months or less (all doses), Outcome 3 FEV1 % predicted.
4.4. Analysis.
Comparison 4 ICS versus placebo, cross‐over studies, 2 months or less (all doses), Outcome 4 Rescue beta‐agonist use (puffs/day).
4.5. Analysis.

Comparison 4 ICS versus placebo, cross‐over studies, 2 months or less (all doses), Outcome 5 Change in post‐bronchodilator FEV1.
Comparison 5. ICS versus placebo, studies of COPD with BHR, greater than 2 months to 6 months (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Salbutamol rescue doses (per month) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Ipratropium rescue doses (per month) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serum cortisol at 6 months (nM/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
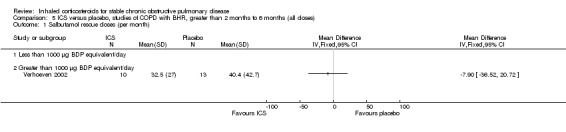
Comparison 5 ICS versus placebo, studies of COPD with BHR, greater than 2 months to 6 months (all doses), Outcome 1 Salbutamol rescue doses (per month).
5.2. Analysis.
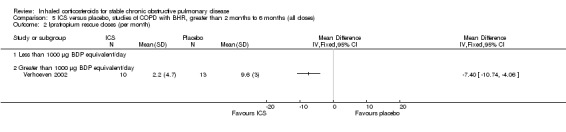
Comparison 5 ICS versus placebo, studies of COPD with BHR, greater than 2 months to 6 months (all doses), Outcome 2 Ipratropium rescue doses (per month).
5.3. Analysis.

Comparison 5 ICS versus placebo, studies of COPD with BHR, greater than 2 months to 6 months (all doses), Outcome 3 Serum cortisol at 6 months (nM/L).
Comparison 6. ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in pre‐bronchodilator FEV1 compared to baseline (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in pre‐bronchodilator VC compared to baseline (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in FEV1 before terbutaline as % baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in FEV1 after terbutaline as % baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in log10 PC20 histamine | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in log10 citric acid threshold | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change in morning peak expiratory flow rate (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in evening peak expiratory flow rate (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Change in dyspnoea score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Change in cough score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Change in sputum score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Change in rescue bronchodilator usage (puffs/day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Less than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Greater than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Change in post‐bronchodilator FEV1 (mL/yr) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Less than 1000 µg BDP equivalent/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Greater than 1000 µg BDP equivalent/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
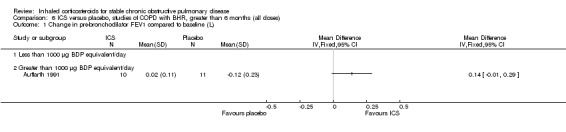
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 1 Change in pre‐bronchodilator FEV1 compared to baseline (L).
6.2. Analysis.
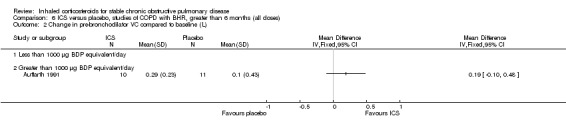
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 2 Change in pre‐bronchodilator VC compared to baseline (L).
6.3. Analysis.
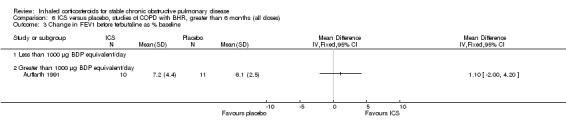
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 3 Change in FEV1 before terbutaline as % baseline.
6.4. Analysis.
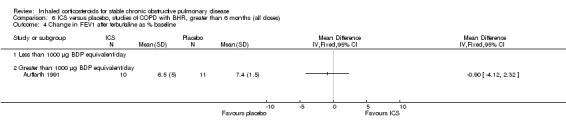
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 4 Change in FEV1 after terbutaline as % baseline.
6.5. Analysis.
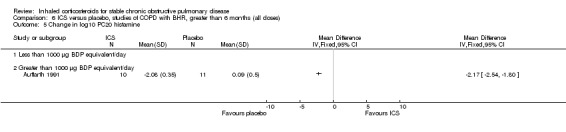
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 5 Change in log10 PC20 histamine.
6.6. Analysis.
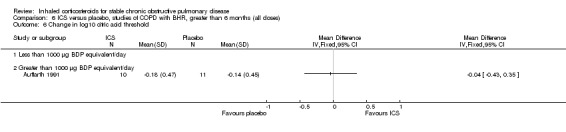
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 6 Change in log10 citric acid threshold.
6.7. Analysis.
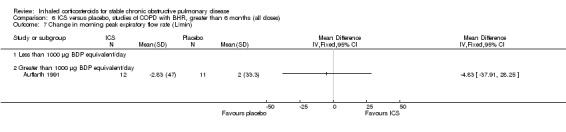
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 7 Change in morning peak expiratory flow rate (L/min).
6.8. Analysis.
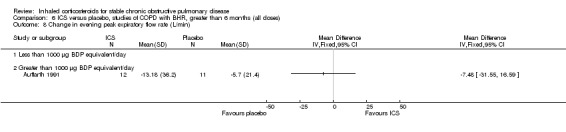
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 8 Change in evening peak expiratory flow rate (L/min).
6.9. Analysis.
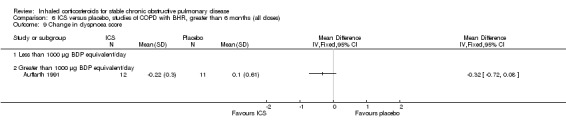
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 9 Change in dyspnoea score.
6.10. Analysis.
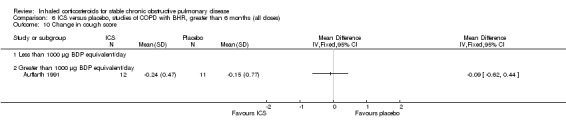
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 10 Change in cough score.
6.11. Analysis.
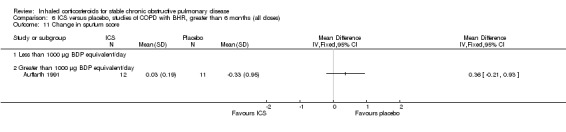
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 11 Change in sputum score.
6.12. Analysis.
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 12 Change in rescue bronchodilator usage (puffs/day).
6.13. Analysis.
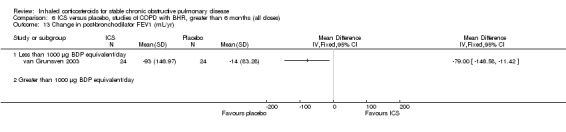
Comparison 6 ICS versus placebo, studies of COPD with BHR, greater than 6 months (all doses), Outcome 13 Change in post‐bronchodilator FEV1 (mL/yr).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Auffarth 1991.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: single‐centre study, hospital outpatient clinic Number eligible: not stated Number enrolled: 24 Number in treatment group: 12 Number in control group: 12 Number of withdrawals (treatment/control): 2/1 Number completing trial (treatment/control): 10/11 Age range: 40 to 70 years Sex: 23 M, 1 F Ethnicity: not stated COPD diagnosis: FEV1 30% to 75% predicted, with bronchial hyper‐responsiveness to histamine (defined in inclusion criteria) Severity of COPD: FEV1 52.5% predicted (BUD group), 54.1% predicted (placebo group) Inclusion criteria: smoking at least 1 cigarette a day for at least 5 years, reversibility in terms of the difference between FEV1 % predicted before and after 0.5 mg inhaled terbutaline of < 20%, PC20 to histamine < 16 mg/mL Exclusion criteria: positive skin prick test or specific IgE to house dust mite, total serum IgE >= 470 IU/mL, peripheral blood eosinophil count >= 0.2 x 10 E6/L, upper respiratory tract infection or oral corticosteroids in the 2 months prior Baseline characteristics of treatment/control groups: comparable | |
| Interventions | BUD 200 µg, 4 puffs, 2 times a day (1600 µg/d) Placebo 4 puffs, 2 times a day Nebuhaler 8 weeks |
|
| Outcomes | FEV1 Bronchodilator response PC20 histamine Citric acid threshold (cough) Morning peak expiratory flow rate Evening peak expiratory flow rate Symptoms of cough Symptoms of dyspnoea Sputum volume Number of rescue bronchodilator (ipratropium) inhalations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocated at random" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind design" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Boothman‐Burrell 1997.
| Methods | Design: cross‐over, 4 weeks washout Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: single‐centre study, research clinic Number eligible: not stated Number enrolled: 22 Number in treatment group: 22 (cross‐over study) Number in control group: 22 (cross‐over study) Number of withdrawals (treatment/control): 4 (cross‐over study) Number completing trial (treatment/control): 18 (cross‐over study) Age range: > 40 years Sex: numbers not stated Ethnicity: not stated COPD diagnosis: FEV1 < 80% predicted, FEV1/FVC < 65% Severity of COPD: FEV1 52.4% predicted Inclusion criteria: smokers or ex‐smokers of > 10 pack‐years, reversibility to salbutamol < 25%. 5 patients had bronchodilator reversibility of 15% to 25%. 2 patients had PC20 methacholine < 16 mg/mL Exclusion criteria: childhood asthma, eczema, allergic rhinitis, other current respiratory disorder, taking oral prednisone, other major disease, major exacerbation in previous 2 months Baseline characteristics of treatment/control groups: cross‐over study | |
| Interventions | BDP 1000 µg, 2 times a day (2000 µg/d) Placebo 2 times a day Metered‐dose inhaler 3 months each treatment period (cross‐over) |
|
| Outcomes | Post‐bronchodilator FEV1 Methacholine challenge Symptom scores (cough, dyspnoea, wheeze, sputum production) Early morning PEFR Adverse events Exacerbations | |
| Notes | 3 months each treatment period (cross‐over) Oral steroid reversibility trial (prednisone 30 mg/day) for 10 days after each treatment or placebo period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 withdrawals (cross‐over study) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Bourbeau 1998.
| Methods | Design: parallel‐group Randomisation: yes, sealed envelopes, block of 4 patients Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: single‐centre study, Canada, hospital outpatient clinic, home recordings Number eligible: 140 Number enrolled: 79 Number in treatment group: 39 Number in control group: 40 Number of withdrawals (treatment/control): 3/10 Number completing trial (treatment/control): 36/30 Age range: >= 40 years Sex: 62 M, 17 F Ethnicity: not stated COPD diagnosis: pre‐bronchodilator FEV1 < 65% predicted, FEV1/FVC < 0.65, post‐bronchodilator FEV1 < 80% Severity of COPD: mean post‐bronchodilator FEV1 43% predicted in intervention group, 43% predicted in placebo group Inclusion criteria: smokers or ex‐smokers, regular treatment with at least one bronchodilator, non‐response to oral steroid trial (prednisolone 40 mg 2 weeks, taper over 1 week) defined as increase in pre‐bronchodilator FEV1 < 15% and < 200 mL compared to baseline or placebo Exclusion criteria: allergic asthma during childhood or adulthood, exacerbation in respiratory symptoms during 2 months prior to study, other active lung disease, diabetes, active peptic ulcer disease, uncontrolled high blood pressure, congestive heart failure, disease other than COPD that interferes with quality of life Baseline characteristics of treatment/control groups: more women and current smokers in placebo group | |
| Interventions | BUD 800 µg, 2 times a day (1600 µg/d) Placebo 2 times a day Dry powder inhaler (Turbuhaler) 6 months |
|
| Outcomes | Change from baseline in pre‐bronchodilator FEV1 Change from baseline in pre‐bronchodilator FVC Change from baseline in post‐bronchodilator FEV1 Change from baseline in post‐bronchodilator FVC Change from baseline in 6‐minute walk distance Change from baseline in 6‐minute walk visual analogue score Quality of life questionnaire (CRQ) Morning PEFR Evening PEFR Shortness of breath score Cough score | |
| Notes | Run‐in phase (trial of oral steroids): oral placebo 2 weeks then prednisolone 40 mg 2 weeks, taper over 1 week. Oral steroid non‐responders were enrolled in the RCT of budesonide versus placebo. Intention‐to‐treat analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was carried out in blocks of 4 patients", "sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was carried out in blocks of 4 patients", "sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals: 3 from BUD group, 10 from placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Bourbeau 2007.
| Methods | Design: parallel‐group Randomisation: Yes. central computer‐generated list of random numbers which was stratified by centre and which used a block size of 6 Blinding: double‐blind Withdrawals: stated |
|
| Participants | Setting: 2 respiratory centres (Montreal Chest Institute and Hospital Laval, Canada) Number eligible: 62 Number enrolled: 60 Number in treatment groups: SFC 19, FP 20 Number in control group: 21 Number of withdrawals: SFC 0, FP 3, placebo 9 Number completing trial: SFC 19, FP 17, placebo 15 Mean age: SFC 62, FP 64, placebo 66 Sex (M/F): SFC 19/0, FP 15/5, placebo 17/4 Ethnicity: not stated COPD diagnosis: as stated below Severity of COPD: as stated below Inclusion criteria: age > 40 and (75 years; smoking history (> 10 pack‐years); post‐bronchodilator FEV1 > 25% of predicted value and FEV1/forced vital capacity (FVC) ≤ 0.70; no history of asthma, atopy (as assessed by an allergy skin prick test during screening) or any other active lung disease Exclusion criteria: home oxygen or with raised carbon dioxide tension (> 44 mm Hg), alpha1‐antitrypsin deficiency, recent exacerbation (in the last 4 weeks), uncontrolled medical condition or hypersensitivity to inhaled corticosteroids and bronchodilators Baseline characteristics of treatment/control groups: no females and greater pack‐year history smoking in SFC group | |
| Interventions | 4 weeks washout period from ICS and LABA. 12 weeks treatment with salmeterol xinafoate/fluticasone propionate 50/500 µg twice daily, fluticasone propionate 500 µg twice daily, or placebo twice daily | |
| Outcomes | Primary: number of CD8+ T cells and CD68+ macrophages Secondary: number neutrophils and eosinophils, FEV1, CRQ score |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computed generated randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts stated, equal |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Brightling 2005.
| Methods | Design: cross‐over, 4 week run‐in, 4 weeks washout Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: single‐centre study, respiratory outpatient clinic, Leicester Number eligible: 95 Number enrolled: 60 Number in treatment group: 30 Number in control group: 30 Number of withdrawals (treatment/control): 5/6 Number completing trial (treatment/control): 23/26 Age range: 66 to 68 years Sex: 35 M; 25 F Ethnicity: not stated COPD diagnosis: post‐bronchodilator FEV1 of < 70% predicted and FEV1/FVC ratio of < 70%, no significant improvement in FEV1 after inhaled salbutamol Severity of COPD: Inclusion criteria: COPD Exclusion criteria: clinical diagnosis of asthma; history of childhood respiratory problems; variability in symptoms not associated with infections; a history of acute wheeze, breathlessness or deterioration associated with allergens; an exacerbation within 6 weeks of trial entry; taking regular oral corticosteroids Baseline characteristics of treatment/control groups: comparable | |
| Interventions | Mometasone furoate 800 µg, 1 time a day (800 µg/day) Placebo | |
| Outcomes | Change in post‐bronchodilator FEV1 Total CRQ Sputum characteristics ‐ eosinophils, neutrophils, macrophages, lymphocytes, histamine, IL8, ECP VAS scores for dyspnoea, cough, sputum production, wheeze | |
| Notes | Intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 2 MF, 1 placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Burge 2000.
| Methods | Design: parallel‐group Randomisation: yes, computer‐generated, stratified by centre Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: multicentre study, UK, hospital outpatient clinics Number eligible: 990 Number enrolled: 751 Number in treatment group: 376 Number in control group: 375 Number of withdrawals (treatment/control): 164/200 Number completing trial (treatment/control): 212/175 Age range: 40 to 75 year Sex: 560 M, 191 F Ethnicity: not stated COPD diagnosis: post‐bronchodilator FEV1 >= 0.8 L and < 85% predicted, FEV1/FVC < 70% Severity of COPD: mean post‐bronchodilator FEV1 50.3% predicted in intervention group, 50.0% predicted in placebo group Inclusion criteria: current or former smokers Exclusion criteria: asthma, FEV1 increase > 10% predicted with 400 µg salbutamol, life expectancy < 5 years from concurrent diseases, use of beta‐blockers Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 500 µg, 2 times a day (1000 µg/day) Placebo 2 times a day Metered‐dose inhaler (identical) and spacer device 3 years |
|
| Outcomes | Change in post‐bronchodilator FEV1 over time Exacerbations Changes in health status (SGRQ) Withdrawals because of respiratory disease Morning serum cortisol Adverse events | |
| Notes | Run‐in phase: 8‐week: withdrawal from oral or inhaled steroids | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals: 43% FP, 53% placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Calverley 2003a.
| Methods | Design: parallel‐group Randomisation: yes, computer‐generated Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: multicentre study, hospital outpatient clinic Number eligible: 1974 Number enrolled: 1465 (all treatment arms) Number in treatment group: 374 FP arm Number in control group: 361 placebo arm Number of withdrawals (treatment/control): 108/140 Number completing trial (treatment/control): 266/221 Age range: mean 63 years Sex: 529 M, 206 F Ethnicity: not stated COPD diagnosis: pre‐bronchodilator FEV1 25% to 70% predicted, acute bronchodilator reversibility < 10% predicted FEV1 with salbutamol, pre‐bronchodilator FEV1/FVC ratio <= 70% Severity of COPD: mean FEV1 45% predicted FP, 44% predicted placebo Inclusion criteria: >= 10 pack‐years smoking, chronic bronchitis, at least 1 episode of acute COPD symptom exacerbation per year in the previous 3 years, at least one exacerbation in the year immediately before trial entry that required treatment with oral steroids, antibiotics or both Exclusion criteria: respiratory disorders other than COPD; regular oxygen treatment; systemic steroids, high dose ICS or antibiotics in the 4 weeks prior Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 500 µg, 2 times a day (1000 µg/d) Salmeterol/FP 50/500 µg, 2 times a day Salmeterol 50 µg, 2 times a day Placebo in identical inhaler Multidose dry powder inhaler (Diskus or Accuhaler) 1 year | |
| Outcomes | Pre‐bronchodilator FEV1, FVC Post‐bronchodilator FEV1, FVC Peak flow Use of relief medication Symptoms score Night‐time awakenings Exacerbations SGRQ Adverse events | |
| Notes | Study of combined salmeterol and fluticasone, versus salmeterol, versus fluticasone, versus placebo. For this review, the comparison of fluticasone versus placebo was analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation schedule generated by the PACT program" |
| Allocation concealment (selection bias) | Low risk | Quote: "unaware of the allocated treatment" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 108 FP, 140 placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Calverley 2003b.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: multicentre study, hospital outpatient clinic Number eligible: not stated Number enrolled: 1141 (all treatment arms) Number in treatment group: 257 (BUD) Number in control group: 256 (placebo) Number of withdrawals (treatment/control): 102 BUD/106 placebo Number completing trial (treatment/control): 155 BUD/150 placebo Age range: mean 64 years BUD, mean 65 years placebo Sex: 74% M BUD, 75% M placebo Ethnicity: not stated COPD diagnosis: GOLD stages III and IV Severity of COPD: FEV1 36% predicted Inclusion criteria: GOLD defined COPD (stages III and IV); >= 40 years; COPD symptoms > 2 years; smoking history >= 10 pack‐years; FEV1/VC <= 70% pre‐BD; FEV1 <= 50% predicted; use of SABAs as reliever medication; >= 1 COPD exacerbation requiring OCS/antibx 2 to 12 months before 1st clinic visit Exclusion criteria: history of asthma/rhinitis before 40 years of age; any relevant cardiovascular disorders; exacerbation of COPD requiring medical intervention within 4 weeks of run‐in/during run‐in phase; non‐allowed medications: O2 therapy; ICS (aside from study medication), disodium cromoglycate, leukotriene‐antagonists, 5‐LO inhibitors, BD (other than study medication and prn terbutaline 0.5 mg), antihistamines, medication containing ephedrine, ß‐blocking agents Baseline characteristics of treatment/control groups: comparable | |
| Interventions | BUD 400 µg bd
Formoterol 9 µg bd
BUD 320 µg/formoterol 9 µg bd
Placebo bd Turbuhaler 12 months |
|
| Outcomes | Time to first exacerbation Change in post‐bronchodilator FEV1 Number of exacerbations Time to and number of OCS‐treated episodes PEFR am and pm Slow VC HRQL Symptoms Use of reliever medication Adverse effects | |
| Notes | Study of combined budesonide and formoterol, versus budesonide, versus formoterol, versus placebo For this review, the comparison of budesonide versus placebo was analysed Run‐in phase: all participants received 30 mg oral prednisolone bd and 2 x 4.5 mg formoterol bd (2 weeks) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 102 BUD, 106 placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Calverley 2003c.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: not stated (placebo‐controlled) Withdrawals: not stated | |
| Participants | Setting: multicentre study Number eligible: not stated (abstract) Number enrolled: 631 Number in treatment group: 318 Number in control group: 313 Number of withdrawals (treatment/control): not stated Number completing trial (treatment/control): not stated Age range: >= 40 years Sex: not stated Ethnicity: not stated COPD diagnosis: criteria not stated in abstract, history of smoking Severity of COPD: post‐bronchodilator FEV1 = 1.37 to 1.39 L, % predicted FEV1 = 47%, reversibility = 3.54 to 3.72 % predicted FEV1 (from abstract) Inclusion criteria: not stated Exclusion criteria: not stated Baseline characteristics of treatment/control groups: not stated | |
| Interventions | Mometasone furoate once daily 800 µg DPI Placebo 52 weeks | |
| Outcomes | Changes from baseline in post‐bronchodilator FEV1 Changes from baseline in COPD symptom scores % of patients with > 1 COPD exacerbation Time to first COPD exacerbation | |
| Notes | Details from Calverley 2003b abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised" (abstract) |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
Calverley 2007.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 3096 Number enrolled: 3058 Number in treatment group: 1534 Number in control group: 1524 Number of withdrawals (treatment/control): 587/673 Number completing trial (treatment/control): 947/851 Age range: placebo (mean) 65 years (8.2); ICS (mean) 65 years (8.4) Sex: placebo 1163 M, 361 F; ICS 1157 M, 377 F Ethnicity: placebo 82% white; ICS 82% white COPD diagnosis: FEV1 < 60% predicted, < 10% reversibility in predicted FEV1, FEV1/FER ratio < 70% Severity of COPD: not stated Inclusion criteria: male or female aged 40 to 80 years; current or ex‐smokers with smoking history of > 10 pack‐years; established Hx of COPD Exclusion criteria: current diagnosis of asthma or respiratory disorders other than COPD; chest radiograph indicating diagnosis other than COPD; had a lung‐volume reduction surgery and/or lung transplant; requirement of LTOT at start of study > 12 hour/day; receiving long‐term oral corticosteroid therapy; serious, uncontrolled disease likely to interfere with study and/or cause death within the 3‐year study period Baseline characteristics of treatment/control groups: not stated |
|
| Interventions | FP 500 µg, 2 times a day (1000 µg/day) Placebo | |
| Outcomes | All‐cause mortality St George Respiratory Questionnaire Exacerbation rate Serious adverse events Fatal SAEs | |
| Notes | TORCH study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised" ‐ computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Quote: "Neither the subject nor the investigator will know to which treatment arm a subject has been allocated" ‐ supplementary protocol |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 38% FP, 44% placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Calverley 2008.
