Abstract
Sarcopenia is defined by the loss of muscle mass and function. In aging sarcopenia is due to mild chronic inflammation but also to fiber-intrinsic defects, such as mitochondrial dysfunction. Age-related sarcopenia is associated with physical disability and lowered quality of life. In addition to skeletal muscle, the nervous tissue is also affected in elderly people. With aging, type 2 fast fibers preferentially undergo denervation and are reinnervated by slow-twitch motor neurons. They spread forming new neuro-muscular junctions with the denervated fibers: the result is an increased proportion of slow fibers that group together since they are associated in the same motor unit. Grouping and fiber type shifting are indeed major histological features of aging skeletal muscle. Exercise has been proposed as an intervention for age-related sarcopenia due to its numerous beneficial effects on muscle mechanical and biochemical features. In 2013, a precursor study in humans was published in the European Journal of Translation Myology (formerly known as Basic and Applied Myology), highlighting the occurrence of reinnervation in the musculature of aged, exercise-trained individuals as compared to the matching control. This paper, entitled «Reinnervation of Vastus lateralis is increased significantly in seniors (70-years old) with a lifelong history of high-level exercise», is now being reprinted for the second issue of the «Ejtm Seminal Paper Series». In this short review we discuss those results in the light of the most recent advances confirming the occurrence of exercise-mediated reinnervation, ultimately preserving muscle structure and function in elderly people who exercise.
Key Words: Sarcopenia, denervation, muscle atrophy, skeletal muscle, physical activity
Ethical Publication Statement
I confirmthat I have read the Journal’s position on ethical publication issues and affirms that this report is consistent with those guidelines.
The definition of sarcopenia, initially meant to indicate the progressive loss of skeletal muscle mass associated with aging, has recently being updated by stressing the importance of the loss of muscle strength and physical performance.1 Indeed, functional assessments are now part of the definition of sarcopenia, similarly to other forms of muscle atrophy such as cachexia,2 which reckons the importance of muscle functionality in defining a muscle disease state. The association of sarcopenia with a significant impairment of function such as slowing of movement and loss of neuromuscular control is linked to decreased quality of life and increased risk of falls.3 With the global aging population, sarcopenia becomes increasingly prevalent, rising major concerns for the health-related authorities,4 and an increased awareness of the importance of research aimed to improve the preservation of the muscle mass and its related function in elderly individuals. A major avenue of research in the field of age-related sarcopenia deals with the process of muscle denervation in aging and the countermeasures that prevent this phenomenon or favor recovery in the elderly. A pioneer report on this subject is the article by Mosole et al.,5 which is re-published in the current issue of European Journal of Translational Myology. Their findings on the beneficial effects of exercise on muscle reinnervation with age have been confirmed and extended by many others 6–9 and are discussed in this mini-review in the light of the more recent literature.
Muscle fiber type and fiber shifting
Fiber diversity reflects the plethora of muscle functional and metabolic roles. Myosin has a prominent position both as a marker for fiber typing and as a molecular motor characterizing the function of the muscle tissue. The conventional class II of muscle myosin encompasses 18 genes driving the expression of several myosin heavy chain (MyHC) isoforms.10 These genes are evolutionary conserved in mammals and expressed to a variable extent and in different combinations in adult striated muscles, while special isoforms are expressed during embryogenesis, perinatal life, and muscle regeneration (Table 1). Distinct myosin gene expression determines four major muscle fiber types, as exhaustively highlighted by Schiaffino and Reggiani.10 Briefly: there are two types of fast-contracting fibers, called type 2A and 2B, which differ for being fatigue-resistant and fatigable, respectively, the type 1 fibers predominant in slow-twitch muscles, the most resistant to fatigue, which form mostly postural muscles; a fourth type of fiber, the 2X, has twitch properties (contraction and half-relaxation time) similar to those of 2A and 2B units, and has an intermediate resistance to fatigue.10,11 These fiber types are determined during development by hormonal and neurogenic factors but are susceptible of extensive remodeling in postnatal life. The default phenotype of a muscle fiber is the fast one,10 as confirmed by the fact that denervated, regenerating muscle fibers activate the default expression of fast-like myosin expression.12 Worth noting, regenerating muscle fibers following trauma or pathological cues re-enter a developmental program in which they re-express embryonic or perinatal MyHC isoforms, such as the embryonic, fetal or neonatal myosins.13–16 Regenerating muscle fibers also share morphological features with pre-natal muscle fibers, such as the central nuclei,17 and ultimately mature acquiring features that are not necessarily those prior to damage, which accounts for muscle plasticity. The final MyHC isoform expressed by an adult, regenerated muscle fiber is ultimately conditioned by the microenvironment, including the type of motoneuron that innervates the fiber.18 Muscle fiber types also differ with respect to resistance to fatigue and numerous additional features, as highlighted by the differential content of succinate dehydrogenase (SDH) and other metabolic enzymes. Interestingly, fiber shifts in MyHC with aging are associated to a remodulation of these enzymatic activities.19 Additional factors, such as the increase in resting muscle stiffness, affect age-related muscle impairment and will not be discussed here.20
Table 1.
