Abstract
Background:
Metformin improves insulin action, but feasibility in treating low milk supply is unknown.
Research aim:
To determine the feasibility of a metformin-versus-placebo definitive randomized clinical trial in women with low milk production and signs of insulin resistance.
Methods:
Pilot trial criteria included: Mother 1–8 weeks postpartum (ideally 1–2 weeks), low milk production, and ≥1 insulin resistance sign; and singleton, healthy, term infant. Eligible mothers were randomly assigned 2:1 (metformin:placebo) and instructed in frequent milk removal for 28 days with option to stop at 14 days.
Results:
From 02/2015 through 06/2016, we screened 114 women, completed baseline assessments on 46, and trialed 15 (median, 36 days postpartum). Comparing metformin-assigned (n = 10) to placebo (n = 5), 70% versus 80% continued to day 28; peak median change in milk output was +8 versus −58 mL/24 hr (p = .31) and 80% peaked at Day 14 for both groups; 0% versus 20% desired to continue assigned drug after study completion; 44% versus 0% reported nausea/vomiting. Post-hoc, median peak change in milk output was +22 (metformin completers, n = 8) versus −58 mL/24 hr (placebo + non-completers, n = 7, p = .07). At baseline assessment, median milk production was significantly lower in those with (n = 31), versus those without (n = 15) signs of insulin resistance (p = .002).
Conclusions:
Although results trend toward hypothesized direction, trial feasibility concerns include late enrollment and only 20% of metformin-assigned participants sustaining improved milk output to Day 28, with none perceiving metformin worthwhile. Better tools are needed to identify and treat metabolically-driven low milk production. Registered at ClinicalTrials.gov (NCT02179788) on 02/JUL/2014.
Keywords: Breastfeeding, human milk production, insufficient milk, lactation, maternal health Randomized Controlled Trials
Background
Maternal obesity is consistently associated with delayed lactogenesis (Chapman & Perez-Escamilla, 1999; Nommsen-Rivers, Chantry, Peerson, Cohen & Dewey, 2010) and shortened duration of breastfeeding (Baker, Michaelsen, Sorensen & Rasmussen, 2007; Li, Jewell, & Grummer-Strawn, 2003; Winkvist, et al., 2015). Recently, investigators elucidated that the explanatory factor, ‘insufficient milk’ significantly mediated the association between maternal obesity and lower prevalence of exclusive breastfeeding among participants in the Infant Feeding Practices II study (O’Sullivan, Perinne & Rasmussen, 2015). A physiologic hallmark of obesity is insulin resistance (Biddinger & Kahn, 2006). While it was previously believed that insulin did not play a direct role in mammary gland function (Neville, McFadden & Forsyth, 2002), beginning in 2009 a progression of animal (Berlato & Doppler, 2009; Menzies, Lefevre, Macmillan & Nicholas, 2009; Neville et al., 2013) and human (Lemay et al., 2013) studies have revealed the mammary gland to be differentially sensitive to insulin during pregnancy and lactation. A 2016 review summarizes the emerging clinical research corroborating the importance of healthy insulin action in lactation (Nommsen-Rivers, 2016). Recently published studies further support the importance of healthy insulin action in lactation. In a multi-ethnic cohort of 616 women receiving prenatal care in Norway, 190 women with diabetes during pregnancy ended predominant breastfeeding significantly earlier than 426 women without diabetes in pregnancy, even after adjustment for sociodemographic, obstetric, and newborn variables (Baerug et al., 2018). In a secondary analysis of metabolic data from a cohort of U.S. women with diabetes during pregnancy, it was reported that women with several markers of poor metabolic health were significantly more likely to report early, undesired weaning (Glover, Berry, Schwartz & Stuebe, 2018).
Thus, we hypothesize that an intervention targeting insulin action may be effective in improving milk production in women with insufficient milk output despite regular breast emptying. Interestingly, the herbs fenugreek and goat’s rue are traditionally used as galactagogues (Mortel & Mehta, 2013) and both are also rich in insulin-sensitizing biguanides (Perla & Jayanty, 2013). Metformin is a biguanide that was originally derived from goat’s rue (Bailey & Day, 2004). Metformin’s mechanism of action is not completely understood, but it is believed to increase glucose uptake in the liver, improve insulin sensitivity in target tissues, and improve beta cell function in the pancreas (Bailey, 2017; Yang, Xu, Zhang, Cai & Zhang, 2017). Metformin is a first-line, FDA-approved drug for treating Type 2 diabetes (American Diabetes Association, 2012) and is safely used to improve insulin action in women with polycystic ovary syndrome (PCOS) (Domecq et al., 2013; Tang, Lord, Norman, Yasmin & Balen, 2012) and prediabetes (Knowler et al., 2002).
Metformin is considered compatible with lactation, with milk concentrations ranging from 0.1 to 0.4 mg/L in mothers taking 1.5–2.0 g/d (dose to infant <0.5% of mother’s weight-adjusted dosage), (National Institutes of Health, 2015) and undetectable (<0.005 mg/L) to very low detection of metformin (<0.08 mg/L) in the serum of breastfed infants (National Institutes of Health, 2015). One large prospective study found no adverse events or difference in growth or development in the breastfed (n = 50) as compared to formula-fed (n = 61) offspring of mothers taking metformin over the first 6 months postpartum (Glueck, Salehi, Sieve & Wang, 2006). Lactic acidosis is the most concerning side effect of metformin. However, in a review of 18 clinical studies of metformin treatment for PCOS, no reports of lactic acidosis occurred over a combined 482 (non-pregnant) patient-years of exposure, supporting the safety of metformin use in non-diabetic women of reproductive age (Domecq et al., 2013).