| Methods | Design: parallel‐group (3 groups ‐ QD treatment, bd treatment, placebo) Randomisation: yes, computer generated code Blinding: double‐blind, dosing regimens (QD or bd) were not blinded Withdrawals: stated |
|
| Participants | Setting: multicentre study (95 sites), multiple country (11) Number enrolled: 911 Number in treatment group: 616 (308 QD, 308 bd) Number in control group: 295 Number of withdrawals (treatment/control): 194 (94 QD, 100 bd)/125 Number completing trial (treatment/control): 422 (214 QD, 208 bd)/170 Age range: ≥ 40, mean 65 Sex: M 622, F 289 Ethnicity: white = 787, non‐white = 124 COPD diagnosis: pre‐bronchodilator FEV1/FVC ratio ≤ 70%, post‐bronchodilator FEV1 30% to 70% predicted, low post‐bronchodilator FEV1 reversibility (< 10% of predicted normal) Severity of COPD: FEV1 50% to < 80% predicted = 266 (29%), FEV1 30% to < 50% predicted = 455 (44%), FEV1 < 30% predicted = 194 (21%) Inclusion criteria: diagnosis of COPD based on currently accepted criteria, and were current smokers who failed a mandatory smoking cessation program or self reported ex‐smokers who had stopped smoking ≥ 12 months before the study Exclusion criteria: clinical history of asthma or any other clinically significant medical illness other than COPD, COPD exacerbation within 3 months before the baseline visit, ventilator support for respiratory failure within the past year, lobectomy, pneumonectomy, lung volume reduction surgery, lung cancer within the past 5 years, nasal CPAP or oxygen use > 2 hours per day, initiation of pulmonary rehabilitation within the past 3 months, treatment with chronic or prophylactic antibiotics, inability to use the MF‐DPI inhaler, and < 80% adherence in recording diary data between screening and baseline Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | MF‐DPI 800 µg QD PM MF‐DPI 400 µg bd Placebo |
|
| Outcomes | Pulmonary function ‐ pre‐ and post‐bronchodilator FEV1, pre‐ and post‐bronchodilator FEF 25% to 75%, pre‐ and post‐bronchodilator FVC Exacerbations ‐ number and severity (hospitalisations, use of both oral steroid and antibiotic, or of oral steroids alone, as opposed to use of antibiotics alone) Symptom scores Health status ‐ SGRQ, SF‐36 Safety ‐ adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated code" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinding but dosing regimens not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts stated with reasons ‐ similar numbers |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Culpitt 1999.
| Methods | Design: cross‐over, 2 weeks washout Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: single‐centre study, hospital outpatient clinic Number eligible: 25 Number enrolled: 20 Number in treatment group: 20 Number in control group: 20 Number of withdrawals (treatment/control): 7 (cross‐over) Number completing trial (treatment/control): 13 (cross‐over) Age range: 43 to 73 years Sex: 13 M, 8 F Ethnicity: not stated COPD diagnosis: FEV1/FVC < 0.7, post‐bronchodilator FEV1 < 85% predicted, reversibility < 15% predicted FEV1 Severity of COPD: mean FEV1 49.5 % predicted at baseline Inclusion criteria: stable COPD, smoking history >= 20 pack‐years Exclusion criteria: use of inhaled or oral steroids, or exacerbation in previous 6 weeks, asthma, variability of symptoms, atopy Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 500 µg, 2 times a day (1000 µg/day) Placebo 2 times a day Metered‐dose inhaler via a spacer 4 weeks cross‐over |
|
| Outcomes | PEFR Use of reliever inhaler Dyspnoea score Cough score Sputum production Sputum colour Spirometry Sputum cytokine and enzyme assays | |
| Notes | Run‐in period 2 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 7 (cross‐over) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Derenne 1995.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: not stated | |
| Participants | Setting: multicentre study Number eligible: not stated Number enrolled: 194 (152 eligible for van Grunsven meta‐analysis) Number in treatment group: 81 Number in control group: 71 Number of withdrawals (treatment/control): not stated Number completing trial (treatment/control): not stated Age range: not stated Sex: not stated Ethnicity: not stated COPD diagnosis: FEV1 30% to 60% predicted Severity of COPD: not stated Inclusion criteria: age <= 75 years, "chronic bronchitis", FEV1 30% to 60% predicted, FEV1 reversibility < 10% predicted, PaO2 > 55 mmHg, usual treatment without corticosteroid, no exacerbation in the last 3 months Exclusion criteria: other pulmonary diseases, corticosteroids past 15 days, IgE > 200 IU/mL, eosinophils > 500 x 10E3/mL Baseline characteristics of treatment/control groups: not stated | |
| Interventions | BDP 1500 µg/d MDI Placebo 24 months | |
| Outcomes | Level of FEV1 Level of PEFR Duration of corticosteroid course | |
| Notes | Abstract only Details from van Grunsven meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Van Grunsven 1999 |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
Ferreira 2001.
| Methods | Design: cross‐over, 2 weeks washout Randomisation: yes, sealed envelopes Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: single‐centre study, hospital outpatient clinic Number eligible: not stated Number enrolled: 20 Number in treatment group: 20 (cross‐over) Number in control group: 20 (cross‐over) Number of withdrawals (treatment/control): 1 (cross‐over) Number completing trial (treatment/control): 19 (cross‐over) Age range: mean 69 years Sex: not stated Ethnicity: not stated COPD diagnosis: ATS guidelines Severity of COPD: mean post‐bronchodilator FEV1 55% predicted Inclusion criteria: >= 20 pack‐year smoking history and abstinence for >= 6 months Exclusion criteria: respiratory tract infection in 6 weeks before study, clinical instability (increased need for medication, emergency care or hospitalisation), other significant medical illnesses affecting eNO, systemic steroids in the month preceding, asthma Baseline characteristics of treatment/control groups: cross‐over | |
| Interventions | BDP 500 µg, 2 times a day (1000 µg/d) Matching placebo Metered‐dose inhaler 2 weeks | |
| Outcomes | Spirometry Exhaled nitric oxide Exhaled breath condensate: hydrogen peroxide | |
| Notes | ICS were withdrawn during run‐in, if patients were on ICS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 withdrawal |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Ferreira 2003.
| Methods | Design: cross‐over group Randomisation: yes, method not stated Blinding: double‐blind, double‐dummy Withdrawals: not stated | |
| Participants | Setting: single centre study Number eligible: 40 Number enrolled: 40 Number in treatment group: 20 Number in control group: 20 Number of withdrawals (treatment/control): not stated Number completing trial (treatment/control): not stated Age range: not stated Sex: not stated Ethnicity: not stated COPD diagnosis: not stated Severity of COPD: not stated Inclusion criteria: not stated Exclusion criteria: not stated Baseline characteristics of treatment/control groups: not stated | |
| Interventions | FP 1000 µg, 1 time a day (1000 µg/day) Placebo | |
| Outcomes | Exhaled nitric oxide levels FEV1 CRQ ‐ dyspnoea, fatigue, emotional function, master 6‐minute walk test | |
| Notes | Poster ATS 99th Conference 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random" (abstract) |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "triple blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
GSK 2005 (FCO30002).
| Methods | Design: parallel‐group, 2 weeks run‐in and 12 weeks treatment Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated |
|
| Participants | Setting: multicentre (32), Germany Number eligible: 210 Number enrolled: 207 Number in groups: FP 68, prednisolone‐FP 70, placebo 69 Number of withdrawals: FP 12, prednisolone‐FP 8, placebo 10 Number completing trial: FP 56, prednisolone‐FP 62, placebo 59 Mean age: FP 61, prednisolone‐FP 61, placebo 63 Sex (number F:M): FP 26:40, prednisolone‐FP 20:49, placebo 19:47 Ethnicity: not reported COPD diagnosis: not stated Inclusion criteria: documented history of COPD, age 40 to 79, FEV1 40% to 80% predicted, FEV1/FVC < 70%, increase of FEV1 < 10% at 30 minutes post salbutamol, symptomatic on ≥ 5 days and symptom score > 5 and/or salbutamol required, able to use Mini‐Wright peak‐flow‐meter and Diskus inhaler correctly Exclusion criteria: long‐term oxygen therapy, use of inhaled or systemic corticosteroids during 8 weeks prior to study entry, acute exacerbation or antibiotic treatment or hospital stay within 4 weeks before study entry, use of beta‐blockers within 2 weeks before study entry Baseline characteristics: comparable |
|
| Interventions | Run‐in period: all participants received salmeterol 50 μg bd as bronchodilator treatment and salbutamol MDI as rescue medication Treatment‐period: salmeterol 50 μg bd was continued throughout the study. At visit 2, participants were randomised into one of 3 groups and received (in addition to salmeterol) either Placebo tablets for 2 weeks plus fluticasone 500 μg bd for 12 weeks OR Prednisolone tablets (20 to 40 mg per day, depending on body weight) plus placebo Diskus™ for 2 weeks, then switch to Fluticasone 500 μg bd for the following 10 weeks OR Placebo tablets for 2 weeks plus Placebo Diskus™ for 12 weeks | |
| Outcomes | Primary: Change in FEV1 (L) after 12 weeks of treatment compared with baseline at randomisation Secondary: participants' self assessment of exercise capacity (oxygen cost diagram) Morning serum cortisol concentrations |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" ‐ method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals stated; similar |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
GSK 2005 (FLTA3025).
| Methods | Design: parallel‐group, 2 weeks placebo run in then 6 months treatment Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated |
|
| Participants | Setting: 55 centres in the United States Number eligible: unknown Number enrolled: 640 Number in groups: placebo 206, FP 200 216, FP 500 218 Number of withdrawals: placebo 79, FP 250 76, FP 500 71 Number completing trial: placebo 127, FP 250 140, FP 500 147 Mean age: placebo 64.8, FP 250 65.2, FP 500 63.3 Sex (number MF:M): placebo 66:140, FP 250 60:156, FP 500 74:144 Ethnicity (white, n): placebo 196, FP 250 204, FP 500 206 COPD diagnosis: not stated Inclusion criteria: COPD diagnosis; at least 40 years old; current or prior 20 pack‐years smoking; productive cough most days for at least 3 months of year, for at least 2 years, and not attributable to another disease process; baseline FEV1 < 65% predicted normal but > 0.70 litres (L) or FEV1 ≤ 0.70 L and > 40% of predicted normal and FEV1/forced vital capacity (FVC) ratio of < 0.70; score of ≥ 2 on the Modified Medical Research Council (MMRC) Dyspnea Scale at screening and a score of ≥ 4 on the CBSQ at randomisation, and had not received systemic corticosteroids or high‐dose inhaled corticosteroid therapy for at least 6 months prior to screening Exclusion criteria: current diagnosis of asthma, concurrent participation in a pulmonary rehabilitation programme, a respiratory disease other than COPD or other significant concurrent disease, an abnormal and clinically significant ECG at screening, and the occurrence of a moderate or severe COPD exacerbation during the run‐in period. Concurrent use of the following respiratory medications was not allowed: beta‐agonists (other than salbutamol), cromolyns, corticosteroids (oral, inhaled and intranasal), anti‐leukotrienes and ipratropium. Use of systemic corticosteroids for the treatment of a COPD exacerbation required subject withdrawal. Baseline characteristics: comparable |
|
| Interventions | Following the 2‐week placebo run‐in period, eligible participants were randomised (1:1:1) to 1 of 3 treatments administered via the DISKUS™ multidose powder inhaler for 6 months (24 weeks): FP 250µg bd, FP 500µg bd or placebo bd. Salbutamol was provided as supplemental medication for the duration of the study. | |
| Outcomes | Primary: morning pre‐dose FEV1 at endpoint. Endpoint was defined as the last pre‐dose FEV1 during the treatment period for each participant. Secondary: Chronic Bronchitis Symptoms Questionnaire (CBSQ) score; Transition Dyspnea Index (TDI) score; exacerbations of COPD (incidence, severity and time to first exacerbation); participant‐recorded daily morning peak expiratory flow rate (PEFR); supplemental salbutamol use; and night‐time awakenings requiring salbutamol. Quality of life was assessed by the Chronic Respiratory Disease Questionnaire (CRDQ). A mild exacerbation was defined as use of more than 12 puffs or 4 nebules of salbutamol on 2 consecutive days. A moderate exacerbation was defined as use of either antibiotics and/or oral or inhaled corticosteroids for treatment of worsening COPD symptoms, and a severe exacerbation was an exacerbation requiring in‐patient hospitalisation. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double blind" ‐ method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts stated and reasons given ‐ similar numbers |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Guenette 2011.
| Methods | Design: cross‐over group Randomisation: yes, method not stated Blinding: double‐blind, placebo‐controlled Withdrawals: stated | |
| Participants | Setting: hospital outpatient clinic Number eligible: not stated Number enrolled: 17 Number in groups: 17 (cross‐over) Number of withdrawals: 0 Number completing trial: 17 Age range: > 40 years old Sex: 12 M, 5 F Ethnicity: not stated (multicentre) COPD diagnosis: clinically stable COPD patients Severity of COPD: mean FEV1 54% predicted Inclusion criteria: > 40 years with a clinical diagnosis of COPD for at least 1 year, smoking > 20 pack‐years, FEV1 < 70% predicted, FEV1/FVC < 0.7, FRC > 120% predicted and moderate to severe chronic activity‐related dyspnoea as evidenced by a modified baseline Dyspnoea Index focal score < 6 Exclusion criteria: asthma, other condition leading to dyspnoea, hospitalised or lower respiratory tract infection 4 week prior, oxygen saturation < 80% during exercise Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 500 µg twice daily Placebo twice daily 2 weeks (with 2 weeks washout) |
|
| Outcomes | Borg dyspnoea score during exercise, cycle endurance, spirometry, lung volumes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Hanania 2003.
| Methods | Design: parallel‐group Randomisation: yes, method not stated (stratified by reversibility and site) Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: multicentre study, hospital outpatient clinic Number eligible: 1489 Number enrolled: 723 Number in groups: FP/SM 178, FP 183, SM 177, placebo 185 Number of withdrawals: FP/SM 53, FP 49, SM 57, placebo 59 Number completing trial: FP/SM 125, FP 134, SM 120, placebo 126 Age range: 40 to 84 years for FP versus placebo Sex: 247 M, 121 F Ethnicity: not stated (multicentre) COPD diagnosis: ATS criteria, FEV1/FVC ratio <= 70%, FEV1 < 65% predicted and > 0.70 L Severity of COPD: mean FEV1 42% predicted Inclusion criteria: current or former smokers with >= 20 pack‐year history, chronic bronchitis, moderate dyspnoea Exclusion criteria: current asthma, oral steroids in previous 6 weeks, abnormal clinically significant ECG, long‐term oxygen therapy, moderate or severe exacerbation in run‐in, significant medical disorder Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 250 µg, 2 times a day (500 µg/d) Salmeterol 50 µg, 2 times a day FP 250 µg/salmeterol 50 µg, 2 times a day Placebo Diskus 24 weeks | |
| Outcomes | Predose FEV1 2 hour post dose FEV1 Morning PEFR Dyspnoea (Transitional Dyspnoea Index) Supplemental salbutamol use Health status (CRDQ) Symptoms of chronic bronchitis (CBSQ) Exacerbations Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 27% FP, 32% placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Hattotuwa 2002.
| Methods | Design: parallel‐group Randomisation: yes, random number table Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: hospital outpatient clinic Number eligible: not stated Number enrolled: 37 Number in treatment group: 17 Number in control group: 19 Number of withdrawals (treatment/control): 1/5 + 1 insufficient biopsy Number completing trial (treatment/control): 16/14 Age range: 40 to 75 years Sex: 26 M, 4 F Ethnicity: not stated COPD diagnosis: FEV1 25% to 80% of predicted Severity of COPD: mean FEV1 % predicted FP 46.2%, placebo 45.5% Inclusion criteria: current or ex‐smokers > 20 pack‐years Exclusion criteria: atopy, acute bronchodilator reversibility, severe concurrent medical problems, chest infection within 8 weeks before study Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 500 µg, 2 times a day (1,000 µg/d) Placebo Multidose dry powder inhaler (Accuhaler) 3 months | |
| Outcomes | Peak flow Symptom score Spirometry Exhaled carbon monoxide Bronchoscopy Exacerbations | |
| Notes | Run‐in 8 weeks Had steroid trial (prednisolone 30 mg, 2 weeks) after cessation of FP for 1 month |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number table" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals: 1 FP, 5 placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
John 2005.
| Methods | Design: cross‐over group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: not stated | |
| Participants | Setting: not stated Number eligible: not stated Number enrolled: 22 Number in treatment group: 11 Number in control group: 11 Number of withdrawals (treatment/control): not stated Number completing trial (treatment/control): 11/11 Age range: placebo: mean 51.36 years; ICS: mean 61.82 years Sex: 10 M, 12 F Ethnicity: not stated COPD diagnosis: GOLD guidelines (5 patients mild, 6 patients moderate) Severity of COPD: placebo FEV1 99.9% predicted; ICS FEV 77% predicted Inclusion criteria: COPD, clinically stable, no previous hospital admission or treatment change in the last 3 months, none received oral corticosteroids in the preceding 8 weeks Exclusion criteria: current or past Dx of asthma, RTI in past 2 weeks, cancer, thyroid disease, severe liver disease, chronic heart failure Baseline characteristics of treatment/control groups: comparable | |
| Interventions | BDP 400 µg, 2 times a day (800 µg/day) Placebo Short acting β2 agonists or theophylline for symptom relief | |
| Outcomes | SGRQ score ‐ symptom, activity, impact Pulmonary function ‐ FEV1, VC, FVC, PEF, TLC Peripheral blood monocytes IL‐10, IFN‐γ, MIP‐1, GM‐CSF Serum cortisol levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Kerstjens 1992.
| Methods | Design: parallel‐group Randomisation: yes, computer‐generated Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: multicentre study, hospital outpatient clinic Number eligible: not stated Number enrolled: 274 (whole study); 182 in subgroup of BDP versus placebo Number in treatment group: 91 (BDP) Number in control group: 91 Number of withdrawals (treatment/control): 12/44 Number completing trial (treatment/control): 79/47 Age range: 18 to 60 years Sex: 117 M, 65 F Ethnicity: Caucasian COPD diagnosis: patients with obstructive airways disease and bronchial hyper‐responsiveness to histamine (asthma, COPD, asthmatic bronchitis or undefined) were recruited. COPD was diagnosed in current or former smokers without a history of asthmatic attacks who reported either chronic cough with or without sputum production or dyspnoea when walking quietly on level ground, or both Severity of COPD: pre‐bronchodilator FEV1 64.6% predicted (BDP group), 63.3% predicted Inclusion criteria: FEV1 between 4.5 and 1.64 residual standard deviations (SDs) below predicted, or the ratio of FEV1 to inspiratory VC was less than 1.64 residual SDs below predicted provided that the TLC was more than 1.64 residual SDs below predicted; bronchial hyper‐responsiveness: PC20 (histamine) <= 9 mg/mL Exclusion criteria: presence of major illnesses Baseline characteristics of treatment/control groups: comparable, except that the BDP group was slightly less hyper‐responsive | |
| Interventions | Terbutaline 250 µg, 2 puffs, 4 times a day with either:
BDP 100 µg, 2 puffs, 4 times a day (800 µg/day)
or ipratropium 20 µg, 2 puffs, 4 times a day
or placebo Placebo, 2 puffs, 4 times a day Metered‐dose inhaler 3 years |
|
| Outcomes | FEV1 Bronchodilator reversibility PC20 to histamine | |
| Notes | Run‐in period of 4 weeks Study was terminated early because of predefined, significant differences in the withdrawal rate, FEV1 and PC20 Only the ICS versus placebo comparison is analysed in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was performed by telephoning an independent center that used a computerized minimization method with stratification" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was performed by telephoning an independent center that used a computerized minimization method with stratification" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind regimens" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rate of withdrawals in corticosteroid group (12 patients) differed from the placebo group (44 patients) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Lapperre 2009.
