To the editor.
Sickle cell disease (SCD) is a group of hereditary red cell disorders determining a multisystem disease and clinical manifestations of variable severity. Patients with compound heterozygosity for HbS and β+-thalassaemia mutation generally present a mild-moderate form of the disease; however, they seem to be more subjected to fat embolism syndrome (FES).1 This is a rare and devastating complication of SCD resulting from massive bone marrow necrosis and is associated with multiorgan dysfunction and high mortality. In this letter, we report the case of a drug-resistant nonconvulsive status epilepticus (NCSE), probably triggered by a cerebral fat embolism (CFE), in a HbS/β+-thalassaemia patient.
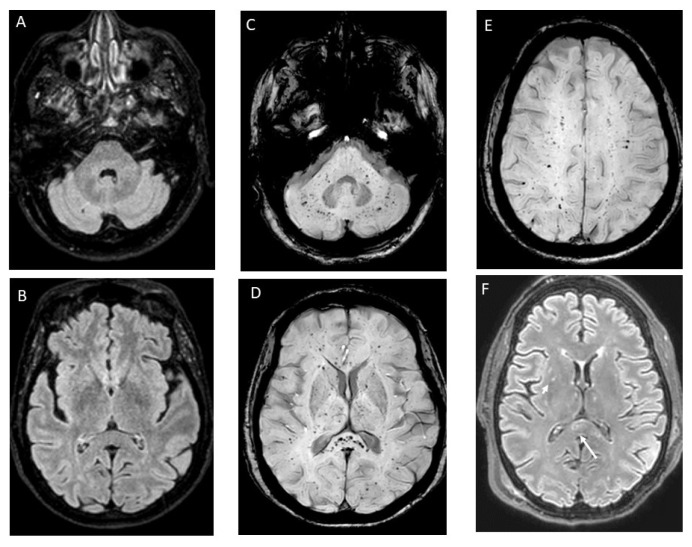
A Caucasian 61-year-old woman with HbS/β+-thalassaemia (βS/β+-IVS-I-6; α+832 G>Aα/αα) presented to the Emergency Department (ED) with severe precordial chest pain, dyspnoea, oxygen desaturation, and a normal chest x-ray. Diagnosis of SCD was made during childhood but, until the age of 50, she had only a few uncomplicated vaso-occlusive crises (VOCs). Two years before this episode, neurological and cardiological studies had been performed following a suspected transient ischemic attack with negative results, except for the presence of a patent foramen ovale (PFO), resulting in right-left shunt without surgical indications. Thus, she had started Hydroxyurea (8.6 mg/kg/d, maximum tolerated dose due to thrombocytopenia at higher dosage), and prophylaxis with acetylsalicylic acid (100 mg/d). At the ED, acute chest syndrome (ACS) associated with VOC was suspected. Intravenous rehydration, low-flow oxygen, opioid analgesia, and top-up transfusion were initiated, and she was admitted. Unfortunately, due to poor suitable vascular access, eritroexchange (EEX) transfusion therapy was delayed over 24 hours. The following day, dyspnoea, oxygen saturation, and mental status worsened (Glasgow Coma Scale, GCS, 11: E4, V2, M5). Electrocardiogram (ECG) showed minor alterations, and Doppler ultrasonography of the lower limbs was not evocative for deep venous thrombosis. Brain-Computer Tomography (CT) was unremarkable, and naloxone, administered suspecting an opioid intoxication, showed no benefits. Blood tests reported a drop in platelets and leukocytes count and increased inflammatory and haemolytic markers, coagulation and liver function tests, cardiac enzyme, and N-terminal prohormone of brain natriuretic peptide. Microbiological analyses were negative but, due to a diffuse bilateral parenchymal infiltrate at the chest X-ray, empirical antibiotic therapy with piperacillin/tazobactam was started. Despite adequate EEX transfusions (target Hb 10–11 g/dl and HbS <30%) being finally established, she became unresponsive (GCS 8: E2, V2, M4), requiring intubation for airway protection. Urgent brain and neck CT angiography was negative for haemorrhages and acute vascular occlusions. Continuous Electroencephalogram (EEG) monitoring showed recurrent epileptiform discharges and triphasic waves in the temporal-central regions bilaterally consistent with NCSE; therapy with benzodiazepine, levetiracetam, and valproic acid was started. Cerebral spinal fluid (CSF) analyses for viral and bacterial infections resulted negative. Brain magnetic resonance imaging (MRI) showed widespread, non-confluent areas of ischemia in multiple anterior and posterior vascular distributions with microhaemorrhages. These alterations detected with susceptibility-weighted imaging (SWI), specific MRI sequences sensible to compounds distorting the local magnetic field (such as iron from haemoglobin), were consistent with a “starfield appearance” with a “walnut kernel microbleed” pattern” (Figure 1). Transthoracic as well as transesophageal echocardiography (TEE) were negative for ventricular dysfunction, reduced ejection fraction, valvular vegetations, or intracardiac thrombi, but confirmed the presence of a