Abstract
Although several dosage adjustment regimens have been proposed, there is little quantitative information to guide the initiation of ceftazidime therapy in patients who are receiving continuous renal replacement therapy. To determine the clearance of ceftazidime by continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodialysis (CVVHD), we performed controlled clearance studies with stable hemodialysis patients with three hemofilters: a 0.6-m2 acrylonitrile copolymer (AN69; Hospal) filter, a 2.1-m2 polymethylmethacrylate filter (PMMA; Toray) filter and a 0.65-m2 polysulfone (PS; Fresenius) filter. Subjects received 1,000 mg of ceftazidime intravenously prior to the start of a clearance study. The concentration of ceftazidime in multiple plasma and dialysate or ultrafiltrate samples was determined by high-performance liquid chromatography. The diffusional clearances (CIdiffusion) and sieving coefficients of ceftazidime were compared by a mixed-model repeated-measures analysis of variance with filter and blood, dialysate inflow, or ultrafiltration rate as the main effect and the patient as a random effect. The fraction of ceftazidime bound to plasma proteins was 17% ± 7% (range, 10 to 25%). The clearances of ceftazidime, urea, and creatinine by CVVHD were essentially constant at blood flow rates of 75 to 250 ml/min for all three filters. Significant linear relationships (P < 0.0001) were observed between CIdiffusion of ceftazidime and clearance of urea for all three filters: AN69 (slope = 0.83), PMMA (slope = 0.89), and PS (slope = 1.03). Ceftazidime clearance was membrane independent during CVVH and CVVHD. CVVH and CVVHD can significantly augment the clearance of ceftazidime. Dosing strategies for initiation of ceftazidime therapy in patients receiving CVVH and CVVHD are proposed.
Continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodialysis (CVVHD) are frequently utilized to manage hemodynamically unstable patients who are volume overloaded or have acute renal failure (4, 13, 20). Continuous venovenous hemodiafiltration (CVVHDF), which employs diffusion as well as convection, may also be utilized, particularly for hypercatabolic patients (23). Drug clearance by CVVH is dependent on the ultrafiltration rate and the sieving coefficient (SC) for the particular solute or drug of interest (3, 16). The clearance of medications by CVVHD is predominantly dependent on the dialysate flow rate, since solute and/or drug removal is primarily diffusive (16). In addition to the ultrafiltration rate and dialysate flow rate, the removal of solutes or drugs by CVVH, CVVHD, or CVVHDF may be dependent on the type of hemofilter utilized (24). The clearance of some small and large molecules has been reported to vary markedly between hemofilters, even when all other procedural variables are held constant (14, 17, 19). Finally, the use of pump-driven systems (i.e., CVVH and CVVHD) may enhance drug clearance due to the consistency of blood flow compared to continuous arteriovenous hemofiltration and continuous arteriovenous hemodialysis.
Although low-to-intermediate-molecular-mass (500 to 1,000 Da) drugs are removed with cellulosic dialyzers, their removal may be enhanced when synthetic filters are utilized (1, 25). Ceftazidime is a prototypical low-to-intermediate-molecular-mass drug (547 Da). Ceftazidime is approximately 17% protein bound and has a volume of distribution of about 0.25 liter/kg of body weight (range, 0.22 to 0.27 liter/kg) (2). The fraction of ceftazidime excreted unchanged by the kidney is ∼90% in subjects with normal renal function. Thus, the half-life of ceftazidime increases significantly in patients with renal insufficiency (2). The reported clearance of ceftazidime during CVVH, CVVHD, or CVVHDF or their arterial derivatives ranges from 4.2 to 24.0 ml/min (3, 6, 12, 13, 27). The methods utilized to determine the clearance of ceftazidime, however, were often not provided or consisted of multiple assumptions (e.g., normal degree of protein binding and consistency of blood, dialysate, and ultrafiltrate flow rates). Unfortunately, many of these studies had limited statistical power due to small sample size (n = 2 to 5), poorly defined continuous renal replacement therapy (CRRT) conditions (i.e., dialysate, ultrafiltration, and blood flow rates; hemofilter type; length of therapy), or the lack of documentation of adequacy of removal of a reference solute (i.e., urea or creatinine). This study was, therefore, designed to rigorously evaluate the extracorporeal clearance of the prototype middle molecule, ceftazidime, by CVVH and CVVHD in stable end-stage renal disease (ESRD) patients in order to assess the influence of critical procedural variables on drug clearance. This study was performed with ESRD patients, since it is difficult if not impossible to conduct rigorous structured studies that may require modification of the prescribed therapeutic CVVH or CVVHD regimen for critically ill patients.