| Methods | Design: parallel‐group Randomisation: yes, performed by independent randomisation centre Blinding: double‐blind Withdrawals: stated, reasons not stated |
|
| Participants | Setting: primary care, Netherlands Number eligible: 4617 Number enrolled: 114 Number in treatment group: 26 (FP 30 months), 31 (FP 6 months, then placebo), 28 (FP/salmeterol) Number in control group: 29 Number of withdrawals: 4 (FP 30 months), 3 (FP 6 months, then placebo), 4 (FP/salmeterol), 4 (placebo) Number completing trial: 22 (FP 30 months), 23 (FP 6 months, then placebo), 21 (FP/salmeterol), 20 (placebo) Age range: 45 to 75 Sex (M/F): 20/4 (FP 30 months), 22/4 (FP 6 months, then placebo), 23/3 (FP/salmeterol), 22/3 (placebo) Ethnicity: not stated COPD diagnosis: Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages II and III Severity of COPD: as above Inclusion criteria: age 45 to 75, current or former smokers, smoked for 10 or more pack‐years, lung function levels compatible with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages II and III Exclusion criteria: asthma and receipt of ICS within 6 months before random assignment Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 500µg twice daily for the first 6 months followed by placebo twice daily for 24 months; FP 500 µg twice daily for 30 months; FP 500 µg twice daily and salmeterol 50 µg twice daily in a single inhaler for 30 months; or placebo twice daily for 30 months | |
| Outcomes | Primary: inflammatory cell counts in bronchial biopsies and induced sputum Secondary: post‐bronchodilator spirometry and hyper‐responsiveness to methacholine PC20, dyspnoea (modified Medical Research Council dyspnoea scale), health status (St George's Respiratory Questionnaire, Clinical COPD Questionnaire) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised by independent randomisation center" |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts stated ‐ similar |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Laptseva 2002.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: not stated | |
| Participants | Setting: not stated Number eligible: 49 Number enrolled: 49 Number in treatment group: 25 Number in control group: 24 Number of withdrawals (treatment/control): ? Number completing trial (treatment/control): 25/24 Age range: 45 to 65 years Sex: not stated Ethnicity: not stated COPD diagnosis: FEV1 40% to 60% of predicted normal, FEV1/VC < 55%, bronchodilator reversibility of < 15% Severity of COPD: not stated Inclusion criteria: COPD as defined above Exclusion criteria: not stated Baseline characteristics of treatment/control groups: comparable | |
| Interventions | BUD 400 µg, 2 times a day (800 µg/d) Placebo | |
| Outcomes | Number and severity of exacerbations FEV1 FVC FEV50 Diary card symptoms PEFR | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available (Abstract) |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
LHS 2000.
| Methods | Design: parallel‐group Randomisation: yes, computer generated ‐ correspondence from Melissa Skeans 11 July 2002 ‐ (participants and staff unaware of treatment allocation), stratified by centre and smoking status Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: multicentre study, USA and Canada, hospital outpatient clinics Number eligible: 1347 Number enrolled: 1116 Number in treatment group: 559 Number in control group: 557 Number of withdrawals (treatment/control): 28/38 Number completing trial (treatment/control): 531/519 Age range: 40 to 69 years Sex: 704 M, 412 F Ethnicity: non‐white race 6.3% of intervention group, 4.1% of placebo group COPD diagnosis: FEV1/FVC < 0.7, FEV1 30% to 90% predicted Severity of COPD: mean post‐bronchodilator FEV1 68.5% predicted in intervention group, 67.2% predicted in placebo group Inclusion criteria: current smokers or quit smoking within previous 2 years Exclusion criteria: medical conditions such as cancer, recent myocardial infarction, alcoholism, heart failure, IDDM, neuropsychiatric disorders; use of bronchodilators, oral or inhaled steroids in previous year Baseline characteristics of treatment/control groups: comparable | |
| Interventions | TAA 600 µg, 2 times a day (1200 µg/day) Placebo 2 times a day Metered‐dose inhaler (identical) Mean duration 40 months |
|
| Outcomes | Change in pre‐bronchodilator FEV1 over time Change in pre‐bronchodilator FVC over time Change in post‐bronchodilator FEV1 over time Change in post‐bronchodilator FVC over time Daily cough and phlegm >= 3 months/year Highest dyspnoea level Highest wheezing level No. of new or increased respiratory symptoms categorised as moderate or severe No. of hospitalisations No. of emergency department visits, not resulting in hospitalisation No. of outpatient physician visits No. of health care visits Cause‐specific morbidity and mortality PC20 methacholine Health‐related quality of life (SF‐36) Side effects Bone mineral density | |
| Notes | Intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation....randomly assigned...according to center" |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants and clinical center staff were unaware of study‐drug assignments" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 28 TAA, 38 placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Llewellyn‐Jones 1996.
| Methods | Design: parallel‐group Randomisation: yes, computer‐generated (information from GSK, 13 May 2002) Blinding: double‐blind, double dummy (information from GSK, 13 May 2002) Withdrawals: stated | |
| Participants | Setting: single‐centre study, UK hospital clinic, home diary Number eligible: not stated Number enrolled: 17 Number in treatment group: 8 Number in control group: 9 Number of withdrawals (treatment/control): 0/1 (infective exacerbation) Number completing trial (treatment/control): 8/8 Age range: 50 to 75 years Sex: 8 M, 8 F Ethnicity: not stated COPD diagnosis: smoking‐related chronic bronchitis and emphysema Severity of COPD: mean RV/TLC 58.3% predicted, mean KCO 43.4% predicted in placebo group; mean RV/TLC 51.2% predicted, mean KCO 52.7% predicted in treatment group Inclusion criteria: chronic bronchitis and clinical evidence of emphysema (radiological hyperinflation, airflow obstruction, hyperinflation, reduced gas transfer) Exclusion criteria: inhaled or oral steroids in preceding 3 months Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 750 µg, 2 times a day (1500 µg/day) Placebo 2 times a day Inhaler, volumatic spacing device 8 weeks |
|
| Outcomes | Daily symptoms of breathlessness, cough, general well‐being Morning peak flow rate at study completion Evening peak flow rate at study completion Sputum volume and colour (reported) Sputum volume (4‐hour collection) Sputum chemotactic activity, elastase inhibitory capacity, albumin concentration, myeloperoxidase activity, fluticasone propionate concentration Blood neutrophil function Serum albumin concentration Acute infective exacerbations Spirometry | |
| Notes | No difference in spirometry reported between intervention and control groups at study completion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 withdrawal from placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Loppow 2001.
| Methods | Design: cross‐over, 4 weeks washout Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: single‐centre study, Germany, hospital outpatient clinic Number eligible: not stated Number enrolled: 19 Number in treatment group: 19 (cross‐over) Number in control group: 19 (cross‐over) Number of withdrawals (treatment/control): 0/0 Number completing trial (treatment/control): 19/19 Age range: 31 to 77 years Sex: 12 M, 7 F Ethnicity: not stated COPD diagnosis: chronic bronchitis (ATS criteria, cough and sputum production), current or ex‐smokers > 20 pack‐years Severity of COPD: mean FEV1 83.4% predicted (2 patients had no airflow obstruction) Inclusion criteria: as for COPD diagnosis; 14 patients had bronchial hyper‐responsiveness (PC20 methacholine < 8 mg/mL) Exclusion criteria: use of inhaled or systemic corticosteroids in previous 3 months, respiratory tract infection in previous 4 weeks Baseline characteristics of treatment/control groups: cross‐over study | |
| Interventions | FP 1000 µg/day Placebo Delivery device not stated 4 weeks each treatment period (cross‐over) |
|
| Outcomes | FEV1 VC Exhaled NO Induced sputum cell count Induced sputum fluid‐phase markers (LCH, ECP, elastase, IL‐8, iNOS) | |
| Notes | Chronic bronchitis patients included, not only COPD with airflow obstruction 14/19 patients had bronchial hyper‐responsiveness (PC20 MCh < 8 mg/mL) and 6/19 had positive skin prick test |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Mahler 2002.
| Methods | Design: parallel‐group Randomisation: yes, method not stated (stratified by centre and reversibility) Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: multicentre study, hospital outpatient clinic Number eligible: 1352 Number enrolled: 691 (645 with evaluable data) Number in groups: SM/FP 165, SM 160, FP 168, placebo 181 Number of withdrawals: SM/FP 52, SM 46, FP 69, placebo 69 Number completing trial: SM/FP 113, SM 114, FP 99, placebo 112 Age range: 42 to 90 years for FP versus placebo Sex: 239 M, 110 F Ethnicity: not stated (multicentre) COPD diagnosis: ATS criteria, FEV1/FVC <= 70%, FEV1 < 65% predicted and more than 0.70 L Severity of COPD: mean 41% predicted Inclusion criteria: current or former smokers with >= 20 pack‐year history, chronic bronchitis, dyspnoea Exclusion criteria: current asthma, oral steroid use in previous 6 weeks, abnormal clinically significant ECG, long‐term oxygen therapy, moderate or severe exacerbation during run‐in, clinically significant medical disorder Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 500 µg, 2 times a day (1000 µg/day) Salmeterol 50 µg, 2 times a day FP 500 µg/salmeterol 50 µg, 2 times a day Placebo Diskus 24 weeks | |
| Outcomes | Change in predose FEV1 Change in 2 hour post dose FEV1 Morning PEFR Supplemental salbutamol use Dyspnoea (Transition Dyspnoea Index) Chronic Bronchitis Symptom Questionnaire Exacerbations Chronic Respiratory Disease Questionnaire Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 40% FP, 38% placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Mirici 2001.
| Methods | Design: parallel‐group Randomisation: yes, computer‐generated Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: single‐centre study, hospital outpatient clinic Number eligible: 96 Number enrolled: 50 Number in treatment group: 25 Number in control group: 25 Number of withdrawals (treatment/control): 5/5 Number completing trial (treatment/control): 20/20 Age range: mean 52 year BUD, mean 54 year placebo Sex: 30 M, 10 F Ethnicity: not stated COPD diagnosis: FEV1 < 70% predicted with no self reported asthma Severity of COPD: mean 64.1% predicted BUD, mean 59.9% predicted placebo Inclusion criteria: FEV1 reversibility with bronchodilator < 15%, smokers Exclusion criteria: long‐term treatment with oral or inhaled corticosteroids within 6 months of study entry, respiratory tract infection in previous 3 months, pregnancy or lactation, other serious systemic diseases Baseline characteristics of treatment/control groups: comparable | |
| Interventions | BUD 400 µg, 2 times a day (800 µg/day) Placebo Turbuhaler 12 weeks | |
| Outcomes | Sputum cell count Spirometry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation masked, computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation masked, computer generated" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 withdrawals in both groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Nishimura 1999.
| Methods | Design: cross‐over, no washout Randomisation: yes, computer‐generated (correspondence from Dr Koyama, 3 June 2002) Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: single‐centre study, Japan, hospital outpatient clinic Number eligible: not stated Number enrolled: 34 Number in treatment group: 34 (cross‐over) Number in control group: 34 (cross‐over) Number of withdrawals (treatment/control): 4 withdrawals Number completing trial (treatment/control): 30 (cross‐over) Age range: > 55 years Sex: 29 M, 1 F (of the 30 who completed the study) Ethnicity: not stated COPD diagnosis: smoking > 20 pack‐years, chest radiographs showing hyperinflation, post‐bronchodilator FEV1/FVC < 0.7, FEV1 < 80% predicted Severity of COPD: mean FEV1 37.4% predicted Inclusion criteria: stable (no acute exacerbation of airflow obstruction within last 3 months) Exclusion criteria: asthma, heart disease, any other significant medical condition, use of inhaled or oral steroids in last 3 weeks Baseline characteristics of treatment/control groups: cross‐over | |
| Interventions | BDP 750 µg, 4 times a day (3000 µg/day) Placebo 4 times a day Metered‐dose inhaler with spacer device 4 weeks each treatment period (cross‐over) |
|
| Outcomes | Change from baseline pre‐bronchodilator FEV1 Change from baseline pre‐bronchodilator FVC Change from baseline post‐bronchodilator FEV1 Change from baseline post‐bronchodilator FVC Peak flow (last 14 days of 4‐week period) Symptoms (last 14 days of 4‐week period) Adverse effects Serum osteocalcin Serum cortisol | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 4 (cross‐over) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Ozol 2005.
| Methods | Design: parallel‐group Randomisation: yes, computer‐generated Blinding: double‐blind Withdrawals: stated |
|
| Participants | Setting: single‐centre study, Turkey Number eligible: not stated Number enrolled: 26 Number in treatment group: 13 Number in control group: 13 Number of withdrawals (treatment/control): 1/3 Number completing trial (treatment/control): 12/10 Age range: mean 65 years Sex: 18 M, 4 F Ethnicity: not stated COPD diagnosis: stable mild to moderate COPD (GOLD criteria) (mild = > 80% predicted FEV1; moderate = 50% to 80% predicted FEV1) Severity of COPD: FEV1 61.1% predicted (BUD); FEV1 57.3% predicted (placebo) Inclusion criteria: FEV1/FVC < 70%, FEV1 > 50% predicted; reversibility of < 200 mL with inhaled salbutamol or less than 12% predicted FEV1; stable COPD; no other systemic or pulmonary disease; no therapy with inhaled or systemic corticosteroids within 3 months; no history asthma or atopy Exclusion criteria: not stated Baseline characteristics of treatment/control groups: comparable | |
| Interventions | BUD 400 µg, 2 times a day (800 µg/day) Placebo | |
| Outcomes | Spirometry BAL cell counts and via bronchoscopy IL8 count Weekly diary ‐ change in cough, dyspnoea, sputum production noted | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised by computer‐generated, blinded randomisation list" |
| Allocation concealment (selection bias) | Low risk | Quote: "treatment randomly assigned" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 1 BUD, 2 placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Paggiaro 1998.
| Methods | Design: parallel‐group Randomisation: yes, computer‐generated, sealed envelopes Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: multicentre study, hospital outpatient clinics: Europe, New Zealand, South Africa Number eligible: 365 entered run‐in (of which 84 withdrew before randomisation) Number enrolled: 281 Number in treatment group: 142 Number in control group: 139 Number of withdrawals (treatment/control): 19/27 Number completing trial (treatment/control): 123/112 Age range: 50 to 75 years Sex: M, F Ethnicity: not stated COPD diagnosis: European Respiratory Society definition: decreased maximum expiratory flow and slow forced emptying of lung, slowly progressive, irreversible, not changing markedly over several months Severity of COPD: mean pre‐bronchodilator FEV1 59% predicted in intervention group, 55% predicted in placebo group Inclusion criteria: current or ex‐smokers (>= 10 pack‐years), chronic bronchitis, at least one exacerbation each year for previous 3 years, high expectation of experiencing exacerbation during study period, regular productive cough, FEV1 35% to 90% predicted, FEV1/FVC <= 70%, FEV1 reversibility < 15% after 400 µg (MDI) or 800 µg (Diskhaler) salbutamol (or > 15% but < 200 mL) At end of 2‐week run‐in: required total symptom score of 4 or more from at least 4 of 14 days of run‐in to be included Exclusion criteria: abnormal chest radiograph; current use of fluticasone; within last 4 weeks: use of oral or depot steroids, inhaled steroids of > 500 µg/day, antibiotics or hospital admission Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 500 µg, 2 times a day (1000 µg/day) Placebo 2 times a day Metered‐dose inhaler (identical), spacers in some patients 6 months |
|
| Outcomes | Exacerbations (defined as worsening of COPD symptoms, requiring changes to normal treatment: mild, self managed; moderate, treatment by family physician or as hospital outpatient; severe, admission to hospital) Change in FEV1 compared to baseline Change in FVC compared to baseline Treatment efficacy 6‐minute walk distance Borg score before and after 6‐minute walk Control of symptoms (4‐point scale) Morning peak flow rate Evening peak flow rate Symptom scores for cough, breathlessness, sputum volume, sputum colour Use of rescue medications Serum cortisol | |
| Notes | Intention‐to‐treat analysis Total number of exacerbations in intervention and control groups described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random numbers were computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 19 in fluticasone group, 27 in placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Pauwels 1999.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: multicentre study, Europe, hospital outpatient clinics Number eligible: 2157 Number enrolled: 1277 Number in treatment group: 634 Number in control group: 643 Number of withdrawals (treatment/control): 176/189 Number completing trial (treatment/control): 458/454 Age range: 30 to 65 years Sex: 923 M, 354 F Ethnicity: not stated COPD diagnosis: post‐bronchodilator FEV1 50% to 100% predicted, pre‐bronchodilator FEV1/VC ratio < 70%, increase in FEV1 < 10% predicted after 1 mg terbutaline (dry‐powder inhaler) Severity of COPD: mean pre‐bronchodilator FEV1 76.8% predicted in intervention group, 76.9% predicted in placebo group Inclusion criteria: currently smoking at least 5 cigarettes/day, smoked for at least 10 years or smoking history at least 5 pack‐years, change in FEV1 between end of 1st and end of 2nd 3 month run‐in phases < 15%, > 75% compliance with inhaler Exclusion criteria: history of asthma, allergic rhinitis, allergic eczema; use of oral steroids > 4 weeks during preceding 6 months Baseline characteristics of treatment/control groups: comparable | |
| Interventions | BUD 400 µg, 2 times a day (800 µg/day) Placebo 2 times a day Dry powder inhaler (Turbuhaler) 3 years |
|
| Outcomes | Change in post‐bronchodilator FEV1 over time Adverse events Skin bruises > 50 mm diameter Vertebral fractures on radiographs Bone mineral density | |
| Notes | Run‐in phase: 3‐month smoking cessation programme Continuing smokers had further 3 months of inhaled medication to check compliance Intention‐to‐treat analysis Data reported as non‐normal Results: 0 to 6 month: FEV1 improved by median 17 mL/year in intervention group, declined by median 81 mL/year in placebo group (P < 0.001) 9 to 36 month: FEV1 declined by median 57 mL/year in intervention group, declined by median 69 mL/year in placebo group (P = 0.39) Stratification by smoking history: <= 36 pack‐years: FEV1 declined by median 120 mL in 3 year in intervention group, declined by median 190 mL in 3 year in placebo group (P < 0.001) > 36 pack‐years: FEV1 declined by median 150 mL in 3 year in intervention group, declined by median 160 mL in 3 year in placebo group (P = 0.57) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 176 BUD, 189 placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Renkema 1996.
| Methods | Design: parallel‐group Randomisation: yes, computer‐generated, stratified for smoking Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: single‐centre study, The Netherlands, hospital outpatient clinic Number eligible: not stated Number enrolled: 39 (of the 58 in the 3 arms of the study) Number in treatment group: 21 Number in control group: 18 Number of withdrawals (treatment/control): 2/5 Number completing trial (treatment/control): 19/13 Age range: adult, < 70 years Sex: 58 M, 0 F Ethnicity: not stated COPD diagnosis: clinical diagnosis of COPD based on history (persistent dyspnoea, mainly on exertion, without sudden attacks of dyspnoea), FEV1 < 80% predicted, RV > 100% predicted, Severity of COPD: mean FEV1 67% predicted in intervention group, 60% predicted in placebo group Inclusion criteria: smokers or ex‐smokers, stable phase of disease, specific compliance post‐bronchodilator > 100% predicted (or < 100% allowed if air trapping > 1.5 L) Exclusion criteria: allergy (positive skin prick test, total serum IgE > 200 IU/mL, blood eosinophils > 250 x 10^3/mL), older than 70 years, receiving continuous steroid therapy, severe concomitant disease, abnormal alpha1‐antitrypsin serum levels Baseline characteristics of treatment/control groups: comparable | |
| Interventions | BUD 800 µg, 2 times a day (1600 µg/day) Placebo 2 times a day Metered‐dose inhaler and spacer (Nebuhaler) 2 years |
|
| Outcomes | Change in pre‐bronchodilator FEV1 over time Cough score Sputum score Wheeze score Dyspnoea score Complaint score Exacerbations Plasma cortisol | |
| Notes | Run‐in period: 3 months Third treatment arm: BUD 800 µg, 2 times a day (1600 µg/d) plus oral prednisolone 5 mg once daily (data not included for this review) Steroid responsiveness assessed by oral prednisolone 40 mg/day for 8 days Medians presented for decline in FEV1, due to skewness and large spread of distribution |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "allocated blindly (by computerized randomisation stratified for smoking)" |
| Allocation concealment (selection bias) | Low risk | Quote: "allocated blindly (by computerized randomisation stratified for smoking)" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals from BUD group, 5 withdrawals from placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Robertson 1986.
