Abstract
Background and Objectives
Limbic-predominant age-related Tar DNA binding protein 43 (TDP-43) encephalopathy neuropathologic change (LATE-NC) is present in ≈25% of older persons' brains and is strongly associated with cognitive impairment. Hippocampal sclerosis (HS) pathology is often comorbid with LATE-NC, but the clinical and pathologic correlates of HS in LATE-NC are not well understood.
Methods
This retrospective autopsy cohort study used data derived from the National Alzheimer's Coordinating Center Neuropathology Data Set, which included neurologic status, medical histories, and neuropathologic results. All autopsies were performed in 2014 or later. Among participants with LATE-NC, those who also had HS pathology were compared with those without HS with regard to candidate risk factors or common underlying diseases. Statistical significance was set at nominal p < 0.05 in this exploratory study.
Results
A total of 408 participants were included (n = 221 were LATE-NC+/HS−, n = 145 were LATE-NC+/HS+, and n = 42 were LATE-NC−/HS+). Most of the included LATE-NC+ participants were severely impaired cognitively (83.3% with dementia). Compared to HS− participants, LATE-NC+ participants with HS trended toward having worse cognitive status and scored lower on the Personal Care and Orientation domains (both p = 0.03). Among LATE-NC+ participants with Braak neurofibrillary tangle (NFT) stages 0 to IV (n = 88), HS+ participants were more impaired in the Memory and Orientation domains (both p = 0.02). There were no differences (HS+ compared with HS−) in the proportion with clinical histories of seizures, stroke, cardiac bypass procedures, diabetes, or hypertension. The HS+ group lacking TDP-43 proteinopathy (n = 42) was relatively likely to have had strokes (p = 0.03). When LATE-NC+ participants with or without HS were compared, there were no differences in Alzheimer disease neuropathologies (Thal β-amyloid phases or Braak NFT stages) or Lewy body pathologies. However, the HS+ group was less likely to have amygdala-restricted TDP-43 proteinopathy (LATE-NC stage 1) and more likely to have neocortical TDP-43 proteinopathy (LATE-NC stage 3) (p < 0.001). LATE-NC+ brains with HS also tended to have more severe circle of Willis atherosclerosis and arteriolosclerosis pathologies.
Discussion
In this cohort skewed toward participants with severe dementia, LATE-NC+ HS pathology was not associated with seizures or with Alzheimer-type pathologies. Rather, the presence of comorbid HS pathology was associated with more widespread TDP-43 proteinopathy and with more severe non–β-amyloid vessel wall pathologies.
Tar DNA binding protein 43 (TDP-43) proteinopathy has been detected in autopsy studies of >15 different neurologic diseases.1 TDP-43 is a nucleic acid–binding protein that is predominantly nonphosphorylated in healthy cells where it is located mostly in cell nuclei. In TDP-43 proteinopathy, the protein becomes phosphorylated and mislocalized to cytoplasm and neurites, as recognized by immunohistochemistry.2
Recommendations were published recently for terminology and classification referent to the most common known subtype of TDP-43 proteinopathy: limbic-predominant age-related TDP-43 encephalopathy (LATE) and its underlying neuropathologic changes (LATE-NC).3 The brains of approximately one-half of persons with a clinical diagnosis of dementia harbor LATE-NC, alone or in combination with the hallmark lesions of Alzheimer disease neuropathologic changes (ADNC).3,4 In LATE-NC, TDP-43 proteinopathy preferentially targets medial temporal lobe structures, including the hippocampus.3 Many (but not all) brains with LATE-NC also are diagnosed with hippocampal sclerosis (HS)5 pathology.
The study of LATE-NC and HS is a fast-moving field that has generated some controversy,6,7 partly due to diagnostic ambiguities. HS pathology is diagnosed routinely with hematoxylin & eosin stains at autopsy, and HS has been defined as “severe pyramidal cell loss and gliosis in CA1 and subiculum of the hippocampal formation that is out of proportion to ADNC in the same structures”.8 This definition lacks rigorous practical criteria or sampling specifications, leaving room for substantial differences in diagnostic approaches among individual neuropathologists, and there is a spectrum of relevant histopathologic alterations. In hippocampi affected by severe HS, the normal neuronal components are largely replaced by reactive astrocytes, the neuropil becomes rarefied, and hippocampal atrophy can be extreme9; however, in some individuals with LATE-NC, the neuronal cell dropout is patchier (seen in some portions of the hippocampus but not others).10 Furthermore, the HS pathology was shown to be unilateral in ≈40% to 50% of individuals.10-12
The term HS also has divergent connotations in the clinical literature, having originated more than a century ago to describe brain changes associated with epilepsy.13 This terminology is still used in the seizure disorder clinical practice and scientific literature. HS pathology also is associated with other disease processes, including hypoxia, hypoglycemia, particular infections that affect the hippocampus, and various neurodegenerative conditions.3 To be clear, brains with HS but lacking TDP-43 pathology do not represent LATE-NC. For example, HS associated with hypoxia or epilepsy is usually negative for TDP-43 proteinopathy12,14,15 and does not fulfill criteria for LATE-NC.3
Although other underlying diseases may induce neuropathologic features meriting the diagnostic label of HS, HS pathology in a cognitively impaired elderly individual is a strong indication that LATE-NC is probably also present.15 HS was historically the first pathologic feature that distinguished LATE-NC from other dementia-inducing conditions such as Alzheimer disease5 and has also been associated with cognitive impairment in older individuals independently of other brain pathologies.4,16-19 In the clinical setting, the presence of MRI features linked to HS (more severe hippocampal atrophy than pure ADNC) has also been associated with cognitive impairment.11,20-22
It is not understood why some persons with LATE-NC develop HS whereas others with LATE-NC do not develop HS. There are also unanswered questions about medical and pathologic comorbid conditions that are associated with HS in the context of LATE-NC. The present exploratory study was designed with the goal of elucidating the factors that are (or are not) associated with the pathologic diagnosis of HS among persons with autopsy-proven LATE-NC.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Clinical and pathologic data were obtained from the National Alzheimer's Coordinating Center (NACC), which is the data repository for past and present Alzheimer’s Disease Research Centers (ADRCs) funded by the National Institute on Aging.23,24 ADRCs obtained written informed consent from each included participant (or guardians of participants) in the study, and each institution maintained its own separate Institutional Review Board review process. However, all the analyses for the present study were performed on anonymized/deidentified participants' data, such that these analyses do not technically represent Human Participants Research according to the NIH (exemption 425).