Major myosin isoforms expressed in mammal muscle
| Gene | Protein | Functional properties | Expression pattern |
|---|---|---|---|
| MYH1 | MyHC-2X | Fast contracting | Multiple skeletal muscles, Type 2X fibers |
| MYH2 | MyHC-2A | Fast contracting | Multiple skeletal muscles, Type 2A fibers |
| MYH3 | MyHC-emb | Slow contracting87 | Developing and regenerating muscle |
| MYH4 | MyHC-2B | Fast contracting | Not in humans, multiple skeletal muscles, Type 2B fibers |
| MYH6 | MyHC-alpha | Intermediate speed88 | Cardiac and skeletal muscle of the jaws |
| MYH7 | MyHC-beta/slow | Slow contracting | Cardiac and slow skeletal muscles, Type 1 fibers |
| MYH8 | MyHC-neo | NA | Developing and regenerating muscle |
| MYH13 | MyHC-EO | Superfast cont.89 | Extraocular (EO) skeletal muscles |
| MYH14* | MyHC-slow/tonic | Slow contracting90 | Muscle spindles, Extraocular skeletal muscles |
| MYH15 | MyHC-15 | Slow contracting90 | Muscle spindles, Extraocular skeletal muscles |
| MYH16 | MyHC-M | Not in humans,° Translational Myolog it is evident Skeletal muscle of the jaws |
The table summarizes the nomenclature and expression pattern of the myosin heavy chain (MyHC) genes (MYH) and the corresponding sarcomeric protein product in mammals. The expression pattern in the striated muscles, as well as the functional properties in terms of contraction speed, are also reported. Hybrid fibers containing two MyHC
Effects of aging on the skeletal muscle
Aging is characterized by a progressive decline in skeletal muscle mass, ultimately leading to decreased strength and functionality.1 The two major mechanisms underpinning the decline in muscle mass are muscle fiber atrophy 21 and muscle fiber loss, even though there is not consensus on the latter.22 Type 2 fibers are preferentially sensitive to atrophy in aging.23 Two additional phenomena characteristic of aging are fiber type grouping and shifting. Grouping is the presence of clusters of fibers of the same type, defined as a group of fibers with at least one enclosed fiber.24 Grouping typically occurs for type 1 fibers in aging 24 and is associated to an enrichment in the percentage of type 1 fibers in the musculature.25 The latter is a paradoxical phenomenon, since, as state above, the default muscle fiber phenotype is the fast, type 2 fiber 12 therefore, aging actively and selectively promote the appearance of type 1 fibers in the musculature. Indeed, in murine models it has been shown that regenerating fibers express by default the fast MyHC-2X and -2B transcripts in absence of innervation, even in slow muscle such as the Soleus. If slow nerve activity is restored or mimicked by electrodes, this induces a switch toward a slow, MyHC-beta/slow isoform in the targeted muscle fibers,26 through a pathway involving RAS and ERK activation.27 Thus, the fiber type essentially depends on the innervation of the muscle fibers themselves. Taken together these reports helped to state the hypothesis that, with aging, type 2, fast fibers preferentially undergo denervation to then form new NMJ by reinnervation from slow-twitch motor neurons. The result is an increased proportion of slow fibers, which also group with each other, since they are associated in the same motor unit. These phenomena, in our opinion, would also explain the slower movements typical of elderly individuals. If reinnervation is insufficient, then fibers undergo atrophy or apoptosis.28
The sarcopenia-associated changes in motor unit numbers has been demonstrated using electromyographic motor unit recordings through surface-recorded compound muscle action potentials (CMAP) and indirectly through MRI and histochemistry (reviewed by Piasecki et al.).29 Motor unit loss and the alterations characterizing the aging neurons are superbly summarized in a recent review by Larsson et al. and will not be further discussed here, for lack of space.3
In addition to denervation and atrophy, other alterations characterize the aging muscle, such as a reduced regenerative capacity and fiber-intrinsic defects: protein oxidation, organelle dysfunction (including lysosomes and mitochondria), changes in sarcomeres and endoplasmic reticulum, ultimately leading to defects in calcium handling.30 Collectively, these events synergize, leading to loss of muscle function during aging.30 For instance, calcium homeostasis, which is very important for the myogenic development and muscle regeneration,31,32 is also essential in the adult muscle fiber and calcium leakage is a major responsible for the diminished contraction capacity observed in aging.33
In the 2010 European consensus article “Report of the European working group on Sarcopenia in older people”1 the authors described the major muscle issues associated with aging and proposed strategies that include treatments and changes in lifestyle in order to prevent age-associated sarcopenia. These interventions are as diverse as hormone therapy and exercise/physical activity, caloric intake control and lowering inflammation.3 The findings of Mosole et al. summarized below, paved the way to exercise-based interventions to spare muscle wasting and pinpointed the peculiar role of denervation-reinnervation processes.
Major findings by Mosole et al. and more recent developments
In the seminal work by Mosole et al. the group demonstrated that long-term high-level exercise promotes muscle reinnervation with age5 (republished in the current issue of Eur J Trans Myol). Indeed, this has been the focus of their research activities for years and led to three publications - first in 2013 and then, extending the original findings, in 2014 and 2016 - that are discussed in details below. Researchers at the University of Padua are among those who propose to apply strictu sensu the concept of “use it or lose it” to sarcopenic muscles.8 Since the 90s there was an interest on skeletal muscle in master athletes and Westerterp in 2000 was probably one of the very first to extensively demonstrate and discuss the benefits deriving from physical activity for elderly individuals.34,35 Nonetheless, Mosole and coworkers were the first ones to demonstrate a clear effect of exercise on muscle fiber reinnervation, thus paving the road for a new avenue of investigation. Indeed, in 2013 Mosole et al. compared muscle biopsies from sedentary seniors, physically active (i.e. sportsmen) seniors and young people in order to prove that exercise protects against age-related denervation.5 In this study they clearly demonstrated the occurrence of selective denervation in the aged muscle, as highlighted by the occurrence of angular, atrophic muscle fibers, a phenomenon prevented in life-long exercised muscles. In addition, the authors demonstrated that senior sportsmen show a greater level of type 1-fiber grouping, as if their muscle were undergoing a higher rate of reinnervation.5 One year later, in 2014, Mosole et al. further developed this idea, by exploiting a technically improved approach to analyze skeletal muscle biopsies, consisting in immunofluorescence for MyHC types.36 They clearly showed the effect of exercise on bona fide denervation markers (such as N-CAM expression by the muscle fibers), as well as fiber type transition (by double immunofluorescence showing the co-expression of fast and slow MyHC). Worth noting, while histology was comparable between the two studies, in the 2014 article the force of the quadriceps was measured, instead of that of the vastus lateralis that was analyzed in the 2013 article. The authors also compared slow fibers with the type of training undertaken by the physically active seniors but they did not establish a correlation. The authors concluded that a long-term, intense exercise promotes reinnervation of muscle