MATERIALS AND METHODS
Eight patients with ESRD who were receiving conventional maintenance hemodialysis participated in this study after granting written informed consent. The Biomedical Institutional Review Board and the General Clinical Research Center Committee of the University of Pittsburgh approved the study and consent document. The clearance of ceftazidime by CVVH and CVVHD was determined during a 12-h procedure (see details below) for each of the three hollow fiber hemofilters evaluated. These included a 0.6-m2 acrylonitrile and sodium methallyl sulfonate copolymer (AN69) hemofilter (Hospal Multiflow 60; CGH Medical), a 2.1-m2 polymethylmethacrylate (PMMA) hemofilter (Filtryzer B1-2.1U; Toray Industries), and a 0.65-m2 polysulfone (PS) hemofilter (Fresenius F40; Fresenius AG). A total of five clearance procedures were performed with each hemofilter. Each 12-h CRRT procedure was performed in addition to the patient's regularly scheduled hemodialysis treatments.
CRRT procedure.
Venous access was obtained by cannulation of the patient's hemodialysis arteriovenous fistula or polytetrafluoroethylene graft. The inlet and outlet ports of the filter were connected to the patient via CVVH tubing. Blood flow rate was regulated by the use of a roller pump (Sarns, Ann Arbor, Mich.). An air detector with an automatic pump shut-off was located distal to the drip chamber on the venous return. Dialysate was pumped countercurrent to blood by utilizing linear peristaltic pumps which controlled both the inflow and outflow rates (Flowgard 6300; Baxter Healthcare Corp., Deerfield, Ill.). These pumps allowed a maximal delivery rate of 1,999 ml/min. Hemodiafiltration fluid (Baxter Healthcare Corp.) was used as a dialysate. No replacement fluids were administered during the CVVH or CVVHD clearance studies. Heparin was infused through a prehemofilter port with initial dosages corresponding to the rate prescribed during the patients' conventional hemodialysis session. The heparin infusion rate was monitored during the procedure and titrated to achieve an activated clotting time of between 120 and 180 s.
Clearance studies.
The patients received a 1,000-mg intravenous dose of ceftazidime administered as a 1-h infusion. The minimum time between the end of the infusion and the commencement of the clearance study of 1 h ensured that the clearance evaluations were performed during the postdistributive phase. Study participants were admitted to the Clinical Research Center outpatient facility the morning of the clearance study. All clearance studies were performed under controlled dialysate, blood, and ultrafiltrate conditions as described below.
The effect of dialysate inflow rate on clearance was determined by increasing the dialysate flow rate incrementally at hourly intervals from 8.3 to 16.7, 25, and 33.3 ml/min while nominal blood and ultrafiltration flow rates were held constant at 100 and 0 ml/min, respectively. The effect of blood flow rate on clearance was determined by increasing the blood flow rate hourly from 75 to 125, 150, and 250 ml/min while the dialysate and ultrafiltration flow rates were held constant at 33.3 and 0 ml/min, respectively. The SC and CVVH clearance were assessed at nominal ultrafiltrate flow rates of 500 and 1,000 ml/h while maintaining blood and dialysate flow rates of 100 and 0 ml/min, respectively. CVVH clearances of ceftazidime, urea, and creatinine at the two ultrafiltration rates were determined during two 15-min periods, after an initial 15-min equilibration period. Each CVVHD clearance study period consisted of an initial 20-min equilibration period and two 20-min clearance determinations. Blood samples were collected at the midpoint of each dialysate or ultrafiltrate collection period.
Analytical.
The concentrations of urea and creatinine in the plasma and dialysate or ultrafiltrate specimens were determined with an Ektachem 700 XRC autoanalyzer (Eastman Kodak, Rochester, N.Y.). The total (bound and unbound) concentrations of ceftazidime in plasma and dialysate were determined by reverse-phase high-performance liquid chromatography (HPLC) with UV detection. Plasma proteins were precipitated with perchloric acid to release drug from its binding sites, and an aliquot of the supernatant was injected into the HPLC system. Separation was achieved with a Microsorb MV C18 column (100 by 4.6 mm; 3 μm) and a mobile phase consisting of 18% methanol and phosphate buffer. The assay was linear over the concentration range of 5.0 to 200 μg/ml in plasma and dialysate. The inter- and intraday coefficients of variation were less than 10% in plasma ultrafiltrate and dialysate.
The fraction of ceftazidime bound to plasma proteins was determined by filtration. Plasma (0.5 ml) was incubated at 37°C for 1 h and then placed in a Centrifree filtration device (molecular weight cutoff, 30,000; Amicon, Beverly, Mass.) and centrifuged for 30 min in a fixed-angle centrifuge. The protein-free filtrate was collected and then analyzed as described above. The concentration of drug in the filtrate represents the portion of the plasma concentration that is unbound.
Pharmacokinetic analysis.