Representative Photomicrographs
Representative photomicrographs of autopsied participants' brains were taken to convey the histopathologic features of interest. These were from research volunteers in the University of Kentucky ADRC autopsy cohort using methods previously described.26
NACC Data: Inclusion and Exclusion
Participants were assessed with the standardized Uniform Data Set (UDS) approximately annually at their local ADRC. The UDS collects a robust set of data, including participant demographics, health history, physical and neurologic examinations, Alzheimer disease and related dementias symptomology, the Clinical Dementia Rating (CDR) scale Dementia Staging Instrument plus NACC frontotemporal lobar degeneration (FTLD) Behavior and Language Domains, and a neuropsychological test battery. Participants who met the eligibility criteria of the study were selected from the March 2021 data freeze, and we included cross-sectional data from the participant's most recent UDS visit before death.
Standardized data collection on neuropathologic features present at the time of death was available for participants who consented to autopsy. The goals of the current study—to compare between HS and non-HS subgroups among persons with autopsy-proven LATE-NC—guided the inclusion and exclusion criteria. The NACC Neuropathology form is used by the ADRCs and provides guidance that is based on established criteria for evaluation of presence of β-amyloid (Aβ), tau, TDP-43, α-synuclein, and cerebrovascular pathologies, as well as unusual conditions such as Huntington disease. Included participants who had Neuropathology data were ≥75 years of age at death. Participants with rare pathologies were excluded (such as Down syndrome, multiple system atrophy, amyotrophic lateral sclerosis, and trinucleotide repeat disease), as were individuals with malformation of cortical development, metabolic/storage disorder of any type, unusual white matter disease (e.g., leukodystrophy, multiple sclerosis), traumatic brain injury (acute or chronic), brain neoplasm (primary or metastatic), brain infection (encephalitis, abscess, etc), prion disease, or motoneuron disease. Also excluded were participants with missing data on HS, the presence/absence of TDP-43 inclusions in the hippocampus, or the presence/absence of FTLD-TDP.
Version 10 of the NACC Neuropathology form,23 implemented in January 2014, introduced the routine assessment of TDP-43 immunoreactive inclusions in the spinal cord, amygdala, hippocampus, entorhinal/inferior temporal cortex, and neocortex (the last generally referring to middle frontal gyrus). All included participants had Neuropathology Version 10 data available. The evaluation of TDP-43 proteinopathy follows center-specific protocols at ADRCs as described previously.27 In the current study, LATE-NC was defined as the presence of TDP-43 inclusions in the amygdala, hippocampus, or neocortex and the absence of an overall clinical-pathological diagnosis of FTLD-TDP. LATE-NC negativity was defined as having an absence of TDP-43 inclusions in the amygdala, hippocampus, and neocortex.
Statistical Analyses
To compare demographic characteristics, clinical measures and symptoms, and neuropathologic features between participants with LATE-NC and comorbid HS (LATE-NC+/HS+), participants with LATE-NC and no HS (LATE-NC+/HS−), and participants who did not have LATE-NC and had comorbid HS (LATE-NC−/HS+), we used the Pearson χ2 or Fisher exact tests for the categorical variables and 2-sample t tests for the continuous variables. The Cochran-Armitage trend test was used to examine the significance of severity trends in LATE-NC stage, Thal Aβ phase (categorized as A0 = 0, A1 = 1/2, A2 = 3, and A3 = 4/5) and Braak neurofibrillary tangle (NFT) stage (categorized as B0 = 0, B1 = I/II, B2 = III/IV, and B3 = V/VI), neuritic amyloid plaque density, cerebral amyloid angiopathy (CAA), and atherosclerosis of the circle of Willis. In this exploratory study, statistical significance was set at nominal p < 0.05, and no corrections were made for multiple comparisons.
Demographic characteristics were compared among the LATE-NC+/HS+, LATE-NC+/HS−, and LATE-NC−/HS+ groups and included age at death, presence of the APOE ε4 allele, years of education, time between the last UDS visit and death, and sex. Among these 3 groups, the clinical measures explored were medical comorbid conditions commonly associated with HS, including body mass index (BMI) at most recent UDS visit, smoking status, and history of cardiac arrest, atrial fibrillation, cardiac bypass procedure, pacemaker or defibrillator, congestive heart failure, stroke, diabetes, hypertension, hypercholesterolemia, thyroid disease, seizures, and depression, including Geriatric Depression Scale score at the most recent UDS visit. The group with LATE-NC−/HS+ pathology was included to test whether the HS+ phenotype has differing implications without comorbid LATE-NC. Cognitive symptoms were compared among LATE-NC+/HS+ and LATE-NC+/HS− participants with a CDR global score of 0 or 0.5 at their most recent UDS visit before death and included impaired memory, executive function, language, visuospatial function, and attention, as well as the duration of these cognitive symptoms. Additional comparisons between these 2 groups were explored with cognitive status at the most recent UDS visit before death, including CDR domain scores.