Even though metformin is considered safe for use during lactation, its feasibility and acceptability in improving milk production is not known. Given its known ability to improve insulin action and its favorable safety profile, we conducted a pilot RCT of metformin to improve milk production in women with signs of insulin resistance and who were experiencing insufficient milk production despite regular breast emptying. Specifically, our objectives were to: (1) describe acceptability, feasibility, tolerance, and safety of testing metformin versus placebo in lactating mothers and their infants; (2) obtain variance estimates and test for a trend (p < .10) toward greater improvement in milk output in the metformin group; and (3) examine the relation between signs of insulin resistance and milk output among all women who underwent baseline eligibility assessment.
Methods
Design
The study was designed to be a pilot RCT. The FDA (IND #119134) and the Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board (IRB) reviewed and approved the study protocol and amendments. The University of Cincinnati agreed for CCHMC to be the IRB of record. The study is registered at clinicaltrials.gov (trial number NCT02179788). A Data and Safety Monitoring Board was assembled to review the study protocol prior to initiating recruitment and to periodically review study progress and results thereafter.
Setting
Between February 2015 and June 2016, we asked local inpatient and outpatient lactation support providers to refer mothers who desired to exclusively breastfeed and who appeared to be managing breastfeeding well, but were struggling with insufficient milk production without an apparent cause.
Sample
We screened mothers via telephone for these initial inclusion criteria: 1–8 weeks postpartum (but ideally 1–2 weeks postpartum), ≥20 years, and a healthy singleton infant ≥ 37 weeks gestation; and exclusion criteria: insufficient frequency of breastfeeding and/or breast pumping, unwilling to continue frequent breast emptying for 2–4 weeks, living outside the catchment area, lack of established pediatric care for infant, history of breast surgery, maternal Type 1 or Type 2 diabetes, current nipple or breast infection, and current metformin use.
We screened 114 women over the 18 months of recruitment. Of the 51 who met telephone screening criteria, 46 completed baseline measurements and 31 of these women exhibited at least one sign of insulin resistance, of which 15 were RCT-eligible and randomized to metformin (n = 10) or placebo (n = 5; Figure 1). RCT-enrolled participants were referred to the study from hospital-affiliated breastfeeding clinic IBCLCs (n = 9), pediatric office IBCLCs (n = 3), home visiting maternity nurses (n = 2), and a private practice IBCLC (n = 1).
Figure 1.
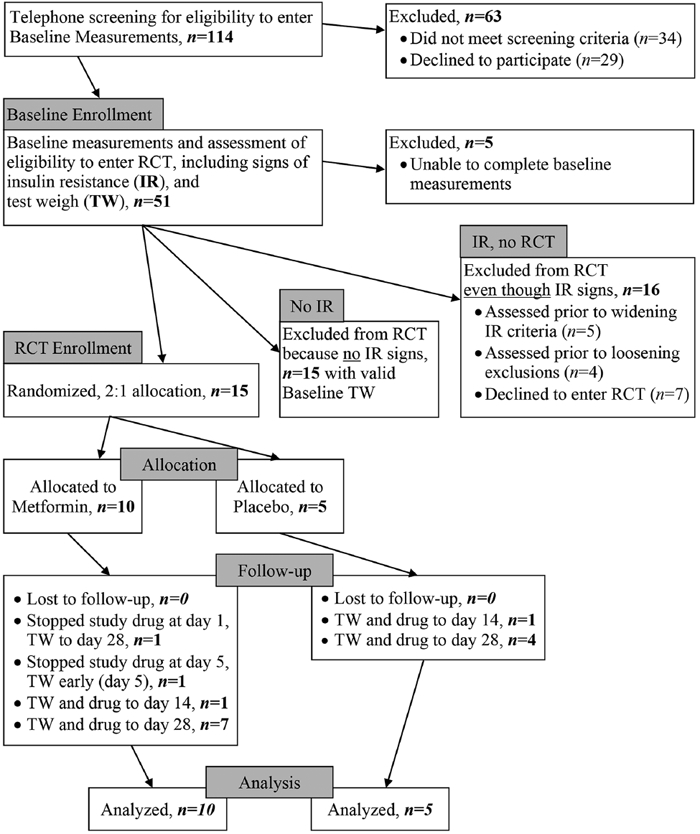
Participant Flow.
Sample size estimation was based on detecting greater improvement in milk production in the metformin group versus placebo at a pilot study level of significance (p < .10). We assumed baseline 24-hr milk production to be ~300 ± 120 mL (normal intake, ~750 mL/day) and hypothesized an average post-intervention increase of ≥90 mL (30%) versus 0 mL (0%) in metformin versus placebo. Accordingly, n = 20 metformin and n = 10 placebo would achieve 79% power to detect (Volume change from baseline) metformin - (Volume change from baseline) placebo ≥90, with a significance level of 0.10 using a two-sided t-test. We estimated that at least 50% of those who underwent baseline assessment would meet the pilot trial eligibility criteria and thus we budgeted for assessing 60 lactating women at baseline over 18 months of recruitment.
Measurements
We measured breast milk production by having participants weigh their infant on a specialized scale (Tanita BD-815U, provided) immediately before and after feeding on each breast (test-weighs) and weighing all milk expressed over 24 continuous hours (Dewey, Lovelady, Nommsen-Rivers, McCrory & Lonnerdal, 1994). LNR observed each participant conducting practice test weighs during home visits, and continued to work with each participant until she demonstrated competency in the technique. We contacted each participant during the test-weighing to answer questions, confirm valid results, and troubleshoot implausible results. If necessary, mothers restarted the test-weigh after correcting their test-weighing technique. We reviewed the test-weigh record within 1–3 days of completion and considered the record invalid if any of the following a priori criteria were met: <20 hr of continuous measurement, >200 g output from a single breast feeding or pumping without explanation, >5 g weight loss after breastfeeding or breast pumping without explanation, or fabricated data. Regarding the latter, the study scales display results in 2 g increments (weights <6,000 g) or 5 g increments (weights >6,000 g); thus, recordings falling outside these parameters were considered erroneous. Participants were asked to repeat the test-weigh if we detected invalid results.