The clearance of urea, creatinine, and ceftazidime (total and unbound) was calculated during each CVVHD period as CL = (QDO × CDO)/CPmid, where CL is solute clearance during CVVHD, QDO is hemofilter outflow rate, CDO is concentration of solute in the hemofilter outflow, and CPmid is concentration of solute in the plasma at the midpoint of the collection period.
The SCs of ceftazidime (total and unbound) were calculated during each CVVH period as SC = CUF/CP, where ultrafiltrate concentration (CUF) and plasma drug concentration (CP) were determined from simultaneously collected specimens. The clearance of urea, creatinine, and ceftazidime was calculated during the four CVVH observation periods as CLCVVH = (CUF · QUF) /CPmid, where CUF is the concentration of solute in the ultrafiltrate and QUF is the ultrafiltrate flow rate.
Dosing regimens for ceftazidime were calculated from the observed CVVH and CVVHD clearance data by assuming a nonrenal clearance of 10.6 ml/min for total ceftazidime (18). The residual renal clearances of total ceftazidime associated with creatinine clearances (CLcr) of 0 to 120 ml/min were assumed to be 1.15 · CLcr (18). The best predictor of bacterial killing and, thereby, clinical efficacy of cephalosporins, is the time within a dosage interval that plasma drug concentrations exceed the MIC for the infecting organism (5, 26). Projected dosage regimens were, therefore, derived by the Tozer method (21). In this scenario, after the administration of a normal loading dose, the projected maintenance dose was reduced in proportion to the patient's degree of renal insufficiency and administered at a practical clinical value of every 12 h to maximize the time above the MIC. An intravenous dose of 1 g every 8 h was utilized as the “normal” maintenance dose for ceftazidime. This should result in the maintenance of unbound serum drug concentrations above the MIC at which 90% of susceptible organisms (4 mg/liter) are inhibited (MIC90) (7, 11) for over 80% of the dosing interval.
Statistics.
The demographic characteristics of the three filter groups for each drug were compared by analysis of variance (ANOVA). The total and unbound clearance of ceftazidime, urea, and creatinine by the three filters during CVVH and CVVHD were compared by a mixed-model repeated-measures ANOVA with filter and flow rate as the main effects and with the patient as a random effect. We determined that a sample size of five subjects per group would allow for the detection of an effect size of 1.0 for within-filter comparisons and 2.0 for between-filter comparisons. This translates into the ability to detect a 25% difference in ceftriaxone clearance within filter and a 75% difference in clearance between filters with 80% power at the 0.05 level of significance. Linear regression analysis was performed to determine the relationship between dialysate, blood, or ultrafiltration rate and CVVHD and CVVH clearance of urea, creatinine, and ceftazidime, respectively. Regression lines were compared by using t tests for common slopes. Results were calculated as means ± standard deviations. Computations were performed with version 6.12 of Statistical Analysis Software (SAS Institute, Cary, N.C.), and P < 0.05 was considered to be statistically significant.
RESULTS
The patients in each of the three hemofilter groups were similar with regard to age, gender, race, weight, and pertinent laboratory measurements (Table 1). The residual renal function of the patients was not characterized, since the aim of the study was to ascertain the extracorporeal clearance of ceftazidime. None of the patients experienced any adverse events while participating in this study.
TABLE 1.
Clinical characteristics of the patients who participated in this studya
| Filter | Age (yr) | Wt (kg) | Race (no. African- American/no. Caucasian) | Gender (no. male/ no. female) |
|---|---|---|---|---|
| AN69 | 39.2 (9.4) | 77.4 (18.8) | 4/1 | 1/4 |
| PMMA | 41.8 (14.5) | 80.0 (23.4) | 4/1 | 2/3 |
| PS | 50.2 (7.9) | 69.4 (11.7) | 4/1 | 2/3 |
Age and weight data are means with standard deviations in parentheses.
CVVH clearance.
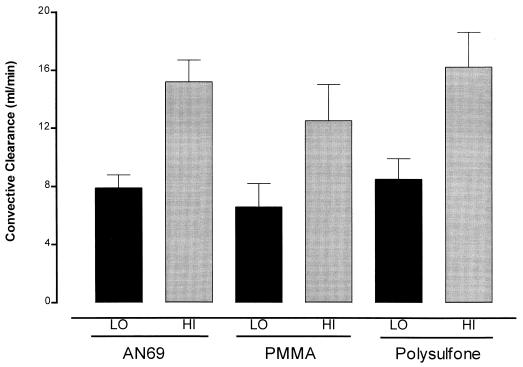
Ceftazidime was minimally protein bound in these ESRD subjects (free fraction ranged from 0.75 to 0.90). No significant differences in fraction unbound to plasma proteins (fup) were noted between the three groups of patients (AN69, fup = 0.80 ± 0.04; PS, fup = 0.85 ± 0.03; PMMA, fup = 0.83 ± 0.06). The SCs of ceftazidime for the PMMA (0.80 ± 0.19), AN69 (0.97 ± 0.11), and PS (0.97 ± 0.13) filters were not significantly different (P = 0.279) from each other or from the fup of their respective group. The convective clearances of urea and ceftazidime at ultrafiltration rates of 500 and 1,000 ml/h for the AN69, PS, and PMMA filters are depicted in Fig. 1. The convective clearance of ceftazidime by each filter was significantly increased at the higher ultrafiltration rate (P = 0.0001).
