Objectives
This is a protocol for a Cochrane Review (intervention). The objectives are as follows:
To assess the effects (benefits and harms) of ventilation tubes (grommets) for otitis media with effusion in children.
Background
Description of the condition
Otitis media with effusion (OME) is a common condition in early childhood. The condition, also known as 'glue ear' and serous otitis media, is defined as "the presence of fluid in the middle ear without signs or symptoms of acute infection" (Rosenfeld 2016).
A key clinical feature of OME is hearing loss, due to decreased mobility of the tympanic membrane and consequent loss of sound conduction (Rosenfeld 2016). When hearing loss persists, this may affect speech and language development, and lead to behavioural problems in some children (NICE 2008). Other symptoms that may be attributable to OME include balance (vestibular) problems and ear discomfort (Rosenfeld 2016). When symptoms persist, they may lead to poor school performance and affect a child's daily activities, social interactions and emotions, possibly leading to a poorer quality of life for the child (Rosenfeld 2000).
It is thought that up to 80% of children have had OME by the age of four years but a decline in prevalence is observed for children beyond six years of age (Williamson 2011). Most episodes of OME in children resolve spontaneously within three months, however approximately 35% of children will have more than one episode of OME and, furthermore, 5% to 10% of episodes will last for more than a year (Rosenfeld 2016). Children with OME following an episode of untreated acute otitis media (AOM) have a 59% rate of resolution by one month, rising to 74% by three months, while children with newly diagnosed OME of unknown duration demonstrate a resolution rate of 28% by three months and up to 42% by six months (Rosenfeld 2003). The condition is more prevalent in children with Down syndrome or cleft palate (Flynn 2009; Maris 2014). Atopy has been considered a potential risk factor for OME in children (Kreiner‐Møller 2012; Marseglia 2008; Zernotti 2017).
Diagnosis of OME is typically by clinical examination including (pneumatic) otoscopy and/or tympanometry in primary care. Following diagnosis, there will often be a period of active observation, for at least three months. During the observation period the care provider may offer a non‐surgical intervention such as hearing aids or autoinflation. NICE and AAO‐HNS do not currently recommend the use of antibiotics, antihistamines, decongestants or corticosteroids for OME as there is insufficient evidence to suggest they are effective treatments (NICE 2008; Rosenfeld 2016). If OME has not resolved within the three‐month observation period, the child may be referred for further management/active intervention. This may include hearing aid provision or review by an ENT surgeon for consideration for myringotomy, ventilation tubes insertion and/or adenoidectomy. The choice of active intervention varies considerably. Earlier active intervention may be considered for children at increased risk of developmental difficulties (see Rosenfeld 2016 for a list of 'at‐risk' factors).
This Cochrane Review will focus on insertion of ventilation tubes as treatment for OME in children. This review forms part of a suite of five reviews of OME treatment that will address those interventions identified in a prioritisation exercise as being most important and in need of up‐to‐date Cochrane Reviews: namely, adenoidectomy, autoinflation, topical and oral steroids, and antibiotics (Cochrane ENT 2020).
Description of the intervention
NICE describes myringotomy and insertion of ventilation tubes (with or without adenoidectomy) as the most common surgical option for OME (NICE CKS 2021). Ventilation tubes (grommets) are tiny plastic tubes inserted in the tympanic membrane (under general anaesthetic in children). The procedure, undertaken by an ENT surgeon, involves making a small incision in the tympanic membrane (myringotomy), aspirating middle ear fluid as necessary and inserting the tube. The ventilation tube promotes middle ear ventilation and provides a passage for drainage of middle ear fluid. Generally, ventilation tubes eventually extrude into the external ear canal and the tympanic membrane closes (Venekamp 2018). In certain cases, early extrusion of the ventilation tubes occurs and they may need replacing. While aspiration is common practice, there is little evidence to suggest that it is of benefit prior to ventilation tube insertion (Laina 2006).
Myringotomy can be performed alone without insertion of ventilation tubes, however when undertaken using 'cold steel' incision with a blade it results in rapid healing without maintenance of benefit. When undertaken using a laser to create a circular perforation in the tympanic membrane, healing and closure of the myringotomy perforation may take longer with more persisting benefits akin to a ventilation tube.
The role of adenoidectomy in addition to ventilation tubes has been assessed in a separate Cochrane Review (van den Aardweg 2010); this evidence will be updated as part of the new suite of five Cochrane Reviews of OME treatments and thus will not be assessed in this review.
How the intervention might work
For children with OME who suffer from hearing loss, the insertion of ventilation tubes helps the middle ear fluid to drain, aerates the middle ear and balances the pressures on each side of the tympanic membrane (Vanneste 2019), allowing for normal mobility and conduction of sound and thus improving the child's ability to hear. The improvement in hearing is immediate in the majority of cases but occasionally complete resolution takes days to weeks. Ventilation tubes usually remain working within the tympanic membrane for 12 months on average (Rosenfeld 2016), and usually spontaneously extrude with healing of the tympanic membrane. Following this the child may remain free from OME, however in a proportion of children OME can return and persist, requiring repeat insertion. Factors that can limit the effectiveness of ventilation tubes include blockage of the tube (with blood), difficulty or inability to place the tubes due to narrow ear canals (Down syndrome and cleft palate) and early extrusion.
A common problem with ventilation tubes is ear discharge (otorrhoea) (Schilder 2016), and in around 2% of cases when the ventilation tube is extruded the tympanic membrane does not heal and a perforation persists. There is some evidence that insertion of ventilation tube may also result in long‐term damage to the tympanic membrane, such as tympanosclerosis or atrophy, and hearing loss (de Beer 2004; de Beer 2005).