| Methods | Design: cross‐over, 2 weeks washout Randomisation: yes, method not stated (correspondence from Professor Burge ‐ 15 October 2002: double‐blind, allocation concealment used) Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: single‐centre study, hospital outpatient clinic Number eligible: 83 Number enrolled: 83 Number in treatment group: 83 (cross‐over) Number in control group: 83 (cross‐over) Number of withdrawals (treatment/control): not stated Number completing trial (treatment/control): not stated Age range: mean 61 years (SD 10) Sex: 66 M, 17 F Ethnicity: no stated COPD diagnosis: FEV1 < 70% predicted Severity of COPD: mean FEV1 44% predicted Inclusion criteria: COPD of at least 5 years duration, chronic bronchitis on MRC questionnaire, 92% were current or ex‐smokers Exclusion criteria: asthma, steroid therapy in previous 6 months Baseline characteristics of treatment/control groups: comparable (cross‐over) | |
| Interventions | BDP 500 µg, 3 times a day (1500 µg/day) Placebo 3 times a day Metered‐dose inhaler 2 weeks each treatment period (cross‐over) |
|
| Outcomes | FEV1 in treatment period compared to baseline or placebo FVC in treatment period compared to baseline or placebo PEFR in treatment period compared to baseline or placebo | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correspondence from Professor Burge ‐ double‐blind, allocation concealment used Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Correspondence from Professor Burge ‐ double‐blind, allocation concealment used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Correspondence from Professor Burge ‐ double‐blind, allocation concealment used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | Outcomes all reported |
Rutgers 1998.
| Methods | Design: parallel‐group Randomisation: yes, computer‐generated (correspondence from Dr Rutgers, 2 June 2002) Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: single‐centre study, The Netherlands, hospital outpatient clinic Number eligible: 49 Number enrolled: 44 Number in treatment group: 22 Number in control group: 22 Number of withdrawals (treatment/control): 1/4 Number completing trial (treatment/control): 21/18 Age range: 45 to 75 years Sex: 35 M, 9 F Ethnicity: not stated COPD diagnosis: American Thoracic Society criteria Severity of COPD: mean FEV1 58% predicted in intervention group, 62% predicted in placebo group Inclusion criteria: current smoker, FEV1 and FEV1/VC < % predicted minus 1.64 residual SD, increase in FEV1 < 10% predicted after 1 mg terbutaline via Turbuhaler, hyper‐responsiveness to MCh (PC20 MCh < 8.0 mg/mL) and AMP (PC20 AMP <= 80 mg/mL) Exclusion criteria: history of atopy, positive skin test, for aeroallergens, specific serum IgE for aeroallergens, serum eosinophil count > 400 x 10^9/mL; in 1 month prior to study: acute upper respiratory tract infection, use of oral or inhaled steroids, antibiotics, mucolytics, theophylline Baseline characteristics of treatment/control groups: comparable | |
| Interventions | BUD 1600 µg/day Placebo Dry powder inhaler (Turbuhaler) 6 weeks |
|
| Outcomes | Pre‐bronchodilator FEV1 MCh challenge AMP challenge Symptom score Bronchodilator use Morning peak flow Evening peak flow Serum IL‐8 Serum histamine | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated (correspondence from Dr Rutgers, 2/6/2002) |
| Allocation concealment (selection bias) | Unclear risk | Computer generated (correspondence from Dr Rutgers, 2/6/2002) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind, double‐dummy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 1 BUD, 4 placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Schermer 2009.
| Methods | Design: parallel‐group Randomisation: yes, randomisation list generated by statistician Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: 44 general practices, Netherlands Number eligible: 442 Number enrolled: 300 Number in groups: FP 94, NAC 96, placebo 96 Number of withdrawals: FP 39, NAC 44, placebo 40 Number completing trial: FP 55, NAC 55, placebo 56 Mean age: FP 58.4, NAC 59.2, placebo 59.6 % male: FP 69, NAC 75, placebo 65 Ethnicity: not stated Inclusion criteria: age 35 to 75 years; current or former smoker; chronic dyspnoea, sputum production and cough for at least 3 consecutive months per year during the previous 2 years; post‐bronchodilator FEV1 < 90% of the predicted value, and/or post‐bronchodilator FEV1/FVC (forced vital capacity) of the predicted value < 88% for men and < 89% for women Exclusion criteria: post‐bronchodilator FEV1 < 40% of predicted and/or a history of asthma, allergic rhinitis or allergic eczema Baseline characteristics of treatment/control groups: comparable | |
| Interventions | 2 week run‐in with oral prednisolone, then 3 years fluticasone propionate 500 mg twice daily administered as dry powder inhalation by Diskus, oral N‐acetylcysteine 600 mg once daily in the morning, or matching placebo treatment | |
| Outcomes | Primary: exacerbation rate, quality of life measured with the Chronic Respiratory Questionnaire (CRQ) Secondary: FEV1 decline, respiratory symptoms |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation list generated by statistician |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts stated ‐ similar numbers |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
SCO30002 2005.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: multicentre study, hospital outpatient clinic Number eligible: 300 Number enrolled: 256 Number in treatment group: 131 FP arm Number in control group: 125 Number of withdrawals (treatment/control): 34/40 Number completing trial (treatment/control): 97/85 Age range: mean age 64.6 years FP arm, 65.7 placebo arm Sex: 209 M, 47 F Ethnicity: 100% Caucasian COPD diagnosis: pre‐bronchodilator baseline FEV1/VC < 88% for men and < 89% for women of predicted normal and FEV1 < 70% of predicted normal, but > 800 mL Inclusion criteria: aged > 40 years, established clinical history of COPD, poor reversibility of airflow obstruction (< 10% increase of FEV1) after bronchodilator, current or ex‐smokers with smoking history of at least 10 pack‐years Exclusion criteria: not stated Baseline characteristics of treatment/control groups: comparable | |
| Interventions | FP 500 µg, 2 times a day (1000 µg/day) Placebo | |
| Outcomes | No of COPD exacerbations Number of withdrawals due to COPD exacerbations FEV1 pre‐bronchodilator FEV1/VC FVC Record card symptoms ‐ cough, breathlessness Use of relief bronchodilator PEFR Shuttle walking test Borg scale St George Respiratory Questionnaire Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 26% FP, 32% placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Senderovitz 1999.
| Methods | Design: parallel‐group Randomisation: yes, method not stated, separately randomised groups based on oral prednisolone response (groups: reversible FEV1 response > 15% and < 30%, irreversible FEV1 < 15%) Blinding: double‐blind, double‐dummy Withdrawals: stated for the reversibility trial and reversible participants, not clearly described for irreversible participants | |
| Participants | Setting: multicentre study, Denmark, hospital outpatient clinics Number eligible: 40 Number enrolled: 37 (35 with no steroid reversibility, 2 with steroid reversibility) Number in treatment group: awaiting information Number in control group: awaiting information Number of withdrawals (treatment/control): awaiting information Number completing trial (treatment/control): 14/12 (no steroid reversibility arm) Age range: 18 to 75 years Sex: 14 M, 12 F (in the steroid‐irreversible group randomised) Ethnicity: not stated COPD diagnosis: FEV1/FVC < 0.7, post‐bronchodilator FEV1 > 40% and < 70% predicted, increase in FEV1 < 15% after 0.5 mg terbutaline via Turbuhaler Severity of COPD: median FEV1 1.46 L in the intervention group, median 1.63 in the placebo group Inclusion criteria: stable COPD Exclusion criteria: clinical evidence of asthma (e.g. pollen season‐related symptoms, exercise‐induced symptoms only, significantly elevated levels of blood eosinophils and IgE), history of atopy (hay fever and/or atopic dermatitis); treatment with oral steroids, sodium cromoglycate or nedocromil in last 4 weeks; other systemic disease making compliance and participation difficult; pregnancy and breast feeding; increase in FEV1 < 30% of baseline after 2 week of prednisolone Baseline characteristics of treatment/control groups: comparable | |
| Interventions | BUD 400 µg, 2 times a day (800 µg/day) Placebo 2 times a day Dry powder inhaler (Turbuhaler) 6 months |
|
| Outcomes | Spirometry Exacerbations Symptom score Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals in BUD or placebo arms |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Shaker 2009.
| Methods | Design: parallel‐group Randomisation: yes, computer‐generated sequence Blinding: double‐blind Withdrawals: stated | |
| Participants | Setting: single‐centre study, Denmark Number eligible: unknown Number enrolled: 278 Number in treatment group: 127 Number in control group: 127 Number of withdrawals (treatment/control): 55/62 Number completing trial (treatment/control): 72/65 Mean age (treatment/control): 63.6/63.6 Sex (% male treatment/control): 62/54 Ethnicity: not stated COPD diagnosis: FEV1 ≤ 70% predicted, FEV1/FVC ≤ 60% and no reversibility to β2‐agonists and oral corticosteroids Severity of COPD: FEV1 % of predicted treatment/control: 51/53 Inclusion criteria: aged 50 to 80 years; current smokers; clinical diagnosis of COPD for not less than 2 years; significant smoking history of at least 10 cigarettes per day during the last 6 months and a previous history of at least 20 pack‐years; FEV1 35% to 70% of predicted (pre‐bronchodilator) and FEV1/forced vital capacity (FEV1/FVC) ≤ 60% Exclusion criteria: ex‐smokers; reversibility of ≥ 12% and 200 mL in FEV1 from baseline values, 15 minutes after inhalation of 1 mg terbutaline or ≥ 15% and 300 mL after 2 weeks on oral prednisolone (25 mg); severe concomitant disease; exacerbation within 30 days prior to the first visit; received oral steroids for more than 4 weeks within 6 months of the first visit; long‐term oxygen therapy Baseline characteristics of treatment/control groups: comparable | |
| Interventions | 2‐week run‐in period on oral prednisolone (25 mg once daily). Patients with reversibility less than 15% or 300 mL from baseline FEV1 values were then randomly assigned to twice‐daily treatment with either 400 μg of budesonide (Pulmicort Turbuhaler) or placebo. | |
| Outcomes | Primary: change over time in the 15th percentile density (PD15) on CT scan (a measure of lung density) Secondary: change over time in the relative area of emphysema at a threshold of –910 Hounsfield units (RA‐910), FEV1 and dif‐ fusion capacity (DLCO) and the number of exacerbations, which was defined as a combination of 2 of the 3 following criteria: increased dyspnoea, increased sputum production and change in sputum colour |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated code" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts stated ‐ similar numbers |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Sin 2004.
| Methods | Design: parallel‐group, 4‐week run‐in Randomisation: yes, method not stated Blinding: double‐blind, double‐dummy Withdrawals: not stated | |
| Participants | Setting: single centre study, Canada Number eligible: 43 Number enrolled: 41 Number in treatment group: 15 Number in control group: 12 Number of withdrawals (treatment/control): 0/0 Number completing trial (treatment/control): 15/12 Age range: 64 yr 9 Sex: 29 M, 12F Ethnicity: Not stated COPD diagnosis: post‐bronchodilator FEV1 25‐90%; FEV1/FVC <75% Severity of COPD: FP: FEV1 1.83 L; 56% pred; Placebo: 1.79 L; 61% pred Inclusion criteria: Stable COPD symptoms 3 months prior; History of at least 10 pack‐years of smoking or prolonged exposure to noxious gases Exclusion criteria: Not stated Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | FP 500 µg, 2 times a day (1000 µg/d) Placebo |
|
| Outcomes | Serum CRP Cytokine levels ‐ IL6, MCP‐1 FEV1 as % baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Sin 2008.
| Methods | Design: parallel‐group with run‐in period Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated |
|
| Participants | Setting: multicentre (11 centres) Number eligible: 356 Number enrolled: 289 Number in groups: 45 placebo, 87 FP 500 bd, 92 FP/salmeterol 500 bd Number of withdrawals: 77 Number completing trial: 212 Age range: mean 69.3 +/‐ 9.3yrs Sex: 63% male Ethnicity: not stated COPD diagnosis: GOLD criteria Severity of COPD: FEV1 47.4 +/‐ 15.9% pred, FVC 74.4 +/‐ 16.3% pred Inclusion criteria: not specified Exclusion criteria: not specified Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Run‐in phase of FP 500mg bd for 4 weeks, followed by a medication withdrawal phase wherein ICS, LABAs, and theophylline products were withdrawn for 4 weeks. All other medications, including short‐acting b2‐adrenoceptor agonists, anticholinergics, and tiotropium, were permitted during all phases of the study. Participants were then randomly assigned to one of three arms: placebo, inhaled FP 500 mg bd or inhaled FP/salmeterol combination 500/50 mg bd. | |
| Outcomes | Primary endpoint ‐ C‐reactive protein (CRP) level. Secondary endpoints ‐ IL‐6, surfactant protein D (SP‐D), SGRQ, FEV1 % predicted, FVC % predicted | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar withdrawal rates between arms |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Szafranski 2003.
| Methods | Design: parallel group Randomisation: yes, method not stated Blinding: double blind, double dummy Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient clinic Number eligible: 980 Number enrolled: 812 Number in groups: Combined 208, BUD 198, FM 201, placebo 205 Number of withdrawals: Combined 59, BUD 62, FM 64, control 90 Number completing trial: Combined 149, BUD 136, FM 137, control 115 (total 537) Age range: mean 64 yr Sex: 639M, 173F Ethnicity: not stated (multicentre) COPD diagnosis: GOLD guidelines Severity of COPD: mean FEV1 36% predicted Inclusion criteria: outpatients aged >=40 yr, COPD symptoms >=2 yr, smoking history >=10 pack‐yrs, FEV1/VC <=70%, FEV1 <50% predicted, total symptom score >=2/day during at least 7 days of run‐in, use of short‐acting inhaled bronchodilators, >=1 severe COPD exacerbation within 2‐12 months before study Exclusion criteria: asthma, seasonal allergic rhinitis before age 40, relevant cardiovascular disorders, current respiratory tract diseases or disorders, regular oxygen therapy, exacerbation during run‐in, patients in whom it was considered unethical to withdraw inhaled steroids Baseline characteristics of treatment/control groups: |
|
| Interventions | budesonide/formoterol 160/4.5 µg, 2 inhalations, 2 times a day (640 µg/d of BUD) BUD 200 µg, 2 inhalations, 2 times a day (800 µg/d) formoterol 4.5 µg, 2 times a day placebo Turbuhaler 12 months |
|
| Outcomes | Exacerbations Morning and evening COPD symptoms short‐acting beta‐agonist use PEFR Spirometry SGRQ adverse events |
|
| Notes | Trial of combined therapy versus monotherapy versus placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Relatively high withdrawal rates |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Tashkin 2008.
| Methods | Design: parallel group Randomisation: yes, computer generated Blinding: double‐blind, double‐dummy Withdrawals: stated |
|
| Participants | Setting: 194 centres in the US, Czech Republic, the Netherlands, Poland and South Africa Number eligible: 1942 Number enrolled: 1704 Number in groups: BUD/FM 320/9mcg 277, BUD/FM 160/9mcg 281, BUD 320mcg + FM 9mcg 287, BUD 320mcg 275, FM 9mcg 284, placebo 300 Number of withdrawals: BUD/FM 320/9mcg 39, BUD/FM 160/9mcg 38, BUD 320mcg + FM 9mcg 48, BUD 320mcg 63, FM 9mcg 61, placebo 77 Number completing trial: BUD/FM 320/9mcg 238, BUD/FM 160/9mcg 243, BUD 320mcg + FM 9mcg 239, BUD 320mcg 212, FM 9mcg 223, placebo 223 Age range: BUD/FM 320/9mcg 41‐86, BUD/FM 160/9mcg 40‐90, BUD 320mcg + FM 9mcg 40‐84, BUD 320mcg 40‐90, FM 9mcg 42‐89, placebo 40‐86 Sex (% male): BUD/FM 320/9mcg 67.9, BUD/FM 160/9mcg 64.4, BUD 320mcg + FM 9mcg 74.2, BUD 320mcg 67.6, FM 9mcg 65.5, placebo 69.0 Ethnicity (% white): BUD/FM 320/9mcg 94.2, BUD/FM 160/9mcg 93.2, BUD 320mcg + FM 9mcg 92.0, BUD 320mcg 94.2, FM 9mcg 92.3, placebo 94.7 COPD diagnosis: GOLD criteria Severity of COPD: as below Inclusion criteria: aged ≥40; symptoms for >2 years; history of at least one COPD exacerbation treated with a course of oral corticosteroids and/or antibacterials within 1–12 months before screening; documented use of an inhaled short acting bronchodilator as rescue medication; moderate to very severe COPD with a pre‐bronchodilator FEV1 of ≤50% of predicted normal and a pre‐bronchodilator FEV1/forced vital capacity of <70%; smoking history of ≥10 pack‐years; score of ≥2 on the Modified Medical Research Council dyspnoea scale at the time of screening; breathlessness, cough and sputum scale (BCSS) score of ≥2 per day for at least half of the 2‐week run‐in period Exclusion criteria: history of asthma; history of allergic rhinitis before 40 years of age; significant/unstable cardiovascular disorder; clinically significant respiratory tract disorder other than COPD; homozygous α‐1 antitrypsin deficiency or any other clinically significant co‐morbidities that could preclude participation in the study or interfere with the study results, as determined by the investigator; required additions or alterations to their usual COPD maintenance therapy or an increment in rescue therapy due to worsening symptoms within 30 days before screening or during the run‐in period; oral or ophthalmic non‐cardioselective β‐ adrenoceptor antagonists; oral corticosteroids; pregnancy; breast‐feeding Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | After 2 weeks of treatment based on previous therapy (ICSs and short‐acting bronchodilators allowed during the run‐in period), patients received one of the following treatments administered twice daily, for 26 weeks: budesonide/formoterol pMDI 160/4.5 μg × two inhalations (320/9 μg); budesonide/formoterol pMDI 80/4.5 μg × two inhalations (160/9 μg); budesonide pMDI 160 μg × two inhalations (320 μg) plus formoterol DPI 4.5 μg × two inhalations (9 μg); budesonide pMDI 160 μg × two inhalations (320 μg); formoterol DPI 4.5 μg × two inhalations (9 μg); or placebo. | |
| Outcomes | Primary: Pre‐dose forced expiratory volume in 1 second (FEV1), 1‐hour post‐dose FEV1 Secondary: morning and evening PEF (L/min) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". Computer generated code |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of withdrawal between ICS and placebo arms |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Thompson 1992.
| Methods | Design: parallel group Randomisation: yes, table of random numbers Blinding: double blind, double dummy Withdrawals: stated |
|
| Participants | Setting: community Number eligible: not stated Number enrolled: 31 Number in treatment group: 31 in total in treatment or control groups Number in control group: 31 in total in treatment or control groups Number of withdrawals (treatment/control): 1 withdrawal (unspecified as to which group) Number completing trial (treatment/control): 20/10 Age range: mean age 50.6 yr in intervention group, mean age 47.0 yr in placebo group Sex: 15M, 15F Ethnicity: not stated COPD diagnosis: chronic bronchitis (chronic productive cough for most days of each month for at least 2 consecutive years) Severity of COPD: mean FEV1 72.6% predicted in intervention group, mean FEV1 72.0% predicted in the placebo group Inclusion criteria: current cigarette smoking, airflow obstruction with FEV1/FVC <75%, improvement of FEV1/FVC to not more than 75% with bronchodilator Exclusion criteria: seasonal or episodic dyspnoea, wheezing, atopy, other active lung disease, DLCO <50%, infiltrates on chest xray; use of oral or inhaled steroids or inhaled cromolyn within previous 3 months; carbon dioxide retention; cardiac disease or other contraindication to bronchoscopy Baseline characteristics of treatment/control groups: higher smoking history in intervention group (with similar exhaled carbon monoxide levels) |
|
| Interventions | BDP µg, 4 times a day ( µg/d) Placebo 4 times a day metered‐dose inhaler 6 weeks |
|
| Outcomes | FEV1 FVC MMEFR PEFR Sputum production Exhaled carbon monoxide levels Bronchoscopy visual bronchitis index Bronchoalveolar lavage cell count and parameter Rescue bronchodilator usage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Thompson 2002.
| Methods | Design: crossover, no washout Randomisation: yes, computer generated Blinding: double blind, double dummy Withdrawals: stated |
|
| Participants | Setting: single centre study, hospital outpatient clinic Number eligible: not stated Number enrolled: 52 Number in treatment group: 52 (crossover) Number in control group: 52 (crossover) Number of withdrawals (treatment/control): 4/12 Number completing trial (treatment/control): 36 (crossover) Age range: 48 to 80 yr Sex: 36M Ethnicity: not stated COPD diagnosis: ATS guidelines Severity of COPD: pre‐bronchodilator FEV1 1.1L Inclusion criteria: >=30 pack‐year smoking, FEV1/FVC <60%, pre‐bronchodilator FEV1<80% predicted, daily use of beta‐agonists and/or ipratropium Exclusion criteria: inhaled or systemic steroids in 30 days prior; family or personal history of asthma; atopy, allergic rhinitis, nasal polyposis, pulmonary disease other than COPD, heart failure, lung cancer Baseline characteristics of treatment/control groups: crossover |
|
| Interventions | FP 220 µg, 2 puffs, 2 times a day (880 µg/d) identical‐appearing placebo inhaler metered‐dose inhaler 3 months |
|
| Outcomes | lung function tests arterial blood gases QOL (Chronic Respiratory Questionnaire) exacerbation respiratory symptoms adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12 withdrawals from placebo, 4 withdrawals from ICS |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
van Grunsven 1999.
| Methods | Meta‐analysis of Derenne, Kerstjens and Renkema | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded studies |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
van Grunsven 2003.