Trained anthropometrists from the Schubert Research Clinic Bionutrition Core measured maternal midsection circumferences, height, and weight; and infant length, weight, and head circumference. All measurements were performed in duplicate and repeated when a preset tolerance was exceeded. A research nurse measured maternal vital signs (body temperature, pulse rate, blood pressure and respiration rate) according to a defined research protocol.
Fasting blood samples were immediately placed on ice, processed into plasma, and sent to the clinical lab for STAT measurement of plasma glucose (in duplicate), lipids, and creatinine via automated colorimetric analyses (Roche 311).
Data Collection
Baseline Assessment.
Women meeting initial baseline eligibility criteria via telephone screening underwent baseline evaluation, including at-home assessments of breastfeeding/lactation and clinical assessment of metabolic health as part of RCT eligibility screening.
During the home visit, LNR, who is an International Board-Certified Lactation Consultant (IBCLC), administered informed consent and recorded participants’ reports of their low milk production history, presence of other breastfeeding concerns, and current approach to improving milk output. During the home visit, when prompted by the infant’s display of feeding cues, LNR evaluated a breastfeeding session, which included observation of infant breastfeeding behavior, maternal comfort, amount of milk transferred during the feeding, amount of milk that could be expressed via breast pumping after the breastfeeding session, and nipple or infant oral cavity concerns. During the same home visit, LNR trained the participant to measure her 24-hr milk production.
During the same home visit LNR provided the participant with a hospital-grade breast pump and properly fitted breast flange (Ameda Platinum, Ameda, Inc., Buffalo Grove, Illinois), and counseled her in the standard approach to increasing milk output (Academy of Breastfeeding Medicine, 2009). LNR worked with each participant to ensure competency in thorough breast emptying via breastfeeding, breast pumping, and hands-on pumping. Hands-on pumping was also advised during breastfeeding if it helped to keep the infant feeding at the breast (Morton, et al., 2009). LNR tailored emphasis on frequent breastfeeding, or breast pumping, or both, based on how well the infant drained the breast, and encouraged participants to begin the standard protocol right away, as it would not be ethical to delay standard care pending baseline data collection.
Within one week of completing the home visits, each participant came to the Schubert Research Clinic for morning collection of fasting blood samples, anthropometry, and measurement of vital signs. Research staff obtained demographics, medical history, medication history and current use, and breastfeeding history. The medical history provided a baseline for the evaluation of adverse events during follow-up.
RCT-eligibility confirmation.
The study obstetrician (AT) reviewed baseline data and, in consultation with LNR and the pediatric MD/IBCLC co-investigators (SR and LPW), evaluated participants for RCT eligibility. The review assured that: (1) the participant successfully completed a valid 24-hr test weigh; (2) the test weigh demonstrated a deficit in production compared to infant milk intake; (3) the test weigh verified that breast emptying occurred ≥ 6 times per breast per 24 hr; (4) the home visit summary, medical history, medication record, and clinical labs did not reveal an explanation for the low milk supply (e.g., inadequate milk transfer during breastfeeding, poor management of breastfeeding, disruption to regular breast emptying since giving birth, serious maternal illness, or ingestion of medication known to impair lactation such as estrogens or pseudoephedrine); (5) the participant agreed to continue with frequent breast emptying for at least another two weeks and agreed to maintain a consistent dose of any herbal supplements that were consumed during baseline milk output measurements; (6) baseline data did not reveal any contraindication to taking metformin; and (7) at least one sign of insulin resistance was present.
Eligibility criteria were expanded after the first 6 months of recruitment. Initially, our sole criterion for establishing evidence of insulin resistance was elevated fasting plasma glucose (≥95 to <126.0 mg/d (Knowler et al., 2002)), and we initially excluded all participants with abnormal liver or kidney function tests (aspartate aminotransferase >37 U/L, alanine aminotransferase >78 U/L, or bilirubin >1.1 mg/dL for liver function; estimated Glomerular Filtration Rate < 60 mL/min for kidney function). It quickly became apparent that these criteria were excessively narrow and would limit generalizability of a definitive RCT. Many potential participants with otherwise strong signs of insulin resistance may not exhibit elevated plasma glucose, and individuals with signs of insulin resistance are also likely to have some degree of fatty liver/elevated liver enzymes. Given that eligibility was determined before randomization, expanding eligibility did not bias study drug allocation but did expand generalizability, enabling us to leverage more insight from this feasibility trial. After the first 6 months of recruitment we obtained IRB approval to expand the insulin resistance criteria to include gestational diabetes (Catalano, Kirwan, Haugel-de Mouzon & King, 2003), PCOS (Tang, Lord, Norman, Yasmin & Balen, 2012), or central obesity (waist circumference ≥90 cm and BMI ≥30 kg/m2, Alberti et al., 2009). After the first year of recruitment we obtained IRB approval and FDA clearance to revise liver and kidney function exclusions to align with National Institutes of Health-funded trials using metformin (aspartate aminotransferase <74 U/L, alanine aminotransferase <156 U/L, serum creatinine <1.4 mg/dL, Knowler et al., 2002).
Study drug.
RCT-eligible participants were given an investigational prescription for metformin or placebo that was prepared by our investigational pharmacy according to a computer-generated randomized block scheme with 2:1 metformin:placebo allocation. This ratio enabled greater insights into tolerance and safety of metformin. The statistician who generated the allocation scheme and the Investigational Pharmacy were the only members of the study team not masked to allocation until RCT data analysis was complete.