Why it is important to do this review
A Cochrane Review assessing ventilation tubes for hearing loss associated with OME was published in 2010 (Browning 2010), updating an earlier review published in 2005. The 2010 review included 10 studies, three of which were randomised by ear (unilateral ventilation tube) and seven were randomised by child (bilateral ventilation tube or no ventilation tube). The authors concluded that the effect of ventilation tubes on hearing was small and diminished after six to nine months (by which time the hearing of children without ventilation tubes had improved due to natural resolution). The authors found few data on other outcomes, and identified a lack of trials conducted in children with established speech, language, learning or developmental problems. Since publication of the Cochrane Review in 2010 there have been two Health Technology Assessment (HTA) reports that include ventilation tubes (Berkman 2013; Steele 2017), and four other systematic reviews (Berkman 2013; Cheong 2012; Wallace 2014; Williamson 2011). Scoping searches for randomised controlled trials (RCTs) of ventilation tubes, which were last undertaken in January 2020, identified 12 abstracts of interest published since the last Cochrane Review. A prioritisation exercise undertaken in 2020 identified a review of ventilation tubes as a top priority (Cochrane ENT 2020). It is therefore timely to update the evidence.
Objectives
To assess the effects (benefits and harms) of ventilation tubes (grommets) for otitis media with effusion in children.
Methods
Criteria for considering studies for this review
Types of studies
We will include randomised controlled trials (RCTs) and quasi‐randomised trials (where studies were designed as RCTs, but the sequence generation for allocation of treatment used methods such as alternative allocation, birth dates and alphabetical order). We will include studies that randomised participants by ear, by participant or by cluster. Due to the self‐limiting nature of the condition, studies that use a cross‐over design are unlikely to be appropriate. However, if we do identify any such studies, we will use data from the first phase only.
Types of participants
The population of interest is children aged 6 months to 12 years with unilateral or bilateral otitis media with effusion. If a study includes children aged younger than 6 months and older than 12 years, we will only include the study if the majority of children fit our inclusion criteria or only if the trialists present outcome data by age group. We will include all children regardless of any comorbidity such as Down syndrome or cleft palate.
Clinical diagnosis of OME will be confirmed by oto(micro)scopy or tympanometry or both. We will include studies where children have had OME for at least three months. We will include children who have previously had ventilation tubes inserted.
Types of interventions
Intervention
Insertion of ventilation tube performed either unilaterally or bilaterally. We will not assess different types of ventilation tubes or surgical approaches to insertion.
Comparator
We are interested in the following six comparisons:
bilateral ventilation tubes versus no treatment/watchful waiting;
bilateral ventilation tubes versus hearing aids;
bilateral ventilation tubes versus non‐surgical treatment;
bilateral ventilation tubes versus myringotomy alone;
unilateral ventilation tubes versus no treatment/watchful waiting;
unilateral ventilation tubes versus myringotomy alone in the other ear/other children.
If study participants have received other treatments, for example, adenoidectomy, intranasal steroids, oral steroids, antibiotics, mucolytics or decongestants, we will include these studies if both arms received identical treatment.
Types of outcome measures
We will analyse the following outcomes in the review, but we will not use them as a basis for including or excluding studies. We will assess all outcomes in the very short term (< 6 weeks for postoperative adverse events), short term (</= 3 months), medium term (> 3 months to </= 1 year) and long term (> 1 year).
Primary outcomes
-
Hearing, measured as:
proportion of children whose hearing has returned to normal;
mean final hearing threshold (determined for the child or ear, depending on the unit of analysis);
change in hearing threshold from baseline (determined for the child or ear, depending on the unit of analysis).
It is anticipated that trial data for these outcomes may be derived from a variety of assessment methods and subject to a variety of definitions. To avoid loss of important evidence, we will extract all such data for analysis. However, we will give consideration to the appropriateness of pooling different types of data in meta‐analysis. Our selection of primary outcomes is based principally upon clinical importance, but also permits applicability across a variety of age‐appropriate assessment methods, and considers the types of outcome data that are most likely to be available. Accordingly, we regard the proportion of participants whose hearing has returned to normal as the most important measure of hearing impact. We consider medium‐ and long‐term outcome data as the most clinically important. Normality will be defined by the trialists.
-
Disease‐specific quality of life measured using a validated instrument, for example:
OM8‐30 (Haggard 2003);
Otitis Media‐6 (Rosenfeld 1997).
Adverse event ‐ persistent perforation.
Secondary outcomes
Presence/persistence of OME.
-
Adverse events ‐ measured by the number of participants affected.
-
Tympanic membrane changes, such as:
atrophy;
atelectasis or retraction;
myringosclerosis;
tympanosclerosis.
-
Tube‐related, such as:
blockage;
extrusion;
granulation tissue formation;
otorrhoea/perforation;
displacement of the ventilation tube into the middle ear space.
-
Patient‐related, such as:
vomiting;
diarrhoea;
dry throat;
nasal stinging;
cough;
long‐term hearing loss;
postsurgical haemorrhage;
pain.
-
-
Receptive language skills, measured using a validated scale, for example:
Peabody Picture Vocabulary Test ‐ Revised (Dunn 2007);
relevant domains of the Reynell Developmental Language Scales (Reynell 1985);
relevant domains of the Preschool Language Scale (PLS) (Zimmermann 1992);
relevant domains of the Sequenced Inventory of Communication (SCID) (Hedrick 1984).
-
Speech development, or expressive language skills, measured using a validated scale, for example:
Schlichting test (Schlichting 2010);
Lexi list (Schlichting 2007);
relevant domains of the Reynell Developmental Language Scales (Reynell 1985);
relevant domains of the PLS (Zimmermann 1992);
relevant domains of the SCID (Hedrick 1984).
-
Cognitive development, measured using a validated scale, for example:
Griffiths Mental Development Scales (Griffiths 1996);
McCarthy General Cognitive Index (McCarthy 1972);
Bayley Scales of Infant and Toddler Development (Bayley 2006).
-
Psychosocial outcomes, measured using a validated scale, for example:
the Social Skills Scale of the Social Skills Rating System (Gresham 1990);
Child Behavior Checklist (Achenbach 2011);
Strengths and Difficulties Questionnaire (Goodman 1997);
Pediatric Symptom Checklist (Jellinek 1988).