fibers, with positive consequences on the muscle structure and function, ultimately leading to a delay in mobility decline.36 Mosole’s 2013 article has been inspirational and the reference for several other papers over the years, confirming that heavy training improves skeletal muscles in very old individuals. In particular, the pivotal role of motor units in the maintenance of muscle mass with aging has been confirmed, on the basis of observations in both male and female master athletes.37 To this regard, the group of Holm demonstrated a very innovative idea, i.e. that just 12 weeks of heavy resistance training are enough to improve muscle strength in old individuals and, suggesting that lifelong exercise may not be strictly required to generate beneficial effects.38 Messi et al. extended these observations to the obese population – indeed, sarcopenic obesity is a common condition observed in a major percentage of elderly individuals, characterized by the combination of being significantly overweight with the age-related loss of muscle mass and strength.39 In particular, Messi et al. showed that resistance training diminished the expression of N-CAM (a denervation marker) in muscle fibers and affected fiber grouping even in obese patients. This group expanded their analysis to muscle stem cells, which account for the remarkable skeletal muscle plasticity, showing that exercise does not modify either the total number of satellite cells or their relative abundance in every fiber type.40 The histological examination alone may not reveal the full extent of ageing-related motor unit remodeling; for instance, it was reported that the fiber grouping did not correlate with aging. Taken alone, these data would indicate that the examined population did not show a major feature of sarcopenia,24 further suggesting the importance of the functional characterization of the aged musculature. Notably, Messa et al. examined the vastus lateralis in athletes, albeit aged, which is a major difference in respect to the previous studies, that focused on very active people, but not athletes. A study by Kletzien et al. reported changes in thyroarytenoid muscle associated to both aging and to exercise training in rats. These changes, affecting MyHC isoforms, were consistent with a glycolytic to oxidative shift in muscle fiber type.41 The conclusion by the authors is that because thyroarytenoid muscle is active during vocalization – it is actually part of the laryngeal muscle structure - the approach of doing exercise is crucial in order to prevent “vocal function deficits, dysphagia, and aspiration observed in elderly people”.41 In addition, we believe that this study represents a striking evidence that the upper airways muscles are affected by treadmill running, even though they are not directly involved, offering a proof of principle of the occurrence of systemic effects of physical activity on muscle fiber type shifting. Another breakthrough article was the comparison of muscle status and performance on monozygotic twins with decades of discordant exercise habits. Here, the authors showed that the trained twins exhibited better endurance features, more slow-twitch MyHC, and increased level of pro-myogenic markers than their counterpart. They concluded that skeletal muscle as a high plasticity depending on lifestyle which overrides the genetic background.42 Intriguingly, an indirect but solid confirmation of Mosole’s data comes from the group of Carraro and co-workers that in several studies have actually exploited Functional Electrical Stimulation (FES) to modify “muscle fibers by increasing contractions per day”43,44 in the absence of physical activity. While primarily used to counteract neurogenic atrophy,45 FES has been successfully applied to age-related sarcopenia, demonstrating that muscle contraction per se is beneficial against atrophy.46 Current trials are optimizing protocols for neuromuscular electrical stimulation in humans.47 Whether FES is sufficient to promote reinnervation has not been established yet. 44 However, very promising results have been recently reported on the use of FES to promote regeneration of a transplanted nerve and muscle functional recovery in a rat model.48 Based on all of the above, we conclude that muscle contraction, induced by physical activity or by electrical stimulation, protects motoneurons from age-related sarcopenia and that peripheral reinnervation might occur due to sprouting of slow motoneurons, ultimately preserving muscle structure and function, in elderly, exercised individuals (Figure 1).
Fig 1.
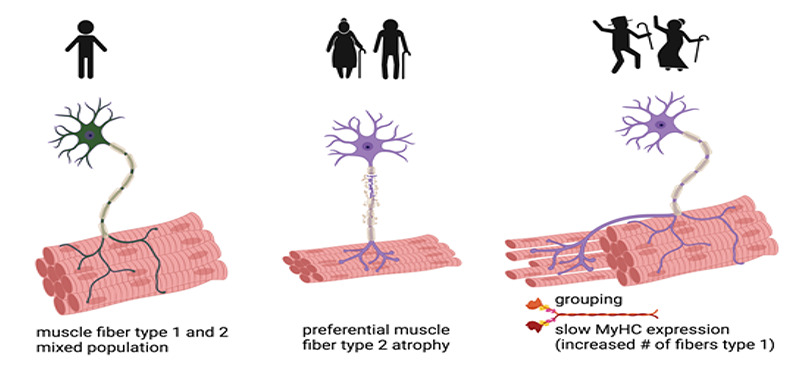
Denervation in age-related sarcopenia and effects of exercise on reinnervation. Defects in neuromuscular junctions and loss of motoneurons occurring with aging decrease the number and size of type 2 fibers and, to a lesser extent, type 1 fibers, ultimately leading to decreased muscle mass and strength (sarcopenia). Exercise favors the sprouting of surviving motor fibers which innervate the orphan muscle fibers, enlarging type 1 motor units. Repeated cycles of denervation and re-innervation eventually lead to changes in fiber-type composition, with a proportional increase in type I fibers and grouping. Created with BioRender.com
The dark side of the moon: sensory nerves and intrafusal fibers in muscle spindles
The skeletal muscle possesses nociceptors49 that are believed to mediate muscle pain via group III and IV afferent fibers. In addition, muscle has proprioceptive neurons, that innervate intrafusal fibers, in the muscle spindles, conveying length information to the central nervous system via primary type Ia and secondary type II sensory fibers.50,51 Very little is known on the effects of aging on the sensory fibers and nociceptors in the aging muscle. Nonetheless, research in this field is of the utmost importance. While being the effector system, the musculoskeletal system participate in the integration of information responsible for balance which also declines with aging involving major social and clinical consequences.52 Such decline in balance is likely due to both reduced proprioception and ability of the cortex to process sensory information,53 resulting in an increased likelihood of falling when fatigued. The relevance of leg proprioception in postural control and its age-related alterations have been notably summarized by Henry and Baudry.54 Aging alters the intrafusal fibers by increasing their number and modifying the content of intrafusal slow and developmental isoforms of MyHC.55 Interestingly, intrafusal muscle fibers undergo degeneration and regeneration cycles during aging in rodents,56 so one would expect the nervous fibers innervating the muscle spindle to also be affected by aging. While it has been reported that bone sensory fibers are spared by aging,57 how aging affects proprioception in muscle remains poorly explored. Recently, it has been shown that, similarly to what happens in the bulk muscle fiber population, proprioceptive sensory neurons degenerate prior to atrophy of the intrafusal muscle fibers with aging.58 However, not all the sensory neurons are affected by aging to the same extent: in aged rats the discharge frequency in response to muscle stretch (dynamic sensitivity) diminishes,59,60 whereas the static sensitivity does not exhibit such an effect. While the effects of exercise on position sensing, including differential effects induced by different muscle contraction types, have been investigated in detail - for a review, see Fortier 2012,61 - to the best of our knowledge, nothing is known on whether exercise has any effect on aged nociceptive or proprioceptive fibers. This issue definitely deserves further investigation.