FIG. 1.
Convective clearance of ceftazidime at low (LO) and high (HI) ultrafiltration rates. The increment in ultrafiltrate flow rate resulted in a significant increase in ceftazidime clearance with all three filters (P = 0.0001). The data are means ± standard deviations.
CVVHD clearance versus dialysate inflow rate.
Urea and creatinine clearance increased linearly with dialysate inflow rates for all three filters (Table 2). The regression lines for urea clearance for the filters when plotted against dialysate inflow rate had similar slopes of 0.84 (r2 = 0.980, P = 0.0001), 0.82 (r2 = 0.933, P = 0.0001), and 0.83 (r2 = 0.937, P = 0.0001) for the AN69, PS, and PMMA filters, respectively (P > 0.05). The regression analysis for creatinine clearance yielded similar results.
TABLE 2.
Ceftazidime, creatinine, and urea clearance in relation to dialysate inflow for AN69, PS, and PMMA filtersa
| Type of clearanceb | Clearance at dialysate inflow rate (ml/min) ofc:
|
|||
|---|---|---|---|---|
| 8.3 | 16.7 | 25.0 | 33.3 | |
| Total ceftazidime | ||||
| AN69 filter | 8.4 (0.6) | 13.5 (1.1) | 18.3 (1.9) | 21.6 (2.2) |
| PS filter+ | 8.6 (1.3) | 16.6 (2.4) | 23.2 (2.9)# | 27.5 (3.7)## |
| PMMA filter+ | 7.3 (1.7) | 14.5 (4.5) | 20.1 (4.9) | 24.2 (5.2) |
| Unbound ceftazidime | ||||
| AN69 filter+ | 10.5 (0.5) | 16.9 (1.8) | 22.8 (1.2) | 26.9 (1.5) |
| PS filter+ | 10.1 (1.5) | 19.5 (2.9) | 27.3 (3.3) | 32.4 (4.5) |
| PMMA filter+ | 8.9 (2.4) | 17.5 (5.7) | 24.4 (7.0) | 29.5 (7.9) |
| Urea | ||||
| AN69 filter+ | 7.6 (0.6) | 13.1 (3.0) | 21.5 (2.6) | 28.1 (4.2) |
| PS filter++ | 6.2 (1.6) | 14.0 (4.0) | 20.7 (5.9) | 26.9 (8.8) |
| PMMA filter+++ | 6.7 (1.0) | 16.1 (5.4) | 19.8 (6.3) | 27.5 (6.6) |
| Creatinine | ||||
| AN69 filter+ | 6.7 (0.6) | 11.3 (2.5) | 18.0 (3.2) | 22.7 (4.0) |
| PS filter° | 5.6 (1.5) | 12.7 (3.5) | 19.2 (5.9) | 23.3 (7.8) |
| PMMA filter°° | 5.9 (1.0) | 14.5 (4.8) | 18.3 (6.6) | 24.4 (5.5) |
Data are means with standard deviations in parentheses.
Within-filter comparisons. +, clearance at dialysate inflow rate = 33.3 > 25.0 > 16.7 > 8.3 (P = 0.0001); ++, clearance at dialysate inflow rate = 33.3 > 16.7 > 8.3 and 25 > 8.3 (P = 0.0007); +++, clearance at dialysate inflow rate = 33.3 > 25 and 16.7 > 8.3 (P = 0.0001); °, clearance at dialysate inflow rate = 33.3 > 16.7 > 8.3 and 25 > 8.3 (P = 0.0027); °°, clearance at dialysate inflow rate = 33.3 and 25 and 16.7 > 8.3 and 33.3 > 16.7 (P = 0.002).
Between-filter comparisons. #, ceftazidime clearance by PS > AN69 (P = 0.027); ##, ceftazidime clearance by PS > AN69 (P = 0.0083).