Listening skills, for example listening to stories and instructions effectively. Given that there are few validated scales to assess listening skills in children with OME, we will include any methods used by trialists.
-
Generic health‐related quality of life assessed using a validated instrument, for example:
EQ‐5D (Rabin 2001);
TNO AZL Children's QoL (TACQOL) (Verrips 1998);
TNO AZL Pre‐school children QoL (TAPQOL) (Fekkes 2000);
TNO AZL Infant Quality of Life (TAIQOL) (TNO 1997);
Infant Toddler Quality of Life Questionnaire (ITQOL) (Landgraf 1994);
Child Heath Questionnaire (CHQ) (Landgraf 1996).
-
Parental stress, measured using a validated scale, for example:
Parenting Stress Index (Abidin 1995).
-
Vestibular function:
balance;
co‐ordination.
Number of doctor‐diagnosed AOM episodes within a specified time frame.
These outcomes were identified as the most important in two studies that aimed to develop a core outcome set for children with OME (Bruce 2015; Liu 2020). As this review forms part of a suite of reviews of interventions for OME, not all outcomes will be relevant for all reviews.
Search methods for identification of studies
The Cochrane ENT Information Specialist will conduct systematic searches for randomised controlled trials and controlled clinical trials. There will be no language, publication year or publication status restrictions. We may contact original authors for clarification and further data if trial reports are unclear and we will arrange translations of papers where necessary.
Electronic searches
The Cochrane ENT Information Specialist will search the following databases from their inception to identify published, unpublished and ongoing RCTs:
the Cochrane ENT Register (search via the Cochrane Register of Studies to date);
the Cochrane Central Register of Controlled Trials (CENTRAL) (search via the Cochrane Register of Studies to date);
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to date);
Ovid EMBASE (1974 to date);
Web of Science, Web of Science (1945 to date);
-
ClinicalTrials.gov, www.clinicaltrials.gov:
search via the Cochrane Register of Studies to date;
search via www.clinicaltrials.gov to date;
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), https://trialsearch.who.int:
search via the Cochrane Register of Studies to date;
search via https://apps.who.int/trialsearch/ to date.
The subject strategies for databases will be modelled on the search strategy designed for CENTRAL, Ovid MEDLINE and Ovid Embase (Appendix 1). The search strategies were designed to identify all relevant studies for a suite of reviews on various interventions for otitis media with effusion. Where appropriate, these will be combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Technical Supplement to Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions version 6.1; Lefebvre 2020).
Searching other resources
We will scan the reference lists of identified publications for additional trials and contact trial authors if necessary. In addition, the Information Specialist will search Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we can scan their reference lists for additional trials. The Information Specialist will also run non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We will not perform a separate search for adverse effects. We will consider adverse effects described in the included studies only.
We will contact original authors for clarification and further data if trial reports are unclear and we will arrange translations of papers where necessary.
Data collection and analysis
Selection of studies
We will consider using Cochrane's Screen4Me workflow to help assess the search results, depending on the number of results retrieved from the database searches. Screen4Me comprises three components:
Known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as 'a RCT' or as 'not a RCT'.
The machine learning classifier (RCT model) (Wallace 2017), available in the Cochrane Register of Studies (CRS‐Web), which assigns a probability of being a true RCT (from 0 to 100) to each citation. For citations that are assigned a probability score below the cut‐point at a recall of 99% we will assume these to be non‐RCTs. For those that score on or above the cut‐point we will either manually dual screen these results or send them to Cochrane Crowd for screening.
Cochrane Crowd is Cochrane's citizen science platform where the Crowd help to identify and describe health evidence. For more information about Screen4Me and the evaluations that have been done, please go to the Screen4Me website on the Cochrane Information Specialist's portal and see Marshall 2018, McDonald 2017, Noel‐Storr 2018 and Thomas 2017.
At least two review authors will independently screen the remaining titles and abstracts to identify potentially relevant studies. At least two review authors will independently evaluate the full text of each potentially relevant study to determine whether it meets the inclusion/exclusion criteria for this review. Any differences will be resolved by discussion and consensus, with the involvement of a third author where necessary.
Screening eligible studies for trustworthiness
Two review authors will appraise all studies meeting our inclusion criteria for trustworthiness using a screening tool developed by Cochrane Pregnancy and Childbirth. This tool includes specified criteria to identify studies that are considered sufficiently trustworthy to be included in the review (see Appendix 2). If any studies are assessed as being potentially 'high‐risk', we will attempt to contact the study authors to obtain further information or address any concerns. If we are unable to contact the authors, or there is persisting uncertainty about the study, then it will not be included in the review. The study will remain in 'awaiting classification' with the reasons for concern, and communication with the authors will be described in full. The process is outlined in Figure 1. We will perform a sensitivity analysis to assess the effect on our findings of including/excluding studies considered at high risk of lack of trustworthiness.
1.
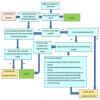
The Cochrane Pregnancy and Childbirth Trustworthiness Screening Tool
Data extraction and management
Two review authors will independently extract outcome data from each study using a standardised data collection form. Where a study has more than one publication, we will retrieve all publications to ensure complete extraction of data. Any discrepancies in the data extracted by the two authors will be checked against the original reports, and differences will be resolved through discussion and consensus, with recourse to a third author where necessary. If required, we will contact the study authors for clarification of any unclear or missing data. We will include key characteristics of the studies, such as the study design and in particular whether randomised by individual or by body part (see Unit of analysis issues), setting, sample size, population and the methods for defining or collecting outcome data in the studies.
We will extract data on study findings according to treatment assignment, irrespective of whether study participants complied with treatment or received treatment to which they were randomised.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we will extract the following summary statistics for each trial and outcome:
For continuous data: the mean values, standard deviation and number of patients for each treatment group at the different time points for outcome measurement. Where endpoint data are not available, we will extract the values for change‐from‐baseline data instead. If values for the individual treatment groups are not reported, where possible we will extract summary statistics (e.g. mean difference) from the studies.