The pharmacy encapsulated Glucophage XR (Bristol Myers Squibb) and placebo (methylcellulose USP Powder supplied by PCCA, Houston TX) in matching #00 opaque capsules and packaged into bottles labeled “Glucophage or placebo” with these titration instructions:
Days 1–7, one 750 mg capsule with evening meal (750 mg/day)
Days 8–14, three 500 mg capsules with evening meal (1500 mg/day)
Days 14–28 (or to study end), four 500 mg capsules with evening meal (2000 mg/day)
The above titration schedule is steeper than the manufacturer’s recommendation in the first 2 weeks of treatment, which is to increase in 500 mg increments/week, but the final dose used in the study (2000 mg/day) is in alignment with the manufacturer’s recommendation. We elected for the steeper increase in the first 2 weeks because this was viewed as less burdensome to participants than prolonging the time period that they would need to follow the protocol while waiting for the drug dose to reach the minimum effective dose of 1500 mg/day (Bristol Myers Squibb, 2008). Participants were instructed to call us if they were experiencing difficulty with perceived side effects so that dosing adjustments could be made. We also issued a daily multivitamin supplement given metformin’s possible effects on nutrient absorption (Gatford et al., 2013; Xu et al., 2013).
Follow-up procedures in RCT-enrolled.
RCT-enrolled participants maintained a daily log of study drug and other medications, symptoms experienced (participant and her infant), and breastfeeding/breast expression frequency. We contacted participants at Days 7, 14, 21, and 28 (+/− 3 days) to review the study log, obtain a pill count, document naked infant weight (from maternal or staff measurement), record symptoms and adverse events, and record details regarding breastfeeding and breast expression (frequency, problems, and concerns).
At the Day 14 and Day 28 encounters, instructions for completing the 24-hr test weigh were reviewed. Participants were given the option of ending the study after the day 14 measurements (if they decided to not continue with regular breast emptying for another two weeks) or continuing to day 28.
The trial ended with a final visit to the Schubert Research Clinic after the Day 28 test weigh (or after the Day 14 test weigh, for those who elected to end the study early), where we repeated baseline measurements and clinical labs.
Data Analysis
The primary objectives were to determine enrollment rates, dropout rates and reasons, adverse event rates, and success rates in completing study measurements. In addition, we conducted a preliminary comparison of maximum change in milk output between post-intervention (VolP) and baseline (VolB), based on the maximum of Day 14 and 28 test-weigh days. When only one follow-up test-weigh was available, it served as the post-intervention milk output value.
All symptoms and adverse events were reviewed and coded for relatedness to study drug or study procedure, expectedness, and severity. Data were entered into a secure database with range checks (Medidata Rave, New York, NY). All test-weigh data entries were verified for accuracy by both the project manager and the study monitor.
A statistician, masked to allocation, calculated breast milk output standardized to 24 hr as follows: For each breast, the test-weigh time interval was the end time of the first breast emptying session (either breastfeeding or breast pumping) up to the end time of the last breast emptying session on the test-weigh diary (thus, the interval started with the breast emptied, and ended after the breast was emptied approximately 24 hr later). Hourly milk output from each breast was calculated by summing weights for all emptying sessions during the interval and dividing by hours in the interval. The results were summed for both breasts and multiplied by 24 to derive the total 24-hr milk output. One gram of milk is equivalent to 1 mL of milk volume; thus, milk output is reported as mL/24 hr. Breast emptying frequency was also standardized to 24 hr using the same approach as described above.
We compared baseline variables, adverse events, and outcome variables in metformin versus placebo groups using Fisher’s exact test to detect differences in frequency distribution and the Wilcoxon rank-sum test to detect differences in relative ranking between allocation groups (i.e., intent-to-treat principal). Since this was a pilot study, two-sided p-values < .10 were considered statistically significant, and we also denote when two-sided p-values fell between 0.10–0.20. In secondary post hoc analysis, we compared peak change in milk output metformin-completers (to at least day 14) versus placebo + non-completers among RCT-enrolled participants. We also compared baseline milk output in those with signs of insulin resistance versus those without, among all baseline respondents assessed.
Results
Eight of the ten mothers in the metformin group, and all five of the mothers in the placebo group, took the study drug at least until Day 14 (80 and 100% drug-compliant completion, respectively). One mother in the metformin group stopped taking the study drug one day after randomization due to concerns raised by her infant’s pediatrician, but she continued with regular breast emptying and completed test-weighs at Day 14 and Day 28. Another mother in the metformin group stopped taking the study drug at Day 5 due to side effects (moderate diarrhea and severe abdominal cramping); she completed an early test-weigh at Day 5, and decided to stop lactating soon after, reporting that she was not seeing any improvement with the pumping intervention. Two other mothers (one metformin and one placebo) elected to stop study participation at Day 14 because they decided to stop lactating, reporting that the small amount of milk that they were producing was not worth the effort required, resulting in 70 and 80% drug-compliant completion to Day 28 in metformin and placebo groups, respectively.
One placebo participant had an invalid Day 14 test-weigh but went on to complete a valid Day 28 test-weigh. Another placebo participant completed an invalid Day 28 test-weigh but had a valid test-weigh on Day 14. Thus, 25 out of 27 attempted test-weighs (93%) were valid (Figure 1).
All 15 RCT-enrolled participants completed at least one valid post-intervention test-weigh enabling intent-to-treat analysis without losses to follow-up.
Tables 1A and 1B show RCT participant characteristics at baseline.
Table 1A.
Comparison of the Baseline Characteristics in Placebo Versus Metformin Groups (N = 15).
| Variables | Total Median [Q1–Q3] |
Placebo (n=5) Median [Q1–Q3] |
Metformin (n=10) Median [Q1–Q3] |
Z | p * |
|---|---|---|---|---|---|
| Maternal age, years | 33 [26–36] | 35 [32–40] | 33 [26–34] | 0.91 | .36 |
| Body Mass Index, kg/m2 | 39.6 [33.4–41.8] | 39.7 [38.5–44.2] | 37.5 [29.3–41.2] | 1.10 | .27 |
| Postpartum day started study drug | 36 [16–52] | 44 [36–52] | 20 [16–41] | 1.04 | .30 |
| Test weigh to drug start interval, days | 3 [3–5] | 4 [3–6] | 3 [3–4] | 0.75 | .45 |
| Breast emptying, total/24 hr (each side counted separately) | 17 [15–21] | 15 [15–19] | 18 [15–23] | −0.31 | .76 |
| Milk output, mL/24 hr | 159 [85–253] | 266 [192–281] | 148 [65–170] | 2.02 | .04 |
Note. Q1–Q3=interquartile range.