Neuropathologic features investigated include hippocampal atrophy (noted grossly), LATE-NC stage,3 ADNC score,8 Thal Aβ phase,28 Braak NFT stage,29 Consortium to Establish a Registry for Alzheimer's Disease neuritic plaque density,30 Lewy bodies,31 infarcts or lacunes, microinfarcts, CAA, and atherosclerosis of the circle of Willis.23 These features were explored among the LATE-NC+/HS+ and LATE-NC+/HS− groups. We additionally explored the association between brain arteriolosclerosis and HS with multivariable logistic regressions that were adjusted for age at death, sex, years of education, hypertension, diabetes, and APOE ɛ4 carrier status and accounted for center clustering.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
The focus of the current study was autopsy-confirmed LATE-NC with and without HS pathology. Specific examples of the pathologic hallmarks of those conditions are depicted in Figure 1. Some brains with LATE-NC lack comorbid HS (Figure 1, A and B). However, among individuals with LATE-NC, comorbid HS pathology is seen far more often than in non-LATE-NC brains3,15 (Figure 1, C and D). LATE-NC stage >0 (i.e., in the amygdala, hippocampus, and/or neocortex) was the criterion used for the designation of LATE-NC+ in the current study.
Figure 1. Representative Photomicrographs of Human Hippocampi Depicting the Main Neuropathologic Features Analyzed in the Current Study: LATE-NC + Pathology Without (A and B) or With (C and D) Comorbid HS Pathology.
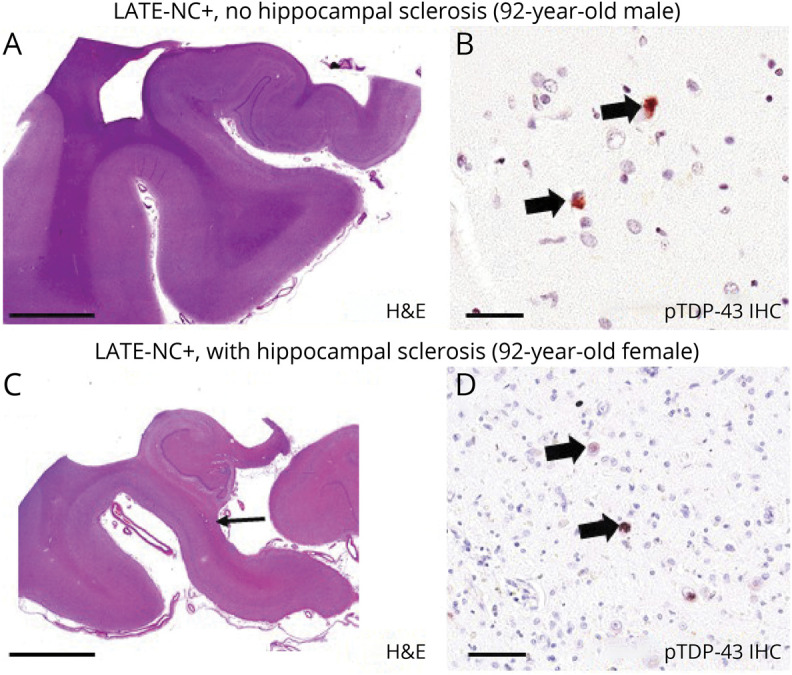
Each photomicrograph depicts anterior hippocampi dissected in the coronal plane. (A and B) Hematoxylin & eosin (H&E) and phospho (p)–TAR DNA binding protein 43 (TDP-43) immunohistochemistry (IHC), respectively, from a man who died at 92 years of age. Autopsy revealed limbic-predominant age-related TDP-43 encephalopathy neuropathologic changes (LATE-NC) stage 2 but no hippocampal sclerosis (HS) pathology. (C and D) Results from a woman who also died at 92 years of age. In her case, the autopsy revealed comorbid LATE-NC stage 2 and HS pathology. Note the relatively fulsome hippocampal profile in panel A compared to panel C (same scale bar); the HS+ profile in panel C shows thinning in CA1 and subiculum (arrow). Higher-magnification assessment confirmed that there was substantial neuronal cell dropout and robust astrocytosis (not shown). pTDP-43–positive intraneuronal inclusions are highlighted with arrows in panels B and D. These representative photomicrographs were from research participants of the University of Kentucky Alzheimer’s Disease Research Center. Scale bar = 2 mm in panels A and C, 70 μm in panel B, and 100 μm in panel D.
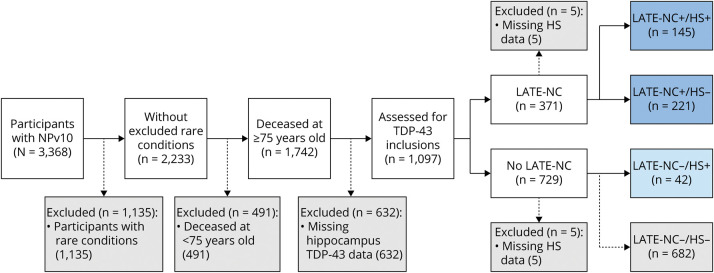
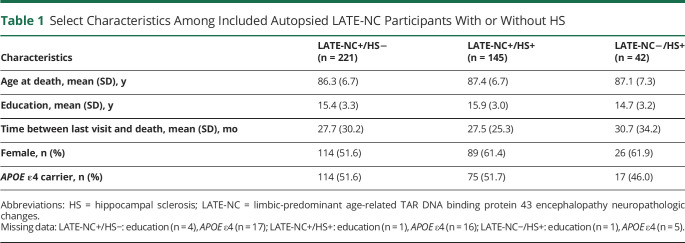
The present study involved analyses of participants in the NACC Neuropathology data set with version 10 data23 available; a total of 3,368 research volunteers are in that dataset. The participants included and excluded and the reasons for exclusion are shown in Figure 2, resulting in the following numbers of included participants: 221 with LATE-NC+/HS−, 145 with LATE-NC+/HS+, and 42 with LATE-NC−/HS+. The groups had similar mean years of education and time between the last UDS visit and death and had similar proportions of women and APOE ɛ4 carriers (Table 1).
Figure 2. Included and Excluded Research Participants in the NACC NPv10 Dataset, Along With Criteria and Missingness for the Current Study.
HS = hippocampal sclerosis; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy neuropathologic changes; NACC = National Alzheimer's Coordinating Center; NPv10 = Neuropathology version 10; TDP-43 = TAR DNA binding protein 43.
Table 1.