Countermeasures: which type of exercise is more efficacious against sarcopenia?
Physical activity has been shown to decrease with age with only an estimated 28-34% of adults age 65 and older participating in any leisure time physical activity.62 In regard to current trends in age-related changes in physical activity, the COVID-19 pandemic has demonstrated that the young and elderly population have experienced the greatest decline in physical activity.63 The link between loss of muscle strength and mass is clearly dependent on physical inactivity,64 which is a modifiable risk factor that can partially reverse skeletal muscle dysfunction related to age.65 Physical activity, and more specifically resistance training, has been demonstrated to be a modality to enhance muscle function in older sarcopenic adults.66 Overall the generalized recommendation in resistance training for sarcopenia includes dynamic movements involving the facilitation of both concentric and eccentric contractions of major muscle groups.67 These movements can be accomplished by a variety of exercise modalities which include strength training, muscular endurance training, power training, and high intensity interval training. Some of the key factors that distinguish the difference between these modalities are the intensity and volume prescribed. In order for resistance training to induce a physiological response that will improve muscle mass and strength, a training load of greater than 70% of the one-repetition max (1-RM) is needed.68 The adaptations from strength training has been demonstrated to increase muscle strength and muscle mass. However, increases in muscular strength or mass may not always relate to a direct improvement in functional muscle performance.65 Worth noting, power training seems to not only elicit a positive effect on muscle mass37 but may more directly improve muscle function.69 There is also evidence of aerobic exercise, balance training and flexibility to have beneficial effects in sarcopenia70,71 and recent findings demonstrate that endurance exercise induces the appearance of hybrid fiber alterations in seniors consistent with what previously observed Mosole et al.72 Sarcopenia is a condition with multiple factors involved67,73 and aerobic exercise seems to be very efficient in eliciting a plethora of beneficial effects on the organism.74 Indeed, a major advantage of endurance exercise are the metabolic and biochemical adaptations specifically linked to this type of exercise, for instance the stimulation of the muscle endocrine activity.75–78 The combination of various modes of exercise may benefit elderly individuals, as this provides variety and allows the exercise practitioner to periodize the training program in order to improve the physiological factors associated with sarcopenia.
Aging is typically associated with burden of late-life diseases.79 In many cases, such as cachexia,2,80 sarcopenia is associated with a primary disease and is possibly exacerbated by aging in a synergistic manner. By using exercise, in principle, it is possible to target the morbidity deriving from the primary disease and the muscle wasting occurring at the same time.77,81,82 However, it is not known whether exercise promotes reinnervation in pathological conditions but still is characterized by NMJ loss in age-related sarcopenia, as it is seen in cancer cachexia.15,83,84
Conclusions
Sarcopenia is often associated to aging, likely due to undergoing mild inflammation,85 but also to more subtle phenomena, such as selective denervation of the fast fibers, ultimately accounting for pronounced muscle fiber atrophy, functional deficit and the acquisition of a slow phenotype of the musculature in elderly people. Since the publication of the seminal work by Mosole et al. republished in this issue of European Journal of Translational Myology it is evident that physical activity promotes muscle reinnervation, thus preserving not only muscle mass but also the capability of the muscle to contract. Therefore, both endurance and resistance training are recommended throughout life or at least for elderly individuals, including patients suffering from various pathologies. The recommendations for exercise should be a multimodal approach as to maximize the benefits from the physical activity.86
Acknowledgments
None.
List of acronyms
- CMAP
Compound Muscle Action Potentials
- MyHC
Myosin Heavy Chain
- N-CAM
Neuronal-Cell Adhesion Molecule
- SDH
Succinate Dehydrogenase
Funding Statement
Funding: DC is funded by Emergence SiRIC - CURAMUS 2020 Sorbonne Université and Fondi di Ateneo 2019 Sapienza University of Rome.
Contributor Information
Gilberto F Acosta, Email: gacost14@calstatela.edu.
Stefan Keslacy, Email: skeslac@calstatela.edu.
Dario Coletti, Email: ccolett@calstatela.edu.
References
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010. Jul;39(4):412-23. doi: 10.1093/ageing/afq034. Epub 2010 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berardi E, Madaro L, Lozanoska-Ochser B, Adamo S, Thorrez L, Bouche M, Coletti D. A Pound of Flesh: What Cachexia Is and What It Is Not. Diagnostics (Basel). 2021. Jan 12;11(1):116. doi: 10.3390/diagnostics11010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol Rev. 2019. Jan 1;99(1):427-511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodds RM, Roberts HC, Cooper C, Sayer AA. The Epidemiology of Sarcopenia. J Clin Densitom. 2015 Oct-Dec;18(4):461-6. doi: 10.1016/j.jocd. 2015.04.012. Epub 2015 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosole S, Rossini K, Kern H, Löfler S, Simone Fruhmann H, Vogelauer M, Burggraf S, Grim-Stieger M, Cvečka J, Hamar D, Sedliak M, Šarabon N, Pond A, Biral D, Carraro U, Zampieri S. Reinnervation of Vastus lateralis is increased significantly in seniors (70-years old) with a lifelong history of high-level exercise. Eur J Transl Myol Basic Appl Myol. 2013;23(4):205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Power GA, Dalton BH, Gilmore KJ, Allen MD, Doherty TJ, Rice CL. Maintaining Motor Units into Old Age: Running the Final Common Pathway. Eur J Transl Myol. 2017. Mar 24;27(1):6597. doi: 10.4081/ejtm.2017.