For binary data: we will extract information on the number of participants experiencing an event, and the number of participants assessed at that time point. If values for the individual treatment groups are not reported, where possible we will extract summary statistics (e.g. risk ratio) from the studies.
For ordinal scale data: we do not anticipate identifying ordinal data which is of relevance for our outcomes. However, if this is identified and if the data appear to be normally distributed, or if the analysis performed by the investigators indicates that parametric tests are appropriate, then we will treat the outcome measure as continuous data. Alternatively, if data are available, we will convert these to binary data for analysis.
We have pre‐specified time points of interest for the outcomes in this review. Where studies report data at multiple time points, we will take the longest available follow‐up point within each of the specific time frames. For example, if a study reports an outcome at 4 months, 8 months and 12 months of follow‐up then the 12‐month data will be included for the time point > 3 months to </= 1 year. For adverse events, some studies may report frequency data for events and it may not be possible to determine whether these events occurred in one participant on one occasion or more than one occasion. In such circumstances we will report the data narratively.
Assessment of risk of bias in included studies
Two authors will undertake assessment of the risk of bias of the included studies independently, with the following taken into consideration, as guided by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other sources of bias.
We will use the Cochrane risk of bias tool in RevMan 5.4 (RevMan 2020), which involves describing each of these domains as reported in the study and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias.
Measures of treatment effect
We will summarise dichotomous data, such as presence of OME, as risk ratios (RR) and 95% confidence intervals (CI) and we will summarise continuous data as mean difference (MD) and 95% CI. For the outcomes to be presented in the summary of findings tables, we will provide both the relative and absolute measures of effect. Where the same outcome has been assessed using different scales we will present continuous data as standardised mean difference (SMD). If individual patient data (IPD) are available we will use these in our analyses.
Unit of analysis issues
For this review we may identify relevant studies that randomise either by participant or by ear. We will assess whether randomisation was conducted at the level of the participant or the ear, and for those studies that randomise by participant we will assess whether the study included one or two ears from each participant. Given that there are likely to be some carry‐over effects of disease and treatment from one ear to the other in a child, we will analyse the outcomes separately for randomisation by ear or by child. For studies that randomise by ear, we will only assess the outcomes of hearing, adverse events, presence of OME and number of AOM episodes. The remaining outcomes are only relevant for studies randomised by child where we can consider the more global effect of hearing difficulty.
Dealing with missing data
We will attempt to contact study authors by email where data on an outcome of interest to the review are not reported but the methods described in the paper suggest that the outcome was assessed. We will do the same if not all data required for meta‐analysis have been reported. If standard deviation data are not available, we will approximate these using the standard estimation methods from P values, standard errors or 95% CIs if these are reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Assessment of heterogeneity
We will assess clinical heterogeneity by examining the included studies for potential differences between them in the types of participants recruited, interventions or controls used, and the outcomes measured. We will assess statistical heterogeneity by considering both the I² statistic, which calculates the percentage of variability that is due to heterogeneity rather than chance with values over 50% suggesting substantial heterogeneity, and the P value from the Chi² test (Higgins 2021).
Assessment of reporting biases
We will assess reporting bias as within‐study outcome reporting bias and between‐study publication bias.
Outcome reporting bias (within‐study reporting bias)
We will assess within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol or trial registry, when this can be obtained. If the protocol or trial registry entry is not available, we will compare the outcomes reported to those listed in the methods section of the published report. If results are mentioned but not reported in a way that allows analysis (e.g. the report only mentions whether the results were statistically significant or not), we will seek further information from the study authors. If no further information can be found, we will note this as being a 'high' risk of bias. If there is insufficient information to judge the risk of bias we will note this as an 'unclear' risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
If we are able to pool 10 or more studies in a single analysis, we will produce a funnel plot to explore possible publication biases. We will test for asymmetry using Egger's test (Egger 1997).
Data synthesis
Where two or more studies report the same outcome we will perform a meta‐analysis using Review Manager 5 (RevMan 2020). We will report pooled effect measures for dichotomous outcomes as a risk ratio (RR) using the Mantel‐Haenszel methods. For continuous outcomes measured using the same scales we will report the mean difference (MD) and if studies have assessed the same outcomes using different scales we will report the standardised mean difference (SMD). We will use a random‐effects model.
Where it is not possible to pool the findings from studies in a meta‐analysis, we will present the results of each study and provide a narrative synthesis of findings. We will use the SWiM guidelines to guide us through this process (Campbell 2020). We will group the studies according to what seem to be appropriate groupings once we have identified included studies that do not provide data suitable for meta‐analysis. We will then identify the standardised metric for each outcome and calculate an intervention effect using the appropriate transformation.
Subgroup analysis and investigation of heterogeneity
We propose the following subgroup analyses if sufficient data are available in study reports:
children with mild hearing loss versus moderate or worse;
children with allergy versus those without (using the trialists' own definition);
children aged up to four years versus children aged four years and over;
children with previous ventilation tubes versus those without ventilation tubes;
children with cleft palate versus children without;
children with Down syndrome versus children without;
conventional cold steel versus other methods of myringotomy.
Unless studies report these subgroups, it will be necessary to carry out the subgroup analysis at the study level, i.e. group the studies according to the characteristics of the majority of their participants.
Sensitivity analysis
We will carry out sensitivity analyses to assess whether our findings are robust to decisions made regarding analyses and inclusions of studies. We will perform sensitivity analyses to assess the following:
impact of model chosen: we will compare the results using a random‐effects versus a fixed‐effect model;
inclusion of studies at high risk of risk of bias: we will compare the results including all studies versus excluding studies at overall high risk of bias, that is four or more of the seven domains of bias are rated as high risk (see Assessment of risk of bias in included studies);
inclusion of studies considered at high risk of trustworthiness, as assessed by the trustworthy tool (Figure 1).