Select Characteristics Among Included Autopsied LATE-NC Participants With or Without HS
The number of participants included in the current study, stratified by individual ADRCs where they were autopsied, is shown in eTable 1, links.lww.com/WNL/B799. Each ADRC was given an anonymous parameter name for this data table. These data include readouts of LATE-NC and HS status, including whether the HS pathology was reported to be unilateral or bilateral or if only 1 side (left or right hemisphere) of the hippocampi was assessed. The LATE-NC+/HS− and LATE-NC+/HS+ participants (n = 366) were derived from 26 different ADRCs, with a median of 10 participants contributed per ADRC (range 1–64 participants per ADRC).
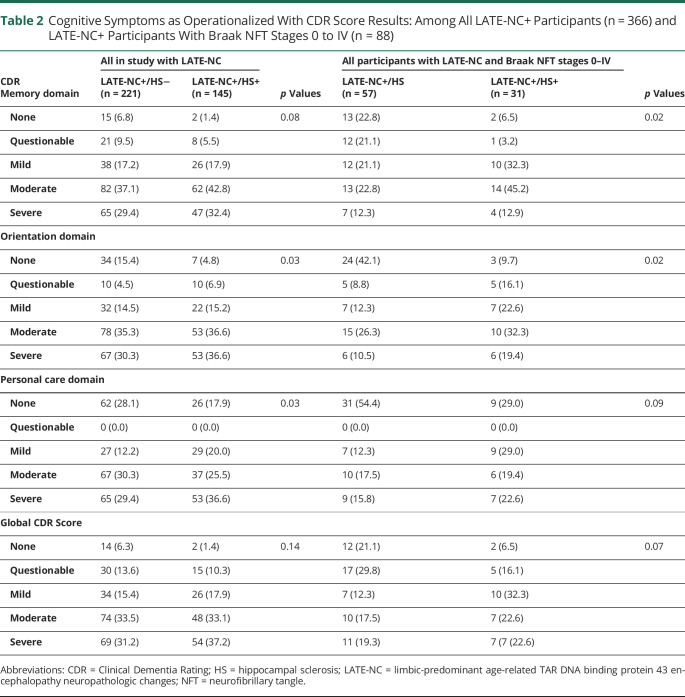
The cognitive symptoms of LATE-NC+/HS− and LATE-NC/HS+ participants were compared and showed a consistent trend toward LATE-NC/HS+ participants being relatively impaired (Table 2). This difference could be discerned despite a skew in this cohort toward a high degree of impairment (83.3% of LATE-NC+ cases had dementia). HS+ participants were more likely to score worse on the Orientation and Personal Care domains of the CDR (both p = 0.03). Among LATE-NC+ participants without severe ADNC (Braak NFT stages 0–IV, n = 88), there were 31 with HS and 57 without HS at autopsy. In this subset of participants, the HS+ group scored lower in the Memory and Orientation cognitive domains (both p = 0.02). Although the trend for the HS+ participants to have more cognitive impairment was consistent and encompassed multiple cognitive domains, the nominal statistical significance at 0.05 for any given test would not have survived a correction for multiple comparisons.
Table 2.
Cognitive Symptoms as Operationalized With CDR Score Results: Among All LATE-NC+ Participants (n = 366) and LATE-NC+ Participants With Braak NFT Stages 0 to IV (n = 88)
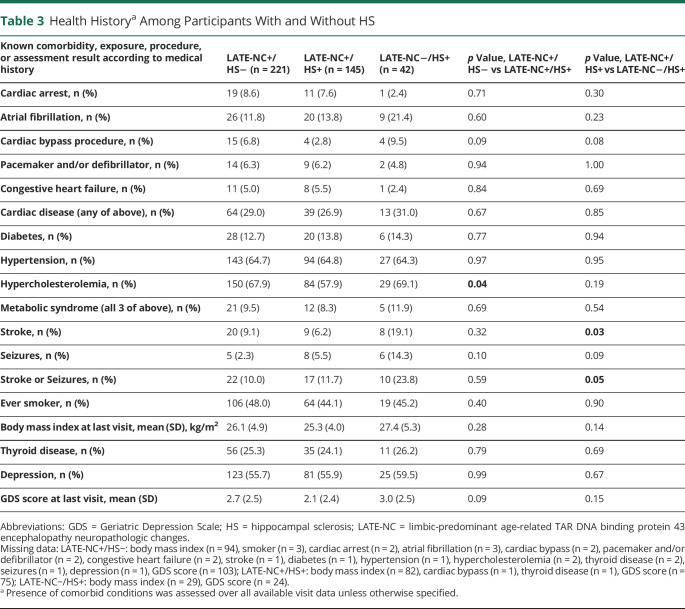
When comparing clinical and medical comorbidity measures among the LATE-NC+/HS− and LATE-NC+/HS+ groups, we found that LATE-NC+ participants with comorbid HS were less likely than LATE-NC+ participants lacking comorbid HS to have a history of a cardiac bypass procedure (2.8% vs 6.8%, p = 0.09) and hypercholesterolemia (57.9% vs 67.9%, p = 0.04) (Table 3). No differences were observed between these 2 groups in stroke or seizures, smoking status, BMI, thyroid disease, or depression. Participants without LATE-NC but with autopsy-confirmed HS were more likely to have a history of stroke (19.1% vs 9.1%, p = 0.03), and there was a trend for the LATE-NC-HS+ cases to have had cardiac bypass procedure (9.5% vs 2.8%, p = 0.08). Again, no group-level differences were observed between HS+ and HS− participants in measures indicating metabolic syndrome (diabetes, hypertension, or hypercholesterolemia), smoking status, BMI, thyroid disease, or depression.
Table 3.