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zampieri S, Mosole S, Löfler S, Fruhmann H, Burggraf S, Cvečka J, Hamar D, Sedliak M, Tirptakova V, Šarabon N, Mayr W, Kern H. Physical Exercise in Aging: Nine Weeks of Leg Press or Electrical Stimulation Training in 70 Years Old Sedentary Elderly People. Eur J Transl Myol. 2015. Aug 25;25(4):237-42. doi: 10.4081/ejtm.2015.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosole S, Carraro U, Kern H, Loefler S, Zampieri S. Use it or Lose It: Tonic Activity of Slow Motoneurons Promotes Their Survival and Preferentially Increases Slow Fiber-Type Groupings in Muscles of Old Lifelong Recreational Sportsmen. Eur J Transl Myol. 2016. Nov 25;26(4):5972. doi: 10.4081/ejtm.2016.5972. eCollection 2016 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galloza J, Castillo B, Micheo W. Benefits of Exercise in the Older Population. Phys Med Rehabil Clin N Am. 2017. Nov;28(4):659-669. doi: 10.1016/j.pmr.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011. Oct;91(4):1447-531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 11.Larsson L, Edström L, Lindegren B, Gorza L, Schiaffino S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. Am J Physiol. 1991. Jul;261(1 Pt 1):C93-101. doi: 10.1152/ ajpcell.1991.261.1.C93. [DOI] [PubMed] [Google Scholar]
- 12.Esser K, Gunning P, Hardeman E. Nerve-dependent and -independent patterns of mRNA expression in regenerating skeletal muscle. Dev Biol. 1993. Sep;159(1):173-83. doi: 10.1006/dbio.1993.1231. [DOI] [PubMed] [Google Scholar]
- 13.Coletti D, Daou N, Hassani M, Li Z, Parlakian A. Serum Response Factor in Muscle Tissues: From Development to Ageing. Eur J Transl Myol. 2016. Jun 22;26(2):6008. doi: 10.4081/ejtm.2016.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coletti D, Teodori L, Lin Z, Beranudin JF, Adamo S. Restoration versus reconstruction: cellular mechanisms of skin, nerve and muscle regeneration compared. Regen Med Res. 2013. Oct 1;1(1):4. doi: 10.1186/2050-490X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daou N, Hassani M, Matos E, De Castro GS, Costa RGF, Seelaender M, Moresi V, Rocchi M, Adamo S, Li Z, Agbulut O, Coletti D. Displaced Myonuclei in Cancer Cachexia Suggest Altered Innervation. Int J Mol Sci. 2020. Feb 6;21(3):1092. doi: 10.3390/ijms21031092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grifone R, Saquet A, Desgres M, Sangiorgi C, Gargano C, Li Z, Coletti D, Shi DL. Rbm24 displays dynamic functions required for myogenic differentiation during muscle regeneration. Sci Rep. 2021. May 3;11(1):9423. doi: 10.1038/s41598-021-88563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzotti AL, Coletti D. The Need for a Consensus on the Locution "Central Nuclei" in Striated Muscle Myopathies. Front Physiol. 2016. Nov 23;7:577. doi: 10.3389/fphys.2016.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaauw B, Schiaffino S, Reggiani C. Mechanisms modulating skeletal muscle phenotype. Compr Physiol. 2013. Oct;3(4):1645-87. doi: 10.1002/ cphy.c130009. [DOI] [PubMed] [Google Scholar]
- 19.Punkt K, Krug H, Huse J, Punkt J. Age-dependent changes of enzyme activities in the different fibre types of rat extensor digitorum longus and gastrocnemius muscles. Acta Histochem. 1993. Sep;95(1):97-110. doi: 10.1016/S0065-1281(11)80395-8. [DOI] [PubMed] [Google Scholar]
- 20.Marcucci L, Reggiani C. Increase of resting muscle stiffness, a less considered component of age-related skeletal muscle impairment. Eur J Transl Myol. 2020. Jun 17;30(2):8982. doi: 10.4081/ ejtm.2019.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018. Nov;47:123-132. doi: 10.1016/ j.arr.2018.07.005. Epub 2018 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein CS, Marsh GD, Petrella RJ, Rice CL. Muscle fiber number in the biceps brachii muscle of young and old men. Muscle Nerve. 2003. Jul;28(1):62-8. doi: 10.1002/mus.10386. [DOI] [PubMed] [Google Scholar]
- 23.Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013. May;48(5):492-8. doi: 10.1016/j.exger.2013.02.012. Epub 2013 Feb 17. [DOI] [PubMed] [Google Scholar]
- 24.Messa GAM, Piasecki M, Rittweger J, McPhee JS, Koltai E, Radak Z, Simunic B, Heinonen A, Suominen H, Korhonen MT, Degens H. Absence of an aging-related increase in fiber type grouping in athletes and non-athletes. Scand J Med Sci Sports. 2020. Nov;30(11):2057-2069. doi: 10.1111/sms. 13778. Epub 2020 Aug 28. [DOI] [PubMed] [Google Scholar]
- 25.Kelly NA, Hammond KG, Stec MJ, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve. 2018. Jan;57(1):E52-E59. doi: 10.1002/mus.25711. Epub 2017 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerkovic R, Argentini C, Serrano-Sanchez A, Cordonnier C, Schiaffino S. Early myosin switching induced by nerve activity in regenerating slow skeletal muscle. Cell Struct Funct. 1997. Feb;22(1):147-53. doi: 10.1247/csf.22.147. [DOI] [PubMed] [Google Scholar]
- 27.Murgia M, Serrano AL, Calabria E, Pallafacchina G, Lomo T, Schiaffino S. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat Cell Biol. 2000. Mar;2(3):142-7. doi: 10.1038/ 35004013. [DOI] [PubMed] [Google Scholar]