| Methods | Design: parallel group Randomisation: yes, method not stated Blinding: Not Specified, Placebo controlled Withdrawals: Stated |
|
| Participants | Setting: hospital outpatient clinic, single centre, the Netherlands Number eligible: 74 Number enrolled: 48 Number in treatment group: 24 Number in control group: 24 Number of withdrawals (treatment/control): 6/6 Number completing trial (treatment/control): 18/18 Age range: mean 46.5yr Sex: 25M, 23F Ethnicity: Not specified COPD diagnosis: EARLY COPD Severity of COPD: FEV1 98% pred (FP); FEV1 99% pred (Placebo) Inclusion criteria: Chronic cough sputum production at least 3 consecutive months and annual decline in pre‐bronchodilator FEV1 40‐80mL Exclusion criteria: Previous Dx of pulmonary condition; co‐morbid condition with reduced life expectancy; intolerance for inhaled 2‐agonists; use of 2‐blocking agents; inability to use inhalation devices or peak‐flow meters Baseline characteristics of treatment/control groups: Comparable |
|
| Interventions | FP 250 µg, 2 times a day (500 µg/day) Placebo | |
| Outcomes | Annual decline of post‐bronchodilator FEV1 Decline of pre‐bronchodilator FEV1 PC20 Histamine Exacerbation rate Number of episodes with aggravated symptoms Use of rescue bronchodilators Symptom score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 25% withdrawal rate overall |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Verhoeven 2002.
| Methods | Design: parallel group Randomisation: yes, method not stated Blinding: double blind, double dummy Withdrawals: stated |
|
| Participants | Setting: single centre study, hospital outpatient clinic Number eligible: not stated Number enrolled: 23 COPD; also studied 6 asymptomatic smokers Number in treatment group: 10 Number in control group: 13 Number of withdrawals (treatment/control): 0/0 Number completing trial (treatment/control): 10/13 Age range: 42 to 67 yr Sex: 19M, 4F Ethnicity: not stated COPD diagnosis: chronic productive cough, FEV1 <=70% predicted Severity of COPD: mean FEV1 66% predicted FP, mean FEV1 61% predicted placebo Inclusion criteria: non‐specific BHR (PC20 histamine <=8 mg/ml), current smoker, FEV1 reversibility <10% after terbutaline, normal serological examination (Phadiatop test), negative skin prick tests for aeroallergens Exclusion criteria: asthma, respiratory tract infection in previous 4 weeks, serious or unstable concomitant disease Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | FP 500 µg, 2 times a day (1,000 µg/d) placebo Diskhaler 6 months |
|
| Outcomes | BHR methacholine Bronchial biopsies Lung function tests Serum cortisol | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Vestbo 1999.
| Methods | Design: parallel group Randomisation: yes, computer generated Blinding: double blind, double dummy Withdrawals: stated |
|
| Participants | Setting: population cohort (Copenhagen City Heart Study), Denmark Number eligible: 1118 (of which 828 were excluded during screening) Number enrolled: 290 Number in treatment group: 145 Number in control group: 145 Number of withdrawals (treatment/control): 36/51 Number completing trial (treatment/control): 109/94 Age range: 30 to 70 yr Sex: 175M, 115F Ethnicity: all subjects living in Copenhagen COPD diagnosis: FEV1/VC ratio <=0.7 and no self‐reported asthma Severity of COPD: mean post‐bronchodilator FEV1 86.2% in intervention group, 86.9% in placebo group Inclusion criteria: age 30‐70 yr, FEV1/VC ratio <=0.7, FEV1 reversibility <15% baseline with 1 mg terbutaline from Turbuhaler, oral steroid response (15 mg prednisolone 10 days) <15% baseline Exclusion criteria: long term treatment (>2 episodes of >4 wk) with oral or inhaled steroids within previous 6 month, pregnancy or lactation, intention to become pregnant, other serious systemic disease, chronic alcohol or drug use Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | BUD 800 µg morning, 400 µg evening for 6 month (1200 µg/d), then
BUD 400 µg, 2 times a day for 30 month (800 µg/d) Placebo 2 times a day Dry powder inhaler (Turbuhaler) (identical) 3 years |
|
| Outcomes | FEV1 decline rate Symptoms Exacerbations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation sequence generated by computer" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was masked" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of withdrawal between arms. Withdrawals: 36 BUD, 51 placebo |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Weiner 1995.
| Methods | Design: crossover, 4 weeks washout Randomisation: yes, method not stated Blinding: double blind, double dummy Withdrawals: stated |
|
| Participants | Setting: single centre study, hospital outpatient clinic Number eligible: 30 (of whom 22 were bronchodilator non‐responders, defined as >20% increase in FEV1) Number enrolled: 30 (of whom 22 were bronchodilator non‐responders) Number in treatment group: 22 bronchodilator non‐responders (crossover, included in this review) Number in control group: 2 bronchodilator non‐responders (crossover, included in this review) Number of withdrawals (treatment/control): 0 Number completing trial (treatment/control): 30 Age range: 55 to 77 yr Sex: 14M, 8F Ethnicity: not stated COPD diagnosis: chronic airflow limitation Severity of COPD: mean FEV1 1.39L Inclusion criteria: stable condition, smoking history >30 pack‐yrs, FEV1<50% predicted, FEV1/FVC<60% Exclusion criteria: asthma, seasonal or episodic dyspnoea or wheezing, family history of asthma, improvement of FEV1/FVC to more than 70%, use of oral or inhaled steroids within previous 3 months Baseline characteristics of treatment/control groups: crossover | |
| Interventions | BUD 400 µg, 2 times a day (800 µg/d) Placebo 2 times a day metered‐dose inhaler via spacer 6 weeks each treatment period (crossover) |
|
| Outcomes | Change in FEV1 from baseline Rescue bronchodilator usage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Weiner 1999.
| Methods | Design: crossover, 4 weeks washout Randomisation: yes, method not stated Blinding: double blind, double dummy Withdrawals: stated |
|
| Participants | Setting: single centre study, Israel, hospital outpatient clinic Number eligible: not stated Number enrolled: 168 (124 bronchodilator non‐responders, 44 bronchodilator responders) Number in treatment group: 124 bronchodilator non‐responders (crossover, included in this review) Number in control group: 124 bronchodilator non‐responders (crossover, included in this review) Number of withdrawals (treatment/control): 7 withdrawals Number completing trial (treatment/control): 117 (crossover) Age range: mean 64.4 yr (bronchodilator non‐responders) Sex: 102M, 66F Ethnicity: not stated COPD diagnosis: chronic airflow limitation on spirometry, without evidence of asthma Severity of COPD: mean post‐bronchodilator FEV1 1.34L Inclusion criteria: stable, smoking >30 pack‐yr, FEV1 <50% predicted, FEV1/FVC <60% Exclusion criteria: physician diagnosis of asthma, seasonal or episodic dyspnoea or wheezing, family history of asthma, atopy (history of allergy and positive skin prick test to common antigens), improvement of FEV1/FVC to >70% with inhaled beta‐agonist, use of oral or inhaled steroids within last 3 month Baseline characteristics of treatment/control groups: crossover |
|
| Interventions | BUD 400 µg, 2 times a day (800 µg/d) Placebo 2 times a day Metered dose inhaler via spacer device 6 weeks each treatment period (crossover) |
|
| Outcomes | FEV1 Rescue bronchodilator usage | |
| Notes | Second phase of study: inhaled BUD 800 µg 2 times a day versus BUD 400 µg 2 times a day for 6 wk, crossover (not included in this review) Third phase of study: oral prednisolone 40 mg/d versus placebo 6 wk, crossover (not included in this review) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Weir 1990a.
| Methods | Design: crossover, 2 weeks washout Randomisation: yes, method not stated Blinding: double blind, double dummy Withdrawals: stated |
|
| Participants | Setting: single centre study, hospital outpatient clinic Number eligible: not stated Number enrolled: 127 Number in treatment group: 127 Number in control group: 127 Number of withdrawals (treatment/control): 20 withdrawals Number completing trial (treatment/control): 107 (crossover) Age range: mean 62.9 yr (SD 9.0) Sex: 82M, 25F Ethnicity: not stated COPD diagnosis: adult onset chronic airflow obstruction of at least 5 yr duration and FEV1<70% predicted Severity of COPD: mean FEV1 44.2% predicted Inclusion criteria: as above, 95/107 were current or ex‐smokers Exclusion criteria: asthma, respiratory symptoms in childhood, variability in symptoms except in association with infections, acute attacks of wheezing and breathlessness, deterioration after exposure to specific allergens, use of oral or inhaled steroids in previous 6 month Baseline characteristics of treatment/control groups: crossover |
|
| Interventions | BDP 500 µg, 3 times a day (1500 µg/d) Placebo 3 times a day metered‐dose inhaler 2 weeks each treatment period (crossover) |
|
| Outcomes | Spirometry Mean PEFR TLCO Serum IgE levels | |
| Notes | Significant order effect was observed: data included here are from the first treatment period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double blind, double dummy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar withdrawals in both arms |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Weir 1999.
| Methods | Design: parallel group Randomisation: yes, method not stated Blinding: double blind, double dummy Withdrawals: stated |
|
| Participants | Setting: multicentre study, UK, hospital outpatient clinic Number eligible: not stated Number enrolled: 98 Number in treatment group: 49 Number in control group: 49 Number of withdrawals (treatment/control): 39 total Number completing trial (treatment/control): 59 total Age range: adult (mean 65.5 yr in intervention group, 67.6 yr in control group Sex: 73M, 15F Ethnicity: not stated COPD diagnosis: clinical diagnosis of COPD, adult onset airflow obstruction , FEV1 <70% predicted, FEV1/FVC <65% Severity of COPD: Mean pre‐bronchodilator FEV1 39.7% in intervention group, 41.4% in control group Inclusion criteria: as for COPD diagnosis Exclusion criteria: clinical diagnosis of asthma (including clinical significant bronchodilator reversibility, acute attacks of breathlessness with recovery between attacks), significant improvement with steroid treatment in the past, steroid treatment clinically indicated, use of steroids >3 month in last 1 yr or during last 4 wk Baseline characteristics of treatment/control groups: more females in the intervention group |
|
| Interventions | BDP 750 µg, 2 times a day (1500 µg/d) for weight <50 kg, BDP 1000 µg, 2 times a day (2000 µg/d) for weight >50 kg Placebo 2 times a day Metered dose inhaler (identical) via spacer device 2 years |
|
| Outcomes | Change in pre‐bronchodilator FEV1 Change in pre‐bronchodilator FVC Change in post‐bronchodilator FEV1 Change in post‐bronchodilator FVC PC20 histamine Exacerbations Dyspnoea index CRQ (subgroup) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Wempe 1992.
| Methods | Design: crossover, no washout Randomisation: yes, method not stated Blinding: double blind, double dummy Withdrawals: stated |
|
| Participants | Setting: single centre study Number eligible: not stated Number enrolled: 10 Number in treatment group: 10 (crossover) Number in control group: 10 (crossover) Number of withdrawals (treatment/control): 2 in total Number completing trial (treatment/control): 8 in total Age range: 49 to 66 yr Sex: 8M, 2F Ethnicity: not stated COPD diagnosis: dyspnoea continuously or on exertion, with BHR Severity of COPD: FEV1 % predicted range 44 to 79% Inclusion criteria: current or former smokers, FEV1 40 to 80% predicted, PC20 to histamine <8 mg/ml Exclusion criteria: asthma, respiratory infection or exacerbation in previous 2 months, positive skin prick tests, elevated total IgE Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | BUD 1,600 µg/d Prednisolone 40 mg daily Placebo Metered‐dose inhaler with spacing device 3 weeks each treatment period (crossover study) |
|
| Outcomes | FEV1 response to cumulative doubling doses of bronchodilators | |
| Notes | 4 study days after each treatment period; no actual washout; no carry‐over effects observed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Yildiz 2004.
| Methods | Design: parallel group Randomisation: yes, method not stated Blinding: double blind Withdrawals: Stated |
|
| Participants | Setting:
single centre study,
hospital outpatient clinic Number eligible: 38 Number enrolled: 38 Number in treatment group: 20 Number in control group: 18 Number of withdrawals (treatment/control): 0/0 Number completing trial (treatment/control): 20/18 Age range: 67yr 8.2Y Sex: 38M 0F Ethnicity: Not stated COPD diagnosis: GOLD II (Prebronchodilator FEV1 30‐80% of predicted, FEV1/FVC <70% of predicted Severity of COPD: ICS: 51% 22 predicted; P: 40% 14 predicted Inclusion criteria: Irreversible airway obstruction <10% improvement in FEV1 post‐bronchodilator, Smoking history of >20 pack years, no exacerbation of respiratory tract infection in previous 4 weeks Exclusion criteria: Asthma, clinical signs of right heart failure, recent hospitalisation or admission to emergency department because of exacerbation, requirement for regular use of oxygen therapy, had used inhaled or oral ICS in the last 6 weeks Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | BUD 800µg, 1 time a day (800µg/d) Placebo Both groups received combined bronchodilator therapy: Formoterol + Ipratropium bromide |
|
| Outcomes | St Georges Respiratory Questionnaire Score FEV1 FVC FEV1/FVC PaO2 PaCO2 SaO2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Abbreviations:
ATS: American Thoracic Society; bd: twice a day; BAL: bronchoalveolar lavage; BDP: beclomethasone dipropionate; BHR: bronchial hyper‐responsiveness; BUD: budesonide; COPD: chronic obstructive pulmonary disease; CPAP: continuous positive airway pressure; CRQ: Chronic Respiratory Questionnaire; CT: computed tomography; DPI: dry powder inhaler; Dx: diagnosis; ECP: Eosinophil Cationic Protein; eNO: exhaled nitric oxide; FEV1: forced expiratory volume in one second; FP: fluticasone propionate; FVC: forced vital capacity; GOLD: Global Initiative for Obstructive Lung Disease; HRQL: health‐related quality of life; Hx: History; ICS: inhaled corticosteroids; LABA: long‐acting beta2‐agonist; LTOT: long‐term oxygen therapy; MF: mometasone furoate; MMEFR: maximal mid‐expiratory flow rate; MRC: Medical Research Council; OCS: oral corticosteroids; PC20 MCh: Methacholine challenge test; PEFR: peak expiratory flow rate; pMDI pressurised metered dose inhaler; qd: quaque die, a Latin phrase meaning "every day"; QOL: quality of life; RCT: randomised controlled trial; RTI: respiratory infection; SABA: short‐acting beta2‐agonist; SAE: serious adverse event; SD: standard deviation; SFC: salmeterol/fluticasone propionate; SGRQ: St George's Respiratory Questionnaire; RTI: respiratory tract infection; RV: residual volume; TAA: triamcinolone acetonide; TLC: total lung capacity; VAS: visual analogue scale; VC: vital capacity; vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albers 2004 | Excluded: subjects had rapid decline in lung function, who were at risk of COPD |
| Anonymous 1999 | Excluded: summary of RCT (Pauwels 1999), not original RCT |
| Anonymous 2000 | Excluded: review, not a clinical trial |
| Balbi 2000 | Excluded: Open trial of BDP in COPD examining bronchoalveolar lavage parameters, not RCT |
| Bensch 2003 | Excluded: asthma |
| Burge 1999 | Excluded: letter referring to a previous RCT (Burge, in: Bronchitis V. Postma DS and Gerritsen J, eds. 1994) |
| Chan 1993 | Excluded: not randomised; randomisation was changed without notifying the chief investigators. Double‐blind, placebo controlled crossover trial of BUD 1600 µg/day versus placebo in 20 COPD patients; All patients received placebo for 4 weeks, then BUD for 8 weeks, without a washout period between placebo and BUD. |
| Confalonieri 1998 | Excluded: open trial of BDP versus no treatment, not double‐blind. Randomised, parallel‐group trial of BDP 500 µg 3 times a day versus no treatment for 2 months in 34 COPD patients. Markers of airway inflammation measured in induced sputum |
| Corda 2008 | Excluded: focused on alpha‐1 antitrypsin deficiency patients only |
| Cox 1999 | Excluded: RCT of BDP versus placebo in smokers with normal FEV1 (> 70% predicted), not COPD with airflow obstruction |
| Dompeling 1992 | Excluded: trial of adding BDP to bronchodilators in asthma or COPD patients, not RCT of ICS versus placebo |
| Dompeling 1993 | Excluded: trial of adding BDP to bronchodilators in asthma or COPD patients, not RCT of ICS versus placebo |
| Egan 1999 | Excluded: bone density study in asthmatics |
| Engel 1989 | Excluded: RCT of BUD in smokers with chronic bronchitis and BHR to histamine; mostly normal lung function; not COPD patients with airflow obstruction |
| Fattore 2005 | Excluded: cost analysis |
| Fazio 1986 | Excluded: Single dose, double‐blind study of BDP in patients with COPD. 5 received BDP, 5 received placebo. Mucociliary clearance measured, no other outcomes |
| Guleria 2003 | Excluded: single inhalation study |
| Keatings 1997 | Excluded: sequential single blind, 2 week crossover study of BUD in COPD patients, with double‐blind assessment of inflammatory markers. Demonstrated no improvement in lung function, symptom scores or inflammatory indices. |
| Kozak‐Skzopek 1997 | Excluded: English title ‐ "Inhaled budesonide therapy for chronic bronchitis". Double‐blind study, no randomisation described. Intervention: BUD 200 µg, 3 times per day. Authors did not respond when contacted regarding randomisation. |
| Matlin 1976 | Abstract only: Results reported were: 17 patients with COPD were treated in a double‐blind, crossover study with triamcinolone 800 mcg/day versus placebo for 2 weeks in random sequence. 6 of the 17 patients had at least a 40% increase of FEV1 on triamcinolone whereas the maximum increase with placebo was 33%. Presented at American Thoracic Society meeting 1976. No subsequent publication. Author not contactable. |
| Melani 1999 | Excluded: nebuliser used to deliver ICS. Randomised, double‐blind cross‐over study of 20 severe COPD patients, nebulised BDP 2 mg bd versus placebo for 4 weeks. |
| Moller 1999 | Excluded: case series, not RCT (translated from German) |
| Nava 2000 | Excluded: RCT of FP versus placebo in ventilator‐dependent COPD patients, 5 days duration, cross‐over, FEV1 performed through tracheostomy (information provided by first author) |
| Nishimura 2000 | Excluded: oral corticosteroids added to inhaled corticosteroids, not RCT of inhaled corticosteroids versus placebo |
| O'Brien 2001 | Excluded: withdrawal study |
| Ouyang 1998 | Excluded: (English abstract): single‐blind trial, not double‐blind. Randomised, placebo‐controlled trial of BDP 1000 µg daily for 6 week in 61 stable non‐asthmatic COPD patients. |
| Roth 1996 | Excluded: Review of study by G Eichler, "Inhaled corticosteroids are effective and well tolerated". Not placebo‐controlled. Open, multicentre, randomised study of 1 mg flunisolide versus 800 µg BUD for 12 weeks in COPD patients. Limited data reported. (Translated from German) |
| Sandrini 2003 | Excluded: withdrawal study |
| Sapey 2000 | Excluded: review, not a clinical trial |
| Schuurmans 2001 | Excluded: review, not a clinical trial (translated from German) |
| Spicuzza 2004 | Excluded: acute study |
| Tsang 1999 | Excluded: letter referring to a previous RCT in bronchiectasis |
| Turker 2004 | Excluded: add on of theophylline |
| van den Boom 2001 | Excluded: cost analysis in obstructive airways disease |
| van der Valk 2002 | Excluded: withdrawal study |
| van Grunsven 2000 | RCT of FP versus placebo in subjects with "early COPD" (FEV1 decline of >0.04L/yr), not patients with airflow limitation |
| van Schayck 1995 | Excluded: trial of adding BDP to bronchodilators in COPD, not RCT of ICS versus placebo |
| Vestbo 2000 | Excluded: translation of Vestbo 1999 (Lancet; 353(9167):1819‐23) (Translated from Danish) |
| Watson 1992 | Excluded: RCT of BUD versus placebo in 14 smokers with BHR to histamine and mild airways obstruction; most subjects had normal spirometry, not COPD patients with airflow limitation |
| Weiner 1997 | Excluded: (abstract): subgroup of COPD patients who were responders to beta‐agonist, reported in Weiner 1999 (Journal of Internal Medicine 1999;245(1):83‐9) |
| Weir 1993 | Excluded: single blind trial of BDP at different doses versus placebo, not double‐blind |
| Wesseling 1991 | Excluded: RCT of BUD versus placebo in chronic bronchitis patients with FEV1 % pred >=70% (mean 96%, SD 17%), not COPD with airflow obstruction |
| Whittaker 2000 | Excluded: review, not a clinical trial |
| Wilcke 1997 | Excluded: RCT of BUD versus placebo in alpha1‐antitrypsin deficiency patients |
| Williamson 2009 | Excluded: Randomised crossover study of two doses of FP vs placebo |
| Yildiz 2000 | Excluded: Information from first author ‐ single blinded study, not double‐blinded (clinician aware of treatment allocation; patient and differential cell count technician not aware). Randomised trial of FP 500 µg 3 times a day vs placebo for 2 months in 18 COPD patients. |
bd: twice a day; BDP: beclomethasone dipropionate; BHR: bronchial hyper‐responsiveness; BUD: budesonide; COPD: chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Questionnaire; FEV1: forced expiratory volume in one second; FP: fluticasone propionate; ICS: inhaled corticosteroids; OCS: oral steroids; RCT: randomised controlled trial; SABAs: short‐acting beta‐agonists; SD: standard deviation
Contributions of authors
Ian Yang, Toby Lasserson, Peter Black and Kwun Fong designed the initial review strategy and selected the studies for inclusion for the original 2007 version. Ian Yang, Esther Sim and Toby Lasserson extracted and entered the data for the original 2007 version.