Health Historya Among Participants With and Without HS
In a comparison of LATE-NC participants with and those without HS in terms of neuropathologic features (Table 4), there were no differences in ADNC (Aβ plaque distribution operationalized by Thal Aβ phases, neuritic amyloid plaque severity according to Consortium to Establish a Registry for Alzheimer's Disease, or Braak NFT stages) or CAA. Because the Fisher exact test returned values of p < 0.2 for Thal Aβ phases and Braak NFT stages, we followed up with trend tests (for which we binned ADNC categories), which returned p = 0.64 for Thal Aβ phases and p = 0.81 for Braak NFT stages, again indicating no correlation between HS pathology and AD-type amyloid plaques or NFTs in persons with comorbid LATE-NC.
Table 4.
Neuropathologic Features at Autopsy Among LATE-NC+ Participants With and Without HS
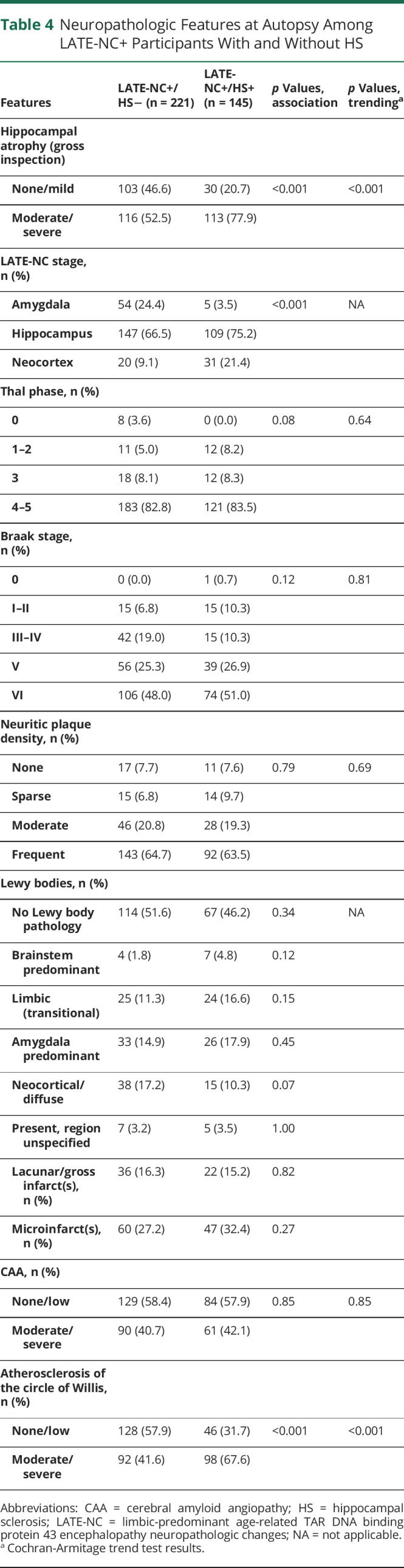
In contrast to the lack of group-level differences in ADNC severity, LATE-NC+ participants with HS were more likely to have a higher LATE-NC stage (less likely to have amygdala-only TDP-43 proteinopathy and more likely to have TDP-43 inclusions in the neocortex, p < 0.001).
In a sensitivity analysis in which LATE-NC stage 1 participants were excluded (n = 307 participants with hippocampal TDP-43 inclusions, i.e., LATE-NC stage >1, were included), most of the associations that included LATE-NC stage 1 cases held (eTable 2, links.lww.com/WNL/B799). However, in this smaller sample, those with HS were also more likely than those without HS to have limbic or amygdala predominant Lewy bodies (35.3% vs 22.8%, p = 0.02). We underscore that, among all LATE-NC+ participants (including those with LATE-NC stage 1), no difference was detected in Lewy body pathologies in the HS+ and HS− groups (Table 4).
In terms of cerebrovascular pathologies other than CAA (see above), there was a robust tendency for LATE-NC+ participants with HS (compared to HS− participants) to have moderate to severe atherosclerosis of the circle of Willis (67.6% vs 41.6%, p < 0.001). Testing the associations between LATE-NC+HS and atherosclerosis with regression models that factored in demographic and copathology parameters did not affect the outcomes (eTable 3, links.lww.com/WNL/B799). No differences between HS+ and HS− LATE-NC+ groups were observed in lacunar/gross infarcts or microinfarcts.
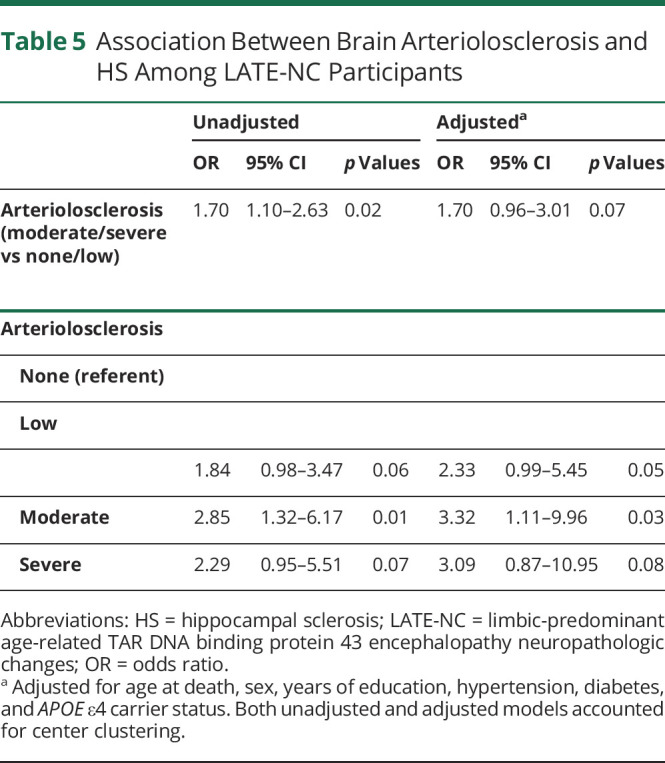
Last, we examined the association between brain arteriolosclerosis pathology and HS in LATE-NC+ patients (Table 5). Participants with moderate to severe arteriosclerosis more often had HS than participants with no or low arteriolosclerosis. After adjustment for age at death, sex, years of education, hypertension, diabetes, and APOEɛ4 carrier status, this association became nonsignificant (odds ratio [OR] 1.70, 95% CI 0.96–3.01). After application of this model to compare the odds of low, moderate, or severe arteriolosclerosis (reference: no arteriolosclerosis), participants with HS pathology were more likely to have moderately severe arteriolosclerosis (compared to those with no arteriolosclerosis; OR 3.32, 95% CI 1.11–9.96). Participants with severe arteriolosclerosis (again compared to those with no arteriolosclerosis) trended toward higher likelihood of having HS, but the CI was wider (OR 3.09, 95% CI 0.87–10.95) in this smaller group, and the test result was not statistically significant.