- 28.Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995. Apr;45(5):397-458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- 29.Piasecki M, Ireland A, Jones DA, McPhee JS. Age-dependent motor unit remodelling in human limb muscles. Biogerontology. 2016. Jun;17(3):485-96. doi: 10.1007/s10522-015-9627-3. Epub 2015 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demontis F, Piccirillo R, Goldberg AL, Perrimon N. Mechanisms of skeletal muscle aging: insights from Drosophila and mammalian models. Dis Model Mech. 2013. Nov;6(6):1339-52. doi: 10.1242/dmm.012559. Epub 2013 Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naro F, De Arcangelis V, Coletti D, Molinaro M, Zani B, Vassanelli S, Reggiani C, Teti A, Adamo S. Increase in cytosolic Ca2+ induced by elevation of extracellular Ca2+ in skeletal myogenic cells. Am J Physiol Cell Physiol. 2003. Apr;284(4):C969-76. doi: 10.1152/ajpcell.00237.2002. Epub 2002 Dec 18. [DOI] [PubMed] [Google Scholar]
- 32.De Arcangelis V, Coletti D, Canato M, Molinaro M, Adamo S, Reggiani C, Naro F. Hypertrophy and transcriptional regulation induced in myogenic cell line L6-C5 by an increase of extracellular calcium. J Cell Physiol. 2005. Mar;202(3):787-95. doi: 10.1002/jcp.20174. [DOI] [PubMed] [Google Scholar]
- 33.Lamboley CR, Wyckelsma VL, McKenna MJ, Murphy RM, Lamb GD. Ca(2+) leakage out of the sarcoplasmic reticulum is increased in type I skeletal muscle fibres in aged humans. J Physiol. 2016. Jan 15;594(2):469-81. doi: 10.1113/JP271382. Epub 2015 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westerterp KR. Daily physical activity and ageing. Curr Opin Clin Nutr Metab Care. 2000. Nov;3(6):485-8. doi: 10.1097/00075197-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Meijer EP, Westerterp KR, Verstappen FT. Effect of exercise training on physical activity and substrate utilization in the elderly. Int J Sports Med. 2000. Oct;21(7):499-504. doi: 10.1055/s-2000-7419. [DOI] [PubMed] [Google Scholar]
- 36.Mosole S, Carraro U, Kern H, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, Mayr W, Krenn M, Paternostro-Sluga T, Hamar D, Cvecka J, Sedliak M, Tirpakova V, Sarabon N, Musarò A, Sandri M, Protasi F, Nori A, Pond A, Zampieri S. Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol. 2014. Apr;73(4):284-94. doi: 10.1097/NEN.0000000000 000032. [DOI] [PubMed] [Google Scholar]
- 37.Drey M, Sieber CC, Degens H, McPhee J, Korhonen MT, Müller K, Ganse B, Rittweger J. Relation between muscle mass, motor units and type of training in master athletes. Clin Physiol Funct Imaging. 2016. Jan;36(1):70-6. doi: 10.1111/cpf.12195. Epub 2014 Oct 24. [DOI] [PubMed] [Google Scholar]
- 38.Bechshøft RL, Malmgaard-Clausen NM, Gliese B, Beyer N, Mackey AL, Andersen JL, Kjær M, Holm L. Improved skeletal muscle mass and strength after heavy strength training in very old individuals. Exp Gerontol. 2017. Jun;92:96-105. doi: 10.1016/j. exger.2017.03.014. Epub 2017 Mar 28. [DOI] [PubMed] [Google Scholar]
- 39.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018. Sep;14(9):513-537. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messi ML, Li T, Wang ZM, Marsh AP, Nicklas B, Delbono O. Resistance Training Enhances Skeletal Muscle Innervation Without Modifying the Number of Satellite Cells or their Myofiber Association in Obese Older Adults. J Gerontol A Biol Sci Med Sci. 2016. Oct;71(10):1273-80. doi: 10.1093/gerona/glv 176. Epub 2015 Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kletzien H, Russell JA, Connor NP. The effects of treadmill running on aging laryngeal muscle structure. Laryngoscope. 2016. Mar;126(3):672-7. doi: 10.1002/lary.25520. Epub 2015 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bathgate KE, Bagley JR, Jo E, Talmadge RJ, Tobias IS, Brown LE, Coburn JW, Arevalo JA, Segal NL, Galpin AJ. Muscle health and performance in monozygotic twins with 30 years of discordant exercise habits. Eur J Appl Physiol. 2018. Oct;118(10):2097-2110. doi: 10.1007/s00421-018-3943-7. Epub 2018 Jul 14. [DOI] [PubMed] [Google Scholar]
- 43.Carraro U, Kern H, Gava P, Hofer C, Loefler S, Gargiulo P, Mosole S, Zampieri S, Gobbo V, Ravara B, Piccione F, Marcante A, Baba A, Schils S, Pond A, Gava F. Biology of Muscle Atrophy and of its Recovery by FES in Aging and Mobility Impairments: Roots and By-Products. Eur J Transl Myol. 2015. Aug 25;25(4):221-30. doi: 10.4081/ ejtm.2015.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carraro U, Kern H, Gava P, Hofer C, Loefler S, Gargiulo P, Edmunds K, Árnadóttir ÍD, Zampieri S, Ravara B, Gava F, Nori A, Gobbo V, Masiero S, Marcante A, Baba A, Piccione F, Schils S, Pond A, Mosole S. Recovery from muscle weakness by exercise and FES: lessons from Masters, active or sedentary seniors and SCI patients. Aging Clin Exp Res. 2017. Aug;29(4):579-590. doi: 10.1007/s40520-016-0619-1. Epub 2016 Sep 3. [DOI] [PubMed] [Google Scholar]
- 45.Carraro U, Coletti D, Kern H. The Ejtm Specials "The Long-Term Denervated Muscle". Eur J Transl Myol. 2014. Mar 27;24(1):3292. doi: 10.4081/ejtm.2014.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kern H, Hofer C, Loefler S, Zampieri S, Gargiulo P, Baba A, Marcante A, Piccione F, Pond A, Carraro U. Atrophy, ultra-structural disorders, severe atrophy and degeneration of denervated human muscle in SCI and Aging. Implications for their recovery by Functional Electrical Stimulation, updated 2017. Neurol Res. 2017. Jul;39(7):660-666. doi: 10.1080/01616412.2017.1314906. Epub 2017 Apr 13. [DOI] [PubMed] [Google Scholar]
- 47.Kern H, Hofer C, Loefler S, Zampieri S, Gargiulo P, Baba A, Marcante A, Piccione F, Pond A, Carraro U. Atrophy, ultra-structural disorders, severe atrophy and degeneration of denervated human muscle in SCI and Aging. Implications for their recovery by Functional Electrical Stimulation, updated 2017. Neurol Res. 2017. Jul;39(7):660-666. doi: 10.1080/01616412.2017.1314906. Epub 2017 Apr 13. [DOI] [PubMed] [Google Scholar]
- 48.Wang P, Li Y, Zhang Z, Lin Y, Jiang Z, Ding X, Yang L. Effects of functional electrical stimulation on neuromuscular function after targeted muscle reinnervation surgery in rats. Annu Int Conf IEEE Eng Med Biol Soc. 2020. Jul;2020:3823-3826. doi: 10.1109/EMBC44109.2020.9175836. [DOI] [PubMed] [Google Scholar]