Ian Yang and Melissa Clarke selected the studies for inclusion for the 2011 update, and extracted and entered the data for the 2011 update.
All current reviewers prepared the update of this review and approved its final version.
Sources of support
Internal sources
No sources of support supplied
External sources
National Health and Medical Research Council, Australia.
The Prince Charles Hospital Foundation, Australia.
Cochrane Airways Group Scholarship, Australia.
Declarations of interest
Ian Yang, Kwun Fong, Melissa Clarke, Esther Sim: none declared.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Auffarth 1991 {published data only}
- Auffarth B, Postma DS, Monchy JG, Mark TW, Boorsma M, Koeter GH. Effects of inhaled budesonide on spirometric values, reversibility, airway responsiveness, and cough threshold in smokers with chronic obstructive lung disease. Thorax 1991;46(5):372‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boothman‐Burrell 1997 {published data only}
- Boothman‐Burrell D, Delany SG, Flannery EM, Hancox RJ, Taylor DR. The efficacy of inhaled corticosteroids in the management of non asthmatic chronic airflow obstruction. New Zealand Medical Journal 1997;110(1053):370‐3. [PubMed] [Google Scholar]
Bourbeau 1998 {published data only}
- Bourbeau J, Rouleau MY, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax 1998;53(6):477‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bourbeau 2007 {published data only}
- Bourbeau J, Christodoulopoulos P, Maltais F, Yamauchi Y, Olivenstein R, Hamid Q. Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: a randomised controlled trial. Thorax 2007;62(11):938‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brightling 2005 {published data only}
- Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005;60(3):193‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burge 2000 {published data only}
- Bale G, Martinez‐Camblor P, Burge PS, Soriano JB. Long‐term mortality follow‐up of the ISOLDE participants: causes of death during 13 years after trial completion. Respiratory Medicine 2008;102(10):1468‐72. [DOI] [PubMed] [Google Scholar]
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA. Prednisolone response in patients with chronic obstructive pulmonary disease: results from the ISOLDE study. Thorax 2003;58(8):654‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000;320(7245):1297‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003;58(8):659‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PM, Spencer S, Willits L, Burge PS, Jones PW. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest 2003;124(4):1350. [DOI] [PubMed] [Google Scholar]
- D'Urzo AD, Calverley PMA. Withdrawal of treatment in the ISOLDE study [letter]. Chest 2004;125(6):2368. [DOI] [PubMed] [Google Scholar]
- Jarad NA, Wedzicha JA, Burge PS, Calverley PM. An observational study of inhaled corticosteroid withdrawal in stable chronic obstructive pulmonary disease. ISOLDE Study Group. Respiratory Medicine 1999;93(3):161‐6. [DOI] [PubMed] [Google Scholar]
- Jones PW, Willits LR, Burge PS, Calverley PMA. Disease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbations. European Respiratory Journal 2003;21(1):68‐73. [DOI] [PubMed] [Google Scholar]
- Spencer S, Calverley PM, Burge PS, Jones PW. Health status deterioration in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;163(1):122‐8. [DOI] [PubMed] [Google Scholar]
- Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. European Respiratory Journal 2004;23(5):698‐702. [DOI] [PubMed] [Google Scholar]
Calverley 2003a {published data only}
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9356):449‐56. [DOI] [PubMed] [Google Scholar]
- Calverley PMA, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):242s [P1572]. [Google Scholar]
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol / fluticasone propionate combination in differing severities of COPD [A035] [Poster D50]. http://www.abstracts2view.com 2003.
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/fluticasone propionate combination for one year provides greater clinical benefit than its individual components [A98] [Poster 306]. http://www.abstracts‐on‐line.com/abstracts/ATS 2002.
- Hunjan MK, Chandler F. Numbers needed to treat (NNT) to avoid an exacerbation in patients with chronic obstructive pulmonary disease (COPD) using salmeterol/fluticasone propionate combination (SFC) and associated costs [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:D22 Poster 503.
- Hunjan MK, Williams DT. Costs of avoiding exacerbations in patients with chronic obstructive pulmonary disease (COPD) treated with salmeterol/fluticasone propionate combination (Seretide) and salmeterol. European Respiratory Journal 2004;24(Suppl 48):291s. [Google Scholar]
- Hunjan MK, Williams DT. Salmeterol/fluticasone propionate combination is clinically effective in avoiding exacerbations in patients with moderate/severe COPD. European Respiratory Journal 2004;24(Suppl 48):513s. [Google Scholar]
- Jones PW, Edin HM, Anderson J. Salmeterol/fluticasone propionate combination improves health status in COPD patients. Proceedings of the 98th International American Thoracic Society Conference [A39] [Poster K39]. http://www.abstracts‐on‐line.com/abstracts/ATS 2002.
- Jones PW, Ståhl E. Budesonide/formoterol sustains clinically relevant improvements in health status in COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1352. [Google Scholar]
- Jones PW, Vestbo J, Pauwels RA, Calverley PMA, Anderson JA, Spencer MD. Informative drop out in COPD studies. Investigation of health status of withdrawals in the TRISTAN study. 13th ERS Annual Congress. 2003:P1593.
- Nitschmann S. Inhalational combination therapy in chronic obstructive lung disease. TRISTAN study. German Internist 2004;45(6):727‐8. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Calverly PMA, Vestbo J, Jones PW, Pride N, Gulsvik A, et al. Reduction of exacerbations with salmeterol/fluticasone combination 50/500 mcg bd in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):240 [P1569]. [Google Scholar]
- Pauwels RA, Vestbo J, Calverley PMA, Jones PW, Pride NB, Gulsvik A. Characterization of exacerbations in the TRISTAN study of salmeterol / fluticasone propionate (SFC) combination in moderate to severe COPD. http://www.abstracts2view.com 2003.
- SFCB3024. A multicentre, randomised, double‐blind, parallel group, placebo‐controlled study to compare the efficacy and safety of the salmeterol/FP combination product at a strength of 50/500mcg bd with salmeterol 50mcg bd alone and FP 500mcg bd alone, delivered via the DISKUS™/ACCUHALER™, in the treatment of subjects with chronic obstructive pulmonary disease (COPD) for 12 months. GlaxoSmithKline Clinical Trials Register (http://ctr.gsk.co.uk) 2005.
- Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics 2005;23(6):619‐37. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Karia N, Anderson J. The clinical significance of treatment benefits with the salmeterol/fluticasone propionate 50/500mcg combination in COPD. European Respiratory Journal 2004;24(Suppl 48):290s. [Google Scholar]
- Vestbo J, Calverley PMA, Pauwels R, Jones P, Pride N, Gulsvik A, et al. Absence of gender susceptibility to the combination of salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):240 [P1570]. [Google Scholar]
- Vestbo J, Pauwels R, Anderson JA, Jones P, Calverley P. Early onset of effect of salmeterol and fluticasone propionate in chronic obstructive pulmonary disease. Thorax 2005;60(4):301‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestbo J, Pauwels RA, Calverley PMA, Jones PW, Pride NB, Gulsvik A. Salmeterol/fluticasone propionate combination produces improvement in lung function detectable within 24 hours in moderate to severe COPD. http://www.abstracts2view.com 2003.
- Vestbo J, Soriano JB, Anderson JA, Calverley P, Pauwels R, Jones P. Gender does not influence the response to the combination of salmeterol and fluticasone propionate in COPD. Respiratory Medicine 2004;98(11):1045‐50. [DOI] [PubMed] [Google Scholar]
Calverley 2003b {published data only}
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference, May 21‐26 2004;(no volume):C22 Poster 505. [Google Scholar]
- Calverley PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(6):912‐9. [DOI] [PubMed] [Google Scholar]
- Calverley PMA, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P436. [DOI] [PubMed] [Google Scholar]
- Calverley PMA, Kuna P, Olsson H. COPD exacerbations are reduced by budesonide/formoterol in a single inhaler [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P1587. [Google Scholar]
- Calverley PMA, Olsson H, Symbicort International COPD Study Group. Budesonide/formoterol in a single inhaler sustains improvements in lung function over 12 months compared with monocomponents and placebo in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024 Poster 418.
- Calverley PMA, Peterson S. Combining budesonide/formoterol in a single inhaler reduces exacerbation frequency in COPD [abstract]. American Thoracic Society 99th International Conference. 2003:D092 Poster 211.
- Calverley PMA, Stahl E, Jones PW. Budesonide/formoterol improves the general health status of patients with COPD [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B93 Poster 303.
- Calverley PMA, Thompson NC, Olsson H. Budesonide/formoterol in a single inhaler sustains lung function improvements in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P435. [Google Scholar]
- Halpin D, Ståhl E, Lundback B, Anderson F, Peterson S. Treatment costs and number needed to treat (NNT) with budesonide/formoterol to avoid one exacerbation of COPD [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:D22 Poster 525.
- Halpin DMG, Larsson T, Calverley PMA. How many patients with COPD must be treated with budesonide/formoterol compared with formoterol alone to avoid 1 day of oral steroid use? [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B93 Poster 314.
- Jones PW, Ståhl E. Budesonide/formoterol in a single inhaler improves health status in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024 Poster 419.
- Jones PW, Ståhl E. Budesonide/formoterol sustains clinically relevant improvements in health status in COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1352. [Google Scholar]
- Jones PW, Ståhl E. Reducing exacerbations leads to a better health‐related quality of life in patients with COPD. 13th ERS Annual Congress, 27th September, Vienna. 2003:P1586.
- Lofdahl CG. Reducing the impact of COPD exacerbations: clinical efficacy of budesonide/formoterol. European Respiratory Review 2004;13(88):14‐21. [Google Scholar]
- Lofdahl CG, Andreasson E, Svensson K, Ericsson A. Budesonide/formoterol in a single inhaler improves health status in patients with COPD without increasing healthcare costs [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P433. [Google Scholar]
- Lofdahl CG, Ericsson A, Svensson K, Andreasson E. Cost effectiveness of budesonide/formoterol in a single inhaler for COPD compared with each monocomponent used alone. Pharmacoeconomics 2005;23(4):365‐75. [DOI] [PubMed] [Google Scholar]
Calverley 2003c {unpublished data only}
- Calverley P, Pauwels R, Nieminem M, Stryszak P, Staudinger H, Lee T. Once‐daily mometasone furoate dry powder inhaler preserves lung function, reduces symptoms, and delays exacerbations in patients with COPD previously maintained on ICS. 13th ERS Annual Congress, 27 September, 2003, Vienna. 2003:P155.
Calverley 2007 {unpublished data only}
- Calverley P, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The TOwards a Revolution in COPD Health (TORCH) study: salmeterol/fluticasone propionate improves survival in COPD over three years [Abstract]. Respirology 2006;11(Suppl 5):A149 [PS‐3‐8]. [Google Scholar]
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW et al and TORCH = Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax 2010;65(8):719‐25. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine 2007;356(8):775‐89. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, Vestbo J, et al. The towards a revolution in COPD health (TORCH) study: fluticasone propionate/salmeterol improves survival in COPD over three years [Abstract]. Chest 2006;130(4):122s. [Google Scholar]
- Calverley PMA, Celli B, Andersen JA, Ferguson GT, Jenkins C, Jones PW, et al. The TORCH (towards a revolution in COPD health) study salmeterol/fluticasone propionate (SFC) improves survival in COPD over three years [Abstract]. European Respiratory Journal 2006;28(Suppl 50):34s [E311]. [Google Scholar]
- Celli B, Calverley PM, Anderson JA, Ferguson GT, Jenkins C, Jones P, et al. The TOwards a Revolution in COPD Health (TORCH) Study: salmeterol/fluticasone propionate reduces the rate of exacerbations over three years [Abstract]. Respirology 2006;11(Suppl 5):A140 [O‐9‐2]. [Google Scholar]
- Celli B, Calverley PM, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The towards a revolution in COPD health (TORCH) study: fluticasone propionate/salmeterol reduces the rate of exacerbations over 3 years [Abstract]. Chest 2006;130(4):177s. [Google Scholar]
- Celli B, Calverley PMA, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The TORCH (towards a revolution in COPD health) study salmeterol/fluticasone propionate (SFC) improves health status reduces exacerbations and improves lung function over three years [Abstract]. European Respiratory Journal 2006;28(Suppl 50):34s [E312]. [Google Scholar]
- Celli B, Ferguson GT, Anderson JA, Jenkins CR, Jones PW, Vestbo J, et al. Salmeterol/fluticasone propionate (SFC) improves lung function and reduces the rate of decline over three years in the TORCH survival study [Abstract]. American Thoracic Society International Conference. San Francisco, California, USA, May 18‐23, 2007:A763.
- Celli B, Vestbo J, Jenkins CR, Jones PW, Ferguson GT, Calverley PMA, et al. Sex differences in mortality and clinical expressions of patients with chronic obstructive pulmonary disease: the TORCH experience. American Journal of Respiratory and Critical Care Medicine 2011;183(3):317‐22. [DOI] [PubMed] [Google Scholar]
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. American Journal of Respiratory and Critical Care Medicine 2008;178(4):332‐8. [DOI] [PubMed] [Google Scholar]
- Ferguson GT, Calverley PM, Anderson JA, Celli B, Jenkins C, Jones PW, et al. The towards a revolution in COPD health (TORCH) study: fluticasone propionate/salmeterol is well tolerated in patients with COPD over 3 years [Abstract]. Chest 2006;130(4):178s. [Google Scholar]
- Ferguson GT, Calverley PM, Anderson JA, Celli B, Jenkins CR, Jones PW, et al. Effect of salmeterol/fluticasone propionate (SFC) on bone mineral density (BMD) and eye disorders over three years in the TORCH trial [Abstract. American Thoracic Society International Conference. San Francisco, California, USA, May 18‐23, 2007:A763.
- Ferguson GT, Calverley PM, Anderson JA, Jenkins CR, Celli B, et al. Prevalence and progression of osteoporosis in patients with COPD results from the towards a revolution in COPD health study. Chest 2009;136(6):1456‐65. [DOI] [PubMed] [Google Scholar]
- Ferguson GT, Calverley PMA, Anderson JA, et al. The TORCH (TOwards a Revolution in COPD Health) study: salmeterol/fluticasone propionate (SFC) improves survival in COPD over three years. European Respiratory Journal 2006;28(Suppl 50):34s. [Google Scholar]
- Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo‐controlled TORCH study. Respiratory Research 2009;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RS, Kathman SJ, Daley‐Yates PT, Cahn T, Beerahee M, Kunka RL, et al. Pharmacokinetics and pharmacodynamics in COPD patients following long‐term twice‐daily treatment with salmeterol/fluticasone propionate (SFC) 50/500mg and the individual components [Abstract]. American Thoracic Society International Conference. San Francisco, California, USA, May 18‐23, 2007:Poster #A41.
- SCO30003. A multicentre, randomised, double‐blind, parallel group, placebo‐controlled study to investigate the long‐term effects of salmeterol/fluticasone propionate (SERETIDE®/VIANI®/ADVAIR®) 50/500mcg bd, salmeterol 50mcg bd and fluticasone propionate 500mcg bd, all delivered via the DISKUS®/ACCUHALER® inhaler, on the survival of subjects with chronic obstructive pulmonary disease (COPD) over 3 years of treatment. www.ctr.gsk.co.uk 2006.
- The TORCH study group. The TORCH (TOwards a Revolution in COPD Health) survival study protocol. European Respiratory Journal 2004;24(2):206‐10. [DOI] [PubMed] [Google Scholar]
Calverley 2008 {published data only}
- Calverley PM, Rennard S, Nelson HS, Karpel JP, Abbate EH, Stryszak P, et al. One‐year treatment with mometasone furoate in chronic obstructive pulmonary disease. Respiratory Research. 2008/11/19 2008; Vol. 9:73. [1465‐993X: (Electronic)] [DOI] [PMC free article] [PubMed]
- Nelson H, Karpel JP, Staudinger H, Busse WW. Mometasone furoate dry powder inhaler (MF‐DPI) improves FEV1, symptoms, quality of life and reduces exacerbations in patients with COPD. Chest 2004;236(4 Suppl):709s‐10s. [Google Scholar]
Culpitt 1999 {published data only}
- Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1999;160(5 Pt 1):1635‐9. [DOI] [PubMed] [Google Scholar]
Derenne 1995 {published data only}
- Derenne JP. Effects of high dose inhaled beclomethasone in the rate of decline in FEV1 in patients with chronic obstructive pulmonary disease: results of a 2 years prospective multicentre study. American Journal of Respiratory and Critical Care Medicine 1995;151:A463. [Google Scholar]
- Grunsven PM, Schayck CP, Derenne JP, Kerstjens HA, Renkema TE, Postma DS, et al. Long term effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a meta‐analysis. Thorax 1999;54(1):7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferreira 2001 {published data only}
- Ferreira IM, Hazari MS, Gutierrez C, Zamel N, Chapman KR. Exhaled nitric oxide and hydrogen peroxide in patients with chronic obstructive pulmonary disease: effects of inhaled beclomethasone. American Journal of Respiratory and Critical Care Medicine 2001;164(6):1012‐5. [DOI] [PubMed] [Google Scholar]
Ferreira 2003 {unpublished data only}
- Ferreira IM, Sandrini A, Zamel N, Balter M, Chapman KR. Effects of inhaled fluticasone propionate (FP) on Exhaled Nitric Oxide (ENO), functional exercise capacity and quality of life in stable patients with COPD. American Thoracic Society 99th International Conference. 2003:B024 Poster 404.
GSK 2005 (FCO30002) {unpublished data only}
- FCO30002. A multicentre, randomised, placebo‐controlled, double‐blind comparison with 3 parallel groups to investigate the efficacy and safety of inhaled glucocorticoid fluticasone (500 μg bd via Diskus™) vs. oral glucocorticoid therapy vs. placebo in subjects with chronic obstructive airway disease (COPD) under therapy with Salmeterol (50 μg bd). GlaxoSmithKline Clinical Trial Register 2005.
GSK 2005 (FLTA3025) {unpublished data only}
- FLTA3025. A randomized, double‐blind, parallel‐group, comparative trial of inhaled fluticasone propionate 250mcg BID, 500mcg BID, and placebo BID via the DISKUS in subjects with chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trial Register 2005.
Guenette 2011 {published data only}
Hanania 2003 {published data only}
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 micro g)/salmeterol (50 micro g) combined in the Diskus inhaler for the treatment of COPD. Chest 2003;124(3):834‐43. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the Diskus. American Journal of Respiratory and Critical Care Medicine 2001;163(Suppl 5):A279. [Google Scholar]
- Horstman D, Darken P, Davis S, Lee B. Improvements in FEV1 and symptoms in poorly reversible COPD patients following treatment with salmeterol 50mcg/fluticasone propionate 250mcg combination [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P434. [Google Scholar]
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD) [Abstract]. National COPD Conference; Arlington, Virginia. 2003:1081.
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to salmeterol/fluticasone propionate combination therapy in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P429. [Google Scholar]
- SFCA3007. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 250mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 250mcg BID (SFC 50/250) compared to placebo in COPD subjects. http://ctr.gsk.co.uk 2004.
Hattotuwa 2002 {published data only}
- Gizycki MJ, Hattotuwa KL, Barnes N, Jeffery PK. Effects of fluticasone propionate on inflammatory cells in COPD: an ultrastructural examination of endobronchial biopsy tissue. Thorax 2002;57(9):799‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattotuwa KL, Gizycki MJ, Ansari TW, Jeffery PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;165(12):1592‐9. [DOI] [PubMed] [Google Scholar]
John 2005 {published data only}
- John M, Bosse S, Oltmanns U, Schumacher A, Witt C. Effects of inhaled HFA beclomethasone on pulmonary function and symptoms in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2005;99(11):1418‐24. [DOI] [PubMed] [Google Scholar]
- John M, Bosse S, Schumacher A, Oltmanns U, Witt C. Effects of inhaled beclomethasone HFA 134 (qvar/ventoiair) on health related quality of life in patients with chronic obstructive pulmonary disease [Abstract]. American Thoracic Society 2005 International Conference. 2005:Poster A43.