Summary of findings and assessment of the certainty of the evidence
Two independent authors will use the GRADE approach to rate the overall certainty of evidence using GRADEpro GDT (https://gradepro.org/). The certainty of evidence reflects the extent to which we are confident that an estimate of effect is correct and we will apply this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high certainty of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low certainty implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision; and
publication bias.
We will include a summary of findings table, constructed according to the recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), for the following comparisons:
bilateral ventilation tubes versus no treatment/watchful waiting;
bilateral ventilation tubes versus hearing aids;
bilateral ventilation tubes versus non‐surgical treatment;
bilateral ventilation tubes versus myringotomy alone;
unilateral ventilation tubes versus no treatment/watchful waiting;
unilateral ventilation tubes versus myringotomy alone in the other ear/other children.
We will include the following four outcomes in the summary of findings table:
hearing;
disease‐specific quality of life;
presence/persistence of OME;
adverse event ‐ persistent perforation.
What's new
| Date | Event | Description |
|---|---|---|
| 8 April 2022 | Amended | Amendment published to ensure figures display correctly. |
History
Protocol first published: Issue 3, 2022
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
The authors are grateful to Anne Littlewood, Information Specialist with Cochrane Oral Health, for providing peer review comments on the draft search methods.
We are also grateful to Dr Richard Rosenfeld for providing editorial sign‐off of this protocol.
Appendices
Appendix 1. Draft search strategies
The search strategies were designed to identify all relevant studies for a suite of reviews on various interventions for otitis media with effusion.
| CENTRAL (CRS) | MEDLINE (Ovid) | Embase (Ovid) |
| 1 MESH DESCRIPTOR Otitis Media with Effusion EXPLODE ALL AND CENTRAL:TARGET 39
2 ("otitis media" adj6 effusion):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 730
3 (OME):TI,TO AND CENTRAL:TARGET 0
4 (Secretory otitis media):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 264
5 (Serous otitis media):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 49
6 (Middle‐ear effusion):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 238
7 (glue ear):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 62
8 (middle‐ear perfusion):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 1
9 MESH DESCRIPTOR Otitis Media AND CENTRAL:TARGET 784
10 (otitis media):TI,TO AND CENTRAL:TARGET 1653
11 #9 OR #10 AND CENTRAL:TARGET 1911
12 (((effusion or Recurrent or persistent or serous or secretory or perfusion) adj3 otitis)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 1010
13 #11 AND #12 AND CENTRAL:TARGET 766
14 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #13 AND CENTRAL:TARGET 1066
|
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to present 1 exp Otitis Media with Effusion/ 5807 2 ("otitis media" adj6 effusion).ab,ti. 3451 3 OME.ti. 469 4 Secretory otitis media.ab,ti. 953 5 Serous otitis media.ab,ti. 567 6 Middle‐ear effusion.ab,ti. 1444 7 Glue ear.ab,ti. 303 8 middle‐ear perfusion.ab,ti. 3 9 Otitis Media/ 17663 10 "otitis media".ti. 11554 11 9 or 10 21726 12 ((effusion or Recurrent or persistent or serous or secretory or perfusion) adj3 otitis).ab,ti. 6178 13 11 and 12 4299 14 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 13 8976 15 randomized controlled trial.pt. 542809 16 controlled clinical trial.pt. 94373 17 randomized.ab. 533045 18 placebo.ab. 221237 19 drug therapy.fs. 2370147 20 randomly.ab. 365421 21 trial.ab. 567106 22 groups.ab. 2243598 23 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 5110951 24 exp animals/ not humans.sh. 4882975 25 23 not 24 4445451 26 14 and 25 2367 |
Embase 1974 to present 1 exp secretory otitis media/ 5885 2 ("otitis media" adj6 effusion).ab,ti. 3999 3 OME.ti. 540 4 Secretory otitis media.ab,ti. 1051 5 Serous otitis media.ab,ti. 615 6 Middle‐ear effusion.ab,ti. 1627 7 glue ear.ab,ti. 351 8 middle‐ear perfusion.ab,ti. 3 9 otitis media/ 21684 10 otitis media.ti. 12337 11 9 or 10 27117 12 ((effusion or Recurrent or persistent or serous or secretory or perfusion) adj3 otitis).ab,ti. 7383 13 11 and 12 5219 14 1 or 2 or 4 or 5 or 6 or 7 or 8 or 13 9824 15 (random* or factorial* or placebo* or assign* or allocat* or crossover*).tw. 2194998 16 (control* adj group*).tw. 724788 17 (trial* and (control* or comparative)).tw. 701811 18 ((blind* or mask*) and (single or double or triple or treble)).tw. 288542 19 (treatment adj arm*).tw. 22835 20 (control* adj group*).tw. 724788 21 (phase adj (III or three)).tw. 67227 22 (versus or vs).tw. 2371156 23 rct.tw. 43341 24 crossover procedure/ 68008 25 double blind procedure/ 187232 26 single blind procedure/ 43636 27 randomization/ 91740 28 placebo/ 370427 29 exp clinical trial/ 1625769 30 parallel design/ 14463 31 Latin square design/ 394 32 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 5531900 33 exp ANIMAL/ or exp NONHUMAN/ or exp ANIMAL EXPERIMENT/ or exp ANIMAL MODEL/ 29428557 34 exp human/ 22679343 35 33 not 34 6749214 36 32 not 35 4834087 37 14 and 36 1820 |
Appendix 2. Tool for screening eligible studies for scientific integrity/trustworthiness
This screening tool has been developed by Cochrane Pregnancy and Childbirth. It includes a set of predefined criteria to select studies that, based on available information, are deemed to be sufficiently trustworthy to be included in the analysis.