- 49.Mense S. Muscle Nociceptors, Neurochemistry. In: Gebhart GF, Schmidt RF, editors. Encyclopedia of Pain. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. p. 1944–50. doi:10.1007/978-3-642-28753-4_2529. [Google Scholar]
- 50.Giuriati W, Ravara B, Porzionato A, Albertin G, Stecco C, Macchi V, De Caro R, Martinello T, Gomiero C, Patruno M, Coletti D, Zampieri S, Nori A. Muscle spindles of the rat sternomastoid muscle. Eur J Transl Myol. 2018. Dec 13;28(4):7904. doi: 10.4081/ejtm.2018.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravara B, Gobbo V, Incendi D, Porzionato A, Macchi V, Caro R, Coletti D, Martinello T, Patruno M. Revisiting the peculiar regional distribution of muscle fiber types in rat Sternomastoid Muscle. Eur J Transl Myol. 2018. Mar 1;28(1):7302. doi: 10.4081/ejtm.2018.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konrad HR, Girardi M, Helfert R. Balance and aging. Laryngoscope. 1999. Sep;109(9):1454-60. doi: 10.1097/00005537-199909000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Piitulainen H, Seipäjärvi S, Avela J, Parviainen T, Walker S. Cortical Proprioceptive Processing Is Altered by Aging. Front Aging Neurosci. 2018. Jun 14;10:147. doi: 10.3389/fnagi.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henry M, Baudry S. Age-related changes in leg proprioception: implications for postural control. J Neurophysiol. 2019. Aug 1;122(2):525-538. doi: 10.1152/jn.00067.2019. Epub 2019 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosant C, Nagel MD, Pérot C. Aging affects passive stiffness and spindle function of the rat soleus muscle. Exp Gerontol. 2007. Apr;42(4):301-8. doi: 10.1016/j.exger.2006.10.007. Epub 2006 Nov 21. [DOI] [PubMed] [Google Scholar]
- 56.Desaki J, Nishida N. A further observation of muscle spindles in the extensor digitorum longus muscle of the aged rat. J Electron Microsc (Tokyo). 2010;59(1):79-86. doi: 10.1093/jmicro/dfp038. Epub 2009 Jul 31. PMID: 19648233. [DOI] [PubMed] [Google Scholar]
- 57.Chartier SR, Mitchell SAT, Majuta LA, Mantyh PW. The Changing Sensory and Sympathetic Innervation of the Young, Adult and Aging Mouse Femur. Neuroscience. 2018. Sep 1;387:178-190. doi: 10.1016/j.neuroscience.2018.01.047. Epub 2018 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaughan SK, Stanley OL, Valdez G. Impact of Aging on Proprioceptive Sensory Neurons and Intrafusal Muscle Fibers in Mice. J Gerontol A Biol Sci Med Sci. 2017. Jun 1;72(6):771-779. doi: 10.1093/gerona/glw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miwa T, Miwa Y, Kanda K. Dynamic and static sensitivities of muscle spindle primary endings in aged rats to ramp stretch. Neurosci Lett. 1995. Dec 8;201(2):179-82. doi: 10.1016/0304-3940(95) 12165-x. [DOI] [PubMed] [Google Scholar]
- 60.Kim GH, Suzuki S, Kanda K. Age-related physiological and morphological changes of muscle spindles in rats. J Physiol. 2007. Jul 15;582(Pt 2):525-38. doi: 10.1113/jphysiol.2007.130120. Epub 2007 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fortier S, Basset FA. The effects of exercise on limb proprioceptive signals. J Electromyogr Kinesiol. 2012. Dec;22(6):795-802. doi: 10.1016/j.jelekin.2012.04.001. Epub 2012 May 11. [DOI] [PubMed] [Google Scholar]
- 62.Nascimento CM, Ingles M, Salvador-Pascual A, Cominetti MR, Gomez-Cabrera MC, Viña J. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. 2019. Feb 20;132:42-49. doi: 10.1016/j.freeradbiomed.2018.08.035. Epub 2018 Aug 31. [DOI] [PubMed] [Google Scholar]
- 63.Wilke J Mohr L Tenforde AS Edouard P Fossati C González-Gross M Sánchez Ramírez C Laiño F Tan B Pillay JD Pigozzi F Jimenez-Pavon D Novak B Jaunig J Zhang M van Poppel M Heidt C Willwacher S Yuki G Lieberman DE Vogt L Verhagen E Hespanhol L Hollander K.. A Pandemic within the Pandemic? Physical Activity Levels Substantially Decreased in Countries Affected by COVID-19. Int J Environ Res Public Health. 2021. Feb 24;18(5):2235. doi: 10.3390 /ijerph18052235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Denison HJ, Cooper C, Sayer AA, Robinson SM. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging. 2015. May 11;10:859-69. doi: 10.2147/CIA.S55842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phu S, Boersma D, Duque G. Exercise and Sarcopenia. J Clin Densitom. 2015. Oct-Dec;18(4):488-92. doi: 10.1016/j.jocd.2015.04. 011. Epub 2015 Jun 10. [DOI] [PubMed] [Google Scholar]
- 66.Papadopoulou SK. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients. 2020. May 1;12(5):1293. doi: 10.3390/nu 12051293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giallauria F, Cittadini A, Smart NA, Vigorito C. Resistance training and sarcopenia. Monaldi Arch Chest Dis. 2016. Jun 22;84(1-2):738. doi: 10.4081/monaldi.2015.738. [DOI] [PubMed] [Google Scholar]
- 68.Lichtenberg T, von Stengel S, Sieber C, Kemmler W. The Favorable Effects of a High-Intensity Resistance Training on Sarcopenia in Older Community-Dwelling Men with Osteosarcopenia: The Randomized Controlled FrOST Study. Clin Interv Aging. 2019. Dec 16;14:2173-2186. doi: 10.2147/CIA.S225618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freiberger E, Sieber C, Pfeifer K. Physical activity, exercise, and sarcopenia - future challenges. Wien Med Wochenschr. 2011. Sep;161(17-18):416-25. doi: 10.1007/s10354-011-0001-z. Epub 2011 Jul 29 [DOI] [PubMed] [Google Scholar]