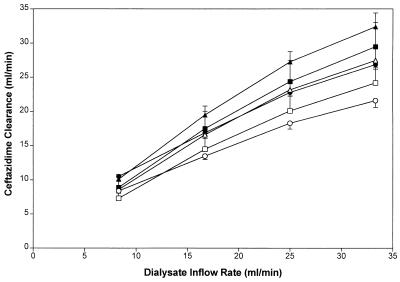
The clearance of total ceftazidime by the PS filter was similar to that by the PMMA filter, but exceeded the values observed with the AN69 filter at a dialysate inflow rate of 25.0 and 33.3 ml/min, (P = 0.027 and 0.0083, respectively) (Table 2). Ceftazidime total and unbound clearance was significantly correlated with the dialysate inflow rate for all three filters (Fig. 2). The slopes of the relationships between dialysate inflow rate and unbound and total ceftazidime with the PMMA, AN69, and PS filters were similar.
FIG. 2.
Ceftazidime clearance in relation to dialysate inflow rate for the AN69 (total, open circles; unbound, solid circles), PS (total, open triangles; unbound, solid triangles), and PMMA (total, open squares; unbound, solid squares) filters at a constant blood flow rate of 100 ml/min. Values are means ± standard errors.
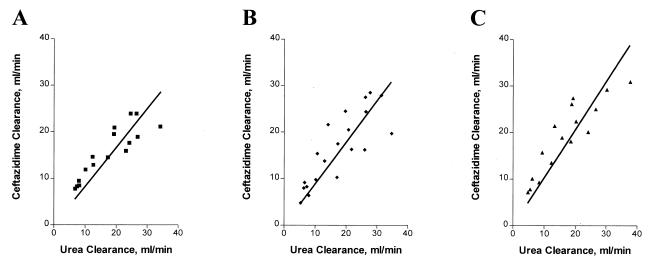
Regression analysis revealed significant linear relationships between total ceftazidime clearance and urea clearance for all three filters: AN69 (slope = 0.83, r2 = 0.956, P = 0.0001), PS (slope = 1.03, r2 = 0.951, P = 0.0001), and PMMA (slope = 0.89, r2 = 0.933, P = 0.0001) (Fig. 3). The slopes were not significantly different. The relationships between clearance of unbound ceftazidime and urea clearance were also similar. Although the slopes were higher for all three filters, they were not significantly different from the total ceftazidime clearance versus urea clearance relationships: AN69 (slope = 1.04, r2 = 0.968, P = 0.0001), PS (slope = 1.21, r2 = 0.943, P = 0.0001), and PMMA (slope = 1.08, r2 = 0.919, P = 0.0001).
FIG. 3.
Relationship between ceftazidime clearance and urea clearance for the AN69 filter (clearance = 0.832 [urea clearance], r2 = 0.956) (A), the filter (clearance = 1.03 [urea clearance], r2 = 0.951), (B) and the PMMA filter (clearance = 0.892 [urea clearance], r2 = 0.933) (C).
CVVHD clearance versus blood flow.
Ceftazidime, urea, and creatinine clearances were also measured at a constant dialysate inflow rate of 33.3 ml/min, while the blood flow rate was increased from 75 to 250 ml/min. The clearances of urea and creatinine were essentially constant at all blood flow rates for all three filters. The clearance of ceftazidime with the AN69 filter, however, did increase significantly from 18.7 ml/min at a blood flow rate of 75 ml/min to 23.9 ml/min at a blood flow rate of 250 ml/min (P = 0.031).
Comparison of membranes.
The CVVH clearances of urea and creatinine were similar for all three filter membranes, and the SCs of ceftazidime were not significantly different. No significant differences in the clearances of urea and creatinine were noted between filters at any level of nominal dialysate inflow rate. The CVVHD clearance of total ceftazidime with the PS filter was significantly higher at dialysate inflow rates of 25 and 33.3 ml/min than values with the AN69 filter (Table 2).
DISCUSSION
The disposition of ceftazidime (6, 12, 27) during CVVH has been reported in single case reports or as a series of clinical cases. Unfortunately, it is difficult, if not impossible, to control the critical variables that may affect the clearance of ceftazidime in acutely ill patients. In this study, we prospectively measured the SC and protein binding of ceftazidime in stable ESRD patients undergoing controlled CVVH with three different hemofilters. The clearances of ceftazidime and two reference solutes (urea and creatinine) determined at two ultrafiltration flow rates confirmed the dependence of CVVH clearance on QUF; the clearance of each solute increased significantly at the higher ultrafiltration rate (P = 0.0001). No clinically significant difference in urea, creatinine, or ceftazidime clearance was attributed to the type of membrane utilized for CVVH. The mean fup of ceftazidime in these patients was similar to those in previous reports in normal volunteers (2) and infected patients (9, 15). The mean measured fup of ceftazidime in these patients (n = 15) of 0.83 ± 0.05 was very consistent and did not significantly differ from the observed mean SC of 0.91 ± 0.17. These values are quite comparable to the earlier report of an SC of 0.86 with an AN69 filter (27). This confirms the dependence of ceftazidime CVVH clearance on fup.