Wilcoxon Rank Sum Test two-sided p-value.
Table 1B.
Comparison of the Baseline Characteristics in Placebo Versus Metformin Groups: Categorical Measures (N = 15).
| Variables | Total n (%) |
Placebo (n=5) n (%) |
Metformin (n=10) n (%) |
Fisher’s Exact Test |
p |
|---|---|---|---|---|---|
| Primiparity | 8 (53) | 3 (60) | 5 (50) | 1.00 | .39 |
| Signs of Insulin Resistancea | |||||
| Gestational Diabetes | 5 (33) | 1 (20) | 4 (40) | .60 | .35 |
| Polycystic Ovary Syndrome | 4 (27) | 3 (60) | 1 (10) | .07 | .08 |
| Fasting glucose >=95 mg/dL | 4 (27) | 3 (60) | 1 (10) | .07 | .08 |
| Abdominal obesity | 12 (80) | 5 (100) | 7 (70) | .51 | .26 |
| Fenugreek use | 7 (47) | 1 (20) | 6 (60) | .28 | .16 |
Categories within signs of insulin resistance are not mutually exclusive.
Table 2 shows maximum change in milk output and related variables according to the study group over 14–28 days of follow-up. Maximum post-intervention milk output was based on Day 5 milk production for one study participant assigned metformin, Day 14 milk production for 11 study participants (seven assigned metformin and four assigned a placebo), and Day 28 milk production for three study participants (two assigned metformin and one assigned a placebo). Maximum milk output improved from baseline in 60% of the participants who were assigned metformin and in 20% of the placebo group (Fisher’s exact two-tailed p = .28). Baseline variables that were most strongly associated with improvement in milk output were (1) earlier study enrollment (Spearman correlation, r = −.44, p = .11) and (2) lower baseline milk output (r = .43, p = .11).
Table 2.
Maximum Change in Milk Output and Breast Emptying Frequency (maximum value over 2–4 weeks of follow-up minus baseline value), Placebo versus Metformin groups (N = 15).
| Variables | Placebo (n=5) Median [Q1–Q3] |
Metformin (n=10) Median [Q1–Q3] |
Z-statistic | p-value* |
|---|---|---|---|---|
| Maximum improvement in milk output over 2–4 weeks of follow-up (mL/24 hr) | −58 [−62 to −1] | + 8 [−23 to 33] | −1.04 | .31 |
| Change in breast emptying frequency (between baseline and follow-up time point of maximum milk output) | −3 [−6 to 0] | −2 [−5 to 0] | 0.06 | .93 |
Note. Q1–Q3=interquartile range.
Placebo versus Metformin, Wilcoxon Rank Sum Test two-sided p-value.
Overall, mean (± SD) change in milk output (Follow-up–Baseline) was −9 (±70) mL/24 hr (n = 15). Given the actual variance in change in milk output in study participants, we had 80% power to detect 98 mL/24 hr difference in improvement in milk output in metformin versus placebo groups at our preset pilot study p-value of .10. Our original goal was 90 mL difference in improvement between the two groups, and we had 73% power to detect this difference as significant.
Table 3 summarizes adverse events that occurred over the course of the study. There were no serious adverse events (defined as unexpected and severe) in mothers or their infants. Six of the nine mothers who took metformin for 5 days or more reported at least one gastrointestinal symptom (nausea, vomiting, diarrhea, or loose stools, 67% prevalence). In particular, the metformin group more frequently reported experiencing nausea/vomiting (44% versus 0% of placebo) but this was not statistically significant (p = .22). The frequency of symptoms reported in the infant were similar between groups.
Table 3.
Comparison of the Most Common Adverse Events Between Metformin (Glucophage XR) and Placebo Groups (N = 15).
| Adverse events | Metformin (n = 9) n (%) |
Placebo (n = 5) n (%) |
Fisher’s Exact Test | p |
|---|---|---|---|---|
| Maternal | ||||
| Diarrhea / loose stools | 5 (56) | 2 (40) | .37 | 1.00 |
| Nausea/Vomiting | 4 (44) | 0 (0) | .13 | .22 |
| Either of the above symptoms | 6 (67) | 2 (40) | .28 | .58 |
| Abdominal pain/ cramping / bloating | 4 (56) | 3 (60) | .37 | 1.00 |
| Headache | 3 (33) | 0 (0) | .23 | .26 |
| Other | 2 (22) | 4 (80) | .06 | .09 |
| Infant | ||||
| Gastric reflux | 3 (33) | 0 (0) | .23 | .26 |
| Gas | 2 (22) | 2 (40) | .36 | .58 |
| Constipation | 1 (11) | 1 (20) | .49 | 1.00 |
| Diarrhea / loose stools | 1 (11) | 2 (40) | .25 | .51 |
| Any of the above symptoms | 5 (56) | 4 (80) | .31 | .58 |
| Insufficient weight gain | 1 (11) | 0 (0) | .64 | 1.00 |
| Runny nose / cold symptoms | 2 (22) | 0 (0) | .40 | .51 |
| Irritability | 2 (22) | 0 (0) | .40 | .51 |
| Other | 1 (11) | 1 (20) | .49 | 1.00 |
Note. Metformin formulation used in this study was Glucophage XR; n=9 represents only those who took the study drug (the one participant assigned metformin did not take the study drug). Fisher’s Exact Test Table Probability and two-sided p-value.