Kerstjens 1992 {published data only}
- Kaptein AA, Brand PL, Dekker FW, Kerstjens HA, Postma DS, Sluiter HJ. Quality‐of‐life in a long‐term multicentre trial in chronic nonspecific lung disease: assessment at baseline. The Dutch CNSLD Study Group. European Respiratory Journal 1993;6(10):1479‐84. [PubMed] [Google Scholar]
- Kerstjens HA, Brand PL, Quanjer PH, Bruggen‐Bogaarts BA, Koeter GH, Postma DS. Variability of bronchodilator response and effects of inhaled corticosteroid treatment in obstructive airways disease. Dutch CNSLD Study Group. Thorax 1993;48(7):722‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstjens HA, Brand PL, Jong PM, Koeter GH, Postma DS. Influence of treatment on peak expiratory flow and its relation to airway hyperresponsiveness and symptoms. The Dutch CNSLD Study Group. Thorax 1994;49(11):1109‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstjens HA, Overbeek SE, Schouten JP, Brand PL, Postma DS. Airways hyperresponsivenes, bronchodilator response, allergy and smoking predict improvement in FEV1 during long‐term inhaled corticosteroid treatment. Dutch CNSLD Study Group. European Respiratory Journal 1993;6(6):868‐76. [PubMed] [Google Scholar]
- Kerstjens HA, Postma DS, Doormaal JJ, Zanten AK, Brand PL, Dekhuijzen PN, et al. Effects of short‐term and long‐term treatment with inhaled corticosteroids on bone metabolism in patients with airways obstruction. Dutch CNSLD Study Group. Thorax 1994;49(7):652‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstjens HA, Schouten JP, Brand PL, Schoonbrood DF, Sterk PJ, Postma DS. Importance of total serum IgE for improvement in airways hyperresponsiveness with inhaled corticosteroids in asthma and chronic obstructive pulmonary disease. The Dutch CNSLD Study Group. American Journal of Respiratory and Critical Care Medicine 1995;151(2 Pt 1):360‐8. [DOI] [PubMed] [Google Scholar]
- Kerstjens HAM, Brand PLP, Hughes MD, Robinson NJ, Postma DS, Sluiter HJ, et al. A comparison of bronchodilator therapy with or without inhaled corticosteroid therapy for obstructive airways disease. New England Journal of Medicine 1992;327(20):1413‐9. [DOI] [PubMed] [Google Scholar]
- Overbeek SE, Kerstjens HA, Bogaard JM, Mulder PG, Postma DS. Is delayed introduction of inhaled corticosteroids harmful in patients with obstructive airways disease (asthma and COPD)? The Dutch CNSLD Study Group. The Dutch Chronic Nonspecific Lung Disease Study Groups. Chest 1996;110(1):35‐41. [DOI] [PubMed] [Google Scholar]
- Rutten‐van Molken MP, Doorslaer EK, Jansen MC, Kerstjens HA, Rutten FF. Costs and effects of inhaled corticosteroids and bronchodilators in asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1995;151(4):975‐82. [DOI] [PubMed] [Google Scholar]
- Grunsven PM, Schayck CP, Derenne JP, Kerstjens HA, Renkema TE, Postma DS, et al. Long term effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a meta‐analysis. Thorax 1999;54(1):7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lapperre 2009 {published data only}
- Lapperre TS, Snoeck‐Stroband JB, Gosman MM, Jansen DF, Schadewijk A, Thiadens HA, et al. Groningen Leiden Universities Corticosteroids in Obstructive Lung Disease Study Group. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2009;151(8):517‐27. [DOI] [PubMed] [Google Scholar]
Laptseva 2002 {published data only}
- Laptseva IM, Laptseva EA, Borshchevsky VV, Gurevich G, Kalechits O. Inhaled budesonide in the management of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):244s. [Google Scholar]
LHS 2000 {published data only}
- Anthonisen NR, Connett JC, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. American Journal of Respiratory and Critical Care Medicine 2002;166(5):675‐9. [DOI] [PubMed] [Google Scholar]
- Eichenhorn MS, Wise RA, Madhok TC, Gerald LB, Bailey WC, Tashkin DP, et al. Lack of long‐term adverse adrenal effects from inhaled triamcinolone: Lung Health Study II. Chest 2003;124(1):57‐62. [DOI] [PubMed] [Google Scholar]
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343(26):1902‐9. [DOI] [PubMed] [Google Scholar]
- Scanlon PD, Connett JE, Wise RA, Tashkin DP, Madhok T, Skeans M, et al. Loss of one density with inhaled triamcinolone in Lung Health Study II. American Journal of Respiratory and Critical Care Medicine 2004;170(12):1302‐9. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Murray HE, Skeans M, Murray RP. Skin manifestations of inhaled corticosteroids in COPD patients. Chest 2004;126(4):1123‐33. [DOI] [PubMed] [Google Scholar]
Llewellyn‐Jones 1996 {published data only}
- Llewellyn‐Jones CG, Harris TA, Stockley RA. Effect of fluticasone propionate on sputum of patients with chronic bronchitis and emphysema. American Journal of Respiratory and Critical Care Medicine 1996;153(2):616‐21. [DOI] [PubMed] [Google Scholar]
Loppow 2001 {published data only}
- Loppow D, Schleiss MB, Kanniess F, Taube C, Jorres RA, Magnussen H. In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammation. Respiratory Medicine 2001;95(2):115‐21. [DOI] [PubMed] [Google Scholar]
Mahler 2002 {published data only}
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD). http://www.abstracts2view.com 2003.
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;166(8):1084‐91. [DOI] [PubMed] [Google Scholar]
- SFCA3006. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 500mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 500mcg BID (SFC 50/500) compared to placebo in COPD subjects. http://ctr.gsk.co.uk 2004.
Mirici 2001 {published data only}
- Mirici A, Bektas Y, Ozbakis G, Erman Z. Effect of inhaled corticosteroids on respiratory function tests and airway inflammation in stable chronic obstructive pulmonary disease. Clinical Drug Investigation 2001;21(12):835‐42. [Google Scholar]
Nishimura 1999 {published data only}
- Nishimura K, Koyama H, Ikeda A, Tsukino M, Hajiro T, Mishima M, et al. The effect of high‐dose inhaled beclomethasone dipropionate in patients with stable COPD. Chest 1999;115(1):31‐7. [DOI] [PubMed] [Google Scholar]
Ozol 2005 {published data only}
- Ozol D, Aysan T, Solak ZA, Mogulkoc N, Veral A, Sebik F. The effect of inhaled corticosteroids on bronchoalveolar lavage cells and IL‐8 levels in stable COPD patients. Respiratory Medicine 2005;99(12):1494‐500. [DOI] [PubMed] [Google Scholar]
Paggiaro 1998 {published data only}
- FLIT97. A multi‐centre, randomised, double‐blind, parallel group study of the efficacy and safety of inhaled fluticasone propionate 1000µg daily with placebo in chronic obstructive pulmonary disease. http://ctr.gsk.co.uk 2005.
- Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo‐controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet 1998;351(9105):773‐80. [DOI] [PubMed] [Google Scholar]
Pauwels 1999 {published data only}
- Johnell O, Pauwels R, Löfdahl C‐G, Laitinen LA, Postma DS, Pride NB, et al. Bone mineral density in patients with chronic obstructive pulmonary disease treated with budesonide Turbuhaler. European Respiratory Journal 2002;19(6):1058‐63. [DOI] [PubMed] [Google Scholar]
- Lofdahl CG, Postma DS, Laitinen LA, Ohlsson SV, Pauwels RA, Pride NB. The European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): recruitment methods and strategies. Respiratory Medicine 1998;92(3):467‐72. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long‐term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. New England Journal of Medicine 1999;340(25):1948‐53. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Lofdahl CG, Pride NB, Postma DS, Laitinen LA, Ohlsson SV. European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): hypothesis and design. European Respiratory Journal 1992;5(10):1254‐61. [PubMed] [Google Scholar]
Renkema 1996 {published data only}
- Renkema TE, Schouten JP, Koeter GH, Postma DS. Effects of long‐term treatment with corticosteroids in COPD. Chest 1996;109(5):1156‐62. [DOI] [PubMed] [Google Scholar]
- Grunsven PM, Schayck CP, Derenne JP, Kerstjens HA, Renkema TE, Postma DS, et al. Long term effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a meta‐analysis. Thorax 1999;54(1):7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 1986 {published data only}
- Robertson AS, Gove RI, Wieland GA, Burge PS. A double‐blind comparison of oral prednisolone 40 mg/day with inhaled beclomethasone dipropionate 1500 ug/day in patients with adult onset chronic obstructive airways disease. European Journal of Respiratory Diseases 1986;146 Suppl:565‐9. [PubMed] [Google Scholar]
Rutgers 1998 {published data only}
- Rutgers SR, Koeter GH, Mark TW, Postma DS. Short‐term treatment with budesonide does not improve hyperresponsiveness to adenosine 5'‐monophosphate in COPD. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Pt 1):880‐6. [DOI] [PubMed] [Google Scholar]
Schermer 2009 {published data only}
- Chavannes NH, Schermer TR, Wouters EF, Akkermans RP, Dekhuijzen RP, Muris JW, et al. Predictive value and utility of oral steroid testing for treatment of COPD in primary care: the COOPT study. International Journal of COPD 2009;4:431‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N‐acetylcysteine in primary care patients with COPD or chronic bronchitis. Respiratory Medicine 2009;103(4):542‐51. [DOI] [PubMed] [Google Scholar]
SCO30002 2005 {unpublished data only}
- SCO 30002. A multicentre, randomised, double‐blind, parallel group, placebo‐controlled study to compare the efficacy and safety of inhaled salmeterol/fluticasone propionate combination product 25/250 µg two puffs bd and fluticasone propionate 250µg two puffs bd alone, all administered via metered dose inhalers (MDI), in the treatment of subjects with chronic obstructive pulmonary disease (COPD) for 52 weeks. GlaxoSmithKline Clinical Trial Register 2005.
Senderovitz 1999 {published data only}
- Senderovitz T, Vestbo J, Frandsen J, Maltbaek N, Norgaard M, Nielsen C, et al. Steroid reversibility test followed by inhaled budesonide or placebo in outpatients with stable chronic obstructive pulmonary disease. Respiratory Medicine 1999;93(10):715‐8. [DOI] [PubMed] [Google Scholar]
Shaker 2009 {published data only}
- Shaker SB, Dirksen A, Ulrik CS, Hestad M, Stavngaard T, Laursen LC, Maltbaek N, et al. The effect of inhaled corticosteroids on the development of emphysema in smokers assessed by annual computed tomography. COPD: Journal of Chronic Obstructive Pulmonary Disease 2009;6(2):104‐11. [DOI] [PubMed] [Google Scholar]
- Shaker SB, Stavngaard T, Laursen LC, Stoel BC, Dirksen A. Rapid fall in lung density following smoking cessation in COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease 2011;8(1):2‐7. [DOI] [PubMed] [Google Scholar]
Sin 2004 {published data only}
- Sin DD, Lacy P, York E, Man SFP. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;170(7):760‐5. [DOI] [PubMed] [Google Scholar]
Sin 2008 {published data only}
- Sin DD, Man SF, Marciniuk DD, Ford G, FitzGerald M, Wong E, York E, et al. ABC (Advair, Biomarkers in COPD) Investigators. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2008;177(11):1207‐14. [DOI] [PubMed] [Google Scholar]
Szafranski 2003 {published data only}
- Anderson P. Budesonide/formoterol in a single inhaler (Symbicort) provides early and sustained improvement in lung function in moderate to severe COPD. Thorax 2002;57(Suppl III):iii43. [Google Scholar]
- Calverley PMA. Effect of budesonide/formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax 2002;BTS Winter Meeting 2002:S145. [Google Scholar]
- Calverley PMA, Thompson NC, Olsson H. Budesonide/formoterol in a single inhaler sustains lung function improvements in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P435. [Google Scholar]
- Campbell LM, Szafranski W. Budesonide/formoterol in a single inhaler (Symbicort) provides sustained relief from symptoms in moderate to severe COPD. Thorax. 2002; Vol. BTS Winter Meeting 2002:S143.
- Campbell LW, Szafranski W. Budesonide/formoterol in a single inhaler (Symbicort) reduces severe exacerbations in patients with moderate‐severe COPD. Thorax. 2002; Vol. BTS Winter Meeting 2002:S141.
- Dahl R, Cukier A, Olsson H. Budesonide/formoterol in a single inhaler reduces severe and mild exacerbations in patients with moderate to severe COPD. European Respiratory Journal 2002;20(Suppl 38):242 [P1575]. [Google Scholar]
- Egede F, Menga G. Budesonide/formoterol in a single inhaler provides sustained relief from symptoms and night‐time awakenings in moderate‐severe COPD: results from symptoms and night‐time awakenings in moderate to severe COPD: results from a 1‐year study. European Respiratory Journal 2002;20(Suppl 38):242 [P1574]. [Google Scholar]
- Halpin D, Stahl E, Lundback B, Anderson F, Peterson S. Treatment costs and number needed to treat (NNT) with budesonide/formoterol to avoid one exacerbation of COPD [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:D22 Poster 525.
- Jones PW, Stahl E, Svensson K. Improvement in health status in patients with moderate to severe COPD after treatment with budesonide/formoterol in a single inhaler. European Respiratory Journal 2002;20(Suppl 38):250 [P1613]. [Google Scholar]
- Korsgaard J, Sansores R. Budesonide/formoterol (single inhaler) provides sustained relief from shortness of breath and chest tightness in a 1‐year study of patients with moderate to severe COPD. European Respiratory Journal 2002;20(Suppl 38):242 [P1577]. [Google Scholar]
- Lange P, Saenz C. Budesonide/formoterol in a single inhaler is well tolerated in patients with moderate to severe COPD: results of a 1 year study. European Respiratory Journal 2002;20(Suppl 38):242 [P1573]. [Google Scholar]
- Lofdahl CG. Reducing the impact of COPD exacerbations: clinical efficacy of budesonide/formoterol. European Respiratory Review 2004;13(88):14‐21. [Google Scholar]
- Milanowski J, Nahabedian S. Budesonide/formoterol in a single inhaler acts rapidly to improve lung function and relieve symptoms in patients with moderate to severe COPD. European Respiratory Journal 2002;20(Suppl 38):242 [P1576]. [Google Scholar]
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. European Respiratory Journal 2003;21:74‐81. [DOI] [PubMed] [Google Scholar]
- Szfranski W, Ramirez A, Peterson S. Budesonide/formoterol in single inhaler provides sustained improvements in lung function in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress. 2002.
Tashkin 2008 {published data only}
- Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE, et al. SHINE (Division of Pulmonary, Critical Care Medicine. University of California, Los Angeles, California, USA). Efficacy and safety of budesonide and formoterol in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6‐month randomized clinical trial. Drugs 2008;68(14):1975‐2000. [DOI] [PubMed] [Google Scholar]
Thompson 1992 {published data only}
- Thompson AB, Mueller MB, Heires AJ, Bohling TL, Daughton D, Yancey SW, et al. Aerosolized beclomethasone in chronic bronchitis. Improved pulmonary function and diminished airway inflammation. American Review of Respiratory Disease 1992;146(2):389‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thompson 2002 {published data only}
- Thompson WH, Carvalho P, Souza JP, Charan NB. Controlled trial of inhaled fluticasone propionate in moderate to severe COPD. Lung 2002;180(4):191‐201. [DOI] [PubMed] [Google Scholar]
van Grunsven 1999 {published data only}
- Grunsven PM, Schayck CP, Derenne JP, Kerstjens HA, Renkema TE, Postma DS, et al. Long term effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a meta‐analysis. Thorax 1999;54(1):7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Grunsven 2003 {published data only}
- Grunsven P, Schermer T, Akkermans R, Albers M, Boom G, Schayck O, et al. Short‐ and long‐term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respiratory Medicine 2003;97(12):1303‐12. [DOI] [PubMed] [Google Scholar]
Verhoeven 2002 {published data only}
- Verhoeven GT, Garrelds IM, Hoogsteden HC, Zijlstra FJ. Effects of fluticasone propionate inhalation on levels of arachidonic acid metabolites in patients with chronic obstructive pulmonary disease. Mediators of Inflammation 2001;10(1):21‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven GT, Hegmans JP, Mulder PG, Bogaard JM, Hoogsteden HC, Prins JB. Effects of fluticasone propionate in COPD patients with bronchial hyperresponsiveness. Thorax 2002;57(8):694‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven GT, Wijkhuijs AJ, Hooijkaas H, Hoogsteden HC, Sluiter W. Effect of an inhaled glucocorticoid on reactive oxygen species production by bronchoalveolar lavage cells from smoking COPD patients. Mediators of Inflammation 2000;9(2):109‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vestbo 1999 {published data only}
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999;353(9167):1819‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weiner 1995 {published data only}
- Weiner P, Weiner M, Azgad Y, Zamir D. Inhaled budesonide therapy for patients with stable COPD. Chest 1995;108(6):1568‐71. [DOI] [PubMed] [Google Scholar]
Weiner 1999 {published data only}
- Weiner P, Weiner M, Rabner M, Waizman J, Magadle R, Zamir D. The response to inhaled and oral steroids in patients with stable chronic obstructive pulmonary disease. Journal of Internal Medicine 1999;245(1):83‐9. [DOI] [PubMed] [Google Scholar]
Weir 1990a {published data only}
- Weir DC, Gove RI, Robertson AS, Burge PS. Corticosteroid trials in non‐asthmatic chronic airflow obstruction: a comparison of oral prednisolone and inhaled beclomethasone dipropionate. Thorax 1990;45(2):112‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir DC, Gove RI, Robertson AS, Burge PS. Response to corticosteroids in chronic airflow obstruction: relationship to emphysema and airways collapse. European Respiratory Journal 1991;4(10):1185‐90. [PubMed] [Google Scholar]
- Weir DC, Robertson AS, Gove RI, Burge PS. Time course of response to oral and inhaled corticosteroids in non‐asthmatic chronic airflow obstruction. Thorax 1990;45(2):118‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weir 1999 {published data only}
- Weir DC, Bale GA, Bright P, Sherwood Burge P. A double‐blind placebo‐controlled study of the effect of inhaled beclomethasone dipropionate for 2 years in patients with nonasthmatic chronic obstructive pulmonary disease. Clinical & Experimental Allergy Supplement 1999;29(2):125‐8. [DOI] [PubMed] [Google Scholar]
Wempe 1992 {published data only}
- Wempe JB, Postma DS, Breederveld N, Kort E, Mark TW, Koeter GH. Effects of corticosteroids on bronchodilator action in chronic obstructive lung disease. Thorax 1992;47(8):616‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yildiz 2004 {published data only}
- Yildiz F, Basyigit I, Yildirim E, Boyaci H, Ilgazli A. Does addition of inhaled steroids to combined bronchodilator therapy affect health status in patients with COPD?. Respirology 2004;9(3):352‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albers 2004 {published data only}
- Albers M, Schermer T, dan Boom G, Akkermans R, Schayck C, Herwaarden C, et al. Efficacy of inhaled steroids in undiagnosed subjects at high risk for COPD: results of the detection, intervention, and monitoring of COPD and asthma program. Chest 2004;126(6):1815‐24. [DOI] [PubMed] [Google Scholar]
Anonymous 1999 {published data only}
- Anonymous. Inhaled glucocorticoids do not help smokers with mild chronic obstructive pulmonary disease. Modern Medicine of Australia 1999;42(12):9. [Google Scholar]
Anonymous 2000 {published data only}
- Anonymous. Inhaled corticosteroids: their role in chronic obstructive pulmonary disease. MeReC Bulletin 2000;11(6):21‐4. [Google Scholar]
Balbi 2000 {published data only}
- Balbi B, Majori M, Bertacco S, Convertino G, Cuomo A, Donner CF, et al. Inhaled corticosteroids in stable COPD patients: do they have effects on cells and molecular mediators of airway inflammation?. Chest 2000;117(6):1633‐7. [DOI] [PubMed] [Google Scholar]
Bensch 2003 {published data only}
- Bensch G, Hampel F, Sachs H. Once‐daily, evening administration of mometasone furoate dry powder inhaler improves pulmonary function in patients with mild to moderate persistent asthma. European Respiratory Journal 2003;22(Suppl 45):282s. [Google Scholar]
Burge 1999 {published data only}
- Burge PS. Inhaled corticosteroids in COPD. Thorax 1999;54(7):655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 1993 {published data only}
- Chan CHS, Cohen M, Pang J. The effects of inhaled corticosteroids on chronic airflow limitation. Asian Pacific Journal of Allergy and Immunology 1993;11(2):97‐101. [PubMed] [Google Scholar]
Confalonieri 1998 {published data only}
- Confalonieri M, Mainardi E, Della Porta R, Bernorio S, Gandola L, Beghe B, et al. Inhaled corticosteroids reduce neutrophilic bronchial inflammation in patients with chronic obstructive pulmonary disease. Thorax 1998;53(7):583‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corda 2008 {published data only}
- Corda L, Bertella E, Piana GE, Boni E, Redolfi S, Tantucci C. Inhaled corticosteroids as additional treatment in alpha‐1‐antitrypsin‐deficiency‐related COPD. Respiration 2008;76(1):61‐8. [DOI] [PubMed] [Google Scholar]
Cox 1999 {published data only}
- Cox G, Whitehead L, Dolovich M, Jordana M, Gauldie J, Newhouse MT. A randomised controlled trial on the effect of inhaled corticosteroids on airways inflammation in adult cigarette smokers. Chest 1999;115(5):1271‐7. [DOI] [PubMed] [Google Scholar]
Dompeling 1992 {published data only}
- Dompeling E, Schayck CP, Molema J, Folgering H, Grunsven PM, Weel C. Inhaled beclomethasone improves the course of asthma and COPD. European Respiratory Journal 1992;5(8):945‐52. [PubMed] [Google Scholar]
Dompeling 1993 {published data only}
- Dompeling E, Schayck CP, Grunsven PM, Herwaarden CLA, Akkermans R, Molema J, et al. Slowing the deterioration of asthma and chronic obstructive pulmonary disease observed during bronchodilator therapy by adding inhaled corticosteroids. Annals of Internal Medicine 1993;118(10):770‐8. [DOI] [PubMed] [Google Scholar]
Egan 1999 {published and unpublished data}
- Egan JJ, Maden C, Kalra S, Adams JE, Eastell R, Woodcock AA. A randomised, double blind study comparing the effects of beclomethasone and fluticasone on bone density over 2 years. European Respiratory Journal 1999;13(6):1267‐75. [PubMed] [Google Scholar]
Engel 1989 {published data only}
- Engel T, Heinig JH, Madsen O, Hansen M, Weeke ER. A trial of inhaled budesonide on airway responsiveness in smokers with chronic bronchitis. European Respiratory Journal 1989;2(10):935‐9. [PubMed] [Google Scholar]
Fattore 2005 {published data only}
- Fattore G, Torbica A, Mangone M. Cost‐analysis of four treatment strategies in the management of moderate‐to‐severe COPD: an application non‐parametric bootstrap. Pharmacoeconomics Italian Research Articles 2005;72(2):135‐43. [Google Scholar]
Fazio 1986 {published data only}
- Fazio F, Lafortuna CL. Beclomethasone dipropionate does not affect mucociliary clearance in patients with chronic obstructive lung disease. Respiration 1986;50(1):62‐5. [DOI] [PubMed] [Google Scholar]
Guleria 2003 {published data only}
- Guleria R, Singh TR, Sinha S, Padhy K, Gupta K, Pande JN. Effect of single inhalation of a salbutamol, ipratropium bromide and beclomethasone on mucociliary clearance in patients with chronic obstructive airway disease. Indian Journal of Chest Diseases & Allied Sciences 2003;45(4):241‐6. [PubMed] [Google Scholar]
Keatings 1997 {published data only}
- Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. American Journal of Respiratory and Critical Care Medicine 1997;155:542‐8. [DOI] [PubMed] [Google Scholar]
Kozak‐Skzopek 1997 {published data only}
- Kozak‐Skzopek E, Ulmer WT. Inhalative Budesonid‐Therapie bei chronischer Bronchitis. Atemweg und Lungenerkrankheiten 1997;23(9):542‐6. [Google Scholar]
Matlin 1976 {unpublished data only}
- Matlin RA. The efficacy of steroid aerosol in chronic obstructive pulmonary disease (COPD). American Review of Respiratory Disease 1976;113(4):184. [Google Scholar]
Melani 1999 {published data only}
- Melani AS, Gregorio A. Four‐week nebulized beclomethasone dipropionate in stable COPD patients with exertional dyspnoea. Monaldi Archives for Chest Disease 1999;54(3):224‐7. [PubMed] [Google Scholar]
Moller 1999 {published data only}
- Moller M, Haller S, Kiebart M, Cloes R. AeroBec Autohaler in patients with reversible chronic‐obstructive respiratory diseases. Atemwegs‐ Und Lungenkrankheiten 1999;25(3):160‐7. [Google Scholar]
Nava 2000 {published data only}
- Nava S, Compagnoni ML. Controlled short‐term trial of fluticasone propionate in ventilator‐dependent patients with COPD. Chest 2000;118(4):990‐9. [DOI] [PubMed] [Google Scholar]
Nishimura 2000 {published data only}
- Nishimura K, Ikeda A, Koyama H, Zhang M, Tsukino M, Hajiro T, et al. Additive effects of prednisolone and beclomethasone dipropionate in patients with stable chronic obstructive pulmonary disease. Pulmonary Pharmacology & Therapeutics 2000;13(5):225‐30. [DOI] [PubMed] [Google Scholar]
O'Brien 2001 {published data only}
- O'Brien A, Russo‐Magno P, Karki A, Hiranniramol S, Hardin M, Kaszuba M, et al. Effects of withdrawal of inhaled steroids in men with severe irreversible airflow obstruction. American Journal of Respiratory and Critical Care Medicine 2001;164(3):365‐71. [DOI] [PubMed] [Google Scholar]
Ouyang 1998 {published data only}
- Ouyang R, Yin B. Clinical study of inhaled corticosteroid in non‐asthmatic chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 1998;21(8):497‐9. [PubMed] [Google Scholar]
Roth 1996 {published data only}
- Roth R. Inhaled corticosteroids are effective and well tolerated [Inhalative Glukokortikoide sind wirksam und gut verträglich]. Therapiewoche 1996;46(24):1380. [Google Scholar]
Sandrini 2003 {published data only}
- Sandrini A, Plit M, Glaville A, Bryant D, Yates DH. Effect of inhaled steroid withdrawal on exhaled nitric oxide in COPD: preliminary data [Abstract]. Thorax 2003;58(Suppl 3):iii86. [Google Scholar]
Sapey 2000 {published data only}
- Sapey E, Langford NJ, Kendall MJ. Inhaled corticosteroids and chronic obstructive pulmonary disease. Journal of Clinical Pharmacy & Therapeutics 2000;25(4):235‐8. [DOI] [PubMed] [Google Scholar]
Schuurmans 2001 {published data only}
- Schuurmans M, Soler M. COPD ‐ Therapie: Bronchodilatatoren und Steroide. Therapiewoche 2001;17(1):28‐32. [Google Scholar]
Spicuzza 2004 {published data only}
- Spicuzza L, Scuderi V, Prosperini G, Balsamo R, DiMara GU, Polosa R. Acute effect of inhaled fluticasone on airway hyperresponsiveness to adenosine 5'‐monophosphate in asthma and in COPD. American Thoracic Society 100th International Conference. 2004; Vol. A58 Poster K33.