CVVHD allows independent regulation of blood, dialysate, and ultrafiltrate flow rates, and clearance of solute during CVVHD is comprised of both a diffusive component and a convective (or ultrafiltration) component (23). Since net QUF ranged from 3.0 to 9.2% of nominal QDI during the CVVHD segment of this study, the observed ceftazidime clearances predominantly reflect the effects of alterations in blood and dialysate flow on diffusion across the membrane. Unfortunately, the previously published studies of ceftazidime clearance during CVVHD did not control ultrafiltration rate to this degree, and thus their results cannot be directly compared to our observations (6, 27). Furthermore, ceftazidime clearance by CVVHD has been previously evaluated with only the AN69 filter. Thus, this study is the first rigorous investigation of the determinants of ceftazidime clearance by CVVHD.
Increasing nominal dialysate inflow rate from 8.3 to 33.3 ml/min produced a linear increase in the clearance of urea and creatinine with each of the three hemofilters. The slopes of the urea clearance to QDI relationship with the AN69 filter during the two segments of the study of ceftazidime (0.84) were similar to the values previously reported by Joy et al. (14) (0.77) and Relton et al. (19) (0.88). Similar congruity in the urea relationships was evident for the PS membrane; Ifediora et al. (10) reported a slope of 0.85 for the Renal Systems HF-500 filter and 0.91 for the Fresenius F-8 filter, while Joy et al. (14) observed a value of 0.80 with a Fresenius F-40 filter. These data suggest that the choice among these three filter membranes is not a critical determinant of CVVHD performance for control of azotemia (23).
The clearance of total and unbound ceftazidime increased significantly as the nominal QDI was increased (Fig. 2). Total ceftazidime clearance by the PS filter exceeded clearance by the AN69 and PMMA filters at all dialysate inflow rates. However, the slopes of the relationship between total ceftazidime clearance and urea clearance did not significantly differ between the three filters (Fig. 3). If diffusive clearance of ceftazidime by CVVHD were flow limited, then one would anticipate that increasing blood flow rate would result in an increase in ceftazidime clearance. Within-filter comparisons revealed that, when the dialysate inflow rate was constant, there was no significant difference in urea or creatinine clearance as blood flow rate was increased from 75 to 250 ml/min (P = 0.066). However, at a blood flow rate of 250 ml/min, ceftazidime clearance with the AN69 filter was significantly greater than the value observed at the lowest blood flow rate (P = 0.031).
On the basis of these data, one can project that CVVH and CVVHD therapy can significantly augment the clearance of ceftazidime. The total body clearance of ceftazidime in patients with acute renal failure is comprised of residual renal clearance and a nonrenal component. In patients with normal renal function, the nonrenal clearance of ceftazidime is approximately 10.6 ml/min (18). The total body clearance of ceftazidime for a patient with a residual creatinine clearance of 10 ml/min would thus be 22.1 ml/min. The initiation of CVVH with an ultrafiltration rate of 1.0 liter/h would thus result in an increase of 60 to 73% in the total body clearance of ceftazidime. The contribution of CVVHD is even more dramatic and dependent on the dialysate inflow rate employed and the patient's residual renal function. One could anticipate a maximal increase in the ceftazidime total body clearance of 121.7 to 146.6% for a patient with a residual creatinine clearance of 10 ml/min. In those patients with a higher degree of residual renal function, the dosage regimen will need to be progressively increased.
Maintenance dosage recommendations for patients receiving CVVH with any of the three filters evaluated in this study after the initiation of ceftazidime therapy with a loading dose of 1,000 mg are listed in Table 3. These regimens should result in the maintenance of serum concentrations above the MIC90 of susceptible organisms for over 80% of the dosing interval. Since some clinicians have proposed that the concentrations of cephalosporins in serum only need to exceed the MIC90 for 40% of the dosage interval, once-a-day administration of the doses in Tables 3 and 4 may be feasible (5). Furthermore, if combination antibiotic therapy is initiated or subsequently added, the desired clinical outcomes may be achieved with an even lower percentage of time above MIC for the cephalosporin (22).
TABLE 3.
CVVH dosage guidelines for ceftazidime
| Residual renal function (creatinine clearance in ml/min) | Maintenance dose (mg) for a ultrafiltration rate (ml/min) ofa:
|
|||
|---|---|---|---|---|
| 5 | 16.7 | 33.3 | 50 | |
| 0 | 250 | 250 | 500 | 500 |
| 5 | 250 | 250 | 500 | 500 |
| 10 | 250 | 500 | 500 | 750 |
| 15 | 250 | 500 | 500 | 750 |
| 20 | 500 | 500 | 500 | 750 |
Maintenance dose to be administered every 12 h.