Of the nine participants in the metformin group who took the study drug for 5 days or more, none reported perceiving that it had increased their milk supply to the degree they had hoped: Two reported a slight increase in their milk supply, five reported that it had remained the same, and two reported a decrease. None reported interest in taking metformin after the completion of the study.
Of the original 46 women who completed baseline screening measurements, median [interquartile range] milk output was significantly lower in the 31 who exhibited at least one of the study-defined signs of insulin resistance as compared to the 15 with none of these signs (192 [119–312] versus 394 [295–589] mL/24 hr, respectively, Wilcoxon p-value = .002).
Discussion
Our objectives in this pilot RCT were to determine the feasibility and acceptability of a definitive metformin versus placebo RCT for improving milk output. Our results align with existing data, in that the most common side effects of metformin are gastrointestinal in nature (Cicero, Tartagni & Ertek, 2012). In our study, most mothers with these symptoms reported the intensity as mild or moderate, but one mother did rate her vomiting and diarrhea as severe, and one of the mothers reported cramping severe enough to stop taking the study drug after 5 days. Two of five placebo participants reported diarrhea. This could be due to non-digestible cellulose being used as the placebo. If acceptability is gauged by willingness to tolerate medication side effects as a trade-off for improved milk production, it can be argued that none of the mothers assigned metformin found the study drug acceptable, as none expressed a desire to continue on metformin after the trial ended. While it is likely that a gentler titration of metformin may have been better tolerated, none of the nine women who actually consumed metformin perceived any improvement in their milk supply. This perception is unlikely to be more favorable with gentler titration, especially considering that for 80% of the metformin group, milk output peaked after 2 weeks of the intervention despite continuing with metformin and frequent breast emptying to week four post-intervention.
Median change in milk production was 68 mL greater in participants assigned metformin as compared to placebo participants, a non-statistically significant difference. In post hoc analysis, we examined the distribution of maximal change in milk output stratified by a placebo, by participants who had taken metformin to at least Day 14 (n = 8), and by participants who stopped taking metformin before Day 14 (n = 2, Figure 2). Peak milk production generally showed a small increase in participants who had completed the metformin course, while production in the placebo group and non-completers generally declined: Median change [interquartile range], +22 [−5 to +54] mL/24 hr versus −58 [−83 to −1] mL/24 hr, metformin versus placebo + non-completers, Wilcoxon rank-sum p-value = .07. These post hoc results lend support to the concept that an intervention aimed at improving insulin sensitivity could improve milk production. However, from a practical perspective, absolute milk output remained very low even in the participants who completed the metformin course, and only 20% of participants assigned metformin sustained improved milk output to Day 28.
Figure 2.
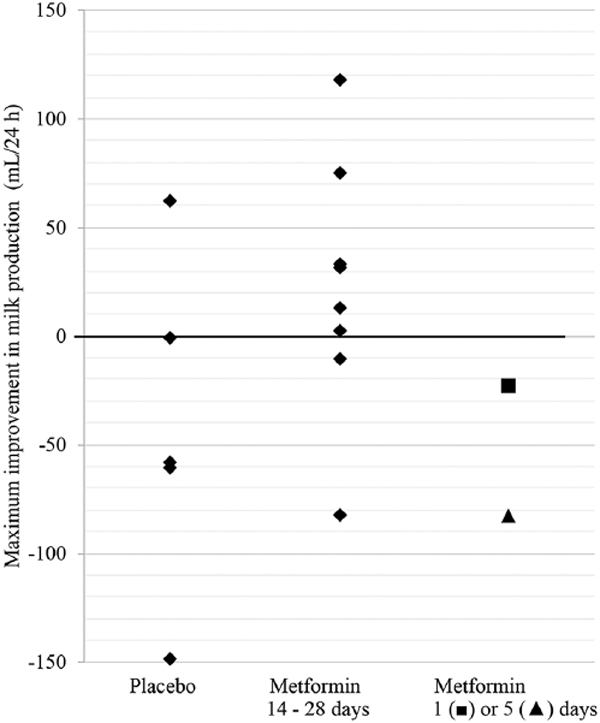
Maximum Improvement in Milk Production from Baseline.
We did not discern any baseline features that were more prevalent in the three metformin participants and one placebo participant who increased milk production >30%; one had marked breast asymmetry and PCOS, another was exclusively pumping, and two were diagnosed with gestational diabetes. Notably, these participants had some of the largest declines in breast emptying frequency. Conversely, one participant who had completed the metformin course experienced a decline of 83 mL/24 hr (253 to 170 mL/24 hr) despite sustaining 25 breast-emptying episodes/24 hr.
Limitations
As a pilot trial, our results revealed concerns regarding the feasibility of a definitive RCT. Many mothers preferred to delay enrollment until first seeing if milk supply improved through non-pharmacological interventions. Consequently, median enrollment was 3 weeks later than our target ideal enrollment of 1–2 weeks postpartum. Also, some otherwise eligible mothers ultimately declined RCT participation either because of hesitancy to take a medication while breastfeeding, or discouragement after learning their baseline milk output was extremely low, and this contributed to our inability to meet our original goal of 30 RCT participants.
This pilot RCT revealed at least three important directions for future research. First, we employed very general criteria for determining insulin resistant status and we cannot rule out unidentified non-metabolic causes of mothers’ low milk production. Insulin resistance is not a condition with definitive diagnostic criteria; rather, it is a spectrum of associated clinical features. Our central hypothesis was that improving insulin action would improve milk production in mothers with no apparent cause for their insufficient production but who exhibited signs of insulin resistance. Low milk supply is often multifactorial. No matter what other unknown contributors there may be, we hypothesized that among women with no apparent explanation for their insufficient milk output but who are exhibiting signs of insulin resistance, the latter is an additional factor dampening their milk production. Our finding of significantly lower milk output in the baseline-screened mothers with signs of insulin resistance as compared to those without signs of insulin resistance, support this line of reasoning. In subsequent analyses we will examine relationships between glucose intolerance, metabolic health features, and milk volume among all 46 baseline-enrolled participants to better identify the most at-risk metabolic phenotypes.