Table 5.
Association Between Brain Arteriolosclerosis and HS Among LATE-NC Participants
Discussion
We examined how individuals with autopsy-proven LATE-NC and HS differed in potential risk factors and clinical and pathologic correlates compared to those with LATE-NC lacking HS. A substantial minority of LATE-NC+ participants (145 of 366, 39.6%) were reported to have comorbid HS pathology. LATE-NC participants with comorbid HS pathology tended to be more cognitively impaired than those lacking HS pathology. HS affects >10% of individuals >80 years of age.32 Thus, LATE-NC with HS has an extremely large impact on public health. We sought clues as to why some but not other LATE-NC+ individuals develop comorbid HS pathology.
Data were analyzed from the NACC Neuropathology dataset, curated data on autopsied research volunteers sourced from multiple research centers. This dataset is not population representative, being largely clinic based and enriched for highly-educated APOE ε4+ White individuals with dementia.23,24 The relative lack of diverse and underserved populations is regrettable, and we hope it will be addressed better in the future. A challenge for studying HS, particularly in the context of a multicenter study, is that individual neuropathologists apply different criteria to generate a diagnosis at autopsy. Prior detailed studies of HS in aging have been performed, and guidelines for evaluating HS pathologically were suggested,33,34 but none of the proposed diagnostic methodologies have achieved universal acceptance. This practical fact, along with other differences in ADRC workflow (e.g., each has a different model for research volunteer recruitment), increased variance in the data and helps to explain the ADRC-to-ADRC differences in the prevalence of HS pathology (eTable 1, links.lww.com/WNL/B799). In this exploratory study, we applied the threshold of nominal significance at p < 0.05 and did not correct statistically for multiple comparisons. The findings will thus need to be corroborated in other autopsy samples using more focused hypothesis-testing methods or larger and more representative populations.
For all the limitations, the multicenter nature of the NACC database confers complementary benefits. The findings incorporate dozens of different experts' methodologies rather than being dependent on the practices of a single research group. The database also provides an unusually large set of autopsy-confirmed research participants without FTLD with TDP-43 proteinopathy, all worked up diagnostically by experts in the field after January 2014, with granular clinical and pathologic data.
Prior work has established that HS pathology is associated with cognitive impairment, factoring in other copathologies.16-18 The current study was not designed for optimal clinical-pathological correlation to evaluate the cognitive impact of HS because of the skew toward participants (almost 85%) with dementia before death. (Community-based cohorts do not see this preponderance of clinical impairment.16,17) Hence, rather than only comparing cognitive profiles among all the included participants, this study was also oriented toward highlighting medical and pathologic correlates that may indicate the biological factors underlying or occurring in parallel with HS.
Previous studies have also reported links between severe LATE-NC and HS and between cerebral vascular pathologies and HS. In both the Mayo Clinic and Rush University Medical Center, autopsy cohorts that developed TDP-43 staging systems based on the anatomical distribution of TDP-43 proteinopathy,3,35 the participants with more widespread TDP-43 proteinopathy were the ones with the highest prevalence of comorbid HS. The finding of increased arteriolosclerosis has also been associated with comorbid LATE-NC and HS.27,36-38 These positive results increase confidence about using this dataset for the evaluation of other clinical and pathologic correlates of HS in the context of LATE-NC.
The NACC dataset enabled analyses related to multiple subtypes of comorbid conditions. Our findings supported the null hypothesis with respect to several hypotheses about the development of HS pathology in LATE-NC: persons with LATE-NC and HS did not have more severe ADNC, were not more likely to have a history of clinically overt seizures, and did not more often have histories of cardiac or systemic vascular risk factors.
Unlike the tendency for more widespread distribution of TDP-43 proteinopathy to be associated with increased risk for HS pathology, ADNC pathologic hallmarks were not more frequent or widespread in HS+ participants: neither Aβ plaque distribution (Thal Aβ phases28) nor tau tangle distribution (Braak NFT stages29) was more severe in LATE-NC+ brains with comorbid HS pathology. There are indications from multiple different autopsy cohorts that there are pathogenetic synergies between ADNC and LATE-NC16,39,40; i.e., individuals with abundant Aβ plaques and NFTs are relatively likely to also have TDP-43 proteinopathy. In contrast, among LATE-NC+ brains, the presence or severity of ADNC did not seem to promote the hippocampal formation neuronal cell dropout seen in HS. In other words, ADNC does seem to promote TDP-43 proteinopathy; however, once that TDP-43 proteinopathy is present, the evolution of HS pathology appears to be largely determined by other factors.