| Criteria questions | Assessment | Comments and concerns | |
| High risk | Low risk | ||
| Research governance | |||
| Are there any retraction notices or expressions of concern listed on the Retraction Watch Database relating to this study? | Yes | No | |
| Was the study prospectively registered (for those studies published after 2010) If not, was there a plausible reason? | No | Yes | |
| When requested, did the trial authors provide/share the protocol and/or ethics approval letter? | No | Yes | |
| Did the trial authors engage in communication with the Cochrane Review authors within the agreed timelines? | No | Yes | |
| Did the trial authors provide IPD data upon request? If not, was there a plausible reason? | No | Yes | |
| Baseline characteristics | |||
| Is the study free from characteristics of the study participants that appear too similar? (e.g. distribution of the mean (SD) excessively narrow or excessively wide, as noted by Carlisle 2017) |
No | Yes | |
| Feasibility | |||
| Is the study free from characteristics that could be implausible? (e.g. large numbers of women with a rare condition (such as severe cholestasis in pregnancy) recruited within 12 months) | No | Yes | |
| In cases with (close to) zero losses to follow‐up, is there a plausible explanation? | No | Yes | |
| Results | |||
| Is the study free from results that could be implausible? (e.g. massive risk reduction for main outcomes with small sample size)? | No | Yes | |
| Do the numbers randomised to each group suggest that adequate randomisation methods were used (e.g. is the study free from issues such as unexpectedly even numbers of women ‘randomised’ including a mismatch between the numbers and the methods, if the authors say ‘no blocking was used’ but still end up with equal numbers, or if the authors say they used ‘blocks of 4’ but the final numbers differ by 6)? | No | Yes | |
| For abstracts only: | |||
| Have the study authors confirmed in writing that the data to be included in the review have come from the final analysis and will not change? | No | Yes | |
Contributions of authors
Samuel MacKeith: drafted the protocol. He will screen the search results and select relevant studies, extract data, carry out statistical analyses, draft the review and edit the review.
Caroline A Mulvaney: drafted the protocol. She will screen the search results and select relevant studies, extract data, carry out statistical analyses, draft the review and edit the review.
Kevin Galbraith: drafted the protocol. He will screen the search results and select relevant studies, extract data, carry out statistical analyses, draft the review and edit the review.
Tal Marom: reviewed the protocol. He will review the findings of the analyses.
Mat Daniel: reviewed the protocol. He will review the findings of the analyses.
Roderick P Venekamp: co‐wrote and edited the protocol. He will interpret the results, and co‐write and edit the review.
Maroeska M Rovers: reviewed the protocol. She will review the findings of the analyses.
Anne GM Schilder: co‐wrote and edited the protocol. She will interpret the results, and co‐write and edit the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research, UK
Infrastructure funding for Cochrane ENT
Declarations of interest
Caroline A Mulvaney: none known.
Kevin Galbraith: none known.
Samuel MacKeith: treats patients with OME in his NHS and private practice and is Assistant Co‐ordinating Editor of Cochrane ENT but has not been involved in the editorial process for this protocol.
Tal Marom: has no conflict of interests to declare.
Mat Daniel: has a financial interest in Aventamed, a company that produces a ventilation tube insertion device.
Roderick P Venekamp: is an Editor for the Cochrane Acute Respiratory Infections Group and Cochrane ENT, but had no role in the editorial process for this protocol.
Maroeska M Rovers: has no financial conflicts of interest. She has previously performed a randomised controlled trial and individual patient data meta‐analysis on the effect of ventilation tubes, and has acted as a member of the Dutch guideline committee on otitis media.
Anne GM Schilder: Professor Anne Schilder was joint Co‐ordinating Editor of Cochrane ENT until April 2020, but had no role in the editorial process for this review. Her evidENT team at the UCL Ear Institute is supported by the National Institute of Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC), with research projects being supported by the NIHR, Wellcome Trust, RNiD, ENT UK and industry. She is the National Specialty Lead for the NIHR Clinical Research Network ENT and Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Research Initiative. In her role as director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, she advises CRO, biotech and pharma companies in the hearing field on clinical trial design and delivery.
Edited (no change to conclusions)
References
Additional references
Abidin 1995
- Abidin RR.Parenting Stress Index: Professional Manual. 3rd edition. Odessa, FL: Psychological Assessment Resources, 1995. [Google Scholar]
Achenbach 2011
- Achenbach TM.Child Behavior Checklist. In: Kreutzer JS, DeLuca J, Caplan B, editors(s). Encyclopedia of Clinical Neuropsychology. New York, NY: Springer, 2011. [DOI: 10.1007/978-0-387-79948-3_1529] [DOI] [Google Scholar]
Bayley 2006
- Bayley N.Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio, TX: Harcourt Assessment, Inc, 2006. [Google Scholar]
Berkman 2013
- Berkman ND, Wallace IF, Steiner MJ, Harrison M, Greenblatt AM, Lohr KN, et al.Otitis Media with Effusion: Comparative Effectiveness of Treatments. Vol. Report No.: 13-EHC091-EF. Rockville (MD): Agency for Healthcare Research and Quality (US), 2013. [PMID: ] [PubMed] [Google Scholar]
Browning 2010
- Browning GG, Rovers MM, Williamson I, Lous J, Burton MJ.Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD001801. [DOI: 10.1002/14651858.CD001801.pub3] [DOI] [PubMed] [Google Scholar]
Bruce 2015
- Bruce I, Harman N, Williamson P, Tierney S, Callery P, Mohiuddin S, et al.The management of Otitis Media with Effusion in children with cleft palate (mOMEnt): a feasibility study and economic evaluation. Health Technology Assessment 2015;19(68):1-374. [DOI: 10.3310/hta19680] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S et al.Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline . BMJ 2020;368:16890. [DOI: 10.1136/bmj.l6890] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheong 2012
- Cheong KH, Hussain SS.Management of recurrent acute otitis media in children: systematic review of the effect of different interventions on otitis media recurrence, recurrence frequency and total recurrence time. Journal of Laryngology & Otology 2012;126(9):874-85. [DOI] [PubMed] [Google Scholar]
Cochrane ENT 2020
- Cochrane ENT.Otitis media with effusion: a project to prioritise Cochrane systematic reviews. https://ent.cochrane.org/otitis-media-effusion-ome-glue-ear 2020.