Second, study mothers had the triple burden of breastfeeding, breast pumping, and providing supplemental feeds to their newborns (i.e. “triple feeding”), and in this context test-weighing is very burdensome. More efficient methods for assessing milk production are needed for future interventions of this nature. Hartmann and Lai’s hourly milk production rate (Lai, Hale, Simmer & Hartmann, 2010) is one such possibility that we are now exploring.
Third, an intervention initiated during pregnancy and/or in the immediate postpartum might be more effective in optimizing milk production as compared to initiating treatment after low milk production is well-established. In humans, secretory differentiation occurs in the latter half of pregnancy, and secretory activation occurs in the first few days postpartum (Neville, McFadden & Forsyth, 2002). Rodent and human research shows insulin-sensitive gene expression to be strongly upregulated at both stages of lactogenesis (Berlato & Doppler, 2009; Lemay et al., 2013; Neville et al., 2013).
Given the physical and emotional burden of ‘triple feeding,’ it is imperative that future research aims to expediently identify mothers with low milk supply who are very unlikely to benefit from this commonly advised intervention. Unfortunately, no randomized trial to date has been successful in ‘curing’ low milk production. For example, the EMPOWER trial (mothers who delivered preterm and were randomly assigned to domperidone) reported that milk output in the intervention group was only 50 mL greater than placebo at follow up, and absolute milk production remained low in both groups (267±189 versus 217±168 mL/24 hr, p = .20, Asztalos et al., 2017), which is quite similar to our follow up results. In post hoc power analysis we determined that we did have 80% power to detect 98 mL/day difference in improvement in milk output in the metformin group as compared to the placebo group at our preset pilot study p-value of .10. We had more power than anticipated because our variance was less than estimated. From our observations in working with study participants, improvement of less than about 98 mL/day is unlikely to be considered worthwhile to mothers struggling with low milk supply. An improvement of 98 mL/day translates to a 6 mL increase in milk output from each breast at each breast expression, assuming an expression frequency of 8 times per day. Thus, even though we did not achieve our sample size goal, we did enroll enough mothers to detect a clinically important difference 73–80% of the time, if one existed.
Key Messages.
Mothers with insulin-resistant conditions such as gestational diabetes, obesity, and polycystic ovary syndrome are at increased risk for insufficient milk production.
Metformin is a first-line drug for improving insulin sensitivity but its efficacy in improving milk production is unknown.
Several results of this pilot trial, including challenges with enrollment, challenges with sustaining an intensive breast pumping intervention, and only 20% of metformin-assigned participants sustaining any improved milk output to Day 28 of the intervention, raise concerns about the feasibility of scaling up to a definitive randomized trial.
Despite the lack of encouraging trial results, we did observe a very strong negative association between signs of insulin resistance and baseline milk output, pointing to the need for further research toward more effective breastfeeding support for metabolically at-risk women.
Acknowledgments
We thank Scott Powers, PhD, ABPP, for superb RCT mentorship; we also thank Cincinnati Children’s Investigational Pharmacy, Schubert Research Clinic, and the Biostatistical Consulting Unit for contributions to the conduct of the study. Finally, most of all, we thank the study mothers for giving generously of their time and effort.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH 5 K12 HD051953 (PI, Tsevat), Bridging Interdisciplinary Research Careers in Women’s Health (BIRCWH award to LN-R); and NIH 5UL1TR001425-03 (PI, Heubi), Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Ameda, Inc., loaned the study hospital-grade breast pumps, which were returned upon study completion.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Academy of Breastfeeding Medicine. (2011). ABM clinical protocol #9: Use of galactogogues in initiating or augmenting the rate of maternal milk secretion (First Revision January 2011). Breastfeeding Medicine, 6(1), 41–49. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, … The International Association for the Study of Obesity. (2009). Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 120(16), 1640–1645. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. (2012). Standards of medical care in diabetes. Diabetes Care, 35(Suppl 1), S11–S63. doi: 10.2337/dc12-s011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztalos EV, Campbell-Yeo M, da Silva OP, Ito S, Kiss A, Knoppert D, … Empower Study Collaborative Group. (2017). Enhancing human milk production with domperidone in mothers of preterm infants. Journal of Human Lactation, 33(1), 181–187. [DOI] [PubMed] [Google Scholar]
- Baerug A, Sletner L, Laake P, Fretheim A, Løland BF, Waage CW, … Jenum AK (2018). Recent gestational diabetes was associated with mothers stopping predominant breastfeeding earlier in a multi-ethnic population. Acta Paediatrica, 107(6), 1028–1035. [DOI] [PubMed] [Google Scholar]
- Bailey CJ (2017). Metformin: Historical overview. Diabetologia. 60(9), 1566–1576. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, & Day C (2004). Metformin: Its botanical background. Practical Diabetes International, 21(3), 115–117. [Google Scholar]
- Baker JL, Michaelsen KF, Sorensen TI, & Rasmussen KM (2007). High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. American Journal of Clinical Nutrition, 86(2), 404–411. [DOI] [PubMed] [Google Scholar]
- Berlato C, & Doppler W (2009). Selective response to insulin versus insulin-like growth factor-I and -II and up-regulation of insulin receptor splice variant B in the differentiated mouse mammary epithelium. Endocrinology, 150(6), 2924–2933. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, & Kahn CR (2006). From mice to men: Insights into the insulin resistance syndromes. Annual Review of Physiology, 68, 123–158. [DOI] [PubMed] [Google Scholar]