HS pathology was not associated with increased likelihood of a history of seizures in LATE-NC participants. Among included participants, 4.7% (19 of 408) overall had a clinical seizure history—a result consistent with expectations among the elderly.41 The current study did not include highly sensitive workups for subclinical seizure activity in each research volunteer; however, the majority of these research volunteers had been evaluated (often over a number of years) by trained behavioral neurologists. Thus, it seems unlikely that overt seizures are what drive HS (or vice versa) in LATE-NC. This result can be compared with the positive findings among persons with HS but lacking LATE-NC: even in this small sample, the HS+ persons without TDP-43 proteinopathy were as a group relatively likely to have either stroke (particularly) or seizure clinical history. These findings are overall compatible with prior studies finding that acute hypoxia or seizure disorders can produce HS that lacks TDP-43 proteinopathy.12,14,15
In this study, there was an association between HS pathology and increased cerebral blood vessel pathologies, but there were no detected associations between HS and conventional cardiovascular risk factors. The vascular pathologies associated with autopsy-confirmed HS were mostly referent to blood vessel walls (arteriosclerosis and atherosclerosis) rather than parenchymal pathologies (microinfarcts, lacunar, or large infarcts). These findings are consistent with prior studies. We previously reported increased arteriolosclerosis in LATE-NC/HS brains,35,42,43 whereas other researchers described associations between HS or LATE-NC with circle of Willis atherosclerosis pathology.16,44 Prior studies also implicated vascular diseases in HS dementia, now considered a subtype of LATE-NC.5,45 Notably, none of the upstream vascular risk factors (diabetes, hypertension, hypercholesterolemia, etc) or cardiac disease readouts in the NACC dataset showed positive association with HS in LATE-NC+ participants.36 If known systemic or cardiac diseases are not contributing to HS pathology, then other factors related to the vasculature, in combination with TDP-43 proteinopathy, may instead promote HS.
Genetics research may help explain the phenomena described above. In a recent study of autopsy cohort data combined with genetic information, associations were confirmed between HS risk and TMEM106B, ABCC9, GRN, and APOE gene variants.46 Unlike the other HS risk genes, ABCC9 genetic variation was not associated with LATE-NC but was associated only with the risk for HS pathology among individuals with LATE-NC.46 The ABCC9 genetic variation may contribute pathogenetically by causing differential vulnerability for vascular pathologies that are upstream of HS in a mechanistic sense. Indeed, multiple lines of evidence implicate ABCC9 in blood vessel functions in healthy states and in cerebrovascular disease. First, ABCC9 plays normal physiologic roles in modulating cerebral blood flow and in ischemic preconditioning.42,47 Second, the HS risk-related allele was associated with lower expression of ABCC9 in blood vessels.46 Third, ABCC9 variations have already been linked to cerebrovascular pathologies: ABCC9 gain-of-function variations cause Cantu syndrome, a multifaceted condition often accompanied by tortuous cerebral blood vessels,48 whereas separate ABCC9 loss-of-function variations lead to ABCC9-related intellectual disability myopathy syndrome, another complex phenotype that includes white matter hyperintensities detected on MRI even in adolescents.49 Thus, the HS/ABCC9 link may be a clue to help explain why vascular pathologies are more severe in brains with HS pathology than in non-HS brains,27,36–38.50 despite the lack of associations between HS pathology and traditional cardiovascular risk factors. It remains an unanswered question whether ABCC9 genotypes also help to explain differential vulnerability to HS pathology among persons without LATE-NC such as in patients with FTLD-TDP.19
The present study argues for a re-examination of some commonly held hypotheses related to dementia and hippocampal pathologies. Hippocampal atrophy seen on MRI is generally presumed to indicate the presence of underlying Alzheimer-type pathology, and many clinicians associate HS with a history of seizures or acute hypoxia. However, among persons with LATE-NC in this study (the usual context in which HS pathology is seen at autopsy in older people), the presence of HS pathology was not correlated with ADNC presence or severity or with a history of seizures, cardiac disease, traditional cardiovascular risk factors, or stroke. In terms of positive findings among participants with LATE-NC, the presence of comorbid HS was associated with more widespread TDP-43 pathology and with relatively severe atherosclerosis and arteriolosclerosis pathologies.
Acknowledgment
The authors are grateful to the research volunteers, families and caregivers, and their colleagues at the National Institute on Aging–funded ADRCs. They thank Dr. Karin B. Nelson (NIH) for reviewing and editing the manuscript for intellectual content.
Glossary
- Aβ
β-amyloid
- ADNC
Alzheimer disease neuropathologic changes
- ADRC
Alzheimer’s Disease Research Center
- BMI
body mass index
- CAA
cerebral amyloid angiopathy
- CDR
Clinical Dementia Rating
- FTLD
frontotemporal lobar degeneration
- HS
hippocampal sclerosis
- LATE
limbic-predominant age-related TDP-43 encephalopathy
- NACC
National Alzheimer's Coordinating Center
- NFT
neurofibrillary tangle
- OR
odds ratio
- TDP-43
TAR DNA binding protein 43
- UDS
Uniform Data Set
Appendix. Authors
Study Funding
The NACC database is funded by National Institute on Aging/NIH grant U01 AG016976. NACC data are contributed by the National Institute on Aging–funded ADRCs: P30 AG019610 (principal investigator [PI] Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD). Dr. Nelson is supported by R01 AG061111, RF1 NS118584, R01AG057187, R01AG054060, and UK-ADC P30AG028383 from the National Institute on Aging.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Chornenkyy Y, Fardo DW, Nelson PT. Tau and TDP-43 proteinopathies: kindred pathologic cascades and genetic pleiotropy. Lab Invest. 2019;99(7):993-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130-133. [DOI] [PubMed] [Google Scholar]
- 3.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2020;79(3):305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015;77(6):942-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson DW, Davies P, Bevona C, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88(3):212-221. [DOI] [PubMed] [Google Scholar]
- 6.Josephs KA, Mackenzie I, Frosch MP, et al. LATE to the PART-y. Brain. 2019;142(9):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson PT, Dickson DW, Trojanowski JQ, et al. Reply: LATE to the PART-y. Brain. 2019;142(9):e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol (Berl). 2007;113:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ighodaro ET, Jicha GA, Schmitt FA, et al. Hippocampal sclerosis of aging can Be segmental: two cases and review of the literature. J Neuropathol Exp Neurol. 2015;74(7):642-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarow C, Weiner MW, Ellis WG, Chui HC. Prevalence, laterality, and comorbidity of hippocampal sclerosis in an autopsy sample. Brain Behav. 2012;2(4):435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134(pt 5):1506-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer W. Erkrankung des Ammon's horn als aetiologis ches moment der epilepsien. Arch Psychiatr Nurs. 1880;10:631-675. [Google Scholar]
- 14.Lee EB, Lee VM, Trojanowski JQ, Neumann M. TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system. Acta Neuropathol. 2008;115(3):305-311. [DOI] [PubMed] [Google Scholar]
- 15.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyle PA, Wang T, Yu L, et al. To what degree is late life cognitive decline driven by age-related neuropathologies? Brain. 2021;144(7):2166-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20(1):66-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray ME, Cannon A, Graff-Radford NR, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014;128(3):411-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenowitz WD, Monsell SE, Schmitt FA, Kukull WA, Nelson PT. Hippocampal sclerosis of aging is a key Alzheimer's disease mimic: clinical-pathologic correlations and comparisons with both alzheimer's disease and non-tauopathic frontotemporal lobar degeneration. J Alzheimers Dis. 2014;39(3):691-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josephs KA, Murray ME, Tosakulwong N, et al. Pathological, imaging and genetic characteristics support the existence of distinct TDP-43 types in non-FTLD brains. Acta Neuropathol. 2019;137(2):227-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One. 2011;6(10):e26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63(1):72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besser LM, Kukull WA, Teylan MA, et al. The revised National Alzheimer's Coordinating Center's Neuropathology form: available data and new analyses. J Neuropathol Exp Neurol. 2018;77(8):717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mock C, Teylan M, Beecham G, et al. The utility of the National Alzheimer's Coordinating Center's database for the rapid assessment of evolving neuropathologic conditions. Alzheimer Dis Assoc Disord. 2020;34:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NIH. NIH exempt human subjects research. grants.nih.gov/sites/default/files/exemption_infographic_v8_508c_1-15-2020.pdf.