PFO. Prolonged ECG monitoring did not record arrhythmia. Given the negative results of the diagnostic work-up for alternative embolic sources and the specific MRI findings, CFE was suspected. The persistence of the NCSE prompted the initiation of add-on therapy with lacosamide and propofol, but only after administering thiopentone, seizure control was achieved. Antiepileptic therapy was progressively tapered until discontinuation and the patient continued levetiracetam as maintenance therapy. At discharge, acetylsalicylic acid was reintroduced while Hydroxyurea was definitively replaced by regular EEX sessions. After six months, she was seizure-free and showed full physical recovery at the follow-up visit. Considering her clinical history, the cerebral lesions, and the right-left shunt severity, the PFO was closed.
Figure 1.
Pre- and post-cerebral fat embolism (CFE) brain magnetic resonance imaging (MRI) with susceptibility-weighted sequences. Normal brain fluid-attenuated inversion recovery (FLAIR) MRI sequences performed 6 months before the index event (left side, A and B). Susceptibility-weighted imaging (SWI) sequences performed 24 hours after intubation. Multiple, punctuate, widespread, hypointense lesions are distributed in the cerebellum (C), basal ganglia, splenium of corpus callosum (D), and subcortical white matter bilaterally (E), (walnut kernel pattern). These alterations are consistent with the accumulation of haemosiderin in the context of diffuse microhaemorrhages from small-vessel occlusion by fat emboli. FLAIR images at 24 hours after intubation show scattered, monomorphic, hyperintense lesions in the deep grey structures (short arrow) and splenium of the corpus callosum (long arrow) not present in the pre-CFE brain imaging (A and B).
Fat embolism syndrome is a rare complication of SCD. Recently, Tsitsikas et al.2 identified 87 cases reported in the literature. This syndrome seems to affect mainly mild forms of SCD and mortality is highly variable from 33% to 66% depending on the timeliness and the type of transfusion regimen established.
Pathophysiological mechanisms causing massive bone marrow necrosis resulting in systemic fat embolism in sickle cell patients are not completely understood. Sickling red blood cells during a VOC could result in bone marrow necrosis and subsequent release of fat emboli into the venous circulation.3 Fat globules primarily embolize the lungs, leading to ACS, and thereafter can enter the arterial circulation through two mechanisms. First, small fat globules can pass through lung capillaries and reach arterial blood; second, they can cross a right-left shunt through a PFO (paradoxical embolism).4 Thus, although not necessary for FES, PFO may represent an additional risk for CFE in SCD patients and its closure may be considered. An additional pathogenic mechanism of FES includes the hydrolyzation of fat emboli into circulating free fatty acids (by phospholipase A2), inducing tissue injury by a secondary production of inflammatory cytokines.2
Typical clinical presentations of FES include pain of unusual severity, fever, respiratory distress, and altered mental status with or without other organ involvements.5 A definitive diagnosis of FES requires histological confirmation of bone marrow necrosis or demonstration of fat globules in different organs.6 However, clinical tools – such as Gurd and Wilson criteria and Shonfeld Fat Embolism Index – represent valid approaches to diagnose FES in SCD.7 Particularly, this diagnosis should be considered when leukoerythroblastosis, drop in haemoglobin and platelet count, increased LDH, C reactive protein, ferritin, and markers of organ failure are detected.8 In addition, brain imaging supports the diagnosis of CFE when the typical “starfield pattern” on MRI with SWI is detected.9 Of note, given the low sensitivity of brain CT in the acute phase of CFE,9 MRI with SWI or gradient echo is superior in the differential diagnosis.