TABLE 4.
Ceftazidime dosage guidelines during CVVHD
| Residual renal function (creatinine clearance in ml/min) | Maintenance dose (mg) for a dialysate inflow rate ofa:
|
|||||
|---|---|---|---|---|---|---|
| 1.0 liter/h
|
2.0 liters/h
|
|||||
| Ultrafiltration rate (liter/h)
|
Ultrafiltration rate (liters/h)
|
|||||
| 0.5 | 1.0 | 2.0 | 0.5 | 1.0 | 2.0 | |
| 0 | 500 | 500 | 500 | 500 | 500 | 750 |
| 5 | 500 | 500 | 750 | 500 | 500 | 750 |
| 10 | 500 | 500 | 750 | 500 | 750 | 1,000 |
| 15 | 500 | 750 | 750 | 750 | 750 | 1,000 |
| 20 | 750 | 750 | 1,000 | 750 | 750 | 1,000 |
Maintenance dose to be administered every 12 h.
Projected ceftazidime dosage requirements for patients receiving CVVHD are listed in Table 4. Although combinations of convective and diffusive transport may be beneficial in many clinical settings, the impact of increasing convection on diffusive clearance was not explicitly evaluated. At the flow rates clinically utilized, the clearances by the two processes are likely to be additive (8). Thus, for CVVHD prescriptions, the urea clearance during CVVHD could be measured and the dosage adjustment of ceftazidime could be individualized on the basis of the estimated ceftazidime clearance from the relationships in Fig. 2 and 3, plus the patient's residual renal and nonrenal clearance as described previously.
In summary, these data indicate that the removal of ceftazidime by CVVH is dependent on the fup of the patient and the delivered ultrafiltration rate. No filter membrane effect was observed to be statistically or clinically significant for ceftazidime. Ceftazidime dosage regimens can be initiated on the basis of the proposed dosing recommendations, and the contribution of CVVH or CVVHD clearance to the patient's residual drug clearance can be subsequently utilized to individualize the antibiotic regimen on the basis of measured urea clearances.
ACKNOWLEDGMENTS
This work was supported in part by grant 5M01 RR00056 from the National Institutes of Health National Center for Research Resources/General Clinical Research Center, Bethesda, Md.
At the time of this investigation, M.S.J. was a Postdoctoral Fellow of the School of Pharmacy, University of Pittsburgh.
REFERENCES
- 1.Amin N B, Padhi L D, Touchette M A, Patel R V, Dunfee T P, Frinak S, Besarab A, Anandan J V. Gentamicin removal by the F-80 membrane in patients with endstage renal disease. Am J Kidney Dis. 1999;34:222–227. doi: 10.1016/s0272-6386(99)70347-1. [DOI] [PubMed] [Google Scholar]
- 2.Benet L Z, Williams R L. Design and optimization of dosage regimen: pharmacokinetic data. In: Gilman A G, Rolt T W, Nies A S, Taylor P, editors. Goodman and Gilman's pharmacologic basis of therapeutics. 8th ed. Elmsford, N.Y: Pergamon Press; 1990. pp. 1650–1735. [Google Scholar]
- 3.Bresolle F, Kinowski J M, de la Coussaye J E, Wynn N, Eledjam J J, Galtier M. Clinical pharmacokinetics during continuous haemofiltration. Clin Pharmacokinet. 1994;26:457–471. doi: 10.2165/00003088-199426060-00004. [DOI] [PubMed] [Google Scholar]
- 4.Conger J D. The role of continuous renal replacement therapy in the future treatment of acute renal failure. Am J Kidney Dis. 1996;28(Suppl.3):S108–S113. [Google Scholar]
- 5.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 6.Davies S P, Lacey L F, Kox W J, Brown E A. Pharmacokinetics of cefuroxime and ceftazidime in patients with acute renal failure treated by continuous arteriovenous haemodialysis. Nephrol Dial Transplant. 1991;6:971–976. doi: 10.1093/ndt/6.12.971. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs P C, Barry A L, Brown S D AST Surveillance Group. Survey of antimicrobial activity of four commonly used third generation cephalosporins tested against recent bacterial isolates from ten American medical centers and assessment of disk diffusion test performance. Diagn Microbiol Infect Dis. 1996;24:213–219. doi: 10.1016/0732-8893(96)00028-4. [DOI] [PubMed] [Google Scholar]