- 70.Carraro U. Thirty years of translational research in Mobility Medicine: Collection of abstracts of the 2020 Padua Muscle Days. Eur J Transl Myol. 2020. Apr 1;30(1):8826. doi: 10.4081/ejtm.2019.8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furst T, Massaro A, Miller C, Williams BT, LaMacchia ZM, Horvath PJ. β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J Int Soc Sports Nutr. 2018. Jul 11;15(1):32. doi: 10.1186/s12970-018-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moreillon M, Conde Alonso S, Broskey NT, Greggio C, Besson C, Rousson V, Amati F. Hybrid fiber alterations in exercising seniors suggest contribution to fast-to-slow muscle fiber shift. J Cachexia Sarcopenia Muscle. 2019. Jun;10(3):687-695. doi: 10.1002/jcsm.12410. Epub 2019 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia M, Seelaender M, Sotiropoulos A, Coletti D, Lancha AH. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition. 2019. Apr;60:66-69. doi: 10.1016/j.nut.2018.09.031. Epub 2018 Oct 7. [DOI] [PubMed] [Google Scholar]
- 74.Barbieri E, Agostini D, Polidori E, Potenza L, Guescini M, Lucertini F, Annibalini G, Stocchi L, De Santi M, Stocchi V. The pleiotropic effect of physical exercise on mitochondrial dynamics in aging skeletal muscle. Oxid Med Cell Longev. 2015;2015:917085. doi: 10.1155/2015/917085. Epub 2015 Apr 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Felice V, Coletti D, Seelaender M. Editorial: Myokines, Adipokines, Cytokines in Muscle Pathophysiology. Front Physiol. 2020. Oct 23;11:592856. doi: 10.3389/fphys.2020.592856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coletti D, Aulino P, Pigna E, Barteri F, Moresi V, Annibali D, Adamo S, Berardi E. Spontaneous Physical Activity Downregulates Pax7 in Cancer Cachexia. Stem Cells Int. 2016; 2016: 6729268. Published online 2015 Dec 20. doi: 10.1155/ 2016/6729268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alves de Lima E Jr, Teixeira AAS, Biondo LA, Diniz TA, Silveira LS, Coletti D, Busquets Rius S, Rosa Neto JC. Exercise Reduces the Resumption of Tumor Growth and Proteolytic Pathways in the Skeletal Muscle of Mice Following Chemotherapy. Cancers (Basel). 2020. Nov 20;12(11):3466. doi: 10.3390/cancers12113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Consitt LA, Dudley C, Saxena G. Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adults. Nutrients. 2019. Nov 3;11(11):2636. doi: 10.3390/nu11112636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018. Sep;561(7721):45-56. doi: 10.1038/s41586-018-0457-8. Epub 2018 Sep 5. [DOI] [PubMed] [Google Scholar]
- 80 de Castro GS, Simoes E, Lima JDCC, Ortiz-Silva M, Festuccia WT, Tokeshi F, Alcântara PS, Otoch JP, Coletti D, Seelaender M. Human Cachexia Induces Changes in Mitochondria, Autophagy and Apoptosis in the Skeletal Muscle. Cancers (Basel). 2019. Aug 28;11(9):1264. doi: 10.3390/cancers 11091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pigna E, Berardi E, Aulino P, Rizzuto E, Zampieri S, Carraro U, Kern H, Merigliano S, Gruppo M, Mericskay M, Li Z, Rocchi M, Barone R, Macaluso F, Di Felice V, Adamo S, Coletti D, Moresi V. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci Rep. 2016. May 31;6:26991. doi: 10.1038/srep26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brunjes DL, Kennel PJ, Christian Schulze P. Exercise capacity, physical activity, and morbidity. Heart Fail Rev. 2017. Mar;22(2):133-139. doi: 10.1007/s10741-016-9592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sartori R, Hagg A, Zampieri S, Armani A, Winbanks CE, Viana LR, Haidar M, Watt KI, Qian H, Pezzini C, Zanganeh P, Turner BJ, Larsson A, Zanchettin G, Pierobon ES, Moletta L, Valmasoni M, Ponzoni A, Attar S, Da Dalt G, Sperti C, Kustermann M, Thomson RE, Larsson L, Loveland KL, Costelli P, Megighian A, Merigliano S, Penna F, Gregorevic P, Sandri M. Perturbed BMP signaling and denervation promote muscle wasting in cancer cachexia. Sci Transl Med. 2021. Aug 4;13(605):eaay9592. doi: 10.1126/scitranslmed.aay 9592. [DOI] [PubMed] [Google Scholar]
- 84.Huot JR, Pin F, Bonetto A. Muscle weakness caused by cancer and chemotherapy is associated with loss of motor unit connectivity. Am J Cancer Res. 2021. Jun 15;11(6):2990-3001 [PMC free article] [PubMed] [Google Scholar]
- 85.Bouché M, Muñoz-Cánoves P, Rossi F, Coletti D. Inflammation in muscle repair, aging, and myopathies. Biomed Res Int. 2014;2014:821950. doi: 10.1155/2014/821950. Epub 2014 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beckwée D, Delaere A, Aelbrecht S, Baert V, Beaudart C, Bruyere O, de Saint-Hubert M, Bautmans I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J Nutr Health Aging. 2019;23(6):494-502. doi: 10.1007/s12603-019-1196-8. [DOI] [PubMed] [Google Scholar]
- 87.Racca AW, Beck AE, Rao VS, Flint GV, Lundy SD, Born DE, Bamshad MJ, Regnier M. Contractility and kinetics of human fetal and human adult skeletal muscle. J Physiol. 2013. Jun 15;591(12):3049-61. doi: 10.1113/jphysiol.2013.252650. Epub 2013 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wall CE, Holmes M, Soderblom EJ, Taylor AB. Proteomics and immunohistochemistry identify the expression of α-cardiac myosin heavy chain in the jaw-closing muscles of sooty mangabeys (order Primates). Arch Oral Biol. 2018. Jul;91:103-108. doi: 10.1016/j.archoralbio.2018.01.019. Epub 2018 Feb 3. [DOI] [PubMed] [Google Scholar]
- 89.Briggs MM, Schachat F. The superfast extraocular myosin (MYH13) is localized to the innervation zone in both the global and orbital layers of rabbit extraocular muscle. J Exp Biol. 2002. Oct;205(Pt 20):3133-42. doi: 10.1242/jeb.205.20.3133. [DOI] [PubMed] [Google Scholar]
- 90.Rossi AC, Mammucari C, Argentini C, Reggiani C, Schiaffino S. Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol. 2010. Jan 15;588(Pt 2):353-64. doi: 10.1113/jphysiol.2009.181008. Epub 2009 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mosole S, Rossini K, Kern H, Löfler S, Simone Fruhmann H, Vogelauer M, Burggraf S, Grim-Stieger M, Cvečka J, Hamar D, Sedliak M, Šarabon N, Pond A, Biral D, Carraro U, Zampieri S. Reinnervation of Vastus lateralis is increased significantly in seniors (70-years old) with a lifelong history of high-level exercise (2013, revisited here in 2022). Eur J Transl Myol. 32(1): 10420, 2022. doi: 10.4081/ejtm.2022.10420. [DOI] [PMC free article] [PubMed] [Google Scholar]