Although the lack of histological data does not allow a definitive diagnosis of FES, as in our case, the association between acute respiratory and neurologic symptoms in a patient with SCD, the specific neuroradiological pattern on brain imaging, as well as the negative work-up for other embolic sources, should lead to consider this diagnosis.
Eritroexchange in FES can be lifesaving and data from the literature2 indicate that it should be started as soon as FES is suspected of limiting morbidity and mortality.
The institution of a regular EEX regimen after recovery is debated; however, it is recommended in case of neurological sequelae.2
Seizures are a possible neurologic complication of SCD, but status epilepticus in SCD patients is anecdotal.10 Its diagnosis depends on a high level of suspicion and the use of electrodiagnostic monitoring. Prolonged status epilepticus is associated with high mortality and requires supportive care, antiepileptic drugs, sedation, and the management of the causative condition.
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Tsitsikas DA, Gallinella G, Patel S, Seligman H, Graves P, Amos RJ. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: Increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev. 2014;28:23–30. doi: 10.1016/j.blre.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Tsitsikas DA, Vize J, Abukar J. Fat Embolism Syndrome in Sickle Cell Disease. J Clin Med. 2020;9(11):3601. doi: 10.3390/jcm9113601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dang NC, Johnson C, Eslami-Farsani M, Haywood LJ. Bone marrow embolism in sickle cell disease: A review. Am J Hematol. 2005;79:61–67. doi: 10.1002/ajh.20348. [DOI] [PubMed] [Google Scholar]
- 4.Mijalski C, Lovett A, Mahajan R, Sundararajan S, Silverman S, Feske S. Cerebral Fat Embolism: A Case of Rapid-Onset Coma. Stroke. 2015;46:e251–e253. doi: 10.1161/STROKEAHA.115.011440. [DOI] [PubMed] [Google Scholar]
- 5.Tsitsikas DA, May JE, Gangaraju R, Abukar J, Amos RJ, Marques MB. Revisiting fat embolism in sickle syndromes: diagnostic and emergency therapeutic measures. Br J Haematol. 2019;186:e112–e115. doi: 10.1111/bjh.15941. [DOI] [PubMed] [Google Scholar]
- 6.Kammeyer R, Devnani R, Mehta R. Cerebral fat embolism syndrome mimicking thrombotic thrombocytopenic purpura in a patient with hemoglobin SC disease. Am J Hematol. 2016;91:539–542. doi: 10.1002/ajh.24286. [DOI] [PubMed] [Google Scholar]
- 7.Bailey K, Wesley J, Adeyinka A, Pierre L. Integrating Fat Embolism Syndrome Scoring Indices in Sickle Cell Disease: A Practice Management Review. J Intensive Care Med. 2019;34:797–804. doi: 10.1177/0885066617712676. [DOI] [PubMed] [Google Scholar]
- 8.Gangaraju R, May JE, Williams LA, Ill, Reddy VB, MacLennan P, Marques MB. Fat embolism syndrome due to bone marrow necrosis in patients with hemoglobinopathies: A life-threatening complication mimicking thrombotic thrombocytopenic purpura. Am J Hematol. 2019;94:e64–e66. doi: 10.1002/ajh.25363. [DOI] [PubMed] [Google Scholar]
- 9.Kuo KH, Pan YJ, Lai YJ, Cheung WK, Chang FC, Jarosz J. Dynamic MR Imaging Patterns of Cerebral Fat Embolism: A Systematic Review with Illustrative Cases. Am J Neuroradiol. 2014;35:1052–1057. doi: 10.3174/ajnr.A3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguirre-Bermeo H, Millón J, Martínez-Domeño A, Marruecos-Sant L. Status epilepticus in a patient with sickle cell disease. Med Clin. 2011;137:428–429. doi: 10.1016/j.medcli.2010.12.015. [DOI] [PubMed] [Google Scholar]