de Beer 2004
- Beer B, Schilder AGM, Zielhuis GA, Ingels K, Graamans K.Hearing loss in young adults who had ventilation tube insertion in childhood. Annals of Otology, Rhinology, and Laryngology 2004;113(6):438-44. [DOI: 10.1177/000348940411300604] [DOI] [PubMed] [Google Scholar]
de Beer 2005
- Beer BA, Schilder AGM, Zielhuis GA, Graamans K.Natural course of tympanic membrane pathology related to otitis media and ventilation tubes between ages 8 and 18 years. Otology & Neurotology 2005;26(5):1016-21. [DOI: 10.1097/01.mao.0000185058.89586.ed] [DOI] [PubMed] [Google Scholar]
Dunn 2007
- Dunn LM, Dunn DM.Peabody Picture Vocabulary Test (PPVT™-4). 4th edition. Pearson Education, 2007. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C.Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fekkes 2000
- Fekkes M, Theunissen NC, Brugman E, Veen S, Verrips EGH, Koopman HM, et al.Development and psychometric evaluation of the TAPQOL: a health-related quality of life instrument for 1–5-year-old children. Quality of Life Research 2000;9:961-72. [DOI: 10.1023/a:1008981603178] [DOI] [PubMed] [Google Scholar]
Flynn 2009
- Flynn T, Möller C, Jönsson R, Lohmander A .The high prevalence of otitis media with effusion in children with cleft lip and palate as compared to children without clefts. International Journal of Pediatric Otorhinolaryngology 2009;73:1441-6. [DOI: 10.1016/j.ijporl.2009.07.015] [DOI] [PubMed] [Google Scholar]
Goodman 1997
- Goodman R.The Strengths and Difficulties Questionnaire: a research note. Journal of Child Psychology and Psychiatry 1997;38:581-6. [DOI: 10.1111/j.1469-7610.1997.tb01545.x] [DOI] [PubMed] [Google Scholar]
Gresham 1990
- Gresham FM, Elliott SN.Social Skills Rating System. Circle Pines, MN: American Guidance Service, 1990. [Google Scholar]
Griffiths 1996
- Griffiths R.The Griffiths mental development scales from birth to two years, manual, the 1996 revision. Henley: Association for Research in Infant and Child Development, Test Agency, 1996. [Google Scholar]
Haggard 2003
- Haggard MP, Smith SC, Nicholls EE.Quality of life and child behaviour. In: Rosenfeld RM, Bluestone CD, editors(s). Evidence-based Otitis Media. 2nd edition. Hamilton, Ontario: BC Decker Inc, 2003:401-29. [https://researchonline.lshtm.ac.uk/id/eprint/15108] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hedrick 1984
- Hedrick DL, Prather EM, Tobin AR.Sequenced Inventory of Communication Development. Seattle, WA: University of Washington Press, 1984. [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Jellinek 1988
- Jellinek MS, Murphy JM, Robinson J, Feins A, Lamb S, Fenton T.Pediatric Symptom Checklist: screening school-age children for psychosocial dysfunction. Journal of Pediatrics 1988;112(2):201-9. [DOI: 10.1016/s0022-3476(88)80056-8] [DOI] [PubMed] [Google Scholar]
Kreiner‐Møller 2012
- Kreiner-Møller E, Chawes BL, Caye-Thomasen P, Bønnelykke K, Bisgaard H.Allergic rhinitis is associated with otitis media with effusion: a birth cohort study. Clinical and Experimental Allergy 2012;42(11):1615-20. [DOI: 10.1111/j.1365-2222.2012.04038.x] [DOI] [PubMed] [Google Scholar]
Laina 2006
- Laina V, Pothier DD.Should we aspirate middle‐ear effusions prior to insertion of ventilation tubes? Journal of Laryngology and Otology 2006;120:818-21. [DOI: 10.1017/S0022215106002118] [DOI] [PubMed] [Google Scholar]
Landgraf 1994
- Landgraf JM.The Infant/Toddler Child Health Questionnaire: conceptual framework, logic content, and preliminary psychometric results. Boston: Health Act, 1994. [Google Scholar]
Landgraf 1996
- Landgraf JL, Abetz L, Ware JE.The CHQ User’s Manual. Boston: The Health Institute, New England Medical Center, 1996. [Google Scholar]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Technical Supplement to Chapter 4: Searching for and selecting studies. Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Liu 2020
- Liu PZ, Ismail-Koch H, Stephenson K, Donne AJ, Fergie N, Derry J, et al.A core outcome set for research on the management of otitis media with effusion in otherwise-healthy children. International Journal of Pediatric Otorhinolaryngology 2020 ;134:Article 110029. [DOI: 10.1016/j.ijporl.2020.110029] [DOI] [PubMed]
Maris 2014
- Maris M, Wojciechowski M, Van de Heyning P, Boudewyns A.A cross-sectional analysis of otitis media with effusion in children with Down syndrome. European Journal of Pediatrics 2014;173:1319-25. [DOI: 10.1007/s00431-014-2323-5] [DOI] [PubMed] [Google Scholar]
Marseglia 2008
- Marseglia GL, Pagella F, Caimmi D, Caimmi S, Castellazzi AM, Poddighe D, et al.Increased risk of otitis media with effusion in allergic children presenting with adenoiditis. Otolaryngology – Head and Neck Surgery 2008;138(5):572-5. [DOI: 10.1016/j.otohns.2008.01.020] [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall J, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC.Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI: 10.1002/jrsm.1287] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 1972
- McCarthy D.Manual for the McCarthy Scales of Children's Abilities. New York: Psychological Corp, 1972. [Google Scholar]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J.Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 Sep 13-17; Cape Town, South Africa. 2017.
NICE 2008
- NICE.Otitis media with effusion in under 12s: Otitis media with effusion in under 12s: surgery (CG60). Available at: https://www.nice.org.uk/guidance/cg60 2008.