- 8.Golper T A, Cigarran-Guldris S, Jenkins R D, Brier M E. The role of convection during simulated continuous arteriovenous hemodialysis. Contrib Nephrol. 1991;93:146–148. doi: 10.1159/000420206. [DOI] [PubMed] [Google Scholar]
- 9.Golper T A, Marx M A. Drug dosing adjustments during continuous renal replacement therapies. Kidney Int. 1998;53:S-165–S-168. [PubMed] [Google Scholar]
- 10.Ifediora O C, Teehan B P, Sigler M H. Solute clearance in continuous venovenous hemodialysis: a comparison of cuprophane, polyacrylonitrile, and polysulfone membranes. ASAIO J. 1992;38:M697–M701. [PubMed] [Google Scholar]
- 11.Jones R N, Pfaller M A, Doern G V, Erwin M E, Hollis R J the Cefepime Study Group. Antimicrobial activity and spectrum investigation of eight broad-spectrum β-lactam drugs: a 1997 surveillance trial in 102 medical centers in the United States. Diagn Microbiol Infect Dis. 1998;30:215–228. doi: 10.1016/s0732-8893(97)00234-4. [DOI] [PubMed] [Google Scholar]
- 12.Joos B, Schmidli M, Keusch G. Pharmacokinetics of antimicrobial agents in anuric patients during continuous venovenous haemofiltration. Nephrol Dial Transplant. 1996;11:1582–1585. [PubMed] [Google Scholar]
- 13.Joy M S, Matzke G R, Armstrong D A, Marx M, Zarowitz B J. A primer on continuous renal replacement therapy for critically ill patients. Ann Pharmacother. 1998;32:362–375. doi: 10.1345/aph.17105. [DOI] [PubMed] [Google Scholar]
- 14.Joy M S, Matzke G R, Frye R F, Palevsky P M. Determinants of vancomycin clearance by CVVH and CVVHD. Am J Kidney Dis. 1998;31:1019–1027. doi: 10.1053/ajkd.1998.v31.pm9631848. [DOI] [PubMed] [Google Scholar]
- 15.Kroh U F. Drug administration in critically ill patients with acute renal failure. New Horizons. 1995;3:748–759. [PubMed] [Google Scholar]
- 16.Kroh U F, Hall T J, Steinhauber W. Management of drug dosing in continuous renal replacement therapy. Semin Dial. 1996;9:161–165. [Google Scholar]
- 17.Kronfol N O, Lau A H, Colon-Rivera J, Libertin C L. Effect of CAVH membrane types on drug-sieving coefficients and clearances. ASAIO Trans. 1986;32:85–87. [PubMed] [Google Scholar]
- 18.Leroy A F, Leguy F, Borsa F, Spencer G R, Fillastre J P, Humbert G. Pharmacokinetics of ceftazidime in normal and uremic subjects. Antimicrob Agents Chemother. 1984;25:638–642. doi: 10.1128/aac.25.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Relton S, Greenberg A, Palevsky P M. Dialysate and blood flow dependence of diffusive solute clearance during CVVHD. ASAIO J. 1992;38:M691–M696. doi: 10.1097/00002480-199207000-00127. [DOI] [PubMed] [Google Scholar]
- 20.Ronco C. Continuous renal replacement therapies: evolution towards a new era. Semin Dial. 1996;9:215–221. [Google Scholar]
- 21.Rowland M, Tozer T N. Clinical pharmacokinetics: concepts and applications. 3rd ed. Philadelphia, Pa: Lea & Febiger; 1995. [Google Scholar]
- 22.Schentag J J, Strenkoski-Nix L C, Nix D E, Forrest A. Pharmacodynamic interactions of antibiotics alone and in combination. Clin Infect Dis. 1998;27:40–46. doi: 10.1086/514621. [DOI] [PubMed] [Google Scholar]
- 23.Sigler M H. Transport characteristics of the slow therapies: implications for achieving adequacy of dialysis in acute renal failure. Adv Renal Replacement Ther. 1997;4:68–80. doi: 10.1016/s1073-4449(97)70018-9. [DOI] [PubMed] [Google Scholar]
- 24.Sigler M H, Manns M. Membranes and devices in continuous renal replacement therapy. Semin Dial. 1996;9:98–106. [Google Scholar]
- 25.Toffelmire E B, Reymond J P, Brouard R, Hansonzadeh M K, Schoenfeld P, Gambertoglio J, Tozer T N. Dialysis clearance in high flux hemodialysis with reuse using ceftazidime as the model drug. Clin Pharmacol Ther. 1988;45:160. . (Abstract). [Google Scholar]
- 26.Turnidge J D. The pharmacodynamics of β-lactams. Clin Infect Dis. 1998;27:10–22. doi: 10.1086/514622. [DOI] [PubMed] [Google Scholar]
- 27.Vos M C, Vincent H H, Yzerman E P F, Vogel M, Mouton J W. Drug clearance by continuous haemodiafiltration: results with the AN69 capillary hemofilter and recommended dose adjustments for seven antibiotics. Drug Investig. 1994;7:315–322. [Google Scholar]