- Bristol Myers Squibb. (2008). Glucophage, Glucophage XR. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020357s031,021202s016lbl.pdf
- Catalano PM, Kirwan JP, Haugel-de Mouzon S, & King J (2003). Gestational diabetes and insulin resistance: Role in short- and long-term implications for mother and fetus. Journal of Nutrition, 133(5), 1674S–1683S. [DOI] [PubMed] [Google Scholar]
- Chapman DJ, & Perez-Escamilla R (1999). Identification of risk factors for delayed onset of lactation. Journal of the American Dietetic Association, 99(4), 450–456. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Tartagni E, & Ertek S (2012). Metformin and its clinical use: New insights for an old drug in clinical practice. Archives of Medical Science, 8(5), 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey KG, Lovelady CA, Nommsen-Rivers LA, McCrory MA, & Lonnerdal B (1994). A randomized study of the effects of aerobic exercise by lactating women on breast-milk volume and composition. New England Journal of Medicine, 330(7), 449–453. [DOI] [PubMed] [Google Scholar]
- Domecq JP, Prutsky G, Mullan RJ, Sundaresh V, Wang AT, Erwin PJ, … Murad MH (2013). Adverse effects of the common treatments for polycystic ovary syndrome: A systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism, 98(12), 4646–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatford KL, Houda CM, Lu ZX, Coat S, Baghurst PA, Owens JA, … Hague WM (2013). Vitamin B12 and homocysteine status during pregnancy in the metformin in gestational diabetes trial: Responses to maternal metformin compared with insulin treatment. Diabetes Obesity and Metabolism, 15(7), 660–667. [DOI] [PubMed] [Google Scholar]
- Glover AV, Berry DC, Schwartz TA, & Stuebe AM (2018). The association of metabolic dysfunction with breast-feeding outcomes in gestational diabetes. American Journal of Perinatology. doi: 10.1055/s-0038-1626713 [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Salehi M, Sieve L, & Wang P (2006). Growth, motor, and social development in breast- and formula-fed infants of metformin-treated women with polycystic ovary syndrome. Journal of Pediatrics, 148(5), 628–632. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, … Diabetes Prevention Program Research Group. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine, 346(6), 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CT, Hale TW, Simmer K, & Hartmann PE (2010). Measuring milk synthesis in breastfeeding mothers. Breastfeeding Medicine, 5(3), 103–107. [DOI] [PubMed] [Google Scholar]
- Lemay DG, Ballard OA, Hughes MA, Morrow AL, Horseman ND, & Nommsen-Rivers LA (2013). RNA sequencing of the human milk fat layer transcriptome reveals distinct gene expression profiles at three stages of lactation. PLoS One, 8(7), e67531. doi: 10.1371/journal.pone.0067531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Jewell S, & Grummer-Strawn L (2003). Maternal obesity and breast-feeding practices. American Journal of Clinical Nutrition, 77(4), 931–936. [DOI] [PubMed] [Google Scholar]
- Menzies KK, Lefevre C, Macmillan KL, & Nicholas KR (2009). Insulin regulates milk protein synthesis at multiple levels in the bovine mammary gland. Functional and Integrative Genomics, 9(2), 197–217. [DOI] [PubMed] [Google Scholar]
- Mortel M, & Mehta SD (2013). Systematic review of the efficacy of herbal galactogogues. Journal of Human Lactation, 29(2), 154–162. [DOI] [PubMed] [Google Scholar]
- Morton J, Hall JY, Wong RJ, Thairu L, Benitz WE, & Rhine WD (2009). Combining hand techniques with electric pumping increases milk production in mothers of preterm infants. Journal of Perinatology, 29(11), 757–764. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. (2015). Drugs and lactation database. Retrieved from http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm
- Neville MC, McFadden TB, & Forsyth I (2002). Hormonal regulation of mammary differentiation and milk secretion. Journal of Mammary Gland Biology and Neoplasia, 7(1), 49–66. [DOI] [PubMed] [Google Scholar]
- Neville MC, Webb P, Ramanathan P, Mannino MP, Pecorini C, Monks J, … MacLean P (2013). The insulin receptor plays an important role in secretory differentiation in the mammary gland. American Journal of Physiology-Endocrinology and Metabolism, 305(9), E1103–E1114. doi: 10.1152/ajpendo.00337.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nommsen-Rivers LA (2016). Does insulin explain the relation between maternal obesity and poor lactation outcomes? An overview of the literature. Advances in Nutrition, 7(2), 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, & Dewey KG (2010). Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. American Journal of Clinical Nutrition, 92(3), 574–584. [DOI] [PubMed] [Google Scholar]
- O’Sullivan EJ, Perrine CG, & Rasmussen KM (2015). Early breastfeeding problems mediate the negative association between maternal obesity and exclusive breastfeeding at 1 and 2 months postpartum. Journal of Nutrition, 145(10), 2369–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perla V, & Jayanty SS (2013). Biguanide related compounds in traditional antidiabetic functional foods. Food Chemistry, 138(2–3), 1574–1580. [DOI] [PubMed] [Google Scholar]
- Tang T, Lord JM, Norman RJ, Yasmin E, & Balen AH (2012). Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database System Review, 5, CD003053. doi: 10.1002/14651858.CD003053.pub5 [DOI] [PubMed] [Google Scholar]
- Winkvist A, Brantsaeter AL, Brandhagen M, Haugen M, Meltzer HM, & Lissner L (2015). Maternal prepregnant body mass index and gestational weight gain are associated with initiation and duration of breastfeeding among Norwegian mothers. Journal of Nutrition, 145(6), 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Huang Z, He X, Wan X, Fang D, & Li Y (2013). Adverse effect of metformin therapy on serum vitamin B12 and folate: Short-term treatment causes disadvantages? Medical Hypotheses, 81(2), 149–151. [DOI] [PubMed] [Google Scholar]
- Yang X, Xu Z, Zhang C, Cai Z, & Zhang J (2017). Metformin, beyond an insulin sensitizer, targeting heart and pancreatic beta cells. Biochimica et Biophysica Acta, 1863(8), 1984–1990. [DOI] [PubMed] [Google Scholar]