NICE CKS 2021
- NICE.Otitis media with effusion (Clinical Knowledge Summary). Available at: https://cks.nice.org.uk/topics/otitis-media-with-effusion/ 2021.
Noel‐Storr 2018
- Noel-Storr AH.Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 Jun 18-20; Oxford, UK. 2018.
Rabin 2001
- Rabin R, Charro F.EQ-5D: a measure of health status from the EuroQol Group. Annals of Medicine 2001;33(5):337-43. [DOI: 10.3109/07853890109002087] [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan).Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Reynell 1985
- Reynell JH.Reynell Development Language Scales Manual. 2nd edition. Windsor, UK: NFER-NELSON, 1985. [Google Scholar]
Rosenfeld 1997
- Rosenfeld RM, Goldsmith AJ, Tetlus L, Balzano A.Quality of life for children with otitis media. Archives of Otolaryngology - Head & Neck surgery 1997;123:1049-54. [DOI: 10.1001/archotol.1997.01900100019002] [DOI] [PubMed] [Google Scholar]
Rosenfeld 2000
- Rosenfeld RM, Bhaya MH, Bower CM, Brookhouser PE, Casselbrant ML, Chan KH, et al.Impact of tympanostomy tubes on child quality of life. Archives of Otolaryngology - Head & Neck Surgery 2000;126:585-92. [DOI] [PubMed] [Google Scholar]
Rosenfeld 2003
- Rosenfeld RM, Kay D.Natural history of untreated otitis media. Laryngoscope 2003;113:1645-57. [DOI: 10.1097/00005537-200310000-00004] [DOI] [PubMed] [Google Scholar]
Rosenfeld 2016
- Rosenfeld RM, Shin JJ, Schwartz SR, Coggins R, Gagnon L, Hackell JM, et al.Clinical practice guideline: otitis media with effusion (update). Otolaryngology - Head & Neck Surgery 2016;154:S1-S41. [DOI: 10.1177/0194599815623467] [DOI] [PubMed] [Google Scholar]
Schilder 2016
- Schilder AGM, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, et al.Otitis media. Nature Reviews Disease Primers 2016;2(1):16063. [DOI: 10.1038/nrdp.2016.63] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schlichting 2007
- Schlichting JEPT, Lutje Spelberg HC.Lexilijst Begrip: An Instrument to Investigate Language Comprehension in Children aged 15-25 Months in the Context of Early Identification. Amsterdam: Pearson Assessment & Information BV, 2007. [Google Scholar]
Schlichting 2010
- Schlichting JEPT, Lutje Spelberg HC.Schlichting Test for Language Comprehension; Instruction Manual. Woooden: Bohn Stafleu van Loghum, 2010. [Google Scholar]
Steele 2017
- Steele D, Adam GP, Di M, Halladay C, Pan I, Coppersmith N, et al.Tympanostomy tubes in children with otitis media. Comparative Effectiveness Review No. 185 (Prepared by the Brown Evidence-based Practice Center under Contract No. 290-2015-00002-I.). AHRQ Publication No. 17-EHC003-EF. Rockville, MD: Agency for Healthcare Research and Quality, 2017. [DOI: 10.23970/AHRQEPCCER185] [DOI] [PubMed] [Google Scholar]
Thomas 2017
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al, Living Systematic Review Network.Living systematic reviews 2: combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. [DOI: 10.1016/j.jclinepi.2017.08.011] [DOI] [PubMed] [Google Scholar]
TNO 1997
- TNO—Prevention and Health/LUMC.TAIQOL—Questionnaire for parents of children aged 1—5 years. Leiden, The Netherlands: Leiden University Medical Center, 1997. [Google Scholar]
van den Aardweg 2010
- den Aardweg MTA, Schilder AGM, Herkert E, Boonacker CWB, Rovers MM.Adenoidectomy for otitis media in children. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD007810. [DOI: 10.1002/14651858.CD007810.pub2] [DOI] [PubMed] [Google Scholar]
Vanneste 2019
- Vanneste P, Page C.Otitis media with effusion in children: pathophysiology, diagnosis, and treatment. A review. Journal of Otolaryngology - Head & Neck Surgery 2019;14(2):33-9. [DOI: 10.1016/j.joto.2019.01.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venekamp 2018
- Venekamp RP, Mick P, Schilder AGM, Nunez DA.Grommets (ventilation tubes) for recurrent acute otitis media in children. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD012017. [DOI: 10.1002/14651858.CD012017.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verrips 1998
- Verrips GH, Vogels AGC, Verloove-Vanhorick SP, Fekkes M, Koopman HM, Kamphuis RP, et al.Health-related quality of life measure for children - the TACQOL. Journal of Applied Therapeutics 1998;1(4):357-60. [Google Scholar]
Wallace 2014
- Wallace IF, Berkman ND, Lohr KN, Harrison MF, Kimple AJ, Steiner MJ.Surgical treatments for otitis media with effusion: a systematic review. Pediatrics 2014;133(2):296-311. [DOI: 10.1542/peds.2013-3228] [DOI] [PubMed] [Google Scholar]
Wallace 2017
- Wallace BC, et al.Identifying reports of randomized controlled trials (RCTs) via a hybrid machine learning and crowdsourcing approach. J Am Med Inform Assoc 2017;24(6):1165-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 2011
- Williamson I.Otitis media with effusion in children. BMJ Clinical Evidence 2011;2011:0502. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Zernotti 2017
- Zernotti ME, Pawankar R, Ansotegui I, Badellino H, Croce JS, Hossny E, et al.Otitis media with effusion and atopy: is there a causal relationship? World Allergy Organization Journal 2017;10(1):37. [DOI: 10.1186/s40413-017-0168-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmermann 1992
- Zimmerman IL, Steiner VG, Pond RE.Preschool Language Scale-3. San Antonio, TX: The Psychological Corporation, 1992. [Google Scholar]