Tsang 1999 {published data only}
- Tsang KW. Inhaled corticosteroids in COPD. Thorax 1999;54(2):186. [PubMed] [Google Scholar]
Turker 2004 {published data only}
- Turker H, Karakurt Z, Durucu K, Sulu E, Boga S, Yavsan M. High dose inhaler corticosteroids in patients with COPD. European Respiratory Journal 2004;24(Suppl 48):289s. [Google Scholar]
van den Boom 2001 {published data only}
- Boom G, Rutten‐Van Molken MPMJ, Molema J, Tirimanna PRS, Weel C, Schayck CP. The cost effectiveness of early treatment with fluticasone propionate 250 mcg twice a day in subjects with obstructive airway disease: results of the DIMCA program. American Journal of Respiratory and Critical Care Medicine 2001;164(11):2057‐66. [DOI] [PubMed] [Google Scholar]
van der Valk 2002 {published data only}
- Valk P, Monninkhof E, Phalen J, Zielhuis G, Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. American Journal of Respiratory and Critical Care Medicine 2002;166(10):1358‐63. [DOI] [PubMed] [Google Scholar]
van Grunsven 2000 {published data only}
- Grunsven PM, Schayck CP, Deuveren M, Herwaarden CL, Akkermans RP, Weel C. Compliance during long‐term treatment with fluticasone propionate in subjects with early signs of asthma or chronic obstructive pulmonary disease (COPD): results of the Detection, Intervention, and Monitoring Program of COPD and Asthma (DIMCA) Study. Journal of Asthma 2000;37(3):225‐34. [DOI] [PubMed] [Google Scholar]
van Schayck 1995 {published data only}
- Schayck CP, Dompeling E, Rutten MP, Folgering H, Boom G, Weel C. The influence of an inhaled steroid on quality of life in patients with asthma or COPD. Chest 1995;107(5):1199‐205. [DOI] [PubMed] [Google Scholar]
Vestbo 2000 {published data only}
- Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in patients with mild to moderate chronic obstructive lung disease. The Østerbro Study [Langtidsvirkningen af inhaleret budesonid hos patienter med mild og moderat kronisk obstruktiv lungesygdom. Østerbro lunge studie]. Ugeskrift for Læger 2000;162(4):493‐7. [PubMed] [Google Scholar]
Watson 1992 {published data only}
- Watson A, Lim TW, Joyce H, Pride NB. Failure of inhaled corticosteroids to modify bronchoconstrictor or bronchodilator responsiveness in middle‐aged smokers with mild airflow obstruction. Chest 1992;101(2):350‐5. [DOI] [PubMed] [Google Scholar]
Weiner 1997 {published data only}
- Weiner P, Zamir D, Beckerman M. Inhaled budesonide for chronic obstructive pulmonary disease. Harefuah 1997;132(11):823. [PubMed] [Google Scholar]
Weir 1993 {published data only}
- Weir DC, Burge PS. Effects of high dose inhaled beclomethasone dipropionate, 750 mcg and 1500 mcg twice daily, and 40 mg per day oral prednisolone on lung function, symptoms, and bronchial hyperresponsiveness in patients with non‐asthmatic chronic airflow obstruction. Thorax 1993;48(4):309‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wesseling 1991 {published data only}
- Wesseling GJ, Quaedvlieg M, Wouters EFM. Inhaled budesonide in chronic bronchitis. Effects on respiratory impedance. European Respiratory Journal 1991;4(9):1101‐5. [PubMed] [Google Scholar]
Whittaker 2000 {published data only}
- Whittaker AJ, Spiro SG. Inhaled steroid therapy in chronic obstructive pulmonary disease. Current Opinion in Pulmonary Medicine 2000;6(2):104‐9. [DOI] [PubMed] [Google Scholar]
Wilcke 1997 {published data only}
- Wilcke JT, Dirksen A. The effect of inhaled glucocorticosteroids in emphysema due to alpha1‐antitrypsin deficiency. Respiratory Medicine 1997;91(5):275‐9. [DOI] [PubMed] [Google Scholar]
Williamson 2009 {published data only}
Yildiz 2000 {published data only}
- Yildiz F, Kaur AC, Ilgazli A, Celikoglu M, Kacar Ozkara S, Paksoy N, et al. Inhaled corticosteroids may reduce neutrophilic inflammation in patients with stable chronic obstructive pulmonary disease. Respiration 2000;67(1):71‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Agarwal 2010
Alsaeedi 2002
- Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomised placebo‐controlled trials. American Journal of Medicine 2002;113(1):59‐65. [DOI] [PubMed] [Google Scholar]
Barnes 2000
- Barnes PJ. Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(2):342‐4. [DOI] [PubMed] [Google Scholar]
Barnes 2004
- Barnes PJ, Ito K, Adcock IM. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet 2004;363(9410):731‐3. [DOI] [PubMed] [Google Scholar]
Bonay 2002
- Bonay M, Bancal C, Crestani B. Benefits and risks of inhaled corticosteroids in chronic obstructive pulmonary disease. Drug Safety 2002;25(1):57‐71. [DOI] [PubMed] [Google Scholar]
Bonay 2005
- Bonay M, Bancal C, Crestani B. The risk/benefit of inhaled corticosteroids in chronic obstructive pulmonary disease. Expert Opinion on Drug Safety 2005;4(2):251‐71. [DOI] [PubMed] [Google Scholar]
Burge 2001
- Burge S. Should inhaled corticosteroids be used in the long term treatment of chronic obstructive pulmonary disease?. Drugs 2001;61(11):1535‐44. [DOI] [PubMed] [Google Scholar]
Burge 2003a
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA. Prednisolone response in patients with chronic obstructive pulmonary disease: results from the ISOLDE study. Thorax 2003;58(8):654‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burge 2003b
- Burge PS, Lewis SA. So inhaled steroids slow the rate of decline in FEV1 in patients with COPD after all?. 2003 Thorax;58:911‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calverley 1999
- Calverley PM. Re‐assessing the evidence about inhaled corticosteroids in chronic obstructive pulmonary disease. Thorax 1999;54(1):3‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calverley 2000
- Calverley PM. Inhaled corticosteroids are beneficial in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(2):341‐2. [DOI] [PubMed] [Google Scholar]
Calverley 2003d
- Calverley PM, Spencer S, Willits L, Burge PS, Jones PW. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest 2003;124(4):1350. [DOI] [PubMed] [Google Scholar]
Calverley 2005
- Calverley PM. The role of corticosteroids in chronic obstructive pulmonary disease. Seminars in Respiratory and Critical Care Medicine 2005;26(2):235‐45. [DOI] [PubMed] [Google Scholar]
Calverley 2011
Celli 2008
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. American Journal of Respiratory and Critical Care Medicine 2008;178(4):332‐8. [DOI] [PubMed] [Google Scholar]
Crim 2009
Donaldson 2002
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57(10):847‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donaldson 2006
- Donaldson GC, Wedzicha JA. COPD exacerbations. 1: Epidemiology. Thorax 2006;61(2):164‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drummond 2008
- Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta‐analysis. JAMA. 2008/11/27 2008; Vol. 300, issue 20:2407‐16. [1538‐3598: (Electronic)] [DOI] [PMC free article] [PubMed]
Eichenhorn 2003
- Eichenhorn MS, Wise RA, Madhok TC, Gerald LB, Bailey WC, Tashkin DP, et al. Lack of long‐term adverse adrenal effects from inhaled triamcinolone: Lung Health Study II. Chest 2003;124(1):57‐62. [DOI] [PubMed] [Google Scholar]
Epstein 2003
- Epstein PE. Inhaled corticosteroids and chronic obstructive pulmonary disease: are we barking up the wrong tracheobronchial tree?. Annals of Internal Medicine 2003;138(12):1001‐2. [DOI] [PubMed] [Google Scholar]
Ferguson 2006
- Ferguson GT, Calverley PMA, Anderson JA, et al. The TORCH (TOwards a Revolution in COPD Health) study: salmeterol/fluticasone propionate (SFC) improves survival in COPD over three years. European Respiratory Journal 2006;28(Suppl 50):34s. [Google Scholar]
Gan 2005
- Gan WQ, Man SF, Sin DD. Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta‐analysis. BMC Pulmonary Medicine 2005;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gartlehner 2006
- Gartlehner G, Hansen RA, Carson SS, Lohr KN. Efficacy and safety of inhaled corticosteroids in patients with COPD: a systematic review and meta‐analysis of health outcomes. Annals of Family Medicine 2006;4(3):253‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gizycki 2002
- Gizycki MJ, Hattotuwa KL, Barnes N, Jeffery PK. Effects of fluticasone propionate on inflammatory cells in COPD: an ultrastructural examination of endobronchial biopsy tissue. Thorax 2002;57(9):799‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Highland 2003
- Highland KB, Strange C, Heffner JE. Long‐term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease: a meta‐analysis. Annals of Internal Medicine 2003;138(12):969‐73. [DOI] [PubMed] [Google Scholar]
Highland 2004
- Highland KB. Inhaled corticosteroids in chronic obstructive pulmonary disease: is there a long‐term benefit?. Current Opinion in Pulmonary Medicine 2004;10(2):113‐9. [DOI] [PubMed] [Google Scholar]
Hudson 1990
- Hudson LD, Monti CM. Rationale and use of corticosteroids in chronic obstructive pulmonary disease. Medical Clinics of North America 1990;74(3):661‐90. [DOI] [PubMed] [Google Scholar]
Ito 2005
- Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. New England Journal of Medicine 2005;325(19):1967‐76. [DOI] [PubMed] [Google Scholar]
Johnell 2002
- Johnell O, Pauwels R, Löfdahl CG, Laitinen LA, Postma DS, Pride NB, et al. Bone mineral density in patients with chronic obstructive pulmonary disease treated with budesonide Turbuhaler. European Respiratory Journal 2002;19(6):1058‐63. [DOI] [PubMed] [Google Scholar]
Jones 2003
- Jones PW, Willits LR, Burge PS, Calverley PMA. Disease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbations. European Respiratory Journal 2003;21(1):68‐73. [DOI] [PubMed] [Google Scholar]
Kanner 2001
- Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex‐smokers with mild chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;164(3):358‐64. [DOI] [PubMed] [Google Scholar]
Loke 2011
Macie 2006
- Macie C, Wooldrage K, Manfreda J, Anthonisen NR. Inhaled corticosteroids and mortality in COPD. Chest 2006;130(3):640‐6. [DOI] [PubMed] [Google Scholar]
Man 2005a
- Man SF, Sin DD. Effects of corticosteroids on systemic inflammation in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2005;2(1):78‐82. [DOI] [PubMed] [Google Scholar]
Man 2005b
- Man SF, Sin DD. Inhaled corticosteroids in chronic obstructive pulmonary disease: is there a clinical benefit?. Drugs 2005;65(5):579‐91. [DOI] [PubMed] [Google Scholar]
Mapel 2006
- Mapel DW, Hurley JS, Roblin D, Roberts M, Davis KJ, Schreiner R, et al. Survival of COPD patients using inhaled corticosteroids and long‐acting beta agonists. Respiratory Medicine 2006;100(4):595‐609. [DOI] [PubMed] [Google Scholar]
Mapp 2000
- Mapp CE. Inhaled glucocorticoids in chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343(26):1960‐1. [DOI] [PubMed] [Google Scholar]
Postma 1999
- Postma DS, Kerstjens HA. Are inhaled glucocorticosteroids effective in chronic obstructive pulmonary disease?. American Journal of Respiratory and Critical Care Medicine 1999;160(5):s66‐s71. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Riancho 2002
- Riancho JA, Cubian I, Portero I. Effectiveness of inhaled corticosteroids in chronic obstructive lung disease: systematic review. Medica Clinica 2002;118(12):446‐51. [DOI] [PubMed] [Google Scholar]
Scanlon 2004
- Scanlon PD, Connett JE, Wise RA, Tashkin DP, Madhok T, Skeans M, et al. Loss of bone density with inhaled triamcinolone in Lung Health Study II. American Journal of Respiratory and Critical Care Medicine 2004;170(12):1302‐9. [DOI] [PubMed] [Google Scholar]
Scott 2006
- Scott S, Walker P, Calverley PM. COPD exacerbations. 4: Prevention. Thorax 2006;61(5):440‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Selroos 2004
- Selroos O. The place of inhaled corticosteroids in chronic obstructive pulmonary disease. Current Medical Research and Opinion 2004;20(10):1579‐93. [DOI] [PubMed] [Google Scholar]
Sin 2001
- Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;164(4):580‐4. [DOI] [PubMed] [Google Scholar]
Sin 2003a
- Sin DD, Man SF. Inhaled corticosteroids and survival in chronic obstructive pulmonary disease: does the dose matter?. European Respiratory Journal 2003;21(2):260‐6. [DOI] [PubMed] [Google Scholar]
Sin 2003b
- Sin DD, Man SF. Inhaled corticosteroids in the long‐term management of patients with chronic obstructive pulmonary disease. Drugs & Aging 2003;20(12):867‐80. [DOI] [PubMed] [Google Scholar]
Sin 2003c
- Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 2003;290(17):2301‐12. [DOI] [PubMed] [Google Scholar]
Sin 2005
- Sin DD, Wu L, Anderson JA, Anthonisen NR, Buist AS, Burge PS, et al. Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax 2005;60(12):992‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sin 2009
Sin 2010
- Sin DD, Man SFP. Steroids in COPD: still up in the air?. European Respiratory Journal 2010;35(4):949‐51. [DOI] [PubMed] [Google Scholar]
Singh 2009
Soriano 2003
- Soriano JB, Kiri VA, Pride NB, Vestbo J. Inhaled corticosteroids with/without long‐acting beta‐agonists reduce the risk of rehospitalization and death in COPD patients. American Journal of Respiratory and Critical Care Medicine 2003;2(1):67‐74. [DOI] [PubMed] [Google Scholar]
Soriano 2007
- Soriano JB, Sin DD, Zhang X, Anderson JA, Anthonisen NR, Buist AS, et al. A pooled analysis of FEV1 decline in COPD patients randomized to inhaled corticosteroids or placebo. Chest 2007;131:682‐9. [DOI] [PubMed] [Google Scholar]
Spencer 2001
- Spencer S, Calverley PM, Burge PS, Jones PW. Health status deterioration in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;163(1):122‐8. [DOI] [PubMed] [Google Scholar]
Suissa 2003
- Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: immortal time bias in observational studies. American Journal of Respiratory and Critical Care Medicine 2003;168(1):49‐53. [DOI] [PubMed] [Google Scholar]
Suissa 2004
- Suissa S. Inhaled steroids and mortality in COPD: bias from unaccounted immortal time. European Respiratory Journal 2004;23(3):391‐5. [DOI] [PubMed] [Google Scholar]
Suissa 2006
- Suissa S. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2006;173(8):842‐6. [DOI] [PubMed] [Google Scholar]
Suissa 2008
Suissa 2008a
- Suissa S, Ernst P, Vandemheen KL, Aaron SD. Methodological issues in therapeutic trials of COPD. European Respiratory Journal 2008;31:927‐33. [DOI] [PubMed] [Google Scholar]
Sutherland 2003
- Sutherland ER, Allmers H, Ayas NT, Venn AJ, Martin RJ. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta‐analysis. Thorax 2003;58(11):937‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Schayck 1996
- Schayck CP, Grunsven PM, Dekhuijzen PN. Do patients with COPD benefit from treatment with inhaled corticosteroids?. European Respiratory Journal 1996;9(10):1969‐72. [DOI] [PubMed] [Google Scholar]
Verhoeven 2000
- Verhoeven GT, Wijkhuijs AJ, Hooijkaas H, Hoogsteden HC, Sluiter W. Effect of an inhaled glucocorticoid on reactive oxygen species production by bronchoalveolar lavage cells from smoking COPD patients. Mediators of Inflammation 2000;9(2):109‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verhoeven 2001
- Verhoeven GT, Garrelds IM, Hoogsteden HC, Zijlstra FJ. Effects of fluticasone propionate inhalation on levels of arachidonic acid metabolites in patients with chronic obstructive pulmonary disease. Mediators of Inflammation 2001;10(1):21‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vestbo 2011
Wedzicha 2005
- Wedzicha JA, Seemungal TA. Inhaled corticosteroids in COPD: a light at the end of the tunnel?. Thorax 2005;60(12):977‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weir 1990b
- Weir DC, Robertson AS, Gove RI, Burge PS. Time course of response to oral and inhaled corticosteroids in non‐asthmatic chronic airflow obstruction. Thorax 1990;45(2):118‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weir 1991
- Weir DC, Gove RI, Robertson AS, Burge PS. Response to corticosteroids in chronic airflow obstruction: relationship to emphysema and airways collapse. European Respiratory Journal 1991;4(10):1185‐90. [PubMed] [Google Scholar]