- 26.Nelson PT, Gal Z, Wang WX, et al. TDP-43 proteinopathy in aging: associations with risk-associated gene variants and with brain parenchymal thyroid hormone levels. Neurobiol Dis. 2019;125:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsumata Y, Fardo DW, Kukull WA, Nelson PT. Dichotomous scoring of TDP-43 proteinopathy from specific brain regions in 27 academic research centers: associations with Alzheimer's disease and cerebrovascular disease pathologies. Acta Neuropathol Commun. 2018;6(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H. The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowledge Environ. 2006;2006(6):re1. [DOI] [PubMed] [Google Scholar]
- 29.Braak E, Braak H, Mandelkow EM. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 1994;87(6):554-567. [DOI] [PubMed] [Google Scholar]
- 30.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18:S91-S94. [DOI] [PubMed] [Google Scholar]
- 31.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124. [DOI] [PubMed] [Google Scholar]
- 32.Nelson PT, Smith CD, Abner EL, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol. 2013;126(2):161-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauramaa T, Pikkarainen M, Englund E, et al. Consensus recommendations on pathologic changes in the hippocampus: a postmortem multicenter inter-rater study. J Neuropathol Exp Neurol. 2013;72(6):452-461. [DOI] [PubMed] [Google Scholar]
- 34.Hatanpaa KJ, Raisanen JM, Herndon E, et al. Hippocampal sclerosis in dementia, epilepsy, and ischemic injury: differential vulnerability of hippocampal subfields. J Neuropathol Exp Neurol. 2014;73(2):136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Josephs KA, Murray ME, Whitwell JL, et al. Updated TDP-43 in Alzheimer's disease staging scheme. Acta Neuropathol. 2016;131(4):571-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neltner JH, Abner EL, Baker S, et al. Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain. 2014;137(pt 1):255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agrawal S, Yu L, Kapasi A, et al. Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change and microvascular pathologies in community-dwelling older persons. Brain Pathol. 2021;31(3):e12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison WT, Lusk JB, Liu B, et al. Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is independently associated with dementia and strongly associated with arteriolosclerosis in the oldest-old. Acta Neuropathol. 2021;142(5):917-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith VD, Bachstetter AD, Ighodaro E, et al. Overlapping but distinct TDP-43 and tau pathologic patterns in aged hippocampi. Brain Pathol. 2017;28:264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol. 2014;127(6):811-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beghi E, Giussani G. Aging and the epidemiology of epilepsy. Neuroepidemiology. 2018;51(3-4):216-223. [DOI] [PubMed] [Google Scholar]
- 42.Nelson PT, Jicha GA, Wang WX, et al. ABCC9/SUR2 in the brain: implications for hippocampal sclerosis of aging and a potential therapeutic target. Ageing Res Rev. 2015;24(pt B):111-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neltner JH, Abner EL, Jicha GA, et al. Brain pathologies in extreme old age. Neurobiol Aging. 2016;37:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez OL, Kofler J, Chang Y, et al. Hippocampal sclerosis, TDP-43, and the duration of the symptoms of dementia of AD patients. Ann Clin Transl Neurol. 2020;7(9):1546-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leverenz JB, Lipton AM. Clinical aspects of hippocampal sclerosis. Handb Clin Neurol. 2008;89:565-567. [DOI] [PubMed] [Google Scholar]
- 46.Dugan A, Nelson PT, Katsumata Y, et al. Analysis of genes (TMEM106B, GRN, ABCC9, KCNMB2, and APOE) implicated in risk for LATE-NC and hippocampal sclerosis provides pathogenetic insights: a retrospective genetic association study. Acta Neuropathol Commun. 2021;9(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440(7083):470-476. [DOI] [PubMed] [Google Scholar]
- 48.Leon Guerrero CR, Pathak S, Grange DK, et al. Neurologic and neuroimaging manifestations of Cantu syndrome: a case series. Neurology. 2016;87:270-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smeland MF, McClenaghan C, Roessler HI, et al. ABCC9-related intellectual disability myopathy syndrome is a KATP channelopathy with loss-of-function mutations in ABCC9. Nat Commun. 2019;10:4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ighodaro ET, Abner EL, Fardo DW, et al. Risk factors and global cognitive status related to brain arteriolosclerosis in elderly individuals. J Cereb Blood Flow Metab. 2017;37(1):201-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.